Abstract
Brain microdialysis is a well-established technique used to monitor the chemistry of the extracellular space in the brain during neurointensive care. Microdialysis may be useful in severe cases of traumatic brain injury, stroke, and hypoxic brain injury in which monitoring of intracranial pressure and cerebral perfusion pressure is required. The parenchymal concentrations of glucose, lactate, pyruvate, glutamate, and glycerol can be measured at the bedside. As the primary source of energy, glucose is an important marker of changes in cerebral metabolism and reflects systemic supply, which is influenced by capillary perfusion, ischemia, and blood glucose concentration. The lactate–pyruvate (L/P) ratio is a sensitive marker of changes in the redox state of cells brought about by ischemia. The glutamate concentration is an indirect marker of cell damage or ischemia. Glycerol concentration reflects cell membrane damage, as glycerol is an integral component of cell membranes. Loss of energy due to ischemia eventually leads to an influx of calcium and a decomposition of cell membranes, which liberates glycerol into the interstitial fluid. Microdialysis, when used with other brain monitoring techniques, may be a useful means of preventing and relieving secondary ischemic injury, predicting outcome and guiding therapy after severe brain damage. However, the value of microdialysis as a tool in routine neurointensive care decision-making remains unclear.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Brain microdialysis monitoring can detect adverse neurochemical conditions involving hypoxia/ischemia and seizure activity in subarachnoid hemorrhage (SAH), traumatic brain injury (TBI), thromboembolic stroke, and epilepsy. The measurement of parenchymal concentration of glucose, lactate, and pyruvate is used to quantify disturbances of cerebral glucose metabolism, and techniques are being developed to quantify excitotoxicity, cell membrane degradation, cellular edema, and blood–brain barrier dysfunction, although these need additional validation. The clinical utility of microdialysis depends on the choice of biomarkers, their sensitivity, specificity, and predictive value for secondary neurochemical events.
2 Principles of Microdialysis
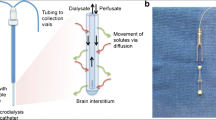
Brain microdialysis requires a specialized catheter to be placed in the brain parenchyma. It is tipped with a semipermeable dialysis membrane, usually with a 20 kDa molecular weight cutoff (Fig. 17.1). The microdialysis catheter can be placed in areas of interest, which is of particular value when therapy is directed at attenuating secondary insults around the brain tissue at risk. The catheter tip should be located in the right frontal lobe after global hypoxic injury (e.g., post-cardiac arrest syndrome or diffuse TBI), the penumbral area of an ischemic stroke, the vascular territories of a ruptured cerebral aneurysm, or the pericontusional area of a focal TBI (Fig. 17.2). The microdialysis catheter is constantly perfused with a cerebrospinal fluid-like solution at a rate of 0.3 μL/min, thereby allowing regular (usually hourly) sampling of the patient’s brain extracellular fluid into microvials and subsequent analysis at the bedside using a proprietary device [1].
3 Clinical Application of Microdialysis and Interpretation of Results
The time taken to analyze the samples means that in practice, the first results are not available until at least 1 h after catheter insertion, but thereafter, new technology allows online monitoring of dynamic changes in patients’ neurochemistry. The small molecules demonstrated to have clinical utility as neurochemical markers used in the management of secondary cerebral injury are glucose, lactate, pyruvate (and the ratio between them, known as the L/P ratio), glutamate, and glycerol. Microdialysate glucose concentration depends on blood glucose and the blood supply to the region of interest. The L/P ratio is a sensitive marker of changes in the redox state of cells caused by ischemia (Fig. 17.3). Microdialysate glucose, lactate and pyruvate concentrations, and L/P ratio may be indicators of secondary complications caused by persistent hypoxia or ischemia. Changes in the L/P ratio are classified into type 1 (in the presence of ischemia, implying anaerobic glycolysis) and type 2 (without ischemia, implying dysfunctional glycolysis; Fig. 17.4). Glutamate is a marker of ischemia and reflects excitotoxicity in the brain. Microdialysate glycerol concentration is a marker of cell membrane disruption and cell lysis, but may be affected by the use of glycerol as an intravenous osmotic diuretic.
Abnormal concentrations of molecules of interest in microdialysate fluid are considered to be: glucose < 0.7–1 mmol/L, glutamate 10–20 μmol/L, and glycerol 100 μmol/L. An L/P ratio >35–40 is also considered abnormal (Table 17.1) [2, 3]. Biochemical changes observed in neurocritical care, including nonischemic glycolysis (Fig. 17.4) [4], are summarized in Table 17.2 [1]. A typical ischemic pattern includes a marked decrease in microdialysate glucose concentration, an increase in L/P and lactate/glucose ratios, and a moderate increase in brain lactate and a decrease in brain pyruvate concentrations [1]. Persistent episodes (>25 min) of profound brain tissue hypoxia (brain tissue oxygen tension [PbtO2] <10 mmHg) are associated with marked metabolic changes (including decreased microdialysate glucose concentration and elevated L/P ratio) [5].
