Abstract
Cyanobacteria are known to produce diverse secondary metabolites that are toxic to aquatic ecosystems and human health. However, data about the cyanotoxins occurrence and cyanobacterial diversity in Pakistan’s drinking water reservoirs is scarce. In this study, we first investigated the presence of microcystin, saxitoxin, and anatoxin in 12 water bodies using an enzyme-linked immunosorbent assay (ELISA). The observed cyanotoxin values for the risk quotient (RQ) determined by ELISA indicated a potential risk for aquatic life and human health. Based on this result, we made a more in-depth investigation with a subset of water bodies (served as major public water sources) to analyze the cyanotoxins dynamics and identify potential producers. We therefore quantified the distribution of 17 cyanotoxins, including 12 microcystin congeners using a high-performance liquid chromatography-high-resolution tandem mass spectrometry/mass spectrometry (HPLC-HRMS/MS). Our results revealed for the first time the co-occurrence of multiple cyanotoxins and the presence of cylindrospermopsin in an artificial reservoir (Rawal Lake) and a semi-saline lake (Kallar Kahar). We also quantified several microcystin congeners in a river (Panjnad) with MC-LR and MC-RR being the most prevalent and abundant. To identify potential cyanotoxin producers, the composition of the cyanobacterial community was characterized by shotgun metagenomics sequencing. Despite the noticeable presence of cyanotoxins, Cyanobacteria were not abundant. Synechococcus was the most abundant cyanobacterial genus found followed by a small amount of Anabaena, Cyanobium, Microcystis, and Dolichospermum. Moreover, when we looked at the cyanotoxins genes coverage, we never found a complete microcystin mcy operon. To our knowledge, this is the first snapshot sampling of water bodies in Pakistan. Our results would not only help to understand the geographical spread of cyanotoxin in Pakistan but would also help to improve cyanotoxin risk assessment strategies by screening a variety of cyanobacterial toxins and confirming that cyanotoxin quantification is not necessarily related to producer abundance.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cyanobacteria are gram-negative photosynthetic prokaryotes that can be found in diverse environments (Filatova et al. 2020) occupying both aquatic and terrestrial biotopes and are well-known as blue-green algae (Briand et al. 2003). Cyanobacteria are well-known to produce harmful blooms that negatively impact lake ecosystems (Buratti et al. 2017; Huisman et al. 2018) through generating various toxic secondary metabolites, e.g., cyanotoxins, potentially generating hypoxic environments upon their decay (Codd et al. 1999; Wicks & Thiel 1990) and intensifying decline in water quality via internal loading (Szlag et al. 2015).
Cyanotoxins are toxicologically and chemically diverse compounds (Cheung et al. 2013). There are different approaches to classifying them, depending on their chemical structure and mode of action (alkaloids, cyclic peptides, and lipopolysaccharides) or by the cyanobacterial species that produce them (Diez-Quijada et al. 2021). Cyanotoxins are therefore categorized as hepatotoxins (e.g., microcystins, nodularin), neurotoxins (anatoxin-a, anatoxin-a(s), and saxitoxin), cytotoxin (cylindrospermopsin), and dermatotoxins or skin irritants (e.g., lyngbiatoxin, lipopolysaccharides, and aplysiatoxin) (Falconer & Humpage 2005). Microcystins are hepatotoxic and one of the most studied cyanotoxins. So far, more than 300 congeners of microcystin have been described (Bouaïcha et al. 2019; Mehinto et al. 2021) where microcystin LR (MC-LR) is the most common and toxic variant, followed by MC-YR and MC-RR (Díez-Quijada et al. 2019). The World Health Organization (WHO), alongside various countries and authorities, have established microcystin regulatory concentrations (1 μg/L) for drinking water (Arman & Clarke 2021, Kasan et al. 2021) and advisory concentrations (recreational thresholds of 2–20 μg/L) for recreational exposure. Cyanobacterial neurotoxins include anatoxins, saxitoxins, beta-N-methylamino-L-alanine (BMAA), and ciguatoxins (Aráoz et al. 2010; Buratti et al. 2017). Regardless of the harmful effects, most of these cyanotoxins are less studied in freshwater environments (Cerasino & Salmaso 2020). Neurotoxin threshold values have been adopted in national legislation in New Zealand and Australia regulating anatoxin and saxitoxin at 3.3 μg/L (Filatova et al. 2021). Cylindrospermopsin (CYN) is another highly relevant toxin that can cause neurotoxicity and dermatoxicity (Skafi et al. 2021) and has a recommended threshold in drinking water at 1 µg/L (Thawabteh et al. 2023). The stability of these different cyanotoxins in the environment might vary depending on biotic factors presence (e.g., biodegraders) and environmental conditions (e.g., light, pH, or temperature). Microcystins are for example known to be relatively stable, even outside of the cell, and could persist in water weeks after the end of a bloom (Pierce &Schnetzer 2023, Zastepa et al. 2014). These cyanotoxins could be released by a wide range of planktonic and benthic cyanobacterial species (Chorus & Welker 2021). The most common producers include Microcystis, Anabaena, Planktothrix (Ferrão-Filho & Kozlowsky-Suzuki 2011), Cylindrospermopsis, Aphanizomenon, and Dolichospermum (Farrer et al. 2015) as well as some picocyanobacteria (e.g., Synechococcus and Aphanocapsa) (Bernard et al. 2017, Chorus &Welker 2021).
Water pollution is a major environmental concern (Fahad & Wang 2020) in developing countries like Pakistan, Bangladesh, and India (Ahmed & Shafique 2019). In Pakistan, numerous resources could contaminate drinking water. Among them, pathogen contamination is the most serious issue affecting both urban and rural areas of the country (Abbasi & Abbasi 2012). The majority of freshwater lakes are needed for recreation (Pandhal et al. 2018), swimming, livestock drinking, washing, and irrigation, all of which increase the risk of direct exposure to pathogens (Van Apeldoorn et al. 2007). To date, risk management strategies for emerging cyanotoxins (Huang et al. 2007) have been ineffective due to a lack of experimental data (Ibelings et al. 2014; Welker et al. 2005). Few studies have reported the presence of cyanotoxins and their potential producer in Pakistan. In a small-scale study carried out in Sindh Keenjhar and Manchar Lake, the authors reported toxic blooms and identified (by microscopy) 14 toxic phytoplanktonic Cyanobacteria species belonging to order Chroococcales. Toxic blooms and Cyanobacteria were observed along the coasts of Karachi (Mahar et al. 2000) and in Sheikhpura water storage tanks and canals, where microcystins were also detected (Badar et al. 2015). All these studies primarily used ELISA (enzyme-linked immunosorbent assay) kits to identify and measure cyanotoxin concentrations.
In this study, we performed two fields of sampling. Samples were initially collected in 2020 from multiple locations in Punjab and Khyber Pakhtunkhwa (KPK) (Fig. 1) These reservoirs, such as Rawal Lake (Islamabad), Kallar Kahar Lake (Chakwal), Head Rasul barrage (Mandi Bahauddin), Head Marala headwork (Sialkot), Panjnad (Uch Sharif), Warsak Dam (Peshawar), and Tarbela Dam (Haripur) were chosen because they serve as major public water sources and are also used for irrigation or recreation (e.g., northern area). The freshwater quality of reservoirs has also been affected by activities such as agriculture, sewage discharge, and the deposition of corrosive materials that impact ecosystems (Jin et al. 2018; Oberholster et al. 2004). The sampling was performed during different seasons (summer and winter) to gain insight into water quality, variability in cyanotoxin concentrations, and their potential risks in water bodies. Collected water samples were analyzed by ELISA to estimate the presence of total microcystin, saxitoxin, and anatoxin. We found that microcystin and anatoxin were prevalent in most sampling sites, including northern areas.
