Abstract
Although significant inconsistencies remain to be clarified, a role for neurogenesis in hippocampal functions, such as cognition, has been suggested by several reports. Yet, investigation in various species of mammals, including humans, revealed that rates of hippocampal neurogenesis are steadily declining with age. The very low levels of hippocampal neurogenesis persisting in the aged brain have been suspected to underlie the cognitive deficits observed in elderly. However, current evidence fails to support the hypothesis that decrease of neurogenesis along normal ageing leads to hippocampal dysfunction. Nevertheless, current studies are suggestive for a distinct role of hippocampal neurogenesis in young versus adult and old brain.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Age-Related Neurogenesis Decrease
Although the presence of neurogenesis in the adult central nervous system has been documented in several species of mammals already in the mid-1960s (see for examples early works from Altman and Das 1965a, b, 1966, 1967), true excitement in adult neurogenic processes arose following the confirmation that neurogenesis also takes place in the human adult brain, even beyond the seventh decade (Eriksson et al. 1998). Based on these and other observations, intense effort has been deployed in the recent years to take advantage of the neural stem cells' population sustaining adult neurogenesis to develop regenerative intervention against neuronal loss.
Since the early reports regarding adult neurogenesis, a plethora of studies mainly conducted in rodents revealed that rates of neurogenesis are not steady. Instead, various physiological and molecular regulators have been shown to significant up- and down-regulate neurogenesis by acting on neural stem and progenitor cell proliferation, differentiation and/or maturation (for a review see Ming and Song 2011). Importantly, of all adverse physiological factors acting on neurogenesis, ageing is probably the most potent one. The first detailed report from Kuhn and colleagues revealed that the decrease in hippocampal neurogenesis during ageing is so pronounced that in the dentate gyrus of 21-month-old rats the number of newly added neurons represented only 10 % of the amount detectable in younger rats of 6 months of age (Kuhn et al. 1996). Kuhn and others demonstrated in follow-up studies that the number of newly generated neurons is steadily declining with ageing.
The pronounced age-related decrease in rates of hippocampal neurogenesis observed by Kuhn and colleagues has been meanwhile documented in a broad range of mammalian species, from rodents to human and non-human primates (Amrein et al. 2011; Cameron and McKay 1999a; Couillard-Despres et al. 2009; Gould et al. 1999; Leuner et al. 2007; Lucassen et al. 2010). According to a metaprojection across species, approximately 5 % of all granular cells are proliferating in the mammalian dentate gyrus shortly after birth (Amrein et al. 2011). However, the frequency of proliferating granular cells already declined below 0.5 % within the first 3 months of life according to a logarithmic mode. Moreover, according to the projections of Amrein and colleagues, the pace of decrease of hippocampal neurogenesis is similar across species, irrespective to their lifespan. In consequence, by the time humans transit from adolescence to adult life, proliferating cells in the dentate gyrus would represent < 0.1 % of all granular cells. Hence, the comparatively low level of neurogenesis observed in adult human and primates, as compared to adult rodents, does not imply that postnatal neurogenesis became less relevant along evolution, but simply reflects the longer duration of neurogenesis decrement.
The presence of neurogenesis in the hippocampus constitutes an exquisite form of neuronal plasticity. As compared to other forms, such as synaptic rearrangement, it requires more resources and is more complex in its implementation. Therefore, it can be assumed that the hippocampus has special plasticity requirement justifying such an investment. Importantly, the bottleneck of neurogenesis’ rate lays on a population of neural stem cells, which are by definition unspecialised cells and remain mostly quiescent.
Along ageing, the number of neural stem cells being caught in cell division decreases drastically. Two non-exclusive phenomena could be at the origin of this marked decline. First, the population of neural stem cells might get progressively exhausted due to cell death or differentiation into a specialised cell type. Although, it might be possible to compensate the net output of neurogenesis temporarily by increasing the proliferation of newly generated neuronal precursors or by improving the survival rate of new cells, with the disappearance of neural stem cells, neurogenesis inexorably tappers off. Alternatively, or in addition, the decrease in neurogenesis might be due to unfavourable alterations of the cellular and molecular stem cell niche composition, thereby gradually decreasing the likelihood of stem cells to initiate mitosis with age. In this situation, reinstatement of an adequate environment, for example through a pharmacological intervention, would restore neurogenesis to levels normally associated with youth.
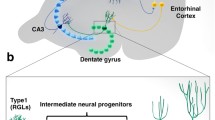
Although the identity of the “real” adult neural stem cell remains elusive, in the dentate gyrus, this function has been ascribed to a group of radial astrocytes (also referred to as type-1 or B cells) spanning the granular layer (Alvarez-Buylla et al. 2002; Kronenberg et al. 2003). Comparison of immunohistological preparations from mouse or rat at different ages reveals that this cell population progressively vanishes (Encinas and Sierra 2012; Encinas et al. 2011). The dispute about the existence of adult neural stem cell maintenance along ageing and the potential to replenish the stem cell population was recently reinflamed by two reports, that came to diametrically opposite conclusions (Bonaguidi et al. 2011; Encinas et al. 2011).
