Abstract
Polymer-based nanocomposites have emerged as a new class of hybrid materials which find many promising bioapplications, ranging from tissue engineering, drug delivery, to various biotechnological applications. The fundamental understanding of structure, properties, polymer–matrix interaction and further modifications based on fundamental requirements are essential for their designing and development. Additionally, the control over physical and chemical properties along with assessment of biological interactions is instrumental for their potential applications. This chapter discusses emerging biomedical and biotechnological applications of polymer-based nanocomposites.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
- Graphene Oxide
- Tissue Engineering
- Polymer Nanocomposites
- Nanocomposite Hydrogel
- Thermally Induce Phase Separation
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
4.1 Introduction
Polymer matrix–based nanocomposites have emerged as a new class of hybrid materials since last two decades and have attracted significant interest of researchers. Polymer composites represent efficient strategies to upgrade the structural and functional properties of polymers. The nanoparticles-reinforced polymer composites often show significant improvements in properties that cannot be achieved by using the polymer alone. Polymer nanocomposites are formed by combination of polymers and inorganic/inorganic nanofillers. Nanofillers are nanostructures which have dimension less than 100 nm and possess high interfacial area per volume [12Ray]. Most commonly, nanofillers are carbon nanotubes, silicates, metals, metal oxide, and ceramics. These fillers consist of different properties such as mechanical, chemical, thermal, electrical, etc. In this regard, the fundamental understanding and knowledge of the nanostructures are required for fabrication of materials for desired applications. The interaction between nanofillers and polymer matrix is the basis of mechanical and functional properties of polymer nanocomposites and by tuning various parameters and controlling the interaction between nanostructures and polymers, unique properties combinations can be achieved [07Lif, 11Gah].
By exploiting the inherent properties of nanomaterials and selecting suitable polymer matrix, a variety of nanocomposites materials have been generated with improvised properties. Current opportunities for polymer nanocomposites arise from the multitude of applications based on different functional requirements [08Pau]. The polymer-based nanocomposites have generated huge interest in many biomedical and biotechnological applications [14Goe]. Biomaterials based on polymer nanocomposites are field of interdisciplinary research which brings together collaborative efforts of materials science, nanotechnology, and biological science. A synergistic combination of physical, chemical, and biological properties of nanocomposites provides an exciting platform for designing and development of improved materials for biomedical applications [09Mit, 10Sat].
Polymer nanocomposites show improved mechanical properties, and thus can be used to replicate high performance materials such bone and silk [08Lee]. Nature offers strategies to mimic biological tissues containing hard and soft components. Biologically inspired materials can be fabricated by unique combination of soft polymer matrix and hard nanostructure; thus, these new composite materials are being developed by dispersing hard inclusions in polymer matrix [08Vai, 09Mit]. However, one of the key challenges faced during fabrication of polymer-based nanocomposites is uniform dispersion of nanofillers into polymer matrix for which different processing technologies have been used by researchers [05Dun]. Another key issue which needs to be addressed while fabricating nanocomposites for biomedical applications is biocompatibility factor. Thus by balancing mechanical properties, functionalities, and biocompatibility factors, polymer nanocomposites are finding many interesting applications in the emerging technologies such as tissue engineering, biomedical imaging, sutures, surgical implants, drug delivery-based applications, etc. [14Goe].
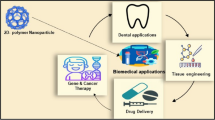
In tissue engineering, scaffold serves as a template and structural support for cell adhesion, proliferation, differentiation, and extracellular matrix formation [10Arm]. Replicating some physical properties of natural tissue and reproducing their complexity and efficiency are very challenging; thus, scaffold matrices have to be designed which not only resemble the structural and mechanical properties of a natural tissue but also imitate the signaling environment of the natural extracellular matrix (ECM). In this regard, polymer-based nanocomposites [14Ser] have been widely explored. These materials are applicable for hard and soft tissue engineering due to their unique mechanical and electrical properties. Apart from tissue engineering, polymer nanocomposites are also considered as potential candidates for drug delivery as they provide possibility to deliver large and controlled doses of therapeutic agents at the action site. The polymer-based polymer composites have been utilized in a myriad of bioapplications (Scheme 1). To achieve different functional requirements, these polymer-based nanocomposites are tailored with functionalities which open new possibilities in developing advanced biomaterials for various biomedical and biotechnological applications.
Here, in this chapter we focus on biomedical applicability of carbon nanotube (CNT), graphene, and nanoclay-based polymer nanocomposites. This chapter highlights applications of these polymer nanocomposites in emerging biomedical fields such as tissue engineering, drug delivery, etc.
4.2 Nanofillers for Polymer-Based Nanocomposites
Polymer-based nanocomposites for bioapplications are fabricated by reinforcing nanofillers into biocompatible polymers which include both natural and synthetic polymers. This section presents three different nanofillers which are extensively used for the preparation of polymer-based nanocomposites:
-
1.
Carbon nanotubes as fillers
-
2.
Graphene as fillers
-
3.
Nanoclay as fillers
4.2.1 Carbon Nanotubes as Fillers
Carbon nanotubes (CNTs) are allotropic forms of carbon discovered in late 1950s; however, the synthesis was first reported by Lijima [91Lij] and Bacon [60Bac]. The major methods adopted for synthesis of carbon nanotubes include laser ablation [96The], arc-discharge [97Jou], template-directed synthesis, chemical vapor deposition (CVD) [99Cas], and catalyst chemical vapor deposition (CCVD). However, CVD is the most commonly used method due to its low setup cost and high production yield. CNTs are either composed of monolayered graphene sheet (Single-walled carbon nanotubes, SWCNTs) or several concentric graphitic layers with multiwalled structure (multiwalled carbon nanotubes, MWCNTs). CNTs have fascinating electrical, mechanical, as well as thermal properties [14Ser]. They are known to have exceptional mechanical properties such as high Young’s modulus and tensile strengths up to 63 GPa (10–100 times higher than steel) [96Tre, 00Yu], as well as thermal stability up to 2800 °C under vacuum [01Tho]. Due to their unique properties, these nanosized structures find wide application in many areas of material science including nanocomposites [12Wan].
