Abstract
When it comes to imaging of the injured athlete’s elbow, there is a vast array of image modalities to choose from, including conventional radiographs, ultrasound (US), computed tomography (CT), magnetic resonance imaging (MRI), and arthrography (CTA, MRA). Choosing the appropriate imaging technique is of vital importance for quick diagnosis and adequate treatment. This chapter will discuss the role of each image modality in the diagnostic workup for pathology around the elbow commonly encountered in overhead athletes. Specific conditions of the elbow will be discussed in detail with a focus on image findings.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
4.1 Imaging of the Elbow in General
When it comes to imaging of the injured athlete’s elbow, there is a vast array of image modalities to choose from, including conventional radiographs, ultrasound (US), computed tomography (CT), magnetic resonance imaging (MRI), and arthrography (CTA, MRA). Choosing the appropriate imaging technique is of vital importance for quick diagnosis and adequate treatment. This chapter will discuss the role of each image modality in the diagnostic workup for pathology around the elbow commonly encountered in overhead athletes. Specific conditions of the elbow will be discussed in detail with a focus on image findings.
4.1.1 Conventional Radiography
Radiography is the first choice in imaging of elbow injuries [1, 2]. It is common practice to depict at least two standard projections of the elbow: a lateral and an anteroposterior (AP) view. The lateral view is obtained with the elbow flexed at 90° angle and the forearm in neutral position (thumb up). The anteroposterior view requires the elbow in full extension with the forearm supinated. In this position, the medial and lateral epicondyles are optimally visualized and the carrying angle can be estimated (normally slightly in valgus) [1, 2]. A radiocapitellar view can additionally be applied to optimally visualize the radiocapitellar joint. It resembles the lateral view with the elbow in 90° of flexion, yet the X-ray tube is angulated 45° anteriorly toward the joint. This view is particularly useful in the evaluation of osteochondral fractures of the capitellum or injuries to the radial head and neck [3, 4]. When evaluating the elbow on radiographic images, the following aspects should be assessed [1, 5]:
-
Radiocapitellar line
The radiocapitellar line is an imaginary line parallel to the long axis of the radial neck on a lateral view and should pass through the center of the capitellum [6]. If not, dislocation of the radius is implied [1, 7, 8]. However, in a Monteggia injury (see below), the radiocapitellar line may seem normal, even if the radial head is almost always dislocated. Careful evaluation of the total alignment of the elbow is therefore mandatory in all cases [5].
-
Cortex of radial head and neck (in adults)
The appearance of the cortex of the proximal radius is smooth on standard lateral and AP views in the normal situation. If injury is present, the outlines of the cortex can display crinkles, steps, or irregularities due to (subtle) fracture lines [5].
-
Anterior humeral line (in children)
On a lateral view, the anterior humeral line can be drawn along the anterior cortex of the distal humeral shaft and should bisect the middle third of the capitellum [6]. If less than one third of the capitellum lies anterior to this line, a supracondylar fracture with posterior displacement is highly probable [5].
-
Ossification centers
Secondary ossification centers, also referred to as apophyses, serve as attachment sites for muscle-tendon units. Ossification centers are primarily composed of maturing chondrocytes which are biomechanically less resistant than musculotendinous structures. As a result, traction forces on an ossification center may result in an apophyseal avulsion injury [9]. During childhood, a total of six ossification centers develop in a set order: capitellum, radial head, medial epicondyle, trochlea, olecranon, and lateral epicondyle [10–12]. Being familiar with the pattern and appearance of these ossification centers is essential in differentiating normal anatomy from pathology on standard radiographs of the pediatrics elbow. Note that the exact timing of ossification shows great variability among young individuals [11, 12].
-
Fat pads
On a lateral view, the anterior fat pad is visible as a dark streak along the anterior side of the distal humerus. The posterior fat pad is never visible, unless intracapsular abnormalities are present. Joint effusion, for example, causes displacement of both the anterior and posterior fat pads, resulting in a positive fat pad sign. This makes the presence of a fracture more likely, but absence of a visible fat pad does not completely exclude a fracture [5]. The fat pad sign is specifically relevant in pediatric cases, as it can indicate fractures of the immature cartilaginous components of the elbow [1] (Table 4.1).
Table 4.1 Essential aspects of radiographic evaluation of the elbow joint
4.1.2 Magnetic Resonance Imaging and Magnetic Resonance Arthrography
Magnetic resonance imaging (MRI) is considered the next step in the imaging workup. Appropriate patient positioning, coil selection, and sequence technique are of vital importance in proper imaging of the elbow. The anatomical position with the patient lying supine, the elbow in full extension and the forearm in supination, is the most comfortable and a widely used position. Note that with this position, the elbow is located off-center of the scanner’s magnetic field. This will reduce the signal-to-noise ratio and may introduce inhomogeneous fat suppression. For this reason, fat suppression by means of inversion recovery sequences is preferred over frequency-selective fat suppression techniques when the anatomical position is applied [13]. An alternative is the “superman position,” where the patient lies prone with the elbow over the head and the forearm in pronation. This will bring the elbow closer to the center of the magnet which will increase overall image quality at the cost of markedly reduced patient comfort. In any case, a dedicated surface coil should be used for optimal imaging of the elbow [14]. Obtaining cross-sectional images in all three orthogonal planes will allow for adequate assessment of all relevant structures around the elbow.
T1-weighted (T1W) images are useful for illustrating anatomical detail, whereas fat-saturated T2-weighted (T2W) images or short-tau inversion recovery (STIR) images are suitable for detecting pathological changes manifesting as fluid or edema. Furthermore, proton density-weighted (PDW) images can provide additional anatomical detail. Gradient-echo sequences are not routinely indicated but may enhance the visibility of intra-articular loose bodies [14, 15]. However, detecting loose bodies without intra-articular contrast remains difficult. Gadolinium is a contrast agent used in MR imaging that can be injected intravenously or directly into a joint, known as MR arthrography (MRA) (see below). Indirect MRA by means of intravenous administration of gadolinium may aid in the detection of post-traumatic disorders affecting the synovium. Direct MRA by means of intra-articular injection of gadolinium may provide superior visualization of disorders commonly encountered in throwing athletes, including partial capsular and ligamentous (ulnar collateral ligament) tears, intra- articular loose bodies, instability, and osteochondritis dissecans [16, 17].
For MRA, approximately 5–10 mL of gadolinium diluted in sterile saline (1:250) is injected with a 20- or 23-gauge needle into the elbow joint. The elbow joint space can be accessed via the standard lateral or posteromedial approach under fluoroscopy. For the lateral approach, the needle is inserted vertically at the superior third of the radiocapitellar joint line while the patient is lying prone with the elbow in 90° flexion and the forearm in supination. A disadvantage of this lateral approach is the possible extravasation of contrast agent around the radial collateral ligaments. For this reason, the alternative posteromedial approach can be employed with the patient lying supine on the fluoroscopic table, with the elbow over the head in 30° flexion and the forearm in pronation. The needle is then inserted between the olecranon and the medial epicondyle, approximately 1 cm lateral to the medial epicondyle to avoid damaging the ulnar nerve. Subsequently, the needle is advanced in anterolateral fashion into the olecranon fossa. Fat- saturated T1W and T2W sequences should be obtained immediately after contrast injection [18] (Table 4.2).
4.1.3 Computed Tomography and Computed Tomographic Arthrography
CT scans of the elbow are mainly used in the acute setting for assessing osseous abnormalities such as occult fractures and loose bodies, for further characterisation, and for support in preoperative planning [19, 20]. Current multi-detector CT scans allow for high-resolution images, multi-planar reconstruction, and fast scanning times. Typically, a section thickness of 1 mm is used with a matrix size of 512 × 512, and scanning is performed in the axial plane [21, 22]. The patient is scanned in the prone position with the elbow resting above the head at about 90° flexion [23–25].
In order to perform CTA, iodinated contrast agent is injected into the elbow joint. As in magnetic resonance arthrography (MRA), 5–10 mL of contrast agent is injected under fluoroscopic guidance through the lateral and, in some cases, the posteromedial approach. In addition to iodinated contrast agent, air can be injected into the elbow. This is defined as double-contrast arthrography. CT scans should be obtained within 30 min of contrast administration [26].
CTA is particularly useful in the evaluation of osteochondritis dissecans, osteochondral lesions, and loose bodies [27]. However, in the diagnostic workup of the athlete’s injured elbow, MRA has essentially replaced the role of CTA. The main reasons for this are the absence of ionizing radiation in MRA and the fact that MRA is superior in the detection of concomitant soft tissue injury [18]. Nonetheless, CTA can be used as an alternative in patients with contraindications for MRA such as pacemakers, implanted devices, or gadolinium-based contrast allergies [28].
4.1.4 Ultrasound
The major advantage of ultrasound (US) is that it provides a low-cost, noninvasive, and dynamic evaluation of elbow structures, without ionizing radiation [29–31]. However, this imaging modality is highly operator-dependent and thus requires sufficient experience of the assessor. US can assist clinicians in the assessment of a wide variety of elbow injuries, including overuse syndromes, traumatic changes, inflammatory diseases, and neuropathies [31]. Transverse and longitudinal images of all four aspects (posterior, anterior, medial, and lateral) of the elbow in both flexion and extension are necessary for a complete examination [31].
Echogenicity is the characteristic ability of an elbow structure to return a signal in US examination; each tissue has its own characteristic appearance. A practical order of echogenicity in musculoskeletal ultrasound can be depicted as bone, ligament, tendon, nerve, and muscle [29]. In general, bone and gas-like substances are hyperechoic and fully reflect the sound waves, which is represented by a more intense appearance on US images. Muscles and fluids are less echogenic (hypoechoic) and are represented darker.
Ultrasound plays a major role in the examination of traumatic changes to ligaments and tendons of the elbow [29, 32]. Although these structures have a similar appearance, they can be distinguished because ligaments are slightly more echogenic than tendons. Moreover, the echogenicity of the fibrillar tendinous pattern increases when the tendon is being held under tension. Pathologic degeneration and partial tearing of a tendon are visualized as a structural hypoechoic gap. In case of a complete tear, the fibrillar pattern is completely absent. In addition, US may demonstrate intra-articular effusion due to a fracture even when the undisplaced fracture line is not detected on plain radiographs. Fractures can also be detected directly by US through depiction of irregularities or interruption of the hyperechoic bone cortex [31].
4.2 Osseous and Osteochondral Injury of the Elbow
4.2.1 Fractures of the Elbow
Elbow fractures in overhead athletes are most often caused by low energy trauma, such a fall onto an outstretched hand (FOOSH) and hyperextension or hyperflexion injuries [33]. Nontraumatic upper extremity fractures related to throwing are rare [34, 35]. However, stress fractures arising from repetitive microtrauma are not uncommon. In the following section, a description of fractures of the distal humerus, proximal ulna, and proximal radius, with associated characteristics on imaging, will be given.
4.2.1.1 Outline Pediatric Osseous Injury
4.2.1.1.1 In General
The immature skeleton contains growth plates, which appear as a radiolucency similar to cartilage on radiographs. Understanding of the developmental anatomy of the pediatric elbow is essential to distinguish normal ossification centers from a fracture fragment in radiography, since misinterpretation is not uncommon [36]. The mnemonic CRITOE is a helpful tool in analyzing pediatric elbow injury. It represents the sequential order of appearance of the ossification centers of the elbow: capitellum, radial head, internal (medial) epicondyle, trochlea, olecranon, and external (lateral) epicondyle [1, 5]. This sequential order extends over the period from 1 year to 12 years of age [37].
Pediatric osseous injury differs in many aspects from adult osseous injury due to the differences in bone composition between children and adults [8, 38]. The thick periosteum of the immature skeleton, for example, inhibits displacement of a fracture. However, supracondylar fractures with posterior displacement occur frequently and are thus an exception to this rule. Finally, children’s bones tend to be more flexible which can result in plastic bowing, torus, or greenstick fractures, mostly affecting the radius or ulna in FOOSH or hyperextension injury [5].
4.2.1.1.2 Physeal Injury
Since the cartilaginous physis is a more vulnerable structure than the surrounding ligaments and muscle tendons, injuries affecting the physis are common in childhood [2]. Fractures of the epiphysis and/or metaphysis are classified according to the Salter-Harris classification, which relates the radiographic appearance to the clinical importance of the fracture (see Table 4.3) [39]. Nevertheless, MRI is considered superior for evaluating fractures of the cartilaginous epiphysis in children [40].
4.2.1.2 Fractures of the Distal Humerus
Fractures of the distal humerus can broadly be categorized into supracondylar, transcondylar, or intercondylar fractures (above the olecranon fossa, through the olecranon fossa, or between the condyles, respectively) [41, 42]. More specific and commonly used is the AO classification system, in which type A describes an extra-articular fracture, type B an intra-articular fracture of a single column, and type C an intra-articular fracture of both columns with no portion of the joint contiguous with the shaft (see Table 4.4) [41]. Each type is subdivided into three subtypes to classify the degree of comminution, with subtype 3 being the highest degree of comminution. Anteroposterior, lateral, and oblique views in plain radiography can be used to confirm the presence and location of distal humeral fractures [42].
