Abstract
Hypoxia is a characteristic of most solid tumors. This makes hypoxia an obvious target for cancer therapy. Both in vitro and in vivo studies have identified a range of different approaches that can reduce or eliminate tumor hypoxia and improve radiation response. Several of these have been tested clinically and in particular in combination with radiotherapy targeting hypoxia has proven beneficial. In order to target hypoxia specifically, a range of clinically applicable techniques have been tested. These include measures of hypoxia either directly as physiological oxygen, using exogenous markers such as nitroimidazoles or endogenous molecular markers regulated by hypoxia. Substantial improvement in outcome following radiation therapy is obtained in particular in head and neck cancer; and in Denmark, the Danish head and neck cancer cooperative group (DAHANCA) has used the hypoxic cell radiosensitizer nimorazole in the treatment of head and neck cancer for several years. Currently, this concept has been adopted in confirmatory clinical trials worldwide as the strategy is refined using hypoxia gene classifiers to target the right patients. Thus, extensive preclinical and clinical studies are ongoing in this area, and hopefully it is a matter of time before the hypoxia problem is eliminated. This chapter reviews experimental and clinical evidence for the role of tumor hypoxia in radiotherapy.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Tumor hypoxia
- Radiation therapy
- Prognostic and predictive assays
- Hypoxia gene classifier
- Hypoxic modification
- Radiation sensitizer
- Bioreductive drugs
1 Introduction
1.1 Tumor Hypoxia and Radiation Resistance
The significance of hypoxia in influencing outcome to radiotherapy clinically was first indicated from a study in which the radiation response of skin was markedly decreased if the blood flow to the irradiated area was reduced by compression (Schwarz 1909). Another early report showed that tissues in which blood flow was stimulated by diathermia had a more prominent response to radiation (Müller 1910). At that time, neither study actually attributed the effects to an oxygen dependency. Later, cell culture experiments showed that cells lacking oxygen (and glucose) were relatively insensitive to irradiation (Mottram 1936) and the role of oxygen deficiency as a major source of radiation resistance was hypothesized by Gray based on experimental observations in Chinese hamster cells irradiated in culture, showing that the radiation sensitivity declined at oxygen tensions below about 30 mmHg and declined steeply at very low oxygen tensions from 3 mmHg down to zero (Gray 1953).
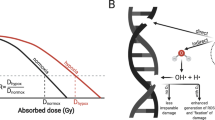
As shown in Fig. 12.1, X-rays interact with biological material and create fast charged particles and free radicals that break chemical bonds and cause damage directly to the target molecule, mostly the DNA, or indirectly to other cellular molecules and from there to the critical target, named R*. The extent of this damage depends on the presence of oxygen because molecular oxygen reacts with R* to eventually form ROOH, a chemically stable composition that consolidates damage to the DNA and limits DNA repair mechanisms.
Radiobiologically relevant hypoxia can be determined in different ways in vitro and in vivo. In a classical assay, tumors in vivo are irradiated under normal air-breathing conditions or when the blood supply to the tumor is shut down by clamping. The tumors are then excised and the viability of the neoplastic cells is assessed by the clonogenic cell survival assay. The clamped dose–response curve represents total hypoxia. Air breathing produces a biphasic curve where the initial part of the survival curve is determined by the aerobic cells in the tumor. At a higher dose, the hypoxic tumor cells dominate the response and thus, the curve begins to parallel the clamped result. As the clamped curve represents 100 % hypoxia, the amount by which the curve in case of the air-breathing animals is displaced allows one to determine the percentage of hypoxia.
Two other ways include the clamped tumor growth delay assay, where measurements are taken of the time taken for tumors to reach a specific size after treatment, and the clamped tumor control assay, in which the percentage of animals showing local tumor control at a certain time after treatment is recorded (Moulder and Rockwell 1984; Horsman et al. 1993). This latter assay produces full radiation dose–response curves under air-breathing and fully anoxic (clamped tumor) conditions, and the hypoxic fractions can then be calculated from the displacement of the dose–response curves .
Evidence from exactly such classical radiobiology assays, used to determine hypoxic clonogenic radiation-resistant cells, suggests a possible link between hypoxia and cancer stem cells (Baumann et al. 2008) as stem cells possess clonogenic potential and hypoxia may affect cancer stem cell generation and maintenance through the upregulation of hypoxia-induced factors (Baumann et al. 2008; Hill et al. 2009). Preclinical studies have shown an inverse correlation between hypoxia and local tumor control after irradiation (Horsman et al. 1993; Yaromina et al. 2006), which could suggest that hypoxia may actually protect the cancer stem cells from the lethal effects of radiation. Using treatments that eliminate hypoxia should, therefore, be an effective method for enhancing the radiosensitivity of cancer stem cells .
2 Targeting Tumor Hypoxia in Patients During Radiotherapy
2.1 High-Oxygen-Content Gas Breathing
A simple method that was tested to eliminate the hypoxic cell population, and thereby improve the therapeutic potential of radiation therapy, involved allowing the tumor-bearing host to breathe high-oxygen-content gas mixtures before and during irradiation . Both oxygen and carbogen (95 % oxygen + 5 % carbon dioxide) breathing could substantially enhance the response of murine tumors to radiation (Du Sault 1963; Suit et al. 1972) and a superior effect was seen when the gasses were inspired under hyperbaric rather than normobaric conditions (Du Sault 1963; Suit et al. 1972). However, normobaric oxygen or carbogen improved the radiosensitizations quite substantially (Siemann et al. 1977; Rojas 1991; Grau et al. 1992; Mortensen et al. 2012).
