Abstract
More than fifty years ago, a number of studies reported the reduction of neuron numbers related to brain aging. However, later studies have concluded that the neuronal loss due to aging is limited to specific regions of the nervous system and its significance is irrelevant in both humans and non-human mammals. Instead, several other mechanisms that may underlie brain aging have attracted attention in the field of neuroscience research. Namely, some papers have indicated the relationship between memory impairment and decline in adult neurogenesis in the hippocampus during aging. It has also been reported that abnormalities of oligodendrogenesis in the mature brain may be involved in age-related cognitive decline. We herein briefly review recent findings on age-related changes in adult neurogenesis and oligodendrogenesis, and discuss their functional significance from the view point of topography of the hippocampus. Namely, the hippocampus has shown to be structurally and functionally differentiated along the longitudinal and transverse axes. In the rodent brain, the dorsal (septal) hippocampus is involved in cognition, learning, and memory, while the ventral (temporal) hippocampus contributes to regulation of emotion, mood, and anxiety. Nevertheless, the question of how topographic differentiation of the hippocampus might be affected by aging still remains largely unanswered. Our latest studies have shown that the waning of adult neurogenesis and oligodendrogenesis during aging is more relevant in the ventral hippocampus than in the dorsal hippocampus. We therefore hypothesize that the ventral-dominant decline in hippocampal neurogenesis and oligodendrogenesis may partly explain why major depression frequently precedes dementia in elderly people. These findings provide new insights into aging of the hippocampus.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
More than fifty years ago, much attention was given to changes in neuron numbers related to brain aging , and many papers reported the significant neuron loss occurred in the aged brain (Brody 1955). However, later studies have concluded that neuronal loss due to aging is limited to specific regions of the nervous system, and is irrelevant (probably no more than 10 %) in both humans and non-human animals (Curcio and Coleman 1982; Peters and Sethares 1993; Pakkenberg and Gundersen 1997). Considering that the variance of neuron numbers in humans with normal cognitive function is large, it is unlikely that a 10 % loss in neurons that is not ubiquitous is the significant factor causing various symptoms that often accompany normal aging (Pannese 2011). To date, several other mechanisms causing brain aging have attracted attention in the field of neuroscience research. For instance, there is a substantial amount of data showing that the rate of adult neurogenesis in the hippocampus radically wanes during aging (Merkley et al. 2014). It has also been suggested that age-related decline in adult neurogenesis is an important factor influencing cognitive performance (Artegiani and Calegari 2012). Similar to neurogenesis, oligodendrogenesis and white matter homeostasis might also be affected by aging (Sim et al. 2002; Miyamoto et al. 2013). We herein briefly review recent findings on age-related changes in adult neurogenesis and oligodendrogenesis , and discuss their functional significance from the view point of topography of the hippocampus .
2 Differentiation of the Hippocampus
The rodent hippocampus has an elongated shape with its major axis extending in a C-shaped fashion from the septal nuclei of the basal forebrain to the temporal lobe. Importantly, the structure and function of the rodent hippocampus is differentiated along the longitudinal (dorsoventral) and transverse axes (Fanselow and Dong 2010). Earlier anatomical studies have reported the topographic differentiation of the hippocampus along the dorsoventral axis (Gaarskjaer 1978). The dorsal hippocampus sends massive projections to the retrosplenial cortex and mammillary complex (Ishizuka 2001; van Groen and Wyss 2003). The ventral hippocampus has intimate reciprocal connections with the amygdala and strong projections to the nucleus accumbens (Pitkänen et al. 2000). The dorsal dentate gyrus (DG) receives afferents both from the lateral and medial area of the entorhinal cortex, whereas the ventral DG receives projections from the medial area of the entorhinal cortex (Witter et al. 1989). In a series of our studies, we have demonstrated the existence of dorsoventral differences in the cellular architecture of the mouse hippocampus (Jinno and Kosaka 2006, 2010). Functional differentiation along the dorsoventral axis has also been reported in the past two decades. Injuries to the dorsal hippocampus impair spatial learning (Moser et al. 1993), but lesions of the ventral hippocampus affect anxiety-related behavior, and have no effect on spatial learning (Bannerman et al. 2003). Genes expressed in the dorsal hippocampus are associated with the brain regions related to cognitive information-processing, while genes expressed in the ventral hippocampus are associated with regions involved in emotional behaviors (Dong et al. 2009). To date, it has been widely accepted that the dorsal hippocampus plays a preferential role in cognition, learning and memory , while the ventral hippocampus is involved in regulation of emotion, mood, and anxiety. Interestingly, the differentiation of the hippocampus is evolutionarily conserved in rodents, monkeys (Colombo et al. 1998) and humans (Small et al. 2011).
