Abstract
Most of high corrosion resistance advanced structural and functional metals and alloys are protected by passive films, that is oxide and/or hydroxide films of few nm in thickness. In this chapter, properties of oxide/hydroxide film formed on metals and alloys are briefly summarized, then the procedure of quantitative analysis of passive film using X-ray photoelectron spectroscopy is described in detail as a one of characterization technique. Passive films on Cr containing high corrosion alloys, such as stainless steels, high nickel alloys, Co–Cr alloys, consist of highly Cr enriched oxide layer and covering hydroxide layer. Assuming the duplex model, the thickness and the content including chemical states in both layers, are quantitatively estimated from deconvoluted XPS spectra acquired without any sputtering technique.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Properties of surface oxide and/or hydroxide films on metals and alloys are important for understanding their corrosion property, because corrosion resistance of metals and alloys are usually derived from surface oxide films. Therefore, many kinds of surface characterization techniques, such as, ellipsometry [1–3], Raman spectroscopy [4, 5], electrochemical impedance spectroscopy [6], modulated UV–vis reflectometry [7, 8], photoelectrochemical response spectroscopy [9, 10], Auger electron spectroscopy [11, 12], and X-ray photoelectron spectroscopy [13–15] are developed in order to discuss corrosion behaviour. In this chapter, role of oxide/hydroxide films on metal and alloys are explained mainly for passive films on high corrosion materials. Then, quantitative X-ray photoelectron spectroscopy (XPS) will be described in detail.
2 Surface Oxide and/or Hydroxide Films on Metals and Alloys
Metals and alloys are usually covered with oxide and/or hydroxide films that are formed by reaction of substrate metal or alloy with surrounding environment. Oxide or hydroxide layers formed on metals and alloys are generally classified in to three categories; thick rust layers, anodizing films and passive films.
Thick rust layer is often observed; red rust on iron and steel [16], patinated copper and brass, for instance. Such rust layers are formed as accumulation of colloidal deposit. Metallic ions released into water excess solubility in water, then precipitate as colloid particle of hydroxide or oxide. Such colloidal deposits are accumulated on the surface, and dehydrated to be deposited layer. Rust layer is usually water and ions permeable, therefore basically not protective. The rust layers formed on weathering steels [17] and bronze exceptionally exhibit high corrosion resistance.
Anodizing oxide films [18, 19] are formed by anodic polarization of Al, Ti, Mg, etc. Anodizing oxide is usually dense and insulating, therefore protective for substrate metal. Anodizing oxide film is able to grow proportionally depending on applied potential, occasionally reaches to few μm. Most of structural materials made of Al alloys are used with anodizing oxide films. Al, Ta, Nb covered with insulating anodized oxide films are applied for electric capacitor.
Passive films [20–22] are very thin oxide and/or hydroxide formed naturally on metals and alloys. For example, passive film on iron is formed in aqueous solution containing some oxidant without harmful anion such as Cl− and SO 2−4 . Passive films are formed on most of metals and alloys, usually 1–5 nm in thickness and protective for corrosion. Figure 6.1 [23] shows variation of the thickness of passive film on pure iron as a function of applied potential. As shown in this figure, the thickness of passive film on iron is in the range from 1 to 4 nm. In the ordinary environment, potential is controlled by oxidant, such as dissolved oxygen and H+ ions and is in the range of horizontal axis of this Figure. Above a critical potential, Fe is more oxidized into soluble FeO 2−4 ions. Similar behavior is observed for Ni [24, 25], Cr [12], Co [26], etc.
Thickness of passive film on Fe formed at various applied potentials [23]
3 Passive Films on Stainless Steels
High corrosion resistant alloys containing Cr as alloying elements, such as stainless steels, high nickel alloys and Co–Cr alloys are protected by Cr enriched passive films. Figure 6.2 [27] shows Cr cation fraction in the passive film and Cr content of substrate alloy underneath the passive film measured by X-ray photo electron spectroscopy as a function of Cr content in Fe–Cr alloys. Cr content in passive film abruptly increases with increasing Cr content in the alloy above 12% Cr, then reaches around 80% at 20% Cr in the alloy. On the other hand Cr content underneath passive film is the same with alloy composition. Therefore, it is concluded that Cr enrichment is due to selective dissolution of Fe into aqueous solution and preferential oxide/hydroxide formation of Cr. Similar enrichment of Cr is observed for Ni–Cr alloys [28–30] and Co–Cr alloys [31, 32]. Properties of passive film, especially Cr content, depend on the aqueous environment and affects corrosion resistance, therefore characterization of passive film is important for considering corrosion behavior.
