Abstract
Cerebral oscillation is a neurophysiological phenomenon related to cerebral rhythmic activity that reflects neural activity. It changes depending on oscillatory frequency, reflecting various brain functions including sensory, motor, and language functions. These oscillatory changes are used as decoding features of brain-machine interfaces. Recently, it has become clear that these oscillations in the different frequency bands are synchronized with each other. This phenomenon is called cross-frequency coupling. We propose a hypothetical model: brain networks have a low-frequency oscillatory property and high γ activity in local circuits that are modulated by cross-frequency coupling between the phase of the low-frequency oscillation of the brain network and the amplitude of the high γ activity of local circuits.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Cerebral Oscillation
1.1 Cerebral Oscillatory Changes
Synchronous oscillations in specific frequency bands such as alpha waves are well known as basic brain rhythms. These basic rhythms change signal power due to brain activation. Event-related desynchronization (ERD) is an attenuation of the oscillation amplitude of a specific frequency band that occurs in relation to specific neural activities [1]. The opposite phenomenon, event-related synchronization (ERS), is an increase in that amplitude [2]. The best-known frequency band is α (8–13 Hz). So-called α waves are detected from the parieto-occipital area during relaxed wakefulness. The α wave increases when the eyes are closed and decreases when opened. This α blocking is one of the most typical examples of ERDs. In addition to the α band, frequency bands are divided depending on neurophysiological properties as follows: δ (1–4 Hz), θ (4–8 Hz), β (13–25 Hz), low γ (25–50 Hz), and high γ (>50 Hz) frequency bands.
Synchronous oscillations can be measured using EEG, MEG, and electrocorticography (ECoG) which is a method of recording neural activity from electrodes directly placed on the cortical surface. Of these three brain signals, ECoGs are the most precise with respect to both spatial and temporal resolution, and they provide a good and typical sample of time-frequency spectrograms for cerebral oscillatory changes including high-frequency components (Fig. 17.1). In Fig. 17.1, ECoGs were recorded during right-hand grasping from subdurally placed grid electrodes placed over the left sensorimotor areas in an intractable epilepsy patient. ERDs are observed in the α and β bands over the sensorimotor areas and are broadly distributed, whereas ERSs are observed in the high γ band in the sensorimotor areas and are more focally distributed [3, 4]. Regarding time domain, ERDs occur 500–1000 ms prior to muscle contraction and are sustained even after the end of muscle contraction, whereas ERSs are more restricted to the periods of muscle contraction. ERS in the high γ band is known to reflect functional localization of the brain better than ERD in the α and β bands. These oscillatory changes during movements are referred to as movement-related cerebral oscillatory changes. Cerebral oscillatory changes are observed not only during movements but also during language activities, sensory processing, and mental concentration.
Cerebral oscillatory changes recorded from subdural grid electrodes. (a) Electrode configuration on the brain. (b) Time-frequency spectrograms of the grid electrodes. ERS in the γ band (red color) is focally distributed, whereas ERD in the α and β bands (blue color) is broadly distributed. (c) A time-frequency spectrogram (upper) and a cortical potential graph (lower) of electrode 8
ECoG provides us with precise information on neural activities directly from brain surface electrodes, but electrode placement requires brain surgery, whereas MEG is noninvasive as well as precise in functional localization. However, compared to ECoG, MEG does not consistently detect high γ band activity, unless time-locked stimuli or tasks are used in the case of the primary sensory or motor areas. Therefore, MEG is generally inferior to ECoG regarding functional localization.
1.2 Somatosensory Processing
Typical neuromagnetic somatosensory responses are observed when we stimulate major peripheral nerves of the body. We measured neuromagnetic responses using a whole-head type axial gradiometer equipped with 64 SQUID sensors. Figure 17.2a shows the averaged waveforms of the somatosensory evoked neuromagnetic fields recorded from all 64 SQUID sensors for 100 electrical stimuli to the right median nerve at the wrist of a healthy subject. Typical neuromagnetic components are clearly observed at 20 ms, 26 ms, 39 ms, and 51 ms after the stimuli. Isomagnetic field maps clearly show a pair of inflow and outflow neuromagnetic fields (Fig. 17.2b). A current dipole equivalent to the magnetic field at 20 ms after the stimuli is localized just in the contralateral postcentral gyrus (Fig. 17.2c). The postcentral gyrus is well known as the primary somatosensory area, where somatosensory processing such as touch and vibration sensation of the body is undertaken.
