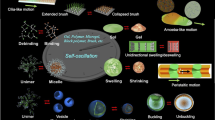
Abstract
Stimuli-responsive polymer gels and their application to smart materials have been widely studied. On the other hand, as a novel biomimetic gel, we developed gels with an autonomous self-oscillating function like a heart muscle, which was firstly reported in 1996. We designed the self-oscillating gels by utilizing the oscillating reaction, called the Belousov-Zhabotinsky (BZ) reaction which is recognized as a chemical model of the TCA cycle in organisms. The self-oscillating gel is composed of a poly(N-isopropylacrylamide) network in which the metal catalyst for the BZ reaction is covalently bonded. In a closed solution containing the reactants other than the catalyst, the gel undergoes spontaneous cyclic swelling–deswelling changes without any on–off switching of external stimuli. Their potential applications include several kinds of functional material systems, such as biomimetic soft-actuators and autonomous mass transport systems. Here recent progress on the novel polymer gels is introduced.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
As mentioned in the other chapters, many kinds of stimuli-responsive polymers and gels and their applications to soft actuators (artificial muscles) inspired by living systems have been studied. Other than stimuli-responsive function, one of characteristic and important behaviors in living systems is autonomous oscillation, that is, spontaneous changes with temporal periodicity (called “temporal structure”) such as heartbeat, brain waves, pulsatile secretion of hormone, cell cycle, and biorhythm. However, there are few studies on polymer and gel systems undergoing self-oscillation under constant condition without any on-off switching of external stimuli. If such autonomous systems can be realized by using completely synthetic polymers, unprecedented biomimetic materials may be created.
From this viewpoint, we have studied polymer gels with autonomous function. As one method, we designed a coupling system of pH responsive gel and pH-oscillating reaction. By soaking pH-responsive gels in a solution of pH-oscillating reaction in a continuous stirred tank reactor (CSTR), autonomous and periodic swelling-deswelling changes of the gel were realized [1, 2]. In this system, however, oscillating outer solution is created by CSTR and swelling-deswelling changes of the gel occur in response to the oscillating outer condition. Therefore, we attempted to develop a novel gel that provides mechanical oscillation by itself without external control in a complete closed and non-oscillating outer solution. We succeeded in developing such a self-oscillating polymer and gels by incorporating oscillating chemical reaction in polymer network, i.e., by constructing built-in circuit of energy conversion cycle producing mechanical oscillation within polymer network itself. In contrast to conventional stimuli-responsive gels, we have developed “self-oscillating” polymer gels that autonomously undergo periodic swelling/deswelling oscillation without on-off switching of external stimuli in a closed solution (Fig. 5.1).
2 Design of Self-Oscillating Polymer Gel
2.1 Oscillating Chemical Reaction: The Belousov-Zhabotinsky Reaction
For the design of such a gel, the Belousov-Zhabotinsky (BZ) reaction [3–6], which is well-known as an oscillating reaction which spontaneously exhibits temporal rhythm and spatial pattern, was focused. The overall process is the oxidation of an organic substrate, such as malonic acid (MA) or citric acid, by an oxidizing agent (typically bromate ion) in the presence of a catalyst under acidic condition. Metal ions or metal complexes with high redox potentials (1.0–1.4 V/SHE), such as cerium ion, ferroin, or ruthenium tris(2,2′-bipyridine) (Ru(bpy)3 2+) are widely used as catalysts. While the reaction proceeds, the catalyst undergoes spontaneous redox oscillation, showing periodical changes in color of the solution under stirring condition and concentric or spiral wave patterns under stationary condition. The wave of oxidized state propagating in the medium is called a “chemical wave”. The understanding of the BZ reaction in terms of the Field-Körös-Noyes (FKN) mechanism [5] allows us to divide the overall reaction into three main processes: consumption of Br− ions (process A), autocatalytic formation of HBrO2 accompanying oxidation of catalyst (process B), and formation of Br− ions accompanying reduction of catalyst (process C). These processes proceeds cyclically with the arrow of time; A → B → C → A → … The elementary reactions which constitute each process have been elucidated by many researchers. The BZ reaction is often analogically compared with the TCA cycle which is a key metabolic process taking place in the living body, and it is recognized as a chemical model for understanding several autonomous phenomena in biological systems.
2.2 Mechanism of Self-Oscillation
We attempted to convert the chemical oscillation of the BZ reaction into a mechanical change in gels and generate an autonomous swelling-deswelling oscillation under non-oscillatory outer conditions. For this purpose, we prepared the gel composed of poly(N-isopropylacrylamide) (PNIPAAm) and Ru(bpy)3 which is covalently bonded to the PNIPAAm network (Fig. 5.2). When the poly(NIPAAm-co-Ru(bpy)3) gel is immersed in the catalyst-free BZ solution containing the substrates (MA, NaBrO3 and nitric acid), the reaction occurs in the gel by the catalytic function of the polymerized Ru(bpy)3. The redox changes of the polymerized catalyst moiety (Ru(bpy)3 2+ ⇄ Ru(bpy)3 3+) change the volume phase transition temperature of the gel as well as the swelling ratio because the hydrophilicity of the polymer chains increases at the oxidized Ru(III) state and decreases at the reduced Ru(II) state. As a result, the gel exhibits an autonomous swelling-deswelling oscillation with the redox oscillation in the closed solution under constant condition. We reported the “self-oscillating” gel in 1996 for the first time [7], and since then we have developed many kinds of biomimetic or smart material systems (Fig. 5.3) [8–44].
