Abstract
O-glycans are the cell-surface carbohydrates attached to membrane proteins through serine (Ser) or threonine (Thr). Recently, extensive investigation on O-glycans in cancer cells has revealed that there is a certain type of O-glycan that can promote cancer cell metastasis. Cancer cells expressing the O-glycans called core2 O-glycans exhibit a high ability to evade host tumor rejection systems by natural killer (NK) cells. Evasion from the NK tumor rejection systems by using core2 O-glycans facilitates the cancer cells to survive longer in host circulation. Core2 O-glycans expressing cancer cells thus increase the chance to invade other organs, thereby resulting in the promotion of metastasis. In this chapter, it is illustrated how core2 O-glycans in cancer cells evade host immune systems to promote metastasis.
Access provided by Autonomous University of Puebla. Download reference work entry PDF
Similar content being viewed by others
Keywords
- NK tumor immunity
- Immune evasion mechanism
- NK receptor
- Tumor necrosis factor-related apoptosis-inducing ligand (TRAIL)
- Ligand masking
- Molecular shield
Introduction
Cancer cells transit from the primary site via host blood circulation to form metastases in distant organs. During this process, cancer cells encounter numbers of environmental challenges such as tumor rejection systems by innate immunity. These systems profoundly impact the metastatic potential of the cancer cells. In the tumor rejection systems, natural killer (NK) cells play a critical role to suppress metastasis. NK cells are innate lymphocytes that can recognize and lyse cancer and infected cells without presensitization. It was known that some cancer cells establish metastasis by exerting several evasion strategies from the NK tumor rejection systems. It has been recently revealed that there is a certain type of O-glycan (carbohydrates attached to cell-surface glycoproteins through serine or threonine residues) that provides evasion function from the NK tumor rejection systems. Cancer cells expressing such O-glycans survive longer in host circulation, thereby exhibiting highly metastatic phenotypes. This chapter illustrates two evasion mechanisms from NK tumor immunity by using the O-glycans.
O-Glycans and Core Structures
O-glycans have numbers of important functions in biologically fundamental phenomena including cell differentiation, development, and immunity (Tsuboi and Fukuda 2001). There are four common O-glycans core structures in mammalian tissues, namely, core1, core2, core3, and core4, which depend on the combination of sugars added (Fig. 1). Among them, O-glycan mainly involved in numbers of immunological processes is core2 O-glycan. Core2 O-glycans contain a core2 branch (GlcNAcβ1 → 6(Galβ1 → 3)GalNAcα1 → Ser/Thr) and the branch is formed by core2 β-1,6-N-acetylglucosaminyltransferase (C2GnT). Three C2GnTs (C2GnT-1, C2GnT-2, and C2GnT-3) that can form core2 branch are known. Only C2GnT-2 also has the ability to form core4 branch (Fig. 1). The core2 branch serves as a scaffold for the subsequent production of lactosamine disaccharide (Galβ1 → 4GlcNAc) n unit called poly-N-acetyllactosamine on O-glycans (Tsuboi 2013; Tsuboi et al. 2012; Fig. 1).
Biosynthesis pathways of O-glycans. The first step to synthesize core1–core4 O-glycans is the transfer of N-acetylgalactosamine (GalNAc) to a serine (Ser) or threonine (Thr) residue in a polypeptide by the peptide GalNAc transferase (GalNAc-T). GalNAcα1-Ser/Thr is then converted to core1 (Galβ1 → 3GalNAcα1 → Ser/Thr). Core1 is converted to core2 (GlcNAcβ1 → 6(Galβ1 → 3)GalNAcα1 → Ser/Thr by β-1,6-N-acetylglucosaminyltransferases (C2GnT-1, C2GnT-2, and C2GnT-3). Core2 O-glycans are a scaffold for the subsequent extension of lactosamine disaccharide unit, (Galβ1 → 4GlcNAc) n called poly-N-acetyllactosamine by β-1,4-galactosyltransferase IV (β1 → 4Gal-T IV) together with β-1,3-N-acetylglucosaminyltransferase (β1 → 3GalNAc-T V). The number of Galβ1 → 4GlcNAc unit repeats varies depending on the carrier molecules and cell types. Core1 is also converted to core3. Core3 is then converted to core4. C2GnT expression was closely correlated with high-metastatic phenotypes. In contrast to C2GnT, core3 synthase expression is generally observed in normal tissues
Immune Evasion Mechanisms by Core2 O-Glycans
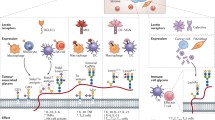
C2GnT-1 (as C2GnT hereafter) is a key enzyme for the formation of core2 O-glycans containing poly-N-acetyllactosamine. It was reported that the expression of C2GnT was closely correlated with high-metastatic phenotypes of several types of cancer such as colorectal cancer, lung cancer, prostate cancer, testicular germ cell tumors, and bladder cancer (Tsuboi 2012; Tsuboi et al. 2012). Those pathological observations led us to postulate that C2GnT-expressing cancer cells have an ability to evade host tumor rejection systems. One of the most critical players in tumor rejection systems is natural killer (NK) cell. NK cells represent one of the effector cells which can kill cancer cells in host circulation. The molecular mechanisms of the immune evasion from NK tumor rejection systems by C2GnT-expressing cancer cells have recently unraveled. It is known that the poly-N-acetyllactosamine formed by C2GnT is a ligand for galectin-3 (Tsuboi 2012; Tsuboi et al. 2012; Fig. 2a). This galectin-3 binding to poly-N-acetyllactosamine is a key for the molecular mechanisms of the immune evasion.
