Abstract
Tumor hypoxia is a clinically relevant cause of radiation resistance. Direct measurements of tumor oxygenation have been performed predominantly with the Eppendorf histograph and these have defined the reduced prognosis after radiotherapy in poorly oxygenated tumors, especially head-and-neck cancer, cervix cancer and sarcoma. Exogenous markers have been used for immunohistochemical detection of hypoxic tumor areas (pimonidazole) or for positron-emission tomography (PET) imaging (misonidazole). Overexpression of hypoxia-related proteins such as hypoxia-inducible factor-1α (HIF-1α) has also been linked to poor prognosis after radiotherapy and such proteins are considered as potential endogenous hypoxia markers.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Intratumoural hypoxia is a clinically relevant condition causing radiation resistance of tumour cells. The insufficient supply of fast-growing tumours with blood vessels and the pathologic structure of intratumoural vessels, respectively, have been associated with two mechanisms of tumour hypoxia. Specifically, these are diffusion-limited hypoxia caused by long distances of individual tumour cells from the nearest blood vessel versus perfusion-limited tumour hypoxia which can also occur close to (non-perfused) blood vessels due to the presence of vascular leaks, thrombosis or shunts. These two types have also been referred to as “chronic” and “acute” hypoxia, respectively. However, oxygenation of a tumour is considered a dynamic process, and in many tissues, intermittent changes of oxygen concentration are observed (“cyclic hypoxia”).
The classic explanation of hypoxia-associated radioresistance in tumour cells is the interference of molecular oxygen with DNA repair. Oxygen has been shown to bind to sites of radiation-induced DNA damage, thereby causing fixation of DNA lesions and preventing repair. In in vitro experiments, an oxygen-enhancement ratio (OER) of 2–3 has been determined, meaning that compared to a reference dose of ionizing radiation under normoxic conditions, 2–3 times this dose has to be given to achieve equivalent cell kill under anoxic (0 % oxygen) conditions.
In vivo, an additional mechanism causing treatment resistance of hypoxic tumours is the selection of tumour cells with increased anti-apoptotic, proliferative and metastatic characteristics by conditions of hypoxia (Graeber et al. 1996). This chapter will discuss currently available methods to detect intratumoural hypoxia and their clinical applicability.
2 Direct Measurements of Tumour Oxygenation
Probably, the most direct method for identifying hypoxia in tumours involves inserting electrodes into the tissue and monitoring the actual oxygenation status. This approach was first applied to human tumours in the 1960s using “home-made” glass electrodes. These early polarographic electrodes were, however, generally cumbersome and fragile, and only a few pO2 values 3–4 mm below the surface of the tumour were possible. Nevertheless, clinical data were obtained in cervix (Kolstad 1968) and head-and-neck (Gatenby et al. 1988) cancer patients that clearly demonstrated a relationship between the oxygenation measurements and outcome to radiation therapy, in that those patients with tumours that were better oxygenated had a significantly superior local response to irradiation.
This whole area was revolutionized with the development of the Eppendorf histograph, which had two distinct improvements. The first was having a more robust electrode with the oxygen sensor protected inside a metal needle. A second improvement was the attachment of this needle to a stepping motor that allowed for multiple measurements along the needle track through the tumour; thus, detailed oxygen partial pressure (pO2) distributions were possible. Numerous clinical studies were thus undertaken in a variety of human tumour types. The results clearly showed that hypoxia was a characteristic feature of virtually all human tumours investigated, although the degree of hypoxia could be variable (Vaupel et al. 1989). Probably, the most significant finding from these studies was the confirmation that hypoxia influenced outcome to therapy. This has been reported for head and neck (Nordsmark et al. 1996, 2005; Brizel et al. 1997; Stadler et al. 1999; Rudat et al. 2001), cervix (Hoeckel et al. 1993, 1996; Knocke et al. 1999; Fyles et al. 1998, 2006; Lyng et al. 2000), soft tissue sarcomas (Brizel et al. 1996; Nordsmark et al. 2001), and prostate (Turaka et al. 2011). Examples of the typical results obtained in each of these clinical sites are illustrated in Fig. 1 and clearly show that patients with more hypoxic tumours had a poorer outcome to therapy. Perhaps the most striking results were those made in cervix and sarcomas that showed hypoxia to influence outcome in patients in which surgery was the primary or only treatment (Hoeckel et al. 1996; Nordsmark et al. 2001), suggesting that hypoxia could also influence malignant progression, especially metastatic spread. In fact, one other study in cervix was able to show that the primary tumours of patients with metastases were indeed more poorly oxygenated than those of patients without metastases (Sundfør et al. 1998).
Relationship between tumour oxygenation estimated prior to therapy using the Eppendorf histograph and eventual outcome to that therapy. a Overall survival for 89 cervix patients given surgery or radiotherapy with curative intent in which the median pO2 values were above or below 10 mmHg. b Freedom from distant failure in 22 patients with soft tissue sarcomas receiving preoperative irradiation and hyperthermia in which the tumour median pO2 values were above or below 10 mmHg. c Overall survival for 397 head-and-neck cancer patients after primary radiation therapy in which the percentage of pO2 values less than or equal to 2.5 mmHg was above (more hypoxic) or below (less hypoxic) the median value of 19 %. d Freedom from biochemical failure for 57 prostate patients treated with brachytherapy in which the prostate/muscle (P/M) mean pO2 ratio was above or below 0.10. Composite figure derived from Hoeckel et al. (1996) (a); Brizel et al. (1996) (b); Nordsmark et al. (2005) (c); and Turaka et al. (2011) (d)
Today, the Eppendorf electrode is no longer commercially available, and there are a number of reasons for this. Without using concurrent imaging during the oxygen measurements, it was impossible to state whether the values obtained were from viable tissue, and even where this was done one could not state whether the cells in the hypoxic regions were clonogenic; the tumours themselves had generally to be easily accessible; and the technique was invasive. Furthermore, despite the positive findings between the Eppendorf measurements and treatment outcome, the machine was never predictive of response on a patient-to-patient basis. This was clearly illustrated in one of the first clinical studies using head-and-neck cancer patients (Nordsmark et al. 1996). Here, the 35 patients in which tumour oxygenation measurements were performed could be separated into two distinct groups with those patients having the more hypoxic tumours showing a significantly lower local tumour control following conventional radiation therapy. However, some 40 % of the patients did not fall within the correct category; they were hypoxic but controlled or had no hypoxia yet failed. Despite the various limitations, the results obtained from the Eppendorf studies must still be considered positive in that it supplied us with a tremendous level of information about tumour hypoxia and its importance.
Another approach that may have the potential to measure tumour oxygenation status involves the use of fibre optic probes. Unlike the Eppendorf histogram electrode, these do not consume oxygen with each measurement; thus; continuous observations of oxygenation status in the same tumour region is possible (Griffiths and Robinson 1999). Preclinical studies comparing the commercially available Oxford-Optronix OxyLite sensor with the polarographic techniques (Collingridge et al. 1997; Braun et al. 2001; Seddon et al. 2001; Wen et al. 2008), or classical paired survival curve estimates of radiobiological hypoxia (Urano et al. 2002), reported similarities and differences depending on the tissue type, tumour size or the changes observed using various modifiers of tumour hypoxia. Although fibre optic probes have the potential to not only measure the pretreatment level of tumour hypoxia, but also to monitor tumour oxygenation status continuously during and after treatment, there has as yet been no clinical application in cancer.
Other less invasive attempts to directly measure tumour oxygenation have involved phosphorescence tomography- or magnetic resonance (MR)-based approaches. The former requires the infusion of water-soluble phosphor probes into the vasculature (Vikram et al. 2007) and has been used to map oxygen concentration in preclinical tumour models (Wilson and Cerniglia 1992; Fukumura et al. 2001), but again has not been used in patients. The MR approaches include monitoring oxygen-sensitive reporter molecules (19F-oximetry). Several such molecules have been developed including perfluorochemical emulsions and hexafluorobenzene (Pacheco-Torres et al. 2011). The latter approach allowed for actual quantification of the MR signals and conversion into oxygen concentrations at the pixel level (Zhao et al. 2005). However, systemic toxicity required the imaging agent to be injected directly into tumours limiting its potential clinical application. An alternative MR method is electron paramagnetic resonance (EPR), which detects paramagnetic materials that have been injected into tissues (Krishna et al. 2012). It can provide quantitative and repeated 3D estimates of oxygenation and has been extensively used in preclinical studies and even in patients for a range of different clinical problems (Swartz et al. 2004). Although many of the preclinical studies have focused on tumour hypoxia, the clinical application of EPR in cancer has, however, been somewhat limited (Krishna et al. 2012).