3 Topography of Adult Hippocampal Neurogenesis
Throughout adulthood, new granule cells are continuously generated in the subgranular zone of the DG (Altman and Das 1967; Kaplan and Hinds 1977; Cameron et al. 1993). Recent studies indicated that adult neurogenesis is involved in various hippocampal functions (Balu and Lucki 2009). Drug-induced inhibition of cell proliferation impaired learning of a hippocampus -dependent spatial memory task (Shors et al. 2001). Addition and removal of adult-born granule cells in the DG might influence spatial learning and memory (Drapeau et al. 2003; Dupret et al. 2007; Farioli-Vecchioli et al. 2008).
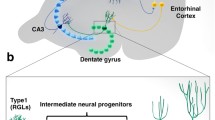
In our recent study (Jinno 2011a), we examined the topography of adult neurogenesis along the longitudinal (dorsal vs. ventral) and transverse (suprapyramidal vs. infrapyramidal) axes of the DG of young adult mice using endogenous neurogenesis markers (Fig. 14.1): brain lipid binding protein (BLBP), doublecortin (DCX), calretinin (CR), proliferation cell nuclear antigen (PCNA) and Ki-67. BLBP belongs to the fatty acid binding protein family (Feng et al. 1994), and is expressed in radial-glia like progenitors and intermediate progenitors, i.e., neural stem cell s (NSCs). DCX is a microtubule-associated phosphoprotein (Gleeson et al. 1999; Francis et al. 1999), which labels both lineage-restricted neuronal progenitors and immature granule cells, i.e., neural lineage cells (NLCs) (Brown et al. 2003; Rao and Shetty 2004). CR belongs to the EF-hand calcium-binding protein family, and is transiently expressed in granule cells at early postmitotic stage (Liu et al. 1996). PCNA (Celis and Celis 1985) and Ki-67 (Gerdes et al. 1984) can label dividing cells. Using combinations of these markers, the cells at specific stages of adult neurogenesis can be identified (Kempermann et al. 2004).
Topography of the rodent hippocampus and endogenous markers of the adult neurogenesis . (a) The hippocampus (shaded) can be divided into the dorsal, middle and ventral regions according to the longitudinal axis. (b) Inverted image of 4′,6-Diamidino-2-phenylindole, dihydrochloride (DAPI)-stained transverse section of the dorsal DG. Arrowhead indicates the crescent. (c) Stages and endogenous markers of the adult neurogenesis in the hippocampus. Scale bar in b = 200 μm (applies to b). Amy amygdala, CC corpus callosum, dDGi infrapyramidal blade in dorsal DG, dDGs suprapyramidal blade in dorsal DG, FX fornix (Modified and reproduced from Jinno (2011a), with permission of the publisher)
It was reported that adult neurogenesis may be more active in the dorsal hippocampus than in the ventral hippocampus (Snyder et al. 2009). In adult male gerbils, the number of 7-day-old bromodeoxyuridine (BrdU)-labeled granule cells was larger in the dorsal DG than in the ventral DG (Dawirs et al. 1998). Similarly, the number of 14-day-old BrdU-labeled granule cells was larger in the dorsal hippocampus than in the ventral hippocampus in adult male C57BL/6J mice (Ferland et al. 2002). In agreement with these studies, our recent study has shown that the numerical densities (NDs) of DCX+ NLCs and BLBP+ NSCs are significantly higher in the dorsal DG than in the ventral DG in young adult male C57BL/6J mice (Jinno 2011a). Considering the functional differentiation of the hippocampus , these results suggest that newly generated granule cells may play different roles in regulation of cognition (dorsal) and emotion (ventral).
Currently, functional dissociations along the transverse axis of the hippocampus are less intensively examined, but earlier studies reported some structural differences between two blades in the DG. For instance, the mossy fibers from the suprapyramidal blade cross directly through the stratum radiatum to reach the distal part of the stratum lucidum, whereas those arising from the infrapyramidal blade travel through the hilus and contact the proximal part of CA3 area in the rat (Claiborne et al. 1986). With regard to the inter-blade differences in the adult neurogenesis , Ambrogini et al. (2000) showed that the number of 15-day-old BrdU+ granule cells was larger in the suprapyramidal blade than in the infrapyramidal blade in rats. Similarly, we have found that the NDs of DCX+ NLCs are relatively higher in the suprapyramidal blade in the dorsal DG (Jinno 2011a). Further research is necessary to tie these findings together and elucidate the functional significance of inter-blade difference in adult neurogenesis .
