Abstract
Cerebral microdialysis (CMD) is a tool increasingly used in neurocritical care units in the management of patients with severe traumatic brain injury and aneurysmal subarachnoid hemorrhage who are comatose. In the International Multidisciplinary Consensus Conference on Multimodality Monitoring in Neurocritical Care, CMD monitoring was recommended in patients with or at risk of cerebral ischemia, hypoxia, energy failure, and glucose deprivation. CMD allows knowing, in near real-time, the dynamic changes in the extracellular brain tissue levels of the metabolites involved in cerebral energy substrate delivery and energy metabolism (glucose, pyruvate, and lactate) and to carry out, with some temporal limitations, bedside sequential measurements of ions and various molecules of interest in the injured brain. At present, this technique has progressed from the laboratory to the clinic and progressively is being introduced in the monitoring of neurocritical patients since it offers important neurometabolic information complementary to the variables that are routinely monitored in these patients (intracranial pressure, cerebral perfusion pressure, and cerebral oximetry). In this chapter, we review the rationale and fundamentals of microdialysis techniques, describe basic methodological aspects, and update the possibilities of this powerful brain monitoring technique. CMD combined with methods of brain oxygen monitoring allows a systematic approach to the different classes of brain hypoxia and the disrupted metabolic profiles found after acute brain injury.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Brain metabolism
- Cerebral microdialysis
- Head injury
- Multimodal neuromonitoring
- Neurocritical patient
- Neuromonitoring
1 Introduction and the Concept of Cerebral Microdialysis
One of the fundamental objectives of the treatment of patients with a severe traumatic brain injury (TBI) is the prevention of secondary brain lesions, hence the importance of being able to make early detection of cerebral tissue ischemia. This concept can be extended to the context of other neurocritical patients. Microdialysis (MD) is a technique based on the principle of solute exchange through a semipermeable membrane, which emulates the functioning of a blood capillary [1,2,3]. MD is an extremely sensitive technique that can provide early metabolic information about the establishment of a tissue lesion. Although complex, this technique provides much better information than any other monitoring system, since it allows one to monitor: (1) the tissue availability of different metabolites, such as glucose; (2) the elements released by the cells; and (3) the cellular consequences of tissue hypoxia-ischemia.
Cerebral MD (CMD) was introduced in 1966 by Bito et al. for in vivo dialysis of the canine brain [4] and is now used for an extensive array of applications that explore the regional chemistry of the human brain. The first known application of CMD in humans was reported in 1990 by Meyerson et al., who implanted MD probes during thalamotomy procedures in patients with Parkinson’s disease [1]. In the same year, the first report of changes in energy-related metabolites during frontal lobe resection in five human patients was published [5]. Since that time, CMD has been increasingly used as a neuromonitoring technique in neurocritical patients with TBI, middle cerebral artery infarction (MCAI), and spontaneous subarachnoid hemorrhage (SAH) to monitor cerebral energy metabolism during the acute phase after injury or stroke [6]. Despite the great potential of this monitoring technique, in 2012, Kitagawa et al. showed that only 42 centers worldwide used MD for clinical decision making in the management of neurocritical patients [7]. In addition to its clinical utility; however, it is important to note that CMD is a unique research tool that allows in-depth analysis of the complex physiological derangements that occur in acute brain injuries.
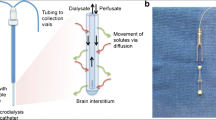
The semipermeable membrane is located at the distal end of the MD catheter implanted in the brain (Fig 7.1) and through it the solutes are exchanged between a solution of known composition and the extracellular space fluid. In the brain, the placement of an MD catheter allows the analysis and quantification of the changes that occur in various “energetic” metabolites, such as lactate, pyruvate, adenosine, inosine, or hypoxanthine. It also allows studying the release of neurotransmitters and neuromodulators (e.g., glutamate, aspartate, GABA, and taurine) or the release of products of inflammatory origin (cytokines) or resulting from tissue degradation (glycerol, potassium). Although CMD catheters allow a large number of molecules and ions to be obtained, in clinical practice, neurochemical monitoring is limited to sequential quantification of four or six metabolites, the maximum number allowed by the analyzer equipment used at the patient’s bedside (CMA-600 or ISCUSflex, CMA Microdialysis, Stockholm, Sweden).
CMD involves the insertion of a catheter, of fine caliber (0.62-mm external diameter) and double lumen, into the extracellular space of the brain parenchyma. One of the “lumens” of the catheter is connected to a small precision pump that infuses saline or ringer serum without lactate at extremely low speeds (0.1–5 μL/min). Because there is a concentration gradient between the two spaces, there is a passage of molecules contained in the extracellular space into the catheter, which depends on the diameter of the pores of the semipermeable membrane. Through the second lumen of the MD catheter, the solution loaded with tissue metabolites is collected in a microvial that is replaced every 10–60 min. The microvials obtained are analyzed at the patient’s bedside, which allows four to six analytes to be sequentially quantified by enzymatic techniques. However, for this brain information to be valid and useful, it must be contrasted with the information provided by an additional catheter placed in the subcutaneous tissue. The latter provides information on systemic (extracerebral) metabolism.
2 Basic Methodological Aspects of the Cerebral Microdialysis
The application of the CMD includes the use of five key elements: the MD catheters, a semipermeable membrane, infusion micropumps, special microvials, and a portable analyzer placed within the same ICU (bedside). Microdialysis catheters (CMA-70 or CMA-71, CMA Microdialysis, Stockholm, Sweden) are flexible, with a small diameter (<1 mm), and contain a double lumen with a semipermeable membrane at the distal end (Fig. 7.1). Small solutes can pass freely across this membrane due to an osmotic gradient. The internal lumen of the catheter contains a metabolite-free solution (Ringer solution without lactate or an isotonic saline serum). The catheter is attached to a continuous infusion micropump (CMA-106, CMA Microdialysis) that infuses the solution at a constant and predetermined velocity. At the distal extreme of the catheter, and through the semipermeable membrane, there is an exchange of solutes of a particular molecular weight (<20 kDa in standard catheters [CMA-70] and up to 100 kDa in high-resolution catheters [CMA-71]). The microdialysate obtained contains molecules from the extracellular space and flows through the external lumen of the catheter. This microdialysate is recovered through special microvials, which are replaced periodically. A portable analyzer (CMA-600 or ISCUSflex) analyzes the microdialized by enzymatic and fluorometric techniques (urea) and quantifies changes in the composition of the initial solution (Fig. 7.1).