The first clinical use of high-oxygen-content gas breathing was tested in the late 1960s (Churchill-Davidson 1968). These trials were fairly small, and suffered from the use of unconventional radiation fractionation schemes, but the effect of hyperbaric oxygen was superior to radiotherapy given in air, in particular when few and large fractions were applied (Churchill-Davidson 1968; Dische 1979; Dische et al. 1983). Large multicenter clinical trials conducted by the UK Medical Research Council gave significant improvements in local tumor control and survival for both advanced head and neck and uterine cervix cancer (Dische 1979; Dische et al. 1993; Henk et al. 1977; Henk and Smith 1977; Watson et al. 1978; Overgaard 1989) but not in the case of bladder cancer (Overgaard 1989). The use of hyperbaric oxygen was stopped not only because of patient compliance and safety concerns but also because of access to chemical agents that mimic oxygen (Hypoxic Radiation Sensitizers, see below).
Experimental studies showed that the gas-breathing time factor during normobaric high-content-oxygen exposure was critical for the enhancement of radiation damage (Suit et al. 1972; Siemann et al. 1977; Rojas 1991; Chaplin et al. 1993). In clinical studies, breathing normobaric high-oxygen-content gas failed to show an improvement in radiation response (Bergsjø and Kolstad 1968; Rubin et al. 1979). This could possibly be due to the failure to achieve the optimum preirradiation gas-breathing time. Studies of carbogen breathing in head and neck cancer showed conflicting results; a benefit was reported when carbogen breathing was combined with accelerated radiation and nicotinamide (ARCON) (Kaanders et al. 2002b) but there was no improvement in radiation response (Mendenhall et al. 2005). The concept of ARCON included administration of accelerated radiotherapy, to overcome accelerated repopulation of tumor cells during fractionated radiotherapy, and nicotinamide to deal with fluctuating hypoxia (Petersen et al. 2001). Experimental studies had demonstrated that the vitamin B3 analog, nicotinamide, enhanced radiation damage in murine tumor models using both single and fractionated treatments (Horsman 1995). The ARCON therapy was tested in a phase II trial with promising local tumor control data (Kaanders et al. 2002c) followed by a phase III head and neck trial showing locoregional benefit from ARCON with acceptable toxicity, but no gain in overall survival (Janssens 2012a, b; Peters and Rischin 2012) . A modified ARCON version with radiotherapy and concurrent carbogen and nicotinamide (CON) was tested in a phase III bladder cancer trial. In this study, toxicity was acceptable, and there was a modest improvement in cystoscopic tumor control and significant improvement in local relapse, risk of death, and overall survival in favor of radiotherapy and CON (Hoskin et al. 2010) .
2.2 Hypoxia Radiation Sensitizers
Hypoxic cell sensitizers replaced oxygen-content gas breathing and became probably the most extensively investigated method for overcoming hypoxic cell radioresistance. It was found that the efficiency of radiosensitization was directly related to electron affinity (Adams and Cooke 1969). This led to in vitro studies demonstrating preferential radiosensitization of hypoxic cells by highly electron-affinic nitroaromatic compounds (Asquith et al. 1974; Adams et al. 1976). These chemical agents mimic oxygen but, unlike oxygen, they are slowly metabolized by the tumor cells and, therefore, by diffusion they reach all cells in the tumor.
The first clinical studies of radiosensitizers were conducted using metronidazole in brain tumors (Urtasun et al. 1976) followed by trials exploring the effect of misonidazole (Dische 1985; Overgaard et al. 1989; Overgaard 1994). Most of these trials failed to improve radiation response, although a benefit was seen in the DAHANCA 2 study (Overgaard et al. 1989). Unfortunately, dose-limiting toxicities of misonidazole were significant and misonidazole was replaced by more efficient or less toxic hypoxic sensitizers such as pimonidazole, etanidazole, and nimorazole. Among these studies, a European pimonidazole trial in uterine cervix cancer was disappointingly closed prematurely due to poor performance in the experimental arm (Dische et al. 1993). Two multicenter trials in head and neck cancer using etanidazole showed no benefit (Lee et al. 1995; Eschwége et al. 1997) but a highly significant, improved locoregional tumor control and disease-free survival was shown in the DAHANCA 5 study in patients with supraglottic and pharynx carcinomas treated with the less toxic drug nimorazole (Overgaard et al. 1998). A recent trial by the International Atomic Energy Agency (IAEA) with the 3-nitrotriazole compound, sanazole (AK-2123), in uterine cervical cancer also demonstrated a significant improvement in both local tumor control and overall survival (Dobrowsky et al. 2007).
As presented in Fig. 12.2, a meta-analysis of all randomized clinical studies in a range of tumor sites with hypoxic modification during radiotherapy showed significant improvement in locoregional tumor control (Overgaard 2007; Overgaard 2011) . In particular, the administration of hypoxic radiosensitizers with primary radiotherapy showed that radiosensitizer modification of tumor hypoxia significantly improved the locoregional tumor control (the overall odds ratio was 0.76 (0.70–0.83). This treatment’s benefit was mainly driven by the result of an improved response in head and neck cancer studies with an odds ratio of 0.73 (0.64–0.82) but to a lesser extent in bladder cancer, while no significant effect was observed in other tumor sites (cervix, lung, central nervous system, and esophagus). The overall gain was in the order of a 5–10 % improvement in local control, and although small, such gains are relevant to pursue because they seem to be associated with a similar improvement in survival .
Results from a meta-analysis of hypoxic modification trials by Overgaard 2007 and 2011. The effect of three different ways of modifying tumor hypoxia during radiotherapy, with significant effect of hyperbaric oxygen and hypoxic sensitizers. The gain of hypoxic modification during radiotherapy was most pronounced in head and neck cancer
2.3 Bioreductive Drugs
Another type of hypoxia-targeting drugs applied during radiotherapy are bioreductive drugs. These are agents which were relatively nontoxic to cells under normal oxygenated conditions, but when reduced showed to be more cytotoxic under hypoxia (Hall and Roizin-Towle 1975). This led to the development of various types of such bioreductive drugs that preferentially killed the radiation-resistant hypoxic tumor cell population. These drugs can be divided into three major groups: the quinones, nitroaromatics, and N-oxides (McKeown et al. 2007) .