The degree of metabolic distress or crisis is reflected by the extent of the difference between energy supply and demand. Metabolic distress is commonly defined as an L/P ratio >40, whereas metabolic crisis comprises a combination of L/P ratio >40 and microdialysis glucose concentration <0.7 mmol/L (Table 17.1) [1].
4 Microdialysis in Post-Cardiac Arrest Brain Injury
In our experience of microdialysis in post-cardiac arrest brain injury, sustained increases in brain glycerol concentration and L/P ratio were observed in patients with unfavorable outcomes, even with the use of therapeutic hypothermia (unpublished data, Fig. 17.5). The increase in L/P ratio during rewarming could be explained by the concomitant restoration of cerebral metabolic demand and associated lack of balance between delivery and consumption of substrate and oxygen. We also found that microdialysate glycerol concentration increased transiently after intravenous infusion of glycerol as an osmotic diuretic, suggesting that it had crossed a permeable blood–brain barrier.
Glycerol and lactate–pyruvate ratio in a case of post-cardiac arrest brain injury treated with therapeutic hypothermia. Repeated transient increases in glycerol concentration were likely caused by intravenous infusion of glycerol as an osmotic diuretic. Abbreviation: ROSC return of spontaneous circulation
The concentration of glucose in microdialysate fluid correlates with that of in the blood (unpublished data, Fig. 17.6). Increased glycolysis and glucose utilization is frequently observed in patients who have suffered global cerebral ischemia [6], potentially leading to reduced availability of the brain’s main brain substrate, glucose [7]. The critical threshold for microdialysate glucose concentration is generally considered to be 0.7 mmol/L. Multimodal neuromonitoring studies have shown that tight glycemic control may be associated with metabolic crisis in severely brain-injured patients [8]. Insulin therapy may decrease brain glucose concentration despite normoglycemia [9]. Combined monitoring of microdialysate and blood glucose concentrations is particularly helpful for the management of insulin infusion and glucose control in neurocritical care and allows glucose targets to be tailored to individual patients [8, 10].
5 Microdialysis in Traumatic Brain Injury
In a large cohort study of patients who had sustained a TBI, elevated L/P ratio was found to be associated with poor neurological recovery [11]. Poor outcome is also reportedly associated with elevated brain lactate and glutamate concentrations, raised L/P ratio, and low brain glucose concentration in TBI patients [7]. In our experience of TBI, sustained increases and fluctuations in L/P ratio are often observed in cases that ultimately have an unfavorable outcome (Fig. 17.7) [12].
6 Microdialysis in Subarachnoid Hemorrhage
Simultaneous elevation of brain L/P ratio and glutamate concentration has been used as an early indicator of delayed cerebral ischemia in patients with poor-grade SAH [13, 14]. Brain biochemistry may predict neurologic deterioration secondary to cerebral vasospasm hours before symptoms are manifest [15]. Microdialysis can be used in combination with PbtO2 for the detection of delayed ischemia and to guide setting of blood pressure targets and transfusion requirements after SAH [16–18].
Poor outcome has been associated with elevated brain lactate and glutamate concentrations, raised L/P ratio, and low brain glucose concentration in patients with SAH [19].
7 Microdialysis and Anesthesia
Recently, Bossers and colleagues reported that induction of anesthesia with propofol and subsequent tracheal intubation may cause an increase in L/P ratio and microdialysate glycerol concentration, which contrasts with the well-recognized phenomenon of general anesthesia suppressing brain metabolism. Microdialysis may become a useful tool to examine which anesthetic strategies might be best suited to preventing secondary brain injury [20].
Microdialysis, in conjunction with other techniques such as intracranial pressure and PbtO2 monitoring, may be useful in preventing and relieving secondary ischemic injury, predicting outcome, and guiding therapy after severe brain damage. The value of microdialysis as a tool in routine neurointensive care decision-making, however, remains unclear.