2.3 Self-Oscillating Behavior on Several Scales
The self-oscillation can be induced on several scales from the order of polymer chain to bulk gel (Fig. 5.4). In the case of the uncrosslinked linear polymer, the polymer solution exhibits self-oscillations of optical transmittance and viscosity due to spontaneous cyclic soluble-insoluble changes of the polymer (Fig. 5.4a). When the gel is much smaller than chemical wavelength, redox changes occur homogeneously in the gel without pattern formation [9]. Then the swelling-deswelling change of the gel becomes isotropic (Fig. 5.4b). On the other hand, when the gel size is larger, chemical waves spontaneously evolve and propagate in the gel by coupling with diffusion of intermediates [10–12]. Then peristaltic motion of the gel is created. Figure 5.4c shows time course of peristaltic motion of the cylindrical gel in a solution of the BZ substrates [10]. The green and orange colors correspond to the oxidized and reduced states of the Ru moiety in the gel, respectively. The chemical waves propagate in the gel at a constant speed in the direction of the gel length. Considering the orange (Ru(II)) and green (Ru(III)) zones represent simply the shrunken and swollen parts respectively, the locally swollen and shrunken parts move with the chemical wave, like the peristaltic motion of living worm or intestine.
Self-oscillation on several scales. (a) Self-oscillating profiles of optical transmittance for poly(NIPAAm-co-Ru(bpy)3) solution. (b) Periodic redox changes of the miniature cubic poly(NIPAAm-co-Ru(bpy)3) gel (lower) and the swelling-deswelling oscillation (upper) at 20 °C. Color changes of the gel accompanied by redox oscillations (orange: reduced state, light green: the oxidized state) were converted to 8-bit grayscale changes (dark: reduced, light: oxidized) by image processing. Transmitted light intensity is expressed as an 8-bit grayscale value. (c) Time course of peristaltic motion of poly(NIPAAm-co-Ru(bpy)3-co-AMPS) gel in a solution of the BZ substrates at 18 °C. The green and orange colors correspond to the oxidized and reduced states of the Ru moiety in the gel, respectively
3 Control of Self-Oscillating Chemomechanical Behaviors
3.1 Concentration and Temperature Dependence of Oscillation
Oscillating behavior of the BZ reaction changes depending on concentration and temperature as characteristics of chemical reaction. Typically, the oscillation period increases with a decrease in the initial concentration of substrates. Further, in general, the oscillation frequency (the reciprocal of the period) of the BZ reaction tends to increase as the temperature increases, in accordance with the Arrhenius equation. The swelling-deswelling amplitude of the gel increases with an increase in the oscillation period and amplitude of the redox potential changes [9]. Therefore the swelling-deswelling amplitude of the gel is controllable by changing the initial concentration of substrates as well as temperature.
3.2 On–Off Regulation of Self-Oscillation by External Stimuli
Further, on-off regulation of the self-oscillation by external stimuli such as temperature change [13], addition and removal of organic acid [14], photo-irradiation is possible [15–17]. In particular, Ru(bpy)3 has photo-sensitivity at the reduced state. By photo-irradiation, it creates the other reaction pathway to stop or cause the oscillation by producing an intermediate to act as an inhibitor (Br−) or an activator (HBrO2), depending on the composition of the BZ solution [6]. By utilizing such photochemical characteristics of the BZ reaction, photo-regulation of self-oscillating motion of the gel was experimentally demonstrated [15, 16]. Balazs et al. [45, 46] theoretically demonstrated light-guided motility of the gel by model simulation.
In this photo-regulation system utilizing the photo-sensitivity of Ru(bpy)3, there is no difference in hydrated state of the polymer chains (consequently swelling state of the gel) between photo-irradiated and non-irradiated conditions. On the other hand, we design photo-regulated self-oscillating systems based on hydration and dehydration change of the polymer by photo-irradiation. For this purpose, spirobenzopyran (Sp) was introduced into the poly(NIPAAm-co-Ru(bpy)3) as a photochromic site [17]. Even under acidic condition necessary for the BZ reaction, photochromism of the spirobenzopyran occurs and the lower critical solution temperature (LCST) of the polymer solution shifts to lower temperature with isomerization from McH to Sp by photo-irradiation. As a result, on-off switching of the soluble-insoluble self-oscillation for the polymer solution is possible by photo-irradiation. Theoretical simulation of chemomechanical behaviors corresponding to this photoregulation system is also demonstrated [47].
3.3 Control of Self-Oscillating Behaviors by Designing Chemical Structure of Gel
The self-oscillating behaviors can also be controlled by designing chemical and physical structures of the gels. There are several variations of the gels with different chemical components. For example, it was found to be effective to copolymerize 2-acrylamido-2′-methylpropanesulfonic acid (AMPS) to a poly(NIPAAm-co-Ru(bpy)3) gel network to generate a large amplitude of volume change of the gel because the gel had a microphase-separated structure due to the effect of the poor solvent in the polymerization process (Fig. 5.5a) [18].
Control of interior morphology of gels for fast response and increasing swelling-deswelling amplitude. (a) Microgel-aggregated gel structure formed by solvent effect. Chemical structure of poly(NIPAAm-co-Ru(bpy)3-co-AMPS) gel (right) and the SEM photograph of the gel (left). (b) Crosslinking after assembling gel particles prepared by precipitation polymerization
The operating conditions for the self-oscillation are limited to conditions under which the BZ reaction occurs. For potential applications as functional bio- or biomimetic materials, it is necessary to design a self-oscillating polymer which acts under biological environments. To induce self-oscillation of polymer systems under physiological conditions, BZ substrates other than organic ones (MA, citric acid, etc.) must be built into the polymer system itself. For this purpose, we have synthesized a quarternary copolymer which includes both pH-control and oxidant-supplying sites in the poly(NIPAAm-co-Ru(bpy)3) chain at the same time [19]. By using this polymer, self-oscillation by adding only the organic acid (i.e., MA) was actually observed.
