Abstract
Toxin-producing cyanobacteria are a worldwide threat to both human and animal health. Microcystins (MCs) are the most commonly occurring toxins produced by bloom-forming cyanobacteria, especially Microcystis sp. This study describes the occurrence of bloom-forming toxigenic Microcystis aeruginosa MBDU 626 from Manjalar Dam, Theni District, Tamil Nadu, South India. Two microcystin (MC) variants, MC-LR and [D-Asp3] MC-LR were identified from the isolated strain using high-performance liquid chromatography and gas chromatography coupled mass spectrometry (GC/MS). Four peptides such as aeruginosin, microginin, kasumigamide and anabaenopeptin were also co-produced along with these MC variants. Our results show that the presence of cyanobacterial toxins in essential water resources requires rapid remedial action and needs to develop a national program for regular monitoring of toxigenic blooms in freshwater bodies of South India, in general, Tamil Nadu, in particular.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
Introduction
Cyanobacteria (blue-green algae) are the prominent cause of water blooms in eutrophic lakes and drinking water reservoirs worldwide (Carmichael 1994; Sivonen 1996). Toxic bloom-forming cyanobacteria have been reported causing animal death and also adversely affecting human health (Carmichael 1994, 2001; Codd et al. 1997). Microcystins (MCs) are the most commonly encountered cyanotoxins (Sivonen 1996). Microcystis, Anabaena, Planktothrix, Nostoc, Hapalosiphon, Anabaenopsis, etc., are common MC-producing cyanobacterial genera (Carmichael 1992; Sivonen and Jones 1999). However, majority of the MCs-producing blooms are dominated by Microcystis (Codd 1999; Kabernick et al. 2000; Lehman et al. 2005; Li et al. 2007; Dai et al. 2008; Xu et al. 2008).
The general structure of MC is cyclo-(D-alanine-X-D-MeAsp-Z-Adda-D-glutamate-Mdha), where D-MeAsp is D-erythro-β-methyl-aspartic acid, and Mdha is N-methyldehydroalanine (Mdha). X and Z are variable L-amino acids (Sivonen and Jones 1999). To date, more than 90 structural variants of MCs have been reported (Zurawell et al. 2005; Wood et al. 2008). The most common MC congener (MC-LR) is characterized by the presence of leucin (L) and arginin (R) as L-amino acids in positions 2 and 4 (Xaa2 = L: Ala, Yaa4 = R: Arg) (Gulledge et al. 2002). Since the first elucidation of MC structure by Botes et al. (1984), extensive structural characterizations of other MC variants have been the subject for many studies (Sivonen et al. 1990; Namikoshi et al. 1992a, b; Luukkainen et al. 1994; Namikoshi et al. 1995; Sano and Kaya 1995, 1998; Beattie et al. 1998) and resulted in the identification of different structural variants of MCs to date. MC-LR is the most toxic and widely encountered MC variant for which World Health Organization (WHO) set a guideline value of 1 μg L−1 for drinking water (WHO 1998). Based on the review of all the toxicity data, the International Agency for Research on Cancer (IARC) classified MC-LR as a potential carcinogen (Group 2B) (Grosse et al. 2006).
Beside MCs, various other linear and cyclic oligopeptides such as aeruginosins, anabaenopeptilides, cyanopeptolins, anabaenopeptins and microginins are found within the genus Microcystis (Namikoshi and Rinehart 1996). As like MCs, the structures of these peptides generally include unusual amino acids residues, such as 3-(4-hydroxyphenyl)-lactic acid (Hpla) and 2-carboxy-6-hydroxy-octahydroindole (Choi) in aeruginosins, or 3-amino-6-hydroxy-2-piperidone (Ahp) in cyanopeptolins, β-amino-α-hydroxy-decanoic acid in the linear microginins (Neumann et al. 1997; Fukuta et al. 2004; Harada 2004; Welker et al. 2004a, b).
In fact, no consistent hypothesis has been developed so far to explain the high structural variability and patchy distribution of cyanopeptides (Welker et al. 2006). This is partly due to the still very limited knowledge on the occurrence of individual peptides and peptide classes in environmental samples (Fastner et al. 2001). These peptides have been found to exhibit a wide range of biochemical and pharmacological activities (Fastner et al. 2001; Bister et al. 2004; Welker et al. 2004a).
While there have been lengthy investigations regarding the occurrence of toxic cyanobacteria in many countries, there are only a few reports on their occurrence in India (Prakash et al. 2009). This might be due to the prevalence of less toxic variants like MC-RR, or in some cases, a lack of awareness and knowledge to correlate properly the toxicity with the prevailing cyanobacterial blooms (Sangolkar et al. 2009). Cyanobacterial blooms that produce MC-LR, MC-RR and its demethylated variant have been reported in India (Agrawal et al. 2006; Prakash et al. 2009), and adjacent tropical countries including Korea (Kim et al. 1999; Oh et al. 2001) and Thailand (Wang et al. 2002). In this study, we have reported the investigations into the occurrence of MC-producing Microcystis sp. in Theni District, Tamil Nadu, South India.
Materials and Methods
Bloom Sampling and Strain Culture Conditions
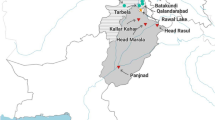
Cyanobacterial bloom sample was collected from Manjalar Dam (10°11′37.15″N 77°37′55.86″E), Theni District, Tamil Nadu, India (Fig. 1). The sample was identified as containing primarily of Microcystis aeruginosa. Generic assignment of the isolate was based on morphological criteria (Rippka et al. 1979). The bloom sample was initially grown in BG-11 medium with nitrate source. The culture was incubated under constant light intensity (50 μE m−2 S−1) for up to 10 days at 25 °C. No bacterial contamination was detected during microscopic observation of the culture.