CNTs have been recognized as effective reinforced materials for polymer nanocomposites because of their excellent mechanical, electrical, and surface properties such as high aspect ratio and enormous surface area. Incorporation of CNT significantly increases the mechanical strength of polymer matrix. In addition to the role of carbon nanotubes, the mechanical behavior of polymer nanocomposites are also dependent on polymer nature (amorphous/crystalline/semicrystalline). Also the adhesion between carbon nanotubes and the polymer matrix, and the homogenous dispersion of nanotubes in the polymer matrix are equally important in improving the properties [02Coo, 03Won] which is influenced by fabricating methods (melt blending/latex technology/in situ polymerization/solvent casting/). Integration of CNT as nanofiller in polymer matrix not only improves the physicochemical properties such as strength and flexibility [10Spi] but also adds new functionalities due to which they find diverse applications [14Ser]. Sometimes CNTs are functionalized to explore their potential in chemical and biological applications [12Mac]. They have been exploited for various biological applications such as the preparation of tips for atomic force microscopy, fuel-powered artificial muscles, and nanosurgical needles and biosensors, etc. [03Wan, 14Ser].
4.2.2 Graphene as Fillers
Graphene is a single layer two-dimensional material with a hexagonal-packed lattice having many unique properties such as high carrier mobility at room temperature [04Nov], high Young’s modulus [08Lee], and excellent conductivity [08Bal]. Graphene can be synthesized by chemical vapor deposition (CVD) growth, mechanical exfoliation of graphite, or exfoliation of graphite oxide [10Dre]. Currently, graphene-based polymer nanocomposites are an exciting area of research due to the superior properties of graphene compared to polymers [09Xu]. Due to its high thermal and electrical conductivity, it is reported to be better nanofiller than other carbon-based polymer composites. The distribution of graphene layers in the polymer matrix and interfacial bonding between the graphene layers and polymer matrix are very important in determining the physicochemical properties of the nanocomposite. Since pristine graphene is not compatible with organic polymers and does not form homogeneous composites, graphene oxide (GO) sheets are more preferred [07Sta] as nanofiller for polymer nanocomposites. The remarkable property of graphene provides possibilities for various fields.
One such interesting area of research is biomedical applications of graphene-based polymer nanocomposites. Indeed, the number of publications on bioapplications of graphene-based nanocomposites has grown exponentially in recent years. Graphene-based polymer composites have been utilized in a myriad of bioapplications.
4.2.3 Nanoclay as Fillers
Nanoparticles derived for clay minerals have been considered as very effective reinforcing materials for designing polymeric nanocomposites. Due to their abundant nature, low cost, and interesting properties such as high strength, stiffness and high aspect ratio of individual platelets, clay-based polymer composites are used in different fields. Clay minerals include both natural clays (e.g., montmorillonite, hectorite, and saponite) and synthesized clays. Depending upon the nature of the components and processing conditions, layered clays filled into a polymer matrix form either conventional composite or nanocomposite. In case of conventional composite, polymer cannot intercalate into the galleries of clay minerals, whereas nanocomposites can further be divided into two types called as intercalated nanocomposites and exfoliated or delaminated nanocomposite.
In case of intercalated nanocomposites, monolayer of extended polymer chains is impregnated into the gallery of clay minerals resulting in an ordered multilayer structure with alternate polymer layers and clay platelets, whereas in exfoliated nanocomposites clay platelets are completely and uniformly dispersed in a continuous polymer matrix resulting in enhanced polymer–clay interaction [10Kil]. Clay-based nanocomposites exhibit strong interfacial interactions between the dispersed clay layers and the polymer matrix which leads to enhanced mechanical, thermal, and barrier properties over the virgin polymer.
The incompatibility between hydrophilic clay and hydrophobic polymer is one of the shortcomings, which often causes agglomeration of clay mineral in the polymer matrix. Therefore, surface modification of clay minerals is the most important step to achieve polymer nanocomposites. The unique layered structure and high intercalation capabilities of clay minerals allow them to be chemically modified to be compatible with polymers which are essential for development of clay-based polymer nanocomposites The selection of clay for polymer nanocomposites depends upon on their targeted applications.
One of the promising applications of these nanocomposites is in the field of biomedical and biotechnological arenas. Clay-based polymer nanocomposites have been investigated in many biomedical and biotechnological applications such as tissue engineering, drug delivery, biosensors, and biomedical devices. However, some of the major challenges to consider while developing materials for biomedical relevance include long-term biocompatibility and biodegrability issues.
4.3 Bioapplications of Polymer Nanocomposites
4.3.1 Biomedical Applications of CNT Polymer Nanocomposites
Recently carbon nanostructures-reinforced polymer nanocomposites have increasingly drawn attention of researchers for their use in biotechnology and biomedical field. They have been exploited for variety of biomedical applications. Particularly CNT-based polymer composites are widely investigated [14Ser]. A significant amount literature is available on nanocomposite made from polymers and nanotubes as biomaterials.
4.3.1.1 Tissue Engineering
The use of CNTs/polymeric composites as scaffolds for bone engineering has recently become a subject of interest. The scaffolds used for tissue engineering serves as substrates for cell adhesion, proliferation, differentiation, extracellular matrix (ECM) formation, and to guide tissue regeneration. The ideal scaffold for tissue regeneration should possess sufficient mechanical properties. In this regard, carbon nanotubes have the potential in providing the needed structural reinforcement for tissue scaffold [10Arm]. The much anticipated use of CNTs in these scaffolds is mainly to improve their overall mechanical properties and to promote and guide bone tissue regeneration. By integrating a small fraction of carbon nanotubes into polymer matrix, significant improvements in the mechanical strength of the composite is observed [05Che]. Wang et al. have shown that MWCNTs blended with chitosan significantly improved the mechanical properties compared with those of chitosan [05Wan]. The biological properties of CNT scaffolds have been confirmed by in vitro studies. Several cells types have been successfully grown on carbon nanotubes-based polymer composites. Jell et al. have reported synthesis of porous thermoplastic polyurethane–multiwalled carbon nanotubes (CNTs) foams by thermally induced phase separation (TIPS) method [08Jel]. They have shown that CNT incorporation significantly improved the compression strength and stiffness of the nanocomposite scaffold. Through in vitro studies, they found that osteoblast production of the potent angiogenic factor VEGF (vascular endothelial growth factor) increased in proportion to CNT loading which verifies the potential influence of the nanocomposite scaffolds. Shi et al. fabricated porous nanocomposite scaffolds using a thermal-cross-linking and particulate-leaching technique) [07Shi]. Through in vitro cultures, they confirmed that mesenchymal stem cells (MSCs) adhere and proliferate on all the PPF-SWNT scaffolds. Sitharaman et al. [08Sit] tested the biocompatibility of porous PPF-SWNT scaffolds in a rabbit model. They found that implants made of PPF-SWNTs displayed only mild inflammatory responses. The PPF-SWNT nanocomposite scaffolds showed significant bone ingrowth after 12 weeks of implantation with increased collagen matrix production (Figs. 4.1 and 4.2).
