Abstract
Anterior and posterior corneal astigmatism must both be considered when calculating toric intraocular lenses (IOLs). The purpose of the two studies discussed in this chapter was to assess the relationship between anterior and posterior corneal astigmatisms and to evaluate the clinical impact of posterior corneal astigmatism on surgical outcomes following implantation of toric IOLs. Anterior and posterior corneal astigmatisms (CAant and CApost, respectively) were measured using the Galilei combined Placido dual Scheimpflug analyzer, and the correlation between them and age was investigated. In addition, pre- and postoperative corneal astigmatism prediction errors were calculated for the IOLMaster, Lenstar, Atlas, manual keratometer, and Galilei. The mean magnitude of CAant was 1.20 ± 0.79 D (diopters) (standard deviation) and of CApost was −0.30 ± 0.15 D. With increasing age, the anterior corneal steeper meridian shifted from vertical to horizontal, while the posterior corneal steeper meridian maintained a vertically aligned steeper meridian. The IOLMaster, Lenstar, Atlas, and manual keratometry had a mean corneal astigmatism prediction error of 0.5–0.6 D of with-the-rule (WTR) astigmatism in eyes with WTR corneal astigmatism and of 0.2–0.3 D of WTR astigmatism in eyes with against-the-rule (ATR) corneal astigmatism. These errors are attributable to posterior corneal astigmatism. In conclusion, ignoring the posterior corneal astigmatism when planning astigmatic correction during cataract surgery may lead to overcorrection in eyes with WTR anterior corneal astigmatism and undercorrection in eyes with ATR anterior corneal astigmatism.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
8.1 Introduction
Astigmatism is a key factor to consider when planning cataract surgery, once postsurgical residual astigmatism can compromise visual acuity. It has been estimated that 30 % of cataract patients have more than 0.75 diopters (D) of corneal astigmatism, that 22 % have more than 1.50 D, and that 8 % have more than 2.00 D [1, 2]. There are several methods to surgically treat corneal astigmatism, including adjustment of wound size and location, corneal relaxing incisions, opposite clear corneal cataract incisions, laser refractive surgery, and toric intraocular lens (IOL) implantation. Toric IOLs correct corneal astigmatism at the time of cataract surgery and are a predictable and permanent treatment [3].
Accurate measurement of corneal astigmatism is mandatory for choosing toric IOL power and planning optimal alignment. Various measuring methods are available, such as manual keratometry (manual K), automated keratometry, reflection methods of corneal topography, slit-scanning technology, optical coherence tomography, and Scheimpflug imaging. The first three methods measure the anterior corneal surface only. They assume a fixed posterior/anterior corneal curvature ratio to calculate total corneal power and astigmatism using a standardized corneal refractive index, most commonly 1.3375. Conversely, slit-scanning technology, optical coherence tomography, and Scheimpflug imaging measure the anterior and posterior corneal surfaces. Therefore, they provide total corneal power and astigmatism based on the measured anterior and posterior corneal data.
Manual and automated keratometry and corneal topography have been traditionally used to assess astigmatism for planning cataract surgery, since it has been assumed that the posterior cornea contributes with negligible amounts of astigmatism to the total corneal astigmatism. However, studies with toric IOLs have shown significant residual astigmatism after surgery [4, 5] and documented that postoperative anterior corneal astigmatism is not the only factor determining the amount of residual refractive astigmatism [6]. In addition, studies using a range of devices have investigated the posterior cornea and have shown that posterior corneal astigmatism ranges from 0.26 D to 0.78 D [7–10]. Thus, two recent studies were conducted to assess the importance of posterior corneal astigmatism in planning cataract surgery, and these are the studies that will be analyzed and discussed in this chapter [11, 12]. The first study used a combined Placido and dual Scheimpflug analyzer to investigate the contribution of posterior corneal astigmatism to total corneal astigmatism and the accuracy in estimating total corneal astigmatism from measurements of the anterior corneal surface [11]. The second study used five devices to evaluate the clinical impact of posterior corneal astigmatism on outcomes of cataract surgery with toric IOLs and provided a nomogram to guide the selection of the appropriate toric IOL power, factoring in posterior corneal astigmatism [12].
8.2 Methods
An institutional review board approval was obtained for both studies, and they followed the tenets of the Declaration of Helsinki.