Among the quinones, the prototype bioreductive drug is mitomycin C (MMC), which has been used for many years in patients as a chemoradiosensitizer. It is activated by bioreduction to form products that cross-link DNA (Kennedy et al. 1980). In two randomized clinical trials in patients with squamous cell carcinoma of the head and neck, MMC improved radiation-induced local tumor control without any enhancement of radiation reactions in normal tissues (Weissberg et al. 1989; Haffty et al. 1993). However, in two other trials no major influence on response or survival was seen (Dobrowsky et al. 1995; Grau et al. 2003). This lack of response may be because the drug was only given once during the radiation schedule. Further, preclinical data show little differential between aerobic and hypoxic cell killing (Stratford and Stephens 1989; Hall 1994).
More efficient quinones such as porfiromycin and apaziquone (EO9) have been developed (McKeown et al. 2007). EO9 has only gone through phase I/II testing and back to the laboratory (Phillips et al. 2012), while porfiromycin was included in a prospective randomized trial in combination with radiation therapy in head and neck cancer but was found to be no better than MMC (Haffty et al. 2005) .
Numerous efforts were made to find other nitroimidazole radiosensitizers that were effective as hypoxic cell cytotoxins. Along that line RSU 1069 was developed. This compound has the classic 2-nitroimidazole radiosensitizing properties, but an aziridine ring at the terminal end of the chain giving the molecule substantial potency as a hypoxic cell cytotoxin. Although the drug was found to have substantial activity in hypoxic cells in vitro and in vivo (Stratford et al. 1986), large animal studies indicated dose-limiting gastrointestinal toxicity. A less toxic prodrug, RB 6145, which is reduced to RSU 1069 in vivo, also showed potent antitumor activity in experimental systems but was dropped when toxicity studies revealed that it induced blindness in dogs. Other nitroaromatic compounds are in the pipeline, including NLCQ-1, CB1954, SN-23862, and PR-104 (McKeown et al. 2007) .
A more promising group of bioreductive drugs is the organic nitroxides, of which the benzotriazene di-N-oxide, tirapazamine, is the lead compound. The parent moiety shows limited toxicity towards aerobic cells but after reduction under hypoxic conditions, a product is formed which has been shown to be highly toxic to cells in vitro and can substantially enhance radiation damage in tumors in vivo (Zeman et al. 1988). Most clinical studies have involved combining tirapazamine with chemotherapy, although there have been a few trials with radiation + chemotherapy (McKeown et al. 2007). The results from the phase II trials generally showed promise, but in the few randomized trials that have been completed the results have been somewhat disappointing. Other N-oxides currently under development include chlorambucil N-oxide and AQ4N (banoxantrone), the latter being combined with radiation in a number of phase II trials (McKeown et al. 2007).
2.4 Hemoglobin and Erythropoietin
The potential benefit of increasing hemoglobin by blood transfusion prior to radiotherapy has been investigated in a number of studies (Thomas 2002). The first clinical investigation of this approach was in advanced squamous cell carcinoma of the uterine cervix (Evans and Bergsjø 1965). Transfusion to patients with low hemoglobin levels resulted in an increased tumor oxygen tension, as measured superficially in the tumor using first-generation oxygen-consuming electrodes. The same study was also the first to show that transfusion to a hemoglobin level of 11 g/dL or higher was significantly related to improved survival. A Canadian retrospective study of 605 cervix cancer patients showed that the negative influence of low hemoglobin on prognosis could be overcome by transfusion (Grogan et al. 1999). However, these observations were not supported by data from a prospective phase III trial, from the Danish Head and Neck Cancer (DAHANCA) study group, showing no benefit of transfusion in patients with low hemoglobin levels (Hoff et al. 2011a, b; Hoff 2012) .
The concentration of hemoglobin can also be increased by stimulation with the hormone erythropoietin (EPO). Preclinical studies have shown that anemia in animals could be corrected by serial injection with EPO and that this EPO treatment also overcame the anemia-induced radiation resistance (Thews et al. 1998; Stuben et al. 2003). The concept of using EPO to correct anemia was tested in a number of clinical trials, and although low hemoglobin levels can be effectively and safely improved by EPO (Lavey and Dempsey 1993; Hoskin et al. 2009), a number of studies in patients undergoing treatment for head and neck cancer failed to show any benefit (Henke et al. 2003; Machtay et al. 2007; Overgaard et al. 2007; Hoskin et al. 2009). In fact, those patients who actually received EPO during radiation therapy did significantly worse than those patients who did not receive EPO and, as a result, all EPO and radiation trials have been stopped .
3 Measuring Hypoxia and Predicting Radiation Response
Methods to detect hypoxic treatment-resistant cells have continuously evolved. Tumor hypoxia can be assessed directly by measurements of oxygen partial pressure (pO2) distributions with polarographic electrodes, or by the use of exogenous markers that are injected into the host and bind specifically to viable hypoxic tumor cells, or by assessments of endogenous markers that are genes/proteins regulated under hypoxia. Other more indirect approaches determine vascularization, perfusion, hemoglobin, or energy metabolism.
3.1 Direct Measures of Oxygen Partial Pressure
Direct measures of physiological oxygen in tumors became feasible with the introduction of the Eppendorf pO2 Histograph, a polarographic oxygen-sensitive probe. This assay allows to sample hundreds of pO2 values rapidly at multiple sites within the tumor (Nordsmark et al. 1994; Nordsmark 1997) in comparison with the old manually moved glass-sealed oxygen-consuming probes allowing only a few pO2 values obtained 3–4 mm under the surface of a tumor (Kolstad 1963; Bergsjø and Kolstad1968; Gatenby et al. 1988). The Eppendorf pO2 Histograph device is used preclinically and clinically for evaluation of tissue oxygen tension. A set of measurements can be obtained from a tumor and related normal tissue within 10–20 min and is generally well tolerated by patients even though it is invasive. It is relevant for the interpretation of such measurements to understand that the catchment range of one pO2 value is between about 60 and 100 µm and is averaged over multiple cells that are malignant, normal; viable, or nonviable (Nordsmark et al. 1994; Nordsmark 1997).