Based on the findings, several locations serving as major sources of public water were then chosen for toxin quantification with better resolution to account for the diversity of toxic secondary metabolites, particularly microcystin congeners. Hence, a second field sampling was conducted in 2021 at Rawal Lake (Islamabad), Kallar Kahar Lake (Chakwal), Head Rasul barrage (Mandi Bahauddin), Head Marala headwork (Sialkot), Panjnad (Uch Sharif), Warsak Dam (Peshawar), and Tarbela Dam. A total of 17 cyanotoxins including 12 microcystin congener profiles ((cylindrospermopsin (CYN), anatoxin-a (ATX-a), microcystin-RR (MC-RR), desmethylated microcystin-RR (dmMC-RR), microcystin-YR (MC-YR), microcystin-HtyR (MC-HtyR), microcystin-LR (MC-LR), desmethylated microcystin-LR (dmMC-LR), microcystin-HilR (MC-HilR), microcystin-WR (MC-WR), microcystin-LA (MC-LA), microcystin-LY (MC-LY), microcystin-LF (MC-LF), microcystin-LW (MC-LW), homoanatoxin-a (HATX-a), anabaenopeptin A (Apt-A), and anabaenopeptin B (Apt-B)) were quantified by high-performance liquid chromatography-high-resolution tandem mass spectrometry/mass spectrometry (HPLC-HRMS/MS). Cyanotoxins were recorded in both summer and winter, with higher concentrations observed in summer. HPLC-HRMS/MS results revealed for the first time the presence of several microcystin congeners and cylindrospermopsin in Pakistani water bodies.
To identify potential producers of these cyanotoxins, we used a shotgun metagenomics approach to characterize the cyanobacterial populations. Despite the presence of cyanotoxins, Cyanobacteria were found at very low abundance, Synechococcus being the most abundant cyanobacterial genus. Moreover, when we looked at genes level resolution, most of the toxic genes were absent.
The findings of this investigation will improve our understanding of the presence of cyanobacterial bloom-impacted areas in Pakistan and provide further evidence that the cyanotoxins quantification does not always correlate with producers’ high abundance. To our knowledge, this is the first study to provide insight into the prevalence of cyanotoxins in multiple freshwater bodies of Pakistan with such analytical resolution.
Methods
Study sites and sample collection
Cyanobacteria usually grow in large numbers during the summers (Wicks &Thiel 1990). However, heavy monsoon rains significantly reduced cyanobacterial numbers so a more episodic sampling regime was adopted. Here, discrete sampling was performed over 2 years (2020–2021). Sampling sites were selected from two provinces Punjab and Khyber Pakhtunkhwa (KPK) in Pakistan with different geographical landscapes (Fig. 1). The targeted water bodies included a variety of habitats ranging from a freshwater lake (Saiful Muluk), an artificial reservoir (Rawal Lake), a semi-saline lake (Kallar Kahar), rivers (Panjnad, Swat), dams (Warsak, Tarbela), headwork and barrage (Head Marala and Head Rasul), and recreational ponds (Batakundi, Kawai, and Qalandarabad), which vary depending on latitude, altitude, regional climate, and geographical location. In 2020 we initially collected water samples from 12 sites, including northern areas (Swat, Abbottabad, Mansehra, and Balakot region) (Table S1. Supplementary) to get an overview of cyanotoxin counts across different locations. Before sampling, three rinses with surface water from the sampling site were done on collection bottles; after that bottles were brimmed to the top. Water samples were collected in pre-cleansed 500-mL HDPE bottles (Skafi et al. 2021) at different depths of (0.04–0.5 m) from the surface and up to 6.15 m beneath. In addition, chlorinated and tap water were sampled from the University of the Punjab, Lahore. In the chlorinated water samples, the residual chlorine was removed by treatment with ascorbic acid.
To evaluate the water quality of major drinking water sources in Pakistan with an emphasis on understanding distribution of noxious cyanobacterial taxa, environmental factors influencing cyanotoxin dynamics or monitoring changes over time, multiple water bodies ((Rawal Lake (Islamabad), Kallar Kahar Lake (Chakwal), Head Rasul barrage (Mandi Bahauddin), Head Marala headwork (Sialkot), Panjnad (Uch Sharif), Warsak Dam (Peshawar), and Tarbela Dam (Haripur)) located in Punjab and Khyber Pakhtunkhwa were resampled in 2021 (Table S1. Supplementary).
Cyanotoxin quantification by online SPE-UHPLC-HRMS and ELISA
The toxin stability in water samples is critical to accurately measure toxin concentrations (Wiltsie et al. 2018). Following sample collection, all water samples were brought to the laboratory immediately under refrigerated conditions (stored on ice) at the Institute of Microbiology and Molecular Genetics, University of the Punjab, Pakistan. Samples were kept frozen till analysis. Enzyme-linked immunosorbent assay (ELISA) was performed to quantify total microcystin, saxitoxin, and anatoxin in multiple water bodies from the regions of Punjab and Khyber Pakhtunkhwa during 2020. ELISA kits were kept at 4 °C until processed. A calibration curve was formulated for each ELISA plate. Every single calibration curve demonstrated a linear relationship between B/B0% (sample mean absorbance/mean absorbance of blank or zero standard). Abraxis provided standard concentrations (0–5.0 μg/L) for total microcystin and anatoxin whereas 0–0.4 ng/mL for saxitoxin. A quality control standard (0.75 ± 1.85 μg/L for anatoxin, 0.75 ± 0.015 μg/L for saxitoxin, and 0.75 ± 0.19 μg/L for microcystin) was utilized in every standard curve to authenticate the quantitation of total microcystin, saxitoxin, and anatoxin in freshwater samples (Christophoridis et al. 2018). After three freeze–thaw cycles, samples were filtered through a 0.2-µm nylon syringe filter to remove debris and release the intracellular toxins. The assay was performed in triplicate for each sample, and the procedure followed the manufacturer’s instructions accordingly (Birbeck et al. 2019).
To confirm that microcystin variants were present in freshwater bodies, the advanced and improved online SPE-UHPLC-MS/MS technique was used for the first time. A total of seven freshwater ecosystems in Punjab and Khyber Pakhtunkhwa provinces were resampled in 2021 and shipped to the University de Montreal in Montreal, Canada for analysis of total microcystin and 17 cyanobacterial secondary metabolites via online SPE–UHPLC-MS/MS methodology as a complementary method (Fayad et al. 2015). Online SPE-UHPLC-MS/MS approach required nominal sample preparation and less sample intake after 1 h of the Lemieux-von Rudloff oxidation step to generate the 2-methyl-3-methoxy-4-phenylbutyric acid (MMPB) moiety; collected water samples were quenched, filtered, and analyzed directly for total microcystin. The process was completed through solid phase extraction (SPE) coupled to online ultrahigh performance liquid chromatography and electrospray ionization triple stage quadrupole mass spectrometry. The online SPE was performed by a hypersil gold aQ via C18 column selectivity (20 mm 2 mm, 12 mm particle size), and chromatographic isolation was achieved by maintaining a temperature of 55 °C through a hypersil gold with C18 column selectivity included (100 mm 2.1 mm, 1.9 mm particle size). Ionization of the particular eluting toxins was obtained using a heated electrospray ionization source (HESI-II) attached to a quantum ultra AM triple quadrupole mass spectrometer operating in selected reaction monitoring (SRM) technique for cyanotoxin detection and quantification. Five milliliters of sample volume was loaded to SPE-UHPLC-MS/MS, and the recorded method run time was just 8 min with a limit of quantification up to (0.5 ng/L) for total microcystin. A wash volume of 3 mL was used for online desalting as well as appropriate method robustness (Munoz et al. 2017). For individual cyanotoxins and microcystin variants (cylindrospermopsin, anatoxin-a, anabaenopeptin A, homoanatoxin-a, anabaenopeptin B and microcystins YR, HtyR, dm-RR, RR, LR, dm-LR, WR, HilR, LF, LA, LW, and LY) a 5 mL aliquot of water sample was filtered through a syringe filter; an internal standard mix was added to a sub-aliquot and analyzed by online SPE-UHPLC-HRMS. The injection volume for the sample was 2 mL, and the instrument provided an analysis time of 8 min. This methodology allowed high throughput screening of cyanotoxins with a detection limit of up to (8–53 ng/L). All samples were tested in duplicate. ELISA results were reported as total microcystin, anatoxin, and saxitoxin.
