Abstract
The recurrent laryngeal nerve contains myelinated nerve fibers mostly measuring 6–10 μm in diameter and many unmyelinated nerve fibers as well. The previously reported view that nerve fibers innervating the abductor muscle and those innervating the adductor muscle separately form fiber fascicles is not supported by current evidence. These nerve fibers are known to be present, sporadically, in nerve fascicles. We have revealed for the first time that the canine recurrent laryngeal nerve contains noradrenaline (NA)-ergic and substance P (SP)-ergic nerve fibers. In addition, we have also demonstrated that the inferior laryngeal nerve, which is the terminal branch of the recurrent laryngeal nerve in the dog, has acetylcholine-, NA-, calcitonin gene-related peptide-, SP-, neuropeptide Y-, and nitric oxide-ergic nerve fibers.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
Introduction
It has long been known that the recurrent laryngeal nerve controls vocal cord motion. This was first discovered by Galenos (Galen in English) in the era of the Roman Empire. Galenos was born in Pergamum (currently in Turkey) in 129 AD and later became monarch Aurelius’s doctor. In the era prior to Galenos, the brain was considered to harbor mental functions, while motions were controlled by the thoracic region. Galenos demonstrated that the brain controls motions, by showing in public that a struggling and grunting pig kept struggling without voice when the recurrent laryngeal nerve was cut [1]. The term “recurrens” was first used for the recurrent laryngeal nerve (nervus laryngeus recurrens) by the Belgian anatomist Vesalius in the sixteenth century; a description of the recurrent laryngeal nerve is found in his classic text entitled “Fabrica”.
The recurrent laryngeal nerve has branches going to the esophagus and the trachea in the cervical region, eventually reaching the larynx. The terminal branch to the larynx is called the inferior laryngeal nerve. The inferior laryngeal nerve controls vocal cord motion by innervating four types of intrinsic laryngeal muscles other than the cricothyroid muscle (i.e., the thyroarytenoid, lateral cricoarytenoid, arytenoid, and posterior cricoarytenoid muscles). This nerve is known to contain autonomic nerve fibers [2] and sensory nerve fibers [3], in addition to motor nerve fibers.
The recurrent laryngeal nerve usually divides into two nerve branches (anterior and posterior), and the posterior branch forms Galen’s anastomosis with the internal branch of the superior laryngeal nerve [4–8].
Nerve Fiber Composition
Many investigators have studied the fiber composition of the recurrent laryngeal nerve in order to indirectly confirm the issue of the conduction velocity of motor nerve fibers innervating intrinsic laryngeal muscles and the presence of mechanoreceptors such as muscle spindles in intrinsic laryngeal muscles. However, early studies failed to obtain consistent results because of species differences and various issues in the research methodology. However, studies carried out in the 1950s and thereafter revealed that the diameter of motor nerve fibers of the recurrent laryngeal nerve is generally smaller than that of the motor nerve fibers of limb muscles, although diameters vary markedly among motor nerve fibers in the recurrent laryngeal nerve (mostly 6–10 μm, with some thick fibers measuring 20 μm). This finding corroborates the observation that the conduction velocity (30–40 m/s) of the motor nerve fibers innervating the intrinsic laryngeal muscle is lower than that for limb muscles (50–60 m/s). The finding of only a few thick nerve fibers was also consistent with muscle spindles being scarce in intrinsic laryngeal muscles [9].
Gacek and Lyon [10], who used an electron microscope for their study, reported that the cat recurrent laryngeal nerve contains 565 and 482 myelinated nerve fibers on average on the right and left sides, respectively. They also reported that there were 827 and 680 unmyelinated nerve fibers on average on the right and left sides, respectively, although considerable variation among individual cats was observed. They speculated the reason why there were more myelinated nerve fibers on the right would be that sensory nerve fibers on the left terminate at the esophagus, while those on the right reach the trachea, terminating at the trachea and esophagus. They also carried out a nerve section experiment on the same occasion and speculated that unmyelinated nerve fibers in the recurrent laryngeal nerve would be sympathetic or parasympathetic fibers that have no relationships with motor function. The right-left difference in the number of nerve fibers was later examined in the rat by Dahlqvist et al. [11] and in humans and the giraffe by Harrison [8], and they reported that no such difference was detected. As to conduction velocity, the right-left difference in the thickness of nerve fibers has been studied. The left recurrent laryngeal nerve is longer than its right counterpart by 10 cm in human subjects [12], by 13 cm in the dog [13], and by 30 cm in the giraffe [8]. Therefore, based on the difference in conduction velocity, it is said that nerve fibers constituting the left recurrent laryngeal nerve are generally thicker than those on the right [12, 14].
