Abstract
The occurrence of distant metastases is the main cause of death for breast cancer patients. However, central factors forcing cancer cells to migrate and grow outside of the primary organ are still not well understood [1]. An association of breast cancer and bone metastasis was previously described in 1889 by Steven Paget’s theory of seed and soil [2]. Rohr and Hegglin suggested the breast cancer-related metastasis in bone marrow (BM) [3] and also recognized metastatic cells in BM biopsies by hematoxylin and eosin staining. The first single disseminated tumor cells in BM smears was also screened out in nonmetastatic breast cancer patients [4], when only a few reports dealt with micrometastasis [5]. Furthermore, morphological criteria were not satisfactory to undoubtfully distinguish single epithelial tumor cells from BM cells, especially because of the extensive variety of morphologically uneven hematopoietic and mesenchymal stem as well as progenitor cells [6].
Significant progress in the field of BM micrometastasis arose from the introduction of immunocytochemical staining procedures using antibodies against epithelial-specific markers (EMA, cytokeratins) that were not expressed on the neighboring BM cells [7]. There is increasing evidence that the presence of disseminated and circulating tumor cells (DTCs/CTCs) and several novel molecular biomarkers is associated with an unfavorable prognosis related to metastatic progression in the bone and other organs. Using these methods and markers, it became more and more established during the last two decades that BM is a common homing and surviving organ for breast cancer cells [8]. These cells are likely to escape from the host immune system in a dormant state until internal and/or external signals might facilitate them to move and grow out to overt metastases at different organs [9].
In the present chapter, we will focus on recent advancements and investigations in the field of liquid biopsy-based biomarkers, especially DTCs and CTCs, along with the evolution of many fluid-based molecular biomarkers which have the capability to behave as potential biomarkers in metastasizing breast cancer.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Disseminated tumor cell
- Circulating tumor cell
- Metastasis
- Breast cancer
- Molecular biomarker
- Glycans
- Tissue interstitial fluid
Introduction
Breast cancer results from multistep carcinogenesis. The transforming process from normal to malignant cells is linked with multiple complex factors. The existence of a specific breast cancer in a specific individual relies on complex, vibrant interaction between the tumor and the host. Breast cancer is the most frequently occurring cancer in females worldwide with an age-standardized incidence rate (ASR) of 39.0 per 100,000, and it is the most common cause of cancer mortality as it comprises 16 % of cancer deaths in adult women [10]. Incidence rates of breast cancer are increasing in most countries, and the changes are usually maximum where rates were previously low [11].
Breast cancer is the second most common cancer in all Indian women, according to current data from the Atlas of Cancer in India project—a study to assess nationwide patterns of cancer incidence across urban and rural parts of the country suggests that breast cancer is the frequent cancer in metropolitan cities and is predicted to be the most common type of cancer in the coming decade. Data from the Atlas project suggest that certain districts display even higher rates (for instance, Chandigarh 39.5 per 100,000; North Goa 36.8 per 100,000) than those reported by the population-based registry in New Delhi (28.9 per 100,000). In Bangalore, Chennai, Delhi, Mumbai, and Kolkata, the age-adjusted incidence rates are 30.9, 33.0, 31.4, 29.3, and 20.6 per 100,000, respectively [12].
A recent report by the Indian Council of Medical Research forecasts the number of breast cancer cases in India to rise to 106,124 in 2015 and to 123,634 in 2020 (Cancer Incidence Rates 1982–2005). According to the National Cancer Registry Programme projections, the number of breast cancer deaths in India will rise to 106,124 in 2015 and to 123,634 in 2020 (Cancer Incidence Rates 1982–2005).
Treatment of breast cancer depends on few well-established prognostic and predictive factors, screening, surveillance, and intervention, but many individuals will die from progressive, advanced breast cancer due to late manifestation of symptoms. Breast cancer morbidity increases significantly if it is not detected early in its progression. Early detection of breast cancer before symptoms appear is the most effective restraint of breast cancer. It is estimated that between 15 and 25 % of women with early-stage breast cancer are currently missed by widely used diagnostic procedures such as mammography. The real challenge is to deal with the inherent limitations of breast cancer detection by identifying new breast cancer markers that can be imaged and detected in the blood by noninvasive procedures. Detection of circulating tumor cells (CTCs) in peripheral blood and disseminated tumor cells (DTCs) in bone marrow of tumor patients has become an active area of translational cancer research, with several groups developing new diagnostic assays and more than 200 clinical trials incorporating CTC counts as a biomarker in patients with various types of solid tumors. Among these activities, breast cancer has played the most prominent role as a “key player” of research on CTCs/DTCs. The clinical importance of DTCs is already well established and has been set by different large-scale studies. CTC analysis could play a role as a “liquid biopsy,” which will allow physicians to follow cancer changes over time and tailor treatment, and it represents a promising new diagnostic field for advanced-stage patients; the sensitive CTC detection platforms allow monitoring of disease and treatment efficacy.
Current research on CTCs is focusing on the identification of novel diagnostic and therapeutic biomarkers expressed by these cells. However, we need to find new strategies with higher sensitivity and specificity for more accurate recognition of breast cancer. This chapter focuses on the presentation of recent data showing that CTCs/DTCs can be used as novel tumor biomarkers together with some novel robust molecular biomarkers for prognostic and predictive purposes in breast cancer.
Screening Methods of Breast Cancer
To date, a few proteins have been suggested as possible markers for the early detection of breast cancer; these include the carbohydrate antigen CA15.3 [13–18], CA 27.29 [19, 20], carcinoembryonic antigen (CEA) [13, 15, 16, 18], clusterin [21], and alpha-1-antichymotrypsin [22]. Due to lack of specificity and/or sensitivity for early disease, however, none of these markers is of value for the detection of early breast cancer [15]. Consequently, novel, highly sensitive, and specific biomarkers for the early detection of breast cancer are urgently needed.
Although the progress in screening and the treatment of breast cancer is satisfactory, about 40 % of patients still surrender to the disease. The development of distant metastases is the main cause of these deaths. Breast cancer is generally no longer curable once metastases are detected by “classical” means: clinical manifestations of the spread, imaging methods, and serum marker assays, such as those based on carcinoma antigen 15.3 (CA15.3) or carcinoembryonic antigen (CEA). According to an established hypothesis, breast cancer dissemination should involve a succession of clinical and pathological stages starting with carcinoma in situ, progressing into invasive lesion, and culminating in metastatic disease. Further, it was thought for decades that metastasizing breast cancer cells (BCC) first disseminated to the lymph nodes before reaching peripheral blood and distant locations, including the bone marrow. Sadly, it has now become clear that metastatic spreading occurs in about 50 % of cases with apparently localized breast cancer and that up to 30 % of patients with lymph node-negative disease will grow distant metastases within 5 years [23]. Hence, recurrence is most likely due to the establishment of micrometastases before primary locoregional treatment. That BCC seem rarely able to shed from the primary lesion very early in the natural history of tumors, and that a direct hematogenous dissemination route is expected to exist that bypasses the lymphogenous one, robustly supports the search for techniques and tumor markers able to unmistakably identify DTCs. This should allow examining the potential of these DTCs in predicting the development of metastases and monitoring the response of patients to various therapies.
Breast cancer screening includes three methods of early detection: (1) breast self-exams (monthly) starting when a woman is in her 20s, (2) clinical breast exams (every 3 years) starting in a woman’s 20s, and (3) mammographic screening (annually) starting at the age of 40 years. Mammography seems quite satisfactory in reducing breast cancer mortality in women who are screened annually or biannually. Consequently, mammography is currently the only accepted screening procedure to discover (measured as the sensitivity) and to exclude (measured as the specificity) the presence of breast cancer in women who are asymptomatic [24, 25]. Even though mammography has reduced breast cancer mortality significantly, it suffers from some limitations: sensitivity ranges from 90 % to as low as 75 %, it leads to overdiagnosis and overtreatment, and it is inadequate for detecting the disease at a very early stage, that is, before the tumor starts to manifest its malignant potential [26, 27]. In addition, it has low-positive prognostic usefulness in younger women [28].
When breast cancer is detected at a localized stage and has less than 10 mm in size, the 5-year survival rate is 98 %. If the lesion is larger, it has often spread to nearby lymph nodes (regional disease), and the 5-year survival rate drops to 50–80 %. If the cancer has spread (metastasized) to distant organs such as the lungs, bone marrow, or liver, the 5-year survival rate is less than 25 %. Hence, it is crucial to develop more sensitive diagnostic tools that will not only complement mammography but also enable the detection and diagnosis of breast cancer much earlier than is currently possible, allowing therapy that is less invasive, thus causing less morbidity in patients while being more effective. The ideal screening approach would involve the development of a panel of highly specific and sensitive biomarkers that can be used to screen high-risk groups, detect recurrence, and monitor treatment using a simple blood-based test that can be performed by general physicians. Currently, the development and progression in exploration of CTCs and DTCs, along with genomics-, transcriptomics-, metabolomics-, and proteomics-based biomarkers, are currently promising to be better markers in the screening of various stages of breast cancer. A fluid-based, i.e., liquid biopsy, approach which utilizes serum/plasma should be pinpointed to determine disease status and progression earlier so the management would be better for breast cancer patients. This chapter will explore the current progress and development in the field of liquid biopsy-based molecular biomarkers in metastasizing breast cancer.
Molecular Biomarker-Based Breast Cancer Classification and Characterization
Recent technological advances have allowed the simultaneous evaluation of multiple RNAs (DNA microarrays) or proteins (tissue arrays) in tumor samples. These studies have revealed that breast tumors could be categorized into very few classes characterized by the high level of expression of specific groups of genes/proteins [29, 30]. According to these studies, about two-thirds of tumors express features reminiscent of the luminal epithelial component of the breast. These lesions are often well differentiated, have a low grade, and display relatively high levels of steroid receptors; cytokeratins KRT8, KRT18, and KRT19; BCL2, CDH1, MUC1; and the transcription factors GATA3, FOXA1, XBP1 [31], TFF1, TFF3, SLC39A6, CDKN1A, CDKN1B, and CCND1. In contrast to the “luminal epithelial-like” lesions, about 15 % of tumors have a low level of the abovementioned markers, whereas they express relatively high levels of cytokeratins KRT5 and KRT17, CDH3, EGFR, FOXC1, KIT, SERPINB5, TRIM29, GABRP, MMP7, SLPI, and various proliferation markers. Most of these “basal/myoepithelial-like” tumors are poorly differentiated and have a high grade [32]. Some of them are associated with the rare medullary carcinomas [33] and mutations in the familial cancer susceptibility BRCA1 gene [34]. Tumors overexpressing ERBB2 as a consequence of gene amplification may be sorted into a separate class (ERBB2 subtype), more closely related to the basal/myoepithelial-like than to the luminal epithelial-like lesions. Of interest, the luminal epithelial-like, basal/myoepithelial-like, and ERBB2 classes are also found in breast cancer cell lines [35], most of which are derived from DTC (obtained in most cases from pleural effusions).
It must be noted that among the markers listed above, many are relatively associated to a specific class. EGFR, SERPINB5, and GABRP are mostly expressed by basal/myoepithelial-like tumors, while high ERBB2 levels are noticeably expressed in lesions of the ERBB2 class. ESR1, TFF1, and TFF3, the expression of which is closely correlated, are found at high levels only in luminal epithelial-like tumors. Other markers related to this well-differentiated, low-grade class are the secreted proteins PIP, SCGB2A1, SCGB2A2, and SCGB2D1, as well as the mucins MUC1 and SBEM, the transcription factor SPDEF and ANKRD30A represent a stable portrait of breast cancer during progression, despite increasing genetic complexity. The existence of breast tumor classes defined by gene/protein signatures suggests that any tumor biology reflects to a large extent the biology of the cell of origin at the time of initiation. Tumors originating from more undifferentiated epithelial cells have a rapid growth pattern and more aggressive behavior and outcome compared with those beginning in a more differentiated epithelial cells. Therefore, the “portrait” of tumors seems to be stable during progression.
It is now clear—based on previous research and a number of data regarding breast cancer biology, pathology, and genetics—that during progression to metastasis, although undergoing increasing genetic alterations, most breast tumors largely maintain their portrait (luminal epithelial-like, basal-/myoepithelial-like, ERBB2). Indeed, the grade (I–III) and the expression of markers, such as ESR1, PGR, TFF1, EGFR, ERBB2, P53, and various proliferation markers, are generally concordant between primaries and metastases [36]. In fact, gene signatures underlying these portraits are preserved throughout the metastatic process of breast cancer [37]. This counters to the classical view, according to which tumor progression is commonly connected with some degree of dedifferentiation (i.e., loss of ER) and is expected to make a deep change in the biological status of cancer cells. One outcome is that DTCs are expected to express the same markers and, likely, the same properties (for instance, sensitivity or resistance to chemotherapeutic agents) than tumor cells in the corresponding primaries. While the portrait of tumors appears stable, their progression from in situ to metastasis is associated by an increasing genetic complexity. This probably results from the gathering of various minor (low-frequency) genetic or epigenetic events at many different sites of the genome, giving rise to a number of different blueprints, each restricted to a small cell subpopulation. This genetic microheterogeneity has small effects on the global portrait but will eventually modify the molecular balances controlling cell adhesion, migratory ability, proteolysis, and angiogenesis and, possibly, allow DTCs to colonize distant organs and produce secondary tumors [36].
Although genetic complexity is a hallmark of breast cancer, recent studies have allowed subclassifying tumors into a few categories, based on array-CGH analysis. Among breast tumors, DNA gains in chromosome 1q and loss in 16q appear to be the most common alterations. Some ER-positive, low-grade tumors have very few copy number alterations in addition to gain of 1q and loss of 16q and are associated with the finest patient outcome. At the other extreme of genome instability are tumors with many low-level copy number aberrations. Copy number losses involving chromosomes 3p, 4, 5q, 11p, 14q, 15q, 17q, and 18q are more ubiquitous in this group, which are composed mainly of ER-negative, high-grade lesions from patients experiencing significantly poorer outcome [38].
Fridlyand et al. have recognized an additional subgroup comprised of both ER-positive and ER-negative tumors and characterized by the presence of low-level gains and losses and recurrent amplifications [38]. The more commonly seen amplifications in this group, which occurred mostly in the ER-positive tumors, involved 8p, including FGFR1, 11q13, CCND1, and regions of 20q, including ZNF217. It is well known that specific gene amplification occurs commonly in breast cancer. For instance, ERBB2, EGFR, MYC, CCND1, MDM2, NCOA3/AIB1, FGFR1, TOP2A, CTTN/EMS1, FGF3, AKT2, and ZNF217 are genes for which amplification has been depicted in previous breast cancer studies [39]. The amplification of some of these genes has been connected more or less clearly to the degree of tumor aggressiveness. For instance, ERBB2 and MYC amplifications have been linked to reduced survival, while ERBB2/MYC-coamplified cancers have a poorer prognosis than tumors with only one of these amplifications [39]. Therefore, a decrease of survival is observed with increasing genome instability in primary tumors, but specific DNA gains/losses combinations as well as gene amplifications appear to have more weight in this regard.
