Abstract
Clean and high efficient energy development has long been hunted to solve energy and environmental crisis. Fuel cells, which convert the chemical energy in fuel straight into electrical energy, are the key empowering technology of this era with an outstanding long-term performance. The future energy source is concerned with two of the most advanced fuel cells—direct methanol fuel cell (DMFC) and proton exchange membrane fuel cell (PEMFC). The motivation of this work is to develop mathematical models for investigating the best operating conditions and comparing the performance of PEMFC and DMFC. Two-dimensional fuel cell models were simulated based on physical laws to foresee the performance of the cell under several operating conditions. Taguchi method has been used to design experiments to study the effect of fuel and oxidant concentration, reactants’ flow direction, and membrane properties. Validating and running case studies of these models have been completed to present a comprehensive viewpoint of modeling. Lastly, comparing performance in term of current and power density between PEMFC and DMFC has been achieved. PEMFC has better performance compared to DMFC. A significant outcome of 2D simulations conducted was expected to maximize the fuel cells’ performance to be used in the transportation sector and portable applications.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Introduction
The fuel cell is a device that converts the chemical energy from a fuel into electricity through a redox reaction with oxygen or other oxidizing agent. Hydrogen is the most common fuel; natural gas and alcohols such as methanol are frequently used. Fuel cells are considered as green, reliable, and highly efficient power generation technology in this century. Among various types of fuel cells, proton exchange membrane fuel cells (PEMFCs) and direct methanol fuel cells (DMFCs) are commonly used because of low weight, high current density, easy design, and low CO2 emission. These are the most promising power sources for transportable electronic devices and transportation applications [1].
Fuel cells are used for primary and backup power for commercial, industrial, and residential buildings [2]. The energy efficiency of a fuel cell is generally between 40 and 60 % or up to 85 % if waste heat is captured for use. It is much higher than a combustion engine, which is usually 30 % efficient.
Lately, there has been a growing concern about acid rain and greenhouse gas releases which have made renewable technologies an attractive option [3]. Continuing work to meet increasing energy demand and also to preserve the global environment, the development of fuel cell systems with readily available fuels, high efficiency, and minimal environmental impact is urgently required. But the problem rising now is how to maximize the performance in terms of current density (A/m2) produced by PEMFC and DMFC.
In fact, it can be maximized by manipulating operating parameters and design system variables simultaneously. These variables/parameters can be classified into two which are processed variable and design variable in fuel cells. Process variable includes the direction flow of reactants, pressure and velocity of reactants’ flow, operating temperature, structure design, and so on, while design variable includes channel length and width, type and porosity of membrane, and other design variations of a fuel cell as well. Hence, PEMFC and DMFC were simulated in this work to identify the best operating condition to deliver the best performance of a fuel cell system.
The objective of this work is to maximize the current density of a fuel cell by investigating the effects of fuel and oxidant concentration, membrane properties, and direction flow of reactants in fuel cells by using Taguchi method.
Taguchi method for design of experiment (DOE), analysis of mean (ANOM), and analysis of variance (ANOVA) is applied to measure the significance of 4 factors, to determine the optimum configurations of 4 factors, and to estimate and validate optimal current density. Taguchi method has been effectively applied in many engineering disciplines. There are four parameters that need to be investigated with two levels in this work. These parameters include fuel concentration at anode channel, oxidant concentration at cathode channel, directional flow of reactants, and membrane properties. So, L8 of Taguchi method has been chosen in this work. Initially, there are 24 which are 16 experiments needed to be carried out for each fuel cell. But by using Taguchi method, the number of experiments for each fuel cell is 8. So the amount of experimentation can be reduced to half; thus, it saves time and resource at all.