3 Exogenous Markers of Hypoxia
The oxygen mapping techniques described above involve injecting exogenous agents to directly ascertain oxygen values that are low and equivalent to hypoxia. A more widely studied method for indirectly detecting tumour hypoxia involves the administration of exogenous compounds that under hypoxic conditions undergoes a chemical change from a non-reactive species to a highly reactive product that then binds to macromolecules within the cell. Subsequent application of techniques that can identify this bound product will allow us to demonstrate the presence of hypoxia. The most popular agents used in this context have been 2-nitroimidazole-based markers. These nitroimidazole compounds were originally developed as hypoxic cell radiosensitizers, with the 2-nitroimidazoles being the most effective agents for enhancing radiation response in preclinical models (Adams and Cooke 1969). Such compounds are characterized by having an NO2 grouping attached to the imidazole ring structure. This NO2 group can undergo a 6-electron intracellular reduction to produce NH2, and although the NO2 and NH2 moieties are generally inactive, one of the formed intermediates is highly reactive and can bind to any macromolecule within the cell (Horsman et al. 2012). In the presence of oxygen, typically above 10 mmHg, the first electron reduction species formed reacts with oxygen and returns to the NO2 moiety with the subsequent production of oxygen radicals that ultimately form hydrogen peroxide, and it is this lack of further reduction that gives rise to the hypoxia specificity. The bound product formed under hypoxia can be identified either using an antibody to the product or by labelling the original compound with a radioactive tracer such as 1H or 14C. The most commonly used nitroimidazole is pimonidazole, the binding of which in preclinical studies was found to correlate with radiobiological hypoxia (Raleigh et al. 1999). Additional clinical studies showed that the degree of pimonidazole binding was related to radiation-induced local tumour control in head-and-neck cancer (Kaanders et al. 2002), but not cervix (Nordsmark et al. 2006). Similar positive findings for head-and-neck cancer patients were reported between radiotherapy outcome and the degree of hypoxia estimated using another nitroimidazole marker, EF5 (Evans et al. 2007).
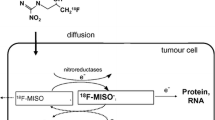
By labelling the nitroimidazole compound with 18F will allow for the hypoxia produced bound product to be identified using positron emission tomography (PET). The first tracer developed for hypoxia PET imaging was a [18F]-fluorinated version of the radiosensitizer misonidazole (FMISO) and was found to be capable of identifying hypoxia in a range of human tumours (Rasey et al. 1996). It was followed by a group of compounds based on another radiosensitizer, etanidazole (i.e. EF3/5). These markers have a relatively high lipophilicity which allows for easy penetration of cell membranes and diffusion into tumour tissue, but simultaneously limited the clearance of unbound tracer, thus leading to relatively low tumour-to-reference tissue ratios. Other fluorinated nitroimidazole compounds have been developed which are more water soluble than FMISO and therefore easier to clear from non-hypoxic tissue. These have included fluoroetanidazole (FETA), fluoroerythronitroimidazole (FETNIM), fluoroazomycinarabinofuranoside (FAZA) and HX4, of which the latter two are currently in clinical evaluation (Schuetz et al. 2010; van Loon et al. 2010). It is difficult to say whether one tracer is superior to another in identifying tumour hypoxia, since there has never been any systematic examination of all the 2-nitroimidazoles tracers in the same tumour model or patient population. The ideal tracer would be one in which clearance of unbound tracer is complete at the time of imaging; thus, only bound material indicative of hypoxia is measured. This can take many hours and even days to achieve, but such measurements have to take into account decay of the radioactive marker and normal clinical schedules. As a result, static scans are typically made 2–4 h after tracer administration, which results in low inter-tissue and intratumour contrast. An alternative approach involves labelling the nitroimidazoles with long-lived radionuclides, for example [124I]-iodoazomycin arabinoside (124I-AZA) and [124I]-iodoazomycin galactoside (124I-AZG), allowing for delayed scans up to 24 and 48 h after tracer administration. Unfortunately, the results have been disappointing with no improvement in image contrast and poor counting statistics (Rischin et al. 2006; Reischi et al. 2007), and it is unlikely that the problems inherent to hypoxia PET can be solved exclusively by better tracers. One small study in head-and-neck cancer patients (Thorwarth et al. 2005) demonstrated that pharmacokinetic analysis of the shape of tumour time activity curves (TACs) obtained from dynamic PET scans increased prognostic accuracy compared to traditional analysis of static PET images. However, dynamic scans are cumbersome and expensive, and cause inconvenience to patients; the analysis is complex; and different estimates of hypoxia can be obtained depending on the kinetic model and tumour type used.
Regardless of whether static or dynamic assessment is applied, one of the major issues with PET markers is that the cells must be hypoxic for significant time periods to be detected, which means that such markers are more likely to identify diffusion-limited chronic hypoxia rather than acute hypoxia resulting from transient fluctuations in blood flow (Horsman et al. 2012). Another significant problem facing the application of PET hypoxia markers is one of resolution in which the voxel sizes identified in the PET scans are much larger than most of the hypoxic structures (Horsman et al. 2012). Thus, the actual PET image does not accurately reflect the true hypoxia heterogeneity at the microregional level.
Several of the nitroimidazole-based PET markers have undergone clinical evaluation with respect to correlating the hypoxia estimates with outcome to radiation therapy. The majority of studies involved FMISO measurements in head-and-neck cancer patients (Rajendran et al. 2006; Rischin et al. 2006; Thorwarth et al. 2006; Eschmann et al. 2007; Dirix et al. 2009; Lee et al. 2009; Kikuchi et al. 2011; Zips et al. 2012). Two other studies in head and neck used either FETNIM (Lehtio et al. 2004) or FAZA (Mortensen et al. 2012). Results from the latter study using FAZA as the imaging agent are illustrated in Fig. 2 and clearly show that like all the other head-and-neck studies, patients with hypoxic tumours had a significantly poorer outcome. Similar findings were found using FETNIM in lung (Li et al. 2010) and oesophagus (Yue et al. 2012), FAZA in sarcomas (Khamly et al. 2008) and cervix (Scheutz et al. 2010) and FMISO in central nervous system tumours (Spence et al. 2008). Despite the positive findings obtained with all these nitroimidazole-based hypoxia PET markers, the situation remains the same as seen with the oxygen electrode methods in that although we can verify the presence of hypoxia in human tumours and demonstrate that it influences outcome to therapy, we still cannot use the results to select those patients that are hypoxic on an individual basis.