DTCs and CTCs as Important Players in Breast Cancer Biology
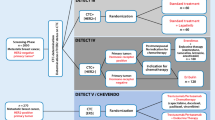
Metastasis is a multistage complex process that selects for CTCs that can infiltrate, survive in, and colonize distant organs [8]. Recent advances in this field are encouraging for the early dissemination model of metastasis, through the observation that DTCs isolated from the bone marrow or lymph nodes exhibit diverse changes on all levels of genomic resolution as compared to primary tumor cells [40]. Cancer cell dissemination may be followed by a dormancy period before relapse in one or more organs [41]. Research on DTCs and CTCs presents a challenge, as these cells are well-defined targets for understanding tumor biology and tumor cell dissemination in cancer patients, and will open new paths for the early detection of metastatic spread and its successful treatment. CTCs are rare, comprising a few cells per 106 hematologic cells in the blood of patients with metastasis; hence, their isolation presents a remarkable technical challenge [42]. DTCs and CTCs can now be detected and characterized at the single-cell level [43]. In Table 22.1, a comparison of CTC and DTC detection in breast cancer is represented.
Dissemination Sites: Lymph Nodes, Peripheral Blood, and Bone Marrow
Lymph Nodes (LN)
In the past, the detection of DTC is most important in pathological staging of lymph node (LN) specimens. In the last few years, the existence of DTC in bone marrow has also been shown to provide prognostic information. Promising detection strategies for DTC in peripheral blood (PB) are also being examined. Regarding LN in breast cancer, the risk of metastatic disease is classically estimated by factors such as tumor size, tumor grade, estrogen (ESR1) and progesterone (PGR) receptor status, ploidy, ERBB2 (HER2/neu), cytokines, MMPs, NF-KB overexpression, and the number of positive axillary lymph nodes (ALN). Several studies have shown that the presence of DTC in ALN is the most powerful prognostic factor, being associated with significantly poor disease-free (DFS) and overall survival [1]. During the past few years, the theory of sentinel lymph node (SLN) has emerged. SLN biopsy gears mapping of one or two LNs that primarily drain the tumor (the sentinel nodes) and therefore are most likely to harbor the metastatic disease. SLN examination is now widely performed in breast cancer, as it can provide prognostic value with minimal associated morbidity in contrast to complete ALN dissection.
The prescreening of SLN with highly sensitive detection methods for micrometastases thus represents a promising strategy. Considering that significant numbers of LN-negative patients develop metastatic disease, the dependability of current staging procedures to detect DTC in LN has been uncertain.
Peripheral Blood (PB)
Peripheral blood (PB) is historically one of the most potent diagnostic specimens. For example, circulating tumor markers have been evaluated in serum for years to give indicative values about metastatic or budding primary breast cancer. Serum markers may be good indications for tumor load, yet in most cases, they fail to provide information about minimal residual disease thus not up to mark. Technically speaking, PB appears as an ideal source for the monitoring of DTC. In fact, PB sampling is relatively trouble-free and can be done at frequent intervals (for instance, to permit an assessment of the patient’s recovery or potential to develop metastases). Several reports have demonstrated the presence of DTC in PB of patients with early-stage cancer without overt metastases [1, 24].
Bone Marrow (BM)
Contrary to PB sampling, blood marrow (BM) aspiration during surgery appears time-consuming and uncomfortable for the patient. However, among the distant organs, BM is a normal homing site for DTCs derived from breast cancer and other primary carcinomas, even in the absence of LN metastases or clinical signs of overt distant metastases [1]. Indeed, the screening rate of DTC in BM from nonmetastatic breast cancer patients has been demonstrated to be in the range from 0 % [44] to 100 % [45], and this corresponds to the variability of results obtained by the use of different techniques or marker genes. In a recent, large (more than 3,500 cases) study of stages I through III breast cancer patients, the incidence of DTC in BM detected by immunocytochemistry (ICC) ranged from 13 to 43 % [46]. The presence of DTC in BM may be supportive not only in predicting the development of bone metastases but also in predicting the development of metastases in other remote organs, such as the lung and liver. At present, however, it remains unsolved whether BM is a reservoir that allows for DTC to adapt and disseminate later into other organs or whether the presence of DTC in BM might reflect the general tendency of these cells to disseminate and survive in organs, rather than just in the BM. Until methods are developed to detect the presence of DTC in organs, such as the lung or liver, it will not be possible to distinguish between these two possibilities. The BM could serve as a reservoir in breast cancer and is supported by the presence of epithelial (cytokeratin-positive) cells in the PB of patients with overt remote metastases years after the removal of the primary tumor. This suggests that tumor cells could break from bone metastases to recirculate and disseminate to secondary tissues [1]. This “two-step” metastasis model could explain why the DTC in patients with overt metastases closely resemble each other genetically [47].
According to Ring et al. [48], in studies using antibody-based (cytometric) assays, cells with the characteristics of tumor cells have been shown in the PB of between 0 and 100 % of patients with operable (stages I through IIIa) breast cancer and in the PB of between 3 and 100 % of patients with metastatic disease. Several reports with nucleic acid-based techniques have shown cells with the characteristics of tumor cells in the PB of 0–88 % of patients with operable (stages I through IIIa) breast cancer and in 0–100 % of patients with metastatic disease. Along the same line, in a survey on a total of more than 3,500 stages I through III breast cancer patients, the incidence of DTCs in BM detected by ICC ranged from 13 to 43 % [46]. In fact, the detection rate of DTCs in BM from nonmetastatic breast cancer patients has been reported to be in the range from 0 % [44] to 100 % [45]. The variability of results obtained in DTC detection results from dramatic variations in methodology. Factors that may influence the results as heterogeneity of the studied populations may be:
-
1.
Stage. The number of positive patients and the absolute numbers of DTCs per patient rise as clinical stage rises [49].
-
2.
Interval of time separating surgery from the obtaining of DTCs. Surgery may increase the number of breast cancer DTCs (from 0 to 8,000 cells/ml) in the PB, which persist for varying length of times in different patients [50].
-
3.
Metastasis location. The separation of populations into those with early and metastatic breast cancer is probably simplistic. Moreover, metastasis sites could be missed when DTCs are obtained, leading to a misclassification of the patient in the “early breast cancer” category.
Other factors such as sample handling and preparation, delay between collection and analysis, conditions of sample storage, and contamination with normal epithelial cells may influence the results. The introduction of skin cells into a PB sample at the time of venipuncture could lead to false-positive results. Many researchers advocate that the first few milliliters of sampled PB are discarded to avoid such contamination. It has also been suggested recently that false-positivity of SLN could result from iatrogenic displacement and transport of benign epithelial cells in patients with breast carcinoma [51]. Clearly, such epithelial cells do not represent metastasis.
Detection of DTCs/CTCs in Bone Marrow
Current models of breast cancer metastasis hold up the possibility of early dissemination of cells from primary tumors and the direct release of DTCs into the blood and BM, bypassing, in some cases, the lymphatic system. DTCs are rare with only 10–20 cells among millions of BM cells. In order to increase the opportunity to screen DTCs in this organ, procedures had to be developed for their enrichment prior to detection and further characterization. For this, different density gradient centrifugation methods such as Ficoll-based assays or the OncoQuick approach, as well as positive or negative immunomagnetic enrichment procedures and simple filtration methods separating tumor cells by their size, have been recognized [9]. Currently, there are two different methods to detect BM aspirates for DTCs/CTCs—namely, cytologic/cytometric (antibody-based) and molecular approaches and nucleic acid-based approaches. The current technologies for CTC detections are summarized in Table 22.2, and a list of commonly used markers in assays to detect disseminated tumor cells by antibody- or nucleic acid-based techniques is summarized in Table 22.3.
Antibody-Based Techniques
Approaches by fluorescence microscopy (FM), ICC, and flow cytometry (FC) analysis aim to isolate and enumerate individual tumor cells. ICC is still a gold standard for DTC detection, and most of the available clinical data have been gathered by ICC screening, especially in BM [23]. An advantage of this approach is that it may permit further characterization of the cells at a molecular level, in terms of expression of key biological markers, such as ERBB2 (ERBB2 gene amplification estimated by FISH analysis) and morphological cell investigation. However, identification of intracellular targets, such as cytokeratins, by antibodies needs cell permeabilization. As a consequence, cell viability is lost, making the important discrimination of dead and viable DTC impossible. Since only viable cells might lead to metastasis, this valuable information cannot be evaluated [23].
Like IHC, FM and ICC are labor intensive and time-consuming, making these techniques too expensive for routine implementation. When compared with conventional, essentially qualitative FM and ICC, FC offers the advantage of a fully automated technique permitting quantitative measurements with high sensitivity, good resolution, speed, reproducibility, and statistical reliability. For breast tumors, the most used targets for antibody-based techniques are the cytokeratins. ERBB2, MUC1, and TACSTD1, the latter two being known under a variety of names, have also been used as antibody targets to isolate and/or identify DTC.
Two-color ELISPOT, an immunological assay based on enzyme-linked immunosorbent assay, has been recently used to detect DTC-secreting cathepsin D (CTSD) and mucin-1 (MUC1). However, antibody-based techniques have limitations. Many of the antibodies directed at epithelial and breast cancer cells are known to also stain hematopoietic cells, including cytokeratins (KRT19), TACSTD1, and MUC1. Nonspecific staining of plasma cells can also occur due to alkaline phosphatase reaction against the k and l light chains on the cell surface [52]. According to the antibody used, a false-positive detection rate of 1–3 % can be estimated [23]. Since tumor- and epithelial-specific cell marker antigens are expressed differentially in DTCs, the use of a panel of monoclonal antibodies may help to enrich DTCs and facilitate their finding [53].
Nucleic Acid-Based Techniques
PCR, either qualitative or quantitative, has been used to identify and characterize DTCs through the detection of genetic (allele-specific expression, microsatellite instability, loss of heterozygosity) and epigenetic alterations (methylation status) that are exclusively linked with cancer cells [54]. This includes the search for tumor-associated point mutations in oncogenes or tumor suppressors. This latter PCR approach, however, is complex by the substantial degree of genetic variability between tumors. For instance, TP53, the gene coding for p53, is mutated in about 25 % of breast tumors; however, more than 1,400 different mutations of this gene have been observed [55]. Of note, PCR has been used to screen free DNA within plasma. For instance, the analysis of DNA methylation status of specific genes (ESR1, APC, HSD17B4, HIC1, and RASSF1A) in serum of breast cancer patients has been shown to be of prognostic value [56]. The PCR-based measurement of RASSF1A methylation has been used for examining efficacy of adjuvant tamoxifen therapy [57]. However, this use of PCR is imperfect by poor specificity. This is due in part to the high stability of DNA in plasma when compared with mRNA [58]. As a result, it is unclear whether the free DNA that is amplified from plasma is from DTCs present in plasma or if the DNA is being shed from primary tumors, metastatic tumors, or from normal tissue [48]. To identify DNA gains and losses in single DTC, the technique of comparative genomic hybridization (CGH) is increasingly used [59].
Reverse transcription (RT)-PCR has been used to identify DTC through their expression of epithelial or breast cancer-associated mRNA transcripts. RT-PCR is generally more sensitive than antibody-based techniques but has also been hampered by false-positive results in samples from normal volunteers and from patients with hematological malignancies [48]. These false-positives stem from multiple sources, including issues with laboratory technique, primer selection, illegitimate expression of the target genes in normal cells, the existence of pseudogenes, or contamination (KRT19/CK19). When using assays based on RT-PCR for detection of DTCs, the balance between sensitivity and specificity must be considered. Generally, specificity decreases with the increase in sensitivity, and vice versa. One way to resolve this problem is to examine multiple tumor markers in samples. As mentioned below, multiplex RT-PCR assays have revealed a higher efficacy (in both sensitivity and specificity) in comparison with the assessment of single markers. To recover the reliability, especially the specificity of RT-PCR assays, quantitative RT-PCR (qRT-PCR) may be used. In addition, qualitative marker information, qRT-PCR uses cutoff values of marker transcript numbers, below which transcripts can be considered as tumor cell-derived. Moreover, when compared with conventional RT-PCR, qRT-PCR relies not only on primers but also on internal probes that specifically hybridize to the amplified sequences. In addition, due to the continuous measurement of the amplified signal, false-positive results, which could produce an abnormally shaped, nonlinear amplification curve, could be easily identified and removed [23]. Variations of the RT-PCR technique, such as nested RT-PCR and competitive nested RT-PCR, have also been used [60].
Fluorescence in situ hybridization (FISH) allows the detection of gene amplifications, for instance, ERBB2 amplification in breast cancer. FISH has been used to analyze genetic aberrations in DTC in BM. Considering the importance of ERBB2 as a novel target for successful antibody-based therapy, the use of FISH to identify ERBB2 amplification in DTC appears promising [61]. Among the cytologic methods that allow isolation and enumeration of individual cells, immunocytochemistry is the most widely used approach. Because of the absence of tumor-specific target antigens—most commonly antibodies against various epithelium-specific antigens such as cytoskeleton-associated cytokeratins—surface adhesion molecules or growth factor receptors are used for the screening of carcinoma cells [62]. The main advantage of cytologic methods is the opportunity to combine immunostaining with the morphology of the cells so that cell size and shape as well as the nucleusplasma relation might be predictable and illicit expression of the protein of interest in BM cells can be excluded.
The detection of DTCs in BM is not yet a routine part of the tumor staging in the clinical practice, but rising data anticipate a future role of DTC screening for risk estimation and therapeutic monitoring of breast cancer patients [63]. However, the detection rates of DTCs in BM from nonmetastatic breast cancer patients vary significantly [45]. This might reflect the different sensitivity, but also specificity, of the numerous detection methods and marker genes/proteins used thus far. The newly defined consensus concept for the detection of DTCs in BM, signifying enrichment of mononuclear cells from BM by Ficoll density gradient centrifugation and immunocytochemical detection of cytokeratin expression as standard procedure, should help overcome these troubles and provide the basis for future multicentric clinical trials. The researchers recommend the pan-anti-cytokeratin antibodies A45-B/B3 or AE1/AE3 against a wide spectrum of cytokeratins as standard application, thereby ensuring detection of DTCs also in cells that have downregulated the expression of individual cytokeratins in the course of epithelial–mesenchymal transition [42]. Microscopic screening of large amounts of immunostained cytologic preparations is accomplished by automatic microscopes using sophisticated imaging approaches. Criteria to examine morphology and staining results have also been defined to avoid false-positive and false-negative results [42].
Although there are existing recommendations for standard operation procedures, there are still restrictions to the standardization of immunocytochemical methods with respect to reproducibility of the staining procedure itself as well as microscopic interpretations. Therefore, both intra- and interlaboratory evaluation of the methods is required to ensure reliability of the results [64].
Besides immunocytochemical methods, very sensitive nucleic acid-based techniques now allow the detection of DTCs at the single-cell level. The main advantage of these methods is the nearly unlimited availability of primers for almost every gene of interest. Although numerous genetic alterations have been described in breast cancer cells, heterogeneity is enormous, so that at present no universally applicable DNA marker exists for the primary screening of a wide range of DTCs [9]. Further efforts have been made to detect free circulating DNA or epigenetic alterations of circulating DNA such as methylation in BM and blood plasma, but the results are still preliminary [65]. Therefore, the measurement of epithelium-specific or more organ-specific mRNA species such as cytokeratin 19 or mammaglobin mRNA by RT-PCR has been proven to be a promising approach to detect DTCs in BM samples [66]. Because of the lack of tumor-specific markers, the main disadvantage of using surrogate tissue-specific markers is false-positive results due to illegitimate low-level transcription of epithelial or breast tissue-specific genes in normal cells [48]. Furthermore, heterogeneity in the expression of particular genes is not recognizable and the expression level of a gene of interest per cell cannot be estimated. At present, analyses are mainly performed by quantitative real-time RT-PCR, ensuring the discrimination between different levels of expression. Moreover, multimarker real-time RT-PCRs have the potential to improve the method even in the case of downregulation of the expression of a single gene [45]. However, storage and sample preparation have to be performed under conditions avoiding RNA degradation, one of the major problems of RT-PCR approaches [66].
