Abstract
This chapter describes invasomes, which represent novel vesicular carriers for enhanced skin delivery. Invasomes are composed of unsaturated phospholipids, small amounts of ethanol and terpenes, and water. Different penetration studies performed in vitro in human skin will be represented in order to show the penetration-enhancing ability of invasomes. The first used invasomes or standard invasomes contained a terpene mixture, composed of cineole:citral:d-limonene = 45:45:10 v/v and were shown to be more efficient in delivering highly lipophilic and hydrophobic drugs into the skin, such as cyclosporine A (CsA) and temoporfin (mTHPC), compared to conventional liposomes and the ethanolic solution of drugs. It was also shown that invasomes significantly increased the permeation of hydrophilic drugs, like calcein and carboxyfluorescein, compared to conventional and deformable liposomes. Furthermore, also other terpene mixtures and single terpenes can be used to formulate invasomes, and dependent on the added terpene or terpene mixture, invasomes may enhance or retard the drug penetration into the skin compared to liposomes without terpenes. The presence of 1 % w/v citral, 1 % w/v cineole, or 1 % w/v standard terpene mixture in invasomes resulted in the formation of highly effective skin delivery systems for mTHPC, especially in the case of invasomes with 1 % w/v cineole providing high amounts of mTHPC not only in the SC but also in the deeper skin layers. As to the therapeutic effectiveness, the effectiveness of CsA invasomes containing 2 % w/v standard terpene mixture was confirmed in the treatment of alopecia areata in the Dundee Experimental Bald Rat (DEBR), while mTHPC invasomes containing 1 % w/v standard terpene mixture provided a slower tumor growth in mice bearing the subcutaneously located human colorectal HT29 carcinoma. The key factor for the high penetration-enhancing ability of invasomes is assumed to be their high fluidity confirmed by electron spin resonance, differential scanning calorimetry, and cryoelectron microscopy. However, as no direct correlation between the fluidity of invasomes and their ability to improve skin penetration of drugs was found, it is proposed that besides the fluidity, also other phenomena might be involved in the mechanism of the skin penetration enhancement induced by invasomes.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Invasomes
- Liposomes
- Penetration-enhancing ability
- Skin delivery
- Terpenes
- Transdermal
- Temoporfin
- Cyclosporine A
5.1 Introduction
The major problem in both topical and transdermal drug delivery is the low permeability of the most apical layer of the skin, the stratum corneum (SC). Due to this, topical formulations encounter the problem of inefficient penetration of drugs into the skin which results in a low dose reaching the site of action and therefore limited local activity. Transdermal drug delivery, i.e., drug absorption into systemic circulation, is even more difficult to achieve, and currently, only a limited number of drugs with low molecular weight (MW), such as scopolamine, clonidine, nitroglycerin, fentanyl, nicotine, estradiol (alone or in combination with levonorgestrel or norethisterone), and some others are used in transdermal patches (Benson 2005; Prausnitz and Langer 2008). Therefore, in order to penetrate easily the SC, a molecule should posses certain physicochemcial properties, such as low MW (<600 Da), adequate solubility in oil and water, intermediate partition coefficient (log K octanol/water of 1–3), and low melting point (Barry 2001; Williams 2003).
If the drug does not match these ideal characteristics, different penetration enhancement techniques can be used to overcome the barrier properties of the SC. These methods include manipulation of the drug or vehicle to enhance drug diffusion (Barry 2004), use of nanocarriers (Benson 2005), as well as methods which modify the SC, such as iontophoresis (Lopez et al. 2003), electroporation (Wang et al. 2009), ultrasound (Cancel et al. 2004), and chemical penetration enhancement using different penetration enhancers (Ahad et al. 2009). The successful use of these methods is well documented in literature (Barry 2001; Benson 2005; Rizwan et al. 2009) and is described in detail in separate chapters of this book.
Among these different methods, nanocarriers have been frequently used, especially liposomes (Cevc et al. 2008a, b; Song and Kim 2006). Liposomes have been intensively studied as drug carrier systems for both the topical (dermal) and transdermal drug delivery (Cevc and Blume 2001, 2004; Cevc 2003; El Maghraby and Williams 2009).
5.2 Liposomes
Liposomes are used as topical drug delivery systems in dermato-pharmacotherapy, as there is a need for a drug delivery system that enhances penetration of the active ingredient into the skin, localizes the drug at the site of action, and reduces percutaneous absorption. Liposomes indeed have the potential to enhance drug penetration into the skin (El Maghraby et al. 2001a; Verma et al. 2003a, b; Betz et al. 2005), improve therapeutic effectiveness (Seth et al. 2004; Mura et al. 2007), decrease side effects (Seth et al. 2004), and act as a local depot for sustained release of dermally active components (Schreier and Bouwstra 1994). For achieving these localizing drug effects in the skin, conventional liposomes typically composed of phospholipids and cholesterol are used. For a review of studies reflecting the localizing effect of liposomes, which is used in local skin treatments with corticosteroids, antimycotics, local anesthetics, and others, the reader should refer to Chap. 2 in this book.
As it has been generally agreed that conventional liposomes are of little or no value as carriers for transdermal drug delivery, because they do not deeply penetrate the skin, but rather remain confined to the upper layer of the SC, there was a need for the development of more potent novel vesicles, which could potentially be used as transdermal drug delivery systems. Thus, these novel vesicles should be used for drug delivery into the deepest skin layers of the SC (dermal effect) or the subcutaneous tissue (regional effect) or the systemic circulation (transdermal effect) dependent on the indication. In order to develop such vesicles, the lipid composition of liposomes was modified. As the key parameter affecting drug permeation across the SC and the interactions with the SC is the thermodynamic state of the liposomes’ bilayers which depend on their lipid composition (i.e., the transition temperature (Tm) of their lipids), the latter was modified. Bilayers may be either in a liquid crystalline state, which is characterized by the fluid state of bilayers, or in a gel thermodynamic state, characterized by rigid bilayers (Riaz et al. 1989). Since conventional liquid-state vesicles have proven to be superior to gel-state vesicles (Van Kuijk-Meuwissen et al. 1998a, b; El Maghraby et al. 1999, 2001a), while elastic vesicles have been shown to be superior to conventional gel-state and even liquid-state vesicles in terms of interactions with human skin (van den Bergh et al. 1999) and enhanced drug penetration (El Maghraby et al. 1999), a series of liquid-state vesicles with elastic membranes were developed.
The first elastic or deformable vesicles, termed Transfersomes® (Idea AG, Germany), were introduced in the 1990s by Cevc et al. (1995). These vesicles contained phosphatidylcholine (PC) and edge activators (sodium cholate, polysorbate 80, or polysorbate 20) to impart deformability to the carrier, being responsible for improved dermal/transdermal drug delivery (Cevc et al. 1998; 2002; 2008a, b, c; Cevc and Blume 2001, 2003, 2004). These vesicles provided a tenfold higher diclofenac amount in the subcutaneous tissue compared to a commercial diclofenac gel (Cevc and Blume 2001), as well as a higher ketoprofen accumulation in deep subcutaneous tissues with Diractin® (gel with ketoprofen-loaded Transfersomes®) compared to conventional gels (Gabrilen® gel, Togal® Mobil Gel, Fastum® gel) (Cevc et al. 2008c). Further, in vivo studies in mice and humans revealed that Transfersomes® enabled a systemic delivery of insulin and that the efficiency of the formulation was comparable to that obtained after a subcutaneous (s.c.) injection of the same preparation, but with a longer lag time (Cevc et al. 1995, 1998).