One of the careful considerations while fabricating carbon nanotube scaffold is uniform dispersion of carbon nanotube in polymer matrix to achieve desired electrical conductivity and mechanical strength. The uniform distribution of CNT in polymer matrix transfers the load from the matrix to the nanotube which is essential for enhancing the mechanical and electrical properties of the composite. Different techniques have been explored to improve the CNT dispersion in different polymer matrix [10Arm]. Functionalization of CNT is also an effective route to increase the dispersing ability. Furthermore, the functionalization of CNTs increases water miscibility and biocompatibility of CNT. Covalent or noncovalent addition of various chemical functional groups can be done to the side walls and tips of CNTs to control the interaction between polymer and carbon nanotube. The nature of the functional group at the CNT surface plays a determinant role in the mechanism of interaction with cells. Lin et al. fabricated nanocomposites by incorporating carboxyl-functionalized multiwalled carbon nanotube (c-MWCNT) into poly(lactic-co-glycolic acid) (PLGA) matrix [11Lin]. Their observations revealed that c-MWCNTs gave a better dispersion than unmodified MWCNTs in the PLGA matrix with increased mechanical properties of the nanocomposites. They have shown that 7-week period in vitro degradation test accelerated the hydrolytic degradation of PLGA. In addition, the cells could adhere to and spread on films via cytoplasmic processes. Their results demonstrated that c-MWCNT-modified PLGA films were beneficial for promoting cell growth and inducing MSCs to differentiate into osteoblasts (Fig. 4.3).
Shi et al. fabricated nanocomposite scaffolds by incorporating single-walled carbon nanotubes (SWNTs) in poly(propylene fumarate) (PPF) matrix. They showed that the functionalization of single-walled carbon nanotubes (SWNTs) increases the interaction between nanotubes and polymer matrix which in turn enhances mechanical properties [06Shi].
The electrical conductivity of CNS-based nanocomposites is used to direct cell growth due to their ability to conduct electricity stimulus into the tissue healing process. Khang et al. used the electrical conductivity of CNTs to increase the cell density on the on CNTs–polycarbonate urethane nanocomposite surface via electrical stimulation [08Kha]. Supronowicz et al. have provided evidence that electrical stimulation delivered through nanocomposites of poly(lactic acid) and MWCNTs promotes osteoblast functions [02Sup].
For the preparation of 2D CNT films and fibers, the techniques involved are mainly layer-by-layer (LbL) deposition and solution–evaporation technique, [05Wan, 10Byr], whereas for the preparation of 3D CNT-based scaffolds the methods such as freeze-casting and electrospinning [14Ser] have been opted which generally produce scaffolds with controlled and interconnected porosity. Concerning nerve tissue repair, Kabiri et al. reported CNTs/PLLA fiber scaffolds as a as potential candidates for neural tissue regeneration [12Kab].
4.3.1.2 Other Bioapplications
Other biomedical and biotechnological applications of CNT-based polymer nanocomposite include drug delivery, wound healing development of chemiresistors [10Cho], dental composites [13Bor], and microcatheters [05End].
4.3.2 Graphene-Based Polymer Nanocomposites
Graphene has been exploited for diverse applications due to its unique properties. Of particular interest is a biological application of graphene which is well documented. Graphene oxide (GO) is more widely used as compared to graphene due to the presence of carboxylic, epoxy, and hydroxide groups, which provide opportunity for functionalization. GO has been functionalized with many biocompatible polymers for requisite biological applications. Graphene-based polymer composites have been investigated for various biomedical applications. They are anticipated to be promising materials to be used for tissue-engineered scaffolds, drug delivery vehicles, biosensors, etc.
4.3.2.1 Tissue Engineering
Due to enhanced mechanical and electrical properties, graphene-reinforced polymer composites hold immense potentials for tissue engineering scaffolds. The graphene-based polymer nanocomposites have been reported to be biocompatible and beneficial for the growth of the cells [11Par]. Sayyar et al. prepared graphene/polycaprolactone composites by covalently linking the polymer to the graphene chains which resulted in improved conductivity and mechanical properties. The growth of Fibroblast (L-929), neural (PC-12) and muscle (C2C12) cell lines on cPCl–CCG materials were assessed by comparing with growth of these cell types on pristine PCl [13Say]. They found that all cell lines proliferated on PCl, cPCl–CCG 0.5% and 5 % in a similar way to tissue culture plastic suggesting the potential applications of fabricated nanocomposite for tissue engineering (Fig. 4.4). In a similar approach GO–chitosan hydrogel scaffolds prepared by covalent linking chitosan with graphene exhibited better mechanical properties and lower degradation rate. In addition, there was significant improvement in cell adhesion, differentiation, proliferation, and calcium phosphate deposition of mouse preosteoblast MC3T3-E1 cells on the hydrogel [11Dep]. Zhang et al. reported that the tensile strength and compressive strength of PVA-based hydrogels were significantly enhanced by incorporating GO without affecting their cytocompatibility [11Zha].
