Abstract
Aortic atresia is defined as complete occlusion of the aortic valve. Its isolated form is combined with a nonrestrictive ventricular septal defect (VSD) and usually a normal mitral valve. Hypoplastic left heart syndrome describes a spectrum of malformations in which the left ventricle is unable to support the systemic circulation due to its small size. Its adjacent structures are also severely hypoplastic.
The diagnosis is made by echocardiography. Cardiac catheterization or magnetic resonance imaging (MRI) is necessary only in rare cases.
The systemic circulation and hence survival is depending on a patent ductus arteriosus. Decreasing pulmonary vascular resistance soon leads to increased diastolic runoff and pulmonary congestion. Therefore, preoperative management is based on maintaining an open ductus and on systemic afterload reduction.
The two principal therapeutic options are multistage reconstruction or primary transplantation.
The multistage reconstruction pathway consists of three stages: Norwood procedure, bidirectional Glenn operation, and Fontan completion.
-
Stage 1 – The Norwood procedure is based on three principles: unrestricted blood flow from the right ventricle to the systemic circulation, restricted pulmonary blood flow, and unrestricted pulmonary venous return. These goals are achieved by an anastomosis between the main pulmonary artery and the aorta usually using an enlarging patch, an RV-PA conduit, or a modified Blalock-Taussig shunt as the source of pulmonary blood flow and the excision of the interatrial septum. This operation is timed around day 5.
-
Stage 2 – A bidirectional cavopulmonary anastomosis replaces the primary source of pulmonary blood flow thus unloading the ventricle. The interval between stage I and stage II varies from center to center. We prefer an interval of 3–4 months and perform a simple bidirectional Glenn anastomosis (see Chap. xy, functional univentricular heart and Fontan operation).
-
Stage 3 – The Fontan operation leads to complete separation of the systemic and pulmonary circulation. The total cavopulmonary anastomosis can be achieved by an extracardiac method using PTFE prosthesis or by an intracardiac technique. As aortic cross clamping can be avoided and postoperative arrhythmias might be minimized, we prefer the extracardiac type of the Fontan operation (operative technique see Chap. xy). We usually perform this operation at about 3 years of age, when the patient has a weight of at least 12 kg.
Results of the multistage reconstruction: Total mortality comprises the mortality of each procedure plus the interstage mortality. Survival rates of the Norwood procedure increased from 50 to 70 % in the early days up to more than 90 % nowadays. Our own early survival over the last 10 years in 206 cases was 87.4 %. Interstage mortality rates between stage I and stage II vary between 10 and 16 % and are influenced by the shunt type. Mortality rates between 0 and 2 % are reported for the bidirectional Glenn shunt as well as for the Fontan completion.
The so-called Hybrid procedure postpones the Norwood procedure by initially performing a ductal stent and bilateral pulmonary artery banding. The Norwood operation is done later together with the bidirectional Glenn operation.
Alternatives to the multistage reconstruction are primary heart transplantation or fetal aortic valve intervention if critical aortic stenosis is the primary lesion. The latter method is still in discussion.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
1 Cardiac Pathoanatomy
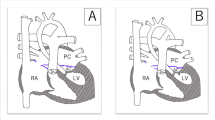
Aortic atresia is defined by a complete occlusion of the aortic valve and a hypoplastic ascending aorta. The isolated form with two normally developed ventricles is extremely rare and exclusively combined with a nonrestrictive ventricular septal defect and a normally developed mitral valve.
The term «hypoplastic left heart syndrome» was introduced by Noonan and Nadas in 1958. It describes a spectrum of malformations in which the left ventricle is unable to support the systemic circulation due to its size. In these cases, not only the left ventricle but also its adjacent structures, mitral valve, aortic valve, and ascending aorta, are severely hypoplastic. A combination of hypoplastic AV and semilunar valve features (mitral stenosis or atresia, aortic stenosis or atresia) defines the hypoplasia of the left ventricle and its adjacent structures.
Traditionally four subtypes are differentiated:
-
Mitral atresia, aortic atresia
-
Mitral hypoplasia/stenosis, aortic atresia
-
Mitral atresia, aortic hypoplasia/stenosis
-
Mitral hypoplasia/stenosis, aortic hypoplasia/stenosis
Further lesions leading to hypoplasia of the left ventricle during fetal live are:
-
Critical aortic coarctation
-
Asymmetric septation in unbalanced AV canal
-
Straddling mitral valve
-
Complex ventricular septal defect (VSD) and hypoplastic or interrupted aortic arch
-
Multiple stenotic lesions in series in the left ventricular system (Shone syndrome)
-
Isolated Severe Mitral Stenosis
Borderline cases without a primary valvular lesion were defined as hypoplastic left heart complex by C. Tchervenkov in 1998.
In hypoplastic left heart syndrome, the ascending aorta is usually very thin walled and fragile. Especially in aortic atresia with retrograde perfusion, functionally the hypoplastic ascending aorta can be looked at as a common coronary artery. A diameter of less than 2 mm is most frequent in these cases. If there is antegrade blood flow (aortic stenosis), it is usually bigger in diameter. Aortic arch and aortic isthmus may also be hypoplastic, however, not as severely as the ascending aorta, as they receive the blood flow for the supra aortic vessels.
Pulmonary artery and ductus arteriosus are big and well developed. The septum primum is typically muscularized and thickened. Sometimes it is shifted toward the left atrium: atrial septum malposition. Without severe restriction at the level of the foramen ovale, the left atrium is smaller than the right one.
In most cases, the leading lesion is a valvular hypoplasia/obstruction either of the mitral or of the aortic valve. As a consequence of the reduced blood flow, growth of the left ventricle and its adjacent structures is reduced or had stopped at some stage. This has been proven in chicken embryos. By obstructing their mitral valve, hypoplastic left heart syndrome could be produced (Harh et al. 1973). The same process could be observed by fetal echocardiography in aortic stenosis in humans, originally described by L. Allan and coworkers at Guy’s Hospital in London (Allan et al. 1989). They found a critical aortic stenosis with severely impaired left ventricular contractility in a fetus in the 22nd week of gestation. At 32 weeks of gestation, the ventricle was not grown but small and highly echogenic, as in typical hypoplastic left heart syndrome. Its dimensions were the same as in the 22nd week of gestation. Our group made the same observations in our fetal cardiac program. The hypothesis of a valvular lesion as the reason for ventricular hypoplasia is the basis for fetal valvular interventions. If such an intervention (balloon valvuloplasty) is done early, an otherwise lost ventricle could be rehabilitated (Makikallio et al. 2006)
This also explains that there is no strict cutoff line between critical aortic stenosis and hypoplastic left heart syndrome with a spectrum of borderline cases concerning the size of the left ventricle.
