Abstract
The foundation for the development of a broad spectrum of stress-related disorders is laid early in development. Early-life stress, including prenatal stress exposure, increases individual susceptibility for the development of mental disorders and physical diseases across the lifespan. Persistent alterations in the brain as well as in endocrine, immune, and metabolic systems underlie this developmental programming of disease susceptibility. Thus, stress exposure in early developmental stages results in neurobiological traces or “scars” in the central nervous system that render individuals susceptible to developing a broad range of diseases throughout the lifespan. Recent evidence suggests that this risk can be passed on to subsequent generations. Genetic factors and the developmental timing of adverse exposures moderate the clinical and biological consequences of early-life stress as well as individual vulnerability to disease and course of disease. A better understanding of the neurobiological mechanisms that link exposure to early life stress with disease risk will allow for the identification of measurable parameters to help identify individuals at risk of disease and susceptibility to a specific intervention. A precise understanding of the processes of biological embedding of early life stress will further enable the development of mechanism-derived targets and time windows for interventions and prevention strategies.
English Translation for:
Roth, Heinz, Walter (Eds.). Psychoneurowissenschaften. Springer-Verlag.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
The foundation for the development of a broad spectrum of stress-related disorders is laid early in development. Early-life stress, including prenatal stress exposure, increases individual susceptibility for the development of mental disorders and physical diseases across the lifespan. Persistent alterations in the brain as well as in endocrine, immune, and metabolic systems underlie this developmental programming of disease susceptibility. Thus, stress exposure in early developmental stages results in neurobiological traces or “scars” in the central nervous system that render individuals susceptible to developing a broad range of diseases throughout the lifespan. Recent evidence suggests that this risk can be passed on to subsequent generations. Genetic factors and the developmental timing of adverse exposures moderate the clinical and biological consequences of early-life stress as well as individual vulnerability to disease and course of disease. A better understanding of the neurobiological mechanisms that link exposure to early life stress with disease risk will allow for the identification of measurable parameters to help identify individuals at risk of disease and susceptibility to a specific intervention. A precise understanding of the processes of biological embedding of early life stress will further enable the development of mechanism-derived targets and time windows for interventions and prevention strategies.
FormalPara Learning ObjectivesThis chapter summarizes current findings from human clinical studies investigating the mechanisms by which early life stress affects neurobiological systems, as well as regulatory outflow systems of the brain, and influences susceptibility for psychiatric disorders and a wide range or physical diseases. The reader will be introduced to the concept of developmental programming of disease vulnerability and neurobiological changes resulting from early-life stress.
7.1 Early-Life Stress
The foundation for health and disease is laid early in development. Early life stress (ELS) is one of the most potent and pervasive risk factors for the development of psychiatric disorders and predicts a broad spectrum of physical diseases and increased mortality across the lifespan (Gilbert et al. 2009; Grummitt et al. 2021). Early life stress involves adverse experiences during childhood, such as exposure to various forms of severe stressors, including parental loss, unstable family situations, inadequate parental care due to mental or physical illness, and poverty. The most salient form of ELS may arguably be maltreatment, which encompasses neglect of care or supervision (emotional and physical neglect) and emotional, physical, and sexual abuse in childhood.
Exposure to ELS is alarmingly common in our society. Globally, prevalence estimates for maltreatment range from 13% for sexual abuse to 36% for emotional abuse (Stoltenborgh et al. 2015).When other forms of ELS are considered, prevalence estimates rise to nearly 50% of children affected and various types of ELS often coexist (for an overview, see Heim et al. 2019).
Childhood Maltreatment
The Centers for Disease Control and Prevention (CDC) define childhood maltreatment as “any act or series of acts of commission (i.e., abuse) or omission (i.e., neglect) by a parent or other caregiver that results in harm, potential for harm, or threat of harm to a child”. In this definition, harm to a child may not be the intended consequence of these acts, however, the act itself has to be deliberate and intentional (Leeb et al. 2008).
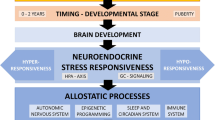
Research demonstrates that ELS exerts pronounced effects on neural systems, as well endocrine, immune, and metabolic regulatory systems, that are fundamental to the organism’s adaptation to stress. Changes in these systems that occur as a function of ELS may mediate increased risk for stress-related diseases (◘ Fig. 7.1). The developing brain is particularly susceptible to the organizing effects of experiences. According to the concept of developmental programming, neural plasticity is particularly pronounced during early developmental periods (Lupien et al. 2009): During such times of heightened plasticity, positive and nurturing social-emotional experiences may be required for an optimal development of neural circuits that mediate adaptation to stress and emotional regulation, whereas any type of ELS occurring within such developmental periods may promote disruptions in the development of these circuits, leading to long-term “scars”, which may result in maladaptive regulation upon further stress exposure an increased susceptibility for stress-related diseases across the lifespan.
ELS, biological effects and disease risk. (Reprinted from Heim et al. 2019)
Disease Vulnerability
An individual’s susceptibility for developing disease across the lifespan. The extent of disease vulnerability may be dependent on whether the exposure occurs during critical periods of development during which ELS has a particularly strong and specific effect on the brain and its regulatory outflow systems. Vulnerability is dependent on interactions of ELS and genetic factors and such gene-environment interactions may be mediated by epigenetic programming (see Heim and Binder 2012).
The well-established link between ELS and increased risk for disease across the lifespan raises several questions: How does ELS exposure get “under the skin”? Which mechanisms mediate the increased long-term susceptibility to various stress-related diseases? Will each individual with a history of ELS develop some form of stress-related disease over the life course or do some individuals demonstrate resilience to the lasting consequences of ELS? Advances from neuroscience and molecular biology research have provided compelling answers to these questions, which are summarized in the following sections.
7.2 Clinical Consequences of ELS
Clinical and epidemiological research demonstrates a robust and substantial increase in the risk for both psychiatric disorders and physical diseases following ELS exposure (for an overview see Heim and Binder 2012). A particularly strong association can be found for affective and anxiety disorders, including post-traumatic stress disorder (PTSD). Further, there are established dose-response relationships between childhood adversity and psychiatric disorders in adulthood (Edwards et al. 2003). Early life stress is not only associated with increased prevalence rates of these disorders, but also predicts earlier onset, chronic course, and greater severity of disease as well as poor treatment response (see Heim and Nemeroff 2001; Nanni et al. 2012). Moreover, ELS is a consistent risk factor for suicidality across disorders (see Heim and Binder 2012). In addition to its adverse effects on mental health, ELS is associated with markedly increased risk for chronic physical diseases, including cardiovascular, immune-related and respiratory diseases, diabetes and obesity, chronic pain, and reduced longevity (Felitti et al. 1998; Norman et al. 2012; Shonkoff et al. 2012). Individuals with a history of ELS often exhibit multiple comorbid psychiatric and physical disorders, suggesting the existence of an ELS-related core “lesion” across neural and peripheral regulatory systems that promotes maladaptation and disease.