Extraction and Analysis of Microcystins
Toxin was extracted as described previously (Frias et al. 2006). Briefly, late log phase culture (15 days old) of Microcystis aeruginosa MBDU 626 was centrifuged at 5,000 × g for 15 min at 4 °C (Remi, India), and the pellets were freeze dried and stored at −20 °C until further analysis. MC was extracted with MeOH/H2O (3:1, v/v) from frozen samples (~1 g) submitted to sonic disruption for 25 min. Extract was centrifuged (10,000 × g for 15 min) and the supernatant collected. The pellet obtained was re-extracted. The supernatant was combined and evaporated to dryness in a rotary evaporator (40 °C). The dried material was resuspended in MeOH and partitioned with CHCl3:MeOH:H2O (7:6:3, v/v/v) to remove hydrophobic compounds and pigments. The hydro-alcoholic phase was evaporated and dissolved in 1 ml of MeOH/H2O (7:3 v/v). The extract was filtered through 0.45 μm millipore membranes and injected into the HPLC system.
HPLC Analysis
A high-performance liquid chromatography equipped with a constant flow pump (Shimadzu LC 8A, Japan) was used. Separation was accomplished under reversed phase isocratic conditions with (Shim-Pack CLC-Octa decyl silane) ODS-C18 column (4.6 mm ID × 25 cm) and guard column (Shim-Pack G-ODS) (4 mm ID × 1 cm) and mobile phase of 100 % methanol. The flow rate was 1 ml/min for analysis, and UV absorbance at 254 nm was used as detector.
Acid Hydrolysis and Derivatization of the Toxin
The isolated compound was mixed with 6 N HCl (100/900 μl) and heated at 110 °C for 22 h. The reaction mixture was cooled to room temperature and evaporated in a stream of N2, then equal volume (200 μl) of dichloromethane (CH2Cl2) and trifluoroacetic anhydride (TFAA) was added, and again the mixture was heated at 110 °C for 5 min, then evaporated by N2 (Namikoshi et al. 1992b). The residue was dissolved in MeOH for GC/MS analysis.
GC/MS Analysis
GC/MS was performed with an Agilent gas chromatograph coupled to a JEOL GC/MS II MATE ion trap mass spectrometer. HP5 fused silica capillary column (30 m × 0.25 mm × 0.25 μm) was operated in a split less mode, and the injector temperature was 220 °C. The carrier gas (He) flow was adjusted to 1 ml min−1. Samples of 1 μl in MeOH were injected into the GC/MS. The program rate for the analysis of amino acid derivatives was 80–250 °C at 20 °C/min.
Molecular Analysis
Total genomic DNA was isolated from the tested cyanobacterial strain following the previously described method (Neilan 1995) and was used as a template in PCR; 16S rDNA was amplified from the genomic DNA using the cyanobacterial specific primers (Wilmotte et al. 1993; Nelissen et al. 1994). Purified PCR product was sequenced using the BigDye Terminator Cycle Sequencing v2.0 kit on an ABI 310 automatic DNA sequencer (Applied Biosystems, CA, USA). The 16S rDNA gene sequence determined in this study was deposited in the GenBank database under the accession number JN542384.
Results
Strain Characterization
The results of the present study revealed the occurrence of M. aeruginosa in Manjalar dam bloom samples. The isolated strain was characterized by both morphological and 16S rDNA sequence analysis. Figure 2 shows the typical morphology of M. aeruginosa strain isolated from the sampling site. 16S rDNA sequence analysis revealed that the isolated strain was having 95 % similarity to M. aeruginosa LMECYA 106 (EU078498) and M. aeruginosa UWOCC 019 (AF139295), confirming its identity.
GC/MS Analysis of Microcystin
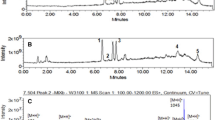
MCs were generally detected as singly protonated molecular ions. GC/MS analysis revealed the presence of two different MC isoforms. Both isoforms showed the characteristic fragment ion peak 135 [M + H+] (Tables 1 and 2), the Adda side-chain [PhCH2CH(OMe)+], which is a key indicator for the presence of MCs. Further investigation into the fragment ion peaks enabled the identification of the isoforms as MC-LR and [D-Asp3] MC-LR.
Product assignation of the fragment at m/z 994.5[M + H+] in GC/MS spectrum revealed the presence of MC-LR. The detected fragment ions at m/z 86 and 112 show the presence of immonium ions. These m/z values indicate the presence of Leu and Arg residues, and this result is also corroborated by the molecular mass of 994 Da. The ion fragment at m/z 553.4 corresponds to [Mdha-Ala-Leu-MeAsp-Arg + H]+ evidencing the presence of other amino acid residue characteristic of MCs, Mdha and also indicating the presence of the residues Leu and Arg at positions 2 and 4, respectively. Additionally, the following molecular ion species have provided full confirmation of MC-LR identity: [Glu-Mdha + H]+ at m/z 213.1, [M + H-Adda]+ at m/z 861.5, [Arg-Adda-Glu + H]+ at m/z 599.8, [M + H-Glu]+ at m/z 866.6 and [C11H14O-Glu-Mdha]+ at m/z 375.2. A complete list of the detected fragment ion peaks for MC-LR is shown in Table 1.
