Abstract
Crickets have emerged as ideal model systems for investigating the mechanisms controlling intraspecific aggressive behaviour. As in many animals, male aggression in crickets is shaped by numerous experiences including physical exertion, past wins and defeats and the acquisition of resources. This chapter reviews work revealing that neuromodulators, primarily octopamine and nitric oxide, mediate such experience-dependent plasticity by modulating the relative behavioural thresholds to fight and to flee. Octopamine, the invertebrate analogue of noradrenaline, promotes the tendency to fight by mediating the effects of flying, winning and shelter occupancy and thus represents the motivational component of aggression. The gaseous neuromodulator nitric oxide, on the other hand, mediates the decision to flee and induces a period of prolonged submissiveness, which is characteristic for social defeat in many animals. Accumulating evidence also suggests a role for serotonin, dopamine and selected peptides in controlling insect aggression. The roles for neuromodulators in insect aggression are in essence similar to those emerging for corresponding signalling molecules in mammals, where their specific behavioural functions are less clear.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
- Agonistic behaviour
- Octopamine
- Serotonin
- Nitric oxide
- Motivation
- Experience-dependent plasticity
- Decision-making
- Assessment
- Social behaviour
- Gryllus bimaculatus
1 Introduction
1.1 Crickets as Model Organisms for the Study of Aggression
Over the past few years, crickets have advanced to the status of a model organism for studying the mechanisms underlying aggressive behaviour (reviews: Stevenson and Rillich 2012; Stevenson and Schildberger 2013; Simpson and Stevenson 2015). But why study crickets? For one, as insects, their miniature brains contain comparatively few, individually identifiable neurons, but they are nonetheless equipped with the capacity to generate sophisticated social interactions (Huber et al. 1989; Guirfa 2012). Fights between crickets are impressive, highly ritualised affairs (Adamo and Hoy 1995; Stevenson et al. 2000, 2005) and accordingly relatively simple to evaluate. On the other hand, their fighting behaviour, as in mammals, is influenced by a wealth of experiences including age and time of day (Dixon and Cade 1986), physical exertion (Hofmann and Stevenson 2000), winning (Rillich and Stevenson 2011), losing (Iwasaki et al. 2006; Stevenson and Rillich 2013), the presence of shelters (Rillich et al. 2011), food (Nosil 2002) or females (Brown et al. 2006; Tachon et al. 1999), courtship and mating (Killian and Allen 2008; Judge et al. 2010), their song (Brown et al. 2007; Rillich et al. 2009; DiRienzo et al. 2012), social isolation and crowding (Adamo and Hoy 1995; Iba et al. 1995; Stevenson and Rillich 2013). They are thus ideal models for investigating mechanisms underlying experience-dependent plasticity of aggressive behaviour, and this will be the main focus of this account. In some quarters, there is a growing tendency to attribute insects with experiencing conscious emotions (see Mendl et al. 2011 for a rational commentary). While this is hard to prove and practically impossible to refute, the experimental data summarised here illustrate that crickets are able to integrate the influences of ongoing and past experiences to generate adaptive aggressive behaviour – without necessitating rational, conscious emotions or reason – simply by exploiting the basic principles of neuromodulation, and this is perhaps the greatest advantage of studying these fascinating insects.
1.2 Understanding Aggression
All animals must cope with a simple biological fact: conspecifics are their greatest natural competitors. They compete for the same territories, shelters, food and sexual partners. For this reason, intraspecific aggression is common throughout the animal kingdom, from the lowest multicellular organisms (Brace and Purvey 1978) to our own species (Albert et al. 1993). Aggression can thus be considered as a behavioural strategy for securing some limited resource. However, since aggression is an inherently dangerous activity, it must be exercised with restraint, so that the costs do not exceed the potential benefits. It is, therefore, generally agreed that competing animals must in some way be able to equate potential costs and benefits of aggression, in order to decide whether it would be more opportune to fight or to flee (cf. Archer 1988). The underlying control mechanisms are, however, barely understood. Game theory predicts that animals adopt evolutionarily stable fighting strategies (Maynard Smith and Price 1973), which typically take the form of stereotyped, gradually escalating contests, involving the ritualised exchange of agonistic signals . This is thought to convey increasingly accurate information on the contestants’ abilities to secure the disputed resource (“resource holding potential ”), from which each individual bases its decision when best to stand and fight or turn and flee (cf. Hurd 2006; Elwood and Arnott 2012). Numerous studies have illustrated that an animal’s resource holding potential not only depends on physique (size, strength, weaponry) but also on an animal’s “willingness” to invest energy in fighting, i.e. its “aggressive motivation ”, a factor determined by a wide variety of experiences such as winning, losing and the possession and value of disputed resources (review: Stevenson and Rillich 2012). These largely theoretical considerations (summarised in Fig. 12.1) provide a convenient framework to explain the observed outcomes of animal contests. But what are the proximate mechanisms? How do different experiences and circumstances control aggressive motivation? How is this encoded in the nervous system? How exactly do animals decide whether to fight or flee?
Experience-dependent plasticity of aggression (modified from Stevenson and Rillich 2012). An individual’s changes of winning an aggressive encounter is given by its “resource holding potential” (RHP) which depends on physical factors (size, strength, weight, weaponry) as well as on “aggressive motivation” (see Hurd 2006), a factor determined by numerous experiences (physical exertion, winning, losing and presence and value of resources such as territory, food and potential mates). In crickets, flying, winning and shelter occupancy are potentially rewarding experiences that promote aggressiveness via the action of octopamine, which represents the motivational component of aggression (Stevenson et al. 2005; Rillich et al. 2011; Rillich and Stevenson 2011). On confronting a competitor, the agonistic signals exchanged during escalating ritualised fighting are evaluated to assess RHP and to decide when it would be more opportune to persist in fighting or to flee. Crickets conform to the cumulative assessment model (Payne 1998), in that they flee the moment the sum of the opponent’s agonistic signals surpasses some critical level (Rillich et al. 2007). The impact of these signals is mediated via activation of the NO/cGMP signalling pathway (Stevenson and Rillich 2015)
1.3 Biogenic Amines: Modulators of Aggressive Behaviour
Traditionally nervous systems were thought to generate each behaviour by virtue of the activity of a discrete dedicated circuit of interneurons that control a set of motor neurons and muscles in what David McFarland called the “behavioural final common path” (McFarland and Sibly 1975). It has since become realised (cf. Simpson and Stevenson 2015) that a single given physical circuit can function as a “polymorphic network” (Getting and Dekin 1985) subject to continued functional reconfiguration by the action of numerous neuromodulators and their blood-borne equivalents, neurohormones (Marder 2012). A neuromodulator can be generally defined as any substance released naturally in nervous tissue that alters the efficacy of “classical” synaptic transmission between a pre- and a postsynaptic cell by acting on dedicated metabotropic receptors. Hence, compared to neurotransmitters, the actions of neuromodulators are slower, but longer lasting, and span a far broader variety of effects that depend on the functional types and localities of the targeted receptors.
Neurochemicals with neuromodulator functions include primarily the biogenic amines along with numerous neuropeptides, but also more unconventional signalling molecules such as the gas nitric oxide. Crickets and other insects employ essentially the same neuronal signalling molecules as mammals, and they possess evolutionarily and pharmacologically related receptors (cf. Nagao and Tanimura 1993; Blenau and Baumann 2001; Homberg 2002; Hauser et al. 2006). Regarding neuromodulators, insects employ different though often structurally related neuropeptides and the same or at least analogous and structurally related amines. As in mammals, dedicated insect neurons can synthesise and release the catecholamine, dopamine, indolamine, serotonin (5-hydroxytryptamine) and histamine. Crickets are not known to release noradrenaline and adrenaline, which occur in only trace amounts in insects and other protostomes (Pflüger and Stevenson 2005). Instead, insects convert the catecholamine substrate amino acid L-tyrosine first to the amines tyramine and then to octopamine , which are known only as “trace amines” in the mammalian brain (Evans 1985).
Biogenic amines have long been attributed with influencing the expression of aggressive behaviour. Notably, the adrenergic/noradrenergic system is traditionally viewed as preparing vertebrate animals for fight or flight . However no consistent relationship with aggression has been found, although most recent data points towards promoting effects (Nelson and Trainor 2007; Haden and Scarpa 2007). This paucity in knowledge is at least partly attributable to the fact that research on biogenic amines and aggression in vertebrates has focused almost entirely on serotonin due to its reputed suppressing influence on the expression of aggression in humans and other mammals (Nelson and Trainor 2007).
These generalised actions of amines were thought to be reversed in invertebrates. For example, in crustaceans , serotonin was found to promote aggressiveness, while the invertebrate adrenergic analogue, octopamine, promotes submissiveness (Kravitz and Huber 2003). However, it is now clear that insects do not fit within this scheme. While the role of serotonin in insect aggression is still conjectural (see 1.6 below), work on crickets was the first to show that octopamine promotes aggression in an insect (Stevenson et al. 2000, 2005), and this was later verified for fruit flies (Baier et al. 2002; Hoyer et al. 2008; Zhou et al. 2008) and more recently in ants (Aonuma and Watanabe 2012) and stalk-eyed flies (Bubak et al. 2014). Hence, studies of insect model systems have already made a significant contribution to current understanding of how intraspecific aggression is controlled in the animal kingdom. Before turning to details, the following provides a brief summary of fighting behaviour in crickets.
