Abstract
Breast cancer metastasis is a complex multistep process during which tumor cells undergo structural and functional changes that allow them to move away from the primary tumor and disseminate to distant organs and tissues. Despite the inefficiency of this process, some populations of circulating tumor cells (CTCs), which are those cells responsible of metastases formation, are able to survive in blood circulation and grow into secondary tumors. Metastatic breast cancer remains an incurable disease, and the phenomenon of metastasis represents the larger cause of death in these patients. The application of liquid biopsy techniques and the advancements in the field have shown the prognostic value of CTCs, suggesting the importance that CTCs analyses may have in the clinic. However, their implementation in routine clinic has not been yet achieved due to the yet small body of evidence showing their clinical utility. This introductory chapter will revise the key aspects of breast cancer metastasis and discuss the importance of CTC analyses in the management of breast cancer patients.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1.1 Breast Cancer Metastasis
Breast cancer (BC) is the most prevalent cancer among women worldwide. Surgical resection of the primary tumor has an elevated successful rate in early-stage BC, however 20–30% of patients will eventually develop disseminated disease or metastasis [1], resulting in the leading cause of cancer deaths. Despite advances in screening, diagnosis and treatment, a significant proportion of patients is diagnosed in advanced stages, with a median survival of ranging from 2 to 3 years for stage IV disease [2], depending on type and site of disease.
Breast cancer is divided into different subtypes according to the molecular profile of the tumor; the estrogen receptor positive (ER+)/progesterone receptor positive (PR+) subtype, also known as luminal; the HER2+ (human epidermal growth factor receptor positive) subtype, and the triple-negative (TNBC) subtype, which lacks the expression of ER, PR and HER2. The different molecular subtypes have implications on the systemic treatment planning. Thus, patients with hormone receptor positive tumors would benefit from endocrine therapy targeting ER; patients with HER2+ tumors are treated with the targeted therapy trastuzumab or the receptor tyrosine kinase inhibitors lapatanib; and TNBC patients lack approved targeted therapies and they are commonly treated with systemic chemotherapy [3].
Breast carcinogenesis is a complex process in which epithelial cells acquire genetic alterations within a permissive microenvironment that allows them to progress to a malignant neoplasm and subsequently metastasize to distant organs [4]. This tumor shows a great molecular and phenotypic heterogeneity, both at an inter- and intra-tumoral level, mainly governed by Darwinian selection driving tumor evolution [5,6,7,8]. Moreover, evidence demonstrate that subclonal populations of cancer cells may exist across different geographical regions within the same tumor, known as “spatial heterogeneity”, and these populations may evolve over time differentiating the primary tumor from subsequent local or distant recurrence, known as “temporal heterogeneity” [9]. This high heterogeneity bears important implication in BC therapeutics, partially explaining the limited efficacy of targeted therapies in this tumor type.
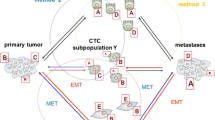
Breast cancer metastasis is characterized by a multistep cascade which can be subdivided in different steps. (i) Detachment of tumor cells from the primary tumor, probably after undergoing epithelial-mesenchymal transition (EMT); (ii) Migration and infiltration of tumor cells into adjacent tissue; (iii) Transendothelial migration of tumor cells into vessels (known as intravasation), entering the blood as circulating tumor cells (CTCs); (iv) Survival of CTCs in the circulatory system; (v) Arrest of CTCs at secondary sites and extravasation as disseminated tumor cells (DTCs); (vi) proliferation of DTCs in distant tissues allowing colonization and growth of metastases [10].
Despite of the large number of tumor cells that are shed daily into circulation, experimental data suggest that only a tiny fraction of these cells are able to form macroscopic metastases, indicating that metastasis is a very inefficient process [11]. It is widely assumed that the reason for that inefficiency are the destruction of CTCs in the bloodstream by shear stress forces and immune attack, as well as a slow rate of extravasation and proliferation in the stroma at a secondary site [12]. Thus, only those cancer cells with the capability to survive in the bloodstream, adapt to the distant tissue and new microenvironment, and induce angiogenesis, will successfully seed metastases.
1.1.1 Escape from Primary Tumor and Infiltration of Neighboring Tissue
In the first steps of the metastasis breast tumor cells, either as individual cells or clusters, detach from the primary tumor invading into the surrounding tissue. In order to do so, carcinoma cells of epithelial origin loss cell polarity and modify cell-to-cell adhesion and cell-matrix adhesion escaping anoikis, a form of apoptosis that occurs in anchorage-dependent cells when they detach from the surrounding extracellular matrix (ECM) [13]. Some of the adhesion molecules involved in cell-to-cell interactions are cadherins, claudins, or plakoglobin. In particular, cadherins play an important role in mediating these interactions [14], as down-regulation of their expression is required to initiate metastatic outgrowth of BC [15]. The molecular and structural alterations needed for tumor cell invasion are mediated by a differentiation process known as epithelial-to-mesenchymal transition (EMT), consisting in a genetic reprograming of epithelial tumor cells by which they attained mesenchymal characteristics and characteristics resembling to those of cancer stem cells (CSCs) [16, 17]. Certain mesenchymal markers such as fibronectin, vimentin, and N-cadherin are activated during EMT, enhancing migration and favoring cell-to-stroma interactions [18, 19]. Under physiological conditions, EMT can be triggered by paracrine signaling of TGF-beta, WNT, platelet-derived growth factors, or interleukin-6 (IL-6) [20]. Recent studies indicate that EMT is required for the dissemination of CTCs from breast tumors, however, EMT is not an on/off binary switch, therefore resulting in hybrid or intermediate phenotypes [21]. It is now believed that these diverse phenotypes provide tumor cells the ability to adapt to the different microenvironments confronted along the metastatic process [22]. In addition to invasion of the surrounding tissue, the characteristics acquired by tumor cells trough EMT are also important for intravasation into the bloodstream and to induce the activation of proteases involved in the degradation of the ECM (including matrix metalloproteinases (MMPs)), thus EMT plays a major role in tumor progression [23]. However, there is evidence that EMT is not essential for metastasis [24, 25]. The importance of EMT in BC progression will be discussed in depth in a separate chapter of this book.
