Abstract
Transdermal delivery of systemic therapeutics is an attractive alternative to conventional needle-based methods. Several physical and chemical strategies for skin permeabilization have been proposed to facilitate the transport of drug molecules across the skin barrier. Validation of a particular method and demonstration of its efficacy require the selection of a representative skin model as well as an appropriate system to measure drug transport. This review summarizes different skin models used to study drug transport across the skin as well as measurement techniques used to quantify the amount of transport. A careful consideration of both choices in development of transdermal delivery systems is critical to success.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Commonly employed delivery systems include injections, pills, and to some extent topical and mucosal formulations. Oral delivery is by far the easiest and most convenient way of delivering drugs especially when repeated or routine administration is required (Chen and Langer 1998). This advantage, however, is offset for protein and peptide-based drugs sensitive to enzymatic degradation in the gastrointestinal tract. Drugs based on proteins and peptides now form a significant fraction of the therapeutic spectrum, primarily due to accelerated advances in understanding protein chemistry and drug interactions. Thus, while the bygone drug delivery systems have been dominated by the oral route, the next millennia of health care will demand more accommodating delivery systems for sensitive drug classes.
Injections comprise the next most commonly used method for administering therapeutics into humans. The World Health Organization (WHO) estimates that 12 billion injections are given annually (Kermode 2004). Despite the common use, needle-based drug administration has several limitations. Needle phobia is a significant issue in adults and children alike (Nir et al. 2003) and makes drug administration stressful (Breau et al. 2001). Accidental needle sticks also add to the limitations of needle use in developed and developing countries alike (Kane et al. 1999; Miller and Pisani 1999). Further, hepatic metabolism results in rapid clearance of active drug from the blood plasma making repeated administration inevitable. This only aggravates the problem of needle pain especially for patients requiring multiple administrations on a daily basis.
It is thus sufficiently obvious that as we move toward the next era of health care, compliant, noninvasive, and sustained delivery will become the key features desirable of any drug delivery system. Several advances to this effect have been made in the last two to three decades and novel drug delivery systems have been brought to the forefront (Drachman 1989; Vanbrunt 1989; Langer 1990). A large contribution to these novel systems appeared as modifications to the active drug or formulation excipients to modulate drug pharmacokinetics, safety, efficacy, and metabolism. A more radical approach has been to explore newer interfaces on the body for introducing therapeutics. One such approach, transdermal drug delivery, makes use of human skin as a port of entry for systemic delivery of drug molecules (Guy 1996; Prausnitz 1997; Barry 2001a, b; Pillai et al. 2001; Prausnitz et al. 2004; Thomas and Finnin 2004).
1.1 Transdermal Drug Delivery
Transdermal drug delivery (TDD) offers an advantageous mode of drug administration by eliminating first-pass hepatic metabolism and providing sustained drug release for a prolonged period of time. It is painless compared to needles and therefore offers superior patient compatibility. However, skin is the first line of defense of an organism and the last barrier separating the organism from its hostile environment of viruses, pathogens, and toxics. Evolved to impede the flux of exogenous molecules into the body, the skin naturally offers a very low permeability to the movement of foreign molecules across it. A unique hierarchical structure of lipid-rich matrix with embedded keratinocytes in the upper strata (15 μm) of skin, stratum corneum (SC), is responsible for this barrier (Bouwstra 1997). In addition to its role as a barrier, both physical and biological, skin performs a complimentary role, which is that of a transport regulator. Skin routinely regulates the flux of water molecules into and out of the body. It also permits the influx of a variety of small molecules that are fairly lipophilic (partition coefficient, log P >1.5) and have molecular weight (MW) less than 500 Da (Bos and Meinardi 2000). As a result there has been a natural bias of transdermal delivery systems to cash in on therapeutics that meet these requirements. Drug molecules currently administered via the transdermal route fall within a narrow range of MW and lipophilicity. They are typically characterized by high log P (>1.5) and low MW (<500 Da), thereby taking advantage of the natural selectivity of skin membrane. A large fraction of drug molecules lie outside these bounds. These are mostly peptide- and protein-based drugs that will become the key therapeutics in the future. The biggest challenge in transdermal drug delivery today is to open the skin safely and reversibly to these high molecular weight hydrophilic drugs.
Several technological advances have been made in the past couple of decades to overcome this challenge. These advances can be broadly divided into two categories: (1) physical approaches including but not limited to iontophoresis (Panchagnula et al. 2000; Delgado-Charro and Guy 2001), sonophoresis (Mitragotri and Kost 2004; Ogura et al. 2008), microneedles (McAllister et al. 2000; Prausnitz 2004; Sivamani et al. 2007), and electroporation (Pliquett 1999; Denet et al. 2004) that use some form of physical energy to modulate the SC ultrastructure, and (2) chemical approaches that employ chemical formulations to modulate skin transport barrier (Sinha and Kaur 2000; Williams and Barry 2004). Each of these methods has its individual benefits and limitations.
1.2 Scope of Review
The early 1990s of the last century brought the first transdermal patch to the market. The market for patch-based therapeutics has since grown to $4 billion per annum worldwide, a small but significant proportion of the total revenues from pharmaceuticals (Barry 2001a, b). After more than a quarter of a century since the introduction of the first patch, the number of marketed transdermal patches has not exceeded beyond a couple of dozen. Another four dozen are in the developmental phases. Although somewhat satisfactory on the face value, these numbers are misleading since a huge fraction is made up of generic replicas of similar drugs. Only 11 independent drugs make up these 80 odd products, almost all exclusively below 500 Da and characteristically lipophilic in nature. Most efforts to push the envelope on molecular weight have shown limited success. Only one physical method (i.e., iontophoresis) has successfully entered the market share of transdermal delivery technologies but it is being used to deliver a low molecular weight drug, lidocaine (235 Da). On the other hand, laurocapram (Azone®), the most widely studied chemical permeation enhancer with high expectations, has failed to gain clinical acceptance in transdermal delivery due to its skin irritation (Okamoto et al. 1988; Lashmar et al. 1989; Wong et al. 1989). The landscape of transdermal delivery opportunities seems grim and one cannot help but ask, “Is transdermal drug delivery research still important today?” (Barry 2001a, b). The concept of transdermal drug delivery is rooted in strong scientific acumen even though the practical realization of it has been less fascinating than expected. A sound engineering approach coupled with fundamental understanding of a complex biological tissue is required. Equally important is adoption of the right platform of models, methods, and measurement techniques to evaluate new and traditional transdermal delivery strategies in light of the knowledge gained in the last five decades in this field of research. The number of original publications with “transdermal delivery” in the title has well approached 1000 with the numbers rising rapidly. These numbers form one metric to indicate that new scientists continue to be attracted to this field. This review aims to provide a synopsis of the “nuts and bolts” in transdermal drug delivery research to a new scientist. A short introduction on skin structure and constituents is followed by a description of different model systems and methods employed in this area.
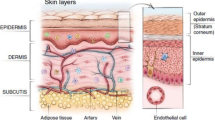
2 Structure of Skin
Elucidation of skin structure, especially in relation to its barrier function, has drawn countless researchers since the early 1950s (Blank 1952; Breathnach et al. 1973; Elias and Friend 1975). The human skin is a sandwich of two layers: a thin layer of epidermis stacked upon a much thicker substrate, the dermis. The dermis is highly vascularized and permeable, consisting predominantly of a fibrous collagen meshwork that is sparsely populated with cells. It houses sweat glands, sebaceous glands, hair follicles, and a network of capillaries supported by the connective tissue. The dermis provides most of the bulk and toughness of the skin. The epidermis is devoid of blood vessels, receiving all of its nutrients and disposing of its waste products by diffusional exchange with the dermis. It is maintained by continuous cell division in the germinative basal layer. Differentiating daughter cells, the keratinocytes move outward toward the surface of skin. During this process, there is a change in the morphology and composition of keratinocytes. Ultimately, the keratinocytes undergo terminal differentiation, forming dead, flattened corneocytes; 20–30 layers of corneocytes embedded in a matrix of lipid, extruded from the cells immediately before cornification, form the SC. Corneocytes continually exfoliate from the SC to maintain a constant thickness of this layer at ~20–30 μm (Elias and Friend 1975; Odland 1983; Matoltsy 1986; Downing 1992). In the last 60 years of close scrutiny of the skin structure, the SC has received by far the most attention. And not surprisingly, since this superficial layer is where the barrier property of skin resides. The seminal work of Scheuplein et al. conclusively summarized the locus and origin of the molecular impermeability of skin and established it to be a passive rather than biologically active property (Scheuplein 1965, 1966, 1967, 1972, 1978; Blank and Scheuplein 1973). Through their studies of the permeability of excised human skin in vitro to a large number of permeants, they were able to show conclusively that the principal barrier to permeation is provided by the SC. Separating the epidermis from the underlying dermis by heat stripping followed by enzymatic removal of the live epidermal layer, Scheuplein et al. measured the permeability of the residual SC and dermis independently. These measurements indicated that the SC is at least three, and frequently as much as five, orders of magnitude less permeable to most substances than the dermis. Moreover, the permeability of the entire epidermis was found to be indistinguishable from that of the SC alone. This prompted Scheuplein to model the skin as a three-layer laminate of SC, epidermis, and dermis, with permeation occurring by Fickian diffusion of the penetrating species through the three layers in series. Since the dominant resistance to permeation of most compounds is offered by the SC, the gradient in penetrant concentration across the entire skin is, for all practical purposes, localized within the SC.
2.1 Ultra-structure of Stratum Corneum
Several models have been proposed for the structure of the SC. These include the classic “brick and mortar” model of Michaels, the “domain mosaic” model of Forslind, the “single gel phase” model of Norlen, “molecular lipid lamellae” models of Swartzendruber and Fenske, and “membrane folding” model of Norlen (Michaels et al. 1975; Swartzendruber et al. 1989; Fenske et al. 1994; Forslind 1994; Kitson et al. 1994; Engström et al. 1995; Menon and Elias 1997; Bouwstra et al. 1998; Menon et al. 1998; Norlen 2001; Norlén 2001). A comprehensive all encompassing model seems to be elusive as newer observations continually require modulating the physical picture of this membrane (Wertz et al. 1987; Norlén et al. 1998). While newer models are being proposed to accommodate minor nuances, the coarse macroscopic–microscopic structure is well agreed upon. The most simplistic model in this respect is still the “brick and mortar” model of Michaels et al.; the term itself coined by Elias (Elias and Friend 1975; Michaels et al. 1975). This model treats the skin barrier as a simplified two-compartment system with discontinuous protein pockets embedded in a continuous, homogeneous lipid matrix. Proteins held within corneocyte lipid envelopes thus form the bricks held by the mortar of a continuous lipid phase in the “brick and mortar” model. The bricks occupy, by far, the larger volume of this assembly. Early solvent extraction experiments indicated that lipids, especially polar lipids, play a critical role in the barrier (Matoltsy et al. 1968; Sweeney and Downing 1970). The freeze fracture studies of the SC established conclusively that lipids form multiple broad bilayers filling the corneocyte intercellular spaces (Breathnach et al. 1973). These bilayers, shown to exist throughout the SC, provide the barrier to water permeability as determined by Elias and Squier through freeze-fracture, thin-section, and tracer studies (Squier 1973; Elias et al. 1977; Madison et al. 1987; Wertz et al. 1987).
A general observation of mammalian cells indicates that their membranes do not provide a formidable barrier to water or water-soluble molecules. These membranes are typically composed of phosphoglycerides, sphingomyelin, and cholesterol where the lipid fatty acyl chains extend to 16 through 20 carbons with a varied degree of unsaturation (Fettiplace and Haydon 1980). Occasionally, methyl branching is also observed on the interior of the fatty acyl chains. This methyl branching coupled with unsaturation in the interior of the chains inhibits formation of a highly ordered membrane. The membrane is disordered or fluid with high permeability to small hydrophilic solutes and water. Interesting to note is the lack of any phospholipids or the usual fatty acyl chain structures in the SC (Yardley and Summerly 1981; Yardley 1983; Wertz 1986). Instead, the SC bilayers are made of cholesterol, fatty acids, and ceramides (Wertz et al. 1987; Hedberg et al. 1988). The molecular structure and composition of these constituents play an important role in defining the barrier properties of the bilayers and in turn of the SC membrane.
Subsequent sections provide a brief description of the corneocyte proteins and the lipids of the SC.
2.2 Lipids of Stratum Corneum
2.2.1 Ceramides
Ceramides (1–6) constitute ~42 % of the material in the bilayers followed by cholesterol (~40 % along with cholesteryl sulfate and cholesteryl esters) and fatty acids (~13 %) (Wertz and Downing 1983a, b; Abraham et al. 1985; Long et al. 1985; Wertz et al. 1985). The ceramides include both sphingosines (ceramides 1, 2, 4, and 5) and phytosphingosines (ceramides 3 and 6). Also, the amide-linked fatty acids include nonhydroxy acids (ceramides 2 and 3), α-hydroxy acids (ceramides 4, 5, and 6), and ω-hydroxy acids (ceramide 1). In addition, ester-linked fatty acids (ceramide 1) are also present in the epidermal ceramides. The ceramides are straight and saturated with the exception of ceramide 1. Also the unsaturation is placed exclusively at the polar end of these ceramide chains thus providing little room for formation of kinks. This architecture is well poised to provide a highly ordered structure to the membrane formed from these ceramides. In addition, there is a considerable chain length variation in the ceramides, i.e., from 15 to 48. This provides room for interdigitation of the hydrocarbon chains, an interaction highly favorable during bilayer formation. Also, the lipids are characteristically amphiphilic in nature capable of extensive hydrogen bonding, once again a primo in formation of self-assembled lamellae.
2.2.2 Cholesterol
Free cholesterol is the second most abundant lipid in the SC, amounting to 25 % of extractable lipid. In addition, 15 % of the SC lipids are made of cholesteryl sulfate and cholesteryl esters. Cholesterol plays a key role in providing barrier property of the SC. This was shown conclusively by Feingold et al. in 1990 based on their observation that barrier recovery was severely inhibited in skin treated with an enzyme that inhibits cutaneous cholesterol synthesis (Feingold et al. 1990). Later Takahashi et al. showed that cholesterol at high concentrations (>30 % molar basis) promotes lamellar structures, regarded generally to provide superior barrier properties (Takahashi et al. 1996). Cholesterol also increases the chain mobility of lipids in the gel state making them more pliable and thus, potentially, more resistant to mechanical stresses (de Kruyff et al. 1974). In addition, cholesterol broadens phase transition regions or in some cases may entirely abolish subtransitions between gel phases thereby stabilizing them (McMullen and McElhaney 1995; Takahashi et al. 1996).
2.2.3 Fatty Acids
Fatty acids make up ~13 % of the SC lipids. The origin of these fatty acids is not completely understood, although it is believed that some of them are a result of hydrolysis of ceramides. The composition of the mixture of fatty acids is unusual in consisting predominantly of very long chain (20–28 carbons) saturated acids, with only 6 % of monounsaturated and 1 % of diunsaturated acids (Wertz et al. 1987; Downing 1992). Presence of fatty acids along with cholesterol and ceramides is essential to the barrier property of the SC. In addition to providing structural integrity to the SC, free fatty acids are also responsible for providing a low pH or acidic surface (Blank 1939; Draize 1942; Beare et al. 1958; Baden and Pathak 1967; Qiang et al. 1993). This may be critical to the antimicrobial activity of the SC thereby making it a physical as well as physiological barrier (Fluhr et al. 2001).
2.3 Proteins of Stratum Corneum
Protein pockets, the bricks in the “brick and mortar” model, form the second important component of the SC. These pockets are included in flat, hexagonal, and physiologically dead corneocytes. The SC proteins are typically composed of keratin. Keratins are a family of α-helical polypeptides ranging from 40 to 70 kDa in size (Green et al. 1982; Wertz and Downing 1989). They are relatively poor in cysteine, rich in serine and glycine, and contain N-acetylserine at the amino terminus (Steinert and Cantieri 1983). Keratins accumulate throughout epidermal differentiation and represent the major component of the SC as well as of epidermal appendages such as hair and nails (Baden et al. 1973). Earlier in epidermal differentiation, low molecular weight keratins predominate, whereas higher molecular weight polypeptides are found in the SC (Skerrow and Hunter 1978). Individual keratin molecules aggregate to form superhelices, the detailed structures of which are still under investigation (Steinert and Cantieri 1983). They are stabilized by disulfide bridges that can be solubilized only by reducing agents. The keratin in the SC is probably responsible for maintaining the hexagonal shapes of the corneocytes and may contribute to the toughness and flexibility of the SC (Wertz and Downing 1989).
The corneocyte envelope enclosing the keratin filaments is made of two layers. The inner portion of the envelope consists of cross linked proteins, predominantly involucrin and at least six other soluble and membrane-associated proteins (Rice and Green 1977; Watt and Green 1981; Simon and Green 1984). The outer portion is made of ester-linked ω-hydroxyacylsphingosines. These hydroxyceramide molecules contain mainly 30–34 carbon ω-hydroxyacyl chains and represent 2 % of the dry weight of the SC (Wertz and Downing 1989). At least 50 % of the hydroxyceramides are linked to the protein envelope through the ω-hydroxy terminus. This helps the sphingosine moieties to interdigitate with the lipid lamellae (Wertz and Downing 1987). This may explain why unlike other membranous structures the lipid envelope persists even after extensive extraction with methanol–chloroform mixture (Swartzendruber et al. 1987; Wertz and Downing 1987). The lipid envelope hydroxyceramides anchor the corneocytes to the intercellular lipids. As a result, even when all the intercellular lipids are extracted the covalently bound hydroxyceramides can interdigitate in a zip-like manner to close the intercellular space and thus maintain the integrity of the SC.