In another study, graphene-reinforced chitosan films did not show any toxicity when tested on murine fibrosarcoma L929 cell culture. The mechanical properties were also enhanced [10Fan]. Recently, Shin et al. fabricated 3D composite scaffolds from gelatin methacrylate and GO [13Shi]. The incorporation of GO into hydrogels enhanced their mechanical and electrical properties without affecting encapsulated fibroblast cells. Lu et al. [12Lu] fabricated chitosan–PVA nanofibrous scaffolds containing graphene. Further, they explored wound healing property of composite with and without graphene along with control (no scaffold) and found that the samples containing graphene healed completely and at a faster rate in both mice and rabbit. The wound healing property of graphene composite was attributed to free electron in graphene which inhibits the prokaryotic cell multiplication. This was further confirmed by performing antibacterial study on E. coli.
4.3.2.2 Drug Delivery
In addition to tissue engineering, the graphene-based polymer composites were found to be potential candidates for other biomedical applications including drug delivery. They are exploited for loading poorly soluble drugs due to their high surface area, π–π stacking, and hydrophobic interactions of graphene.
A number of studies have been reported on applications of chitosan and graphene nanocomposite for loading various drugs like 5-fluorouracil [11Ran] and camptothecin (CPT) [11Bao]. Liu et al. successfully loaded doxorubicine (DOX), an anticancer drug, into graphene nanosheets (GS) using gelatin as a reducing and functionalizing agent [11Liu]. Gelatin–GS–DOX exhibited higher drug loading capacity due to large surface area and relatively higher π interactions. The Gelatin–GS–DOX complex also showed high toxicity towards MCF-7 cells through endocytosis. Sun et al. [08Sun] fabricated targeted delivery system by conjugating rituxan (CD20+ antibody) with polyethylene glycol–nanographene oxide (PEG–NGO). Loading of doxorubicin (DOX) onto PEG–NGO conjugate was favored due to the noncovalent π–π stacking and in vitro pH‐dependent drug release was studied.
Kim et al. developed stimuli-responsive nanocarrier for intracellular cytosolic delivery of DOX by functionalizing rGO with PEG and branched polyethylenimine (BPEI) [13Kim]. DOX was released in response to near infrared (NIR), acidic pH, and high intracellular levels of glutathione (GSH). In another study [12Dem], DOX-loaded PEG–GO nanocomposites were developed and released in response to GSH.
Miao et al. [13Mia] demonstrated successful codelivery of anticancer drug doxorubicin (DOX) and photosensitizer (Ce6) using polyethylene glycol-grafted graphene oxide (pGO). They revealed that pGO nanosheets increased the cellular uptake as well as tumor tissue accumulation of Ce6, compared to treatment with free drugs (Fig. 4.5).
In vivo biodistribution of pGO nanophysisorplexes. SCC7-bearing mice were systemically treated with pGO, free Ce6, Ce6/pGO, or with Ce6/Dox/pGO (Ce6 10 mg/kg and Dox 5 mg/kg). After 1 h (a), 24 h (b), and 48 h (c), the in vivo distributions of Ce6 fluorescence were visualized using a molecular imaging system. (d) Optical images are provided for location of tumors (Adapted from Miao 2013)
4.3.2.3 Gene Delivery and Bioimaging
Graphene-based polymer nanocomposites have also been explored for other biomedical and biotechnological applications such as gene delivery. For the purpose of gene delivery, Chen et al. functionalized graphene with cationic polymer polyethylenimine (PEI) [11Che]. PEI acts as a nonviral gene vector due to its strong electrostatic interactions with negatively charged phosphates of RNA and DNA. However, it shows low biocompatibility and high toxicity which limits its use. It was revealed that PEI–GO exhibited gene delivery high transfection efficiency and lower cytotoxicity compared to PEI alone. Kim et al. complexed PEG–BPEI–rGO with plasmid DNA for photothermally controlled gene delivery. The complex did not show any cytotoxicity to PC3 and NIH/3 T3 cells. The presence of rGO accelerated endosomal membrane disruption led to higher transfection efficacy when subjected to NIR irradiation [13Kim].
Graphene-based nanocomposites have also been fabricated for bioimaging purposes. Shen et al. [12She] prepared a multifunctional nanocomposite as MRI probe by combining PEG–functionalized GO complex and gadolinium–diethylenetriamine-pentaacetic acid–poly(diallyldimethylammonium)chloride (Gd–DTPA–PDDA). GO–IONP (superparamagnetic iron oxide nanoparticles) functionalized with PEG have been successfully used for drug delivery and bioimaging applications. [12Ma].
4.3.3 Clay-Based Nanocomposites
Silicate-based polymer nanocomposites have been anticipated as the next-generation materials for various biomedical applications due to the enhanced surface interactions of silicate nanoparticles and polymer chains [12Gah].
4.3.3.1 Tissue Engineering
Nanoclays (synthetic silicates) are widely used to reinforce polymer to improve physical and mechanical properties of polymeric matrix [10Wu] due to their anisotropic and high aspect ratio morphology. The physical and chemical properties of nanocomposite matrix can be controlled by addition of nanoclay to polymeric matrix. Nitya et al. have shown that the incorporation of halloysite nanoclay within the PCL scaffolds not only increased the mechanical strength but also the protein adsorption and cell adhesion of the nanocomposite [12Nit]. Their results indicated that the human mesenchymal stem cells (hMSCs) seeded on these scaffolds proliferated faster than in PCL scaffolds (Fig. 4.6).
Gaharwar et al. have they have shown that silicate nanoparticles (Laponite RD) can be used to effectively control the adhesion, spreading, and proliferation of fibroblast and preosteoblast cells on silicate cross-linked PEO surfaces [10Gah]. In a similar study, they have revealed through in vitro cell culture studies that increase in silicate concentration in silicate cross-linked poly(ethylene oxide) (PEO) nanocomposites enhanced the attachment and proliferation of human mesenchymal stem cells significantly [12Gah].
In a recent study, nanoclay has been shown to induce osteogenic differentiation in hMSCs without using any growth factors [13Gah]. Ambre et al. have shown that MMT clay modified with 5-aminovaleric acid increases interlayer spacing and improves biocompatibility with human osteoblasts. They used MMT clay modified with 5-aminovaleric acid for preparing chitosan/polygalacturonic acid (ChiPgA) composite scaffolds [10Amb] In a similar approach, in another study they have reported fabrication of biopolymer (ChiPgA) scaffolds and films by intercalated nanoclays containing organomodified MMT clay with HAP (in situ HAPclay) for bone tissue engineering. These HAPclay scaffolds were able to promote osteogenic differentiation of hMSCs [13Amb] (Fig. 4.7).