4 Age-Related Changes in Adult Neurogenesis
There is a substantial amount of data showing that the rate of new cell production in the hippocampus radically wanes during aging (Seki and Arai 1995; Cameron and McKay 1999). However, the mechanism underlying age-related decline in adult neurogenesis is still controversial. Namely, Alonso (2001) showed that the reduction of neurogenesis in aged rats was attributable to the decline in proliferation of primary progenitors. Hattiangady and Shetty (2008) also reported that aging did not alter the number of primary progenitors, and suggested that age-related decline might be an outcome of increased quiescence of progenitors in the neurogenic region of the rat hippocampus . By contrast, Olariu et al. (2007) claimed that the decreased neurogenesis in aged rats was attributable to loss of primary progenitor cells. Aizawa et al. (2011) reported that decline in primary progenitors defined by Sox2, GFAP, and BLBP expression was specific for aged primates, and there were no alterations in the number of these cells in aged (2-year-old) ICR mice. Walter et al. (2011) reported that age-related reduction in proliferation was not only caused by a general reduction in total number of progenitor subtypes but also by a subtype-specific alteration of the proliferation rate.
In our recent study (Jinno 2011b), we examined the age-related differences in adult neurogenesis between young adult (2-month-old) and middle-aged (10-month-old) mice using endogenous markers (see, Fig. 14.2). The age-related reductions in BLBP+ NSCs were significantly larger in the ventral DG (76 % decrease) than in the dorsal DG (56 % decrease). The age-related reductions in DCX+ NLCs were more drastic than those of NSCs accompanying with a similar dorsoventral gradient: the ventral DG (95 % decrease), dorsal DG (91 % decrease). In the field of geriatric psychiatry, major depressive disorder and dementia are common conditions in old age, and frequently occur concurrently (Korczyn and Halperin 2009). These two clinical entities have a very complicated relationship, and accurate mechanisms underlying their co-occurrence are largely unclear. Some studies hypothesize that depressive disorder is a risk factor for developing dementia (Kessing and Nilsson 2003; Ownby et al. 2006), and suggest that depression is an early prodromal phase of dementia state (Schweitzer et al. 2002). Our findings indicate that hippocampal neurogenesis wanes faster in the ventral hippocampus than in the dorsal hippocampus during aging . Interestingly, exposure to chronic mild stress results in decreased cell proliferation in the ventral but not in the dorsal hippocampus (Jayatissa et al. 2006). Chronic treatment with agomelatin, an antidepressant, increases neurogenesis only in the ventral DG (Banasr et al. 2006). Together, these findings provide some key to understand why depression frequently precedes dementia in aged people. Future studies addressing this issue will inform us on how age-related alterations in neurogenesis are involved in late onset depression and dementia.
Schematic illustration of the age-related changes in adult neurogenesis and oligodendrogenesis in the rodent hippocampus . (a) The dorsal hippocampus is mainly involved in cognition, learning and memory . In this region, the number of NSCs and NLCs halves from YA (young adult) to MA (middle-aged). The number of quiescent OPCs decline with age, and the fraction of mitotic OPCs remains invariant during aging . The number of residual OLs declines with age, while the fraction of newly generated OLs increases during aging. As a result, the number of OLs remain unchanged. (b) The hippocampus contributes to the regulation of emotion, mood and anxiety. The number of NSCs and NLCs drops sharply from YA to MA. The number of quiescent OPCs declines with age, and the fraction of mitotic OPCs remains invariant during aging. The number of residual OLs declines with age, and the fraction of newly generated OLs remains unchanged (Modified and reproduced from Jinno (2011b), with permission of the publisher)
At this time, only a few attempts have been made at the functional significance of age-related changes in transverse differentiation of the DG. In our study, we have reported that there were no inter-blade differences in neurogenesis in middle-aged (10-month-old) mice (Jinno 2011b), while the number of BLBP+ NLCs and DCX+ NLCs were significantly higher in the suprapyramidal blade than in infrapyramidal blade in young adult (2-month-old) mice (Jinno 2011a). In this regard, there is an interesting report showing that deposition and maturation of granule cells begin near the lateral tip of the suprapyramidal blade and proceed into the infrapyramidal blade, establishing the suprapyramidal to infrapyramidal morphogenic gradient in the DG (Angevine 1965). These results indicate that dentate neurogenesis might be more active in the suprapyramidal blade than in the infrapyramidal blade only during adolescence and young adulthood, and also suggest that larger number of new granule cells in the suprapyramidal blade could be involved in the higher cognitive performance of young animals.
5 Oligodendrogenesis and Myelin Homeostasis
Oligodendrocytes (OLs) synthesize myelin, which is required for fast saltatory conduction of nerve impulses. The majority of myelinating OLs are born in the early postnatal period by differentiation of oligodendrocyte precursor cells (OPCs). Recent evidence shows that impaired paranode structure and function can impact neural circuitry, leading to downstream effects related to emotion and potentially to mood regulation in human psychiatric disorders (Edgar and Sibille 2012). Histological analysis using Kluver–Barrera staining method revealed that the staining intensity of deep white matter in the dorsolateral prefrontal cortex was significantly less intense in subjects who suffered from major depression (Regenold et al. 2007). A decrease in OL density has been reported in the frontopolar cortex of major depressive disorder subjects (Hayashi et al. 2011).