Immune evasion mechanisms of NK cell tumor rejection systems by core2 O-glycans. (a) Modification of MICA O-glycans. Core2 O-glycans are a scaffold for the subsequent extension of lactosamine disaccharide unit, (Galβ1 → 4GlcNAc)n called poly-N-acetyllactosamine. The number of Galβ1 → 4GlcNAc unit repeats varies depending on the carrier molecules and cell types. A β-galactose-binding protein, galectin-3, binds to MICA and MUC1 through this poly-N-acetyllactosamine. (b) NK cells are activated by the interaction of NKG2D with MICA expressed on the cancer cell surface. Activated NK cells secrete perforin and granzyme B to induce apoptosis of target cancer cells. (c) Galectin-3 binds to the NKG2D-binding site of MICA through poly-N-acetyllactosamine. Modification of MICA with poly-N-acetyllactosamine and galectin-3 reduces the affinity of MICA for NKG2D, impairing NK cell activation. This masking increases survival of the cancer cells in host blood circulation, promoting metastasis. (d) TRAIL expressed in NK cells interacts with death receptors (DR4) expressed in cancer cells to induce apoptosis of target cancer cells. (e) Modification of MUC1 with poly-N-acetyllactosamine and galectin-3 interferes with the access of TRAIL to DR4, impairing TRAIL-mediated killing of target cancer cells. This shielding also increases survival of the cancer cells in host blood circulation, promoting metastasis
Ligand Masking
There are two major tumor rejection systems by NK cells. One is the NK cell receptor-tumor ligand interaction-mediated killing. Numbers of NK receptor s are involved in tumor rejection. Among them, the interaction of the NK-activating receptor, natural killer group 2 member D (NKG2D), with the cancer-cell-expressing ligand, MHC class I-related chain A (MICA), is critical for eliminating cancer cells. The NKG2D-MICA interaction activates NK cell to secrete several apoptosis-inducing substances such as granzyme B and perforin, thereby killing target cancer cells (Fig. 2b).
The immune evasion mechanism of this first tumor rejection system by using core2 O-glycans in C2GnT-expressing cancer cells is as follows (Fig. 2c):
-
1.
The NKG2D-binding site in MICA is modified with core2 O-glycans.
-
2.
The core2 branch in core2 O-glycans serves as a scaffold for the subsequent production of poly-N-acetyllactosamine consisting of the repeat of a disaccharide unit (Galβ1 → 4GlcNAc) n .
-
3.
Galectin-3 binds to the poly-N-acetyllactosamine on the O-glycans in NKG2D-binding site of MICA.
-
4.
Modification of MICA O-glycans with poly-N-acetyllactosamine and galectin-3 masks the NKG2D-binding site in MICA.
-
5.
This masking of NKG2D-binding site of MICA reduces the affinity of MICA for NKG2D.
-
6.
Reduction of the MICA binding affinity for NKG2D severely impairs NK cell activation.
In a manner described above, core2 O-glycans on MICA finally prevent NK cell-mediated killing of C2GnT-expressing cancer cells (Tsuboi et al. 2011; Fig. 2c).