2 Extracardiac Malformations
Congenital cardiac malformations are frequently combined with extracardiac malformations. For hypoplastic left heart syndrome, rates reported are between 12 and 37 %.
While one post mortem study shows an overall malformation rate in hypoplastic left heart syndrome (HLHS) of 28 % (Natowicz et al. 1988), in another autopsy series, cerebral malformations alone were found in 29 % of patients. However, severe cerebral malformations like agenesis of the corpus callosum were seen only in 10 % (Glauser et al. 1990). In more than half of the cases in Natowicz’s series, genetic disorders were described either chromosomal anomalies or autosomal dominant or recessive lesions. Malformations of all organ systems are described. Among our own series of 233 patients with hypoplastic left heart syndrome, we saw one Goldenhar syndrome and one VACTERL syndrome. There was one case each of anal atresia, intestinal malrotation, growth retardation, and several kidney malformations. The overall malformation rate in our own series is 5.6 % (unpublished data). Glauser’s study also demonstrated that the risk for cerebral malformations is higher if hypoplastic left heart syndrome is part of a malformations syndrome. Among our own patients, there was one with agenesis of the corpus callosum.
3 Pathophysiologic Considerations
The principal problem is a ductus-dependent systemic circulation. This means antegrade blood flow in the ductus arteriosus – from the main pulmonary artery into the descending aorta and retrograde flow into the aortic arch and into the great cervical arteries as well as into the ascending aorta. In aortic atresia, the ascending aorta merely is a single coronary artery. The duct has to be kept patent on prostaglandin infusion preoperatively. In cases with aortic stenosis, there might be antegrade blood flow in the ascending aorta and in the proximal aortic arch. During diastole, blood runs from the aorta (systemic circulation) into the pulmonary arteries – diastolic runoff, like in a nonrestrictive aortopulmonary shunt. While pulmonary vascular resistance decreases, shunt volume increases and leads to pulmonary congestion and volume load of the right ventricle. Systemic diastolic blood pressure decreases and perfusion of the coronaries, brain, kidneys, and intestinal organs worsens. This increases the risk of cerebral injury and necrotizing enterocolitis.
4 Clinical Diagnosis
Nowadays, diagnosis of hypoplastic left heart syndrome is made almost exclusively by echocardiography alone. In many cases, diagnosis is made by echocardiography during fetal live. This is possible after the 16th week of gestation. Fetal diagnosis is extremely helpful for the newborn as emergency situations like ductal closure can be avoided. All aspects, which are important for the operation, can be seen echocardiographically, at best immediately after birth. Cardiac catheterization is required and justified only in exceptional cases. There may be a therapeutic indication, as in hybrid procedure. If the anatomy of the pulmonary veins cannot be completely clarified by echocardiography or in cases with borderline left ventricle, MRI may be helpful. The decision, whether a left ventricle and its mitral valve are suitable for biventricular repair, may be very difficult, especially in borderline cases of critical aortic stenosis. Different authors developed different criteria – usually morphologic criteria –for this decision (Rhodes et al. 1991).
Usually there is no problem with cardiopulmonary circulation during fetal period, except in the situation of an obstructive or near atretic foramen ovale. Newborns with hypoplastic left heart syndrome are generally of unremarkable appearance; sometimes a mild degree of cyanosis may be noticed. A systolic-diastolic murmur can be heard, due to the persisting ductus arteriosus. If there is no fetal diagnosis, the first symptoms may be severe metabolic acidosis, shock, and cardiovascular collapse due to ductal closure.
5 Preoperative Management
As the preoperative condition of the newborn with hypoplastic left heart syndrome is an important prognostic factor, postnatal preoperative treatment is very important (Tworetzky et al. 2001).
Preoperative management is based upon maintaining the ductus arteriosus patent. This is achieved by infusion of Prostaglandin E. A further goal is to avoid severe congestive heart failure due to nonrestrictive pulmonary blood flow secondary to decreasing pulmonary vascular resistance.
That means all pulmonary vasodilating agents should be avoided: no additional oxygen, no hyperventilation, and no intubation if tolerated. Vascular resistance in systemic circulation should be kept low: afterload reduction with sodium nitroprusside or other vasodilators. If possible, no vasopressors should be used. An optimal situation: The diagnosis is made during fetal life. The baby is delivered at or close to the tertiary center, where the operation is performed, so that no extensive transport is necessary. Intensive care treatment is started immediately after birth.
5.1 Timing of the Operation
This depends on the pathophysiology of an unrestrictive aortopulmonary shunt. If pulmonary overcirculation and decompensation of the systemic ventricle can be avoided, patients are operated around day 5 of life. If there are serious signs of congestive heart failure (fluid retention), the operation has to be scheduled earlier.
6 Operative Therapy
6.1 History and Principal Pathways
Since the early 1970s, case reports are published about successful palliation of hypoplastic left heart syndrome (Cayler et al. 1970; Doty and Knott 1977). Cayler and colleagues published a case of mitral atresia and aortic atresia, in which they did a successful palliation already in 1970. However in none of these early cases a Fontan procedure as a subsequent, more definite procedure could be performed. It was in 1982 at Children’s Hospital Boston, when the first patient with hypoplastic left heart syndrome underwent a Fontan procedure. This patient had an initial palliation prior to the Fontan procedure, described by William I Norwood, his surgeon, as a stage I palliation (Norwood et al. 1980, 1983).