Once tumor cells are liberated from the originating tumor tissue and become motile, they can migrate either individually or collectively [26]. Main differences between these two forms of migration are the need for tumor cells to maintain stable cell-to-cell adhesion and multicellular coordinated movement in order to collectively migrate, whereas individual migration requires losing cell-to-cell adhesion [27]. While individual migration can be either of a mesenchymal or an amoeboid type of movement, collective migration may require leader cells exhibiting mesenchymal features and therefore mesenchymal migration [28]. Inner cells within these groups may retain an epithelial phenotype. In keeping with this, clusters of CTCs found in the blood of BC patients can show both epithelial- and mesenchymal-like phenotype [29]. An important feature of the collective migration is the protection of inner cells from insults such as immune attack and shear forces while in circulation.
In addition to this well described active migration, mobile tumor cells can also migrate through a passive mechanism by which they are “pushed” into blood circulation [12]. A hypothesis suggests that due to the effects of tumor growing, tumor cells can be shed into fragile and leaky tumor blood vessels that are being formed (by angiogenesis), accidentally ending up in the circulation [30]. An indication for this is the fact that a large fraction of epithelial CTCs found in the blood of patients with advanced BC are apoptotic [31], although it is difficult to determine whether apoptosis takes place before or after intravasation.
A critical factor for the progression of ‘in situ’ BC to metastatic breast cancer (MBC) is the interplay between tumor cells and tumor microenvironment [32]. This tumor microenvironment is comprised by many types of cells such as macrophages, fibroblast, endothelial cells and immune cells, together with the ECM, and it may be determinant on tumor progression. Indeed, in BC, evidences support a role for cancer associated fibroblasts (CAFs) aiding tumor cells migration [33, 34], as well as a role for tumor associated macrophages (TAMs) on promoting tumor growth, angiogenesis and immune response suppression [35].
1.1.2 Intravasation
Invading tumor cells have the ability to penetrate basement membranes and endothelial walls and lymphatic vessels, becoming CTCs, in order to spread to secondary sites for metastases formation. To complete this process, angiogenesis and/or lymphangiogenesis are previously required [36], and tumor cells ought to disrupt the endothelial wall by mechanisms common to both the intravasation and extravasation processes [37, 38]. The vessels generated by tumor present weak cell-to-cell junctions which facilitate intravasation and the passive shedding of CTCs into the circulation [39]. However, active disruption of endothelial integrity increases the number of cells entering in blood or lymphatic vessels and therefore increasing metastasis [40]. In BC, dissemination can occur via the hematogenous and lymphatic systems. Lymphatic dissemination plays an important role in BC tumor cell spread [41]. Determining whether the cancer has spread to the regional lymph nodes is critical in staging a newly diagnosed patient, and the affectation of regional lymph nodes is considered to be a strong predictor of recurrences and survival [42]. Tumor cells disseminated to lymph nodes eventually exit via the efferent lymphatic vessels and make use of the hematogenous system that irrigates lymph nodes to reach the blood circulation and disseminate to secondary organs.
1.1.3 Survival of CTCs in Circulation
As previously mentioned, during their transit in the circulation, CTCs encounter several obstacles such as shear forces, collisions with blood cells, attack of the immune system, and oxidative stress [43], which ultimately affect their survival and capacity to establish metastatic foci. The mechanical forces experienced by CTCs in the vasculature are a major interference with the survival of CTCs. Interestingly, experimental evidences show that CTCs which underwent EMT are more resistant against these insults than epithelial CTCs [44]. Moreover, CTCs have to resist anoikis and survive in the circulation in the absence of cell-to-matrix interactions which provide proliferation signals. In this regard, mesenchymal CTCs may have an advantage since they do not require these interactions for survival [10]. In support of this, it is the finding of CTCs with mesenchymal characteristics in the blood of patients with BC [29]. Also, some CTCs acquire anoikis resistance mechanisms such as the autocrine BCL2-dependent resistance mechanisms [45], or the activation of tropomyosin-related kinase B (TrkB), that enable cells to survive in suspension [13]. In addition, CTC clusters prevent tumor cells from anoikis by maintaining strong cell-to-cell interactions, promoting their survival in the circulation system [46, 47]. Another obstacle faced by CTCs in the blood circulation is the attack of the immune system, particularly from natural killer (NK) cells [48]. In order to evade the antitumoral surveillance, CTCs cooperate with platelets inducing their aggregation, what acts as a physical shield that protects CTCs [49, 50]. All these hurdles are responsible for the low survival rate of CTCs in the bloodstream, making metastasis a very inefficient process. It has been estimated that only <0.01% of CTCs with high metastatic potential give rise to distant metastasis [51], and that in BC, CTCs survive only a few hours in the circulation [52].