In order to induce self-oscillation while maintaining a larger amplitude at higher temperatures and around body temperature for potential applications to biomaterials, etc., we prepared a self-oscillating gel composed of a thermosensitive N,N′-ethylmethylacrylamide (EMAAm) polymer exhibiting a higher LCST than that of PNIPAAm [20]. The self-oscillating behavior of the poly(EMAAm-co-Ru(bpy)3) gel was investigated by comparing against gels composed of a thermosensitive NIPAAm polymer with a lower LCST or non-thermosensitive N,N′-dimethylacrylamide (DMAAm) polymer. The design concept of self-oscillation at higher temperatures without a decrease in swelling-deswelling amplitude was demonstrated by utilizing a thermosensitive polymer exhibiting a higher LCST.
3.4 Remarkable Swelling-Deswelling Changes by Assembled Self-Oscillating Microgels
Typically, there are several strategies to improve the response of gels, such as introducing porous microstructures, decreasing the size of the gels. And also, in a living muscle, there exists hierarchical structure to amplify the microscopic movements of actin-myosin to macroscopic displacements. Such a hierarchical structure was introduced into the self-oscillating gel to get large amplitude of swelling/deswelling oscillation [21]. The soft actuators assembled from self-oscillating submicron-sized particles (microgels) were fabricated (Fig. 5.5b). Firstly, the microgels composed of NIPAAm, Ru(bpy)3 and N-(3-aminopropyl) methacrylamide hydrochloride (APMA) were prepared by aqueous free radical precipitation polymerization. Then the microgels were assembled and crosslinked by using the glutaric dialdehyde to induce a chemical reaction between the amino groups located on microgel exterior. The resulting macrogels exhibitied large displacements due to cooperative dispersing/flocculating motion of constituent microelements.
3.5 Comb-Type Self-Oscillating Gel
Further, as another method to improve the response of gels, it is effective to introduce grafted side chains with freely mobile ends on the backbone networks of gels. For example, it was demonstrated that the comb-type grafted PNIPAAm gels exhibited a fast deswelling response to temperature change by the guiding effect of hydrophobic interaction between side chains that have freely mobile ends [48, 49]. These network-arranged designs are expected to be applicable for improving the swelling/deswelling kinetics to the redox change in the self-oscillating gel.
We designed a novel comb-type self-oscillating gel that has Ru(bpy)3 on both the main and side chains (Fig. 5.6) [22]. Different from the conventional type of self-oscillating gel in which Ru(bpy)3 is immobilized only on main chains, faster swelling/deswelling changes are obtained because the grafted side chains with freely mobile ends can respond to the redox change of Ru(bpy)3 more quickly than the main chains. As a result, self-oscillation with a larger swelling-deswelling amplitude was achieved.
4 Design of Biomimetic Soft-Actuators
4.1 Ciliary Motion Actuator Using Self-Oscillating Gel (Artificial Cilia)
By utilizing such swelling-deswelling oscillation of the gel, novel biomimetic actuators may be created. As applications to autonomous biomimetic actuators, ciliary motion actuators (artificial cilia) [23, 24] and self-walking gels [25], etc. were realized. Recently, microfabrication technologies such as photolithography are also attempted for preparation of microgels. Since any shape of gel can be created by these methods, application as a new manufacturing method for soft microactuator, microgel valve, gel display, etc. is expected. One of the promising fields of the MEMS is micro actuator array or distributed actuator systems. The actuators, which have a very simple actuation motion such as up and down motion, are arranged in an array form. If their motions are random, no work is extracted from this array. However, by controlling them to operate in a certain order, they can generate work as a system. A typical example of this kind of actuation array is a ciliary motion micro actuator array. There have been many reports on this system. Although various actuation principles have been proposed, all the previous work is based on the concept that the motion of actuators is controlled by external signals. If a self-oscillating gel plate with a micro projectaion structure array on top were realized, it would be expected that the chemical wave propagation would create dynamic rhythmic motion of the structure array. This proposed structure could exhibit spontaneous dynamic propagating oscillation producing a ciliary motion array [23, 24].
A gel plate with micro projection array was fabricated by molding. First, moving mask deep-X-ray lithography was utilized to fabricate a PMMA plate with a truncated conical shape microstructure array. This step was followed by evaporation of a Au seed layer and subsequent electroplating of nickel to form the metal mold structure. Then, a PDMS mold structure was duplicated from the Ni structure and utilized for gel molding. The formation of gel was carried out by vacuum injection molding. A structure with a height of 300 μm and bottom diameter of 100 μm was successfully fabricated by the described process (Fig. 5.7). The propagation of chemical reaction wave and dynamic rhythmic motion of the micro projection array were confirmed by chemical wave observation and displacement measurements. Motion of the top with 5 μm range in both lateral and vertical directions, and elliptical motion of the projection top were observed. The feasibility of the new concept of the ciliary motion actuator made of self-oscillating polymer gel was successfully confirmed. The actuator may serve as a micro-conveyer to transport objects on the surface.
