Abstract
In this chapter, we focus on the use of robotic fish in animal behavior studies. Specifically, we describe the design and control of a low-cost robot along with accompanying enabling technologies for use in animal experiments. The robotic fish appearance and movement are inspired by the zebrafish animal model. The robot is capable of autonomous underwater operation. Two behavioral studies demonstrate the use of the robotic fish to test hypotheses on zebrafish social behavior. In the first study exploring zebrafish preference in a binary choice test, we find that the robot is able to elicit attraction in both individuals and small shoals when the other alternative is an empty compartment. At the same time, between conspecifics and the robot, zebrafish prefer the former, highlighting design choices that need further improvement. The second study describes the interaction between the robot and shoals of zebrafish in a free-swimming environment. The robot swims autonomously along predefined circular trajectories at three different speeds, corresponding to increasing tail-beat frequency. The robot is found to modulate zebrafish shoal cohesion, confirming expectations from the preference study result. In summary, the robotic fish platform described in this chapter provides a viable and fully controllable three-dimensional interactive tool for animal behavior experiments.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Ionic Polymer Metal Composite
- Proportional Integral Derivative
- Robot Interaction
- Robotic Platform
- Proportional Integral Derivative Control
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Introduction
Animals possess high interindividual differences in their behavioral response to the same environment, even within the same species [1]. Obtaining a consistent response in behavioral studies where live stimuli are used as independent variables is therefore a challenging task. In this respect, robots constitute a valid tool for testing hypotheses that would otherwise require extensive animal training and use [2, 3]. As controllable machines can be made to look [4], sound [5], or even smell [6] like animals, robots can be assigned a set of repeatable behaviors to elicit consistent response from subjects [7], thus contributing to our understanding of animal behavior [8, 9]. Furthermore, with frequent field deployments and greater degree of autonomy [10], robots hold the promise of assisting behavioral studies in the wild.
Fish-like robots have been used to gain insight into quorum sensing [4], leadership [11], swimming hydrodynamics [12], and the effects of psychotropic drugs on the regulation of emotions [7, 13]. Because fish in a majority of these studies use vision as their primary sensory modality, the robot’s morphology plays an important role in obtaining a consistent response. Studies in [4, 11] have shown that it is possible to regulate fish behavior with a life-sized rigid replica that is maneuvered inside a tank with a mobile magnetic base. At the same time, investigations using a bioinspired robotic fish with undulating body parts that mimic fish locomotion have established that body movement plays an important role in fish perception of their robotic analogs [12, 14, 15]. The bioinspired robotic fish used in these studies has the swimming mechanism onboard, making it a viable alternative for autonomous operation [15].
In this chapter, we describe the design of a low-cost, modular, bioinspired robotic fish platform including the accompanying enabling technologies that are used to quantify animal behavior and response. The original design of the bioinspired robotic fish is inspired by zebrafish, a model organism frequently used in neurobehavioral, developmental, and preclinical research [16–18]. Since its inception, several versions of the robotic fish platform have been used in a range of experimental studies to investigate spatial preference, boldness and shyness, anxiety-related response, hydrodynamic implications of swimming, information flow during social interactions, effect of color morphs on courtship, and collective behavior in fish [7, 12–15, 19–30]. Here, we summarize two of those studies. The first study focuses on the preference of zebrafish individuals and shoals for an anchored version of the robotic fish [20], and the second study focuses on the response of small shoals to an autonomous version of the robotic fish [15].
2 Customizable Robotic Platform for Lab Fish Studies
In this section, we describe the hardware components of the robotic platform and the enabling technologies used in behavioral studies [15, 31]. We designed the robotic platform for low-cost assembly, customization, and ease of implementation. The robot has the actuation and control mechanism onboard and can be controlled to perform specific maneuvers, similar to other prototypes [32–40]. Fish–robot interactions and fish behavior are quantified using standard methods of data collection and data assimilation that require minimal user training.