Based on the positive results obtained with Transfersomes®, a series of novel deformable vesicles was developed by modifying the composition of vesicles. These vesicles include ethosomes, vesosomes, penetration enhancer-containing vesicles (PEVs), invasomes, etc. (Touitou et al. 2000; Verma 2002; Mishra et al. 2006; Mura et al. 2009; Mura et al. 2011). The aim of this chapter is to introduce invasomes as novel nanocarriers, and for other vesicles, one should refer to other chapters in Part 2 of this book.
5.3 Invasomes
5.3.1 Development of Invasomes
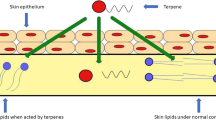
Invasomes (as termed by the inventors) were introduced by the group of Professor Alfred Fahr (Verma 2002). They were composed of unsaturated soybean lecithin (with high % PC), small amount of ethanol, and small amount of a mixture of terpenes (cineole, citral, and d-limonene). Unsaturated phospholipids were chosen as they, due to their low Tm, lead to the formation of liposomes being in liquid crystalline thermodynamic state. The purpose of using terpenes was to impart deformability to the carrier. It was supposed that terpenes, which are used as penetration enhancers, as they increase the fluidity of SC lipid bilayers (Cornwell et al. 1994), would also increase the fluidity of vesicles’ bilayers. Namely, terpenes have been shown to be potent enhancers for a variety of drugs, such as nicardipine (Krishnaiah et al. 2003), lorazepam, clonazepam (Puglia et al. 2001), haloperidol (Vaddi et al. 2002), nicardipine, carbamazepine, tamoxifen (El-Kattan et al. 2001), etc. Investigations employing differential scanning calorimetry (DSC) and x-ray diffraction revealed that terpenes increase drug permeation by disrupting lipid packaging of the SC and/or disturbing the stacking of the bilayers (Cornwell et al. 1994). Moreover, the enhanced skin penetration of various drugs is supposed to be a result of increased drug solubility in the SC treated by terpenes. The lipophilic drugs show increased penetration due to their increased partition coefficient SC/vehicle, and their penetration increases proportionally to their solubility in the enhancer. Regarding hydrophilic drugs, their penetration is assumed to be improved due to their increased diffusion coefficient (Williams and Barry 1991a, b; Cornwell et al. 1996; Moghimi et al. 1997). Ethanol was also added to liposomes, besides the assumption that ethanol is detrimental to liposomes and is therefore removed from ethanolic solutions of phospholipids during the preparation of liposomes. Ethanol was added as it was believed that it would fluidize the vesicles’ bilayers in the same manner as it fluidizes the SC lipid bilayers. This assumption was already confirmed by Touitou et al. (2000) who developed ethosomes – vesicles containing besides phospholipids and water also high amounts of ethanol (>30 %) – which have proven to be able to deliver drugs to the deep skin layers and/or the systemic circulation (Godin and Touitou 2003; Ainbinder and Touitou 2005; Dubey et al. 2010). Hence, it has been shown that ethanol is a potent penetration enhancer not only in combination with other chemical or physical penetration enhancers (Srinivasan et al. 1990; Kobayashi et al. 1994; Bhatia and Singh 1999), but also in combination with liposomes due to their synergistic effect (Touitou et al. 2000; Verma and Fahr 2004).
In conclusion, the inventors of invasomes assumed that these potent penetration enhancers, ethanol and terpenes, would act synergistically on the fluidity and deformability of the vesicles’ bilayers, as well as on disturbing the SC lipid bilayers. Further, these enhancers could act synergistically with liposomes in enhancing the drug penetration into the skin.
5.3.2 Penetration-Enhancing Ability of Invasomes
Verma (2002) performed the first studies with invasomes using cyclosporine A (CsA, CyA) as a drug. The aim was to enhance the penetration of CsA into the skin, being a challenge as CsA does not possess favorable physicochemical properties to penetrate the skin. CsA is a lipophilic drug with MW of 1202.61 Da and a partition coefficient of 4000 and, hence, shows a poor penetration into the skin. The topical use of CsA would be advantageous in treating psoriasis and dermatological diseases that are thought to have an inflammatory T-cell-mediated pathogenesis. Further, due to its stimulating effect on hair growth, CsA has been considered for the treatment of alopecia areata, as well as for the treatment of androgenetic alopecia.
Verma prepared CsA-loaded invasomes containing 10 % w/v unsaturated soybean PC; 3.3 % w/v ethanol; 0.5, 1.0, and 1.5 % w/v of a terpene mixture composed of citral, cineole and d-limonene (cineole:citral:d-limonene = 45:45:10 v/v = standard terpene mixture); and phosphate buffer saline (PBS) up to 100 % w/v. In vitro studies in human skin revealed (Fig. 5.1) that invasomes provided a significantly higher amount of CsA in the deeper layers of human skin (viable epidermis and dermis) compared to conventional liquid-state liposomes (without ethanol and terpenes) and the aqueous/ethanolic drug solution (Verma 2002). In addition, increasing the amount of terpenes from 0.5 to 1.5 % increased the amount of CsA recovered in the SC and deeper skin layers, indicating a direct correlation between the amount of added terpenes and the amount of drug found in the skin. However, there was no statistically significant difference between invasomes containing 1 or 1.5 % standard terpene mixture. This study proved the penetration-enhancing ability of invasomes and revealed that invasomes present an effective carrier system for delivering the highly lipophilic CsA to the deeper skin layers where it should exert its therapeutic effect.
Skin depth profile of CsA after 6 h nonocclusive application of different formulations onto human abdominal skin (expressed as cumulative % of dose applied/cm2 ± SE, n = 3–6). PE penetration enhancer, i.e., standard terpene mixture (Verma 2002)
Invasomes have also been investigated for their influence on the skin delivery of temoporfin (mTHPC), which is an interesting candidate for topical photodynamic therapy (PDT) of cutaneous malignant and nonmalignant diseases. Unfortunately, mTHPC (Dragicevic-Curic et al., 2008a) like CsA possesses unfavorable properties to easily penetrate the skin. It has a MW of 680 Da and is highly hydrophobic (octanol/water partition coefficient of 9.4) (Kelbauskas 2003). Consequently, mTHPC exhibits low percutaneous absorption and there are no topical formulations with mTHPC at the market. It has been to date applied only intravenously in PDT of skin cancers (Kübler et al. 1999). Hence, it was very important to develop a formulation enabling the skin delivery of mTHPC, i.e., enhancing its penetration into the skin and depositing sufficiently high amounts of mTHPC in the deeper skin layers, which would ensure a positive outcome in PDT of different skin diseases. The topical use of mTHPC would simplify PDT, since the skin is readily accessible for topical treatment, increase the drug concentration in the skin, enhance patient compliance, and restrict the residual photosensitivity only to the site of application.