The application of multimarker assays might also compensate for low mRNA amounts due to the low number of tumor cells. There are numerous excellent reviews listing the marker genes currently used in RT-PCR approaches to detect DTCs in BM or CTCs in blood from breast cancer patients [48]. The methods explained above are not able to discriminate between viable and apoptotic DTCs. A new technique, designated EPISPOT (epithelial immunospot), offers the advantage of detecting viable tumor cells by their ability to secrete individual proteins. In a newly published study, it was demonstrated that BM samples from metastatic and nonmetastatic breast cancer patients contain viable tumor cells which secret Muc-1 and/or cytokeratin 19 in about 90 and 50 % of cases, respectively, whereas in controls from healthy women, cells secreting these proteins could not be detected [9].
Clinical Relevance of DTCs in Bone Marrow (BM)
A large number of studies have documented DTCs in BM from patients with most types of epithelial cancers [1]. Within the last 15 years, several studies have confirmed that detection of DTCs in BM of breast cancer patients is accompanied by a substantially worse prognosis [63]. In a pooled analysis evaluating the results from 9 different European centers, including a total of 4,703 patients, Braun et al. have reported that approximately 30 % of women with primary breast cancer have DTCs in BM, and in a multivariate analysis, the 10-year follow-up of these patients revealed a significantly decreased overall survival, when compared to patients without DTCs [67]. The presence of DTCs in BM was significantly associated with higher tumor stage, worse differentiation, lymph node metastasis, and negativity in hormone receptor expression. Prognostic relevance was shown for all subgroups, even among those patients with small tumors and without lymph node metastasis. While using different antibodies and detection methods, almost all investigators participating in this pooled analysis used anti-cytokeratin antibodies to screen for DTCs in the BM [67].
Bone Marrow of DTCs Replaceable by Blood CTCs?
Aspiration of bone marrow (BM) is invasive, time-consuming, and in many cases painful or at least uncomfortable for patients, precluding repeated samplings necessary for therapy-monitoring studies. Moreover, BM aspiration is more difficult to standardize with regard to the required volume and quality. Consequently, recent efforts have concentrated on the detection of CTCs in peripheral blood (PB) of cancer patients [48], but the clinical usage of CTCs has not yet been implemented for routine clinical practice. Furthermore, there are only a limited number of studies comparing BM and PB examinations performed at the same time points, and the clinical significance of CTCs in PB is less clear than that for DTCs in BM. In all studies published thus far, there was a higher frequency of BM-positive than blood-positive samples from the same patients [68], probably due to the fact that BM might provide conditions for homing and survival of DTCs, thus contributing to their accumulation in this compartment. Although both Pierga et al. [68] and Muller et al. reported about a significant number of patients with concordant results concerning BM and blood analysis [69], in the Pierga study, only the presence of DTCs in BM and not that of CTCs in blood had prognostic relevance for disease-free survival in nonmetastatic breast cancer patients [68]. Interestingly, the presence of both DTCs in BM and CTCs in blood in a subgroup of patients resulted in an especially poor prognosis [70]. While all studies referred above applied immunocytochemical methods, real-time RT-PCR detection of DTCs in BM also had superior prognostic significance in comparison with CTCs in patients with breast cancer. A study analyzed cytokeratin 19 and mammaglobin mRNA levels by quantitative RT-PCR [71]. Currently, the results obtained by comparative studies do not hold a replacement of BM by blood analysis, but CTC detection might have supplementary value.
There are an increasing number of studies demonstrating clinical relevance of CTCs in blood detected by real-time RT-PCR, identifying either only cytokeratin 19 mRNA or multiple markers [62]. Recently, analyzing cytokeratin 19 mRNA by real-time RT-PCR [72], they detected CTCs in 22 % of blood samples from 167 node-negative breast cancer patients as significantly associated with overall and disease-free survival. A correlation of the presence of CTCs in blood to the lymph node status was found in 2007 [73], when CTCs were detected with the help of a multimarker real-time RT-PCR in 39 of 90 (43 %) stage I through III breast cancer patients, but not in normal healthy volunteers.
Significant progress in this field arose from the development of an automated enrichment and immunocytochemical detection system for CTCs (CellSearch™) [74]. This system consists of an automated instrument for the enrichment of epithelial cells by ferrofluids coated with anti-EpCAM antibodies followed by immunostaining of captured cells with fluorescently labeled anti-cytokeratin and anti-CD45 antibodies (AutoPrep), and a semiautomated microscope for scanning and reading results (CellSpotter Analyzer). Using this system, Cristofanilli et al. [74] demonstrated in a prospective study that CTC detection provided important prognostic information for patients with metastatic breast cancer. Additionally, Hayes et al. demonstrated that CTCs at each follow-up time point during therapy of these metastatic breast cancer patients predict progression-free and overall survival. The CellSearch system has been cleared by the FDA for regular clinical use in metastatic breast cancer patients. Validation data from three independent laboratories and high interinstrument accordance confirmed the reliability of this system for CTC measurements in PB from metastatic breast cancer patients.
Also, it was shown that samples can be shipped at room temperature and CTC counts are stable for at least 72 h, which facilitates testing at central laboratories or remote sites requiring transportation [75]. There are also several reports about the detection of CTCs in patients with primary breast cancer, however, mostly with lower frequencies and varying results concerning both the number of positive patients and the number of CTCs in individual patients [76].
Molecular Characterization of DTCs in Bone Marrow and CTCs in Blood
The characterization of DTCs/CTCs is aimed to (1) provide proof for their malignant origin and (2) identify further diagnostically and therapeutically related features of these cells, which might permit a more targeted and individualized antimetastatic therapy. This characterization is hindered by the fact that DTCs/CTCs can exhibit features distinct from the primary tumors, but on the other hand, this could help to identify cancer patients for additional targeted therapies. By multiple fluorescence in situ hybridization analysis, it was shown that the vast majority of CTCs in blood from breast cancer patients are aneusomic and derived from the primary tumor [42]. By single-cell comparative genomic hybridization, further study indicated that DTCs might be gnomically unstable and heterogeneous [77]. Moreover, research also suggests that DTCs from BM of breast cancer patients disseminate in a less progressed genomic state and might acquire genomic alterations typical for metastatic cells later [78].
In order to escape from the dormant state into the dynamic phase of metastasis arrangement, dormancy has to be disturbed by both genetic and epigenetic changes in the DTCs/CTCs as well as in the surrounding microenvironment or premetastatic niche [79]. Transcriptional analyses of EpCAM-enriched BM and blood cells resulted in gene expression profiles that may be used to differentiate normal donors from cancer patients [80]. Further studies have to reveal whether individual genes, the expression of which is changed in these cell populations, might become markers to recognize recurrence in breast cancer patients early [80].
Interestingly, TWIST1, a transcription factor that in the past has been recognized to play an important role in metastasis by accelerating epithelial–mesenchymal transition [81], was part of the gene expression signature identified in EpCAM-enriched cells from BM of breast cancer patients after chemotherapy [80]. TWIST1 expression, which was not observed in EpCAM-enriched cells of BM from healthy volunteers, linked with the occurrence of remote metastasis and local progression, even in pretreatment BM samples [80].
DTCs/CTCs seem to be heterogeneous with regard to the expression of growth factor receptors, adhesion molecules, proteases, and their inducers and receptors, major histocompatibility complex antigens, or signaling kinases [47]. Of particular attention is the epidermal growth factor receptor HER2, the expression of which in primary tumors forms the basis of Herceptin treatment decisions for breast cancer patients.
As shown by Braun et al., HER2 overexpression on DTCs in BM was predictive for a poor clinical outcome of stage I through III breast cancer patients [82]. While a study of 27 breast cancer patients showed that the HER2 status remained relatively stable between primary tumors and BM micrometastases in most cases [83], there is also increasing proof for discrepancies between the HER2 status in primary tumors and DTCs in BM [84]. They noticed HER2-positive DTCs in 12 of 20 BM samples from patients with HER2-negative primary tumors. Although HER2 expression was heterogeneous in DTCs from individual patients, HER2-positive DTCs might recognize additional patients who can benefit from Herceptin therapy. The HER2 status of CTCs from PB might also be different from that of the corresponding primary tumors as reported [85]. These authors presented a significant number of patients whose primary tumors were HER2 negative, whereas CTCs were HER2 positive before surgery. Moreover, in this study the recognition of HER2-positive CTCs correlated significantly with disease-free and overall survival [85]. It remains to be explored whether high levels of HER2-positive CTCs reflect the activity of the tumor and have predictive value for an improved response of the patients to Herceptin treatment [85]. Although Meng et al. reported a high agreement (97 %) of the HER2 status between primary tumors and CTCs in 31 cases, during tumor progression, HER2-positive CTCs could be detected in 9 of 24 breast cancer patients in spite of HER2-negative primary tumors. These CTCs might have acquired HER2 gene amplifications. Four of these patients received Herceptin therapy and three of them responded to this therapy [61].
In the study shown by Apostolaki et al., adjuvant chemotherapy eliminated HER2 mRNA-positive CTCs in 16 of 45 patients. The detection of HER2 mRNA-positive CTCs after chemotherapy was linked with a reduced disease-free survival. Moreover, in 8 of 161 patients with HER2-negative primary tumors, HER2 mRNA-positive CTCs could be noticed [86]. Therefore, the detection of HER2 mRNA-positive CTCs after adjuvant chemotherapy in the PB of stage I and II breast cancer patients might provide information about the usefulness of chemotherapy and the prognosis of the patients and identify patients in need of additional Herceptin therapy [86].
During the past few years, the number of single markers that have been assessed for DTC detection, mainly by nucleic acid-based techniques, has noticeably increased. For a detailed description of these studies, the reader is encouraged to consult the current reports published by Gilbey et al. [60] and Ring et al. [48]. In this chapter, the same name will be used for the gene and the corresponding protein. For instance, regardless of the fact that the terms NY-BR-1 and B726P are bumped into in the literature, the name of the corresponding gene, ANKRD30A, will also preferentially be used to cite the protein. SCGB2A2 will be used instead of mammaglobin, ESR1 rather than estrogen receptor-a (ERa), etc.
An ideal marker should be universally, but exclusively, expressed on all breast cancer cells. It should be easily noticeable, with little variance, and bear clinical relevance. Since no single, precise marker that meets these criteria has been recognized, attempts are now made to develop assays with multiple tumor markers, of which some are preferably highly specific to breast tissue or breast tumors. The aim is to avoid both false-positive (detection of non-tumor cells, due to the fact that the majority of potential markers have some baseline expression in normal tissues) and false-negative (non-detection of tumor cells, due to the use of high-threshold levels for positivity) cases.
Multimarker assays have been used by various researchers [48, 60, 87] and have shown a higher efficacy (sensitivity and specificity) in comparison with the assessment of single markers. Markers with low breast (cancer) specificity cytokeratins (KRTs) regarding epithelial tumors, the cytoskeleton components KRTs have become the markers of choice for DTC recognition. They belong to a large multigene family of more than 30 identified members. They are expressed at various levels and compositions in all epithelial tumors, but hardly ever in other tissues. For antibody-based studies, most use a combination of several monoclonal antibodies that distinguish various cytokeratin antigens or a broad-spectrum anti-cytokeratin monoclonal antibody that recognizes a single epitope that is frequent to most cytokeratins [1, 48]. For nucleic acid-based studies, cytokeratin 19 (KRT19) and, to a lesser extent, cytokeratin 20 (KRT20) have been commonly used as markers. KRT19 presents an illustration of the possible sources of false-positivity in DTC detection.
Due to its high sensitivity, KRT19 is the widely used marker for finding DTCs in breast cancer patients [48, 60]. Depending on the assays, KRT19 has been discovered to be both a specific and a nonspecific marker. In fact, KRT19 is an outstanding candidate to demonstrate the potential sources of false-positivity in RT-PCR studies: illegitimate transcription, hematological disorders, the presence of pseudogenes, and sample contamination. Illegitimate transcription explains the expression in normal tissues of small amounts of mRNA by genes that have no actual physiological role in these cells. It can be estimated that every promoter could be activated by ubiquitous transcription factors, which leads to an estimated expression level of one tumor marker gene transcript in 500–1,000 non-tumor cells [23]. For hematological disorders, KRT19 expression can be induced in PB by cytokines and growth factors, which circulate at higher concentrations in inflammatory conditions and neutropenia [48]. As a consequence, false-positive results are more expected under these circumstances. The presence of pseudogenes, two KRT19 pseudogenes, KRT19a and KRT19b [88], have been identified, which have significant sequence homology to KRT19 mRNA. Consequently, attempts to identify the expression of the authentic KRT19 may result in the detection of either or both of these pseudogenes. To avoid pseudogene amplification, it is suggested to carefully design the primers used for RT-PCR analysis. Regarding contamination, it has been suggested that PB sampling for subsequent analysis could introduce contaminating epithelial cells expressing the KRT19 mRNA into the blood sample. Possible contamination could be reduced or prevented by discarding the first sample of blood taken.
In conclusion, KRT19 emerges to be a very sensitive tumor marker, whose use, however, is often held back by low specificity. It is useful in detecting disseminated epithelial cells but is not a true breast cancer marker.
KRT20
KRT20 is found in breast cancer cells [89]. However, its expression is less linked to breast tissue and more related to gastric and intestinal epithelium, urothelium, and Merkel cells [23]. Additionally, KRT20 expression has been established in granulocytes [90]. Due to its lower specificity when compared with KRT19, the use of KRT20 is not suggested in breast cancer patients. KRT8 and KRT18 have been hardly ever used for DTC detection. In fact, the expression patterns of these epithelial cytokines are very similar to that of KRT19 and they are not expected to provide more specificity than KRT19. Of note, KRT8, KRT18, and KRT19 are expressed in the breast epithelium but at higher levels in the luminal than in the basal component. In view of recent observations that breast tumors may be classified into subtypes, or classes, including luminal epithelial-like and basal epithelial-like, one can believe that these cytokeratins will be less easily distinguished in DTCs originating from basal-like tumors.
CEACAM5
Commonly known as CEA, it functions in several biological roles, including cell–cell adhesion. It is one of the most commonly expressed markers in breast, as well as in various other, cancer cells [48, 60]. Therefore, it suffers from low specificity, as also seen with KRT19, and can likewise be induced in peripheral blood (PB) by cytokines and growth factors [48].
TACSTD1
This epithelial cell–cell adhesion protein is known under a range of names, of which GA733-2 and EpCAM are the most commonly used. Ubiquitously expressed on the surface of epithelial cells, it has been normally used as a target for positive IMS to enrich DTC for RT-PCR analysis [23]. Monoclonal antibodies against this antigen have been widely developed for diagnostic, but also therapeutic, approaches. Although highly sensitive for epithelial malignancies, including breast cancer, its use is, however, hindered by the fact that it is expressed in low amounts in PB cells [91].
