Abstract
Despite years of research, a neurological etiology for sdolescent idiopathic scoliosis is still being explored. The task is complicated by the difficulty in differentiating a characteristic of scoliosis as a primary etiologic factor or an effect that is secondary to the spinal deformity. Here, we provide an overview of the accumulating data pointing to the involvement of neurological causes in the onset for adolescent idiopathic scoliosis.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
7.1 Introduction
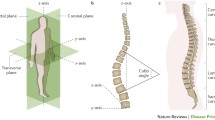
Idiopathic scoliosis (IS) is a three-dimensional deformity of the spine with lateral curvature combined with vertebral rotation. The primary lesion, however, lies in the sagittal plane, taking the form of lordosis. It was first described by Hippocrates, and the term “scoliosis” was first coined by Galen (AD 131–201). IS, therefore, refers to a structural scoliosis with lordosis and vertebral rotation. This is the most common and clincally important type of idiopathic scoliosis, which affects more offen in girls. The spine curvature progresses during the growing years, particularly during the preadolescent and adolescent growth spurt, and halts as growth ceases. Although the clinical manifestations of scoliosis have been well described, its etiology and pathogenesis of IS have not been clearly elucidated.
IS is a multifactorial disease which involves intrinsic factors such as the growth of vertebral bodies, abnormal morphometry of the posterior elements, and asymmetrical growth of the neurocentral cartilage. Other multiple factors have been proposed such as genetics, extra-spinal left-right asymmetrical body growth and development, imbalance of muscle structures, dysfunction of the nervous, somatosensory and vestibular functions, length discrepancy between spine and spinal cord, abnormal platelet calmodulin, and abnormality in melatonin metabolism [1]. Recently, research of IS etiology has focused on the structural elements of the spine such as musculature and collagenous structures, systemic factors such as endocrine and central nervous system, and also genetics [2] (Fig. 7.1), but none has shown convincing evidence for the cause of IS. Results of various animal experimental models and clinical studies have indicated possible anatomical or functional components as a cause of IS, but many of these may be epiphenomena rather than the cause.
This chapter is a review of the neurological causative factors thus far proposed for IS and a discussion of where the research is heading in order to find the etiology of IS.
7.2 Erect Posture and Paravertebral Muscles
Bipedalism with an upright posture predisposed humans to a series of three-dimensional changes in the spine and trunk, affecting the sagittal shape pelvic-spine rotation/counter-rotation in the horizontal plane, and trunk broadening in the coronary plane. The fully erect posture, which is unique to humans, seems to be a prerequisite for the development of IS. From a biomechanical standpoint of view, this transformation could be economical and functional, but it tends to weaken the spinal column resulting in a form of “backward shear stress (dorsal shear force)” to which the vertebrae are not accustomed. The effects of dorsal shear force can be further enhanced by the rotational instability associated with the hypokyphosis or lordosis caused by the relative anterior spinal overgrowth (RASO) and the axial spinal movements unique to humans. Consequently, the upright bipedal posture may contribute to the rotator instability from which scoliosis originates. This has been confirmed by the experimental rat studies of Machida et al., in which experimental scoliosis developed only in pinealectomized bipedal, but not in quadrupedal, rats [3, 4]. These results support the notion that the bipedal posture may play an important role in the development of scoliosis (Fig. 7.2).
The idea that erector spine asymmetry might be the source of scoliosis is by no means new; tenotomy of the multifidus muscle had been suggested as far back as the nineteenth century a treatment for scoliosis [5]. The idea is evidently related to the high frequency of scoliosis in childhood neuromuscular disease. A few decades later another connection between scoliosis and muscle function was proposed by Eulenberg, in which asymmetrical muscle action between the concave and convex sides contributes to the deformity formation. Objecting to this idea, Adams thought that the role of the musculature in scoliosis was secondary by the overreaction of the convex paraspinal muscles attempting to stabilize the spine and prevent aggravation of the curvature [6]. He described fatty degeneration in the paraspinal muscle of the concave side of the curve, but focused on a similar pathology in the convex side of musculature at later stages with advanced deformity. Malgaigne had already suggested that scoliotic deformity was due to weakness of the muscle on the convex side in association with weak ligaments. Trontelj et al. also demonstrated asymmetrical muscle tone in the rotators of the spine on the two sides of the curve [7]. Conceivably this could be either due to an altered descending control of the spinal neurons or to a segmental neurogenic lesion involving one or a few segments that lead to asymmetric muscle weakness.
During the last century, research was focused on the role of neuromuscular factors in the causation and progression of IS. Spencer and Eccles were the first to describe the presence of two types of muscle fibers in the paraspinal muscles of AIS patients [8]. They differentiated between type-I (slow-twitch) and type-II (fast-twitch) fibers and noted that the number of type-II fibers was lower in AIS patients, suggesting a myopathic etiology for IS. Bylund et al. described a normal distribution of type-I and type-II fibers on the convexity of the curve but a lower frequency of type-I fibers on the concave side [9]. By studying biopsy samples of the paraspinal muscles in AIS patients, Slager and Hsu confirmed a decrease in the number and size of type-II fibers with no preference for either the convex or the concave [10]. In an effort to support a global myopathy, Yarom et al. found similar findings in muscle from distant sites and concluded that this represented a myopathic process [11, 12]. The myopathy differs from the known forms of congenital myopathies by its lack of specific morphology, asymmetry, and mildness.
The first studies using the electromyography (EMG) were reported by Riddles and Roaf [13]. Surface electrodes overlying the paraspinal muscles at the apex of the scoliotic curve were studied, although some of their studies including the deeper layers of the spinal muscles using needle electrodes were performed. They surmised that the unopposed activity of the deep spinal muscles would give rise to vertebral rotation as the first stage of developing scoliosis and suggested that weakening the muscle on the convex side might have stopped curve progression. Henssge studied the needle EMG potentials of paraspinal muscles in IS patients and concluded that there was evidence of muscle denervation in the deep layers in certain types of curvature [14]. In contrast, Badger found no evidence of increased EMG activity at the convex side and suggested the presence of a primary myopathic process [15]. However, Walfe reported that the EMG findings revealed variable degrees of myopathy in the deltoids of the concave side [16].
Trontelj et al. studied segmental spinal reflexes (stretch reflex) in patients with scoliosis [7]. The proprioceptive responses to the phasic stretch of the paraspinal muscles were asymmetric in all patients and were higher in the convex side. The increase in reflex response of the superficial muscles on the convex side can be attributed to diminished reciprocal inhibition from the weak and deep muscles. They concluded that a segmental neurogenic disorder predominantly involving the deep paraspinal muscles of the convex side may be the primary lesion responsible for the development of scoliosis.
Trontelj later reported the results of evaluation of single-fiber EMG (SFEMG) studies in IS patients [17]. He found an increase in fiber density, correlating with the fiber type grouping noted on histomorphometric examinations. There was increased “jitter” which was defined as abnormal conduction across the terminal axon and end plate, which is observed in both neurogenic and myopathic muscle pathologies. He also reported changes in conduction time, which correlate with changes in fiber size. Fernandez also studied the SFEMG in 51 patients with moderate idiopathic juvenile scoliosis [18]. A mild but significant neuromuscular transmission abnormality and a moderate prolonged mean of interspike latency were observed in extensor digitorum communis (EDC) musculus. The paraspinal and intercostal muscles at the apex of the scoliosis curvature in the same patients showed similar abnormalities. The study suggested the existence of a subclinical and systemic neuromuscular disorder in patients with IS, which might have a pathogenic significance. These studies suggested the presence of a systemic neuromuscular conduction defect may be the cause, rather than the effect, of the deformity. This theory was further supported in patients with a strong family history of IS who showed unilateral EMG abnormalities as early as 6 months before any radiological signs of scoliosis became evident [19]. Based on these studies, there appears to be little doubt that asymmetric EMG abnormalities exist in the paraspinal muscles of IS patients, especially in the erect posture.
Whether the EMG asymmetry results from hyperactivity on the convex side or from reduced activity on the concave side remains elusive, and there is little evidence to confirm or deny the primary importance of this EMG asymmetry contributing for the pathogenesis of IS. Although the abnormality of the paraspinal muscles has long been considered as a cause of IS, Yarom and Robin found myofilament disarray, central core formation, fiber splitting, and a marked increase in muscle calcium not only in spinal muscles but also in distal muscles like the gluteus maximus [20]. High calcium concentration was also found in the tongue muscles of IS patients with mild curvature. They attributed this to a generalized membrane defect—namely, an impaired calcium pump. Following this line of thought, low bone quality has been observed in IS patients, with changes in bone density at sites remote from the spine.