Typically, human tumors are more hypoxic than normal tissues, and there is substantial inter- and intraheterogeneity. The most frequently reported parameters are the median pO2, the proportion of pO2 values ≤ 2.5 mmHg (HP2.5), ≤ 5 mmHg (HP5), ≤ 10 mmHg (HP10), or the hypoxic subvolume, defined as HP5 multiplied by the total tumor volume.The result of these parameters (HP2.5, HP5, and HP10) are given as instantly printed values delivered by the Eppendorf pO2 Histograph and they comply conveniently with the radiobiological rationale (Nordsmark et al. 1994).
The measurement of pO2 distributions with polarographic electrodes is probably the most direct method for estimating tumor hypoxia and one that has showed to correlate with radiobiological hypoxic fractions based on direct analysis of the radiation response under normal and modified conditions in vivo in mouse C3H mammary carcinomas . Tumor hypoxia measured by the Eppendorf electrode was also correlated with tissue-based quantification of pimonidazole staining and the radiobiological hypoxic fraction in an experimental model. In a preclinical study using the mouse C3H model, it was shown that pretreatment pO2 was predictable of tumor control probability following radiotherapy when tumors were grouped as more or less hypoxic ( Fig. 12.3a–b). A similar observation was done in advanced head and neck cancer, where pretreatment oxygen measurements were used to classify patients into more hypoxic or less hypoxic and thereby predict local tumor control ( Fig. 12.3c–d). Numerous such clinical studies have now clearly shown the ability of hypoxia to influence outcome in squamous cell carcinomas of the cervix and head and neck (Hoeckel et al. 1993; Fyles et al. 1998; Fyles et al. 2002; Brizel et al. 1997; Nordsmark et al. 1996; Nordsmark 2005).
a Cumulative frequency of mouse tumors as function of pO2 values ≤ 2.5 mmHg measured by the Eppendorf electrodes under different gas-breathing conditions. b Local tumor control of tumors irradiated by 55 Gy single dose under different gas-breathing conditions and divided into “less hypoxic” and “more hypoxic” based on pO2 values ≤ 2.5 mmHg measured by the Eppendorf electrodes. c Cumulative frequency of head and neck tumors as a function of pO2 values ≤ 2.5 mmHg measured by the Eppendorf electrodes. d Locoregional tumor control in 63 head and neck cancer patients divided by median tumor pO2 into “less hypoxic” and “more hypoxic.” (a–b modified from Mortensen et al. 2011; d Nordsmark, with permission)
Direct measurement in the tumor using an oxygen electrode is often referred to as a gold standard due to its direct measurement principle and its ability to identify patients with poor prognosis. These measurements have served as standard reference in the development of other new and hopefully more clinically applicable hypoxia-specific assays such as positron emission tomography (PET) hypoxia imaging and hypoxia-specific gene classifiers. Ironically, the device is no longer commercially available.
3.2 Nitroimidazole Reduction
Clinically attractive techniques to measure tumor hypoxia are available, with special focus on the detection of injectable exogenous tracers that accumulate in hypoxic cells . These tracers can subsequently be detected in biopsies using immunohistology (Raleigh et al. 1996; Olive and Aquino-Parsons 2004) or alternatively they can be labeled with a radionuclide prior to injection allowing noninvasive detection using PET (Rasey et al. 1996) and single-photon emission computed tomography (SPECT) (Urtasun et al. 1996) or detected by magnetic resonance spectroscopy (Seddon et al. 2003). The exogenous immunodetectable markers mainly belong to the 2-nitroimidazole compounds and enter cells by diffusion. Once inside the cell, the molecule undergoes a single electron reduction to form a nitro radical anion which is immediately reoxidized when pO2 is sufficiently high. However, when pO2 drops below a threshold (~ 10 mmHg) the molecule is further reduced to form highly reactive intermediates that bind to various macromolecules in the cells, in particular, thiol groups in peptides and protein.
The most commonly used nitroimidazole for detection of hypoxia is pimonidazole, which was originally developed as a hypoxia radiosensitizer under the name Ro 03-8799 (Williams et al. 1982). Although pimonidazole failed as a useful sensitizer, it was further developed as a marker of tissue hypoxia and is now commercially available as Hypoxyprobe™. Pimonidazole and related exogenous hypoxia probes (e.g., EF5) can be visualized on tissue sections prepared from biopsies or surgically resected tumor tissue from animals or patients previously administered with the probes. Since hypoxic cells are difficult to reach, tracer diffusion and accumulation is a slow process, and tracers are normally allowed to circulate for hours or even days before tissue sampling. During the staining procedure, the remaining unbound tracer (not hypoxia related) is washed away resulting in highly specific staining of viable cells with a pO2 below ~ 10 mmHg. Pimonidazole staining was correlated with the radiobiological hypoxic fraction in experimental models (Raleigh et al. 1999) and in accordance, locoregional control rates are lower in head and neck cancer patients with high uptake of pimonidazole than in patients with low uptake (Kaanders et al. 2002a). Recently, it was further demonstrated that regional control following hypoxia-targeting ARCON treatment (see Sect. 9.3.4) in head and neck cancer patients was improved specifically in patients with tumors with high retention of pimonidazole (Janessens et al. 2012a, b). In support of the usefulness of nitroimidazole-based compounds, (Evans et al. 2007) showed that elevated EF5 binding was significantly associated with poor outcome in patients with head and neck cancer receiving various treatments. In contrast, there was no correlation between pimonidazole and locoregional tumor control or overall survival in cervix carcinoma patients treated with radiotherapy (Nordsmark et al. 2006). Likewise in patients with bladder cancer treated with radiotherapy concurrent with carbogen and nicotinamide (CON), no clear correlations between pimonidazole staining and local control or metastases-free survival were observed (Hoskin et al. 2003), but conversely this could simply suggest that CON eliminated the negative influence of hypoxia effectively. Immune-detectable markers require a biopsy and provide an assessment of hypoxia in a small tumor area, which may result in sampling errors due to intratumoral heterogeneity in hypoxia. Weak or absent correlations between clinical endpoints and tracer retention quantification in small tumor subvolumes may indeed relate to this heterogeneity. Finally, invasive tissue-based analysis provides no three-dimensional (3-D) information on the distribution of hypoxia within the tumor, which is required for some treatments like hypoxic dose painting (see Chap. 9.3.5).