There are two principal pathways in surgical treatment of hypoplastic left heart syndrome:
-
Multistage reconstruction
-
Primary transplantation
Today in most centers, the reconstructive approach is the standard therapeutic pathway. The final goal is the Fontan operation with a right ventricle as systemic ventricle. This goal, originally aimed at in two stages, nowadays is achieved by a staged palliation consisting of three operations:
-
Stage I: Norwood procedure
-
Stage II: bidirectional cavopulmonary anastomosis (modified Glenn anastomosis)
-
Stage III: total cavopulmonary anastomosis (Fontan-type operation)
The Norwood procedure produces a balanced univentricular physiology with restricted pulmonary and unrestricted systemic blood flow. In stage II, the source of the pulmonary blood flow (Blalock-Taussig shunt or RV-PA conduit) is replaced by a bidirectional Glenn shunt at the age of 3–6 months. The completion to Fontan operation as stage III is achieved by connecting the inferior vena cava to the right pulmonary artery. As we use the extracardiac version of this procedure employing PTFE tube prosthesis, we usually perform stage III at the age of 2–3 years.
6.2 Stage I: Norwood Procedure
Hypoplastic left heart syndrome comprises univentricular hemodynamics with nonrestrictive pulmonary blood flow and ductus-dependent, potentially restrictive, systemic circulation. The goal of the Norwood procedure is a balanced univentricular physiology which is not dependent on a persisting ductus arteriosus.
It is based mainly on three principles:
-
1.
Unrestricted flow from the right ventricle into the systemic circulation
-
2.
Restrictive pulmonary blood flow (Blalock-Taussig shunt or RV-PA conduit)
-
3.
Nonrestrictive pulmonary venous runoff to the tricuspid valve
6.2.1 Operative Technique
Surgical access and dissection
A median sternotomy, slightly extended upward into the jugulum is used as access. The thymus is resected, leaving part of the cervical extensions in place. Right, left, and main pulmonary arteries are dissected. The right pulmonary artery is temporarily occluded by a tourniquet, thus reducing pulmonary blood flow, unloading the ventricle, and increasing the systemic blood pressure. The aortic arch with the supraaortic branches and the first 10–15 mm of the descending aorta are dissected and mobilized, as well as the ductus arteriosus. Marking stitches are placed on the ascending aorta and the main pulmonary artery at the planned anastomotic sites to avoid distortion.
If it is planned to perfuse via the brachiocephalic trunk, it has to be decided whether this is done by direct cannulation or through a small PTFE tube prosthesis (Pigula et al. 1999). Heparin is now administered. In case prosthesis is used for cannulation, the end-to-side anastomosis between a 3.5 mm PTFE prosthesis and the brachiocephalic trunk is now performed.
6.2.2 Cardiopulmonary Bypass, Perfusion
Deep hypothermic circulatory arrest
This is the classical form of extracorporeal circulation used for stage I palliation in this malformation. Cannulation is simple. Originally we placed a soft arterial cannula into the most distal part of the main pulmonary artery and a big solitary venous cannula into the right atrium. If this method is used, aortic arch reconstruction and atrioseptectomy are done in circulatory arrest. Even with enough training and exercise, one can get quickly into borderline duration of circulatory arrest and beyond the borders of safety. Therefore, we abandoned this method 13 years ago and introduced what we feel is a safer form of perfusion in these patients.
Antegrade cerebral perfusion (regional low flow perfusion)
This method was introduced by Pigula and coworkers in 1999. Cannulation is done in the brachiocephalic artery, either directly or via a 3.5-mm PTFE prosthesis. A single venous cannula is put into the right atrium as usual. In our experience, cooling to a rectal temperature of 22 °C is enough (Hofer et al. 2005). During aortic arch reconstruction, flow is reduced to 30 ml/kg/min. The descending aorta is cross clamped and the cervical vessels are controlled by tourniquets. Circulatory arrest is necessary only for excision of the atrial septum.
Moderate hypothermia and additional cannulation of the descending aorta (Imoto et al. 2001)
This method is used in our unit now for 10 years. Initial cannulation is performed as described above. Both pulmonary arteries are controlled by tourniquets. The posterior pericardium is incised, the left pleural space is opened, the inferior pulmonary ligament is divided, and the aorta is carefully dissected out. A second arterial cannula is brought into the supradiaphragmatic aorta (◘ Fig. 17.1). Using this method of perfusion, cooling to a rectal temperature of 25 °C is enough.
The ductus arteriosus is closed with a typical purse string suture at the aortic side. At the pulmonary artery end, it is excised completely and sewn over transversely to the left pulmonary artery.
The main pulmonary artery is divided close to its bifurcation. As we have seen kinking in some cases due to excess length of the pulmonary arteries, we don’t use a patch for closing the main pulmonary artery. We perform direct closure by a suture line exactly transverse to the right and left pulmonary artery.
Cardioplegia
Brachiocephalic artery, left common carotid artery, and left subclavian artery are occluded by tourniquets. The descending aorta is cross clamped with an atraumatic vascular clamp. The residual stump of the ductus arteriosus is excised. Aortic arch and superior part of the ascending aorta are incised longitudinally at the concave side of the arch up to a level of 6–7 mm superior to the marked anastomotic site. Cold crystalloid cardioplegic solution is administered via a small olive shaped cannula. After that, the incision is continued to the anastomotic site.
Reconstruction of the aortic arch
When excising the residual ductal stump, attention must be drawn not to leave any ductal tissue at this level, which is usually seen as a rim on the opposite side of the ductus. If there is any rim, the whole isthmus should be resected and the continuity of the aorta is reestablished by a typical extended end-to-end anastomosis between the incised distal part of the aortic arch and the descending aorta. After almost completing the anastomosis posteriorly, the incision in the arch should be extended for about 5–10 mm into the descending aorta (◘ Fig. 17.2).
Anastomosis between the pulmonary artery and aorta
If the ascending aorta is of sufficient size and if a good sized anastomosis between descending aorta and distal arch has been performed, a direct anastomosis between the aorta and pulmonary artery can be done. However one has to pay attention not to compress the pulmonary artery that is underneath the aorta and above the left bronchus. Compression of the pulmonary artery is a very unfavorable situation, which has a considerable negative influence on short- and long-term prognosis of the patient. So if there is any doubt about space for the pulmonary artery, a patch should be used (◘ Fig. 17.3).