Biochemical analyses of the muscles in IS patients have found on tissue enzyme actovity and protein synthesis. Gibson et al. analyzed protein synthesis in paraspinal muscle biopsy specimens obtained bilaterally from the top, bottom, and apex of the curve in IS patients using the stable isotope-labeled l-leucine [21] and found no difference between the two sides of the spine. However, at the apex of the curve, protein synthesis was higher on the convexity than on the concavity, and muscle ribonucleic acid activity was lower on the concavity than on the convexity. They believed that these results were consistent with the effects on muscle protein turnover secondary to increased muscle contraction and functional immobilization that occurs on the concave aspect of the curve.
Although the idea that an abnormality of the paraspinal muscles might be the cause of IS has been proposed many times throughout the years, there is insufficient data to back this claim. Morphological changes were most pronounced in the concave paraspinal muscles, but similar changes were found in other muscles such as the gluteus and deltoid muscles. Whatever the etiology of the myopathy and its asymmetry, these muscle changes may be more effectual than the causal in the pathogenesis of IS; most of the abnormalities that have been noted in the muscles may be more likely secondary to the deformity itself than the cause.
7.3 Calmodulin and Melatonin
There is a close relationship between calcium and muscle contraction with the binding of calcium to troponin on contractile protein structure being the initiating factor for contraction. Troponin found by Ebashi and Endo in 1968 inhibits the interaction of myosin and actin, and the inhibition is removed by Ca++ [22]. Since elevated calcium stimulates ATPase function and leads to contraction, the presence of an increased sarcoplasmic calcium concentration in IS patients has suggested a possible true role of calcium-related myopathy in IS pathogenesis.
Calmodulin, another calcium-binding receptor protein, is a critical mediator of eukaryotic cellular calcium function and a regulator of many important enzymatic systems. Calmodulin regulates the contractile properties of muscles and platelets through its interaction with actin and myosin and regulates calcium fluxes from the sarcoplasmic reticulum. Several studies on platelet abnormalities in IS were reported in the 1980s based on the similarity between the cytoskeleton of skeletal muscle cells and platelet cells. An abnormal platelet aggregation in IS patients with a large Cobb angle has been confirmed, and increased platelet calmodulin levels have been shown to be associated with the progression of AIS. Cohen et al. found a 2.5- to 3-fold increase in the calmodulin activity in platelets of AIS patients [23] and suggested that the platelet calmodulin levels may be a better predictor for scoliosis progression than the Risser sign. Kindsfater et al. showed that platelet calmodulin levels were significantly higher in patients with a progressive curve than in patients with stable curvature [24]. Using calmodulin as a systemic mediator of contracting tissue, the relationship between platelet calmodulin level changes and Cobb angle changes in AIS patients suggested that altered paraspinal muscle activity may be a cause of scoliosis. Asymmetric distribution of calmodulin reported in another study further supports this hypothesis [25]. Lowe et al. noted that AIS through bracing or spinal fusion resulted in a decrease of the platelet calmodulin levels in many patients with AIS [26, 27], but the cause of this decrease in platelet calmodulin is unknown.
Recent evidence suggests that melatonin which binds to calmodulin with high affinity and acts as a calmodulin antagonist may modulate calcium-activated calmodulin. Since melatonin is to be tightly associated with the sleep-wake cycle, it may diurnally modulate many cellular functions involving calcium transport [28, 29]. Although there were some reports that platelet calmodulin may be related to muscle disorders, the high levels of calmodulin in the brain suggests that the role of calmodulin in IS may more likely be related to an abnormality in the central nervous system rather than abnormal skeletal muscles.
Thillard was the first to report the development of scoliosis in pinealectomized chickens [30], and her findings were confirmed by Dubousset and Machida [31,32,33,34,35] (Fig. 7.3). As melatonin is a neuromodulator which is present in the pineal gland, we put forward the hypothesis that the pathogenic mechanism is associated with a postural equilibrium induced by melatonin deficiency. We also investigated the effect of pinealectomy in rats. We performed pinealectomy not only in quadrupedal rats but also in bipedal rats created by resection of the forearms and tails. It was found that scoliosis developed in all bipedal rats (Fig. 7.4) but not in quadrupedal rats. Based upon these results, we postulated that the scoliotic deformity of the fibroelastic and bony structures of the spine in humans was associated with the bipedal condition [3]. An inherited disorder of neuromodulators and/or neurohormones affecting melatonin synthesis produces a systemic imbalance that generates an abnormal force leading to scoliosis. Furthermore, bipedal C57BL/6J mice, which have in a reduced plasma and pineal melatonin levels due to a natural knockout in a major enzyme required for melatonin synthesis (NAT gene), developed scoliosis without resection of the pineal gland [36] (Fig. 7.5). Supplementation of melatonin or pineal gland transplantation in melatonin-deficient chickens, rats, and mice prevented the development of scoliosis [3, 33, 36]. Later, Chung et al., investigating the effects of pinealectomy in monkeys, which are much closer to human beings, demonstrated that melatonin deficiency in nonhuman primates did not induce scoliosis [37]. They concluded that the factors producing scoliosis in lower animals, such as chickens and rats, are different from the etiological factors in nonhuman and human primates, but we strongly disagree with their conclusion, because monkeys are mostly of quadrupedal gait, though they are capable of bipedal standing.
The pictures of the thoracic spine in a pinealectomized chicken. The scoliotic deformity is lordoscoliosis with vertebral rotation. The vertebral body is compressed downward on the concave side leading to a wedge-shaped deformity. The posterior elements of a scoliotic vertebra show much more complicated deformity
Utilizing a [125I]iodomelatonin-binding assay in the experimental scoliosis model of melatonin-deficient C57BL/6J mice, we recently found that the responsible site for scoliosis development was likely to be the melatonin receptor in the paraventricular thalamic nucleus [38] (Fig. 7.6). There are melatonin receptors at the cerebral cortex, striatum, hippocampus, thalamus, paraventricular thalamic nucleus, hypothalamus, midbrain, and cerebellum in the brain. Mice treated with melatonin had uniformly lower total binding of [125I]iodomelatonin, but mice that did not receive melatonin treatment had an unproportionately high density of melatonin receptors in the paraventricular thalamic nucleus compared to other sites of the brain. In contrast, mice treated with melatonin had a comparable density of melatonin receptors throughout the brain.
(a, b) Distribution of [125I]iodomelatonin binding sites in the bipedal C57BL/6J mouse with and without melatonin treatment. The binding density at the paraventricular thalamic nucleus (PTN) which was extremely high without melatonin treatment is uniformly lower in mice treated with melatonin. (c) An HE stain of the brain level in which the PTN is located. (d) Brain map
In agreement with the view that melatonin is deficient in IS patients, decreased melatonin levels have been observed in adolescent carriers of scoliosis [39,40,41]. We also reported that the melatonin concentration throughout a 24-h period was significantly lower in patients with progressive scoliosis than in patients with stable curves and normal controls (Fig. 7.7). Changes in melatonin receptor binding have also been mentioned as a possible factor in the development of IS [42, 43]. Along with this line of thought, we studied a group of 40 AIS patients with moderate to severe scoliosis, and we investigated the correlation between serum melatonin levels and curve progression and studied the effects of melatonin therapy in patients with reduced levels of endogenous melatonin. Our findings suggested that transient melatonin deficiency may be associated with deterioration of scoliosis and that melatonin levels may serve as a useful predictor for progression of spine curvature in patients with IS. Our results also suggested that melatonin treatment may provide some benefit in preventing the progression of scoliosis in melatonin-deficient patients, if the curves do not exceed 35° [41] (Fig. 7.8). Moreau et al. reported that the progression character of scoliosis is associated with a membrane anomaly in the melatonin receptor [44, 45]. However, recent studies in patients with AIS have shown that no evidence of mutations in the melatonin gene receptor [42, 43]. Asymmetric expression of melatonin receptor mRNA in the paraspinal muscles and melatonin signaling dysfunction in osteoblasts have been demonstrated in AIS, but it is not clear whether these findings are causative or secondary changes [44, 46]. Lowe et al. concluded that IS does not result from a simple absence of melatonin but rather an alteration in the system of melatonin production with direct or indirect consequences upon growth mechanisms [2] (Fig. 7.9).
7.4 Nervous System
Scoliosis is seen in various neurological disorders at different levels of the nervous system, from the peripheral nerves up to the central nervous system, but the abnormalities in the central nervous system have long been thought to play a role in the pathogenesis of AIS. The studies carried out at Tokushima University in Japan since 1965 were primarily designed to investigate the effect of scoliosis on postural tone, i.e., a reflex function depending on afferent stimuli from somatic proprioceptive organs onto the vestibular mechanism of the inner ears and the visual input from the eyes [47,48,49,50,51] (Fig. 7.10). At the 2000 Scoliosis Research Society Symposium, Lowe et al. concluded that there is a defect of central control or processing in the central nervous system affecting the growth of spine; the most consistent clinical studies point to the pontine and hindbrain regions as the most likely sites of primary pathology [2].