4.2 Self-Walking Gel
Further, we successfully developed a novel biomimetic walking-gel actuator made of self-oscillating gel [25]. To produce directional movement of gel, asymmetrical swelling-deswelling is desired. For these purposes, as a third component, hydrophilic 2-acrylamido-2′-methylpropanesulfonic acid (AMPS) was copolymerized into the polymer to lubricate the gel and to cause anisotropic contraction. During polymerization, the monomer solution faces two different surfaces of plates; a hydrophilic glass surface and a hydrophobic Teflon surface. As the thickness of the spacer is thin (0.5 mm), the surface property of the plate may affect the distribution of the monomer in the solution. Since Ru(bpy)3 2+ monomer is hydrophobic, it easily migrates to the Teflon surface side. As a result, a non-uniform distribution along the height is formed by the components, and the resulting gel has gradient distribution for the content of each component in the polymer network.
In order to convert the bending and stretching changes to one-directional motion, we employed a ratchet mechanism. A ratchet base with an asymmetrical surface structure was fabricated. On the ratchet base, the gel repeatedly bends and stretches autonomously. Figure 5.8 shows successive profiles of the “self-walking” motion of the gel like a looper in the BZ substrate solution under constant temperature. During stretching, the front edge can slide forward on the base, but the rear edge is prevented from sliding backwards. Oppositely, during bending, the front edge is prevented from sliding backwards while the rear edge can slide forward. This action is repeated, and as a result, the gel walks forward. The walking velocity of the gel actuator was approximately 170 μm/min. Since the oscillating period and the propagating velocity of chemical wave change with concentration of substrates in the outer solution, the walking velocity of the gel can be controlled. By using the gel with gradient structure, other type of actuator which generates a pendulum motion is also realized [26].
Time course of self-walking motion of the gel actuator in a solution of the BZ substrates at 18 °C. During stretching, the front edge can slide forward on the base, but the rear edge is prevented from sliding backwards. Oppositely, during bending, the front edge is prevented from sliding backwards while the rear edge can slide forward. This action is repeated, and as a result, the gel walks forward
4.3 Self-Propelled Motion
Further, self-propelled motion of gels was theoretically demonstrated and experimentally realized by utilizing the gels [27]. The cylindrical self-oscillating gel with a gradient in crosslinking density was prepared by photo-polymerization. To introduce gradient structure, during the gelation, light was irradiated from one side to the pre-gel solution in a grass capillary. The resulting gel has denser crosslinking at the photo-irradiated side, and bends to the direction at the reduced state. In the catalyst-free BZ solution, the gel repeated bending and stretching motion, and the position of the center of the gel apparently shifts slowly upwards (towards the region with lower cross-link density) (Fig. 5.9).
Control of the motion by preparing a composite gel made of self-oscillating and non-active gels was also attempted [28]. For this purpose, heterogeneous BZ gel was prepared. The disk-shaped self-oscillating gels were arranged around the corners of a polygonal sheet of poly(acrylamide) (PAAm) gel. Wave propagation was controlled by changing the patch size, catalyst content of the BZ gel, and spacing between the patches.
4.4 Theoretical Simulation of the Self-Oscillaiting Gel
The self-oscillating gels construct provides unique material systems which contain reaction–diffusion and chemomechanical motion. Since the chemical reaction and the mechanical motion are affected each other with a feedback control, the oscillating behaviors of gels become more complicated compared with conventional stimuli-responsive gels. Then theoretical simulation becomes effective tool for understanding the chemomechanical behaviors. Balazs et al. [45–47, 50–53] developed a mathematical model for simulating chemomechanical behaviors of the self-oscillating gels. Since reported in 2006 [50], they have demonstrated several aspects of the self-oscillating behavior for the gel by theoretical simulation. Many interesting phenomena and possible actuating behaviors, some of which were experimentally realized, were demonstrated theoretically. The investigations from theoretical studies will provide helpful guidelines for creating autonomously moving objects, which can be used for robotic applications.
5 Design of Autonomous Mass Transport Systems
5.1 Self-Driven gel Conveyer: Autonomous Transportation on the Self-Oscillating Gel Surface by Peristaltic Motion
In order to realize self-driven gel conveyer as novel autonomous mass transport system, we attempted to transport an object by utilizing the peristaltic motion of the self-oscillating gel. A model object, a cylindrical or spherical PAAm gel was put on the surface of self-oscillating gel sheet [18, 29–31]. It was observed that the object was transported on the gel surface with the propagation of the chemical wave as it rolled (Fig. 5.10a). The velocity and the inclination angle of the chemical wave were changed by changing the concentrations of the outer solution. For the controlled chemical waves with several inclination angles and velocities, whether the cylindrical gel could be transported or not was estimated. It was found that the cylindrical PAAm gel was not transported when the inclination angle was less than approximately 3°. The mass transportability did not depend on the velocity of the chemical wave, but on the diameter of the cylindrical PAAm gel and the inclination angle of the wave front. For analysing this result, we have proposed a model to describe the mass transport phenomena based on the Hertz contact theory, and the relation between the transportability and the peristaltic motion was investigated. As a result of calculation from the theoretical equation, the minimum inclination angle was 3° and it was the same as the angle resulted from the experiment. It was supported by the model that the sheer wave front of the peristaltic motion was necessary to transport cylindrical gels.
Autonomous mass transport with wave propagation on the surface of self-oscillating gel (gel conveyor). (a) Schematic illustration of mass transport on the peristaltic surface and observed transport of cylindrical PAAm gel on the self-oscillating gel sheet in a solution of the BZ substrates. (b) The grooved self-oscillating gel sheet prepared by the PDMS template. Schematic illustration of mass transport on the peristaltic surface and transport of poly(AAm-co-AMPS) gel beads on the grooved surface
Then, the surface figure capable of transporting microparticles in one direction was designed to fabricate more versatile self-driven gel conveyer. The self-oscillating gel having a grooved surface was fabricated by using PDMS templete and the effectiveness of the surface design was investigated. Poly(AAm-co-AMPS) gel beads with the diameter of several hundred μm to several mm were transported on the grooved surface of the self-oscillating gel by its autonomous peristaltic motions (Fig. 5.10b) [29]. It was found that the traveling direction of the peristaltic motion could be confined to the direction along the grooves by designing the groove-distance shorter than the wavelength of the chemical wave. Consequently, several gel beads were transported in parallel.