Localizations of Nerve Fibers Innervating the Abductor and Adductor Muscles
The greatest interest in the field of laryngology from the end of the nineteenth century through the middle of the twentieth century focused on vocal cord position during recurrent laryngeal nerve paralysis. This interest was elicited by the report of Semon in 1881 [15]. He pointed out that nerve fibers innervating the abductor muscle are more subject to injury than nerve fibers innervating the adductor muscle in the case of recurrent laryngeal nerve injury and explained this by hypothesizing that fibers in the recurrent laryngeal nerve are arranged in a concentric fashion, with the nerve innervating the adductor muscle being located in the center [16]. However, in 1952, Sunderland and Swaney [17] morphologically studied the distributions of nerve fibers at various levels of the recurrent laryngeal nerve and reported that nerve fibers innervating the abductor muscle and those innervating the adductor muscle did not form separate fiber fascicles. The hypothesis of Semon regarding vocal cord position during recurrent laryngeal nerve paralysis was also later ruled out by various studies using the electrophysiological approach or other methods.
In recent years, Gacek et al. [18] have studied the distribution of labeled fibers in the recurrent laryngeal nerve electron microscopically at the level 1–2 cm caudal to the orifice of the larynx, after injecting horseradish peroxidase into the feline thyroarytenoid and posterior cricoarytenoid muscles. Their results made it apparent that nerve fibers innervating each of these muscles were scattered throughout the nerve fascicle. Therefore, nerve fibers innervating the abductor muscle and those innervating the adductor muscle were not located separately, instead being mixed, in the nerve fascicle.
Neurotransmitters Contained in the Recurrent Laryngeal Nerve
Malmgren and Gacek [19] classified cholinergic nerve fibers into two groups, in terms of the stainability and diameter of cat and human recurrent laryngeal nerve fibers as determined by acetylcholinesterase staining. One is a group of motor nerve fibers that were strongly stained and measured 4–12 μm, the other a group of nerve fibers measuring 1–5 μm in diameter with strong or moderate stainability, which the authors speculated were either sensory or autonomic nerve fibers. It is well known that motor nerve and parasympathetic preganglionic fibers are cholinergic nerve fibers. Our present study confirmed that recurrent laryngeal nerves are also cholinergic. The results of this study raise the possibility that cholinergic sensory nerve fibers are present as well.
In 1982, we first demonstrated, employing the Falck-Hillarp method, that adrenergic nerve fibers were contained in the canine recurrent laryngeal nerve [2]. In addition, in 1985, we also demonstrated for the first time, using an immunohistochemical method, that there were nerve fibers containing substance P (SP) [20]. Thereafter, Hauser-Kronberger et al. [21] reported that neuropeptide Y (NPY)-, vasoactive intestinal polypeptide (VIP)-, and calcitonin gene-related peptide (CGRP)-positive nerve fibers were contained in the human recurrent laryngeal nerve. Because it was difficult to identify neurotransmitters in the nerve fiber without employing ligation or crush processing, we later used gold-labeled cholera toxin B (CTBG) as a tracer and identified neurotransmitters contained in the innervating nerve fibers. As a result, it became apparent that the inferior laryngeal nerve, which is the terminal branch of the recurrent laryngeal nerve in the dog, has acetylcholine (Ach), noradrenaline (NA), CGRP-, SP-, NPY-, and nitric oxide (NO)-ergic nerve fibers [22].