Molecular Shield
The second NK tumor rejection system is the tumor necrosis factor (TNF)-related apoptosis-inducing ligand (TRAIL )-mediated killing. TRAIL expressed on the NK cell surface directly stimulates cancer cell-expressing death receptors such as DR4, thereby inducing apoptosis of target cancer cells (Fig. 2d).
The immune evasion mechanism of this second tumor rejection system by using core2 O-glycans in C2GnT-expressing cancer cells is as follows (Fig. 2e):
-
1.
One of the cell-surface mucins (MUC1) is heavily modified with core2 O-glycans.
-
2.
The core2 branch in the MUC1 core2 O-glycans serves as a scaffold for the subsequent production of poly-N-acetyllactosamine.
-
3.
Galectin-3 binds to the poly-N-acetyllactosamine on the MUC1 O-glycans.
-
4.
Modification of MUC1 O-glycans with poly-N-acetyllactosamine and galectin-3 shields against the access of TRAIL on NK cells to DR4 on cancer cells.
In a manner described above, core2 O-glycans on MUC1 finally prevent NK cell TRAIL -mediated killing of C2GnT-expressing cancer cells (Okamoto et al. 2013; Suzuki et al. 2012; Fig. 2e).
These two immune evasion mechanism s (Fig. 2c, e) facilitate C2GnT-expressing cancer cells to survive longer in the host circulation and to increase the chance to invade other organs, finally promoting metastasis.
Concluding Remarks
This chapter summarizes the newly identified molecular mechanisms of the immune evasion from NK tumor rejection systems by using cell-surface carbohydrates. There are two major NK cell-mediated tumor rejection systems. To evade each rejection system, C2GnT-expressing cancer cells exert two different types of immune evasion strategies by using core2 O-glycans. At present, these two novel evasion strategies have been demonstrated in bladder and prostate cancers (Okamoto et al. 2013; Tsuboi et al. 2011). However, considering that the correlation between C2GnT expression and a high-metastatic phenotypes was observed in several other types of cancers (Tsuboi 2012; Tsuboi et al. 2012), it is highly likely that those cancers also employ the same evasion strategies.
In the future, our current understanding of the molecular mechanisms of the immune evasion strategies may lead to the development of new therapeutic methods or agents for preventing metastasis. Reduction of C2GnT activity or downregulation of C2GnT expression using specific inhibitors or siRNA-based drugs could restore susceptibility to NK cell attack, promoting tumor rejection and suppressing metastasis.
References
Okamoto T, Yoneyama Sutoh M, Hatakeyama S et al (2013) Core2 O-glycan-expressing prostate cancer cells are resistant to NK cell immunity. Mol Med Rep 7:359–364
Suzuki Y, Sutoh M, Hatakeyama S et al (2012) MUC1 carrying core2 O-glycans functions as a molecular shield against NK cell attack, promoting bladder tumor metastasis. Int J Oncol 40:1831–1838
Tsuboi S (2012) Tumor defense systems using O-glycans. Biol Pharm Bull 35:1633–1636
Tsuboi S (2013) Immunosuppressive functions of core2 O-glycans against NK immunity. Trends Glycosci Glycotech 25:117–123
Tsuboi S, Fukuda M (2001) Roles of O-linked oligosaccharides in immune responses. Bioessays 23:46–53
Tsuboi S, Sutoh M, Hatakeyama S et al (2011) A novel strategy for evasion of NK cell immunity by tumours expressing core2 O-glycans. EMBO J 30:3173–3185
Tsuboi S, Hatakeyama S, Ohyama C et al (2012) Two opposing roles of O-glycans in tumor metastasis. Trends Mol Med 18:224–232
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer Japan
About this entry
Cite this entry
Tsuboi, S. (2015). Glycans Against NK Tumor Immunity. In: Taniguchi, N., Endo, T., Hart, G., Seeberger, P., Wong, CH. (eds) Glycoscience: Biology and Medicine. Springer, Tokyo. https://doi.org/10.1007/978-4-431-54841-6_190
Download citation
DOI: https://doi.org/10.1007/978-4-431-54841-6_190
Received:
Accepted:
Published:
Publisher Name: Springer, Tokyo
Print ISBN: 978-4-431-54840-9
Online ISBN: 978-4-431-54841-6
eBook Packages: Biomedical and Life SciencesReference Module Biomedical and Life Sciences