Supracondylar (type A) fractures are common and account for more than half of all elbow fractures in children, but are relatively uncommon in adults [6]. Pediatric supracondylar fractures are classified according to the classification of Gartland [43]. Type I fractures are non-displaced, type II fractures are partially displaced (with intact posterior cortex) and type III fractures are completely displaced. The anterior humeral line in particular can be used to assess the direction of the displacement, which is commonly posterior [5]. A rare, but important complication of pediatric supracondylar fractures is the fishtail deformity (see Sect. 4.2.2) [44, 45].
Transcondylar (type B) fractures include fractures of the lateral and medial humeral condyle. Fractures of the lateral condyle are the most common fractures in children under the age of 7 years [5]. When only the cartilaginous part of the distal humeral epiphysis is involved, this fracture equals a Salter-Harris type IV epiphyseal fracture. A specific type of transcondylar fractures of the capitellum and trochlea are coronal shear fractures. These fractures occur when the radial head impacts into the anterior articular cortex of the distal humerus and both the capitellum and the lateral ridge of the trochlea are sheared off. Indicative for this injury is the double-arc sign on lateral view radiographs [46, 47]. This sign represents an increased radiographic density due to overprojection of the subchondral bone of the displaced capitellum and the lateral trochlear ridge. Coronal shear fractures can also be visualized with a radial head-capitellum view [48].
Regarding other imaging modalities, two- and three-dimensional CT images have been shown to be of particular benefit in preoperative decision making and planning of the operative treatment [49]. Nonoperative treatment (i.e., immobilization and bracing) is only recommended in case of non-displaced fractures. Patients with displaced, comminuted, or highly unstable distal humeral fractures should be referred to an orthopedic surgeon, since surgical intervention is the standard treatment [41, 42].
4.2.1.3 Fractures of the Proximal Ulna
Olecranon process fractures can be the result of a direct trauma to the elbow, for example a fall on the elbow with the arm flexed. As a consequence, the olecranon collides with the distal humerus and is often comminuted [50]. These fractures occur more frequently in adults than in children, as the immature olecranon is relatively stronger than the distal humerus (which also explains the higher occurrence of supracondylar fractures in children). Indirect forces are mostly due to a FOOSH injury together with forceful contraction of the triceps which may show transverse or short oblique fractures on plain radiographs [50, 51]. Undisplaced, simple fractures are easily assessed on plain radiographs. Displaced or comminuted fractures require two- and three-dimensional CT imaging in support of surgery [52].
In addition to traumatic injury, the olecranon process is the most common location for stress fractures in throwers [2]. During throwing, repetitive forces in valgus load are applied through excessive pulling of the triceps on the olecranon, which may result in posteromedial osseous stress syndrome. This comprises trabecular collapse and transverse or short oblique stress fractures. Since plain radiographs may not show significant alterations in the appearance of the proximal ulna, accurate assessment is justified [53, 54]; progression of small stress fractures to a complete and displaced fracture is possible. Either a hairline fracture or a lucent region surrounded by a sclerotic margin (indicating nonunion and periosteal new bone formation) can be seen. These features can also be detected with CT [2]. However, MR imaging is the most sensitive method for identifying early changes consistent with osseous stress injury, like bone marrow edema and hyperemia [53]. These changes on T1-weighted images consist of poorly defined, patchy areas of low signal intensity in the affected bone.
Fractures of the coronoid process rarely occur isolated. Since the coronoid is responsible for resisting posterior displacement of the ulna, these fractures are often associated with other elbow injuries that increase joint instability. In the O’Driscoll classification, three major traumatic injury patterns are linked to coronoid fractures [55]. This classification can aid in predicting associated injuries of coronoid fractures [56]. Type I includes a small transverse fracture of the coronoid tip. This fracture accounts for one of the three distinct injuries in the terrible triad, the others being a fracture of the radial head and a posterior elbow dislocation [57]. If external rotation forces and valgus stress are loaded axially in a FOOSH injury, the lateral collateral ligament (LCL) is typically torn as well. Type II fractures of the anteromedial facet are often seen with varus posteromedial rotatory instability pattern injuries, occurring after an elbow subluxation. Associated injury includes an LCL avulsion from the lateral epicondyle. Varus stress radiographs often reveal radiocapitellar widening and ulnohumeral narrowing. Type III includes relatively large fractures of the coronoid process, associated with transolecranon fracture-dislocations (anterior or posterior).
4.2.1.4 Fractures of the Proximal Radius
Radial head fractures are the most common type of elbow fracture in athletes and represent 50 % of all elbow fractures in adults [33]. In children, the radial neck is more commonly involved (leading to Salter-Harris II fracture). Based on results of 100 cases of radial head fractures, Mason established a classification system to guide treatment based on the injury pattern [58]. Type I fractures include non-displaced or peripheral fractures of the rim, type II includes displaced fractures of the rim, and type III fractures are comminuted and displaced fractures of the entire radial head. Johnston added a fourth type to this classification, which denotes a fracture of the radial head with associated dislocation [59]. Initially, type I fractures are treated nonoperatively, type II may be treated either nonoperatively or operatively, while types III and IV require surgical management. However, although these guidelines of the Mason-Johnston classification are widely used, there is a paucity of data confirming the outcomes of surgical management [60].
Isolated radial head fractures resulting from a fall with the elbow extended and the forearm pronated occur rarely. Investigation of radial head fractures with MR imaging showed that radial head fractures in three-quarters of cases are associated with soft tissue injuries [61, 62]. Common injuries occurring in association with these fractures are posterior dislocation of the elbow, medial collateral ligament rupture, capitellar fracture, terrible triad injuries, and Monteggia injuries [63]. If a radial head fracture is suspected, anteroposterior and lateral radiographs of the elbow should be obtained. A radiocapitellar view may help delineate the fracture. In addition, computed tomography can identify fractures not visualized in plain radiographs. CT may help in identifying the fracture pattern, the degree of comminution (if present), possible associated injuries and in planning surgical treatment [63, 64].
4.2.2 OCD and Avascular Necrosis Around the Elbow
4.2.2.1 Osteochondritis Dissecans of the Capitellum
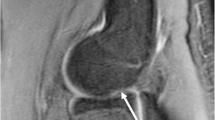
Osteochondritis dissecans (OCD) is an idiopathic disorder of the subchondral bone with dissection of the articular surface and underlying bone of the immature skeleton. OCD is commonly localized in the capitellum of the dominant elbow but can also occur in the trochlea, radial head, and olecranon [65]. It typically affects young, competitive athletes in overhead sports such as baseball or weight-bearing sports like gymnastics, in which repetitive valgus stress is placed on the elbow joint [66, 67]. Patients, most commonly adolescent boys, present with lateral elbow pain, swelling, tenderness, stiffness, and locking of the joint. Although the etiology remains unclear, it is believed that the underlying pathogenesis involves repetitive microtrauma due to compression and shear forces, leading to overuse injury of the vulnerable and relatively hypovascular epiphyseal cartilage [65].
Although prevention is the best treatment for OCD, early detection and classification of the lesion are necessary to protect athletes from developing irreversible damage [68, 69]. The International Cartilage Repair Society (ICRS) divides OCD lesions into four categories. To determine the best treatment option for capitellar OCD in young athletes, it is important to differentiate between stable and unstable lesions: ICRS I and II are classified as stable and ICRS III and IV as unstable [69–72]. However, the major drawback of this classification is that it is based on intraoperative findings. To assess the stability of the OCD lesion in a noninvasive way, the use of ultrasound, radiographs, MRI, or CT is recommended [72]. The characteristics of each imaging modality will be discussed in the following section (Table 4.5).
Ultrasound is useful in the initial examination of cartilaginous changes in capitellar OCD [74]. US can visualize the subchondral bone and overlying articular cartilage simultaneously in one dynamic image [75]. The image should be obtained in both an anterior and a posterior longitudinal view to display the whole capitellum. The normal capitellum is shown as a highly echogenic band with the overlying cartilage as an overlying hypoechoic band. Subchondral bone flattening causes the highly echogenic band to narrow. Moreover, non-displaced or (slightly) displaced bony fragments, marrow gap formation, or complete osteochondral defects can be seen on ultrasound imaging [74]. It is advised to compare findings on ultrasound with MRI and/or radiographic assessment to identify both cartilaginous and bone changes, so the lesion can be more accurately classified [74, 76].
Routine AP radiographic examination of the elbow for detecting capitellar OCD and intra- articular loose bodies has limited sensitivity [77]. However, radiographic images of the capitellum on radiocapitellar view or AP view with the elbow 45° flexed can show the following:
-
Grade I. Localized flattening or subchondral radiolucency
-
Grade II. Non-displaced bone fragment(s)
-
Grade III. Displaced or detached fragment(s)
Takahara et al. [71] proposed a guideline for treatment, based on findings at initial presentation of the patient, supplemented with radiographic findings (Table 4.6) [71]. In stable OCD, an immature capitellum with open growth plate is present with flattening or radiolucency of the subchondral bone (Grade I), but with normal elbow motion. The preferred treatment is conservative; elbow rest and analgesics are recommended. In unstable OCD, the capitellum is mature (the growth plates have closed) and fragments (Grade II or III) may occur. The fragments or loose bodies can lead to restricted elbow motion due to narrowing of the articular space. In this case, surgical treatment is indispensable to prevent further damage. The advantage of this classification system is that it directly links radiographic findings with the ICRS classification and thus is useful in the choice for treatment [78].
Magnetic resonance imaging has been approved as the most sensitive and reliable means for the assessment of osteochondritis dissecans [74, 79]. MRI provides information about size, location, presence of joint effusion, bone marrow change, and loss of continuity or cartilage over the OCD lesion [79]. Cartilage changes in early disease may not be obvious radiographically, but can be visualized with MRI [68]. These early changes of osteochondral defects are detectable on T1-weighted images and appear normal on T2 images [67]. Advanced changes are detectable in both T1 and T2 images. T2-weighted images may show high-signal intensity interfaces between fragments and their beds or reflect the interposition of synovial fluid interposed through the articular cartilage. Focal articular defects may be seen as well [69, 72]. The MRI staging system developed by Itsubo et al. [79] provides evidence regarding the instability of the OCD and the corresponding stages of the ICRS classification, but has not yet been validated in other studies [79].
It should be noted that the literature on imaging of capitellar OCD by computed tomography (CT) is limited. The general consensus on the advantages of CT over radiography or MRI is that CT can aid in defining the subchondral bone condition and that it is often used to determine the extent of the osseous lesion and the presence of ossified loose bodies [80]. However, CT should not be used to detect cartilaginous change at the lesion; for this purpose, computed tomographic arthrography (CTA) is more suitable. CTA favors examination of the overlying cartilage and can confirm the intra-articular position of calcified loose bodies, yet this can also be achieved with MRI [73, 81].
4.2.2.2 Panner’s Disease
It is important to distinguish Panner’s disease from OCD of the capitellum. Although the presentation and clinical features may be similar, Panner’s disease is a self-limiting condition of the epiphysis and will resolve with rest and conservative treatment [65]. In general, it affects a younger age group (mainly boys under the age of 10 years) and it is not necessarily related to sports. The characteristic appearance of Panner’s disease on radiographs is the initially subchondral rarefaction, which is in a later stage followed by translucency and fragmentation of the entire capitellum. Magnetic resonance imaging shows low T1 signal and high T2 signal of the entire capitellum. Loose bodies are seldom seen [65].
4.2.2.3 Hegemann’s Disease
In the continuum of disorders of endochondral ossification like OCD and Panner’s disease, in 1951 Hegemann described a total of 15 cases of avascular osteonecrosis of the humeral trochlea [82]. Since then, reports on this disease have been limited. This condition seems to affect predominantly preadolescent boys and is seldom accompanied by pain. Swelling and decreased range of motion are more often described [83]. In contrast to OCD, there is no locking of the joint and radiography shows rarefaction of the entire epiphyseal center of the trochlea (instead of the subchondral bone only) [84]. Another condition that strongly resembles Hegemann’s disease is the fishtail deformity of the trochlea, a late complication of pediatric supracondylar fractures [45]. Claessen et al. [85] provided an overview of the most recent knowledge on the etiology, radiographic findings, and treatment options of both these rare conditions [85].
4.2.3 Apophysitis and Apophysiolysis: Little Leaguer’s Elbow
The apophysis is a secondary ossification center located outside the joint surface. Injury of the medial epicondylar apophysis occurs almost exclusively in young athletes performing overhead sports and is referred to as the clinical diagnosis Little Leaguer’s elbow [86–88]. The medial epicondyle is relatively weak compared to the increasing muscle strength in adolescents. Therefore, apophysiolysis or apophyseal avulsion fractures are often the consequence of sustained valgus stress forces with traction of the common origins of the flexor muscles at the apophysis, due to repetitive overhead throwing [5, 89, 90]. Moreover, avulsion fractures can also be the consequence of an acute traumatic event such as a dislocation due to FOOSH injury [91].
AP and lateral radiographic images with comparative views of the unaffected side should be used in the initial evaluation [1]. Although these images appear normal in 85 % of cases, they may reveal a hypertrophic medial epicondyle with bony fragmentations and apophyseal widening or complete avulsion from the underlying humerus, with possible entrapment of the fragment in the joint [87, 89].