Changes in composition of passive film and substrate alloy beneath passive film on Fe–Cr alloys polarized at 500 and 100 mV (SCE) in 1 M H2SO4 as a function of Cr faction in substrate alloy [27]
4 Principle and Characteristics of X-ray Photoelectron Spectroscopy (XPS)
Photoelectron is yielded from occupied electron orbitals around atoms by X-ray adsorption. The kinetic energy, E k, of photoelectron emitted is described as follows:
where hν is photon energy of irradiated X-ray, E b binding energy of electron in a specific orbital of an atom and Φ work function of a photoelectron analyser. The binding energy of electron in the core levels slightly shifts depending on the chemical state of the atom. Therefore, XPS is also called Electron Spectroscopy for Chemical Analysis (ESCA). Photoelectron emitted from the most surface of a solid is captured by an analyser. However, photoelectron from inside of solid is inelastically scattered to be back ground noise. In another words, the electron generated inside of material is attenuated before emitted from the surface. The attenuation length, λ, which depends on kinetic energy and materials, is defined as the length in a material after passes through which the intensity of photoelectron without inelastic scattering becomes 1/e. Typical attenuation length for photoelectron generated by commonly used X-ray source, such as AlKα and MgKα, is few nm. Therefore, XPS is very surface sensitive characterization technique. XPS is widely utilized for surface characterization in research areas such as metallurgy, catalyst, corrosion, and surface treatment etc. In the following, the procedure of quantitative analysis of passive films on corrosion resistant metals and alloys will be described.
5 Procedure of Quantitative Analysis of Layered Surface
Intensity of photoelectron, i z,0, yielded from atoms, z, existing inside an infinitesimal volume, dv, is given by:
where X is intensity of irradiated X-ray, C z mol concentration of the element, z, for an unit volume, and σ z photoionization cross section which is the sensitivity factor to yield photoelectron. When dv is located inside of some solid, s, at a depth of y, as shown in Fig. 6.3(a), the intensity of photoelectron of z which emitted from the surface of the solid is described as;
where \( \lambda_z^s \) is the attenuation length of photoelectron of the atom, z, in the solid, s. As shown in Fig. 6.3(b), when z is distributed homogeneously in the solid s, the photo electron emitted from a unit area (v = 1 × 1 × y) and thickness of d is a summation of i z :
If photo electron is emitted from bulk solid, that is d = ∞, as shown in Fig. 6.3(c),
The counts of photoelectron of z yielded in a bulk solid and detected by an analyzer is described as:
where Ψ is an acquisition constant which is specific to apparatus and usually changes with E k. If photo electron is emitted from a thin layer of thickness d,
where θ is photoelectron take off angle measured from normal to surface as shown in Fig. 6.4. The term cosθ is applied, because the path inside the solid where photoelectron passes through increases by a factor of 1/cosθ as described in Fig. 6.4.
If the infinite or limited thickness layer is covered with another solid sc with thickness of t, the photoelectron is attenuated during passing through the covering layer by a factor of exp(−t/λ scz ), where λ scz is the attenuation length of photoelectron of z through the solid sc.
Quantitative analysis of XPS spectra is proceeded using above equations. If subjected material, s, consists of a single layer, Eq. (6.6) or (6.7) directly provides concentration and thickness, d s, as described in the following. When a solid, s, consists of components, z1–zn, Eq. (6.7) is applicable for all components, including various chemical states;
For the layer of s which consists of z1, z2, … zn, the following equation is obtained:
Where M z1, M z2, … M zn are atomic weight of element z1, z2, … zn, respectively, ρ s is density of the layer of s. C z1, C z2, … C zn in Eq. (6.11) are substituted by Eqs. (6.8)–(6.10). As a result, only d s becomes unknown variable in Eq. (6.11). Therefore, thickness d of the layer s and concentration of each elements (states) C zn are obtained.
On the other hand, if a sample consists of multi layers, some structural model is to be assumed. Therefore, an appropriate procedure for quantitative analysis should be developed for each subject. In the following, the quantitative analysis on passive films formed on high corrosion alloys is described in detail.