According to a model presented by Encinas and colleagues, adult neural stem cells can be conceived as “disposable stem cells” (Encinas et al. 2011; Encinas and Sierra 2012). This model predicts that once neural stem cells enter proliferation, they undergo successive divisions, generally limited to 2–3 cell cycles, to generate proliferative neuronal precursors. Thereafter, they would terminally differentiate into mature astrocytes. Encinas could not detect evidence for symmetrical division of neural stem cells, i.e. “self-maintenance”. In contrast, Bonaguidi and colleagues presented data showing that neural stem cells could, although seldom, divide symmetrically, thereby supporting the tantalising hypothesis that stem cell population might eventually be replenished by a stimulation of neural stem cells promoting symmetrical division (Bonaguidi et al. 2011). A previous report using retroviral infection to follow the fate of Sox2+ cells in the dentate gyrus came to a similar conclusion (Suh et al. 2007). Although Encinas could not exclude that a small population of infrequent proliferating neural stem cells with the capacity of self-renewal might exist, current evidence suggests that under physiological conditions the loss of neural stem cells along ageing is constant and irremediable.
Supplemental decrease of neurogenesis can be observed during neurodegenerative disorders. However, although some age-dependent processes might lead to neurodegenerative disorder, the latters should not be regarded as accelerated forms of ageing, but discrete physiological disorders. Hence, whereas specific neuronal populations are lost during neurodegenerative disorders, e.g. pyramidal neurons of the CA1 region in the hippocampus of individuals suffering of Alzheimer’s disease, no significant granular or pyramidal neuronal loss could be detected along normal ageing in the dentate gyrus and the Ammon’s horn (West 1993; West et al. 1994).
Many significant differences in the microenvironment surrounding the neurogenic regions can be documented between the young and the aged brains. Altered levels of neurotransmitter release, the higher corticosteroid concentrations or accumulation of molecules typically associated with inflammation and allergy constitute only few examples of factors that could inhibit neurogenesis and impair hippocampal functions (Cameron and McKay 1999b; Huber et al. 2011; Nichols et al. 2001; Popa-Wagner et al. 2011; Villeda et al. 2011). For example, in cases of stress and ageing, high levels of corticosteroids are thought to inhibit neurogenesis. The fact that adrenalectomized rats, young or old, displayed significant increases of hippocampal neurogenesis supports this assumption (Cameron and McKay 1999b; Spanswick et al. 2011). However, exposition of mice to an enriched environment has been also reported to strongly augment levels of neurogenesis, although levels of corticosterone were threefolds higher than in standard-housed animals (Kempermann et al. 2002). Clearly, regulation of neurogenesis is a complex process summing multiple components. In addition, it must be kept in mind that factors modulating neurogenesis can also act on mature neurons and glia, and thereby profoundly influence hippocampal functions in a neurogenesis-independent fashion.
2 Age-Related Neurogenesis Decrease and Cognition
Should we be concerned by the age-related decrease of neurogenesis within our hippocampi? Whereas the role of neurogenesis in olfaction got somewhat marginal for human survival, processes carried out in the hippocampus, namely cognition and emotion, are the very essence of who we are. At the level of cognitive performances, it is well documented that, on average, human and animal models at advanced age have poorer scores than young adults. However, if neurogenesis levels were directly linked with cognitive performances, the most rapid and pronounced cognitive decline would be observed in first life interval, meaning before puberty, rather than in the elderly population. Several studies attempted to decipher the complex correlation between the age-associated decrease of neurogenesis and the aged-dependent cognitive performance weaknesses. However, no definite evidence has been provided so far that neurogenesis decline in normal ageing causes cognitive deficits.
Despite the fact that ageing is associated with a severe decrease in the rate of hippocampal neurogenesis, in rats, careful analysis in the course of normal ageing ruled out significant neuronal loss (Rapp and Gallagher 1996). In their report, Rapp and Gallagher used unbiased stereology to quantify the number of neurons within the different hippocampal structures in groups of young and old rats. The latter group was further stratified into groups of good and bad learners according to learning and memory tests (e.g. Morris Water Maze). Yet, despite significant differences in test performances between the three groups analysed, Rapp and Gallagher could not detect any significant difference in the number of neurons as a function of age or cognitive status in the hippocampal regions investigated. Similar histological studies performed in tissues from aged human also came to the conclusion that the number of hippocampal neurons within the dentate gyrus and the Ammon’s horn remains stable during normal ageing, although decreases were observed within the hilus and in the subiculum (West 1993; West et al. 1994). These observations substantiate other reports suggesting an equilibrium between cell death and the addition of new neurons within the hippocampus (Biebl et al. 2000; Cameron and McKay 1999b). Hence, the age-associated decrease of hippocampal neurogenesis is most likely compensated by a proportional decrease of cell removal through apoptosis, therefore resulting in a “neuronal steady state”.