Results from four different clinical trials showing the relationship between hypoxia imaging and outcome to therapy. a Disease-free survival in 40 head-and-neck cancer patients based on the preradiation therapy estimate of hypoxia as determined by a tumour-to-muscle ratio of ≥1.4 from [18F] FAZA PET measurements. b Progression-free survival in 38 cervical cancer patients receiving radiotherapy and chemoradiotherapy in which the tumour/muscle (T/M) levels of 60Cu-ATSM were above or below the threshold dose of 3.5. c Overall survival for 32 non-small-cell lung cancer patients in which the tumour-to-normal tissue (T/N) ratio measured with 99mTc-HL91 SPECT before radiation therapy was above/below 1.47. d Disease-specific survival in 98 cancer patients based on the level of perfusion measured with DCE-MRI either before or before and during radiation treatment. Composite figure derived from Mortensen et al. (2012) (a); Dehdashti et al. (2008) (b); Li et al. (2006) (c); and Mayr et al. (2010) (d)
A chemically different group of putative PET markers that may have the potential to identify tumour hypoxia includes Cu-ATSM [Cu(II)-diacetyl-bis(N 4-methylthiosemicarbazone)], which can be labelled with a variety of positron-emitting isotopes of copper. Cu-ATSM has been shown to have high membrane permeability and fast tumour uptake, thus allowing for rapid imaging after injection (Dearling et al. 1998), but its exact retention mechanism is still not completely understood. The hypoxia specificity is believed to occur because while the Cu2+ moiety can easily pass across cell membranes, under low oxygen conditions the Cu2+ is converted to Cu1+ which is then trapped within cells (Vävere and lewis 2007). A good correlation between Cu-ATSM and pO2 measurements (Lewis and Welch 2001; O’Donoghue et al. 2005) and nitroimidazole-based hypoxia markers (O’Donoghue et al. 2005; Dence et al. 2008) have been shown in preclinical studies, although these effects are time- and tumour-dependent (O’Donoghue et al. 2005). Measurements in patients with lung (Dehdashti et al. 2003), cervix (Dehdashti et al. 2008), rectal (Dietz et al. 2008) or head-and-neck (Minagawa et al. 2011) cancer support the possibility of using Cu-ATSM as a marker of outcome to radiotherapy. The results from the cervix trial (Dehdashti et al. 2008) are shown in Fig. 2 and clearly demonstrate that those patients with a higher tumour uptake of Cu-ATSM, and presumably more hypoxic, had a lower progression-free survival. However, additional studies have shown Cu-ATSM to be affected by mechanisms other than hypoxia and that it is also insensitive to treatments that modify tumour oxygenation (Yuan et al. 2006); thus, its potential to be used as a specific marker for tumour hypoxia remains unclear.
The use of alternative radioactive labels allows for the possibility to use other non-invasive imaging techniques to identify hypoxia in tumours. Such an approach has been achieved with a number of [123I/125I]-iodoazomycin derivatives which can be detected using single-photon emission computer tomography (SPECT). Of these, only [123I]-iodoazomycin arabinoside (IAZA) has undergone clinical evaluation (Urtasun et al. 1996), and it was found that head-and-neck cancer patients with positive IAZA scans had a poorer outcome to radiotherapy than those patients with negative scans. Other potential SPECT markers for hypoxia that have been developed include 99mTechnetium-labelled compounds, such as BMS 181321 and BRU59-21, and complex ligands, specifically HL91. BRU59-21 was investigated in a phase I study in patients with head-and-neck cancer, and a significant correlation was found with pimonidazole binding (Hoebers et al. 2002). HL91 uptake was studied in non-small-cell lung cancer patients prior to radiation therapy, and the results, as shown in Fig. 2, demonstrated that those patients with the highest uptake had a significantly poorer response (Li et al. 2006). Various [19F]-labelled nitorimidazole compounds have also been developed which can be detected using MR. To-date, two [19F]-labelled nitroimidazoles have been developed, namely [19F]-EF5 and [19F]-SR 4554. Of these, only [19F]-SR 4554 underwent some clinical evaluations (Seddon et al. 2003), but there has not been any real follow-up.
An alternative MR approach that utilizes measurements made after injecting an exogenous marker is dynamic contrast-enhanced magnetic resonance imaging (DCE-MRI). This involves intravenously injecting a contrast agent and then monitoring its extravasation over several minutes from a region of interest (Nielsen et al. 2012). The focus with these estimates is actually on tumour perfusion, but this may still be an excellent method for identifying tumour hypoxia; oxygen delivery occurs via the vascular supply so the measurements could reflect chronic hypoxia, and changes in perfusion are clearly responsible for fluctuating hypoxia (Horsman et al. 2012). Clinical studies have been performed with DCE-MRI and reported that the parameters obtained actually correlated with oxygen electrode measurements in cervix (Cooper et al. 2000; Lyng et al. 2001), pimonidazole binding in head and neck (Newbold et al. 2009; Donaldson et al. 2011) and [18F]-FMISO uptake in glioblastoma multiforme (Swanson et al. 2009) and head-and-neck nodal metastases (Jansen et al. 2010). Several studies have also attempted to correlate the DCE-MRI measurements with radiotherapy outcome in patients with cervical cancer (Loncaster et al. 2002; Mayr et al. 2010; Andersen et al. 2012) and reported that those patients with supposedly more hypoxic tumours had a poorer response to radiotherapy, as illustrated in Fig. 2. Tumour perfusion can also be estimated with PET following administration of [15O]-labelled water. However, the clinical studies that used this method in head-and-neck cancer have produced conflicting results. One study reported that the [15O]-labelled water perfusion estimates correlated reasonably well with [18F]-EF5 measurements of hypoxia (Komar et al. 2008), but another study found that poor local tumour control and survival after radiation therapy were associated with high blood perfusion rather than the low perfusion one would expect to be indicative of hypoxia (Lehtio et al. 2004).
4 Endogenous Markers of Hypoxia
As an alternative to invasive electrode measurements or exogenously applied hypoxia markers, molecules expressed under (patho)physiological conditions of low oxygenation have been studied as potential “endogenous” or “intrinsic” hypoxia markers. Most of these studies are related to hypoxia-inducible factor-1α (HIF-1α), a transcription factor subunit considered to be the main regulator of the hypoxia response in mammalian cells. HIF-1α protein accumulates in the nucleus under hypoxic conditions and binds to hypoxia-responsive elements in the promoter regions of hypoxia-regulated genes, including erythropoietin (EPO), carbonic anhydrase IX (CA IX), glucose transporter 1 (GLUT1) and vascular endothelial growth factor (VEGF). Therefore, both HIF-1α protein itself and mRNA or protein of HIF-1-regulated genes could serve as indicators of tumour hypoxia.
An appropriate marker of hypoxic radioresistance should become overexpressed or accumulate at a level of hypoxia which is relevant for cellular radiosensitivity. The half-maximal oxygen effect on radiosensitivity is observed at about 0.5 % O2 (Hall 1988). HIF-1α protein has been shown to be detectable in HeLa cells after 4 h at 20 % O2 with a moderate increase between 20 and 6 %, a strong increase below 6 % and a maximum expression at 0.5 % (Jiang et al. 1996). In U87 MG human glioma cells, a constant increase in HIF-1α protein could be shown between 2 and <0.02 % O2, for in vitro hypoxia durations between 1 and 18 h (Vordermark and Brown 2003).
The temporal response of HIF-1α protein response to hypoxia (and reoxygenation) has been described as rather rapid: in HeLa cells, induction of HIF-1α protein was observed after 2 min of hypoxia, a maximum expression was seen after 1 h, and after 32 min of reoxygenation, the protein was again undetectable (Jewell et al. 2001). These data indicate that HIF-1α protein expression in tissue can occur already at higher oxygen concentrations where radiosensitivity of cells is not yet compromised and that care must be taken in processing of surgical/biopsy tissue to account for the immediate response to changes in oxygenation. Colocalization studies of HIF-1α and injectable hypoxia markers in xenograft tumours have supported the assumption that HIF-1-related hypoxia markers accumulate at higher O2 concentrations (i.e. closer to perfused blood vessels) than exogenous markers such as EF5 (Vukovic et al. 2001).
Among the HIF-1-regulated genes, the membrane enzyme carbonic anhydrase IX (CA IX) has received the most attention as a potential endogenous hypoxia marker for use in radiotherapy. In vitro, a continuous increase of CA IX protein expression has been described in A-549 lung carcinoma cells exposed to decreasing O2 concentrations from 5 to 0.1 % (Wykoff et al. 2000). Long-term hypoxia (24 h at 1.5 % O2) was found to result in higher CA IX levels than exposure for up to 10 h, suggesting that CA IX indicates predominantly prolonged exposure of cells to hypoxia (Lal et al. 2001). In FaDu head-and-neck squamous cell carcinoma, expression of CA IX increased over a radiosensitivity-relevant range of oxygen concentrations, resulting in a correlation of CA IX protein and cellular radioresistance (Vordermark et al. 2005).