8.2.1 Study 1 [11]
This retrospective study included consecutive patients who were screened for cataract or refractive surgery and had corneal measurements made using the dual Scheimpflug analyzer (Galilei, Ziemer Ophthalmic Systems AG, Port, Switzerland) between January 2008 and March 2011 at the Cullen Eye Institute, Baylor College of Medicine. Exclusion criteria were a history of previous ocular surgery or trauma, any ocular diseases that might affect the cornea or good fixation, contact lens wear within 2 weeks of the measurement, and image quality below “good quality.”
The Galilei combines dual Scheimpflug cameras and a Placido disk to assess both the anterior and posterior corneal surfaces. It derives the anterior corneal measurements from the combination of the Placido and Scheimpflug data and the posterior corneal measurements from the Scheimpflug data.
From the four corneal astigmatic values (CA) that were investigated in the study, we will assess two in this chapter:
-
CAant: Corneal astigmatism from the anterior corneal surface, which derives from the CA from simulated keratometry (SimK) [CASimK over the 1.0- to 4.0-mm zone. It is based only on anterior corneal measurement]. The CAant is calculated by multiplying the CASimK by (1.376–1.0)/(1.3375–1.0), assuming that the refractive index of the air is 1.0, the refractive index of the cornea is 1.376, and the standardized corneal refractive index is 1.3375. The CAant meridian is the steep SimK meridian.
-
CApost: Corneal astigmatism from the posterior corneal surface over the 1.0- to 4.0-mm zone, which is calculated with the indices of refraction of the cornea (1.376) and the aqueous humor (1.336), assuming that the rays approach the posterior corneal surface parallel to each other.
8.2.1.1 Data Analysis
We calculated the (1) mean magnitude, standard deviation (SD), and range of CAant and CApost, (2) the percentage of eyes with corneal astigmatism magnitudes up to 0.25 D, 0.50 D, 0.75 D, and 1.00 D, and (3) the correlation of magnitude and alignment of astigmatism on the anterior and posterior corneal surfaces. The eyes were subdivided based on the patients’ age at the time of the Galilei exam. To assess the changes in location of the steep meridian over time, the percentages of eyes with the steep meridian aligned vertically (60–120°), obliquely (30–60° or 120–150°), and horizontally (0–30° or 150–180°) on the anterior and posterior corneal surfaces were calculated for each age group. Chi-square test was used to compare the proportion data between age groups, and a Bonferroni correction was used for multiple comparisons. SPSS for Windows software (version 15.0, SPSS, Inc.) was used for statistical analysis. A P value less than 0.05 was considered statistically significant.
8.2.2 Study 2 [12]
This prospective study enrolled patients of the Cullen Eye Institute, Baylor College of Medicine, from July 2011 to September 2012. To be included, patients were required to have AcrySof toric IOL implantation without postoperative decentration/tilt under the slit lamp examination and good-quality preoperative and 3-week postoperative scans of the following devices: IOLMaster (Carl Zeiss Meditec AG, Jena, Germany), Lenstar (Haag-Streit, Koeniz, Switzerland), Atlas Corneal Topographer (Carl Zeiss Meditec AG, Jena, Germany), manual K (Bausch and Lomb, Rochester, New York, USA), and Galilei. Exclusion criteria were a history of previous ocular surgery or trauma, any ocular diseases that might affect the cornea or good fixation, contact lens wear within 2 weeks of the measurement, and poor image quality with each device.
Also, subjects with oblique corneal astigmatism (steep corneal meridian at 30–60° or 120–150°) measured by the IOLMaster were excluded, due to the small number of eyes.
8.2.2.1 Corneal Astigmatism Measurements
The following five devices were used to measure corneal astigmatism in this study:
-
1.
IOLMaster: Measures automated keratometry (K) based on a hexagonal array of 6 points reflected off the surface of the cornea at a diameter of approximately 2.3 mm, depending on the corneal curvature.
-
2.
Lenstar: Keratometry is calculated from an array of 32 light reflections projected off the anterior corneal surface. These lights are arranged in two rings at diameters of approximately 1.65 mm and 2.3 mm, depending on the corneal curvature.
-
3.
Atlas: The Atlas is a Placido-disk-based corneal topographer and provides SimK values along the steepest and flattest meridians at the 3-mm annular zone.
-
4.
Manual K: This is the conventional method for measuring corneal power at a diameter of approximately 3 mm, depending on the corneal curvature.
-
5.
Galilei: Calculates the total corneal power (TCP) by tracking the path of incident light rays through the anterior and posterior corneal surface using ray-tracing method and Snell’s law.