6 Procedure of the Quantitative Analysis of Passive Films
As mentioned before, passive films on stainless steels formed in aqueous environment consist of oxide layer and covering hydroxide layer. Therefore, passive films are assumed to be composed of two layers [33, 34]. Actually, specimens exposed to atmosphere must be covered with contaminant hydrocarbon layer. Moreover, the substrate alloy beneath the passive films occasionally changes the composition because of dealloying. Therefore a multi-layers model which is composed of contaminant/hydroxide/oxide/substrate alloy is assumed as shown in Fig. 6.5.
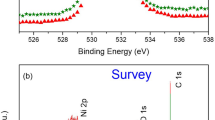
In order to analyse passive films on stainless steel, photoelectron spectra of Fe2d, Cr2d, Ni2d, O1s, and C1s orbitals are measured. If minor element like Mo is required to be analysed, spectra of such elements are also measured.
Typical spectra obtained for passive film on Type304 stainless steel are shown in Fig. 6.6 Spectra of contaminant carbon are integrated to an area I C,con. Eq. (6.7) is applied for contaminant carbon, Ccon:
where I C,gra is area of C1s spectrum measured for pure Carbon, for example graphite. λ graC is escape length of photoelectron yielded from carbon through graphite. These equations are modified into:
As shown above, thickness of contaminant hydrocarbon layer is estimated.
Spectrum of Fe is deconvoluted into metallic: Femet, ferrous oxide: Fe2+,ox, ferrous oxide: Fe3+,ox, and hydroxide: Fehyd states as shown in Fig. 6.6(a). Similarly, spectra of Cr, Ni and other metallic elements are also deconvoluted into metallic, oxide and hydroxide. Intensities, that are area of spectrum of each element and state in hydroxide layer are described as:
The last term in Eqs. (6.16)–(6.19) refers to attenuation of photo electron during passing through the contaminant layer which covers hydroxide layer. C Fe,hyd, C Cr,hyd, C Ni,hyd, and C O,hyd in Eq. (6.20) are replaced by Eqs. (6.16)–(6.19). Accordingly,
The thickness of d hyd is thus obtained, because d hyd is only unknown variable in Eq. (6.22). Then, substituting d hyd in Eqs. (6.16)–(6.19) gives values of concentration of each element, C z, in the hydroxide layer.
A similar relation can be applied for the oxide layer:
From Eq. (6.22) the thickness of oxide layer, d ox, is calculated. With same procedure as hydroxide layer, concentrations of each element, C z, in the oxide layer are obtained.
When the thickness of passive film is fairly thin for detection of photoelectron yielded from the substrate steel, spectra of metallic states are detected and their intensities are described as:
These equations provide concentration of each element, C z.
7 Parameters Adopted for Quantitative Analysis
In the previous section, process of calculation to provide the chemical composition and thickness of passive film is described in detail. In the process, many parameters that are specific to elements and substances are required.
7.1 Photoionization Cross Section
Sensitivity parameter for yielding photo electron of an element, z, is usually termed photoionization cross section, σ z. Theoretically calculated values are available in the literature [35]. However, such value is not necessarily applicable for quantitative analysis. Therefore, σ z is estimated from the intensity of photoelectron, I z, measured for some standard specimen. The process to obtain σ z from I z is described later.
σ z of metallic element is measured for Ar+ ion sputtered specimen. For example, σ z of elements consisting a stainless steel is obtained using the sputtered stainless steel, the chemical composition of which is known. Value of σ z is regarded as proportional to atomic concentration. Therefore, usage of alloy is convenient, because σ z of several elements is obtained for a specimen with same measurement conditions that are intensity of X-ray, sensitivity of photoelectron detector and etc. Since σ z may change depending on the photon energy of irradiated X-ray, it is recommended to measure σ z using the same X-ray source which is used for measurement of unknown specimen.
Photoionization cross section of carbon, σ C, is usually necessary, because most of specimen is covered with contaminant hydrocarbon layer of few nm in thickness. σ C is usually measured using graphite.
Determination of photoionization cross section of oxygen, σ O, is most difficult but crucial for quantitative analysis of passive films with high reliability. Since pure material of oxygen is not available, standard specimen of some compound with exact stoichiometry is required. Thermally oxidized pure metals could be candidates for such purpose. SiO2, Cr2O3, Al2O3 etc. are used as standard. On the other hand, Asami et al. reported that they adopted oxides and hydroxide of metal, M, with several valences that are commercially available as high purity chemicals for standard. They measured intensity of photoelectron of them to obtain averaged ratio of σ O/σ M [36–38]. Reliable empirically obtained sensitivity parameters as a form of σ z λ z are reported [39].