Relevant studies can be divided into two major groups: neuromorphologic studies and neurophysiologic studies. Utilizing the high resolution of magnetic resonance imaging (MRI) and advanced computer-aided images to compare the central nervous system of AIS patients and normal adolescents, the studies were concluded in regional brain volumes, the white matter in the corpus callosum and internal capsule [52], and the vestibular system morphology, particularly in the alignment of the semicircular canals [53,54,55]. Apart from the investigation of neuromorphology, functional studies in AIS subjects have shown varieties of abnormalities in the multiple systems/functions as follows: postural balance, somatosensory function, equilibrium [56,57,58,59], proprioceptive function [60], oculovestibular function [61], lateral gaze palsy [62], electromyography [63], somatosensory evoked potentials [64,65,66,67], and even electroencephalography [68]. There were also contradictory findings regarding vibratory responses [60, 69], transcranial magnetic stimulation [70, 71] and motor cortex hyperexcitability [72], and gait studies [73,74,75,76].
7.4.1 Spinal Cord
Experimentally induced spinal cord scoliosis has been produced in mammals through microsurgical dorsal rhizotomies [77,78,79]. All animals in which three or more roots were transected developed a progressive scoliosis. Histological examination of the spinal cord in the scoliosis animals showed no evidence of anterior horn damage, infarct, or ischemic change. Dorsal rhizotomies were believed to lead to spinal deformity due to the proprioceptive deficit that followed the transection of the nerve roots. Pincott et al. also reported the development of scoliosis after transection of the dorsal roots in a series of primate animals. They found that the frequency of scoliosis was higher when more roots were transected and that the addition of transecting a ventral root showed no effect on the production of a spinal deformity. In all animals, the spinal deformity convexity was on the side of the experimental lesions [80]. This supported the hypothesis that the scoliosis could be created by asymmetrical spinal muscle weakness due to loss of proprioception.
In another series of animal experiments, experimental scoliosis was produced in animals by both anterior and posterior spinal rhizotomies over several levels [81]. Scoliosis also occurred when the posterior horn cells along with the central posterior gray matter and adjacent Clarke columns were damaged on the convex side of the spinal cord. Injection of attenuated polio virus into the spinal cords of monkeys also produced scoliosis [82]. Although there was obvious spinal cord damage, scoliosis was thought not to be due to poliomyelitis because the anterior horn was not involved. This experiment provided a useful animal model, but the scoliosis that developed was similar to a paralytic or neuropathic deformity than IS, since rotation deformity was not detected.
Segmental destruction of the vascular supply in the spinal cord of rabbits gave rise to a progressive scoliotic deformity, radiologically similar to IS. In these experiments, the nerve damage was seen mainly in the posterior and lateral columns along with anterior horn, suggesting that segmental damage to the proprioceptive control of spinal musculature could have occurred [83]. Barrios et al. also produced scoliotic deformity in rabbits by using laser or electrocoagulation to induce focal damage to the posterior horn of the spinal cord [84]. This preparation produced scoliotic deformity. The areas of neuronal necrosis and/or gliosis were limited entirely to the posterior horn and the contiguous parts of the posterior and lateral columns. The scoliosis created was always convex on the side of the cord lesion. In a clinical study, Dubousset et al. reported spinal deformities in all 30 cases that received excision of spinal cord neuroblastomas, with the spinal curvature always on the convex side. The ligation or coagulation of the intercostal neurovascular bundle within foramen might have resulted in posterior horn damage of the spinal cord [85].
Scoliosis has been induced in animals through selective destruction of the posterior column at the spinal cord level, the brainstem, and the posterior hypothalamus. Clinical studies assessing posterior column function have consistently shown significant differences between scoliotic subjects and controls, and numerous studies have shown that AIS patients display abnormal postural equilibrium. Proprioceptive input, a prominent component of postural equilibrium, has been hypothesized to play a significant role in the etiology of AIS, and multiple clinical studies have supported this theory. Since proprioceptive and vibratory inputs are conducted through the posterior column system, reports of significant differences in somatosensory evoked potentials (SEPs) between AIS and normal controls imply posterior column dysfunction [86]. However, whether abnormal postural equilibrium is the primary etiologic factor or whether it is secondary to spinal deformity cannot be determined. Whitecloud et al. showed deceased sensitivity to vibration in the lower but not the upper extremities in patients with congenital scoliosis, demonstrating the presence of a sensory deficit that is secondary to the congenital curve itself or an associated subclinical spinal cord lesion; they argued that the sensory deficit is secondary to the spinal deformity rather than a primary etiologic factor. Furthermore, equilibrium dysfunction was shown to be correlated with the progression of scoliosis, regardless of whether the curve was idiopathic or congenital in origin [87] and suggested that equilibrium dysfunction was secondary to the curve but not the primary etiological factor. Since no significant asymmetry in vibration response was found between the concave and convex sides in the IS patients or the right and left sides in the control subjects, it is unlikely that a lesion of the posterior column is the primary cause for IS.
The advent of MRI has led to renewed interest in abnormal neuroanatomy seen in condition such as Chiari malformation or tethered spinal cord, both of which have been linked to scoliosis. In patients with IS, there is a substantially higher prevalence of cervicothoracic syrinxes associated with Chiari type I malformations at the foramen magnum particularly in those who exhibit a juvenile onset of this disorder [88,89,90]. A review of the literature revealed that the prevalence of a syrinx in a comparable series of patients with scoliosis ranged from 10% to 47% [90] [91]. The site and extent of the curve showed no differences from those found in IS without a syrinx, but there was a greater prevalence of left-sided thoracic deformity in patients with a syrinx. Neurologic function was normal in most patients, although some patients showed asymmetrical superficial abdominal reflexes. It is not yet known whether this is secondary to the syrinx formation as the first sign of syringomyelia or whether this asymmetrical reflex might reflect a more proximal hindbrain or midbrain lesion. A brain lesion could be linked to or even cause the syrinx, the tonsillar prolapse, and the scoliosis. Alternatively, the Chiari malformation and the syrinx could be the result of traction acting upon the medulla distally through the foramen magnum. It is also possible that growth of the spinal cord is slower in patients with IS, resulting in a cord that is shorter than the more rapidly growing spinal canal [92]. Reduced growth of the cord may also be due to pineal dysfunction, perhaps involving a melatonin deficiency, leading to a discrepancy between the lengths of the spinal column and the spinal cord in the normal spine [93].
In 1968, Roth proposed that shortening of the spinal cord can lead to IS. He explained the patho-mechanism of IS by a spring-string model and believed that the disturbed symmetry of nerve tension causes lateral flexion of the spine toward the side of increased tension [92]. Later, Porter, analyzing the axial lengths of the spinal column and the spinal canal in normal and IS spines, found no significant difference between the two groups in the lengths of the spine [94]. He hypothesized that a short spinal canal could tether the posterior elements, and the continuing growth of the vertebral bodies would result in buckling and rotation of the spine around the axis of the spinal cord; he called this the theory of “uncoupled neuro-osseous growth” [95]. Recently, Chu et al. reported on relative overgrowth of the spinal column in AIS without a corresponding increase in the length of the spinal cord, postulating that the fixed spinal cord could act as a functional tether [96]. The role of the spinal cord itself in the development of AIS, however, remains controversial.
7.4.2 Brainstem
It is conceivable that lateral displacement of the vertebrae requires an altered postural tone to maintain the balance of the body, which is regulated by a postural reflex mechanism: this mechanism originates from the proprioceptors, the vestibules of the internal ears, and the optic organs, ending at the equilibrium center of the brainstem. If disturbances occur at any level of the postural reflex circuit system, the stability of the axis skeleton and spine is at risk and may contribute to development of scoliosis (Yamamoto [47]) (Fig. 7.11). The imbalance may be in the primary or secondary afferent systems, but there are also clinical and experimental evidences that brainstem dysfunction may contribute to the etiology of IS [97].
(a) Experimental scoliosis produced by stereotaxic destruction of the brainstem in a young bipedal rat. (b) Site of lesion (arrow) in the brainstem. Reprinted from Yamamoto H. A postural disequilibrium as an etiological factor in idiopathic scoliosis. 213. Pathogenesis of Idiopathic Scoliosis. Edit by Jacobs RR. Published Scoliosis Research Society 1984. Courtesy from Professor Hiroshi Yamamoto (Kochi University)
The spinal column, the main axis of posture, is maintained and dynamically regulated by a postural reflex mechanism in the gamma system which operates between the peripheral proprioceptive organs and the brainstem center. Much of the input concerning postural equilibrium is coordinated with proper motor commands at the supraspinal center through a continuous integration of the impulses from a different receptor system.
Magnus performed a series of experiments in which he excised one labyrinth in dogs and found not only that a spinal deviation ensued but also that this deviation could be corrected by excising the contralateral labyrinth at a later stage [98]. These experiments were in fact the first demonstration of the effects of conduction disturbances between the inner ear and spinal posture, although no evidence was presented as to whether true structural curvatures of the spine were produced or whether they were only postural deformities. The latter possibility was substantiated by the findings in Xenopus laevis, an amphibian species established previously as a model for developmental and lesion-induced plasticity of the vestibular reflex [99, 100]. After unilateral labyrinthectomy at the larval stages, adult Xenopus frogs exhibited a postural asymmetry and skeletal deformation with many characteristics reminiscent of human AIS [101]. However, this model resulted in kyphoscoliosis and not lordoscoliosis, which would cause an asymmetric and tonic contraction of the bilateral axial muscles and a continuous pull on the mostly cartilaginous skeletal elements during early ontogeny.
