Abstract
Lactosylceramide (LacCer), which is abundantly expressed on plasma membranes and stored in granules of mature human neutrophils, forms membrane microdomains together with the Src family tyrosine kinase Lyn. We previously demonstrated that LacCer-enriched membrane microdomains mediate superoxide generation, migration, and phagocytosis, suggesting that LacCer functions as a pattern recognition receptor (PRR) in the innate immune response of neutrophils. Glycosphingolipid–receptor cis interactions have been found to be critical in mediating various physiological functions. The interactions of GM3 with insulin receptor and epidermal growth factor receptor have been found to regulate these receptor-mediated functions. We also found that the interaction of LacCer with αMβ2 integrin (CD11b/CD18) is essential for CD11b/CD18-mediated neutrophil phagocytosis of non-opsonized microorganisms. We describe here the significance of the LacCer–CD11b/CD18 interaction in the innate immune response of human neutrophils.
Access provided by Autonomous University of Puebla. Download reference work entry PDF
Similar content being viewed by others
Keywords
Introduction
Glycosphingolipid (GSL) expressed on various cells interacts with other receptors on plasma membranes to regulate signal transduction. The ganglioside GM3 is associated with insulin receptor (IR) in adipocytes (Kabayama et al. 2007), and the interaction of GM3 with EGFR also regulates the function of the latter (Coskun et al. 2011). These GM3–receptor interactions are mediated by GM3 binding of lysine residues in the membrane-proximal regions of both IR and EGFR. The binding of laminin-1 to GM1, the most abundant glycosphingolipid in brain and neurons, has been shown to facilitate the formation of a focal microdomain with the NGF receptor TrkA in the membrane (Ichikawa et al. 2009). In addition, laminin 1-binding to GM1 has been found to enhance signal transduction, which promotes neurite outgrowth by linking NGF-TrkA signaling with the laminin–integrin signaling pathways (Ichikawa et al. 2009). Taken together, these findings suggest that the interactions between GSL and other receptors may control a wide range of receptor-mediated cellular functions.
Professional phagocytes, such as neutrophils , macrophages, and dendritic cells, are responsible for the innate immune response. This response is initiated by the binding of pathogen-associated molecular patterns (PAMPs) to pattern-recognition receptors (PRRs) expressed on phagocytes, forming phagosomes and resulting in the fusion of lysosomes to phagosomes. PAMPs are directly recognized by PRRs, such as TLRs, C-type lectin receptor (CLR), and αMβ2 integrin (CD11b/CD18). PAMPs can also be sensed by GSLs, which constitute specialized membrane microdomains . The cooperation between GSLs and PRRs seems to be a key step in innate immune responses, playing crucial roles in phagocytosis and the activation of inflammation. Toll-like receptors (TLRs) can sense microbial structures, triggering the production of proinflammatory cytokines. GSLs are able to interact with TLRs. For example, the binding of bacterial flagellin to asialoGM1 and TLR5 expressed on human lung epithelial cells has been found to induce the autocrine release of ATP (McNamara et al. 2006). This released ATP binds to and activates ATP receptors on plasma membranes, leading to Ca2+ mobilization and Erk1/2 phosphorylation. GD1a on human monocytes binds to the subunit of type IIb Escherichia coli enterotoxin, promoting its interaction with the TLR2/TLR1 signaling complex and activating NF-κB activation (Liang et al. 2007). Thus, these findings suggest that GSL–receptor interactions may be involved in various aspects of innate immune responses resulting from the binding of PAMPs. We describe here GSL–cell surface receptor interactions, which modulate innate immune response. We especially focus on our findings that lactosylceramide (LacCer, CDw17, Galβ4Glcβ1Cer) interacts with CD11b/CD18 integrin in non-opsonic phagocytosis in human neutrophils.
The Functional Roles of LacCer in the Innate Immune Response
Of the several types of β-glucans, the β-1,6 long glucosyl side chain-branched β-glucans, such as Candida albicans-derived β-glucan (CSBG), were observed to bind specifically to LacCer expressed on human neutrophils (Sato et al. 2006). CSBG was found to induce neutrophil migration, which was completely inhibited by LacCer liposomes and by the Src family kinase inhibitor PP1. Anti-LacCer antibodies were also shown to induce neutrophil production of superoxide, a response also blocked by PP1 (Iwabuchi and Nagaoka 2002). In addition, Lyn molecules in LacCer-enriched membrane microdomains were phosphorylated by anti-LacCer antibodies (Iwabuchi and Nagaoka 2002), CSBG-derived β-glucan (Sato et al. 2006), and non-opsonized zymosans (Nakayama et al. 2008). Using immunoelectron microscopy and biochemical methods, we recently showed that LacCer is closely associated with Lyn on plasma membranes of human neutrophils (Iwabuchi et al. 2008). LacCer is expressed on neutrophil plasma membranes as clusters around 45 nm in diameter, with 24 % of these clusters associated with Lyn. LacCer-enriched membrane microdomains are mainly composed of LacCer, sphingomyelin, phosphatidylcholine, and cholesterol, along with signal transduction molecules, and can be isolated from detergent-resistant membrane fractions (DRM) of neutrophils using the anti-LacCer monoclonal antibody Huly-m13. These results suggest that Lyn-coupled LacCer-enriched membrane microdomains are responsible for human neutrophil migration, non-opsonic phagocytosis, and superoxide production. Thus, LacCer functions as a PRR to mediate the innate immune responses of neutrophils.