7.3 Long-Term Biological Consequences of ELS
The precise mechanisms that mediate the detrimental effects of ELS on disease risk have been subject to basic and clinical investigation over the past decades. Lasting effects of ELS on the brain and its regulatory outflow systems, including the autonomic, endocrine, immune, and metabolic systems, may lead to increased sensitivity to stress and risk for a range of psychiatric and physical diseases. Studies in animal models involving maternal separation or natural variation of the quality of maternal care provide causal evidence that ELS leads to structural and functional changes in neural circuits that are involved in the mediation of stress responses, autonomic and neuroendocrine control, emotion regulation, and fear conditioning. These neurobiological changes promote exaggerated behavioral and physiological reactivity to stressors later in life (stress sensitization). For instance, adult rodents exposed to maternal separation or naturally occurring low maternal care in early life exhibit hyperactivity of the central stress-mediating neuropeptide corticotrophin releasing hormone (CRH) system and sensitization of the hypothalamic-pituitary-adrenal (HPA) axis as well as behavioral responses reminiscent of symptoms of depression and anxiety (see Heim and Binder 2012; Heim et al. 2019).
In accordance with findings from animal models, adult women with a history of ELS exhibit markedly increased pituitary-adrenal and autonomic responses to psychosocial laboratory stress, induced by the Trier Social Stress Test (TSST). This effect was particularly pronounced in abused women with concurrent major depression. Neuroendocrine alterations following ELS were demonstrated at multiple levels of stress regulation, including reduced adrenal capacity and dysregulated negative feedback of the HPA axis due to relative glucocorticoid receptor resistance as measured with the dexamethasone/CRH challenge test. Furthermore, increased cerebrospinal fluid (CSF) concentrations of CRH and decreased CSF levels of the neuropeptide oxytocin were reported as a function of severity of ELS, suggesting that stress-mediating systems are upregulated as a consequence of ELS whereas stress-buffering neuropeptide systems are downregulated. Taken together, these findings suggest a sensitization of the endocrine and autonomic stress responses and a disturbed balance between stress-mediating and stress-protective neural systems after ELS exposure, converging into increased stress vulnerability (see Heim et al. 2008).
The neuroendocrine system is tightly linked to the immune system and systemic inflammation is one of the most replicated biological correlates of ELS. Adults exposed to ELS exhibit significantly increased plasma levels of interleukin-6 (IL-6) and C-reactive protein (CRP), particularly those with depression (Baumeister et al. 2016). Notably, studies in 12 year-old and 3- to 5-year-old children suggest that the effect of ELS on inflammation emerges already in childhood and in the immediate aftermath of exposure (Danese et al. 2011; Entringer et al. 2020). One potential pathway through which ELS can induce an increased release of these inflammatory mediators may involve the above-described dysfunction of the glucocorticoid receptor (GR), a key regulator of the immune response (see Raison et al. 2006). In addition, ELS is associated with metabolic dysregulation. Elevated inflammatory levels and cortisol secretion following ELS may lead to decreased sensitivity to insulin contributing to the development of metabolic disorders, such as type 2 diabetes or metabolic syndrome. Inflammatory processes and metabolic abnormalities may also promote atherosclerosis progression contributing to the development of cardiovascular disease (see Danese and McEwen 2012).
At the central nervous system level, exaggerated concentrations of cortisol or inflammatory cytokines may exert neurotoxic effects on brain structures that are implicated in stress and emotion regulation. During early developmental periods of pronounced plasticity, ELS may shape the development of these brain regions (◘ Table 7.1). Volumetric alterations as a function of ELS have been shown specifically in cortical and subcortical regions that are particularly sensitive to glucocorticoid exposure and hence vulnerable the detrimental effects of stress, including the hippocampus (see Teicher et al. 2016). The hippocampus is critically involved in contextual aspects of fear conditioning and one of the most plastic central regions, exhibiting a high degree of synaptic reorganization and neurogenesis across the lifespan. With a high density of GRs, the hippocampus exerts an inhibitory control of hypothalamic CRH neurons. Several magnetic resonance imaging (MRI) studies in adults have demonstrated a small hippocampal volume in association with ELS (hippocampal atrophy), suggesting a dysfunctional inhibition of the stress response. Region-specific investigations of the hippocampus with high-resolution imaging demonstrate a pronounced decrease in volume in the CA3 region, the dentate gyrus and the left subiculum in ELS-exposed individuals (Teicher et al. 2012). Furthermore, structural and functional changes in cortico-limbic circuits have been reported as a function of ELS. The prefrontal cortex (PFC) is critically involved in executive functioning, regulation of goal-directed behavior, and impulse inhibition. The medial PFC is particularly relevant for emotion regulation via structural connections to the anterior cingulate cortex (ACC) and the amygdala. ELS has consistently been associated with volume loss in the PFC, including the medial PFC and ACC (see Heim et al. 2019). In addition, neuroimaging studies suggest structural and functional changes of the amygdala following ELS. The amygdala plays a key role in fear conditioning, emotion processing, as well as evaluating potentially threatening stimuli and eliciting an appropriate stress response. Whereas findings on volumetric alterations of the amygdala are inconsistent, functional MRI studies consistently demonstrate a sustained hyperactivity of the amygdala in response to emotionally threatening stimuli following ELS (Dannlowski et al. 2013). With the PFC exerting inhibitory and the amygdala exerting excitatory regulation of hypothalamic CRH neurons (Ulrich-Lai and Herman 2009), these findings may reflect a dysfunctional “top down” control of emotion regulation, fear conditioning, and stress responses that promotes disease risk.