1.1.4 Extravasation to a Secondary Site to form Micrometastasis
CTCs must eventually extravasate and leave the circulation systems. In order to do so, CTCs slow down in small capillaries, attach to the endothelium lining of blood vessels, and finally undergo transendothelial migration [53]. Two main mechanisms for CTC extravasations have been proposed, (i) physical occlusion in capillaries of smaller diameter than CTCs, and (ii) cell adhesion to the endothelium in capillaries of higher diameter thanks to the expression of ligands and receptors on both CTCs and endothelial cells [10].
Recent evidences indicate that shear forces play an important role in this process, determining the place in the body where CTCs will extravasate from the blood [54]. Also platelet aggregation can aid CTCs by enhancing their adhesion to the vasculature, facilitating transmigration trough the endothelial barrier [50, 55]. In addition, BC CTCs increase the permeability of the vasculature by secreting soluble factors, such as TGF-β-induced Angiopoietin-like 4 [56], Angiopoietin-2 [57], and VEGF [58].
Upon extravasation in secondary sites, and in order to re-gain proliferation, DTCs must undergo a mesenchymal-epithelial transition (MET) reverting to an epithelial phenotype [59]. They must also scape immune surveillance, mainly mediated by cytotoxic T cells and natural killer (NK) cells [60]. But mandatorily, DTCs must adapt to the new microenvironment. Although the factors determining the adaptation of tumor cells in secondary sites are not well understood, the interactions between them and the microenvironment seem to be key [61]. The success of tumor cells in forming metastatic foci will be greatly determined by the microenvironmental niche, also known as metastatic niche. This metastatic niche represents a complex interplay among DTCs and resident cells (osteoblasts in the bone, hepatocytes in the liver, astrocytes in the brain, etc.), the ECM, and infiltrating cells such as immune cells [62]. But tumor cells have the capacity to establish a “premetastatic niche” [63] by which primary tumors release systemic signals (cytokines, exosomes, extracellular-matrix-remodeling enzymes) that allow a more permissive and friendly microenvironment where DTCs can grow [43].
1.1.5 Breast Cancer Tropism and Dormancy
As already mentioned, in BC the initial dissemination of tumor cells is through the lymphatic system, reaching the sentinel lymph node from where they enter the blood circulation by exiting via the efferent lymphatic vessels. This tumor type metastasizes in an organ-specific manner preferentially to the bone and lungs and less frequently to other organs such as the liver and brain (95). Specifically, 47–60% of breast cancers metastasize to bone [64], 19–20% to the liver [65], 16–34% to the lung [66], and 10–16% to the brain [67]. This pattern of dissemination or organ tropism is mainly explained by circulation patterns that guide CTCs through the capillary bed where they arrest due to size restrictions of vessels whose diameter is too small to allow their passage [68]. Nevertheless, some CTCs are able to bypass this initial filter to reach other organs through the arterial circulation [43]. However, some BC CTCs may show preferences for specific tissues that favor their trapping through ligand-receptor interactions [69]. Indeed, several genes mediating preferential metastasis of breast tumor cells to bone, lung and brain have been identified [66, 70, 71].
The detection of metastases can occur many years and even decades after surgical resection of the primary tumor, indicating that CTCs extravasated in secondary organs as DTCs may remain in a dormant state. In BC, 20% of clinically disease-free patients relapse 7–25 years after mastectomy [72], suggesting the existence of a phenomena of early dissemination. Indeed, experimental evidences and clinical observations show that DTCs are detected in the bone marrow of people with no evidence of metastatic disease, in support of the idea that tumor cells can disseminate early, even before primary tumors become overtly invasive [61] or before the tumor is diagnosed. Thus, DTCs may give rise to occult micrometastases which mandatorily will undergo a growth restriction, and although the mechanisms of dormancy are still not clear, it is believed that tumor cells can survive in a quiescent state, in which they withdraw from the cell cycle, or by keeping a tight balance between cell proliferation at a slow rate and cell death [73]. The mechanisms and clinical implication of BC dormancy will be discussed in depth in a separate chapter of this book. It is only in the presence of the appropriate signals that DTCs may reenter into a proliferative state, colonize the secondary site, and eventually give rise to the formation of clinically relevant overt metastases [43].
1.2 CTCs Analyses, a Tool to Understand Breast Cancer Progression
Depending on the different subtypes, early-stage BC is amenable to curative tumor resection surgery. However, detection of early disease at the pre-symptomatic stages is very challenging. Once the local disease is detected, diagnosis based on small tumor samples or biopsies may result incomplete or incorrect given the high degree of heterogeneity of this tumor, which will consequently turn into a treatment which may be directed against targets not expressed throughout the entire tumor [74,75,76]. Likewise, in the metastatic setting, biopsies from metastatic lesion are usually not accessible, and therefore unable to guide therapy decision; and even when they are available, discordance between the primary tumor and recurrent metastasis in ER, PR and HER2 status has been shown [77, 78], with discordances of 6–40%, 21–41% and 1–43%, respectively [77]. Therefore, the study of CTCs through liquid biopsy techniques represents an optimal approach to address the heterogeneity of breast tumors, since they may represent the diverse molecular complexity of the tumors, and to limit the negative impact of heterogeneity in treatment selection. Moreover, CTC analysis will allow a more comprehensive understanding of the metastatic cascade, as they can originate either from primary sites on route to metastatic niches, or from established metastases.