The IOLMaster, Lenstar, Atlas, and manual K measure anterior corneal curvature only, and their astigmatism values are the differences between the anterior corneal steep K and flat K. The Galilei provides a TCP astigmatism value, which is the difference between the steep TCP and flat TCP at the 1.0- to 4.0-mm central zone.
Biometry was done using the IOLMaster and Lenstar. The Holladay 1 formula was used for toric IOL power calculation. Selection of the toric lens power and alignment was determined by the surgeons based on all data available and on Study 1 [11]. The axis of the toric IOL alignment was recorded at the time of surgery and at the slit lamp exam in the 3-week postoperative visit. Manifest refraction was performed 3 weeks after surgery.
8.2.2.2 Data Analysis
Based on the anterior corneal steep meridian measured by the IOLMaster, the eyes were divided into two groups: (1) with-the-rule (WTR) group with corneal steep meridian between 60 and 120° and (2) against-the-rule (ATR) group with corneal steep meridian between 0–30° and 150–180°.
Vector analysis was used in all calculations [13]. To account for the impact of IOL power and anticipated effective lens position, the effective toric power of the IOL at the corneal plane was calculated using the Holladay 2 Consultant program. The assumed “actual” corneal astigmatism was calculated as the difference between the postoperative manifest refraction corrected to the corneal plane and the effective toric power. The corneal astigmatism prediction error for each device, or the deviation from actual corneal astigmatism, was obtained by subtracting the “actual” corneal astigmatism from the corneal astigmatism measured by each device.
Analysis of aggregate corneal astigmatism prediction errors was performed. Using pre- and postoperative corneal astigmatism measurements, both pre- and postoperative corneal astigmatism prediction errors were assessed for each device. The corneal astigmatism prediction errors were further analyzed as follows: (1) WTR/ATR prediction errors, i.e., the magnitudes of errors along the 90- and 180-degree meridians, with negative values indicating WTR prediction errors, and positive values indicating ATR errors and (2) oblique prediction errors, in which positive values indicate oblique astigmatism prediction errors along 45° and negative values along 135°.
8.2.2.3 Statistical Analysis
We aimed at detecting corneal astigmatism prediction error of >0.2 D. To achieve a significance level of 5 % and a test power of 80 %, a minimum sample size of 32 eyes was required.
To assess whether the prediction errors were WTR/ATR or oblique, a one sample t-test was performed to evaluate if the mean vector component values were significantly different from zero. Bonferroni correction was used for multiple comparisons. SPSS for Windows software (version 15.0, SPSS, Inc.) was used for statistical analysis. A P value less than 0.05 was considered statistically significant.
8.3 Results
8.3.1 Study 1 [11]
This first study included 715 eyes of 435 patients, with a mean age of 55 ± 20 years (range, 20–89 years). When subdivided by age, 101 (14.1 %) eyes were from patients with 20–29 years, 104 (14.5 %) eyes were from patients with 30–39 years, 101 (14.1 %) eyes were from patients with 40–49 years, 101 (14.1 %) eyes were from patients with 50–59 years, 101 (14.1 %) eyes were from patients with 60–69 years, 105 (14.7 %) eyes were from patients with 70–79 years, and 102 (14.3 %) eyes were from patients with 80–89 years.
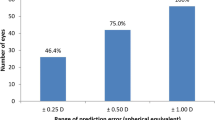
The mean magnitude of CAant was 1.20 ± 0.79 D (range, 0.02–4.90 D). The percentages of eyes with CAant ≤0.25 D, ≤0.50 D, ≤0.75 D, and ≤1.00 D were 4.3 %, 18.2 %, 31.6 %, and 46.9 %, respectively. Regarding CApost, the mean magnitude was −0.30 ± 0.15 D (range, −0.01 to −1.10 D). The percentages of eyes with CApost ≤0.25 D, ≤0.50 D, ≤0.75 D, and ≤1.00 D were 43.1 %, 91.0 %, 99.3 %, and 99.9 %, respectively.
The anterior cornea had the steep meridian aligned vertically in 364 (50.9 %) eyes, while the posterior cornea had a vertical steep meridian in 619 (86.8 %) eyes. There was a moderate correlation between anterior and posterior corneal astigmatism when the steep anterior meridian was aligned vertically (r = 0.56, P < 0.001). There was a weak correlation when it was oriented obliquely (r = 0.37, P < 0.001), and there was no correlation when it was aligned horizontally (r = −0.08, P = 0.26).