2.1 Hardware
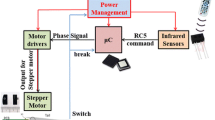
The robotic fish body is modeled in a computer aided design (CAD) software (Fig. 1). We selected the body size to (a) match the aspect ratio of a zebrafish, and (b) to contain sufficient room for housing the electronics needed for autonomous operation. The robot itself consists of two parts, a body and a movable tail. A flexible caudal fin attached to the tail provides the necessary propulsion for swimming underwater. The length, width, and height of the robot body are 15.4 cm, 4.8 cm, and 2.6 cm, respectively. This size permits inserting a servomotor in the tail section; the body section can hold a microcontroller for controlling the servomotor, a transceiver to send and receive the control signals, and a rechargeable battery that can hold charge for up to an hour of regular operation.
The total cost to manufacture a single robot including the electronics is less than 100 USD [41]. The robotic fish is fabricated in a 3D prototyping machine using ABS plastic. A single-cell rechargeable lithium polymer battery, an Arduino Pro mini microcontroller (Sparkfun Electronics, Boulder, Colorado, USA), and an nRF2401A transceiver chip (Nordic Semiconductor, Oslo, Norway) are all assembled outside the body and can be replaced if needed. A Hitec HS-55 servomotor (Hitec RCD 155 USA Inc., Poway, California, USA) in the tail section actuates the body–tail joint and propels the robot in a carangiform/subcarangiform movement. The robot is remotely controlled using an Arduino Duemilanove microcontroller (Sparkfun Electronics, Boulder, Colorado, USA) that interfaces with a computer and an nRF2401A transceiver chip. The onboard microcontroller is used to control the tail-beat frequency, amplitude, and offset, which in turn controls the robot speed and turning rate [31]. The robot can be controlled in real time to perform interactive experiments. In [14], for example, the platform is augmented to allow the robotic fish to beat its tail in response to fish position. This is achieved by tracking the fish position in real time to control the tail-beat frequency of the anchored robot.
The robotic platform is highly customizable. Besides its use in laboratory experiments, we have extensively used the robotic fish in outreach programs that encourage the involvement of K-12 students in science, technology, engineering, and mathematics (STEM) fields [42–45]. For example, students in an outreach activity have been tasked with designing the geometry of the caudal fin to maximize the swimming thrust based on observations of animal morphology at the New York Aquarium [42–44]. The modular design also permits disassembly and changing of individual parts on-the-go making the platform ideal for testing hardware performance [41]. The caudal fin can be attached and removed easily to test the effect of different shapes and sizes on swimming performance [42]. The body can be painted with nontoxic pigments to match the color pattern of a zebrafish. Finally, to make the robot accessible for users in a wide age-group, such as those who are likely to participate in a public event, we custom designed a smart phone application to replace the remote control unit [44].
2.2 Enabling Technologies
Animal behavior studies often entail the continuous observation of live animals over a considerable amount of time [46]. Quite often, this task is performed by human observers and is prone to bias and fatigue, whereby an experimenter may involuntarily score a behavior that is not present. In this respect, a data assimilation workflow that allows automatic quantification of fish behavior would overcome all these limitations. Enabling technologies for such a workflow include a video multitarget tracking system and behavioral analysis scripts that can calculate behavioral measures directly from video data [47–49]. Running in real time, these tools offer the capability to control the robot to perform specific maneuvers [15, 27] as well as to respond to fish behavior [14].