The possibility to detect HIF-1α protein and related proteins such as CA IX in archival tumour material from patients with an already known clinical course of disease has motivated researchers to analyse the relationship of marker expression on immunohistochemistry and clinical outcome (e.g. survival and local control) after cancer treatment, especially radiotherapy. In early histopathological series investigating a number of different tumour types, HIF-1α has been detected in 40–82 % of prostate cancers, 80–100 % of colon adenocarcinomas and 29–83 % of breast adenocarcinomas (Zhong et al. 1999; Talks et al. 2000), with respective corresponding numbers for CA IX protein of 0, 100 and 26 % (Ivanov et al. 2001).
The HIF-1α immunohistochemical staining pattern observed in sections of human tumour material is not consistent across tumour types and individual studies. In oropharyngeal carcinoma, a typical staining pattern of “diffusion-limited hypoxia” (i.e. positive cells in ring shape at a distance from a central blood vessel) was described in 65 % of positive tumours, with more diffuse patterns in the remainder (Aebersold et al. 2001). Other authors characterized the area of HIF-1α-positive cells as close to a blood vessel (compatible with perfusion-related or “acute” hypoxia) versus distant from a vessel versus unspecific pattern (Haugland et al. 2002). A so-called perinecrotic staining pattern (i.e. accumulation of the marker in the zone most distant from a blood vessel, near regions of necrosis) was also reported in studies of CA IX expression in cervical carcinoma, head-and-neck cancer and non-small-cell lung cancer (Beasley et al. 2001; Giatromanolaki et al. 2001; Loncaster et al. 2001). The use of the term “endogenous hypoxia marker” has been criticized, since immunohistochemical studies of pO2 electrode measurement tracks in cervix cancer tumour tissue have shown no direct correlation of overexpression of the proteins HIF-1α, CA IX or GLUT1 with the corresponding pO2 reading (Mayer et al. 2006). This suggests that in vivo additional mechanisms other than the mere oxygenation level modulate expression of the putative endogenous hypoxia markers and that their expression is at least not hypoxia-specific.
However, a vast body of clinical literature suggests that a high level of expression of HIF-1-related proteins is related to poor outcome after cancer treatment. In head-and-neck cancer, several groups reported an association of HIF-1α overexpression and reduced overall survival or disease-specific survival following surgery or radiotherapy or combination treatment (Aebersold et al. 2001; Beasley et al. 2002; Winter et al. 2006). The association of high CA IX expression with poor prognosis in head-and-neck cancer was seen to a lesser extent in comparable studies, and some groups found this marker to be prognostic only in combination with other potential hypoxia markers (Hui et al. 2002; De Schutter et al. 2005) or not at all (Eriksen and Overgaard 2007; Nordsmark et al. 2007).
Cervix cancer, the other tumour entity with strong evidence from oxygen electrode studies of a relationship between tumour oxygenation and clinical response to radiotherapy, has been studied extensively regarding endogenous hypoxia marker expression. In three of the largest series treated with radiotherapy and/or surgery, HIF-1α expression was also significantly associated with overall survival or disease-specific survival on multivariate analysis (Birner et al. 2000; Bachtiary et al. 2003; Burri et al. 2003). Similar associations were found in some, but not all studies of CA IX expression in cervix cancer (Loncaster et al. 2001; Lee et al. 2007).
Other cancer types have been studied extensively regarding endogenous hypoxia marker expression, but with a stronger therapeutic focus on surgery and less impact of radiotherapy, among them breast cancer and lung cancer. Predominant associations of high marker expression and poor survival were observed here as well (review in Bache et al. 2008).
5 Plasma Hypoxia Markers
In theory, the measurement of a hypoxia-related protein secreted from hypoxic tumour cells into the plasma could permit an integrated assessment of both the total tumour burden (“number of cells”) and their oxygenation level (“percentage of hypoxic tumour cells”). The best-studied secreted hypoxia-related protein is osteopontin (OPN), a tumour-associated glycoprotein secreted into bodily fluids and in the plasma of tumour patients. Plasma OPN level was shown by Le et al. (2003) to correspond with Eppendorf electrode measurements of tumour oxygenation in patients with head-and-neck cancer, suggesting a role for OPN as an endogenous marker of tumour hypoxia. In a landmark study, Overgaard et al. (2005) showed that only patients with high plasma levels of OPN (upper tertile) significantly benefitted from the addition of nimorazole, a hypoxic radiosensitizer, compared to standard radiotherapy in patients with head-and-neck cancer. OPN may therefore serve as a marker by which to select head-and-neck cancer patients for intensified, hypoxia-specific, treatment. A molecular mechanism for the intracellular accumulation of OPN under hypoxia has been described (Sorensen et al. 2005; Zhu et al. 2005), although secretion of OPN may require additional steps (Said et al. 2005; Lukacova et al. 2006).
Elevated plasma or serum levels of OPN have been reported for several human cancer types including pancreatic, hepatocellular, colon, breast, prostate and lung cancer (Fedarko et al. 2001; Koopmann et al. 2004; Zhang et al. 2006). An association of high OPN plasma levels with poor prognosis has been established for different clinical situations. For instance, Isa et al. (2009) demonstrated that low plasma OPN measured before treatment correlated with improved overall survival and progression-free interval after chemotherapy for non-small-cell lung cancer. Mack et al. (2008) reported an association of elevated OPN plasma levels and inferior overall survival after carboplatin-/paclitaxel-based chemotherapy for advanced non-small-cell lung cancer. Blasberg et al. (2010) could show that OPN plasma levels significantly decreased after resection of early stage lung cancer and increased with later relapse, supporting the potential value of OPN as a biomarker for monitoring the treatment response in lung cancer. Recently, a pilot study of plasma hypoxia markers has also suggested a prognostic role of osteopontin response to radiotherapy of locally advanced non-small-cell lung cancer (Ostheimer et al. 2013)
Given the strong association with both electrode-measured oxygenation (Le et al. 2006) and response of tumour to surgery or chemotherapy in non-small-cell lung cancer, the prognostic potential of plasma OPN in radiotherapy of non-small-cell lung cancer was recently studied. Ostheimer et al. (2013) found in a pilot study of 55 patients with locoregionally advanced non-small-cell lung cancer that a panel of plasma biomarkers (OPN, CA IX and VEGF) in combination was an independent prognostic factor for overall survival in multivariate analysis. Early data on repeated measurements of OPN suggest a prognostic value of increasing plasma OPN over time in patients undergoing radiotherapy. In malignant glioma patients, OPN plasma levels did not decrease after surgical resection which may in part be explained by the fact that resection of such tumours is microscopically incomplete by nature, but also suggests that tumour-unrelated factors, such as wound healing, may contribute to total plasma osteopontin levels (Güttler et al. 2013). Nevertheless, a low plasma level of osteopontin at the end of postoperative radiotherapy identified patients with significantly improved overall survival in this pilot study. In patients with head-and-neck cancer, pretreatment OPN plasma levels were evaluated for outcome after surgery, radiotherapy, combined chemoradiation or sequential combinations. An adverse effect of high pretreatment OPN levels on survival and tumour control was confirmed for the different treatment arms (Petrik et al. 2006). In a separate series, lower pretreatment OPN levels were in favour of better tumour response and superior survival in head-and-neck cancer patients after radiotherapy alone (Snitcovsky et al. 2009).
6 Conclusions and Future Perspectives
Hypoxia is a characteristic feature of human tumours that has a major negative influence on determining tumour response to conventional therapy and is also an important factor in influencing malignant progression, both in terms of the aggressive growth of the primary tumour and its ability for metastatic spread. What is now needed is a method that allows us to identify those individual patients that have tumours containing significant levels of hypoxia, so that we can predict their outcome to therapy and where necessary select alternative treatments to eliminate that hypoxia.
Of course, one of the problems here is that hypoxia is often considered as a single entity when in fact we know that the degree and type of hypoxia found in tumours are very heterogenous (Horsman et al. 2012). At the very least, we have chronic and acute hypoxia, and it is not known whether both types respond equally well to therapy. Even if we could identify regions of both chronic and acute hypoxia within tumours, it is often difficult to state whether the cells in those hostile environments are actually viable and clonogenic. The situation becomes even more complicated because we also know that other microenvironmental factors such as intermediate hypoxia, low pH and glucose deprivation can influence malignant progression and thus outcome. The ideal imaging method must be able to identify all the critical factors. Furthermore, it must also give accurate, reliable and reproducible measurements, be easy to use on a routine basis and be applicable to any tumour type regardless of location. A non-invasive method would also be preferable. Clearly, no one technique that is currently available can achieve all these criteria. We must, therefore, either rely on measurements made with the best method there is or begin to combine modalities that can give us a better indication of the relevant parameters.