As to the ability of invasomes to enhance the penetration of mTHPC, the results revealed their superiority, especially of invasomes with 1 % w/v terpene mixture compared to other formulations (Fig. 5.2) (Dragicevic-Curic et al., 2008a). Invasomes with 1 % w/v terpene mixture delivered an about 3.5-, 2.7-, 2-, and 1.7-fold higher mTHPC amount to the SC than conventional liposomes, liposomes containing 3.3 % w/v ethanol, ethanolic solution of mTHPC, and invasomes containing 0.5 % w/v terpenes, respectively. Invasomes with 1 % w/v terpene mixture delivered mTHPC also into the deeper skin layers (viable epidermis and dermis). However, as to the deeper skin layers, they were only superior to the ethanolic solution of mTHPC. It is assumed that the mTHPC amounts delivered to the SC and the deeper skin layers by all formulations, except the mTHPC ethanolic solution, may be sufficient for an effective PDT (according to unpublished data from biolitec AG, Germany). The highest total penetration-enhancing effect was ascribed to mTHPC-loaded invasomes containing the highest amount of terpenes, i.e., 1 % w/v standard terpene mixture. This was in accordance with the previous study with CsA-loaded invasomes (Verma 2002).
Skin depth profile of mTHPC after 6 h nonocclusive application of different formulations onto human abdominal skin (expressed as cumulative % of dose applied/cm2 ± SE, n = 3). Standard terpene mixture was used (Dragicevic-Curic et al. 2008a)
In order to increase further the penetration-enhancing ability of invasomes containing 1 % w/v terpene mixture regarding the skin delivery of mTHPC, the ratio between d-limonene, citral, and cineole was varied in the standard terpene mixture and also single terpenes were used as additives (Dragicevic-Curic et al. 2009). As a result, seven new mTHPC-loaded invasome dispersions were obtained, which were investigated for their penetration-enhancing ability.
Obtained results revealed that, dependent on the added terpene or terpene mixture, invasomes may enhance or retard the drug penetration into the skin compared to liposomes without terpenes. The addition of 1 % w/v citral, 1 % w/v cineole, or 1 % w/v standard terpene mixture to liposomes (containing also 3.3 % w/v ethanol) resulted in the formation of highly effective skin delivery systems for mTHPC. Among these three formulations, invasomes with 1 % w/v citral provided the highest overall mTHPC amount in the skin, while invasomes with 1 % w/v cineole provided a smaller total mTHPC amount in the skin than invasomes with 1 % w/v citral, but delivered, besides the high mTHPC amount in the SC, also a high mTHPC amount in the deeper skin layers, representing therefore the optimal formulation. Invasomes with 1 % w/v standard terpene mixture provided high mTHPC amounts in the SC and the deeper skin layers, but lower than invasomes with 1 % w/v cineole. Thus, these three mTHPC-loaded invasome systems might be promising for the topical PDT of cutaneous disorders.
An interesting finding in this study was that invasomes containing high amounts of d-limonene in the terpene mixture or only d-limonene exhibited low enhancement ratios, which was unexpected and difficult to explain. Limonene as a lipophilic terpene enhanced the skin delivery of the following lipophilic drugs: estradiol (Williams and Barry 1991b), indomethacin (Ogiso et al. 1995), hydrocortisone (El-Kattan et al. 2001), midazolam (Ota et al. 2003), and others. The structure–activity relationship of terpenes was also confirmed by Hori et al. (1991) and Moghimi et al. (1997), who found that hydrophilic terpenes (alcohols, ketones, and oxide terpenes, like fenchone and thymol) are more effective in enhancing the permeation of hydrophilic drugs (propranolol), while hydrocarbon terpenes (like limonene and cymene) are more effective in improving the permeation of lipophilic drugs (diazepam). The high lipophilicity of terpenes was thought to be an important property for enhancing the permeation of lipophilic drugs (Ghafourian et al. 2004). However, the addition of d-limonene failed to induce a high transport of mTHPC across the skin. This could be explained by the fact that mTHPC cannot be considered as having a high affinity to the invasomal phospholipid membrane or the skin lipids (membrane-philic or highly lipophilic), but rather as a highly hydrophobic drug.
In contrast, the highest enhancement ratio of invasomes with 1 % w/v citral was not surprising, since citral is lipophilic and should therefore enhance the skin delivery of lipophilic substances, according to Williams and Barry (1991b), Hori et al. (1991), and Moghimi et al. (1997).
As to invasomes with 1 % w/v cineole, the obtained results showing such high mTHPC penetration into the SC and deeper skin layers were unexpected, since as aforementioned, some studies showed that hydrophilic terpenes are less effective in enhancing the permeation of lipophilic drugs and were even ineffective in enhancing the permeation of the lipophilic drug indomethacin (Nagai et al. 1989; Okabe et al. 1989). However, El-Kattan et al. (2001) showed that cineole provided among 12 different terpenes the highest amount of the lipophilic hydrocortisone in the skin, which would agree with the results obtained in this study. In addition, El-Kattan et al. (2001) reported that there was no correlation between the lipophilicity of terpenes and the amount of hydrocortisone in the skin. The study with different terpene mixtures showed that besides the standard terpene mixture, also other terpene mixtures or single terpenes can be used to formulate invasomes possessing high penetration-enhancing ability.
Invasomes were shown to be able to enhance the penetration also of hydrophilic substances into the skin. Namely, invasomes and core-multishell (CMS) nanotransporters were compared regarding their ability to enhance the skin delivery of the spin label 2,2,5,5-tetramethyl-1-pyrrolidinyl-oxy-3-carboxylic acid (PCA) (Haag et al. 2011). The study has shown that CMS nanotransporters provided higher amounts of the agent in the upper layers of the SC, whereas invasomes delivered the agent into the deeper SC layers. Moreover, compared to the solution of PCA, CMS nanotransporters delivered a 2.5-fold, while invasomes delivered 1.9-fold higher PCA amount to the skin.
Moreover, Chen et al. (2011) reported that invasomes and ethosomes, regardless if they were applied in finite or infinite doses, compared to non-vesicular systems, can significantly improve the delivery of the hydrophilic dye carboxyfluorescein (CF) into the deep skin layers or across the skin. In contrast, the authors showed that in the case of mTHPC applied in finite or infinite dose, most of the drug was accumulated in the superficial skin layers, for both vesicular systems and non-vesicular systems.
As to hydrophilic drugs, it was shown among different vesicles that conventional liposomes enhanced calcein flux 1.2 times, Transfersomes® about 1.8 times, and invasomes 7.2 times compared to the calcein aqueous solution (Ntimenou et al. 2012). Permeation (drug flux) and elasticity values for vesicles were correlated by rank order, but not linearly, indicating that elasticity can be used only as a crude predictive tool to evaluate the potential of vesicles to enhance the penetration of the hydrophilic drug through the skin. Hence, other vesicle-related properties besides elasticity may also influence the penetration-enhancing ability of vesicles. Since the cumulative calcein amount (calcein permeated up to 10 h) provided by vesicles was not significantly different among the different elastic vesicles investigated, the authors assumed that it was possible for penetration enhancers to diffuse out from invasomes and permeate through the skin lipids to enhance drug transport. Thus, besides vesicle elasticity, also other factors are most probably influencing the transport of hydrophilic drug molecules through the skin (Ntimenou et al. 2012). Drug encapsulation efficiency was not found to be an important factor influencing drug penetration into the skin.