Cyanotoxins data treatment
Data analysis for online SPE-UHPLC-MS/MS and SPE–UHPLC-HRMS was carried out using the Xcalibur 3.0 Software (Thermo Fisher Scientific, Waltham, MA, USA). Chromatographic peaks of targeted cyanotoxins were integrated using the Xcalibur Quan browser by their retention time and experimental mass (m/z). Sample quantification and calibration curves were subjected to area ratios of native compounds to their comparable internal standard (Roy-Lachapelle et al. 2019).
Measures of environmental parameters
Cyanobacteria often cause cyanobacterial blooms when pH, temperature, and nutrient levels are high. Hence, cyanotoxin occurrence was investigated with the physicochemical parameters of water. The study sites were evaluated for nine different physicochemical properties ((conductivity (EC, µs/cm), pH (unit), ORP (mV), depth (m), total dissolved solids (TDS, mg/L), salinity (Sal, PSU), DO (% Sat), dissolved oxygen (DO, mg/L), and temperature (°C)) measured directly at sampling points over 2 years using an AQUA METER (AP-2000) probe (Table S2. Supplementary).
DNA extraction and sequencing
Before DNA extraction, 250–300 mL of each water sample (obtained from second field sampling of seven freshwater ecosystems) was filtered through a 0.22-µm nitrocellulose filter membrane. Filters were kept at − 20 °C until analysis. DNA extraction was performed by DNeasy® PowerWater® Kit (Qiagen) following manufacturer instructions (Sanseverino et al. 2022). Each DNA sample was quantified with a Qubit v.2.0 fluorometer and sequenced with the Genome Québec sequencing platform. Library was prepared with the NEB Ultra II kit (New England Bio labs) and sequenced with NovaSeq 6000 S4, (2 × 150 bp; Illumina Inc., San Diego, CA, USA). Low-quality reads and adapter sequences were filtered using fastp (v0.20.1) (Chen et al. 2018). We obtained an average of 63 million reads per sample.
Microbial analysis—a read-based approach
To characterize the microbial community, the cleaned metagenomic reads were classified with Kraken2 (v2.1.2) using the confidence = 0.1 parameter and the standard kraken2 database (64 GB, 12/9/2022) (Wood et al. 2019). Bracken (v2.6.0) (Lu et al. 2017) was then used with -t10 (minimum of ten counts) to estimate the abundance at genus and species level.
Cyanotoxin-encoding genes analysis
Further to evaluate the presence and abundance of genes coding for cyanobacterial secondary metabolites, reads were mapped using KMA (v1.3.25) (Clausen et al. 2018) with the following parameters: -1t1 -mem_mode -and -apm f -ef -p 0.01 -ID 70 to a home-made database of biosynthesis gene clusters (BGCs) found in cyanobacteria and associated bacteria. Mapped reads were then normalized taking into account gene size and total number of reads (reads per kilo base per million reads). This database collected 94 BGCs and 460 genes. BGCs were predicted using ANTISMASH (v5.1.2) (Blin et al. 2019), and the corresponding reference sequences (of nucleotides and amino acids) were retrieved from the NCBI or ANTISMASH databases.
Sequencing data analysis
R package phyloseq (V.1.28.0) was used to analyze the community composition (McMurdie & Holmes 2013). The R packages vegan and ggord (v1.1.7) were used to create the redundancy analysis (RDA) plot. The model, axes, and variables were tested using anova.cca from vegan R package. The Spearman correlation coefficient was calculated to measure the association between microbial taxa and cyanotoxins using R package corrplot (v0.92), (Foster et al. 2017). P-values were corrected using the Benjamini–Hochberg Procedure.
Results
Cyanotoxin quantification in Pakistani water bodies
Analyzing spatio-temporal variations in cyanotoxin levels with ELISA
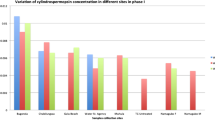
To determine the cyanotoxin prevalence, samples from seven freshwater reservoirs and five recreational sites were first analyzed by ELISA. Figure 2 represents the total analyzed toxins and average concentration recorded for individual water reservoirs. Of 36 samples from freshwater reservoirs and six samples from tap and chlorinated water, 85.7% displayed measurable cyanotoxin concentration. 41.6% of the total samples had microcystin concentrations higher than the guideline values recommended by WHO (World Health Organization) for drinking water ((microcystin > 1 μg/L)), and 8.3% of samples contained higher anatoxin values above the provisional limit ((anatoxin > 3 µg/L)) (Moraes 2020). Samples collected during summer (May to August) from Tarbela and Rawal Lake showed relatively high levels of microcystin and anatoxin concentrations detected by ELISA. Anatoxin was the predominant toxin detected during winter, with concentrations ranging from 0.3 to 0.75 µg/L. Overall, microcystin risks were greater in summer at recreational areas with concentrations ranging from 0.47 to 2.82 µg/L than in winter at most sites (dams and barrage). Microcystin had also been detected in tap water. ELISA findings indicated that cyanotoxins were found with a broad distribution pattern that was independent of geographic landscapes as they all lie in the temperate zone.
Location-specific concentration of total microcystin, anatoxin, and saxitoxin in freshwater samples calculated from ELISA is highlighted. Averages of MC total (total microcystin, μg/L), ANA (anatoxin, µg/L), and STX (saxitoxin, ng/mL) were recorded during the 2020 sampling in temperate and recreational waters for spring/summer and winter seasons. The error bars represent standard deviation (SD), and the data are means of three replicates. MCs were recorded at the majority of the sampling sites, including northern areas (River Swat, Lake Saiful Muluk, Batakundi, Kawai, and Qalandarabad)
We also found that multiple cyanotoxins could co-occur at the same sampling site, i.e., Rawal Lake, Tarbela, River Swat, and Panjnad. Multiple cyanotoxins MC, ANA, and STX were detected in high concentrations in Rawal Lake. For other sites, one type of cyanotoxin usually predominated, MC in summer and ANA in winter, the exception being Tarbela, which had higher toxin levels (> 2 µg/L) (Table S3. Supplementary).
High-resolution analysis of multiclass cyanotoxins
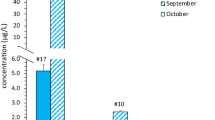
To validate the presence of cyanotoxins in the 2020 sampled freshwater reservoirs, a subset of 14 samples were measured using UHPLC-MS/MS (Fig. 3). Online SPE-UHPLC-MS/MS detected cyanotoxins in 57.14% (eight out of 14) of samples spreading over 2021. When we determined the total microcystin, Rawal Lake and Head Rasul showed significant values of 0.33 and 0.83 μg/L. Microcystin concentrations in Panjnad were measured up to (3.2 µg/L) exceeding the proposed guideline value by WHO (World Health Organization) in drinking water ((microcystin > 1 μg/L)) (chromatographic peaks illustration in Fig S1). Online SPE-UHPLC-HRMS identified three major microcystin congeners (MC-RR, MC-LR, and dmMC-RR) and one minor MC variant (MC-WR). The levels of microcystin-RR were higher than microcystin-LR (chromatographic peaks illustration in Fig S2. supplementary). However, most of the targeted congeners were not found in freshwater samples including anatoxin and saxitoxin. Instead, Cylindrospermopsin was found at three selected sites Panjnad, Rawal Lake, and Kallar Kahar with the highest concentration up to 0.13 μg/L in Kallar Kahar (Table S4. Supplementary). The positive finding of cyanotoxin variants was based on their retention time compared with that of the reference standards. UHPLC-MS/MS indicated that cyanotoxin was detected mainly in water bodies in Punjab and not in Khyber Pakhtunkhwa. Additionally, both cylindrospermopsin and total microcystin co-occurred at multiple water bodies (Rawal Lake, Head Rasul, and Panjnad).