In order to overcome some of the limitations of invasive quantification of hypoxia marker retention, labeling 2-nitroimidazoles with positron emitters (in particular fluorine-18) has received a lot of attention. Such labeling allows noninvasive assessment of global as well as regional tumor tracer retention using PET scans. Tracer retention can be quantified in numerous ways but typically, tracer activity in the tumor is compared to tracer activity in a nonhypoxic reference tissue such as muscle or blood. However, slow distribution, uptake, and clearance of nitroimidazoles result in a significant presence of image-contaminating unbound tracer (unlike the invasive assays where it is washed away during staining), which leads to a relatively limited tissue contrast even 2–4 h after tracer administration when a typical scan is performed. The formation of unwanted metabolites, which varies substantially among different tracers, may further deteriorate image quality and hypoxia specificity. The low-contrast problem is further exacerbated by the relatively low resolution of PET (several millimeters in clinical scanners), which is much lower than the dimensions of the hypoxic structures that are studied. Taken together, these inherent limitations increase the risk of missing small hypoxic foci, especially in areas where hypoxia and necrosis are intermixed (Busk et al. 2008). Still, hypoxia imaging has shown some promising results (Horsman et al. 2012).
18F-misonidazole (FMISO) was the first tracer developed for hypoxia PET imaging (Rasey et al. 1989). Despite its nonideal pharmacokinetics which results in low image contrast, FMISO has shown prognostic value in several studies in different tumor types including head and neck cancer (Kikuchi et al. 2011; Rajendran et al. 2006), renal cell carcinoma (Hugonnet et al. 2011), and non-small-cell lung cancer (NSCLC) (Eschmann et al. 2005) . In addition, FMISO scans were able to identify those head and neck cancer patients who benefitted from treatment with the hypoxia-activated prodrug tirapazamine (Rischin et al. 2006). Intriguingly, a recent study showed that FMISO hypoxia PET conducted 1–2 weeks after initiation of treatment with radiotherapy was more strongly linked to therapeutic response than pretreatment scans (Zips et al. 2012).
Several second-generation hypoxia tracers with supposedly better pharmacokinetic characteristics than FMISO have been developed and are currently being tested. Fluoroazomycin arabinoside (FAZA) is less lipophilic than FMSIO and may therefore clear faster from nonhypoxic tissue. FAZA proved highly superior to FMISO in tumor-bearing mice in terms of tumor-to-reference tissue ratios (Piert et al. 2005), but unexpectedly FAZA was slightly inferior to FMISO in tumor-bearing rats (Sorger et al. 2003), suggesting that conclusions obtained in one species/model cannot uncritically be extrapolated to other species/models. A preclinical study showed that FAZA retention was able to predict tumor control probability following radiotherapy in tumor-bearing mice (Mortensen et al. 2011; Fig. 12.4a–b). Similarly, it was shown that FAZA could identify mice that benefit from hypoxia-directed radiochemotherapy using tirapzamine (Beck et al. 2007). FAZA is currently undergoing clinical testing, and it was recently shownthat FAZA retention was correlated to disease-free survival in patients treated with radiotherapy (Fig. 12.4c–d;Mortensen et al. 2012).
a Cumulative frequency distribution of tumor hypoxia measured by 18F FAZA PET in experimental mouse tumors under different gas-breathing conditions. b Local tumor control of tumors irradiated by 55 Gy single dose under different gas-breathing conditions and divided into “less hypoxic” and “more hypoxic” based on 18F FAZA PET. c Cumulative frequency of 40 head and neck tumors as a function of hypoxia measured by 18F FAZA PET prior to radiotherapy. d Disease-free survival of patients with hypoxic versus nonhypoxic tumors measured by 18F FAZA PET (a and b modified from Mortensen et al. 2011; c and d adapted from Mortensen et al. 2012)
3.3 Molecular Markers, Gene Expression, and the Therapeutic Opportunity of Targeting Hypoxia During Radiotherapy
The endogenous markers of hypoxia include a number of molecular molecules regulated by low oxygen content. These can be measured from biopsy material using protein immunohistochemistry or gene expression, or proteins identified from blood samples. Although endogenous markers have been correlated with outcome to radiation therapy in some studies, it is not a universal finding. This probably reflects the fact that many of these endogenous markers are not hypoxic specific rather than being any indication that hypoxia does not play a role in influencing radiation response (Bussink et al. 2003) . In an attempt to confirm how well these endogenous markers express radiobiologically relevant hypoxia, several studies that have compared tumor oxygenation with the expression level of the hypoxia marker hypoxia inducible factor-1α (HIF-1α) (Mayer et al. 2004; Haugland et al. 2002; Jankovic et al. 2006), carbonic anhydrase IX (CAIX) (Loncaster et al. 2001; Mayer et al. 2004; Hedley et al. 2003; Jankovic 2006), and Glut-1 (Mayer et al. 2005). These studies showed mixed observations in uterine cervix cancer. In head and neck cancer, oxygen tension was compared with HIF-1α, CAIX, osteopontin (OPN) plasma, and OPN tumor and showed no mutual correlation and had different prognostic values (Nordsmark et al. 2006; Nordsmark et al. 2007) . In primary NSCLC, there was a correlation between the tumor/normal lung tissue pO2 ratio and the expression of OPN measured in plasma by enzyme-linked immunosorbent assay (ELISA) (r = 0.53, p = 0.02) and CAIX measured by immohistochemistry, (p = 0.006) (Le et al. 2006).