Patch material and shape
The goal of the reconstruction is a symmetrically curved arch without any kinking, a so-called roman arch. Some important technical aspects must be considered:
-
The patch must not be too long, especially on its concavity. Otherwise this would result in kinking and «gothic deformation.»
-
As the aortic arch forms a curvature of 180°, the patch has to be naturally curved in two planes: cross section and longitudinal section.
The best patch material, fulfilling these criteria, is the right-sided part of a pulmonary artery homograft bifurcation and its adjacent parts of the main and right pulmonary arteries (◘ Fig. 17.4). Alternatively, there is a PTFE prosthesis available, which has a curved and trumpet formed distal end. From this prosthesis, a similar looking patch can be cut out and used for aortic arch reconstruction. We have used this in more than 40 cases with excellent anatomic results. We don’t use aortic arch homografts, as they calcify heavily, due to their huge amount of elastic fibers. The patch is inserted with 6/0 non-absorbable monofilament material. If a PTFE patch is used, it is sewn in with a PTFE thread.
More recently, Emile Bacha in New York and James Tweddell in Milwaukee started to employ bioengineered porcine small bowel submucosa for reconstruction with no long-term results available yet.
Great care is taken not to get any stenosis in the proximal part of the ascending aorta. This is avoided best by using only a few (three to four) interrupted stitches of 7/0 non-absorbable monofilament material in this area.
Excision of the atrial septum
This is usually done via the cannulation site in the right atrium. If the abovementioned newer techniques of perfusion are used, this is the only part of the operation done in circulatory arrest.
It is of particular importance that no residual septal tissue is left. This guarantees an uninhibited pulmonary venous runoff. To be absolutely sure, the coronary sinus can be unroofed additionally.
6.2.3 Reestablishment of Pulmonary Blood Flow- Two Options
-
(A)
Modified Blalock-Taussig shunt. This shunt arises from the brachiocephalic artery and goes to the right pulmonary artery. It is made with PTFE tube prosthesis. A diameter of 3.5 mm is usually adequate for a newborn.
-
(B)
RV-PA conduit. This method was described first by Norwood (Norwood et al. 1981). However, at that time valved conduits were used, and they were too big for this purpose. The smaller non-valved RV-PA conduit – a 5 mm PTFE prosthesis – was introduced by Sano in 2001 (Sano et al. 2003, 2004). Technically this conduit can be placed on the left side between infundibulum and distal main pulmonary artery. However we prefer to place the conduit on the right side of the ascending aorta (◘ Fig. 17.5) between the infundibulum and right pulmonary artery (Griselli et al. 2006). The original method of Sano with the conduit on the left side of the reconstructed aortic arch leads to a lateral shift of the distal conduit anastomosis and to stretching of the right pulmonary artery. In our experience this frequently results in stenosis of the right pulmonary artery. Since we do the distal anastomosis on the right side and on the right pulmonary artery, this problem has become extremely rare. Furthermore the Glenn procedure is easier, if you don’t have to dissect on the left side. In general we use 5 mm prostheses.
The right pulmonary artery is incised longitudinally between two stay sutures over a length of about 7-8 mm. Usually, there is no clamping necessary. A simple end-to-side anastomosis is done between a 5 mm PTFE prosthesis and the pulmonary artery with a 6/0 polypropylene thread. The site of the oblique incision in the infundibulum is marked by two stay sutures. A ventricular pacemaker is applied and fibrillation is induced. An oblique incision of about 10 mm in length is performed. The edges are undermined slightly to avoid proximal conduit stenosis. The prosthesis is cut obliquely to the correct length. Kinking and pressure upon the aorta must be avoided. The anastomosis is performed with a continuous PTFE suture.
Modified Blalock-Taussig shunt versus RV-PA conduit
The hemodynamics differ in that the shunt patients show a diastolic runoff in their systemic circulation, while the conduit patients have the hemodynamics of a banded pulmonary artery with pulmonary regurgitation.
Patients with an RV-PA conduit have a higher diastolic blood pressure by about 10 mmHg than patients with a modified Blalock-Taussig shunt (Mair et al. 2003). This should lead to an improved organ perfusion predominantly in the coronaries but also in brain, kidneys, and intestinal organs. However for the RV-PA conduit, a ventriculotomy in the infundibulum is necessary. This causes a scar in a univentricular heart and may worsen ventricular function. The question arising from this is whether the improved coronary perfusion weighs out the local infundibular scar. The multicentric study of R. Oyhe and coworkers showed that transplantation free survival at 12 months was higher in patients with an RV-PA conduit compared to a Blalock-Taussig shunt. After this interval no significant difference in transplantation free survival could be detected between these two groups. In the same study, RV-PA conduits showed more reinterventions and complications than the BT shunt group (Oyhe et al. 2010). The physiologic difference between these two methods however has an impact on the early postoperative management of these patients. Whereas shunt physiology generally demands a low resistance strategy, patients with an RV-PA conduit are not that sensitive to an increase in systemic vascular resistance. Vasopressors are better tolerated by these patients. So the decision between RV-PA conduit and modified Blalock-Taussig shunt is not a purely surgical one. The ability and routine of the intensive care team with the management of shunt physiology should influence the surgeon’s decision as well.
Delayed chest closure
If there is any doubt in hemodynamic stability, chest closure is delayed and the chest is closed temporarily with a waterproof membrane. This is sewn in with a simple continuous non-absorbable suture in between the skin edges. We drape the patient additionally with a sterile adhesive membrane. Criteria for definitive chest closure are completely stable hemodynamic and respiratory parameters and a negative fluid balance during 2–3 days. Delayed chest closure is usually done at the intensive care unit. Signs of pulmonary hyperfusion, especially in shunt patients, may require urgent chest closure.
6.3 Stage II: Bidirectional Glenn Shunt
In the late 1980s, it was observed that patients with a univentricular heart had better results after a Fontan operation, when they had a bidirectional cavopulmonary anastomosis as an intermediate step after stage I palliation than patients without this operation. This was not influenced by the type of stage I palliation that was used (aortopulmonary shunt, pulmonary artery banding or stage I Norwood procedure). The highest benefit had patients, who were not optimal candidates for a Fontan operation, either due to anatomic or physiologic reasons (Bridges et al. 1990).