Quantified concentration of MC total (total microcystin, μg/L) by online SPE-UHPLC-MS/MS, microcystin variants (MC-RR, MC-LR, dmMC-RR, MC-WR, µg/L), and CYN (cylindrospermopsin, μg/L) via HPLC-HRMS/MS methodology in water bodies during 2021. The Y-axis shows the average value of observed concentration in environmental samples. The X-axis indicates only those sampling points where toxin concentration was recorded. HPLC-HRMS/MS results indicated the presence of multiple MC congeners in Panjnad ((MC-LR, MC-RR, MC-WR, and dmMC-RR)) at low concentration with total MCs up to (3.2 μg/L). Cylindrospermopsin was recorded at Panjnad, Rawal Lake, and Kallar Kahar. We did not find any cyanotoxin at Head Marala and Tarbela
This is the first time that microcystin congeners and cylindrospermopsin have been found in freshwater reservoirs in Pakistan.
Environmental drivers of cyanotoxins
Our results indicated that temperature, ORP, and TDS differed between reservoirs (Table S2. Supplementary). The surface water of freshwater bodies was warmer in summers and better oxygenated in winters. pH values were mostly alkaline. ORP appeared lower in Head Marala and Panjnad showing a reducing environment. TDS measurements were high at some sampling stations and low at others, regardless of the season, and conductivity displayed its maximum levels in winter at Panjnad and Head Marala for 2 years (an indicator of nutrient enrichment and water pollution). With the exception of Panjnad and Warsak, most of the samples were taken from the surface. We presumed that the combination of high temperature, alkaline pH, and high conductivity observed in the study areas may create a favorable environment for cyanotoxin production.
To identify the relationship between environmental parameters and cyanotoxins, redundancy analysis (RDA) was performed (Fig S3. Supplementary) using ELISA quantifications as it involved more sampling information. Environmental variables such as water temperature, EC, DO, and depth were the only significant variables explaining cyanotoxin variation (analysis of variance (ANOVA) test, p < 0.05). RDA also showed a positive correlation between water temperature and total MC and anatoxin concentration (Fig S3. Supplementary). On the contrary, total MC was negatively correlated with depth and DO. However, it seems that the axis 1, which is the only one significant (ANOVA test, p < 0.05) is mainly driven by Rawal lake sample where the highest concentrations of cyanotoxins were found.
Following cyanotoxin identification and determining environmental parameters, we then looked at potential cyanotoxin producers.
Identification of potential cyanotoxins producers
We used shotgun metagenomics sequencing to characterize the cyanobacterial population and to identify the potential cyanotoxin producers. As we observed a co-occurrence of different cyanotoxins, we expected to observe different cyanobacterial producers being co-abundant in the same samples. We first analyzed the whole bacterial community and found that Proteobacteria and Actinobacteria were, by far, the two most abundant phyla. Cyanobacteria were only found at a low abundance in the total bacterial community (Fig. 4).
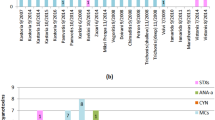
Despite the low abundance, we explored the cyanobacterial composition in the samples and found that the orders of Synechococcales, Nostocales, and Chroococcales were common in all environmental metagenomes. The two most frequently observed cyanobacterial genera were Synechococcus and Cyanobium (Fig. 5), although Cyanobium is not recognized as a potential cyanotoxins producer. Anabaena (known as a potential producer) was widely distributed in Rawal Lake compared to other cyanobacterial genera. Head Marala and Head Rasul also showed an abundance of Anabaena. Microcystis was detected in Rawal Lake, Warsak, and Kallar Kahar, while Nostoc was only detected in Head Marala.
We then analyzed cyanobacterial prevalence (based on relative abundance) to outline common genera shared by all sampling sites (Fig S4. Supplementary, Table S5). We identified Synechococcus (79%), Cyanobium (11%), Anabaena (6%), and Microcystis (4%) as the most prevalent cyanobacterial genera. We used a correlation analysis to link cyanotoxin concentration to cyanobacterial general relative abundance. To reduce the potential false positive, we corrected the p value and still found several positive correlations between microcystin variants (dmMC-RR, MC-LR, MC-WR, MC-RR) and Geminocytis and between cylindrospermopsin and Moorena. However, it is not clear if these genera could produce toxic secondary metabolites (Fig S5. Supplementary).
To validate if potential cyanotoxin-producing genera may possess the necessary gene clusters to produce cyanotoxins, we estimated the presence of toxic genes involved in cyanotoxin (MC, CYN, STX, and ANA) production, by mapping the reads to a home-made database of 94 biosynthetic gene cluster that we generated in a previous study (Ramalho et al. 2022). We were not able to find the full cyanotoxins operon, and only one gene from the microcystin mcy operon was found (Fig S6. Supplementary).
Identification of potential cyanobacterial predators
To explain such a low abundance of Cyanobacteria, we investigated the presence of potential predators (bacterial species with the ability to inhibit algal growth or destroy cells). We first compared the relative abundance of multiple potential algicidal bacteria (Wang et al. 2020) with the most abundant Cyanobacteria and the known potential cyanotoxin producers. Bacillus and Pseudomonas predominated but not at the same location. Pseudomonas was abundant in Rawal Lake and Panjnad, while Bacillus was found in Warsak. Flavobacterium was most abundant in Kallar Kahar, Head Marala, and Head Rasul. Pseudomonas, Flavobacterium, and Bacillus were nearly all prevalent in Rawal Lake (Fig S7. Supplementary).
Discussion
Microcystin and other cyanotoxins such as anatoxin, cylindrospermopsin, and saxitoxin have been reported in tropical regions (Habtemariam et al. 2021) of Africa, Asia, Australia, and America (You et al. 2022) with studies showing widespread microcystin prevalence (Preece et al. 2021; Walter et al. 2018). However, data on cyanotoxin distribution and cyanobacterial diversity in Pakistani drinking water reservoirs is limited. Several different freshwater ecosystems were analyzed in this study. We targeted dams, lakes, barrages, and canals to examine the overall distribution of cyanotoxins in different regions of Pakistan. A complementary set of analytical techniques (ELISA, UHPLC/MS/MS) (Gaget et al. 2017) was used for the first time to determine whether cyanotoxins were present in freshwater bodies. The robustness of these two techniques (UHPLC-MS/MS and ELISA) was evaluated based on their reproducibility, accuracy, linearity, method detection limit, and method quantification limit (MDL, MQL) (Montiel-León et al. 2018). Almost all sampling sites contained cyanotoxins detected by ELISA, while LC–MS detected cyanotoxins in (8/14) 57.14% of samples.
ELISA quantification showed variable cyanotoxin concentrations between sampling sites and seasons. Microcystin appeared more frequently in the summer of 2020 and was especially more abundant in Rawal Lake, River Swat, and Qalandarabad. During the winter of 2020, anatoxin predominated, with the highest concentration at Head Marala. The highest microcystin concentrations were found in lakes, recreational ponds, and rivers. This could be explained by the different levels of nutrient enrichment in the freshwater bodies (Kalaitzidou et al. 2021). The seasonal shift observed between microcystin and anatoxin could be related to seasonal changes in the composition of the cyanobacterial population (Davis et al. 2009).
Online SPE-UHPLC-MS/MS was then used to identify microcystin and its congeners with higher resolution in 2021. Other cyanotoxins, anatoxin-a, homoanatoxin-a (HANA-a), cylindrospermopsin (CYN), and anabaenopeptin (AP-A, AP-B), were also quantified. Online SPE-UHPLC-HRMS identified cylindrospermopsin for the first time in Pakistani freshwaters and several MC congeners (MC-WR, MC-LR, MC-RR, and dmMC-RR) in Panjnad. The total microcystin concentration is generally expected to be low for rivers (Bustillos-Guzmán et al. 2020). However, in this present study, the total microcystin concentration in Panjnad reached up to 3.25 μg/L, exceeding the WHO guideline value for drinking water (Mehinto et al. 2021). The LC–MS results indicated that most of the cyanotoxins were detected in Punjab, a subtropical region (Tariq & Qin 2023).