While human-assisted behavioral phenotyping was adopted in the first study discussed in this chapter, a multitarget tracking software was developed in MATLAB (R2011a, Mathworks, Natick, Massachusetts, USA) for the second study. The input to the tracking system was video from an overhead camera view. The output was two-dimensional position and velocity estimates of each fish (and the robot, if present) in the tank at each frame. The tracking algorithm included a measurement extraction procedure where, in each frame, individual fish were segmented as blobs after background subtraction. An optimal filtering algorithm called a Kalman filter was used to estimate the position and velocity of each fish. A global optimal assignment algorithm [50] was used to preserve fish identities in scenarios where the fish swam close to each other. In a recent version of the tracking system [51], fish occlusions are resolved automatically as follows: a normal distribution of fish size in pixels is created and updated at each frame until five hundred points are available. Once the distribution is constructed, each blob on the frame is checked to ensure that it is less than two standard deviations of the average size. If the size of the blob is larger, an expectation–maximization (EM) algorithm is used to split the large blob into individual blobs. In particular, the EM algorithm optimally fits multiple Gaussian distributions to the occluded blob so that individual fish shapes are approximated as two-dimensional ellipses [52]. The tracking system consists of a graphical user interface, also developed in MATLAB, which is used to manually verify and repair fish trajectories. In the event of an unresolved occlusion, missed detection, or a false detection, a user can switch, add, and delete tracks projected on the video.
Trajectory data from the tracking system is stored in the form of text files that can be further processed for behavioral analysis. For example, the following select behavioral measures (Fig. 2) can be automatically computed from the tracking system output:
Trajectory data is used to classify fish behavior into a preference for the robot (R) compared to the empty side (0) in a tripartitioned tank; b cohesion, where left group is more cohesive than the one on the right; c polarization, where the group on the left is more polarized than the one on the right; and d freezing, which is the percentage of experimental time when the fish stays within a radius of 2 cm for 2 s or more
Preference: the experimental tank is virtually divided into three parts and the time spent by the fish in each part is recorded. Preference for a given choice is then computed as the ratio of the time spent near one end of the tank and the total time spent by the subject in the two parts near the tank ends. For a shoal, preference is computed for each fish individually and averaged over the experimental time.
Cohesion: the degree of cohesion of zebrafish shoals is computed using individual fish positions in terms of the average nearest neighbor distance (ANND). Given the two-dimensional position of fish i at time k as r i [k], the ANND at k is
where N is the total number of fish and \(\left\| \cdot \right\|\) denotes the standard Euclidean norm. Another measure of cohesion is the average pairwise distance (APD), which is computed by averaging the distances between all pairs of shoal members within the focal group.
Polarization: the degree of group coordination is calculated using fish velocity in terms of polarization that quantifies the degree of alignment in fish motion. Given the two-dimensional velocity v i of fish i at time k, polarization is computed as
where \({\hat{\mathbf{v}}}_{i} \left[ k \right] = \frac{{{\mathbf{v}}_{i} [k]}}{{\left\| {{\mathbf{v}}_{i} [k]} \right\|}}\) is the direction of motion. Polarization varies between 0 and 1, with a value of 1 corresponding to all fish moving in the same direction and close to 0 if the fish move in randomly distributed directions.
Freezing: fish stress is measured directly from trajectory data in terms of the amount of time spent freezing during each trial. A fish is considered freezing if it spends two continuous seconds within a ball of radius 2 cm [14].
3 Fish Behavioral Studies
3.1 Zebrafish Animal Model
Behavioral research rests upon our understanding of model organisms that share similarities with mammals at developmental, genetic, and behavioral levels [53]. Among such organisms, zebrafish (Danio rerio) is rapidly emerging as a valid animal model [54–59] due to its elevated degree of homology with human genes, ability to rapidly absorb psychoactive compounds with minimal invasiveness, short intergeneration time, and high stocking densities [16].
Adult zebrafish are typically 3–4 cm in size and are characterized by a striped color pattern on their body (Fig. 1), hence the name “zebrafish” [60]. They exhibit strong shoaling behavior that has been associated with improved foraging efficiency and predator detection [61]. Zebrafish are especially useful in robotics-based research due to their propensity to form groups based dominantly on vision [16, 17, 62], a sensory modality that can be preferentially exploited by designing the robot morphology to match that of the fish shape and color pattern.