2 Stereotyped Fighting and Its Initiation
2.1 Role of the Antennae
When two crickets meet, they are faced with the choice to court, fight or flee, and the outcome is largely controlled by information exchanged during antennal contact (Adamo and Hoy 1995; Hofmann and Schildberger 2001; Rillich and Stevenson 2015; see also Fernandez et al. 2010 on Drosophila). Species and sex are perceived by the pheromone signature (Iwasaki and Katagiri 2008), which induces males to court conspecific females. Antennal contact between males, which takes the form of “fencing”, is a sufficient and necessary releasing stimulus for initiating cricket aggression and involves both mechanical and olfactory components (Hofmann and Schildberger 2001; Iwasaki and Katagiri 2008; Sakura and Aonuma 2013; Rillich and Stevenson 2015).
Agonistic responses, such as mandible spreading, can be evoked by simply lashing the antennae with a bristle (Alexander 1961; Rillich and Stevenson 2015) or by contact with male pheromones (Iwasaki and Katagiri 2008), which have been specifically identified in fruit flies (Wang and Anderson 2010). The antennal afferent pathways in the cricket brain are known in some detail (Staudacher et al. 2005; Yoritsune and Aonuma 2012) along with descending interneurons that are directly excited by mechanical antennal stimulation (Schöneich et al. 2011). These interneurons descend to thoracic motor centres; some respond to cricket song (Staudacher and Schildberger 1998) and can initiate walking, turning (Böhm and Schildberger 1992; Zorovic and Hedwig 2012) and possibly escape or backward walking as found for homologous neurons in other insects (Comer and Baba 2011; Bidaye et al. 2014).
2.2 Levels of Escalating Aggression
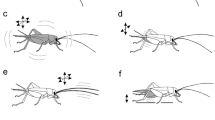
Once initiated, aggressive interactions between male crickets follow a stereotyped sequence of levels to which a fight escalates, before one contestant retreats (Hofmann and Stevenson 2000; Fig. 12.2). Antennal contact is always followed by mandible spreading, then mandible engagement and pushing, which culminates in a grappling contest. Most fights involve physical contact without injury and last only several seconds, but fights can go on for minutes and result in the loss of an antenna or leg. The end of a fight is marked by the loser retreating, after which the winner frequently starts to sing a characteristic rivalry song and produce erratic jerking body movements. Winners repeatedly attack losers, while losers actively avoid contact with other males for hours after defeat. There is no firm evidence for individual recognition . Losers will also retreat from unfamiliar opponents (Hofmann and Stevenson 2000), although in Drosophila , losers appear to fight differently against familiar and unfamiliar opponents (Yurkovic et al. 2006).
Escalating levels of aggression for adult male crickets. Level 0 mutual avoidance: no aggressive interaction. Level 1 pre-established dominance: one cricket attacks, the other retreats. Level 2 antennal fencing. Level 3 mandible spreading (by one): one cricket displays spread mandibles. Level 4 mandible spreading (both): both crickets display spread mandibles. Level 5 mandible engagement: the mandibles interlock and the animals push against each other. Level 6 grappling: an all out fight, the animals may disengage and reengage to bite other body parts. Fights can be concluded at any level by one opponent, the loser, retreating, the established winner typically produces the rival song and body jerking movements (Modified from Stevenson et al. 2000, in part redrawn from Huber et al. 1989)
Female crickets rarely interact, but fight vigorously in the presence of a courting male or his courtship song, and winning females have a greater probability of receiving the male’s spermatophore (Rillich et al. 2009). In contrast, Drosophila males and females adopt different fighting strategies, whereby the males but not the females establish dominance relationships (Nilsen et al. 2004). The sexually dimorphic fighting patterns in fruit flies are specified by sex-specific splicing of the fruitless gene (Vrontou et al. 2006) and controlled by specific subsets of neurons expressing the male form of fruitless proteins (Chan and Kravitz 2007).
2.3 Amines and the Initiation of Fighting
Biogenic amines have frequently been shown to have the capacity of initiating selected motor behaviours by directly activating the underlying central pattern generators. The classical example in insects is initiation of flight in locusts (Sombati and Hoyle 1984; Stevenson and Kutsch 1987). Recent studies, however, indicate that a cholinergic, rather than aminergic, mechanism is necessary for initiating flight (Buhl et al. 2008), while amines act as accessory neuromodulators that promote release and fine-tune the motor score (Rillich et al. 2013).
Studies in crickets indicate a similar principle for aggression. The octopamine , dopamine and serotonin content of the cricket central nervous system can be effectively depleted by treatment with reserpine (Stevenson et al. 2000), which blocks the molecular transporter that loads free amines from the cytoplasm into storage vesicles in nerve terminals for subsequent release (Henry and Scherman 1989). Reserpinised crickets are extremely lethargic, and have severely depressed escape responses. However, they are still capable of exhibiting all major elements of aggressive behaviour, albeit they often need to be coaxed to do so by repeated mechanical stimulation of their antennae (Stevenson et al. 2000). Essentially the same response also occurs following semi-selective depletion of octopamine and dopamine using the competitive synthesis inhibitor alpha-methyl-p-tyrosine (AMT) . In contrast, crickets with nervous systems depleted of serotonin by treatment with alpha-methyltryptophan (AMTP) exhibit hyperactivity, and enhanced escape responses, but seemingly unchanged aggressive behaviour (Stevenson et al. 2000). On the other hand, the tendency of male crickets to court rather than fight other males after antennectomy (Hofmann and Schildberger 2001) was suggested to result from loss of serotonin in the brain following this operation (Murakami and Itoh 2003). This seems unlikely, however, since antennectomy had no effect on the intensity of serotonin-like immunoreactivity in the cricket brain, and drugs that selectively block serotonergic, octopaminergic or dopaminergic signalling had no effect on the efficacy of antennal stimulation as an aggression-releasing stimulus (Rillich and Stevenson 2015).
Taken together, current evidence thus suggests that biogenic amines are not essential for initiating the basic motor elements of aggressive behaviour. Interestingly, however, prior antennal stimulation with a fresh cut, male antenna is followed by elevated expression of aggression in subsequent encounters with a male, via a mechanism that is dependent on the amine octopamine, but not serotonin or dopamine (Rillich and Stevenson 2015). This priming effect of octopamine is only one example where this amine acts as a neuromodulator to promote the expression of aggression (Rillich and Stevenson 2015). In the following we outline other examples in more detail.
3 Experience-Dependent Promotion of Aggression
3.1 Octopamine, Physical Exertion and the Flight Effect
Physical exertion , stress, challenge and fighting are frequently accompanied by fluctuations in the brain- or blood-content of neuromodulators, neurohormones and hormones (Wingfield et al. 1990; Bhatia et al. 2011). In vertebrates, stress-induced discharges of adrenalin/noradrenalin are thought to underlie the classical fight or flight response described originally by Walter Bradford Cannon (1915). Causal relationships between such changes and behaviour have, however, rarely been established. Insects exhibit a similar response (Lihoreau et al. 2009; Sokolowski 2010), whereby stressful and a variety of other experiences can lead to an almost tenfold elevation in the haemolymph content of octopamine, the invertebrate analogue of noradrenaline (Davenport and Evans 1984; Evans 1985). In crickets, increases in octopamine levels in the haemolymph or central nervous system are known to occur following a variety of experiences that influence aggressive behaviour (Fig. 12.3), including male antennal contact, copulation, fighting, flying (Adamo et al. 1995), grouping (Iba et al. 1995) and exposure to a mock predator (Adamo and Baker 2011).
Experience-dependent changes in levels of octopamine. (a) Haemolymph content of adult male crickets (pg/ul, circles median, bars interquartile range) at rest (base) after running (run), copulation (sex), fighting (fight, data for winners and losers), flying (flight) and touching the antennae (touch) with either a probe or male or female antenna (Adopted from data generated by Adamo et al. 1995). (b) Brain content (pmol/brain, bar mean, whisker standard error of mean) of adult crickets that were kept either in isolation or grouped (Adopted from data generated by Iba et al. 1995)
Studies in crickets were the first to draw a correlation between activity-dependent promotion of octopaminergic signalling and aggression. Cricket fighting has been a popular pastime for centuries in China, where aficionados recommend “punishing” poor fighters by shaking and launching them in the air several times (Hofmann 1996), a treatment similar to that used to evoke stress-induced release of octopamine (Davenport and Evans 1984). This treatment in fact works surprisingly well, but it proved to be far more effective to simply induce the animals to fly for a minute or so (Hofmann and Stevenson 2000). After flying, crickets become extremely aggressive, and their fights nearly always escalate to the highest level of aggression (6) and last two to three times longer than usual. These effects occurred without enhancing general excitability as evaluated from the animals’ startle responses and clearly depended on the execution of flight motor activity, but not on wind stimulation alone.