MRI is not warranted in the initial imaging workup, but can be justified to outline the surrounding structures [92]. MR images in such cases may show bone marrow edema in the apophysis (or distal in the humerus) and tendinopathy of the common flexor tendon. Contrary to previous literature, there is a growing consensus that the ulnar collateral ligament (UCL) is not involved in the pathology of the Little Leaguer’s elbow, but solely associated with valgus extension overload in adult patients (see Sect. 4.3.1) [92].
4.2.4 Degeneration, Osteophytosis, and Loose Bodies
While traumatic injury may precipitate secondary degenerative arthritis in the elbow, primary degeneration is not associated with acute elbow trauma or rheumatologic disease. Primary degenerative arthritis of the elbow is a relatively rare condition, but occurs to a greater extent in overhead athletes at whom excessive stress on the elbow joint is placed [93, 94]. The pathologic changes that occur in both the radiohumeral and ulnohumeral compartments of the elbow can be divided in three stages [95]. The first stage involves loss and fragmentation of the cartilage due to repetitive impaction of the coronoid process and the tip of the olecranon against the olecranon fossa membrane. As a response to this erosion, hypertrophic bone and cartilage formation results in so-called osteophytes and loose bodies. Osteophytes or bone spurs reduce the amount of joint space needed for a full, pain-free range of motion, giving rise to symptoms as pain, locking, or reduced elbow motion. In the final stage, the impingement caused by these small protuberances (particularly in the olecranon fossa) leads to distortion and in most severe cases to contracture of the elbow joint. Arthroscopic intervention with removal of the eroded bone and its fragments is the best treatment option to prevent further degeneration of the elbow [96]. Plain radiography and computed tomography are the modalities of choice when assessing the condition of the elbow.
Two views in plain radiography are usually sufficient for the initial evaluation of primary osteoarthritis. Standard lateral radiographs allow identification of the most frequent features of the osteoarthritic elbow (i.e., osteophytes of all involved bony structures, thickening of the olecranon fossa membrane, and joint space narrowing). The anteroposterior view in addition enables the assessment of the olecranon fossa membrane [97].
In preoperative planning, computed tomography (CT) is favorable when heterotopic ossification or intra-articular loose bodies are suspected [93]. More advanced three-dimensional CT scans can specifically determine the size, location, and bony architecture of the hypertrophic bone spurs and loose bodies [97, 98].
4.2.5 Goalkeeper’s Elbow
Shot blocking of a ball with the forearm fully extended induces repeated hyperextension trauma of the elbow, mostly seen in goalkeepers of handball and soccer [99]. The injury pattern resembles elbow lesions in overhead athletes: repeated impaction of the posteromedial olecranon leads to arthritic changes with cartilage damage, osteophyte formation, and intra-articular loose bodies [100]. The presence of these pathological alterations can be confirmed by radiological evaluation. Soft tissue lesions can be visualized by US or MRI and may comprise bilateral thickening of the medial collateral ligament, flexor-pronator tendon, triceps tendon, and ulnar nerve [100].
4.3 Ligamentous Injury of the Elbow
Various osseous and soft tissue constraints provide static and dynamic stability to the elbow joint, respectively. Primary stabilization is provided by the ulnohumeral articulation as well as by the medial (ulnar) and lateral (radial) collateral ligament complexes. The medial ulnar collateral ligament (MCL/UCL) complex comprises anterior, posterior, and transverse bundles, of which the anterior bundle is the primary restraint against valgus stress. The lateral ligament complex includes the radial collateral ligament, the lateral collateral ligament (LCL), and the annular ligament, of which the LCL provides both varus- and posterolateral stability [101]. The radiocapitellar articulation, the common extensor tendon, the flexor-pronator tendon, and the joint capsule all contribute to secondary stabilization [102].
Ligamentous injury of the elbow in athletes can be caused by repetitive overhead activities or by an acute traumatic event like an elbow dislocation. Timely recognition of injuries to these structures is very important; disruption of the ligaments may threaten elbow stability and can possibly be career ending for an athlete [102, 103]. MR imaging is indispensable in the assessment of the ligaments, since it provides superior soft tissue contrast and allows for simultaneous evaluation of bony structures in a single examination [104]. In the following section, an overview of elbow ligament injuries and their appearance on various imaging methods are provided.
4.3.1 Ulnar Collateral Ligament Injury and Valgus Extension Overload
Valgus extension overload is a spectrum of symptoms that are commonly seen in competitive overhead athletes [105]. Large valgus and extension forces in the acceleration phase of throwing lead to major tensile stress on medial structures, compressive forces on the lateral structures (see Sect. 4.2.2), and shear forces posteriorly (see Sect. 4.2.4). These chronic tensile forces lead to inflammation, microtearing, and laxity of the ligament, which may progress into disruption of the UCL. Less commonly, the UCL may be injured after traumatic elbow dislocation [105].
Plain radiographs may not provide any direct information on ligamentous injuries, but can be indirectly supportive if focal calcifications of the UCL are present [106, 107]. When compared to the normal appearance of the UCL on US, UCL sprains show thickening, decreased echogenicity, and hyperechoic areas demonstrating local calcifications [31, 107]. A completely ruptured UCL appears as a hypoechoic band surrounded by fluid.
On normal axial MR images, the anterior band of the UCL has uniform low signal intensity on T1W and T2W images. However, a completely normal UCL on MRI in a competitive throwing athlete is rarely seen [108]. Adaptations in response to forces in throwing include thickening of the anterior band of the UCL and posteromedial subchondral sclerosis of the trochlea. Therefore, MRI ought to be used to differentiate between acute versus chronic injury and to observe the degree of remodeling of the chronic ligament deformity [109]. Ruptures, sprains, laxity, or other irregularities manifest as a discontinuity with hyperintense fluid filling the hiatus on both T1W and T2W images [14, 104]. Avulsion fracture of the medial epicondyle may be present.
MR arthrography may be of particular benefit when partial-thickness tearing is suspected, since it improves the sensitivity of detecting such tears [18]. In case of a partial-thickness tear, the so-called T-sign may demonstrate increased signal intensity at the distal insertion near the sublime tubercle [14, 110].
4.3.2 Dislocation of the Elbow Joint
Dislocation of the elbow is the most common dislocation in children and the second most common dislocation in adults (after dislocation of the shoulder) [111]. The elbow owes its stability to the osseous architecture of the ulnohumeral joint, which provides the most stability in the anteroposterior direction. The surrounding capsuloligamentous and musculotendinous aspects (including the collateral ligaments, joint capsule, and adjacent muscles) provide further stability. If these components are disrupted by trauma, elbow dislocation may result.
Dislocations of the elbow can either be simple or complex depending on the absence or presence of associated bony injury, respectively. Simple dislocations are described by the direction of the dislocated ulna relative to the humerus. Posterior displacement occurs in over 90 % of cases, with posterolateral dislocation as its most common subtype [112]. The injury mechanism is considered to be a combination of axial compression, supination, and valgus stress, often seen in FOOSH-type injuries [103]. Lateral and anterior displacements are rare and may result from a direct posterior blow to a flexed elbow [113]. Bony injuries of the olecranon and avulsion of the medial and lateral condyles and epicondyles can be present. Complex dislocations with combined fractures of the radial head or neck and the coronoid process are referred to as the terrible triad (see Sect. 4.2.1) [57, 102].
Accompanying ligamentous and capsular disruption can be described according to the Horii circle [103]. Stage 1 involves disruption of the LUCL with posterolateral rotatory subluxation of the ulna. In stage 2, the coronoid places on the trochlea (i.e., incomplete dislocation) and the other adjacent lateral ligaments are torn, including anterior and posterior aspects of the joint capsule. Finally in stage 3, the elbow is completely dislocated with the coronoid located posteriorly to the humerus. The MCL may be disrupted only posteriorly (stage 3A) or completely (stage 3B). Thus, elbow dislocation is the result of a posterolateral rotatory subluxation followed by a total disruption of the surrounding soft tissue from the lateral to the medial side [102].
Posterolateral dislocation can lead to permanent valgus instability that correlates with a worse overall clinical and radiographic result. All treatment options are therefore primarily aimed at restoring functional elbow stability [102]. Simple dislocations may be treated nonoperatively after reduction under adequate muscular relaxation and appropriate analgesia. To prevent joint contractures, definitive management involves limited mobilization and early active range of motion [114]. Complex fracture-dislocations require operative management with fixation of fractures and repair of damaged surrounding soft tissues. Damage to the brachial artery or median and ulnar nerve must be ruled out, although neurovascular injury is uncommon in the setting of a FOOSH injury [103].
Anteroposterior, lateral, and oblique radiographs should be obtained to determine the direction of the dislocation and the potential presence of associated fractures. An intact radiocapitellar line should be evident on all views, since this is no longer aligned in posterior elbow dislocations [8]. Post-reduction radiographs are required to ensure correct positioning of the elbow.
Concerning preoperative planning after complex elbow dislocation, CT can be used to delineate fractures, and MR imaging is helpful to visualize the extent of the soft tissue disruption [57, 115, 116].
4.3.3 Chronic Insufficiency of the LCL: Posterolateral Rotatory Instability
Elbow dislocation from a FOOSH trauma poses a substantial risk for recurrent elbow instability, since the stabilizing architecture of the surrounding ligaments, the radial head, and the coronoid process can be significantly disrupted. This condition has also been reported following coronoid insufficiency, radial head excision, or steroid injections for lateral epicondylitis [18].
Several criteria are used to classify the degree of the instability: the articulation(s) involved, the direction of the displacement (valgus, varus, anterior or posterolateral), the degree of displacement (subluxation or dislocation), the timing of displacement (acute, chronic or recurrent), and the presence or absence of associated fractures [103]. The most common type of chronic elbow instability is posterolateral rotatory instability (PLRI) [117]. PLRI implies a dislocation by which external rotation of the radius and the ulna relative to the distal humerus results in posterior displacement of the radial head relative to the capitellum. Contrary to isolated dislocation of the radial head, the radioulnar joint does not dislocate because the annular ligament is not affected [118].
The lateral ligament complex limits external rotation of the radius and ulna relative to the humerus and is therefore considered the weakest link in the pathogenesis of PLRI [102]. However, the medial collateral ligament may contribute as well [119, 120].
The diagnosis is made clinically based on the patient’s history and physical examination. Patients with PLRI often have a history of ulnohumeral dislocation; recurrent symptoms of lateral pain, locking, clicking, snapping, or popping can be present. The feeling of instability mostly occurs when the elbow is actively brought from flexion into extension with the forearm in supination. Several specific apprehension tests are available to provoke these symptoms [118, 121]. During the lateral pivot-shift maneuver, the elbow is in supine position and mild valgus stress is applied while the elbow is flexed. The test is positive if apprehension or frank subluxation of the radius and the ulna (rotating away from the humerus) occurs [122]. The posterolateral rotatory drawer test involves overhead placement of the elbow in 40° of flexion. Subsequent application of an anteroposterior force on the ulna and the radius (with the forearm in external rotation) will subluxate the forearm away from the humerus on the lateral side, pivoting on the intact medial ligaments [122]. A more adequate evaluation of instability by these tests may be performed with the patient under anesthesia. The radial head then visibly subluxates posteriorly, whereas apprehension occurs when the patient is awake.
The primary treatment goal in patients with PLRI is to restore elbow stability. Nonoperative measures are applied in the first days after reduction. These measures include both splinting of the arm as well as rehabilitation to strengthen the surrounding musculature [123]. If unsatisfactory results are yielded by conservative management, surgical treatment may be considered. The majority of surgically treated patients encounter satisfactory outcomes regarding elbow stability [118]. Surgical management aimed at the reconstruction of ligaments can be performed either open or arthroscopically [123]. Deficiency of the radial head or coronoid may require bony reconstructions. In that case, computed tomography is of particular use to delineate complex fracture patterns and to assist in surgical planning [115].
Plain radiographs are used to demonstrate changes in the alignment of the elbow by reviewing the integrity of the radial head, coronoid process, and capitellum. The drop sign, indicative for PRLI, represents ulnohumeral separation on lateral radiographs [124]. Posterior displacement of the radial head in relation to the capitellum may be visible as well.
Although MRI has been well established as an effective method for the assessment of ligamentous injury to the LCL, the role of MRI in the diagnosis of PLRI remains questionable [121, 125]. However, examination through MR arthrography is advantageous if uncertainty about the diagnosis remains even though PLRI is suspected [123]. Arthrography reveals laxity of the LCL, widening of the lateral joint space, and osteochondral lesions at the radiocapitellar joint [18, 118].
4.3.4 Monteggia Injury of the Forearm
The ulna and the radius act as a single functional unit through binding via the interosseous membrane and ligaments in the forearm. As a consequence, hyper-pronation injury with fracture of the ulna is often accompanied by a dislocation of the proximal radioulnar joint. This combination of injuries was first described by Monteggia in 1814 and further classified by Bado [126]. Depending on the location of displacement of the radial head, four types can be distinguished (see Table 4.7).