Several studies suggest a specific impact of ELS on sensory representation areas implicated in the perception of the very nature of the abusive experience. Using whole mantle cortical thickness analysis in adults, we observed pronounced cortical thinning of the somatosensory genital field as a function of childhood sexual abuse. Emotional abuse was specifically associated with cortical thinning in the precuneus, a region that is relevant for self-awareness and self-evaluation, as well as thinning in the anterior cingulate cortex, which is relevant for emotional regulation (Heim et al. 2013; see ◘ Fig. 7.2). These findings suggest that experience-dependent plasticity leads to effects of ELS on sensory and associative processing areas in a highly region-specific manner. This specific cortical thinning in sensory processing areas may represent the most adaptive and protective response of the developing brain that may “shield” the child living under these conditions from the abusive experience, similar to sensory gating. In later life, these neurostructural changes may represent a direct biological substrate for behavioral disorders, such as sexual dysfunction. Of note, Teicher and colleagues report similar findings for other sensory modalities, including thinning of the visual cortex after witnessing domestic violence and thinning of the auditory cortex after verbal abuse (see Teicher et al. 2016).
7.4 Molecular Consequences of ELS: Epigenetic Programming and Telomere Biology
A major research question in the field of ELS research concerns the molecular effects by which early environmental exposures interact with genetic factors to produce a neurobiological phenotype with elevated vulnerability for stress and disease. In this context, epigenetic alterations as a mechanism underlying the biological embedding of the above-described stable physiologic and behavioral changes that result from ELS exposure have received much attention (Box: Epigenetic Programming, see Anacker et al. 2014).
Epigenetic Programming
The process by which the environment regulates DNA transcriptional activity without altering DNA sequence. Epigenetic changes are produced by DNA (de-) methylation, histone modifications, and non-coding RNAs (for an overview, see Jaenisch and Bird 2003).
Over the past decade, numerous studies have investigated whether exposure to ELS can leave persistent epigenetic marks in the genome, thereby altering gene expression and ultimately neurobiological substrates (Parade et al. 2021; Provençal and Binder 2015). Initial studies have typically employed a candidate gene approach, focusing on epigenetic variation in specific stress-relevant genes, including the GR gene, NR3C1, and FKBP5, a key modulator of glucocorticoid signaling. For instance, ELS has been associated with increased DNA methylation of the promoter region of NR3C1 in the human postmortem hippocampus. This hypermethylation was associated with decreased NGFI-A transcription factor binding and reduced gene transcription (McGowan et al. 2009; Perroud et al. 2011). ELS has also been linked with DNA demethylation in functional glucocorticoid response elements of FKBP5 in carriers of the “risk”-allele of a functional polymorphism within the FKBP5 gene, resulting in an increased risk of developing affective disorders in adulthood (Klengel et al. 2013). More recently, research interest has shifted away from candidate gene approaches toward investigating epigenetic variation across the entire epigenome in a hypothesis-free design (Epigenome-Wide Association Studies, EWAS). EWAS have consistently demonstrated presence of methylation changes in individuals exposed to ELS (e.g., Cicchetti et al. 2016; O’Donnell et al. 2018), however, to date no clear patterns of functional enrichment within the epigenome-wide methylation profiles associated with ELS have emerged (Cecil et al. 2020; Parade et al. 2021). A recent study from our group suggests that epigenetic modifications associated with ELS may, in part, also reflect co-occurring concurrent adversities and prenatal exposures (Martins et al. 2021). Epigenetic marks can also be used to estimate a form of biological ageing that takes into account time-dependent changes in DNA methylation at specific sites in the genome. The difference between this epigenetic age and the chronological age can be interpreted as accelerated or decelerated biological ageing. Our group recently observed accelerated epigenetic ageing in children with internalizing problems, but only if they had been exposed to ELS, whereas no acceleration of ageing was observed in children with internalizing disorder who had no history of ELS, suggesting that these molecular alterations may already be present shortly after the exposure and are associated with health status (Dammering et al. 2021).
Cellular ageing is another molecular process and form of biological ageing that has the potential to mediate the ELS-associated increased risk of developing both physical and mental health problems across the lifespan. Cellular ageing is a function of the integrity of telomeres, DNA-protein complexes at the end of chromosomes promoting chromosomal stability (Box: Telomere Biology). Telomeres shorten with each cell replication cycle until a critical limit is reached and the cell enters a state of senescence or undergoes apoptosis. Telomere length can be maintained by the enzyme telomerase, however, in most somatic tissues, telomerase levels and activity are very low so that telomeres cannot be maintained indefinitely. Thus, telomere length can serve as a biomarker for biological ageing and time until senescence and research suggests that it may also be a risk marker for a variety of age-related diseases (e.g., cancer, hypertension) as well as shorter lifespan (Price et al. 2013).
Telomere Biology
A system that plays a central role in maintaining the integrity of the genome and the cell. Telomere biology refers to the structure and function of two related entities: telomeres, a complex of non-coding double-stranded repeats of guanine-rich DNA sequences and the shelterin protein structures that serve to protect the ends of chromosomes, as well as the enzyme telomerase, a ribonucleoprotein that adds telomeric DNA to the ends of chromosomes and thus elongates and maintains telomeres (for an overview, see Entringer et al. 2018).
In addition to chronological age, telomere length is also influenced by a wide range of environmental and behavioral factors, including stress. Chronic psychological stress, such as ELS, may shape the biochemical milieu of the cellular environment, promoting conditions of inflammation and oxidative stress which can lead to telomere damage (Barnes et al. 2019). The telomere system has been shown to play an important role in the development of depression and other psychiatric disorders (Ridout et al. 2016). As such, reduced telomere length and/or telomerase activity may be underlying, in part, the association between ELS and adverse mental and physical health outcomes (see Entringer et al. 2018).
In the last decade, numerous studies have provided evidence linking ELS with reduced telomere length (see meta-analysis by Ridout et al. 2018). The developmental timing of ELS exposure appears to influence the size of the effect, with adversity earlier in development showing greater negative associations with telomere length (Ridout et al. 2018). These findings are consistent with the neurobiological findings in that they suggest that early childhood seems to be a particularly sensitive period in which stress exposure can exert long-lasting effects on various systems, including the telomere system.