Subsequent studies demonstrated that this flight effect is mediated by octopamine. It can be mimicked by treatment with the tissue-permeable octopamine agonist, chlordimeform , and abolished following octopamine/dopamine depletion with AMT or after selective blockade of octopamine receptors (Stevenson et al. 2000, 2005). Flying also modulates cricket courtship behaviour (Dyakonova and Krushinskii 2008) and the responsiveness of identified neurons to sensory stimuli in the same way as chlordimeform (Jung et al. 2011). Whether flying confers any advantage on migrant crickets over residents in securing territory is not known.
While flying leads to a pronounced surge of octopamine in the haemolymph (Adamo et al. 1995; Adamo and Baker 2011), the concentration is too low to pass the insect “blood-brain” barrier and will hence be without effect on the nervous control of aggressive behaviour (cf. Stevenson et al. 2005). However, this surge is largely due to heightened activation of central neurons (cf. Roeder 1999) which probably include specific dorsal and ventral unpaired median (DUM/VUM) neurons such as those activated during flight in locusts (Duch et al. 1999). DUM/VUM neurons are comparatively large and accessible neurons that are typically located on the dorsal, but occasionally ventral, midline of all ventral ganglia of all orthopterans and many other winged insects (Stevenson and Sporhase-Eichmann 1995; Bräunig and Pflüger 2001; Pflüger and Stevenson 2005). Though absent in the brain, a small group of several DUM/VUM neurons in the anterior of the suboesophageal ganglia ascend via the connectives to the brain, where they form widespread projections within all major brain neuropiles (e.g. locusts, Bräunig 1991; honeybees, Schröter et al. 2007; fruit flies, Busch and Tanimoto 2010), including regions where Franz Huber demonstrated, in his now classic experiments, that local electrical stimulation can elicit components of aggressive behaviour (Huber 1955, 1960). Elegant genetic techniques in fruit flies led to the identification of a small subset of octopaminergic cells, possibly VUM type, in the suboesophageal ganglion that are “functionally important for expressing aggression” (Zhou et al. 2008). The precise function of these neurons in aggression remains, however, to be established. Another subset of VUM cells in fruit flies express the sex-determining factor fruitless, and these appear to be involved in mediating the choice between courtship and aggression (Certel et al. 2007, 2010). Very little is known about DUM/VUM neurons in crickets (Gras et al. 1990; Bräunig et al. 1990; Stevenson and Sporhase-Eichmann 1995).
3.2 Octopamine and the Winner Effect
In practically all animals investigated, winning an aggressive encounter against a conspecific promotes an individual’s aggressiveness, thereby rendering it more likely to win a subsequent encounter (Hsu et al. 2005). Although comparatively little is known of the proximate causes, recent studies implicate the involvement of androgens in rodents (Fuxjager and Marler 2010) and octopamine in crickets (Rillich and Stevenson 2011; Fig. 12.4).
Octopamine and the winner effect. (a) Level of aggression and (b) fight duration (circles medians, bars interquartile ranges) for encounters between pairs of weight-matched male crickets that were both either socially inexperienced (naive, N), winners of one previous encounter (W1) or winners of two previous encounters (W2) for an inter-fight interval of 5 min. Before the initial fight, the animals were injected with either vehicle (white bars), β-adrenergic blocker propranolol (green bars), tyramine (TA) blocker yohimbine (blue bars), dopamine (DA) blocker fluphenazine (brown bars) or octopamine (OA) blocker epinastine (red bars). Numbers in parenthesis in (a) give the pairs for each round. Significant differences between tournament rounds are indicated (Kruskal-Wallis one-way variance test, ** p < 0.01, n.s. not significant) (Adopted from Rillich and Stevenson 2011)
In crickets, winning increases the probability of a cricket subsequently defeating an inexperienced opponent (Khazraie and Campan 1999) and is also associated with increased mating success (Tachon et al. 1999). When winners are matched against each other in knockout tournaments, the fights become progressively more severe and longer with each win scored (Rillich and Stevenson 2011). This winner effect is transient and persists for less than 20 min after winning, which is far shorter than in rodents, where it can last for days (Fuxjager and Marler 2010). Changes in social status in crickets are thus not necessarily associated with (long term) learning and memory, as suggested for fruit flies (Yurkovic et al. 2006). As found for the flight effect, the cricket winner effect is mediated by the amine octopamine. It is prohibited by treatment with the selective octopamine receptor blocker epinastine (cf. Roeder et al. 1998), but not by propranolol, a ß-adrenergic receptor antagonist, by yohimbine, an insect tyramine receptor blocker, nor by fluphenazine, an insect dopamine receptor blocker (Rillich and Stevenson 2011).
Insights into what actually constitutes a win were gained by interrupting fights between two contestants before either won (Rillich and Stevenson 2011). In a subsequent encounter, these crickets exhibit hyperaggressiveness, indicating that a winner effect can result alone from the physical exertion of fighting, without actually scoring a win. This is feasible considering that octopaminergic neurons are activated during walking and by a wide variety of mechanosensory signals (Gras et al. 1990; Morris et al. 1999) and that fighting itself leads to an almost fivefold increase in haemolymph levels of octopamine in winners and losers alike (Adamo et al. 1995). On the other hand, a winner effect also develops in crickets that experience wins against submissive opponents that retreat prior to any physical engagement (Rillich and Stevenson 2011). While it is known in humans that merely watching previous victories can elevate levels of hormones with aggression-promoting properties (Carré and Putnam 2010), this is a surprising finding for crickets. The following gives a further example of an essentially non-physical experience with aggression-promoting effects.
3.3 Octopamine and the Residency Effect
Regardless of species, animals that possess a key resource are more likely to win disputes against contenders, but it is hotly debated how this is controlled (reviews: Kemp and Wiklund 2004; Hsu et al. 2005). For male field crickets, burrows are valuable assets offering shelter from predators and an aid in attracting females. Females mate preferentially with burrow owners, and these owners zealously fight off any intruding male (Alexander 1961; Simmons 1986; Rodriguez-Munoz et al. 2011). Cricket species with burrowing males also tend to be more aggressive than non-burrowing species (Bertram et al. 2011).
In the laboratory, crickets that are submissive after having just lost a fight become highly aggressive when given an artificial shelter to occupy, and frequently defeat aggressive intruders (Rillich et al. 2011). This residency effect does not depend on the initial sensory experience of shelter acquisition, since it becomes first evident after 2–15 min of residency and declines within 15 min after taking the shelter away, i.e. on a similar time course as the winner effect. Furthermore, shelters of wire or with a transparent roof are far less effective, and darkness alone ineffective (personal observations). It seems the cricket must reside in a dark, burrow-like structure. Whatever its proximate cause, the residency effect is clearly octopamine-dependent. It is not evident in crickets depleted of octopamine and dopamine, while being unaffected by serotonin depletion, but selectively blocked by treatment with octopamine receptor antagonists (Rillich et al. 2011; Fig. 12.5).
Octopamine and the residency effect. (a) Level of aggression and (b) fight duration (circles medians, bars interquartile ranges) for initial fights between weight-matched socially inexperienced (naive) male crickets and for a subsequent interaction between winners and losers which previously remained in the arena without a shelter for 15 min (winners vs. control losers) or occupied a shelter in the arena for 15 min (winners vs. resident losers, grey background). Prior to the initial fight, the crickets were treated with either a vehicle (white bars), a β-adrenergic blocker (green bars), a tyramine (TA) blocker (blue bars), an α-adrenergic blocker (violet bars) or an octopamine (OA) blocker (red bars). The number of contests evaluated (n) is given in parenthesis beneath each column, excepting initial fight, which is pooled. Asterisks denote statistically significant differences (Mann–Whitney U-test *, **, ***: p < 0.05, 0.01, 0.001, respectively) (Adopted from Rillich et al. 2011)
3.4 Octopamine and Reward
The paradoxical question posed by our studies is how experiences as diverse as physical exertion (flying), fighting and residency – which span the entire range of energy expenditure – all promote aggression via a seemingly common mechanism involving octopamine? An intriguing idea is that all these experiences are in some way associated as being positive or in some way rewarding (Rillich et al. 2011; Rillich and Stevenson 2011). Physical exercise in mammals, including humans, seems to be equated with reward (Raichlen et al. 2011) and can act as a mood elevator that alleviates symptoms of depression by invoking changes in a variety of neurotransmitter systems including dopamine (Craft and Perna 2004). Aggression in mammals also leads to increased activity in dopaminergic pathways and increases the expression of androgen receptors in regions of the brain mediating motivation and reward (O’Connell and Hofmann 2011). In insects, rewarding experiences are primarily associated with octopamine (review: Perry and Barron 2013). In honeybees the value of food sources appears to be encoded by octopamine modulating associative reward pathways (Barron et al. 2010). Octopamine is known to convey reward signals in appetitive learning paradigms in honeybees (Hammer and Menzel 1995), fruit flies (Schwärzel et al. 2003) and crickets (Mizunami et al. 2009). It has even been demonstrated that the activity of only one of the group of 15 DUM/VUM neurons (“VUMmx1”) in honeybees (cf. Schröter et al. 2007) can substitute for the sucrose reward in an associative, appetitive learning paradigm (Hammer 1993). Considering the possible role of members of this cell group in the expression of aggression and courtship in fruit flies (Zhou et al. 2008; Certel et al. 2007, 2010), we clearly now need to learn more about the related DUM/VUM neurons in the suboesophageal ganglion of crickets.