In the healthy adult brain, OPCs continue to divide and generate new OLs (Richardson et al. 2011). It has been shown that OPCs express NG2 proteoglycan (so they are also known as NG2 cells) and the platelet-derived growth factor receptor-alpha (PDGFαR). NG2 and PDGFαR play a critical role in proliferation , migration and survival of OPCs (Noble et al. 1988; Barres et al. 1993; Hill et al. 2013; Binamé et al. 2013). Recent studies have indicated that OPCs are not just progenitors, but are also involved in regulation of neuronal circuits, because these cells receive synaptic inputs from neurons and respond to neurotransmitters released at synapses (Bergles et al. 2000; Wigley et al. 2007). Particularly, OPCs have AMPA-type glutamate receptors, which are activated by neural activity (Lin et al. 2005). In addition, OPCs sense fine changes in extracellular K+ concentrations during physiological neuronal activity (Maldonado et al. 2013). The authors suggest that OPCs possibly remove the excess K+ caused by neuronal K+ efflux at specific sites devoid of astrocytes via Kir4.1 channels. Moreover, OPCs are considered to release soluble factors, which promote neuronal survival, maintain axonal structure, and support synaptic plasticity (Wilkins et al. 2003; Sun et al. 2013). It is conceivable that OPCs sense the “state of health” of their partner neurons over the neuron-glial synapse and respond accordingly by release of neuroprotective substances (Sakry et al. 2011).
6 Topography of Age-Related Changes in Hippocampal Oligodendrogenesis
Until now, many papers reported the involvement of microglia and astrocytes in brain aging (Bronson et al. 1993; Sheng et al. 1996; Wu et al. 2005). Recently, age-related changes in OLs have also been well documented. For instance, several studies have shown that abnormalities of OLs and myelin may be involved in age-related cognitive decline (Peters and Kemper 2012; Kohama et al. 2012). The NDs of OLs in the rat hippocampus showed a significant aging -dependent reduction (Tanaka et al. 2005). The intensity of immunostaining for 2′,3′-cyclic nucleotide 3′-phosphodiesterase (a marker of OLs) in the rat hippocampus declined with age (Hayakawa et al. 2007). The number of OLs in the rat subcortical white matter also showed an aging -dependent reduction (Chen et al. 2011). Interestingly, the number of OLs in the mouse anterior commissure began to decline between 9 and 12 months and remained fairly low between 15 and 22 months, before rising sharply to above the 9 month level between 22 and 25 months and thereafter remaining constant (Sturrock 1987). In rhesus monkeys, the number of OLs in the optical nerve showed an age-dependent increase (Sandell and Peters 2002), while there were no significant age-related changes in the number of OLs in the occipital and prefrontal cortices (Peters and Sethares 2002).
In our recent study (Yamada and Jinno 2014), we estimated the age-related changes in oligodendrogenesis of young adult (2-month-old) and middle-aged (10-month-old) mouse hippocampi. To identify OPCs and OLs, we used a set of molecular markers, oligodendrocyte lineage transcription factor (Olig2) and PDGFαR. Intracellular dye injection shows that PDGFαR+/Olig2+ cells and PDGFαR-/Olig2+ cells can be defined as OPCs and OLs, respectively (Fig. 14.3). The quantitative analysis showed that the number of OLs declined with age in the ventral hippocampus , but they were not compromised in the dorsal hippocampus (Fig. 14.2). In this regard, it is necessary to consider two possibilities here. The first is the dorsoventral difference in death of OLs. The vulnerability of OLs increases with increasing age at differentiation as later-differentiating cells myelinate increasing the number of axonal segments (Bartzokis 2004). We thus hypothesized that mature OLs in the hippocampus might be more susceptible to aging in the ventral than the dorsal region. The second is the dorsoventral differences in production of OLs. Differently from neurogenesis , previous studies have shown the rather controversial effects of aging on oligodendrogenesis . Namely, in the murine spinal cord, oligodendrogenesis was not only preserved, but it also increased during aging (Lasiene et al. 2009). In the rostral migratory stream of mice, the number of proliferative OPCs and new OLs remained unchanged during aging (Capilla-Gonzalez et al. 2013). Here we observed that the number of BrdU+ mitotic OPCs in the Ammon’s horn were not compromised with age both in the dorsal and ventral hippocampus . It should also be noted that the number of BrdU+ newly generated OLs in the Ammon’s horn significantly increased with age in the dorsal hippocampus , but remained unchanged in the ventral hippocampus . Together, these findings suggest that the number of OLs in the dorsal Ammon’s horn may be compensatory maintained by increased generation of OLs.