The second MC isoform identified in the strain tested was [D-Asp3] MC-LR at m/z 981.5[M + H+]. This MC isoform has a molecular weight of 981 Da. The detected fragment ion peak at m/z 539[Arg-Asp-Leu-Ala-Mdha + H]+ is characteristic for this demethylated MC-LR isoform (Table 2). Indeed, the fragmentation pattern of the m/z ion completely matched with that expected from [D-Asp3] MC-LR. Table 2 shows the product assignation of the fragment produced in GC/MS. The m/z ions at 213.2[Glu-Mdha + H]+, 155.2[Mdha-Ala + H]+ and 446.3[C11H14O-Glu-Mdha-Ala + H]+ indicated the presence of Mdha and Ala in position 7 and 1, respectively. On the other hand, the m/z ions at 289.1[(Arg + NH2)-Asp + 2H]+, 272.1[Asp-Arg + H]+, 553.3[Asp-Arg-(Adda-MeOH) + H]+ and 682.4[Asp-Arg-(Adda-MeOH)-Glu + H]+ strongly indicated the presence of Asp instead of MeAsp in position 4, proving the demethylated MC to be [D-Asp3] MC-LR. The mass spectrum of both MC-LR and [D-Asp3] MC-LR MC isoforms detected in this study shown in Figs. 3 and 4, respectively.
GC/MS spectrum of MC-LR detected from Microcystis aeruginosa MBDU 626. (See Table 1 for fragment ion identifications)
GC/MS spectrum of [D-Asp3] MC-LR detected from Microcystis aeruginosa MBDU 626. (See Table 2 for fragment ion identifications)
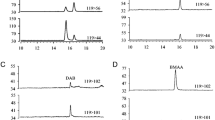
It is interesting to note that the tested M. aeruginosa MDBU 626 had shown five peptides identical to microginin, aeruginosin 602, aeruginosin 101, anabaenopeptin and kasumigamide at 698.3 m/z, 603.3 m/z, 645.6 m/z, 851.5 m/z and 788.6 m/z, respectively. Microginin are linear peptides with a characteristic N-terminal 3-amino-2 hydroxydecanoic acid (Ahda). The fragment ions at m/z 698.3 (Ahda-Thr-Pro-Tyr-Trp) from the side chain of Ahda were observed with the same ions in the mass spectrum (Fig. 6).
The other peptide aeruginosin is linear tetrapeptides with the unique moiety 2-carboxy-6-hydroxy-octahydroindole (Choi) and a C-terminal Arg derivative. Fragment spectra of two peptides characteristically show an intense mass signal detected at m/z 140 Da, the Choi-immonium ion which is indicative of aeruginosins. A peptide with [M + H]+ at m/z 645.36 could also be identified as an aeruginosin (aeruginosin 644) with a number of fragments identical to fragments of aeruginosin 602: m/z 86, 140, 250, 266 and 350 Da. The fragment ions at m/z 86.2 (Leu- or Ile-immonium ions), m/z 140.1 (Choi-immonium ions), m/z 250.1 (Hpla-Leu-Choi-Argal), m/z 266.2 (Choi-Arginal-CH3N2–H2O + H) and m/z 350.4 (Choi-Ac Argininal −NH2 +H) were observed together with the same ions in the mass spectrum (Fig. 5). The predominant fragment ions were observed in the MS spectra of the related aeruginosin, which are summarized in the Table 3.
GC/MS spectrum of aeruginosin peptide detected from Microcystis aeruginosa MBDU 626; peak at m/z 603 and m/z 642 corresponding to aeruginosin 602 and aeruginosin 101; peak at m/z 788 identified as kasumigamide. (See Table 3 for fragment ion identifications)
Kasumigamide, a linear tetrapeptide containing an N-terminal α-hydroxyl acid with m/z 787.38 (Pla-βAla-Ahipa-Arg-phSer) having the C-terminal moiety, that is, hydroxy-group of phenyl-serine was observed in the mass spectrum (Fig. 5).
Anabaenopeptins, a group of cyclic hexapeptides are characterized by a 19-membered peptide ring that is formed by cyclization between the C-terminal amino acid and the ε-amine of a lysine residue. The α-amine of the lysine is further linked through an ureido group to a side-chain amino acid. Two anabaenopeptin variants with similar mass have been identified in this study. Fragmentation spectrum by GC/MS was indicated that the peak at m/z 851[M + H+] corresponded to two isobaric anabaenopeptin variants, that is, anabaenopeptin B1 and F. The fragment ions at m/z 57 (MAla-Immonium ion) m/z 70 (Arg-/Lys-related ions), m/z 84 (Lys-Immonium ion), m/z 112 and 129 (Arg-Immonium ion), m/z 201 (CO + Arg) (side chain) m/z 233 (MAla + Phe + H), m/z 286 (Lys + CO + Arg-CN2H2), m/z 291(HTry + Ile + H), m/z 376 (MAla + HTyr + Ile + H), m/z 417 (HTyr + Ile + H), m/z 538 (Lys + Phe + MAla + HTyr + 2H) and m/z 651 (Lys + Phe + MAla + HTyr + Ile + 2H) were observed together with the same ions in the mass spectrum (Fig. 6 and Table 4).