7.5 Sensitive Periods for the Effects of ELS
The above-referenced findings give rise to the question as to whether or not there are sensitive periods during human childhood, where the brain is particularly sensitive to the environment, including the effects of ELS. Of note, one important factor that max contribute to variability of the outcomes of ELS between individuals may depend on the developmental timing of the ELS exposure within childhood. In other words, there may exist discrete sensitive periods during development, during which adverse exposures may be specifically detrimental, leading to longterm change. Little is known to date about such circumscribed time windows for the effects of ELS. Teicher and colleagues have conducted statistical analyses to identify sensitive periods for the effects of ELS on brain regional development. They report that the amygdala is particularly sensitive to the effects of abuse at the age of around 10 years, whereas the hippocampus has heightened sensitivity at an earlier age and the prefrontal cortex seems to be particularly amenable around puberty (see Teicher et al. 2016). Whether or not such temporally differential effects of ELS on brain regions are associated with specific symptom constellations remains poorly understood. A precise identification of sensitive periods for the effects of ELS may enable the development of timing-specific interventions that make use of sensitive periods to induce positive change with lasting effects. Further, a precise understanding of the mechanisms that determine the opening and closing of sensitive time windows of developmental plasticity may have therapeutic benefit, enabling the development of novel pharmacological targets for augmenting effects of psychotherapy by increasing plasticity. Of note, rodent models of maternal separation or naturally occurring low care typically focus on the first 2 weeks of life, which developmentally corresponds to fetal life in humans. Hence, it is conceivable that prenatal stress has profound impact on adult health and adaptation in humans, as discussed next.
7.6 Fetal Programming of Health and Disease
While most research has been conducted on ELS occurring postnatally, the intrauterine period of life represents another sensitive developmental window during which stress exposure can have long-term or even permanent consequences for health and disease susceptibility (Box: Fetal Programming; for an overview see Entringer et al. 2015).
Fetal Programming
The concept of fetal programming describes the process by which the embryo/fetus seeks, receives, and responds to signals from the gestational environment to incorporate this information into its development. The concept is based on the assumption that the rapid and foundational nature of developmental processes occurring during intrauterine life render them particularly vulnerable to environmental perturbations with lifelong consequences for disease susceptibility (see Entringer et al. 2015; Gluckman and Hanson 2004). The research field has its origins in a set of epidemiological studies, demonstrating that a person’s birth weight is associated with the risk for cardiovascular disease, as well as a variety of other conditions, including depression, obesity, and diabetes, later in life (see Wadhwa et al. 2009). In this context, birth weight is assumed to reflect the developmental environment in the womb, which, in interaction with the genetic make-up, elicits context-dependent and long-term adaptations in cells, tissues, organ systems and homeostatic set points of the developing embryo/fetus.
In recent years, an impressive body of research has collected evidence suggesting that maternal psychosocial stress and emotional state during pregnancy may affect child neurodevelopment as well as social-emotional and cognitive development and thus may increase risk for a variety of adverse physical and mental health outcomes, including mood disorders, attention problems, asthma, and obesity (Entringer et al. 2015; Lautarescu et al. 2020; Madigan et al. 2018). Since there are no direct vascular or neural connections between the maternal and fetal compartments all exchange of signals and communication is mediated by biological processes via the placenta. Stress-related biological processes appear to play a role as key sensors, transducers and effectors of maternal stress on the developing fetus. It is important to note that these stress-related biological mediators participate directly or indirectly in the process of phenotypic specification of the brain and other organ systems and should not be considered as developmental disruptors.
Several studies characterize alterations in brain structure and function in association with exposure to maternal stress-related biological mediators. For instance, elevated maternal cortisol concentrations have been associated with amygdala volume, microstructure and connectivity in newborns and children, with consequences for internalizing and affective problems (Buss et al. 2012; Graham et al. 2019). The pro-inflammatory cytokine interleukin-6 (IL-6) is another potential stress-related biological mediator of environmental conditions with an important role in fetal brain development. Several studies support a link between maternal IL-6 concentrations and offspring amygdala volume, structural and functional connectivity as well as connectivity within and between networks involved in sensory processing and higher order cognition (see Heim et al. 2019).
Maternal cortisol concentrations during pregnancy have also been repeatedly associated with offspring body composition and adiposity (Entringer et al. 2017; Van Dijk et al. 2012), suggesting that cortisol may also be involved in the prenatal programming of metabolism. Alterations in metabolic function appear to be present very early in postnatal life as demonstrated by one study showing that cortisol production particularly during the third trimester of pregnancy was associated with a greater change in infant percent body fat from 1 to 6 months assessed with Dual-energy X-ray absorptiometry imaging (Entringer et al. 2017).
Furthermore, prenatal stress exposure seems to have an effect on the child’s telomere length. Maternal psychosocial stress during pregnancy has been associated with shortened offspring telomeres in young adulthood and in the newborn period, whereas maternal psychological resiliency during pregnancy has been linked to increased newborn telomere length (for an overview, see Heim et al. 2019; Verner et al. 2021). The effects of maternal stress on fetal telomere biology may be mediated by alterations in gestational biology, including increased cortisol concentrations and a higher pro-inflammatory milieu (Bosquet Enlow et al. 2019; Lazarides et al. 2019). The initial telomere length at birth may have lifelong implications for telomere biology and health, the precise relationship of which remains to be explored.
Taken together, this evidence suggests that in utero exposure to maternal psychosocial stress may confer increased long-term risk of a range of negative health outcomes mediated by adaptations in organ systems during intrauterine development in response to stress-related biological signals. The presence of alterations in brain structure and function and in body composition in association with maternal biological mediators of stress so close to birth provides compelling evidence for a programming effect of the gestational environment that is independent of postnatal environmental influences.
7.7 Intergenerational Transmission of the Effects of Early Life Stress
Over the last decade, the notion that the deleterious consequences of ELS may be transmitted across generations has received increasing attention. A steadily growing body of studies have explored the effects of ELS exposure in the parental generation on neurodevelopmental outcomes in the offspring generation and observed many of the same sequelae that are well-established consequences of ELS in exposed individuals (Box: Intergenerational Transmission, Buss et al. 2017; Moog et al. 2022).
Empirical evidence points to an increased risk for a range of behavioral and emotional problems, including internalizing and externalizing problems, conduct disorder, self-regulation difficulties, and anxiety disorders as well as neurodevelopmental disorders, including autism and attention-deficit hyperactivity disorder in children of mothers who experienced ELS. In addition, maternal ELS is associated with an increased offspring risk of developing physical health problems and risk factors, including asthma, allergy and obesity (see Moog et al. 2022). As in the directly exposed generation, structural and functional alterations in the brain as well as dysregulations in autonomic, endocrine and immune system may be underlying these mental and physical health problems. We showed that infants of mothers with experiences of ELS had less cortical gray matter, which contributed to an overall lower brain volume compared to infants of mothers without ELS (Moog et al. 2018).