The influences of these surface properties on the transport behaviors were also investigated [30]. Instead of the homopolymer gel of PAAm, several kinds of copolymer gels consisting of AAm with AMPS, N-(3-aminopropyl)methacrylamide hydrochloride (APMA), N-(hydroxymethyl)acrylamide (HMAAm), and methyl methacrylate (MMA) were prepared as model cargos with different surface properties; positive or negative charge, more hydrophilicity or hydrophobicity, respectively. It was found that the adhesion force between the cylindrical poly(AAm-co-MMA) gel and the self-oscillating gel sheet was too strong to apply the transport model because of their hydrophobic interaction. The adhesive force to prevent transportation is not significant for the other gels, which agrees with the prediction from swelling, zeta potential, and contact angle measurements. Then the effect of surface roughness on the transportation was investigated. It was found that higher surface roughness is more effective in transporting the loaded gel because frictional force increases and the moment of force of the rotational motion increases.
5.2 Autonomous Intestine-Like Motion of Tubular Self-Oscillating Gel
Further, to construct autonomous mechanical pumping systems like an intestine, we fabricated the self-oscillating gel in a tubular shape [32] (Fig. 5.11). Tubular self-oscillating gels were fabricated by photopolymerization. Several kinds of tubular self-oscillating gels that exhibit autonomous peristaltic motion were prepared. First, a tubular poly(NIPAAm-co-Ru(bpy)3) gel adhered to the inner wall of a glass capillary was prepared and the periodic inner diameter changes during the BZ reaction were analyzed. Second, by removing the gel from the glass capillary, a tubular gel that can swell and deswell freely without a mechanical restraint was prepared. Then, a tubular gel with interpenetrating network structure composed of self-oscillating and non-oscillating polymers was prepared. It was shown that these tubular self-oscillating gels exhibited various behaviors of peristaltic motions.
(a) Schematic illustration of autonomous mass transport by peristaltic pumping of a tubular self-oscillating gel. (b) Time course images of the peristaltic motion of the tubular poly(NIPAAm-co-Ru(bpy)3-co-AMPS) gel. (c) The behavior of the autonomous transport of a CO2 bubble in the gel tube by peristaltic pumping. (d) Change in the position of the bubble. (e) The velocity of the bubble
In addition, it was demonstrated that an object was autonomously transported in the gel tube by the peristaltic pumping motion similar to an intestine. Figure 5.11c shows the behavior of a CO2 bubble in the tubular poly(NIPAAm-co-Ru(bpy)3) gel. When the chemical wave reaches the contact point, the bubble is squashed and deformed by swelling of the gel layer at the point. Then the bubble is mechanically pushed forward by the peristaltic pumping mechanism. After the wave passes through, the gel layer deswells and the squashed bubble returns back to the initial round shape. Due to a decrease in pushing force and a negative pressure, the bubble moves backward slightly. After that, the movement of the bubble stops for a while. As a result, the movement was intermittent. By repeating this process, the bubble is transported in the gel tube. Figure 5.11d, e shows the changes in the position and the velocity of the bubble, respectively. The velocity was calculated by differentiating the position with time. It is obvious that a net movement of the bubble occurs by repeating backward and forward movements. Potential applications to artificial intestines, artificial digestive tracts, etc. can be expected. Furthermore, there is a possibility of autonomous flow of an inner fluid. We are investigating an application to a novel micropump for microfluidic systems.
5.3 Self-Oscillating Polymer Brushes
Recently, surface modification techniques for polymer chains have made a lot of progress with the development of new polymer synthesis methods. In particular, surface-initiated atom transfer radical polymerization (SI-ATRP) is one of the most effective modification methods for preparing a well-defined dense polymer brush structure, or polymer brush, on substrates. Thus, a self-oscillating polymer brush prepared by SI-ATRP can be expected to create a new self-oscillating surface with autonomic function like a cilia, which will lead to potential applications to transporting systems for nano-materials or flow control in micro fluidics.
We prepared the self-oscillating polymer brush on glass substrates through SI-ATRP (Fig. 5.12) [33]. The self-oscillating polymer was grafted onto the inner surface of a glass capillary. Figure 5.12b shows the image observed by fluorescence microscope, which shows that the self-oscillating polymer brush was successfully modified onto the inner surface of the capillary. Then the catalyst-free BZ solution was fed into the capillaries and the BZ reaction on the inner surface was observed by fluorescence microscope. Spatiotemporal image analyses were performed in different locations (1–4) of the glass capillary and the oscillating profiles of the fluorescence intensity were compared (Fig. 5.12c). It was found that oscillations occurred at each position with a phase difference. This suggests that the chemical wave propagates in the self-oscillating polymer brush layer on the inner surface of the glass capillary. A self-oscillating surface to generate spontaneous periodic changes was able to be demonstrated by using synthetic polymers, as a novel autonomous functional surface which has potential applications in systems such as nano-transport systems.