This is now possible thanks to the development of highly sensitive technologies able to capture these cells from a simple blood drawn from a cancer patient, in a serial and non-invasive manner. However, the detection of CTCs is not exempt of difficulties. CTCs are found in the bloodstream of patients but very rarely found in healthy individuals [79]. It has been estimated that there is 1–10 CTCs per mL of whole blood in patients with metastatic disease [80]. To date, more than 50 assays have been developed for the identification, enumeration, and even molecular characterization of CTCs. Apart from the very low frequency of CTCs, the main challenges that technologies face to detect tumor cells in bloodstream are to distinguish them from the large background of blood cells, and the phenotypical and molecular heterogeneity of CTCs. In this regard different strategies for CTCs identification and enrichment have been adopted. Generally speaking, the strategies developed can classify CTC enrichment technologies in two main groups, the ones that take advantage of the biological properties of the cells (surface marker expression), or the ones that take advantage of physical properties of the cell (i.e. size and density) [81], although a growing number of newer technologies combine both strategies. The first group comprises immunoaffinity-based methods that exploit the positive recognition of protein markers in the surface of the CTCs (“positive selection”) by antibodies; being the most used marker the epithelial cell adhesion molecule (EpCAM). Among them, the CellSearch® system is the most frequently used for the isolation, enrichment and enumeration of CTCs in BC, and the only technology cleared by the U.S. FDA for clinical use in breast, prostate and colorectal cancer. All other technologies are available for clinical research. However, the CellSearch® system, and all technologies based on EpCAM recognition, present a major drawback, the downregulation or loss of expression of this epithelial marker during EMT [82], process that might be required for metastasis formation. The different technologies used for the isolation and enrichment of CTCs, together with the main advantages and disadvantages, will be discussed in a separate chapter.
Based on CTC counts provided by the CellSearch® system, CTCs are detectable in about 20–25% of patients with localized non-metastatic BC at the time of diagnosis [83], while these figures reach and even surpass a 65% in patients with MBC [84]. These numbers put on evidence the challenge that represents to detect CTCs in the blood of BC patients, and in particular at an early stage cancer, which would mean the possibility to monitor and even prevent cancer relapse. Despite this, the clinical validity of CTCs in BC has been clearly demonstrated, and CTC levels have been proven to be a valuable tool to predict prognosis in BC patients. Thus, in MBC a count ≥5 CTCs per 7.5 mL of blood is associated with significantly inferior progression-free survival (PFS) and overall survival (OS) [84]. Similarly, although with a lower threshold, in non-metastatic BC a CTC count ≥1 cells per 7.5 mL of blood is associated with decreased PFS and OS [83]. In addition to the prognostic value, CTC counts also enable prediction of treatment efficacy in patients with MBC [85,86,87]. Therefore, CTC enumeration is an effective prognostic and predictive biomarker, allowing early detection of metastasis development and monitoring of therapies efficacy. However, despite these clinical evidences, CTCs have not been included yet into the clinical guidelines, and their clinical utility, meaning the capacity to guide therapy decision and improve patient outcomes, remains to be determined in clinical trials. This topic will be further discussed across different chapters of the book.
In addition to enumeration, CTCs isolated from the blood of cancer patients can be characterized at the molecular and genomic level through the use of methods based on DNA, RNA, and protein analysis, either as a pool of cells or at single cell level. This characterization would enable the possibility to identify therapeutic targets and resistance mechanisms to targeted therapies [88]. Furthermore, it would bring the opportunity to adapt therapeutic strategies and to improve treatment selection, which would lastly translate into individualized treatments and a personalized medicine [89]. Interestingly, the characterization of CTCs at protein, RNA and DNA level is already providing relevant information for the identification of therapeutic targets and resistance mechanisms in BC [90, 91].
In summary, CTC analyses have the potential to elucidate the dynamics of the progression from localized BC to MBCs, changing our understanding about the metastatic process, and identifying the characteristics of the cells with the capacity to initiate cancer metastasis. Moreover, the analysis of CTCs bears a great potential to improve the management of BC patients, changing the landscape of BC treatment and even preventing the progression towards the metastatic disease. Specifically, it will be instrumental for the identification of new therapeutic targets in order to prevent metastatic recurrence, and to monitor treatment and understand the mechanisms of drug resistance, hereby representing a key tool to achieve a more personalize management of breast cancer patients.
References
Redig AJ, McAllister SS. Breast cancer as a systemic disease: a view of metastasis. J Intern Med. 2013;274(2):113–26. https://doi.org/10.1111/joim.12084.
Cardoso F, Costa A, Senkus E, Aapro M, Andre F, Barrios CH, et al. 3rd ESO-ESMO International Consensus Guidelines for Advanced Breast Cancer (ABC 3). Ann Oncol. 2017;28(1):16–33. https://doi.org/10.1093/annonc/mdw544.
Fragomeni SM, Sciallis A, Jeruss JS. Molecular subtypes and local-regional control of breast cancer. Surg Oncol Clin N Am. 2018;27(1):95–120. https://doi.org/10.1016/j.soc.2017.08.005.
Mao J-H, PJv D, Perez-Losada J, Snijders AM. Revisiting the impact of age and molecular subtype on overall survival after radiotherapy in breast cancer patients. Sci Rep. 2017;7(1):12587. https://doi.org/10.1038/s41598-017-12949-5.
Gerlinger M, Horswell S, Larkin J, Rowan AJ, Salm MP, Varela I, et al. Genomic architecture and evolution of clear cell renal cell carcinomas defined by multiregion sequencing. Nat Genet. 2014;46(3):225–33. https://doi.org/10.1038/ng.2891.