The metabolites dissolved in the fluid of the extracellular brain space come from the tissue capillaries, neurons, and adjacent glial cells [2]. The passage of substances to the MD catheter depends on its molecular weight (the semipermeable membrane of the usual catheter only allows the passage of ions and molecules of molecular weight less than 20,000 Da), the infusion rate of the perfusion fluid, the length of the membrane, and the diffusion coefficient of each substance in the tissue to be studied [8]. The recovery of the different metabolites is always a percentage of the actual content that exists in the study tissue, which introduces the concept of “relative recovery,” which is explained in detail in the following section.
3 The Recovery Principle
The dialyzing properties of the MD membrane are routinely expressed as its recovery for a particular solute [9]. The recovery of a certain metabolite is defined as the concentration of this element that contains the microvial divided by the actual concentration in the interstitial space, a fraction expressed as a relative recovery (RR) [2], which is calculated as a simplification of the following equation: RR = (Cout—Cin) / (Cex–Cin), where Cout is the concentration of a given substance in the dialysate, Cex is the concentration of the same substance in the extracellular fluid , and Cin is the concentration of the solute in the perfusion fluid. RR is expressed as a percentage. The concentration of the solute in dialysate will always be lower than that in the extracellular fluid. Therefore, RR will always be below 100%, except when the flow rate equals zero. In this particular situation—and for a low molecular weight solute—the concentrations equilibrate in both sides of the dialyzing membrane [10].
The optimal recovery of any metabolite should be 100%; that is, the information provided by the MD catheter should accurately reflect the composition of the extracellular space. Hutchinson et al. analyzed the influence of various methodological aspects on the recovery of analytes, such as glucose, lactate, pyruvate, and glutamate in brain neurosurgical patients [11]. These authors confirmed that the concentrations of these metabolites obtained from adjacent catheters were practically identical [11].
In the same study, when the influence of dialysis membrane length was analyzed, metabolite recovery was found to be much higher if 30 mm dialysate membranes were used instead of 10 mm. The authors also verified that the perfusion rate of the dialysate fluid had a significant influence, with 0.3 μL/min performing best. Using a dialysis membrane of 10 mm and an infusion rate of 0.3 μL/min, these authors obtained an approximate recovery of 70% of all the metabolites studied [11]. Coinciding with these results, Ungerstedt et al. observed that with a flow rate of 0.3 μL/min and a membrane length of 30 mm, the recovery was almost 100% [3].
Another important aspect reported in the Hutchinson et al.’s study was that the samples analyzed at the bedside (online analysis with the CMA-600 analyzer) showed an excellent correlation, with minimal differences in absolute values, with off-line determinations using chromatographic techniques [11]. Likewise, the freezing of the microdialysis samples at a temperature of −70 °C over a period of 3 months also did not significantly alter the determinations obtained, confirming that the differences in all the elements studied were less than 5%. Finally, these authors also confirmed that the use of Ringer solutions without lactate or isotonic saline did not influence the final concentration of glucose, lactate, pyruvate, or glutamate [11]. However, Ungerstedt et al. recommend the use of Ringer without lactate as a fluid to be perfused, based on the fact that the use of saline causes a depletion of calcium and potassium that can alter neurotransmission in the areas near the implanted catheter [3].
4 How to Implant a Cerebral Microdialysis Catheter
For placement in the brain of MD catheters, stereotaxy techniques were initially used. However, these techniques, which might be ideal in certain experimental studies, constitute an important limitation in the clinical context of neurocritical patients. At present, CMD catheters are inserted by placing them under direct vision during surgical procedures or through a burr hole in the operating room, through cranial multilumen bolts (Fig. 7.2), or percutaneously by a twist drill craniotomy [12]. Despite its greater ease, an important drawback of using bolts is that positioning of the catheter next to focal lesions can be difficult or impossible because bolts are manufactured to allow a fixed-length insertion of the catheter. Therefore, the implantation of catheters around focal lesions requires an independent burr hole to reach the target area. To simplify the insertion of cerebral microdialysis catheters, in our center, we use a percutaneous technique, similar to that used to implant an intraparenchymal ICP or PtiO2 sensor without a bolt [12]. Like the implantation of other brain sensors used to monitor neurocritical patients, this technique can be performed at the bedside in the ICU.
The surgical technique for implanting CMD catheters involves a twist-drill craniotomy, with a small drill hole (2.7-mm in diameter), and subcutaneous catheter tunneling (Fig. 7.3). The patient’s head is shaved and prepared. The craniotomy includes the outer and inner tables of the skull and is followed by a blind duramater perforation using a 14G needle. This maneuver ensures adequate patency of the craniotomy and opening of the dura. Then, a small stylet is inserted in the craniotomy and is introduced slightly into the opened dura to make a small puncture in the pia mater and ensure smooth insertion of the microdialysis catheter. Next, the stylet is removed, and the MD catheter is introduced into the craniotomy following the route created by the stylet. Finally, the dialysis membrane was positioned in the subcortical white matter, the skin is sutured, and the catheter is fixed to the scalp by additional sutures [12]. As with other cranial sensors, CMD catheters are only implanted when coagulation parameters are considered to be normal (platelet count > 100,000, prothrombin time < 14 s, and partial thromboplastin time < 50 s), without the use of antibiotic prophylaxis. Routine precautions against infection are taken during sensor insertion and daily care.