3 Routes of Permeation
There are three major routes of permeation for passive diffusion of a molecule across the SC. These include: (a) diffusion through appendages such as sweat ducts, sebaceous glands, and hair follicles; (b) diffusion through the corneocytes of the SC; and (c) diffusion through the lipids of the SC. Diffusion across the corneocytes and lipids of the intact SC comprises the predominant route through which most molecules penetrate. The appendageal area available for diffusion is significantly lower, ~0.1 %, but has received considerable attention as an important permeation pathway for ions or large polar molecules that have slow permeation across the SC (Barry 2001a, b).
3.1 Skin Appendages
The involvement of skin appendages in transcutaneous permeation has received considerable attention over six decades. Early studies by many investigators implicated skin appendages as important avenues for penetration of topically applied chemicals (Mackee et al. 1945; Shelley and Melton 1949; Fredriksson 1961; Tregear 1961; Vankooten and Mali 1966; Wahlberg 1968; Rutherford and Black 1969; Wallace and Barnett 1978). Using full-thickness mouse skin maintained as short-term organ cultures in an in vitro experimental system, Kao et al. demonstrated that permeation of topically applied benzo[a]pyrene was higher in haired mice skin compared to hairless mice skin (Kao et al. 1988). Histochemical techniques, autoradiographic techniques, and fluorescence microscopy have been used to visualize and quantitate appendageal absorption. These studies revealed that topically applied agents concentrated and persisted in the hair follicles and sebaceous glands (Grasso and Lansdown 1972; Foreman et al. 1979; Holland et al. 1984).
Of all the appendageal routes, the hair follicle has received the most attention as a prominent route of permeation. It also serves as an important cutaneous reservoir for topically applied molecules (Lademann et al. 2006). Hueber et al. and Tenjarla et al. showed that the penetration of corticosteroids is considerably lower in hairless skin compared to haired skin (Hueber et al. 1994; Tenjarla et al. 1999). Hydrocortisone permeability increased in tissue engineered skin on insertion of hair follicles (Michel et al. 1999). Permeation enhancers that specifically target hair follicles have been investigated with great success. Of these, liposomes have been shown to deliver DNA (Li et al. 1993), plasmids (Domashenko et al. 2000), monoclonal antibodies (Balsari et al. 1994), calcein (Lieb et al. 1992), and melanin (Li and Hoffman 1997) to hair follicles. Lee et al. reported that the auxiliary SC associated with the sweat glands has a reduced barrier function (Lee et al. 2001). Following up with elegant immunostaining studies, Wilke et al. proposed that the active permeation barriers in sweat ducts in the epidermis and dermis are functionally and morphologically distinct (Wilke et al. 2005, 2006). The innermost layer of the intra-epidermal duct is completely keratinized (Zelickson 1961; Hashimot et al. 1965). In contrast, the dermal ducts lack the presence of cornified corneocytes but contain luminal tight junctions, which seem to be absent from the epidermal duct lining (Hashimot 1971a, b). Similar to the dermal ducts, the secretory coils of the sweat glands themselves lack cornified layers but are rich in tight junctions as evidenced by the colocalization of occludin and claudin-4 (Hashimot 1971a, b). In light of these observations, Wilke et al. propose that dermal sweat ducts and the sweat glands could serve as potential permeation routes (Wilke et al. 2006). Only a few experimental studies have actually been dedicated to evaluating the contribution of sweat glands and ducts to transcutaneous permeation (Vankoote and Mali 1966).
3.2 Intracellular Route
Certain permeation enhancers can open up the dense keratin structure in corneocytes creating porous pathways for diffusion across them. For example, decylmethyl sulfoxide interacts with keratin and is hypothesized to enhance permeability by opening up aqueous channels within the corneocyte (Cooper 1982). Dimethyl sulfoxide can induce reversible changes in protein structures of isolated corneocytes (Mendelsohn et al. 2006). Hexamethyl sulfoxide and dimethyl sulfoxide convert α-helical keratins in the corneocyte to β-sheets (Oertel 1977). Lee et al. have demonstrated the capability of thioglycolates in depilatory creams in disrupting intracellular keratin matrix and the protein envelope using multiphoton microscopy (Lee et al. 2008). He et al. have shown that N-trimethyl chitosan is capable of increasing transcutaneous permeation by affecting secondary structure of keratins within the corneocytes (He et al. 2008). Azone® can act on the keratin fibers of the corneocytes converting their rigid α-helical conformation to a flexible β-sheet confirmation (Xueqin et al. 2005). Lauric acid enhances the permeability of verapamil by interacting with skin proteins (Shah et al. 1992). Dithiothreitol enhances flux of sucrose and mannitol across the SC exclusively through interactions with corneocyte keratin matrix (Goates and Knutson 1993). Oleic acid and isopropyl myristate increase the permeability of the corneocytes for polar substances after pretreatment of the skin (Eder and Müller-Goymann 1995).
3.3 Intercellular Route
Several chemicals can alter or disrupt the organization of lipid molecules in the SC bilayers thereby facilitating the diffusion of molecules across the SC. Barry postulated different ways in which permeation enhancers can modify SC lipids (Barry 1988, 1991, 2004). Enhancers can act on polar head groups of lipids and modify the hydrogen bonding and ionic forces between them resulting in a disruption of the packing geometry. Fluidity caused at the polar plane due to the disruption of packing geometry accelerates the diffusion of solute molecules across the lipid bilayers. An alternate consequence of disrupting packing geometry of lipid head groups is the creation of aqueous pockets that facilitate diffusion of hydrophilic molecules. In addition to fluidizing bilayers, enhancers that disrupt lipid head group interactions can cause extraction of lipid molecules, phase separation, or micelle formation (Barry 2004). Enhancers can also insert themselves between the hydrocarbon chains of the lipid bilayers and thereby disrupt the packing of lipid molecules. Consequent fluidization of the lipid bilayers facilitates the diffusion of permeants. Disruption in packing of lipid chains can in turn alter the packing of polar head groups of the lipid molecules, thereby accelerating, to a small extent, the diffusion of permeants.
Karande et al. studied permeation enhancers from eight different categories: anionic surfactants, cationic surfactants, zwitterionic surfactants, fatty acids, fatty alcohols, fatty amines, fatty esters, and azone-like molecules, and showed that chemicals in all these categories could be classified, more simply, as lipid extractors or lipid fluidizers (Karande et al. 2005). Lipid extractors increased SC permeability by extracting lipids from bilayers or the corneocyte envelope. Loss of lipids from the SC was monitored as a decrease in the signal intensity of methylene groups of lipid chains in Fourier transform infrared (FT-IR) spectroscopy. Lipid fluidizers increased SC permeability by partitioning themselves in the bilayers and disrupting the bilayers packing structure. Fluidization was monitored as an increase in the signal intensity of methylene groups (from hydrocarbon tails of the enhancer) and disappearance of peaks related to the ordered packing of lipids in FT-IR spectroscopy. Extent of extraction or fluidization correlated very well with the extent of skin permeabilization.
4 In Vitro Skin Models
4.1 Excised Human skin
Human skin is the obvious choice in experiments for determining the permeability of model compounds or therapeutics (Rao and Misra 1994; McCullough et al. 2006; Suppasrivasuseth et al. 2006; Elewski 2007; Kim et al. 2008). Freshly excised skin from autopsies, cadaver skin, or discarded skin from breast reduction procedures are excellent sources of human skin (Bronaugh et al. 1986; Wester and Maibach 1989; Friend 1992). The primary barrier to transport of molecules across the skin is the SC. In comparison the epidermis and dermis offer minimal resistance to passive diffusion of solutes. The SC is composed of lipids and terminally differentiated, fully keratinized corneocytes. It is, therefore, intuitively expected for ex vivo skin to maintain the barrier integrity of the SC for an extended period of time after harvesting when stored under appropriate conditions. Some investigators have indeed verified that skin can be frozen for up to 12 months without significant deterioration of barrier properties (Franz 1975; Harrison et al. 1984). Barry et al. found that human cadaver skin stored at −18 °C for 466 days did not show any significant change in permeability toward tritiated water (Harrison et al. 1984). Interestingly, Barry et al. also found that the skin obtained from an iceman 5000 years old and buried in glacial ice was very well preserved. Several reports have documented the comparison between in vivo and ex vivo SC and have shown that it retains its barrier properties for several days after harvesting (Berenson and Burch 1951; Galey et al. 1976). Wester et al. monitored glucose metabolism in skin as a measure of its viability and showed that the metabolic activity was highest during the first 18 h after the skin was harvested. The metabolic activity showed a decrease by day 2 but stayed steady until day 8 (Wester et al. 1998a).
In spite of the several advantages of using human skin in permeation experiments there are several problems associated with its use such as safety concerns, difficulty in procurement, limited supply, and regulatory considerations. Also the permeability measurements obtained on human skin samples vary greatly between individuals as well as between samples from different anatomical sites on the same individual (Wester and Maibach 1992; Norlen et al. 1999; Robert Peter Chilcott 2000). Chilcott et al. have shown that there is a statistically significant variation in the skin barrier property with relation to gender, chirality, time of the day when measurement was obtained, and to some extent the dietary habits of the individual (Robert Peter Chilcott 2000). Akomeah measured the permeability of caffeine, methyl paraben, and butyl paraben on skin samples from several donors and found interdonor variabilities between 33 % and 44 % (Akomeah et al. 2007). In general, the intersubject skin sample variability in skin permeation was higher than that observed within the same subject. Similar observations have been reported by other investigators (Southwell et al. 1984; Langguth et al. 1986; Rochefort et al. 1986). Further, these permeability measurements show a non-Gaussian distribution (Williams et al. 1992; Cornwell and Barry 1995).
4.2 Excised Animal Skin
In view of the difficulties associated with human skin, animal skin is routinely used as a model for human skin in in vitro experiments (Haigh and Smith 1994). Mouse (Bonina et al. 1993; Roy et al. 1994; Panchagnula et al. 1997; Bhandari et al. 2008; Cho et al. 2008), rat (Panchagnula et al. 1997; Hai et al. 2008; Zhao et al. 2008), guinea pig (Panchagnula et al. 1997; Tipre and Vavia 2003; Pabla and Zia 2007), rabbit (Panchagnula et al. 1997; Ogiso et al. 2001; Artusi et al. 2004; Sebastiani et al. 2005; Elgorashi et al. 2008), porcine (Panchagnula et al. 1997; Karande et al. 2004; Ben-Shabat et al. 2007), monkey (Wester and Maibach 1987; Roy and Degroot 1994; Panchagnula et al. 1997), dog (Sato et al. 1991; Panchagnula et al. 1997; Rohatagi et al. 1997), hamster (Coutelegros et al. 1992; Panchagnula et al. 1997; Bach and Lippold 1998), fish (Watanabe et al. 1989; Masson et al. 2002), snake (Megrab et al. 1995; Suh and Jun 1996; Panchagnula et al. 1997), cow (Panchagnula et al. 1997; Netzlaff et al. 2006b), frog (Dewhurst and Williams 1993; Smith 1993), sheep (Panchagnula et al. 1997), and marmoset (Scott et al. 1991) are some of the animal skin models studied to represent human skin. Animal skin offers advantages over human skin in that the age and sex of the animal can be controlled as well as large quantities of skin can be obtained for experimental purpose (Friend 1992).
One needs to be cautious, however, in extrapolating animal skin data to human skin. Several differences exist and have been documented. Skin from experimental animals is different from human skin in thickness, composition, and constitution of the SC, and distribution and density of appendages such as sweat glands and hair follicles (Schalla and Schaefer 1982; Bronaugh et al. 1983). Panchagnula et al. have documented follicular density, SC thickness, epidermis thickness, and full skin thickness for 16 animal models including human skin (Panchagnula et al. 1997). These parameters vary significantly between the different species studied. For two model compounds used in this study, water and 7-hydroxycoumarin, lag time and permeability varied significantly across the skin models. While both compounds have similar permeabilities across human skin, their permeabilities across other skin models vary drastically. The lipid content of the skin is a major determinant in its barrier potential and differs between species or between sites on the same animal (Elias et al. 1980, 1981). Hairless mouse skin which is commonly used as a model for human skin is comparatively fragile. While permeability of human skin exposed to water increases only twofold in 10 days, hydration can completely disintegrate hairless mouse skin (Bond and Barry 1988a, b, c). A 2-min treatment with acetone has negligible effect on human skin but can increase hairless mouse skin permeability by 15-fold (Bond and Barry 1988a, b, c, d). Hairless mouse skin model overestimates the effect of permeation enhancers on skin permeability by sevenfold (Bond and Barry 1988a, b, c). In contrast, another common model, shed snake skin, underestimates the effect of permeation enhancers on skin permeability when compared to human skin (Rigg and Barry 1990). In general, it has been observed that animal skin permeability is higher than human skin permeability (Panchagnula et al. 1997).
Of all animal skin models studied, porcine skin, and particularly porcine ear skin, is closest to human skin in terms of its biochemical composition and histological features (Gray and Yardley 1975; Dick and Scott 1992; Wester et al. 1998b; Sekkat et al. 2002; Muhammad et al. 2004; Jacobi et al. 2007). Porcine skin resembles human skin most in terms of the SC thickness (Holbrook and Odland 1974; Wester and Maibach 1989; Jacobi et al. 2007), epidermis thickness (Wester and Maibach 1989; Sandby-Moller et al. 2003; Jacobi et al. 2007), follicular structure and density (Jacobi et al. 2007), lipid composition (Gray and Yardley 1975), and the underlying vasculature (Simon and Maibach 2000). As a result, the porcine skin has gained wide acceptance as a representative model for human skin.
4.3 Living Skin Equivalents
Skin samples obtained from different species show varying permeability responses in presence of the same permeation enhancer on account of the differences in their constituents, composition, and microstructure. In addition to an interspecies variation, there is also a variation observed in skin permeability with age and anatomical location within the same species (Bronaugh et al. 1982; Dupuis et al. 1986; Hughes et al. 1994; Duncan et al. 2002). Cell culture or tissue culture-based models of human skin can potentially overcome this problem by offering a more consistent skin representation (Roguet et al. 1998; Faller and Bracher 2002; Lotte et al. 2002). In general, in vitro cell culture models of living tissues offer several advantages such as high reproducibility, rapid assessment of permeability and metabolism of drugs, stricter control over experimental conditions, well-defined end points, and potential time and cost savings when compared to animal use. The biggest advantage of cell culture models, however, is their amenability to high-throughput studies for drug discovery or formulation optimization studies (Audus et al. 1990).
Reconstruction of skin in vitro typically starts with obtaining keratinocytes from full thickness or split thickness skin by enzymatic digestion using trypsin (Larsen et al. 1988), dispase (Green et al. 1979), or thermolysin (Walzer et al. 1989). Basal keratinocytes are isolated and grown at an air–liquid interface on a substrate that is equivalent of the dermis. Dermal equivalents that have been used successfully include permeable synthetic membranes such as nylon mesh (Slivka et al. 1993; Crooke et al. 1996) and polycarbonate membranes (MonteiroRiviere et al. 1997; Poumay et al. 2004; Kandarova et al. 2006), collagen (Fransson et al. 1998; Flamand et al. 2006), collagen lattices (Bell et al. 1981), glycated collagen (Pageon and Asselineau 2005), collagen-glycosaminoglycan matrices (Boyce et al. 1988), chitosan cross-linked collagen-glycosaminoglycan matrices (Shahabeddin et al. 1990), fibrin (Holland et al. 2008), dead de-epidermized dermis (Regnier et al. 1998; Rehder et al. 2004), synthetic scaffolds (Shakespeare 2001; Mansbridge 2002), biodegradable scaffolds (El Ghalbzouri et al. 2004), or combinations thereof (Slivka et al. 1993; Lee et al. 2000; Barker et al. 2004; Sobral et al. 2007). Keratinocytes receive nutrients from the lower surface of the culture while being pushed upward in a process of progressive differentiation. In 14–21 days, the topmost layer achieves terminal differentiation and manifests characteristics remarkably similar to those of normal SC, i.e., completely cornified cells surrounded by a lipid intercellular matrix (Nabila Sekkat 2001). Today, several cell culture-based skin models are commercially available for ready use in skin permeation or skin toxicity studies. These include TestSkin® and TestSkin® II by Organogenesis, Canton, MA (Davis 1990; Moody et al. 1995; Elyan et al. 1996; Rodriguez et al. 2004; Shibayama et al. 2008), EpiDerm™ and EpiDermFT™ (Hayden et al. 2004, 2005; Kandarova et al. 2007; Borgia et al. 2008; Schafer-Korting et al. 2008) by MatTek Corp., Ashland, MA, EpiSkin® and SkinEthic RHE® (Botham 2004; Schafer-Korting et al. 2006, 2008; Luu-The et al. 2007; Netzlaff et al. 2007) by SkinEthic Labs., Nice, France, Vitrolife-Skin (Uchino et al. 2002; Morikawa et al. 2007) by Gunze, Kyoto, Japan. Netzlaff et al. have reviewed the EpiDerm™, EpiSkin®, and SkinEthic® models based on their morphology, lipid composition, biochemical markers, and their applicability in tests for evaluating phototoxicity, corrosivity, irritancy, and transport properties (Netzaff et al. 2005). The architecture, homeostasis, and lipid composition of these models come close to human skin (Ponec and Kempenaar 1995; Ponec et al. 2000, 2002). Faller et al. compared the models in their ability to secrete extracellular enzymes glutamic oxaloacetic transaminase (GOT) and lactate dehydrogenase (LDH), and interleukin-1α on treatment with sodium lauryl sulfate (SLS). EpiDermTM was the most resistant to SLS and most reproducible (Faller and Bracher 2002).