Intergenerational Transmission
The contribution of parental experiences and exposures (e.g., of early life stress) in shaping the development and phenotype of the offspring.
The mechanisms underlying the intergenerational transmission of ELS effects have not been fully clarified. One potential mechanism that has been investigated mainly in animal models is epigenetic inheritance. The term epigenetic inheritance refers to germline transmission of epigenetic information between generations independent of the DNA sequence, either through direct transfer of epigenetic marks or through reconstruction and reestablishment of germline epigenetic alterations in the zygote. As reviewed in ► Sect. 7.4, ELS exposure has been associated with persistent epigenetic alterations in certain tissues, including the germline in humans (Provençal and Binder 2015; Roberts et al. 2018). However, while epigenetic transmission of paternal ELS has been demonstrated in animal models (Gapp et al. 2020), conclusive evidence for the existence of epigenetic inheritance in humans is still lacking. Another pathway that has been debated is based on the observation that ELS-related physiological dysregulations in the stress, immune and metabolic systems may be carried forward into pregnancy and thus affect child health outcomes via a fetal programming mechanism (see ► Sect. 7.6; for an overview see Moog et al. 2022). Lastly, a caregiving environment characterized by maternal psychopathology or difficulty providing high-quality parenting due to ELS-related personal, social and socio-economic constraints is another important potential mediator of the intergenerational transmission of ELS (Plant et al. 2018).
7.8 Gene-Environment (GxE) Interactions
While the increase in disease risk in association with ELS is substantial and alarming, not all children exposed to ELS go on to develop stress-related disorders, even if additional stressors occur later in life. Genetic factors likely play a moderating role in the extent to which environmental conditions, such as ELS exposure, may program neurobiological structures and functions as well as in the individual susceptibility versus resilience to developing stress-related disorders. A variety of studies have identified candidate genes in stress-regulatory systems that moderate the link between ELS and risk for depression and other disorders, including the CRH receptor 1 (CRHR1), GR (NR3C1), the GR-regulating FK506 binding protein 5 (FKBP5), serotonin transporter (SLC6A4), brain-derived neurotrophic factor (BDNF), and oxytocin receptor (OXTR) genes (for an overview, see Heim and Binder 2012). Notably, most functional polymorphisms in these genes that confer risk for depression in combination with ELS are associated with GR resistance or enhanced stress hormone system activity. However, there has been increasing awareness that multiple gene variants likely work together to shape disease risk, such that in recent years the field has moved towards employing polygenic approaches. Polygenic risk scores incorporate the contributions of many common genetic variants and are derived from genome-wide association studies (for an overview, see Halldorsdottir and Binder 2017). For instance, a polygenic risk factor for major depressive disorder has been demonstrated to moderate the association between ELS and depression (Peyrot et al. 2014). In the future, a deeper and comprehensive understanding of GxE interactions is critically important to identify cases that are vulnerable to the pathogenic effects of ELS and require preventive intervention. Genetic markers may also be used to develop more targeted treatments, which will be discussed in the following section.
7.9 Implications for Intervention
Research findings on ELS as a risk factor for a wide range of diseases, as reviewed in this chapter, have the potential to inform the development of novel intervention strategies that target different aspects of biological embedding, disease manifestation and transmission of disease risk across generations (see Heim et al. 2019). Results from our studies demonstrating neurobiological differences and differential responses to drug or psychotherapy as a function of ELS suggest that developmental factors should be included in individual treatment decisions for patients with affective disorders. The development of algorithms based on biomarkers, genetic factors and symptom constellations could lead to personalized interventions as a form of precision medicine in the field of psychiatry. However, we propose that it is even more efficient to take advantage of the high levels of plasticity during early development and intervene before the clinical manifestation of disease to counteract, reverse or compensate biological “scars” of ELS. These interventions target the underlying mechanisms rather than symptoms and may involve “top down” compensatory regulation of the altered neural and physiological systems (e.g., via psychotherapeutic interventions starting as soon as possible after the exposure) or “bottom up” approaches directly counteracting biological embedding effects of ELS (e.g., FKBP51 antagonists). Furthermore, a better understanding of the molecular mechanisms that determine sensitive periods of brain development may enable the development of entirely novel treatment approaches that restore such a state of increased plasticity in order to reverse the programming effects of ELS (Bavelier et al. 2010). It is important to note, however, that biological alterations may not be detrimental per se but can represent adaptations that confer short-term benefits to help the system function under conditions characterized by ELS. Our research on the intergenerational transmission of ELS-related risk suggest that interventions during pregnancy that target gestational physiology, stress reduction, trauma coping and different forms of social and personal constraints that can be consequences of ELS-exposure could minimize the intergenerational effects on the offspring.
7.10 Conclusion
In sum, ELS is a profound and nonspecific risk factor for a wide range of diseases. Exposure to ELS during sensitive developmental periods, including the prenatal period, appears to lead to immediate processes of biological embedding. This biological embedding of ELS may involve epigenetic modifications in stress-regulatory genes, with subsequent dysregulation of endocrine and immune stress response systems, metabolic dysregulation, structural and functional changes in brain regions regulating stress and emotion, as well as accelerated biological ageing. These physiological alterations may lead to manifestation of adverse mental and physical health outcomes, depending also on the presence of additional stressors later in life as well as genetic factors. The phenotypic consequences of ELS may be transmitted into the next generation, thereby multiplying the number of affected individuals. Existing and future research will inform novel approaches that make use of developmental plasticity in order to promote optimal development, health, and longevity in all children.