Gerlinger M, Rowan AJ, Horswell S, Math M, Larkin J, Endesfelder D, et al. Intratumor heterogeneity and branched evolution revealed by multiregion sequencing. N Engl J Med. 2012;366(10):883–92. https://doi.org/10.1056/NEJMoa1113205.
Nash I. Intratumor heterogeneity and branched evolution. N Engl J Med. 2012;366(22):2132–3.; ; discussion 3. https://doi.org/10.1056/NEJMc1204069.
Martin-Pardillos A, Valls Chiva A, Bande Vargas G, Hurtado Blanco P, Pineiro Cid R, Guijarro PJ, et al. The role of clonal communication and heterogeneity in breast cancer. BMC Cancer. 2019;19(1):666. https://doi.org/10.1186/s12885-019-5883-y.
Martelotto LG, Ng CK, Piscuoglio S, Weigelt B, Reis-Filho JS. Breast cancer intra-tumor heterogeneity. Breast Cancer Res. 2014;16(3):210. https://doi.org/10.1186/bcr3658.
Melzer C, Von Der Ohe J, Hass R. Breast carcinoma: from initial tumor cell detachment to settlement at secondary sites. Biomed Res Int. 2017;2017. https://doi.org/10.1155/2017/8534371.
Bystricky B, Mego M. Circulating tumor cells in breast cancer patients. Neoplasma. 2016;63(1):18–29. https://doi.org/10.4149/neo_2016_003.
Bockhorn M, Jain RK, Munn LL. Active versus passive mechanisms in metastasis: do cancer cells crawl into vessels, or are they pushed? Lancet Oncol. 2007;8(5):444–8. https://doi.org/10.1016/s1470-2045(07)70140-7.
Douma S, Van Laar T, Zevenhoven J, Meuwissen R, Van Garderen E, Peeper DS. Suppression of anoikis and induction of metastasis by the neurotrophic receptor TrkB. Nature. 2004;430(7003):1034–9. https://doi.org/10.1038/nature02765.
Li DM, Feng YM. Signaling mechanism of cell adhesion molecules in breast cancer metastasis: potential therapeutic targets. Breast Cancer Res Treat. 2011;128(1):7–21. https://doi.org/10.1007/s10549-011-1499-x.
Wendt MK, Taylor MA, Schiemann BJ, Schiemann WP. Down-regulation of epithelial cadherin is required to initiate metastatic outgrowth of breast cancer. Mol Biol Cell. 2011;22(14):2423–35. https://doi.org/10.1091/mbc.E11-04-0306.
Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan A, Zhou AY, et al. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell. 2008;133(4):704–15. https://doi.org/10.1016/j.cell.2008.03.027.
Ye X, Tam WL, Shibue T, Kaygusuz Y, Reinhardt F, Ng Eaton E, et al. Distinct EMT programs control normal mammary stem cells and tumour-initiating cells. Nature. 2015;525(7568):256–60. https://doi.org/10.1038/nature14897.
Choi Y, Lee HJ, Jang MH, Gwak JM, Lee KS, Kim EJ, et al. Epithelial-mesenchymal transition increases during the progression of in situ to invasive basal-like breast cancer. Hum Pathol. 2013;44(11):2581–9. https://doi.org/10.1016/j.humpath.2013.07.003.
Angelucci C, Maulucci G, Lama G, Proietti G, Colabianchi A, Papi M, et al. Epithelial-stromal interactions in human breast cancer: effects on adhesion, plasma membrane fluidity and migration speed and directness. PLoS One. 2012;7(12):e50804. https://doi.org/10.1371/journal.pone.0050804.
Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139(5):871–90. https://doi.org/10.1016/j.cell.2009.11.007.
Jie XX, Zhang XY, Xu CJ. Epithelial-to-mesenchymal transition, circulating tumor cells and cancer metastasis: mechanisms and clinical applications. Oncotarget. 2017;8(46):81558–71. https://doi.org/10.18632/oncotarget.18277.
Lu W, Kang Y. Epithelial-mesenchymal plasticity in cancer progression and metastasis. Dev Cell. 2019;49(3):361–74. https://doi.org/10.1016/j.devcel.2019.04.010.
Bonnomet A, Brysse A, Tachsidis A, Waltham M, Thompson EW, Polette M, et al. Epithelial-to-mesenchymal transitions and circulating tumor cells. J Mammary Gland Biol Neoplasia. 2010;15(2):261–73. https://doi.org/10.1007/s10911-010-9174-0.
Fischer KR, Durrans A, Lee S, Sheng J, Li F, Wong ST, et al. Epithelial-to-mesenchymal transition is not required for lung metastasis but contributes to chemoresistance. Nature. 2015;527(7579):472–6. https://doi.org/10.1038/nature15748.
Zheng X, Carstens JL, Kim J, Scheible M, Kaye J, Sugimoto H, et al. Epithelial-to-mesenchymal transition is dispensable for metastasis but induces chemoresistance in pancreatic cancer. Nature. 2015;527(7579):525–30. https://doi.org/10.1038/nature16064.
McSherry EA, Donatello S, Hopkins AM, McDonnell S. Molecular basis of invasion in breast cancer. Cell Mol Life Sci. 2007;64(24):3201–18. https://doi.org/10.1007/s00018-007-7388-0.
Friedl P, Gilmour D. Collective cell migration in morphogenesis, regeneration and cancer. Nat Rev Mol Cell Biol. 2009;10(7):445–57. https://doi.org/10.1038/nrm2720.