Graph summarizing the steps for the implantation of a brain microdialysis catheter using a percutaneous technique: (1) a small drill hole (2.7-mm in diameter) was carried out, (2) the duramater was perforated using a 14G needle, (3) the microdialysis catheter was placed within the protective sheath and tunneled, (4) the distal part of the microdialysis catheter was guided toward the white matter. Finally, the microdialysis catheter was fixed on the scalp and covered by a semipermeable membrane, which remained in place throughout the monitoring period
5 Where Should the Cerebral Microdialysis Catheter Be Placed? The Importance of Radiological Control
As in other local monitoring systems, in CMD, clinicians must decide where the catheter should be implanted to obtain the most useful information for the clinical management of the patient. The implantation of a catheter in the “healthy” tissue offers the possibility of monitoring the tissue with greater chances of recovery, while offering us information that we can extrapolate globally to the rest of the uninjured brain. On the other hand, the placement of a catheter in the “penumbra” areas allows the monitoring of potentially recoverable brain regions. We consider ischemic or traumatic “penumbra” as a brain region of normal macroscopic appearance around the ischemic core or traumatic cerebral contusions or hemorrhages with no changes in brain tissue attenuation in a noncontrast CT scan (Fig. 7.4); these regions had to be at least 20 mm away from any brain region with parenchymal abnormalities [10]. The traumatic penumbra is also considered when the probe is located in the brain immediately below any significant extra-cerebral hematoma. To resolve this conflict, and ideally, in the focal lesions, two microdialysis catheters should be implanted in the brain parenchyma, one in healthy tissue and the second in the area of the penumbra. In diffuse lesions, however, the placement of a single cerebral catheter is sufficient. In our patients, we have found that in the focal or ischemic lesions, the information provided by two brain MD catheters can be very different, confirming the pathophysiological complexity of the cerebral lesions (Fig. 7.5).
A microdialysis catheter implanted in a traumatic “penumbra” region (brain region with normal macroscopic appearance around cerebral traumatic contusions or hemorrhages with no changes in brain tissue attenuation in a noncontrast CT scan). These regions had to be at least 20 mm away from any brain region with parenchymal abnormalities
CT scan of a patient with malignant infarction of the middle cerebral artery. Two microdialysis catheters were implanted in the ischemic hemisphere: in the ischemic core and in the penumbra region. Both catheters have a gold tip. Note how the information offered by the two catheters is very different depending on the tissue in which they are inserted
An essential aspect of this type of monitoring is to know the exact position of the MD catheter (e.g., gray matter, white substance, and specific brain territory). Initially, the MD catheters were not radiopaque or equipped with radiological markers, so it was impossible to determine the exact position of the catheter. As an additional difficulty, if we wanted to place the dialysate membrane in a cortical position, the flexibility and small caliber of the catheters favored their migration to the subarachnoid space. At present, there is a consensus in locating the dialysate membrane in the white subcortical matter, and the CMD catheters incorporate a tiny piece of gold at its distal end, which is perfectly visible in radiological controls (Fig. 7.5). Also, the information we obtain can be interfered with by the presence of blood or air around the catheter (Fig. 7.6), so for a proper clinical interpretation of the results, we must rule out these facts.
6 Metabolites to Be Determined: Neurotransmitters, Markers of Ischemia, and Tissue Injury
Although CMD catheters allow a large number of molecules and ions to be obtained, neurochemical monitoring at the bedside is limited to the sequential quantification of four to six metabolites, the maximum number allowed by the analyzers used in the ICU. The standard analyzer equipment (CMA-600) allows the quantification of four analytes per patient and the monitoring of three simultaneous patients. The ISCUSflex, the third generation of MD analyzers, allows the monitoring of up to eight simultaneous patients, with a batch analysis capacity of 16 samples, and the use of six available reagents: glucose, lactate, pyruvate, glycerol, glutamate, and urea. However, if the microvials are preserved at the appropriate temperature (−70 °C), subsequent determinations of additional analytes can be made, with the only limitation being the residual volume of dialysate fluid that the microvial contains.
Glucose is the metabolite most frequently determined in MD. Glucose constitutes the fundamental energy substrate of the brain. Its extracellular concentration depends on the concentration of peripheral blood glucose, local capillary flow, and cell uptake. The latter can vary when cellular metabolism shifts from an aerobic to an anaerobic pathway. The simultaneous use of an MD catheter located in the subcutaneous tissue provides continuous information on the systemic availability of glucose and therefore information for the correct interpretation of brain glucose levels. Thus, when the glucose in the brain descends parallel to the brain tissue O2 (PtiO2), with peripheral glucose being preserved, we can affirm that there is a decrease in capillary blood flow [3]. In other situations, to correctly interpret the metabolic events that take place, the simultaneous quantification of several metabolites in the brain is required [3]. Several studies have established that brain glucose levels are lower than plasma levels [13, 14]. Reinstrup et al. established the traditional clinical upper threshold for MD brain glucose at 0.3 μL/min in awake patients—using the ±1.96 SD method—at 3.5 mmol/L [15]. This upper limit was similar to the values found in our awake and anesthetized patients at the same perfusion rate [14].