The vestibular system is essential for postural control and intimately interacts with the visual and proprioceptive systems. Integration of impulses from those different systems takes place mainly at the brainstem level. When vestibular function is unbalanced, skeletal muscle tone can be influenced reflexively through afferent tracts, resulting in a deviation and rotation of the body toward the side with lower muscle tone. The abnormal asymmetric posture demonstrated by the spine in these circumstances resembles the characteristics of a scoliotic deformity.
Rousie et al. found an abnormal connection between the lymphatic lateral and posterior semicircular canals (LPCC) in 52/95 (55%) of IS patients. They evaluated the functional impact of LPCC by testing the vestibule-ocular reflex (VOR) in the horizontal and vertical planes and found reproducible abnormal responses. They found that ampullofugal displacement of the fluid in the posterior canal unexpectedly produced an upbeat nystagmus and suggested that it could be due to an abnormal congenital process of ossification of the canal [53].
Shi et al. examined vestibular deficits in AIS. Geometric morphological differences were detected by vestibular analysis on the left semicircular canals in AIS patients with a right thoracic curve but not in healthy controls [52]. These morphological changes are likely to cause subclinical postural, vestibular, and proprioceptive dysfunctions. Further longitudinal studies could help to define the link between morphological and functional dysfunctions, which may have important predictive and prognostic values on curvature development and progression.
It is difficult to distinguish the cause from effect when a neurological abnormality is associated with IS. For example, an abnormal sway pattern is one area of proprioception that can be measured with a “stabilimeter,” which has force transducers placed at the center of the four sides of a square platform on which the subject stands. Although sway was originally thought to be a primary abnormality that causes scoliosis [102], it is now considered to be secondary to scoliosis, i.e., a result of the spinal deformity [103]. It tends to be more marked when the central nervous system is still immature [104] and apparently reverts to normal as growth ceases, despite the residual scoliosis [103]. Therefore, the observed abnormality in equilibrium believed to be secondary to scoliosis may actually be a compensatory phenomenon of an immature neurologic system.
Sahlstrand et al. found significantly poorer postural control in 57 patients with AIS compared with normal children [105]. The difference was most pronounced in tests in which the proprioceptive functions were most important for maintaining the postural equilibriums, and the greatest abnormality was observed in the subgroup with small curvatures. They concluded that their results indirectly indicated that postural disequilibrium is a contributory causative factor for AIS. On the other hand, Gregoric et al. reported that IS patients showed no significant difference in postural equilibrium from the control group, although there was a tendency toward increased oscillations in some patients, especially when recorded with the eyes closed. With the mean oscillation frequency practically identical to the control subjects, this study did not support the theory that the pathogenesis of scoliosis is triggered by an impairment of descending postural control [106]. It is worth noting that visual input is important for IS patients to improve their postural stability.
Sahlstrand et al. studied the effects of Barany caloric stimulation on postural sway by comparing IS children and normal controls. They found that stimulation of the labyrinth on the convex side increased the area of sway, as measured on the “stabilimeter,” while concave stimulation had much less effect [105]. In general, the effects of caloric stimulation in the children with scoliosis were much more pronounced than in the normal controls, and the two sides tended to be asymmetric in IS cases, which is usually symmetric in most control cases. Sahlstrand et al. also combined caloric stimulation with electronystagmographic examination with various positions of the trunk [107]. It was noted that the increase in the degree of the spinal curvature was limited to a change from an erect posture that did not affect the increased caloric response on the convex side of the curvature. This suggested that the degree of spinal curvature was not the primary reason for the abnormal vestibular response but rather that the labyrinthine asymmetry might be a basic factor of the condition. It was difficult, however, to draw a definite conclusion as to whether the vestibular imbalance was a contributory factor to AIS or whether the vestibular findings were a feedback effect from the deformed spine.
Equilibrium function should develop in pace with the growth of the body and reach maturity around the time of prepuberty. The developmental retardation of the postural reflex during the rapid growth of the body induces a risky situation for stabilizing the juvenile spine. Yamada et al. performed a series of clinical and experimental investigations of postural equilibrium dysfunction in the proprioceptive and/or the ocular reflex. 119 of 150 patients with IS (79%) showed marked equilibrium dysfunction in at least one of the equilibrium function tests while only one of 20 control subjects showed any dysfunction [47]. After cessation of vertebral growth, the equilibrium dysfunction gradually decreases and finally disappears when the subject is about 20 years of age, irrespective of degree of the curve. As for the pattern of the ocular reflex system in IS, equilibrium dysfunction was improved by changing posture from a standing or sitting to a supine position. These results demonstrate that disequilibrium in IS is dependent upon the posture, and equilibrium disorder may be a dysfunction of the reflex system which operates between the peripheral proprioceptive organs and the brainstem. Studies uncovered functional and organic impairments of the brainstem center for the posture equilibrium, suggesting the possibility that progressive scoliosis during the growth stages could be brought about by dysfunction of the postural reflex control that is derived from a functional or organic disorder at the brainstem [47].
Using sophisticated equipment, Herman and MacEwen detected a significant difference in the brainstem function between IS and control cases and between congenital scoliosis and healthy children [108]. Hinoki investigated the ocular motor system and found unstable foveal stabilization in the pursuit movement system which can be interpreted as a functional impairment of the brainstem [109]. Although a different anatomical location of the brainstem is implicated, many researchers agree that dysfunction of the brainstem plays a role in the development of IS. The clinical investigations mentioned previously revealed that most of the patients with IS showed equilibrium dysfunction in the proprioceptive and/or ocular reflex system during growth.
The dysfunction initially appeared to be a secondary phenomenon resulting from a positive feedback mechanism in the postural circuit system. However, the possibility that a brainstem disorder could be a primary etiological factor was implied by the following studies. In order to clarify the effect of brainstem lesions on development scoliosis, Yamamoto and Yamada et al. produced experimental scoliosis in bipedal rats by microscopic electrocoagulation to create stereotactic lesion in the brainstem. Histology of the lesions in the bipedal rats, which all developed scoliosis, revealed lesions in the periaqueductal gray matter, including Schutz’s longitudinal dorsal bundle, at the level of the midbrain and/or the pons, close to the vestibular and periocular nuclei (Fig. 7.11). This experiment suggested that equilibrium dysfunction brought out by a disturbance in the interaction between proprioceptors of the trunk and equilibrium centers within the brainstem caused scoliosis. Yamada et al. explored further the role of the equilibrium center at the brainstem has on development of experimental scoliosis by the recording the optokinetic reflex through electronystagmography. The optokinetic reflex gradually develops in the rat with growth, but when scoliosis is experimentally produced with semicarbazides, the development of the optokinetic nystagmus was inhibited. From these results, they concluded that the scoliosis was caused by a functional disorder of equilibrium center at the brainstem [103] and proposed that there are two specific groups of patients with disequilibrium: one is due to functional disturbance and the other is due to an organic disorder within the brainstem centers. They also stated that the equilibrium disorders, whether induced by proprioceptor reflexes (functional) or brainstem lesions (organic), had a distinct relationship to the progress and the degree of the scoliosis curve. They emphasized that proprioceptors have a positive feedback influence to the equilibrium control centers, but no evidence was provided for this notion [103].
A number of studies have shown an abnormal nystagmus response during caloric testing in patients with IS, suggesting an oculovestibular abnormality. Herman et al. proposed that a dysfunction of the motor cortex that controls disturbing the axial posture due to a sensory input deficiency concerning spatial orientation and concluded that this effect probably results from central proprioceptive sources involving visual and vestibular function [110]. The clinical syndrome of symmetrical horizontal or lateral gaze palsy is often seen in patients with IS [111]. The site of neurological abnormality in this condition is thought to reside in the paramedian pontine reticular formation, which links the periocular motor nuclei and the vestibular nuclei [112].
It has been suggested that brainstem dysfunction may be a cause of IS, but whether the disturbances are indeed primary or whether they develop secondary to the existence of a scoliosis remains to be further elucidated. Equilibrium dysfunction is characteristic in patients with progressive curves, regardless of etiology, implying that it is secondary to the curve rather than a primary event [113]. Equilibrium dysfunction has been previously reported in numerous scoliosis groups reporting multifactorial abnormalities such as impaired postural control [47, 105, 106], spontaneous and positional nystagmus [47, 104, 105], abnormal smooth pursuit [97, 108], and changes in vestibular-induced nystagmus [104, 108]. The idiopathic progressive and congenital progressive patients performed the worst, with abnormal results on all of the above tests [113]. While the idiopathic nonprogressive group also demonstrated abnormalities in postural control, spontaneous and positional nystagmus episodes, and disorders in smooth visual pursuit, the equilibrium and oculovestibular functions were almost uniformly normal. The message from these studies is that equilibrium dysfunction correlates with progression of scoliosis, irrespective of the curve being either idiopathic or congenital in origin. This in turn suggests that the dysfunction is secondary to the curve rather than being a primary event.