3.2 Preference Experiments
The classical preference test utilizes an experimental setup where a fish is observed as it swims between two different choices presented on either end of an experimental tank [63–65]. In our case, this setup serves to test the hypothesis that a robotic fish, which is inspired by zebrafish in its shape, color pattern, and motility, will attract single individuals as well as shoals of zebrafish. The robotic fish is anchored to one side of the tank and actuated externally. The size of the robotic fish allows for housing all the electronics necessary for self-propulsion, toward further implementation beyond choice tests. This permits using the results of this experiment to inform future studies. For example, protocols for free-swimming and field experiments that require the robot to be autonomous and interact with the fish without any tethering can be designed on the basis of the average distance of the fish from the robot quantified from preference studies. Similarly, the color pattern and preferential frequency in one study can be used as a reference condition in the next [15, 21].
The zebrafish used in this study were acquired from local pet stores (Petland Discounts, Brooklyn and New World Aquarium, Manhattan, New York City, USA) and acclimatized for at least two weeks in the housing facility at New York University Polytechnic School of Engineering before use in the experiments. The housing tanks were maintained at 26 ± 1 °C temperature and 7.2 pH. Lighting was controlled according to a 12 h light/day circadian rhythm [66] and the stocking density was maintained at less than 1 fish per liter at all times.
The experimental setup consisted of a 74 cm × 30 cm × 30 cm glass tank with the longest side partitioned into three distinct regions using a perforated transparent plexiglass (Fig. 3). The central region where the experimental subjects were present was 54 cm long and the choice regions on either side of the tank were 10 cm long each. The plexiglass partition permitted physical separation between the subject and the stimuli while preserving visual communication. The tank surface was lit by two 50 W fluorescent lamps on either side to ensure a uniform and diffused lighting. Fish behavior was recorded with a high definition video camera (Canon, Vixia HG20, Japan) mounted approximately 150 cm above the test tank. The test setup was isolated from external disturbances using black curtains on all four sides of the tank.
Schematic of the preference test experimental apparatus. The two choices are the robotic fish and an empty compartment (© IOP Publishing. Reproduced by permission of IOP Publishing and [19]. All rights reserved)
The experimental procedure consisted of sixteen experimental conditionsFootnote 1 that tested different combinations of choices with single individuals and small shoals of zebrafish (Fig. 4). In particular, the choices available for the subjects in the central compartment were Robot (R), one fish (1), ten fish (10), static replica that had no tail actuation (SR), and empty compartment (0). To investigate the effect of noise due to servomotor, the absence of visual cues, one-way visual feedback, and physical separation, the setup was modified so that experiments were performed without lighting (Dark), using one-way glass (Glass), and without the transparent plexiglass (Free). Ten trials were conducted for each condition, where each trial consisted of a 10 min habituation period followed by a 5 min experimental time during which the fish were observed every thirty seconds. The robotic fish, wherever present, was anchored to one side of the tank at an angle of 45° with tail beating at 2.3 Hz frequency at 3 cm amplitude (except in the case of SR when tail-beat was absent). The tail-beat frequency and amplitude were selected on the basis of engineering constraints and biological observations. Experimental trials for each condition were distributed uniformly between 10 am and 7 pm to ensure no bias existed because of the time of the day. In this first exploratory study involving large fish populations, fish were sometimes tested more than once in a randomized protocol.
Fish behavior was quantified in terms of preference for a stimulus, APD and Extended ANND (EANND). Given two choices A and B, and n A and n B the number of instances spent by a subject near the stimulus A and B, the preference for A was computed as n A/(n A + n B). Extended ANND was computed in the same manner as ANND in (1) but including the robotic fish as part of the group and only considering positions along the length of the tank. Fish preference was compared to no preference within a condition using chi-square statistical tests and fish APD (also computed along the length of the tank) and EANND were compared using one-way ANOVA [67]. Post hoc comparisons, wherever significance was found, were performed using Fisher’s protected least significant difference tests. Significance level was set to p < 0.05.