References
Anacker C, O’Donnell KJ, Meaney MJ (2014) Early life adversity and the epigenetic programming of hypothalamic-pituitary-adrenal function. Dialogues Clin Neurosci 16(3):321–333
Barnes RP, Fouquerel E, Opresko PL (2019) The impact of oxidative DNA damage and stress on telomere homeostasis. Mech Ageing Dev 177:37–45. https://doi.org/10.1016/j.mad.2018.03.013
Baumeister D, Akhtar R, Ciufolini S, Pariante CM, Mondelli V (2016) Childhood trauma and adulthood inflammation: a meta-analysis of peripheral C-reactive protein, interleukin-6 and tumour necrosis factor-α. Mol Psychiatry 21(5):Article 5. https://doi.org/10.1038/mp.2015.67
Bavelier D, Levi DM, Li RW, Dan Y, Hensch TK (2010) Removing brakes on adult brain plasticity: from molecular to behavioral interventions. J Neurosci 30(45):14964–14971. https://doi.org/10.1523/JNEUROSCI.4812-10.2010
Bosquet Enlow M, Sideridis G, Bollati V, Hoxha M, Hacker MR, Wright RJ (2019) Maternal cortisol output in pregnancy and newborn telomere length: evidence for sex-specific effects. Psychoneuroendocrinology 102:225–235. https://doi.org/10.1016/j.psyneuen.2018.12.222
Buss C, Poggi Davis E, Shahbaba B, Pruessner JC, Head K, Sandman CA (2012) Maternal cortisol over the course of pregnancy and subsequent child amygdala and hippocampus volumes and affective problems. Proc Natl Acad Sci U S A 109:E1312–E1319. https://doi.org/10.1073/pnas.1201295109
Buss C, Entringer S, Moog NK, Toepfer P, Fair DA, Simhan HN, Heim CM, Wadhwa PD (2017) Intergenerational transmission of maternal childhood maltreatment exposure: implications for fetal brain development. J Am Acad Child Adolesc Psychiatry 56(5):373–382. https://doi.org/10.1016/j.jaac.2017.03.001
Cecil CAM, Zhang Y, Nolte T (2020) Childhood maltreatment and DNA methylation: a systematic review. Neurosci Biobehav Rev 112:392–409. https://doi.org/10.1016/j.neubiorev.2020.02.019
Cicchetti D, Hetzel S, Rogosch FA, Handley ED, Toth SL (2016) An investigation of child maltreatment and epigenetic mechanisms of mental and physical health risk. Dev Psychopathol 28(4pt2):1305–1317. https://doi.org/10.1017/S0954579416000869
Dammering F, Martins J, Dittrich K, Czamara D, Rex-Haffner M, Overfeld J, de Punder K, Buss C, Entringer S, Winter SM, Binder EB, Heim C (2021) The pediatric buccal epigenetic clock identifies significant ageing acceleration in children with internalizing disorder and maltreatment exposure. Neurobiol Stress 15:100394. https://doi.org/10.1016/j.ynstr.2021.100394
Danese A, McEwen BS (2012) Adverse childhood experiences, allostasis, allostatic load, and age-related disease. Physiol Behav 106(1):29–39. https://doi.org/10.1016/j.physbeh.2011.08.019
Danese A, Ouellet-Morin I, Arseneault L (2011) Elevated salivary C-reactive protein in maltreated children. Brain Behav Immun 25:S210. https://doi.org/10.1016/j.bbi.2011.07.110
Dannlowski U, Kugel H, Huber F, Stuhrmann A, Redlich R, Grotegerd D, Dohm K, Sehlmeyer C, Konrad C, Baune BT, Arolt V, Heindel W, Zwitserlood P, Suslow T (2013) Childhood maltreatment is associated with an automatic negative emotion processing bias in the amygdala. Hum Brain Mapp 34(11):2899–2909. https://doi.org/10.1002/hbm.22112
Edwards VJ, Holden GW, Felitti VJ, Anda RF (2003) Relationship between multiple forms of childhood maltreatment and adult mental health in community respondents: results from the adverse childhood experiences study. Am J Psychiatr 160(8):1453–1460. https://doi.org/10.1176/appi.ajp.160.8.1453
Entringer S, Buss C, Rasmussen JM, Lindsay K, Gillen DL, Cooper DM, Wadhwa PD (2017) Maternal cortisol during pregnancy and infant adiposity: a prospective investigation. J Clin Endocrinol Metabol 102(4):1366–1374. https://doi.org/10.1210/jc.2016-3025
Entringer S, Buss C, Wadhwa PD (2015) Prenatal stress, development, health and disease risk: a psychobiological perspective-2015 Curt Richter Award Paper. Psychoneuroendocrinology 62:366–375. https://doi.org/10.1016/j.psyneuen.2015.08.019
Entringer S, de Punder K, Buss C, Wadhwa PD (2018) The fetal programming of telomere biology hypothesis: an update. Philos Trans R Soc Lond Ser B Biol Sci 373(1741):20170151. https://doi.org/10.1098/rstb.2017.0151
Entringer S, de Punder K, Overfeld J, Karaboycheva G, Dittrich K, Buss C, Winter SM, Binder EB, Heim C (2020) Immediate and longitudinal effects of maltreatment on systemic inflammation in young children. Dev Psychopathol 32(5):1725–1731. https://doi.org/10.1017/S0954579420001686
Felitti VJ, Anda RF, Nordenberg D, Williamson DF, Spitz AM, Edwards V, Koss MP, Marks JS (1998) Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults: the Adverse Childhood Experiences (ACE) study. Am J Prev Med 14(4):245–258. https://doi.org/10.1016/S0749-3797(98)00017-8
Gapp K, van Steenwyk G, Germain PL, Matsushima W, Rudolph KLM, Manuella F, Roszkowski M, Vernaz G, Ghosh T, Pelczar P, Mansuy IM, Miska EA (2020) Alterations in sperm long RNA contribute to the epigenetic inheritance of the effects of postnatal trauma. Mol Psychiatry 25(9):Article 9. https://doi.org/10.1038/s41380-018-0271-6
Gilbert R, Widom CS, Browne K, Fergusson D, Webb E, Janson S (2009) Burden and consequences of child maltreatment in high-income countries. Lancet 373(9657):68–81. https://doi.org/10.1016/S0140-6736(08)61706-7
Gluckman PD, Hanson MA (2004) Developmental origins of disease paradigm: a mechanistic and evolutionary perspective. Pediatr Res 56(3):3. https://doi.org/10.1203/01.PDR.0000135998.08025.FB
Graham AM, Rasmussen JM, Entringer S, Ward EB, Rudolph MD, Gilmore JH, Styner M, Wadhwa PD, Fair DA, Buss C (2019) Maternal cortisol concentrations during pregnancy and sex-specific associations with neonatal amygdala connectivity and emerging internalizing behaviors. Biol Psychiatry 85(2):172–181. https://doi.org/10.1016/j.biopsych.2018.06.023