4.3.3.2 Drug Delivery
One of the biggest challenges in developing polymer-based drug delivery system is to control release of encapsulated or entrapped drugs. The therapeutic effects of the drugs and their biological activity can be optimized by controlling release kinetics of drugs. Silicate-based polymer nanocomposites show good barrier properties for diffusion of small molecules and thus can be applied for sustained drug release applications. Various drug delivery systems have been designed based on clay-based polymer nanocomposites for drug delivery-based applications.
Saha et al. [14Sah] fabricated film as well as nanofibrous web form of polyurethane/MMT clay nanocomposite as a delivery system for chlorhexidine acetate (CA), a bactericidal agent. Sustained release of drug was attributed to the presence of bulky and immobilized drug cation in the clay interlayer spacing which hinders the exchange of the cationic species present in the buffer media (Fig. 4.8).
Montmorillonite clay-based polyurethane nanocomposites have been explored as implantable drug delivery system. The therapeutics loaded nanocomposites were implanted in specific organs to control the release of a therapeutic agent to a specific target and to prevent different types of pathological processes [11Sil].
Lee and coworker [03Lee, 04Lee] fabricated clay-based poly (N-isopropylacrylamide) nanocomposite hydrogels and studied their drug release as well as swelling behaviors. It was concluded that clay in nanocomposites led to the decrease in swelling ratio and increase in strength of the nanocomposite hydrogels. In addition, they found that the release behavior of model drugs was largely depended on various factors including the content of clay and its intercalated agents.
Mishra et al. [14Mis] prepared nanocomposites of polyurethane by dispersing organically modified 2-D nanoclay followed by prepolymerization and subsequent chain extension using various chain extenders. These nanocomposites showed sustained release of drug as compared to the pristine PU. They have shown that by increasing the length of chain extender larger crystallites were formed which restricted drug diffusion due to barrier effect (Fig. 4.9).
Microstructure-controlled drug delivery. Crystallite size increases with the increase in aliphatic chain length of the chain extender. The formed with the chain extenders EG, BD, HD, and DD were designated as EG-PU, BD-PU, HD-PU, and DD-PU, respectively. BD-PU, which has a small crystallite size, shows prompt release, whereas DD-PU, which has larger crystallites, shows lower drug release. Moreover, in the case of DD-NC, the delayed diffusion of drug is controlled by crystallite size and 2D nanoclays as compared to that of BD-NC (Adapted from Mishra (2014))
A sustained release of dexamethasone drug was observed when organic modified silicate nanoparticles (Cloisite clay) were added to poly (ethylene-co-vinyl acetate) [56]. The drug release kinetics was suggested to be dependent on degree of dispersion as well as the aspect ratio of the silicate nanoparticles [03Cyp]. Li et al. have proposed Laponite nanoparticle-enriched alginate gels for the controlled delivery of cationic drugs [11Li] [14Mis].
4.3.3.3 Other Biomedical Applications
Clay-based polymer nanocomposites find diverse applications in biomedical field. For example, poly vinyl alcohol-clay-based polymer hydrogels have been exploited for wound dressing [07kok]. Bionanocomposites based on montmorillonite combined with HAP and chitosan have been evaluated as implants [08Kat]. Clay-based polymer composites have been also explored for food packaging applications [07Sor].
4.4 Concluding Remarks
There has been tremendous progress in synthesis of polymer-based nanocomposites due to the unique properties of nanostructured reinforcing materials. The synthesis and functionalization of nanofillers open new avenues for exploring their use in fabrication of polymer-based nanocomposites for bioapplications. These polymer nanocomposites have demonstrated improved properties significantly compared to the virgin polymer; thus, remarkable progress has been achieved in exploiting their use for emerging technologies such as drug/gene delivery and tissue engineering.
This chapter provides insights to multitude of bioapplications of polymer nanocomposites, thus expanding the range of applications from scaffolds for cell growth to biosensors. In spite of numerous efforts that have been taken to prepare polymer-based nanocomposites for bioapplications, there are still many challenges that need to be addressed to reach their full potential. While using these nanocomposites as biomaterial, the requisite criteria include biocompoatibilty and biodegradabilty and a host of other parameters which must be assessed properly with relevant preclinical studies to avoid false expectations. A careful selection of materials for developing nanocomposites and investigating their cellular level interactions will be instrumental for their biological applications.