Changes in vestibular-induced nystagmus including impaired quality, decreased duration of nystagmus, and asymmetric labyrinthine sensitivity have also been associated with IS, and such equilibrium dysfunction has been shown to be characteristic of patients with progressive curves. It is also possible that some of the abnormalities found in IS are related to a delay in maturation of the central nervous system.
7.4.3 Cerebellum
Recent research supports that the theory that scoliosis is a multifactorial disease involving abnormalities in postural disequilibrium [114, 115], vestibular dysfunction [101], and communication between the cerebellum and the vestibular system [116]. Since the posterior cerebellum and floplease cculonodular lobe have extensive afferent and efferent connections with vestibular nuclei [117, 118], the function and morphology of the cerebellum should be examined in AIS research studies.
Previous studies mainly looking into the abnormal morphology of the cerebellum in IS patients have suggested that the cerebellar dysfunction may play a role in AIS development. Shi et al. measured and compared the volume of the cerebellum between AIS patients and normal controls [119] and reported that the volumes of four regions, namely, the right VIIa, right VIIb, left X, and right X, were significantly increased by approximately 7.43–8.25% in the AIS group when compared to the control group. The increase in AIS cerebellar volume is mainly observed in the regions of the prepyramidal/prebiventer and intrabiventer fissures, the intrabiventer and secondary fissures, and the flocculonodular (X)-posterolateral fissures to the inferior hemispheric margin. They concluded that the volume differences could be compensatory consequences of the central nervous system brought out by the patient’s efforts to maintain body balance an asymmetric spine [119].
Further research is required to better understand the role of anatomic changes in the cerebellum of AIS cases, and functional imaging studies to examine the correlation between the structural and functional abnormalities are anticipated. There are a small number of AIS cases reporting the cross-sectional studies, but longitudinal studies are required to further understand the role of anatomic changes in the cerebellum from onset to progression of AIS.
7.4.4 Brain
The brain or cerebrum has been one of the major factors examined in the pathogenesis of AIS. To ascertain the relationship between organic brain lesions and the development of scoliosis, stereotaxic destruction of the hypothalamus using minute electrical charges was performed in the bipedal rats [50, 103]. Marked scoliosis developed in 16 of 103 bipedal rats, and they showed equilibrium dysfunction more frequently than the rats without scoliosis. Histologic examination of the brain in the rats with scoliosis revealed lesions in the posterior hypothalamus, leading Kawata to postulate that a lesion in posterior hypothalamus may disturb the reticular formation of the brainstem and lead to scoliosis in bipedal rats [50]. Machida et al. produced experimental scoliosis in melatonin-deficient C57BL/6J mice and investigated the action sites of melatonin in the brain utilizing morphological analyses with [125I]iodomelatonin. Our results suggest that the site in the brain most responsible for the etiology of scoliosis is likely to be the paraventricular thalamic nucleus (Fig. 7.6) [50].
Recently, various neuroanatomical studies of the brain have been proposed to explore the relationship between brain dysfunction and pathogenesis of AIS. Structural brain MRI analyses, such as region volume [120], cortical thickness [121], comprehensive morphometry of corpus callosum [122], vestibular morpho-anatomy [56], and volume-based morphometry [52], have been used to investigate the morphological changes in the brain of AIS patients. Preliminary analyses of regional brain volume have indicated an anatomical asymmetry in brain regions functionally related to somatic motor control and coordination, and Wang et al. recently reported the thinning of the cerebral cortex in AIS patients and demonstrated that the “small-world” architecture (Walls and Strongatz 1998) and organization of the cortical networks in AIS patients was fully preserved, but there is hemispheric asymmetry in AIS brains. Their results suggested an increased central role of temporal and occipital cortexes and a decreased central role of limbic cortex in AIS patients when compared to normal controls. Furthermore, decreased structural connectivity between the two hemispheres and increased connectivity in several cortical regions within one hemisphere demonstrate an alteration of cortical network in the AIS brain [111].
Shi et al. applied MRI volume-based morphometry to test their hypothesis that there are neuroanatomic changes in patients with AIS [52]. It has been reported that patients with left thoracic curves have a slightly higher prevalence of neuroanatomic brain abnormalities than those with right thoracic curves. White matter attenuation in the corpus callosum and left internal capsule, which are responsible for interhemispheric communication and acts a conduit for corticothalamic projectional fibers, respectively, were found to be slightly lower in left thoracic AIS cases when compared to control subjects; however, this was not evident in right thoracic AIS cases. Lee et al. found no significant difference between left thoracic AIS and controls [123]. Joly et al. investigated the in vivo microstructures of the white matter within the corpus callosum in AIS patients using diffusion magnetic imaging and found that the corpus callosum had significantly lower fractional anisotropy in patients with right thoracic AIS than in controls. In particular, the anterior part of the corpus callosum (region II) revealed significantly lower value in both the voxel- and ROI-based analyses. He hypothesized that defective sensorimotor integration at the cortical level plays a role in AIS [124]. Confirmation of these findings is required in future research to evaluate the relationship of white matter abnormality with curve laterality, pathogenesis, and prognosis in patients with AIS.
To better understand the role of neuroanatomic changes in AIS, functional imaging studies that correlate structural changes with functional impairments are needed. In the past, electroencephalographic (EEG) studies were utilized to localize intracerebral defects in AIS patients. Peterson et al. carried out an EEG investigation in 57 patients with AIS and found that 30% exhibited “increase of low-frequency activity” only or “paroxysmal activity,” compared to 17% in control cases [68]. On further examination, however, it was noted that these abnormalities tended to appear only at rest, and that there was no statistical difference between the scoliotic and the normal children if their EEG recordings included “activation procedures” such as hyperventilation, photic stimulation, or sleep. They concluded that centrally located subcortical structures are involved in the pathologic process in AIS. They also pointed out that the previously noted paroxysmal activity was like an age-dependent phenomenon, and the possibility therefore existed that the “abnormalities” found in scoliotic children were due to cerebral immaturity. However, frequency analysis of the EEG recordings, which is a quantitative measure for determining brain maturity, showed no differences between the scoliotic subjects and the controls (Peterson et al. [68]). In fact, there was an inverse relationship between the degree of curvature and the presence of abnormal paroxysmal EEG waves. This inversed activity in the patients with less advanced deformity was not an age-related phenomenon, since there was no statistical difference between the age of the patients and degree of curvatures. They also noted that there was no correlation between the direction of the curve and any asymmetry of EEG activity.
Data indicating the presence of EEG changes in IS was presented by Lukeschitsch et al. who compared 115 children with IS with 35 children suffering from congenital scoliosis. Fifty of the children with IS showed abnormalities in their EEGs, while the incidence was even greater in the congenital scoliosis group with 20 out of 35. No relationship was found between EEG abnormalities and the degree of curvature or the presence of other neurological changes [125]. In contrast, the study by Peterson showed that only a small number of the IS cases (seven out of 50) were associated with paroxysmal activity. Leonard also carried out an EEG examination in 57 patients with IS and found that 22 patients (38%) were considered to have abnormal EEGs. He postulated that the abnormality was emanating from the midline subcortical structures in the brainstem region [126]. Dretakis et al. also showed an increased frequency of EEG changes in IS patients when compared to normal controls. The EEG changes were even more evident after “activation” by sleep, hyperventilation, or photic stimulation. There were no specific EEG changes in IS, but the data raised the possibility of minor brain trauma during birth both in the children with IS and those with congenital spinal deformity. They also reported that the EEG abnormalities were related to the location of curve with focal EEG changes observed in the brain hemisphere opposite the curve direction in the case with lumbar curves, while EEG changes were observed in the ipsilateral brain hemisphere in cases with thoracolumbar curves. In cases with thoracic curves, EEG abnormalities suggestive of subcortical changes were found in 50% of the patients with abnormal EEGs. Bilateral synchronous abnormalities were predominant in children with thoracic scoliosis, whereas focal EEG changes were more frequently seen in children with other locations of scoliosis. However, there are studies that contradict the presence of EEG abnormalities [127]. Robb et al. performed EEG examinations in 25 children with IS and compared the results with those achieved in 25 age- and sex-matched normal children. All of the IS cases and 24 out of 25 controls had normal EEGs resulting to the view that IS has a central neurologic that can be assessed by EEG [128]. Although there are numerous studies using EEG to evaluate IS pathology, it is important to remember that EEG cannot detect subcortical abnormalities.