MUC1
Mucin-1 is an extensive, polymorphic, and heavily glycosylated mucin. The role of mucins is mainly one of the hydrating and lubricating epithelial linings, but these proteins have also been concerned in modulating both growth factor signaling and cell adhesion. Further, it has been suggested that MUC1 expression at the surface of tumor cells could decrease cell adhesion and favor dissemination [92]. Conversely, MUC1 could play a role in the initial attachment of breast tumor cells to tissue at remote sites, facilitating establishment of metastatic sites [93]. Extensively expressed in normal epithelial tissues, MUC1 is remarkably present on the apical surfaces of breast, bronchial, pancreatic, uterine, salivary, intestinal, and other glandular tissue cells. Like TACSTD1, MUC1 has been commonly used as a target for positive IMS to enrich DTC for RT-PCR analysis [23]. Many studies have reported the expression of MUC1 in a significant proportion of healthy blood donors. Indeed, MUC1 expression has been considerably found in PB cells [23]. Although it has low specificity, the assessment of MUC1 expression in DTC is supported by the increasing interest for MUC1-based immunotherapy [94]. Although MUC1 is expressed in a majority of breast tumors, its overexpression has been associated with a lower grade and a higher ER-positive phenotype [95].
EGFR
A series of RT-PCR-based mono- or multimarker studies have assessed the relevance of this growth factor receptor for DTC detection [96, 97]. EGFR emerges as more specific but less sensitive than KRT19. Unluckily, it has also been found infrequently in the PB of healthy donors [23]. Furthermore, Weigelt et al. [97] have shown that the median expression of EGFR was higher in normal ALN than in DTC-positive ALN! Notably, EGFRvIII, a cancer-specific EGFR variant, has been now used to detect DTC in breast cancer patients. The mutant was seen in the peripheral blood in 30 % of 33 low-risk, early-stage patients, 56 % of 18 patients chosen for neoadjuvant chemotherapy, 63.6 % of 11 patients with disseminated disease, and, remarkably, 0 of 40 control women [98].
ERBB2
Involved in signal transduction, ERBB2 participates in breast tumor biology. Yet, it is not breast specific [99], and weak ERBB2 expression has been found in the PB of healthy women in several studies [23]. However, it is overexpressed in 20–35 % of breast cancer patients, mostly as a result of gene amplification, and this forecasts for reduced survival. Furthermore, in patients with breast cancer, ERBB2 overexpression by DTC in the BM predicts poor clinical outcome [82]. This, as well as the increasing use of ERBB2 as target for immunotherapy (trastuzumab) [94], supports its assessment in DTC, at both the mRNA (RT-PCR) and the DNA (FISH) levels.
Markers with High Breast (Cancer) Specificity
Using molecular biology methods or combinations of techniques, various groups have recognized markers specifically expressed in breast and/or breast cancer tissue or cells, when compared with normal PB, BM, or other human tissues. For instance, genes profusely expressed in breast cancer tissue but absent in normal PB and BM have been identified by serial investigation of gene expression (SAGE).
By order of decreasing SAGE tag frequency, these genes are SBEM, LACRT, TFF3, COL1A1, MGP, KRT8, MUC1, KRT7, CLECSF1, IL6ST, APOC1, SCGB2A2, TFF1, TM4SF1, C6, and KRT19 [100]. A series of genes coding for secreted proteins overexpressed in breast cancer tissue when compared with corresponding normal tissue and/or other (colon, gastric, kidney, liver, lung, ovary, pancreas, prostate) normal tissues were recognized by a combination of annotation/protein sequence analysis, transcript profiling, immunohistochemistry, and immunoassay: HAPLN1, GFRA, SCGB1D2, CXCL10, CXCL11, COL11A1, E2F3, TRMT1, CHST2, SERHL2, ZNF324, SCGB2A2, COX6C, and SCGB2A1 [101]. Gene expression profiling was used to construct a site of origin classifier in order to decide the origin of cancer of unknown primary. From an analysis of 229 primary and metastatic tumors representing 14 tumor types (breast – 34 samples, colorectal, gastric, melanoma, mesothelioma, ovarian, pancreas, prostate, renal, testicular, squamous cell carcinoma, uterine, and lung), a “finest” list of 79 site-specific markers was defined. Genes linked to breast specificity were ACADSB, CCNG2, ESR1, EFHD1, GATA3, SLC39A6, MYB, SCYL3, PIK3R3, PIP, PRLR, RABEP1, TRPS1, and VAV3. Two of them, GATA3 and PIP, were recognized as appearing to be strongly and relatively consistently expressed across the range of breast tumors.
Smirnov et al. [102] achieved PB containing R100 DTC from one metastatic colorectal, one metastatic prostate, and one metastatic breast cancer patient. In a primary step, global gene expression study was performed on these samples, and a list of cancer-specific DTC genes was achieved. Among genes distinguishing between tumor (colorectal, prostate, and breast) and control patients were KRT18, KRT19, TACSTD1, TACSTD2, AGR2, TFF1, and TFF3, all genes known to be linked to the epithelial cell phenotype. Fifty-three genes distinguishing between breast tumor and controls were recognized, including ESR1 and ERBB2.
In a second step, PB samples immunomagnetically enriched for DTC from 74 metastatic patients (30 colorectal, 31 prostate, 13 metastatic breast cancer patients, and 50 normal donors) were used to confirm the DTC-specific expression of selected genes by real-time RT-PCR. The genes most restricted to breast cancer patients, when compared with normal donors and colorectal cancer and prostate cancer patients, were SCGB2A1, SCGB2A2, and PIP. Two additional genes, S100A14 and S100A16, were restricted to breast and colon cancers. Of note, two genes, KRT19 and AGR2, were expressed in the majority of metastatic samples (colorectal and prostate and breast) and not in the control individuals. This validates the interest of KRT19 as an epithelial tumor cell marker.
Yet AGR2 expression has been less frequently examined. Smirnov et al. [102] isolated RNA from a highly metastatic SCGB2A2-overexpressing ALN (only one sample). It was diluted into a pool of normal LN RNA at various ratios. Gene expression (microarray) analysis was performed, and candidate breast cancer-associated genes were then selected based on three criteria: (a) absence of expression in a pool of four normal LN, (b) a high fluorescence signal on microarray, and (c) a fluorescence signal also present in the 1:50 dilution. The 34 genes recognized by criteria (a), (b), and (c) were specified by relative intensity of the signal in the metastatic ALN. The 14 genes were SCGB2A2, TFF1, TFF3, KRT19, SCGB1D2, S100P, FOS, SERPINA3, ESR1, TACSTD2, JUN, PGDS, KRT8, and AFP. Notably, other genes used for molecular finding of micrometastatic disease, such as PIP, SPDEF, TACSTD1, CEACAM5, and SCGB2A1, were not present among the top 15, even though their signal was observed in metastatic ALN. Real-time RT-PCR analysis of pathology-negative ALN (nZ72) demonstrated that of PIP, SCGB2A2, SPDEF, TACSTD1, and TFF1, SCGB2A2 and TFF1 had the highest evident sensitivity for the detection of micrometastatic breast cancer [103].
In a microarray approach, Backus et al. investigated RNA from samples covering normal, benign, and cancerous tissues from breast, colon, lung, ovarian, prostate, and peripheral blood leukocytes from healthy donors. By a combination of this microarray testing and database/literature searching, a series of candidate breast tissue-specific markers and candidate breast cancer status markers were recognized [104]. These potential markers were then submitted to an additional multiuse selection process: some markers were excluded for one of the following reasons: (1) their expression level in white blood cells was too high, (2) their expression in breast cancer was too low, and (3) their expression in lung, colon, and ovarian cancers was too high. The authors finally achieved 14 markers, of which 7, ANKRD30A, GABRP, KRT19, OR4K11P, PIP, SCGB2A2, and SPDEF, were further chosen (the others were CEACAM6, ERBB2, MUC1, S100A7, S100A14, SBEM, and TNNT1). The utility of these markers for identifying clinically utilizable metastases in LN was assessed through RT-PCR analysis of SLN from 254 breast cancer patients. The investigators recognized an optimal two-gene expression (KRT19 and SCGB2A2) marker set for the detection of the actionable metastasis in breast SLN [104].
A series of markers with high breast (cancer) specificity reported so far are now in details.
SCGB2A2
No breast cancer marker has been shown to be never expressed in healthy volunteers, but some markers are hardly ever found in controls. SCGB2A2 [105], widely known as mammaglobin, is one of these markers. It is a member of the secretoglobin superfamily [106], a group of small, secretory, rarely glycosylated, dimeric proteins generally expressed in mucosal tissues that could be involved in signaling, immune response, chemotaxis [107], and, probably, as a carrier for steroid hormones in humans. SCGB2A2 has become a quasi standard in breast DTC detection by RT-PCR-based methods, being the most extensively studied marker after KRT19. It has been used to identify DTC in LN, PB, BM, and even in malignant effusions. SCGB2A2 expression has been noticed, rarely and in low levels, in various normal tissues. This could restrict its prospective use as an immunotherapeutic target [108], due to concerns about autoimmune toxicity.
Zafrakas et al. have found an abundant SCGB2A2 expression in malignant and normal tissues of the breast and in the female genital tract, namely, the cervix, uterus, and ovary, while lower expression levels were hardly ever found in other tumors and normal tissues [109]. These remarks might extend the diagnostic potential of SCGB2A2 to the detection of DTC from gynecologic malignancies. While SCGB2A2 is significantly more breast cancer specific than KRT19, it is less “universal” among these tumors. Indeed, SCGB2A2 expression level is highly changeable in breast tumors, with some of them showing no expression at all. SCGB2A2 expression, estimated at mRNA or protein level, has been reported in 61–93 % of primary and/or metastatic breast cancer biopsies [110–112]. By examining SCGB2A2 gene expression levels in 11 BCC lines, BT-474, Evsa-T, Hs578T, IBEP-1, IBEP-2, IBEP-3 [113], KPL-1, MCF-7, MDA-MB-231, MDA-MB-453, and T-47D, by microarray and RT-PCR, researchers have shown elevated SCGB2A2 mRNA level only in Evsa-T BCC, while mild expression was seen in BT-474 BCC [114]. Notably, most of these BCC lines are of metastatic origin [113].
The function of SCGB2A2 in normal breast and its promising role in breast cancer etiology are unknown. Efforts have been made to find associations between SCGB2A2 expression and various tumor features. High SCGB2A2 expression has been linked with low-grade, steroid receptor-positive tumors from postmenopausal patients [112]. O’Brien et al. [115] have shown that in breast tissue, SCGB2A2 exists in two main forms migrating with an approximate molecular mass of 18 and 25 kDa. The high molecular weight form links positively with hormone receptors and negatively with tumor grade and proliferation rate [115]. Thus, SCGB2A2 has currently the highest diagnostic accuracy for the screening of metastatic breast cancer. However, although tissue specificity is the most essential factor for a marker for circulating cells, sensitivity may not pass. Unluckily, the most aggressive, steroid receptor-negative, high-grade breast tumors and their corresponding DTCs are likely to escape detection using SCGB2A2 as a marker.
SCGB2A1
SCGB2A1 is a protein far more similar to SCGB2A2 than to other proteins, including the other members of the secretoglobin superfamily. In breast tumors, SCGB2A1 exhibits a pattern of expression similar to that of SCGB2A2 [116]. In breast cancer cell lines, SCGB2A1 is greatly expressed in MDA-MB-415 BCC, as also observed for SCGB2A2 [116]. SCGB2A1 has been detected by RT-PCR in 12 out of 30 (40.0 %) SLN from breast cancer patients [117]. Lee et al. performed a large-scale analysis of mRNA co-expression based on 60 diverse large human datasets containing a total of 62.2 million expression measurements distributed among 3,924 microarrays [118]. In line, a strong correlation between SCGB2A2 and SCGB1D2 levels has been identified in breast cancer. SCGB1D2 may bind to SCGB2A2 in an antiparallel manner forming a covalent tetrameric complex. The significance of this interaction is not known, but it appears to be the predominant form of both proteins in breast cancer cells [119].
As also observed with SCGB2A2, abundant SCGB1D2 expression has been found in malignant and normal tissues of the breast and in the female genital tract, namely, the cervix, uterus, and ovary [109]. Briefly, the secretoglobins SCGB2A1, SCGB2A2, and SCGB1D2 are expressed at variable levels in subsets of breast tumors. Despite their relatively high breast specificity, they may also be found in several other tissues, remarkably in glands and steroid-rich organs. Of these secretoglobins, SCGB2A2 has been the most used for DTC detection. Since SCGB2A1, SCGB2A2, and SCGB1D2 are often co-expressed, it is probable that in most cases, DTCs that do not express SCGB2A2 will also be negative for SCGB2A1 and SCGB1D2 expressions.
PIP
Generally known as gross cystic disease fluid protein-15, PIP has been used for years to screen breast cancer and follow breast cancer progression and metastasis. It is a small protein that is considered as a highly specific and sensitive marker of apocrine differentiation [120]. It has been identified in the majority of breast cancer biopsies [121], in correlation with steroid receptor status. In agreement, androgens, estrogens, and glucocorticoids have been found to regulate PIP expression [122]. However, as observed with SCGB2A1, PIP expression levels may noticeably vary among breast tumors, some of them showing no expression at all. By evaluating PIP gene expression levels in 11 BCC lines (see above for SCGB2A2), researchers found elevated PIP mRNA level only in MDA-MB-453 BCC, supporting the global apocrine phenotype of these cells [114]. Therefore, PIP sensitivity in breast cancer may fail. Although being highly breast specific, PIP has also been detected, although usually at low levels, in various other tissues [121].
SBEM
Also known as BS106 [123], SBEM cDNA was identified based on its preferential illustration in libraries prepared from normal breast tissue and breast tumors. SBEM is a small secreted mucin-like protein with strong resemblance to many sialomucins [124]. In a study of 43 normal human tissues, its existence was largely restricted to the mammary and salivary glands. Concerning cancer tissues, SBEM has been identified in breast and prostate [125] MCF-7, T-47D, and ZR-75-1 BCC, but not in the poorly differentiated, ER-negative, basal epithelial-like MDA-MB-231 cells [125].
SBEM expression was noticed in 90 % of invasive ductal carcinomas, although with considerable differences in expression levels, and linked with the expression of SCGB2A2. No close connection was found between SBEM expression and steroid receptor levels or tumor grade [125].
ESR1
Although ESR1 has not been used to distinguish DTCs to date, it represents an essential marker of breast cancer. ESR1 is a transcription factor that permits regulatory functions of female sex steroids, mainly 17b-estradiol, on growth, differentiation, and function in several target tissues, including the female and male reproductive tract, mammary gland, and skeletal and cardiovascular systems. Its central role in the biology and the treatment of breast cancer is well recognized, with the mechanisms underlying its activation and function [126].
ESR1 is expressed in about two-thirds of all breast cancers. In fact, ESR1 is the main discriminator in breast tumor classifications. Its existence is characteristic of a specific class (luminal epithelial-like) of tumors with a well-differentiated, low-grade phenotype. Significant ESR1 expression has also been found in endometroid and ovarian carcinomas. TFF1 and TFF3 both are small cysteine-rich acidic-secreted proteins containing one trefoil domain that has several conserved features, including six cysteine residues with conserved spacing. Trefoil peptides function as “luminal epithelium guardians.” They are involved in the protection of luminal mucosa and mucosal restoration after damage. Rapid repair of mucous epithelia is necessary for preventing inflammation, which is a vital component of cancer progression [127]. Abnormal elevated TFF1 and TFF3 levels have been observed in various neoplastic diseases, including breast cancer. TFF3 is widely co-expressed with TFF1 in ER-positive malignant breast cancer cells [128], and both are geared up by estrogens. TFF3 is also stimulated by growth hormone.