Once we have decided on the most relevant technique for imaging hypoxia, then there is the question of how do we deal with that hypoxia? Numerous preclinical studies have demonstrated a variety of methods that can be applied to eliminate hypoxia (Horsman et al. 2011), and many of these have been successfully applied in the clinic (Horsman and Overgaard 2007; Overgaard 2007), but none is currently in routine clinical use. It has also been suggested that in situations where we can actually image the distribution of hypoxia in tumours, we can then use that information to increase the radiation dose delivered to the tumour (Ling et al. 2000; Søvik et al. 2009; Bentzen and Gregoire 2011). But, whether that should be an increase to the gross tumour volume in which a substantial hypoxic volume has been identified or simply increase the dose to the biological target volume defined by the hypoxic area is not clear.
At present, we have a wealth of information about tumour hypoxia from a variety of clinically relevant techniques. Although we are not yet in a position to use that data to help us predict outcome on an individual patient basis or decide what we should do to tackle the hypoxia problem, the amount of effort being applied to this area would suggest that it is only a matter of time before we achieve those goals and the hypoxia problem eventually becomes obsolete.
References
Adams GE, Cooke MS (1969) Electron–affinic sensitization. I. A structural basis for chemical radiosensitizers in bacteria. Int J Radiat Biol 15:457–471
Aebersold DM, Burri P, Beer KT et al (2001) Expression of hypoxia-inducible factor-1alpha: a novel predictive and prognostic parameter in the radiotherapy of oropharyngeal cancer. Cancer Res 61:2911–2916
Andersen EKF, Hole KH, Lund KV et al (2012) Dynamic contrast-enhanced MRI of cervical cancers: temporal percentile screening of contrast enhancement identifies parameters for prediction of chemoradioresistance. Int J Radiat Oncol Biol Phys 82:485–492
Bache M, Kappler M, Said HM et al (2008) Detection and specific targeting of hypoxic regions within solid tumors: current preclinical and clinical strategies. Curr Med Chem 15:322–338
Bachtiary B, Schindl M, Potter R et al (2003) Overexpression of hypoxia-inducible factor 1alpha indicates diminished response to radiotherapy and unfavorable prognosis in patients receiving radical radiotherapy for cervical cancer. Clin Cancer Res 9:2234–2240
Beasley NJ, Wykoff CC, Watson PH et al (2001) Carbonic anhydrase IX, an endogenous hypoxia marker, expression in head and neck squamous cell carcinoma and its relationship to hypoxia, necrosis, and microvessel density. Cancer Res 61:5262–5267
Beasley NJ, Leek R, Alam M et al (2002) Hypoxia-inducible factors HIF-1alpha and HIF-2alpha in head and neck cancer: relationship to tumor biology and treatment outcome in surgically resected patients. Cancer Res 62:2493–2497
Bentzen SM, Gregoire V (2011) Molecular imaging-based dose painting: a novel paradigm for radiation therapy prescription. Sem Radiat Oncol 21:101–110
Birner P, Schindl M, Obermair A et al (2000) Overexpression of hypoxia-inducible factor 1alpha is a marker for an unfavorable prognosis in early-stage invasive cervical cancer. Cancer Res 60:4693–4696
Blasberg JD, Pass HI, Goparaju CM et al (2010) Reduction of elevated plasma osteopontin levels with resection of non-small-cell lung cancer. J Clin Oncol 28:936–941
Braun RD, Lanzen JL, Snyder SA, Dewhirst MW (2001) Comparison of tumor and normal tissue oxygen tension measurements using Oxylite or microelectrodes in rodents. Am J Physiol Heart Circ Physiol 280:H2533–H2544
Brizel DM, Scully SP, Harrelson JM et al (1996) Tumor oxygenation predicts for the likelihood of distant metastases in human soft tissue sarcoma. Cancer Res 56:941–943
Brizel DM, Sibley GS, Prosnitz LR, Scher RL, Dewhirst MW (1997) Tumor hypoxia adversely affects the prognosis of carcinoma of the head and neck. Int J Radiat Oncol Biol Phys 38:285–289
Burri P, Djonov V, Aebersold DM et al (2003) Significant correlation of hypoxia-inducible factor-1alpha with treatment outcome in cervical cancer treated with radical radiotherapy. Int J Radiat Oncol Biol Phys 56:494–501
Collingridge DR, Young WK, Vojnovic B et al (1997) Measurement of tumor oxygenation: a comparison between polarographic needle electrodes and a time-resolved luminescence-based optical sensor. Radiat Res 147:329–334
Cooper RA, Carrington BM, Loncaster JA et al (2000) Tumour oxygenation levels correlate with dynamic contrast-enhanced magnetic resonance imaging parameters in carcinoma of the cervix. Radiother Oncol 57:53–59
De Schutter H, Landuyt W, Verbeken E et al (2005) The prognostic value of the hypoxia markers CA IX and GLUT 1 and the cytokines VEGF and IL 6 in head and neck squamous cell carcinoma treated by radiotherapy ± chemotherapy. BMC Cancer 5:42
Dearling JL, Lewis JS, Mullen GE, Rae MT, Zweit J, Blower PJ (1998) Design of hypoxia-targeting radiopharmaceuticals: selective uptake of copper-64 complexes in hypoxic cells in vitro. Eur J Nucl Med 25:788–792
Dehdashti F, Mintun MA, Lewis JS et al (2003) In vivo assessment of tumor hypoxia in lung cancer with 60Cu-ATSM. Eur J Nucl Med Mol Imaging 30:844–850
Dehdashti F, Grigsby PW, Lewis JS, Laforest R, Siegel BA, Welch MJ et al (2008) Assessing tumor hypoxia in cervical cancer by PET with 60Cu-labeled diacetyl-bis(N4-methylthiosemicarbazone). J Nucl Med 49:201–205
Dence CS, Ponde DE, Welch MJ, Lewis JS (2008) Autoradiographic and small-animal PET comparisons between (18)F-FMISO, (18)F-FDG, (18)F-FLT and the hypoxic selective (64)Cu-ATSM in a rodent model of cancer. Nucl Med Biol 35:713–720
Dietz DW, Dehdashti F, Grigsby PW et al (2008) Tumor hypoxia detected by positron emission tomography with 60Cu-ATSM as a predictor of response and survival in patients undergoing neoadjuvant chemoradiotherapy for rectal carcinoma: a pilot study. Dis Colon Rectum 51:1641–1648
Dirix P, Vandecaveye V, De Keyzer F, Stroobants S, Hermans R, Nuyts S (2009) Dose painting in radiotherapy for head and neck squamous cell carcinoma: value of repeated functional imaging with (18)F-FDG PET, (18)F-fluoromisonidazole PET, diffusion-weighted MRI, and dynamic contrast-enhanced MRI. J Nucl Med 50:1020–1027
Donaldson SB, Betts G, Bonington SC et al (2011) Perfusion estimated with rapid dynamic contrast-enhanced magnetic resonance imaging correlates inversely with vascular endothelial growth factor expression and pimonidazole staining in head-and-neck cancer: a pilot study. Int J Radiat Oncol Biol Phys 81:1176–1183
Eriksen JG, Overgaard J (2007) Lack of prognostic and predictive value of CA IX in radiotherapy of squamous cell carcinoma of the head and neck with known modifiable hypoxia: an evaluation of the DAHANCA 5 study. Radiother Oncol 83:383–388
Eschmann SM, Paulsen F, Bedeshem C et al (2007) Hypoxia-imaging with (18)F-Misonidazole and PET: changes of kinetics during radiotherapy of head-and-neck cancer. Radiother Oncol 83:406–410