With this approach, we were unable to quantify anatoxin and saxitoxin. This difference between the ELISA and UHPLC-MS/MS finding could be attributed to the distinct cyanobacterial composition in these different water systems, and multiple cyanotoxin producers (involving several MC congeners, cylindrospermopsin, saxitoxin, and anatoxin) are present and concurrent. As observed with ELISA different cyanotoxins (MC and ANA) were found on the same site (Rawal Lake and Panjnad). Microcystin and cylindrospermopsin were found in Panjnad, Head Rasul, and Rawal Lake using UHPLC-MS/MS. The health risks and consequences of co-occurring cyanotoxins (Li et al. 2022) are poorly defined because health thresholds are normally based on exposures to a single cyanotoxin (Rutkowska et al. 2019). Ingestion of freshwater contaminants (toxins) through tainted drinking water or recreational activities may increase the risk of bioaccumulation in humans.
Cyanobacterial community and cyanotoxins are highly variable temporally and spatially in eutrophic waters. This variation is mainly due to variations in environmental factors, predators (e.g., phages, zooplankton), and anthropogenic activities (Tanvir et al. 2021). A redundancy analysis was performed to observe the association between environmental factors and cyanotoxins (Fig S3. Supplementary). Water temperature was positively related to MC total and anatoxin, while depth and DO were negatively correlated. Three water bodies (Rawal Lake, Kallar Kahar, and Tarbela) displayed the highest water temperature (> 30 °C); though they did not show any cyanobacteria bloom, microcystin concentration was higher than the WHO guideline value [microcystin > 1 μg/L) in Rawal lake. Some studies have even shown that total microcystin might decrease with high temperatures (Bui et al. 2018). In the current study, total microcystin seems to be positively driven by temperature, but we also acknowledge that more sampling with higher resolution during the different seasons would help to validate this current observation.
To explain cyanotoxin variability, we used metagenomic analysis of microbial communities in several freshwater bodies in Punjab and KPK (similar to that used for SPE-UHPLC-HRMS). Taxonomic analysis at the Phylum level showed that Proteobacteria were the major taxonomic group in most of the samples, followed by Actinobacteria. Proteobacteria, a phylum containing major food-borne and plant pathogens (Klair et al. 2023), were abundant in six different locations except Warsak. The relative abundance of Proteobacteria was higher in Kallar Kahar and Tarbela. Actinobacteria were the second most frequent phylum, identified mainly in Head Marala and Warsak. Earlier, in Pakistan, a differential metagenomics evaluation of three freshwater lakes (Keenjhar, Rawal, and Saif-ul Maluk) was carried out which also highlighted Proteobacteria as the most predominant phyla (58–79%) followed by Planctomycetes (34%), Cyanobacteria (12%), and Bacteroidetes (15%) (Saleem et al. 2019). In the current investigation, the abundance of Cyanobacteria was low, less than 2% of the total bacterial diversity. The cyanobacterial taxa that predominated in our samples were Synechococcus, Cyanobium, Microcystis, and Anabaena. The genera Nostoc, Planktothrix, Dolichospermum (Hernandez et al. 2022), Microcystis, Oscillatoria, Aphanocapsa, Aphanizomenon, and Raphidiopsis (Carmichael 1992; Turner et al. 2018) are generally identified as potential cyanotoxins producer. Interestingly, we found all the genera except Aphanocapsa and Aphanizomenon in the water bodies.
Picocyanobacteria (e.g., Synechococcus) have recently captured the attention with their ability to produce and release a wide range of biologically active secondary metabolites (Pagliara et al. 2021) including cyanobactines, oligopeptides, geosmin (1, 2, 7, 7-tetramethyl-2-norborneol) (GSM) and 2-methylisoborneol (MIB) (Jakubowska &Szeląg-Wasielewska 2015). In this study, we revealed the presence of complete or potentially complete cyanobacterial genes clusters coding for biosynthesis of lipopolysaccharide (endotoxin), syringolin, viscosin, pyoverdin, kijanimycin, zwittermicinA, orphamid A, and carotenoid (colorful pigments). However, further analyses are needed to confirm their production and to elucidate the distribution of secondary metabolites released by picocyanobacteria, which was dominant in our samples. Among these secondary metabolites, we look at the genes involved in cyanotoxins production to identify the potential producers of the quantified cyanotoxins. No reads mapped to genes involved in anatoxin or cylindrospermopsin production were found, and we got coverage for only one gene from the microcystin mcy operon which is generally composed of ten genes.
To explain the presence of cyanotoxins but no potential producers, we hypothesized that different sampling sites might be composed of a high number of algicidal/predators that could induce lysis of cyanobacterial cells (Fig S7. Supplementary). These taxa are generally more observed at the end of a bloom, often in association with other taxa, known to be involved in the degradation of microcystin. Here, we found several known microcystin degraders (e.g., Sphingomonas, Novosphingobium) present in the top 30 non-cyanobacteria genera (Fig S8. Supplementary). Another explanation is that Cyanobacteria may be relatively rare (eclipsed by Proteobacteria), but their absolute abundance is high enough to produce the toxin, albeit below the limit of detection by metagenomics. This result would confirm what several studies suggested: toxins are not necessarily directly related to the total number of cyanobacterial cells (Bubak et al. 2020; Scherer et al. 2017; Svirčev et al. 2022). Another possible explanation could be the release/production of extracellular toxins from senescent cyanobacterial cells (Zurawell et al. 2005) or epibenthic rather than planktonic Cyanobacteria, which we missed here as we only collected water (Bauer et al. 2023).
Considering the limited information available regarding the prevalence of toxic secondary metabolites from South Asia (Tavakoli et al. 2021), these findings will improve our knowledge and help develop new water management guidelines in the future.
Conclusion
In this study, 2 years of monitoring data (2020–2021) from multiple freshwater reservoirs was analyzed to determine the dynamics of cyanotoxin presence and the importance of local environmental factors. Cyanotoxin concentration was measured using ELISA and liquid chromatography-mass spectrometry. Online SPE-UHPLC-HRMS detected five of the 17 toxic variants in various combinations. Total microcystin and cylindrospermopsin were detected at three sampling sites. Microcystin-RR was detected at higher levels than microcystin-LR. Only Panjnad exceeded drinking water limits for microcystin ((drinking water > 1 μg/L)). Conversely, four water bodies analyzed by ELISA exceeded WHO guideline values for microcystin in drinking water, but none of the samples exceeded recreational guidelines. To our knowledge, this study is the first to confirm the presence of microcystin congeners and cylindrospermopsin in Pakistani freshwater bodies. Our results showed that the cyanotoxin concentration was not necessarily directly related to the total number of cyanobacterial cells. The metagenomics results indicated that the majority of freshwater bodies (dams and lakes) have been dominated by Proteobacteria, regardless of their geographical location. Synechococcus was identified as the dominant genus. We identified Cyanobacteria capable of producing microcystin and cylindrospermopsin, but the corresponding toxic genes were not found. This study showed that even if Cyanobacteria were not abundant, a significant concentration of cyanotoxins could still be quantified in the majority of the samples. Further studies are needed to identify the cyanotoxins producers and understand how toxin dynamics are related to the cyanobacterial population dynamic.
Data availability
Raw metagenomics sequencing data have been deposited at NCBI GenBank under Bio Project ID: PRJNA1016429.