GC/MS spectrum of anabaenopeptin peptide detected from Microcystis aeruginosa MBDU 626; peak at m/z 851 corresponding to a mixture of anabaenopeptin F and [HArg6]-anabaenopeptin B; peak at m/z 699 identified as microginin. (See Table 4 for fragment ion identifications)
Discussion
Occurrence of cyanobacterial blooms and associated animal and human poisoning has been documented from over sixty-five countries (Codd et al. 2005), including India (Agrawal et al. 2006), Sri Lanka (Jayatissa et al. 2006) and Bangladesh (Welker et al. 2005). The warm water temperature in India promotes dense Microcystis growth almost throughout the year (Parker et al. 1997; Agarwal et al. 2001). There have been few reports of MC occurrence in India (Sangolkar et al. 2009), and information about the evidence of bloom formation and toxicity in South Indian water bodies is particularly scarce. During the biodiversity survey of different freshwater ponds of Thanjavur District, Tamil Nadu, it is reported that potentially toxic cyanobacterial blooms are common in the freshwater ponds of Tamil Nadu region (Muthukumar et al. 2007). Out of the five ponds investigated, Dabeerkulam pond showed low diversity of cyanobacteria which was attributed to a massive bloom of Microcystis aeruginosa (Muthukumar et al. 2007). Similarly, this study indicates the presence of toxigenic M. aeruginosa MBDU 626 in the fresh water of Manjalar Dam in Periyakulam, Theni District. The freshwater bodies of South India in general, Tamil Nadu in particular, have so far been given less attention. This work was an extension of our earlier report on the presence of MC-LA-producing Microcystis aeruginosa MBDU 013 in Kuttappar Lake at Tiruchirappalli District, Tamil Nadu (communicated data).
GC/MS method has been developed for screening MCs, in complex samples such as sediments. It is based on the detection of 2-methyl-3-methoxy-4-phenylbutyricacid (MMPB), which is formed when the Adda residue is split following oxidation of MCs (Harada et al. 1996; Kaya and Sano 1999; Tsuji et al. 2001). Mass spectrometry (MS) has proved to be a valuable technique for providing structural information on MCs (Harada 1995; Kondo and Harada 1996; Meriluoto et al. 2000), without need for toxin standards or specific retention times that are required for HPLC analyses (Jungblut et al. 2006).
The [D-Asp3] MC-LR and MC-LR have been shown to form [M + H] + ion of m/z 981 and 995 (Diehnelt et al. 2005; Jungblut et al. 2006; Del Campo and Ouahid 2010). Similar fragment ions for [D-Asp3] MC-LR and MC-LR were reported from an Antarctic cyanobacterial mat community by Q-Star quadrupole-TOF hybrid mass spectrometer (Jungblut et al. 2006). The characteristic fragment ion for MC-LR has also been reported by Diehnelt et al. (2005). In Uttar Pradesh, India, five eutrophic temple ponds in the vicinity of Varanasi city were reported for MC-LR-producing Microcystis blooms (Prakash et al. 2009). In addition to Microcystis, MC-LR forming Nostoc sp. BHU001 was reported from the agricultural pond of Banaras Hindu University, Varanasi, India (Bajpai et al. 2009). Frias et al. (2006) have reported that the occurrence of MC-LR in a bloom in the eutrophic reservoir Billings, Sao Paulo City, Brazil, by ESI–MS/MS analysis. In a similar study, ten out of 12 MCs, including [D-Asp3] MC-LR and MC-LR, were detected from International Culture Collections strains of Microcystis (Del Campo and Ouahid 2010) and reported the fragment ions for [D-Asp3] MC-LR at m/z 155.2, 213.2, 289.1, 553.3 and 682.4. Similar fragment ions were obtained from our experiment (Figs. 4 and 5). [D-Asp3] MC-LR also has been detected in bloom samples from Morocco (Oudra et al. 2001) and the Philippines (Baldia et al. 2003). The characteristic Adda fragment for MCs was seen at 135.2 m/z (Figs. 3 and 4), possibly generated by the α-cleavage at the methoxy group of the Adda β-amino acid moiety (Ortea et al. 2004).
Five peptides were identified as to aeruginosin, microginin, anabaenopeptin and kasumigamide (Figs. 5 and 6), and these were also identified from the m/z of GC/MS analysis. The MS approach was successful in detecting a multitude of known and new peptides from very small samples of cyanobacterial cells. Detectability of individual peptides depends partly on the efficiency with which they can be protonated (Karas et al. 2000). Further information on the identity of oligopeptides was gained from the comparison with published fragmentation data from pure substances and from a fragment database (Haande et al. 2007).
The co-occurrence of MCs and cyanopeptolins in Microcystis spp. dominated field samples was reported previously (Jacobi et al. 1996; Neumann et al. 2000). Many of the substances detected belong to well-known groups of cyanobacterial peptides like MCs, anabaenopeptins, microginins, cyanopeptolins and aeruginosins, of which many have been discovered in Microcystis spp. (Namikoshi et al. 1996). With respect to known peptides, combinations of anabaenopeptins, microginins and aeruginosins were observed, while MCs were found along with aeruginosins. This correlates to the detection of aeruginosins as well as cyanopeptolins in both toxic and nontoxic Microcystis culture strains (Namikoshi et al. 1996; Dittmann et al. 1997).