Identification of OPCs and OLs by intracellular labeling and immunostaining for PDGFαR and Olig2 in the hippocampus . (a, b) Intracellular injection of lucifer yellow (LY) into OPC (a) and OL (b) in the mouse hippocampus. (c) An Olig2+/PDGFαR+ OPC shows short multi-branched processes. (d) An Olig2+/PDGFαR- OL shows long extended processes with ramified structure. Scale bars in b = 20 μm (applies to a and b), in d 3 = 10 μm (applies to c 1–3 and d 1–3) (Modified and reproduced from Yamada and Jinno (2014), with permission of the publisher)
7 Conclusion
Despite the increased number of publications in the field of gerontology, age-related changes in the topography of the hippocampus still remains largely unanswered. As this review summarizes here, the waning of adult neurogenesis and oligodendrogenesis during aging is more relevant in the ventral hippocampus than in the dorsal hippocampus . Because the ventral hippocampus mainly contributes to regulation of emotion, while the dorsal hippocampus has a preferential role in memory, these findings may provide some key to understanding various psychiatric problems seen in elderly people without dementia.
References
Aizawa K, Ageyama N, Terao K, Hisatsune T (2011) Primate-specific alterations in neural stem/progenitor cells in the aged hippocampus. Neurobiol Aging 32:140–150
Alonso G (2001) Proliferation of progenitor cells in the adult rat brain correlates with the presence of vimentin-expressing astrocytes. Glia 34:253–266
Altman J, Das GD (1967) Postnatal neurogenesis in the guinea-pig. Nature 214:1098–1101
Ambrogini P, Cuppini R, Cuppini C, Ciaroni S, Cecchini T, Ferri P, Sartini S, Del Grande P (2000) Spatial learning affects immature granule cell survival in adult rat dentate gyrus. Neurosci Lett 286:21–24
Angevine JB Jr (1965) Time of neuron origin in the hippocampal region. An autoradiographic study in the mouse. Exp Neurol Suppl 2:1–70
Artegiani B, Calegari F (2012) Age-related cognitive decline: can neural stem cells help us? Aging (Albany NY) 4:176–186
Balu DT, Lucki I (2009) Adult hippocampal neurogenesis: regulation, functional implications, and contribution to disease pathology. Neurosci Biobehav Rev 33:232–252
Banasr M, Soumier A, Hery M, Mocaër E, Daszuta A (2006) Agomelatine, a new antidepressant, induces regional changes in hippocampal neurogenesis. Biol Psychiatry 59:1087–1096
Bannerman DM, Grubb M, Deacon RM, Yee BK, Feldon J, Rawlins JN (2003) Ventral hippocampal lesions affect anxiety but not spatial learning. Behav Brain Res 139:197–213
Barres BA, Schmid R, Sendtner M, Raff MC (1993) Multiple extracellular signals are required for long-term oligodendrocyte survival. Development 118:283–295
Bartzokis G (2004) Age-related myelin breakdown: a developmental model of cognitive decline and Alzheimer’s disease. Neurobiol Aging 25:5–18
Bergles DE, Roberts JD, Somogyi P, Jahr CE (2000) Glutamatergic synapses on oligodendrocyte precursor cells in the hippocampus. Nature 405:187–191
Binamé F, Sakry D, Dimou L, Jolivel V, Trotter J (2013) NG2 regulates directional migration of oligodendrocyte precursor cells via Rho GTPases and polarity complex proteins. J Neurosci 33:10858–10874
Brody H (1955) Organization of the cerebral cortex. III. A study of aging in the human cerebral cortex. J Comp Neurol 102:511–516
Bronson RT, Lipman RD, Harrison DE (1993) Age-related gliosis in the white matter of mice. Brain Res 609:124–128
Brown JP, Couillard-Després S, Cooper-Kuhn CM, Winkler J, Aigner L, Kuhn HG (2003) Transient expression of doublecortin during adult neurogenesis. J Comp Neurol 467:1–10
Cameron HA, McKay RD (1999) Restoring production of hippocampal neurons in old age. Nat Neurosci 2:894–897
Cameron HA, Woolley CS, McEwen BS, Gould E (1993) Differentiation of newly born neurons and glia in the dentate gyrus of the adult rat. Neuroscience 56:337–344
Capilla-Gonzalez V, Cebrian-Silla A, Guerrero-Cazares H, Garcia-Verdugo JM, Quiñones-Hinojosa A (2013) The generation of oligodendroglial cells is preserved in the rostral migratory stream during aging. Front Cell Neurosci 7:147
Celis JE, Celis A (1985) Cell cycle-dependent variations in the distribution of the nuclear protein cyclin proliferating cell nuclear antigen in cultured cells: subdivision of S phase. Proc Natl Acad Sci U S A 82:3262–3266