The expression of TFF1 and TFF3 is not established in all breast tumors. Their expression pattern is close to that of ESR1, and the three genes are components of a luminal epithelial signature defining a well-differentiated, low-grade subtype that includes about 65 % of all breast cancers. Therefore, TFF1 and TFF3 may not be viewed as excellent breast tumor markers. In particular, they are unlikely to be informative in the detection of DTC from most aggressive, ER-negative, high-grade tumors.
SPDEF
SPDEF is a member of the “Ets” family. These transcription factors regulate a number of biological processes, including cell proliferation, differentiation, and invasion, and are thought to play an important role in oncogenesis. Unlike the majority of Ets factors, SPDEF is expressed exclusively in tissues with a high epithelial content, such as the prostate and the breast [129]. Moreover, numerous studies showed SPDEF to be one of the most highly overexpressed mRNAs in human and mouse mammary tumors [129, 130]. In breast cancer cells, it has been currently shown that SPDEF could cooperate with ERBB2 to promote motility and invasion. These experimental data suggest that the coevaluation of SPDEF and ERBB2 expressions of DTC could be of high prognostic value [131].
ANKRD30A
ANKRD30A has been earlier recognized as NY-BR-1 [117] or antigen B726P [132]. The protein is regarded as an excellent transcription factor, as it contains a bipartite nuclear localization signal motif and a bZIP site (DNA-binding site followed by leucine zipper motif). Additional structural features include five tandem ankyrin repeats, implying a role for ANKRD30A in protein–protein interactions. Considering its highly restricted expression pattern, ANKRD30A may be considered as a breast differentiation antigen that could represent a suitable target for immunotherapy [133]. In fact, it was found in 80 % of breast cancer specimens, while tumors of other histological types were ANKRD30A negative. ANKRD30A expression was found in 40–50 and 60–70 % of primary and metastatic breast cancer specimens, respectively [134], which has been established by other investigators [135]. Currently, ANKRD30A expression was recognized by immunohistochemistry in breast (60 % of 124 invasive carcinoma lesions), but not in 23 other normal tissues, including prostate and testes, and in breast tumors, but not in lymphoma, seminoma, melanoma, kidney, ovarian, endometrial, prostate, and lung cancers [136].
ANKRD30A has been detected by RT-PCR in 13 out of 30 (43.3 %) SLN from breast cancer patients [117]. Therefore, even though being a highly sensitive marker, ANKRD30A is not constantly expressed by breast cancers. Furthermore, its expression has been significantly associated with the differentiation grade. For instance, in a study of 124 invasive breast carcinoma lesions, 20 out of 26 grade 1 (77 %), 24 out of 38 grade 2 (63 %), and 30 out of 60 grade 3 (50 %) samples were positive. NYBR-1 expression was also considerably associated with LN negativity, presence of ERBB2, amplification, and ER expression [136]. Therefore, ANKRD30A is likely to be detected in well-differentiated tumors and related DTCs.
SERPINB5
Generally known as maspin, it is an epithelial-specific serine protease inhibitor (serpin) that shares extensive homology to the plasminogen activator inhibitors PAI-1 (SERPINE1) and PAI-2 (SERPINB2). SERPINB5 expression has been established in the epithelium of several normal organs, including the mammary gland [137]. In breast tissue, the presence of SERPINB5 seems to be restricted to myoepithelial cells [138], when compared with the luminal epithelial ones, and it has been considered that those myoepithelial cells form a defensive barrier for the progression from ductal carcinoma in situ to more invasive carcinoma [139]. SERPINB5 has also been documented in tumors of various origins, including the breast, although, in most cases, its level was reduced when compared with normal counterparts [137].
Accumulated evidence shows that SERPINB5 may act as a tumor suppressor. Its extracellular form is enough to inhibit tumor cell motility, extracellular matrix degradation, and invasion in vitro and inhibits tumor growth and metastasis in vivo [140]. It also inhibits tumor-induced angiogenesis [141]. Intracellular SERPINB5 is accountable for an increased cellular sensitivity to apoptosis [142]. It has been formerly suggested that SERPINB5 expression in breast tumors turns down with progression and that high SERPINB5 levels were linked to low aggressiveness. For instance, a significant stepwise decrease in maspin expression was shown to occur in the sequence ductal cancer in situ—invasive cancer—lymph node metastasis [138]. According to various studies, however, SERPINB5 overexpression has been seen only in a subset (10–35 %) of breast tumors [138]. In these studies, SERPINB5 levels in breast carcinomas have been directly linked to tumor size, high grade, high S-phase fraction, aneuploidy, positive p53 status, the presence of comedo necrosis and of lymphocyte-rich stroma, inversely correlated to the presence of steroid receptors, and recognized as a strong indicator of poor prognosis, with shorter relapse-free survival (RFS) and OS [143–145]. Therefore, in spite of its tumor-suppressor function, SERPINB5 expression seems to be a characteristic of aggressive tumors, supporting its use for DTC detection.
GABRP
The g-aminobutyric acid (GABA) receptor is a multimeric transmembrane chloride ion channel. Sixteen subtypes of GABA-receptor subunits have been classified within six structural classes (a1–6, b1–3, g1–3, g 3, q, p). These subunits are thought to assemble in different pentameric complexes. GABRP was previously identified by in silico analysis of four million ESTs as a candidate gene differentially expressed in breast cancer. It codes for the p-subunit of the GABA receptor. In a study of 23 normal human tissues, the GABRP expression level was most prominent in the breast. In breast tissue, GABRP is mainly expressed in myoepithelial/basal cells, and it is hypothesized that its function could be linked to tissue contractility. GABRP expression was established to be lower in a majority of primary breast tumors when compared with corresponding normal tissues. Along the same line, strong GABRP expression was examined in normal epithelial and benign papilloma breast cells, but no signal could be noticed in invasive ductal carcinoma, signifying that GABRP is progressively downregulated with tumor progression and that it may be valuable as a prognostic marker in breast cancer [109]. In contrast, in a study of 203 invasive breast cancers, GABRP expression was found high in a subset (16 %) of ER-negative, ERBB2-negative, high-grade tumors with basal-like (undifferentiated) phenotype [146].
Genetic Change in DTCs
There are indications that DTCs may exhibit a significant genetic diversity, reflecting the instability and microheterogeneity observed in primary tumors. Using a procedure involving whole-genome amplification and subsequent CGH of single immunostained cells, it has been observed that cytokeratin-positive DTCs in the bone marrow (BM) of breast cancer patients without clinical signs of overt metastases (stage M0) were genetically heterogeneous [47]. This heterogeneity was reduced with the emergence of clinically evident metastasis (stage M1). The fact that DTC in M1 patients closely resemble each other genetically suggests that cells could separate from lesions at secondary sites (e.g., BM) and recirculate and may cause the appearance of other metastatic sites.
As revealed earlier, it has been hypothesized that BM could serve as a “reservoir” allowing for DTC to adapt and disseminate later into other organs. Investigators using a combination of ICC and FISH found that the pattern of genetic aberrations in BM-derived DTC varied considerably among different breast cancer patients [147]. This is consistent with the CGH-based data of Klein et al. supporting a plethora of different random changes in M0 cells. Schmidt-Kittler et al. [78] also demonstrated a high genetic heterogeneity in M0 cells, although these DTCs displayed fewer chromosomal aberrations than primary tumors or cells from M1-stage patients. Numerous M0 DTCs without detectable aberration (CGH analysis) were also found by these authors.
In M0 cells, genetic aberrations appeared to be randomly generated, while characteristic chromosomal imbalances were observed in M1 cells. This suggests that in breast cancer, tumor cells may disseminate in a far less progressed genomic state than previously thought and that they acquire aberrations typical of metastatic cells thereafter.
Similarly, Gangnus et al. [148] analyzed tumor cells in BM of early-stage breast tumor patients for genomic changes by single-cell CGH. The viable disseminated cancer cells had a plethora of copy number changes in their genome. All evaluated cells showed chromosomal copy number changes with a substantial intercellular heterogeneity and differences to the matching primary tumors. The further development of M0 cells into metastasis, and hence M1 cells, apparently is a matter of mutation and selection, leading to a plausible explanation for tumor dormancy. In this interpretation, dormancy reflects the time needed for M0 cells to acquire the full capacity of unrestrained growth. This selection model is in agreement with the fact that DTCs in patients with overt metastases closely resemble each other genetically [47]. It must be noted that the genetic changes as observed in DTCs from BM [47, 148] and PB [149] confirm the tumoral nature of these DTCs. Since specific DNA gains/losses combinations and genes amplifications in primary tumors are associated with prognosis, it would be helpful to assess whether such changes are also found in DTCs, as well as the probable relationships between their presence in these cells and various parameters (survival of DTCs, time before clinical appearance of metastases, metastasis target organs). For instance, the prognostic value of genomic alterations in breast DTCs has been observed [150]. These authors found considerable correlations between genomic alterations of the DCC and ERBB2 genes in DTCs and relapse-free survival. Moreover, increasing numbers of genomic imbalances measured in DTCs were significantly associated with worse prognosis of recurrent disease. Some of the genes that are frequently amplified in breast tumors encode proteins that are or could be targeted by specific therapies. For instance, Her-2/neu, the product of ERBB2, is targeted by the antibody trastuzumab, while attempts are made to design molecules preventing the interaction between the ubiquitin ligase MDM2 and the p53 oncogene [55]. At term, the identification of specific gene amplifications in DTCs, notably by a combination of array CGH and FISH, could allow the application of specific therapies [151].
Significance of DTCs in Lymph Node, Peripheral Blood, and Bone Marrow
Prognosis and Correlations
Many studies have reported that the presence of disseminated tumor cells (DTCs) in bone marrow (BM), evaluated by ICC or RT-PCR, links strongly with an early relapse of breast cancer and decreased patient survival [71, 152]. As demonstrated by clinical follow-up data on more than 4,000 breast cancer patients studied in prospective trials by several international groups, the presence of DTCs in BM (identified by ICC at primary diagnosis) predicts the postoperative occurrence of overt metastases in bone and other organs [67]. Notably, strong correlations between the presence of BM micrometastases and poor survival have been reported in breast cancer independent from lymph node (LN) metastases [153].
Prognosis of Women with Stage IV Breast Cancer Depends on Detection of CTCs Rather than DTCs
The BM DTC detection rate is noticeably increased in the metastatic setting (59 %) compared with the 15 % detection rate in early breast cancer [154]. No significant difference in BM DTC detection rate was observed between patients in the first line (58 %, n = 110 patients) and second (or more) line of treatment (61 %, n = 28 patients) [155]. For CTC detection, the standard Ficoll technique used in this study was responsible for a lower blood CTC screening rate compared with an epithelial cell adhesion molecule (EpCAM) enrichment method explained previously (40 % versus 61 %) [156]. This lack of sensitivity may be counterbalanced by a higher specificity, i.e., detection of patients with high CTC count, and could explain why CTC detection represented a significant prognostic factor in several studies. Moreover, a study reported that an increased number of DTCs identified in the BM represents an independent prognostic factor in a short series of 33 metastatic breast cancer patients [157]. Further study on much larger number of patients reported that BM DTC detection was of less clinical significance [155]. They also investigated the prognostic value of this parameter according to the two methods of analysis: presence or absence or by defining a cutoff value for the number of tumor cells. None of these analyses was statistically significant for predicting OS in these 138 patients.
Several biological studies assessing the persistence of BM DTCs after adjuvant treatments have specified a possible resistance of these cells to chemotherapy [158]. BM DTC finding has been shown to be predictive for bone metastases in the early breast cancer setting [158]. Bidard et al. showed that the strong link between BM DTC and bone metastasis was maintained after metastatic growth. They also observed a higher frequency of DTCs in patients with lobular carcinoma compared with ductal carcinoma [155]. Their observations indicate that the homing of cancer cells to bone and BM may depend on similar molecular determinants [159]. This is in accordance with the more extensive metastatic spread of lobular carcinoma previously reported by a research group [160]. In contrast, CTCs were not associated with a specific metastatic pattern. Finally, DTCs, detected in the BM (DTC) or in the blood (CTC), can be evaluated at both the early and metastatic stages of breast cancer. Thus, several researchers concluded that BM DTC detection at an early stage appears to be more closely correlated with breast cancer prognosis than CTC [69]. Clinical studies are presently ongoing to define the value of CTCs in the adjuvant setting using more sensitive and specific techniques [161]. Clinical significance of CTCs detection and overall significance in breast cancer is summarized in Table 22.4.
Potential Applications of DTCs
Since tumor cells may in some cases disseminate very early in the natural history of breast cancer, one can envisage the detection of DTCs in women apparently without cancer but who are regularly screened because they are considered at high risk. At present, the selection of patients is based on their statistical risk of developing tumor recurrence, without knowing whether they actually harbor any DTCs. This doubt may lead to overtreatment of patients with cancer with toxic agents that exert severe side effects. For example, only 20–25 % of lymph node (LN)-negative breast cancer patients undergo metastatic relapse within 10 years postsurgery, but more than 90 % of these patients are supposed to receive chemotherapy according to recommendations [162].
DTC recognition in peripheral blood (PB) or bone marrow (BM) may represent an additional clinical marker to identify those LN-negative patients who are cured by surgery alone and need no additional adjuvant systemic therapy. Monitoring the efficacy of a therapy is an important aspect; this might contribute to predicting which patients with early-stage or metastatic disease will recur. This may also possibly support the shift to another treatment, such as monitoring for recurrence after apparently successful adjuvant therapy in patients with early-stage or metastatic disease or destroying DTCs before they develop into metastases. One can consider that the observed moderate rate of response in advanced cancer patients might be caused by the fact that solid metastases form physiological barriers that prevent the access of macromolecules such as antibodies from the circulation in the metastatic lesion [163]. So, DTCs are expected to be more easily accessible for intravenously applied immunoglobulins.
Conclusion and Future Perspective
Advances in modern sciences and technology have allowed the detection of single or small groups of breast cancer cells disseminated in lymph node (LN), peripheral blood (PB), and bone marrow (BM); consequently, the screening and visibility between primary tumors and metastases has become quite easy. Current research and progress in breast tumor biology made it clear that two distinct routes may lead to tumor cell dissemination. Some cells may transit by LN before accessing the PB and BM (lymphogenous route), while other DTCs appear able to directly enter the blood stream (hematogenous route). The mechanism leading to direct hematogenous tumor cell dissemination is not clearly recognized as yet, but it is likely favored by a high microvessel density (MVD) in the primary lesion, as this latter feature has been linked to the presence of DTCs in PB or BM [164, 165].
Screening of DTCs/CTCs according to standardized protocols and subsequent comprehensive phenotypical and molecular characterization of these cells might contribute to an improved identification of patients in need of additional systemic anticancer therapy, in accordance with their present disease status and, finally, to the development of more customized and personalized therapies for breast cancer patients. Last but not least, the various molecular biomarkers with CTCs and DTCs, i.e., fluid biopsy-based strategies, may able to guide the path of early detection and treatment and will open new vistas in understanding the tumor biology of breast cancer; thus, the ultimate goal of better management of breast cancer patients will be possible.