In general, the reconstructed skin models have higher permeabilities compared to excised human skin (Gysler et al. 1999). Schmook et al. compared the permeabilities of four topical dermatological compounds of varying polarity—salicylic acid, hydrocortisone, clotrimazole and terbinafine, across rat, human and pig skin as well as two models of human skin—Graftskin™ LSE™ and Skinethic™ HRE (Schmook et al. 2001). In these studies pig skin performed similar to human skin with comparable flux of solute across both tissues. Graftskin™ LSE™ provided an adequate barrier to salicylic acid, but clotrimazole flux across it was 1000-fold higher and its skin concentration 50-fold higher when compared with human skin. Skinethic™ HRE was approximately sevenfold more permeable compared to human skin for salicylic acid and 900-fold more permeable to clotrimazole. In a similar study Marty et al. reported that trinitroglycerol and estradiol were about 20-fold more permeable across Skinethic™ HRE compared to split-thickness human skin (Marty et al. 1997). In cutaneous bioavailability studies on topical formulations, vehicle effects were observed to be vastly different in EpiDerm™ and EpiSkin® models compared to ex vivo human skin (Dreher et al. 2002). Although the reconstructed human skin models underperform significantly in reproducing the barrier properties of ex vivo human skin they can still be used to rank order the permeabilities of solutes based on their permeabilities. Such a rank order has been shown to match the order obtained on ex vivo human skin for several different molecules. Lotte et al. have shown that the skin absorption and permeability of lauric acid, mannitol, and caffeine follow the same rank order as they would on ex vivo human skin (Lotte et al. 2002). Dreher et al. found that the EpiSkin® and EpiDerm™ models showed the same rank order permeability as human skin for caffeine and α-tocopherol acetate from a water in oil (w/o)-emulsion, an oil in water (o/w)-emulsion, a liposomal dispersion and a hydrogel (Dreher et al. 2002). In addition, a multilab study verified that the permeability ranking across EpiSkin®, EpiDerm™, and Skin Ethic™ RHE models was comparable to the permeation through human epidermis for caffeine and testosterone (Schafer-Korting et al. 2008).
The biggest shortcoming of commercially available skin models is their relatively weak barrier function. Impaired desquamation (Vicanova et al. 1996a, b), impaired transfer of desmosomes (Vicanova et al. 1996a, b), and presence of unkeratinized microscopic foci (Mak et al. 1991) are cited as reasons for this poor performance. Another significant impediment to the use of reconstructed human skin models is their high cost. This has limited the use of such models mostly to industry and out of reach of most academic labs and small enterprises. Furthermore, all commercially available models use proprietary chemically defined media and sources for cells that can put additional constraints on the flexibility of using such models. All three leading models, Epiderm™, EpiSkin®, and SkinEthic™ RHE are based on the epidermis raised on a minimal dermal equivalent such as collagen gel scaffold encapsulating fibroblasts. In contrast, Nakamura et al. report that full-thickness models based on organ cultures of skin explants match the in vivo situation more closely (Nakamura et al. 1990).
4.4 Polymers
Model membrane systems can provide tremendous insight into mechanistic details of solute diffusion and thermodynamics of solute–membrane and solvent–membrane interactions (Corrigan et al. 1980; Flynn 1985; Beastall et al. 1986; Haigh and Smith 1994). Diffusion of a solute molecule across a membrane is governed by physical factors such as molecule size and shape, pore size, pore distribution, path length and tortuosity, and chemical factors such as hydrogen bonding, hydrophobic interactions, and electrostatic interactions. The contribution from each of these factors can potentially be decoupled by a systematic study with model membranes. Synthetic membranes and polymers such as silicone (Hou and Flynn 1997; Cross et al. 2001), cellulose acetate (Barry and Eleini 1976; Barry and Brace 1977; Farinha et al. 2003), poly(dimethylsiloxane) (Cronin et al. 1998; Du Plessis et al. 2002; Farinha et al. 2003; Frum et al. 2007), polyvinylidene difluoride (Olivella et al. 2006), polyvinyl chloride, polyether sulfone (Farinha et al. 2003), ethyl vinyl acetate (Farinha et al. 2003), multimembrane laminates (Scheuplein and Bronaugh 1983; Houk and Guy 1988), and a mixture of isopropyl myristate and silicone oil (Ottaviani et al. 2006) have been used to this end. In spirit of the “fluid—mosaic model” of the skin, organic solvents such as 1-octanol, alkanes, ether, chloroform, esters, and paraffins have also been used to model diffusion through skin (Houk and Guy 1988). Relatively less studied synthetic membrane systems are porous materials. In diffusion studies across model biomembranes, filter supports have typically gained prominence as support membranes. A few studies, however, have used filter supports or filter supports filled with organic liquid to study diffusion of topical agents (Tanaka et al. 1978; Demeere and Tomlinson 1984; Turakka et al. 1984; Viegas et al. 1986). Schramm-Baxter et al. have used polyacrylamide gels to model human skin and study the energetics of liquid jet penetration into skin (Schramm-Baxter et al. 2004). Dyer et al. have tested zeolites as model systems (Dyer et al. 1979). Although such models are simplistic and lack all the functional and structural complexity of skin, they provide several other advantages such as uniformity of structure, sample-to-sample reproducibility, and ease of procurement.
4.5 Lipids
In vitro models based on lipids, model lipids, or mixtures of natural or model lipids have been evaluated for studying percutaneous absorption in humans. An artificial lipid membrane composed of isopropyl myristate (IPM) supported in a rotating diffusion cell has been used to simulate the epidermal barrier. Reasonable correlation was obtained between diffusion of a wide range of compounds across the IPM membrane and excised skin. Transport resistance across the model membrane, however, was 1000-fold lower as compared to excised skin (Hadgraft and Ridout 1987). A three-component mixture of dipalmitoyl phosphatidylcholine, linoleic acid, and tetradecane showed an order of magnitude improvement in transport resistance when compared to IPM membrane (Hadgraft and Ridout 1988). Matsuzaki et al. developed a model skin membrane by fixing liposomes composed of SC lipids: ceramides, palmitic acid, cholesterol, and cholesterol-3-sulfate onto a supporting filter, Biodyne B (Matsuzaki et al. 1993; Miyajima et al. 1994). Drug permeability through this system correlated very well (r = 0.88) with that through guinea pig skin although permeability through the model system was an order of magnitude higher. Moghimi et al. constructed a model lipid matrix from cholesterol, water, and free fatty acids of the SC and their sodium salts (Moghimi et al. 1996). This model matrix was shown to be a good representation of the SC barrier based on the permeability of a model hydrophobic drug, 5-fluorouracil. Using a similar approach, de Jager et al. created a SC substitute (SCS) by applying a mixture of synthetic SC lipids, free fatty acids, and cholesterol on a porous substrate. The composition, organization, and orientation of lipids in the SCS bore high resemblance to that of the intercellular barrier lipids in SC (de Jager et al. 2006a, b). Other groups have reported studies on membranes reconstituted from porcine SC lipids or porcine brain ceramides on porous substrates. These models have been shown to reproduce the permeability of water and some other permeants across intact SC (Abraham and Downing 1989; Friberg and Kayali 1989; Friberg et al. 1990; Kittayanond et al. 1992; Lieckfeldt et al. 1993; Kuempel et al. 1998).
5 Evaluation of Skin Permeability In Vitro
The ability to measure skin permeability is of utmost importance for percutaneous absorption and transdermal delivery applications. Several methods have been proposed to quantify skin permeability.
5.1 Diffusion Measurements
Diffusion cells are by far the oldest and most commonly used apparatus in measuring permeation of solutes across the skin. A typical diffusion cell assembly contains a donor chamber coupled to a receiver chamber by means of a spring clamp or screw. The membrane, in this case skin, whose permeability is to be assessed, is sandwiched between the donor and receiver chambers such that the SC is exposed to the donor and the epidermis/dermis to the receiver. A solute whose permeability across skin is to be measured is placed in the donor chamber by formulating it in a suitable solvent. Appearance of the solute in the receiver chamber is periodically monitored using appropriate analytical methods. The rate of appearance of the solute in the receiver chamber is then expressed as a permeability profile in the form amount vs. time.
5.1.1 Theory
A number of relationships have been used to describe the permeation of drugs across skin. While the basis for these relationships can be complex, the amount of solute (M t ) crossing the skin in time t can be related to skin permeability (P) by a reasonably straightforward relation.
For an infinite dose of solute in the donor,
where C 0 is the concentration of the solute in the donor chamber, K is the partition coefficient, or log P of the solute into skin (SC), D is the effective diffusion coefficient across the skin, L is the path length of diffusion.
At steady state \( \left( t\to \infty \right) \), Eq. (9.1) above can be rewritten in a simpler form as
This represents the permeability profile of a solute diffusing across skin. The slope of this profile provides flux of the solute across skin,
The permeability profile is linear in time but exhibits a lag time, \( {t}_L=\frac{L^2}{6 D} \)
The terms K, D and L are grouped together and defined as a single term, \( P=\frac{KD}{L} \), the skin permeability. Solute permeability can then be estimated from flux of the solute across the skin and its concentration in the donor chamber.
Several simplifying assumptions have been made in deriving this relationship (Foreman and Kelly 1976; Osborne 1986). Nevertheless, Eq. (9.4) above provides a straightforward way of determining skin permeability to different solutes by measuring their flux.
5.1.2 Model Solutes
The solute, whose permeability is to be assessed across the skin, needs to be detected in the receiver chamber by means of appropriate analytical methods. These may include spectrometry, chromatography, biochemical methods such as ELISA, western blots, etc. The solute itself may be labeled using a fluoropore or radioisotope for direct detection. Care needs to be taken that labeling of the solute does not alter its physicochemical properties, which may affect skin permeability resulting in misleading conclusions.
5.1.3 Diffusion Cells
A wide variety of diffusion cell systems have been developed for measuring solute permeation through membranes (Frantz 1990; Bronaugh and Collier 1991; Gummer and Maibach 1991; Friend 1992). The most common configurations are vertical cells, where the donor chamber is atop the receiver chamber separated by the membrane in between, and horizontal diffusion cells where the donor and receiver chamber are arranged side-by-side. Mixing of the chambers to create homogeneous compartments is critical in horizontal diffusion cells. In case of vertical diffusion cells, mixing is not critical. However, a homogeneous well-mixed receiver chamber better mimics in vivo conditions and prevents the formation of a static boundary layer of high-solute concentration in the receiver chamber. Formation of unmixed zones is especially critical when assessing the permeability of a hydrophobic solute in an aqueous receiver compartment (Tsuruta 1977; Bronaugh and Stewart 1984, 1986). Efficient mixing can be obtained using small magnetic stir bars. Flow-through diffusion cells in which the receptor fluid is continuously refreshed to mimic in vivo sink conditions (i.e., metabolism and diffusion into the subdermal vasculature) have also been used successfully (Ainsworth 1960). Temperature control of the diffusion cell can be attained using water jackets or simply submerging the entire cell assembly into a water bath. For studying permeation of highly hydrophobic compounds, solubilizing solvents can be added to the aqueous receiver chamber. These include Triton X-lOO (Bronaugh and Stewart 1984), bovine serum albumin (Brown and Ulsamer 1975), Poloxamer 188 (Hoelgaard and Mollgaard 1982), PEG 400 (Valia et al. 1984), and ethanol (Scott et al. 1986). Caution needs to be exercised when using solubilizing agents that they do not alter inherent membrane properties. Hydration effect on membrane integrity needs to be considered when assessing solute permeability over extended periods of time. Some studies have reported that long-term hydration in rodent skin, and in particular hairless mouse skin, can lead to changes in permeation rates (Whitton and Everall 1973; Bond and Barry 1988a, b, c, d; Hinz et al. 1989). Finally, the area of diffusion of the donor chamber has been shown to have some effects on the permeation rates (Karande and Mitragotri 2003). Water penetration into the skin introduces a lateral strain at the edges of the donor chamber due to swelling. This scales as the strain edge available per unit area and results in higher observed permeation rates in smaller donor chambers.
-
(a)
Horizontal diffusion cells
Several designs have been suggested and used successfully for this type of diffusion cell. These include the T-shape configuration (Washitake et al. 1980), L-shape configuration (Dyer et al. 1979; Tojo et al. 1985a), glass conical flasks configuration (Wurster et al. 1979), vertical membrane, equi-compartment diffusion cell with high area to volume ratio (Flynn and Smith 1971), glass diffusion cells with steel mesh (Southwell and Barry 1983), Valia–Chien cells (Tojo et al. 1985a, b), and flow through system with central inlet and peripheral effluent ports (Astley and Levine 1976). In recent years, several modified versions of these early designs have been used (Morell et al. 1996; Aramaki et al. 2003; Bakand et al. 2006; Soni et al. 2006; Tas et al. 2007).
-
(b)
Vertical cells
Vertical diffusion cells are closer to in vivo situation in obtaining permeability data across the skin (Friend 1992). The Coldman cell represents the earliest of all vertical diffusion cells (Coldman et al. 1969). A glass cell with a side arm for sampling and stir bar for mixing forms the receiver chamber. Skin is sandwiched between the donor and receiver using a clamp. Whitton et al. studied a similar cell with the sampling arm located at the bottom of the receiver (Whitton and Everall 1973). The Franz diffusion cell remains the most widely studied vertical diffusion cell today (Franz 1978). The original design had poor mixing properties which have been addressed in subsequent modifications (Nacht et al. 1981; Loftsson 1982; Kao et al. 1983; Dugard et al. 1984; Hawkins and Reifenrath 1986; Gummer et al. 1987; Tiemessen et al. 1989). In the past two decades several modifications of the Coldman cell have been used successfully. These include the release cells (Morell et al. 1996), enhancer cells (Bosman et al. 1996), Kelder cells in combination with the Automatic Sample Preparation with Extraction Columns system (Bosman et al. 1996), Oak Ridge National Laboratory Skin Permeability Chamber (Holland et al. 1984), and Ussing type chambers (Li et al. 2006; Ito et al. 2007).
-
(c)
Skin Flaps
In addition to the conventional diffusion systems discussed above, novel in vitro systems that measure effect of perfusion rates on solute permeation have also been designed. Isolated Perfused Porcine Skin Flap (IPPSF) is a model system of porcine skin flap perfused by the caudal superficial epigastric artery and its associated veins and mounted on a diffusion cell (Williams et al. 1990; Riviere et al. 1991). Bovine udder is used in a similar fashion for permeation studies (Kietzmann et al. 1993).
5.2 Tape Stripping
Tape stripping is a technique that has been found useful in dermatopathological and dermatopharmacological research for selectively or at times exhaustively removing the SC (Surber et al. 1999). Typically, an adhesive tape is applied to the skin and removed abruptly. This application can be repeated between 10 and 100 times (Sheth et al. 1987; Ohman and Vahlquist 1994). The observation that skin may serve as a reservoir for chemicals was first reported in 1955 (Malkinson and Ferguson 1955). Drugs like corticosteroids were shown to localize within the SC (Vickers 1963; Carr and Wieland 1966). These observations led to the use of the tape stripping technique in investigating the barrier and reservoir function of the skin (Rougier et al. 1983; Tojo and Lee 1989). This technique is now being increasingly used in measuring drug concentration and its concentration profile in the SC (Pershing et al. 1990; Pellett et al. 1997; Shah et al. 1998).
5.2.1 Theory
Solute diffusion in the SC can be described by Fick’s law (Crank 1975) as follows
where D is the average solute diffusion coefficient in the SC, C s is the solute concentration in the SC, and x is the distance from the SC surface. Eq. (9.1) can be solved with the following boundary conditions:
where x = 0 corresponds to the SC surface and x = L corresponds to the end of the SC, K is the average solute partition coefficient in the SC, and C 0 is the donor concentration of the solute. The resulting equation for solute concentration in the SC, C s is given as follows (Crank 1975):
where C ∞ is the solute concentration in SC at steady state (\( {C}_{\infty }=\frac{K{ C}_0}{2} \)). For short times, i.e., low values of \( \frac{Dt}{L^2} \), Eq. (9.6) can be simplified as
Using the definition of permeability P, the above Eq. (9.7) further simplifies to
Equation (9.8) shows that the solute concentration in the SC measured at short times is proportional to its steady-state permeability. Accordingly, solute concentration can be measured via tape stripping in the SC to infer its steady-state permeability. An analytical technique such as high-performance liquid chromatography (HPLC) or an immunoassay or radioimmunoassay is required in conjunction to accurately determine solute concentration. Several studies have successfully applied this method to determine the skin permeability of a wide range of solutes (Stinchcomb et al. 1999; Alberti et al. 2001a, b, c; Moser et al. 2001).
5.3 Impedance Spectroscopy
Methods based on measuring solute diffusion across skin may not always provide the sensitivity required to measure small perturbations in skin permeability or follow permeability changes over short intervals of time. Electrical measurements across the SC provide improved sensitivity (Dugard and Scheuple 1973). Electrical properties of SC parallel those of permeability and play a dominant role in the control of current flow (Lawler et al. 1960; Tregear 1966). A review of factors governing the passage of electricity across skin has been presented by Tregear (Tregear 1966).