References
Abbasi T, Abbasi SA (2012): Water quality indices. Elsevier https://doi.org/10.1016/C2010-0-69472-7
Ahmed A, Shafique I (2019) Perception of household in regards to water pollution: an empirical evidence from Pakistan. Environ Sci Pollut Res 26:8543–8551. https://doi.org/10.1007/s11356-019-04273-4
Aráoz R, Molgó J, de Marsac NT (2010) Neurotoxic cyanobacterial toxins. Toxicon 56:813–828. https://doi.org/10.1016/j.toxicon.2009.07.036
Arman T, Clarke JD (2021) Microcystin toxicokinetics, molecular toxicology, and pathophysiology in preclinical rodent models and humans. Toxins 13:537–539. https://doi.org/10.3390/toxins13080537
Badar M, Khan S, Khokhar I, Batool F (2015) Removal of cyanobacterial toxins from drinking water sources by aluminium sulphate treatment. Braz J Biol 2:135–145
Bauer F, Wolfschlaeger I, Geist J, Fastner J, Schmalz CW, Raeder U (2023) Occurrence, distribution and toxins of benthic cyanobacteria in German lakes. Toxics 11:643–645. https://doi.org/10.3390/toxics11080643
Bernard C, Ballot A, Thomazeau S, Maloufi S, Furey A, Mankiewicz-Boczek J, Pawlik‐Skowrońska B, Capelli C, Salmaso N (2017) Cyanobacteria associated with the production of cyanotoxins. Appendix 2. In: Meriluoto J, Spoof L, Codd GA (eds) Handbook of cyanobacterial monitoring and cyanotoxin analysis. John Wiley & Sons, Chichester, pp 501–525
Birbeck JA, Westrick JA, O’Neill GM, Spies B, Szlag DC (2019) Comparative analysis of microcystin prevalence in Michigan lakes by online concentration LC/MS/MS and ELISA. Toxins 11:13–15. https://doi.org/10.3390/toxins11010013
Blin K, Shaw S, Steinke K, Villebro R, Ziemert N, Lee SY, Medema MH, Weber T (2019) antiSMASH 5.0: updates to the secondary metabolite genome mining pipeline. Nucleic Acids Res 47:W81–W87. https://doi.org/10.1093/nar/gkz310
Bouaïcha N, Miles CO, Beach DG, Labidi Z, Djabri A, Benayache NY, Nguyen-Quang T (2019) Structural diversity, characterization and toxicology of microcystins. Toxins 11:714. https://doi.org/10.3390/toxins11120714
Briand J-F, Jacquet S, Bernard C, Humbert J-F (2003) Health hazards for terrestrial vertebrates from toxic cyanobacteria in surface water ecosystems. Vet Res 34:361–377. https://doi.org/10.1051/vetres:2003019
Bubak I, Śliwińska-Wilczewska S, Głowacka P, Szczerba A, Możdżeń K (2020) The importance of allelopathic picocyanobacterium Synechococcus sp. on the abundance, biomass formation, and structure of phytoplankton assemblages in three freshwater lakes. Toxins 12:259–265. https://doi.org/10.3390/toxins12040259
Bui T, Dao T-S, Vo T-G, Lürling M (2018) Warming affects growth rates and microcystin production in tropical bloom-forming Microcystis strains. Toxins 10:123–126. https://doi.org/10.3390/toxins10030123
Buratti FM, Manganelli M, Vichi S, Stefanelli M, Scardala S, Testai E, Funari E (2017) Cyanotoxins: producing organisms, occurrence, toxicity, mechanism of action and human health toxicological risk evaluation. Arch Toxicol 91:1049–1130. https://doi.org/10.1007/s00204-016-1913-6
Bustillos-Guzmán JJ, Turner A, Hernández-Almeida OU, Band-Schmidt CJ, Romero-Bañuelos CA, Hernández-Sandoval FE, Núñez-Vázquez EJ, Palomino-Hermosillo YA (2020) Presence of cyanotoxins in a Mexican subtropical monomictic crater lake. Appl Sci 10:6719–6721. https://doi.org/10.3390/app10196719
Carmichael W (1992) Cyanobacteria secondary metabolites—the cyanotoxins. J Appl Bacteriol 72:445–459. https://doi.org/10.1111/j.1365-2672.1992.tb01858.x
Cerasino L, Salmaso N (2020) Co-occurrence of anatoxin-a and microcystins in lake Garda and other deep subalpine lakes. Adv Oceanogr Limnol 11:55–58. https://doi.org/10.4081/aiol.2020.8677
Chen S, Zhou Y, Chen Y, Gu J (2018) fastp: an ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 34:i884–i890. https://doi.org/10.1093/bioinformatics/bty560
Cheung MY, Liang S, Lee J (2013) Toxin-producing cyanobacteria in freshwater: a review of the problems, impact on drinking water safety, and efforts for protecting public health. J Microbiol 51:1–10. https://doi.org/10.1007/s12275-013-2549-3
Chorus I, Welker M (2021) Toxic cyanobacteria in water: a guide to their public health consequences, monitoring and management. Taylor & Francis
Christophoridis C, Zervou S-K, Manolidi K, Katsiapi M, Moustaka-Gouni M, Kaloudis T, Triantis TM, Hiskia A (2018) Occurrence and diversity of cyanotoxins in Greek lakes. Sci Reports 8:1–22. https://doi.org/10.1038/s41598-018-35428-x
Clausen PT, Aarestrup FM, Lund O (2018) Rapid and precise alignment of raw reads against redundant databases with KMA. BMC Bioinform 19:1–8. https://doi.org/10.1186/s12859-018-2336-6
Codd GA, Bell SG, Kaya K, Ward CJ, Beattie KA, Metcalf JS (1999) Cyanobacterial toxins, exposure routes and human health. Eur J Phycol 34:405–415. https://doi.org/10.1080/09670269910001736462
Davis TW, Berry DL, Boyer GL, Gobler CJ (2009) The effects of temperature and nutrients on the growth and dynamics of toxic and non-toxic strains of Microcystis during cyanobacteria blooms. Harmful algae 8:715–725. https://doi.org/10.1016/j.hal.2009.02.004
Díez-Quijada L, Prieto AI, Guzmán-Guillén R, Jos A, Cameán AM (2019) Occurrence and toxicity of microcystin congeners other than MC-LR and MC-RR: A review. Food Chem Toxicol 125:106–132. https://doi.org/10.1016/j.fct.2018.12.042
Diez-Quijada L, Benítez-González MdM, Puerto M, Jos A, Cameán AM (2021) Immunotoxic effects induced by microcystins and cylindrospermopsin: a review. Toxins 13:711–715. https://doi.org/10.3390/toxins13100711
Fahad S, Wang J (2020) Climate change, vulnerability, and its impacts in rural Pakistan: a review. Environ Sci Pollut Res 27:1334–1338. https://doi.org/10.1007/s11356-019-06878-1
Falconer I, Humpage A (2005) Health risk assessment of cyanobacterial (blue-green algal) toxins in drinking water. Int J Environ Res Public Health 2:43–50. https://doi.org/10.3390/ijerph2005010043
Farrer D, Counter M, Hillwig R, Cude C (2015) Health-based cyanotoxin guideline values allow for cyanotoxin-based monitoring and efficient public health response to cyanobacterial blooms. Toxins 7:457–477. https://doi.org/10.3390/toxins7020457
Fayad PB, Roy-Lachapelle A, Duy SV, Prévost M, Sauvé S (2015) On-line solid-phase extraction coupled to liquid chromatography tandem mass spectrometry for the analysis of cyanotoxins in algal blooms. Toxicon 108:167–175. https://doi.org/10.1016/j.toxicon.2015.10.010
Ferrão-Filho AdS, Kozlowsky-Suzuki B (2011) Cyanotoxins: bioaccumulation and effects on aquatic animals. Mar Drugs 9:2729–2772. https://doi.org/10.3390/md9122729
Filatova D, Picardo M, Núñez O, Farré M (2020) Analysis, levels and seasonal variation of cyanotoxins in freshwater ecosystems. Tren Environ Anal Chem 26:e00091–e00096. https://doi.org/10.1016/j.teac.2020.e00091
Filatova D, Jones MR, Haley JA, Núñez O, Farré M, Janssen EM-L (2021) Cyanobacteria and their secondary metabolites in three freshwater reservoirs in the United Kingdom. Env Sci Euro 33:1–11. https://doi.org/10.1186/s12302-021-00472-4