Further, in vitro skin permeation and skin deposition studies demonstrated that the permeation profile of the amphiphilic drug ferulic acid through human stratum corneum epidermis membrane and the drug accumulation in the skin were both improved significantly using different liposomal systems, i.e., invasomes, polysorbate 80-based liposomes, conventional liposomes, and ethosomes compared to PBS solution of ferulic acid (Chen et al. 2010). Among all vesicles, ethosomes (with higher drug content) provided the highest skin flux and deposition of ferulic acid in the skin being 75 times and 7.3 times higher than those obtained with saturated PBS (pH 7.4) solution, respectively.
5.3.3 Penetration-Enhancing Ability of Invasomes Combined with Physical Penetration-Enhancing Methods
Invasomes were shown to be more effective in delivering hydrophilic compounds CF and radiolabeled mannitol into and through human skin in vitro compared to the aqueous drug solutions. For this purpose, invasomes containing 1 % standard terpene mixture were used. These invasomes were also applied in combination with skin perforation using a Dermaroller® which further enhanced drug penetration and permeation. Dermarollers with three different microneedle lengths (150, 500, and 1500 μm) were used and the one with a needle length of 500 μm appeared most promising for drug delivery into deeper skin layers or through the skin (Badran et al. 2009) (Fig. 5.3).
Fluorescence microscopic images of the cross section of human abdominal skin after 6 h of incubation with different formulations of the fluorescent dye CF ((a) invasomes, (b) non-extruded multilamellar invasomes (MLV), and (c) buffer solution) and pretreatment with the Dermaroller® of different microneedle length. Scale bars represent 100 μm (Badran et al. 2009)
Trauer et al. (2014) investigated ex vivo using Franz diffusion cells the influence of massage and of occlusion, on the follicular penetration depth of invasomes and conventional rigid liposomes being loaded with a hydrophilic and a lipophilic dye. Massage, as a physical penetration-enhancing method, increased significantly follicular penetration of both conventional liposomes and invasomes while occlusion increased follicular penetration depth only of rigid liposomes. The finding that invasomes did not penetrate more effectively if occlusion was applied was expected as for deformable liposomes, transepidermal water gradient is needed for an efficient skin penetration (Cevc et al. 1998). The results confirmed that massage is a potent tool for increasing follicular penetration of vesicles.
5.3.4 In vivo and In Vitro Therapeutic Effectiveness of Invasomes
In order to prove the therapeutic effectiveness of CsA-loaded invasomes, Verma et al. (2004) performed a study using 0.5 % CsA invasomes containing 2 % w/v standard terpene mixture in the treatment of alopecia areata in the Dundee Experimental Bald Rat (DEBR) model. CsA-loaded invasomes were compared to conventional liquid-state CsA-loaded liposomes and to the CsA ethanolic solution. Hence, 15 rats were divided into three groups. All rats were treated with CsA twice a day for 6 weeks within a bald flank, while the contralateral flank received an equivalent control formulation. The obtained results suggested that CsA liposomes with (i.e., invasomes) and without terpenes have promising potential as a topical treatment for alopecia areata, showing hair regrowth, reduced inflammatory infiltrate, and improved hair follicle morphology on treated sites. Invasomes induced a faster visible hair regrowth on the drug-treated site than conventional liposomes (see Fig. 5.4 for the effect of invasomes). In contrast, the ethanolic solution of CsA showed neither visible signs of hair growth nor reduction of hair follicle inflammation. The results indicated that CsA in liposomes provided a localized hair growth-promoting effect, while the synergistic effect of ethanol, terpenes, and phospholipids might be an explanation for the enhanced delivery of CsA by invasomes and any accumulation of the vesicles in the hair follicle (Verma et al. 2004).
Hair growth before and after treatment of DEBR with CsA invasomes. At the start of therapy, complete loss of hair on the flanks can be seen within and beyond the marked area, as well as hair loss on head and shoulders (a, b). After 6 weeks of topical application of CsA invasomes, the treated area is fully rehaired with some hair fibers longer than would be expected with a normal rat pelage (c). Control-treated skin did not show any growth of hair (d) as compared to the drug-treated skin (c, e) (Verma and Fahr 2004)
Two mTHPC invasome dispersions, invasomes with 1 % w/v citral and invasomes with 1 % w/v terpene mixture, and the ethanolic mTHPC solution were used for PDT of mice bearing the subcutaneously (s.c.) implanted human colorectal carcinoma HT29 (Dragicevic-Curic et al. 2008 b). This pilot PDT study was performed with the aim to test whether the chosen mTHPC-loaded invasomes can reduce tumor size by PDT or at least slow down tumor growth compared to the control group (mice without any treatment). mTHPC invasomes containing 1 % w/v terpene mixture, showing the best results, were not able to reduce the tumor size. However, their application induced a slower tumor growth compared to the control despite the high invasivity, intermediate sensitivity to PDT, and subcutaneous localization of the HT29 carcinoma, which limited the success of PDT. Thus, these results, despite not showing a tumor decrease, are promising and indicate that mTHPC invasomes might be a good modality for the PDT treatment of skin disorders which are more sensitive to PDT, less invasive, and more accessible, like psoriasis or different superficial skin tumors (Bowen’s disease, basal cell carcinoma).
These mTHPC-loaded invasomes and the ethanolic solution, which were used in the PDT of mice bearing the s.c. implanted tumor HT29 (Dragicevic-Curic et al. 2008 b), were further investigated for their photodynamic efficacy in vitro in two tumor cell lines, i.e., in the human epidermoid carcinoma cell line A431 and the human colorectal carcinoma cell line HT29 (Dragicevic-Curic et al. 2010). The results revealed that invasomes and the ethanolic solution used at a 2 μM mTHPC concentration and photoirradiation at 20 J/cm2 were able to reduce survival of HT29 cells and especially of A431 cells, being more sensitive to PDT. In contrast to HT29 cells, where there was not a significant difference between cytotoxicity of the mTHPC ethanolic solution and mTHPC invasomes, in A431 cells, mTHPC invasomes were more cytotoxic (Fig. 5.5). Survival of about 16 % of A431 cells treated with invasomes is very promising, since it demonstrates invasomes’ potential to be used in topical PDT of cutaneous malignant diseases.
Dark and photocytotoxicity of three mTHPC formulations used at different mTHPC concentrations against A431 and HT29 tumor cells after 24 h of incubation. For the photocytotoxicity study, tumor cells were photoirradiated with a light dose of 20 J/cm2. (a) A431 cells and (b) HT29 cells. PE penetration enhancer = standard terpene mixture (Dragicevic-Curic et al. 2010)
5.3.5 Fluidity of Invasomes
Among all properties, the fluidity, i.e., thermodynamic state of liposomes’ bilayers, influences at most their penetration-enhancing ability. As liquid-state (fluid) vesicles have been found to be superior over gel-state (rigid) vesicles in terms of increasing the drug penetration into the skin (van Kuijk-Meuwissen et al. 1998a, b; El Maghraby et al. 1999, 2001a, b), it was assumed that invasomes, showing high penetration-enhancing ability (Dragicevic-Curic et al. 2008a, 2009), possess high membrane fluidity.