Over more than a decade, several studies aimed to elucidate the function of hippocampal neurogenesis via a targeted depletion of the latter. These studies have been conducted almost exclusively in young animals, which typically display high levels of hippocampal neurogenesis. The first depletion strategies attempted were rather coarse, e.g. cytotoxic agents or X-rays irradiations (see for example Madsen et al. 2003; Shors et al. 2001). The reported learning deficits resulting from these neurogenesis eradications were received with interest, yet with some scepticism because treatments used provoked additional damages and inflammation apart from depleting neurogenesis. Following these pioneer studies, however, numerous works addressed the role of hippocampal neurogenesis using increasingly refined neurogenesis deletion methods and elaborated behavioural testing (for an exhaustive review Deng et al. 2010). Despite all experiments, no clear conclusion on the function of adult hippocampal neurogenesis in cognition can be drawn to date. Although many studies reported correlation between cognitive deficits and total or partial ablation of hippocampal neurogenesis, important discrepancy remains.
Taken that confounding side effects cannot be ruled out for any intervention aiming to deplete neurogenesis, investigation of animal models in which hippocampal neurogenesis is naturally low or absent constitutes an elegant alternative. The cyclin D2 knockout mouse, that lacks adult neurogenesis, is an example of such a useful animal model. However, caution in the interpretation of behavioural tests in this model must be taken since the deletion of cyclin D2 also affects the overall brain development (Jaholkowski et al. 2009; Jedynak et al. 2012; Kowalczyk et al. 2004). In the hippocampus of the cyclin D2 deficient mouse line, only 10 % of the expected number of neuronal precursors (DCX+ cells) can be detected. Noteworthy, the integration of the few neuronal precursors as new mature neurons appears to be completely abolished. Surprisingly, according to a battery of learning-dependent paradigms used by Filipkowski and colleagues, the absence of hippocampal neurogenesis did not overtly affect learning or anxiety levels in these knockout mice as compared to their wild-type littermates (Jaholkowski et al. 2009; Jedynak et al. 2012). Nevertheless, significant abnormalities in the cyclin D2 deficient mice were detected in some learning-independent behavioural tests, such as nest construction, exploration and motor activities. Taken together, the analysis of the cyclin D2 deficient mouse model strongly suggests that learning is not dependent on the creation of new neuronal circuitries generated in the course of adult neurogenesis. However, it cannot be excluded that normal hippocampal function is supported by the few neuronal precursors present or that more refined behavioural testing would be required to put the cognitive deficits in evidence.
As the cyclin D2 knockout mouse model is also an artificial model, one might rather consider “natural” models of neurogenesis deficiency. For example, some species of shrews and bats are particularly interesting in this respect. Although neurogenesis at the subventricular zone appears to remain life-long in these species, hippocampal neurogenesis can be detected only very early in adulthood and vanishes afterward (Amrein et al. 2007; Bartkowska et al. 2008). Despite the absence of hippocampal neurogenesis, these animals can adapt to an environment in constant mutation, and can memorise precisely the new locations of food supplies, which are spread over large territories in cases of bats. Hence, it appears from these animal models deficient in hippocampal neurogenesis that the generation of new neurons is not required for learning, and in particular spatial learning such as frequently assessed in laboratory setting using variants of the Morris Water Maze task.
Evidence thus indicates that neurogenesis is not essential for learning, yet it might still act as a facilitator. Immature neurons are more readily excitable upon stimuli as compared to their mature counterpart (Couillard-Després et al. 2006; Ge et al. 2007; Schmidt-Hieber et al. 2004). During adult hippocampal neurogenesis, immature neurons are generated in the granular layer of the dentate gyrus, which is the entryway of signals in the hippocampal formation. Hence, due to their strategic position, neuronal precursors could have a significant impact on the sensitivity and response intensity of the dentate gyrus to incoming signals.
Over the last years, several groups developed abstract and biology-based computational models to investigate the roles of the dentate gyrus and neurogenesis in hippocampal functions. One process repeatedly ascribed to the dentate gyrus is the so-called pattern separation (see Aimone and Gage 2011 and Yassa and Stark 2011 for reviews). Pattern separation consists in the generation of contrasted outputs from similar incoming inputs to allow the hippocampus to recognise but distinguish similar, yet different inputs. With ageing, in animal models as well as in human, the efficacy of pattern separation appears to gradually diminish, i.e. greater differences between similar inputs are required to properly recognise the later as “similar” and not “same” (Yassa et al. 2011). The extent of the dentate gyrus’ contribution to pattern separation is currently a matter of debates. Nevertheless, it is very unlikely that pattern separation of the constant flow of inputs is performed by the very few new neurons residing in the adult dentate gyrus. Nevertheless, considering their high excitability and their insensitivity to GABAergic inhibition, the immature neurons could in spite of their poor activation specificity maintain the dentate gyrus and the downward hippocampal circuitry away from an inhibitory stare by “overreacting” to the inputs. Moreover, in contrast to a model system bearing a subpopulation of resident neurons having properties similar to those of young neurons, the solution based on continuous neurogenesis offers the possibility to modulate the number of intervening immature neurons according to the needs.