In the brain, interstitial lactate arises as an intermediate metabolite in aerobic glycolysis and is generated in large quantities in anaerobic glycolysis, in an attempt to increase the production of ATP through a less efficient metabolic pathway. Therefore, when high levels of lactate are found in the brain, it can come from an increase in aerobic metabolism (situation of cellular hypermetabolism) or from a situation of tissue hypoxia, ischemic or nonischemic, in which glycolysis is fundamentally anaerobic . In patients with spontaneous SAH, Oddo et al. found that brain lactate elevations (>4 mmol/L) were more often caused by cerebral hyperglycolysis than by brain hypoxia and that hypoxic lactate was associated with increased mortality, whereas hyperglycolysis was a predictor of a good outcome [16]. The differential diagnosis between these situations, conceptually opposed, can be made with the simultaneous determination of pyruvate and the calculation of the lactate/pyruvate ratio (LPR) . An increase in lactate parallel to an increase in pyruvate, with a normal LPR, indicates a situation of cellular hyperglycolysis. In contrast, an increase in lactate accompanied by a decrease in pyruvate and an increase in the LPR are indicators of ischemic or nonischemic brain hypoxia or mitochondrial dysfunction [17]. According to the results of our study of reference levels in CMD [14], a pragmatic upper limit for the LPR in both awake and anesthetized patients appears to be 35, and not the limit of 25 that we have used in a classical way [17].
Glycerol is one of the structural components of the lipid bilayer of the cell membrane. Located in the outermost part of the cell membrane (hydrophilic portion), glycerol emerges from this structure in situations of lack of cellular energy, constituting a biochemical marker of tissue injury [18]. In situations of excitotoxicity, mediated by massive glutamate releases in the synaptic cleft, or in a situation of lack of energy, uncontrolled entry of calcium into the cell occurs. Intracellular calcium activates certain phospholipases and generates the formation of free radicals of O2, which are responsible for the phenomenon of lipid peroxidation. Lipid peroxidation destroys the cell membrane, with the consequent release of fatty acids and glycerol. In clinical practice, there are several situations that can generate cellular “suffering,” such as increases in ICP (Fig. 7.7). However, it is not clearly established whether the increase in glycerol is associated with the destruction of the cell membrane, with secondary death of the cell, or if it constitutes a marker of “cell suffering,” with the possibility of reversal of the process, without destruction of the cell. In a small cohort of TBI patients, Peerdeman et al. found that values of glycerol >150 μmol/L in the normal-appearing regions of the brain had a positive predictive value of 100% for an unfavorable outcome [19]. In a series of patients, we found a significant increase in cerebral glycerol in both the ischemic and traumatic core, but the glycerol levels were always below the upper reference threshold in both the normal-appearing brain and the traumatic penumbra. Our findings reinforce the idea that glycerol is a marker of tissue injury [10]. However, some studies have pointed out that maneuvers as simple and routine as the application of a glycerol enema in a patient can greatly increase the concentrations of this substance, which might raise questions about its validity as a marker element of tissue injury and leads us back to the need for systemic information to properly interpret the information offered by CMD catheters [20].
Glutamate is the most abundant excitatory neurotransmitter in the mammalian nervous system, followed in importance by aspartate. Glutamate is distributed practically throughout all brain regions, and its action is essential in normal neuronal transmission. When the neuron is depolarized, glutamate is released into the synaptic cleft, exerting its action on a variable set of receptors. The function of this neurotransmitter depends fundamentally on the type of receptor on which it acts. In severe TBI, there are certain situations (e.g., hypoxia, ischemia, mechanical injury with rupture of cell membranes, and the release of blood content) in which large amounts of glutamate and aspartate are released into the extracellular space. In these circumstances, both neurotransmitters, although especially glutamate, can exert a repeated and uncontrolled exciting action on neurons, leading them to a state of repetitive depolarization that can condition cellular self-destruction (excitotoxicity phenomenon). Since ischemic phenomena cause a massive release of this neurotransmitter, its determination can also be used as a marker of tissue injury.
As with any high-precision technique and on which clinical decisions might be based, in CMD, it is essential to have a system capable of identifying artifactual information that might be included among valid values. Ronne-Engstrom et al. [21] proposed the use of urea to detect problems in the recovery of MD catheters, since this molecule acts as an endogenous reference compound. An endogenous reference compound is a substance naturally present in the organism that, once synthesized , is not metabolized and can diffuse freely through biological membranes, reaching a similar and stable concentration in all systemic fluids. Urea is a molecule of low molecular weight (60 Da) and polar structure, but without an electric charge, characteristics that make it suitable for use as an endogenous reference compound [22], provided there is a sufficient period to allow a homogeneous distribution of the molecule.
In mammals, urea is the main end product of nitrogen catabolism. Almost all the urea present in blood and urine is synthesized in the liver and excreted through the kidney, without its concentration showing sharp oscillations. Assuming that the concentration of urea is similar in the extracellular space fluid of the brain and subcutaneous tissue, the ratio between cerebral urea and subcutaneous urea of MD samples constitutes a good quality control in the application of this technique. The ratio between cerebral and subcutaneous urea usually ranges between 0.5 and 1 in different patients. This variability is due to the different physicochemical and physiological conditions that occur in the tissue in which each catheter is inserted and that directly affects the amount of urea collected by each of the catheters [23]. However, the value of the urea ratio brain/subcutaneous measured between a pair of specific catheters should be constant in a given patient. Thus, if the ratio of cerebral urea versus subcutaneous urea is constant, it can be said that the two MD catheters are functioning correctly. When the ratio is constant, the oscillations in the concentration of the other analytes studied simultaneously would be a correct reflection of the real oscillations of these analytes in the interstitial space. An increase or decrease in the ratio between cerebral urea and subcutaneous urea will indicate dysfunction of one of the two catheters . Urea should not be used as a reference substance in cases of renal insufficiency, since in this situation, its concentration may suffer sharp oscillations [21].
7 Reference Values of Brain Metabolites
One of the fundamental problems of MD lies in establishing the normal values of different metabolites. Given the ethical and methodological impossibility of monitoring normal subjects, the reference values should be obtained from experimental studies or from patients with intracranial pathology, with the limitations that this implies. The reference values of the different elements or metabolites studied that we have used for years were obtained from neurological and neurosurgical patients, awake and anesthetized, inserting the cerebral catheter into uninjured tissue [15]. In most cases, these patients had benign tumors of the posterior fossa, and the catheter was implanted in an uninjured frontal region without cerebral edema [Table 7.1] [15]. Other authors have reported brain metabolite levels in the normal brains of patients with central nervous system tumors [24], in awake epileptic patients [13, 25], and in patients with spontaneous SAH [26].