LacCer–CD11b/CD18 Interactions in Non-opsonic Phagocytosis
CD11b/CD18 plays a pivotal role in neutrophil adhesion and phagocytosis. However, the cytoplasmic domain of this integrin receptor does not contain a signaling motif that mediates outside–in signals through the receptor. We recently showed that LacCer-enriched membrane microdomains mediated CD11b/CD18-dependent signaling upon ligand binding to this integrin (Nakayama et al. 2008). During phagocytosis of non-opsonized zymosans, CD11b/CD18 and Lyn-coupled LacCer-enriched membrane microdomains accumulated and colocalized in actin-enriched phagocytic cup regions. Along with stimulation of CD11b/CD18 by the CD11b-activating antibody VIM12, LacCer formed large clusters and colocalized with CD11b on plasma membranes in human neutrophils. In addition, Lyn molecules, which were coimmunoprecipitated by Huly-m13 from VIM12-stimulated neutrophil lysates, were highly activated. These observations suggest that CD11b/CD18-dependent outside–in signaling is mediated by Lyn molecules in LacCer-enriched membrane microdomains.
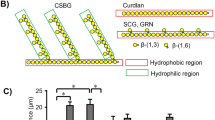
We also focused on functional and significant interactions between LacCer and CD11b/CD18. The monoclonal antibody Huly-m13 did not immunoprecipitate CD11b or CD18 subunit from the DRM fraction of plasma membranes of resting neutrophils (Nakayama et al. 2008). However, after phagocytosis of zymosans, Huly-m13 was able to immunoprecipitate CD18, but not CD11b, from phagosomal-membrane DRM fractions. These findings suggested that CD11b/CD18 on phagosomal membranes is associated with LacCer-enriched membrane microdomains via CD18. To evaluate this hypothesis, we examined the effects of three different anti-CD18 monoclonal antibodies on VIM12-induced Lyn phosphorylation: TS1/18, which recognizes an epitope localized to residues 123–163 in the N-terminal portion and residues 303–344 in the C-terminal portion of the conserved domain (Nakayama et al. 2008; Fig. 1a); CLB-LFA-1/1, which recognizes the C-terminal α-helix of the I-like ligand binding domain; and MEM-48, which recognizes epitopes localized to the C-terminal portion of the cysteine-rich repeat three domains. Of these three monoclonal antibodies, only MEM-48 inhibited VIM12-induced phosphorylation of Lyn molecules coimmunoprecipitated by Huly-m13 from neutrophils. A schematic image of LacCer–CD11b/CD18 interactions in neutrophil phagocytosis is shown in Fig. 1. Similar to the interactions of GM3 with IR and EGFR, CD11b/CD18 was apparently directly associated with LacCer via the proximal membrane region of CD18 on the neutrophil plasma membrane (Fig. 1b). These findings suggest that LacCer-mediated CD11b/CD18-dependent phagocytosis by professional phagocytes is an important aspect of innate immune responses, and is crucial in the first line of defense against a wide range of pathogens.
(a) CD18 subdomains recognized by MEM-48. PSI domain, N-terminal cysteine-rich plexin/semaphorin/integrin domain. (b, c) Two models of LacCer–CD11b/CD18 interactions during non-opsonic phagocytosis. (b) Direct interaction of CD18 and LacCer. Upon binding to CD11b of PAMPs expressed on pathogens, such as CSBG-derived β-glucan, CD11b/CD18 is activated and undergoes a conformational change, resulting in actin rearrangement induced by the accumulation of cytoskeletal proteins, such as talin and α-actinin. The CD11b/CD18 subsequently translocates into LacCer-enriched membrane microdomains, allowing CD11b/CD18 to transmit stimulatory signals to Lyn through the direct interaction of LacCer with residues 514–553 in the C-terminal portion of the conserved domain of CD18 that are recognized by MEM-48. (c) Indirect interaction of CD18 and LacCer. When CD11b/CD18 translocates into LacCer-enriched membrane microdomains upon ligand binding to CD11b as described above, an adaptor molecule X is needed to mediate the interaction between CD18 and LacCer
The mechanism by which LacCer interacts with CD18, however, has not yet been determined. The glycosylphosphatidylinositol (GPI)-anchored, neutrophil-specific receptor NB1 (CD177), which presents the autoantigen proteinase 3 (PR3) on the plasma membranes of neutrophils, has been shown to colocalize with CD11b/CD18 within membrane microdomains (Jerke et al. 2011). These results suggested that NB1–CD11b/CD18 interactions are involved in neutrophil activation mediated by complexes formed by PR3 and anti-neutrophil cytoplasmic auto-antibodies (ANCA). Thus, some adaptors may be required for interactions between LacCer-enriched membrane microdomains and CD18 (Fig. 1c).