Identifying neurologic deficits in IS not only helps in defining possible etiologies but may also provide useful clinical information. Schneider et al. examined the spinal vertebrae and the spinal cord in eight IS patients that exhibited abnormal somatosensory evoked potentials (SEPs) and found that six children showed structural abnormalities in the spine or the spinal cord. Based on these findings, they recommend SEPs as a screening method for hidden lesions of the central nervous system [129]. We studied SEPs in 20 chickens with experimentally induced scoliosis after pinealectomy and found that SEPs after leg stimulation were significantly delayed in the scoliosis group compared to the controls (Fig. 7.12). We then compared SEPs taken from 100 patients with AIS and 20 age-matched healthy youngsters without scoliosis and found that the latency of cortical potential (N37) after stimulation of the tibial nerve was longer in the scoliosis group than in the control group (Fig. 7.13). Analysis of individual patients revealed SEPs abnormalities in all except for three patients. Our findings in both experimental and clinical studies strongly support the hypothesis that IS results from dysfunction, somewhere within the central nervous system related to sensory pathway [64].
(a) Waveform somatosensory evoked potentials (SEPs) in control chicken after stimulation of the leg consists of an initial positive peak followed by subsequent negative and positive peaks. The initial positive peak originates in the brainstem, and negative peak arises from the cortex. Note that identical SEP latencies are observed bilaterally. (b) Waveform SEPs in pinealectomized chicken reveal a normal initial positive potential but lacks subsequent negative peak
Hausman et al. also reported an increase in SEPs latencies after stimulation of the tibial nerve in 68 of 100 patients with AIS [66]. Pathological SEPs are indicative of functional abnormalities with subclinical involvement of the recorded neuronal pathways. Chau et al. suggested that the site of SEP abnormalities in AIS originates from a level above the cervical spine and is likely to involve disturbances along the spinocortical pathway [130]. Other studies involving late reflexes and SEPs in patients undergoing surgery for AIS have demonstrated abnormal and asymmetrical latencies, correlating with the side and indeed the progression of the scoliosis [131]. They concluded that these findings suggested a problem of the midbrain and/or brainstem, where primary neurological pathogenesis might cause a functional asymmetry in balance and consequently result in scoliosis [131]. Whether these findings are primary etiological factors or secondary to the spinal deformity is not known.
In order to find the etiology and pathogenesis of AIS, as shown in this review, extensive studies have been conducted exploring entire neurological systems, from peripheral nerve to the brain. Although many possible findings were discovered in multiple organs and systems, none showed conclusively the true etiology of AIS. Continued researches to find the true etiology of AIS is imperative in order to provide appropriate and successful treatment for AIS.
References
Kouwenhoven JW, Castelein RM. The pathogenesis of adolescent idiopathic scoliosis: review of the literature. Spine. 2008;33:2898–908.
Low TG, Edgard M, Margulies JY, et al. Etiology of idiopathic scoliosis: current trends in research. J Bone Joint Surg. 2000;82A:1157–68.
Machida M, Murai I, Miyashita Y, et al. Pathogenesis of idiopathic scoliosis: experimental study in rats. Spine. 1999;24:1985–9.
Machida M, Saito M, Dubousset J, et al. Pathological mechanism of idiopathic scoliosis: experimental scoliosis in pinealectomized rats. Eur Spine J. 2005;14:843–8.
Guerin J. Letter sur le traitement des deviations laterals de l’epine par la section sous-cutanee des muscles de dos et de la colonne vertebrale. Gaz Med Paris. 1839;7:403–4.
Adams W. Lectures on the pathology and treatment of lateral and other forms of curvature of the spine. London: Churchill & Sons; 1865.
Trontelj JV, Pecak F, Dimitrijevic MR. Segmental neurophysiological mechanisms in scoliosis. J Bone Joint Surg. 1979;61B:310–3.
Spencer GSG, Eccles MJ. Spinal muscle in scoliosis. Part 2. The proportion and size of type I and type II skeletal muscle fibers measured using a computer controlled microscope. J Neurol Sci. 1976;30:143–54.
Bylund P, Jansson E, Dahlberg E, et al. Muscle fiber types in thoracic erector spinae muscles. Fiber types in idiopathic and other forms of scoliosis. Clin Orthop. 1987;214:222–8.
Slager UT, Hsu JD. Morphometry and pathology of the paraspinous muscles in idiopathic scoliosis. Dev Med Child Neurol. 1986;28:749–56.
Yarom R, Robin GC, Gorodetsky R. X-Ray fluorescence analysis of muscle in scoliosis. Spine. 1978;3:142–5.
Yarom R, Robin GC. Studies on spinal and peripheral muscles from patients with scoliosis. Spine. 1979;4:12–21.
Riddle HVF, Roaf R. Muscle imbalance in the causation of scoliosis. Lancet. 1955;1:1245–7.
Henssge J. Are signs of denervation of muscles of the spine primary or secondary findings in cases of scoliosis? J Bone Joint Surg. 1968;50B:882.
Badger VM. Correlation studies on muscle in scoliosis: histochemistry, EMG, EM and quantitative enzyme estimation. J Bone Joint Surg. 1969;51A:204.
Walf E, Robin GC, Yarom R, et al. Myopathy of deltoid muscle in patients with idiopathic scoliosis. Electromyogr Clin Neurophysiol. 1982;22:357–69.
Trontelj JV. The motor unit in idiopathic scoliosis. Acta Orth Jugosl. 1984;15:7–14.
Fernandez JM. Single fiber EMG in juvenile idiopathic scoliosis. Muscle Nerve. 1988;11:297–300.
Valentino B, Maccauro L, Mango G. Electromyography in the investigation and early diagnosis of scoliosis. Anat Clin. 1985;7:55–9.
Yarom R, Robin GC. Muscle pathology in idiopathic scoliosis. Isr J Med Sci. 1979;15:917–24.
Gibson JN, McMaster MJ, Scrimgeour CM, et al. Rates of muscle protein synthesis in paraspinal muscle: lateral disparity in children with idiopathic scoliosis. Clin Sci. 1988;75:79–83.
Ebashi S, Endo M. Calcium and muscle contraction. Prog Biophys Mol Biol. 1968;18:123–83.
Cohen DS, Solomons CS, Lowe TG. Altered platelet calmodulin activity in AIS. Orthop Trans. 1985;9:106.
Kindsfater K, Lowe T, Lawellin D, et al. Levels of platelet calmodulin for the prediction of progression and severity of adolescent idiopathic scoliosis. J Bone Joint Surg. 1994;76A:1186–92.
Acaroglu RE, Akel I, Alanay A, et al. Comparison of the melatonin and calmodulin in paraventral muscle and platelets of patients with or without adolescent idiopathic scoliosis. Spine J. 2009;34:E659–63.
Lowe TG, Lawellin D, Smith D, et al. Platelet calmodulin levels in adolescent idiopathic scoliosis: do the levels correlate with curve progression and severity? Spine. 2002;27:768–75.
Lowe TG, Buewell RG, Dangerfield PH. Platelet calmodulin levels in adolescent idiopathic scoliosis (AIS): can they predict curve progression and severity? Summary of an electronic focus group debate of the IBSE. Eur Spine J. 2004;13:257–65.
Benitex-King G, Anton-Tay F. Calmodulin mediates melatonin cytoskeletal effects. Experientia. 1993;49:635–41.
Brezeniski AA. melatonin in humans. New Engl J Med. 1997;336:186–9.
Thillard MJ. Deformation de la colonne vertebrale consecutives a l’epiphysectomie chez le poussin. Extrait Compt Rendus Assoc Anat. 1959;XLVI:22–6.
Dubousset J, Queneau P, Thillard MJ. Experimental scoliosis induced by pineal and dicephalic lesions in young chickens: its relation with clinical findings in idiopathic scoliosis. Orthop Trans. 1983;7:7.
Machida M, Dubousset J, Imamura Y, et al. An experimental study in chickens for the pathogenesis of idiopathic scoliosis. Spine. 1993;18:1609–15.
Machida M, Dubousset J, Imamura Y, et al. Role of melatonin deficiency in the development of scoliosis in pinealectomised chickens. J Bone Joint Surg. 1995;77B:134–8.
Dubousset J, Machida M. Possible role of the pineal gland in the pathogenesis of idiopathic scoliosis. Experimental and clinical studies. Bull Acad Natl Med. 2001;185:593–602.
Machida M, Dubousset J, Sato T, et al. Pathological mechanism of experimental scoliosis in pinealectomized chickens. Spine. 2001;26:E385–91.
Machida M, Dubousset J, Yamada T, et al. Experimental scoliosis in melatonin-deficient C57BL/6J mice without pinealectomy. J Pineal Res. 2006;41:1–7.
Cheung KM, Wang T, Poon AM, et al. The effect of pinealectomy in scoliosis development in young nonhuman primates. Spine. 2005;30:2009–13.