References
Pantel K, Brakenhoff RH. Dissecting the metastatic cascade. Nat Rev Cancer. 2004;4:448–56.
Paget S. The distribution of secondary growths in cancer of the breast. Lancet. 1889;1:571–3.
Rohr K, Hegglin R. Tumorzellen im sternalpunktat. Dtsch Arch Klin Med. 1936;179:61–79.
Schreiber D. Demonstration of micrometastases in the bone marrow of clinically undiagnosed primary tumor. Z Arztl Fortbild (Jena). 1954;48:389–92.
Frey U, Senn HJ. Demonstration of osseous tumor micrometastases: comparison of the value of bone marrow cytology and histology. Schweiz Med Wochenschr. 1978;108:82–91.
Bauer K. Das Krebsproblem. Berlin: Springer; 1946.
Sloane JP, Ormerod MG, Neville AM. Potential pathological application of immunocytochemical methods to the detection of micrometastases. Cancer Res. 1980;40:3079–82.
Pantel K, Woelfle U. Micrometastasis in breast cancer and other solid tumors. J Biol Regul Homeost Agents. 2004;18:120–5.
Alix-Panabieres C, Vendrell JP, Pelle O, Rebillard X, Riethdorf S, Müller V, et al. Detection and characterization of putative metastatic precursor cells in cancer patients. Clin Chem. 2007;53:537–9.
Ferlay J Shin H, Bray F, Forman D. GLOBOCAN v1.2, Cancer incidence and mortality worldwide: IARC cancer base. 2008; No. 10. 2010. Cited 25 Oct 2011, 2011.
Parkin DM, Bray F, Devesa S. Cancer burden in the year 2000: the global picture. Eur J Cancer. 2001;37:S4–66.
RainaV, Tyagi BB, Manoharan N. Two year report of the population based cancer registries, 2004–2005. Incidence and distribution of cancer. New Delhi: National Cancer Registry Programme, Indian Council of Medical Research; 2009. p. 63–5. Available at: https://canceratlasindia.org
Hayes DF. Serum (circulating) tumor markers for breast cancer. Recent Results Cancer Res. 1996;140:101–13.
Martin A, Corte MD, Alvarez AM, Rodriguez JC, Andicoechea A, Bongera M, et al. Prognostic value of pre-operative serum CA 15.3 levels in breast cancer. Anticancer Res. 2006;26:3965–71.
Duffy MJ. Serum tumor markers in breast cancer: are they of clinical value? Clin Chem. 2006;52:345–51.
Uehara M, Kinoshita T, Hojo T. Long-term prognostic study of carcinoembryonic antigen (CEA) and carbohydrate antigen 15–3 (CA 15–3) in breast cancer. Int J Clin Oncol. 2008;13:447–51.
Kim HS, Park YH, Park MJ, Chang MH, Jun HJ, Kim KH, et al. Clinical significance of a serum CA15-3 surge and the usefulness of CA15-3 kinetics in monitoring chemotherapy response in patients with metastatic breast cancer. Breast Cancer Res Treat. 2009;118:89–97.
Dede DS, Arslan C, Altundag K, et al. Serum levels of CEA and CA 15-3 in triple-negative breast cancer at the time of diagnosis. Med Oncol. 2010;27(4):1429.
Gion M, Mione R, Leon AE, Lüftner D, Molina R, Possinger K, et al. CA 27.29: a valuable marker for breast cancer management. A confirmatory multicentric study on 603 cases. Eur J Cancer. 2001;37:355–63.
Lumachi F, Basso SM. Serum tumor markers in patients with breast cancer. Expert Rev Anticancer Ther. 2004;4:921–31.
Yom CK, Woo HY, Min SY, Kang SY, Kim HS. Clusterin overexpression and relapse-free survival in breast cancer. Anticancer Res. 2009;29:3909–12.
Doustjalali SR, Yusof R, Yip CH, Looi LM, Pillay B, Hashim OH. Aberrant expression of acute-phase reactant proteins in sera and breast lesions of patients with malignant and benign breast tumors. Electrophoresis. 2004;25:2392–401.
Zieglschmid V, Hollmann C, Böcher O. Detection of disseminated tumor cells in peripheral blood. Crit Rev Clin Lab Sci. 2005;42:155–96.
Elmore JG, Armstrong K, Lehman CD, Fletcher SW. Screening for breast cancer. JAMA. 2005;293:1245–56.
Nothacker M, Duda V, Hahn M, Warm M, Degenhardt F, Madjar H, et al. Early detection of breast cancer: benefits and risks of supplemental breast ultrasound in asymptomatic women with mammographically dense breast tissue. A systematic review. BMC Cancer. 2009;9:335.
Timins JK. Controversies in mammography. N J Med. 2005;102:45–9.
Pisano E. Issues in breast cancer screening. Technol Cancer Res Treat. 2005;4:5–9.
Gillet D, Kennedy C, Carmalt H. Breast cancer in young women. Aust N Z J Surg. 1997;67:761–4.
Brenton JD, Carey LA, Ahmed AA, Caldas C. Molecular classification and molecular forecasting of breast cancer: ready for clinical application? J Clin Oncol. 2005;23:7350–60.
Hu Z, Fan C, Oh DS, Marron JS, He X, Qaqish BF, et al. The molecular portraits of breast tumors are conserved across microarray platforms. BMC Genomics. 2006;7:96.
Lacroix M, Leclercq G. About GATA3, HNF3A, and XBP1, three genes co-expressed with the oestrogen receptor-alpha gene (ESR1) in breast cancer. Mol Cell Endocrinol. 2004;219(1–2):1–7.
Nielsen TO, Hsu FD, Jensen K, Cheang M, Karaca G, Hu Z, et al. Immunohistochemical and clinical characterization of the basal-like subtype of invasive breast carcinoma. Clin Cancer Res. 2004;10:5367–74.
Bertucci F, Finetti P, Cervera N, Charafe-Jauffret E, Mamessier E, Adélaïde J, et al. Gene expression profiling shows medullary breast cancer is a subgroup of basal breast cancer. Cancer Res. 2006;66:4636–44.
Lacroix M, Leclercq G. Hereditary breast cancer: an update on genotype and phenotype. In: Yao AP, editor. New breast cancer research. New York: Nova Science Publishers; 2006. p. 27–51.
Charafe-Jauffret E, Ginestier C, Monville F. Gene expression profiling of breast cell lines identifies potential new basal markers. Oncogene. 2006;25:2273–84.
Lacroix M, Toillon RA, Leclercq G. Stable ‘portrait’ of breast tumors during progression: data from biology, pathology and genetics. Endocr Relat Cancer. 2004;11:497–522.
Weigelt B, Hu Z, He X, Livasy C, Carey LA, Ewend MG, et al. Molecular portraits and 70-gene prognosis signature are preserved throughout the metastatic process of breast cancer. Cancer Res. 2005;65:9155–8.
Fridlyand J, Snijders AM, Ylstra B, Li H, Olshen A, Segraves R, et al. Breast tumor copy number aberration phenotypes and genomic instability. BMC Cancer. 2006;6:96.
Al-Kuraya K, Schraml P, Torhorst J. Prognostic relevance of gene amplifications and co amplifications in breast cancer. Cancer Res. 2004;64:8534–40.
Klein CA. Parallel progression of primary tumours and metastases. Nat Rev Cancer. 2009;9:302–12.
Aguirre-Ghiso AJ. Models, mechanisms and clinical evidence for cancer dormancy. Nat Rev Cancer. 2007;7:834–46.
Fehm T, Braun S, Muller V, Janni W, Gebauer G, Marth C, et al. A concept for the standardized detection of disseminated tumor cells in bone marrow from patients with primary breast cancer and its clinical implementation. Cancer. 2006;107:885–92.
Hartmann CH, Klein CA. Gene expression profiling of single cells on large-scale oligonucleotide arrays. Nucleic Acids Res. 2006;34:e143.
Fetsch PA, Cowan KH, Weng DE, Freifield A, Filie AC, Abati A. Detection of circulating tumor cells and micrometastases in stage II, III and IV breast cancer patients utilizing cytology and immunohistochemistry. Diagn Cytopathol. 2000;22:323–8.
Slade MJ, Singh A, Smith BM, Tripuraneni G, Hall E, Peckitt C, et al. Persistence of bone marrow micrometastases in patients receiving adjuvant therapy for breast cancer: results at 4 years. Int J Cancer. 2005;114:94–100.
Braun S, Naume B. Circulating and disseminated tumor cells. J Clin Oncol. 2005;23:1623–6.
Klein CA, Blankenstein TJ, Schmidt-Kittler O, Petronio M, Polzer B, Stoecklein NH, et al. Genetic heterogeneity of single disseminated tumour cells in minimal residual cancer. Lancet. 2002;360:683–9.
Ring A, Smith IE, Dowsett M. Circulating tumour cells in breast cancer. Lancet Oncol. 2004;5:79–88.
Ben Hsieh H, Marrinucci D, Bethel K, et al. High speed detection of circulating tumor cells. Biosens Bioelectron. 2006;21:1893–9.
Hu XC, Loo WT, Chow LW. Surgery-related shedding of breast cancer cells as determined by RT-PCR assay. J Surg Oncol. 2003;82:228–32.
Bleiweiss IJ, Nagi CS, Jaffer S. Axillary sentinel lymph nodes can be falsely positive due to iatrogenic displacement and transport of benign epithelial cells in patients with breast carcinoma. J Clin Oncol. 2006;24:2013–8.
Smerage JB, Hayes DF. The measurement and therapeutic implications of circulating tumour cells in breast cancer. Br J Cancer. 2006;94:8–12.
Hager G, Cacsire-Castillo Tong D, Schiebel I, Rezniczek GA, Watrowski R, Speiser P, et al. The use of a panel of monoclonal antibodies to enrich circulating breast cancer cells facilitates their detection. Gynecol Oncol. 2005;98:211–6.
Sidransky D. Nucleic acid-based methods for the detection of cancer. Science. 1997;278:1054–9.
Lacroix M, Toillon RA, Leclercq G. P53 and breast cancer: an update. Endocr Relat Cancer. 2006;13:293–325.
Muller HM, Widschwendter A, Fiegl H. DNA methylation in serum of breast cancer patients: an independent prognostic marker. Cancer Res. 2003;63:7641–5.
Fiegl H, Millinger S, Mueller-Holzner E, Marth C, Ensinger C, Berger A, et al. Circulating tumor-specific DNA: a marker for monitoring efficacy of adjuvant therapy in cancer patients. Cancer Res. 2005;65:1141–5.
Silva JM, Garcia JM, Dominguez G, Silva J, Miralles C, Cantos B, et al. Persistence of tumor DNA in plasma of breast cancer patients after mastectomy. Ann Surg Oncol. 2002;9:71–6.
Schardt JA, Meyer M, Hartmann CH. Genomic analysis of single cytokeratin positive cells from bone marrow reveal early mutational events in breast cancer. Cancer Cell. 2005;8:227–39.
Gilbey AM, Burnett D, Coleman RE, Holen I. The detection of circulating breast cancer cells in blood. J Clin Pathol. 2004;57:903–11.
Meng S, Tripathy D, Shete S. HER-2 gene amplification can be acquired as breast cancer progresses. Proc Natl Acad Sci U S A. 2004;101:9393–8.
Lacroix M. Significance, detection and markers of disseminated breast cancer cells. Endocr Relat Cancer. 2006;13:1033–67.
Braun S, Kentenich C, Janni W, Hepp F, de Waal J, Willgeroth F, et al. Lack of effect of adjuvant chemotherapy on the elimination of single dormant tumor cells in bone marrow of high-risk breast cancer patients. J Clin Oncol. 2000;18:80–6.
Borgen E, Pantel K, Schlimok G, Müller P, Otte M, Renolen A, et al. A European interlaboratory testing of three well-known procedures for immunocytochemical detection of epithelial cells in bone marrow. Results from analysis of normal bone marrow. Cytometry B Clin Cytom. 2006;70:400–9.
Schwarzenbach H, Muller V, Beeger C, Gottberg M, Stahmann N, Pantel K. A critical evaluation of loss of heterozygosity detected in tumor tissues, blood serum and bone marrow plasma from patients with breast cancer. Breast Cancer Res. 2007;9:R66.
Becker S, Becker-Pergola G, Fehm T, Wallwiener D, Solomayer EF. Time is an important factor when processing samples for the detection of disseminated tumor cells in blood/bone marrow by reverse transcription-PCR. Clin Chem. 2004;50:785–6.
Braun S, Vogl FD, Naume B, Janni W, Osborne MP, Coombes RC, et al. A pooled analysis of bone marrow micrometastasis in breast cancer. N Engl J Med. 2005;353:793–802.
Pierga JY, Bonneton C, Vincent-Salomon A, de Cremoux P, Nos C, Blin N, et al. Clinical significance of immunocytochemical detection of tumor cells using digital microscopy in peripheral blood and bone marrow of breast cancer patients. Clin Cancer Res. 2004;10:1392–400.
Müller V, Stahmann N, Riethdorf S, Rau T, Zabel T, Goetz A, et al. Circulating tumor cells in breast cancer: correlation to bone marrow micrometastases, heterogeneous response to systemic therapy and low proliferative activity. Clin Cancer Res. 2005;11:3678–85.
Wiedswang G, Borgen E, Schirmer C, Kåresen R, Kvalheim G, Nesland JM, et al. Comparison of the clinical significance of occult tumor cells in blood and bone marrow in breast cancer. Int J Cancer. 2006;118:2013–9.
Benoy IH, Elst H, Philips M, Wuyts H, Van Dam P, Scharpé S, et al. Real-time RT-PCR detection of disseminated tumour cells in bone marrow has superior prognostic significance in comparison with circulating tumour cells in patients with breast cancer. Br J Cancer. 2006;94:672–80.
Xenidis N, Perraki M, Kafousi M, Apostolaki S, Bolonaki I, Stathopoulou A, et al. Predictive and prognostic value of peripheral blood cytokeratin-19 mRNA-positive cells detected by real-time polymerase chain reaction in node-negative breast cancer patients. J Clin Oncol. 2006;24:3756–62.
Nakagawa T, Martinez SR, Goto Y, Koyanagi K, Kitago M, Shingai T, et al. Detection of circulating tumor cells in early-stage breast cancer metastasis to axillary lymph nodes. Clin Cancer Res. 2007;13:4105–10.
Cristofanilli M, Hayes DF, Budd GT, Ellis MJ, Stopeck A, Reuben JM, et al. Circulating tumor cells: a novel prognostic factor for newly diagnosed metastatic breast cancer. J Clin Oncol. 2005;23:1420–30.
Hayes DF, Cristofanilli M, Budd GT, Ellis MJ, Stopeck A, Miller MC, et al. Circulating tumor cells at each follow-up time point during therapy of metastatic breast cancer patients predict progression-free and overall survival. Clin Cancer Res. 2006;12:4218–24.
Pachmann K, Camara O, Kavallaris A, Schneider U, Schünemann S, Höffken K. Quantification of the response of circulating epithelial cells to neoadjuvant treatment for breast cancer: a new tool for therapy monitoring. Breast Cancer Res. 2005;7:R975–9.
Klein CA, Seidl S, Petat-Dutter K, Offner S, Geigl JB, Schmidt-Kittler O, et al. Combined transcriptome and genome analysis of single micrometastatic cells. Nat Biotechnol. 2002;20:387–92.