4 Experience-Dependent Promotion of Submission
4.1 Opponent Assessment and the Decision to Flee
Fights are concluded the moment one contestant submits or retreats. The events leading to submission and its maintenance are, however, poorly understood. Various behavioural theories agree that information from ritualised agonistic signals exchanged during fighting are assessed to determine when to fight or flee, but differ regarding which individual evaluates these cues (sender, receiver or both: Payne 1998; Elwood and Arnott 2012).
By experimentally manipulating information exchange, it was revealed that crickets evaluate only the opponent’s signals and that these signals promote the “decision to flee ” (Rillich et al. 2007). In one key experiment, crickets with either blackened eyes (“blinded”) or lamed mandibles were found to fight against untreated, equally sized opponents with almost unabated vigour and chance of winning, whereas the “blinded” crickets won practically all fights against crickets with lamed mandibles (Fig. 12.6). This unusual finding is fully conformed to the core prediction of the cumulative assessment model of Payne (1998) that an animal persists in fighting until the accumulated sum of the opponent’s actions surpasses some critical threshold to flee. Hence, the “blinded” cricket persists since it receives no visual and only limited physical input from the opponent with lamed mandibles, whereas the latter accumulates the full brunt of his adversary’s actions and thus becomes the first to flee (Rillich et al. 2007). The cumulative assessment model also accounts for effects of physical disparities (e.g. size strength and weaponry: Dixon and Cade 1986; Judge and Bonanno 2008; Hall et al. 2010) or energy status (Briffa 2008) on fight outcome. For example, an animal with any physical or energetic advantage will have a greater sensory impact on its opponent, which will hence be more likely to flee first. But how do crickets add up the sensory impact of their opponents, and how does the perception of this lead to retreat?
Effects of impaired agonistic signalling and nitridergic drugs on cricket aggression: (a) level of aggression, (b) fight duration (circles medians, bars interquartile ranges) and (c) win chances (%). In each case male crickets deprived of visual information (blind) were matched against weight-matched males with disabled mandibles (disarmed), but one opponent (indicated by pictogram and # in the x-axis label) received either control solutions (grey bars, ringer or DNAME) or the NO donor S-nitroso-N-acetyl-DL-penicillamine (SNAP, red bars) or the NO synthase inhibitor nitro-L-arginine methyl ester hydrochloride (LNAME, blue bars). The number of fighting pairs is given above the top axis. Asterisks in (c) denote statistically significant differences (chi-square test **, ***: p < 0.01, 0.001, respectively) (Adopted from Stevenson and Rillich 2015)
4.2 Adding Up the Odds: The Role of Nitric Oxide
Recent studies have revealed that the gaseous neuromodulator nitric oxide (NO) plays a key role in mediating the effect of an opponent’s agonistic actions (Stevenson and Rillich 2015). In both mammals and insect, this unconventional neuromodulator can be synthesised by neurons bearing the enzyme nitric oxide synthase and, once produced, traverses by diffusion to activate the intracellular receptor molecule soluble guanylyl cyclase, which in turn initiates production of the second messenger cyclic guanosine monophosphate (cGMP , review: Müller 1997). In mammals NO can act to suppress aggression, at least partly by influencing serotonergic signalling (Nelson and Trainor 2007), but its specific behavioural function in normal aggressive behaviour is unknown. Earlier work on crickets indicated that NO can either promote or inhibit the expression of aggression depending on circumstances. For example, inhibition of NO synthesis has been reported to prohibit the aggression-promoting effects of flying (Dyakonova and Krushinskyii 2006) or have no effect on socially naive crickets, but increase aggression in submissive losers (Iwasaki et al. 2007). More recently, we found that inhibitors of the NO/cGMP pathway increase aggressiveness in socially naive crickets while activators suppress it (Stevenson and Rillich 2015), i.e. in effect NO suppresses aggression as in mammals. However, rather than simply suppressing the tendency to fight, i.e. aggressive motivation, the application of nitridergic drugs to animals with manipulated signalling abilities revealed that NO mediates the impact of the opponent’s aggressive signals during fighting. To take one example, when treated with an NO donor, crickets deprived of visual inputs (blinded) escalate and persist normally, but no longer have a win advantage over opponents rendered unable to inflict force with their mandibles. Conversely, when treated with an NOS inhibitor, crickets with lamed mandibles no longer have a win disadvantage against blinded crickets (Fig. 12.6; for supporting data, see Stevenson and Rillich 2015). Taken together, the data suggest that any aversive stimulus perceived in the context of aggression leads to activation of the NO signalling pathway, which in turn increases the probability of fleeing in response to further aversive stimuli.
4.3 The Loser Effect
Social defeat , i.e. losing an agonistic dispute with a conspecific, is followed by a period of suppressed aggressiveness in many animal species (Hsu et al. 2005; Rutte et al. 2006) and is generally regarded as a major stressor, which in humans may play a role in psychiatric disorders (Huhman 2006). Although accompanied by numerous changes in brain chemicals and gene expression (Miczek et al. 2011), the underlying cause of the loser effect is unknown.
Once a cricket has decided to flee, it will subsequently retreat on contact with any conspecific male (Alexander 1961; Adamo and Hoy 1995; Khazraie and Campan 1999; Hofmann and Stevenson 2000) and requires on average some 3 h to fully regain its initial level of aggressiveness (Stevenson and Rillich 2013). Confirming work of earlier authors (Iwasaki et al 2007), treatment with nitridergic drugs revealed that this loser effect in crickets results from activation of the NO/cGMP pathway (Stevenson and Rillich 2015). In males treated with nitridergic agonists, recovery was delayed by up to 24 h, whereas the majority of those receiving antagonists recovered far earlier (within 15 min). It is important to stress that socially subjugated crickets are still potentially aggressive. Losers will often attack other losers when they retreat first, and they will fight vigorously when their eyes are blackened, which eliminates the visual impact of the approaching opponent (Rillich et al. 2007). Losers are also equally responsive to antennal stimulation, the releasing stimulus for aggression, as socially naive crickets (Rillich and Stevenson 2015). Accordingly, losers are more susceptible to aversive stimulation, rather than motivationally depressed.
Nitric oxide is unlikely to act alone in controlling the decision to flee and loser submissiveness – for one it occurs in neurons that can be expected to contain more conventional neuromodulators (see e.g. Bullerjahn et al. 2006), and amines in particular are likely to be involved. Recovery from the loser effect is prohibited in crickets following depletion of octopamine and dopamine after treatment with the synthesis inhibitor AMT, and octopamine or dopamine receptor agonists are sufficient to fully restore aggression (Rillich and Stevenson 2014). However, while loser crickets still regain their aggressiveness after octopamine receptor blockade, they are prevented from doing so by dopamine receptor blockade. Hence, dopaminergic signalling is necessary for the normal recovery of aggression after social defeat in crickets (Rillich and Stevenson 2014). Finally, a mathematical model for recovery from defeat has been developed for crickets on the assumption that serotonin is involved (Yano et al. 2012), but solid experimental evidence for this is lacking (see also Sect. 6 on serotonin below).
5 Social Isolation, Biogenic Amines and Aggression
Social isolation results in dramatic behavioural and physiological changes in a wide variety of animal species from insects to man (Cacioppo and Hawkley 2009; Lihoreau et al. 2009; Simpson and Sword 2009; Sokolowski 2010). A wealth of studies have noted that isolation leads to increased aggressive behaviour in vertebrates (Hsu et al. 2005) and in insects such as solitary wasps (Pfennig and Reeve 1989), fruit flies (Zhou et al. 2008; Johnson et al. 2009) and crickets (Alexander 1961; Adamo and Hoy 1995; Iba et al. 1995). Isolation and crowding in insects are also associated with dramatic changes in the levels of biogenic amines (Iba et al. 1995; Rogers et al. 2004; Wada-Katsumata et al. 2011). However, a recent study on crickets revealed that reduced aggression of grouped individual results from social subjugation and resultant submissive behaviour of most group members by one or two dominant males, while heightened aggression in isolates is simply due to recovery from the loser effect and a return to a default aggressive state (Stevenson and Rillich 2013). While the effects of social isolation in different animal groups will no doubt differ depending on social structure, the possibility that recovery from social subjugation may contribute to heightened aggressiveness in social isolates appears to have been neglected in many studies.