5.3.1 Theory
Flow of ions across skin under an electric field is analogous to diffusion of solutes under a chemical gradient. Current across skin can thus be related to permeability of skin. Skin and its appendages can be represented by an equivalent circuit containing a resistance R shunted by capacitance C (Lackermeier et al. 1999). The impedance, Z, of this equivalent skin model can be represented as
where f is the frequency of the applied alternating current (AC) signal. A formal porous pathway theory based on Nernst–Planck flux equations and the Nernst–Einstein relations for ideal solutions has been developed that relates skin impedance to skin permeability (Lakshminarayanaiah 1965; Srinivasan and Higuchi 1990; Li et al. 1998, 1999; Tezel et al. 2003).
A simplified correlation between skin resistivity, R, and skin permeability, P, is provided by Tang et al. (2001) as
where C is dependent on the properties of the solute and the solvent in which it is dissolved.
The impedance of intact skin is in the range of several hundred kilo ohms (kΩ). As the skin is permeabilized, impedance drops finally attaining a value of ~1 kΩ, which corresponds to removal of the entire barrier (Naik et al. 2001). Skin impedance measurements have been used to study the effect of sonophoresis (Mitragotri et al. 1996; Tezel et al. 2001; Paliwal et al. 2006), iontophoresis (Burnette and Bagniefski 1988; Kalia et al. 1996; Kumar and Lin 2008), and chemical enhancers on skin permeability (Karande et al. 2004; Karande et al. 2006a, b).
In comparison to diffusion measurements of solute permeability in diffusion cells, electrical impedance measurements are relatively simpler, faster, and more sensitive. Impedance-based permeability assessment provides direct readout of barrier integrity and does not require subsequent analysis, which may be tedious, time consuming, and expensive. Also, since these measurements can be performed rapidly and by use of automated systems the throughput of impedance-based assays is significantly larger compared to diffusion cells. Karande et al. have described the design of an impedance based high-throughput assay to determine the effect of chemical permeation enhancers on skin permeability (Karande et al. 2004, 2006a, b). This system, in vitro impedance Guided High Throughput (INSIGHT) screen, is capable of assessing thousands of formulations per day for their ability to modulate skin permeability. The authors discovered synergistic formulations of permeation enhancers using this screen, which were capable of delivering a biologically active hormone across the skin at therapeutically relevant doses. One downside of using impedance to assess skin permeability is that impedance serves only as a surrogate measure of actual skin permeability. The actual flux of a solute needs to be assessed by conventional diffusion methods.
5.4 Infrared Spectroscopy
Transport of solute molecules in the skin can be studied using spectroscopic techniques. Fourier transform infrared (FTIR) spectroscopy has been used extensively in determining skin structure, properties, hydration, and effect of permeation enhancers (Potts and Francoeur 1993; Moore and Rerek 1998; Karande et al. 2005; Mendelsohn et al. 2006).
5.4.1 Theory
FTIR spectroscopy is used to track solute molecules in the epidermis by recording their molecular vibrations. The integrated absorbance of these vibrations is directly proportional to the amount of solute present in the skin. Depth-dependant profiling of the solute in the skin can be achieved by attenuated total reflectance (ATR)-FTIR spectroscopy. ATR-FTIR spectroscopy generally provides information in the superficial 1–2 μm layer of the skin. Repeated tape stripping can be used to scan successive layers of the skin for solute penetration. Transport properties for the solute can then be obtained from an unsteady state solution for Eq. (9.5) given as (Pirot et al. 1997)
where C(x) is the concentration of solute at a depth x from skin surface and C 0 is the concentration of the solute at the skin surface. L is the total thickness of skin and D is the effective diffusivity of the solute in skin. The rate per unit area at which the solute diffuses out of the skin can be obtained by differentiation of Eq. (9.11) as \( - D{\left(\frac{\partial C}{\partial x}\right)}_{x=0} \). Further, integration of this rate with time yields the total amount of solute diffusing across skin in a given time. This information can be used to obtain the permeability profile of the solute and hence its permeability across the skin. Several assumptions have been made in deriving this correlation that is discussed in Pirot et al. (1997). One downside of using ATR-FTIR spectroscopy is that the solute to be monitored needs to be IR active and have a signature distinct from those of the SC components. This limitation can, in theory, be overcome using spectral correction techniques (Naik et al. 2001).
Several other methods have been used to study permeation and absorption of material into and across skin. Xiao et al. have used Raman microscopy and imaging to track penetration of phospholipids in skin (Xiao et al. 2005a, b). Williams et al. provide a critical comparison of different Raman spectroscopy techniques and their application in vivo (Williams et al. 1993). Sonavane et al. have used a combination of UV-vis spectroscopy, X-ray spectroscopy, and inductive coupled mass spectroscopy to track permeation of gold nanoparticles in the rat skin (Sonavane et al. 2008). Other groups have used photoacoustic spectroscopy for studying permeation of Carbopol 940 and transdermal gels (Christ et al. 2001; Rocha et al. 2007; Rossi et al. 2008). Remittance spectroscopy and photothermal deflection are a few of the other methods that have been suggested in this area (Gotter et al. 2008).
5.5 Trans Epidermal Water Loss
Mammalian skin has evolved to regulate the transport of material into and out of the body. One of the primary functions of skin is to regulate the loss of water from the body. It follows, then, that the quantitative measure of water loss from the skin is indicative of its barrier integrity. A device, such as an evaporimeter, that can adequately and accurately measure the water vapor flux at the skin surface, and hence the rate of trans-epidermal water loss (TEWL), can be used to assess barrier integrity of the skin. A quantitative correlation between TEWL and skin permeability has been reported (Rougier et al. 1989) and is found to be consistently reproducible in vivo and in vitro (Pinnagoda et al. 1989, 1990). Several studies have reported the use of TEWL to assess the effect of permeation enhancers on skin permeability (Loden 1992; Kanikkannan and Singh 2002; Luzardo-Alvarez et al. 2003; Tokudome and Sugibayashi 2004). A comprehensive review of their findings is provided by Levin and Maibach (Levin and Maibach 2005). This review also sheds light on possible reasons why two particular studies did not observe quantitative correlation between percutaneous absorption and TEWL (Tsai et al. 2001; Chilcott et al. 2002). Recent literature continues to provide opposing views on the use of TEWL as a measure of skin barrier integrity (Fluhr et al. 2006; Netzlaff et al. 2006a). Nevertheless, several commercial devices based on this principle are readily available today for laboratory work.
6 Evaluation of Skin Permeability In Vivo
In vivo methods for quantification of solute permeation across the skin serve as a gold standard in transdermal drug delivery. Such methods can potentially eliminate variables associated with using excised human or animal skin, surrogate endpoints as well as faithfully reproduce metabolic, pharmacokinetic, and pharmacodynamic behavior of the drug molecule. Wherever possible, and practically feasible, in vivo measurements are considered reliable and superior to measurements made on model systems in vitro.
6.1 Diffusion Measurements
Diffusion cells have been designed that can be filled with drug solution and strapped on to an animal in vivo or the arm of a human volunteer (Wurster and Kramer 1961; Quisno and Doyle 1983; Leopold and Lippold 1992). Such a system allows one to study the passive permeation of a solute across the skin in vivo in much the same way as diffusion cells with excised skin or model membranes. Different endpoints can then be used to quantify permeation rates across the skin.
6.1.1 Systemic Bioavailability
A solute whose percutaneous absorption is to be measured is applied topically to the skin and its concentration measured in blood plasma or urine over a period of time. These amounts generally tend to be very low and hence a sensitive detection technique such as radiolabeling with 3H or 14C is necessary (Wester et al. 1983; Gschwind et al. 2008). Caution needs to be exercised when using such techniques if the drug is susceptible to metabolism. Metabolism can lead to by-products that have significantly different pharmacokinetics and pharmacodynamics than the parent compound and can give misleading results.
6.1.2 Surface Loss
Alternate approach to determine in vivo percutaneous absorption is to measure the loss of solute from the surface as it penetrates the skin. This can be achieved when all residual material, contained in a reservoir, can be recovered. Difference between concentration or amount at time zero and at time of recovery provides an estimate of amount absorbed. Generally, the method used to detect the residual amount needs to be sensitive enough, especially in supersaturated systems, as the reservoir can be infinitely large when compared to the amount absorbed. Also, this method assumes that the skin does not act as a reservoir, which in itself can be a poor assumption.
6.2 Pharmacological Response
A good method to assess percutaneous absorption is to measure the biological or pharmacological response to the drug. Vasoconstriction can be used as an endpoint to measure transdermal delivery of topically applied corticosteroids (Barry 1983) and vasodilation as an endpoint for topically applied nicotinates (Le and Lippold 1995). Laser Doppler flowmetry (LDF) has been used to study absorption of prostaglandin E1 (PGE1) (Foldvari et al. 1998), methylnicotinate (Wilkin et al. 1985; Poelman et al. 1989), and minoxidil (Wester et al. 1984) in vivo by measuring changes in cutaneous microcirculation. Reduction in blood glucose levels has been used as an endpoint to determine the efficacy of transdermal delivery of insulin (Mitragotri et al. 1995; Chen et al. 2006). The downside of this method is that it works only for drugs that have a detectable biological endpoint and can tend to be more qualitative than quantitative.
6.3 Other Approaches
Several of the methods discussed above for in vitro skin barrier assessment can be adopted for in vivo measurements with little or no modification at all. Tape stripping and TEWL measurements in particular are attractive for in vivo measurements as they are relatively straightforward and minimally invasive or noninvasive. The amount of solute that penetrates the SC can be quantified in vivo, in humans, by tape stripping with an appropriate adhesive tape (Tsai et al. 1991). In case of animal studies, in vivo, it is possible to quantify penetration in deeper skin layers by sacrificing the animal and harvesting its skin. The concentration profiles can then be used to determine solute permeability as suggested in Eq. (9.8). Umemura et al. have used tape stripping in vivo in healthy human subjects to determine the pharmacokinetics of topically applied maxacalcitol from an ointment and lotion (Umemura et al. 2008). Puglia et al. have used tape stripping to quantify skin penetration of lipid nanoparticles (Puglia et al. 2008). A review of tape-stripping methods in determining percutaneous absorption in vivo is provided by Herkenne et al. (Herkenne et al. 2008). Several reports have documented the use of TEWL to assess the barrier integrity after treatment with physical or chemical enhancers of skin permeability (Atrux-Tallau et al. 2008; Kolli and Banga 2008). Maibach et al. have documented the effect of successive tape strippings on TEWL in vivo in human subjects as well as compared two different configurations for devices measuring TEWL (Zhai et al. 2007). Xhauflaire-Uhoda et al. have used TEWL measurements to study barrier repair after the application of miconazole nitrate and tape stripping (Xhauflaire-Uhoda et al. 2006). Among the various spectroscopic techniques, ATR-FTIR spectroscopy has been used extensively in tracking solute permeation in the skin (Ayala-Bravo et al. 2003; Tsai et al. 2003; Curdy et al. 2004; Escobar-Chavez et al. 2005; Remane et al. 2006). A comprehensive review of ATR-FTIR spectroscopy and its use in in vivo studies appears in Naik et al. (Naik et al. 2001). Raman spectroscopy, similar in principle to FTIR spectroscopy, has also been used in studying transdermal solute penetration in vivo. A big advantage of Raman spectroscopy is that it does not require tape stripping of the skin for depth profiling and is thus a truly noninvasive technique. Stamatas et al. have used in vivo confocal Raman microspectroscopy to study the uptake of vegetable oils and paraffin oil in infants (Stamatas et al. 2008). Pudney et al. have demonstrated the use of Raman spectroscopy to obtain depth profiles of trans-retinol in the epidermis for 10 h after application in an in vivo setting (Pudney et al. 2007). Caspers et al. have used confocal Raman spectroscopy to detect dimethyl sulfoxide in the SC (Caspers et al. 2002). For both techniques, ATR-FTIR and Raman spectroscopy, the molecule of interest should have an active IR signature of sufficient intensity that is distinct from the signatures of skin components. An additional drawback of using Raman spectroscopy is that it can only provide relative concentrations as against absolute amounts of the diffusing solute in different layers of the skin. Impedance spectroscopy is a highly sensitive and relatively straightforward technique that can be used in vivo to assess skin barrier integrity. Curdy et al. have used impedance spectroscopy to follow barrier recovery after the application of iontophoresis (Curdy et al. 2002). Dujardin et al. describe the use of impedance measurements to assess effects of electroporation on barrier function in vivo in rats (Dujardin et al. 2002). Kalia et al. have looked at the effect of surfactant treatment and iontophoresis on skin impedance in vivo (Kalia and Guy 1997).
In addition, several other techniques have been utilized to quantify in vivo transdermal delivery (Herkenne et al. 2008). These include creation of suction blisters and punch biopsies. Although relatively straightforward, these are painful and invasive procedures that are less popular in studying percutaneous absorption of solutes, especially in human subjects. Recently, microdialysis has been suggested as a novel technique to measure the diffusion of solutes across the skin (Kreilgaard 2002; Mathy et al. 2005). A thin probe perfused with a physiological solution is implanted under the dermis where, on equilibration, it can exchange material with the extracellular tissue components by passive diffusion. The perfusate from the probe can then be analyzed for the solute diffusing across the skin. A time-based concentration profile of the solute diffusing across the skin into the probe can be used to determine the pharmacokinetics of the solute. A practical challenge associated with this method is the careful and reproducible insertion of the probe in the deeper layers of the skin. Also, extremely sensitive detection methods are required to analyze the small amounts of material obtained from the perfusate.
Conclusion
The field of transdermal drug delivery research has come of age and is rich with opportunities and promises. A combination of improved representative systems that meet regulatory considerations and capture relevant biophysical properties of the skin, reliable and accurate quantification methods, as well as innovative skin permeabilization strategies will expedite the appearance of transdermal delivery systems in this new century.