Foster ZS, Sharpton TJ, Grünwald NJ (2017) Metacoder: An R package for visualization and manipulation of community taxonomic diversity data. PLoS Comput Biol 13:e1005404–e1005408. https://doi.org/10.1371/journal.pcbi.1005404
Gaget V, Lau M, Sendall B, Froscio S, Humpage AR (2017) Cyanotoxins: which detection technique for an optimum risk assessment? Water Res 118:227–238. https://doi.org/10.1016/j.watres.2017.04.025
Habtemariam H, Kifle D, Leta S, Beekman W, Lürling M (2021) Cyanotoxins in drinking water supply reservoir (Legedadi, Central Ethiopia): implications for public health safety. SN Appl Sci 3:1–10. https://doi.org/10.1007/s42452-021-04313-0
Hernandez BY, Zhu X, Risch HA, Lu L, Ma X, Irwin ML, Lim JK, Taddei TH, Pawlish KS, Stroup AM (2022) Oral cyanobacteria and hepatocellular carcinoma cyanobacteria and HCC. Cancer Epidemiol Biomarkers Prev 31:221–229. https://doi.org/10.1158/1055-9965.EPI-21-0804
Huang W-J, Lai C-H, Cheng Y-L (2007) Evaluation of extracellular products and mutagenicity in cyanobacteria cultures separated from a eutrophic reservoir. Sci Total Environ 377:214–223. https://doi.org/10.1016/j.scitotenv.2007.01.075
Huisman J, Codd GA, Paerl HW, Ibelings BW, Verspagen JMH, Visser PM (2018) Cyanobacterial blooms. Nat Rev Microbiol 16:471–483. https://doi.org/10.1038/s41579-018-0040-1
Ibelings BW, Backer LC, Kardinaal WEA, Chorus I (2014) Current approaches to cyanotoxin risk assessment and risk management around the globe. Harmful Algae 40:63–74. https://doi.org/10.1016/j.hal.2014.10.002
Jakubowska N, Szeląg-Wasielewska E (2015) Toxic picoplanktonic cyanobacteria. Mar Drugs 13:1497–1518. https://doi.org/10.3390/md13031497
Jin C, Mesquita MM, Deglint JL, Emelko MB, Wong A (2018) Quantification of cyanobacterial cells via a novel imaging-driven technique with an integrated fluorescence signature. Sci Reports 8:1–12. https://doi.org/10.1038/s41598-018-27406-0
Kalaitzidou MP, Nannou CI, Lambropoulou DA, Papageorgiou KV, Theodoridis AM, Economou VK, Giantsis IA, Angelidis PG, Kritas SK, Petridou EJ (2021) First report of detection of microcystins in farmed mediterranean mussels Mytilus galloprovincialis in Thermaikos gulf in Greece. J Biol Res (thessalon) 28:1–14. https://doi.org/10.1186/s40709-021-00139-4
Kasan NA, Yusof SZM, Manan H, Khairul WM, Zakeri HA (2021) Inhibitory effect of thiourea derivatives on the growth of blue-green algae. J Environ Manage 294:113008–113010. https://doi.org/10.1016/j.jenvman.2021.113008
Klair D, Dobhal S, Ahmad A, Hassan ZU, Uyeda J, Silva J, Wang K-H, Kim S, Alvarez AM, Arif M (2023) Exploring taxonomic and functional microbiome of Hawaiian stream and spring irrigation water systems using Illumina and Oxford Nanopore sequencing platforms. Front Microbiol 14:1039292–1039294. https://doi.org/10.3389/fmicb.2023.1039292
Li H, Gu X, Chen H, Mao Z, Shen R, Zeng Q, Ge Y (2022) Co-occurrence of multiple cyanotoxins and taste-and-odor compounds in the large eutrophic Lake Taihu, China: dynamics, driving factors, and challenges for risk assessment. Environ Pollut 294:118594–118596. https://doi.org/10.1016/j.envpol.2021.118594
Lu J, Breitwieser FP, Thielen P, Salzberg SL (2017) Bracken: estimating species abundance in metagenomics data. PeerJ Comput Sci 3:e104–e109. https://doi.org/10.7717/peerj-cs.104
Mahar M, Baloch W, Jafri S (2000) Diversity and seasonal occurrence of planktonic rotifers in manchar lake, Sindh, Pakistan. Pak J Fish 1:25–32
McMurdie PJ, Holmes S (2013) phyloseq: an R package for reproducible interactive analysis and graphics of microbiome census data. PLoS ONE 8:e61217–e61219. https://doi.org/10.1371/journal.pone.0061217
Mehinto AC, Smith J, Wenger E, Stanton B, Linville R, Brooks BW, Sutula MA, Howard MD (2021) Synthesis of ecotoxicological studies on cyanotoxins in freshwater habitats—evaluating the basis for developing thresholds protective of aquatic life in the United States. Sci Total Environ 795:148864–148866. https://doi.org/10.1016/j.scitotenv.2021.148864
Montiel-León JM, Duy SV, Munoz G, Amyot M, Sauvé S (2018) Evaluation of on-line concentration coupled to liquid chromatography tandem mass spectrometry for the quantification of neonicotinoids and fipronil in surface water and tap water. Anal Bioanal Chem 410:2765–2779. https://doi.org/10.1007/s00216-018-0957-2
Moraes MdAB 2020: Spatial and temporal variability of cyanobacteria in two subtropical reservoirs: community composition, molecular and cyanotoxin analyses, Universidade de São Paulo, 1500–1515 https://doi.org/10.11606/T.18.2020.tde-09062021-103549
Munoz G, Duy SV, Roy-Lachapelle A, Husk B, Sauvé S (2017) Analysis of individual and total microcystins in surface water by on-line preconcentration and desalting coupled to liquid chromatography tandem mass spectrometry. J Chromatogr A 1516:9–20. https://doi.org/10.1016/j.chroma.2017.07.096
Oberholster P, Botha A, Grobbelaar J (2004) Microcystis aeruginosa: source of toxic microcystins in drinking water. Afr J Biotechnol 3:67–69. https://doi.org/10.5897/AJB2004.000-2029
Pagliara P, De Benedetto GE, Francavilla M, Barca A, Caroppo C (2021) Bioactive potential of two marine picocyanobacteria belonging to Cyanobium and Synechococcus Genera. Microorganisms 9:2048–2050. https://doi.org/10.3390/microorganisms9102048
Pandhal J, Siswanto A, Kuvshinov D, Zimmerman WB, Lawton L, Edwards C (2018) Cell lysis and detoxification of cyanotoxins using a novel combination of microbubble generation and plasma microreactor technology for ozonation. Front Microbiol 9:678–682. https://doi.org/10.3389/fmicb.2018.00678
Pierce EF, Schnetzer A (2023) Microcystin concentrations, partitioning, and structural composition during active growth and decline: a laboratory study. Toxins 15:684–686. https://doi.org/10.3390/toxins15120684
Preece EP, Hobbs W, Hardy FJ, O’Garro L, Frame E, Sweeney F (2021) Prevalence and persistence of microcystin in shoreline lake sediments and porewater, and associated potential for human health risk. Chemosphere 272:129581–129584. https://doi.org/10.1016/j.chemosphere.2021.129581
Ramalho TP, Chopin G, Pérez-Carrascal OM, Tromas N, Verseux C (2022) Selection of Anabaena sp. PCC 7938 as a cyanobacterium model for biological ISRU on Mars. Appl Environ Microbiol 88:e00594-e622. https://doi.org/10.1128/aem.00594-22
Roy-Lachapelle A, Duy SV, Munoz G, Dinh QT, Bahl E, Simon DF, Sauvé S (2019) Analysis of multiclass cyanotoxins (microcystins, anabaenopeptins, cylindrospermopsin and anatoxins) in lake waters using on-line SPE liquid chromatography high-resolution Orbitrap mass spectrometry. Anal Methods 11:5289–5300. https://doi.org/10.1039/C9AY01132C
Rutkowska M, Płotka-Wasylka J, Majchrzak T, Wojnowski W, Mazur-Marzec H, Namieśnik J (2019) Recent trends in determination of neurotoxins in aquatic environmental samples. Trends Analyt Chem 112:112–122. https://doi.org/10.1016/j.trac.2019.01.001