Expression of CAIX has been related with poor outcome in some head and neck cancer studies (Buffa et al. 2004) but not in others (Nordsmark et al. 2007; Eriksen and Overgaard 2007).
OPN is a secreted arginine–glycine–aspartic acid-containing phosphoprotein that was correlated with tumor hypoxia (Le et al. 2003) . OPN is inversely correlated with Von Hippel–Lindau (VHL) gene expression, and the VHL protein drives the proteasome-mediated proteolysis of HIF-1α under normoxic conditions. In concordance with these results, there was a weak but significant correlation between median tumor pO2 and plasma OPN in patients with advanced head and neck cancer (Nordsmark et al. 2007).
High levels of the plasma marker OPN were associated with poor local tumor control and survival after radiotherapy in 320 head and neck cancer patients who were included in a randomized double-blind placebo-controlled trial between radiation therapy alone and radiation combined with the hypoxia sensitizer nimorazole (Overgaard 1998; Overgaard et al. 2005) . This was the first proof of principle that modifiable hypoxia is predictable. However, high plasma OPN was not predictive of hypoxic modification by cisplatin and tirapazamin in patients with locoregionally advanced head and neck squamous cell carcinoma treated in the TROG 02.02 phase III trial (Lim et al. 2012), and more work is need before OPN is safely established as a predictive assay for hypoxic modification .
More promising results relate to a cohort of patients from the same randomized double-blind placebo-controlled trial between radiation therapy alone and radiation combined with the hypoxia sensitizer, nimorazole, where a hypoxia gene classifier showed to be predictive for hypoxic modification by nimoraxole (Toustrup et al. 2012) . The gene classifier was developed stepwise in vitro (Soerensen et al. 2005; Soerensen et al. 2010) and in vivo (Busk et al. 2011a) and patient selection for the final gene classifier was based on tumor pO2 measured by the Eppendorf pO2 Histograph in a sample of head and neck tumors (Nordsmark et al. 2007; Toustrup et al. 2011). This hypoxia gene classifier is currently refined and tested in a new cohort of head and neck tumors and in other tumor sites as well, in order to advance targeting tumor hypoxia during radiotherapy on an individual patient basis .
4 Conclusions and Perspectives
It has been proposed that “targeted therapies” are the future of cancer therapy. In that context, hypoxia must be considered the ultimate target (Overgaard 2011; Peters and Rischin 2012). Hypoxia is a fundamental feature of most solid tumors, whether animal or human, which can be identified by a range of clinically applicable techniques. There is definitive evidence that its existence in specific tumor types will have a significant negative impact on cancer treatment, especially radiotherapy. Numerous preclinical studies have identified a range of different approaches that can reduce or eliminate tumor hypoxia and preferentially improve radiation response, and many of these approaches have undergone clinical testing and substantial improvements in outcome following radiation therapy . However, except for Denmark where the hypoxic cell radiosensitizer nimorazole is routinely used in the treatment of head and neck cancer, none of these hypoxic modifiers has become established as a standard therapy with radiation. Nevertheless, extensive preclinical and clinical studies are ongoing in this area, so hopefully, it is simply a matter of time before the hypoxia problem is eliminated.
References
Adams GE, Cooke MS (1969) Int J Radiat Biol 15:457–471
Adams GE, Flockhart IR, Smithen CE et al (1976) Radiat Res 67, 9–20
Asquith JC, Watts ME, Patel K et al (1974) Radiat Res 60:108–118
Baumann M, Krause M, Hill R (2008) Nature Reviews Cancer 8:545–554
Beck R, Roper B, Carlsen JM et al (2007) Journal of Nuclear Medicine 48:973–80
Bergsjø P, Kolstad P (1968) Scand J Clin Lab Inv 106:167–171
Brizel DM, Sibley GS, Prosnitz LR et al (1997) Int J Radiat Oncol Biol Phys 38:285–289
Buffa FM, Bentzen SM, Daley FM et al. (2004) Clinical Cancer Research 10 (11):3745–3754
Busk M, Horsman MR, Overgaard J (2008) Acta Oncologica 47:1201–10
Busk M, Toustrup K, Soerensen BS et al. (2011) BMC Cancer 9(11):63
Bussink J, Kaanders JHAM, Kogel AJ van der (2003) Radiother Oncol 67:3–15
Chaplin DJ, Horsman MR, Siemann DW (1993) Br J Cancer 68:269–273
Churchill-Davidson I (1968) Front Radiat Ther Oncol 1:1–15
Dische S (1979) Br J Radiol 51:888–894