Chen L, Lu W, Yang Z, Yang S, Li C, Shi X, Tang Y (2011) Age-related changes of the oligodendrocytes in rat subcortical white matter. Anat Rec (Hoboken) 294:487–493
Claiborne BJ, Amaral DG, Cowan WM (1986) A light and electron microscopic analysis of the mossy fibers of the rat dentate gyrus. J Comp Neurol 246:435–458
Colombo M, Fernandez T, Nakamura K, Gross CG (1998) Functional differentiation along the anterior-posterior axis of the hippocampus in monkeys. J Neurophysiol 80:1002–1005
Curcio CA, Coleman PD (1982) Stability of neuron number in cortical barrels of aging mice. J Comp Neurol 212:158–172
Dawirs RR, Hildebrandt K, Teuchert-Noodt G (1998) Adult treatment with haloperidol increases dentate granule cell proliferation in the gerbil hippocampus. J Neural Transm 105:317–327
Dong HW, Swanson LW, Chen L, Fanselow MS, Toga AW (2009) Genomic-anatomic evidence for distinct functional domains in hippocampal field CA1. Proc Natl Acad Sci U S A 106:11794–11799
Drapeau E, Mayo W, Aurousseau C, Le Moal M, Piazza PV, Abrous DN (2003) Spatial memory performances of aged rats in the water maze predict levels of hippocampal neurogenesis. Proc Natl Acad Sci U S A 100:14385–14390
Dupret D, Fabre A, Döbrössy MD, Panatier A, Rodríguez JJ, Lamarque S, Lemaire V, Oliet SH, Piazza PV, Abrous DN (2007) Spatial learning depends on both the addition and removal of new hippocampal neurons. PLoS Biol 5:e214
Edgar N, Sibille E (2012) A putative functional role for oligodendrocytes in mood regulation. Transl Psychiatry 2:e109
Fanselow MS, Dong HW (2010) Are the dorsal and ventral hippocampus functionally distinct structures? Neuron 65:7–19
Farioli-Vecchioli S, Saraulli D, Costanzi M, Pacioni S, Cinà I, Aceti M, Micheli L, Bacci A, Cestari V, Tirone F (2008) The timing of differentiation of adult hippocampal neurons is crucial for spatial memory. PLoS Biol 6:e246
Feng L, Hatten ME, Heintz N (1994) Brain lipid-binding protein (BLBP): a novel signaling system in the developing mammalian CNS. Neuron 12:895–908
Ferland RJ, Gross RA, Applegate CD (2002) Differences in hippocampal mitotic activity within the dorsal and ventral hippocampus following flurothyl seizures in mice. Neurosci Lett 332:131–135
Francis F, Koulakoff A, Boucher D, Chafey P, Schaar B, Vinet MC, Friocourt G, McDonnell N, Reiner O, Kahn A, McConnell SK, Berwald-Netter Y, Denoulet P, Chelly J (1999) Doublecortin is a developmentally regulated, microtubule-associated protein expressed in migrating and differentiating neurons. Neuron 23:247–256
Gaarskjaer FB (1978) Organization of the mossy fiber system of the rat studied in extended hippocampi. I. Terminal area related to number of granule and pyramidal cells. J Comp Neurol 178:49–72
Gerdes J, Lemke H, Baisch H, Wacker HH, Schwab U, Stein H (1984) Cell cycle analysis of a cell proliferation-associated human nuclear antigen defined by the monoclonal antibody Ki-67. J Immunol 133:1710–1715
Gleeson JG, Lin PT, Flanagan LA, Walsh CA (1999) Doublecortin is a microtubule-associated protein and is expressed widely by migrating neurons. Neuron 23:257–271
Hattiangady B, Shetty AK (2008) Aging does not alter the number or phenotype of putative stem/progenitor cells in the neurogenic region of the hippocampus. Neurobiol Aging 29:129–147
Hayakawa N, Kato H, Araki T (2007) Age-related changes of astorocytes, oligodendrocytes and microglia in the mouse hippocampal CA1 sector. Mech Ageing Dev 128:311–316
Hayashi Y, Nihonmatsu-Kikuchi N, Yu X, Ishimoto K, Hisanaga SI, Tatebayashi Y (2011) A novel, rapid, quantitative cell-counting method reveals oligodendroglial reduction in the frontopolar cortex in major depressive disorder. Mol Psychiatry 16:1155–1158
Hill RA, Patel KD, Medved J, Reiss AM, Nishiyama A (2013) NG2 cells in white matter but not gray matter proliferate in response to PDGF. J Neurosci 33:14558–14566
Ishizuka N (2001) Laminar organization of the pyramidal cell layer of the subiculum in the rat. J Comp Neurol 435:89–110
Jayatissa MN, Bisgaard C, Tingström A, Papp M, Wiborg O (2006) Hippocampal cytogenesis correlates to escitalopram-mediated recovery in a chronic mild stress rat model of depression. Neuropsychopharmacology 31:2395–2404