Nevertheless, the true reference limits of the different metabolites that we usually monitor in clinical practice are still unknown. Recently, we published the results of an in vitro and a human study in which the reference limits for glucose, lactate, pyruvate, and glycerol were determined in a cohort of 19 patients who were observed twice: while anesthetized and while fully awake [14]. In anesthetized patients, the extrapolation to the zero-flow rate method was used. In awake patients, we employed perfusion at a constant infusion speed of 0.3 μL/min, as recommended by a recent consensus conference on neuromonitoring [27]. The reference intervals reported in this study provide additional support for the thresholds suggested by the most recent consensus conference on MD neuromonitoring [27], highlighting the importance of lactate in brain energetics and raising the need to reconsider traditional definitions of metabolic disturbances observed in neurocritical patients. This study emphasizes the importance of using different thresholds for patients that are awake and those under anesthesia or deep sedation [14]. Table 7.1 summarizes the main results of this study.
8 Clinical Indications of Cerebral Microdialysis and Evidence-Based Medicine
Although CMD has been used in the monitoring of patients with epilepsy, brain tumors, and different types of neurosurgical interventions, at present the clinical applications of this technique mainly focus on the neuromonitoring of patients with a severe TBI, SAH, or brain injury of ischemic origin.
In the latest edition (4th) of the clinical practice guidelines of the Brain Trauma Foundation, the authors recognize that the goal of the medical management of severe TBI is to ensure that nutrient delivery to the brain is optimized through the period of abnormal physiology and brain swelling that follows the injury, and that the only way to be assured that this is being achieved to the greatest extent possible is to measure brain metabolites, which provide reassurance that the needs of oxidative metabolism are being met [28]. Another statement is that the use of advanced monitoring techniques, such as CMD, in tandem with ICP and CPP monitoring, adds to the assessment of brain metabolic needs and the effects of therapies to meet them [28]. However, they only offer recommendations (with a level III of evidence) on the use of jugular bulb monitoring of arteriovenous oxygen content difference [28]. Of the 51 new studies on all advanced monitoring tools that are reviewed in this latest edition, methodological limitations eliminate 42, and of the nine remaining, only one refers to MD. This corresponds to the Chamoun study in which it is observed that patients with glutamate levels that tend to normalize within 120 h of trauma have lower mortality than those in which glutamate remains high [29]. Therefore, indications and recommendations on the use of CMD are based only on the result of expert consensus conferences.
In agreement with the conclusions of an expert panel meeting in the Karolinska Institute in Stockholm in 2002, published in 2004 [30], the patients who will derive the greatest benefit from the inclusion of CMD in neuromonitoring are those with a severe TBI or SAH. In both types of patient, the aim of MD monitoring is the same: the early detection of metabolic changes suggesting the development of tissue ischemia and monitoring of the effect of the therapeutic maneuvers applied to treat the ischemia. In TBI, one or more brain catheters should be applied according to the type of lesion. In diffuse lesions, the implantation of a single brain catheter in the right frontal region was recommended. In focal lesions, two catheters were recommended, one in the macroscopically nonlesioned region and the other in the “area of penumbra” (the brain area surrounding a focal lesion, which is considered at greatest risk). The consensus conference concluded that the information that might be provided by placing an additional catheter in an established lesion does not add important information for patient management. In patients with SAH, a single brain catheter was recommended , although it should be implanted in the vascular area at greatest risk. The analytes recommended, and the relative importance of each, in both entities were as follows: (a) in SAH, glutamate and the lactate/pyruvate ratio and (b) in TBI, lactate/pyruvate ratio, glucose, glycerol, and glutamate.
In the second consensus conference on neuromonitoring in neurocritical patients made by the NICEM (Neuro-Intensive Care and Emergency Medicine), a section of the ESICM (European Society of Intensive Care Medicine) [31], the expert panel concluded that:
-
Despite the increase in the application of cerebral microdialysis in the clinical management of patients with a severe TBI, class I evidence on the routine use of this technique is lacking.
-
MD is the only technique that allows continuous monitoring of the biochemical characteristics of the extracellular space of the brain parenchyma. This information is much more reliable than any other biomarker obtained from peripheral blood.
-
MD can help in the differential diagnosis of the distinct types of nonischemic hypoxia.
-
High-resolution CMD allows the recovery of additional substances contained in the extracellular space, such as cytokines, interleukins, and other inflammatory molecules, which will allow a greater understanding of the physiopathology of acute neurological lesions.
-
This technique is ideal to obtain direct information on the passage of drugs through the blood–brain barrier and on their metabolic repercussions, which will allow studies of neuroprotective drugs to be more rational and effective.
In 2015, the conclusions of the last consensus statement from the 2014 International Microdialysis Forum were published [27]. In this document, regarding the clinical indications of CMD, the conclusions are very similar to the previous recommendations. The most obvious change is the recommendation of where to place the MD catheter or catheters. In patients with SAH, two situations are considered: (1) when the patient does not present a risk for any specific vascular territory, in which case the catheter would be placed in the watershed anterior cerebral artery–middle cerebral artery territory (frontal lobe) and (2) when the monitoring is implanted in a deferred way in a patient who has deterioration, in which case the catheter should be placed in the vascular territory of risk of infarction [27]. In TBI, a distinction is made between diffuse lesions (in which a single catheter would be placed in the frontal region of the nondominant hemisphere) and focal lesions (in which one or more catheters could be placed, depending on what is of interest to monitor: area of penumbra or macroscopically normal brain tissue) [27]. In this consensus conference, additional recommendations were made about methodology, interpretation of CMD, and core data reporting required in the publication of microdialysis papers [27]. The conclusions of this consensus conference are summarized in Table 7.2.