References
Bacon, R.: Growth, Structure and Properties of Graphite Whiskers. J. App. Physiol. 31, 283 (1960)
Iijima, S.: Helical microtubules of graphitic carbon. Nature 354, 56 (1991)
Thess, A., Lee, R., Nikolaev, P., Dai, H.J., Petit, P., Robert, J., Xu, C., Lee, Y.H., Kim, S.G., Rinzler, A.G., Colbert, D.T., Scuseria, G.E., Tománek, D., Fischer, J.E., Smalley, R.E.: Crystalline Ropes of Metallic Carbon Nanotubes. Science 273, 483 (1996)
Treacy, M.M.J., Ebbesen, T.W., Gibson, J.M.: Exceptionally high Young’s modulus observed for individual carbon nanotubes. Nature 381, 67 (1996)
Journet, C., Maser, W.K., Bernier, P., Loiseau, A., Lamydela, C.M., Lefrant, S., Deniard, P., Lee, R., Fischer, J.E.: Large-scale production of single-walled carbon nanotubes by the electric-arc technique. Nature 388, 75699 (1997)
Cassell, A.M., Raymakers, J.A., Kong, J., Dai, H.J.: Large Scale CVD Synthesis of Single-Walled Carbon nanotubes Cassell. J. Phys. Chem. B 103, 6484 (1999)
Yu, M.F., Lourie, O., Dyer, M.J., Moloni, K., Kelly, T.F., Ruoff, R.S.: Strength and breaking mechanism of multiwalled carbon nanotubes under tensile load. Science 287, 637 (2000)
Thostenson, E.T., Ren, Z., Chou, T.W.: Advances in the science and technology of carbon nanotubes and their composites: a review. Compos. Sci. Technol. 61, 1899 (2001)
Cooper, C.A., Ravich, D., Lips, D., Mayer, J., Wagner, H.D.: Distribution and alignment of carbon nanotubes and nanofibrils in a polymer matrix. Compos. Sci. Technol. 62, 1105 (2002)
Supronowicz, P.R., Ajayan, P.M., Ullmann, K.R., Arulanandam, B.P., Metzger Bizios, D.W., Biomed, R.: Novel current-conducting composite substrates for exposing osteoblasts to alternating current stimulation. J. Mater. Res. 59, 499 (2002)
Cypes, S.H., Saltzman, W.M., Giannelis, E.P.: Organosilicate-polymer drug delivery systems: controlled release and enhanced mechanical properties. J. Control. Release 90, 163 (2003)
Lee, W.F., Fu, Y.T.: Effect of montmorillonite on the swelling behavior and drug-release behavior of nanocomposite hydrogels. J. Appl. Polym. Sci. 89, 3652 (2003)
Wang, J., Musameh, M., Lin, Y.H.: Solubilization of Carbon Nanotubes by Nafion toward the Preparation of Amperometric Biosensors. J. Am. Chem. Soc. 125, 2408 (2003)
Wong, M., Paramsothy, M., Xu, X., Ren, Y., Li, S., Liao, K.: Physical interactions at carbon nanotube-polymer interface. Polymer 44, 7757 (2003)
Lee, W.F., Lou, L.L.: Effect of the intercalation agent content of montmorillonite on the swelling behavior and drug release behavior of nanocomposite hydrogels. J. Appl. Polym. Sci. 94, 74 (2004)
Novoselov, K.S., Geim, A.K., Morozov, S.V., Jiang, D., Zhang, Y., Dubonos, S.V., Grigorieva, I.V., Firsov, A.A.: Electric Field Effect in Atomically Thin Carbon Films. Science 306, 666 (2004)
Chen, G.X., Kim, H.S., Park, B.H., Yoon, J.S.: Controlled Functionalization of Multiwalled Carbon Nanotubes with Various Molecular-Weight Poly(l-lactic acid). J. Phys. Chem. B 109, 22237 (2005)
Dundigalla, A., Lin-Gibson, S., Ferreiro, V., Malwitz, M.M., Schmidt, G.: Unusual Multilayered Structures in Poly(ethylene oxide)/Laponite Nanocomposite Films. Macromol. Rapid Commun. 26, 143 (2005)
Endo, M., Koyama, S., Matsuda, Y., Hayashi, T., Kim, Y.A.: Thrombogenicity and Blood Coagulation of a Microcatheter Prepared from Carbon Nanotube–Nylon-Based Composite. Nano Lett. 5, 101–105 (2005)
Wang, S.-F., Shen, L., Zhang, W.-D., Tong, Y.-J.: Preparation and Mechanical Properties of Chitosan/Carbon Nanotubes Composites. Biomacromolecules 6, 3067 (2005)
Shi, X., Hudson, J.L., Spicer, P.P., Tour, J.M., Krishnamoorti, R., Mikos, A.G.: Injectable Nanocomposites of Single-Walled Carbon Nanotubes and Biodegradable Polymers for Bone Tissue Engineering. Biomacromolecules 7, 2237 (2006)
Kokabi, M., Sirousazar, M., Hassan, Z.M.: PVA–clay nanocomposite hydrogels for wound dressing. Eur. Polym. J. 43, 773 (2007)
Liff, S.M., Kumar, N., McKinley, G.H.: High-performance elastomeric nanocomposites via solvent-exchange processing. Nat. Mater. 6, 76 (2007)
Shi, X.B., Sitharaman, Q.P., Pham, F., Liang, K., Wu, K., Billups, W.E., Wilson, L.J., Mikos, A.G.: Fabrication of porous ultra-short single-walled carbon nanotube nanocomposite scaffolds for bone tissue engineering. Biomaterials 28, 4078 (2007)
Sorrentino, A., Gorrasi, G., Vittoria, V.: Potential perspectives of bio-nanocomposites for food packaging applications. Trends Food Sci. Technol 18, 84 (2007)
Stankovich, S., Dikin, D.A., Piner, R.D., Kohlhaas, K.A., Kleinhammes, A.: Synthesis of graphene-based nanosheets via chemical reduction of exfoliated graphite oxide. Carbon 45, 1558 (2007)
Balandin, A.A., Ghosh, S., Bao, W., Calizo, I., Teweldebrhan, D., Miao, F., Lau, C.N.: Superior Thermal Conductivity of Single-Layer Graphene. Nano Lett. 8, 902 (2008)
Jell, G., Verdejo, R., Safinia, L., Shaffer, M.S.P., Stevens, M.M., Bismarck, A.: Carbon nanotube-enhanced polyurethane scaffolds fabricated by thermally induced phase separation. J. Mater. Chem. 18, 1865 (2008)
Katti, K.S., Katti, D.R., Dash, R.: Synthesis and characterization of a novel chitosan/montmorillonite/hydroxyapatite nanocomposite for bone tissue engineering. Biomed. Mater. 3, 034122 (2008)