A fragment ion m/z at 698.3[M + H]+, characteristics of microginin, was reported from bloom material of lake Tegamura, Japan (Kodani et al. 1999). Our experiments identified similar fragment ions from the tested organism. A peptide with a molecular mass of m/z 603[M + H]+ is probably a new variant of an aeruginosin-type peptide, as suggested by the fragment ion of m/z 140, indicating the presence of the unusual amino acid Choi, which is unique to aeruginosin-type molecules (Murakami et al. 1995; Matsuda et al. 1997; Erhard 1999). Ishida et al. (1999) have reported that aeruginosin 101 was originally isolated from Microcystis aeruginosa (NIES 101). Agarwal et al. (2006) have reported the presence of aeruginosin by MALDI-TOF/MS analysis, in the Microcystis blooms from Gosalpur Lake of Jabalpur in Central India. Kasumigamide, a novel antialgal peptide which shows a characteristic fragment m/z at 787.3[M + H]+ was originally isolated from freshwater cyanobacterium, Microcystis aeruginosa (NIES-87) (Ishida and Murakami 2000). Microcystis colonies isolated from lakes Müggelsee, Pehlitzsee and Parsteiner See in and around Berlin, Germany, were shown to possess mainly of kasumigamide linear peptide (Welker et al. 2004a).
The co-occurrence of both MCs and anabaenopeptins in natural populations has been well documented (Kodani et al. 1999; Fastner et al. 2001; Grach-Pogrebinsky et al. 2003). In the samples dominated by Microcystis spp., anabaenopeptins were found only when MCs also were present (Gkelis et al. 2005) and similar results have been reported from natural population samples studied (Kodani et al. 1999; Fastner et al. 2001; Grach-Pogrebinsky et al. 2003). However, it is still unclear whether cyanobacterial strains produce both types of peptides simultaneously or produce only MCs. Our results support the hypothesis of the coexistence of toxic MC with nontoxic peptides.
This study reinforces the earlier investigations into cyanobacterial blooms in Central India on the occurrence of toxigenic species in freshwater bodies of Indian ecosystem and states that major concern should be given for the screening program at least for those freshwater bodies used for animal or human consumption.
For a variety of reasons, the harmful impact of cyanobacteria on human health was always been a topic of interest (Falconer 1996, 1997). Concern about the MCs health risk to humans through drinking water, led the WHO to develop and suggest a provisional guideline level of MC-LR at 1 μg/L−1. Up to now, this value has been considered as a safe level in drinking water (Falconer et al. 1999). Further research and data analysis are needed to generate the information on MC occurrence, diversity and distribution with reference to climatic zones, namely temperate, tropical and subtropical regions (Sangolkar et al. 2009). This study clearly revealed that toxigenic Microcystis strains are present in the freshwater bodies of Southern Indian region and major attention should be given for the effective screening and mitigation strategies.
References
Agrawal MK, Bagchi D, Bagchi SN (2001) Acute inhibition of protease and suppression of growth in zooplankter, Moina macrocopa, by Microcystis blooms collected in Central India. Hydrobiologia 464:37–44
Agrawal MK, Ghosh SK, Bagchi D, Weckesser J, Erhard M, Bagchi SN (2006) Occurrence of microcystin-containing toxic water blooms in Central India. J Microbiol Biotechnol 16:212–218
Bajpai R, Sharma NK, Lawton LA, Edwards C, Rai AK (2009) Microcystin producing cyanobacterium Nostoc sp. BHU001 from a pond in India. Toxicon 53:587–590
Baldia SF, Conaco MCG, Nishijima T, Imanishi S, Harada KI (2003) Microcystin production during algal bloom occurrence in Laguna de Bay, the Philippines. Fish Sci 69:110–116
Beattie KA, Kaya K, Sano T, Codd GA (1998) Three dehydrobutyrine-containing microcystins from Nostoc. Phytochemistry 47:1289–1292
Bister B, Keller S, Baumann HI, Nicholson G, Weist S, Jung G, Sussmuth RD, Juttner F (2004) Cyanopeptolin 963A, a chymotrypsin inhibitor of Microcystis PCC 7806. J Nat Prod 67:1755–1757
Botes DP, Tuinman AA, Wessel PL, Viljoen CC, Kruger H, Williams DH, Santikarn S, Smith RJ, Hammond SJ (1984) The structure of cyanoginosin-LA, a cyclic hepatopeptide toxin from the cyanobacterium Microcystis aeruginosa. J Chem Soc Perkin Trans 1:2311–2318
Carmichael JV (1992) The male librarian and the feminine image: a survey of stereotype, status, and gender perceptions. Libr Inform Sci Res 14:411–447
Carmichael WW (1994) The toxins of cyanobacteria. Sci Amer 270:64–72
Carmichael WW, Azevedo SMFO, An JS (2001) Human fatalities from cyanobacteria: chemical and biological evidence for cyanotoxins (Caruaru syndrome). Environ Health Persp 109:663–668