Along the same line, in a recent in vitro experiment, Stephens and colleague reported that the addition of immature neurons into a mature and stable network led to a rejuvenation of the network activity pattern as a whole (Stephens et al. 2011). According to this study, the activity pattern adopted by the network following addition of immature neurons would facilitate synaptic plasticity and the establishment of LTP, two processes tightly linked to memory formation. Therefore, implantation of neuronal precursors should not, or not only, be regarded as a mean to establish new networks, but also as functional modifier for mature hypoactive or even damaged networks (Stephens et al. 2011). It is worth notice that during ageing, the quantitative decrease of neurons production might be partly compensated by their slower maturation, which de facto prolonged the duration of high excitability of the few new cells in the system (Nyffeler et al. 2010; Rao et al. 2005). In 3-month-old mice for example, we reported the presence of NeuN, a marker for mature neurons, in roughly 90 % of granular neurons generated 4 weeks before (Couillard-Després et al. 2006). In contrast, this proportion of NeuN-expressing young neurons was decreased to 8 % when labelling was performed in 20-month-old mice (Kempermann et al. 2002).
3 Neurogenesis Relevance in the Aged Brain
Hence, although neurogenesis rates are continuously decreasing with age, cognitive performances remain rather constant during the adulthood in human and animal models, only showing decline in advanced ages. For example, Cuppini and colleagues investigated groups of rats having 2, 5 and 12 months of age using trace fear conditioning, which assesses hippocampal-dependent learning (Cuppini et al. 2006). As expected, levels of hippocampal neurogenesis was significantly reduced in the two older groups of rats, as compared to the 2-month-old rats, with reduction roughly representing 50 and 95 %, respectively. Notwithstanding the massive depletion of hippocampal neurogenesis in older rats, no significant differences in their test performance could be detected between the three groups (Cuppini et al. 2006). Intriguingly, a decrease of approximately 50 % of hippocampal neurogenesis in young adult rats via the application of a cytostatic agent, methylazoxymethanol (MAM), resulted in a significantly poorer test performance (Shors et al. 2001). How can this behavioural discrepancy be explained? Are poorer performances resulting from a broad cytotoxic effect upon MAM application, or do young and old animals differ in their requirement of neurogenesis for hippocampal functions?
As the functions of adult neurogenesis are not yet deciphered, speculation on the consequence of neurogenesis modulation such as during ageing is particularly precarious. Evidence accumulated so far from the various investigations on hippocampal neurogenesis argues against the simple correlation “the more, the better”. Moreover, the fact that sites of integration for newly generated neurons are also sites of apoptosis, suggests that mere addition of supernumerary neurons does not constitute the aim of adult neurogenesis. Instead, the possibility to integrate new neurons could enable the continuous selection for neurons that are functionally better suited for the current spectrum of tasks to be accomplished and can be envisaged as the capacity of original circuitries to become gradually optimised.
In order to better pinpoint the impact of neurogenesis on hippocampal function, it is advantageous to minimise the number of intervening variables, such as age or treatment and rely only on the interindividual variation of neurogenesis rates. Using such a strategy, Bizon and colleagues came in 2004 to the astonishing observation that the levels of neurogenesis in the population of older rats were inversely proportional to their learning index, i.e. good learners had less hippocampal neurogenesis than bad learners (Bizon et al. 2004). In a more recent report, this finding was readdressed by Nyffeler and colleagues who looked at ongoing neurogenesis by quantifying the population of neuronal precursors expressing DCX in rats of three different age groups (3, 6 and 24 months of age) (Nyffeler et al. 2010). At first sight, comparing all groups at once, a correlation appeared between learning performance and higher neurogenesis levels. However, following partition of rats within their respective age groups a completely different picture arose. Hence, replotting the data generated by Nyffeler, Lazic observed that direct correlation between learning and neurogenesis could only be detected in the 3-month-old rat group, whereas in the 6- and 24-month-old groups, an inverse correlation was observed, consistent with the report of Bizon and colleagues (Lazic 2010).