The results from statistical comparisons are summarized in Table 1 and Fig. 5. These results show that both individuals and small shoals tend to join larger shoals when given a choice between a shoal of ten conspecifics and one. Comparison between an empty compartment and robotic fish preference indicate that both individuals and small shoals of zebrafish tend to prefer the bioinspired robotic fish, and that this preference is independent of the existence of a physical barrier between them. At the same time, when given a choice between the robotic fish and live conspecifics, the subjects preferred the live fish, indicating that the robotic fish is not perceived as a conspecific. Control conditions show that the noise from the servomotor in the dark has a negative effect on fish preference whereby they spend significantly more time near the empty compartment when unable to see the robotic fish. The presence of holes or visual feedback did not have a significant effect on fish preference. Finally, robot body movement played an important role as shown by the increased preference for the robot moving its tail over a static replica.
Graphical synopsis of preference data: histograms of position data points for zebrafish position frequency for each tested pair of conditions reported in Table 1. Column a presents main experiments on single individuals, column b presents control experiments on individuals, and column c presents main experiments on shoals. In the condition labels, R is the bioinspired robot and SR is the static replica. In addition, Free refers to the free-swimming scenario, Dark to the experiments in the dark, and Glass to the use of one-way glass partitions. Note that the region available for fish to swim in the free-swimming case is larger than all other conditions (© IOP Publishing. Reproduced by permission of IOP Publishing and [20]. All rights reserved.)
3.3 Free-Swimming Experiments
The results from preference experiments demonstrate that zebrafish individuals and shoals preferred the robotic fish to an empty compartment. The robotic fish design permits autonomous operation using onboard electronics. In a second study, we allow the robotic fish to swim autonomously with the help of the online tracking system to test the hypothesis that robot fish spatial movement will modulate the behavior of small shoals of zebrafish.
Zebrafish used in this study were acquired from an online aquarium (LiveAquaria.com, Rhinelander, Wisconsin, USA) and maintained in 37.8 l tanks at a stocking density of at most 1 fish per two liters. The fish were acclimatized for at least 10 days to the new environment before starting the experiments. Fish tanks were lit under a 12 h light/day circadian rhythm [66]. Water temperature and pH in the holding and experimental tanks were maintained at 27 ± 1 °C and 7.2, respectively.
The experimental setup comprised a large square water tank of 120 cm side length and 20 cm high mounted on an aluminum frame (Fig. 6). The water level was maintained at 10 cm during the experiments. A Web camera (Life Cam, Microsoft Corp., Seattle, Washington, USA) was mounted 150 cm above the water surface to film an overhead view of the tank. The tank was lit by diffused light from four 150 W fluorescent tubes mounted 100 cm above the water surface. The multitarget tracking and control algorithm was run on a 2.5 GHz Pentium dual core desktop computer with 3 GB memory. The experimental setup was isolated using dark curtains on all sides of the tank.
Schematic of the free-swimming test experimental apparatus. The experimental apparatus consisted of a square shallow tank and an overhead camera for real-time tracking (Reproduced with permission from [15])
The control algorithm consisted of a Proportional Integral Derivative (PID) controller to maneuver the robotic fish in fixed-size circular trajectories in the presence of groups of zebrafish (Fig. 7). The control signal was sent to the robot via the transceiver every three-fifths of a second to follow a set of sixteen waypoints, w s , s = 1, …, 16, spaced equally on a 40 cm circle centered in the tank in a clockwise motion. In particular, the control input to attain the desired direction of motion \({\hat{\mathbf{v}}}_{R}^{d} [k]\) was computed using estimates of robot position r R [k] and velocity v R [k] at each k as
where w s [k] is the waypoint-to-reach at the current time-step. At frame k′, when the robot was within a threshold distance (15 cm) of the current waypoint-to-reach, the value of the current waypoint was updated. The control input was computed as a function of the error e[k] = sin(θ[k]), where \(\theta \left( k \right) = \arg ({\hat{\mathbf{v}}}_{R} \left[ k \right] - {\mathbf{v}}_{R}^{d} \left[ k \right])\) is the angle between the robot direction of motion and the desired direction of motion. The resulting PID control was
where K p , K i , and K d are the proportional, integral, and derivative control gains and ∆e[k] = e[k] − e[k − 1]. The control gains were tuned so that the robot followed the circle closely for over five minutes in the test trials. Figure 8 shows the robot trajectory in comparison to the waypoints on the tank region.