Since that time, the bidirectional cavopulmonary connection became standard stage II palliation between Norwood procedure and Fontan Operation.
6.3.1 Physiologic Considerations
The heart is unloaded by the bidirectional cavopulmonary anastomosis. The blood of the superior vena cava bypasses the heart and goes through the lungs directly following the pressure gradient between superior vena cava and the common atrium. Once shunt or conduit is closed, the ventricle does no longer pump into the pulmonary circulation. Systemic oxygen saturation after a bidirectional Glenn shunt is usually between 80 and 85 %. This is similar to the situation after stage I palliation, but it is achieved with a significantly smaller workload of the ventricle. So the efficiency of heart labor is optimized and the ventricle should be better prepared for Fontan completion.
6.3.2 Timing of the Procedure
The timing of the bidirectional cavopulmonary anastomosis varies from center to center. As the flow through the pulmonary vessels is reduced by the Glenn procedure, it is mainly an outweighing between potential further growth of the pulmonary arteries on the one side and early unloading the ventricle to keep the ventricular performance and possibly reduce interstage mortality on the other side. Many authors recommend an interval of 6 months between stage I and stage II. In our unit, we think it is important to unload the systemic ventricle early as it is a right ventricle. Regarding the pulmonary vascular resistance, the earliest possible time would be after 6 weeks. We prefer an interval of 3–4 months between stage I and stage II.
6.3.3 Operative Technique
As a rule, we generally perform a simple bidirectional Glenn shunt. We do not perform Hemi-Fontan techniques. Surgery is described in ► Chapter «Definite Palliation of Functional Single Ventricle» Sect. 13.7.2.1 and Sect. 13.7.2.2. A special feature in hypoplastic left heart syndrome with aortic atresia is that great attention must be paid in order to not compress the small aorta, while clamping the right pulmonary artery during the bidirectional cavopulmonary anastomosis. This could result in severe ventricular failure.
Technique after a Blalock-Taussig shunt
Besides the abovementioned aspects, there is no difference to other univentricular hearts with aortopulmonary shunts.
Technique after RV-PA conduit
The RV-PA conduit is cross clamped and divided. The proximal end is sewn over very close to the infundibular anastomosis, so that no blind stump is left. If the distal conduit anastomosis has been made on the left side, this region has to be dissected and the distal end of the conduit is sewn over transversely. If the distal anastomosis has been made on the right side, the conduit must be removed completely. The site of the anastomosis can be used for the bidirectional cavopulmonary anastomosis. Usually it has to be enlarged by 2–3 mm. In case of a stenosis of the pulmonary arteries, this has to be repaired simultaneously either by a patch plasty or, if there is compression under the aortopulmonary truncus, with a stent placed intraoperatively.
While the Glenn anastomosis can be performed off pump in cases with a left-sided conduit anastomosis, shunt or conduit anastomoses on the right pulmonary artery at stage I surgery require normothermic extracorporeal circulation (beating heart) for stage II.
6.4 Stage III: Fontan Operation
The completion of the bidirectional cavopulmonary anastomosis, namely, from partial to total cavopulmonary connection, is the third step in definite surgical palliation for univentricular hearts. The operative technique is described in ► Chapter «Definite Palliation of Functional Single Ventricle» Sect. 13.7.3. The age of operation depends to some extent on the method used for this Fontan completion (lateral tunnel or extracardiac conduit). As we prefer a fenestrated extracardiac conduit, we usually perform the procedure during the third year of life. The patient should weigh 12 kg, so that PTFE tube prosthesis of 20 mm in diameter can be used. The following aspects made the extracardiac conduit the method of choice for us.
In this version, we avoid major suture lines on the atrial wall. Only when closing the junction of the inferior vena cava (IVC), atrial tissue may be incorporated in the suture line. In addition, no part of the atrial wall is under the elevated systemic venous pressure employing this technique. This should minimize rhythm problems in this cohort of patients.
Using an extracardiac conduit, the aorta is not to be cross clamped, and there is no period of ischemia for the heart in this procedure. We deem this to be especially important in face of a right ventricle supporting the systemic circulation. Furthermore, an extensive dissection would be necessary to cross clamp the aorta after a Norwood procedure if at all possible after complex neoaortic reconstruction.
6.5 Results of Univentricular Palliation
The total mortality of univentricular palliation comprises the mortalities of each surgical stage as well as the interstage mortality.
Before the Norwood operation (stage I), neonates and infants with hypoplastic left heart syndrome suffer from univentricular circulation with increasingly nonrestrictive pulmonary blood flow resulting in diastolic systemic runoff and consequently poor systemic perfusion.
The result of a successful Norwood procedure is a balanced univentricular circulation. Pulmonary blood flow is restricted, either in form of shunt physiology (when a BT shunt is used) or in form of a functional pulmonary artery banding (PAB) (using an RV-PA conduit). This leads to some relief of ventricular volume load; however, the ventricle has still to be considered volume overloaded until stage II surgery is performed. The problems of balancing the two circulations after extensive palliative surgery on extracorporeal circulation attribute to the higher mortality compared to corrective neonatal surgery.
In the early days of Norwood procedures performed, the survival rate was somewhere between 50 and 70 %. Today, several centers come up with rates above 90 %. In our group, the survival rate of the Norwood procedure improved from initially 66 % in 1998 to 87.4 % in a consecutive series of 206 unselected patients since 2003 (complete body perfusion by add. cannulation of the descending aorta). As independent risk factors for mortality after the Norwood procedure, we identified age older than 20 days at time of operation, the anatomic subgroup of unbalanced AV canal and former trial of biventricular repair.
Apart from the learning curve of the surgeon and other responsible team members, we identified the following factors to have influenced the improvements of our results:
-
Prenatal diagnostics. Prenatally identified patients usually come to the specialized center in better condition than those who get diagnosed only once severe cardiac failure or circulatory shock after ductal closure occurred. The preoperative status clearly influences postoperative outcome.