Grummitt LR, Kreski NT, Kim SG, Platt J, Keyes KM, McLaughlin KA (2021) Association of childhood adversity with morbidity and mortality in US adults: a systematic review. JAMA Pediatr 175(12):1269–1278. https://doi.org/10.1001/jamapediatrics.2021.2320
Halldorsdottir T, Binder EB (2017) Gene × environment interactions: from molecular mechanisms to behavior. Annu Rev Psychol 68(1):215–241. https://doi.org/10.1146/annurev-psych-010416-044053
Heim C, Binder EB (2012) Current research trends in early life stress and depression: review of human studies on sensitive periods, gene–environment interactions, and epigenetics. Exp Neurol 233(1):102–111. https://doi.org/10.1016/j.expneurol.2011.10.032
Heim CM, Entringer S, Buss C (2019) Translating basic research knowledge on the biological embedding of early-life stress into novel approaches for the developmental programming of lifelong health. Psychoneuroendocrinology 105:123–137. https://doi.org/10.1016/j.psyneuen.2018.12.011
Heim CM, Mayberg HS, Mletzko T, Nemeroff CB, Pruessner JC (2013) Decreased cortical representation of genital somatosensory field after childhood sexual abuse. Am J Psychiatry 170(6):616–623. https://doi.org/10.1176/appi.ajp.2013.12070950
Heim C, Nemeroff CB (2001) The role of childhood trauma in the neurobiology of mood and anxiety disorders: preclinical and clinical studies. Biol Psychiatry 49(12):1023–1039. https://doi.org/10.1016/s0006-3223(01)01157-x
Heim C, Newport DJ, Mletzko T, Miller AH, Nemeroff CB (2008) The link between childhood trauma and depression: insights from HPA axis studies in humans. Psychoneuroendocrinology 33(6):693–710. https://doi.org/10.1016/j.psyneuen.2008.03.008
Jaenisch R, Bird A (2003) Epigenetic regulation of gene expression: how the genome integrates intrinsic and environmental signals. Nat Genet 33(Suppl):245–254. https://doi.org/10.1038/ng1089
Klengel T, Mehta D, Anacker C, Rex-Haffner M, Pruessner JC, Pariante CM, Pace TW, Mercer KB, Mayberg HS, Bradley B, Nemeroff CB, Holsboer F, Heim CM, Ressler KJ, Rein T, Binder EB (2013) Allele-specific FKBP5 DNA demethylation mediates gene-childhood trauma interactions. Nat Neurosci 16(1):33–41. https://doi.org/10.1038/nn.3275
Knop A, Spengler S, Bogler C, Forster C, Brecht M, Haynes JD, Heim C (2022) Sensory-tactile functional mapping and use-associated structural variation of the human female genital representation field. J Neurosci 42:1131–11402. https://doi.org/10.1523/JNEUROSCI.1081-21.2021
Lautarescu A, Craig MC, Glover V (2020) Prenatal stress: effects on fetal and child brain development. In: International Review of Neurobiology, vol 150. Elsevier, pp 17–40. https://doi.org/10.1016/bs.irn.2019.11.002
Lazarides C, Epel ES, Lin J, Blackburn EH, Voelkle MC, Buss C, Simhan HN, Wadhwa PD, Entringer S (2019) Maternal pro-inflammatory state during pregnancy and newborn leukocyte telomere length: a prospective investigation. Brain Behav Immun 80:419–426. https://doi.org/10.1016/j.bbi.2019.04.021
Leeb, R., Paulozzi, L., Melanson, C., Simon, T., & Arias, I. (2008). Child maltreatment surveillance: uniform definitions for public health and recommended data elements. Centers for Disease Control and Prevention (CDC)
Lupien SJ, McEwen BS, Gunnar MR, Heim C (2009) Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat Rev Neurosci 10(6):6. https://doi.org/10.1038/nrn2639
Madigan S, Oatley H, Racine N, Fearon RMP, Schumacher L, Akbari E, Cooke JE, Tarabulsy GM (2018) A meta-analysis of maternal prenatal depression and anxiety on child socioemotional development. J Am Acad Child Adolesc Psychiatry 57(9):645–657.e8. https://doi.org/10.1016/j.jaac.2018.06.012
Martins J, Czamara D, Sauer S, Rex-Haffner M, Dittrich K, Dörr P, de Punder K, Overfeld J, Knop A, Dammering F, Entringer S, Winter SM, Buss C, Heim C, Binder EB (2021) Childhood adversity correlates with stable changes in DNA methylation trajectories in children and converges with epigenetic signatures of prenatal stress. Neurobiol Stress 15:100336. https://doi.org/10.1016/j.ynstr.2021.100336
McGowan PO, Sasaki A, D’Alessio AC, Dymov S, Labonté B, Szyf M, Turecki G, Meaney MJ (2009) Epigenetic regulation of the glucocorticoid receptor in human brain associates with childhood abuse. Nat Neurosci 12(3):342–348. https://doi.org/10.1038/nn.2270
Michels L, Mehnert U, Boy S, Schurch B, Kollias S (2010) The somatosensory representation of the human clitoris: an fMRI study. NeuroImage 49:177–184. https://doi.org/10.1016/j.neuroimage.2009.07.024
Moog NK, Entringer S, Rasmussen JM, Styner M, Gilmore JH, Kathmann N, Heim CM, Wadhwa PD, Buss C (2018) Intergenerational effect of maternal exposure to childhood maltreatment on newborn brain anatomy. Biol Psychiatry 83(2):120–127. https://doi.org/10.1016/j.biopsych.2017.07.009
Moog NK, Heim CM, Entringer S, Simhan HN, Wadhwa PD, Buss C (2022) Transmission of the adverse consequences of childhood maltreatment across generations: focus on gestational biology. Pharmacol Biochem Behav 215:173372. https://doi.org/10.1016/j.pbb.2022.173372
Nanni V, Uher R, Danese A (2012) Childhood maltreatment predicts unfavorable course of illness and treatment outcome in depression: a meta-analysis. Am J Psychiatr 169(2):141–151. https://doi.org/10.1176/appi.ajp.2011.11020335
Norman RE, Byambaa M, De R, Butchart A, Scott J, Vos T (2012) The long-term health consequences of child physical abuse, emotional abuse, and neglect: a systematic review and meta-analysis. PLoS Med 9(11):e1001349. https://doi.org/10.1371/journal.pmed.1001349