Future Perspectives
Although the mechanisms of pathogenesis and clinical presentations of intracellular pathogens, such as Salmonella, Legionella, and Mycobacteria, differ, they share a common feature, i.e., the molecular complex of PRRs and GSL-enriched membrane microdomains. Therefore, determination of the structural and molecular mechanisms underlying the interactions of GSL with PRRs in the immune system may lead to the development of new pharmacological reagents to treat many types of infectious disease. We have elucidated the molecular mechanism by which GSL–receptor cis interactions mediate physiological functions in innate immune response. More recently, we showed that a new type of glycolipid, phosphatidylglucoside (PtdGlc), forms membrane microdomains, which are functionally distinct from LacCer-enriched membrane microdomains, and that PtdGlc interacts with Fas (CD95) on plasma membranes to mediate neutrophil apoptosis (Kina et al. 2011). These findings indicate that interactions between glycolipids and their partner molecules may be ubiquitously important for transducing specific signals from outside to inside cells. Further studies using advanced technologies, such as stimulated emission depletion (STED) microscopy, may enhance understanding of the mechanisms of association between GSL and partner molecules in different aspects of pathogenesis, providing novel therapeutic targets for many types of disease.
References
Coskun U, Grzybek M, Drechsel D et al (2011) Regulation of human EGF receptor by lipids. Proc Natl Acad Sci U S A 108:9044–9048
Ichikawa N, Iwabuchi K, Kurihara H et al (2009) Binding of laminin-1 to monosialoganglioside GM1 in lipid rafts is crucial for neurite outgrowth. J Cell Sci 122:289–299
Iwabuchi K, Nagaoka I (2002) Lactosylceramide-enriched glycosphingolipid signaling domain mediates superoxide generation from human neutrophils. Blood 100:1454–1464
Iwabuchi K, Prinetti A, Sonnino S et al (2008) Involvement of very long fatty acid-containing lactosylceramide in lactosylceramide-mediated superoxide generation and migration in neutrophils. Glycoconj J 25:357–374
Jerke U, Rolle S, Dittmar G et al (2011) Complement receptor Mac-1 is an adaptor for NB1 (CD177)-mediated PR3-ANCA neutrophil activation. J Biol Chem 286:7070–7081
Kabayama K, Sato T, Saito K et al (2007) Dissociation of the insulin receptor and caveolin-1 complex by ganglioside GM3 in the state of insulin resistance. Proc Natl Acad Sci U S A 104:13678–13683
Kina K, Masuda H, Nakayama H et al (2011) The novel neutrophil differentiation marker phosphatidylglucoside mediates neutrophil apoptosis. J Immunol 186:5323–5332
Liang S, Wang M, Tapping RI et al (2007) Ganglioside GD1a is an essential coreceptor for Toll-like receptor 2 signaling in response to the B subunit of Type IIb enterotoxin. J Biol Chem 282:7532–7542
McNamara N, Gallup M, Sucher A et al (2006) AsialoGM1 and TLR5 cooperate in flagellin-induced nucleotide signaling to activate Erk1/2. Am J Respir Cell Mol Biol 34:653–660
Nakayama H, Yoshizaki F, Prinetti A et al (2008) Lyn-coupled LacCer-enriched lipid rafts are required for CD11b/CD18-mediated neutrophil phagocytosis of nonopsonized microorganisms. J Leukoc Biol 83:728–741
Sato T, Iwabuchi K, Nagaoka I et al (2006) Induction of human neutrophil chemotaxis by Candida albicans-derived β-1,6-long glycoside side-chain-branched β-glucan. J Leukoc Biol 80:204–211
Acknowledgments
This study was supported by a Grant-in-Aid (S0991013) from the Foundation of Strategic Research Projects in Private Universities from the Ministry of Education, Culture, Sports, Science, and Technology, Japan, MEXT KAKENHI Grant Number 70514933, and the Naito Foundation.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer Japan
About this entry
Cite this entry
Nakayama, H., Iwabuchi, K. (2015). Glycosphingolipid-Receptor Interactions in the Innate Immune Response. In: Taniguchi, N., Endo, T., Hart, G., Seeberger, P., Wong, CH. (eds) Glycoscience: Biology and Medicine. Springer, Tokyo. https://doi.org/10.1007/978-4-431-54841-6_141
Download citation
DOI: https://doi.org/10.1007/978-4-431-54841-6_141
Received:
Accepted:
Published:
Publisher Name: Springer, Tokyo
Print ISBN: 978-4-431-54840-9
Online ISBN: 978-4-431-54841-6
eBook Packages: Biomedical and Life SciencesReference Module Biomedical and Life Sciences