-
Introduction of the RV-PA conduit. The severe changes in pathophysiology, creating a physiology similar to a PAB, lead to a more stable condition especially in the early postoperative period. We are well aware that the RV-PA conduit is not shown to be superior in larger studies; however, it clearly helped us to improve our results.
-
Evolution of extracorporeal circulation. We replaced deep hypothermic circulatory arrest initially by selective antegrade cerebral perfusion and later on by complete body perfusion via an additional arterial cannula in the supradiaphragmatic aorta. This change in perfusion technique makes the operation comparable to other procedures using extracorporeal circulation and reduces the detrimental effects of intraoperative ischemia.
Summarizing these changes, the significant improvement of the results over time can be explained.
Postoperative morbidity can be caused by aortic arch stenosis, which can be kept low by using the right patch form. In our series, aortic arch reoperations were required in 2.5 %, and cathlab interventions in 6 % of patients. Stenoses of the pulmonary arteries seem to be more common in the RV-PA conduit group (about 12 % in our series) than with Blalock-Taussig shunts. They can usually be addressed at the time of the bidirectional cavopulmonary anastomosis.
The bidirectional cavopulmonary anastomosis is technically a rather straightforward procedure that reduces ventricular volume load. The mortality of the Glenn shunt ranges between 0 and 2 %. This operation contributes only minimally to the overall mortality of the univentricular palliation pathway.
This consideration is also valid for the completion to the total cavopulmonary connection. The extracardiac version of the Fontan palliation can be safely performed without cross clamping the aorta, gaining near normal arterial saturation by the same cardiac workload. Mortality rate is between 0 and 2 %.
Additionally, the death rate between stage I and II has to be considered. Numbers vary between 10 and 16 % («interstage mortality»).
Causes for death between stage I and stage II can be:
-
Residual or recurrent anatomic lesions like a restrictive interatrial septum, obstruction of the aortic arch or stenosis of the BT shunt, RV-PA conduit or pulmonary artery.
-
Trivial gastrointestinal infections can suddenly lead to dehydration and circulatory instability.
-
Respiratory infections can rapidly cause severe respiratory insufficiency and death.
A strict home monitoring program looking at certain parameters (pulse oximetry, weight gain, food habits) was introduced by the Milwaukee group and showed significant advantage (Ghanayem et al. 2003). The early performance of the bidirectional cavopulmonary anastomosis at the age of 3–4 months is also commonly seen as an advancement and is practiced at our unit. Our own experience showed that the – what we call – banded physiology of the RV-PA conduit was able to lower the interstage mortality in comparison to the BT shunt group.
Only few reliable data are published about the interstage mortality between stages II and III. Death can be caused by compromised ventricular function, cardiac failure due to multiple aortopulmonary collaterals, or rhythm disturbances. Overall, lethal events seem to be rare in this period.
6.6 Alternatives to the Norwood Procedure: Ductal Stenting and Bilateral Pulmonary Artery Banding (Hybrid Procedure)
The principal goal of the Norwood procedure, to create unrestricted ejection of the right ventricle into systemic circulation, and restrictive pulmonary blood flow (balanced univentricular circulation) with unrestricted pulmonary venous return (unrestrictive atrial septal defect (ASD)), can be achieved also by a hybrid procedure (conventional surgery combined with interventional techniques).
A stent is placed in the ductus arteriosus in catheter technique and both pulmonary arteries are banded. If the foramen ovale is restrictive, an additional stent may be placed there (Akintürk et al. 2002; Michel-Behnke et al. 2003; Bacha et al. 2006). This procedure could be applied as an alternative to the stage I Norwood procedure.
Aortic arch repair, aortopulmonary anastomosis, and atrial septectomy are done together with the bidirectional cavopulmonary anastomosis at stage II, which now may be called comprehensive stage II. The number of centers applying this procedure, as well as the numbers of cases, is still small. Therefore, a fair comparison with the Norwood procedure is difficult.
It is the advantage of this method that it avoids cardiopulmonary bypass, deep hypothermia, and circulatory arrest in a newborn for a palliative procedure only. Consequently, this approach lessens the surgical trauma for stage I palliation. This could be of increased importance in high-risk patients especially in low birth weight or immature neonates.
In some centers, it is therefore used predominantly in this high-risk group of patients.
Pizarro and coworkers however found out that there is no significant difference in survival in high-risk patients whether a Norwood procedure or a hybrid approach is used (Pizarro et al. 2007). They also reported that between stage I and stage II procedures, a substantial number of reinterventions concerning stents and pulmonary artery band were necessary. The comprehensive stage II in this group of patients has also a considerable morbidity and mortality rate.
This might be attributed to some inherent problems of this method reported by the applying surgeons and interventional cardiologists themselves.
The stent in the ductus arteriosus may lead to retrograde preductal aortic coarctation (Bacha et al. 2006). In aortic atresia, this could cause serious perfusion problems of the coronaries and the brain.
The interatrial stent, which is necessary sometimes, might be difficult to place.
During the comprehensive stage II procedure, an aortic arch repair has to be performed in a redo situation and an ingrown ductal stent has to be removed. The latter can be very demanding, at least in our experience.
Furthermore, if the new perfusion methods are used, as they are described above, the avoidance of cardiopulmonary bypass is the only remaining advantage of this procedure, as neither deep hypothermia nor circulatory arrest are absolutely necessary for stage I Norwood palliation.
Regarding all these aspects, it is presently difficult to define the advantages of this procedure over the Norwood operation either with modified Blalock-Taussig shunt or RV-PA conduit.
6.7 Alternatives to Univentricular Palliation
6.7.1 Heart Transplantation
Primary heart transplantation for hypoplastic left heart syndrome was first performed in 1984 by L. Bailey et al. (1986) and Mavroudis et al. (1988). The donor heart for this patient came from a baboon weighing 3.8 kg. In addition to standard heart transplantation (► Chapter «Heart and Heart-Lung Transplantation», Sect. 37.2.2), an extensive aortic arch repair is necessary in hypoplastic left heart syndrome. This can be done usually with the donor aortic arch. The availability of donor hearts is an unsolved problem in this therapeutic pathway and sometimes this requires prolonged waiting time. Furthermore, the patient needs lifelong immunosuppression. Coronary sclerosis in donor hearts is also a well-described problem.