Typical somatosensory evoked neuromagnetic fields (SEFs) induced by peripheral nerve stimulation. (a) Averaged waveforms of the SEFs for 100 electrical stimuli to the right median nerve. (b) Isomagnetic field maps showing a pair of inflow and outflow of neuromagnetic fields. (c) A current dipole equivalent to the magnetic field at 20 ms localized in the contralateral postcentral gyrus
Beamformer analysis provides us with additional information about the spatiotemporal distribution of oscillatory activity of somatosensory processing. The ERS is focally localized in the contralateral postcentral gyrus in the high γ band, whereas the ERDs are more broadly localized over the bilateral postcentral gyri in the α band, lateralized to the contralateral side (Fig. 17.3) [5]. The high γ ERS is suggested to reflect activation of the primary somatosensory area, whereas α ERDs are suggested to reflect the modulatory responses of somatosensory processing.
Clinically, high γ ERS induced by somatosensory stimulation is useful to identify the central sulcus as well as the dipole of neuromagnetic responses at 20 ms evoked by median nerve stimulation.
1.3 Motor Processing
Similar oscillatory changes are observed in the motor cortex during motor execution, as described above. These oscillatory changes during motor execution can also be observed using MEG. ERDs are observed in the α and β bands, broadly localized over the bilateral primary sensorimotor areas, lateralized to the contralateral side [6, 7]. Beta ERD is generally more anteriorly localized than α ERD, suggesting that β ERD reflects motor processing, while α ERD reflects somatosensory processing [7, 8]. In contrast, ERSs in the high γ band are focally localized on the contralateral precentral gyrus, well reflecting the activation of the contralateral primary motor area [9]. However, it is difficult to consistently detect high γ ERS using MEG. Therefore, β ERD is often used to evaluate the location of the primary motor area (Fig. 17.4). Although it is difficult to detect high γ ERS on a single trial basis with MEG, ECoG can detect high γ ERS on a single trial basis. Using this trial-by-trial high γ ERS as a decoding feature, hand movements can be differentiated, and a robotic arm can be controlled in real time [10, 11] (Fig. 17.5).
1.4 Language Processing
Compared to somatosensory processing and motor processing, neuromagnetic responses to language processing are more complex. We use a silent reading task to avoid noise contamination due to muscle contraction during phonation. A three-character hiragana word was presented on a display for 3 s. Subjects were instructed to silently read the words once, as soon as the words were presented. A total of 100 words were presented serially every 6 s. Figure 17.6a shows the averaged waveforms for neuromagnetic responses of all 64 SQUID sensors for visually presented hiragana words in a healthy subject. They have later latency components and isofield maps indicate more complex inflow and outflow distributions than those of somatosensory processing (Fig. 17.6b). Single dipole analyses managed to localize a dipole until 330 ms, but represented only part of the complex neuromagnetic fields. Multiple dipole analyses failed to localize stable dipoles with sufficient goodness of fit.
Beamformer analyses provide us with information on the complex spatial distribution of cerebral oscillatory changes during silent reading. Figure 17.7 shows the results of group analysis for 14 healthy right-handed subjects. It is noteworthy that the spatial distribution is dependent on the frequency bands of the oscillatory changes. ERDs in the α band are distributed in the posterior (receptive) language area, whereas ERDs in the low γ band are distributed in the frontal (expressive) language area [12]. In addition, we found that the left and right lateralization of ERD in the frontal area in the low γ band well corresponds to language dominance [13]. Using this property, we established a method to evaluate language dominance noninvasively with beamforming MEG analyses. Consistency is approximately 85 % with the standard but invasive method for evaluating language dominance (the Wada test), which requires the injection of anesthetic agents intra-arterially at the carotid artery [12]. Beamforming MEG analysis is considered to be an alternative to the Wada test in selected cases. Figure 17.8 shows an example of language dominance evaluated by this method.