6 Serotonin and Aggression
The actions of octopamine in arthropods are often functionally antagonised by serotonin . Examples of this antagonism can be seen in antennal scanning in honeybees (Erber et al. 1993), escape in cockroaches Goldstein and Camhi 1991) and crickets (Dyakonova et al. 1999), mating interval in male crickets (Nagao et al. 1991) and aggression in crustaceans (Kravitz and Huber 2003). Serotonin is renowned for its restraining effect on aggression in numerous animals including man (Kravitz and Huber 2003; Nelson and Trainor 2007; Passamonti et al. 2012). In locusts it clearly promotes grouping and swarm formation by subduing mutual avoidance or promoting attraction (Anstey et al. 2009). The role of serotonin in insect aggression is, however, not yet clear.
A promoting effect of serotonin on cricket aggression is suggested by the observation that reduced aggression, after losing and antennal ablation (cf. Hofmann and Schildberger 2001), is correlated with decreased serotonin brain content (Murakami and Itoh 2001, 2003). However, the loss of serotonin from the cricket nervous system following treatment with the synthesis inhibitor alpha-methyltryptophan (AMTP) has been found to induce hyperactivity and enhances startle responses in crickets, but does not have any obvious effects on aggression (Stevenson et al. 2000, 2005; Rillich and Stevenson 2015). Serotonin depletion does, however, appear to reduce the chance of winning (Dyakonova et al. 1999), though this may be a non-selective effect of hyperactivity. Nonetheless, a more recent study (Dyakonova and Krushinskii 2013) revealed clear, but in part functionally conflicting, effects of elevating serotonin levels by treatment with its precursor 5-hydroxytryptophan (5HTP) . On the one hand, 5HTP induces a raised “aggressive-like” body posture (see also Kravitz and Huber 2003 on crustaceans), enhanced general activity, more frequent rival song production and longer fights that do not resolve clear losers. On the other hand 5HTP-treated crickets exhibit a delayed latency to spread their mandibles, launch fewer attacks and have an unchanged chance of winning.
Similarly conflicting findings have been reported for fruit flies. While Baier et al. (2002) reported that aggression in fruit flies was unaffected by blockade of serotonin biosynthesis, or 5HTP treatment, Dierick and Greenspan (2007) found aggression was promoted by 5HTP. Similarly, Alekseyenko et al. (2010), using molecular genetic techniques, report that acute activation of serotonergic neurons resulted in flies that escalated faster and fought at higher intensities, while selective disruption of serotonergic neurotransmission yielded flies that fought with reduced ability to escalate fights.
These inconsistencies can be expected to at least partly result from serotonin acting on different receptor subtypes. For example, pharmacological activation of 5HT2-type receptors reduces total aggression in Drosophila, while activating 5HT1A-type receptors increased it (Johnson et al. 2009). Also in mammals, different serotonin receptor subtypes influence different aspects of the total aggressive behavioural repertoire (de Boer and Koolhaas 2005). These and other findings challenge the dogmatic view of serotonin acting simply to suppress aggression in mammals, where it is currently thought to limit impulsivity (Nelson and Trainor 2007) or promote the drive to withdraw (Tops et al. 2009). An analogous scenario is conceivable for crickets, where evidence suggests that serotonergic signalling depresses escape responses in aggressive individuals (Dyakonova et al. 1999), while losers show enhanced escape behaviour due to lower brain levels of serotonin after defeat (Murakami and Itoh 2001). In crayfish, the effects of serotonin on escape and aggressive–submissive body posture change with social status due to a shift in the relative expression of different serotonin receptor subtypes to a pattern more appropriate for the new status (Cattaert et al. 2010). In conclusion, some features of dominant behaviour involve activation of the serotonergic system, while a decrease in serotonergic signalling is functionally important for the control of loser behaviour (Dyakonova and Krushinsky 2013). Considering findings in other animal groups, it is conceivable that the different actions of serotonin are mediated via different receptor subtypes, which may change in their relative expression after social defeat.
7 Neuropeptides and Aggression
Compared to vertebrates very little is known about the roles of peptides in controlling aggression in invertebrates. In crickets, treatment with the opiate antagonist naloxone elevates aggressiveness in losers, without affecting winners or socially naive animals (Dyakonova et al. 2002). In Drosophila aggression is increased following genetic silencing of circuitry employing neuropeptide F , the invertebrate homologue of neuropeptide Y (Dierick and Greenspan 2007).
8 Conclusions and Future Directions
Work on crickets have revealed that biogenic amines and nitric oxide signalling play key roles in mediating the effects of a wide variety of experiences on the expression of aggression. In this respect the cricket has advanced to the status of a model system for investigating experience-dependent plasticity of social behaviour.
Octopamine, the invertebrate analogue of noradrenaline, increases aggression by promoting the tendency to fight (Stevenson et al. 2005) and exhibition of agonist behaviours such as lunging (Hoyer et al. 2008; Zhou et al. 2008) and mandible spreading (Rillich and Stevenson 2011). Experiences as diverse as physical exertion (flying, fighting), winning and possession of resources (shelter) may all be evaluated as being in some way positive or rewarding, and these experiences promote aggression via a mechanism dependent on activation of the octopaminergic system (reviews: Stevenson and Rillich 2012; Stevenson and Schildberger 2013; Simpson and Stevenson 2015). In this respect, octopamine can be considered as representing the motivational component of aggression. Candidate neurons for mediating these effects are members of the group of DUM/VUM neurons with somata in the suboesophageal ganglion that project to the brain. Related neurons have already been shown to mediate reward in associative learning of honeybees and are possibly important for the expression of aggression in fruit flies (Zhou et al. 2008). The function of these cells in crickets will not be easy to analyse due to their irregular and variable localization (Sporhase-Eichmann et al. 1992; Stevenson and Spörhase-Eichmann 1995) as well as the current lack of genetic silencing and activation techniques that have been firmly established for fruit flies.
Submissive behaviour and the timing of the decision to flee result from the assessment of agonistic signals exchanged during fighting. Crickets conform to the cumulative assessment hypothesis of Payne (1998) in that they persist in fighting until the sum of the perceived adversary’s actions surpasses some threshold to flee (Rillich et al. 2007). Recent studies have revealed that aversive stimuli, such as an opponent’s agonist signals, promote the tendency to flee via activation of the NO/cGMP pathway (Stevenson and Rillich 2015). Although defeated crickets have a reduced tendency to fight, they are still potentially aggressive (Rillich et al. 2007). It appears now that losing increases the tendency to flee rather than reduces aggressiveness per se (Stevenson and Rillich 2015). Activation of the NO/cGMP pathway also results in the reduced expression of aggression after losing. While both octopamine and dopamine can each readily restore aggressiveness in losers, alone dopamine is necessary for the normal recovery of aggressiveness (Rillich and Stevenson 2014). The effects of social subjugation have been shown to be responsible for the reduced aggressiveness of grouped individuals, while recovery from the loser effect is the main cause of heightened aggressiveness in isolates (Stevenson and Rillich 2013).
While the role of serotonin in aggression remains unclear, recent findings indicate that this amine may promote some aspects of dominant behaviour while also being functionally important for controlling submissive behaviour after social defeat (Dyakonova and Krushinskii 2013). In view of findings in other animal species, the different actions of serotonin may be mediated via different receptor subtypes, which may have different patterns of expression depending on social status. Genes encoding serotonin receptor subtypes have recently been identified in crickets (Watanabe et al. 2011; Watanabe and Aonuma 2012) and we now need to know more of their distribution in nervous tissue and their specific pharmacology. Candidate serotonergic neurons for influencing aggression have only been identified in Drosophila (Alekseyenko and Kravitz 2015).
In addition to biogenic amines, the expression of aggressive behaviour in insects is also modulated by some peptides. Further work is necessary to understand the exact roles of such modulators and how they interact with aminergic pathways. Peptides and nitric oxide often occur as co-transmitters in aminergic neurons (cf. Bullerjahn et al. 2006), so we also need to know more about their distribution in relation to biogenic amines in the cricket brain. We in fact know very little about the neuronal substrates for aggression in crickets, and future effort must be devoted to performing chronic recordings from different regions of the brain during behaviour.
It should also be mentioned that the effects of experiences on aggressive behaviour outlined here are relatively short lived, and yet aggression can have longer-term changes on the operation of the nervous system than those discussed here. Agonistic behaviour can trigger neurogenesis (Ghosal et al. 2009) and FOS-like protein expression in the male cricket brain (Ghosal et al. 2010), but it is not known whether this leads to changes in behaviour. A hint of the possible complexities involved is given by the finding that aggressive behaviour in Drosophila is affected by over 50 novel genes with widespread pleiotropic effects (Edwards et al. 2009).
In effect, the biogenic amine octopamine and nitric oxide control the expression of aggression by modulation the respective behavioural thresholds to fight and to flee. These modulators no doubt operate in concert with the amines dopamine and serotonin as well as selected neuropeptides. In essence, octopamine, serotonin and nitric oxide appear to have similar roles to those emerging for their counterparts in the control of aggression in mammals.