Dische S (1985) Radiother Oncol 3:97–115
Dische S, Anderson PJ, Sealy R et al (1983) Br J Radiol 56:251–255
Dische S, Machin D, Chassagne D (1993) Radiother Oncol 26:93–103
Dobrowsky W, Naude J, Dobrowsky E et al (1995) Acta Oncologica 34:270–272
Du Sault LA (1963) Br J Radiol 36:749–754
Dobrowsky W, Huigol NG, Jayatilake RS et al (2007) Radiother Oncol 82:24–29
Eriksen JG, Overgaard J (2007) Radiother Oncol 83(3):383–388
Eschmann SM, Paulsen F, Reimold M et al (2005) J Nucl Med 46:253–60
Eschwége F, Sancho-Garnier H, Chassagne D et al (1997) Int J Radiat Oncol Biol Phys 39:275–281
Evans JC, Bergsjø P (1965) Radiology 84:709–717
Evans SM, Du KL, Chalian AA et al (2007) Int J Radiat Oncol Biol Phys 69:1024–31
Fyles AW, Milosevic M, Wong R et al. (1998) Radiother Oncol 48(2):149–56
Fyles A, Milosevic M, Hedley D et al. (2002) J Clin Oncol 20(3):680–687
Gatenby RA, Kessler HB, Rosenblum JS et al (1988) Int J Radiat Oncol Biol Phys 14:831–838
Grau C, Horsman MR, Overgaard J (1992) Int J Radiat Oncol Biol Phys 22:415–419
Grau C, Agarwal JP, Jabeen K et al (2003) Radiother Oncol 67:17–27
Gray LH, Conger AD, Ebert M et al (1953) Br J Radiol 26:638–648
Grogan M, Thomas GM, Melamed I et al (1999) Cancer 86:1528–1536
Haffty BG, Son YH, Sasaki CT et al (1993) Int J Radiat Oncol Biol Phys 27:241–250
Haffty BG, Wilson LD, Son YH et al (2005) Int J Radiat Oncol Biol Phys 61:5–6
Hall EJ (1994) Radiobiology for the Radiobiologist, 4th edn. JB Lippincott, Philadelphia
Hall EJ, Roizin-Towle L (1975) Radiology 117:453–457
Haugland HK, Vukovic V, Pintilie M et al (2002) Int J Radiat Oncol Biol Phys 53:854–861
Hedley D, Pintilie M, Woo J et al (2003) Clin Cancer Res 9:5666–5674
Henk JM, Smith CW (1977 July) Lancet 104–105
Henk JM, Kunkler PB, Smith CW (1977 July) Lancet 101–103
Henke M, Laszig R, Rube C et al (2003) Lancet 362:1255–1260
Hill RP, Marie-Egyptienne DT, Hedley DW (2009) Semin Radiat Oncol 19:106–111
Hoeckel M, Knoop C, Schlenger K et al (1993) Radiother Oncol 26:45–50
Hoff CM, Lassen P, Eriksen JG et al. (2011a) Acta Oncologica 50:1006–1014
Hoff CM, Hansen HS, Overgaard M et al. (2011b) Radiother Oncol 98:28–33
Hoff CM (2012) Acta Oncologica 1–14
Horsman MR, Khalil AA, Nordsmark M et al. (1993) Radiother Oncol 28:69–71
Horsman MR (1995) Acta Oncologica 34:571–587
Horsman MR, Mortensen LS, Petersen JB et al. (2012) Nat Rev Clin Oncol 9(12):674–87
Hoskin PJ, Sibtain A, Daley FM et al. (2003) Br J Cancer 89:1290–7
Hoskin PJ, Robinson M, Slevin N et al (2009) J Clin Oncol 27:5751–5756
Hoskin PJ, Rojas A, Bentzen SM (2010) J Clin Oncol 33:4912–4918
Hugonnet F, Fournier L, Medioni J et al (2011) J Nucl Med 52:1048–55
Jankovic B, Aquino-Parsonsquino-Parsons C, Raleigh JA et al (2006) Cytometry B Clin Cytom 70:45–55
Janssen HL, Haustermans KM, Sprong D et al (2002) Int J Radiat Oncol Biol Phys 54:1537–1549
Janssens GO, Rademakers SE, Terhaard CH et al. (2012a) J Clin Oncol 20, 30(15):1777–83
Janssens GO, Terhaard CH, Doornaert PA et al. (2012b) Int J Radiat Oncol Biol Phys 82(2):532–8
Kaanders JH, Wijffels KI, Marres HA et al (2002a) Cancer Res 62:7066–74
Kaanders JH, Bussink J, Kogel AJ van der (2002b) Lancet Oncology 3:728–737
Kaanders JHAM, Pop LAM, Marres HAM (2002c) Int J Radiat Oncol Biol Phys 52:769–778
Kennedy KA, Rockwell S, Sartorelli AC (1980) Cancer Res 40:2356–2360
Kikuchi M, Yamane T, Shinohara S et al (2011) Ann Nucl Med 25:625–33
Kolstad P (1963) Copenhagen Stockholm Göteborg, Scandinavian University Books, 1963
Lavey and Demsey (1993)
Le QT, Sutpin PD, Raychaudhuri S et al (2003) Clin Cancer Res 9:59–67
Le QT, Chen E, Salim A et al. (2006) Clin Cancer Res 12(5):1507–1514
Lee D-J, Cosmatos D, Marcial VA et al (1995) Int J Radiat Oncol Biol Phys 32:567–576
Lim AM, Rischin D, Fisher R et al. (2012) Clin Cancer Res 18(1):301–7
Loncaster JA, Harris AL, Davidson SE et al (2001) Cancer Res 61:6394–6399
Mayer A, Wree A, Hockel M et al (2004) Cancer Res 64:5876–5881
Mayer A, Hockel M, Wree A et al (2005) Clin Cancer Res 11:2768–2773
Machtay M, Pajak T, Suntharalingam M et al (2007) Int J Radiat Oncol Biol Phys 69:1008–1017
Mendenhall WM, Morris CG, Amdur RJ et al. (2005) Cancer 104:333–337
McKeown SR, Cowen RL, Williams KJ (2007) Clin Oncol 19:427–442
Mortensen LS, Busk M, Nordsmark M et al. (2011) Radiother Oncol 99(3):418–423
Mortensen LS, Johansen J, Kallehauge J et al. (2012) Radiother Oncol 105(1):14–20
Mottram JC (1936) Br J Radiol 10:606–614
Moulder JE, Rockwell S (1984) Int J Radiat Oncol Biol Phys 10:695–712
Müller C (1910) Munchener Medizinische Wochenschrift 28:1490–1493