Sliding time-window analyses of beamforming MEG revealed the temporal profiles of cerebral oscillatory changes during silent reading (Fig. 17.9) [14]. We found that the transient ERS first occurred in the occipital visual area and then in the temporo-occipital language areas and finally propagated to the frontal language areas. This transient θ (8–13 Hz) ERS was followed by α ERDs in the temporo-occipital language areas and low γ ERDs in the frontal language areas. High γ ERS was found in the occipital lobe, which reflects visual processing. It seems that transient θ ERS reflects serial processing, while α and low γ ERDs reflect parallel neural processing.
High γ ERSs related to language processing can be detected using ECoGs [15]. Figure 17.10 shows the relationship between the spatial distribution of high γ ERS induced by a verb generation task and the result of cortical electrical stimulation mapping. The localization of high γ ERS is well concordant with that of cortical mapping. Although it is generally difficult to detect high γ ERS related to language processing in individual subjects with MEG, high γ ERS can be detected by MEG by combining group analyses, targeted time windows, and tasks with high task demand to the frontal language area such as a verb generation tasks (Fig. 17.11).
2 Cross-Frequency Coupling and Oscillatory Coupling Network Model
Cross-frequency coupling is a phenomenon where cerebral oscillations in the different frequency bands are synchronized (Fig. 17.12). Cross-frequency coupling has been reported in various cerebral areas [16, 17]. It is well known that the amplitude of the high γ band activity is modulated by the phase of the θ band activity. In general, slow oscillations in the θ, α, and β bands modulate fast oscillations in the high γ band [18]. We found that in using ECoGs, the amplitude in the high γ band is coupled with the phase in the α band more than 2 s before the onset of hand movements and decoupled just before movement onset [17]. This cross-frequency phase-amplitude coupling in the motor cortex might play an important role in control of movement initiation and postures.
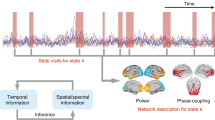
Cerebral network models have been proposed regarding neural networks between the cortical areas and deep brain structures as well as cortico-cortical networks [19]. Based on the above-mentioned cerebral oscillation and cross-frequency coupling, we propose an oscillatory coupling network model of the brain (Fig. 17.13). Sensory networks consisting of thalamocortical loops are dominated by α band activity including α waves. Motor networks consisting of cortico-basal ganglia loops are dominated by α and β band activity including μ waves. Memory networks consisting of hippocampus/amygdala-cortical loops are dominated by θ band activity. Executive networks consisting of parieto-prefrontal circuits are dominated by low γ band activity. High γ band activity is a pivotal activity of local circuits in the cortical and deep brain structures consisting of these networks. Its activity may be modulated by the phase of slow oscillatory activity including those of the θ, α, β, and low γ bands specific to each network.
Proposed oscillatory coupling network model. Sensory network dominated by α band activity, motor network dominated by α and β band activity, memory network dominated by θ band activity, and executive network dominated by low γ band activity. High γ band activity is a pivotal activity of local circuits modulated by the phase of the slow oscillatory activity including those of the θ, α, β, and low γ bands specific to each network
References
Pfurtscheller G, Aranibar A. Event-related cortical desynchronization detected by power measurements of scalp EEG. Electroencephalogr Clin Neurophysiol. 1977;2:817–26.
Pfurtscheller G. Event-related synchronization (ERS): an electrophysiological correlate of cortical areas at rest. Electroencephalogr Clin Neurophysiol. 1992;83:62–9.
Crone NE, Miglioretti DL, Gordon B, Sieracki JM, Wilson MT, Uematsu S, et al. Functional mapping of human sensorimotor cortex with electrocorticographic spectral analysis. I. Alpha and beta event-related desynchronization. Brain. 1998;121(Pt 12):2271–99.
Crone NE, Miglioretti DL, Gordon B, Lesser RP. Functional mapping of human sensorimotor cortex with electrocorticographic spectral analysis. II. Event-related synchronization in the gamma band. Brain. 1998;121(Pt 12):2301–15.
Hirata M, Kato A, Taniguchi M, Ninomiya H, Cheyne D, Robinson SE, et al. Frequency-dependent spatial distribution of human somatosensory evoked neuromagnetic fields. Neurosci Lett. 2002;318(2):73–6.