9 Limitations and Complications
Among the limitations of CMD, it should be noted that it is a local monitoring system, which might not detect metabolic events that take place at points away from the location of the catheter. Also, depending on the duration of the monitoring, local inflammation phenomena have been described that might hinder the passage of molecules from the interstitial space to the MD catheter [23]. However, it has been confirmed that this phenomenon has no clinical relevance during the first week of monitoring [23]. Another limitation of the technique is the type of molecules that can be determined from the use of catheters with dialysis membranes of 20 kDa. However, this limitation has been largely resolved, since, at present, there are already cerebral catheters equipped with dialysate membranes with pores of 100 kDa. These catheters make it possible to determine larger molecules related to neuroinflammation phenomena and other processes involved in the pathophysiology of certain acute neurological lesions.
The complication rate attributed to this monitoring system in the different published series has been much lower than that associated with the placement of an ICP sensor [32]. Brain MD catheters are extremely thin (0.62 mm), which minimizes the possibility of brain injury. No significant hemorrhagic complications or infections attributable to CMD have been described, probably because it is a closed circuit that is not manipulated (except for the exchange of microvials) for the duration of the monitoring. In our series [12], no clinically significant hemorrhages were observed after catheter implantation in 122 implanted MD catheters. In only four of these 122 MD catheters, the follow-up CT scan showed minute blood collections around the tip of the catheter (Fig. 7.6). These findings cannot be considered as true hemorrhagic complications. However, they might affect the validity of the information we obtain from the catheter. In this series, there were no instances of meningitis, empyema, or brain abscess that could be attributed to MD monitoring [12]. Moreover, the rates of complications and catheter malfunction observed after using our percutaneous technique to implant CMD catheters were also very similar to those reported by other studies in which these catheters were implanted using bolts or conventional burr holes [33,34,35]. Fractures of the catheter or microdialysis membrane are almost always due to poor system manipulation and decrease at the end of the learning curve of each center.
10 The Future: Cerebral Microdialysis with High-Resolution Membranes
The appearance of 100 kDa MD membranes (also called “high resolution” membranes) has allowed the recovery, directly from the brain extracellular space in vivo, of larger molecules such as cytokines, interleukins, and matrix metalloproteases, which until now had been detected only in postmortem studies. These molecules are key to understanding and deepening the neuroinflammatory processes that have been observed in the context of the neurotraumatic patient, patients with a massive infarction of the middle cerebral artery, or in other neurocritical patients. These molecules are actively involved in the formation of cerebral edema, disruption of the blood–brain barrier, and in the expression of adhesion proteins and tissue infiltration, events involved in the appearance of secondary brain lesions that can exacerbate the neurological damage of the patient. These membranes have allowed MD to become a powerful tool for the development of translational research projects in neurosciences and other scientific disciplines interested in a wide variety of analytes.
Despite its advantages, some technical limitations associated with the 100 kDa MD membranes that affect the recovery of larger molecules must be considered in the clinical setting. Although their molecular weights are below the nominal cut-off of the membrane, and therefore are potentially recoverable, each molecule has unique physicochemical characteristics (e.g., tertiary/quaternary structure of the molecule, polarity, and hydrophobicity) that will condition its diffusion through the dialysis membrane. On the other hand, preliminary observations made in our unit by scanning electron microscopy of implanted CMD membranes have objectified depositions and adhesions of biological components (protein exudate) that cover the membrane and occlude the pores (Fig. 7.8). The formation of this bioplate (the biofouling phenomena) could also affect the recovery of the membrane progressively throughout the days in which the catheter is implanted. These findings demonstrate the need to perform recovery experiments for each analyte if the aim is to extrapolate the actual concentration in the brain parenchyma.
The importance of the use of this neuromonitoring technique has become evident in the multitude of recent studies that include MD as a fundamental tool to determine the concentrations of various drugs inside the central nervous system [36,37,38,39,40].
11 Final Considerations
CMD is an extremely sensitive technique that can provide early metabolic information on the development of a brain lesion. The information provided by this technique is superior to that provided by any other monitoring system. Given its undeniable current position in research, in all probability, its use will become widespread in the clinical setting in the next few years, providing new knowledge on the physiopathology of neurocritical patients, as well as guidance on the more effective and personalized treatment. However, the introduction of this monitoring system involves a learning curve and requires human and technical resources, which currently limit its use to certain neurocritical units.
References
Meyerson BA, Linderoth B, Karlsson H, Ungerstedt U. Microdialysis in the human brain: extracellular measurements in the thalamus of parkinsonian patients. Life Sci. 1990;46(4):301–8. https://doi.org/10.1016/0024-3205(90)90037-R.
Ungerstedt U. Microdialysis: principles and applications for studies in animals and man. J Intern Med. 1991;230(4):365–73. https://doi.org/10.1111/j.1365-2796.1991.tb00459.x.
Ungerstedt U, Rostami E. Microdialysis in neurointensive care. Curr Pharm Des. 2004;10(18):2145–52. https://doi.org/10.2174/1381612043384105.
Bito L, Davson H, Levin E, Murray M, Snider N. The concentrations of free amino acids and other electrolytes in cerebrospinal fluid, in vivo dialysate of brain, and blood plasma of the dog. J Neurochem. 1966;13(11):1057–67. https://doi.org/10.1111/j.1471-4159.1966.tb04265.x.
Hillered L, Persson L, Ponten U, Ungerstedt U. Neurometabolic monitoring of the ischaemic human brain using microdialysis. Acta Neurochir. 1990;102(3–4):91–7. https://doi.org/10.1007/bf01405420.
Nordstrom CH. Cerebral energy metabolism and microdialysis in neurocritical care. Childs Nerv Syst. 2010;26(4):465–72. https://doi.org/10.1007/s00381-009-1035-z.