References
Adamo SA, Baker JL (2011) Conserved features of chronic stress across phyla: the effects of long-term stress on behavior and the concentration of the neurohormone octopamine in the cricket, Gryllus texensis. Horm Behav 60:478–483
Adamo SA, Hoy RR (1995) Agonistic behavior in male and female field crickets, Gryllus bimaculatus, and how behavioural context influences its expression. Anim Behav 49:1491–1501
Adamo SA, Linn CE, Hoy RR (1995) The role of neurohormonal octopamine during ‘fight or flight’ behaviour in the field cricket Gryllus bimaculatus. J Exp Biol 198:1691–1700
Albert D, Walsh M, Jonik R (1993) Aggression in humans: what is its biological foundation? Neurosci Biobehav Rev 17:405–425
Alekseyenko OV, Kravitz EA (2015) Serotonin and the search for the anatomical substrate of aggression. Fly 8(4):1–6
Alekseyenko OV, Lee C, Kravitz EA (2010) Targeted manipulation of serotonergic neurotransmission affects the escalation of aggression in adult male Drosophila melanogaster. PLoS One 5:e10806
Alexander RD (1961) Aggressiveness, territoriality, and sexual behaviour in field crickets (Orthoptera: Gryllidae). Behavior 17:130–223
Anstey ML, Rogers SM, Ott SR, Burrows M, Simpson SJ (2009) Serotonin mediates behavioral gregarization underlying swarm formation in desert locusts. Science 323:627–630
Aonuma H, Watanabe T (2012) Octopaminergic system in the brain controls aggressive motivation in the ant. Formica Jpn Acta Biol Hung 63(Suppl 2):63–68
Archer J (1988) The behavioural biology of aggression. Cambridge University Press, Cambridge
Baier A, Wittek B, Brembs B (2002) Drosophila as a new model organism for the neurobiology of aggression? J Exp Biol 205:1233–1240
Barron AB, Sovik E, Cornish J (2010) The roles of dopamine and related compounds in reward-seeking behavior across animals phyla. Front Behav Neurosci 4:1–9
Bertram SM, Rook VLM, Fitzsimmons JM, Fitzsimmons LP (2011) Fine- and broad-scale approaches to understanding the evolution of aggression in crickets. Ethology 117:1067–1080
Bhatia N, Maiti PP, Choudhary A, Tuli A, Masih D, Khan MMU et al (2011) Animal models in the study of stress: a review. NSHM J Pharm Healthc Manag 2:42–50
Bidaye SS, Machacek C, Wu Y, Dickson BJ (2014) Neuronal control of Drosophila walking direction. Science 344:97–101
Blenau W, Baumann A (2001) Molecular and pharmacological properties of insect biogenic amine receptors: lessons from Drosophila melanogaster and Apis mellifera. Arch Insect Biochem Physiol 48:13–38
Böhm H, Schildberger K (1992) Brain neurones involved in the control of walking in the cricket Gryllus bimaculatus. J Exp Biol 166:113–130
Brace RC, Purvey J (1978) Size-dependant dominance hierarchy in the anemone Actinia equina. Nature 273:752–753
Bräunig P (1991) Suboesophageal DUM neurons innervate the principal neuropiles of the locust brain. Philos Trans R Soc Lond B 332:221–240
Braunig P, Pflüger HJ (2001) The unpaired median neurons of insects. Adv Insect Physiol 28:185–266
Bräunig P, Allgäuer C, Honegger HW (1990) Suboesophageal DUM neurones are part of the antennal motor system of locusts and crickets. Experientia 46:259–261
Briffa M (2008) Decisions during fights in the house cricket, Acheta domesticus: mutual or self assessment of energy, weapons and size? Anim Behav 75:1053–1062
Brown WD, Smith AT, Moskalik B, Gabriel J (2006) Aggressive contests in house crickets: size, motivation and the information content of aggressive songs. Anim Behav 72:225–233
Brown WD, Chimenti AJ, Siebert JR (2007) The payoff of fighting in house crickets: motivational asymmetry increases male aggression and mating success. Ethology 113:457–465
Bubak AN, Grace JL, Watt MJ, Renner KJ, Swallow JG (2014) Neurochemistry as a bridge between morphology and behavior: perspectives on aggression in insects. Curr Zool 60:778–790
Buhl E, Schildberger K, Stevenson PA (2008) A muscarinic cholinergic mechanism underlies activation of the central pattern generator for locust flight. J Exp Biol 211:2346–2357
Bullerjahn A, Mentel T, Pflüger HJ, Stevenson PA (2006) Nitric oxide: a co-modulator of efferent peptidergic neurosecretory cells including a unique octopaminergic neurone innervating locust heart. Cell Tissue Res 325:345–360
Busch S, Tanimoto H (2010) Cellular configuration of single octopamine neurons in Drosophila. J Comp Neurol 518:2355–2364
Cacioppo JT, Hawkley LC (2009) Perceived social isolation and cognition. Trends Cogn Sci 13:447–454
Cannon WB (1915) Bodily changes in pain, hunger, fear and rage: an account of recent researches into the function of emotional excitement. Appleton, New York
Carre JM, Putnam SK (2010) Watching a previous victory produces an increase in testosterone among elite hockey players. Psychoneuroendocrinology 35:475–479
Cattaert D, Delbecque JP, Edwards DH, Issa FA (2010) Social interactions determine postural network sensitivity to 5-HT. J Neurosci 30:5603–5616
Certel SJ, Savella MG, Schlegel DC, Kravitz EA (2007) Modulation of Drosophila male behavioral choice. Proc Natl Acad Sci U S A 104:4706–4711
Certel SJ, Leung A, Lin CY, Perez P, Chiang AS, Kravitz EA (2010) Octopamine neuromodulatory effects on a social behavior decision-making network in Drosophila males. PLoS One 5:e13248
Chan YB, Kravitz E (2007) Specific subgroups of FruM neurons control sexually dimorphic patterns of aggression in Drosophila melanogaster. Proc Natl Acad Sci U S A 104:9577–9582
Comer C, Baba Y (2011) Active touch in orthopteroid insects: behaviours, multisensory substrates and evolution. Philos Trans R Soc Lond B 366:3006–3015
Craft LL, Perna FM (2004) The benefits of exercise for the clinically depressed. J Clin Psychiatry 6:104–111
Davenport AP, Evans PD (1984) Changes in haemolymph octopamine levels associated with food deprivation in the locust Schistocerca gregaria. Physiol Entomol 9:269–274
de Boer SF, Koolhaas JM (2005) 5-HT1A and 5-HT1B receptor agonists and aggression: a pharmacological challenge of the serotonin deficiency hypothesis. Eur J Pharmacol 526:125–139
Dierick HA, Greenspan RJ (2007) Serotonin and neuropeptide F have opposite modulatory effects on fly aggression. Nat Genet 39:678–682
DiRienzo N, Pruitt JN, Hedrick AV (2012) Juvenile exposure to acoustic sexual signals from conspecifics alters growth trajectory and an adult personality trait. Anim Behav 84:861–868
Dixon KA, Cade WH (1986) Some factors influencing male-male aggression in the field Cricket Gryllus integer (time of day, age, weight and sexual maturity). Anim Behav 34:340–346
Duch C, Mentel T, Pflüger HJ (1999) Distribution and activation of different types of octopaminergic DUM neurons in the locust. J Comput Neurol 403:119–134
Dyakonova VE, Krushinkskii AL (2006) Effects of an NO synthase inhibitor on aggressive and sexual behavior in male crickets. Neurosci Behav Physiol 36:565–571
Dyakonova VE, Krushinkskii AL (2013) Serotonin precursor (5-hydroxytryptophan) causes substantial changes in the fighting behavior of male crickets, Gryllus bimaculatus. J Comp Physiol A 199:601–609
Dyakonova VE, Krushinskii AL (2008) Previous motor experience enhances courtship behaviour in male cricket Gryllus bimaculatus. J Insect Behav 21:172–180
Dyakonova VE, Schurmann F, Sakharov DA (1999) Effects of serotonergic and opioidergic drugs on escape behaviors and social status of male crickets. Naturwissenschaften 86:435–437
Dyakonova VE, Schurmann FW, Sakharov DA (2002) Effects of opiate ligands on intraspecific aggression in crickets. Peptides 23:835–841
Edwards AC, Zwarts L, Yamamoto A, Callaerts P, Mackay TF (2009) Mutations in many genes affect aggressive behavior in Drosophila melanogaster. BMC Biol 7:29
Elwood RW, Arnott G (2012) Understanding how animals fight with Lloyd Morgan’s canon. Anim Behav 84:1095–1102
Erber J, Kloppenburg P, Scheidler A (1993) Neuromodulation by serotonin and octopamine in the honeybee: behaviour, neuroanatomy and electrophysiology. Experientia 49:1073–1083
Evans PD (1985) Octopamine. In: Kerkut GA, Gilbert LI (eds) Comprehensive insect physiology biochemistry and pharmacology. Pergamon, Oxford, pp 499–530
Fernandez MP, Chan YB, Yew JY, Billeter JC, Dreisewerd K, Levine JD et al (2010) Pheromonal and behavioral cues trigger male-to-female aggression in Drosophila. PLoS Biol 8:e1000541