Codd GA (1999) Cyanobacterial toxins: their occurrence in aquatic environments and significance to health. In: Charpy P, Larkum AWD (eds) Marine Cyanobacteria. Bulletin de l’Institut Oce′anographique. Monaco, pp 483–500
Codd GA, Ward CJ, Bell SG (1997) Cyanobacterial toxins: occurrence, modes of action, health effects and exposure routes. In: Seiler JP, Vilanova E (eds) Applied toxicology: approaches through basic science. In: Proceedings of the 1996 EUROTOX meeting, Spain. Archiv Toxicol Suppl 19, Berlin, Springer, pp 399–410
Codd GA, Azevedo SMFO, Bagchi SN, Burch MD, Carmichael WW, Harding WR, Kaya K, Utkilen HC (2005) CYANONET A global network for cyanobacterial bloom and toxin risk management: Initial situation assessment and recommendations. Int Hydrol Progr-VI Tech Doc Hydrol 76, UNESCO, Paris
Dai R, Liu H, Qu J, Ru J, Hou Y (2008) Cyanobacteria and their toxins in Guanting Reservoir of Beijing, China. J Hazard Mater 153:470–477
Del campo FF, Ouahid Y (2010) Identification of Microcystis from three collection strains of Microcystis aeruginosa. Environ poll 158:2906–2914
Diehnelt CW, Peterman SM, Budde WL (2005) Liquid chromatography-tandem mass spectrometry and accurate m/z measurements of cyclic peptide cyanobacteria toxins. TrAC Trends Anal Chem 24:622–634
Dittmann E, Neilan BA, Erhard M, von Dohren H, Borner T (1997) Insertional mutagenesis of a peptide synthetase gene that is responsible for hepatotoxin production in the cyanobacterium Microcystis aeruginosa PCC 7806. Mol Microbiol 26:779–787
Erhard M (1999) Ph.D. thesis. Technische Universitat Berlin, Germany
Falconer IR (1996) Potential impact on human health of toxic cyanobacteria. Phycologia 35:6–11
Falconer IR (1997) Blue-green algae in lakes and rivers: their harmful effects on human health. Austr Biologist 10:107–110
Falconer IR, Bartram J, Chorus I, Kuiper-Goodman T, Utkilen H, Burch M, Codd GA (1999) Safe levels and safe practice. In: Chorus I, Bartram J (eds) Toxic cyanobacteria in water, a guide of their public health consequences, monitoring and management. E&FN Spon WHO, London, pp 155–176
Fastner J, Erhard M, von Dohren H (2001) Determination of oligopeptide diversity within a natural population of Microcystis spp. (Cyanobacteria) by typing single colonies by matrix-assisted laser desorption ionization-time of flight mass spectrometry. Appl Environ Microbiol 67:5069–5076
Frias HV, Mendes MA, Cardozo KHM, Carvalho VM, Tomazela D, Colepicolo P, Pinto E (2006) Use of electrospray tandem mass spectrometry for identification of microcystins during a cyanobacterial bloom event. Biochem Biophys Res Commun 344:741–746
Fukuta Y, Ohshima T, Gnanadesikan V, Shibuguchi T, Nemoto T, Kisugi T, Okino T, Shibasaki M (2004) Enantioselective syntheses and biological studies of aeruginosin 298-A and its analogs: application of catalytic asymmetric phase-transfer reaction. Proc Natl Acad Sci 101:5433–5438
Gkelis S, Harjunpaa V, Lanaras T, Sivonen K (2005) Diversity of hepatotoxic microcystins and bioactive anabaenopeptins in cyanobacterial blooms from Greek freshwaters. Environ Toxicol 20:249–256
Grach-Pogrebinsky O, Sedmak B, Carmeli S (2003) Protease inhibitors from a Slovenian lake Bled toxic water bloom of the cyanobacterium Planktothrix rubescens. Tetrahed 59:8329–8336
Grosse Y, Baan R, Straif K, Secretan B, Ghissassi FE, Cogliano V (2006) Carcinogenicity of nitrate, nitrite, and cyanobacterial peptide toxins. Lancet Oncol 7:628–629
Gulledge BM, Aggen JB, Huang HB, Nairn AC, Chamberlin AR (2002) The microcystins and nodularins: cyclic polypeptide inhibitors of PP1 and PP2A. Curr Med Chem 9:1991–2003
Haande S, Ballot A, Rohrlack T, Fastner J, Wiedner C, Edvardsen B (2007) Diversity of Microcystis aeruginosa isolates (Chroococcales, Cyanobacteria) from East-African water bodies. Arch Microbiol 188:15–25
Harada KI (1995) Chemistry and detection of microcystins. In: Watanabe MF, Harada KI, Carmichael WW, Fujiki H (eds) Toxic microcystis. CRC Press, Boca Raton, pp 103–148
Harada KI (2004) Production of secondary metabolites by freshwater cyanobacteria. Chem Pharm Bull 52:889–899
Harada KI, Murata H, Qiang Z, Suzuki M, Kondo F (1996) Mass spectrometric screening method for microcystins in cyanobacteria. Toxicon 34:701–710
Ishida K, Murakami M (2000) Kasumigamide an antialgal peptide from the cyanobacterium Microcystis aeruginosa. J Org Chem 65:5898–5900
Ishida K, Okita Y, Matsuda H, Okino T, Murakami M (1999) Aeruginosins, protease inhibitors from the cyanobacterium Microcystis aeruginosa. Tetrahed 55:10971–10988
Jacobi C, Rinehart KL, Codd GA, Carmienke I, Weckesser J (1996) Occurrence of toxic water blooms containing microcystins in a German lake over a three year period. J Syst Appl Microbiol 19:249–254
Jayatissa LP, Silva EIL, McElhiney J, Lawton LA (2006) Occurrence of toxigenic cyanobacterial blooms in freshwaters of Sri Lanka. Syst Appl Microbiol 29:156–164