Schmidt-Kittler O, Ragg T, Daskalakis A, Granzow M, Ahr A, Blankenstein TJ, et al. From latent disseminated cells to overt metastasis: genetic analysis of systemic breast cancer progression. Proc Natl Acad Sci U S A. 2003;100:7737–42.
Marches R, Scheuermann R, Uhr J. Cancer dormancy: from mice to man. Cell Cycle. 2006;5:1772–8.
Watson MA, Ylagan LR, Trinkaus KM. Isolation and molecular profiling of bone marrow micrometastases identifies TWIST1 as a marker of early tumor relapse in breast cancer patients. Clin Cancer Res. 2007;13:5001–9.
Kang Y, Massague J. Epithelial-mesenchymal transitions: twist in development and metastasis. Cell. 2004;118:277–9.
Braun S, Schlimok G, Heumos I, Schaller G, Riethdorf L, Riethmüller G, et al. ErbB2 overexpression on occult metastatic cells in bone marrow predicts poor clinical outcome of stage I–III breast cancer patients. Cancer Res. 2001;61:1890–5.
Vincent-Salomon A, Pierga JY, Couturier J, d’Enghien CD, Couturier J, Nos C, Sigal-Zafrani B, et al. HER2 status of bone marrow micrometastasis and their corresponding primary tumours in a pilot study of 27 cases: a possible tool for anti-HER2 therapy management? Br J Cancer. 2007;96:654–9.
Solomayer EF, Becker S, Pergola-Becker G. Comparison of HER2 status between primary tumor and disseminated tumor cells in primary breast cancer patients. Breast Cancer Res Treat. 2006;98:179–84.
Wulfing P, Borchard J, Buerger H, Heidl S, Zänker KS, Kiesel L, et al. HER2-positive circulating tumor cells indicate poor clinical outcome in stage I to III breast cancer patients. Clin Cancer Res. 2006;12:1715–20.
Apostolaki S, Perraki M, Pallis A. Circulating HER2 mRNA-positive cells in the peripheral blood of patients with stage I and II breast cancer after the administration of adjuvant chemotherapy: evaluation of their clinical relevance. Ann Oncol. 2007;18:851–8.
Ring AE, Zabaglo L, Ormerod MG. Detection of circulating epithelial cells in the blood of patients with breast cancer: comparison of three techniques. Br J Cancer. 2005;92:906–12.
Ruud P, Fodstad O, Hovig E. Identification of a novel cytokeratin 19 pseudogene that may interfere with reverse transcriptase-polymerase chain reaction assays used to detect micrometastatic tumor cells. Int J Cancer. 1999;80:119–25.
Corradini P, Voena C, Astolfi M, Delloro S, Pilotti S, Arrigoni G, et al. Maspin and mammaglobin genes are specific markers for RT-PCR detection of minimal residual disease in patients with breast cancer. Ann Oncol. 2001;12:1693–8.
Jung R, Petersen K, Kruger W, Wolf M, Wagener C, Zander A, et al. Detection of micrometastasis by cytokeratin 20 RT-PCR is limited due to stable background transcription in granulocytes. Br J Cancer. 1999;81:870–3.
Zhong XY, Kaul S, Eichler A, Bastert G. Evaluating GA733-2 mRNA as a marker for the detection of micrometastatic breast cancer in peripheral blood and bone marrow. Arch Gynecol Obstet. 1999;263:2–6.
Ligtenberg MJ, Buijs F, Vos HL. Suppression of cell aggregation by high levels of episialin. Cancer Res. 1992;52:2318–24.
Ciborowski P, Finn OJ. Non-glycosylated tandem repeats of MUC1 facilitate attachment of breast tumor cells to normal human lung tissue and immobilized extracellular matrix proteins (ECM) in vitro: potential role in metastasis. Clin Exp Metastasis. 2002;19:339–45.
Emens LA, Reilly RT, Jaffee EM. Breast cancer vaccines: maximizing cancer treatment by tapping into host immunity. Endocr Relat Cancer. 2005;12:1–17.
Rakha EA, Boyce RW, Abd El-Rehim D. Expression of mucins (MUC1, MUC2, MUC3, MUC4, MUC5AC and MUC6) and their prognostic significance in human breast cancer. Mod Pathol. 2005;18:1295–304.
Gradilone A, Gazzaniga P, Silvestri I, Gandini O, Trasatti L, Lauro S, et al. Detection of CK19, CK20 and EGFR mRNAs in peripheral blood of carcinoma patients: correlation with clinical stage of disease. Oncol Rep. 2003;10:217–22.
Weigelt B, Verduijn P, Bosma AJ, Rutgers EJ, Peterse HL, van’t Veer LJ. Detection of metastases in sentinel lymph nodes of breast cancer patients by multiple mRNA markers. Br J Cancer. 2004;90:1531–7.
Silva HA, Abraul E, Raimundo D. Molecular detection of EGFRvIII-positive cells in the peripheral blood of breast cancer patients. Eur J Cancer. 2006;42:2617–22.
Mitas M, Mikhitarian K, Walters C, Baron PL, Elliott BM, Brothers TE, et al. Quantitative real-time RT-PCR detection of breast cancer micrometastasis using a multigene marker panel. Int J Cancer. 2001;93:162–71.
Bosma AJ, Weigelt B, Lambrechts AC. Detection of circulating breast tumor cells by differential expression of marker genes. Clin Cancer Res. 2002;8:1871–7.
Welsh JB, Sapinoso LM, Kern SG, Brown DA, Liu T, Bauskin AR, et al. Large-scale delineation of secreted protein biomarkers overexpressed in cancer tissue and serum. Proc Natl Acad Sci U S A. 2003;100:3410–5.
Smirnov DA, Zweitzig DR, Foulk BW, Miller MC, Doyle GV, Pienta KJ, et al. Global gene expression profiling of circulating tumor cells. Cancer Res. 2005;65:4993–7.
Mikhitarian K, Gillanders WE, Almeida JS, Hebert Martin R, Varela JC, Metcalf JS, et al. An innovative microarray strategy identifies informative molecular markers for the detection of micrometastatic breast cancer. Clin Cancer Res. 2005;11:3697–704.
Backus J, Laughlin T, Wang Y, Belly R, White R, Baden J, et al. Identification and characterization of optimal gene expression markers for detection of breast cancer metastasis. J Mol Diagn. 2005;7:327–36.
Watson MA, Fleming TP. Mammaglobin, a mammary-specific member of the uteroglobin gene family, is overexpressed in human breast cancer. Cancer Res. 1996;56:860–5.
Klug J, Beier HM, Bernard A, Chilton BS, Fleming TP, Lehrer RI, et al. Uteroglobin/Clara cell 10-kDa family of proteins: nomenclature committee report. Ann N Y Acad Sci. 2000;923:348–54.
Brown NM, Stenzel TT, Friedman PN, Henslee J, Huper G, Marks JR. Evaluation of expression based markers for the detection of breast cancer cells. Breast Cancer Res Treat. 2006;97:41–7.
Viehl CT, Tanaka Y, Chen T, Frey DM, Tran A, Fleming TP, et al. Tat mammaglobin fusion protein transduced dendritic cells stimulate mammaglobin-specific CD4 and CD8 T cells. Breast Cancer Res Treat. 2005;91:271–8.
Zafrakas M, Petschke B, Donner A, Fritzsche F, Kristiansen G, Knüchel R, et al. Expression analysis of mammaglobin A (SCGB2A2) and Lipophilin B (SCGB1D2) in more than 300 human tumors and matching normal tissues reveals their co-expression in gynecological malignancies. BMC Cancer. 2006;6:88.
Min CJ, Tafra L, Verbanac KM. Identification of superior markers for polymerase chain reaction detection of breast cancer metastases in sentinel lymph nodes. Cancer Res. 1998;58:4581–4.
Han JH, Kang Y, Shin HC, Kim HS, Kang YM, Kim YB, et al. Mammaglobin expression in lymph nodes is an important marker of metastatic breast carcinoma. Arch Pathol Lab Med. 2003;127:1330–4.
Span PN, Waanders E, Manders P, Heuvel JJ, Foekens JA, Watson MA, et al. Mammaglobin is associated with low-grade steroid receptor-positive breast tumors from postmenopausal patients, and has independent prognostic value for relapse-free survival time. J Clin Oncol. 2004;22:691–8.
Siwek B, Larsimont D, Lacroix M, Body JJ. Establishment and characterization of three new breast-cancer cell lines. Int J Cancer. 1998;76:677–83.
De Longueville F, Lacroix M, Barbuto AM, Bertholet V, Gallo D, Larsimont D, et al. Molecular characterization of breast cancer cell lines by a low-density microarray. Int J Oncol. 2005;27:881–92.
O’Brien N, O’Donovan N, Ryan B. Mammaglobin A in breast cancer: existence of multiple molecular forms. Int J Cancer. 2005;114:623–7.
Becker RM, Darrow C, Zimonjic DB, Popescu NC, Watson MA, Fleming TP. Identification of mammaglobin B, a novel member of the uteroglobin gene family. Genomics. 1998;54:70–8.
Nissan A, Jager D, Roystacher M, Prus D, Peretz T, Eisenberg I, et al. Multimarker RT-PCR assay for the detection of minimal residual disease in sentinel lymph nodes of breast cancer patients. Br J Cancer. 2006;94:681–5.
Lee HK, Hsu AK, Sajdak J, Qin J, Pavlidis P. Coexpression analysis of human genes across many microarray data sets. Genome Res. 2004;14:1085–94.
Carter D, Douglass JF, Cornellison CD, Retter MW, Johnson JC, Bennington AA, et al. Purification and characterization of the mammaglobin/lipophilin B complex, a promising diagnostic marker for breast cancer. Biochemistry. 2002;41:6714–22.
Jones C, Damiani S, Wells D, Chaggar R, Lakhani SR, Eusebi V. Molecular cytogenetic comparison of apocrine hyperplasia and apocrine carcinoma of the breast. Am J Pathol. 2001;158:207–14.
Clark JW, Snell L, Shiu RP. The potential role for prolactin-inducible protein (PIP) as a marker of human breast cancer micrometastasis. Br J Cancer. 1999;1:1002–8.
Murphy LC, Lee-Wing M, Goldenberg GJ, Shiu RP. Expression of the gene encoding a prolactin-inducible protein by human breast cancers in vivo: correlation with steroid receptor status. Cancer Res. 1987;47:4160–4.
Colpitts TL, Billing P, Granados E, Hayden M, Hodges S, Roberts L, et al. Identification and immunohistochemical characterization of a mucin-like glycoprotein expressed in early stage breast carcinoma. Tumour Biol. 2002;23:263–78.
Hube F, Mutawe M, Leygue E, Myal Y. Human small breast epithelium mucin: the promise of a new breast tumor biomarker. DNA Cell Biol. 2004;23:842–9.
Miksicek RJ, Myal Y, Watson PH. Identification of a novel breast- and salivary gland-specific, mucin-like gene strongly expressed in normal and tumor human mammary epithelium. Cancer Res. 2002;62:2736–40.
Leclercq G, Lacroix M, Laïos I, Laurent G. Estrogen receptor alpha: impact of ligands on intracellular shuttling and turnover rate in breast cancer cells. Curr Cancer Drug Targets. 2006;6:39–64.
Hoffmann W. Trefoil factors TFF (trefoil factor family) peptide-triggered signals promoting mucosal restitution. Cell Mol Life Sci. 2005;62:2932–8.
Poulsom R, Hanby AM, Lalani EN. Intestinal trefoil factor (TFF 3) and pS2 (TFF 1), but not spasmolytic polypeptide (TFF 2) mRNAs are co-expressed in normal, hyperplastic, and neoplastic human breast epithelium. J Pathol. 1997;183:30–8.
Mitas M, Mikhitarian K, Hoover L, Lockett MA, Kelley L, Hill A, et al. Prostate-Specific Ets (PSE) factor: a novel marker for detection of metastatic breast cancer in axillary lymph nodes. Br J Cancer. 2002;86:899–904.
Ghadersohi A, Sood AK. Prostate epithelium-derived Ets transcription factor mRNA is overexpressed in human breast tumors and is a candidate breast tumor marker and a breast tumor antigen. Clin Cancer Res. 2001;7:2731–8.
Gunawardane RN, Sgroi DC, Wrobel CN, Koh E, Daley GQ, Brugge JS. Novel role for PDEF in epithelial cell migration and invasion. Cancer Res. 2005;65:11572–80.
Jiang Y, Harlocker SL, Molesh DA, Dillon DC, Stolk JA, Houghton RL, et al. Discovery of differentially expressed genes in human breast cancer using subtracted cDNA libraries and cDNA microarrays. Oncogene. 2002;21:2270–82.
Wang W, Epler J, Salazar LG, Riddell SR. Recognition of breast cancer cells by CD8C cytotoxic T-Cell clones specific for NY-BR-1. Cancer Res. 2006;66:6826–33.
Zehentner BK, Dillon DC, Jiang Y, Xu J, Bennington A, Molesh DA, et al. Application of a multigame reverse transcription-PCR assay for detection of mammaglobin and complementary transcribed genes in breast cancer lymph nodes. Clin Chem. 2002;48:1225–31.
O’Brien N, O’Donovan N, Hill AD. B726P, a gene expressed specifically in breast tissue. Proc Am Assoc Cancer Res. 2003;32:63.
Varga Z, Theurillat JP, Filonenko V, Sasse B, Odermatt B, Jungbluth AA, et al. Preferential nuclear and cytoplasmic NY-BR-1 protein expression in primary breast cancer and lymph node metastases. Clin Cancer Res. 2006;12:2745–51.
Zhang W, Zhang M. Tissue microarray analysis of maspin expression and its reverse correlation with mutant p53 in various tumors. Int J Oncol. 2002;20:1145–50.
Maass N, Teffner M, Rosel F, Pawaresch R, Jonat W, Nagasaki K, et al. Decline in the expression of the serine proteinase inhibitor maspin is associated with tumour progression in ductal carcinomas of the breast. J Pathol. 2001;195:321–6.
Polyak K, Hu M. Do myoepithelial cells hold the key for breast tumor progression? J Mammary Gland Biol Neoplasia. 2005;10:231–47.
Zou Z, Anisowicz A, Hendrix MJ, Thor A, Neveu M, Sheng S, et al. Maspin, a serpin with tumor-suppressing activity in human mammary epithelial cells. Science. 1994;263:526–9.
Zhang M, Volpert O, Shi YH, Bouck N. Maspin is an angiogenesis inhibitor. Nat Med. 2000;6:196–9.
Lockett J, Yin S, Li X, Meng Y, Sheng S. Tumor suppressive maspin and epithelial homeostasis. J Cell Biochem. 2006;97:651–60.
Martin KJ, Kritzman BM, Price LM, Koh B, Kwan CP, Zhang X, et al. Linking gene expression patterns to therapeutic groups in breast cancer. Cancer Res. 2000;60:2232–8.
Kim DH, Yoon DS, Dooley WC, Nam ES, Ryu JW, Jung KC, et al. Association of maspin expression with the high histological grade and lymphocyte-rich stroma in early-stage breast cancer. Histopathology. 2003;42:37–42.
Mohsin SK, Zhang M, Clark GM, Craig Allred D. Maspin expression in invasive breast cancer: association with other prognostic factors. J Pathol. 2003;199:432–5.