Fuxjager MJ, Marler CA (2010) How and why the winner effect forms: influences of contest environment and species differences. Behav Ecol 21:37–45
Getting PA, Dekin MA (1985) Tritonia swimming. A model system for integration within rhythmic motor systems. In: Selverston AI (ed) Model networks and behavior. Plenum Press, New York, pp 3–20
Ghosal K, Gupta M, Killian KA (2009) Agonistic behavior enhances adult neurogenesis in male Acheta domesticus crickets. J Exp Biol 212:2045–2056
Ghosal K, Naples SP, Rabe AR, Killian KA (2010) Agonistic behavior and electrical stimulation of the antennae induces Fos-like protein expression in the male cricket brain. Arch Insect Biochem Physiol 74:38–51
Giurfa M (2012) Social learning in insects: a higher-order capacity? Front Behav Neurosci 6:57
Goldstein RS, Camhi JM (1991) Different effects of the biogenic amines dopamine, serotonin and octopamine on the thoracic and abdominal portions of the escape circuit in the cockroach. J Comp Physiol A 168:103–112
Gras H, Hörner M, Runge L, Schürmann FW (1990) Prothoracic DUM neurons of the cricket Gryllus bimaculatus respond to natural stimuli activity in walking behaviour. J Comp Physiol A 166:901–914
Haden SC, Scarpa A (2007) The noradrenergic system and its involvement in aggressive behaviors. Aggress Violent Behav 12:1–15
Hall MD, McLaren L, Brooks RC, Lailvaux SP (2010) Interactions among performance capacities predict male combat outcomes in the field cricket. Funct Ecol 24:159–164
Hammer M (1993) An identified neurone mediates the unconditioned stimulus in associative olfactory learning in honeybees. Nature 366:59–63
Hammer M, Menzel R (1995) Learning and memory in the honey bee (Review). J Neurosci 15:1617–1630
Hauser F, Cazzamali G, Williamson M, Blenau W, Grimmelikhuijzen CJP (2006) A review of neurohormone GPCRs present in the fruitfly Drosophila melanogaster and the honey bee Apis mellifera. Prog Neurobiol 80:1–19
Henry J, Scherman D (1989) Radioligands of the vesicular monoamine transporter and their use as markers of monoamine storage vesicles. Biochem Pharmacol 28:2395–2404
Hofmann HA (1996) The cultural history of Chinese fighting crickets: a contribution not only to the history of biology. [German]. Biologisches Zbl 115:206–213
Hofmann HA, Schildberger K (2001) Assessment of strength and willingness to fight during aggressive encounters in crickets. Anim Behav 62:337–348
Hofmann HA, Stevenson PA (2000) Flight restores fight in crickets. Nature 403:613
Homberg U (2002) Neurotransmitters and neuropeptides in the brain of the locust. Microsc Res Tech 56:189–209
Hoyer SC, Eckart A, Herrel A, Zars T, Fischer SA, Hardie SL et al (2008) Octopamine in male aggression of Drosophila. Curr Biol 18:159–167
Hsu Y, Earley RL, Wolf LL (2005) Modulation of aggressive behaviour by fighting experience: mechanisms and contest outcomes. Biol Rev Camb Philos Soc 81:33–74
Huber F (1955) Sitz und Bedeutung nervöser Zentren für Instinkthandlungen beim Männchen von Gryllus campestris. Z Tierpsychol 12:12–48
Huber F (1960) Untersuchungen über die Funktion des Zentralnervensystems und insbesondere des Gehirnes bei der Fortbewegung und der Lauterzeugung der Grillen. Z Vergleichende Tierphysiologie 44:60–132
Huber F, Moore TE, Loher W (1989) Cricket behavior and neurobiology. Cornell University, New York
Huhman K (2006) Social conflict models: can they inform us about human psychopathology? Horm Behav 50:640–646
Hurd PL (2006) Resource holding potential, subjective resource value, and game theoretical models of aggressiveness signalling. J Theor Biol 241:639–648
Iba M, Nagao T, Urano A (1995) Effects of population density on growth, behavior and levels of biogenic amines in the cricket, Gryllus bimaculatus. Zool Sci 12:695–702
Iwasaki M, Katagiri C (2008) Cuticular lipids and odors induce sex-specific behaviors in the male cricket Gryllus bimaculatus. Comput Biochem Physiol A 149:306–313
Iwasaki M, Delago A, Nishino H, Aonuma H (2006) Effects of previous experience on the agonistic behaviour of male crickets, Gryllus bimaculatus. Zool Sci 23:863–872
Iwasaki M, Nishino H, Delago A, Aonuma H (2007) Effects of NO/cGMP signaling on behavioral changes in subordinate male crickets, Gryllus bimaculatus. Zool Sci 24:860–868
Johnson O, Becnel J, Nichols CD (2009) Serotonin 5-HT(2) and 5-HT(1A)-like receptors differentially modulate aggressive behaviors in Drosophila melanogaster. Neuroscience 158:1292–1300
Judge KA, Bonanno VL (2008) Male weaponry in a fighting cricket. PLoS One 3:e3980
Judge KA, Ting JJ, Schneider J, Fitzpatrick MJ (2010) A lover, not a fighter: mating causes male crickets to lose fights. Behav Ecol Sociobiol 64:1971–1979
Jung SN, Borst A, Haag J (2011) Flight activity alters velocity tuning of fly motion-sensitive neurons. J Neurosci 31:9231–9237
Kemp DJ, Wiklund C (2004) Residency effects in animal contests. Proc R Soc Lond 271:1707–1711
Khazraie K, Campan M (1999) The role of prior agonistic experience in dominance relationships in male crickets Gryllus bimaculatus (Orthoptera: Gryllidae). Behav Processes 44:341–348
Killian KA, Allen JR (2008) Mating resets male cricket aggression. J Insect Behav 21:535–548
Kravitz E, Huber R (2003) Aggression in invertebrates. Curr Opin Neurobiol 13:736–743
Lihoreau M, Brepson L, Rivault C (2009) The weight of the clan: even in insects, social isolation can induce a behavioural syndrome. Behav Processes 82:81–84
Marder E (2012) Neuromodulation of neuronal circuits: back to the future. Neuron 76:1–11
Maynard Smith J, Price GR (1973) The logic of animal conflict. Nature 246:15–18
McFarland DJ, Sibly RM (1975) The behavioural final common path. Philos Trans R Soc B 270:265–293
Mendl M, Paul ES, Chittka L (2011) Animal behaviour: emotions in invertebrates? Curr Biol 21:R463–R465
Miczek KA, Nikulina EM, Takahashi A, Covington HE III, Yap JJ, Boyson CO et al (2011) Gene expression in aminergic and peptidergic cells during aggression and defeat: relevance to violence, depression and drug abuse. Behav Genet 41:787–802
Mizunami M, Unoki S, Mori Y, Hirashima D, Hatano A, Matsumoto Y (2009) Roles of octopaminergic and dopaminergic neurons in appetitive and aversive memory recall in an insect. BMC Biol 7:46
Morris OT, Duch C, Stevenson PA (1999) Differential activation of octopaminergic (DUM) neurones via proprioceptors responding to flight muscle contractions in the locust. J Exp Biol 202:3555–3564
Müller U (1997) The nitric oxide system in insects. Prog Neurobiol 51:363–381
Murakami S, Itoh MT (2001) Effects of aggression and wing removal on brain serotonin levels in male crickets, Gryllus bimaculatus. J Insect Physiol 47:1309–1312
Murakami S, Itoh MT (2003) Removal of both antennae influences the courtship and aggressive behaviors in male crickets. J Neurobiol 57:110–118
Nagao T, Tanimura T (1993) Distribution of biogenic amines in the cricket central nervous system. Anal Biochem 171:33–40
Nagao T, Tanimura T, Shimozawa T (1991) Neurohormonal control of the mating interval in the male cricket, Gryllus bimaculatus DeGeer. J Comp Physiol A 168:159–164
Nelson RJ, Trainor BC (2007) Neural mechanisms of aggression. Nature reviews. Neuroscience 8:536–546
Nilsen SP, Chan YB, Huber R, Kravitz EA (2004) Gender-selective patterns of aggressive behavior in Drosophila melanogaster. Proc Natl Acad Sci U S A 101:12342–12347
Nosil P (2002) Food fights in house crickets, Acheta domesticus, and the effects of body size and hunger level. Can J Zool 80:409–417
O’Connell LA, Hofmann HA (2011) The vertebrate mesolimbic reward system and social behavior network: a comparative synthesis. J Comp Neurol 519:3599–3639
Passamonti L, Crockett MJ, Apergis-Schoute AM, Clark L, Rowe JB, Calder AJ et al (2012) Effects of acute tryptophan depletion on prefrontal-amygdala connectivity while viewing facial signals of aggression. Biol Psychiatry 71:36–43
Payne RJH (1998) Gradually escalating fights and displays: the cumulative assessment model. Anim Behav 56:651–662
Perry CJ, Barron AB (2013) Neural mechanisms of reward in insects. Annu Rev Entomol 58:543–562
Pfennig DW, Reeve HK (1989) Neighbor recognition and context dependent aggression in a solitary wasp, Sphecius speciosus (Hymenoptera, sphecidae). Ethology 80:1–18