Once more, do requirements in neurogenesis for adequate hippocampal function differ in young and old individuals? Although the cellular composition of the hippocampus appears to remain relatively stable from the time point at which development is completed up to an advanced age, the young hippocampus can still be considered as immature system since it “lacks experience”. A similarity could be perceived with the immune system. In addition to the cellular development of the hippocampus, further maturation will result from the constant flow of inputs coming from the environment. The hippocampal circuitry will “learn”, will get functionally optimised to the needs and will progressively accumulate experience. In this respect, neurogenesis would enable the possibility to select, out of the newly generated population, neurons better suited for hippocampal function. This process might be critical in young immature individuals, but dispensable in the older and already optimised hippocampus.
Similar to the neuronal content, the overall thickness of the molecular layer remains constant over most of the life span, but undergoes qualitative changes. Hence, in aged rats, a significant reduction of the medial molecular layer (incoming projections from the medial entorhinal cortex) to the benefit of the inner molecular layer (commissural/associational inputs arising from hilar neurons) was reported (Rapp et al. 1999). These track volumetric changes along ageing reflect the synaptic reorganization and permanent rewiring of the hippocampus. It is tempting to conclude that along ageing, processing of incoming inputs will increasingly involve association to previously acquired memory. Investigation of the dynamic of such hippocampal circuitry rewiring in species showing very low or no adult neurogenesis might reveal if this constitute a common strategy to overcome the decreased number of immature neurons production.
Similar functional rewiring was suggested by a recent fMRI investigation comparing two groups composed of men with 20 or 60 years of age (Burgmans et al. 2010). Although these two groups were shown to have equivalent cognitive performance according to the tests employed, differences were observed in cortical activation patterns during memory processing. Hence, the aged group revealed in fMRI a weaker activation of the medial temporal areas, as compared to the 20-year-old candidates, whereas the ventral and prefrontal cortex, as well as in some additional frontal and parietal regions presented a stronger activation (Burgmans et al. 2010; Grady 2008). One could speculate that individuals of 60 years of age relied for the execution of cognitive challenges more heavily on association with previously acquired experiences.
It is noteworthy that in numerous studies stronger challenge of the cognitive resources in youth and adulthood appears to lower the risk of cognitive decline during ageing, and reciprocally lower educational attainment is consistently associated with higher risks of dementia (e.g. Tervo et al. 2004). One interpretation could be that intense cognitive stimulation during the phase of high neurogenesis levels would permit a thorough optimisation of hippocampal processing and allow to mask longer functional deficits associated with pathological processes. This postulation would be in line with the cognitive and the neurogenic reserve hypotheses. The cognitive reserve hypothesis states that optimised neuronal circuitries have greater functional capacities and are less susceptible to disruption per se and/or can better compensate for dysfunctional elements (Kempermann 2008; Stern 2006). On the other side, the neurogenic reserve hypothesis implies that the generation of new neurons constitutes a cellular plasticity to incessantly optimise the hippocampal circuitry according to the demand. As younger individuals are relatively “inexperienced” as compared to elders, there is a stronger need for optimisation of input processing and therefore more need for neurogenesis. Once optimised, the network remains more efficient over long periods of time for the processing of similar inputs. Following a context change, e.g. enriched environment, neurogenesis can be stimulated, even in the aged hippocampus (Kempermann et al. 1998). Therewith, increased neurogenesis provides new resources for greater cellular plasticity as required in the new situation. However, as the neurogenesis levels are constantly decreasing along ageing, the reserve available for optimisation inexorably thins out as well.
In any case, interpretation of such a correlation between risk of dementia and educational attainment must be taken with great care. Hence, persons susceptible to develop dementia might be already biased against intensive cognitive challenges long before the appearance of the first symptomatic manifestation.
Since the first reports on age-related neurogenesis decrease, many strategies have been envisaged to counteract neurogenesis decline with pro-neurogenic approaches. In light of actual evidence, however, the pertinence and potential benefit of such treatment must be questioned. The common finding of a decrease in neurogenesis in ageing mammals supports the idea that it does not represent a major evolutionary disadvantage (Amrein et al. 2011). Moreover, would an undue increase of neurogenesis in the aged brain be detrimental to a system that has been optimised over a long period of time? Usefulness of targeted neurogenesis stimulation might be limited to pathological situations in which neuronal loss and dysfunction are involved and require mending. As for normal ageing, it might simply be the best to make good use of available resources today in order to optimise our brain for tomorrow.