References
Abraham W, Downing DT (1989) Permeability studies on model membranes prepared from stratum-corneum lipids. J Invest Dermatol 92(3):393
Abraham W, Wertz PW et al (1985) Linoleate-rich acylglucosylceramides of pig epidermis: structure determination by proton magnetic resonance. J Lipid Res 26:761–766
Ainsworth M (1960) Methods for measuring percutaneous absorption. J Soc Cosmet Chem 11:69–78
Akomeah FK, Martin GP et al (2007) Variability in human skin permeability in vitro: comparing penetrants with different physicochemical properties. J Pharm Sci 96(4):824–834
Alberti I, Kalia YN et al (2001a) Effect of ethanol and isopropyl myristate on the availability of topical terbinafine in human stratum corneum, in vivo. Int J Pharm 219(1–2):11–19
Alberti I, Kalia YN et al (2001b) In vivo assessment of enhanced topical delivery of terbinafine to human stratum corneum. J Control Release 71(3):319–327
Alberti I, Kalia YN et al (2001c) Assessment and prediction of the cutaneous bioavailability of topical terbinafine, in vivo, in man. Pharm Res 18(10):1472–1475
Aramaki Y, Arima H et al (2003) Intradermal delivery of antisense oligonucleotides by the pulse depolarization iontophoretic system. Biol Pharm Bull 26(10):1461–1466
Artusi M, Nicoli S et al (2004) Effect of chemical enhancers and iontophoresis on thiocolchicoside permeation across rabbit and human skin in vitro. J Pharm Sci 93(10):2431–2438
Astley JP, Levine M (1976) Effect of dimethyl-sulfoxide on permeability of human skin in vitro. J Pharm Sci 65(2):210–215
Atrux-Tallau N, Huynh NTT et al (2008) Effects of physical and chemical treatments upon biophysical properties and micro-relief of human skin. Arch Dermatol Res 300(5):243–251
Audus KL, Bartel RL et al (1990) The use of cultured epithelial and endothelial-cells for drug transport and metabolism studies. Pharm Res 7(5):435–451
Ayala-Bravo HA, Quintanar-Guerrero D et al (2003) Effects of sucrose oleate and sucrose laureate on in vivo human stratum corneum permeability. Pharm Res 20(8):1267–1273
Bach M, Lippold BC (1998) Percutaneous penetration enhancement and its quantification. Eur J Pharm Biopharm 46(1):1–13
Baden HP, Pathak MA (1967) The metabolism and function of urocanic acid in skin. J Invest Dermatol 48:11–17
Baden HP, Goldsmith EL et al (1973) A comparative study of the physiochemical properties of human keratinized tissues. Biochim Biophys Acta 322:269–278
Bakand S, Winder C et al (2006) An experimental in vitro model for dynamic direct exposure of human cells to airborne contaminants. Toxicol Lett 165(1):1–10
Balsari AL, Morelli D et al (1994) Protection against doxorubicin-induced alopecia in rats by liposome-entrapped monoclonal-antibodies. FASEB J 8(2):226–230
Barker CL, McHale MT et al (2004) The development and characterization of an in vitro model of psoriasis. J Invest Dermatol 123(5):892–901
Barry BW (1983) Dermatological formulations Percutaneous absorption. Marcel Dekker, New York
Barry BW (1988) Action of skin penetration enhancers – the lipid protein partitioning theory. Int J Cosmet Sci 10(6):281–293
Barry BW (1991) Lipid-protein-partitioning theory of skin penetration enhancement. J Control Release 15(3):237–248
Barry BW (2001a) Is transdermal drug delivery research still important today? Drug Discov Today 6(19):967–971
Barry BW (2001b) Novel mechanisms and devices to enable successful transdermal drug delivery. Eur J Pharm Sci 14(2):101–114
Barry BW (2004) Breaching the skin’s barrier to drugs. Nat Biotechnol 22(2):165–167
Barry BW, Brace AR (1977) Permeation of estrone, estradiol, estriol and dexamethasone across cellulose-acetate membrane. J Pharm Pharmacol 29(7):397–400
Barry BW, Eleini DID (1976) Influence of nonionic surfactants on permeation of hydrocortisone, dexamethasone, testosterone and progesterone across cellulose-acetate membrane. J Pharm Pharmacol 28(3):219–227
Beare JM, Cheeseman EA et al (1958) The pH of the skin surface of children with seborrheic dermatitis compared with unaffected children. Br J Dermatol 70:233
Beastall J, Guy RH et al (1986) The influence of urea on percutaneous-absorption. Pharm Res 3(5):294–297
Bell E, Ehrlich HP et al (1981) Living tissue formed in vitro and accepted as skin-equivalent tissue of full thickness. Science 211(4486):1052–1054
Ben-Shabat S, Baruch N et al (2007) Conjugates of unsaturated fatty acids with propylene glycol as potentially less-irritant skin penetration enhancers. Drug Dev Ind Pharm 33(11):1169–1175
Berenson GS, Burch GE (1951) Studies of diffusion of water through dead human skin – the effect of different environmental states and of chemical alterations of the epidermis. Am J Trop Med 31(6):842–853
Bhandari KH, Lee DX et al (2008) Evaluation of skin permeation and accumulation profiles of a highly lipophilic fatty ester. Arch Pharm Res 31(2):242–249
Blank IH (1939) Measurement of pH of the skin surface. J Invest Dermatol 2:67
Blank IH (1952) Factors which influence the water content of stratum corneum. J Invest Dermatol 18:433–440
Blank IH, Scheuplein RJ (1973) Mechanism of percutaneous absorption. IV. Penetration of nonelectrolytes (alcohols) from aqueous solutions and from pure liquids. J Invest Dermatol 60(5):286–296
Bond JR, Barry BW (1988a) Damaging effect of acetone on the permeability barrier of hairless mouse skin compared with that of human-skin. Int J Pharm 41(1–2):91–93
Bond JR, Barry BW (1988b) Hairless mouse skin is limited as a model for assessing the effects of penetration enhancers in human-skin. J Invest Dermatol 90(6):810–813
Bond JR, Barry BW (1988c) Limitations of hairless mouse skin as a model for in vitro permeation studies through human-skin – hydration damage. J Invest Dermatol 90(4):486–489
Bonina FP, Montenegro L et al (1993) In-vitro percutaneous-absorption evaluation of phenobarbital through hairless mouse, adult and premature human skin. Int J Pharm 98(1–3):93–99
Borgia SL, Schupp P et al (2008) In vitro skin absorption and drug release – a comparison of six commercial prednicarbate preparations for topical use. Eur J Pharm Biopharm 68(2):380–389
Bos JD, Meinardi MHM (2000) The 500 dalton rule for the skin penetration of chemical compounds and drugs. Exp Dermatol 9(3):165–169
Bosman IJ, Lawant AL et al (1996) Novel diffusion cell for in vitro transdermal permeation, compatible with automated dynamic sampling. J Pharm Biomed Anal 14(8–10):1015–1023
Botham PA (2004) The validation of in vitro methods for skin irritation. Toxicol Lett 149(1–3):387–390
Bouwstra JA (1997) The skin, a well organized membrane. Colloids Surf A 123–124:403–413
Bouwstra JA, Gooris GS et al (1998) Role of ceramide 1 in the molecular organisation of the stratum corneum lipids. J Lipid Res 39:186–196
Boyce ST, Christianson DJ et al (1988) Structure of a collagen-gag dermal skin substitute optimized for cultured human epidermal-keratinocytes. J Biomed Mater Res 22(10):939–957
Breathnach AS, Goodman T et al (1973) Freeze fracture replication of cells of stratum corneum of human epidermis. J Anat 114:65–81
Breau LM, McGrath PJ et al (2001) Facial expression of children receiving immunizations: a principal components analysis of the child facial coding system. Clin J Pain 17(2):178–186
Bronaugh RL, Collier S (1991) Preparation of human and animal skin. In: Bronaugh RL, Maibach HI (eds) In vitro percutaneous absorption: principles, fundamentals, and applications. CRC Press, Boca Raton, pp 1–6
Bronaugh RL, Stewart RF (1984) Methods for in vitro percutaneous-absorption studies. 3. Hydrophobic compounds. J Pharm Sci 73(9):1255–1258
Bronaugh RL, Stewart RF (1986) Methods for in vitro percutaneous-absorption studies. 6. Preparation of the barrier layer. J Pharm Sci 75(5):487–491
Bronaugh RL, Stewart RF et al (1982) Methods for in vitro percutaneous absorption studies II. Animal models for human skin. Toxicol Appl Pharmacol 62(3):481–488
Bronaugh RL, Stewart RF et al (1983) Differences in permeability of rat skin related to sex and body site. J Soc Cosmet Chem 34(3):127–135
Bronaugh RL, Stewart RF et al (1986) Methods for in vitro percutaneous-absorption studies. 7. Use of excised human-skin. J Pharm Sci 75(11):1094–1097
Brown DWC, Ulsamer AG (1975) Percutaneous penetration of hexachlorophene as related to receptor solutions. Food Cosmet Toxicol 13(1):81–86
Burnette RR, Bagniefski TM (1988) Influence of constant current iontophoresis on the impedance and passive na + permeability of excised nude-mouse skin. J Pharm Sci 77(6):492–497
Carr RD, Wieland RG (1966) Corticosteroid reservoir in stratum corneum. Arch Dermatol 94(1):81–84
Caspers PJ, Williams AC et al (2002) Monitoring the penetration enhancer dimethyl sulfoxide in human stratum corneum in vivo by confocal Raman spectroscopy. Pharm Res 19(10):1577–1580
Chen H, Langer R (1998) Oral particulate delivery: status and future trends. Adv Drug Deliv Rev 34(2–3):339–350
Chen YP, Shen YY et al (2006) Transdermal protein delivery by a coadministered peptide identified via phage display. Nat Biotechnol 24(4):455–460
Chilcott RP, Dalton CH et al (2002) Transepidermal water loss does not correlate with skin barrier function in vitro. J Invest Dermatol 118(5):871–875
Cho CW, Choi JS et al (2008) Development of the ambroxol gels for enhanced transdermal delivery. Drug Dev Ind Pharm 34(3):330–335
Christ A, Szurkowski J et al (2001) Drug penetration into a membrane investigated by photoacoustic and FTIR-ATR spectroscopy. Anal Sci 17:S371–S373
Coldman MF, Poulsen BJ et al (1969) Enhancement of percutaneous absorption by use of volatile – nonvolatile systems as vehicles. J Pharm Sci 58(9):1098–1102
Cooper ER (1982) Effect of decylmethyl sulfoxide on skin penetration. In: Mittal KL, Fendler EJ (eds) Solution behaviour of surfactants: theoretical and applied aspects. Plenum Press, New York, pp 1505–1516
Cornwell PA, Barry BW (1995) Effects of penetration enhancer treatment on the statistical distribution of human skin permeabilities. Int J Pharm 117(1):101–112
Corrigan OI, Farvar MA et al (1980) Drug membrane-transport enhancement using high-energy drug polyvinylpyrrolidone (PVP) co-precipitates. Int J Pharm 5(3):229–238
Coutelegros A, Maitani Y et al (1992) Combined effects of ph, cosolvent and penetration enhancers on the in vitro buccal absorption of propranolol through excised hamster-cheek pouch. Int J Pharm 84(2):117–128
Crank J (1975) The Mathematics of diffusion. Oxford University Press, Inc., New York
Cronin MTD, Dearden JC et al (1998) An investigation of the mechanism of flux across polydimethylsiloxane membranes by use of quantitative structure-permeability relationships. J Pharm Pharmacol 50(2):143–152
Crooke RM, Crooke ST et al (1996) Effect of antisense oligonucleotides on cytokine release from human keratinocytes in an in vitro model of skin. Toxicol Appl Pharmacol 140(1):85–93
Cross SE, Pugh WJ et al (2001) Probing the effect of vehicles on topical delivery: understanding the basic relationship between solvent and solute penetration using silicone membranes. Pharm Res 18(7):999–1005
Curdy C, Kalia YN et al (2002) Post-iontophoresis recovery of human skin impedance in vivo. Eur J Pharm Biopharm 53(1):15–21
Curdy C, Naik A et al (2004) Non-invasive assessment of the effect of formulation excipients on stratum corneum barrier function in vivo. Int J Pharm 271(1–2):251–256
Davis DA (1990) TestSkin racks up in vitro converts. Drug Cosmet Indust 146(5):40
de Jager M, Groenink W et al (2006a) A novel in vitro percutaneous penetration model: evaluation of barrier properties with P-aminobenzoic acid and two of its derivatives. Pharm Res 23(5):951–960
de Jager M, Groenink W et al (2006b) Preparation and characterization of a stratum corneurn substitute for in vitro percutaneous penetration studies. Biochim Biophys Acta Biomembranes 1758(5):636–644
de Kruyff B, van Dijck PWM et al (1974) Non random distribution of cholesterol in phosphatidylcholine bilayers. Biochim Biophys Acta 356:1–7
Delgado-Charro MB, Guy RH (2001) Transdermal iontophoresis for controlled drug delivery and non-invasive monitoring. STP Pharma Sci 11(6):403–414
Demeere ALJ, Tomlinson E (1984) Physicochemical description of the absorption rate of a solute between water and 2,2,4-trimethylpentane. Int J Pharm 22(2–3):177–196
Denet AR, Vanbever R et al (2004) Skin electroporation for transdermal and topical delivery. Adv Drug Deliv Rev 56(5):659–674
Dewhurst DG, Williams A (1993) Frog-skin – a computer-simulation of experiments performed on frog-skin in-vitro to investigate the epithelial transport of ions. ATLA Altern Lab Anim 21(3):350–358
Dick IP, Scott RC (1992) Pig ear skin as an in vitro model for human skin permeability. J Pharm Pharmacol 44(8):640–645
Domashenko A, Gupta S et al (2000) Efficient delivery of transgenes to human hair follicle progenitor cells using topical lipoplex. Nat Biotechnol 18(4):420–423
Downing DT (1992) Lipid and protein structures in the permeability barrier of mammalian epidermis. J Lipid Res 33:301–313
Drachman D (1989) Novel drug delivery systems-opportunities and caveats. Neurobiol Aging 10(5):632–633
Draize JH (1942) The determination of pH of the skin of man and common laboratory animals. J Invest Dermatol 5:77
Dreher F, Fouchard F et al (2002) Comparison of cutaneous bioavailability of cosmetic preparations containing caffeine or alpha-tocopherol applied on human skin models or human skin ex vivo at finite doses. Skin Pharmacol Appl Skin Physiol 15:40–58
Du Plessis J, Pugh WJ et al (2002) The effect of the nature of H-bonding groups on diffusion through PDMS membranes saturated with octanol and toluene. Eur J Pharm Sci 15(1):63–69
Dugard PH, Scheuple RJ (1973) Effects of ionic surfactants on permeability of human epidermis – electrometric study. J Invest Dermatol 60(5):263–269
Dugard PH, Walker M et al (1984) Absorption of some glycol ethers through human-skin in vitro. Environ Health Perspect 57:193–197
Dujardin N, Staes E et al (2002) In vivo assessment of skin electroporation using square wave pulses. J Control Release 79(1–3):219–227
Duncan EJ, Brown A, Lundy P, Sawyer TW, Hamilton M, Hill I, Conley JD (2002) Site-specific percutaneous absorption of methyl salicylate and VX in domestic swine. J Appl Toxicol 22(3):141–148
Dupuis D, Rougier A et al (1986) In vivo relationship between percutaneous absorption and transepidermal water loss according to anatomic site in man. J Soc Cosmet Chem 37(5):351–357
Dyer A, Hayes GG et al (1979) Diffusion through skin and model systems. Int J Cosmet Sci 1(2):91–100
Eder I, Müller-Goymann CC (1995) In vivo amino acid extraction from human stratum corneum as indicator for penetration enhancing properties of oleic acid and isopropylmyristate. Pharm Pharmacol Lett 1:14–17
El Ghalbzouri A, Lamme EN et al (2004) The use of PEGT/PBT as a dermal scaffold for skin tissue engineering. Biomaterials 25(15):2987–2996
Elewski BE (2007) Percutaneous absorption kinetics of topical metronidazole formulations in vitro in the human cadaver skin model. Adv Ther 24(2):239–246
Elgorashi AS, Heard CM et al (2008) Transdermal delivery enhancement of haloperidol from gel formulations by 1,8-cineole. J Pharm Pharmacol 60(6):689–692
Elias PM, Friend DS (1975) The permeability barrier in mammalian epidermis. J Cell Biol 65:180–191
Elias PM, McNutt NS et al (1977) Membrane alterations during cornification of mammalian squamous epithelia: a freeze-fracture, tracer and thin-section study. Anat Rec 189:577–594
Elias PM, Brown BE et al (1980) The permeability barrier in essential fatty-acid deficiency – evidence for a direct role for linoleic-acid in barrier function. J Invest Dermatol 74(4):230–233
Elias PM, Cooper ER et al (1981) Percutaneous transport in relation to stratum-corneum structure and lipid-composition. J Invest Dermatol 76(4):297–301
Elyan BM, Sidhom MB et al (1996) Evaluation of the effect of different fatty acids on the percutaneous absorption of metaproterenol sulfate. J Pharm Sci 85(1):101–105
Engström,S, Forslind B et al (1995) Lipid polymorphism-a key to the understanding of skin penetration. In: Brain KR, James VJ, Walters KA (eds) Proceedings of prediction of percutaneous penetration, vol 4b. STS Publishing Ltd., Cardiff, pp 163–166
Escobar-Chavez JJ, Quintanar-Guerrero D et al (2005) In vivo skin permeation of sodium naproxen formulated in pluronic F-127 gels: effect of Azone (R) and Transcutol (R). Drug Dev Ind Pharm 31(4–5):447–454
Faller C, Bracher M (2002) Reconstructed skin kits: reproducibility of cutaneous irritancy testing. Skin Pharmacol Appl Skin Physiol 15:74–91
Farinha A, Toscano C et al (2003) Permeation of naproxen from saturated solutions and commercial formulations through synthetic membranes. Drug Dev Ind Pharm 29(4):489–494
Feingold K, Qiang M et al (1990) Cholesterol synthesis is required for cutaneous barrier function in mice. J Clin Invest 86:1738–1745
Fenske DB, Thewalt JL et al (1994) Models of stratum corneum intercellular membranes: 2H NMR of macroscopically oriented multilayers. Biophys J 67:1562–1573
Fettiplace R, Haydon DA (1980) Water permeability of lipid membranes. Phys Rev 60:510–550