Preparation of self-oscillating polymer brush by surface-initiated atom transfer radical polymerization (SI-ATRP). (a) Chemical structure of the self-oscillating polymer brush. (b) Images of glass capillary modified with the self-oscillating polymer observed by fluorescence microscope. (c) Oscillating profile of fluorescence intensity at each position for the self-oscillating polymer brush modified on inner surface of glass capillary. The solution containing MA, NaBrO3 and HNO3 is enclosed in the capillary at 25 °C
6 Self-Oscillating Fluids
6.1 Transmittance and Viscosity Oscillation of Polymer Solution and Microgel Dispersion
In the case of the uncrosslinked linear polymer (poly(NIPAAm-co-Ru(bpy)3), as shown in Fig. 5.4, the polymer undergoes spontaneous cyclic soluble-insoluble changes and the transmittance of the polymer solution oscillates autonomously with redox changes of the copolymerized Ru(bpy)3 [34]. Further, we prepared submicron-sized poly(NIPAAm-co-Ru(bpy)3) gel beads by surfactant-free aqueous precipitation polymerization and analyzed the oscillating behavior of the microgel dispersions [35–38]. The microgel dispersion also exhibited transmittance oscillation due to swelling and deswelling changes of the microgels. With increasing temperature, the oscillation period decreased and the amplitude increased a little. When the temperature increased near to the volume phase transition temperature of the reduced microgel, the microgels showed dispersing/flocculating oscillation as well as swelling-deswelling oscillation (Fig. 5.13) because the reduced and shrunken microgels lost colloidal stability, which resulted in a remarkable increase of amplitude in optical transmittance oscillation. Due to such behaviors, as mentioned before, the macroscopic self-oscillating gel prepared by crosslinking the assembled microgels exhibits a faster response and leads to larger amplitude of swelling-deswelling oscillation [21].
In both cases of the polymer solution and the microgel dispersion, viscosity oscillation was observed with optical transmittance oscillation [39–41]. In the microgel dispersion, it was found that the viscosity oscillation occurs with exhibiting two different waveforms; a simple pulsatile waveform or a complex waveform with two peaks per period. The difference in waveform is due to the difference in the oscillating behavior of the microgels: swelling/deswelling or dispersing/flocculating oscillation. The oscillating behavior can be controlled by changing the concentration of microgels, Ru(bpy)3 contents, crosslinking density, etc. It is expected that these polymer solutions and microgel dispersions are applied as novel functional fluid.
6.2 Autonomous Viscosity Oscillation by Reversible Complex Formation of Terpyridine-Terminated PEG in the BZ Reaction
We realized autonomous viscosity oscillations of a polymer solution based on different mechanisms, that is, an autonomous viscosity oscillation of polymer solutions coupled with metal–ligand association/dissociation between Ru and terpyridine (tpy), driven by the BZ reaction [42, 43] (Fig. 5.14). The tpy ligand for the Ru catalyst was attached to the terminals of poly(ethylene glycol) (PEG) with different numbers of branches (linear-, tetra-, and octa-PEG). It is well known that mono-tpy coordination is stable when Ru is oxidized (Ru(tpy)3+), whereas bis-tpy coordination is stable when the Ru centre is reduced (Ru(tpy)2 2+) [54]. In the oxidized state, these polymers existed as solutions. In contrast, when the Ru centre was reduced, gels were obtained for the tetra- and octa-PEG owing to the formation of a three-dimensional polymer network through Ru–tpy coordination. By increasing the number of PEG branches, we succeeded in decreasing the number of crosslinking points necessary for gelation. This was qualitatively explained by the tree-like structure theory stating a rate of reaction of crosslinking point for a branched polymer at gelation point. Further, the gelation kinetics of the octa-PEG system were approximately four times faster than those of the tetra-PEG system.
The polymer solutions exhibited self-oscillation of absorbance and viscosity when BZ substrates were added to the solutions of Ru2+-tpy-modified tetra-/octa-PEG. This indicated that the Ru(tpy)2 2+ attached to the polymer ends could work as a metal catalyst for the BZ reaction. The viscosity oscillation profiles of the tetra- and octa-PEG were compared under the optimised conditions for each system (Fig. 5.14). As expected, the octa-PEG was much more effective than the tetra-PEG system in providing a large oscillation amplitude as well as a higher viscosity baseline. For the octa-PEG, the viscosity baseline and amplitude were approximately two times higher and ten times larger, respectively, than for the tetra-PEG system. This is likely because the number of crosslinking points necessary for gelation was decreased by increasing the branch number of PEG, as predicted from the tree-like structure theory. As a result, the maximum value of crosslinking points in oscillation is closer to that necessary for gelation. Thus, viscosity oscillation occurred in the region of higher viscosity with a larger amplitude.
6.3 Self-Oscillating Micelles
Further, we prepared a novel block copolymer (“self-oscillating micelle”) that could undergo spontaneous unimer–micelle oscillation under constant conditions [44]. Diblock copolymer (PEO-b-P(NIPAAm-r-Ru(bpy)3) was successfully prepared by RAFT random copolymerization of NIPAAm and vinyl monomer having Ru(bpy)3 side chain from poly(ethylene oxide) (PEO)-based macro-CTA (Fig. 5.15). Rhythmical oscillation of the scattering intensity and hydrodynamic radii of the block copolymer solution driven by the BZ reaction was demonstrated without any on-off switching of external stimuli. This is the first report on a synthetic block copolymer that realizes the novel concept of self-assembly assisted by a dissipative structure.
6.4 BZ Reaction in Protic Ionic Liquids
We realized the BZ reaction using hydrated protic ionic liquid (PIL) as a reaction medium [55]. It was found for the first time that the BZ oscillation reaction occurred in certain hydrated PILs without adding strong acid such as HNO3. Furthermore, unprecedented stable and long-lasting self-oscillation can be realized when concentrations of the BZ substrates are optimized. Investigation of more optimized ion structures for a stable and long-lasting BZ reaction under mild conditions is now in progress for evolution of the self-oscillating gels.