Khang, D., Park, G.E., Webster, T.J.: Enhanced chondrocyte densities on carbon nanotube composites: The combined role of nanosurface roughness and electrical stimulation. J. Biomed. Mater. Res. A 86A, 253 (2008)
Lee, C., Wei, X.D., Kysar, J.W., Hone, J.: Measurement of the Elastic Properties and Intrinsic Strength of Monolayer Graphene. Science 321, 385 (2008)
Lee, S., Spencer, N.D.: Sweet, Hairy, Soft, and Slippery. Science 319, 575 (2008)
Sitharaman, B., Shi, X., Walboomers, X.F., Liao, H., Cuijpers, V., Wilson, L.J., Mikos, A.G., Jansen, J.A.: In vivo biocompatibility of ultra-short single-walled carbon nanotube/biodegradable polymer nanocomposites for bone tissue engineering. Bone 43, 362 (2008)
Paul, D.R., Robeson, L.M.: Polymer nanotechnology: nanocomposites. Polymer 49, 3187 (2008)
Sun, X., Liu, Z., Welsher, K., Robinson, J., Goodwin, A., Zaric, S., Dai, H.: Nano-graphene oxide for cellular imaging and drug delivery. Nano Res. 1, 203 (2008)
Vaia, R., Baur, J.: Adaptive Composites. Science 319, 420 (2008)
Mitragotri, S., Lahann, J.: Nat. Mater. 8, 15 (2009)
Xu, Y., Wang, Y., Jiajie, L., Huang, Y., Ma, Y., Wan, X.: A hybrid material of graphene and poly (3,4-ethyldioxythiophene) with high conductivity, flexibility, and transparency. Nano Res. 2, 343 (2009)
Ambre, A.H., Katti, K.S., Katti, D.R.: Nanoclay Based Composite Scaffolds for Bone Tissue Engineering Applications. J. Nanotechnol. Eng. Med. 1, 031013 (2010)
Armentano, I., Dottori, M., Fortunati, E., Mattioli, S., Kenny, J.M.: Biodegradable polymer matrix nanocomposites for tissue engineering: A review. Polym. Degrad. Stab. 95, 2126 (2010)
Byrne, M.T., Gun’ko, Y.K.: Recent advances in research on carbonnanotube–polymer composites. Adv. Mater. 22, 1672–8810 (2010)
Choi, J., Park, E.J., Park, D.W., Shim, S.E.: MWCNT–OH adsorbed electrospun nylon 6,6 nanofibers chemiresistor and their application in low molecular weight alcohol vapours sensing. Synth. Met. 160, 2664 (2010)
Dreyer, D.R.: From Conception to Realization: An Historial Account of Graphene and Some Perspectives for Its Future. Angew. Chem. Int. Ed. 49, 9336 (2010)
Fan, H., Wang, L., Zhao, K., Li, N., Shi, Z., Ge, Z., Jin, Z.: Fabrication, Mechanical Properties, and Biocompatibility of Graphene-Reinforced Chitosan Composites. Biomacromolecules 11, 2345–2351 (2010)
Gaharwar, A.K., Schexnailder, P., Kaul, V., Akkus, O., Zakharov, D., Seifert, S.: Adv. Funct. Mater. 20, 429 (2010)
Kiliaris, P., Papaspyrides, C.D.: Polymer/layered silicate (clay) nanocomposites: An overview of flame retardancy. Prog. Polym. Sci. 35, 902 (2010)
Satarkar, N.S., Biswal, D., Hilt, J.Z.: Hydrogel nanocomposites: a review of applications as remote controlled biomaterials. Soft Matter 6, 2364 (2010)
Spitalsky, Z., Tasis, D., Papagelis, K., Galiotis, C.: Carbon nanotube–polymer composites: Chemistry, processing, mechanical and electrical properties. Prog. Polym. Sci. 35(35), 357 (2010)
Wu, C.-J., Gaharwar, A.K., Schexnailder, P.J., Schmidt, G.: Development of Biomedical Polymer-Silicate Nanocomposites: A Materials Science Perspective. Materials 3, 2986.1013 (2010)
Bao, H., Pan, Y., Ping, Y., Sahoo, N.G., Wu, T., Li, L., Li, J., Gan, L.H.: Chitosan-Functionalized Graphene Oxide as a Nanocarrier for Drug and Gene Delivery. Small 7, 1569 (2011)
Chen, B., Liu, M., Zhang, L., Huang, J., Yao, J., Zhang, Z.: Polyethylenimine-functionalized graphene oxide as an efficient gene delivery vector. J. Mater. Chem. 21, 7736 (2011)
Depan, D., Girase, B., Shah, J.S., Misra, R.D.K.: Structure–process–property relationship of the polar graphene oxide-mediated cellular response and stimulated growth of osteoblasts on hybrid chitosan network structure nanocomposite scaffolds. Acta Biomater. 7, 3432 (2011)
Gaharwar, A.K., Dammu, S.A., Canter, J.M., Wu, C.-J., Schmidt, G.: Highly Extensible, Tough, and Elastomeric Nanocomposite Hydrogels from Poly(ethylene glycol) and Hydroxyapatite Nanoparticles. Biomacromolecules 12, 1641 (2011)
Li, Y., Maciel, D., Tomas, H., Rodrigues, J., Ma, H., Shi, X.: pH sensitive Laponite/alginate hybrid hydrogels: swelling behaviour and release mechanism. Soft Matter 7, 6231 (2011)
Lin, C., Wang, Y., Lai, Y., Yang, W., Jiao, F., Zhang, H., Ye, S., Zhang, Q.: Incorporation of carboxylation multiwalled carbon nanotubes into biodegradable poly(lactic-co-glycolic acid) for bone tissue engineering. Colloids Surf. B Biointerfaces 83, 367 (2011)
Liu, K., Zhang, J.-J., Cheng, F.-F., Zheng, T.-T., Wang, C., Zhu, J.-J.: Green and facile synthesis of highly biocompatible graphene nanosheets and its application for cellular imaging and drug delivery. J. Mater. Chem. 21, 12034 (2011)
Park, S.Y., Park, J., Sim, S.H., Sung, M.G., Kim, K.S., Hong, B.H.: Enhanced Differentiation of Human Neural Stem Cells into Neurons on Graphene. Mater 23, 23 (2011)
Rana, V.K., Choi, M.-C., Kong, J.-Y., Kim, G.Y., Kim, M.J., Kim, S.-H., Mishra, S., Singh, R.P., Ha, C.-S.: Synthesis and Drug-Delivery Behavior of Chitosan-Functionalized Graphene Oxide Hybrid Nanosheet. Macromol. Mater. Eng. 296, 131 (2011)
Silva, G.R., Da, A., Da, S.C., Behar-Cohen, F., Ayres, E., Or’efice, R.L.: Biodegradable polyurethane nanocomposites containing dexamethasone for ocular route. Mat. Sci. and Eng. C.: 31, 414 (2011)