Kitagawa R, Yokobori S, Mazzeo AT, Bullock R. Microdialysis in the neurocritical care unit. Neurosurg Clin N Am. 2013;24(3):417–26. https://doi.org/10.1016/j.nec.2013.02.002.
Hutchinson PJ, O’Connell MT, Al Rawi PG, Kett-White R, Gupta AK, Kirkpatrick PJ, et al. Clinical cerebral microdialysis: determining the true extracellular concentration. Acta Neurochir Suppl. 2002;81:359–62. https://doi.org/10.1007/978-3-7091-6738-0_91.
Li Z, Cui Z. Application of microdialysis in tissue engineering monitoring. Prog Nat Sci. 2008;18(5):503–11. https://doi.org/10.1016/j.pnsc.2008.02.001.
Martinez-Valverde T, Sanchez-Guerrero A, Vidal-Jorge M, Torne R, Castro L, Gandara D, et al. Characterization of the ionic profile of the extracellular space of the injured and ischemic brain: a microdialysis study. J Neurotrauma. 2017;34(1):74–85. https://doi.org/10.1089/neu.2015.4334.
Hutchinson PJ, O’Connell MT, Al-Rawi PG, Maskell LB, Kett-White R, Gupta AK, et al. Clinical cerebral microdialysis: a methodological study. J Neurosurg. 2000;93(1):37–43. https://doi.org/10.3171/jns.2000.93.1.0037.
Poca MA, Sahuquillo J, Vilalta A, de los Rios J, Robles A, Exposito L. Percutaneous implantation of cerebral microdialysis catheters by twist-drill craniostomy in neurocritical patients: description of the technique and results of a feasibility study in 97 patients. J Neurotrauma. 2006;23(10):1510–7. https://doi.org/10.1089/neu.2006.23.1510.
Abi-Saab WM, Maggs DG, Jones T, Jacob R, Srihari V, Thompson J, et al. Striking differences in glucose and lactate levels between brain extracellular fluid and plasma in conscious human subjects: effects of hyperglycemia and hypoglycemia. J Cereb Blood Flow Metab. 2002;22(3):271–9. https://doi.org/10.1097/00004647-200203000-00004.
Sanchez-Guerrero A, Mur-Bonet G, Vidal-Jorge M, Gandara-Sabatini D, Chocron I, Cordero E, et al. Reappraisal of the reference levels for energy metabolites in the extracellular fluid of the human brain. J Cereb Blood Flow Metab. 2017;37(8):2742–55. https://doi.org/10.1177/0271678X16674222.
Reinstrup P, Stahl N, Mellergard P, Uski T, Ungerstedt U, Nordstrom CH. Intracerebral microdialysis in clinical practice: baseline values for chemical markers during wakefulness, anesthesia, and neurosurgery. Neurosurgery. 2000;47(3):701–9. https://doi.org/10.1097/00006123-200009000-00035.
Oddo M, Levine JM, Frangos S, Maloney-Wilensky E, Carrera E, Daniel RT, et al. Brain lactate metabolism in humans with subarachnoid hemorrhage. Stroke. 2012;43(5):1418–21. https://doi.org/10.1161/STROKEAHA.111.648568.
Sahuquillo J, Merino MA, Sanchez-Guerrero A, Arikan F, Vidal-Jorge M, Martinez-Valverde T, et al. Lactate and the lactate-to-pyruvate molar ratio cannot be used as independent biomarkers for monitoring brain energetic metabolism: a microdialysis study in patients with traumatic brain injuries. PLoS One. 2014;9(7):e102540. https://doi.org/10.1371/journal.pone.0102540.
Hillered L, Valtysson J, Enblad P, Persson L. Interstitial glycerol as a marker for membrane phospholipid degradation in the acutely injured human brain. J Neurol Neurosurg Psychiatry. 1998;64(4):486–91. https://doi.org/10.1136/jnnp.64.4.486.
Peerdeman SM, Girbes AR, Polderman KH, Vandertop WP. Changes in cerebral interstitial glycerol concentration in head-injured patients; correlation with secondary events. Intensive Care Med. 2003;29(10):1825–8. https://doi.org/10.1007/s00134-003-1850-8.
Gliemroth J, Klaus S, Bahlmann L, Klohn A, Duysen K, Reith A, et al. Interstitial glycerol increase in microdialysis after glycerol enema. J Clin Neurosci. 2004;11(1):53–6. https://doi.org/10.1016/s0967-5868(03)00113-9.
Ronne-Engstrom E, Cesarini KG, Enblad P, Hesselager G, Marklund N, Nilsson P, et al. Intracerebral microdialysis in neurointensive care: the use of urea as an endogenous reference compound. J Neurosurg. 2001;94(3):397–402. https://doi.org/10.3171/jns.2001.94.3.0397.
Brunner M, Joukhadar C, Schmid R, Erovic B, Eichler HG, Muller M. Validation of urea as an endogenous reference compound for the in vivo calibration of microdialysis probes. Life Sci. 2000;67(8):977–84. https://doi.org/10.1016/s0024-3205(00)00685-8.
Benveniste H, Diemer NH. Cellular reactions to implantation of a microdialysis tube in the rat hippocampus. Acta Neuropathol (Berl). 1987;74(3):234–8. https://doi.org/10.1007/bf00688186.
Langemann H, Alessandri B, Mendelowitsch A, Feuerstein T, Landolt H, Gratzl O. Extracellular levels of glucose and lactate measured by quantitative microdialysis in the human brain. Neurol Res. 2001;23(5):531–6. https://doi.org/10.1179/016164101101198785.
Cavus I, Kasoff WS, Cassaday MP, Jacob R, Gueorguieva R, Sherwin RS, et al. Extracellular metabolites in the cortex and hippocampus of epileptic patients. Ann Neurol. 2005;57(2):226–35. https://doi.org/10.1002/ana.20380.