As already described, the photoelectron intensity of the elements z in a substance s is measured as
Therefore, the value λ SZ is necessary in order to determine σ z. Ψ, acquisition constant of analyzer is usually provided by vendor of apparatus. Otherwise, Ψσ z is used as a parameter, because Ψ depends not on material examined but only on the kinetic energy of photoelectron. It is to be noted that intensity of irradiated X-ray, X is, also required. Some standard specimen, such as Au, Ag which easily provides pure elemental surface without any compounds could be used as the reference to calibrate intensity of X-ray and kinetic energy of spectrometer. The values of σ z of elements may be summarized as values with respect to that of the reference element.
7.2 Attenuation Length
Attenuation length, λ SZ , is probably most difficult parameter to obtain for quantitative analysis of XPS spectra. The value of λ SZ depends on kinetic energy and the substance in which photoelectron passes through. Recently, values of inelastic mean free path, IMFP, calculated based on the first principal evaluation are available [40–45]. Such data are useful for some specific purposes. It is also mentioned that attenuation length is smaller than inelastic mean free path [40, 46]. According to the author’s experience, the values available in the literature, most of them might be determined empirically, are more reliable than that obtained by first principal calculation for passivity study. It is empirically recognized that attenuation length of photoelectron is roughly described is roughly described as
with binding energy in the range of 70–1,500 eV independent of substance [38]. Therefore, λ Sz is substituted by attenuation length of oxygen which is always contained in passive films, λ SO , as follows.
where E k,z and E k,O are kinetic energy of photoelectron yielded in an metallic element, z, and oxygen, respectively, that are known values. If Eq. (6.27) is introduced in Eqs. (6.21)–(6.23), concentration of each component is able to be determined without absolute value of escape depth.
7.3 Chemical Shift
Extensive data for binding energy, E b, of elements are easily available and usually reliable, especially for metallic elements. However, binding energy of compounds is usually empirically obtained and termed as chemical shift. For the passivity study, chemical shifts as oxide and hydroxide of metallic elements and also of oxygen are necessary. Although chemical shift of oxygen as oxide and hydroxide may depend on metallic cation and pH of solution in which passive film is formed, values of chemical shifts available in the literature [13–15, 27–29, 32, 34, 36–38, 47–53] should be adopted with a careful consideration.
8 Summary
The procedure of the quantitative analysis of XPS spectra for passive films formed on stainless steel is described concretely in detail. This approach is also applicable for any kind of thin surface films with thickness of up to few nm. In order to conduct quantitative analysis, as mentioned in the Sects. 6.6 and 6.7, many parameters specific for each element and substance are necessary. However, no appropriate date base is established for the passivity study. Therefore, photoionization cross section and attenuation length should be carefully collected and selected with careful consideration.
References
Szklarska-Smialovska Z, Kozowski Z (1983) In: Froment M (ed) Passivity of metals and semiconductors. Elsevier, Amsterdam, p 89
Ohtsuka T (1992) Denkikagaku 60:1123
Kirchheim R (1994) In: Marcus P, Baroux B, Keddam M (eds) The institute of metals., p 102
Boucherit N, Hugot-Le Goff A, Joiret S (1989) Thin Solid Films 174:111
Gui J, Devine TM (1994) Corros Sci 36:441
Azumi K, Ohtsuka T, Sato N (1987) J Electrochem Soc 134:1352