Pflüger H, Stevenson P (2005) Evolutionary aspects of octopaminergic systems with emphasis on arthropods. Arthropod Struct Dev 34:379–396
Raichlen DA, Foster AD, Gerdeman GL, Seillier A, Giuffrida A (2011) Wired to run: exercise-induced endocannabinoid signaling in humans and cursorial mammals with implications for the ‘runner’s high’. J Exp Biol 215:1331–1336
Rillich J, Stevenson PA (2011) Winning fights induces hyperaggression via the action of the biogenic amine octopamine in crickets. PLoS One 6:e28891
Rillich J, Stevenson PA (2014) A fighter’s comeback: dopamine is necessary for recovery of aggression after social defeat. Horm Behav 66:696–704
Rillich J, Stevenson PA (2015) Releasing stimuli and aggression in crickets: octopamine promotes escalation and maintenance but not initiation. Front Behav Neurosci 9(95):1–11
Rillich J, Schildberger K, Stevenson PA (2007) Assessment strategy of fighting crickets revealed by manipulating information exchange. Anim Behav 74:823–836
Rillich J, Buhl E, Schildberger K, Stevenson PA (2009) Female crickets are driven to fight by the male courting and calling songs. Anim Behav 77:737–742
Rillich J, Schildberger K, Stevenson PA (2011) Octopamine and occupancy – an aminergic mechanism for intruder-resident aggression in crickets. Proc R Soc Lond B 278:1873–1880
Rillich J, Stevenson PA, Pflüger HJ (2013) Flight and walking in locusts – cholinergic co-activation, temporal coupling and its modulation by biogenic amines. PLoS One 8(5):e62899
Rodriguez-Munoz R, Bretman A, Tregenza T (2011) Guarding males protect females from predation in a wild insect. Curr Biol 21:1716–1719
Roeder T (1999) Octopamine in invertebrates. Prog Neurobiol 59:533–561
Roeder T, Degen J, Gewecke M (1998) Epinastine, a highly specific antagonist of insect neuronal octopamine receptors. Eur J Pharmacol 349:171–177
Rogers SM, Matheson T, Sasaki K, Kendrick K, Simpson SJ, Burrows M (2004) Substantial changes in central nervous system neurotransmitters and neuromodulators accompany phase change in the locust. J Exp Biol 207:3603–3617
Rutte C, Taborsky M, Brinkhof MW (2006) What sets the odds of winning and losing? Trends Ecol Evol 21:16–21
Sakura M, Aonuma H (2013) Aggressive behavior in the antennectomized male cricket Gryllus bimaculatus. J Exp Biol 216:2221–2228
Schöneich S, Schildberger K, Stevenson PA (2011) Neuronal organization of a fast-mediating cephalo-thoracic pathway for antennal-tactile information in the cricket. J Comp Neurol 519:1677–1690
Schröter U, Malun D, Menzel R (2007) Innervation pattern of suboesophageal ventral unpaired median neurones in the honeybee brain. Cell Tissue Res 327:647–667
Schwärzel M, Monastirioti M, Scholz H, Friggi-Grelin F, Birman S, Heisenberg M (2003) Dopamine and octopamine differentiate between aversive and appetitive olfactory memories in Drosophila. J Neurosci 23:10495–10502
Simmons LW (1986) Inter-male competition and mating success in the field cricket, Gryllus bimaculatus (de Geer). Anim Behav 34:567–579
Simpson SJ, Stevenson PA (2015) Neuromodulation of social behaviour in insects. In: Canli T (ed) The Oxford handbook of molecular psychology. Oxford University Press, Oxford, pp 27–51
Simpson JS, Sword GA (2009) Phase polyphenism in locusts: mechanisms, population consequences, adaptive significance and evolution. In: Whitman DW, Ananthakrishnan TN (eds) Phenotypic plasticity of insects, mechanisms and consequences. Science Publishers, Enfield, pp 147–189
Sokolowski MB (2010) Social interactions in “simple” model systems. Neuron 65:780–794
Sombati S, Hoyle G (1984) Generation of specific behaviors in a locust by local release into neuropil of the natural neuromodulator octopamine. J Neurobiol 15:481–506
Spörhase-Eichmann U, Vullings HGB, Buijs RM, Hörner M (1992) Octopamine-immunoreactive neurones in the central nervous system of the cricket Gryllus bimaculatus. Cell Tissue Res 268:287–304
Staudacher E, Schildberger K (1998) Gating of sensory responses of descending brain neurones during walking in crickets. J Exp Biol 201:559–572
Staudacher EM, Gebhardt M, Dürr V (2005) Antennal movements and mechanoreception: neurobiology of active tactile sensors. Adv Insect Physiol 32:49–205
Stevenson PA, Kutsch W (1987) A reconsideration of the central pattern generator concept for locust flight. J Comp Physiol A 161:115–129
Stevenson PA, Rillich J (2012) The decision to fight or flee – insights into underlying mechanism in crickets. Front Neurosci 6(118):1–12
Stevenson PA, Rillich J (2013) Isolation associated aggression in crickets is a result of recovery from social subjugation. Plos ONE 8(9):e74965
Stevenson PA, Rillich J (2015) Adding up the odds – nitric oxide underlies the decision to flee and post conflict depression. Sci Adv 1:e1500060
Stevenson PA, Schildberger K (2013) Mechanisms of experience dependant control of aggression in crickets. Curr Opin Neurobiol 23:318–323
Stevenson PA, Spörhase-Eichmann U (1995) Localization of octopaminergic neurons in insects. Comp Biochem Physiol B 11:203–215
Stevenson PA, Hofmann HA, Schoch K, Schildberger K (2000) The fight and flight responses of crickets depleted of biogenic amines. J Neurobiol 43:107–120
Stevenson PA, Dyakonova VE, Rillich J, Schildberger K (2005) Octopamine and experience-dependent modulation of aggression in crickets. J Neurosci 25:1431–1441
Tachon G, Murray A-M, Gray DA, Cade WH (1999) Agonistic displays and the benefits of fighting in the field cricket, Gryllus bimaculatus. J Insect Behav 12:533–543
Tops M, Russo S, Boksem MA, Tucker DM (2009) Serotonin: modulator of a drive to withdraw. Brain Cogn 71:427–436
Vrontou E, Nilsen SP, Demir E, Kravitz EA, Dickson BJ (2006) Fruitless regulates aggression and dominance in Drosophila. Nat Neurosci 9:1469–1471
Wada-Katsumata A, Yamaoka R, Aonuma H (2011) Social interactions influence dopamine and octopamine homeostasis in the brain of the ant Formica japonica. J Exp Biol 214:1707–1713
Wang L, Anderson DJ (2010) Identification of an aggression-promoting pheromone and its receptor neurons in Drosophila. Nature 463:227–231
Watanabe T, Aonuma H (2012) Identification and expression analyses of a novel serotonin receptor gene, 5-HT2β, in the field cricket, Gryllus bimaculatus. Acta Biol Hung 63:58–62
Watanabe T, Sadamoto H, Aonuma H (2011) Identification and expression analysis of the genes involved in serotonin biosynthesis and transduction in the field cricket Gryllus bimaculatus. Insect Mol Biol 20:619–635
Wingfield JC, Hegner RE, Dufty AM Jr, Ball GF (1990) The challenge hypothesis—theoretical implications for patterns of testosterone secretion, mating systems, and breeding strategies. Am Nat 136:829–846
Yano S, Ikemoto Y, Aonuma H, Asama H (2012) Forgetting curve of cricket, Gryllus bimaculatus, derived by using serotonin hypothesis. Robot Auton Syst 60:722–728
Yoritsune A, Aonuma H (2012) The anatomical pathways for antennal sensory information in the central nervous system of the cricket, Gryllus bimaculatus. Invert Neurosci. doi:10.1007/s10158-012-0137-6
Yurkovic A, Wang, Basu C, Kravitz EA (2006) Learning and memory associated with aggression in Drosophila melanogaster. Proc Natl Acad Sci U S A 103:17519–17524
Zhou C, Rao Y, Rao Y (2008) A subset of octopaminergic neurons are important for Drosophila aggression. Nat Neurosci 11:1059–1067
Zorovic M, Hedwig B (2012) Descending brain neurons in the cricket Gryllus bimaculatus (de Geer): auditory responses and impact on walking. J Comp Physiol A 199:25–34
Acknowledgements
We thank the German Research Council (DFG) for generous funding (DFG Research Group, FOR 1363, grant STE 714/4-1).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer Japan KK
About this chapter
Cite this chapter
Stevenson, P.A., Rillich, J. (2017). Neuromodulators and the Control of Aggression in Crickets. In: Horch, H., Mito, T., Popadić, A., Ohuchi, H., Noji, S. (eds) The Cricket as a Model Organism. Springer, Tokyo. https://doi.org/10.1007/978-4-431-56478-2_12
Download citation
DOI: https://doi.org/10.1007/978-4-431-56478-2_12
Published:
Publisher Name: Springer, Tokyo
Print ISBN: 978-4-431-56476-8
Online ISBN: 978-4-431-56478-2
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)