2 Literature Review
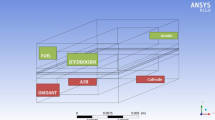
There are numerous categories of fuel cells, but they all comprise of an anode (negative side), a cathode (positive side), and an electrolyte that permits electron to travel between the two sides of the fuel cell [4]. A catalyst oxidizes the fuel at anode, usually hydrogen or other hydrogen compound, converting fuel into a positively charged ion and a negatively charged electron. The electrolyte is a substance specifically designed so ions can pass through it, but the electrons cannot. Electrons are drawn from the anode to the cathode through an external circuit, producing direct current electricity. Chemical redox reactions occur at the interfaces of the three different sectors. The net result of the reactions is that fuel is consumed, water or carbon dioxide (DMFC) is formed, and an electric current is produced, which can be used to power electrical devices as indicated in Fig. 1.
3 Modeling and Methodology
3.1 Proton and Electron Transport Balance
A conductive media DC application mode describes the potential distributions in the three subdomains, as shown in Fig. 2 by using the following equations (a = anode, m for membrane, and c = cathode):
Charge transfer current density expression is generally described by using the Butler–Volmer electrochemical kinetic expression as a boundary condition. For the electrolyte potential equation, this results in a condition where the inward normal ionic current densities at the anode and cathode boundaries, i a and i c, are specified as in below:
3.2 Modeling for Anode and Cathode
The current density can be expressed analytically by solving a combination of the Maxwell–Stefan diffusion equation and the Butler–Volmer electrode kinetic equation for agglomerate with constant electric and ionic potentials. The resulting equations for the current density in the anode and cathode are [6]
The dissolved H2 and O2 at the surface of the agglomerates are related to the molar fractions of the respective species in the gas phase through Henry’s law. The concentration of the species is partial pressure of the species divided by Henry’s constant. Henry’s constant for oxygen is 769.23 L/atm./mol, while 1,282.05 L/atm./mol is for hydrogen:
The potential difference between the cathode and anode current collectors corresponds to the total cell voltage. The total cell voltage as the boundary condition at the cathode current collector is as follows:
3.3 Porous Media Transport Balance
Darcy’s law was being applied to specify the fluid flow through a porous medium. The gas velocity is given by the continuity equation as below:
Darcy’s law for porous media states that the gradient of the pressure, the viscosity of the fluid, and the structure of the porous media determine the velocity:
Combined with these boundary conditions, Darcy’s law determines the gas flow velocity and preserves the total mass conservation in the anode and cathode gas backing. The main assumptions used in the modeling are as follows [5]:
-
1.
Ignore the formations of the CO2 bubbles and water vapor.
-
2.
Membranes are fully hydrated.
-
3.
Methanol in DMFC is fully consumed at the interface of the cathode membrane and the cathode catalyst layer.
-
4.
The flow in the electrolyte channel is laminar flow.
-
5.
The fuel cell is isothermal and operates at steady state.
3.4 Design of Experiment
A symmetric 2D simulation flow diagram is shown in Fig. 3. In this work, 2D models were simulated in COMSOL Multiphysics version 3.5a. First of all, fuel cell geometry has been drawn in COMSOL. A set of equations involved in this 2D simulation has been chosen such as Darcy’s law (fluid flow through a porous medium), Maxwell–Stefan mass transport equation (diffusion in multicomponent systems), Henry’s law (at constant temperature, the amount of gas that dissolves in a liquid is directly proportional to the partial pressure of the gas), and Butler–Volmer kinetics electrochemical kinetic expression as a boundary condition. Next, constants, domain equations, and boundary conditions involved in this simulation were specified in Fig. 2. This model consists of 3 domains which are an anode (Ωa), a membrane (Ωm), and a cathode (Ωc). Each of the porous electrodes is in contact with a gas distributor, which has an inlet channel (∂Ωa, inlet), a current collector (∂Ωa, cc), and an outlet channel (∂Ωa, outlet). The same notation is used for the cathode side.
A mesh model has been generated easily for the specification of each tiny element in this mesh. In this work, the maximum element size for membrane is 50 μm and for both anode and cathode is 10 μm.