References
Hillered L, Vespa PM, Hovda DA (2005) Translational neurochemical research in acute human brain injury: the current status and potential future for cerebral microdialysis. J Neurotrauma 22:3–41
Ståhl N, Mellergård P, Hallström A et al (2001) Intracerebral microdialysis and bedside biochemical analysis in patients with fatal traumatic brain lesions. Acta Anaesthesiol Scand 45:977–985
Reinstrup P, Ståhl N, Mellergård P et al (2004) Intracerebral microdialysis in clinical practice: baseline values for chemical markers during wakefulness, anesthesia, and neurosurgery. Neurosurgery 47:701–709
Vespa P, Bergsneider M, Hattori N et al (2005) Metabolic crisis without brain ischemia is common after traumatic brain injury: a combined microdialysis and positron emission tomography study. J Cereb Blood Flow Metab 25:763–774
Kett-White R, Hutchinson PJ, Al-Rawi PG et al (2002) Cerebral oxygen and microdialysis monitoring during aneurysm surgery: effects of blood pressure, cerebrospinal fluid drainage, and temporary clipping on infarction. J Neurosurg 96:1013–1019
Glenn TC, Kelly DF, Boscardin WJ et al (2003) Energy dysfunction as a predictor of outcome after moderate or severe head injury: indices of oxygen, glucose, and lactate metabolism. J Cereb Blood Flow Metab 23:1239–1250
Vespa PM, McArthur D, O’Phelan K et al (2003) Persistently low extracellular glucose correlates with poor outcome 6 months after human traumatic brain injury despite a lack of increased lactate: a microdialysis study. J Cereb Blood Flow Metab 23:865–877
Oddo M, Schmidt JM, Carrera E et al (2008) Impact of tight glycemic control on cerebral glucose metabolism after severe brain injury: a microdialysis study. Crit Care Med 36:3233–3238
Vespa P, Boonyaputthikul R, McArthur DL et al (2006) Intensive insulin therapy reduces microdialysis glucose values without altering glucose utilization or improving the lactate/pyruvate ratio after traumatic brain injury. Crit Care Med 34:850–856
Vespa P, McArthur DL, Stein N et al (2012) Tight glycemic control increases metabolic distress in traumatic brain injury: a randomized controlled within-subjects trial. Crit Care Med 40:1923–1929
Timofeev I, Carpenter KL, Nortje J et al (2011) Cerebral extracellular chemistry and outcome following traumatic brain injury: a microdialysis study of 223 patients. Brain 134:484–494
Kawai N, Kawakita K, Yano T et al (2010) Use of intracerebral microdialysis in severe traumatic brain injury. No Shinkei Geka 38:795–809
Sarrafzadeh A, Haux D, Sakowitz O et al (2003) Acute focal neurological deficits in aneurysmal subarachnoid hemorrhage: relation of clinical course, CT findings, and metabolite abnormalities monitored with bedside microdialysis. Stroke 34:1382–1388
Sarrafzadeh AS, Haux D, Ludemann L et al (2004) Cerebral ischemia in aneurysmal subarachnoid hemorrhage: a correlative microdialysis-PET study. Stroke 35:638–643
Sarrafzadeh AS, Sakowitz OW, Kiening KL et al (2002) Bedside microdialysis: a tool to monitor cerebral metabolism in subarachnoid hemorrhage patients? Crit Care Med 30:1062–1070
Oddo M, Milby A, Chen I et al (2009) Hemoglobin concentration and cerebral metabolism in patients with aneurysmal subarachnoid hemorrhage. Stroke 40:1275–1281
Ko SB, Choi HA, Parikh G et al (2011) Multimodality monitoring for cerebral perfusion pressure optimization in comatose patients with intracerebral hemorrhage. Stroke 42:3087–3092
Schmidt JM, Ko SB, Helbok R et al (2011) Cerebral perfusion pressure thresholds for brain tissue hypoxia and metabolic crisis after poor-grade subarachnoid hemorrhage. Stroke 42:1351–1356
Helbok R, Schmidt JM, Kurtz P et al (2010) Systemic glucose and brain energy metabolism after subarachnoid hemorrhage. Neurocrit Care 12:317–323
Bossers SM, Peerdeman SM, Oedayrajsingh Varma P et al (2012) Increase in cerebral metabolites during induction of propofol anaesthesia. Br J Anaesth 108:165–167
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer Japan
About this chapter
Cite this chapter
Kuroda, Y., Kawai, N., Kawakita, K. (2015). Role of Microdialysis in Neuroanesthesia. In: Uchino, H., Ushijima, K., Ikeda, Y. (eds) Neuroanesthesia and Cerebrospinal Protection. Springer, Tokyo. https://doi.org/10.1007/978-4-431-54490-6_17
Download citation
DOI: https://doi.org/10.1007/978-4-431-54490-6_17
Publisher Name: Springer, Tokyo
Print ISBN: 978-4-431-54489-0
Online ISBN: 978-4-431-54490-6
eBook Packages: MedicineMedicine (R0)