Identification of NA Fibers in the Recurrent Laryngeal Nerve [2]
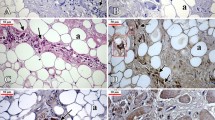
The canine inferior laryngeal nerve consists of the anterior and posterior branches at the laryngeal orifice, and the latter contains numerous unmyelinated fibers (Fig. 5.1). The canine inferior laryngeal nerve was crushed at the laryngeal orifice and processed by the Falck-Hillarp method. Although numerous NA nerve fibers were identified in the posterior branch (Fig. 5.2), only a few were found in the anterior branch. It was also noted that NA nerve fibers initially converged in the marginal region of the posterior branch and then branched off separately (Fig. 5.3a, b). These findings indicate that sympathetic nerve fibers are abundant in the posterior branch of the inferior laryngeal nerve. We can also reasonably speculate that these nerve fibers branch off from the main trunk of the nerve, while forming small nerve fascicles in the vicinity of the larynx, eventually reaching the muscle layer and the mucosa.
Canine inferior laryngeal nerve stained with toluidine blue ((a) anterior branch, (b) posterior branch). The posterior branch can be seen to contain numerous unmyelinated fibers [2]
Posterior branch of the canine inferior laryngeal nerve processed immunohistochemically by the Falck-Hillarp method. Several fluorescent fibers (NA nerve fibers) can be seen [2]
Posterior branch of the canine inferior laryngeal nerve processed immunohistochemically by the Falck-Hillarp method. NA nerve fibers can be visualized as converging in the marginal region (a) and then branching off separately (b) [2]
Immunohistochemical Identification of Neurotransmitters Contained in the Recurrent Laryngeal Nerve [22]
The canine inferior laryngeal nerve was crushed at the laryngeal orifice and subjected to immunohistochemical analysis using anti-SP antibody. No accumulation of SP-positive substances was found in the crushed area, but the presence of a few SP-positive nerve fibers was confirmed. In this regard, cell bodies extending fibers to the interior laryngeal nerve were labeled using CTBG as a tracer, and labeled cells in the nucleus ambiguus, dorsal motor nucleus of the vagus nerve, nodose ganglion, and superior cervical ganglion were examined for neurotransmitters.
CTBG was injected into the canine right inferior laryngeal nerve at the level of the first tracheal ring and subjected to perfusion fixation after 48 hour. The brain stem, nodose ganglia, and superior cervical ganglia were extirpated, and sections were prepared. After visualizing CTBG by silver enhancement, immunohistochemical procedures with various antibodies and the NADPH-diaphorase (NADPHd) histochemical method for identification of NO were carried out. Anti-choline acetyltransferase (ChAT) antibody was used for identification of Ach, and anti-tyrosine hydroxylase (TH) antibody was used for identification of NA. Table 5.1 shows the target substances and the results of analysis at each site.
In the nucleus ambiguous, almost all CTBG-labeled cells were positive for ChAT, and most of the labeled cells were positive for CGRP.
The majority of cells in the dorsal motor nucleus of the vagus nerve were positive for ChAT. CTBG-labeled cells were mostly positive for ChAT, and there were also CGRP-positive cells.
CTBG-labeled cells were scattered throughout the nodose ganglion, and CGRP, SP, TH, and NADPHd-positive cells were present as well (Fig. 5.4). CGRP-positive cells were observed most frequently, followed by NADPHd-positive and SP-positive cells, whereas TH-positive cells were very rare.
In the superior cervical ganglion, CTBG-labeled cells were located mainly on the caudal and medial sides of the ganglion, and TH-positive cells were most frequent, followed by NPY-positive cells. Though VIP-positive cells were also present, they were very rare.
These results indicate that the inferior laryngeal nerve functions not only as a motor nerve but also has sensory and autonomic nerve functions mediated via various transmitters. This very interesting finding suggests that attention should not be focused solely on vocal cord motion disorders when considering the pathological condition of the recurrent laryngeal nerve paralysis.
References
Freeman FR. Galen’s ideas on neurological function. J Hist Neurosci. 1994;3:263–71.
Hisa Y. Fluorescence histochemical studies on the noradrenergic innervation of the canine larynx. Acta Anat. 1982;113:15–25.