Since the long-term range of motion of the elbow is seriously threatened in Monteggia injury, early recognition is important [127]. Pediatric patients may sustain injuries slightly different to Monteggia injury, including plastic deformation, incomplete or greenstick fractures, and ulnar metaphyseal fractures [127]. Although conservative management can be successful in the younger population, operative treatment is warranted for the majority of adults [127, 128]. The treatment goal is to restore the cooperative functioning of the radius, ulna, and their associated articulations.
Radiographic examination should comprise AP, lateral, and oblique views of both the forearm and the wrist. The distal forearm should be evaluated for displacement of the ulna relative to the radius. The radiocapitellar line must accurately be assessed in the proximal forearm, since it may seem normal due to concurrent displacement of the ulnar shaft [5].
4.3.5 Isolated Dislocation of the Radial Head
Isolated dislocation of the proximal radius, also termed nursemaid’s elbow or pulled elbow, is the result of a sudden pull on the arm. This longitudinal traction force with the forearm in pronation and extension pulls the radial head trough the annular ligament. Due to relative laxity of the annular ligament, this injury is common in children aged 0–5 years [129]. After the age of 5 years, the annular ligament is stronger and less likely to tear or be displaced. Generally, the diagnosis is based on the clinical presentation. The injured child is likely to not use the affected arm and holds it in pronation, mild flexion, and abduction against the body. Radiography (AP view) should be considered if the diagnosis is equivocal, if the mechanism of injury other than a pull is suspected, or if reduction attempts are unsuccessful [130].
4.4 Musculotendinous Injury of the Elbow
4.4.1 Epicondylitis
4.4.1.1 Lateral Epicondylitis
Lateral epicondylitis, also known as tennis elbow, is the most common cause of lateral elbow pain [131]. Any sport or occupation that demands repetitive wrist extension can result in this type of injury. Lateral epicondylitis most commonly occurs in the fourth and fifth decades of life, with both sexes affected equally [132]. The common extensor tendon (CET) originates from the anterior aspect of the lateral epicondyle of the elbow and consists of the three conjoining tendons of the extensor carpi radialis brevis (ECRB), the extensor digitorum communis (EDC), and the extensor carpi ulnaris (ECU) muscles [133]. Lateral epicondylitis represents a condition where repetitive contractions of the ECRB, and to a lesser extent the EDC and ECU, lead to microtearing with subsequent degeneration, immature repair, and tendinosis [131, 134]. Tendinopathy or tearing of the ECRB tendon is invariably seen in lateral epicondylitis [132]. Physical examination typically reveals tenderness at the origin of the ECRB tendon and pain exacerbating with active wrist extension [135, 136]. The clinical picture is often sufficient for making the diagnosis. However, when symptoms are atypical or patients do not respond to therapy, imaging may be performed.
In case of suspected lateral epicondylitis, elbow radiographs may show some calcification along the lateral epicondyle. Nevertheless, radiographs are often false-negative and the routine use of plain films does not seem justified in the diagnostic process [137]. Both magnetic resonance imaging (MRI) and ultrasound (US) are useful tools in diagnosing lateral epicondylitis. US provides an inexpensive and fast imaging method, whereas MRI is more expensive and time-consuming. Presently, MRI is considered the golden standard with a diagnostic sensitivity ranging between 90 % and 100 %. The sensitivity for US ranges between 60 % and 80 % [138]. Additional US techniques have no extra benefit over standard gray-scale ultrasonography in detecting abnormal musculoskeletal findings in painful elbows [138].
The CET origin in individuals with lateral epicondylitis shows increased signal intensity on T2-weighted fat-suppressed MR images within the substance of the tendon, most commonly the ECRB, with or without tendon thickening [138–140]. However, CET thickening and increased signal intensity on T2-weighted images have also been observed in asymptomatic high-performance athletes [140]. MRI can be used to categorize epicondylitis into several grades of severity. In mild epicondylitis, the CET is thickened with increased internal signal intensity. In moderate epicondylitis, there is a partial-thickness tear with thinning and focal disruption that does not extend across the full thickness of the tendon. Severe epicondylitis consists of a near-complete or complete tear, characterized as a fluid-filled gap separating the tendon from its origin at the lateral epicondyle [132]. This grading system has a significant role in surgical planning [139].
4.4.1.2 Medial Epicondylitis
Medial epicondylitis, also known as golfer’s elbow, is another common cause of elbow pain among athletes and workers in occupations that demand repetitive flexion of the wrist. In throwing athletes, medial epicondylitis may result from repetitive stress to the flexor-pronator mass, consisting of the pronator teres and flexor carpi radialis muscles [141]. The tendon origin of the flexor-pronator mass attaches to the anterior aspect of the medial epicondyle of the humerus and is most commonly affected in medial epicondylitis [133, 142]. This condition has the same pathogenesis as lateral epicondylitis, repetitive microtrauma at the tendinous insertion of the flexor-pronator mass leading to degeneration, tendinosis, and ultimately tearing [143–145]. Patients most often report a history of activities involving wrist flexion and forearm pronation, as is the case in golf, racket sports, and overhead throwing [142, 146]. Examination typically reveals painful flexion and pronation against resistance, decreased grip strength, and tenderness over the origin of the flexor-pronator mass at the medial epicondyle [147].
When clinical signs are confounding, the diagnosis of medial epicondylitis can be further explored using both US and MRI. Plain radiographs may show calcification or traction osteophytes at the flexor-pronator mass origin, but these findings have overall low sensitivity [148]. US may demonstrate focal hypoechoic or anechoic areas in the tendon, cortical irregularity at the tendinous insertion, tendon thickening, and calcification. Most abnormalities occur in the tendons of the flexor carpi radialis and pronator teres but changes may also be seen inside the tendon of the palmaris longus and flexor digitorum superficialis [30]. MRI is considered more sensitive than US and may demonstrate findings similar to those described in lateral epicondylitis: focal thickening and increased signal intensity within the flexor-pronator tendons accompanied by surrounding soft tissue edema best seen on T2-weighted fat-suppressed MR image series. In both lateral and medial epicondylitis however, clinical evaluation remains the mainstay of the diagnosis and the role of imaging is primarily to confirm the presence of suspected tendon pathology [135].
4.4.2 Tendon Pathology
4.4.2.1 Distal Biceps Tendon
Distal biceps tendon (DBT) pathology is a relatively rare cause of anterior elbow pain and ranges from tendinopathy to partial tearing and complete tears of the DBT. A complete tear of the DBT is the most common entity, followed by partial tearing, with isolated tendinopathy being exceedingly rare [135]. Complete ruptures of the DBT typically occur in male weightlifters and athletes between 40 and 60 years of age [149, 150]. Risk factors include smoking, anabolic steroid use, and a history of previous DBT rupture [151]. Rupture of the DBT is classically an acute injury occurring when a strong eccentric force is applied on the contracted biceps with the elbow in 90° flexion, leading to tear at the insertion site of the DBT into the radial tuberosity [135]. In the case of a full DBT rupture, physical examination often shows a palpable defect within the antecubital fossa and proximal bulging of the biceps muscle due to retraction of the ruptured tendon. Pain over the antecubital fossa and weakness of forearm supination and elbow flexion can be observed in both partial and complete tears [135].
Imaging has an important role in distinguishing partial from complete tears [152–154]. Plain radiographs are not indicated unless concomitant injury of the elbow is suspected [148]. A complete tear can be diagnosed on US as a complete absence of the DBT that is retracted proximally, often more than 10 cm from the insertion at the radial tuberosity [155]. In addition to diagnosing complete tears, MRI is useful for visualizing partial tears of the DBT. A partial rupture of the distal biceps tendon is characterized by the presence of increased signal intensity within the tendon [156, 157]. Secondary MRI findings of partial tears may include the presence of bone marrow edema within the radial tuberosity, indicative of a micro-avulsion at the DBT’s insertion site. Differentiating partial tears from tendinopathy proves to be challenging both clinically and radiologically [158]. As such, MRI is indicated when the presence of a complete versus a partial rupture is uncertain. This distinction is clinically important as complete tears need to be repaired surgically. This is in contrast with partial tears and tendinopathy of the DBT, where conservative treatment is often adequate [148].
4.4.2.2 Distal Triceps Tendon
Tendinosis and rupture of the distal triceps tendon constitute the least common type of elbow tendinopathy [159]. Males are affected twice as often as females and triceps injuries have been reported in professional football players, soccer players, softball players, skiers, and weightlifters [160–162]. In contrast to biceps tendon injuries, triceps injuries are exclusively seen at the distal insertion of the triceps tendon onto the olecranon. Presently, no proximal tendon avulsion of the triceps has been described in the English literature [163]. Several risk factors for triceps tendon pathology have been explored, including chronic renal failure, endocrine disorders, metabolic bone disease, and steroid use [164–166]. The most common mechanism of injury is a fall on an outstretched hand in which a deceleration load is applied to the triceps while it is actively contracting [167]. In case of a complete triceps rupture, the most universal finding on physical examination is the inability to extend the elbow against gravity [168].
Tendinosis and partial tears of the triceps can be more difficult to diagnose on physical examination and this is where imaging comes into play. Plain radiographs often show osseous flakes, also termed the flake sign, which is considered pathognomonic for avulsion injuries of the triceps [169]. Radiographs are indicated in traumatic settings to rule out concomitant injuries of the elbow. Both US and MRI can differentiate between either a partial or full tear of the distal triceps tendon. Moreover, the degree of tearing is of major value in deciding whether surgical repair or conservative treatment is indicated [167]. US may diagnose all types of triceps tendon injury ranging from tendinosis to complete tears along with retraction of the tendon. However, data on sensitivity and specificity have not been documented [170]. MRI is an acknowledged imaging modality for confirming the presence of complete tendon tears and staging partial tears. The triceps tendon is best visualized on sagittal images. Partial ruptures of the triceps tendon are characterized by a small fluid-filled defect within the distal triceps tendon with edema in the surrounding subcutaneous tissue of the posterior elbow. Complete rupture of the triceps tendon is characterized by a large fluid-filled gap between the distal triceps tendon and the olecranon process with a large amount of edema in the adjacent subcutaneous tissue. The distal edges of the torn triceps tendon are frayed and show heterogeneous signal intensity. A variable amount of retraction of the distal triceps tendon is usually present [135, 171].
4.4.2.3 Snapping Medial Head of the Triceps with Subluxating Ulnar Nerve
The medial head of the triceps originates just inferior to the radial sulcus of the humerus, traverses posterior to the medial epicondyle, and inserts into the olecranon process of the ulna. During flexion of the elbow, a portion of the medial head of the triceps may dislocate or “snap” anteriorly over the medial epicondyle [172]. The ulnar nerve is in close relationship with the medial head of the triceps and may also dislocate during flexion. This condition often presents as a combination of medial elbow pain, a single- or double-snapping sensation during flexion of the elbow, and additional symptoms of ulnar nerve irritation [172]. A symptomatic dislocating medial head of the triceps muscle is frequently associated with overhead activities in throwing athletes and with weightlifting in bodybuilders. Predisposing factors include hypertrophy of the triceps musculature, post-traumatic alteration of bone alignment, and congenital predisposition owing to anatomical variations of the triceps [173]. Snapping of the medial head of the triceps is relatively easily observable during physical examination compared to snapping of the ulnar nerve [172].
Because the snapping syndrome is a dynamic and intermittent condition, MRI and CT are unfavorable for confirming the diagnosis. Nonetheless, axial imaging with CT or MRI may demonstrate the structures that dislocate with the elbow positioned in different degrees of flexion [174]. US is the modality of choice and can provide a dynamic assessment of the structures involved during a snapping sensation. With an isolated dislocating ulnar nerve, the nerve and medial triceps will often appear to separate during flexion of the elbow, whereas with a dislocating medial triceps, the ulnar nerve and triceps appear to travel as one unit over the medial epicondyle in the anterior direction [175]. Differentiating between these two entities is of clinical importance as it aids in deciding which type of surgery is indicated [176].
4.4.2.4 Bursitis of the Elbow
Two main bursae can be found in the elbow joint. Anteriorly, the bicipitoradial bursa fills the antecubital fossa. Posteriorly, the olecranon bursa is located just below the skin. The bicipitoradial bursa encases the distal biceps tendon and reduces friction between this tendon and the radial tuberosity during joint movement [177]. Repeated supination and pronation of the forearm is believed to be a possible cause of chronic bicipitoradial bursitis [178]. Due to its close relationship with the distal biceps tendon, bicipitoradial bursitis may be accompanied by tendinopathy of the biceps [179]. In contrast with the bicipitoradial bursa, the olecranon bursa is located more superficially and therefore prone to direct trauma leading to acute, post-traumatic bursitis. Traumatic olecranon bursitis has been reported in athletes who train and play on hard surfaces [180]. In general, bursitis of the elbow in athletes is an aseptic condition [181].