Flamand N, Marrot L et al (2006) Development of genotoxicity test procedures with Episkin®, a reconstructed human skin model: towards new tools for in vitro risk assessment of dermally applied compounds? Mut Res (Genetic Toxicology and Environmental Mutagenesis) 606(1–2):39–51
Fluhr J, Kao J et al (2001) Generation of free fatty acids from phospholipids regulates stratum corneum acidification and integrity. J Invest Dermatol 117:44–51
Fluhr JW, Feingold KR et al (2006) Transepidermal water loss reflects permeability barrier status: validation in human and rodent in vivo and ex vivo models. Exp Dermatol 15(7):483–492
Flynn GL (1985) Mechanism of percutaneous absorption from physicochemical evidence. In: Bronaugh RL, Maibach H (eds) Percutaneous absorption: mechanisms-methodology-drug delivery. Marcel Dekker, New York, pp 17–42
Flynn GL, Smith EW (1971) Membrane diffusion I: design and testing of a new multifeatured diffusion cell. J Pharma Sci 60(11):1713–1717
Foldvari M, Oguejiofor CJ et al (1998) Transcutaneous delivery of prostaglandin E1: in vitro and laser doppler flowmetry study. J Pharm Sci 87(6):721–725
Foreman MI, Kelly I (1976) The diffusion of nandrolone through hydrated human cadaver skin. Br J Dermatol 95(3):265–270
Foreman MI, Picton W et al (1979) Effect of topical crude coal-tar treatment on unstimulated hairless hamster skin. Br J Dermatol 100(6):707–715
Forslind B (1994) A domain mosaic model of the skin barrier. Acta Derm Venereol 74:1–6
Fransson J, Heffler LC et al (1998) Culture of human epidermal Langerhans cells in a skin equivalent. Br J Dermatol 139(4):598–604
Frantz SW (1990) Instrumentation and methodology for in vitro diffusion cells. In: Kemppainen BW, Reifenrath WG (eds) Methods for skin absorption. CRC Press, Boca Raton, pp 35–59
Franz TJ (1975) Percutaneous absorption – relevance of in vitro data. J Invest Dermatol 64(3):190–195
Franz TJ (1978) The finite dose technique as a valid in vitro model for the study of percutaneous absorption in man. Curr Probl Dermatol 7:58–68
Fredriksson T (1961) Studies on percutaneous absorption of parathion and paraoxon. 2. Distribution of 32p-labelled parathion within skin. Acta Derm Venereol 41(5):344
Friberg SE, Kayali I (1989) Water evaporation rates from a model of stratum-corneum lipids. J Pharm Sci 78(8):639–643
Friberg SE, Kayali I et al (1990) Water permeation of reaggregated stratum-corneum with model lipids. J Invest Dermatol 94(3):377–380
Friend DR (1992) In vitro skin permeation techniques. J Control Release 18(3):235–248
Frum Y, Eccleston GM et al (2007) Evidence that drug flux across synthetic membranes is described by normally distributed permeability coefficients. Eur J Pharm Biopharm 67(2):434–439
Galey WR, Lonsdale HK et al (1976) In vitro permeability of skin and buccal mucosa to selected drugs and tritiated-water. J Invest Dermatol 67(6):713–717
Goates CY, Knutson K (1993) Enhanced permeation and stratum-corneum structural alterations in the presence of dithiothreitol. Biochim Biophys Acta 1153(2):289–298
Gotter B, Faubel W et al (2008) Optical methods for measurements of skin penetration. Skin Pharmacol Physiol 21(3):156–165
Grasso P, Lansdown AB (1972) Methods of measuring, and factors affecting, percutaneous absorption. J Soc Cosmet Chem 23(8):481
Gray GM, Yardley HJ (1975) Lipid compositions of cells isolated from pig, human, and rat epidermis. J Lipid Res 16(6):434–440
Green H, Kehinde O et al (1979) Growth of cultured human epidermal-cells into multiple epithelia suitable for grafting. Proc Natl Acad Sci U S A 76(11):5665–5668
Green H, Fuchs E et al (1982) Differentiated structural components of the keratinocyte. Cold Spring Harbor Symp Quant Biol 1:293–301
Gschwind H-P, Waldmeier F et al (2008) Pimecrolimus: skin disposition after topical administration in minipigs in vivo and in human skin in vitro. Eur J Pharm Sci 33(1):9–19
Gummer CL, Maibach HI (1991) Diffusion cell design. In: Bronaugh RL, Maibach HI (eds) In vitro percutaneous absorption: principles, fundamentals, and applications. CRC Press, Boca Raton, pp 7–16
Gummer CL, Hinz RS et al (1987) The skin penetration cell – a design update. Int J Pharm 40(1–2):101–104
Guy RH (1996) Current status and future prospects of transdermal drug delivery. Pharm Res 13(12):1765–1769
Gysler A, Kleuser B et al (1999) Skin penetration and metabolism of topical glucocorticoids in reconstructed epidermis and in excised human skin. Pharm Res 16(9):1386–1391
Hadgraft J, Ridout G (1987) Development of model membranes for percutaneous absorption measurements. I. Isopropyl myristate. Int J Pharm 39(1–2):149–156
Hadgraft J, Ridout G (1988) Development of model membranes for percutaneous absorption measurements. II. Dipalmitoyl phosphatidylcholine, linoleic acid and tetradecane. Int J Pharm 42(1–3):97–104
Hai NT, Kim J et al (2008) Formulation and biopharmaceutical evaluation of transdermal patch containing benztropine. Int J Pharm 357(1–2):55–60
Haigh JM, Smith EW (1994) The selection and use of natural and synthetic membranes for in-vitro diffusion experiments. Eur J Pharm Sci 2(5–6):311–330
Harrison SM, Barry BW et al (1984) Effects of freezing on human-skin permeability. J Pharm Pharmacol 36(4):261–262
Hashimot K (1971a) Demonstration of intercellular spaces of human eccrine sweat gland by lanthanum. 1. Secretory coil. J Ultrastruct Res 36(1–2):249
Hashimot K (1971b) Demonstration of intercellular spaces of human eccrine sweat gland by lanthanum. 2. Duct. J Ultrastruct Res 37(5–6):504
Hashimot K, Gross BG et al (1965) Ultrastructure of skin of human embryos. i. Intraepidermal eccrine sweat duct. J Invest Dermatol 45(3):139
Hawkins GS, Reifenrath WG (1986) Influence of skin source, penetration cell fluid, and partition-coefficient on in vitro skin penetration. J Pharm Sci 75(4):378–381
Hayden PJ, Burnham B et al (2004) Wound healing response or a full thickness in vitro human skin equivalent (EpiDerm-FT 200) after solar UV-irradiation. J Invest Dermatol 122(3):A141
Hayden PJ, Petrali JP et al (2005) Development of a full thickness in vitro human skin equivalent (EpiDerm-FT) for sulfur mustard research. J Invest Dermatol 124(4):A29
He W, Guo XX et al (2008) Transdermal permeation enhancement of N-trimethyl chitosan for testosterone. Int J Pharm 356(1–2):82–87
Hedberg CL, Wertz PW et al (1988) The time course of biosynthesis of epidermal lipids. J Invest Dermatol 91(2):169–174
Herkenne C, Alberti I et al (2008) In vivo methods for the assessment of topical drug bioavailability. Pharm Res 25(1):87–103
Hinz RS, Hodson CD et al (1989) In vitro percutaneous penetration – evaluation of the utility of hairless mouse skin. J Invest Dermatol 93(1):87–91
Hoelgaard A, Mollgaard B (1982) Permeation of linoleic-acid through skin in vitro. J Pharm Pharmacol 34(9):610–611
Holbrook KA, Odland GF (1974) Regional differences in thickness (cell layers) of human stratum-corneum – ultrastructural analysis. J Invest Dermatol 62(4):415–422
Holland JM, Kao JY et al (1984) A multisample apparatus for kinetic evaluation of skin penetration in vitro – the influence of viability and metabolic status of the skin. Toxicol Appl Pharmacol 72(2):272–280
Holland DB, Bojar RA et al (2008) Microbial colonization of an in vitro model of a tissue engineered human skin equivalent – a novel approach. FEMS Microbiol Lett 279(1):110–115
Hou SYE, Flynn GL (1997) Influences of 1-dodecylazacycloheptan-2-one on permeation of membranes by weak electrolytes. 1. Theoretical analysis of weak electrolyte diffusion through membranes and studies involving silicone rubber membranes. J Pharm Sci 86(1):85–91
Houk J, Guy RH (1988) Membrane models for skin penetration studies. Chem Rev 88(3):455–471
Hueber F, Besnard M et al (1994) Percutaneous-absorption of estradiol and progesterone in normal and appendage-free shin of the hairless rat – lack of importance of nutritional blood-flow. Skin Pharmacol 7(5):245–256
Hughes MF, Fisher HL et al (1994) Effect of age on the in-vitro percutaneous-absorption of phenols in mice. Toxicol In Vitro 8(2):221–227
Ito K, Kato Y et al (2007) Involvement of organic anion transport system in transdermal absorption of flurbiprofen. J Control Release 124(1–2):60–68
Jacobi U, Kaiser M et al (2007) Porcine ear skin: an in vitro model for human skin. Skin Res Technol 13(1):19–24
Kalia YN, Guy RH (1997) Interaction between penetration enhancers and iontophoresis: effect on human skin impedance in vivo. J Control Release 44(1):33–42
Kalia YN, Nonato LB et al (1996) The effect of iontophoresis on skin barrier integrity: non-invasive evaluation by impedance spectroscopy and transepidermal water loss. Pharm Res 13(6):957–960
Kandarova H, Liebsch M et al (2006) Assessment of the human epidermis model SkinEthic RHE for in vitro skin corrosion testing of chemicals according to new OECD TG 431. Toxicol In Vitro 20(5):547–559
Kandarova H, Richter H et al (2007) Stratum corneum architecture of reconstructed human skin models monitored by fluorescent confocal laser scanning microscopy. Laser Phys Lett 4(4):308–311
Kane A, Lloyd J et al (1999) Transmission of hepatitis B, hepatitis C and human immunodeficiency viruses through unsafe injections in the developing world: model-based regional estimates. Bull World Health Organ 77(10):801–807
Kanikkannan N, Singh M (2002) Skin permeation enhancement effect and skin irritation of saturated fatty alcohols. Int J Pharm 248(1–2):219–228
Kao J, Hall J et al (1983) Quantitation of cutaneous toxicity – an in vitro approach using skin organ-culture. Toxicol Appl Pharmacol 68(2):206–217
Kao J, Hall J et al (1988) In vitro percutaneous-absorption in mouse skin – influence of skin appendages. Toxicol Appl Pharmacol 94(1):93–103
Karande P, Mitragotri S (2003) Dependence of skin permeability on contact area. Pharm Res 20(2):257–263
Karande P, Jain A et al (2004) Discovery of transdermal penetration enhancers by high-throughput screening. Nat Biotechnol 22(2):192–197
Karande P, Jain A et al (2005) Design principles of chemical penetration enhancers for transdermal drug delivery. Proc Natl Acad Sci U S A 102(13):4688–4693
Karande P, Jain A et al (2006a) Relationships between skin’s electrical impedance and permeability in the presence of chemical enhancers. J Control Release 110(2):307–313
Karande P, Jain A et al (2006b) Synergistic combinations of penetration enhancers and their discovery by high-throughput screening. In: Katdare A, Chaubal M (eds) Excipient development for pharmaceutical, biotechnology, and drug delivery systems. Informa Healthcare, New York/London
Kermode M (2004) Unsafe injections in low-income country health settings: need for injection safety promotion to prevent the spread of blood-borne viruses. Health Promot Int 19(1):95–103
Kietzmann M, Loscher W et al (1993) The isolated-perfused bovine udder as an in-vitro model of percutaneous drug absorption skin viability and percutaneous-absorption of dexamethasone, benzoyl peroxide, and etofenamate. J Pharmacol Toxicol Methods 30(2):75–84
Kim YC, Park JH et al (2008) Synergistic enhancement of skin permeability by N-lauroylsarcosine and ethanol. Int J Pharm 352(1–2):129–138
Kitson N, Thewalt J et al (1994) A model membrane approach to the epidermal permeability barrier. Biochemistry 33(21):6707–6715
Kittayanond D, Dowton SM et al (1992) Development of a model of the lipid constituent phase of the stratum-corneum. 2. Preparation of artificial membranes from synthetic lipids and assessment of permeability properties using in vitro diffusion experiments. J Soc Cosmet Chem 43(5):237–249
Kolli CS, Banga AK (2008) Characterization of solid maltose microneedles and their use for transdermal delivery. Pharm Res 25(1):104–113
Kreilgaard M (2002) Assessment of cutaneous drug delivery using microdialysis. Adv Drug Deliv Rev 54(Suppl 1):S99–S121
Kuempel D, Swartzendruber DC et al (1998) In vitro reconstitution of stratum corneum lipid lamellae. Biochim Biophys Acta Biomembranes 1372(1):135–140
Kumar MG, Lin SS (2008) Transdermal iontophoresis: impact on skin integrity as evaluated by various methods. Crit Rev Ther Drug Carrier Syst 25(4):381–401
Lackermeier AH, McAdams ET et al (1999) In vivo AC impedance spectroscopy of human skin: theory and problems in monitoring of passive percutaneous drug delivery. Ann N Y Acad Sci 873(Electrical Bioimpedance Methods: Applications to Medicine and Biotechnology):197–213
Lademann J, Richter H et al (2006) Hair follicles – a long-term reservoir for drug delivery. Skin Pharmacol Physiol 19(4):232–236
Lakshminarayanaiah N (1965) Transport phenomena in artificial membranes. Chem Rev 65(5):491
Langer R (1990) Novel drug delivery systems. Chem Br 26(3):232
Langguth P, Spahn H et al (1986) Variations of in vitro nitroglycerine permeation through human-epidermis. Pharm Res 3(1):61–63
Larsen CG, Larsen FG et al (1988) Preparation of human epidermal tissue for functional immune studies. Acta Derm Venereol 68(6):474–479
Lashmar UT, Hadgraft J et al (1989) Topical application of penetration enhancers to the skin of nude-mice – a histopathological study. J Pharm Pharmacol 41(2):118–121
Lawler JC, Davis MJ et al (1960) Electrical characteristics of the skin – the impedance of the surface sheath and deep tissues. J Invest Dermatol 34(5):301–308
Le VH, Lippold BC (1995) Influence of physicochemical properties of homologous esters of nicotinic-acid on skin permeability and maximum flux. Int J Pharm 124(2):285–292
Lee D-Y, Ahn H-T et al (2000) A new skin equivalent model: dermal substrate that combines de-epidermized dermis with fibroblast-populated collagen matrix. J Dermatol Sci 23(2):132–137
Lee RS, Watkinson A et al (2001) Barrier function of the axillary stratum corneum. J Invest Dermatol 117(3):810–810
Lee J-N, Jee S-H et al (2008) The effects of depilatory agents as penetration enhancers on human stratum corneum structures. J Invest Dermatol 128:2240–2247
Leopold CS, Lippold BC (1992) A new application chamber for skin penetration studies in vivo with liquid preparations. Pharm Res 9(9):1215–1218
Levin J, Maibach H (2005) The correlation between transepidermal water loss and percutaneous absorption: an overview. J Control Release 103(2):291–299
Li LN, Hoffman RM (1997) Topical liposome delivery of molecules to hair follicles in mice. J Dermatol Sci 14(2):101–108
Li LN, Lishko V et al (1993) Liposome targeting of high-molecular-weight DNA to the hair-follicles of histocultured skin – a model for gene-therapy of the hair-growth processes. In Vitro Cell Dev Biol Anim 29A(4):258–260
Li SK, Suh W et al (1998) Lag time data for characterizing the pore pathway of intact and chemically pretreated human epidermal membrane. Int J Pharm 170(1):93–108
Li SK, Ghanem AH et al (1999) Pore induction in human epidermal membrane during low to moderate voltage iontophoresis: a study using AC iontophoresis. J Pharm Sci 88(4):419–427
Li Q, Tsuji H et al (2006) Characterization of the transdermal transport of flurbiprofen and indomethacin. J Control Release 110(3):542–556
Lieb LM, Ramachandran C et al (1992) Topical delivery enhancement with multilamellar liposomes into pilosebaceous units. 1. In vitro evaluation using fluorescent techniques with the hamster ear model. J Invest Dermatol 99(1):108–113
Lieckfeldt R, Villalain J et al (1993) Diffusivity and structural polymorphism in some model stratum-corneum lipid systems. Biochim Biophys Acta 1150(2):182–188
Loden M (1992) The increase in skin hydration after application of emollients with different amounts of lipids. Acta Derm Venereol 72(5):327–330
Loftsson T (1982) Experimental and theoretical model for studying simultaneous transport and metabolism of drugs in excised skin. Arch Pharm Chem Sci Ed 10:17
Long SA, Wertz PW et al (1985) HUman stratum corenum polar lipids and desquamation. Arch Dermatol Res 277:284–287
Lotte C, Patouillet C et al (2002) Permeation and skin absorption: reproducibility of various industrial reconstructed human skin models. Skin Pharmacol Appl Skin Physiol 15:18–30
Luu-The V, Ferraris C et al (2007) Steroid metabolism and profile of steroidogenic gene expression in Episkin (TM): high similarity with human epidermis. J Steroid Biochem Mol Biol 107(1–2):30–36
Luzardo-Alvarez A, Delgado-Charro MB et al (2003) In vivo iontophoretic administration of ropinirole hydrochloride. J Pharm Sci 92(12):2441–2448
Mackee GM, Sulzberger MB et al (1945) Histologic studies on percutaneous penetration with special reference to the effect of vehicles. J Invest Dermatol 6(1):43–61
Madison KC, Swartzendruber DC et al (1987) Presence of intercellular lipid lamellae in the upper layers of stratum corneum. J Invest Dermatol 88:714–718
Mak VHW, Cumpstone MB et al (1991) Barrier function of human keratinocyte cultures grown at the air-liquid interface. J Invest Dermatol 96(3):323–327
Malkinson FD, Ferguson EH (1955) Percutaneous absorption of hydrocortisone-4-c-14 in 2 human subjects. J Invest Dermatol 25(5):281–283
Mansbridge J (2002) Tissue-engineered skin substitutes. Expert Opin Biol Ther 2(1):25–34