Jinno S (2011a) Topographic differences in adult neurogenesis in the mouse hippocampus: a stereology-based study using endogenous markers. Hippocampus 21:467–480
Jinno S (2011b) Decline in adult neurogenesis during aging follows a topographic pattern in the mouse hippocampus. J Comp Neurol 519:451–466
Jinno S, Kosaka T (2006) Cellular architecture of the mouse hippocampus: a quantitative aspect of chemically defined GABAergic neurons with stereology. Neurosci Res 56:229–245
Jinno S, Kosaka T (2010) Stereological estimation of numerical densities of glutamatergic principal neurons in the mouse hippocampus. Hippocampus 20:829–840
Kaplan MS, Hinds JW (1977) Neurogenesis in the adult rat: electron microscopic analysis of light radioautographs. Science 197:1092–1094
Kempermann G, Jessberger S, Steiner B, Kronenberg G (2004) Milestones of neuronal development in the adult hippocampus. Trends Neurosci 27:447–452
Kessing LV, Nilsson FM (2003) Increased risk of developing dementia in patients with major affective disorders compared to patients with other medical illnesses. J Affect Disord 73:261–269
Kohama SG, Rosene DL, Sherman LS (2012) Age-related changes in human and non-human primate white matter: from myelination disturbances to cognitive decline. Age (Dordr) 34:1093–1110
Korczyn AD, Halperin I (2009) Depression and dementia. J Neurol Sci 283:139–142
Lasiene J, Matsui A, Sawa Y, Wong F, Horner PJ (2009) Age-related myelin dynamics revealed by increased oligodendrogenesis and short internodes. Aging Cell 8:201–213
Lin SC, Huck JH, Roberts JD, Macklin WB, Somogyi P, Bergles DE (2005) Climbing fiber innervation of NG2-expressing glia in the mammalian cerebellum. Neuron 46:773–785
Liu Y, Fujise N, Kosaka T (1996) Distribution of calretinin immunoreactivity in the mouse dentate gyrus. I. General description. Exp Brain Res 108:389–403
Maldonado PP, Vélez-Fort M, Levavasseur F, Angulo MC (2013) Oligodendrocyte precursor cells are accurate sensors of local K+ in mature gray matter. J Neurosci 33:2432–2442
Merkley CM, Jian C, Mosa A, Tan YF, Wojtowicz JM (2014) Homeostatic regulation of adult hippocampal neurogenesis in aging rats: long-term effects of early exercise. Front Neurosci 8:174
Miyamoto N, Pham LD, Hayakawa K, Matsuzaki T, Seo JH, Magnain C, Ayata C, Kim KW, Boas D, Lo EH, Arai K (2013) Age-related decline in oligodendrogenesis retards white matter repair in mice. Stroke 44:2573–2578
Moser E, Moser MB, Andersen P (1993) Spatial learning impairment parallels the magnitude of dorsal hippocampal lesions, but is hardly present following ventral lesions. J Neurosci 13:3916–3925
Noble M, Murray K, Stroobant P, Waterfield MD, Riddle P (1988) Platelet derived growth factor promotes division and motility and inhibits premature differentiation of the oligodendrocyte/type-2 astrocyte progenitor cell. Nature 333:560–562
Olariu A, Cleaver KM, Cameron HA (2007) Decreased neurogenesis in aged rats results from loss of granule cell precursors without lengthening of the cell cycle. J Comp Neurol 501:659–667
Ownby RL, Crocco E, Acevedo A, John V, Loewenstein D (2006) Depression and risk for Alzheimer disease: systematic review, meta-analysis, and metaregression analysis. Arch Gen Psychiatry 63:530–538
Pakkenberg B, Gundersen HJ (1997) Neocortical neuron number in humans: effect of sex and age. J Comp Neurol 384:312–320
Pannese E (2011) Morphological changes in nerve cells during normal aging. Brain Struct Funct 216:85–89
Peters A, Kemper T (2012) A review of the structural alterations in the cerebral hemispheres of the aging rhesus monkey. Neurobiol Aging 33:2357–2372
Peters A, Sethares C (1993) Aging and the meynert cells in rhesus monkey primary visual cortex. Anat Rec 236:721–729
Peters A, Sethares C (2002) The effects of age on the cells in layer 1 of primate cerebral cortex. Cereb Cortex 12:27–36
Pitkänen A, Pikkarainen M, Nurminen N, Ylinen A (2000) Reciprocal connections between the amygdala and the hippocampal formation, perirhinal cortex, and postrhinal cortex in rat. A review. Ann N Y Acad Sci 911:369–391
Rao MS, Shetty AK (2004) Efficacy of doublecortin as a marker to analyse the absolute number and dendritic growth of newly generated neurons in the adult dentate gyrus. Eur J Neurosci 19:234–246