Robot trajectory with reference to waypoints (a) and the error (b) (Reproduced with permission from [15])
We tested four experimental conditionsFootnote 2 covering a range of swimming speeds (0, 2, 3, and 4 cm/s) corresponding to different tail-beat frequencies (0, 1, 2, and 3 Hz) of the robotic fish as it swam in fixed-size circles within the tank. The tail-beat frequencies corresponded to variations about the 2 Hz value used earlier in the preference tests [20]. The remote control unit was programmed to receive control parameters namely tail-beat frequency, tail-beat amplitude, and tail-section offset via a universal serial bus (USB), which were then transmitted wirelessly to the robot. In our experiments, we kept the tail-beat amplitude constant at 20°. The heading of the robot was controlled by varying the tail-section offset from a trim value of zero degrees when the robot body was in line with the tail section. Additional experiments where the fish were observed without a robot in the tank (No Robot), and where the robotic fish was held stationary in a place with its tail beating at 2 Hz (Fixed) were tested to control for the presence of robot in the tank and its body movement. Eight trials were performed for each condition with three experimentally naive fish used per trial.
Fish response to the robot was quantified in terms of average speed, ANND, and polarization. Fish interaction with the robot was quantified using average and minimum distance to the robot, and relative group speed. Freezing behavior was measured in terms of percentage of the total experimental time. All comparisons were made using one-way ANOVA statistical tests and post hoc comparisons were made using Tukey-HSD tests [67]. One-way ANOVA was used to compare the experimental conditions with the control conditions.
The results of the experiment are summarized in Fig. 9. Statistical comparisons of group behavior show that group cohesion (ANND) varied significantly with robot speed (p = 0.0126). The maximum and minimum values of ANND at 14.87 and 3.6 cm were recorded when the robot swam at 3 and 4 cm/s, respectively. Post hoc comparisons with control conditions did not show a significant difference due to the presence of the robot (No robot and 0 Hz, p = 0.099) and the presence of tail-beat movement only (Fixed and 2 Hz, p = 0.243). Group coordination as measured using polarization failed to reach statistical significance (p = 0.123). As with cohesion, the presence of the robot (No robot and 0 Hz, p = 0.172) and tail-beat movement (Fixed and 2 Hz, p = 0.740) did not have an effect on polarization. Although group speed was not affected by the robot speed (p = 0.151), the presence of a robot produced a significant effect (No robot and 0 Hz, p < 0.01). Finally, the time spent freezing was not significantly affected by robot speed (p = 0.171), robot presence (No robot and 0 Hz, p = 0.091), or due to tail-beat movement (Fixed and 2 Hz, p = 0.642).
Group cohesion measured using ANND and group relative speed varied significantly with robot speed (Reproduced with permission from [15])
Fish–robot interactions measured in terms of average (p = 0.067) and minimum (p = 0.093) distance to the robot were seemingly affected by the robot speed but failed to reach statistical significance. While the average distance to the robot stayed more than 45 cm, the 2 Hz condition saw the largest difference of 9.5 cm between average and minimum distance. Relative group speed varied significantly (p = 0.0154) with robot speed. Post hoc comparisons show that the fish tend to match their speed with the robot closely at 2 Hz (3 cm/s) and that this value of relative speed is significantly different from when the robot was stationary or swimming at 1 Hz (2 cm/s).