Saleem F, Azim MK, Mustafa A, Kori JA, Hussain MS (2019) Metagenomic profiling of fresh water lakes at different altitudes in Pakistan. Ecol Inform 51:73–81. https://doi.org/10.1016/j.ecoinf.2019.02.013
Sanseverino I, Pretto P, António DC, Lahm A, Facca C, Loos R, Skejo H, Beghi A, Pandolfi F, Genoni P (2022) Metagenomics analysis to investigate the microbial communities and their functional profile during cyanobacterial blooms in Lake Varese. Microb Ecol 83:850–868. https://doi.org/10.1007/s00248-021-01914-5
Scherer PI, Millard AD, Miller A, Schoen R, Raeder U, Geist J, Zwirglmaier K (2017) Temporal dynamics of the microbial community composition with a focus on toxic cyanobacteria and toxin presence during harmful algal blooms in two South German lakes. Front Microbiol 8:2387–2390. https://doi.org/10.3389/fmicb.2017.02387
Skafi M, Duy SV, Munoz G, Dinh QT, Simon DF, Juneau P, Sauvé S (2021) Occurrence of microcystins, anabaenopeptins and other cyanotoxins in fish from a freshwater wildlife reserve impacted by harmful cyanobacterial blooms. Toxicon 194:44–52. https://doi.org/10.1016/j.toxicon.2021.02.004
Svirčev Z, Chen L, Sántha K, Drobac Backović D, Šušak S, Vulin A, Palanački Malešević T, Codd GA, Meriluoto J (2022) A review and assessment of cyanobacterial toxins as cardiovascular health hazards. Arch Toxicol 96:2829–2863
Szlag DC, Sinclair JL, Southwell B, Westrick JA (2015) Cyanobacteria and cyanotoxins occurrence and removal from five high-risk conventional treatment drinking water plants. Toxins 7:2198–2220. https://doi.org/10.3390/toxins7062198
Tanvir RU, Hu Z, Zhang Y, Lu J (2021) Cyanobacterial community succession and associated cyanotoxin production in hypereutrophic and eutrophic freshwaters. Environ Pollut 290:118056–118058. https://doi.org/10.1016/j.envpol.2021.118056
Tariq A, Qin S (2023) Spatio-temporal variation in surface water in Punjab, Pakistan from 1985 to 2020 using machine-learning methods with time-series remote sensing data and driving factors. Agric Water Manag 280:108228–108230. https://doi.org/10.1016/j.agwat.2023.108228
Tavakoli Y, Mohammadipanah F, Te SH, You L, Gin KY-H (2021) Biodiversity, phylogeny and toxin production profile of cyanobacterial strains isolated from lake Latyan in Iran. Harmful Algae 106:102054–102056. https://doi.org/10.1016/j.hal.2021.102054
Thawabteh AM, Naseef HA, Karaman D, Bufo SA, Scrano L, Karaman R (2023) Understanding the risks of diffusion of cyanobacteria toxins in rivers, lakes, and potable water. Toxins 15:582–584. https://doi.org/10.3390/toxins15090582
Turner A, Dhanji-Rapkova M, O’Neill A, Coates L, Lewis A, Lewis K (2018) Analysis of microcystins in cyanobacterial blooms from freshwater bodies in England. Toxins 10:39–43. https://doi.org/10.3390/toxins10010039
Van Apeldoorn ME, Van Egmond HP, Speijers GJ, Bakker GJ (2007) Toxins of cyanobacteria. Mol Nutr Food Res 51:7–60. https://doi.org/10.1002/mnfr.200600185
Walter JM, Lopes FA, Lopes-Ferreira M, Vidal LM, Leomil L, Melo F, De Azevedo GS, Oliveira R, Medeiros AJ, Melo AS (2018) Occurrence of harmful cyanobacteria in drinking water from a severely drought-impacted semi-arid region. Front Microbiol 9:176–179. https://doi.org/10.3389/fmicb.2018.00176
Wang M, Chen S, Zhou W, Yuan W, Wang D (2020) Algal cell lysis by bacteria: a review and comparison to conventional methods. Algal Res 46:101794–101798. https://doi.org/10.1016/j.algal.2020.101794
Welker M, Chorus I, Fastner J, Khan S, Haque MM, Islam S, Khan NH (2005) Microcystins (cyanobacterial toxins) in surface waters of rural Bangladesh: pilot study. J Water Health 3:325–337. https://doi.org/10.2166/wh.2005.009
Wicks RJ, Thiel PG (1990) Environmental factors affecting the production of peptide toxins in floating scums of the cyanobacterium Microcystis aeruginosa in a hypertrophic African reservoir. Environ Sci Technol 24:1413–1418. https://doi.org/10.1021/es00079a017
Wiltsie D, Schnetzer A, Green J, Vander Borgh M, Fensin E (2018) Algal blooms and cyanotoxins in jordan lake, north carolina. Toxins 10:92–96. https://doi.org/10.3390/toxins10020092
Wood DE, Lu J, Langmead B (2019) Improved metagenomic analysis with Kraken 2. Genome Biol 20:1–13. https://doi.org/10.1186/s13059-019-1891-0
You L, Tong X, Te SH, Tran NH, bte Sukarji NH, He Y, Gin KY-H, (2022) Multi-class secondary metabolites in cyanobacterial blooms from a tropical water body: distribution patterns and real-time prediction. Water Res 212:118129–118131. https://doi.org/10.1016/j.watres.2022.118129
Zastepa A, Pick FR, Blais J (2014) Fate and persistence of particulate and dissolved microcystin-LA from Microcystis blooms. Hum Ecol Risk Assess 20:1670–1686. https://doi.org/10.1080/10807039.2013.854138
Zurawell R, Chen H, Burke JM, Prepas EE (2005) Hepatotoxic cyanobacteria: a review of the biological importance of microcystins in freshwater environments. J Toxicol Environ Health Part B 8:1–37. https://doi.org/10.1080/10937400590889412
Funding
This research was funded by The Higher Education Commission (HEC) of Pakistan to Uzma Batool for her PhD studies (Indigenous PhD scholarship; PIN No. 5, 518–84931-2BS5-007). HEC Pakistan also provided funding to Uzma Batool (PIN No. IRSIP 47 BMS 18) to undertake research at the Department of Biological sciences, University de Montreal, Canada. This work was also funded by the HEC Pakistan under the National Research Programme for Universities (NRPU Grant 6769).
Author information
Authors and Affiliations
Contributions
Uzma Batool, Nicolas Tromas, and Mehboob Ahmed contributed to the study’s conception and design. Data collection was done by Uzma Batool. Data analysis was performed by Nicolas Tromas and Uzma Batool, Materials for online SPE-UHPLC-MS/MS were provided by Sebastian Sauvé, Dana F. Simon in collaboration with the Department of Biological Sciences, University de Montreal. Funds for Shotgun metagenomics were provided by Jesse Shapiro. Funding by the Indigenous PhD scholarship and the International Research Support Initiative Program (IRSIP) was acquired by Uzma Batool. Funding by the NRPU grant was acquired by Mehboob Ahmed. The first draft of the manuscript was written by Uzma Batool, and all authors read and reviewed the manuscript. This research was supervised by Nicolas Tromas and Mehboob Ahmed.
Corresponding author
Ethics declarations
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
All authors agree to publication.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Lotfi Aleya
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Batool, U., Tromas, N., Simon, D.F. et al. Snapshot of cyanobacterial toxins in Pakistani freshwater bodies. Environ Sci Pollut Res 31, 24648–24661 (2024). https://doi.org/10.1007/s11356-024-32744-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-024-32744-w