Wyke BD, Kirchner JA. Neurology of the larynx. In: Hinchcliffe R, Harrison D, editors. Scientific foundations of otolaryngology. London: William Heinemann Medical Books; 1976. p. 546–74.
Dilworth TFM. The nerves of the human larynx. J Anat. 1921;56:48–52.
Lemere F. Innervation of the larynx. II. Ramus anastomoticus and ganglion cells of the superior laryngeal nerve. Anat Rec. 1932;54:389–407.
Armstrong WG, Hinton JW. Multiple divisions of the recurrent laryngeal nerve. Arch Surg. 1951;62:532–9.
Bowden REM. The surgical anatomy of the recurrent laryngeal nerve. Br J Surg. 1955;43:153–63.
Harrison DFN. Fiber size frequency in the recurrent laryngeal nerves of man and giraffe. Acta Otolaryngol. 1981;91:383–9.
Boden RE. Innervation of intrinsic laryngeal muscles. In: Wyke B, editor. Ventilatory and phonatory control system. London: Oxford University Press; 1974. p. 370–91.
Gacek RR, Lyon MJ. Fiber components of the recurrent laryngeal nerve in the cat. Ann Otol Rhinol Laryngol. 1976;85:460–71.
Dahlqvist A, Carlsoo B, Hellstrom S. Fiber components of the recurrent laryngeal nerve of the rat: a study by light and electron microscopy. Anat Rec. 1982;204:365–70.
Shin T, Rabuzzi D. Conduction studies of the canine recurrent laryngeal nerve. Laryngoscope. 1971;81:586–96.
Atkins JP. An electromyographic study of recurrent laryngeal nerve conduction and its clinical application. Laryngoscope. 1973;83:796–807.
Tomasch J, Britton WA. A fiber-analysis of the recurrent laryngeal nerve supply in man. Acta Anat. 1955;23:386–98.
Semon F. Clinical remarks on the proclivity of the abductor fibers of the recurrent laryngeal nerves to become affected sooner than the adductor fibers, or even exclusively, in cases of undoubted central or peripheral injury or disease of the roots or trunks of the pneumogastric, spinal accessory, or recurrent nerves. Arch Laryngol. 1881;2:197–222.
Hirose H. Sir Felix Semon’s original article about the recurrent laryngeal nerve paralysis. Otolaryngol Head Surg (Tokyo). 1967;39:1053–7.
Sunderland S, Swaney WE. The intraneural topography of the recurrent laryngeal nerve in man. Anat Rec. 1952;114:411–26.
Gacek RR, Malmgren LT, Lyon MJ. Localization of adductor and abductor motor nerve fibers to the larynx. Ann Otol Rhinol Laryngol. 1977;86:770–6.
Malmgren LT, Gacek RR. Acetylcholinesterase staining of fiber components in feline and human recurrent laryngeal nerve. Acta Otolaryngol. 1981;91:337–52.
Hisa Y, Sato F, Fukui K, Ibata Y, Mizukoshi O. Substance P nerve fibers in the canine larynx by PAP immunohistochemistry. Acta Otolaryngol. 1985;100:128–33.
Hauser-Kronberger C, Albegger K, Saria A, Hacker GW. Regulatory peptides in the human larynx and recurrent nerves. Acta Otolaryngol. 1993;113:409–13.
Hisa Y, Uno T, Tadaki N, Okamura H, Ibata Y. Neurotransmitters in the canine inferior laryngeal nerve. Larynx Jpn. 1994;6:117–21.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer Japan
About this chapter
Cite this chapter
Uno, T., Hisa, Y. (2016). Recurrent Laryngeal Nerve. In: Hisa, Y. (eds) Neuroanatomy and Neurophysiology of the Larynx. Springer, Tokyo. https://doi.org/10.1007/978-4-431-55750-0_5
Download citation
DOI: https://doi.org/10.1007/978-4-431-55750-0_5
Published:
Publisher Name: Springer, Tokyo
Print ISBN: 978-4-431-55749-4
Online ISBN: 978-4-431-55750-0
eBook Packages: MedicineMedicine (R0)