Although the diagnosis of bursitis is mainly clinical, the affected bursa can be excellently visualized on US. Imaging signs include bursal wall distension with presence of local hypoechoic or anechoic intra-bursal material [182]. Power Doppler is able to demonstrate the presence of pathological signal enhancement in case of active inflammation [170]. An added benefit of US is the possibility to guide the needle into the bursa for direct aspiration and injection of corticosteroids. In case of bicipitoradial bursitis, US can provide information about concomitant radial nerve injury [183]. Both bicipitoradial and olecranon bursitis can be further evaluated on MRI, especially in more severe cases where extensive damage of surrounding structures is suspected and preoperative planning. MRI aspects of olecranon bursitis include hypo-intensity on T1-weighted images and variable signal intensity in T2-weighted sequences over the olecranon, with adjacent soft tissue edema and contrast enhancement of the bursal margins [184]. MRI aspects of bicipitoradial bursitis include increased signal intensity within the lesion on T2-weighted images suggestive of a fluid collection. Furthermore, hypointense septal structures may be observed. A biceps tendon with low signal intensity on both T1- and T2-weighted images can be detected at the anterior edge of the bursa [185].
4.5 Neurological Injury of the Elbow
4.5.1 Cubital Tunnel Syndrome
Next to dislocation of the ulnar nerve, as described in a snapping medial head of the triceps, the ulnar nerve may also become compressed at the cubital tunnel of the elbow. Compression of the ulnar nerve, also known as cubital tunnel syndrome, is the second most common compression neuropathy in the upper limp, following carpal tunnel syndrome [186]. In most instances, the ulnar nerve can become entrapped at the entrance of the cubital tunnel due to a thickened aponeurosis connecting the two heads of the flexor carpi ulnaris muscle [187]. This may lead to ulnar neuropathy with clinical symptoms of paresthesia and weakness of the intrinsic musculature around the fourth and fifth digits and the hypothenar region of the hand [188]. The diagnosis is confirmed with electromyography (EMG), showing a decrease in compound muscle action potential amplitude (CMAP) and slowing of focal conduction along the elbow segment [189]. EMG however is a rather uncomfortable procedure and several other diagnostic approaches have therefore been investigated, including high-resolution ultrasound (HRU) and magnetic resonance imaging (MRI) [190–192].
Qualitatively, US findings suggestive of ulnar neuropathy include abnormal enlargement of the nerve with an abrupt caliber change or loss of the normal fascicular pattern [193]. Numerous quantitative US findings have been investigated, including the ulnar nerve cross-sectional area (UNCSA), nerve diameter, and swelling ratio. The UNCSA measured at the site of greatest enlargement is a useful parameter for diagnosing cubital tunnel syndrome [194]. With the elbow in full extension and supination, the UNCSA measured at the cubital tunnel is significantly elevated in case of suspected cubital tunnel syndrome [195]. HRU may also demonstrate signs of ulnar nerve dedifferentiation consisting of edematous infiltration with a homogeneous hypoechoic aspect of the nerve. These HRU findings correspond well with cubital tunnel syndrome as diagnosed on EMG [195]. In addition, HRU can assess ulnar nerve instability during active flexion and extension of the elbow, one of the causes for ulnar neuropathy [193].
A universal MRI finding of neuropathy involves a hyperintense signal on short-tau inversion recovery (STIR) sequences. However, this finding has low specificity and is occasionally seen in healthy nerves [196]. Diffusion weighted imaging (DWI) is useful for imaging tissues with an organized microstructure such as the peripheral nerves, and the diagnostic value of DWI in median nerve entrapment neuropathy proves to be high [197]. When the ulnar nerve is entrapped, DWI is able to highlight diffusion restriction appreciable as an increase in signal intensity. Contrary to STIR sequences, an increased signal intensity of the ulnar nerve on DWI images is only visible in case of cubital tunnel syndrome as diagnosed with EMG [198].
4.5.2 Median Nerve Entrapment Syndromes
Pronator syndrome (PS) is a rare and controversial diagnosis that was originally coined to describe a compression syndrome of the median nerve between the humeral and ulnar heads of the pronator teres (PT) muscle [199]. Despite its name, compression of the median nerve can occur at several other, less common sites as it travels through the antecubital region into the forearm. Proximally, the median nerve may become entrapped as it dives under the ligament of Struthers, a ligament present in 2–3 % of the population connecting a residual supracondylar process with the medial epicondyle of the humerus [200–202].
The nerve then runs across the antecubital fossa and enters the forearm deep to the bicipital aponeurosis, another potential site of median nerve compression around the elbow. Distal to the elbow, the nerve travels between the two heads of PT muscle and passes beneath the proximal arch of the flexor digitorum superficialis (FDS) muscle [203]. PS is characterized by proximal, volar forearm pain with paresthesias of the first three digits and radial half of the fourth digit but has varying clinical manifestations due to the multiple potential sites of nerve entrapment [204]. Furthermore, the median nerve gives off a branch deep to the FDS muscle which may also become entrapped, resulting in another compression syndrome called the anterior interosseous nerve (AIN) syndrome [203].
Diagnosing median nerve entrapment around the elbow may be challenging and EMG studies are often inconclusive [205]. Conventional elbow radiographs are considered an initial step in the imaging workup and can show a residual supracondylar process of the distal humerus indicative of a Struthers’ ligament [206]. To date, no studies concerning the diagnostic efficacy of MRI and US have been published. However, both MRI and US are useful for ruling out secondary causes of median nerve compression such as ganglion cysts of nerve (sheath) tumors. Moreover, MRI can demonstrate the presence of denervation edema resulting from compression neuropathy when AIN syndrome is suspected. Denervation edema is visible in the muscles enervated by the AIN, mostly the pronator quadratus (PQ) muscle, and presents as a hyperintense signal within the affected muscles on fat-saturated T2-weighted images [207, 208].
Fatty atrophy of the affected muscles, presenting as hyperechogenicity on US, is another characteristic of chronic median nerve entrapment syndromes. However, US and MRI findings of fatty atrophy correlate poorly [209].
4.5.3 Radial Nerve Compression Syndromes
Next to ulnar and median nerve compression neuropathies in the elbow, the radial nerve is the least involved in compression injury with an annual incidence of 0.003 % for radial nerve compression syndromes [210]. As the radial nerve continues along the antecubital fossa, it branches into the motor posterior interosseous nerve (PIN) and sensory superficial radial nerve (SRN). The SRN is a subcutaneous sensory branch of the radial nerve and compression of this nerve is exceedingly rare [211]. More common is entrapment of the PIN as it courses through the radial tunnel and gives rise to either PIN syndrome or radial tunnel syndrome (RTS). Remarkably, RTS and PIN syndrome are both the result of entrapment of the same deep branch of the radial nerve, or PIN, but symptoms of both compression neuropathies show considerable diversity among patients. PIN syndrome is dominated by loss of motor function of the innervated musculature, whereas RTS is dominated by posterolateral forearm pain. This discrepancy in symptoms may be explained by the degree and duration of nerve compression [203].
There are at least five anatomical landmarks responsible for entrapment of the deep branch of the radial nerve along the radial tunnel: fibrous bands between the brachialis and brachioradialis muscles at the level of the radiocapitellar joint; the anastomosing vessels of the radial recurrent artery at the level of the radial neck, also referred to as the leash of Henry; the proximal edge of the extensor carpi radialis brevis (ECRB) muscle; the proximal edge of the supinator muscle, also referred to as the arcade of Fröhse; and the distal edge of the supinator muscle [203]. The arcade of Fröhse or proximal edge of the supinator muscle may undergo tendinous thickening due to repetitive pronosupination and is the most common site for PIN compression, hence its alternative name supinator syndrome [212, 213].
Because motor function is commonly affected in PIN syndrome, nerve conduction studies often reveal abnormal findings and are thus a useful tool for the diagnosis in addition to physical examination. Imaging studies are not routinely indicated in PIN syndrome, but MRI may reveal soft tissue masses responsible for nerve compression. Moreover, reported MRI findings in patients with suspected PIN syndrome include denervation edema of the supinator muscle, marked by an increased signal intensity of the muscle as seen on fluid-sensitive sequences with fat suppression [214]. Ultrasound may show hypoechogenicity, increased diameter of the radial deep branches, and hyperemia of the nerve on power Doppler in PIN syndrome as compared to healthy individuals [215]. However, standardized cutoff values have yet to be developed and sensitivity is relatively poor. Consequently, the role of imaging studies is limited and may be used to further strengthen the diagnosis of suspected PIN syndrome or rule out other pathology. In contrast with PIN syndrome, electrodiagnostic studies are often normal in RTS, which add to the difficulty and controversy of this diagnosis [203]. Symptoms of RTS may mimic those of lateral epicondylitis and this is where ultrasound can be used to rule out epicondylitis [211]. This distinction can usually be made during physical examination, where lateral epicondylitis presents with focal tenderness at the insertion of the ECRB, whereas RTS presents with pain starting a few centimeters more distally from the lateral epicondyle radiating into the forearm [216].




References
Grayson D. The elbow: radiographic imaging pearls and pitfalls. Semin Roentgenol. 2005;40(3):223–47.
Cain E, Dugas J, Wolf R, Andrews J. Elbow injuries in throwing athletes: a current concepts review. Am J Sports Med. 2003;31(4):621–35.
Chen A, Youm T, Ong B, Rafii M, Rokito A. Imaging of the elbow in the overhead throwing athlete. Am J Sports Med. 2003;31(3):466–73.
Greenspan A, Norman A, Rosen H. Radial head-capitellum view in elbow trauma: clinical application and radiographic-anatomic correlation. Am J Roentgenol. 1984;143(2):355–9. doi:10.2214/ajr.143.2.355.
Raby N, Berman L, De Lacey G. Accident and emergency radiology. 3rd ed. London: Bailliere Tindall; 2005.
Rogers L, Malave SJ, White H, Tachdjian M. Plastic bowing, torus and greenstick supracondylar fractures of the humerus: radiographic clues to obscure fractures of the elbow in children. Radiology. 1978;128(1):145–50. doi:10.1148/128.1.145.
Frick M. Imaging of the elbow: a review of imaging findings in acute and chronic traumatic disorders of the elbow. J Hand Ther. 2006;19(2):98–113. doi:10.1197/j.jht.2006.02.007.
Iyer R, Thapa M, Khanna P, Chew F. Pediatric bone imaging: imaging elbow trauma in children. A review of acute and chronic injuries. Am J Roentgenol. 2012;198(5):1053–68. doi:10.2214/AJR.10.7314.
LaBella CR. Common acute sports-related lower extremity injuries in children and adolescents. Clin Pediatr Emerg Med. 2007;8(1):31–42.
Shrader MW. Pediatric supracondylar fractures and pediatric physeal elbow fractures. Orthop Clin N Am. 2008;39(2):163–71. doi:10.1016/j.ocl.2007.12.005. v.
Patel B, Reed M, Patel S. Gender-specific pattern differences of the ossification centers in the pediatric elbow. Pediatr Radiol. 2009;39(3):226–31. doi:10.1007/s00247-008-1078-4.
Cheng JC, Wing-Man K, Shen WY, Yurianto H, Xia G, Lau JT, Cheung AY. A new look at the sequential development of elbow-ossification centers in children. J Pediatr Orthop. 1998;18(2):161–7.
Hayter C, Giuffre B. Overuse and traumatic injuries of the elbow. Magn Reson Imaging Clin N Am. 2009;17(4):617–38. doi:10.1016/j.mric.2009.06.004.
Sampath S, Sampath S, Bredella M. Magnetic resonance imaging of the elbow: a structured approach. Sports Health. 2013;5(1):34–49. doi:10.1177/1941738112467941.
Brunton L, Anderson M, Pannunzio M, Khanna A, Chhabra B. Magnetic resonance imaging of the elbow: update on current techniques and indications. J Hand Surg [Am]. 2006;31(6):1001–11. doi:10.1016/j.jhsa.2006.04.006.
Ouellette H, Bredella M, Labis J, Palmer W, Torriani M. MR imaging of the elbow in baseball pitchers. Skelet Radiol. 2008;37(2):115–21. doi:10.1007/s00256-007-0364-9.
Schwartz M, Al-Zahrani S, Morwessel R, Andrews J. Ulnar collateral ligament injury in the throwing athlete: evaluation with saline-enhanced MR arthrography. Radiology. 1995;197(1):297–9. doi:10.1148/radiology.197.1.7568841.
Delport A, Zoga A. MR and CT arthrography of the elbow. Semin Musculoskelet Radiol. 2012;16(1):15–26. doi:10.1055/s-0032-1304298.
Bohndorf K, Kilcoyne R. Traumatic injuries: imaging of peripheral musculoskeletal injuries. Eur Radiol. 2002;12(7):1605–16. doi:10.1007/s00330-002-1461-8.
Rosas H, Lee K. Imaging acute trauma of the elbow. Semin Musculoskelet Radiol. 2010;14(4):394–411. doi:10.1055/s-0030-1263255.
Zubler V, Saupe N, Jost B, Pfirrmann C, Hodler J, Zanetti M. Elbow stiffness: effectiveness of conventional radiography and CT to explain osseous causes. Am J Roentgenol. 2010;194(6):515–20. doi:10.2214/ajr.09.3741.
Schaeffeler C, Waldt S, Woertler K. Traumatic instability of the elbow – anatomy, pathomechanisms and presentation on imaging. Eur Radiol. 2013;23(9):2582–93. doi:10.1007/s00330- 013-2855-5.