Jurkiewicz MT, Gaetz WC, Bostan AC, Cheyne D. Post-movement beta rebound is generated in motor cortex: evidence from neuromagnetic recordings. Neuroimage. 2006;32(3):1281–9. doi:10.1016/j.neuroimage.2006.06.005.
Taniguchi M, Kato A, Fujita N, Hirata M, Tanaka H, Kihara T, et al. Movement-related desynchronization of the cerebral cortex studied with spatially filtered magnetoencephalography. Neuroimage. 2000;12(3):298–306. doi:10.1006/nimg.2000.0611.
Salmelin R, Hari R. Spatiotemporal characteristics of sensorimotor neuromagnetic rhythms related to thumb movement. Neuroscience. 1994;60:537–50.
Cheyne D, Bells S, Ferrari P, Gaetz W, Bostan AC. Self-paced movements induce high-frequency gamma oscillations in primary motor cortex. Neuroimage. 2008;42(1):332–42. doi:10.1016/j.neuroimage.2008.04.178.
Yanagisawa T, Hirata M, Saitoh Y, Goto T, Kishima H, Fukuma R, et al. Real-time control of a prosthetic hand using human electrocorticography signals. J Neurosurg. 2011;114(6):1715–22. doi:10.3171/2011.1.jns101421.
Yanagisawa T, Hirata M, Saitoh Y, Kishima H, Matsushita K, Goto T, et al. Electrocorticographic control of a prosthetic arm in paralyzed patients. Ann Neurol. 2012;71(3):353–61. doi:10.1002/ana.22613.
Hirata M, Goto T, Barnes G, Umekawa Y, Yanagisawa T, Kato A, et al. Language dominance and mapping based on neuromagnetic oscillatory changes: comparison with invasive procedures. J Neurosurg. 2010;112(3):528–38. doi:10.3171/2009.7.JNS09239.
Hirata M, Kato A, Taniguchi M, Saitoh Y, Ninomiya H, Ihara A, et al. Determination of language dominance with synthetic aperture magnetometry: comparison with the Wada test. Neuroimage. 2004;23(1):46–53. doi:10.1016/j.neuroimage.2004.05.009.
Goto T, Hirata M, Umekawa Y, Yanagisawa T, Shayne M, Saitoh Y et al. Frequency-dependent spatiotemporal distribution of cerebral oscillatory changes during silent reading: a magnetoencephalograhic group analysis. Neuroimage. 2011;54(1):560–7. doi:S1053-8119(10)01105-5 [pii] 10.1016/j.neuroimage.2010.08.023 [doi].
Crone NE, Hao L, Hart Jr J, Boatman D, Lesser RP, Irizarry R, et al. Electrocorticographic gamma activity during word production in spoken and sign language. Neurology. 2001;57(11):2045–53.
Canolty RT, Edwards E, Dalal SS, Soltani M, Nagarajan SS, Kirsch HE, et al. High gamma power is phase-locked to theta oscillations in human neocortex. Science (New York, NY). 2006;313(5793):1626–8. doi:10.1126/science.1128115.
Yanagisawa T, Yamashita O, Hirata M, Kishima H, Saitoh Y, Goto T, et al. Regulation of motor representation by phase-amplitude coupling in the sensorimotor cortex. J Neurosci. 2012;32(44):15467–75. doi:10.1523/jneurosci.2929-12.2012.
Buzsaki G, Wang XJ. Mechanisms of gamma oscillations. Annu Rev Neurosci. 2012;35:203–25. doi:10.1146/annurev-neuro-062111-150444.
Krack P, Hariz MI, Baunez C, Guridi J, Obeso JA. Deep brain stimulation: from neurology to psychiatry? Trends Neurosci. 2010;33(10):474–84. doi:10.1016/j.tins.2010.07.002.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer Japan
About this chapter
Cite this chapter
Hirata, M. (2016). Oscillation and Cross-Frequency Coupling. In: Tobimatsu, S., Kakigi, R. (eds) Clinical Applications of Magnetoencephalography. Springer, Tokyo. https://doi.org/10.1007/978-4-431-55729-6_17
Download citation
DOI: https://doi.org/10.1007/978-4-431-55729-6_17
Published:
Publisher Name: Springer, Tokyo
Print ISBN: 978-4-431-55728-9
Online ISBN: 978-4-431-55729-6
eBook Packages: MedicineMedicine (R0)