6.7.2 Fetal Intervention
If critical aortic stenosis is the primary lesion of a hypoplastic left heart syndrome, it can be diagnosed by fetal echocardiography after the 16th week of gestation. At that time, the left ventricle is still big, distended, and of severely impaired contractility. It is known from many echocardiographic studies that these ventricles stop growing and develop endocardial fibroelastosis and coronary fistulas. If the aortic valve can be opened up at that time and antegrade blood flow through the left ventricle can be reestablished, the ventricle can be rehabilitated. Growth can go on and ventricular function will be preserved.
The first fetal aortic valvuloplasties were performed at Guy’s Hospital in London, England, in the late 1980s by Allan and Maxwell in a small series (Allan et al. 1995). At that time the Norwood procedure already showed encouraging results and became more and more widespread. Therefore this concept of treatment was abolished temporarily. Around 2000 it was restarted in a few places (Makikallio et al. 2006; Tworetzky et al. 2004; Arzt et al. 2011). The experience of our own unit includes now 43 fetal aortic valvuloplasties in 40 fetuses. After an initial decompression the ventricle becomes smaller. During the residual fetal period, almost normal growth was observed. In most cases, aortic valve and left ventricular outflow tract are still stenotic at birth and need reintervention. We are convinced that fetal valvuloplasty is the central part of ventricular rehabilitation in these babies, but the program does not end at birth. To allow normal growth and biventricular physiology, the left ventricle has to be aggressively freed from any possible obstruction. Seventeen of the 40 patients were delivered in our maternity hospital and further managed by our unit. Ten of the 17 patients remained with a biventricular circulation. All of these ten patients needed aortic valvuloplasty in the first days of life. Eight of these patients underwent a Ross-Konno procedure in the newborn period. In four, we did an additional resection of endocardial fibroelastosis. One patient died from a mucormycosis infection due to necrotizing enterocolitis after 40 days.
We think that in critical aortic stenosis, this therapeutic concept is very promising and will lead to a higher rate of biventricular repairs in these babies.
6.8 Treatment of Borderline Cases: Hypoplastic Left Heart Complex (Tchervenkov et al. 1998)
The decision whether a left ventricle is able to support systemic circulation and biventricular repair is suitable, or single ventricle palliation has to be done, is made by morphologic criteria. The most widespread criteria were published by L. Rhodes (Rhodes et al. 1991):
-
Mitral valve area >4.75 cm2/m2
-
Left ventricular long axis dimension to long axis of the heart >0.8
-
Left ventricular mass > 35 g/m2
-
Aortic root diameter 3.5 cm/m2
These parameters are valid only for critical aortic stenosis.
The left ventricular outflow tract however can be enlarged surgically by a Konno or Ross-Konno procedure and is therefore of very limited value as a criteria for biventricular repair in critical aortic stenosis.
These criteria are also of very limited value in aortic coarctation with hypoplastic left ventricle. For coarctation and hypoplastic left heart complex, Tchervenkov and coworkers showed, that even smaller ventricles than those selected by the Rhodes criteria are amenable for biventricular repair, if certain requirements are fulfilled
-
Good left ventricular function
-
No mitral or aortic valve stenosis
-
No endocardial fibroelastosis
-
Antegrade blood flow in the ascending aorta and the proximal aortic arch
Undisturbed antegrade blood flow through the mitral valve is also crucial for the success of biventricular repair in these cases. It is a requirement for left ventricular growth. That means that any ASD should to be closed. Furthermore, all obstructions in the outflow tract – aortic root, ascending aorta, aortic arch, and isthmus – must be removed aggressively.
Different criteria were elaborated by van Son for the repair of the unbalanced AV canal. Van Son and coworkers found that a left ventricular volume index greater than 15 ml/m2 is required for biventricular repair of this malformation. A big VSD component and a dysplastic mitral valve component worsen the prognosis of biventricular repair (Van Son et al. 1997).
6.9 Aortic Atresia and Normally Sized Left Ventricle
This rare form of aortic atresia can only exist with an unrestrictive VSD. A biventricular reconstruction is possible in this malformation. The principles of aortic arch repair and aortopulmonary anastomosis are the same as in a conventional Norwood procedure. An intracardiac baffle is necessary to direct the left ventricular flow through the VSD into the pulmonary artery, similar to Rastelli procedure. A valved or non-valved conduit is used to reestablish the continuity between RV and central pulmonary arteries.
This procedure can be performed as a complete repair in one stage, as we have done it successfully in our unit several times. Other authors recommend a two-stage procedure:
The first step would be a conventional Norwood procedure. In second step, the intracardiac baffle is made and the RV-PA conduit is implanted.