Tables 8.1 and 8.2 show the percentages of eyes with the steep meridian aligned vertically, obliquely, and horizontally on the anterior and posterior corneal surfaces when subdividing the eyes based on the patients’ age. With increasing age, the percentage of eyes with a vertical steep anterior corneal meridian decreased significantly (P < 0.05), while there was a significant increase in eyes with a horizontally oriented anterior corneal steep meridian (P < 0.05). On the posterior corneal surface, the steep meridian had a vertical alignment in the majority of eyes in all ages.
8.3.2 Study 2 [12]
This second study included 41 eyes of 41 patients, with a mean age of 71 ± 9 years (range 46–91 years). Seventeen eyes had WTR astigmatism and 24 had ATR astigmatism. In the group of eyes that had WTR anterior corneal astigmatism, the mean aggregate preoperative astigmatism as measured with the IOLMaster, Lenstar, Atlas, Galilei, and manual keratometry were 1.78 D at 91°, 1.66 D at 92°, 1.66 D at 88°, 1.76 D at 93°, and 1.91 D at 94°, respectively. In the ATR group, the mean aggregate preoperative corneal astigmatism as measured with the IOLMaster, Lenstar, Atlas, Galilei, and manual keratometry were 1.28 D at 1°, 1.24 D at 2°, 1.22 D at 0°, 1.56 D at 1°, and 1.32 D at 8°, respectively. The mean postoperative refractive astigmatism were 0.08 D at 11° in the WTR eyes and 0.12 D at 148° in the ATR eyes.
With the IOLMaster, Lenstar, Atlas, and manual K, the mean pre- and postoperative corneal astigmatism prediction errors in the WTR eyes ranged from 0.27 to 0.62 D, all aligned along the vertical meridian, and in the ATR eyes ranged from 0.17 to 0.37 D, also aligned along the vertical meridian. With the Galilei TCP, the mean pre- and postoperative corneal astigmatism prediction errors were 0.57 D and 0.26 D aligned vertically in the WTR eyes and 0.12 D and 0.18 D aligned horizontally in the ATR eyes.
In the WTR eyes, the WTR/ATR prediction error ranged from −0.60 to −0.26 D, and the oblique prediction error ranged from −0.26 to +0.20 D. There were significant WTR prediction errors of 0.5–0.6 D by all devices, except for the Atlas and Galilei. There was a significant oblique prediction error of −0.26 D by the Lenstar (all P < 0.05).
In the ATR eyes, the WTR/ATR prediction errors ranged from −0.29 to +0.17 D, and the oblique prediction errors ranged from −0.13 to +0.36 D. There were significant WTR prediction errors of 0.2–0.3 D by the IOLMaster, Lenstar, Atlas, and manual K and oblique prediction errors of 0.3–0.4 D by manual K (all P < 0.05). There were no significant WTR/ATR or oblique prediction errors by the Galilei.
8.4 Discussion
The high expectation of patients regarding visual outcomes after cataract surgery and the significant postoperative residual astigmatism seen in many patients have led researchers to investigate the posterior corneal surface in more detail [4, 5, 11, 12]. In the two studies discussed in this chapter, the authors used different devices to assess anterior and posterior corneal astigmatism and to evaluate the clinical impact of posterior corneal astigmatism on outcomes of surgeries with toric IOLs [11, 12].
In the first study, 9 % of eyes had a posterior corneal astigmatism greater than 0.50 D. The mean posterior corneal astigmatism was −0.30 D, which is within the range of −0.26 to −0.78 D reported by other authors using different methodologies [7, 8, 10, 14, 15].
Although there was a moderate positive correlation between the magnitude of corneal astigmatism on the anterior and posterior corneal surfaces when the anterior corneal steep meridian was oriented vertically, this correlation was weak when the steep anterior corneal meridian was aligned obliquely and was not found when it was aligned horizontally [11]. Thus, assuming magnitudes of posterior corneal astigmatism based only on the magnitude on anterior corneal surface without taking into account alignment is a potential source of error in planning toric IOL power and alignment.