7 Future Prospects
As mentioned above, we developed novel “self-oscillating” polymer gels which exhibit autonomous swelling-deswelling oscillation under constant external condition like a heart muscle. Since the first report in 1996 [7], we have systematically studied the polymer gels, and we will continue to advance our research by studying new polymer systems. In this chapter, our recent progress on the self-oscillating polymer gels as functional materials were summarized. As an innovative study to propose novel potential of polymer gels and achieve an autonomous behavior by coupling chemical and mechanical oscillations in polymer systems, the study has attracted much attention in the many research fields of polymer science, material science, physical chemistry, theoretical simulation, biophysics, etc. Robotics is one of those fields, and applications to autonomous soft actuators will be extensively expected. Many similar studies following the same mechanism will be possible in future. But more innovative researches, which are not simple applied researches just only improving or utilizing the gels, will be necessary.
References
Yoshida R, Ichijo H, Hakuta T, Yamaguchi T (1995) Self-oscillating swelling and deswelling of polymer gels. Macromol Rapid Commun 16:305–310
Yoshida R, Yamaguchi T, Ichijo H (1996) Novel oscillating swelling-deswelling dynamic behaviour for pH-sensitive polymer gels. Mater Sci Eng C 4:107–113
Field RJ, Burger M (eds) (1985) Oscillations and traveling waves in chemical systems. Wiley, New York
Epstein IR, Pojman JA (1998) An introduction to nonlinear chemical dynamics: oscillations, waves, patterns, and chaos. Oxford University Press, New York
Field RJ, Körös E, Noyes RM (1972) Oscillations in chemical systems. II. Through analysis of temporal oscillation in the bromate-cerium-malonic acid system. J Am Chem Soc 94:8649–8664
Amemiya T, Ohmori T, Yamaguchi T (2000) An Oregonator-class model for photoinduced behavior in the Ru(bpy)3 2+-catalyzed Belousov-Zhabotinsky reaction. J Phys Chem A 104:336–344
Yoshida R, Takahashi T, Yamaguchi IH (1996) Self-oscillating gel. J Am Chem Soc 118:5134–5135
Yoshida R (2010) Self-oscillating gels driven by the Belousov-Zhabotinsky reaction as novel smart materials. Adv Mater 22:3463–3483
Yoshida R, Tanaka T, Onodera S, Yamaguchi T, Kokufuta E (2000) In-phase synchronization of chemical and mechanical oscillations in self-oscillating gels. J Phys Chem A 104:7549–7555
Maeda S, Hara Y, Yoshida R, Hashimoto S (2008) Peristaltic motion of polymer gels. Angew Chem Int Ed 47:6690–6693
Takeoka Y, Watanabe M, Yoshida R (2003) Self-sustaining peristaltic motion on the surface of a porous gel. J Am Chem Soc 125:13320–13321
Sasaki S, Koga S, Yoshida R, Yamaguchi T (2003) Mechanical oscillation coupled with the Belousov-Zhabotinsky reaction in gel. Langmuir 19:5595–5600
Ito Y, Nogawa N, Yoshida R (2003) Temperature control of the Belousov-Zhabotinsky reaction using a thermo-responsive polymer. Langmuir 19:9577–9579
Yoshida R, Takei K, Yamaguchi T (2003) Self-beating motion of gels and modulation of oscillation rhythm synchronized with organic acid. Macromolecules 36:1759–1761
Shinohara S, Seki T, Sakai T, Yoshida R, Takeoka Y (2008) Chemical and optical control of peristaltic actuator based on self-oscillating porous gel. Chem Commun 4735–4737
Shinohara S, Seki T, Sakai T, Yoshida R, Takeoka Y (2008) Photoregulated wormlike motion of a gel. Angew Chem Int Ed 47:9039–9043
Yamamoto T, Yoshida R (2013) Self-oscillation of polymer and photo-regulation by introducing photochromic site to induce LCST changes. React Func Polym 73:945–950
Murase Y, Maeda S, Hashimoto S, Yoshida R (2009) Design of a mass transport surface utilizing peristaltic motion of a self-oscillating gel. Langmuir 25:483–489
Hara Y, Yoshida R (2008) Self-oscillating polymer fueled by organic acid. J Phys Chem B 112:8427–8429
Hidaka M, Yoshida R (2011) Self-oscillating gel composed of thermosensitive polymer exhibiting higher LCST. J Control Release 150:171–176
Suzuki D, Kobayashi T, Yoshida R, Hirai T (2012) Soft actuators of organized self-oscillating microgels. Soft Matter 8:11447–11449
Mitsunaga R, Okeyoshi K, Yoshida R (2013) Design of comb-type self-oscillating gel. Chem Commun 49:4935–4937
Tabata O, Kojima H, Kasatani T, Isono Y, Yoshida R (2003) Chemo-mechanical actuator using self-oscillating gel for artificial cilia. In: Proceedings of the international conference on MEMS 2003, pp 12–15
Tabata O, Hirasawa H, Aoki S, Yoshida R, Kokufuta E (2002) Ciliary motion actuator using self-oscillating gel. Sens Actuators A 95:234–238
Maeda S, Hara Y, Sakai T, Yoshida R, Hashimoto S (2007) Self-walking gel. Adv Mater 19:3480–3484
Maeda S, Hara Y, Yoshida R, Hashimoto S (2008) Control of dynamic motion of a gel actuator driven by the Belousov-Zhabotinsky reaction. Macromol Rapid Commun 29:401–405