Carpe diem
References
Aimone JB, Gage FH (2011) Modeling new neuron function: a history of using computational neuroscience to study adult neurogenesis. Eur J Neurosci 33(6):1160–1169. doi: 10.1111/j.1460-9568.2011.07615.x
Altman J, Das GD (1965a) Autoradiographic and histological evidence of postnatal hippocampal neurogenesis in rats. J Comp Neurol 124(3):319–335
Altman J, Das GD (1965b) Post-natal origin of microneurones in the rat brain. Nature 207(5000):953–956
Altman J, Das GD (1966) Autoradiographic and histological studies of postnatal neurogenesis. I. A longitudinal investigation of the kinetics, migration and transformation of cells incorporating tritiated thymidine in neonate rats, with special reference to postnatal neurogenesis in some brain regions. J Comp Neurol 126(3):337–389. doi: 10.1002/cne.901260302
Altman J, Das GD (1967) Postnatal neurogenesis in the guinea-pig. Nature 214(5093):1098–1101
Alvarez-Buylla A, Seri B, Doetsch F (2002) Identification of neural stem cells in the adult vertebrate brain. Brain Res Bull 57(6):751–758
Amrein I, Dechmann DKN, Winter Y, Lipp H-P (2007) Absent or low rate of adult neurogenesis in the hippocampus of bats (Chiroptera). PLoS ONE 2(5):e455. doi: 10.1371/journal.pone.0000455
Amrein I, Isler K, Lipp H-P (2011) Comparing adult hippocampal neurogenesis in mammalian species and orders: influence of chronological age and life history stage. Eur J Neurosci 34(6):978–987. doi: 10.1111/j.1460-9568.2011.07804.x
Bartkowska K, Djavadian RL, Taylor JRE, Turlejski K (2008) Generation recruitment and death of brain cells throughout the life cycle of Sorex shrews (Lipotyphla). Eur J Neurosci 27(7):1710–1721. doi: 10.1111/j.1460-9568.2008.06133.x
Biebl M, Cooper CM, Winkler J, Kuhn HG (2000) Analysis of neurogenesis and programmed cell death reveals a self-renewing capacity in the adult rat brain. Neurosci Lett 291(1):17–20
Bizon JL, Lee HJ, Gallagher M (2004) Neurogenesis in a rat model of age-related cognitive decline. Aging Cell 3(4):227–234. doi: 10.1111/j.1474-9728.2004.00099.x
Bonaguidi MA, Wheeler MA, Shapiro JS, Stadel RP, Sun GJ, Ming G-L, Song H (2011) In vivo clonal analysis reveals self-renewing and multipotent adult neural stem cell characteristics. Cell 145(7):1142–1155. doi: 10.1016/j.cell.2011.05.024
Burgmans S, van Boxtel MPJ, Vuurman EFPM, Evers EAT, Jolles J (2010) Increased neural activation during picture encoding and retrieval in 60-year-olds compared to 20-year-olds. Neuropsychologia 48(7):2188–2197. doi: 10.1016/j.neuropsychologia.2010.04.011
Cameron HA, McKay RD (1999a) Restoring production of hippocampal neurons in old age. Nat Neurosci 2(10):894–897. doi: 10.1038/13197
Cameron HA, McKay RD (1999b) Restoring production of hippocampal neurons in old age. Nat Neurosci 2(10):894–897. doi: 10.1038/13197
Couillard-Despres S, Wuertinger C, Kandasamy M, Caioni M, Stadler K, Aigner R, Bogdahn U et al (2009) Ageing abolishes the effects of fluoxetine on neurogenesis. Mol Psychiatry 14(9):856–864. doi: 10.1038/mp.2008.147
Couillard-Després S, Winner B, Karl C, Lindemann G, Schmid P, Aigner R, Laemke J et al (2006) Targeted transgene expression in neuronal precursors: watching young neurons in the old brain. Eur J Neurosci 24(6):1535–1545. doi: 10.1111/j.1460-9568.2006.05039.x
Cuppini R, Bucherelli C, Ambrogini P, Ciuffoli S, Orsini L, Ferri P, Baldi E (2006) Age-related naturally occurring depression of hippocampal neurogenesis does not affect trace fear conditioning. Hippocampus 16(2):141–148. doi: 10.1002/hipo.20140
Deng W, Aimone JB, Gage FH (2010) New neurons and new memories: how does adult hippocampal neurogenesis affect learning and memory? Nat Rev Neurosci 11(5):339–350. doi: 10.1038/nrn2822
Encinas JM, Sierra A (2012) Neural stem cell deforestation as the main force driving the age-related decline in adult hippocampal neurogenesis. Behav Brain Res 227(2):433–439. doi: 10.1016/j.bbr.2011.10.010
Encinas JM, Michurina TV, Peunova N, Park J-H, Tordo J, Peterson DA, Fishell G et al (2011) Division-coupled astrocytic differentiation and age-related depletion of neural stem cells in the adult hippocampus. Stem Cell 8(5):566–579. doi: 10.1016/j.stem.2011.03.010