Evans SM, Du KL, Chalian AA et al (2007) Patterns and levels of hypoxia in head and neck squamous cell carcinomas and their relationship to patient outcome. Int J Radiat Oncol Biol Phys 69:1024–1031
Fedarko NS, Jain A, Karadag A et al (2001) Elevated serum bone sialoprotein and osteopontin in colon, breast, prostate, and lung cancer. Clin Cancer Res 7:4060–4066
Fukumura D, Xu L, Chen Y, Gohongi T, Seed B, Jain RK (2001) Hypoxia and acidosis independently up-regulate vascular endothelial growth factor transcription in brain tumors in vivo. Cancer Res 61:6020–6024
Fyles AW, Milosevic M, Wong R et al (1998) Oxygenation predicts radiation response and survival in patients with cervix cancer. Radiother Oncol 48:149–156
Fyles A, Milosevic M, Pintilie M et al (2006) Long-term performance of interstitial fluid pressure and hypoxia as prognostic factors in cervix cancer. Radiother Oncol 80:132–137
Gatenby RA, Kessler HB, Rosenblum JS et al (1988) Oxygen distribution in squamous cell carcinoma metastases and its relationship to outcome of radiation therapy. Int J Radiat Oncol Biol Phys 14:831–838
Giatromanolaki A, Koukourakis MI, Sivridis E et al (2001) Expression of hypoxia-inducible carbonic anhydrase-9 relates to angiogenic pathways and independently to poor outcome in non-small cell lung cancer. Cancer Res 61:7992–7998
Graeber T, Osmanian C, Jacks T et al (1996) Hypoxia-mediated selection of cells with diminished apoptotic potential in solid tumors. Nature 379(6560):88–91
Griffiths JR, Robinson SP (1999) The oxylite: a fibre-optic oxygen sensor. Br J Radiol 72:627–630
Güttler A, Giebler M, Cuno P et al (2013) Osteopontin and splice variant expression level in human malignant glioma: radiobiologic effects and prognosis after radiotherapy. Radiother Oncol 108:535–540
Hall EJ (1988) Radiobiology for the radiologist. Lippincott, Philadelphia
Haugland HK, Vukovic V, Pintilie M et al (2002) Expression of hypoxia-inducible factor-1alpha in cervical carcinomas: correlation with tumor oxygenation. Int J Radiat Oncol Biol Phys 53:854–861
Hoebers FJP, Janssen HLK, Valdés Olmos RA et al (2002) Phase 1 study to identify tumour hypoxia in patients with head and neck cancer using technetium-99 m BRU 59-21. Eur J Nucl Med 29:1206–1211
Hoeckel M, Knoop C, Schlenger K et al (1993) Intratumoral pO2 predicts survival in advanced cancer of the uterine cervix. Radiother Oncol 26:45–50
Hoeckel M, Schlenger K, Aral B, Mitze M, Schaffer U, Vaupel P (1996) Association between tumor hypoxia and malignant progression in advanced cancer of the uterine cervix. Cancer Res 56:4509–4515
Horsman MR, Overgaard J (2007) Hyperthermia: a potent enhancer of radiotherapy. Clin Oncol 19:418–426
Horsman MR, Overgaard J, Siemann DW (2011) Impact on radiotherapy. In: Siemann DW (ed) Tumor Microenvironment. Wiley, Chichester
Horsman MR, Mortensen LS, Petersen JB, Busk M, Overgaard J (2012) Imaging hypoxia to improve radiotherapy outcome. Nat Rev Clin Oncol 9:674–687
Hui EP, Chan AT, Pezzella F et al (2002) Coexpression of hypoxia-inducible factors 1alpha and 2alpha, carbonic anhydrase IX, and vascular endothelial growth factor in nasopharyngeal carcinoma and relationship to survival. Clin Cancer Res 8:2595–2604
Isa S, Kawaguchi T, Teramukai S et al (2009) Serum osteopontin levels are highly prognostic for survival in advanced non-small cell lung cancer: results from JMTO LC 0004. J Thorac Oncol 4:1104–1110
Ivanov S, Liao SY, Ivanova A et al (2001) Expression of hypoxia-inducible cell-surface transmembrane carbonic anhydrases in human cancer. Am J Pathol 158:905–919
Jansen JFA, Schöder H, Lee NY et al (2010) Noninvasive assessment of tumor microenvironment using dynamic contrast-enhanced magnetic resonance imaging and 18F-fluoromisonidazole positron emission tomography imaging in neck nodal metastases. Int J Radiat Oncol Biol Phys 77:1403–1410
Jewell UR, Kvietikova I, Scheid A et al (2001) Induction of HIF-1alpha in response to hypoxia is instantaneous. FASEB J 15:1312–1314
Jiang BH, Semenza GL, Bauer C et al (1996) Hypoxia-inducible factor 1 levels vary exponentially over a physiologically relevant range of O2 tension. Am J Physiol 271:C1172–C1180
Kaanders JH, Wijffels KI, Marres HA et al (2002) Pimonidazole binding and tumor vascularity predict for treatment outcome in head and neck cancer. Cancer Res 62:7066–7074
Khamly K, Choong P, Ngan S et al (2008) Hypoxia in soft-tissue sarcomas on [18F]-fluoroazomycin arabinoside positron emission tomography (FAZA-PET) powerfully predicts response to radiotherapy and early relapse (abstract 35029). Presented at the 14th Connective Tissue Oncology Society Annual Meeting, November 13–15 (London)
Kikuchi M, Yamane T, Shinoharas S et al (2011) 18F-fluoromisonidazole positron emission tomography before treatment is a predictor of radiotherapy outcome and survival prognosis in patients with head and neck squamous cell carcinoma. Ann Nucl Med 25:625–633
Knocke TH, Weitmann HD, Feldmann HJ, Selzer E, Pötter R (1999) Intratumoral pO2-measurements as predictive assay in the treatment of carcinoma of the uterine cervix. Radiother Oncol 53:99–104
Kolstad P (1968) Intercapillary distance, oxygen tension and local recurrence in cervix cancer. Scand J Clin Lab Invest Suppl 106:145–157
Komar G, Seppänen M, Eskola O et al (2008) 18F-EF5: a new PET tracer for imaging hypoxia in head and neck cancer. J Nucl Med 49:1944–1951
Koopmann J, Fedarko NS, Jain A et al (2004) Evaluation of osteopontin as biomarker for pancreatic adenocarcinoma. Cancer Epidemiol Biomarkers Prev 13:487–491
Krishna MC, Matsumoto S, Yasui H et al (2012) Electron paramagnetic resonance imaging of tumor pO2. Radiat Res 177:376–386
Lal A, Peters H, St Croix B et al (2001) Transcriptional response to hypoxia in human tumors. J Natl Cancer Inst 93:1337–1343
Le QT, Sutphin PD, Raychaudhuri S et al (2003) Identification of osteopontin as a prognostic plasma marker for head and neck squamous cell carcinomas. Clin Cancer Res 9:59–67
Le QT, Chen E, Salim A et al (2006) Evaluation of tumor oxygenation and gene expression in patients with early stage non-small cell lung cancers. Clin Cancer Res 12:1507–1514
Lee S, Shin HJ, Han IO et al (2007) Tumor carbonic anhydrase 9 expression is associated with the presence of lymph node metastases in uterine cervical cancer. Cancer Sci 98:329–333
Lee N, Nehmeh S, Schöder H et al (2009) Prospective trial incorporating pre-/mid-treatment [18F]-misonidazole positron emission tomography for head-and-neck cancer patients undergoing concurrent chemoradiotherapy. Int J Radiat Oncol Biol Phys 75:101–108
Lehtio K, Eskola O, Vijanen T et al (2004) Imaging perfusion and hypoxia with PET to predict radiotherapy response in head-and-neck cancer. Int J Radiat Oncol Biol Phys 59:971–982
Lewis JS, Welch MJ (2001) PET imaging of hypoxia. Quant J Nucl Med 45:183–188
Li L, Yu J, Xing L et al (2006) Serial hypoxia imaging with 99mTc-HL91 SPECT to predict radiotherapy response in non small cell lung cancer. Amer J Clin Oncol 29:628–633
Li L, Hu M, Zhu H, Zhao W, Yang G, Yu J (2010) Comparison of 18F-Fluoroerythronitroimidazole and 18F-fluorodeoxyglucose positron emission tomography and prognostic value in locally advanced non-small-cell lung cancer. Clin Lung Cancer 11:335–340