Schulz MK, Wang LP, Tange M, Bjerre P. Cerebral microdialysis monitoring: determination of normal and ischemic cerebral metabolisms in patients with aneurysmal subarachnoid hemorrhage. J Neurosurg. 2000;93(5):808–14. https://doi.org/10.3171/jns.2000.93.5.0808.
Hutchinson PJ, Jalloh I, Helmy A, Carpenter KL, Rostami E, Bellander BM, et al. Consensus statement from the 2014 international microdialysis forum. Intensive Care Med. 2015;41(9):1517–28. https://doi.org/10.1007/s00134-015-3930-y.
Carney N, Totten AM, O’Reilly C, Ullman JS, Hawryluk GW, Bell MJ, et al. Guidelines for the management of severe traumatic brain injury, fourth edition. Neurosurgery. 2017;80(1):6–15. https://doi.org/10.1227/NEU.0000000000001432.
Chamoun R, Suki D, Gopinath SP, Goodman JC, Robertson C. Role of extracellular glutamate measured by cerebral microdialysis in severe traumatic brain injury. J Neurosurg. 2010;113(3):564–70. https://doi.org/10.3171/2009.12.JNS09689.
Bellander BM, Cantais E, Enblad P, Hutchinson P, Nordstrom CH, Robertson C, et al. Consensus meeting on microdialysis in neurointensive care. Intensive Care Med. 2004;30(12):2166–9. https://doi.org/10.1007/s00134-004-2461-8.
Andrews PJ, Citerio G, Longhi L, Polderman K, Sahuquillo J, Vajkoczy P. NICEM consensus on neurological monitoring in acute neurological disease. Intensive Care Med. 2008;34(8):1362–70. https://doi.org/10.1007/s00134-008-1103-y.
Poca MA, Sahuquillo J, Arribas M, Baguena M, Amoros S, Rubio E. Fiberoptic intraparenchymal brain pressure monitoring with the Camino V420 monitor: reflections on our experience in 163 severely head-injured patients. J Neurotrauma. 2002;19(4):439–48. https://doi.org/10.1089/08977150252932398.
Meixensberger J, Kunze E, Barcsay E, Vaeth A, Roosen K. Clinical cerebral microdialysis: brain metabolism and brain tissue oxygenation after acute brain injury. Neurol Res. 2001;23(8):801–6. https://doi.org/10.1179/016164101101199379.
Sarrafzadeh AS, Kiening KL, Unterberg AW. Neuromonitoring: brain oxygenation and microdialysis. Curr Neurol Neurosci Rep. 2003;3(6):517–23. https://doi.org/10.1007/s11910-003-0057-2.
Skjoth-Rasmussen J, Schulz M, Kristensen SR, Bjerre P. Delayed neurological deficits detected by an ischemic pattern in the extracellular cerebral metabolites in patients with aneurysmal subarachnoid hemorrhage. J Neurosurg. 2004;100(1):8–15. https://doi.org/10.3171/jns.2004.100.1.0008.
Bjorkblom B, Jonsson P, Tabatabaei P, Bergstrom P, Johansson M, Asklund T, et al. Metabolic response patterns in brain microdialysis fluids and serum during interstitial cisplatin treatment of high-grade glioma. Br J Cancer. 2019; https://doi.org/10.1038/s41416-019-0652-x.
Stewart C, Campagne O, Davis A, Zhong B, Nair S, Haberman V, et al. CNS penetration of cyclophosphamide and metabolites in mice bearing group 3 medulloblastoma and non-tumor bearing mice. J Pharm Pharm Sci. 2019;22(1):612–29. https://doi.org/10.18433/jpps30608.
Hosmann A, Wang WT, Dodier P, Bavinzski G, Engel A, Herta J, et al. The impact of intra-arterial Papaverine-hydrochloride on cerebral metabolism and oxygenation for treatment of delayed-onset post-subarachnoid hemorrhage vasospasm. Neurosurgery. 2019; https://doi.org/10.1093/neuros/nyz500.
Wang Q, Ren T, Zhao J, Wong CH, Chan HYE, Zuo Z. Exclusion of unsuitable CNS drug candidates based on their physicochemical properties and unbound fractions in biomatrices for brain microdialysis investigations. J Pharm Biomed Anal. 2020;178:112946. https://doi.org/10.1016/j.jpba.2019.112946.
Havelund JF, Nygaard KH, Nielsen TH, Nordstrom CH, Poulsen FR, Faergeman NJ, et al. In vivo microdialysis of endogenous and (13)C-labeled TCA metabolites in rat brain: reversible and persistent effects of mitochondrial inhibition and transient cerebral ischemia. Metabolites. 2019;9(10) https://doi.org/10.3390/metabo9100204.
Acknowledgments
We would like to thank all the nurses of the neuroICU of the VHUH for their continuous help. This work was supported in part by the Fondo de Investigación Sanitaria (Instituto de Salud Carlos III) with grant PI15/01228, which was co-financed by the European Regional Development and awarded to Dr. J. Sahuquillo and by the grants KidsBrainIT (ERA-NET NEURON), co-financed by the European Regional Development Fund (ERDF), and grant 20172430 from the Marató de TV3, awarded to Dr. J. Sahuquillo and Dr. M.A. Poca, respectively.
Disclosure Statement
The authors report no conflict of interest concerning the materials or methods mentioned in this chapter.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Poca, M.A., Sanchez-Ortiz, D., Baena, J., Sahuquillo, J. (2021). Brain Microdialysis Monitoring. In: Figueiredo, E.G., Welling, L.C., Rabelo, N.N. (eds) Neurocritical Care for Neurosurgeons. Springer, Cham. https://doi.org/10.1007/978-3-030-66572-2_7
Download citation
DOI: https://doi.org/10.1007/978-3-030-66572-2_7
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-66571-5
Online ISBN: 978-3-030-66572-2
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)