Jungblut AD, Hoeger SJ, Mountfort D, Hitzfeld BC, Dietrich DR, Neilan BA (2006) Characterization of microcystin production in Antarctic cyanobacterial mat community. Toxicon 47:271–278
Kaebernick M, Neilan BA, Borner T, Dittmann E (2000) Light and the transcriptional response of the microcystin biosynthesis gene cluster. Appl Environ Microbiol 66:3387–3392
Karas M, Gluckmann M, Schafer J (2000) Ionization in matrix-assisted laser desorption/ionization: singly charged molecular ions are the lucky survivors. J Mass Spectrom 35:1–12
Kaya K, Sano T (1999) Total microcystin determination using erythro-2-methyl-3-[methoxy-(3)]-4-phenylbutyric acid [MMPB-(3)] as the internal standard. Anal Chim Acta 386:107–112
Kim B, Kim HS, Park HD, Choi K, Park JG (1999) Microcystin content of cyanobacterial cells in Korean reservoirs and their toxicity. Korean J Limnol 32:288–294
Kodani S, Suzuki S, Ishida K, Murakami M (1999) Five new cyanobacterial peptides from water bloom materials of lake Teganuma (Japan). FEMS Microbiol Lett 178:343–348
Kondo F, Harada K (1996) Mass-spectrometric analysis of cyanobacterial toxins. J Mass Spectrom Soc Jpn 44:355–376
Lehman PW, Boyer G, Hall C, Waller S, Gehrts K (2005) Distribution and toxicity of a new colonial Microcystis aeruginosa bloom in the San Francisco Bay Estuary, California. Hydrobiologia 541:87–99
Li S, Xie P, Xu J, Zhang X, Qin J, Zheng L, Liang G (2007) Factors shaping the pattern of seasonal variations of microcystins in Lake Xingyun, a subtropical plateau lake in China. Bull Environ Contam Toxicol 78:226–230
Luukkainen R, Namikoshi M, Sivonen K, Rinehart KL, Niemela SI (1994) Isolation and identification of 12 microcystins from four strains and two bloom samples of Microcystis spp: structure of a new hepatotoxin. Toxicon 32:133–139
Matsuda H, Okino T, Murakami M, Yamaguchi K (1997) Aeruginosins 102-A and B, new thrombin inhibitors from the cyanobacterium Microcystis viridis (NIES-102). Tetrahed 52:14501–14506
Meriluoto J, Lawton L, Harada K (2000) Isolation and detection of microcystins and nodularins, cyanobacterial peptide hepatotoxins. Methods Mol Biol 145:65–87
Murakami M, Ishida K, Okino T, Okita Y, Matsuda H, Yamaguchi K (1995) Aeruginosin 98-A and-B, trypsin inhibitors from the blue-green alga Microcystis aeruginosa (NIES-98). Tetrahed Lett 36:2758–2788
Muthukumar C, Vijayakumar R, Muralitharan G, Panneerselvam A, Thajuddin N (2007) Cyanobacterial biodiversity from different freshwater ponds of Thanjavur, Tamil Nadu (India). Acta Bot Malacitana 32:17–25
Namikoshi M, Rinehart KL (1996) Bioactive compounds produced by cyanobacteria. J Ind Microbiol 17:373–384
Namikoshi M, Rinehart KL, Sakai R, Stotts RR, Dahlem AM, Beasley VR, Carmichael WW, Evans WR (1992a) Identification of 12 hepatotoxins from a homer lake bloom of the cyanobacteria Microcystis aeruginosa, Microcystis viridis, and Microcystis wesenbergii: nine new microcystins. J Org Chem 57:866–872
Namikoshi M, Sivonen K, Evans WR, Sun F, Carmichael WW, Rinehart KL (1992b) Isolation and structures of microcystins from a cyanobacterial water bloom (Finland). Toxicon 30:1473–1479
Namikoshi MF, Sun B, Choi BW, Rinehart KL, Carmichael WW, Evans WR, Beasley VR (1995) Seven more microcystins from Homer Lake cells: application of the general method for structure assignment of peptides containing α,β-dehydroamino acid unit(s). J Org Chem 60:3671–3679
Neilan BA (1995) Identification and phylogenetic analysis of toxigenic cyanobacteria by multiplex randomly amplified polymorphic DNA PCR. Appl Environ Microbiol 61:2286–2291
Nelissen B, Wilmotte A, Neefs JM, De Wachter R (1994) Phylogenetic relationships among filamentous helical bacteria investigated on the basis of 16S ribosomal RNA gene sequence analysis. System Appl Microbiol 17:206–210
Neumann U, Forchert A, Flury T, Weckesser J (1997) Microginin FR1, a linear peptide from a water bloom of Microcystis species. FEMS Microbiol Lett 153:475–478
Neumann U, Campos V, Cantarero S, Urrutia H, Heinze R, Weckesser J, Erhard M (2000) Co-occurrence of non-toxic (cyanopeptolin and toxic microcystin) peptides in a bloom of Microcystis sp from a Chilean lake. Syst Appl Microbiol 23:191–197
Oh HM, Lee SJ, Kim JH, Kim HS, Yoon BD (2001) Seasonal variation and indirect monitoring of microcystin concentrations in Daechung reservoir, Korea. Appl Environ Microbiol 67:1484–1489
Ortea PM, Allis O, Healy BM, Lehane M, Ni Shuilleabhain A, Furey A, James KJ (2004) Determination of toxic cyclic heptapeptides by liquid chromatography with detection using ultra-violet, protein phosphatase assay and tandem mass spectrometry. Chemosphere 55:1395–1402
Oudra B, Loudikia M, Sbiyyaa B, Martins R, Vasconcelos V, Namikoshi N (2001) Isolation, characterization and quantification of microcystins (heptapeptides hepatotoxins) in Microcystis aeruginosa dominated bloom of Lalla Takerkoust lake-reservoir (Morocco). Toxicon 39:1375–1381