Eriksson PS, Perfilieva E, Björk-Eriksson T, Alborn AM, Nordborg C, Peterson DA, Gage FH (1998) Neurogenesis in the adult human hippocampus. Nat Med 4(11):1313–1317. doi: 10.1038/3305
Ge S, Yang C-H, Hsu K-S, Ming G-L, Song H (2007) A critical period for enhanced synaptic plasticity in newly generated neurons of the adult brain. Neuron 54(4):559–566. doi: 10.1016/j.neuron.2007.05.002
Gould E, Reeves AJ, Fallah M, Tanapat P, Gross CG, Fuchs E (1999) Hippocampal neurogenesis in adult old world primates. Proc Natl Acad Sci U S A 96(9):5263–5267
Grady CL (2008) Cognitive neuroscience of aging. Ann NY Acad Sci 1124:127–144. doi: 10.1196/annals.1440.009
Huber C, Marschallinger J, Tempfer H, Furtner T, Couillard-Després S, Bauer H-C, Rivera FJ et al (2011) Inhibition of leukotriene receptors boosts neural progenitor proliferation. Cell Physiol Biochem Int J Exp Cell Physiol Biochem Pharmacol 28(5):793–804. doi: 10.1159/000335793
Jaholkowski P, Kiryk A, Jedynak P, Ben Abdallah NM, Knapska E, Kowalczyk A, Piechal A, et al (2009) New hippocampal neurons are not obligatory for memory formation; cyclin D2 knockout mice with no adult brain neurogenesis show learning. Learn Mem (Cold Spring Harbor, N.Y.) 16(7):439–451. doi: 10.1101/lm.1459709
Jedynak P, Jaholkowski P, Wozniak G, Sandi C, Kaczmarek L, Filipkowski RK (2012) Lack of cyclin D2 impairing adult brain neurogenesis alters hippocampal-dependent behavioral tasks without reducing learning ability. Behav Brain Res 227(1):159–166. doi: 10.1016/j.bbr.2011.11.007
Kempermann G (2008) The neurogenic reserve hypothesis: what is adult hippocampal neurogenesis good for? Trends Neurosci 31(4):163–169. doi: 10.1016/j.tins.2008.01.002
Kempermann G, Gast D, Gage FH (2002) Neuroplasticity in old age: sustained fivefold induction of hippocampal neurogenesis by long-term environmental enrichment. Ann Neurol 52(2):135–143. doi: 10.1002/ana.10262
Kempermann G, Kuhn HG, Gage FH (1998) Experience-induced neurogenesis in the senescent dentate gyrus. J Neurosci Off J Soc Neurosci 18(9):3206–3212
Kowalczyk A, Filipkowski RK, Rylski M, Wilczynski GM, Konopacki FA, Jaworski J, Ciemerych MA et al (2004) The critical role of cyclin D2 in adult neurogenesis. J Cell Biol 167(2):209–213. doi: 10.1083/jcb.200404181
Kronenberg G, Reuter K, Steiner B, Brandt MD, Jessberger S, Yamaguchi M, Kempermann G (2003) Subpopulations of proliferating cells of the adult hippocampus respond differently to physiologic neurogenic stimuli. J Comp Neurol 467(4):455–463. doi: 10.1002/cne.10945
Kuhn HG, Dickinson-Anson H, Gage FH (1996) Neurogenesis in the dentate gyrus of the adult rat: age-related decrease of neuronal progenitor proliferation. J Neurosci Off J Soc Neurosci 16(6):2027–2033
Lazic SE (2010) Relating hippocampal neurogenesis to behavior: the dangers of ignoring confounding variables. Neurobiol Aging 31(12):2169–2171. doi: 10.1016/j.neurobiolaging.2010.04.037
Leuner B, Kozorovitskiy Y, Gross CG, Gould E (2007) Diminished adult neurogenesis in the marmoset brain precedes old age. Proc Nat Acad Sci U S A 104(43):17169–17173. doi: 10.1073/pnas.0708228104
Lucassen PJ, Stumpel MW, Wang Q, Aronica E (2010) Decreased numbers of progenitor cells but no response to antidepressant drugs in the hippocampus of elderly depressed patients. Neuropharmacology 58(6):940–949. doi: 10.1016/j.neuropharm.2010.01.012
Madsen TM, Kristjansen PEG, Bolwig TG, Wörtwein G (2003) Arrested neuronal proliferation and impaired hippocampal function following fractionated brain irradiation in the adult rat. Neuroscience 119(3):635–642
Ming G-L, Song H (2011) Adult neurogenesis in the mammalian brain: significant answers and significant questions. Neuron 70(4):687–702. doi: 10.1016/j.neuron.2011.05.001
Nichols NR, Zieba M, Bye N (2001) Do glucocorticoids contribute to brain aging? Brain Res. Brain Res Rev 37(1–3):273–286
Nyffeler M, Yee BK, Feldon J, Knuesel I (2010) Abnormal differentiation of newborn granule cells in age-related working memory impairments. Neurobiol Aging 31(11):1956–1974. doi: 10.1016/j.neurobiolaging.2008.10.014
Popa- Wagner A, Buga A-M, Kokaia Z (2011) Perturbed cellular response to brain injury during aging. Ageing Res Rev 10(1):71–79. doi: 10.1016/j.arr.2009.10.008