The results obtained by electron spin resonance (ESR) measurements revealed that the addition of 1 % w/v of a single terpene or terpene mixture to liposomes containing 3.3 % w/v ethanol in order to obtain invasomes increased significantly the phospholipid fluidity near to the C16 atom of their acyl chains, compared to liposomes without terpenes (conventional liposomes and liposomes containing 3.3 % w/v ethanol) (Dragicevic-Curic et al. 2011). Thus, ESR showed that invasomes indeed represent vesicles of higher membrane fluidity than conventional liposomes. However, the membrane fluidity did not differ markedly among different invasomes. It was not possible to differentiate between the influences of each single terpene/terpene mixture on the invasome fluidity, and hence, there was also no direct correlation between the invasomes fluidity and their penetration-enhancing ability (Dragicevic-Curic et al. 2008a, 2009). However, conventional liposomes (being in fluid thermodynamic state, but significantly lower than invasomes) provided the second lowest mTHPC amount in the skin compared to different invasomes (after invasomes with the terpene mixture containing the highest amount of d-limonene). According to ESR data, the addition of all terpenes/terpene mixtures increased the vesicle fluidity, while not all terpenes/terpene mixtures increased the penetration-enhancing ability of invasomes to improve the skin delivery of mTHPC compared to conventional liposomes or liposomes containing 3.3 % ethanol (Dragicevic-Curic et al. 2009). The ESR results are in agreement with differential scanning calorimetry (DSC), which also showed that the addition of 1 % w/v terpenes/terpene mixtures increased the molecular motional freedom of phospholipid acyl chains in invasome bilayers (Dragicevic-Curic et al. 2011). The ESR and DSC results are also in agreement with the cryoelectron microscopy investigation (Dragicevic-Curic et al. 2008a), which showed that the addition of terpenes had an influence on the shape of vesicles, i.e., besides spherical vesicles, deformed vesicles of different shapes were also present in invasome dispersions and an increase of the terpenes’ amount resulted in their increased number (Fig. 5.6). It was assumed that the addition of terpenes, especially 1 % w/v terpenes, to already liquid-state (fluid) liposomes with 3.3 % w/v ethanol increased further their membrane fluidity. The obtained invasomes were, thus, of very high membrane fluidity compared to liposomes without terpenes, which was confirmed by cryoelectron microscopy, ESR, and DSC measurements.
Cryoelectron microscopy of mTHPC invasomes containing 1 % w/v terpenes (the angle of the goniometer was changed from −25° to +30°). Arrows in the micrographs (a–f) depict same deformed vesicles but viewed from different angles: short arrows represent a deformed vesicle, which shape changed its appearance from a cuplike shape (c) to a shape similar to bilamellar liposomes (f); long arrows represent a deformed vesicle which first looked like a bilamellar vesicle (b), but afterward, it was shown that the vesicle has an invagination (f). (Dragicevic-Curic et al. 2008a)
Since penetration studies, ESR and DSC (Dragicevic-Curic et al., 2008a, 2009, 2011) did not show a direct correlation between fluidity and penetration-enhancing ability of invasomes, it was proposed that besides fluidity, other phenomena might also be involved in the mechanism of the skin penetration enhancement induced by invasomes. Hofland et al. (1994) proposed, for example, two mechanisms for nonionic vesicles to play an important role in the vesicle–skin interactions, leading to an enhanced drug penetration: the penetration-enhancing effect of the surfactant molecules and the effect of the vesicular structure. Therefore, in the case of invasomes, besides the effect of the fluid vesicular structure, also the penetration-enhancing effect of invasome constituents can be assumed. The constituents of invasomes, i.e., phospholipids, ethanol, and terpenes, represent potent penetration enhancers (El-Kattan et al. 2001; Yokomizo and Sagitani 1996a, b; Mutalik et al. 2009), which if released from fragmented invasomes could synergistically fluidize the intercellular lipid layers in the SC and thereby increase the skin penetration of drugs and possibly of small invasomes. However, this is only an assumption. The mechanism by which invasomes induce drug penetration enhancement should be investigated in further experiments.
5.3.6 Mode of Action of Invasomes
Analyzing the results from the aforementioned studies (Verma 2002; Verma and Fahr 2004; Dragicevic-Curic et al. 2008a, 2009, 2011), it is assumed that the reason for the superior behavior of certain invasomes compared to conventional liposomes, liposomes containing small amounts of ethanol and the ethanolic solution, is the presence of terpenes and ethanol in liposomes, i.e., the synergistic effect of ethanol, terpenes, and liposomes. Liposomes have been used for years to enhance the skin delivery of various drugs (El Maghraby and Williams 2009). Further, all constituents of invasomes, i.e., terpenes (Kunta et al. 1997; El-Kattan et al. 2001), as well as ethanol (Berner et al. 1989; Mutalik et al. 2009; Jaimes-Lizcano et al. 2010) and phospholipids (Yokomizo and Sagitani 1996a, b), have also been shown to be very potent penetration enhancers, as aforementioned. As to phospholipids, invasomes were made of PC, which, due to its head group, having a strong enhancing effect, presents a strong penetration promoter (Yokomizo and Sagitani 1996a). In addition, the acyl chains of the PC were unsaturated, which further increased the phospholipids’ penetration-enhancing ability (Yokomizo and Sagitani 1996b). Moreover, synergistic effects of ethanol and terpenes (Puglia et al. 2001; Vaddi et al. 2002; Ota et al. 2003), ethanol and liposomes (Kirjavainen et al. 1999b; Touitou et al. 2000; Verma and Fahr 2004), and ethanol, terpenes, and liposomes (Verma 2002) in enhancing the drug permeation have been reported in literature.
On the basis of gained results in these studies (Verma 2002; Verma and Fahr 2004; Dragicevic-Curic et al. 2008a, 2009, 2011) and studies performed by other authors (Cevc and Blume 1992; Cevc et al. 2002; Honeywell-Nguyen et al. 2002; Verma 2002), the following mechanism of the penetration-enhancing ability of invasomes was proposed. As invasomes were in all studies applied in finite doses under nonocclusion, a number of concomitant processes could take place. Small amounts of ethanol from the invasome dispersion (being outside the vesicles) could be able to fluidize the intercellular SC lipids (Berner and Liu 1995; Barry 2001). Further, a high part of invasomes is probably fragmented in their attempt to penetrate into the upper SC layers, which leads to the release of terpenes, ethanol, and unsaturated phospholipids. This would be in agreement with findings of most authors (Hofland et al. 1994; Zellmer et al. 1995; Kirjavainen et al. 1996, 1999b), who propose that vesicles disintegrate at the skin surface and that vesicle components (i.e., phospholipids) penetrate molecularly dispersed into the intercellular lipid matrix, where they mix with the intercellular lipids of the SC thereby modifying the lipid layers and leading to an enhanced drug penetration. Thus, the released ethanol, terpenes, and unsaturated phospholipids would be free to exert their penetration-enhancing effect. It is proposed that these penetration enhancers would synergistically act on fluidizing the intercellular SC lipids, since all of them act via this mechanism (Berner and Liu 1995; Kirjavainen et al. 1999a; Thakur et al. 2006). This could lead to the formation of microcavities and to an increase of the free volume for drug diffusion (Barry 2001), which could further increase the diffusion coefficient of the drugs released from vesicles. In addition, also the partitioning of the drug into the intercellular lipid bilayers of the SC would be increased by phospholipids (Kirjavainen et al. 1999a), ethanol (Megrab et al. 1995), and terpenes (Williams and Barry 1991b). However, since the constituents of invasomes, being potent penetration enhancers, act also via other mechanisms (see in the book series Percutaneous Penetration Enhancers, the volume Chemical Methods in Penetration Enhancement, Modification of the Stratum Corneum), these mechanisms could also have an influence on the enhanced penetration of the drug. Regarding the penetration of vesicles, a lot of phenomena are included which synergistically might facilitate the penetration of some small intact invasomes into the SC, such as: (1) disturbed organization of the SC lipids, (2) high fluidity of invasomes due to the effect of terpenes and ethanol (confirmed by Dragicevic-Curic et al. 2011), (3) probably high deformability of invasomes (assumed due to the correlation between vesicles’ high fluidity and deformability) (Godin and Touitou 2003), (4) small particle size of vesicles, and (5) presence of the transepidermal osmotic gradient, which is an important driving force for the diffusion of intact deformable vesicles of high hydrophilicity as they tend to follow the hydration gradient across the skin (Cevc and Blume 1992). The release of the drug in the skin layers could be a result of fusion of penetrated vesicles with the intercellular lipids of the SC (Touitou et al. 2000). Verma (2002) proposed the penetration of small invasomes through SC bilayers, disturbed due to the effect of ethanol and terpenes on the SC. Further, according to Verma (2002), the pilosebaceous units appeared to be a major route of invasomes’ penetration into the skin. The penetration of intact deformable vesicles through the skin was proposed by Cevc et al. (2002, 2003). Honeywell-Nguyen et al. (2002) proposed also the penetration of intact elastic vesicles through channel like regions into the deeper layers of the SC. Touitou et al. (2000) assumed that ethosomes could penetrate into the SC bilayers, being disturbed due to the effect of ethanol. However, the penetration of intact vesicles is rejected by most authors (Lasch et al. 1991; Hofland et al. 1994; Zellmer et al. 1995; Kirjavainen et al. 1996, 1999a).