Parker DL, Kumar HD, Rai LC, Singh JB (1997) Potassium salts inhibit growth of the cyanobacteria Microcystis spp. in pond water and defined media: implications for control of microcystin-producing aquatic blooms. Appl Environ Biol 63:2324–2329
Prakash S, Lawton LA, Edwards C (2009) Stability of toxigenic Microcystis blooms. Harmful Algae 8:376–384
Rippka R, Dereuelles J, Waterbury J, Herdman M, Stanier R (1979) Generic assignments, strain histories and properties of pure cultures of cyanobacteria. J Gen Microbiol 111:1–61
Sangolkar LN, Maske SS, Muthal PL, Kashyap SM, Chakrabarti T (2009) Isolation and characterization of microcystin producing Microcystis from a Central Indian water bloom. Harmful Algae 8:674–684
Sano T, Kaya K (1995) Oscillamide Y, a chymotrypsin inhibitor from toxic Oscillatoria agardhii. Tetrahed Lett 36:5933–5936
Sano T, Kaya K (1998) Two new (E)-2-Amino-2-Butenoic Acid (Dhb)-containing microcystins isolated from Oscillatoria agardhii. Tetrahed 54:463–470
Sivonen K (1996) Cyanobacterial toxins and toxin production. Phycologia 35:12–24
Sivonen K, Jones G (1999) Cyanobacterial toxins. In: Chorus I, Bartram J (eds) Toxic cyanobacteria in water: a guide to their public health consequences, monitoring and management. E&FN Spon, London, pp 41–111
Sivonen K, Niemela SI, Niemi RM, Lepisto L, Luoma TH, Rasinen LA (1990) Toxic cyanobacteria (bluegreen algae) in Finnish fresh and coastal waters. Hydrobiologia 190:267–275
Tsuji K, Masui H, Uemura H, Mori Y, Harada KI (2001) Analysis of microcystins in sediments using MMPB method. Toxicon 39:687–692
Wang X, Parkpian P, Fujimoto N, Ruchirawat KM, DeLaune RD, Jugsujinda A (2002) Environmental conditions associating microcystins production to Microcystis aeruginosa in a reservoir of Thailand. J Environ Sci Health Part A-Toxic/Hazard Subst Environ Eng 37:1181–1207
Welker M, Brunke M, Preussel K, Lippert I, von Dohren H (2004a) Diversity and distribution of Microcystis (Cyanobacteria) oligopeptide chemotypes from natural communities studied by single-colony mass spectrometry. Microbiology 150:1785–1796
Welker M, Christiansen G, von Dohren H (2004b) Diversity of co existing Planktothrix (Cyanobacteria) chemotypes deduced by mass spectral analysis of microcystins and other oligopeptides. Arch Microbiol 182:288–289
Welker M, Khan S, Haque MM, Islam S, Khan NH, Chorus I, Fastner J (2005) Microcystins (cyanobacterial toxins) in surface waters of rural Bangladesh: pilot study. J Water Health 3:325–337
Welker M, Marsalek B, Sejnohova L, von Dohren H (2006) Detection and identification of oligopeptides in Microcystis (Cyanobacteria) colonies: toward an understanding of metabolic diversity. Peptides 27:2090–2103
Wilmotte A, Van de Peer Y, Goris A, Chapelle S, De Baere R, Nelissen B, Neefs JM, Hennebert GL, De Wachter R (1993) Evolutionary relationships among higher fungi inferred form small ribosomal subunit RNA sequence analysis. System Appl Microbiol 16:436–444
Wood SA, Mountfort D, Selwood AI, Holland PT, Puddick J, Cary SC (2008) Widespread distribution and identification of eight novel microcystins in Antarctic cyanobacterial mats. Appl Environ Microbiol 74:7243–7251
World Health Organization (1998). Guidelines for drinking water quality. Addendum to Volume 2. Health Criteria and Other supporting information, 2nd edn. World Health Organization, Geneva, pp 95–110
Xu Y, Wu Z, Yu B, Peng X, Yu G, Wei Z, Wang G, Li R (2008) Non-microcystin producing Microcystis wesenbergii (Komárek) Komárek (Cyanobacteria) representing a main water bloom-forming species in Chinese waters. Environ Poll 156:162–167
Zurawell RW, Chen H, Burke JM, Prepas EE (2005) Hepatotoxic cyanobacteria: a review of the biological importance of microcystins in freshwater environments. J Toxicol Environ Health 8:1–37
Acknowledgments
The authors are grateful to the University Grants Commission (UGC), Government of India, for the financial support. AMP Anahas acknowledges the Maulana Azad National Fellowship Scheme (MANF) for the fellowship.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2013 Springer India
About this chapter
Cite this chapter
Anahas, A.M.P., Gayathri, M., Muralitharan, G. (2013). Isolation and Characterization of Microcystin-Producing Microcystis aeruginosa MBDU 626 from a Freshwater Bloom Sample in Tamil Nadu, South India. In: Velu, R. (eds) Microbiological Research In Agroecosystem Management. Springer, India. https://doi.org/10.1007/978-81-322-1087-0_16
Download citation
DOI: https://doi.org/10.1007/978-81-322-1087-0_16
Published:
Publisher Name: Springer, India
Print ISBN: 978-81-322-1086-3
Online ISBN: 978-81-322-1087-0
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)