In this work, PEMFC and DMFC models have been simulated and validated by experimental data. It means that these two models were validated and can work in real life. If these two fuel cell models were not validated, the developer has to go back to the early stage, whereby specifying constants, domain equations, and boundary conditions over again. The next step was running some case studies by manipulating some parameters on fuel cells.
The implementation of Taguchi method can be illustrated as shown in Fig. 4. At the top of that is the formulation of a problem which requires defining a main objective. In this work, the main objective is to maximize the performance of PEMFC and DMFC by using Taguchi method. Furthermore, it consists of factors and levels of experiment. Controllable factors A to D were set up in an L8 (24) orthogonal array. The next step is designing experiments by using orthogonal array. There are four controllable factors at two levels. An L8 array which consists of eight rows and four columns was chosen. The row is representing simulation run, and column represents number of factors in this work. It should be noted that in order to take enough response of all four variables toward object function, only eight simulation runs are needed to be done for each fuel cell.
Step 3 is analyzing the results of simulation. Table 1 shows Taguchi orthogonal array used in this simulation. In this work, there were two statistical tools that have been used which were ANOM and ANOVA. ANOM is an analysis of mean which compares means and variances across several groups of result data, while analysis of variance, ANOVA, is a particular form of statistical hypothesis testing heavily used in the analysis of experimental data. A statistical hypothesis test is a method of making decisions using data. Finally, step four is a validation of experimental result. For each run in Lg orthogonal array, it will yield the highest current density of the particular fuel cell. A preliminary visualization of trend of each factor’s average contribution at all 2 levels can be made through response plot. Response plot is used to identify the optimal design configuration for validating the result obtained from simulation.
4 Results and Discussions
4.1 Data Gathering and Analysis for Fuel Cells
From Fig. 5, PEMFC has better performance (max 3,600 A/m2) than DMFC (max 3,025 A/m2) whereby the current density difference ΔJ e is 575 A/m2 in optimum operating configuration A 2 B 2 C 2 D 2 .
4.2 Analysis of Mean (ANOM)
The analysis of mean (ANOM) is a graphical method for comparing a group data by means to define if any one of the data varies significantly from the overall mean. ANOM is a type of multiple comparison method. For PEMFC, factor B ranks number 1, while factor A ranks number 1 for DMFC. It means that factor B is an important factor for PEMFC and factor A is an important factor for DMFC as shown in Table 2 below.
4.3 Analysis of Variance (ANOVA)
In statistics, analysis of variance (ANOVA) is a collection of statistical data and their associated method, in which the variance in a particular manipulated variable is separated into components attributable to different variations.
Factors are assigned to experimental units by a combination of randomization and blocking to ensure the validity of the results by using Taguchi orthogonal array. Factor B (oxidant concentration) affects the most in PEMFC performance, while factor A (methanol concentration) gives the most impact on DMFC performance. From ANOM plot, A 2 B 2 C 2 D 2 is the best configuration for PEMFC and DMFC as shown in Table 3. It gives the maximum current density for both these fuel cells. Higher fuel and oxidant concentration accelerate redox reaction in fuel cells, counter-current flow of reactants maximizes the transfer rate of heat and mass, and by using Nafion® 211 membrane, there is more hydrogen ion that can pass through the membrane to combine with oxygen to form water and produce electricity.
4.4 Validation of Taguchi Method
For each case study in this project, there are eight combinations of experimental runs for each fuel cell. By simulation design, only one will yield the highest current density. Initial visualization of trends of each factor contribution at all levels is possible through a main effect plot. Here, average values of factor k \( {\overline{x}}_{\mathrm{kl}} \) are plotted against all 2 levels in this project. The effect plot may be used to detect optimum design configuration for the purpose of verifying the results. An additional experiment is run to compare both experimental and calculated outputs (current density). The calculated optimum current density x opt is obtained as shown in Table 4 by summing up global mean \( \overline{x} \) with maximum deviations of average of 4 factors over all 2 levels, \( {\overline{x}}_{\mathrm{kl}} \), from its corresponding average \( {\overline{x}}_{\mathrm{k}} \):
where
5 Conclusion
In conclusion, the main objectives of the project have been successfully achieved. These models enable us to view in two-dimensional and study the effects of fuel and oxidant concentration, reactants’ flow direction, and membrane properties over the full range of operating current densities and performance. There is a good agreement between the results of the 2D simulated models and the experimental data in the validation section in Sect. 4. In this work, oxidant concentration in the cathode channel has much influence on current and power density of PEMFC, while fuel concentration in anode channel is the main effect that affects DMFC’s performance.