Machida M, Dubousset J, Miyake A, et al. The possible pathogenesis in adolescent idiopathic scoliosis based on experimental model of melatonin-deficient C57BL/65J mice. Presented at 51st annual meeting of Scoliosis Research Society in Prague, Sep 2016. Prague: Scoliosis Research Society; 2016. p. 22.
Machida M, Dubousset J, Imamura Y, et al. Melatonin. A possible role in pathogenesis of adolescent idiopathic scoliosis. Spine. 1996;21:1147–52.
Machida M. Cause of idiopathic scoliosis. Spine. 1999;24:2576–83.
Machida M, Dubousset J, Yamada T, et al. Serum melatonin levels in adolescent idiopathic scoliosis prediction and prevention for curve progression – a prospective study. J Pineal Res. 2009;46:344–8.
Morcuende JA, Minhas R, Dolan L, et al. Allelic variants of human melatonin 1A receptor in patients with familial adolescent idiopathic scoliosis. Spine. 2003;28:2025–8.
Qiu XS, Tang NL, Yeung HY, et al. The role of melatonin receptor 1B gene (MTNR1B) in adolescent idiopathic scoliosis – a genetic association study. Stud Health Technol Inform. 2006;123:3–8.
Moreau A, Wang DS, Forget S, et al. Melatonin signaling dysfunction in adolescent idiopathic scoliosis. Spine. 2004;29:1772–81.
Akoume MY, Azeddine B, Turgeon I, et al. Cell-based screening test for idiopathic scoliosis using cellular dielectric spectroscopy. Spine. 2010;35:E601–8.
Wu J, Qiu Y, Zhang L, et al. Association of estrogen receptor gene polymorphisms with susceptibility to adolescent idiopathic scoliosis. Spine. 2006;31:1131–6.
Yamada K, Ikata T, Yamamoto H, et al. Equilibrium function in scoliosis and active corrective plaster jacket for the treatment. Tokushima J Med. 1969;16:1–7.
Tezuka A. Development of scoliosis in cases with congenital organic abnormalities of the brain stem. A report of seven cases. Tokushima J Exp Med. 1971;18:49–62.
Yamamoto H, Yamada K. Equilibrium approach to scoliotic posture. Agressologie. 1976;17:61–6.
Kawata S. Experimental scoliosis produced by stereotaxic destruction of the posterior part of the hypothalamus in bipedal rats. Shikoku Acta Med. 1976;32:125–31.
Yamada K, Yamamoto H. Neuromuscular and neurohormonal approaches to the etiology of idiopathic scoliosis. Orth Trans. 1978;2:277.
Shi L, Wang D, Chu WCW, et al. Volume-based morphometry of brain MR images in adolescent idiopathic scoliosis and healthy control subjects. Am J Neuroradiol. 2009;30:1302–7.
Rousie DL, Hache JC, Pellerin P, et al. Oculomotor, postural, and perceptual asymmetries and asymmetries in vestibular organ anatomy. Ann N Y Acad Sci. 1999;871:439–46.
Chu WC, Shi L, Wang D, et al. Variations of semicircular canals orientation and left-right asymmetry in adolescent idiopathic scoliosis (AIS) comparing with normal controls: MR morphometry study using advanced image computation techniques. Stud Health Technol Inform. 2008;140:333.
Shi L, Wang D, Chu WC, et al. Automatic MRI segmentation and morphoanatomy analysis of the vestibular system in adolescent idiopathic scoliosis. NeuroImage. 2011;54:S180–8.
Guo X, Chau W, Hui-Chan CWY, et al. Balance control in adolescents with idiopathic scoliosis and disturbed somatosensory function. Spine. 2006;31:E437–40.
Lao ML, Chow DH, Guo X, et al. Impaired dynamic balance control in adolescents with idiopathic scoliosis and abnormal somatosensory evoked potentials. J Pediatr Orthop. 2006;28:846–9.
Beaulieu M, Toulotte C, Gatto L, et al. Postural imbalance in nontreated adolescent idiopathic scoliosis at different periods of progression. Eur Spine J. 2009;18:38–44.
Simoneau M, Richer N, Mercier P, et al. Sensory deprivation and balance control in idiopathic scoliosis adolescent. Exp Brain Res. 2006;170:576–82.
Barrack RL, Wyatt MP, Whitecloud TSIII, et al. Vibratory hypersensitivity in idiopathic scoliosis. J Pediatr Orthop. 1988;8:389–95.
Wiener-Vacher SR, Mazda K. Asymmetric otolith vestibule-ocular responses in children with idiopathic scoliosis. J Pediatr. 1998;132:1028–32.
Jen J, Coulin CJ, Bosley TM, et al. Familial horizontal gaze palsy with progressive scoliosis maps to chromosome 11q23-25. Neurology. 2002;59:432–5.
Cheung J, Veldhuizen AG, Jp H, et al. Geometric and myographic assessments in the evaluation of curve progression in idiopathic scoliosis. Spine. 2006;31:322–9.
Machida M, Dubousset J, Imamura Y, et al. Pathogenesis of idiopathic scoliosis: SEPs in chickens with experimentally induced scoliosis and in patients with idiopathic scoliosis. J Pediatr Orthop. 1994;14:329–35.
Cheng JC, Guo X, Sher AH, et al. Correlation between curve severity, somatosensory evoked potentials, and magnetic resonance imaging in adolescents idiopathic scoliosis. Spine. 1999;23:332–7.
Hausmann ON, Boni T, Pfirrmann CW, et al. Preoperative radiological and electrophysiological evaluation in 100 adolescent idiopathic scoliosis patients. Eur Spine J. 2003;12:501–6.
Lao ML, Chow DH, Guo X, et al. Impaired dynamic balance control in adolescents with idiopathic scoliosis and abnormal somatosensory evoked potentials. J Pediatr Orthop. 2008;28:846–9.
Peterson I, Sahlstrand T, Sellden U. EEG investigation of patients with adolescent idiopathic scoliosis. Acta Orth Scand. 1979;50:283–93.
McInnes E, Hill DL, Raso VJ, et al. Vibratory response in adolescents who have idiopathic scoliosis. J Bone Joint Surg. 1991;73A:1208–12.
Kimiskidis VK, Potoupnis M, Papagiannopoulos SK, et al. Idiopathic scoliosis: a transcranial magnetic stimulation study. J Musculoskelet Neuronal Interact. 2007;7:155–60.
Mihailia D, Calancie B. Is corticospinal tract organization different in idiopathic scoliosis? Stud Health Technol Inform. 2008;140:350.
Domenech J, Torms JM, Barrios C, et al. Motor cortical hyperexcitability in idiopathic scoliosis: could focal dystonia be a subclinical etiological factor? Eur Spine J. 2010;19:223–30.
Chockalingam N, Dangerfield PH, Rahmatalla A, et al. Assessment of ground reaction force during scoliotic gait. Eur Spine J. 2004;13:750–4.
Chow DH, Kwok ML, Cheng JC, et al. The effect of backpack weight on the standing posture and balance of schoolgirls with adolescent idiopathic scoliosis and normal controls. Gait Posture. 2006;24:173–81.
Bruyneel AV, Chavet P, Bollini G, et al. Dynamical asymmetries in idiopathic scoliosis during forward and lateral initiation step. Eur Spine J. 2009;18:188–95.
Mahaudens P, Banse X, Mousny M, et al. Gait in adolescent idiopathic scoliosis: kinematic electromyographic analysis. Eur Spine J. 2009;18:512–21.
Liszka O. Spinal cord mechanisms leading to scoliosis in animal experiments. Acta Med Pol. 1961;2:45–63.
MacEwen GD. Experimental scoliosis. In: Zorab PA, editor. Proceedings of the 2nd symposium on scoliosis: causation. Livigstone: Edinburgh; 1968. p. 14–8.
Alexander MA, Bunch WH, Ebbesson SOE. Can experimental dorsal rhizotomy produce scoliosis? J Bone Joint Surg. 1972;54A:1509–13.
Pincott JR, Davies JS, Taffs LF. Scoliosis caused by section of dorsal spinal nerve roots. J Bone Joint Surg. 1984;66B:27–9.
Suk SI, Song HS, Lee CK. Scoliosis induced anterior and posterior rhizotomy. Spine. 1989;14:692–7.
Pincott JR, Taffs LF. Experimental scoliosis in primates. A neurological cause. J Bone Joint Surg. 1982;64B:503–7.
deSalis J, Beguiristain JL, Canadell J. The production of experimental scoliosis by selective arterial ablation. Int Orthop. 1980;3:311–5.
Barrios C, Tunon MT, deSalis JA, et al. Scoliosis induced by medullary damage: an experimental study in rabbits. Spine. 1987;12:433–9.
Dubousset J, Bancel PH, Missenard G. Spinal deformities secondary to the treatment of neuroblastomas in children. Presented at 15th annual meeting of scoliosis research society in Chicago, Sep 1980. Prague: Scoliosis Research Society; 1980. p. 17.
Cheng JC, Guo X, Shea AH. Posterior tibial nerve somatosensory cortical evoked potentials in idiopathic scoliosis. Spine. 1998;23:332–7.