Regenold WT, Phatak P, Marano CM, Gearhart L, Viens CH, Hisley KC (2007) Myelin staining of deep white matter in the dorsolateral prefrontal cortex in schizophrenia, bipolar disorder, and unipolar major depression. Psychiatry Res 151:179–188
Richardson WD, Young KM, Tripathi RB, McKenzie I (2011) NG2-glia as multipotent neural stem cells: fact or fantasy? Neuron 70:661–673
Sakry D, Karram K, Trotter J (2011) Synapses between NG2 glia and neurons. J Anat 219:2–7
Sandell JH, Peters A (2002) Effects of age on the glial cells in the rhesus monkey optic nerve. J Comp Neurol 445:13–28
Schweitzer I, Tuckwell V, O’Brien J, Ames D (2002) Is late onset depression a prodrome to dementia? Int J Geriatr Psychiatry 17:997–1005
Seki T, Arai Y (1995) Age-related production of new granule cells in the adult dentate gyrus. Neuroreport 6:2479–2482
Sheng JG, Mrak RE, Rovnaghi CR, Kozlowska E, Van Eldik LJ, Griffin WS (1996) Human brain S100 beta and S100 beta mRNA expression increases with age: pathogenic implications for Alzheimer’s disease. Neurobiol Aging 17:359–363
Shors TJ, Miesegaes G, Beylin A, Zhao M, Rydel T, Gould E (2001) Neurogenesis in the adult is involved in the formation of trace memories. Nature 410:372–376
Sim FJ, Zhao C, Penderis J, Franklin RJ (2002) The age-related decrease in CNS remyelination efficiency is attributable to an impairment of both oligodendrocyte progenitor recruitment and differentiation. J Neurosci 22:2451–2459
Small SA, Schobel SA, Buxton RB, Witter MP, Barnes CA (2011) A pathophysiological framework of hippocampal dysfunction in ageing and disease. Nat Rev Neurosci 12:585–601
Snyder JS, Radik R, Wojtowicz JM, Cameron HA (2009) Anatomical gradients of adult neurogenesis and activity: young neurons in the ventral dentate gyrus are activated by water maze training. Hippocampus 19:360–370
Sturrock RR (1987) Age-related changes in the number of myelinated axons and glial cells in the anterior and posterior limbs of the mouse anterior commissure. J Anat 150:111–127
Sun Y, Xu CC, Li J, Guan XY, Gao L, Ma LX, Li RX, Peng YW, Zhu GP (2013) Transplantation of oligodendrocyte precursor cells improves locomotion deficits in rats with spinal cord irradiation injury. PLoS One 8:e57534
Tanaka J, Okuma Y, Tomobe K, Nomura Y (2005) The age-related degeneration of oligodendrocytes in the hippocampus of the senescence-accelerated mouse (SAM) P8: a quantitative immunohistochemical study. Biol Pharm Bull 28:615–618
van Groen T, Wyss JM (2003) Connections of the retrosplenial granular b cortex in the rat. J Comp Neurol 463:249–263
Walter J, Keiner S, Witte OW, Redecker C (2011) Age-related effects on hippocampal precursor cell subpopulations and neurogenesis. Neurobiol Aging 32:1906–1914
Wigley R, Hamilton N, Nishiyama A, Kirchhoff F, Butt AM (2007) Morphological and physiological interactions of NG2-glia with astrocytes and neurons. J Anat 210:661–670
Wilkins A, Majed H, Layfield R, Compston A, Chandran S (2003) Oligodendrocytes promote neuronal survival and axonal length by distinct intracellular mechanisms: a novel role for oligodendrocyte-derived glial cell line-derived neurotrophic factor. J Neurosci 23:4967–4974
Witter MP, Van Hoesen GW, Amaral DG (1989) Topographical organization of the entorhinal projection to the dentate gyrus of the monkey. J Neurosci 9:216–228
Wu Y, Zhang AQ, Yew DT (2005) Age related changes of various markers of astrocytes in senescence-accelerated mice hippocampus. Neurochem Int 46:565–574
Yamada J, Jinno S (2014) Age-related differences in oligodendrogenesis across the dorsal-ventral axis of the mouse hippocampus. Hippocampus 24:1017–1029
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer Japan
About this chapter
Cite this chapter
Jinno, S. (2015). Insights into Aging of the Hippocampus: A View from the Topographic Differentiation. In: Mori, N., Mook-Jung, I. (eds) Aging Mechanisms. Springer, Tokyo. https://doi.org/10.1007/978-4-431-55763-0_14
Download citation
DOI: https://doi.org/10.1007/978-4-431-55763-0_14
Publisher Name: Springer, Tokyo
Print ISBN: 978-4-431-55762-3
Online ISBN: 978-4-431-55763-0
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)