Symmans WF, Fiterman DJ, Anderson SK, et al. A single-gene biomarker identifies breast cancers associated with immature cell type and short duration of prior breastfeeding. Endocr Relat Cancer. 2005;12:1059–69.
Solakoglu O, Maierhofer C, Lahr G, Breit E, Scheunemann P, Heumos I, et al. Heterogeneous proliferative potential of occult metastatic cells in bone marrow of patients with solid epithelial tumors. Proc Natl Acad Sci U S A. 2002;99:2246–51.
Gangnus R, Langer S, Breit E, Pantel K, Speicher MR. Genomic profiling of viable and proliferative micrometastatic cells from early-stage breast cancer patients. Clin Cancer Res. 2004;10:3457–64.
Fehm T, Sagalowsky A, Clifford E, Beitsch P, Saboorian H, Euhus D, et al. Cytogenetic evidence that circulating epithelial cells in patients with carcinoma are malignant. Clin Cancer Res. 2002;8:2073–84.
Austrup F, Uciechowski P, Eder C. Prognostic value of genomic alterations in minimal residual cancer cells purified from the blood of breast cancer patients. Br J Cancer. 2000;83:1664–73.
Bussey KJ, Chin K, Lababidi S, Reimers M, Reinhold WC, Kuo WL, et al. Integrating data on DNA copy number with gene expression levels and drug sensitivities in the NCI-60 cell line panel. Mol Cancer Ther. 2006;5:853–67.
Pantel K, Woelfle U. Detection and molecular characterisation of disseminated tumour cells: implications for anti-cancer therapy. Biochim Biophys Acta. 2005;1756:53–64.
Cote RJ, Rosen PP, Lesser ML. Prediction of early relapse in patients with operable breast cancer by detection of occult bone micrometastases. J Clin Oncol. 1991;9:1749–56.
Wiedswang G, Borgen E, Karesen R, et al. Detection of isolated tumor cells in bone marrow is an independent prognostic factor in breast cancer. J Clin Oncol. 2003;21:3469–78.
Bidard FC, Vincent-Salomon A, Sigal-Zafrani B, Diéras V, Mathiot C, Mignot L, et al. Prognosis of women with stage IV breast cancer depends on detection of circulating tumor cells rather than disseminated tumor cells. Ann Oncol. 2008;19:496–500.
Cristofanilli M, Budd GT, Ellis MJ, Stopeck A, Matera J, Miller MC, et al. Circulating tumor cells, disease progression, and survival in metastatic breast cancer. N Engl J Med. 2004;351:781–91.
Janni W, Hepp F, Rjosk D, Kentenich C, Strobl B, Schindlbeck C, et al. The fate and prognostic value of occult metastatic cells in the bone marrow of patients with breast carcinoma between primary treatment and recurrence. Cancer. 2001;92:46–53.
Wiedswang G, Borgen E, Kåresen R, Qvist H, Janbu J, Kvalheim G, et al. Isolated tumor cells in bone marrow three years after diagnosis in disease-free breast cancer patients predict unfavorable clinical outcome. Clin Cancer Res. 2004;10:5342–8.
Kang Y, He W, Tulley S, Gupta GP, Serganova I, Chen CR, et al. Breast cancer bone metastasis mediated by the Smad tumor suppressor pathway. Proc Natl Acad Sci U S A. 2005;102:13909–14.
Ferlicot S, Vincent-Salomon A, Medioni J, Genin P, Rosty C, Sigal-Zafrani B, et al. Wide metastatic spreading in infiltrating lobular carcinoma of the breast. Eur J Cancer. 2004;40:336–41.
Pantel K, Alix-Panabieres C. The clinical significance of circulating tumor cells. Nat Clin Pract Oncol. 2007;4:62–3.
Goldhirsch A, Wood WC, Gelber RD, Coates AS, Thürlimann B, Senn HJ. Meeting highlights: updated international expert consensus on the primary therapy of early breast cancer. J Clin Oncol. 2003;21:3357–65.
Jain RK. Physiological barriers to delivery of monoclonal antibodies and other macromolecules in tumors. Cancer Res. 1990;50:814s–9s.
Gerber B, Krause A, Müller H, Richter D, Reimer T, Makovitzky J, et al. Simultaneous immunohistochemical detection of tumor cells in lymph nodes and bone marrow aspirates in breast cancer and its correlation with other prognostic factors. J Clin Oncol. 2001;19:960–71.
Benoy IH, Salgado R, Elst H, Van Dam P, Weyler J, Van Marck E, et al. Relative microvessel area of the primary tumour, and not lymph node status, predicts the presence of bone marrow micrometastases detected by reverse transcriptase polymerase chain reaction in patients with clinically non-metastatic breast cancer. Breast Cancer Res. 2005;7:R210–9.
Bartkowiak K, Wieczorek M, Buck F, Harder S, Moldenhauer J, Effenberger KE, et al. Two-dimensional differential gel electrophoresis of a cell line derived from a breast cancer micrometastasis revealed a stem/progenitor cell protein profile. J Proteome Res. 2009;8:2004–14.
Banys M, Krawczyk N, Becker S, Jakubowska J, Staebler A, Wallwiener D, et al. The influence of removal of primary tumor on incidence and phenotype of circulating tumor cells in primary breast cancer. Breast Cancer Res Treat. 2012;132(1):121–9.
GEPARQuattro trial [Internet]. Available at: http://www.germanbreastgroup.de/studien/neoadjuvant/geparquattro-/english-summary-html?lang=de_DE.UTF-8%2C+de_CH.U.
Daskalaki A, Agelaki S, Perraki M, Apostolaki S, Xenidis N, Stathopoulos E, et al. Detection of cytokeratin-19 mRNA-positive cells in the peripheral blood and bone marrow of patients with operable breast cancer. Br J Cancer. 2009;101:589–97.
Liu Z, Fusi A, Schmittel A, Tinhofer I, Schneider A, Keilholz U. Eradication of EGFR-positive circulating tumor cells and objective tumor response with lapatinib and capecitabine. Cancer Biol Ther. 2010;10:860–4.
Slade MJ, Payne R, Riethdorf S, Ward B, Zaidi SA, Stebbing J, et al. Comparison of bone marrow, disseminated tumour cells and blood-circulating tumour cells in breast cancer patients after primary treatment. Br J Cancer. 2009;100:160–6.
Krishnamurthy S, Cristofanilli M, Singh B, Reuben J, Gao H, Cohen EN, et al. Detection of minimal residual disease in blood and bone marrow in early stage breast cancer. Cancer. 2010;116:3330–7.
Pantel K, Alix-Panabières C, Riethdorf S. Cancer micrometastases. Nat Rev Clin Oncol. 2009;6:339–51.
Maheswaran S, Sequist LV, Nagrath S, Ulkus L, Brannigan B, Collura CV, et al. Detection of mutations in EGFR in circulating lung-cancer cells. N Engl J Med. 2008;359:366–77.
Stott SL, Lee RJ, Nagrath S, Yu M, Miyamoto DT, Ulkus L, et al. Isolation and characterization of circulating tumor cells from patients with localized and metastatic prostate cancer. Sci Transl Med. 2010;2:25ra23.
Saliba AE, Saias L, Psychari E, Minc N, Simon D, Bidard FC, et al. Microfluidic sorting and multimodal typing of cancer cells in self-assembled magnetic arrays. Proc Natl Acad Sci U S A. 2010;107:14524–9.
Talasaz AH, Powell AA, Huber DE, Berbee JG, Roh KH, Yu W, et al. Isolating highly enriched populations of circulating epithelial cells and other rare cells from blood using a magnetic sweeper device. Proc Natl Acad Sci U S A. 2009;106:3970–5.
Rolle A, Günzel R, Pachmann U, Willen B, Höffken K, Pachmann K. Increase in number of circulating disseminated epithelial cells after surgery for non small cell lung cancer monitored by MAINTRAC(R) is a predictor for relapse: A preliminary report. World J Surg Oncol. 2005;3:18.
Ntouroupi TG, Ashraf SQ, McGregor SB. Detection of circulating tumour cells in peripheral blood with an automated scanning fluorescence microscope. Br J Cancer. 2008;99:789–95.
Deng G, Herrler M, Burgess D, Manna E, Krag D, Burke JF. Enrichment with anti-cytokeratin alone or combined with anti-EpCAM antibodies significantly increases the sensitivity for circulating tumor cell detection in metastatic breast cancer patients. Breast Cancer Res. 2008;10:R69.
Andreopoulou E, Yang LY, Rangel KM. Comparison of assay methods for detection of circulating tumor cells (CTCs) in metastatic breast cancer (MBC): AdnaGen AdnaTest BreastCancer Select/Detect™ versus Veridex Cell Search™ system. Int J Cancer. 2012;130(7):1590–7.
Lu J, Fan T, Zhao Q, Zeng W, Zaslavsky E, Chen JJ, et al. Isolation of circulating epithelial and tumor progenitor cells with an invasive phenotype from breast cancer patients. Int J Cancer. 2010;126:669–83.
Vona G, Sabile A, Louha M, Sitruk V, Romana S, Schütze K, et al. Isolation by size of epithelial tumor cells: a new method for the immunomorphological and molecular characterization of circulating tumor cells. Am J Pathol. 2000;156:57–63.
Somlo G, Lau SK, Frankel P, Hsieh HB, Liu X, Yang L, et al. Multiple biomarker expression on circulating tumor cells in comparison to tumor tissues from primary and metastatic sites in patients with locally advanced/inflammatory, and stage IV breast cancer, using a novel detection technology. Breast Cancer Res Treat. 2011;128(1):155–63.
Gascoyne PR, Noshari J, Anderson TJ. Isolation of rare cells from cell mixtures by dielectrophoresis. Electrophoresis. 2009;30:1388–98.
Tan SJ, Lakshmi RL, Chen P, Lim WT, Yobas L, Lim CT. Versatile label free biochip for the detection of circulating tumor cells from peripheral blood in cancer patients. Biosens Bioelectron. 2010;26:1701–5.
Zach O, Lutz D. Tumor cell detection in peripheral blood and bone marrow. Curr Opin Oncol. 2006;18:48–56.
Reinholz MM, Nibbe A, Jonart LM, Kitzmann K, Suman VJ, Ingle JN, et al. Evaluation of a panel of tumor markers for molecular detection of circulating cancer cells in women with suspected breast cancer. Clin Cancer Res. 2005;11:3722–32.
Harigopal M, Berger AJ, Camp RL, Rimm DL, Kluger HM. Automated quantitative analysis of E-cadherin expression in lymph node metastases is predictive of survival in invasive ductal breast cancer. Clin Cancer Res. 2005;11:4083–9.
Gillanders WE, Mikhitarian K, Hebert R, Mauldin PD, Palesch Y, Walters C, et al. Molecular detection of micrometastatic breast cancer in histopathology-negative axillary lymph nodes correlates with traditional predictors of prognosis. Ann Surg. 2004;239:828–37.
Mikhitarian K, Martin RH, Mitas M, Mauldin PD, Palesch Y, Metcalf JS, et al. Molecular analysis improves sensitivity of breast sentinel lymph node biopsy: results of a multi-institutional prospective cohort study. Surgery. 2005;138:474–81.
Stathopoulou A, Vlachonikolis I, Mavroudis D, Perraki M, Kouroussis C, Apostolaki S, et al. Molecular detection of cytokeratin- 19-positive cells in the peripheral blood of patients with operable breast cancer: evaluation of their prognostic significance. J Clin Oncol. 2002;20:3404–12.
Ignatiadis M, Kallergi G, Ntoulia M, Perraki M, Apostolaki S, Kafousi M, et al. Prognostic value of the molecular detection of circulating tumor cells using a multimarker reverse transcription-PCR assay for cytokeratin 19, mammaglobin A, and HER2 in early breast cancer. Clin Cancer Res. 2008;14:2593–600.
Ignatiadis M, Xenidis N, Perraki M, Apostolaki S, Politaki E, Kafousi M, et al. Different prognostic value of cytokeratin-19 mRNA positive circulating tumor cells according to estrogen receptor and HER2 status in early-stage breast cancer. J Clin Oncol. 2007;25:5194–202.
Xenidis N, Ignatiadis M, Apostolaki S, Perraki M, Kalbakis K, Agelaki S, et al. Cytokeratin-19 mRNA-positive circulating tumor cells after adjuvant chemotherapy in patients with early breast cancer. J Clin Oncol. 2009;27:2177–84.
Saloustros E, Perraki M, Apostolaki S. Cytokeratin-19 mRNA-positive circulating tumor cells during follow-up of patients with operable breast cancer: prognostic relevance for late relapse. Breast Cancer Res. 2011;13:R60.
Bidard FC, Mathiot C, Delaloge S, Brain E, Giachetti S, de Cremoux P, et al. Single circulating tumor cell detection and overall survival in nonmetastatic breast cancer. Ann Oncol. 2010;21:729–33.
Rack B, Schindlbeck C, Andergassen U. Use of circulating tumor cells (CTC) in peripheral blood of breast cancer patients before and after adjuvant chemotherapy to predict risk for relapse: the SUCCESS trial. J Clin Oncol. 2010;28:7s.
Nakamura S, Yagata H, Ohno S, Yamaguchi H, Iwata H, Tsunoda N, et al. Multi-center study evaluating circulating tumor cells as a surrogate for response to treatment and overall survival in metastatic breast cancer. Breast Cancer. 2010;17:199–204.
Gaforio JJ, Serrano MJ, Sanchez-Rovira P, Sirvent A, Delgado-Rodriguez M, Campos M, et al. Detection of breast cancer cells in the peripheral blood is positively correlated with estrogen-receptor status and predicts for poor prognosis. Int J Cancer. 2003;107:984–90.
Ntoulia M, Stathopoulou A, Ignatiadis M, Malamos N, Mavroudis D, Georgoulias V, et al. Detection of Mammaglobin A-mRNA-positive circulating tumor cells in peripheral blood of patients with operable breast cancer with nested RT-PCR. Clin Biochem. 2006;39:879–87.
De Giorgi U, Valero V, Rohren E, Mego M, Doyle GV, Miller MC, et al. Circulating tumor cells and bone metastases as detected by FDG-PET/CT in patients with metastatic breast cancer. Ann Oncol. 2010;21:33–9.
Budd GT, Cristofanilli M, Ellis MJ, Stopeck A, Borden E, Miller MC, et al. Circulating tumor cells versus imaging – predicting overall survival in metastatic breast cancer. Clin Cancer Res. 2006;12:6403–9.
Acknowledgments
The authors are thankful to Mrs. Shashi Dwivedi, Jatin Joshi, Kamla Kant Shukla, Akshay Kumar Sharma, Vinay Vashistha, and Francis Massey Prakash for their support in the manuscript preparation and editing.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer India
About this chapter
Cite this chapter
Dwivedi, S. et al. (2014). Molecular Diagnosis of Metastasizing Breast Cancer Based Upon Liquid Biopsy. In: Barh, D. (eds) Omics Approaches in Breast Cancer. Springer, New Delhi. https://doi.org/10.1007/978-81-322-0843-3_22
Download citation
DOI: https://doi.org/10.1007/978-81-322-0843-3_22
Published:
Publisher Name: Springer, New Delhi
Print ISBN: 978-81-322-0842-6
Online ISBN: 978-81-322-0843-3
eBook Packages: MedicineMedicine (R0)