Guan X. Cancer metastases: challenges and opportunities. Acta Pharm Sin B. 2015;5(5):402–18. https://doi.org/10.1016/j.apsb.2015.07.005.
Yu M, Bardia A, Wittner BS, Stott SL, Smas ME, Ting DT, et al. Circulating breast tumor cells exhibit dynamic changes in epithelial and mesenchymal composition. Science. 2013;339(6119):580–4. https://doi.org/10.1126/science.1228522.
Joosse SA, Gorges TM, Pantel K. Biology, detection, and clinical implications of circulating tumor cells. EMBO Mol Med. 2015;7(1):1–11. https://doi.org/10.15252/emmm.201303698.
Mehes G, Witt A, Kubista E, Ambros PF. Circulating breast cancer cells are frequently apoptotic. Am J Pathol. 2001;159(1):17–20. https://doi.org/10.1016/S0002-9440(10)61667-7.
Place AE, Jin Huh S, Polyak K. The microenvironment in breast cancer progression: biology and implications for treatment. Breast Cancer Res. 2011;13(6):227. https://doi.org/10.1186/bcr2912.
Mego M, Mani SA, Cristofanilli M. Molecular mechanisms of metastasis in breast cancer – clinical applications. Nat Rev Clin Oncol. 2010;7(12):693–701. https://doi.org/10.1038/nrclinonc.2010.171.
Micke P, Ostman A. Exploring the tumour environment: cancer-associated fibroblasts as targets in cancer therapy. Expert Opin Ther Targets. 2005;9(6):1217–33. https://doi.org/10.1517/14728222.9.6.1217.
Gao D, Mittal V. The role of bone-marrow-derived cells in tumor growth, metastasis initiation and progression. Trends Mol Med. 2009;15(8):333–43. https://doi.org/10.1016/j.molmed.2009.06.006.
Alitalo K. The lymphatic vasculature in disease. Nat Med. 2011;17(11):1371–80. https://doi.org/10.1038/nm.2545.
Le Guelte A, Dwyer J, Gavard J. Jumping the barrier: VE-cadherin, VEGF and other angiogenic modifiers in cancer. Biol Cell. 2011;103(12):593–605. https://doi.org/10.1042/BC20110069.
Katt ME, Wong AD, Searson PC. Dissemination from a solid tumor: examining the multiple parallel pathways. Trends Cancer. 2018;4(1):20–37. https://doi.org/10.1016/j.trecan.2017.12.002.
Utoguchi N, Mizuguchi H, Dantakean A, Makimoto H, Wakai Y, Tsutsumi Y, et al. Effect of tumour cell-conditioned medium on endothelial macromolecular permeability and its correlation with collagen. Br J Cancer. 1996;73(1):24–8. https://doi.org/10.1038/bjc.1996.5.
Grisard E, Nicoloso MS. Following MicroRNAs Through the Cancer Metastatic Cascade. Int Rev Cell Mol Biol. 2017;333:173–228. https://doi.org/10.1016/bs.ircmb.2017.04.005.
Brown P. Unlocking the drains. Nature. 2005;436(7050):456–8. https://doi.org/10.1038/436456a.
Rahman M, Mohammed S. Breast cancer metastasis and the lymphatic system. Oncol Lett. 2015;10(3):1233–9. https://doi.org/10.3892/ol.2015.3486.
Massague J, Obenauf AC. Metastatic colonization by circulating tumour cells. Nature. 2016;529(7586):298–306. https://doi.org/10.1038/nature17038.
Mitchell MJ, King MR. Computational and experimental models of cancer cell response to fluid shear stress. Front Oncol. 2013;3:44. https://doi.org/10.3389/fonc.2013.00044.
Oudenaarden CRL, van de Ven RAH, Derksen PWB. Re-inforcing the cell death army in the fight against breast cancer. J Cell Sci. 2018;131(16). https://doi.org/10.1242/jcs.212563.
Zhao Q, Barclay M, Hilkens J, Guo X, Barrow H, Rhodes JM, et al. Interaction between circulating galectin-3 and cancer-associated MUC1 enhances tumour cell homotypic aggregation and prevents anoikis. Mol Cancer. 2010;9:154. https://doi.org/10.1186/1476-4598-9-154.
Fabisiewicz A, Grzybowska E. CTC clusters in cancer progression and metastasis. Med Oncol. 2017;34(1):1–10. https://doi.org/10.1007/s12032-016-0875-0.
Fabisiewicz A, Grzybowska E, Friedl P, Zanker KS, Friedl P, Noble PB, et al. On the development of cancer in the veins, and the transmission of cancer from man to the lower animals. Cancer Res. 2016;55(1):251–3. https://doi.org/10.1007/s12032-016-0875-0.
Jurasz P, Alonso-Escolano D, Radomski MW. Platelet – cancer interactions: mechanisms and pharmacology of tumour cell-induced platelet aggregation. Br J Pharmacol. 2004;143(7):819–26. https://doi.org/10.1038/sj.bjp.0706013.
Gay LJ, Felding-Habermann B. Contribution of platelets to tumour metastasis. Nat Rev Cancer. 2011;11(2):123–34. https://doi.org/10.1038/nrc3004.
Tremblay PL, Huot J, Auger FA. Mechanisms by which E-selectin regulates diapedesis of colon cancer cells under flow conditions. Cancer Res. 2008;68(13):5167–76. https://doi.org/10.1158/0008-5472.CAN-08-1229.