5.4 Conclusion
Invasomes have proven to be an efficient skin delivery system for lipophilic as well as even to a higher degree for hydrophilic drugs. Different single terpenes or terpene mixtures can be used to formulate invasomes. Dependent on the added terpene or terpene mixture, invasomes may enhance or retard the drug penetration into the skin compared to liposomes without terpenes. Thus, besides described terpene mixtures, other mixtures should be investigated, i.e., the composition of invasomes could be further improved, by exploring the possibility of using other terpenes and terpene mixtures as constituents of invasomes. This could lead to the development of novel invasomes being more efficient skin delivery systems than existing invasomes.
References
Ahad A, Aqil M, Kohli K, Chaudhary H, Sultana Y, Mujeeb M, Talegaonkar S (2009) Chemical penetration enhancers: a patent review. Expert Opin Ther Patents 19:969–988
Ainbinder D, Touitou E (2005) Testosterone ethosomes for enhanced transdermal delivery. Drug Deliv 12:297–303
Badran MM, Kuntsche J, Fahr A (2009) Skin penetration enhancement by a microneedle device (Dermaroller®) in vitro: dependency on needle size and applied formulation. Eur J Pharm Sci 36:511–523
Barry B (2001) Novel mechanisms and devices to enable successful transdermal drug delivery. Eur J Pharm Sci 14:101–114
Barry BW (2004) Breaching the skin’s barrier to drugs. Nat Biotechnol 22(2):165–167
Benson HA (2005) Transfersomes for transdermal drug delivery. Expert Opin Drug Deliv 3:727–737
Berner B, Liu P (1995) Alcohols as percutaneous penetration enhancers. In: Smith EW, Maibach HI (eds) Percutaneous penetration enhancers. CRC Press, NewYork
Berner B, Mazzenga GC, Otte JH, Steffens RJ, Juang R-H, Ebert CD (1989) Ethanol: water mutually enhanced transdermal therapeutic system: II. Skin permeation of ethanol and nitroglycerin. J Pharm Sci 78:402–407
Betz G, Aeppli A, Menshutina N, Leuenberger H (2005) In vivo comparison of various liposome formulations for cosmetic application. Int J Pharm 296:44–54
Bhatia KS, Singh J (1999) Effect of linolenic acid/ethanol or limonene/ethanol and iontophoresis on the in vitro percutaneous absorption of LHRH and ultrastructure of human epidermis. Int J Pharm 180:235–250
Cancel LM, Tarbell JM, Ben-Jebria A (2004) Fluorescein permeability and electrical resistance of human skin during low frequency ultrasound application. J Pharm Pharmacol 56:1109–1118
Cevc G (2003) Transdermal drug delivery of insulin with ultradeformable carriers. Clin Pharmacokinet 42:461–474
Cevc G, Blume G (1992) Lipid vesicles penetrate into intact skin owing to the transdermal osmotic gradients and hydration force. Biochim Biophys Acta 1104:226–232
Cevc G, Blume G (2001) New, highly efficient formulation of diclofenac for the topical, transdermal administration in ultradeformable drug carriers. Transfersomes Biochim Biophys Acta 1514:191–205
Cevc G, Blume G (2003) Biological activity and characteristics of triamcinolone-acetonide formulated with the self-regulating drug carriers. Transfersomes Biochim Biophys Acta 1614:156–164
Cevc G, Blume G (2004) Hydrocortisone and dexamethasone in very deformable drug carriers have increased biological potency, prolonged effect, and reduced therapeutic dosage. Biochim Biophys Acta 1663:61–73
Cevc G, Schätzlein A, Blume G (1995) Transdermal drug carriers: basic properties, optimization and transfer efficiency in the case of epicutaneously applied peptides. J Control Rel 36:3–16
Cevc G, Gebauer D, Stieber J, Schätzlein A, Blume G (1998) Ultraflexible vesicles, Transfersomes, have an extremely low pore penetration resistance and transport therapeutic amounts of insulin across the intact mammalian skin. Biochim Biophys Acta 1368:201–215
Cevc G, Schätzlein A, Richardsen H (2002) Ultradeformable lipid vesicles can penetrate skin and other semi-permeable membrane barriers unfragmented. Evidence from double label CLSM experiments and direct size measurement. Biochim Biophys Acta 1564:21–30
Cevc G, Mazgareanu S, Rother M (2008a) Preclinical characterisation of NSAIDs in ultradeformable carriers or conventional topical gels. Int J Pharm 360:29–39
Cevc G, Mazgareanu U, Rother M, Vierl U (2008b) Occlusion effect on transcutaneous NSAID delivery from conventional and carrier-based formulations. Int J Pharm 359:190–197
Cevc G, Vierl U, Mazgareanu S (2008c) Functional characterisation of novel analgesic product based on self-regulating drug carriers. Int J Pharm 360:18–28
Chen M, Liu X, Fahr A (2010) Skin delivery of ferulic acid from different vesicular systems. J Biomed Nanotechnol 6(5):577–585
Chen M, Liu X, Fahr A (2011) Skin penetration and deposition of carboxyfluorescein and temoporfin from different lipid vesicular systems: In vitro study with finite and infinite dosage application. Int J Pharm 408(1-2):223–234
Cornwell PA, Barry BW, Stoddart CP, Bouwstra JA (1994) Wide-angle X-ray diffraction of human stratum corneum: effects of hydration and terpene enhancer treatment. J Pharm Pharmacol 46:938–950
Cornwell PA, Barry BW, Bouwstra JA, Gooris GS (1996) Modes of action of terpene penetration enhancers in human skin; differential scanning calorimetry, small-angle X-ray diffraction and enhancer uptake studies. Int J Pharm 127:9–26
Dragicevic-Curic N, Scheglmann D, Albrecht V, Fahr A (2008a) Temoporfin-loaded invasomes: development, characterization and in vitro skin penetration studies. J Control Release 127(1):59–69
Dragicevic-Curic N, Gräfe S, Albrecht V, Fahr A (2008b) Topical application of temoporfin-loaded invasomes for photodynamic therapy of subcutaneously implanted tumours in mice: a pilot study. J Photochem Photobiol B 91(1):41–50
Dragicevic-Curic N, Scheglmann D, Albrecht V, Fahr A (2009) Development of different temoporfin-loaded invasomes-novel nanocarriers of temoporfin: characterization, stability and in vitro skin penetration studies. Colloids Surf B Biointerfaces 70(2):198–206