Ling CC, Humm J, Larson S et al (2000) Towards multidimensional radiotherapy (MD-CRT): biological imaging and biological conformality. Int J Radiat Oncol Biol Phys 47:551–560
Loncaster JA, Harris AL, Davidson SE et al (2001) Carbonic anhydrase (CA IX) expression, a potential new intrinsic marker of hypoxia: correlations with tumor oxygen measurements and prognosis in locally advanced carcinoma of the cervix. Cancer Res 61:6394–6399
Loncaster JA, Carrington BM, Sykes JR et al (2002) Prediction of radiotherapy outcome using dynamic contrast enhanced MRI of carcinoma of the cervix. Int J Radiat Oncol Biol Phys 54:759–767
Lukacova S, Khalil AA, Overgaard J et al (2006) Relationship between radiobiological hypoxia in a C3H mouse mammary carcinoma and osteopontin levels in mouse serum. Int J Radiat Biol 81:937–944
Lyng H, Sundfør K, Tropé C, Rofstad EK (2000) Disease control of uterine cervical cancer: relationships to tumor oxygen tension, vascular density, cell density, and frequency of mitosis and apoptosis measured before treatment and during radiotherapy. Clin Cancer Res 6:1104–1112
Lyng H, Vorren AO, Sundfør K et al (2001) Assessment of tumor oxygenation in human cervical carcinoma by use of dynamic Gd-DTPA-enhanced MR imaging. J Magn Reson Imaging 14:750–756
Mack PC, Redman MW, Chansky K et al (2008) Lower osteopontin plasma levels are associated with superior outcomes in advanced non-small-cell lung cancer patients receiving platinum-based chemotherapy: SWOG Study S0003. J Clin Oncol 26:4771–4776
Mayer A, Hockel M, Vaupel P (2006) Endogenous hypoxia markers in locally advanced cancers of the uterine cervix: reality or wishful thinking? Strahlenther Onkol 182:501–510
Mayr NA, Wang JZ, Zhang D et al (2010) Longitudinal changes in tumor perfusion pattern during the radiation therapy course and its clinical impact in cervical cancer. Int J Radiat Oncol Biol Phys 77:502–508
Minagawa Y, Shizukuishi K, Koike I et al (2011) Assessment of tumor hypoxia by 62Cu-ATSM PET/CT as a predictor of response in head and neck cancer: a pilot study. Ann Nucl Med 25:339–345
Mortensen LS, Johansen J, Kallehauge J et al (2012) FAZA PET/CT hypoxia imaging in patients with squamous cell carcinoma of the head and neck treated with radiotherapy: results from the DAHANCA 24 trial. Radiother Oncol 105:14–20
Newbold K, Castellano I, Charles-Edwards E et al (2009) An exploratory study into the role of dynamic contrast-enhanced magnetic resonance imaging or perfusion computed tomography for detection of intratumoral hypoxia in head-and-neck cancer. Int J Radiat Oncol Biol Phys 74:29–37
Nielsen T, Wittenborn T, Horsman MR (2012) Dynamic contrast-enhanced magnetic resonance imaging (DCE-MRI) in preclinical studies of antivascular treatments. Pharmaceutics 4:563–589
Nordsmark M, Overgaard M, Overgaard J (1996) Pretreatment oxygenation predicts radiation response in advanced squamous cell carcinoma of the head and neck. Radiother Oncol 41:31–39
Nordsmark M, Alsner J, Keller J et al (2001) Hypoxia in human soft tissue sarcomas: adverse impact on survival and no association with p53 mutations. Br J Cancer 84:1070–1075
Nordsmark M, Bentzen SM, Rudat V et al (2005) Prognostic value of tumor oxygenation in 397 head and neck tumors after primary radiation therapy. An international multi-center study. Radiother Oncol 77:18–24
Nordsmark M, Loncaster J, Aquino-Parsons C et al (2006) The prognostic value of pimonidazole and tumour pO2 in human cervix carcinomas after radiation therapy: a prospective international multi-center study. Radiother Oncol 80:123–131
Nordsmark M, Eriksen JG, Gebski V et al (2007) Differential risk assessments from five hypoxia specific assays: the basis for biologically adapted individualized radiotherapy in advanced head and neck cancer patients. Radiother Oncol 83:389–397
O’Donoghue JA, Zanzonico P, Pugachev A et al (2005) Assessment of regional tumor hypoxia using 18F-fluoromisonidazole and 64Cu(II)-diacetyl-bis(N4-methylthiosemicarbazone) positron emission tomography: Comparative study featuring microPET imaging, Po2 probe measurement, autoradiography, and fluorescent microscopy in the R3327-AT and FaDu rat tumor models. Int J Radiat Oncol Biol Phys 61:1493–1502
Ostheimer C, Bache M, Güttler A et al (2014) Osteopontin, carbonic anhydrase 9 and vascular endothelial growth factor. A pilot study on potential plasma hypoxia markers in the radiotherapy o non-small-cell lung cancer. Strahlenther Onkol 190:276–282
Overgaard J (2007) Hypoxic radiosensitization: adored and ignored. J Clin Oncol 25:4066–4074
Overgaard J, Eriksen JG, Nordsmark M et al (2005) Plasma osteopontin, hypoxia, and response to the hypoxia sensitiser nimorazole in radiotherapy of head and neck cancer: results from the DAHANCA 5 randomised double-blind placebo-controlled trial. Lancet Oncol 6:757–764
Pacheco-Torres J, López-Larrubia P, Ballesteros P, Cerdán S (2011) Imaging tumor hypoxia by magnetic resonance methods. NMR Biomed 24:1–16
Petrik D, Lavori PW, Cao H et al (2006) Plasma osteopontin is an independent prognostic marker for head and neck cancers. J Clin Oncol 24:5291–5297
Rajendran JG, Schwartz DL, O’Sullivan J et al (2006) Tumor hypoxia imaging with [F-18] fluoromisonidazole positron emission tomography in head and neck cancer. Clin Cancer Res 12:5435–5441
Raleigh JA, Chou SC, Arteel GE, Horsman MR (1999) Comparisons among pimonidazole binding, oxygen electrode measurements and radiation response in C3H mouse tumors. Radiat Res 151:580–589
Rasey JS, Koh WJ, Evans ML et al (1996) Quantifying regional hypoxia in human tumors with positron emission tomography of [18F]fluoromisonidazole: a pretherapy study of 37 patients. Int J Radiat Oncol Biol Phys 36:417–428
Reischl G, Dorow DS, Cullinane C et al (2007) Imaging of tumor hypoxia with [124I]IAZA in comparison with [18F]FMISO and [18F]FAZA–first small animal PET results. J Pharm Pharm Sci 10:203–211
Rischin D, Hicks RJ, Fischer R et al (2006) Prognostic significance of [18F]-misonidazole positron emission tomography-detected tumor hypoxia in patients with advanced head and neck cancer randomly assigned to chemoradiation with or without tirapazamine: a substudy of Trans-Tasman Radiation Oncology Group study 98.02. J Clin Oncol 24:2098–2104
Rudat V, Stadler P, Becker A et al (2001) Predictive value of the tumor oxygenation by means of pO2 histography in patients with advanced head and neck cancer. Strahlenther Onkol 177:462–468
Said HM, Katzer A, Flentje M, Vordermark D (2005) Response of the plasma hypoxia marker osteopontin to in vitro hypoxia in human tumor cells. Radiother Oncol 76:200–205
Schuetz M, Schmid MP, Pötter R et al (2010) Evaluating repetitive 18F-fluoroazomycin-arabinoside (18FAZA) PET in the setting of MRI guided adaptive radiotherapy in cervical cancer. Acta Oncol 49:941–947
Seddon BM, Honess DJ, Vojnovic B, Tozer GM, Workman P (2001) Measurement of tumor oxygenation: in vivo comparison of a luminescence fiber-optic sensor and a polarographic electrode in the p22 tumor. Radiat Res 155:837–846