Marty P, Faure C et al (1997) Assessment of human skins obtained by in vitro culture as membrane models for cutaneous permeation tests. In: Brain KR, James VJ, Walters KA (eds) Perspectives in percutaneous penetration. STS Publishing, Cardiff, p 64
Masson M, Sigfusson SD et al (2002) Fish skin as a model membrane to study transmembrane drug delivery with cyclodextrins. J Incl Phenom Macrocycl Chem 44(1–4):177–182
Mathy FX, Ntivunwa D et al (2005) Fluconazole distribution in rat dermis following intravenous and topical application: a microdialysis study. J Pharm Sci 94(4):770–780
Matoltsy AG (1986) The skin of mammals: structure and function of the mammalian epidermis. In: BereiterHahn J, Matoltsy AG, Richards KS (eds) Biology of the integument. 2. Vertebrates. Berlin, Springer, pp 255–277
Matoltsy AG, Downes AM et al (1968) Studies of the epidermal water barrier. Part II. Investigation of the chemical nature of the water barrier. J Invest Dermatol 50:19–26
Matsuzaki K, Imaoka T et al (1993) Development of a model membrane system using stratum-corneum lipids for estimation of drug skin permeability. Chem Pharm Bull 41(3):575–579
McAllister DV, Allen MG et al (2000) Microfabricated microneedles for gene and drug delivery. Annu Rev Biomed Eng 2:289–313
McCullough J, Ramirez J et al (2006) In vitro percutaneous absorption of a novel topical benzoyl peroxide formulation in human cadaver skin layers. J Am Acad Dermatol 54(3):AB29
McMullen TPW, McElhaney RN (1995) New aspects of the interactions of cholesterol with dipalmitoyl phosphatidylcholine bilayers as revealed by high-sensitivity differential scanning calorimetry. Biochim Biophys Acta 1234:90–98
Megrab NA, Williams AC et al (1995) Estradiol permeation across human skin, silastic and snake skin membranes – the effects of ethanol-water cosolvent systems. Int J Pharm 116(1):101–112
Mendelsohn R, Flach CR et al (2006) Determination of molecular conformation and permeation in skin via IR spectroscopy, microscopy, and imaging. Biochim Biophys Acta Biomembranes 1758(7):923–933
Menon GK, Elias PM (1997) Morphologic basis for a pore-pathway in mammalian stratum corneum. Skin Pharmacol 10:235–246
Menon GK, Lee SH et al (1998) Ultrastructural effects of some solvents and vehicles on the stratum corneum and other skin components: evidence for an “extended mosaic partitioning” model of the skin barrier. In: Roberts MS, Walters KA (eds) Dermal absorption and toxicity assessment. Marcel Dekker, New York, pp 727–751
Michaels AS, Chandrasekaran SK et al (1975) Drug permeation through human skin. Theory and in vitro experimental measurements. AIChE J 21(5):985–996
Michel M, L'Heureux N et al (1999) Characterization of a new tissue-engineered human skin equivalent with hair. In Vitro Cell Dev Biol Anim 35(6):318–326
Miller MA, Pisani E (1999) The cost of unsafe injections. Bull World Health Organ 77(10):808–811
Mitragotri S, Kost J (2004) Low-frequency sonophoresis – a review. Adv Drug Deliv Rev 56(5):589–601
Mitragotri S, Blankschtein D et al (1995) Ultrasound-mediated transdermal protein delivery. Science 269(5225):850–853
Mitragotri S, Blankschtein D et al (1996) Transdermal drug delivery using low-frequency sonophoresis. Pharm Res 13(3):411–420
Miyajima K, Tanikawa S et al (1994) Effects of absorption enhancers and lipid-composition on drug permeability through the model membrane using stratum-corneum lipids. Chem Pharm Bull 42(6):1345–1347
Moghimi HR, Williams AC et al (1996) A lamellar matrix model for stratum corneum intercellular lipids. 1. Characterisation and comparison with stratum corneum intercellular structure. Int J Pharm 131(2):103–115
MonteiroRiviere NA, Inman AO et al (1997) Comparison of an in vitro skin model to normal human skin for dermatological research. Microsc Res Tech 37(3):172–179
Moody RP, Nadeau B et al (1995) In-vivo and in-vitro dermal absorption of benzo[a]pyrene in rat, guinea-pig, human and tissue-cultured skin. J Dermatol Sci 9(1):48–58
Moore DJ, Rerek ME (1998) Biophysics of skin barrier lipid organization. J Invest Dermatol 110(4):674
Morell JLP, Claramonte MDC et al (1996) Validation of a release diffusion cell for topical dosage forms. Int J Pharm 137(1):49–55
Morikawa N, Kitagawa T et al (2007) Assessment of the in vitro skin irritation of chemicals using the Vitrolife-Skin™ human skin model. AATEX 14:417–423
Moser K, Kriwet K et al (2001) Permeation enhancement of a highly lipophilic drug using supersaturated systems. J Pharm Sci 90(5):607–616
Muhammad F, Brooks JD et al (2004) Comparative mixture effects of JP-8(100) additives on the dermal absorption and disposition of jet fuel hydrocarbons in different membrane model systems. Toxicol Lett 150(3):351–365
Nabila Sekkat RHG (2001) Biological models to study skin permeation. In: Testa B, van de Waterbeemd H, Folkers G, Guy R (eds) Pharmacokinetic optimization in drug research. Verlag Helvetica Chimica Acta, Postfach, CH-8042 Zürich, Switzerland pp 155–172
Nacht S, Yeung D et al (1981) Benzoyl peroxide – percutaneous penetration and metabolic disposition. J Am Acad Dermatol 4(1):31–37
Naik A, Kalia YN et al (2001) Characterization of molecular transport across human stratum corneum in vivo. J Toxicol Cutan Ocular Toxicol 20(2–3):279–301
Nakamura M, Rikimaru T et al (1990) Full-thickness human skin explants for testing the toxicity of topically applied chemicals. J Invest Dermatol 95(3):325–332
Netzaff F, Lehr CM et al (2005) The human epidermis models EpiSkin (R), SkinEthic (R) and EpiDerm (R): an evaluation of morphology and their suitability for testing phototoxicity, irritancy, corrosivity, and substance transport. Eur J Pharm Biopharm 60(2):167–178
Netzlaff F, Kostka KH et al (2006a) TEWL measurements as a routine method for evaluating the integrity of epidermis sheets in static Franz type diffusion cells in vitro. Limitations shown by transport data testing. Eur J Pharm Biopharm 63(1):44–50
Netzlaff F, Schaefer UF et al (2006b) Comparison of bovine udder skin with human and porcine skin in percutaneous permeation experiments. ATLA Altern Lab Anim 34(5):499–513
Netzlaff F, Kaca M et al (2007) Permeability of the reconstructed human epidermis model Episkin (R) in comparison to various human skin preparations. Eur J Pharm Biopharm 66(1):127–134
Nir Y, Paz A et al (2003) Fear of injections in young adults: prevalence and associations. Am J Trop Med Hyg 68(3):341–344
Norlen L (2001) Skin barrier formation: the membrane folding model. J Invest Dermatol 117(4):823–829
Norlén L (2001) Skin barrier structure and function: the single phase model. J Invest Dermatol 117:830–836
Norlén L, Nicander I et al (1998) A new HPLC-based method for the quantitative analysis of inner stratum corneum lipids with special reference to the free fatty acid fraction. Arch Dermatol Res 290:508–516
Norlen L, Nicander I et al (1999) Inter- and intra-individual differences in human stratum corneum lipid content related to physical parameters of skin barrier function in vivo. J Invest Dermatol 112(1):72–77
Odland GF (1983) Structure of the skin. In: Goldsmith EL (ed) Biochemistry and physiology of the skin. Oxford University Press, New York, pp 3–63
Oertel RP (1977) Protein conformational-changes induced in human stratum-corneum by organic sulfoxides – IR spectroscopic investigation. Biopolymers 16(10):2329–2345
Ogiso T, Hata T et al (2001) Transdermal absorption of bupranolol in rabbit skin in vitro and in vivo. Biol Pharm Bull 24(5):588–591
Ogura M, Pahwal S et al (2008) Low-frequency sonophoresis: current status and future prospects. Adv Drug Deliv Rev 60(10):1218–1223
Ohman H, Vahlquist A (1994) In-vivo studies concerning a ph gradient in human stratum-corneum and upper epidermis. Acta Derm Venereol 74(5):375–379
Okamoto H, Hashida M et al (1988) Structure activity relationship of 1-alkylazacycloalkanone or 1-alkenylazacycloalkanone derivatives as percutaneous penetration enhancers. J Pharm Sci 77(5):418–424
Olivella MS, Debattista NB et al (2006) Salicylic acid permeation: a comparative study with different vehicles and membranes. Biocell 30(2):321–324
Osborne DW (1986) Computational method for predicting skin permeation of chemicals. Pharmaceutical Manufacturing 41–48
Ottaviani G, Martel S et al (2006) Parallel artificial membrane permeability assay: a new membrane for the fast prediction of passive human skin permeability. J Med Chem 49(13):3948–3954
Pabla D, Zia H (2007) A comparative permeation/release study of different testosterone gel formulations. Drug Deliv 15(6):389–396
PageonH, Asselineau D (2005) An in vitro approach to the chronological aging of skin by glycation of the collagen – the biological effect of glycation on the reconstructed skin model. In: Maillard reaction: chemistry at the interface of nutrition, aging, and disease, vol 1043. New York, New York Academy Sciences, pp 529–532
Paliwal S, Menon GK et al (2006) Low-frequency sonophoresis: ultrastructural basis for stratum corneum permeability assessed using quantum dots. J Invest Dermatol 126(5):1095–1101
Panchagnula R, Stemmer K et al (1997) Animal models for transdermal drug delivery. Methods Find Exp Clin Pharmacol 19(5):335–341
Panchagnula R, Pillai O et al (2000) Transdermal iontophoresis revisited. Curr Opin Chem Biol 4(4):468–473
Pellett MA, Roberts MS et al (1997) Supersaturated solutions evaluated with an in vitro stratum corneum tape stripping technique. Int J Pharm 151(1):91–98
Pershing LK, Lambert LD et al (1990) Mechanism of ethanol-enhanced estradiol permeation across human skin in vivo. Pharm Res 7(2):170–175
Pillai O, Nair V et al (2001) Noninvasive transdermal delivery of peptides and proteins. Drugs Future 26(8):779–791
Pinnagoda J, Tupker RA et al (1989) The intra-individual and inter-individual variability and reliability of trans-epidermal water-loss measurements. Contact Dermatitis 21(4):255–259
Pinnagoda J, Tupker RA et al (1990) Guidelines for transepidermal water loss (TEWL) measurement – a report from the standardization group of the European Society of contact dermatitis. Contact Dermatitis 22(3):164–178
Pirot F, Kalia YN et al (1997) Characterization of the permeability barrier of human skin in vivo. Proc Natl Acad Sci U S A 94(4):1562–1567
Pliquett U (1999) Mechanistic studies of molecular transdermal transport due to skin electroporation. Adv Drug Deliv Rev 35(1):41–60
Poelman MC, Piot B et al (1989) Assessment of topical non-steroidal anti-inflammatory drugs. J Pharm Pharmacol 41(10):720–722
Ponec M, Kempenaar J (1995) Use of human skin recombinants as an in-vitro model for testing the irritation potential of cutaneous irritants. Skin Pharmacol 8(1–2):49–59
Ponec M, Boelsma E et al (2000) Lipid and ultrastructural characterization of reconstructed skin models. Int J Pharm 203(1–2):211–225
Ponec M, Boelsma E et al (2002) Characterization of reconstructed skin models. Skin Pharmacol Appl Skin Physiol 15:4–17
Potts RO, Francoeur ML (1993) Infrared spectroscopy of stratum corneum lipids. In: Walters KA, Hadgraft J (eds) Pharmaceutical skin penetration enhancement. Marcel Dekker, New York
Poumay Y, Dupont F et al (2004) A simple reconstructed human epidermis: preparation of the culture model and utilization in in vitro studies. Arch Dermatol Res 296(5):203–211
Prausnitz MR (1997) Transdermal delivery of macromolecules: recent advances by modification of skin’s barrier properties. In: Zahra Shahrokh (ed.) Therapeutic protein and peptide formulation and delivery, vol 675, American Chemical Society symposium series pp 124–153
Prausnitz MR (2004) Microneedles for transdermal drug delivery. Adv Drug Deliv Rev 56(5):581–587
Prausnitz MR, Mitragotri S et al (2004) Current status and future potential of transdermal drug delivery. Nat Rev Drug Discov 3(2):115–124
Pudney PDA, Melot M et al (2007) An in vivo confocal Raman study of the delivery of trans-retinol to the skin. Appl Spectrosc 61(8):804–811
Puglia C, Blasi P et al (2008) Lipid nanoparticles for prolonged topical delivery: an in vitro and in vivo investigation. Int J Pharm 357(1–2):295–304
Qiang M, Engström S et al (1993) Fatty acids are required for epidermal permeability barrier function. J Clin Invest 92:791–798
Quisno RA, Doyle RL (1983) A new occlusive patch test system with a plastic chamber. J Soc Cosmet Chem 34(1):13–19
Rao VU, Misra AN (1994) Effect of penetration enhancers on transdermal absorption of insulin across human cadaver skin. Drug Dev Ind Pharm 20(16):2585–2591
Regnier M, Patwardhan A et al (1998) Reconstructed human epidermis composed of keratinocytes, melanocytes and Langerhans cells. Med Biol Eng Comput 36(6):821–824
Rehder J, Souto LR et al (2004) Model of human epidermis reconstructed in vitro with keratinocytes and melanocytes on dead de-epidermized human dermis. Sao Paulo Med J 122:22–25
Remane Y, Leopold CS et al (2006) Percutaneous penetration of methyl nicotinate from ointments using the laser Doppler technique: bioequivalence and enhancer effects. J Pharmacokinet Pharmacodyn 33(6):719–735
Rice RH, Green H (1977) The cornified envelope of terminally differentiated human epidermal keratinocytes consists of cross linked proteins. Cell 11:417–422
Rigg PC, Barry BW (1990) Shed snake skin and hairless mouse skin as model membranes for human skin during permeation studies. J Invest Dermatol 94(2):235–240
Riviere JE, Sage B et al (1991) Effects of vasoactive drugs on transdermal lidocaine iontophoresis. J Pharm Sci 80(7):615–620
Robert Peter Chilcott RF (2000) Biophysical measurements of human forearm skin in vivo: effects of site, gender, chirality and time. Skin Res Technol 6(2):64–69
Rocha JCB, Pedrochi F et al (2007) Ex vivo evaluation of the percutaneous penetration of proanthocyanidin extracts from Guazuma ulmifolia using photoacoustic spectroscopy. Anal Chim Acta 587(1):132–136
Rochefort A, Druot P et al (1986) A new technique for the evaluation of cosmetics effect on mechanical-properties of stratum-corneum and epidermis in vitro. Int J Cosmet Sci 8(1):27–36
Rodriguez H, O’Connell C et al (2004) Measurement of DNA biomarkers for the safety of tissue-engineered medical products, using artificial skin as a model. Tissue Eng 10(9–10):1332–1345
Roguet R, Cohen C et al (1998) An interlaboratory study of the reproducibility and relevance of Episkin, reconstructed human epidermis, in the assessment of cosmetics irritancy. Toxicol In Vitro 12(3):295–304
Rohatagi S, Barrett JS et al (1997) Selegiline percutaneous absorption in various species and metabolism by human skin. Pharm Res 14(1):50–55
Rossi RCP, de Paiva RF et al (2008) Photoacoustic study of percutaneous absorption of Carbopol and transdermic gels for topic use in skin. Eur Phys J (Special Topics) 153:479–482
Rougier A, Dupuis D et al (1983) Invivo correlation between stratum-corneum reservoir function and percutaneous-absorption. J Invest Dermatol 81(3):275–278
Rougier A, Lotte C et al (1989) In vivo relationship between percutaneous absorption and transepidermal water loss. In: Bronaugh RL, Maibach HI (eds) Percutaneous absorption. Marcel Dekker, New York, pp 175–190
Roy SD, Degroot JS (1994) Percutaneous-absorption of nafarelin acetate, an LHRH analog, through human cadaver skin and monkey skin. Int J Pharm 110(2):137–145
Roy SD, Hou SYE et al (1994) Transdermal delivery of narcotic analgesics – comparative metabolism and permeability of human cadaver skin and hairless mouse skin. J Pharm Sci 83(12):1723–1728
Rutherford T, Black JG (1969) Use of autoradiography to study localization of germicides in skin. Br J Dermatol S81:75
Sandby-Moller J, Poulsen T et al (2003) Epidermal thickness at different body sites: relationship to age, gender, pigmentation, blood content, skin type and smoking habits. Acta Derm Venereol 83(6):410–413
Sato K, Sugibayashi K et al (1991) Species-differences in percutaneous-absorption of nicorandil. J Pharm Sci 80(2):104–107
Schafer-Korting M, Bock U et al (2006) Reconstructed human epidermis for skin absorption testing: results of the German prevalidation study. ATLA Altern Lab Anim 34(3):283–294
Schafer-Korting M, Bock U et al (2008) The use of reconstructed human epidermis for skin absorption testing: results of the validation study. ATLA Altern Lab Anim 36(2):161–187
Schalla W, Schaefer H (1982) Mechanisms of penetration of drugs into the skill. In: Brandau R, Lippold BH (eds) Dermal and transdennal absorption. Wissenschaftlichen Verlag, Stuttgart, pp 41–70
Scheuplein RJ (1965) Mechanism of percutaneous absorption. I. Routes of penetration and the influence of solubility. J Invest Dermatol 45:334–346
Scheuplein RJ (1966) Analysis of permeability data for the case of parallel diffusion pathways. Biophys J 6(1):1–17
Scheuplein RJ (1967) Mechanism of percutaneous absorption. II. Transient diffusion and the relative importance of various routes of skin penetration. J Invest Dermatol 48(1):79–88
Scheuplein RJ (1972) Properties of the skin as a membrane. Adv Biol Skin 12:125–152
Scheuplein RJ (1978) Permeability of the skin: a review of major concepts. Curr Prob Dermatol 7:172–186