Dragicevic-Curic N, Gräfe S, Gitter B, Fahr A (2010) Efficacy of temoporfin-loaded invasomes in the photodynamic therapy in human epidermoid and colorectal tumour cell lines. J Photochem Photobiol B 101(3):238–250
Dragicevic-Curic N, Friedrich M, Petersen S, Scheglmann D, Douroumis D, Plass W, Fahr A (2011) Assessment of fluidity of different invasomes by electron spin resonance and differential scanning calorimetry. Int. J, Pharm
Dubey V, Mishra D, Nahar M, Jain V, Jain NK (2010) Enhanced transdermal delivery of an anti-HIV agent via ethanolic liposomes. Nanomedicine 6:590–596
El Maghraby GM, Williams AC (2009) Vesicular systems for delivering conventional small organic molecules and larger macromolecules to and through human skin. Expert Opin Drug Deliv 6:149–163
El Maghraby GM, Williams AC, Barry BW (1999) Skin delivery of oestradiol from deformable and traditional liposomes: mechanistic studies. J Pharm Pharmacol 51:1123–1134
El Maghraby GM, Williams AC, Barry BW (2001a) Skin delivery of 5-fluorouracil from ultradeformable and standard liposomes in vitro. J Pharm Pharmacol 53:1069–1077
El Maghraby GM, Williams AC, Barry BW (2001b) Skin hydration and possible shunt route penetration in controlled skin delivery of estradiol from ultradeformable and standard liposomes in vitro. J Pharm Pharmacol 53:1311–1322
El-Kattan AF, Asbill CS, Kim N, Michniak B (2001) The effects of terpene enhancers on the percutaneous permeation of drugs with different lipophilicities. Int J Pharm 215:229–240
Ghafourian T, Zandasrar P, Hamishekar H, Nokhodchi A (2004) The effect of penetration enhancers on drug delivery through skin: a QSAR study. J Control Rel 99:113–125
Godin B, Touitou E (2003) Prospects in transdermal delivery. Therapeutic Drug Carrier Systems 20:63–102
Haag SF, Fleige E, Chen M, Fahr A, Teutloff C, Bittl R, Lademann J, Schäfer-Korting M, Haag R, Meinke MC (2011) Skin penetration enhancement of core-multishell nanotransporters and invasomes measured by electron paramagnetic resonance spectroscopy. Int J Pharm 416(1):223–228
Hofland EJH, Geest R, Bodde EH, Junginger EH, Bouwstra AJ (1994) Estradiol permeation from nonionic surfactant vesicles through human stratum corneum in vitro. Pharm Res 5:78–89
Honeywell-Nguyen PL, De Graaff AM, Wouter Groenink HW, Bouwstra JA (2002) The in vivo and in vitro interactions of elastic and rigid vesicles with human skin. Biochim Biophys Acta 1573:130–140
Hori M, Satoh S, Maibach HI, Guy RH (1991) Enhancement of propranolol hydrochloride and diazepam skin absorption in vitro: effect of enhancer lipophilicity. J Pharm Sci 80:32–35
Jaimes-Lizcano YA, Lawson LB, Papadopoulos KD (2011) Oil-frozen W(1)/O/W(2) double emulsions for dermal biomacromolecular delivery containing ethanol as chemical penetration enhancer. J Pharm Sci 100:1398–1406.
Kelbauskas L (2003) Untersuchungen zur Struktur-Eigenschafts-Beziehung selbstassoziierender Photosensibilisatoren mittels zeitaufgelöster Spektroskopie. Friedrich Schiller University, Jena, Ph.D., Thesis
Kirjavainen M, Urti A, Jääskeläinen L, Suhonen TM, Paronen P, Valjakka-Koskela R, Kiesvaara J, Mönkkönen J (1996) Interaction of liposomes with human skin in vitro-the influence of lipid composition and structure. Biochim Biophys Acta 1304:179–189
Kirjavainen BM, Mönkkönen J, Saukkosaari M, Valjakka-Koskela R, Kiesvaara J, Urtti A (1999a) Phospholipids affect stratum corneum lipid bilayer fluidity and drug partitioning into the bilayers. J Control Rel 58:207–214
Kirjavainen M, Urtti A, Valjakka-Koskel R, Kiesvaara J, Mönkkönen J (1999b) Liposome-skin interactions and their effects on the skin permeation of drugs. Eur J Pharm Sci 7:279–286
Kobayashi D, Matsuzawa T, Sugibayashi Y, Morimoto Y, Kimura M (1994) Analysis of the combined effect of l-menthol and ethanol as skin penetration enhancers based on a two-layer skin model. Pharm Res 11:96–103
Krishnaiah YS, Satyanarayana V, Bhaskar P (2003) Enhanced percutaneous permeability of nicardipine hydrochloride by carvone across the rat abdominal skin. Drug Dev Ind Pharm 29:191–202
Kübler AC, Haase T, Staff C (1999) Photodynamic therapy of primary non-melanomatous skin tumors of the head and neck. Laser Surg Med 25:60–68
Kunta JR, Goskonda VR, Brtherton HO, Khan MA, Reddy IK (1997) Effect of menthol and related terpenes on the percutaneous absorption of propranolol across excised hairless mouse skin. J Pharm Sci 86:1369–1373
Lasch J, Laub R, Wohlrab W (1991) How deep do intact liposomes penetrate into human skin? J Control Release 18:55–58
Lopez RF, Bentley MV, Begona Delgado-Charro M, Guy RH (2003) Optimization of aminolevulinic acid delivery by iontophoresis. J Control Rel 88:65–70
Megrab NA, Williams AC, Barry BW (1995) Oestradiol permeation across human skin, silastic and snake skin membranes: the effects of ethanol/water co-solvent system. Int J Pharm 116:101–112
Mishra V, Mahor S, Rawat A, Dubey P, Gupta PN, Singh P, Vyas SP (2006) Development of novel fusogenic vesosomes for transcutaneous immunization. Vaccine 24:5559–5570
Moghimi HR, Williams AC, Barry BW (1997) A lamellar matrix model for stratum corneum intercellular lipids. V. Effect of terpene penetration of the matrix. Int J Pharm 146:41–54
Mura P, Maestrelli F, Gonzalez-Rodrıguez ML, Michelacci I, Ghelardini C, Rabasco AM (2007) Development, characterization and in vivo evaluation of benzocaine-loaded liposomes. Eur J Pharm Biopharm 67:86–95
Mura S, Manconi M, Sinico C, Valenti D, Fadda AM (2009) Penetration enhancer-containing vesicles (PEVs) as carriers for cutaneous delivery of minoxidil. Int J Pharm 380:72–79
Mura S, Manconi M, Valenti D, Sinico C, Vila AO, Fadda AM (2011) Transcutol containing vesicles for topical delivery of minoxidil. J Drug Target 19(3):189–196
Mutalik S, Parekh HS, Davies NM, Udupa N (2009) A combined approach of chemical enhancers and sonophoresis for the transdermal delivery of tizanidine hydrochloride. Drug Deliv 16:82–91
Nagai T, Okabe H, Ogura A, Takayama K (1989) Effect of limonene and related compounds on the percutaneous absorption of indomethacin, Proceedings of the 16th International Symposium on Controlled Release Bioactive Material. USA, Chicago, pp 181–182