References
Smirnov A, Burt A, Celik I (2006) Multi-physics simulations of fuel cells using multi-component modeling. J Power Sources 158(1):295–302
Dyer CK (2002) Fuel cells for portable applications. J Power Sources 106(1–2):31–34
Colpan CO, Funga A, Hamdullahpur F (2012) 2D modeling of a flowing-electrolyte direct methanol fuel cell. J Power Sources 209:301–311
Siegel C (2008) Review of computational heat and mass transfer modeling in polymer-electrolyte-membrane (PEM) fuel cells. J Energy 33(9):1331–1352
Feng L, Cai W, Li C, Zhang J, Liu C, Xing W (2012) Fabrication and performance evaluation for a novel small planar passive direct methanol fuel cell stack. Fuel 94:401–408
Lottin O, Antoine B, Colinart T, Didierjean S, Maranzana G, Moyne C, Ramousse J (2009) Modeling of the operation of polymer exchange membrane fuel cells in the presence of electrodes flooding. Int J Thermal Sci 48(1):133–145
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Nomenclatures
Nomenclatures
- c i, agg :
-
Concentrations of agglomerate surface (mol/m3)
- c i, ref :
-
Reference concentrations (mol/m3)
- D agg :
-
Agglomerate gas diffusivity (m2/s)
- D ij :
-
Maxwell–Stefan diffusivity matrix (m2/s)
- E :
-
“a” (anode) or “c” (cathode)
- F :
-
Faraday’s constant (C/mol)
- i 0a/c :
-
Exchange current densities (A/m2)
- j agg, a/c :
-
Current densities in agglomerate model
- K :
-
Henry’s constant (Pa·m3/mol)
- kp :
-
Electrode’s permeability (m2)
- L act :
-
Active layer’s thickness (m)
- M:
-
Concentration (mol/L)
- n e :
-
“Charge transfer” number (1 is anode, 2 is cathode)
- p :
-
Pressure (Pa)
- R :
-
Gas constant
- R agg :
-
Agglomerate radius (m)
- S :
-
Specific area of the catalyst (m)
- T :
-
Temperature (K)
- u :
-
Velocity (m/s)
- u :
-
Gas velocity (m/s)
- w:
-
Weight fraction
- x:
-
Mole fraction of the species
- εmac :
-
Porosity (the macroscopic porosity)
- η:
-
Gas viscosity (Pa/s)
- κm, κm, eff :
-
Membrane ionic conductivity (S/m)
- κs, eff :
-
Effective electronic conductivity (S/m)
- ρ :
-
Density of the gas phase (kg/m3)
- φm :
-
Potential (V) in the membrane phase
- φs :
-
Potential (V) in the solid phases
Rights and permissions
Copyright information
© 2015 Springer Japan
About this paper
Cite this paper
Ng, TM., Yusoff, N. (2015). An Investigation on Performance of 2D Simulated Fuel Cells Using Taguchi Method. In: Ab. Hamid, K., Ono, O., Bostamam, A., Poh Ai Ling, A. (eds) The Malaysia-Japan Model on Technology Partnership. Springer, Tokyo. https://doi.org/10.1007/978-4-431-54439-5_2
Download citation
DOI: https://doi.org/10.1007/978-4-431-54439-5_2
Published:
Publisher Name: Springer, Tokyo
Print ISBN: 978-4-431-54438-8
Online ISBN: 978-4-431-54439-5
eBook Packages: EngineeringEngineering (R0)