Hara N, Sugimoto K (1991) J Electrochem Soc 138:1594
Hara N, Sugimoto K (1990) Corros Sci 31(197)
Tsuchiya H, Fujimoto S, Shibata T (2004) J Electrochem Soc 151:B39
Fujimoto S (2005) In: Waseda Y, Suzuki S (eds) Characterization of corrosion products on steel surfaces. Springer, Benlin, p 33
Lumsden JB, Staehle RW (1972) Scr Metall 6:1205
Seo M, Saito R, Sato N (1980) J Electrochem Soc 127:1909
Asami K, Hashimoto K, Shimodaira S (1977) Corros Sci 17(713)
Marcus P, Olefjord I (1982) Surf Interface Anal 4:29
Asami K, Ohnuma S, Masumoto T (1998) Surf Interface Anal 26:659
Suzuki S (2005) In: Waseda Y, Suzuki S (eds) Characterization of corrosion products on steel surfaces. Springer, Benlin, p 131
Asami K (2005) In: Waseda Y, Suzuki S (eds) Characterization of corrosion products on steel surfaces. Springer, p 159
Despic A, Parkhutik V (1989) Modern aspects of electrochemistry, vol 20. Plenum, New York, p 401
Habazaki H, Shimizu K, Skeldon P, Thompson GE, Wood GC (1998) J Surf Sci Soc Jpn 19:772
Marcus P, Maurice V (eds) (2006) Passivity of metals and semiconductors, and properties of thin oxide layers. Elsevier, Amsterdam
Ohtsuka T (1995) Mater Jpn 34:1037
Fujimoto S, Shibata T (1998) J Surf Sci Soc Jpn 19:812
Azumi K, Ohtsuka T, Sato N (1983) In: Froment M (ed) Passivity of metals and semiconductor. Elsevier, Amsterdam, p 199
Chao CY, Szklarska-Smialowska Z (1980) Surf Sci 96:426
Hoppe HW, Strehblow HH (1990) Corros Sci 31:167
Ohtsuka T, Sato N (1983) J Electroanal Chem 147:167
Asami K, Hashimoto K, Shimodaira S (1978) Corros Sci 18:151
Asami K, Hashimoto K (1979) Corros Sci 19:427
Kawashima A, Asami K, Hashimoto K (1985) Corros Sci 25:1103
McIntyre MS, Zetaruk DG, Owen D (1979) J Electrochem Soc 126:750
Hanawa T, Hiromoto S, Asami K (2001) Appl Surf Sci 183:68
Milosev I, Strehblow HH (2003) Electrochim Acta 48:2767
Olefjord I, Wegrelius L (1990) Corros Sci 31:89
Yang WP, Costa D, Marcus P (1994) J Electrochem Soc 141:2669
Scofield JH (1976) J Electron Spectros Relat Phenom 8:129
Asami K, Hashimoto K, Shimodaira S (1977) Corros Sci 17:713
Asami K, Hashimoto K (1984) Corros Sci 84:83
Hashimoto K, Asami K (1977) Boshoku Gijyutsu 26:375
Wagner CD, Davis LE, Zeller MV, Taylor JA, Raymound RH, Gale LH (1981) Surf Interface Anal 3:211
Tanuma S, Powell CJ, Penn DR (1988) Surf lnferface Anal 11:577
Tanuma S, Powell CJ, Penn DR (1991) Surf lnferface Anal 17:911
Tanuma S, Powell CJ, Penn DR (1991) Surf lnferface Anal 17:927
Tanuma S, Powell CJ, Penn DR (1993) Surf lnferface Anal 20:77
Tanuma S, Powell CJ, Penn DR (1993) Surf Interface Anal 21:165
Gries WG (1996) Surf Interface Anal 24:38
Olefjord I (1991) In: Proceedings of the symposium on the application of surface analytical methods to environmental/material interactions, PV 91–7, The Electrochemical Society, p 22
Marcus P, Olefjord I (1988) Corros Sci 28:589
De Vito E, Marcus P (1992) Surf lnferface Anal 19:403
Yang WP, Costa D, Marcus P (1994) J Electrochem Soc 141:111
Maurice V, Yang WP, Marcus P (1998) J Electrochem Soc 145:909
Tossi A, Elsener B (1995) Mater Sci Forum 185/188:337
Fujimoto S, Yamada T, Shibata T (1998) J Electrochem Soc 145:L79
Fujimoto S, Shibata T, Yamada T (1998) J Jpn Soc Mater 62:527
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2013 Springer Japan
About this chapter
Cite this chapter
Fujimoto, S. (2013). Advanced Analysis of Surface Films Formed on Passive Metals and Alloys Using X-ray Photoelectron Spectroscopy. In: Kakeshita, T. (eds) Progress in Advanced Structural and Functional Materials Design. Springer, Tokyo. https://doi.org/10.1007/978-4-431-54064-9_6
Download citation
DOI: https://doi.org/10.1007/978-4-431-54064-9_6
Published:
Publisher Name: Springer, Tokyo
Print ISBN: 978-4-431-54063-2
Online ISBN: 978-4-431-54064-9
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)