Kuksenok O, Yashin VV, Kinoshita M, Sakai T, Yoshida R, Balazs AC (2011) Exploiting gradients in cross-link density to control the bending and self-propelled motion of active gels. J Mater Chem 21:8360–8371
Yashin VV, Suzuki S, Yoshida R, Balazs AC (2012) Controlling the dynamic behavior of heterogeneous self-oscillating gels. J Mater Chem 22:13625–13636
Murase Y, Hidaka M, Yoshida R (2010) Self-driven gel conveyer: autonomous transportation by peristaltic motion of self-oscillating gel. Sens Actuators B 149:272–283
Murase Y, Takeshima R, Yoshida R (2011) Self-driven gel conveyer: effect of interactions between loaded cargo and self-oscillating gel surface. Macromol Biosci 11:1713–1721
Yoshida R, Murase Y (2012) Self-oscillating surface of gel for autonomous mass transport. Colloids Surf B: Biointerfaces 99:60–66
Shiraki Y, Yoshida R (2012) Autonomous intestine-like motion of tubular self-oscillating gel. Angew Chem Int Ed 51:6112–6116
Masuda T, Hidaka M, Murase Y, Akimoto AM, Nagase K, Okano T, Yoshida R (2013) Self-oscillating polymer brushes. Angew Chem Int Ed 52:7468–7471
Yoshida R, Sakai T, Ito S, Yamaguchi T (2002) Self-oscillation of polymer chains with rhythmical soluble-insoluble changes. J Am Chem Soc 124:8095–8098
Suzuki D, Sakai T, Yoshida R (2008) Self-flocculating/self-dispersing oscillation of microgels. Angew Chem Int Ed 47:917–920
Suzuki D, Yoshida R (2008) Temporal control of self-oscillation for microgels by cross-linking network structure. Macromolecules 41:5830–5838
Suzuki D, Yoshida R (2008) Effect of initial substrate concentration of the Belousov-Zhabotinsky reaction on self-oscillation for microgel system. J Phys Chem B 112:12618–12624
Suzuki D, Yoshida R (2010) Self-oscillating core/shell microgels. Polymer J 42:501–508
Hara Y, Yoshida R (2008) A viscosity self-oscillation of polymer solution induced by the BZ reaction under acid-free condition. J Chem Phys 128:224904
Suzuki D, Taniguchi H, Yoshida R (2009) Autonomously oscillating viscosity in microgel dispersions. J Am Chem Soc 131:12058–12059
Taniguchi H, Suzuki D, Yoshida R (2010) Characterization of autonomously oscillating viscosity induced by swelling/deswelling oscillation of the microgels. J Phys Chem B 114:2405–2410
Ueno T, Bundo K, Akagi Y, Sakai T, Yoshida R (2010) Autonomous viscosity oscillation by reversible complex formation of terpyridine-terminated poly(ethylene glycol) in the BZ reaction. Soft Matter 6:6072–6074
Ueki T, Takasaki Y, Bundo K, Ueno T, Sakai T, Akagi Y, Yoshida R (2014) Autonomous viscosity oscillation via metallo-supramolecular terpyridine chemistry of branched poly(ethylene glycol) driven by the Belousov-Zhabotinsky reaction. Soft Matter 10:1349–1355
Ueki T, Yoshida R (2013) Self-oscillating micelles. Chem Commun 49:6947–6949
Dayal P, Kuksenok O, Balazs AC (2010) Designing autonomously motile gels that follow complex paths. Soft Matter 6:768–773
Dayal P, Kuksenok O, Balazs AC (2009) Using light guide the self-sustained motion of active gels. Langmiur 25:4298–4301
Kuksenok O, Balazs AC (2013) Modeling the photoinduced reconfiguration and directed motion of polymer gels. Adv Funct Mater 23:4601–4610
Kaneko Y, Sakai K, Kikuchi A, Yoshida R, Sakurai Y, Okano T (1995) Influence of freely mobile grafted chain length on dynamic properties of comb-type grafted poly(N-isopropylacrylamide) hydrogels. Macromolecules 28:7717–7723
Yoshida R, Uchida K, Kaneko Y, Sakai K, Kikuchi A, Sakurai Y, Okano T (1995) Comb-type grafted hydrogels with rapid de-swelling response to temperature changes. Nature 374:240–242
Yashin VV, Balazs AC (2006) Science 314:798–801
Yashin VV, Kuksenok O, Balazs AC (2010) Modeling autonomously oscillating chemo-responsive gels. Prog Polym Sci 35:155–173
Yashin VV, Kuksenok O, Dayal P, Balazs AC (2012) Mechano-chemical oscillations and waves in reactive gels. Rep Prog Phys 75:066601
Yashin VV, Kuksenok O, Balazs AC (2010) Computational design of active, self-reinforcing gels. J Phys Chem B 114:6316–6322
Lohmeijer BGG, Schubert US (2002) Supramolecular engineering with macromolecules: an alternative concept for block copolymers. Angew Chem Int Ed 41:3825–3829
Ueki T, Watanabe M, Yoshida R (2012) Belousov-Zhabotinsky reaction in protic ionic liquids. Angew Chem Int Ed 51:11991–11994
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer Japan
About this chapter
Cite this chapter
Yoshida, R. (2014). Self-Oscillating Gels. In: Asaka, K., Okuzaki, H. (eds) Soft Actuators. Springer, Tokyo. https://doi.org/10.1007/978-4-431-54767-9_5
Download citation
DOI: https://doi.org/10.1007/978-4-431-54767-9_5
Published:
Publisher Name: Springer, Tokyo
Print ISBN: 978-4-431-54766-2
Online ISBN: 978-4-431-54767-9
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)