The anterior cornea’s steeper meridian is commonly oriented vertically in younger individuals, but there is a shift towards the horizontal meridian as patients get older. Similar to the anterior surface, the posterior cornea generally has a steeper vertical meridian in young patients. However, it remains steeper vertically with increasing age. Since the posterior cornea is a negative lens, the vertically aligned steep meridian produces net plus refractive power along the horizontal meridian. Thus, in general, posterior corneal astigmatism partially compensates for anterior corneal astigmatism in young adults and increases total corneal astigmatism in older individuals [11]. In a previous study using the Pentacam device (Oculus, Inc., Lynnwood, WA), a weak correlation was found between the posterior cornea’s shift from being steep vertically to horizontally with age [16]. The percentages of eyes with a horizontally steep posterior cornea were similar to Koch’s et al. [11]. In the former study it increased from 0 % in the 21- to 30-year-old group to 9.1 % in the 71-year-old and older group. In the latter study, it increased from 0 % in the age range of 20 to 29 years to 7 % in the age range of 70 to 79 years.
In the second study assessed in this chapter, the authors evaluated the prediction errors of corneal astigmatism for five devices: four that calculate total corneal astigmatism based on anterior surface measurements only and one that measures both anterior and posterior corneal astigmatisms to provide a total corneal astigmatism value [12].
The IOLMaster, Lenstar, Atlas, and manual K had a mean corneal astigmatism prediction errors of 0.5–0.6 D of WTR astigmatism in eyes with WTR corneal astigmatism and of 0.2–0.3 D of WTR astigmatism in eyes with ATR corneal astigmatism for both pre- and postoperative corneal measurements [12]. Based on the first study discussed, it is known that the posterior cornea generally has a steeper vertical meridian. In addition, the magnitude of posterior corneal astigmatism correlates with the amount of anterior corneal astigmatism in WTR eyes, whereas, in ATR eyes, the mean posterior corneal astigmatism is approximately 0.2 D and does not change with increasing amounts of anterior corneal astigmatism [11]. Therefore, the total corneal astigmatism prediction errors from the IOLMaster, Lenstar, Atlas, and manual K were primarily caused by the posterior corneal astigmatism.
When considering the Galilei TCP values, the WTR group had a significant WTR prediction error of 0.57 D using preoperative corneal astigmatism, while in the ATR group there were no significant WTR/ATR prediction errors. This suggests that the Galilei TCP may underestimate the posterior corneal astigmatism in WTR eyes.
In addition to ours and other studies that directly measured posterior corneal astigmatism, several previous studies reported that the anterior corneal astigmatism is not the only variable determining the total corneal astigmatism [6, 17–19]. The authors from these studies observed that when the topographic astigmatism axis was steeper vertically, the topographic astigmatism magnitude exceeded the refractive astigmatism, while when the axis was steeper horizontally, the refractive astigmatism had a greater value. These findings support the concept that posterior corneal astigmatism is an important contributor to refractive astigmatism.
In the second study reported in this chapter, we provide a toric IOL nomogram that accounts for posterior corneal astigmatism, based on the mean values that we documented clinically (Table 8.3). The refractive target of this nomogram is to leave eyes with a small amount of WTR refractive astigmatism, since most eyes have an ongoing ATR astigmatism shift with age (Table 8.3).
8.5 Conclusions
In conclusion, posterior corneal astigmatism partially compensates for anterior corneal astigmatism in young adults and increases total corneal astigmatism in older individuals. Ignoring posterior corneal astigmatism when planning cataract surgery may lead to overcorrection in eyes with WTR anterior corneal astigmatism and undercorrection in eyes with ATR anterior corneal astigmatism. Devices that calculate total corneal astigmatism based only on anterior corneal measurements overestimate WTR astigmatism by 0.5–0.6 D and underestimate ATR astigmatism by 0.2–0.3 D. These prediction errors are due to the posterior corneal astigmatism and need to be considered in selecting toric IOLs. There is a clear need for widespread availability of devices that accurately measure posterior as well as anterior corneal astigmatism.
References
Hoffmann PC, Hutz WW. Analysis of biometry and prevalence data for corneal astigmatism in 23,239 eyes. J Cataract Refract Surg. 2010;36(9):1479–85. doi:10.1016/j.jcrs.2010.02.025.
Ferrer-Blasco T, Montes-Mico R, Peixoto-de-Matos SC, Gonzalez-Meijome JM, Cervino A. Prevalence of corneal astigmatism before cataract surgery. J Cataract Refract Surg. 2009;35(1):70–5. doi:10.1016/j.jcrs.2008.09.027.