Meng S, Tripathy D, Frenkel EP, Shete S, Naftalis EZ, Huth JF, et al. Circulating tumor cells in patients with breast cancer dormancy. Clin Cancer Res. 2004;10(24):8152–62. https://doi.org/10.1158/1078-0432.CCR-04-1110.
Reymond N, d’Agua BB, Ridley AJ. Crossing the endothelial barrier during metastasis. Nat Rev Cancer. 2013;13(12):858–70. https://doi.org/10.1038/nrc3628.
Follain G, Osmani N, Azevedo AS, Allio G, Mercier L, Karreman MA, et al. Hemodynamic forces tune the arrest, adhesion, and extravasation of circulating tumor cells. Dev Cell. 2018;45(1):33-52 e12. https://doi.org/10.1016/j.devcel.2018.02.015.
Labelle M, Hynes RO. The initial hours of metastasis: the importance of cooperative host-tumor cell interactions during hematogenous dissemination. Cancer Discov. 2012;2(12):1091–9. https://doi.org/10.1158/2159-8290.CD-12-0329.
Padua D, Zhang XH, Wang Q, Nadal C, Gerald WL, Gomis RR, et al. TGFbeta primes breast tumors for lung metastasis seeding through angiopoietin-like 4. Cell. 2008;133(1):66–77. https://doi.org/10.1016/j.cell.2008.01.046.
Huang Y, Song N, Ding Y, Yuan S, Li X, Cai H, et al. Pulmonary vascular destabilization in the premetastatic phase facilitates lung metastasis. Cancer Res. 2009;69(19):7529–37. https://doi.org/10.1158/0008-5472.CAN-08-4382.
Weis S, Cui J, Barnes L, Cheresh D. Endothelial barrier disruption by VEGF-mediated Src activity potentiates tumor cell extravasation and metastasis. J Cell Biol. 2004;167(2):223–9. https://doi.org/10.1083/jcb.200408130.
Chaffer CL, Thompson EW, Williams ED. Mesenchymal to epithelial transition in development and disease. Cells Tissues Organs. 2007;185(1-3):7–19. https://doi.org/10.1159/000101298.
Eyles J, Puaux AL, Wang X, Toh B, Prakash C, Hong M, et al. Tumor cells disseminate early, but immunosurveillance limits metastatic outgrowth, in a mouse model of melanoma. J Clin Invest. 2010;120(6):2030–9. https://doi.org/10.1172/JCI42002.
Pantel K, Speicher MR. The biology of circulating tumor cells. Oncogene. 2015;35:1216. https://doi.org/10.1038/onc.2015.192.
Ursini-Siegel J, Siegel PM. The influence of the pre-metastatic niche on breast cancer metastasis. Cancer Lett. 2016;380(1):281–8. https://doi.org/10.1016/j.canlet.2015.11.009.
Psaila B, Lyden D. The metastatic niche: adapting the foreign soil. Nat Rev Cancer. 2009;9(4):285–93. https://doi.org/10.1038/nrc2621.
Coleman RE, Rubens RD. The clinical course of bone metastases from breast cancer. Br J Cancer. 1987;55(1):61–6. https://doi.org/10.1038/bjc.1987.13.
Selzner M, Morse MA, Vredenburgh JJ, Meyers WC, Clavien PA. Liver metastases from breast cancer: long-term survival after curative resection. Surgery. 2000;127(4):383–9.
Minn AJ, Gupta GP, Siegel PM, Bos PD, Shu W, Giri DD, et al. Genes that mediate breast cancer metastasis to lung. Nature. 2005;436(7050):518–24. https://doi.org/10.1038/nature03799.
Lin NU, Bellon JR, Winer EP. CNS metastases in breast cancer. J Clin Oncol. 2004;22(17):3608–17. https://doi.org/10.1200/JCO.2004.01.175.
Weinberg Robert A, Valastyan S. Tumor metastasis: molecular insights and evolving paradigms. Cell. 2011;147(2):275–92. https://doi.org/10.1016/j.cell.2011.09.024.
Brown DM, Ruoslahti E. Metadherin, a cell surface protein in breast tumors that mediates lung metastasis. Cancer Cell. 2004;5(4):365–74.
Kang Y, Siegel PM, Shu W, Drobnjak M, Kakonen SM, Cordon-Cardo C, et al. A multigenic program mediating breast cancer metastasis to bone. Cancer Cell. 2003;3(6):537–49.
Palmieri D, Bronder JL, Herring JM, Yoneda T, Weil RJ, Stark AM, et al. Her-2 overexpression increases the metastatic outgrowth of breast cancer cells in the brain. Cancer Res. 2007;67(9):4190–8. https://doi.org/10.1158/0008-5472.CAN-06-3316.
Uhr JW, Pantel K. Controversies in clinical cancer dormancy. Proc Natl Acad Sci U S A. 2011;108(30):12396–400. https://doi.org/10.1073/pnas.1106613108.
Willis L, Alarcon T, Elia G, Jones JL, Wright NA, Tomlinson IP, et al. Breast cancer dormancy can be maintained by small numbers of micrometastases. Cancer Res. 2010;70(11):4310–7. https://doi.org/10.1158/0008-5472.CAN-09-3144.
Denisov EV, Litviakov NV, Zavyalova MV, Perelmuter VM, Vtorushin SV, Tsyganov MM, et al. Intratumoral morphological heterogeneity of breast cancer: neoadjuvant chemotherapy efficiency and multidrug resistance gene expression. Sci Rep. 2014;4:4709. https://doi.org/10.1038/srep04709.