Zhang, L., Wang, Z., Xu, C., Li, Y., Gao, J., Wang, W., Liu, Y.: High strength graphene oxide/polyvinyl alcohol composite hydrogels. J. Mater. Chem. 21, 10399 (2011)
Dembereldorj, U., Kim, M., Kim, S., Ganbold, E.-O., Lee, S.Y., Joo, S.-W.: A spatiotemporal anticancer drug release platform of PEGylated graphene oxide triggered by glutathione in vitro and in vivo. J. Mater. Chem. 22, 23845 (2012)
Gaharwar, A.K., Kishore, V., Rivera, C., Bullock, W., Wu, C., Akkus, O., Schmidt, G.: Physically Crosslinked Nanocomposites from Silicate-Crosslinked PEO: Mechanical Properties and Osteogenic Differentiation of Human Mesenchymal Stem Cells, Gaharwar. Macromol. Biosci. 12, 779 (2012)
Kabiri, M., Soleimani, M., Shabani, I., Futrega, K., Ghaemi, N., Ahvaz, H.H., Elahi, E., Doran, M.R.: Neural differentiation of mouse embryonic stem cells on conductive nanofiber scaffolds. Biotechnol. Biotechnol. Lett. 34, 1357 (2012)
Lu, B., Li, T., Zhao, H., Li, X., Gao, C., Zhang, S., Xie, E.: Graphene-based composite materials beneficial to wound healing. Nanoscale 4, 2978 (2012)
Ma, X., Tao, H., Yang, K., Feng, L., Cheng, L., Shi, X., Li, Y., Guo, L., Liu, Z.: A functionalized graphene oxide-iron oxide nanocomposite for magnetically targeted drug delivery, photothermal therapy, and magnetic resonance imaging. Nano Res. 5, 199 (2012)
Macossay, J., Ybarra, A.V.R., Arjamend, F.A., Cantu, T., Eubanks, T.M., Chipara, M., López-Cuéllar, E., Mohamed-Noriega, N.: Electrospun Polystyrene-Multiwalled Carbon Nanotubes: Imaging, Thermal and Spectroscopic Characterization. Des. Monomers Polym. 15, 197 (2012)
Nitya, G., Nair, G.T., Mony, U., Chennazhi, K.P., and Nair, S.V. : J Mater Sci: Mater Med 23, 1749 (2012)
Ray, S.S.: Polylactide-Based Bionanocomposites: A Promising Class of Hybrid Materials. Acc. Chem. Res. 45, 1710 (2012)
Shen, A.-J., Li, D.-L., Cai, X.-J., Dong, C.-Y., Dong, H.-Q., Wen, H.-Y., Dai, G.-H., Wang, P.-J., Li, Y.-Y.: Multifunctional nanocomposite based on graphene oxide for in vitro hepatocarcinoma diagnosis and treatment. J. Biomed. Mater. Res. A 100, 2499 (2012)
Wang, P., Ma, J., Wang, Z., Shi, F., Liu, Q.: Enhanced Separation Performance of PVDF/PVP-g-MMT Nanocomposite Ultrafiltration Membrane Based on the NVP-Grafted Polymerization Modification of Montmorillonite (MMT). Langmuir 28, 4776 (2012)
Ambre, A.H., Katti, D.R., Katti, K.S.: Nanoclays Mediate Stem Cell Differentiation and Mineralized ECM Formation on Biopolymer Scaffolds. J. Biomed. Mater. Res. Part A 101, 2826 (2013)
Borges, A.L.S., Souza, A.C., Paes, Jr, T.J.A., Yoshida, T., Buttino, M.C.: Multiwalled carbon nanotube/nylon-6 nanofiber-reinforced dental composite. Dent. Mater. 29 e34. (2013)
Gaharwar, A.K., Mihaila, S.M., Swami, A., Patel, A., Sant, S., Reis, R.L.: Bioactive Silicate Nanoplatelets for Osteogenic Differentiation of Human Mesenchymal Stem Cells. Adv. Mater 25, 3329 (2013)
Kim, H., Lee, D., Kim, J., Kim, T.I., Kim, W.J.: Photothermally Triggered Cytosolic Drug Delivery via Endosome Disruption Using a Functionalized Reduced Graphene Oxide. ACS Nano 7, 6735 (2013)
Kim, H., Kim, W.J.: Photothermally Controlled Gene Delivery by Reduced Graphene Oxide–polyethylenimine Nanocomposite. Small 10, 117 (2014)
Miao, W., Shim G., Lee, S., Lee S., Choe Y. S., Oh, Y. K .: Biomaterials 34, 3402 (2013)
Sayyar, S., Murray, E., Thompson, B.C., Gambhir, S., Officer, D.L., Wallace, G.G.: Covalently linked biocompatible graphene/polycaprolactone composites for tissue engineering. Carbon 52, 296 (2013)
Shin, S.R., Aghaei-Ghareh-Bolagh, B., Dang, T.T., Topkaya, S.N., Gao, X., Yang, S.Y., Jung, S.M., Oh, J.H., Dokmeci, M.R., Tang, X., Khademhosseini, A.: Cell-laden Microengineered and Mechanically Tunable Hybrid Hydrogels of Gelatin and Graphene Oxide. Adv. Mater. 25, 6385 (2013)
Goenka, S., Sant, V., Shilpa, S.: Graphene-based nanomaterials for drug delivery and tissue engineering. J. Control. Release 173, 75 (2014)
Mishra, A., Singh, S.K., Dash, D., Aswal, V.K., Maiti, B., Misra, M., Maiti, P.: Self-assembled aliphatic chain extended polyurethane nanobiohybrids: Emerging hemocompatible biomaterials for sustained drug delivery. Acta Biomater. 10, 2133 (2014)
Saha, K., Butola, B.S., Joshi, M.: Drug release behavior of polyurethane/clay nanocomposite: Film vs. nanofibrous web. J. Appl. Polym. Sci. 131, 40824 (2014)
Serrano, M.C., Gutiérrez, M.C.: Role of polymers in the design of 3D carbon nanotube-based scaffolds for biomedical applications. Francisco del Monte. Prog. Polym. Sci. 39, 1448 (2014)
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer-Verlag GmbH Germany
About this chapter
Cite this chapter
Tripathy, J. (2017). Polymer Nanocomposites for Biomedical and Biotechnology Applications. In: Tripathy, D., Sahoo, B. (eds) Properties and Applications of Polymer Nanocomposites. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-662-53517-2_4
Download citation
DOI: https://doi.org/10.1007/978-3-662-53517-2_4
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-662-53515-8
Online ISBN: 978-3-662-53517-2
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)