Visser N, Bauer NJ, Nuijts RM. Toric intraocular lenses: historical overview, patient selection, IOL calculation, surgical techniques, clinical outcomes, and complications. J Cataract Refract Surg. 2013;39(4):624–37. doi:10.1016/j.jcrs.2013.02.020.
Sun XY, Vicary D, Montgomery P, Griffiths M. Toric intraocular lenses for correcting astigmatism in 130 eyes. Ophthalmology. 2000;107(9):1776–81. discussion 1781–1772.
Mendicute J, Irigoyen C, Aramberri J, Ondarra A, Montes-Mico R. Foldable toric intraocular lens for astigmatism correction in cataract patients. J Cataract Refract Surg. 2008;34(4):601–7. doi:10.1016/j.jcrs.2007.11.033.
Teus MA, Arruabarrena C, Hernandez-Verdejo JL, Sales-Sanz A, Sales-Sanz M. Correlation between keratometric and refractive astigmatism in pseudophakic eyes. J Cataract Refract Surg. 2010;36(10):1671–5. doi:10.1016/j.jcrs.2010.05.010.
Ho JD, Tsai CY, Liou SW. Accuracy of corneal astigmatism estimation by neglecting the posterior corneal surface measurement. Am J Ophthalmol. 2009;147(5):788–95. doi:10.1016/j.ajo.2008.12.020. 795. e781–782.
Dubbelman M, Sicam VA, Van der Heijde GL. The shape of the anterior and posterior surface of the aging human cornea. Vision Res. 2006;46(6–7):993–1001. doi:10.1016/j.visres.2005.09.021.
Prisant O, Hoang-Xuan T, Proano C, Hernandez E, Awwad ST, Azar DT. Vector summation of anterior and posterior corneal topographical astigmatism. J Cataract Refract Surg. 2002;28(9):1636–43.
Modis Jr L, Langenbucher A, Seitz B. Evaluation of normal corneas using the scanning-slit topography/pachymetry system. Cornea. 2004;23(7):689–94.
Koch DD, Ali SF, Weikert MP, Shirayama M, Jenkins R, Wang L. Contribution of posterior corneal astigmatism to total corneal astigmatism. J Cataract Refract Surg. 2012;38(12):2080–7. doi:10.1016/j.jcrs.2012.08.036.
Koch DD, Jenkins R, Weikert MP, Yeu E, Wang L. Correcting astigmatism with toric intraocular lenses: the effect of posterior corneal astigmatism. J Cataract Refract Surg. 2013;39:1803.
Holladay JT, Moran JR, Kezirian GM. Analysis of aggregate surgically induced refractive change, prediction error, and intraocular astigmatism. J Cataract Refract Surg. 2001;27(1):61–79.
Royston JM, Dunne MC, Barnes DA. Measurement of posterior corneal surface toricity. Optom Vis Sci. 1990;67(10):757–63.
Dunne MC, Royston JM, Barnes DA. Posterior corneal surface toricity and total corneal astigmatism. Optom Vis Sci. 1991;68(9):708–10.
Ho JD, Tsai CY, Tsai RJ, Kuo LL, Tsai IL, Liou SW. Validity of the keratometric index: evaluation by the Pentacam rotating Scheimpflug camera. J Cataract Refract Surg. 2008;34(1):137–45. doi:10.1016/j.jcrs.2007.09.033.
Grosvenor T, Quintero S, Perrigin DM. Predicting refractive astigmatism: a suggested simplification of Javal’s rule. Am J Optom Physiol Opt. 1988;65:292–7.
Alpins NA. New method of targeting vectors to treat astigmatism. J Cataract Refract Surg. 1997;23(1):65–75.
Bae JG, Kim SJ, Choi YI. Pseudophakic residual astigmatism. Korean J Ophthalmol. 2004;18(2):116–20.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer Japan
About this chapter
Cite this chapter
Ventura, B.V., Wang, L., Weikert, M.P., Koch, D.D. (2014). Correction of Corneal Astigmatism with Toric IOLs. In: Bissen-Miyajima, H., Koch, D., Weikert, M. (eds) Cataract Surgery: Maximizing Outcomes Through Research. Springer, Tokyo. https://doi.org/10.1007/978-4-431-54538-5_8
Download citation
DOI: https://doi.org/10.1007/978-4-431-54538-5_8
Published:
Publisher Name: Springer, Tokyo
Print ISBN: 978-4-431-54537-8
Online ISBN: 978-4-431-54538-5
eBook Packages: MedicineMedicine (R0)