Whitecloud TS III, Brinker MR, Barrack RL, et al. Vibratory response in congenital scoliosis. J Pediatr Orthop. 1989;9:422–6.
Isu T, Chono Y, Iwasaki Y, et al. Scoliosis associated with syringomyelia presenting in children. Childs Nerv Syst. 1992;8:97–100.
Arai S, Ohtsuka Y, Moriya H, et al. Scoliosis associated with syringomyelia. Spine. 1993;18:1591–2.
Gupta P, Lenke LG, Bridwell KH. Incidence of neural axis abnormalities in infantile and juvenile patients with spinal deformity. Is a magnetic resonance image screening necessary? Spine. 1998;23:206–10.
Singhal R, Perry DC, Prasad S, et al. The use of routine preoperative magnetic resonance imaging in identifying intraspinal anomalies in patients with idiopathic scoliosis: a 10-year review. Eur Spine J. 2013;22:355–9.
Roth M. Idiopathic scoliosis caused by a short spinal cord. Acta Radiol Diagn (Stockh). 1968;7:257–2571.
Porter RW. Can a short spinal cord produce scoliosis? Eur Spine J. 2001;10:2–9.
Porter RW. Idiopathic scoliosis: the relation between the vertebral canal and the vertebral bodies. Spine. 2000;25:1360–6.
Porter RW. The pathogenesis of idiopathic scoliosis: uncoupled neuro-osseous growth? Eur Spine J. 2001;10:473–81.
Chu WC, Lam WW, Chan YL, et al. Relative shortening and functional tethering of spinal cord in adolescent idiopathic scoliosis?: study with multiplanar reformat magnetic resonance imaging and somatosensory evoked potential. Spine. 2001;10:482–7.
Yamamoto H. A postural disequilibrium as an etiological factor in idiopathic scoliosis. In: Jacobs RR, editor. Pathogenesis of idiopathic scoliosis: proceeding of an international conference. Prague: Scoliosis Research Society; 1983.
Magnus R. Korperstellung. Berlin: Springer; 1925. (cited by Stilwell DL, 1962)
Horn E, Rayer B. Compensation of vestibular lesions in relation to development. Naturwissenscaften. 1978;65:441.
Rayer B, Horn E. The development of the static vestibule-ocular reflex in the Southern Clawed Toad, Xenopus laevis. III Chronic hemilabyrinthectomized tadpoles. J Com Physiol A. 1986;159:887–95.
Lambert FM, Malinvaud D, Glaunes J, et al. Vestibular asymmetry as the cause of idiopathic scoliosis: a possible answer from Xenopus. J Neurosci. 2009;29:12477–83.
Nachemson A, Sahlstrand F. Etiologic factors in adolescent idiopathic scoliosis. Spine. 1977;3:176–82.
Yamada K, Yamamoto H, Nakagawa Y, et al. Etiology of idiopathic scoliosis. Clin Orthop. 1984;184:50–7.
Sahlstrand T, Petrusson B. A study of labyrinthine function in patients with adolescent idiopathic scoliosis. (i) An electronystagmographic study. Acta Orth Scan. 1979;50:759–69.
Sahlstrand T, Ortengren R, Nachemson A. Postural equilibrium in idiopathic scoliosis. Acta Orth Scand. 1978;49:354–65.
Gregoric M, Pecak F, Trontelj JV, et al. Postural control in scoliosis: a statokinesimetric study in patients with scoliosis due to neuromuscular disorders and in patients with idiopathic scoliosis. Acta Orthop Scand. 1981;52:59–63.
Sahlstrand T, Petrusson B. Postural effects on nystagmus response during caloric stimulation in patients with adolescent idiopathic scoliosis. (ii) an electronystagmographic study. Acta Orth Scan. 1979;50:771–5.
Herman R, MacEwen GD. Idiopathic scoliosis: a visio-vesticular disorder of the central nervous system. In: Zorab PA, editor. Scoliosis. Preceeding of the sixth symposium. London: Academic; 1978. p. 61–9.
Hinoki M. Measurement of the slow phase of optokinetic nystagmus in patients with scoliosis. Agressologie. 1979;2D–C:223–4.
Herman R, Mixon J, Fisher A, et al. Idiopathic scoliosis and the central nervous system: a motor control problem. Spine. 1985;10:1–14.
Hamanishi C, Tanaka S, Kasahara Y, et al. Progressive scoliosis associated with lateral gaze palsy. Spine. 1993;18:2545–8.
Herman R, Maulucci R, Stuyck J, et al. Vestibular functioning in idiopathic scoliosis. Orth Trans. 1979;3:218–9.
O’Beirne J, Goldberg C, Dowling E, et al. Equilibrium dysfunction in scoliosis – cause or effect? J Spinal Dis. 1989;2:184–9.
Byl NH, Hollands S, Jurek A, et al. Postural imbalance and vibratory sensitivity in patients with idiopathic scoliosis: implications for treatment. J Orthop Sports Phys Ther. 1997;26:60–8.
Mirovsky Y, Blankstei A, Shlamkovitch N. Postural control in patients with severe idiopathic scoliosis: a prospective study. J Pediatr Orthop. 2006;15B:168–71.
Simoneuau M, Lamothe V, Hutin T, et al. Evidence for cognitive vestibular integration impairment in idiopathic scoliosis patients. BMC Neurosci. 2009;10:102.
Ghez C, Farn S. The cerebellum. In: Kandel ER, Schwartz JH, editors. Principle of neural science. 2nd ed. New York, NY: Elsevier; 1985. p. 502–22.
Barmack NH. Central vestibular system: vestibular nuclei and posterior cerebellum. Brain Res Bull. 2003;60:511–41.
Shi L, Wang D, Hui SCN, et al. Volumetric changes in cerebellar regions in adolescent idiopathic scoliosis compared with healthy controls. Spine J. 2013;
Liu T, Chu WC Yeung G, et al. MR analysis of regional brain volume in adolescent idiopathic scoliosis: neurological manifestation of a systemic disease. J Magn Reson Imaging. 2008;27:732–6.
Wang D, Shi L, Chu WC, et al. Abnormal cerebral cortical thinning pattern in adolescent idiopathic scoliosis. NeuroImage. 2012;59:935–42.
Wang D, Shi L, Chu WCW, et al. A comparison of morphometric techniques for studying the shape of corpus callosum in adolescent idiopathic scoliosis. NeuroImage. 2009;45:738–48.
Lee JS, Kim S-J, Suh KT, et al. Adolescent idiopathic scoliosis may not be associated with brain anomalies. Acta Radiol. 2009;50:941–6.
Joly O, Rousie D, Jissendi P, et al. A new approach to corpus callosum anomalies in idiopathic scoliosis using diffusion tensor magnetic resonance imaging. Eur Spine J. 2014;22:2643–9.
Lukeschitsch G, Meznik F, Feldner-Bustin H. Zerebrale dysfunction bei patenten mit idiopatische skoliose. Ztsch Orth. 1980;118:372–5.
Leonard MA. An investigation into the EEG findings in patients with idiopathic scoliosis. J Bone Joint Surg. 1981;63B:632.
Dretakins EK, Parskevaidis H, Zarkadoulas V, et al. Electroencephalographic study of school children with adolescent idiopathic scoliosis. Spine. 1988;13:143–5.
Robb JE, Conner AN, Stephenson JBT. Normal electroencephalograms in idiopathic scoliosis. Act Orth Scand. 1985;57:220–1.
Schneider E, Niethard FU, Schiek H, et al. Wie idioppathisch ist die idiopathische Skoliose? Ergebnisse neurologischer Untersuchungen mit somatosensorisch evozierten Potentialen bei Kindern und Jugendlichen. Z Orthop. 1991;129:355–61.
Chau WW, Guo X, Fu LL, et al. Abnormal sensory evoked potential (SSEP) in adolescents with idiopathic scoliosis – the site of abnormality. In: Sawatzky BJ, editor. International Research Society of spinal deformities symposium. Vancouver, BC: International Research Society; 2004. p. 279–81.
Maguire J, Madigan R, Wallance S, et al. Intraoperative long-latency reflex activity in adolescent idiopathic scoliosis demonstrates abnormal central processing. A possible cause of adolescent idiopathic scoliosis. Spine. 1993;18:1621–6.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer Japan KK
About this chapter
Cite this chapter
Machida, M. (2018). Neurological Research in Idiopathic Scoliosis. In: Machida, M., Weinstein, S., Dubousset, J. (eds) Pathogenesis of Idiopathic Scoliosis. Springer, Tokyo. https://doi.org/10.1007/978-4-431-56541-3_7
Download citation
DOI: https://doi.org/10.1007/978-4-431-56541-3_7
Published:
Publisher Name: Springer, Tokyo
Print ISBN: 978-4-431-56539-0
Online ISBN: 978-4-431-56541-3
eBook Packages: MedicineMedicine (R0)