Sauser D, Thordarson S, Fahr L. Imaging of the elbow. Radiol Clin N Am. 1990;28(5):923–40.
Franklin P, Dunlop R, Whitelaw G, Jacques EJ, Blickman J, Shapiro J. Computed tomography of the normal and traumatized elbow. J Comput Assist Tomogr. 1988;12(5):817–23.
Frahm R, Wimmer B. The search for joint loose bodies in the elbow joint – conventional or CT arthrography? Radiologe. 1990;30(3):113–5.
Steinbach L, Schwartz M. Elbow arthrography. Radiol Clin N Am. 1998;36(4):635–49.
Waldt S, Bruegel M, Ganter K, Kuhn V, Link T, Rummeny E, Woertler K. Comparison of multislice CT arthrography and MR arthrography for the detection of articular cartilage lesions of the elbow. Eur Radiol. 2005;15(4):784–91. doi:10.1007/s00330-004-2585-9.
Hodge J. Musculoskeletal procedures: diagnostic and therapeutic. Austin: Landes Bioscience; 2003.
Nofsinger C, Konin J. Diagnostic ultrasound in sports medicine: current concepts and advances. Sports Med Arthrosc. 2009;17(1):25–30. doi:10.1097/JSA.0b013e3181982add.
Martinoli C, Bianchi S, Giovagnorio F, Pugliese F. Ultrasound of the elbow. Skelet Radiol. 2001;30(11):605–14. doi:10.1007/s002560100410.
Draghi F, Danesino G, de Gautard R, Bianchi S. Ultrasound of the elbow: examination techniques and US appearance of the normal and pathologic joint. J Ultrasound. 2007;10(2):76–84. doi:10.1016/j.jus.2007.04.005.
Lee KS, Rosas HG, Craig JG. Musculoskeletal ultrasound: elbow imaging and procedures. Semin Musculoskelet Radiol. 2010;14(4):449–60. doi:10.1055/s-0030-1263260.
Badia A, Stennett C. Sports-related injuries of the elbow. J Hand Ther. 2006;19(2):206–27. doi:10.1197/j.jht.2006.02.006.
Miller A, Dodson C, Ilyas A. Thrower’s fracture of the humerus. Orthop Clin N Am. 2014;45(4):565–9. doi:10.1016/j.ocl.2014.06.011.
Curtin P, Taylor C, Rice J. Thrower’s fracture of the humerus with radial nerve palsy: an unfamiliar softball injury. Br J Sports Med. 2005;39(11):40. doi:10.1136/bjsm.2004.016345.
Sofka C, Potter H. Imaging of elbow injuries in the child and adult athlete. Radiol Clin N Am. 2002;40(2):251–65. doi:10.1016/S0033-8389(02)00011-8.
Little K. Elbow fractures and dislocations. Orthop Clin N Am. 2014;45(3):327–40. doi:10.1016/j.ocl.2014.03.004.
Merkel D, Molony J. Recognition and management of traumatic sports injuries in the skeletally immature athlete. Int J Sports Phys Ther. 2012;7(6):691–704.
Salter R, Harris R. Injuries involving the epiphyseal plate. J Bone Joint Surg Am. 1963;45(3):587–622.
Beltran J, Rosenberg Z, Kawelblum M, Montes L, Bergman A, Strongwater A. Pediatric elbow fractures: MRI evaluation. Skelet Radiol. 1994;23(4):277–81.
Seth A, Baratz M. Fractures of the elbow. In: Trumble T, Budoff J, Cornwall R, editors. Hand, elbow, shoulder. Philadelphia: Mosby; 2006. p. 522–31.
Wong A, Baratz M. Elbow fractures: distal humerus. J Hand Surg Am. 2009;34(1):176–90. doi:10.1016/j.jhsa.2008.10.023.
Gartland J. Management of supracondylar fractures of the humerus in children. Surg Gynecol Obstet. 1959;109(2):145–54.
Kim H, Song M, Conjares J, Yoo C. Trochlear deformity occurring after distal humeral fractures: magnetic resonance imaging and its natural progression. J Pediatr Orthop. 2002;22(2):188–93.
Narayanan S, Shailam R, Grottkau B, Nimkin K. Fishtail deformity – a delayed complication of distal humeral fractures in children. Pediatr Radiol. 2014. doi:10.1007/s00247-014-3249-9.
Guitton T, Doornberg J, Raaymakers E, Ring D, Kloen P. Fractures of the capitellum and trochlea. J Bone Joint Surg Am. 2009;91(2):390–7. doi:10.2106/jbjs.g.01660.
Lee J, Lawton J. Coronal shear fractures of the distal humerus. J Hand Surg [Am]. 2012;37(11):2412–7. doi:10.1016/j.jhsa.2012.09.001.
Sen R, Tripahty S, Goyal T, Aggarwal S. Coronal shear fracture of the humeral trochlea. J Orthop Surg (Hong Kong). 2013;21(1):82–6.
Doornberg J, Lindenhovius A, Kloen P, van Dijk C, Zurakowski D, Ring D. Two and three- dimensional computed tomography for the classification and management of distal humeral fractures. Evaluation of reliability and diagnostic accuracy. J Bone Joint Surg Am. 2006;88(8):1795–801. doi:10.2106/jbjs.e.00944.
Newman S, Mauffrey C, Krikler S. Olecranon fractures. Injury. 2009;40(6):575–81. doi:10.1016/j.injury.2008.12.013.
Sahajpal D, Wright T. Proximal ulna fractures. J Hand Surg Am. 2009;34(2):357–62. doi:10.1016/j.jhsa.2008.12.022.
Baecher N, Edwards S. Olecranon fractures. J Hand Surg Am. 2013;38(3):593–604. doi:10.1016/j.jhsa.2012.12.036.
Schickendantz M, Ho C, Koh J. Stress injury of the proximal ulna in professional baseball players. Am J Sports Med. 2002;30(5):737–41.
Stephenson D, Love S, Garcia G, Mair S. Recurrence of an olecranon stress fracture in an elite pitcher after percutaneous internal fixation: a case report. Am J Sports Med. 2012;40(1):218–21. doi:10.1177/0363546511422796.
O’Driscoll S, Jupiter J, Cohen M, Ring D, McKee M. Difficult elbow fractures: pearls and pitfalls. Instr Course Lect. 2003;52:113–34.
Doornberg J, Ring D. Coronoid fracture patterns. J Hand Surg [Am]. 2006;31(1):45–52. doi:10.1016/j.jhsa.2005.08.014.
Ring D, Jupiter J, Zilberfarb J. Posterior dislocation of the elbow with fractures of the radial head and coronoid. J Bone Joint Surg Am. 2002;84(4):547–51.
Mason M. Some observations on fractures of the head of the radius with a review of one hundred cases. Br J Surg. 1954;42(172):123–32. doi:10.1002/bjs.18004217203.
Johnston G. A follow-up of one hundred cases of fracture of the head of the radius with a review of the literature. Ulster Med J. 1962;31(1):51–6.
Struijs P, Smit G, Steller E. Radial head fractures: effectiveness of conservative treatment versus surgical intervention. A systematic review. Arch Orthop Trauma Surg. 2007;127(2):125–30. doi:10.1007/s00402-006-0240-4.
Kaas L, Turkenburg J, van Riet R, Vroemen J, Eygendaal D. Magnetic resonance imaging findings in 46 elbows with a radial head fracture. Acta Orthop. 2010;81(3):373–6. doi:10.3109/17453674.2010.483988.
Itamura J, Roidis N, Mirzayan R, Vaishnav S, Learch T, Shean C. Radial head fractures: MRI evaluation of associated injuries. J Should Elb Surg. 2005;14(4):421–4. doi:10.1016/j.jse.2004.11.003.
Kumar V, Wallace W. Radial head fractures – update on classification and management. Orthop Traumatol. 2012;26(2):124–31. doi:10.1016/j.mporth.2012.04.002.
Pike J, Athwal G, Faber K, King G. Radial head fractures – an update. J Hand Surg Am. 2009;34(3):557–65. doi:10.1016/j.jhsa.2008.12.024.
Baker 3rd C, Romeo A, Baker CJ. Osteochondritis dissecans of the capitellum. Am J Sports Med. 2010;38(9):1917–28. doi:10.1177/0363546509354969.
Ruchelsman D, Hall M, Youm T. Osteochondritis dissecans of the capitellum: current concepts. J Am Acad Orthop Surg. 2010;18(9):557–67.
Kijowski R, De Smet A. MRI findings of osteochondritis dissecans of the capitellum with surgical correlation. Am J Roentgenol. 2005;185(6):1453–9. doi:10.2214/AJR.04.1570.
Bradley J, Petrie R. Osteochondritis dissecans of the humeral capitellum. Diagnosis and treatment. Clin Sports Med. 2001;20(3):565–90.
Takahara M, Ogino T, Takagi M, Tsuchida H, Orui H, Nambu T. Natural progression of osteochondritis dissecans of the humeral capitellum: initial observations. Radiology. 2000;216(1):207–12. doi:10.1148/radiology.216.1.r00jl29207.
van den Ende K, McIntosh A, Adams J, Steinmann S. Osteochondritis dissecans of the capitellum: a review of the literature and a distal ulnar portal. Arthroscopy. 2011;27(1):122–8. doi:10.1016/j.arthro.2010.08.008.
Takahara M, Mura N, Sasaki J, Harada M, Ogino T. Classification, treatment, and outcome of osteochondritis dissecans of the humeral capitellum. J Bone Joint Surg Am. 2007;89(6):1205–14. doi:10.2106/jbjs.f.00622.
Satake H, Takahara M, Harada M, Maruyama M. Preoperative imaging criteria for unstable osteochondritis dissecans of the capitellum. Clin Orthop Relat Res. 2013;471(4):1137–43. doi:10.1007/s11999-012-2462-9.
Brittberg M, Winalski C. Evaluation of cartilage injuries and repair. J Bone Joint Surg Am. 2003;85:58–69.
Takahara M, Ogino T, Tsuchida H, Takagi M, Kashiwa H, Nambu T. Sonographic assessment of osteochondritis dissecans of the humeral capitellum. Am J Roentgenol. 2000;174(2):411–5. doi:10.2214/ajr.174.2.1740411.
Takenaga T, Goto H, Nozaki M, Yoshida M, Nishiyama T, Otsuka T. Ultrasound imaging of the humeral capitellum: a cadaveric study. J Orthop Sci. 2014;19(6):907–12. doi:10.1007/s00776-014- 0637-9.
Harada M, Takahara M, Sasaki J, Mura N, Ito T, Ogino T. Using sonography for the early detection ofelbow injuries among young baseball players. Am J Roentgenol. 2006;187(6):1436–41. doi:10.2214/AJR.05.1086.
Kijowski R, De Smet A. Radiography of the elbow for evaluation of patients with osteochondritis dissecans of the capitellum. Skelet Radiol. 2005;34(5):266–71. doi:10.1007/s00256-005- 0899-6.
Smith M, Bedi A, Chen N. Surgical treatment for osteochondritis dissecans of the capitellum. Sports Health. 2012;4(5):425–32. doi:10.1177/1941738112444707.
Itsubo T, Murakami N, Uemura K, Nakamura K, Hayashi M, Uchiyama S, Kato H. Magnetic resonance imaging staging to evaluate the stability of capitellar osteochondritis dissecans lesions. Am J Sports Med. 2014;42(8):1972–7. doi:10.1177/0363546514532604.
Holland P, Davies A, Cassar-Pullicino V.Computed tomographic arthrography in the assessment of osteochondritis dissecans of the elbow. Clin Radiol. 1994;49(4):231–5. doi:10.1016/S0009-9260(05)81846-X.
Jans L, Ditchfield M, Anna G, Jaremko J, Verstraete K. MR imaging findings and MR criteria for instability in osteochondritis dissecans of the elbow in children. Eur J Radiol. 2012;81(6):1306–10. doi:10.1016/j.ejrad.2011.01.007.
Hegemann G. [Spontaneous aseptic bone necrosis of the elbow] (Die “spontanen” aseptischen Knochennekrosen des Ellenbogengelenkes). Fortschr Geb Rontgenstr. 1951;75(1):89–92.
Beyer W, Heppt P, Gluckert K, Willauschus W. Aseptic osteonecrosis of the humeral trochlea (Hegemann’s disease). Arch Orthop Trauma Surg. 1990;110(1):45–8.
Patel N, Weiner S. Osteochondritis dissecans involving the trochlea: report of two patients (three elbows) and review of the literature. J Pediatr Orthop. 2002;22(1):48–51.
Claessen F, Louwerens J, Doornberg J, van Dijk C, van den Bekerom M, Eygendaal D. Hegemann’s disease and fishtail deformity: aetiopathogenesis, radiographic appearance and clinical outcome. J Child Orthop. 2015. doi:10.1007/s11832-014-0630-z.
Brogdon B, Crow N. Little leaguer’s elbow. Am J Roentgenol Radium Ther Nucl Med. 1960;83:671–5.
Zellner B, May M. Elbow injuries in the young athlete – an orthopedic perspective. Pediatr Radiol. 2013;43 Suppl 1:S129–34. doi:10.1007/s00247-012-2593-x.