Ntimenou V, Fahr A, Antimisiaris SG (2012) Elastic vesicles for transdermal drug delivery of hydrophilic drugs: a comparison of important physicochemical characteristics of different vesicle types. J Biomed Nanotechnol 8(4):613–623
Ogiso T, Iwaki M, Tsuyoshi P (1995) Effects of enhancers on transdermal penetration of indomethacin and urea, and relationship between penetration parameters and enhancement factors. J Pharm Sci 84:482–488
Okabe H, Takayama K, Ogura A, Nagai T (1989) Effect of limonene and related compounds on the percutaneous absorption of indomethacin. Drug Des Deliv 4:313–321
Ota Y, Hamada A, Nakano M, Saito H (2003) Evaluation of percutaneous absorption of midazolam by terpenes. Drug Metab Pharmacokin 18:261–266
Prausnitz MR, Langer R (2008) Transdermal drug delivery. Nat Biotechnol 26(11):1261–1268
Puglia C, Bonina F, Trapani G, Franco M, Ricci M (2001) Evaluation of in vitro percutaneous absorption of lorazepam and clonazepam from hydroalcoholic gel formulations. Int J Pharm 228:79–87
Riaz M, Weiner N, Martin F (1989) Liposomes. In: Lieberman HA, Rieger MM, Banker GS (eds) Pharmaceutical dosage forms: disperse systems, vol 2. Marcel Dekker, New York, Basel, pp 567–603
Rizwan M, Aqil M, Talegaonkar S, Azeem A, Sultana Y, Ali A (2009) Enhanced transdermal drug delivery techniques: an extensive review of patents. Recent Pat Drug Deliv Formul 3:105–124
Schreier H, Bouwstra J (1994) Liposomes and niosomes as topical drug carriers: Dermal and transdermal drug delivery. J Control Rel 30:1–15
Seth AK, Misra A, Umrigar D (2004) Topical Liposomal Gel of Idoxuridine for the treatment of Herpes Simplex: Pharmaceutical and Clinical Implications. Pharm Dev Tech 9:277–289
Song YK, Kim CK (2006) Topical delivery of low-molecular-weight heparin with surface-charged flexible liposomes. Biomaterials 27:271–280
Srinivasan V, Su MH, Higuchi WI, Behl CR (1990) Iontophoresis of polypeptides: effect of ethanol pretreatment of human skin. J Pharm Sci 79:588–591
Thakur RA, Wang Y, Michniak BB (2006) Essential oils and terpenes. In: Smith EW, Maibach HI (eds) Percutaneous penetration enhancers. CRC Press/Taylor & Francis Group/LLC, Boca Raton, pp 159–173
Touitou E, Dayan N, Bergelson L, Godin B, Eliaz M (2000) Ethosomes-novel vesicular carriers: characterization and delivery properties. J Control Rel 65:403–418
Trauer S, Richter H, Kuntsche J, Büttemeyer R, Liebsch M, Linscheid M, Fahr A, Schäfer-Korting M, Lademann J, Patzelt A (2014) Influence of massage and occlusion on the ex vivo skin penetration of rigid liposomes and invasomes. Eur J Pharm Biopharm 86(2):301–306
Vaddi HK, Wang LZ, Ho PC, Chan YW, Chan SY (2002) Terpenes in ethanol: haloperidol permeation and partition through human skin and stratum corneum changes. J Control Rel 81:121–133
Van den Bergh BAI, Vroom J, Gerritsen H, Junginger HE, Bouwstra JA (1999) Interactions of elastic and rigid vesicles with human skin in vitro: electron microscopy and two-photon excitation microscopy. Biochim Biophys Acta 1461:155–173
Van Kuijk-Meuwissen MMJ, Junginger HE, Bouwstra JA (1998a) Interactions between liposomes and human skin in vitro, confocal laser scanning microscopy study. Biochim Biophys Acta 1371:31–39
Van Kuijk-Meuwissen MEMJ, Mougin L, Junginger HE, Bouwstra JA (1998b) Application of vesicles to rat skin in vivo: a confocal laser scanning microscopy study. J Control Rel 56:189–196
Verma DD (2002) Invasomes-novel topical carriers for enhanced topical delivery: characterization and skin penetration properties, PhD thesis. Philipps-University Marburg, Marburg/Lahn
Verma DD, Fahr A (2004) Synergistic penetration enhancement effect of ethanol and phospholipids on the topical delivery of cyclosporine A. J Control Rel 97:55–66
Verma DD, Verma S, Blume G, Fahr A (2003a) Particle size of liposomes influences dermal delivery of substances into skin. Int J Pharm 258:141–151
Verma DD, Verma S, Blume G, Fahr A (2003b) Liposomes increase skin penetration of entrapped and non-entrapped hidrophilic substances into human skin: a skin penetration and confocal laser scanning microscopy study. Eur J Pharm Biopharm 55:271–277
Wang J, Yuan Y, Liu C, Zhu D, Shen X, Yang B (2009) Preparation and pharmaceutical/pharmacodynamic evaluation of topical brucine-loaded liposomal hydrogel. J Mater Sci Mater Med 20:2075–2084
Williams AC (2003) Transdermal and topical drug delivery: from theory to clinical practice. Pharmaceutical Press, London/Chicago, pp 1–49
Williams AC, Barry BW (1991a) Terpenes and the lipid-protein-partitioning theory of skin penetration enhancement. Pharm Res 8:17–24
Williams AC, Barry BW (1991b) The enhancement index concept applied to terpene penetration enhancers for human skin and model lipophilic (oestradiol) and hydrophilic (5-fluorouracil) drugs. Int J Pharm 74:157–168
Yokomizo Y, Sagitani H (1996a) Effects of phospholipids on the percutaneous penetration of indomethacin through the dorsal skin of the guinea pig, in vitro. J Control Rel 38:37–46
Yokomizo Y, Sagitani H (1996b) Effects of phospholipids on the percutaneous penetration of indomethacin through the dorsal skin of the guinea pig, in vitro. 2. The effects of hydrophobic group in the phospholipids and a comparison with general enhancers. J Control Rel 42:37–46
Zellmer S, Pfeil W, Lasch J (1995) Interaction of phosphatidylcholine liposomes with the human stratum corneum. Biochim Biophys Acta 1237:176–182
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
Dragicevic, N., Verma, D.D., Fahr, A. (2016). Invasomes: Vesicles for Enhanced Skin Delivery of Drugs. In: Dragicevic, N., Maibach, H. (eds) Percutaneous Penetration Enhancers Chemical Methods in Penetration Enhancement. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-662-47862-2_5
Download citation
DOI: https://doi.org/10.1007/978-3-662-47862-2_5
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-662-47861-5
Online ISBN: 978-3-662-47862-2
eBook Packages: MedicineMedicine (R0)