Russo M, Siravegna G, Blaszkowsky LS, Corti G, Crisafulli G, Ahronian LG, et al. Tumor heterogeneity and lesion-specific response to targeted therapy in colorectal cancer. Cancer Discov. 2016;6(2):147–53. https://doi.org/10.1158/2159-8290.CD-15-1283.
Schneider G, Schmidt-Supprian M, Rad R, Saur D. Tissue-specific tumorigenesis: context matters. Nat Rev Cancer. 2017;17(4):239–53. https://doi.org/10.1038/nrc.2017.5.
Criscitiello C, André F, Thompson AM, De Laurentiis M, Esposito A, Gelao L, et al. Biopsy confirmation of metastatic sites in breast cancer patients: clinical impact and future perspectives. Breast Cancer Res. 2014;16(2):205. https://doi.org/10.1186/bcr3630.
Karlsson E, Appelgren J, Solterbeck A, Bergenheim M, Alvariza V, Bergh J. Breast cancer during follow-up and progression - A population based cohort on new cancers and changed biology. Eur J Cancer. 2014;50(17):2916–24. https://doi.org/10.1016/j.ejca.2014.08.014.
Allard WJ, Matera J, Miller MC, Repollet M, Connelly MC, Rao C, et al. Tumor cells circulate in the peripheral blood of all major carcinomas but not in healthy subjects or patients with nonmalignant diseases. Clin Cancer Res. 2004;10(20):6897–904. https://doi.org/10.1158/1078-0432.CCR-04-0378.
Miller MC, Doyle GV, Terstappen LW. Significance of Circulating Tumor Cells Detected by the CellSearch System in Patients with Metastatic Breast Colorectal and Prostate Cancer. J Oncol. 2010;2010:617421. https://doi.org/10.1155/2010/617421.
Ferreira MM, Ramani VC, Jeffrey SS. Circulating tumor cell technologies. Mol Oncol. 2016;10(3):374–94. https://doi.org/10.1016/j.molonc.2016.01.007.
Hyun KA, Koo GB, Han H, Sohn J, Choi W, Kim SI, et al. Epithelial-to-mesenchymal transition leads to loss of EpCAM and different physical properties in circulating tumor cells from metastatic breast cancer. Oncotarget. 2016;7(17):24677–87. https://doi.org/10.18632/oncotarget.8250.
Lucci A, Hall CS, Lodhi AK, Bhattacharyya A, Anderson AE, Xiao L, et al. Circulating tumour cells in non-metastatic breast cancer: a prospective study. Lancet Oncol. 2012;13(7):688–95. https://doi.org/10.1016/S1470-2045(12)70209-7.
Cristofanilli M, Budd GT, Ellis MJ, Stopeck A, Matera J, Miller MC, et al. Circulating tumor cells, disease progression, and survival in metastatic breast cancer. N Engl J Med. 2004;351(8):781–91. https://doi.org/10.1056/NEJMoa040766.
Liu MC, Shields PG, Warren RD, Cohen P, Wilkinson M, Ottaviano YL, et al. Circulating tumor cells: a useful predictor of treatment efficacy in metastatic breast cancer. J Clin Oncol. 2009;27(31):5153–9. https://doi.org/10.1200/JCO.2008.20.6664.
Bidard FC, Peeters DJ, Fehm T, Nole F, Gisbert-Criado R, Mavroudis D, et al. Clinical validity of circulating tumour cells in patients with metastatic breast cancer: a pooled analysis of individual patient data. Lancet Oncol. 2014;15(4):406–14. https://doi.org/10.1016/S1470-2045(14)70069-5.
Cristofanilli M, Hayes DF, Budd GT, Ellis MJ, Stopeck A, Reuben JM, et al. Circulating tumor cells: a novel prognostic factor for newly diagnosed metastatic breast cancer. J Clin Oncol. 2005;23(7):1420–30. https://doi.org/10.1200/JCO.2005.08.140.
Alix-Panabieres C, Pantel K. Circulating tumor cells: liquid biopsy of cancer. Clin Chem. 2013;59(1):110–8. https://doi.org/10.1373/clinchem.2012.194258.
Lianidou ES, Mavroudis D, Georgoulias V. Clinical challenges in the molecular characterization of circulating tumour cells in breast cancer. Br J Cancer. 2013;108(12):2426–32. https://doi.org/10.1038/bjc.2013.265.
Alix-Panabieres C, Pantel K. Clinical applications of circulating tumor cells and circulating tumor DNA as liquid biopsy. Cancer Discov. 2016;6(5):479–91. https://doi.org/10.1158/2159-8290.CD-15-1483.
Braun M, Markiewicz A, Kordek R, Sadej R, Romanska H. Profiling of invasive breast carcinoma circulating tumour cells-are we ready for the ‘liquid’ revolution? Cancer. 2019;11(2). https://doi.org/10.3390/cancers11020143.
Acknowledgements
The work of the author is supported by Roche-Chus Joint Unit (IN853B 2018/03) funded by GAIN, “Consellería de Economía, Emprego e Industria”.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Piñeiro, R. (2020). Introduction – Biology of Breast Cancer Metastasis and Importance of the Analysis of CTCs. In: Piñeiro, R. (eds) Circulating Tumor Cells in Breast Cancer Metastatic Disease. Advances in Experimental Medicine and Biology, vol 1220. Springer, Cham. https://doi.org/10.1007/978-3-030-35805-1_1
Download citation
DOI: https://doi.org/10.1007/978-3-030-35805-1_1
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-35804-4
Online ISBN: 978-3-030-35805-1
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)