Banks KP, Ly JQ, Beall DP, Grayson DE, Bancroft LW, Tall MA. Overuse injuries of the upper extremity in the competitive athlete: magnetic resonance imaging findings associated with repetitive trauma. Curr Probl Diagn Radiol. 2005;34(4):127–42.
Hang D, Chao C, Hang Y. A clinical and roentgenographic study of Little League elbow. Am J Sports Med. 2004;32(1):79–84.
Frush T, Lindenfeld T. Peri-epiphyseal and overuse injuries in adolescent athletes. Sports Health. 2009;1(3):201–11. doi:10.1177/1941738109334214.
Davis K. Imaging pediatric sports injuries: upper extremity. Radiol Clin N Am. 2010;48(6):1199–211. doi:10.1016/j.rcl.2010.07.020.
Wei A, Khana S, Limpisvasti O, Crues J, Podesta L, Yocum L. Clinical and magnetic resonance imaging findings associated with Little League elbow. J Pediatr Orthop. 2010;30(7):715–9. doi:10.1097/BPO.0b013e3181edba46.
Biswas D, Wysocki R, Cohen M. Primary and posttraumatic arthritis of the elbow. Arthritis. 2013;2013:1–6. doi:10.1155/2013/473259.
Lim Y, van Riet R, Mittal R, Bain G. Pattern of osteophyte distribution in primary osteoarthritis of the elbow. J Should Elb Surg. 2008;17(6):963–6. doi:10.1016/j.jse.2008.03.012.
Suvarna S, Stanley D. The histologic changes of the olecranon fossa membrane in primary osteoarthritis of the elbow. J Should Elb Surg. 2004;13(5):555–7. doi:10.1016/j.jse.2004.02.014.
Savoie Iii F, Nunley P, Field L. Arthroscopic management of the arthritic elbow: indications, technique, and results. J Should Elb Surg. 1999;8(3):214–9. doi:10.1016/S1058-2746(99)90131-3.
Dalal S, Bull M, Stanley D. Radiographic changes at the elbow in primary osteoarthritis: a comparison with normal aging of the elbow joint. J Should Elb Surg. 2007;16(3):358–61. doi:10.1016/j.jse.2006.08.005.
Nishiwaki M, Willing R, Johnson J, King G, Athwal G. Identifying the location and volume of bony impingement in elbow osteoarthritis by 3-dimensional computational modeling. J Hand Surg [Am]. 2013;38(7):1370–6. doi:10.1016/j.jhsa.2013.03.035.
Tyrdal S, Finnanger A. Osseous manifestations of ‘handball goalie’s elbow’. Scand J Med Sci Sports. 1999;9(2):92–7.
Popovic N, Lemaire R. Hyperextension trauma to the elbow: radiological and ultrasonographic evaluation in handball goalkeepers. Br J Sports Med. 2002;36(6):452–6. doi:10.1136/bjsm.36.6.452.
Bryce C, Armstrong A. Anatomy and biomechanics of the elbow. Orthop Clin N Am. 2008;39(2):141–54. doi:10.1016/j.ocl.2007.12.001.
O’Driscoll S, Jupiter J, King G, Hotchkiss R, Morrey B. The unstable elbow. J Bone Joint Surg Am. 2000;82(5):724.
O’Driscoll S, Morrey B, Korinek S, An K. Elbow subluxation and dislocation. A spectrum of instability. Clin Orthop Relat Res. 1992;(280):186–97.
Kaplan L, Potter H. MR imaging of ligament injuries to the elbow. Magn Reson Imaging Clin N Am. 2004;12(2):221–32. doi:10.1016/j.mric.2004.02.006.
Dugas J, Chronister J, Cain EJ, Andrews J. Ulnar collateral ligament in the overhead athlete: a current review. Sports Med Artrosc Rev. 2014;22(3):169–82. doi:10.1097/jsa.0000000000000033.
Cain EJ, Andrews J, Dugas J, Wilk K, McMichael C, 2nd Walter J, Riley R, Arthur S. Outcome of ulnar collateral ligament reconstruction of the elbow in 1281 athletes: results in 743 athletes with minimum 2-year follow-up. Am J Sports Med. 2010;38(12):2426–34. doi:10.1177/0363546510378100.
Hariri S, Safran M. Ulnar collateral ligament injury in the overhead athlete. Clin Sports Med. 2010;29(4):619–44. doi:10.1016/j.csm.2010.06.007.
Hurd W, Eby S, Kaufman K, Murthy N. Magnetic resonance imaging of the throwing elbow in the uninjured, high school-aged baseball pitcher. Am J Sports Med. 2011;39(4):722–8. doi:10.1177/0363546510390185.
Potter H, Sofka C. Imaging of the athlete’s elbow. In: Altchek D, Andrews J, editors. The athlete’s elbow. Philadelphia: Lippincott-Raven; 2001. p. 59–80.
Timmerman L, Schwartz M, Andrews J. Preoperative evaluation of the ulnar collateral ligament by magnetic resonance imaging and computed tomography arthrography: evaluation in 25 baseball players with surgical confirmation. Am J Sports Med. 1994;22(1):26–32. doi:10.1177/036354659402200105.
Parsons B, Ramsey M. Acute elbow dislocations in athletes. Clin Sports Med. 2010;29(4):599–609. doi:10.1016/j.csm.2010.06.005.
Kuhn M, Ross G. Acute elbow dislocations. Orthop Clin N Am. 2008;39(2):155–61. doi:10.1016/j.ocl.2007.12.004.
Taylor F, Sims M, Theis J, Herbison G. Interventions for treating acute elbow dislocations in adults. Cochrane Database Syst Rev. 2012;4:Cd007908. doi:10.1002/14651858.CD007908.pub2.
McCabe M, Savoie Iii F. Simple elbow dislocations: evaluation, management, and outcomes. Physicians. 2012;40(1):62–71. doi:10.3810/psm.2012.02.1952.
Tarassoli P, McCann P, Amirfeyz R. Complex instability of the elbow. Injury. 2013. doi:10.1016/j.injury.2013.09.032.
Potter H, Schachar J, Jawetz S. Imaging of the elbow. Oper Tech Orthop. 2009;19(4):199–208. doi:10.1053/j.oto.2009.09.002.
O’Driscoll S. Classification and evaluation of recurrent instability of the elbow. Clin Orthop Relat Res. 2000;370:34–43.
Charalambous C, Stanley J. Posterolateral rotatory instability of the elbow. J Bone Joint Surg (Br). 2008;90(3):272–9. doi:10.1302/0301-620x.90b3.19868.
Schreiber J, Potter H, Warren R, Hotchkiss R, Daluiski A. Magnetic resonance imaging findings in acute elbow dislocation: insight into mechanism. J Hand Surg Am. 2014;39(2):199–205. doi:10.1016/j.jhsa.2013.11.031.
Eygendaal D, Verdegaal S, Obermann W, van Vugt A, Poll R, Rozing P. Posterolateral dislocation of the elbow joint. Relationship to medial instability. J Bone Joint Surg Am. 2000;82(4):555–60.
Anakwenze O, Kancherla V, Iyengar J, Ahmad C, Levine W. Posterolateral rotatory instability of the elbow. Am J Sports Med. 2014;42(2):485–91. doi:10.1177/0363546513494579.
O’Driscoll S, Bell D, Morrey B. Posterolateral rotatory instability of the elbow. J Bone Joint Surg Am. 1991;73(3):440–6.
Savoie III F, Field L, Ramsey J. Posterolateral rotatory instability of the elbow: diagnosis and management. Oper Tech Sports Med. 2006;14(2):81–5. doi:10.1053/j.otsm.2006.03.001.
Coonrad R, Roush T, Major N, Basamania C. The drop sign, a radiographic warning sign of elbow instability. J Should Elb Surg. 2005;14(3):312–7. doi:10.1016/j.jse.2004.09.002.
Grafe M, McAdams T, Beaulieu C, Ladd A. Magnetic resonance imaging in diagnosis of chronic posterolateral rotatory instability of the elbow. Am J Orthop. 2003;32(10):501–4.
Bado J. The Monteggia lesion. Clin Orthop Relat Res. 1967;50:71–86.
Beutel B. Monteggia fractures in pediatric and adult populations. Orthopedics. 2012;35(2):138–44. doi:10.3928/01477447-20120123-32.
Dahmoush H, Pollock A. Monteggia fracture-dislocation. Pediatr Emerg Care. 2013;29(3):406–7. doi:10.1097/PEC.0b013e318286495e.
Browner E. Nursemaid’s elbow (annular ligament displacement. Pediatr Rev. 2013;34(8):366–7. doi:10.1542/pir.34-8-366.
Krul M, van der Wouden J, van Suijlekom-Smit L, Koes B. Manipulative interventions for reducing pulled elbow in young children. Cochrane Database Syst Rev. 2012;1:Cd007759. doi:10.1002/14651858.CD007759.pub3.
Faro F, Wolf J. Lateral epicondylitis: review and current concepts. J Hand Surg Am. 2007;32(8):1271–9. doi:10.1016/j.jhsa.2007.07.019.
Walz D, Newman J, Konin G, Ross G. Epicondylitis: pathogenesis, imaging, and treatment. Radiographics. 2010;30(1):167–84. doi:10.1148/rg.301095078.
Blease S, Stoller D, Safran M, Li A, Fritz R. The elbow. In: Stoller D, editor. Magnetic resonance imaging in orthopaedics and sports medicine. 3rd ed. Philadelphia: Lippincott, Williams & Wilkins; 2007. p. 1463–625.
Bunata R, Brown D, Capelo R. Anatomic factors related to the cause of tennis elbow. J Bone Joint Surg Am. 2007;89(9):1955–63. doi:10.2106/jbjs.f.00727.
Kijowski R, Tuite M, Sanford M. Magnetic resonance imaging of the elbow. Part II: abnormalities of the ligaments, tendons, and nerves. Skelet Radiol. 2005;34(1):1–18. doi:10.1007/s00256-004-0854-y.
Wessely M, Grenier J. Elbow MRI: part 2: the imaging of common disorders affecting the elbow region. Clin Chiropr. 2007;10(1):43–9. doi:10.1016/j.clch.2006.12.001.
Pomerance J. Radiographic analysis of lateral epicondylitis. J Should Elb Surg. 2002;11(2):156–7.
Miller T, Shapiro M, Schultz E, Kalish P. Comparison of sonography and MRI for diagnosing epicondylitis. J Clin Ultrasound. 2002;30(4):193–202. doi:10.1002/jcu.10063.
Potter H, Hannafin J, Morwessel R, DiCarlo E, O’Brien S, Altchek D. Lateral epicondylitis: correlation of MR imaging, surgical, and histopathologic findings. Radiology. 1995;196(1):43–6. doi:10.1148/radiology.196.1.7784585.
Martin C, Schweitzer M. MR imaging of epicondylitis. Skelet Radiol. 1998;27(3):133–8.
Grana W. Medial epicondylitis and cubital tunnel syndrome in the throwing athlete. Clin Sports Med. 2001;20(3):541–8.
Ciccotti M, Schwartz M, Ciccotti M. Diagnosis and treatment of medial epicondylitis of the elbow. Clin Sports Med. 2004;23(4):693–705. doi:10.1016/j.csm.2004.04.011.
Nirschl R, Pettrone F. Tennis elbow. The surgical treatment of lateral epicondylitis. J Bone Joint Surg Am. 1979;61(6):832–9.
Nirschl R, Pettrone F. Lateral and medial epicondylitis. Master techniques in orthopedic surgery: the elbow. New York: Raven Press; 1994.
Nirschl R. Prevention and treatment of elbow and shoulder injuries in the tennis player. Clin Sports Med. 1988;7(2):289–308.
Bernard F, Regan W. Elbow and forearm. In: DeLee JC, editor. DeLee and Drez’s orthopaedic sports medicine. 2nd ed. Philadelphia: Saunders; 2003.
Pienimaki T, Siira P, Vanharanta H. Chronic medial and lateral epicondylitis: a comparison of pain, disability, and function. Arch Phys Med Rehabil. 2002;83(3):317–21.
Taylor S, Hannafin J. Evaluation and management of elbow tendinopathy. Sports Health. 2012;4(5):384–93. doi:10.1177/1941738112454651.
Morrey B, Askew L, An K, Dobyns J. Rupture of the distal tendon of the biceps brachii. A biomechanical study. J Bone Joint Surg Am. 1985;67(3):418–21.
Thompson K. Rupture of the distal biceps tendon in a collegiate football player: a case report. J Athl Train. 1998;33(1):62–4.
Safran M, Graham S. Distal biceps tendon ruptures: incidence, demographics, and the effect of smoking. Clin Orthop Relat Res. 2002;404:275–83.
RaAntanen J, Orava S. Rupture of the distal biceps tendon. A report of 19 patients treated with anatomic reinsertion, and a meta-analysis of 147 cases found in the literature. Am J Sports Med. 1999;27(2):128–32.
Aldridge J, Bruno R, Strauch R, Rosenwasser M. Management of acute and chronic biceps tendon rupture. Hand Clin. 2000;16(3):497–503.
Ramsey M. Distal biceps tendon injuries: diagnosis and management. J Am Acad Orthop Surg. 1999;7(3):199–207.