References
Akintürk H, Michel-Behnke I, Valeske K et al. (2002) Stenting of the arterial duct and banding of the pulmonary arteries. Basis for the combined Norwood stage 1 and 2 repair in hypoplastic left heart. Circulation 105:1099–1103
Allan LD, Sharland G, Tynan M (1989) The natural history of hypoplastic left heart syndrome. Int J Cardiol 25:341–343
Allan LD, Maxwell DJ, Carminati M, Tynan MJ (1995) Survival after fetal aortic balloon valvuloplasty. Ultrasound Obstet Gynecol 5:90–91
Arzt W, Wertaschnigg D, Veit I, Klement F, Gitter R, Tulzer G (2011) Intrauterine aortic valvuloplasty in fetuses with critical aortic stenosis: experience and results of 24 procedures. Ultrasound Obstet Gynecol 37(6):689–695
Bacha EA, Daves S, Hardin J et al. (2006) Single-ventricle palliation for high risk neonates: the emergence of an alternative hybrid stage I strategy. J Thorac Cardiovasc Surg 131:163–171
Bailey L, Conception W, Shattuck H, Huang L (1986) Method of heart transplantation for treatment of hypoplastic left heart syndrome. J Thorac Cardiovasc Surg 92:1–5
Bridges ND, Jonas RA, Mayer JE, Flanagan MF, Keane JF, Castaneda AR (1990) Bidirectional cavopulmonary anastomosis as interim palliation for high- risk Fontan candidates. Circulation 82(Suppl IV):170–176
Cayler GG, Smeloff EA, Miller GE (1970) Surgical palliation for hypoplastc left side of the heart. N Engl J Med 282:780–783
Doty DB, Knott HW (1977) Hypoplastic left heart syndrome. Experience with an operation to establish functionally normal circulation. J Thorac Cardiovasc Surg 74:624–630
Ghanayem NS, Hoffman GM, Mussatto KA et al. (2003) Home surveillance program prevents interstage mortality after the Norwood procedure. J Thorac Cardiovasc Surg 126:1367–1377
Glauser TA, Rorke LB, Weinberg PM, Clancy RR (1990) Congenital brain anomalies associated with hypoplastic left heart syndrome. Pediatrics 85:984–990
Griselli M, McGuirk SP, Stümper O et al. (2006) Influence of surgical strategies on outcome after the Norwood procedure. J Thorac Cardiovasc Surg 131:416–426
Harh JY, Paul MH, Gallen WJ et al. (1973) Experimental production of hypoplastic left heart syndrome in the chick embryo. Am J Cardiol 73:51–56
Hofer A, Haizinger B, Geiselseder G, Mair R, Rehak P, Gombotz H (2005) Monitoring of selective antegrade cerebral perfusion using near infrared spectroscopy in neonatal aortic arch surgery. Eur J Anaesthesiol 22:293–298
ImotoY KH, Shiokawa Y, Minami K, Yashui H (2001) Experience with the Norwood procedure without circulatory arrest. J Thorac Cardiovasc Surg 122:879–882
Mair R, Tulzer G, Sames E et al. (2003) Right ventricular to pulmonary artery conduit instead of modified Blalock-Taussig shunt improves postoperative hemodynamics in newborns after the Norwood operation. J Thorac Cardiovasc Surg 126:1378–1384
Makikallio K, McElhinney DB, Levine JC et al. (2006) Fetal aortic valve stenosis and the evolution of hypoplastic left heart syndrome: patient selection for intervention. Circulation 113:1401–1405
Mavroudis C, Harrison H, Klein JB et al. (1988) Infant orthotopic cardiac transplantation. J Thorac Cardiovasc Surg 96:912–924
Michel-Behnke I, Akintuerk H, Marquardt I et al. (2003) Stenting of the ductus arteriosus and banding of the pulmonary arteries: basis for various surgical strategies in newborns with multiple left heart obstructive lesions. Heart 89:645–650
Natowicz M, Chatten J, Clancy R et al. (1988) Genetic disorders and major extracardiac anomalies associated with hypoplastic left heart syndrome. Pediatrics 82:698–706
Noonan JA, Nadas AS (1958) The hypoplastic left heart syndrome: an analysis of 101 cases. Pediatr Clin N Am 5:1029–1056
Norwood WI, Kirklin JK, Sanders SP (1980) Hypoplastic left heart syndrome: experience with palliative surgery. Am J Cardiol 45:87–91
Norwood WI, Lang P, Castaneda AR, Campbell DN (1981) Experience with operations for hypoplastic left heart syndrome. J Thorac Cardiovasc Surg 82:511–519
Norwood WI, Lang P, Hansen DD (1983) Physiologic repair of aortic atresia – hypoplastic left heart syndrome. N Engl J Med 308:23–26
Ohye RG, Sleeper LA, Mahony L, Newburger JW et al. (2010) Comparison of shunt types in the Norwood procedure for single-ventricle lesions. N Engl J Med 362(21):1980–1992
Pigula FA, Siewers RD, Nemoto EM (1999) Regional perfusion of the brain during neonatal aortic arch reconstruction. J Thorac Cardiovasc Surg 117:1023–1024
Pizarro C, Derby CD, Baffa JM, Murdison KA, Radtke WA (2007) Improving outcome of high-risk neonates with hypoplastic left heart syndrome: hybrid procedure or conventional surgical palliation? Eur J Cardiothorac Surg 33(4):613–618
Pizarro C, Murdison KA, Radtke WA, Derby CD (2008) Stage II reconstruction after hybrid palliation for high-risk patients with a single ventricle. Ann Thorac Surg 85(4):1332–1338
Rhodes LA, Colan SD, Perry SB, Jonas RA, Sanders P (1991) Predictors of survival in neonates with critical aortic stenosis. Circulation 84:2325–2335
Sano S, Ishino K, Kawada M et al. (2003) Right ventricle-pulmonary artery shunt in first stage palliation of hypoplastic left heart syndrome. J Thorac Cardiovasc Surg 129:504–509
Sano S, Ishino K, Kado H et al. (2004) Outcome of right-ventricle–to-pulmonary artery shunt in first stage palliation of hypoplastic left heart syndrome: a multi-institutional study. Ann Thorac Surg 78:1951–1957
Tchervenkov CI, Tahta SH, Jutra LC, Beland MJ (1998) Biventricular repair in neonates with hypoplastic left heart complex. Ann Thorac Surg 66:1350–1357
Tworetzky W, McElhinney DB, Reddy VM, Brook MM, Hanley FL, Silverman NH (2001) Improved surgical outcome after fetal diagnosis of hypoplastic left heart syndrome. Circulation 103(9):1269–1273
Tworetzky W, Wilkins-Haug L, Jennings RW et al. (2004) Balloon dilatation of severe aortic stenosis in the fetus: potential for prevention of hypoplastic left heart syndrome: candidate selection, technique, and results of successful intervention. Ciculation 110:2125–2131
Van Son JAM, Phoon CK, Silverman NH, Haas GS (1997) Predicting feasibility of biventricular repair of right dominant unbalanced atrioventricular canal. Ann Thorac Surg 63:1657–1663
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
Mair, R., Sames-Dolzer, E. (2017). Surgery for Aortic Atresia, Hypoplastic Left Heart Syndrome, and Hypoplastic Left Heart Complex. In: Ziemer, G., Haverich, A. (eds) Cardiac Surgery. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-662-52672-9_17
Download citation
DOI: https://doi.org/10.1007/978-3-662-52672-9_17
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-662-52670-5
Online ISBN: 978-3-662-52672-9
eBook Packages: MedicineMedicine (R0)