O’Donnell KJ, Chen L, MacIsaac JL, McEwen LM, Nguyen T, Beckmann K, Zhu Y, Chen LM, Brooks-Gunn J, Goldman D, Grigorenko EL, Leckman JF, Diorio J, Karnani N, Olds DL, Holbrook JD, Kobor MS, Meaney MJ (2018) DNA methylome variation in a perinatal nurse-visitation program that reduces child maltreatment: a 27-year follow-up. Transl Psychiatry 8(1):15. https://doi.org/10.1038/s41398-017-0063-9
Parade SH, Huffhines L, Daniels TE, Stroud LR, Nugent NR, Tyrka AR (2021) A systematic review of childhood maltreatment and DNA methylation: candidate gene and epigenome-wide approaches. Transl Psychiatry 11(1):1–33. https://doi.org/10.1038/s41398-021-01207-y
Perroud N, Paoloni-Giacobino A, Prada P, Olié E, Salzmann A, Nicastro R, Guillaume S, Mouthon D, Stouder C, Dieben K, Huguelet P, Courtet P, Malafosse A (2011) Increased methylation of glucocorticoid receptor gene (NR3C1) in adults with a history of childhood maltreatment: a link with the severity and type of trauma. Translational. Psychiatry 1(12):12. https://doi.org/10.1038/tp.2011.60
Peyrot WJ, Milaneschi Y, Abdellaoui A, Sullivan PF, Hottenga JJ, Boomsma DI, Penninx BWJH (2014) Effect of polygenic risk scores on depression in childhood trauma. Br J Psychiatry J Ment Sci 205(2):113–119. https://doi.org/10.1192/bjp.bp.113.143081
Plant DT, Pawlby S, Pariante CM, Jones FW (2018) When one childhood meets another—maternal childhood trauma and offspring child psychopathology: a systematic review. Clin Child Psychol Psychiatry 23(3):483–500. https://doi.org/10.1177/1359104517742186
Price LH, Kao H-T, Burgers DE, Carpenter LL, Tyrka AR (2013) Telomeres and early-life stress: an overview. Biol Psychiatry 73(1):15–23. https://doi.org/10.1016/j.biopsych.2012.06.025
Provençal N, Binder EB (2015) The effects of early life stress on the epigenome: from the womb to adulthood and even before. Exp Neurol 268:10–20. https://doi.org/10.1016/j.expneurol.2014.09.001
Raison CL, Capuron L, Miller AH (2006) Cytokines sing the blues: inflammation and the pathogenesis of depression. Trends Immunol 27(1):24–31. https://doi.org/10.1016/j.it.2005.11.006
Ridout KK, Levandowski M, Ridout SJ, Gantz L, Goonan K, Palermo D, Price LH, Tyrka AR (2018) Early life adversity and telomere length: a meta-analysis. Mol Psychiatry 23(4):Article 4. https://doi.org/10.1038/mp.2017.26
Ridout KK, Ridout SJ, Price LH, Sen S, Tyrka AR (2016) Depression and telomere length: a meta-analysis. J Affect Disord 191:237–247. https://doi.org/10.1016/j.jad.2015.11.052
Roberts AL, Gladish N, Gatev E, Jones MJ, Chen Y, MacIsaac JL, Tworoger SS, Austin SB, Tanrikut C, Chavarro JE, Baccarelli AA, Kobor MS (2018) Exposure to childhood abuse is associated with human sperm DNA methylation. Translational. Psychiatry 8(1):1. https://doi.org/10.1038/s41398-018-0252-1
Shonkoff JP, Garner AS, The Committee on Psychosocial Aspects of Child and Family Health, C. on E. C, Siegel BS, Dobbins MI, Earls MF, Garner AS, McGuinn L, Pascoe J, Wood DL (2012) The lifelong effects of early childhood adversity and toxic stress. Pediatrics 129(1):e232–e246. https://doi.org/10.1542/peds.2011-2663
Stoltenborgh M, Bakermans-Kranenburg MJ, Alink LRA, van Ijzendoorn MH (2015) The prevalence of child maltreatment across the globe: review of a series of meta-analyses. Child Abuse Rev 24(1):37–50. https://doi.org/10.1002/car.2353
Teicher MH, Anderson CM, Polcari A (2012) Childhood maltreatment is associated with reduced volume in the hippocampal subfields CA3, dentate gyrus, and subiculum. Proc Natl Acad Sci 109(9):E563–E572. https://doi.org/10.1073/pnas.1115396109
Teicher MH, Samson JA, Anderson CM, Ohashi K (2016) The effects of childhood maltreatment on brain structure, function and connectivity. Nat Rev Neurosci 17(10):652–666. https://doi.org/10.1038/nrn.2016.111
Ulrich-Lai YM, Herman JP (2009) Neural regulation of endocrine and autonomic stress responses. Nat Rev Neurosci 10(6):397–409. https://doi.org/10.1038/nrn2647
Van Dijk AE, Van Eijsden M, Stronks K, Gemke RJBJ, Vrijkotte TGM (2012) The relation of maternal job strain and cortisol levels during early pregnancy with body composition later in the 5-year-old child: the ABCD study. Early Hum Dev 88(6):351–356. https://doi.org/10.1016/j.earlhumdev.2011.09.009
Verner G, Epel E, Lahti-Pulkkinen M, Kajantie E, Buss C, Lin J, Blackburn E, Räikkönen K, Wadhwa PD, Entringer S (2021) Maternal psychological resilience during pregnancy and newborn telomere length: a prospective study. Am J Psychiatr 178(2):183–192. https://doi.org/10.1176/appi.ajp.2020.19101003
Wadhwa PD, Buss C, Entringer S, Swanson JM (2009) Developmental origins of health and disease: brief history of the approach and current focus on epigenetic mechanisms. Semin Reprod Med 27(5):358–368. https://doi.org/10.1055/s-0029-1237424
Acknowledgement
This work was funded by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) under Germany’s Excellence Strategy—EXC-2049—390688087 (to CH) and DFG-441735381 (to NM) and a scholarship from the Einstein Center for Neuroscience Berlin (to AJJK).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer-Verlag GmbH, DE, part of Springer Nature
About this chapter
Cite this chapter
Knop, A.J.J., Moog, N.K., Heim, C. (2023). Neurobiological Consequences of Early Life Stress. In: Roth, G., Heinz, A., Walter, H. (eds) Psychoneuroscience. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-662-65774-4_7
Download citation
DOI: https://doi.org/10.1007/978-3-662-65774-4_7
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-662-65773-7
Online ISBN: 978-3-662-65774-4
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)