Seddon BM, Payne GS, Simmons L et al (2003) A phase I study of SR-4554 via intravenous administration for noninvasive investigation of tumor hypoxia by magnetic resonance spectroscopy in patients with malignancy. Clin Cancer Res 9:5101–5112
Snitcovsky I, Leitao GM, Pasini FS et al (2009) Plasma osteopontin levels in patients with head and neck cancer undergoing chemoradiotherapy. Arch Otolaryngol Head Neck Surg 135:807–811
Sorensen BS, Hao J, Overgaard J et al (2005) Influence of oxygen concentration and pH on expression of hypoxia induced genes. Radiother Oncol 76:187–193
Søvik Å, Malinen E, Olsen DR (2009) Strategies for biologic image-guided dose escalation: a review. Int J Radiat Oncol Biol Phys 73:650–658
Spence AM, Muzi M, Swanson KR et al (2008) Regional hypoxia in glioblastoma multiforme quantified with [18F]fluoromisonidazole positron emission tomography before radiotherapy: correlation with time to progression and survival. Clin Cancer Res 14:2623–2630
Stadler P, Becker A, Feldmann HJ et al (1999) Influence of the hypoxic subvolume on the survival of patients with head and neck cancer. Int J Radiat Oncol Biol Phys 44:749–754
Sundfør K, Lyng H, Rofstad EK (1998) Tumour hypoxia and vascular density as predictors of metastasis in squamous cell carcinoma of the uterine cervix. Br J Cancer 78:822–827
Swanson KR, Chakraborty G, Wang CH et al (2009) Complementary but distinct roles for MRI and 18F-fluoromisonidazole PET in the assessment of human glioblastomas. J Nucl Med 50:36–44
Swartz HM, Khan N, Buckey J et al (2004) Clinical applications of EPR: overview and perspectives. NMR Biomed 17:335–351
Talks KL, Turley H, Gatter KC et al (2000) The expression and distribution of the hypoxia-inducible factors HIF-1alpha and HIF-2alpha in normal human tissues, cancers, and tumor-associated macrophages. Am J Pathol 157:411–421
Thorwarth D, Eschmann SM, Scheiderbauer J, Paulsen F, Alber M (2005) Kinetic analysis of dynamic 18F-fluoromisonidazole PET correlates with radiation treatment outcome in head-and-neck cancer. BMC Cancer 5:152
Thorwarth D, Eschmann SM, Holzner F, Paulsen F, Alber M (2006) Combined uptake of [18F]FDG and [18F]FMISO correlates with radiation therapy outcome in head-and-neck cancer patients. Radiother Oncol 80:151–156
Turaka A, Buyyounouski MK, Hanlon AL, Horwitz EM, Greenberg RE, Movsas B (2011) Hypoxic prostate/muscle Po2 ratio predicts for outcome in patients with localized prostate cancer: long-term results. Int J Radiat Oncol Biol Phys 82:e433–e439
Urano M, Chen Y, Humm J, Koutcher JA, Zanzonico P, Ling C (2002) Measurements of tumor tissue oxygen tension using a time-resolved luminescence-based optical oxylite probe: comparison with a paired survival assay. Radiat Res 158:167–173
Urtasun RC, Parliament MB, McEwan AJ et al (1996) Measurement of hypoxia in human tumours by non-invasive spect imaging of iodoazomycin arabinoside. Br J Cancer 74(Suppl.):S209–S212
van Loon J, Janssen MHM, Öllers M et al (2010) PET imaging of hypoxia using [18F] HX4: a phase I trial. Eur J Nucl Med Mol Imaging 37:1663–1668
Vaupel P, Kallinowski F, Okunieff P (1989) Blood flow, oxygen and nutrient supply, and metabolic micro-environment of human tumors: a review. Cancer Res 49:6449–6465
Vävere AL, Lewis JS (2007) Cu-ATSM: a radiopharmaceutical for the PET imaging of hypoxia. Dalton Trans 21:4893–4902
Vikram DS, Zweier JL, Kuppusamy P (2007) Methods for noninvasive imaging of tissue hypoxia. Antioxid Redox Signal 9:1745–1756
Vordermark D, Brown JM (2003) Evaluation of hypoxia-inducible factor-1α (HIF-1α) as an intrinsic marker of tumor hypoxia in U87 MG human glioblastoma: in-vitro and xenograft studies. Int J Radiat Oncol Biol Phys 56:1184–1193
Vordermark D, Kaffer A, Riedl S et al (2005) Characterization of carbonic anhydrase IX (CA IX) as an endogenous marker of chronic hypoxia in live human tumor cells. Int J Radiat Oncol Biol Phys 61:1197–1207
Vukovic V, Haugland HK, Nicklee T et al (2001) Hypoxia-inducible factor-1alpha is an intrinsic marker for hypoxia in cervical cancer xenografts. Cancer Res 61:7394–7398
Wen B, Urano M, Humm JL, Seshan VE, Li GC, Ling CC (2008) Comparison of helzel and oxylite systems in the measurement of tumor partial pressure (pO2). Radiat Res 169:67–75
Wilson DF, Cerniglia GJ (1992) Localization of tumors and evaluation of their state of oxygenation by phosphorescence imaging. Cancer Res 52:3988–3993
Winter SC, Shah KA, Han C et al (2006) The relation between hypoxia-inducible factor (HIF)-1alpha and HIF-2alpha expression with anemia and outcome in surgically treated head and neck cancer. Cancer 107:757–766
Wykoff CC, Beasley NJ, Watson PH et al (2000) Hypoxia-inducible expression of tumor-associated carbonic anhydrases. Cancer Res 60:7075–7083
Yuan H, Schroeder T, Bowsher JE, Hedlund LW, Wong T, Dewhirst MW (2006) Intertumoral differences in hypoxia selectivity of the PET imaging agent 64Cu(II)-diacetyl-bis(N4-methylthiosemicarbazone). J Nucl Med 47:989–998
Yue J, Yang Y, Cabrera AR et al (2012) Measuring tumor hypoxia with 18F-FETNIM PET in esophageal squamous cell carcinoma: a pilot clinical study. Dis Esophagus 25:54–61
Zhang H, Ye QH, Ren N et al (2006) The prognostic significance of preoperative plasma levels of osteopontin in patients with hepatocellular carcinoma. J Cancer Res Clin Oncol 132:709–717
Zhao D, Jiang L, Hahn EW, Mason RP (2005) Tumor physiologic response to combretastatin A4 phosphate assessed by MRI. Int J Radiat Oncol Biol Phys 62:872–880
Zhong H, De Marzo AM, Laughner E et al (1999) Overexpression of hypoxia-inducible factor 1alpha in common human cancers and their metastases. Cancer Res 59:5830–5935
Zhu Y, Denhardt DT, Cao H et al (2005) Hypoxia upregulates osteopontin expression in NIH-3T3 cells via a Ras-activated enhancer. Oncogene 24:6555–6563
Zips D, Zöphel K, Abolmaali N et al (2012) Exploratory prospective trial of hypoxia imaging during radiochemotherapy in patients with locally advanced head-and-neck cancer. Radiother Oncol 105:21–28
Acknowledgements
The authors would like to thank the following organizations for financial support: the Danish Agency for Science Technology and Innovation; the Danish Cancer Society; the EC FP7 project METOXIA (project no. 222741); the German Research Foundation (Deutsche Forschungsgemeinschaft); the Wilhelm Sander Foundation and CIRRO—the Lundbeck Foundation Center for Interventional Research in Radiation Oncology and the Danish Council for Strategic Research.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
Vordermark, D., Horsman, M.R. (2016). Hypoxia as a Biomarker and for Personalized Radiation Oncology. In: Baumann, M., Krause, M., Cordes, N. (eds) Molecular Radio-Oncology. Recent Results in Cancer Research, vol 198. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-662-49651-0_6
Download citation
DOI: https://doi.org/10.1007/978-3-662-49651-0_6
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-662-49649-7
Online ISBN: 978-3-662-49651-0
eBook Packages: MedicineMedicine (R0)