Scheuplein RJ, Bronaugh RL (1983) Percutaneous absorption. In: Goldsmith LA, ed. Biochemistry and Physiology of the Skin, Vol. 2. New York: Oxford University Press 1255–1295
Schmook FP, Meingassner JG et al (2001) Comparison of human skin or epidermis models with human and animal skin in in-vitro percutaneous absorption. Int J Pharm 215(1–2):51–56
Schramm-Baxter J, Katrencik J et al (2004) Jet injection into polyacrylamide gels: investigation of jet injection mechanics. J Biomech 37(8):1181–1188
Scott RE, Leahy DE et al (1986) In vitro percutaneous model for quantitative structure-absorption studies. J Pharm Pharmacol 38(Suppl):66
Scott RC, Corrigan MA et al (1991) The influence of skin structure on permeability: an intersite and interspecies comparison with hydrophilic penetrants. J Invest Dermatol 96(6):921–925
Sebastiani P, Nicoli S et al (2005) Effect of lactic acid and iontophoresis on drug permeation across rabbit ear skin. Int J Pharm 292(1–2):119–126
Sekkat N, Kalia YN et al (2002) Biophysical study of porcine ear skin in vitro and its comparison to human skin in vivo. J Pharm Sci 91(11):2376–2381
Shah HS, Tojo K et al (1992) Enhancement of in vitro skin permeation of verapamil. Drug Dev Ind Pharm 18(13):1461–1476
Shah VP, Flynn GL et al (1998) Bioequivalence of topical dermatological dosage forms – methods of evaluation of bioequivalence. Pharm Res 15(2):167–171
Shahabeddin L, Berthod F et al (1990) Characterization of skin reconstructed on a chitosancross-linked collagen-glycosaminoglycan matrix. Skin Pharmacol 3:107–114
Shakespeare P (2001) Burn wound healing and skin substitutes. Burns 27(5):517–522
Shelley WB, Melton FM (1949) Factors accelerating the penetration of histamine through normal intact human skin. J Invest Dermatol 13(2):61–71
Sheth NV, McKeough MB et al (1987) Measurement of the stratum-corneum drug reservoir to predict the therapeutic efficacy of topical iododeoxyuridine for herpes-simplex virus-infection. J Invest Dermatol 89(6):598–602
Shibayama H, Hisama M et al (2008) Permeation and metabolism of a novel ascorbic acid derivative, disodium isostearyl 2-O-L-ascorbyl phosphate, in human living skin equivalent models. Skin Pharmacol Physiol 21(4):235–243
Simon M, Green H (1984) Participation of membrane associated proteins in the formation of the cross linked envelope of the keratinocyte. Cell 36:827–834
Simon GA, Maibach HI (2000) The pig as an experimental animal model of percutaneous permeation in man: qualitative and quantitative observations – an overview. Skin Pharmacol Appl Skin Physiol 13(5):229–234
Sinha VR, Kaur MP (2000) Permeation enhancers for transdermal drug delivery. Drug Dev Ind Pharm 26(11):1131–1140
Sivamani RK, Liepmann D et al (2007) Microneedles and transdermal applications. Expert Opin Drug Deliv 4(1):19–25
Skerrow D, Hunter I (1978) Protein modification during the keratinization of normal and psoriatic epidermis. Biochim Biophys Acta 537:474–484
Slivka SR, Landeen LK et al (1993) Characterization, barrier function, and drug-metabolism of an in vitro skin model. J Invest Dermatol 100(1):40–46
Smith ICH (1993) Frog skin. ATLA Altern Lab Anim 21(3):392
Sobral CS, Gragnani A et al (2007) Inhibition of proliferation of Pseudomonas aeruginosa by KGF in an experimental burn model using human cultured keratinocytes. Burns 33(5):613–620
Sonavane G, Tomoda K et al (2008) In vitro permeation of gold nanoparticles through rat skin and rat intestine: effect of particle size. Colloids Surf B Biointerfaces 65(1):1–10
Soni J, Baird AW et al (2006) Rat, ovine and bovine Peyer’s patches mounted in horizontal diffusion chambers display sampling function. J Control Release 115(1):68–77
Southwell D, Barry BW (1983) Penetration enhancers for human-skin – mode of action of 2-pyrrolidone and dimethylformamide on partition and diffusion of model compounds water, normal-alcohols, and caffeine. J Invest Dermatol 80(6):507–514
Southwell D, Barry BW et al (1984) Variations in permeability of human-skin within and between specimens. Int J Pharm 18(3):299–309
Squier CA (1973) The permeability of keratinized and nonkeratinized oral epithelium to horseradish peroxidase. J Ultrastr Res 43:160–177
Srinivasan V, Higuchi WI (1990) A model for iontophoresis incorporating the effect of convective solvent flow. Int J Pharm 60(2):133–138
Stamatas GN, de Sterke J et al (2008) Lipid uptake and skin occlusion following topical application of oils on adult and infant skin. J Dermatol Sci 50(2):135–142
Steinert PM, Cantieri JS (1983) Epidermal keratins. In: Goldsmith AL (ed) Biochemistry and physiology of the skin, vol I. Oxford University Press, New York, pp 135–169
Stinchcomb AL, Pirot F et al (1999) Chemical uptake into human stratum corneum in vivo from volatile and non-volatile solvents. Pharm Res 16(8):1288–1293
Suh H, Jun HW (1996) Effectiveness and mode of action of isopropyl myristate as a permeation enhancer for naproxen through shed snake skin. J Pharm Pharmacol 48(8):812–816
Suppasrivasuseth J, Bellantone RA et al (2006) Permeability and retention studies of (−)Epicatechin gel formulations in human cadaver skin. Drug Dev Ind Pharm 32(9):1007–1017
Surber C, Schwarb FP et al (1999) Tape-stripping technique. In: Bronough H, Maibach H (eds) Drugs and the pharmaceutical sciences. Marcel Dekker, New York, pp 395–409
Swartzendruber DC, Wertz PW et al (1987) Evidence that the corneocyte has a chemically bound lipid envelope. J Invest Dermatol 88:709–713
Swartzendruber DC, Wertz PW et al (1989) Molecular models of the intercellular lipid lamellae in mammalian stratum corneum. J Invest Dermatol 92:251–257
Sweeney TM, Downing DT (1970) The role of lipids in the epidermal barrier to diffusion. J Invest Dermatol 55:135–140
Takahashi H, Sinoda K et al (1996) Effects of cholesterol on the lamellar and the inverted hexagonal phases of dielaidoylphosphatidylethanolamine. Biochim Biophys Acta 1289:209–216
Tanaka M, Fukuda H et al (1978) Permeation of drug through a model membrane consisting of millipore filter with oil. Chem Pharm Bull 26(1):9–13
Tang H, Mitragotri S et al (2001) Theoretical description of transdermal transport of hydrophilic permeants: application to low-frequency sonophoresis. J Pharm Sci 90(5):545–568
Tas C, Ozkan Y et al (2007) In vitro and ex vivo permeation studies of etodolac from hydrophilic gels and effect of Terpenes as enhancers. Drug Deliv 14(7):453–459
Tenjarla SN, Kasina R et al (1999) Synthesis and evaluation of N-acetylprolinate esters – novel skin penetration enhancers. Int J Pharm 192(2):147–158
Tezel A, Sens A et al (2001) Frequency dependence of sonophoresis. Pharm Res 18(12):1694–1700
Tezel A, Sens A et al (2003) Description of transdermal transport of hydrophilic solutes during low-frequency sonophoresis based on a modified porous pathway model. J Pharm Sci 92(2):381–393
Thomas BJ, Finnin BC (2004) The transdermal revolution. Drug Discov Today 9(16):697–703
Tiemessen H, Bodde HE et al (1989) A human stratum-corneum silicone membrane sandwich to simulate drug transport under occlusion. Int J Pharm 53(2):119–127
Tipre DN, Vavia PR (2003) Acrylate-based transdermal therapeutic system of nitrendipine. Drug Dev Ind Pharm 29(1):71–78
Tojo K, Lee ARC (1989) A method for predicting steady-state rate of skin penetration in vivo. J Invest Dermatol 92(1):105–108
Tojo K, Masi JA et al (1985a) Hydrodynamic characteristics of an in vitro drug permeation cell. Ind Eng Chem Fund 24(3):368–373
Tojo K, Sun Y et al (1985b) Characterization of a membrane permeation system for controlled drug delivery studies. AIChE J 31(5):741–746
Tokudome Y, Sugibayashi K (2004) Mechanism of the synergic effects of calcium chloride and electroporation on the in vitro enhanced skin permeation of drugs. J Control Release 95(2):267–274
Tregear RT (1961) Relative penetrability of hair follicles and epidermis. J Physiol (London) 156(2):307
Tregear RT (1966) Physical function of skin. Academic, New York
Tsai JC, Weiner ND et al (1991) Properties of adhesive tapes used for stratum-corneum stripping. Int J Pharm 72(3):227–231
Tsai JC, Sheu HM et al (2001) Effect of barrier disruption by acetone treatment on the permeability of compounds with various lipophilicities: implications for the permeability of compromised skin. J Pharm Sci 90(9):1242–1254
Tsai JC, Lin CY et al (2003) Noninvasive characterization of regional variation in drug transport into human stratum corneum in vivo. Pharm Res 20(4):632–638
Tsuruta H (1977) Percutaneous absorption of organic solvents. 2. A method for measuring the penetration rate of chlorinated solvents through excised rat skin. Ind Health 15:131–139
Turakka L, Piepponen T et al (1984) Release of hydrocortisone and hydrocortisone acetate from topical vehicles in vitro. Labo-Pharma Probl Tech 344:540–544
Uchino T, Tokunaga H et al (2002) Effect of squalene monohydroperoxide on cytotoxicity and cytokine release in a three-dimensional human skin model and human epidermal keratinocytes. Biol Pharm Bull 25(5):605–610
Umemura K, Ikeda Y et al (2008) Cutaneous pharmacokinetics of topically applied maxacalcitol ointment and lotion. Int J Clin Pharmacol Ther 46(6):289–294
Valia KH, Chien YW et al (1984) Long-term skin permeation kinetics of estradiol. 1. Effect of drug solubilizer olyethylene-glycol 400. Drug Dev Ind Pharm 10(7):951–981
Vanbrunt J (1989) Novel drug delivery systems. Biotechnology 7(2):127–130
Vankoote WJ, Mali JWH (1966) Significance of sweat-ducts in permeation experiments on isolated cadaverous human skin. Dermatologica 132(2):141
Vankooten WJ, Mali JWH (1966) Significance of sweat-ducts in permeation experiments on isolated cadaverous human skin. Dermatologica 132(2):141
Vicanova J, Mommaas AM et al (1996a) Impaired desquamation in the in vitro reconstructed human epidermis. Cell Tissue Res 286(1):115–122
Vicanova J, Mommaas AM et al (1996b) Transformation of desmosomes is impaired in the in vitro reconstructed human epidermis. J Invest Dermatol 107(4):44
Vickers CFH (1963) Existence of reservoir in stratum corneum – experimental proof. Arch Dermatol 88(1):20
Viegas TX, Kibbe AH et al (1986) An in vitro method of evaluating tolnaftate release from topical powder. Pharm Res 3(2):88–92
Wahlberg JE (1968) Transepidermal or transfollicular absorption – in vivo and in vitro studies in hairy and non-hairy guinea pig skin with sodium (22 Na) and mercuric (203 Hg) chlorides. Acta Derm Venereol 48(4):336
Wallace SM, Barnett G (1978) Pharmacokinetic analysis of percutaneous absorption – evidence of parallel penetration pathways for methotrexate. J Pharmacokinet Biopharm 6(4):315–325
Walzer C, Benathan M et al (1989) Thermolysin treatment – a new method for dermo-epidermal separation. J Invest Dermatol 92(1):78–81
Washitake M, Takashima Y et al (1980) Studies on drug release from ointment. 1. Drug permeation through eggshell membranes. Chem Pharm Bull 28(10):2855–2861
Watanabe Y, Hongo S et al (1989) Evaluation of excised loach skin for studies on transdermal permeation of drugs in vitro. Yakugaku Zasshi (Journal of the Pharmaceutical Society of Japan) 109(9):656–661
Watt F, Green H (1981) Involucrin synthesis is correlated with cell size in human cultures. J Cell Biol 90:738–742
Wertz PW (1986) Lipids of keratinizing tissues. In: BereiterHahn J, Matoltsy AG, Richards KS (eds) Biology of the integument. Springer, Berlin, pp 815–823
Wertz PW, Downing DT (1983a) Acylglucosylceramides of pig epidermis: structure determination. J Lipid Res 24:753–758
Wertz PW, Downing DT (1983b) Ceramides of pig epidermis: structure determination. J Lipid Res 24:759–765
Wertz PW, Downing DT (1987) Covalently bound ω-hydroxyacylceramide in the stratum corneum. Biochim Biophys Acta 917:108–111
Wertz PW, Downing DT (1989) Stratum corneum: biological and biochemical considerations. In: Hadgraft J, Guy RH (eds) Transdermal drug delivery: developmental issues and research initiatives. Marcel Dekker, New York and Basel, pp 1–22
Wertz PW, Miethke MC et al (1985) The composition of the ceramides from human stratum corneum and from comedones. J Invest Dermatol 84:410–412
Wertz PW, Swartzendruber DC et al (1987) The composition and morphology of epidermal cyst lipids. J Invest Dermatol 89:419–425
Wester RC, Maibach HI (1987) Animal models for transdermal drug delivery. In: Kydonieus AF, Berner B (eds) Transdermal delivery of drugs, vol 1. CRC Press, Boca Raton, pp 61–70
Wester RC, Maibach HI (1989) In vivo methods for percutaneous absorption measurements. In: Brounaugh RL, Maibach HI (eds) Percutaneous absorption: mechanisms-methodology-drug delivery. Marcel Dekker, New York, pp 215–237
Wester RC, Maibach HI (1992) Percutaneous-absorption of drugs. Clin Pharmacokinet 23(4):253–266
Wester RC, Noonan PK et al (1983) Pharmacokinetics and bioavailability of intravenous and topical nitroglycerin in the rhesus-monkey – estimate of percutaneous 1st-pass metabolism. J Pharm Sci 72(7):745–748
Wester RC, Maibach HI et al (1984) Minoxidil stimulates cutaneous blood-flow in human balding scalps – pharmacodynamics measured by laser doppler velocimetry and photopulse plethysmography. J Invest Dermatol 82(5):515–517
Wester RC, Christoffel J et al (1998a) Human cadaver skin viability for in vitro percutaneous absorption: storage and detrimental effects of heat-separation and freezing. Pharm Res 15(1):82–84
Wester RC, Melendres J et al (1998b) Percutaneous absorption of salicylic acid, theophylline, 2,4-dimethylamine, diethyl hexyl phthalic acid, and p-aminobenzoic acid in the isolated perfused porcine skin flap compared to man in vivo. Toxicol Appl Pharmacol 151(1):159–165
Whitton JT, Everall JD (1973) Thickness of epidermis. Br J Dermatol 89(5):467–476
Wilke K, Wepf R et al (2005) Initial investigations towards a better understanding of the barrier properties of the sweat gland apparatus. J Invest Dermatol 125(6):A29
Wilke K, Wepf R et al (2006) Are sweat glands an alternate penetration pathway? Understanding the morphological complexity of the axillary sweat gland apparatus. Skin Pharmacol Physiol 19(1):38–49
Wilkin JK, Fortner G et al (1985) Prostaglandins and nicotinate-provoked increase in cutaneous blood-flow. Clin Pharmacol Therap 38(3):273–277
Williams AC, Barry BW (2004) Penetration enhancers. Adv Drug Deliv Rev 56(5):603–618
Williams PL, Carver MP et al (1990) A physiologically relevant pharmacokinetic model of xenobiotic percutaneous-absorption utilizing the isolated perfused porcine skin flap. J Pharm Sci 79(4):305–311
Williams AC, Cornwell PA et al (1992) On the non-Gaussian distribution of human skin permeabilities. Int J Pharm 86(1):69–77
Williams AC, Barry BW et al (1993) A critical comparison of some Raman-spectroscopic techniques for studies of human stratum-corneum. Pharm Res 10(11):1642–1647
Wong O, Huntington J et al (1989) New alkyl N, N-dialkyl-substituted amino acetates as transdermal penetration enhancers. Pharm Res 6(4):286–295
Wurster DE, Kramer SF (1961) Investigation of some factors influencing percutaneous absorption. J Pharm Sci 50(4):288
Wurster DE, Ostrenga JA et al (1979) Sarin transport across excised human-skin. 1. Permeability and adsorption characteristics. J Pharm Sci 68(11):1406–1409
Xhauflaire-Uhoda E, Vroome V et al (2006) Dynamics of skin barrier repair following topical applications of miconazole nitrate. Skin Pharmacol Physiol 19(5):290–294
Xiao CH, Moore DJ et al (2005a) Permeation of dimyristoylphosphatidylcholine into skin – structural and spatial information from IR and Raman microscopic imaging. Vib Spectrosc 38(1–2):151–158
Xiao CH, Moore DJ et al (2005b) Feasibility of tracking phospholipid permeation into skin using infrared and Raman microscopic imaging. J Invest Dermatol 124(3):622–632
Xueqin Z, Jing X et al (2005) Interaction of 1-dodecyl-azacycloheptan-2-one with mouse stratum corneum. J Biomater Sci Polym Ed 16(5):563–574
Yardley HJ (1983) Epidermal lipids. In: Goldsmith AL (ed) Biochemistry and physiology of skin. Oxford University Press, New York, pp 363–381
Yardley HJ, Summerly R (1981) Lipid composition and metabolism in normal and diseased epidermis. Pharmacol Ther 13:357–383
Zelickson A (1961) Electron microscopic study of epidermal sweat duct. Arch Dermatol 83(1):106
Zhai HB, Dika E et al (2007) Tape-stripping method in man: comparison of evaporimetric methods. Skin Res Technol 13(2):207–210
Zhao LG, Fang L et al (2008) Transdermal delivery of penetrants with differing lipophilicities using O-acylmenthol derivatives as penetration enhancers. Eur J Pharm Biopharm 69(1):199–213
Author information
Authors and Affiliations
Corresponding authors
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
Cropek, D.M., Karande, P. (2017). Models, Methods, and Measurements in Transdermal Drug Delivery. In: Dragicevic, N., I. Maibach, H. (eds) Percutaneous Penetration Enhancers Drug Penetration Into/Through the Skin. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-662-53270-6_9
Download citation
DOI: https://doi.org/10.1007/978-3-662-53270-6_9
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-662-53268-3
Online ISBN: 978-3-662-53270-6
eBook Packages: MedicineMedicine (R0)