Nordsmark M, Bentzen SM, Overgaard J (1994) Acta Oncologica 33(4):383–389
Nordsmark M, Overgaard M, Overgaard J (1996) Radiother Oncol 41:31–39
Nordsmark M (1997) PHD thesis Faculty of Health Sciences. University of Aarhus 1:1–121
Nordsmark M, Bentzen SM, Rudat V et al (2005) Radiother Oncol 77:18–24
Nordsmark M, Loncaster J, Aquino-Parsonsquino-Parsons C et al. (2006) Radiother Oncol 80(2):123–131
Nordsmark M, Eriksen JG, Gebski V et al (2007) Radiother Oncol 83:389–397
Olive PL, Aquino-Parsons C (2004) Semin Radiat Oncol 14:241–248
Overgaard J (1989) Int J Radiat Biol 56:801–811
Overgaard J (1994) Oncol Res 6:509–518
Overgaard J (2007) J Clin Oncol 25(26):4066–74
Overgaard J (2011) Radiother Oncol 100(1):22–32
Overgaard J, Hansen HS, Andersen AP et al (1989) Int J Radiat Oncol Biol Phys 16:1065–1068
Overgaard JS, Hansen H, Overgaard M et al (1998) Radiother Oncol 46:135–146
Overgaard J, Eriksen JG, Nordsmark M et al (2005) Lancet Oncol 6(10):757–64
Overgaard J, Hoff C, Sand HH (2007) Eur J Cancer 5 (Suppl 7)
Peters L, Rischin D (2012) J Clin Oncol 30(15):1741–3
Petersen C, Zips D, Krause M et al (2001) Int J Radiat Oncol Biol Phys 51:483–493
Piert M, Machulla HJ, Picchio M et al (2005) J Nucl Med 46:106–113
Phillips RM, Hendriks HR, Peters GJ (2012) Br J Pharmacol Apr 18
Rajendran JG, Schwartz DL, O’Sullivan J et al (2006) Clin Cancer Res 12:5435–41
Raleigh JA, Dewhirst MW, Thrall DE (1996) Semin Radiat Oncol 6:37–45
Raleigh JA, Chou SC, Arteel GE et al. (1999) Radiat Res 151(5):580–9
Rasey JS, Koh WJ, Grierson JR et al. (1989) Int J Radiat Oncol Biol Phys 17:985–91
Rasey JS, Koh WJ, Evans ML et al (1996) Int J Radiat Oncol Biol Phys 36:417–428
Rischin D, Hicks RJ, Fisher R et al (2006) J Clin Oncol 24:2098–104
Rouschop KM, Beucken T van den, Dubois L et al. (2010) J Clin Invest 120(1):127–141
Rojas A (1991) Radiother Oncol 20(Suppl 1):65–70
Roti Roti JL (2004) Int J Hyperther 201:109–114
Rubin P, Hanley J, Keys HM et al (1979) Int J Radiat Oncol Biol Phys 5:1963–1970
Schwarz G (1909) Munchener Medizinische Wochenschrift 24:1–2
Seddon BM, Payne GS, Simmons L et al (2003) Clin Cancer Res 9:5101–5112
Siemann DW, Hill RP, Bush RS (1977) Int J Radiat Oncol Biol Phys 2:903–911
Sorger D, Patt M, Kumar P et al (2003) Nucl Med Biol 30:317–26
Stratford IJ, Stephens MA (1989) Int J Radiat Oncol Biol Phys 16:973–976
Stratford IJ, O’Neill P, Sheldon PW et al (1986) Biochem Pharmacol 35:105–109
Stuben G, Pottgen C, Knuhmann K et al (2003) Int J Radiat Oncol Biol Phys 55:1358–1362
Suit HD, Marshall N, Woerner D (1972) Cancer 30:1154–1158
Sørensen BS, Hao J, Overgaard J et al (2005) Radiother Oncol 76:187–193
Sørensen BS, Toustrup K, Horsman MR et al (2010) Acta Oncologica 49:895–905
Thews O, Koenig R, Kelleher DK, Kutzner J, Vaupel P (1998) Br J Cancer 78:752–756
Thomas GM (2002) Oncology 63:19–28
Toustrup K, Sorensen BS, Nordsmark M et al. (2011) Cancer Res 71(17):5923–5931
Toustrup K, Sorensen BS, Lassen P et al (2012) Radiother Oncol 102:122–129
Urtasun R, Band P, Chapman, JD et al (1976) New Engl J Med 294:1364–1367
Urtasun RC, Parliament MB, McEwan AJ et al (1996) Br J Cancer 74(Suppl):S209–S212
Watson ER, Halnan KE, Dische S et al (1978) Br J Radiol 51:879–887
Weissberg JB, Son YH, Papac RJ et al (1989) Int J Radiat Oncol Biol Phys 17:3–9
Williams MV, Denekamp J, Minchinton AI et al. (1982) Int J Radiat Oncol Biol Phys 8:477–81
Yaromina A, Zips D, Thames HD et al (2006) Radiother Oncol 81:122–129
Zeman EM, Hirst VK, Lemmon MJ, Brown JM (1988) Radiother Oncol 12:209–218
Zips D, Zophel K, Abolmaali N et al (2012) Radiother Oncol 105:21–28
Acknowledgments
The authors would like to thank the following organizations for financial support: the Danish Cancer Society; the EC FP7 project METOXIA (project no. 222741); and CIRRO—the Lundbeck Foundation Center for Interventional Research in Radiation Oncology & the Danish Council for Strategic Research.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer Science+Business Media New York
About this chapter
Cite this chapter
Nordsmark, M., Alsner, J., Busk, M., Overgaard, J., Horsman, M. (2014). Hypoxia and Radiation Therapy. In: Melillo, G. (eds) Hypoxia and Cancer. Cancer Drug Discovery and Development. Springer, New York, NY. https://doi.org/10.1007/978-1-4614-9167-5_12
Download citation
DOI: https://doi.org/10.1007/978-1-4614-9167-5_12
Published:
Publisher Name: Springer, New York, NY
Print ISBN: 978-1-4614-9166-8
Online ISBN: 978-1-4614-9167-5
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)