Tran N, Chow K. Ultrasonography of the elbow. Semin Musculoskelet Radiol. 2007;11(2):105–16. doi:10.1055/s-2007-1001876.
Fitzgerald S, Curry D, Erickson S, Quinn S, Friedman H. Distal biceps tendon injury: MR imaging diagnosis. Radiology. 1994;191(1):203–6. doi:10.1148/radiology.191.1.8134571.
Falchook F, Zlatkin M, Erbacher G, Moulton J, Bisset G, Murphy B. Rupture of the distal biceps tendon: evaluation with MR imaging. Radiology. 1994;190(3):659–63. doi:10.1148/radiology.190.3.8115606.
Williams B, Schweitzer M, Weishaupt D, Lerman J, Rubenstein D, Miller L, Rosenberg Z. Partial tears of the distal biceps tendon: MR appearance and associated clinical findings. Skelet Radiol. 2001;30(10):560–4. doi:10.1007/s002560100397.
Anzel S, Covey K, Weiner A, Lipscomb P. Disruption of muscles and tendons; an analysis of 1.014 cases. Surgery. 1959;45(3):406–14.
Sierra R, Weiss N, Shrader M, Steinmann S. Acute triceps ruptures: case report and retrospective chart review. J Should Elb Surg. 2006;15(1):130–4. doi:10.1016/j.jse.2005.01.004.
Mair S, Isbell W, Gill T, Schlegel T, Hawkins R. Triceps tendon ruptures in professional football players. Am J Sports Med. 2004;32(2):431–4.
Sollender J, Rayan G, Barden G. Triceps tendon rupture in weight lifters. J Should Elb Surg. 1998;7(2):151–3.
Blackmore S, Jander R, Culp R. Management of distal biceps and triceps ruptures. J Hand Ther. 2006;19(2):154–68. doi:10.1197/j.jht.2006.02.001.
Johnson D, Allen A. Biceps and triceps tendon injury. In: Altchek D, Andrews J, editors. The athlete’s elbow. Philadelphia: Lippincott Williams & Wilkins; 2001. p. 105–20.
Stannard J, Bucknell A. Rupture of the triceps tendon associated with steroid injections. Am J Sports Med. 1993;21(3):482–5.
Bach BJ, Warren R, Wickiewicz W. Triceps rupture. A case report and literature review. Am J Sports Med. 1987;15(3):285–9.
Stucken C, Ciccotti M. Distal biceps and triceps injuries in athletes. Sports Med Arthrosc. 2014;22(3):153–63. doi:10.1097/jsa.0000000000000030.
van Riet R, Morrey B, Ho E, O’Driscoll S. Surgical treatment of distal triceps ruptures. J Bone Joint Surg Am. 2003;85(10):1961–7.
Pina A, Garcia I, Sabater M. Traumatic avulsion of the triceps brachii. J Orthop Trauma. 2002;16(4):273–6.
Radunovic G, Vlad V, Micu M, Nestorova R, Petranova T, Porta F, Iagnocco A. Ultrasound assessment of the elbow. Med Ultrason. 2012;14(2):141–6.
Gaines S, Durbin R, Marsalka D. The use of magnetic resonance imaging in the diagnosis of triceps tendon ruptures. Contemp Orthop. 1990;20(6):607–11.
Spinner R, Goldner R. Snapping of the medial head of the triceps: diagnosis and treatment. Tech Hand Upper Extrem Surg. 2002;6(2):91–7.
Spinner R, Goldner R. Snapping of the medial head of the triceps and recurrent dislocation of the ulnar nerve. Anatomical and dynamic factors. J Bone Joint Surg Am. 1998;80(2):239–47.
Spinner R, Hayden FJ, Hipps C, Goldner R. Imaging the snapping triceps. Am J Roentgenol. 1996;167(6):1550–1. doi:10.2214/ajr.167.6.8956595.
Jacobson J, Jebson P, Jeffers A, Fessell D, Hayes C. Ulnar nerve dislocation and snapping triceps syndrome: diagnosis with dynamic sonography – report of three cases. Radiology. 2001;220(3):601–5. doi:10.1148/radiol.2202001723.
Spinner R, O’Driscoll S, Jupiter J, Goldner R. Unrecognized dislocation of the medial portion of the triceps: another cause of failed ulnar nerve transposition. J Neurosurg. 2000;92(1):52–7. doi:10.3171/jns.2000.92.1.0052.
Wenzke D. MR imaging of the elbow in the injured athlete. Radiol Clin N Am. 2013;51(2):195–213. doi:10.1016/j.rcl.2012.09.013.
Bak B. Bicipitoradial bursitis. Ugeskr Laeger. 2008;170(40):3123–4.
Bond J, Sundaram M, Beckenbaugh R. Radiologic case study. Partial tear of the distal biceps tendon with mass-like bicipitoradial bursitis and associated hyperostosis of the radial tuberosity. Orthopedics. 2003;26(4):448–50.
Larson R, Osternig L. Traumatic bursitis and artificial turf. J Sports Med. 1974;2(4):183–8.
Del Buono A, Franceschi F, Palumbo A, Denaro V, Maffulli N. Diagnosis and management of olecranon bursitis. Surgeon. 2012;10(5):297–300. doi:10.1016/j.surge.2012.02.002.
Blankstein A, Ganel A, Givon U, Mirovski Y, Chechick A. Ultrasonographic findings in patients with olecranon bursitis. Ultraschall Med. 2006;27(6):568–71. doi:10.1055/s-2006-926569.
Draghi F, Gregoli B, Sileo C. Sonography of the bicipitoradial bursa: a short pictorial essay. J Ultrasound. 2012;15(1):39–41. doi:10.1016/j.jus.2012.02.003.
Floemer F, Morrison W, Bongartz G, Ledermann H. MRI characteristics of olecranon bursitis. Am J Roentgenol. 2004;183(1):29–34. doi:10.2214/ajr.183.1.1830029.
Hoi T, Lui T. Bicipitoradial bursitis: a review of clinical presentation and treatment. JOTR. 2013;8(1):7–11. doi:10.1016/j.jotr.2013.12.009.
Aliandro P, La Torre G, Padua R, Giannini F, Padua L. Treatment for ulnar neuropathy at the elbow. Cochrane Database Syst Rev. 2012;7:Cd006839. doi:10.1002/14651858.CD006839.pub3.
Tewart J, Shantz S. Perioperative ulnar neuropathies: a medicolegal review. Can J Neurol Sci. 2003;30(1):15–9.
Bordalo-Rodrigues M, Rosenberg Z. MR imaging of entrapment neuropathies at the elbow. Magn Reson Imaging Clin N Am. 2004;12(2):247–63. doi:10.1016/j.mric.2004.02.002.
Zrieli Y, Weimer L, Lovelace R, Gooch C. The utility of segmental nerve conduction studies in ulnar mononeuropathy at the elbow. Muscle Nerve. 2003;27(1):46–50. doi:10.1002/mus.10293.
Aumer P, Dombert T, Staub F, Kaestel T, Bartsch A, Heiland S, Bendszus M, Pham M. Ulnar neuropathy at the elbow: MR neurography – nerve T2 signal increase and caliber. Radiology. 2011;260(1):199–206. doi:10.1148/radiol.11102357.
Eekman R, Schoemaker M, Van Der Plas J, Van Den Berg L, Franssen H, Wokke J, Uitdehaag B, Visser L. Diagnostic value of high-resolution sonography in ulnar neuropathy at the elbow. Neurology. 2004;62(5):767–73.
Yromlou H, Tarzamni M, Daghighi M, Pezeshki M, Yazdchi M, Sadeghi-Hokmabadi E, Sharifipour E, Ghabili K. Diagnostic value of ultrasonography and magnetic resonance imaging in ulnar neuropathy at the elbow. ISRN Neurol. 2012. doi:10.5402/2012/491892.
Bojniewicz A. US for diagnosis of musculoskeletal conditions in the young athlete: emphasis on dynamic assessment. Radiographics. 2014;34(5):1145–62. doi:10.1148/rg.345130151.
Eekman R, Visser L, Verhagen W. Ultrasonography in ulnar neuropathy at the elbow: a critical review. Muscle Nerve. 2011;43(5):627–35. doi:10.1002/mus.22019.
Abusiaux D, Laulan J, Bouilleau L, Martin A, Adrien C, Aubertin A, Rabarin F. Contribution of static and dynamic ultrasound in cubital tunnel syndrome. Orthop Traumatol Surg Res. 2014;100(4 Suppl):S209–12. doi:10.1016/j.otsr.2014.03.008.
Jarvik J, Yuen E. Diagnosis of carpal tunnel syndrome: electrodiagnostic and magnetic resonance imaging evaluation. Neurosurg Clin N Am. 2001;12(2):241–53.
Ba K, Wada T, Tamakawa M, Aoki M, Yamashita T. Diffusion-weighted magnetic resonance imaging of the ulnar nerve in cubital tunnel syndrome. Hand Surg. 2010;15(1):11–5. doi:10.1142/s021881041000445x.
Ltun Y, Aygun M, Cevik M, Acar A, Varol S, Arikanoglu A, Onder H, Uzar E. Relation between electrophysiological findings and diffusion weighted magnetic resonance imaging in ulnar neuropathy at the elbow. J Neuroradiol. 2013;40(4):260–6. doi:10.1016/j.neurad.2012.08.004.
Eyffarth H. Primary myoses in the M. pronator teres as cause of lesion of the N. medianus (the pronator syndrome). Acta Psychiatr Neurol Scand Suppl. 1951;74:251–4.
Newman A. The supracondylar process and its fracture. Am J Roentgenol Radium Ther Nucl Med. 1969;105(4):844–9.
Truthers J. On hereditary supracondyloid process in man. Lancet. 1873;1:231–2.
Lonsdale H. A sketch of his life and writings of Robert Knox, the anatomist. London: Macmillan; 1870.
Ang A, Rodner C. Unusual compression neuropathies of the forearm, part I: radial nerve. J Hand Surg Am. 2009;34(10):1906–14. doi:10.1016/j.jhsa.2009.10.016.
Johnson R, Spinner M, Shrewsbury M. Median nerve entrapment syndrome in the proximal forearm. J Hand Surg Am. 1979;4(1):48–51.
Pinner M, Linscheid R. Nerve entrapment syndromes. In: Morrey B, editor. The elbow and its disorders. 2nd ed. Philadelphia: Saunders; 1993. p. 813–32.
Arnard L, McCoy S. The supra condyloid process of the humerus. J Bone Joint Surg Am. 1946;28(4):845–50.
L-Qattan M. Gantzer’s muscle. An anatomical study of the accessory head of the flexor pollicis longus muscle. J Hand Surg (Br). 1996;21(2):269–70.
Rainger A, Campbell R, Stothard J. Anterior interosseous nerve syndrome: appearance at MR imaging in three cases. Radiology. 1998;208(2):381–4. doi:10.1148/radiology.208.2.9680563.
Iller T, Reinus W. Nerve entrapment syndromes of the elbow, forearm, and wrist. Am J Roentgenol. 2010;195(3):585–94. doi:10.2214/ajr.10.4817.
Atinovic R, Gulliford M, Hughes R. Incidence of common compressive neuropathies in primary care. J Neurol Neurosurg Psychiatry. 2006;77(2):263–5. doi:10.1136/jnnp.2005.066696.
Sai P, Steinberg D. Median and radial nerve compression about the elbow. Instr Course Lect. 2008;57:177–85.
Lavert P, Lutz J, Adam P, Wolfram-Gabel R, Liverneaux P, Kahn J. Frohse’s arcade is not the exclusive compression site of the radial nerve in its tunnel. Orthop Traumatol Surg Res. 2009;95(2):114–8. doi:10.1016/j.otsr.2008.11.001.
Uillain G, Courtellemont R. Role of the supinator in radial nerve paralysis: pathogenesis of a partial radial nerve paralysis in an orchestra conductor. Presse Med. 1905;7:50–2.
Erdinand B, Rosenberg Z, Schweitzer M, Stuchin S, Jazrawi L, Lenzo S, Meislin R, Kiprovski K. MR imaging features of radial tunnel syndrome: initial experience. Radiology. 2006;240(1):161–8. doi:10.1148/radiol.2401050028.
Bodner G, Harpf C, Meirer R, Gardetto A, Kovacs P, Gruber H. Ultrasonographic appearance of supinator syndrome. J Ultrasound Med. 2002;21(11):1289–93.
Roles N, Maudsley R. Radial tunnel syndrome: resistant tennis elbow as a nerve entrapment. J Bone Joint Surg Br. 1972;54(3):499–508.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 ESSKA
About this chapter
Cite this chapter
van Steenkiste, R.L., Opperman, J., Kox, L.S., Maas, M. (2016). Imaging of the Elbow in Overhead Athletes. In: Pederzini, L., Eygendaal, D., Denti, M. (eds) Elbow and Sport. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-662-48742-6_4
Download citation
DOI: https://doi.org/10.1007/978-3-662-48742-6_4
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-662-48740-2
Online ISBN: 978-3-662-48742-6
eBook Packages: MedicineMedicine (R0)