Rao MS, Hattiangady B, Abdel-Rahman A, Stanley DP, Shetty AK (2005) Newly born cells in the ageing dentate gyrus display normal migration, survival and neuronal fate choice but endure retarded early maturation. Eur J Neurosci 21(2):464–476. doi: 10.1111/j.1460-9568.2005.03853.x
Rapp PR, Gallagher M (1996) Preserved neuron number in the hippocampus of aged rats with spatial learning deficits. Proc Nat Acad Sci U S A 93(18):9926–9930
Rapp PR, Stack EC, Gallagher M (1999) Morphometric studies of the aged hippocampus: I. Volumetric analysis in behaviorally characterized rats. J Comp Neurol 403(4):459–470
Schmidt-Hieber C, Jonas P, Bischofberger J (2004) Enhanced synaptic plasticity in newly generated granule cells of the adult hippocampus. Nature 429(6988):184–187. doi: 10.1038/nature02553
Shors TJ, Miesegaes G, Beylin A, Zhao M, Rydel T, Gould E (2001) Neurogenesis in the adult is involved in the formation of trace memories. Nature 410(6826):372–376. doi: 10.1038/35066584
Spanswick SC, Epp JR, Sutherland RJ (2011) Time-course of hippocampal granule cell degeneration and changes in adult neurogenesis after adrenalectomy in rats. NSC 190(C):166–176. doi:10.1016/j.neuroscience.2011.06.023
Stephens CL, Toda H, Palmer TD, DeMarse TB, Ormerod BK (2011) Adult neural progenitor cells reactivate superbursting in mature neural networks. Exp Neurol 234(1):20–30. doi: 10.1016/j.expneurol.2011.12.009
Stern Y (2006) Cognitive reserve and Alzheimer disease. Alzheimer Dis Assoc Disord 20(2):112–117. doi: 10.1097/01.wad.0000213815.20177.19
Suh H, Consiglio A, Ray J, Sawai T, D’Amour KA, Gage FH (2007) In vivo fate analysis reveals the multipotent and self-renewal capacities of Sox2 + neural stem cells in the adult hippocampus. Stem Cell 1(5):515–528. doi: 10.1016/j.stem.2007.09.002
Tervo S, Kivipelto M, H auml nninen T, Vanhanen M, Hallikainen M, Mannermaa A, Soininen H (2004) Incidence and risk factors for mild cognitive impairment: a population-based three-year follow-up study of cognitively healthy elderly subjects. Dement Geriatr Cogn Disord 17(3):196–203. doi: 10.1159/000076356
Villeda SA, Luo J, Mosher KI, Zou B, Britschgi M, Bieri G, Stan TM et al (2011) The ageing systemic milieu negatively regulates neurogenesis and cognitive function. Nature 477(7362):90–94. doi: 10.1038/nature10357
West MJ (1993) Regionally specific loss of neurons in the aging human hippocampus. Neurobiol Aging 14(4):287–293
West MJ, Coleman PD, Flood DG, Troncoso JC (1994) Differences in the pattern of hippocampal neuronal loss in normal ageing and Alzheimer’s disease. Lancet 344(8925):769–772
Yassa MA, Stark CEL (2011) Pattern separation in the hippocampus. Trends Neurosci 34(10):515–525. doi: 10.1016/j.tins.2011.06.006
Yassa MA, Lacy JW, Stark SM, Albert MS, Gallagher M, Stark CEL (2011) Pattern separation deficits associated with increased hippocampal CA3 and dentate gyrus activity in nondemented older adults. Hippocampus 21(9):968–979. doi: 10.1002/hipo.20808
Acknowledgments
The author is grateful to Prof. L. Aigner (Paracelsus Medical University, Salzburg) and Prof. B. Iglseder (Christian Doppler Clinic, Salzburg) for their critical and stimulating comments. This work has been made possible through the support from the State Government of Salzburg (Austria), the German Federal Ministry of Education and Research (BMBF grant 01GN0978) and the foundation Propter Homines (Liechtenstein). The research leading to these publication has received funding from the European Union’s Seventh Framework Programme (FP7/2007-2013) under grant agreement n° HEALTH-F2-2011-278850 (INMiND) and n° HEALTH-F2-2011-279288 (IDEA).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2012 Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
Couillard-Després, S. (2012). Hippocampal Neurogenesis and Ageing. In: Belzung, C., Wigmore, P. (eds) Neurogenesis and Neural Plasticity. Current Topics in Behavioral Neurosciences, vol 15. Springer, Berlin, Heidelberg. https://doi.org/10.1007/7854_2012_232
Download citation
DOI: https://doi.org/10.1007/7854_2012_232
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-642-36231-6
Online ISBN: 978-3-642-36232-3
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)