4 Discussion and Conclusion
The two experimental studies described here demonstrate the capability of using a bioinspired robotic fish to modulate live zebrafish behavior. Results from the first study show that both individual fish and small shoals display a robust attraction toward the robotic fish when given a choice between the robot and an empty compartment. This preference is lost when the choice is between the robot and live zebrafish, showing that the former is not perceived as a conspecific. This same response is confirmed in the second study where the ANND of fish shoals was found to be considerably smaller than their distance to the robot.
The robotic fish in both studies present competing cues that on one hand attract the zebrafish through its color pattern, body movement, and aspect ratio [21]; on the other hand it repels them with its servomotor noise [20]. At the same time, preference due to the possibility of the robot being inspected as a novel object is remote because of the 10 min habituation time [68] that provides ample opportunity for the fish to come close. Fish shoals in the free-swimming test maintain a larger distance from the robot as compared to those in the preference test. The closest analog in the preference test is perhaps the R v 0 (free) condition that allows direct physical contact, where the fish demonstrate significant preference for the robot. Since in the free-swimming test the robot is additionally covering a large experimental region, the presence of relatively large fish–robot distances suggests that the robot spatial movement is unlikely to constitute an attractive stimulus. While we cannot dismiss the possibility that the robot is perceived as a predator in the free-swimming environment, we do not observe a significantly larger freezing response in the presence of the robot. Compared to the preference test setup, a free-swimming environment with a mobile robot presents a complex interplay of cues that require further studies investigating the perception of robot motion by live zebrafish. In this respect, we have conducted experiments to study the effect of number of robots, their speed, and their configuration on zebrafish [27].
The collective behavior of the shoals is modulated by the robotic fish in both the studies. In the preference test, the APD of approximately three body lengths and an EANND of approximately one body length indicates that the preference of the shoal toward the robot is likely a result of one-to-one interaction and not individual preference, which would otherwise result in a ceiling effect. In the free-swimming scenario, though the shoals maintain a relatively larger distance from the robot, the fish exhibit maximum disparity between minimum and average distance to the robot when the robot’s tail is beating at 2 Hz. This is also the frequency at which the fish match their speed closely to the robot, and the shoal is least cohesive, suggesting that visual cues at this combination of speed and body movement are relevant in shaping fish–robot interactions. More importantly, this combination of body and spatial movement of the robotic fish, where the fish tend to explore the unconstrained free-swimming environment, shows that both types of motion differentially modulate zebrafish behavior.
In summary, an ethorobotics approach as described in this chapter presents an important direction for the design of a robotic fish—one that bears direct relevance to how robots may aid biology and in turn benefit their own design. The modular design used here makes the robotic fish easy to customize; the actuation mechanism adds a natural undulating movement that is shown to affect fish preference. The robotic platform is complemented with enabling technologies that allow controlling the robotic fish to perform specific maneuvers in a free-swimming environment, and opens the possibility to actively interact with the fish [14]. Finally, open problems include mitigating the repelling cues such as servomotor noise by using the alternative propulsion techniques such as ionic polymer metal composites and piezoelectric materials [29, 30, 69–73].
Notes
- 1.
The experimental procedure was approved by Polytechnic Institute of New York University (now New York University Polytechnic School of Engineering) Animal Welfare Oversight Committee AWOC-2011-101.
- 2.
Experiments followed protocol numbers AWOC-2012-101 and AWOC-2013-103 that were approved by the Animal Welfare Oversight Committee of the Polytechnic Institute of New York University (now New York University Polytechnic School of Engineering).
References
Boissy A (1995) Fear and fearfulness in animals. Q Rev Biol 70(2):165–191
Krause J, Winfield AFT, Deneubourg J (2011) Interactive robots in experimental biology. Trends Ecol Evol 26(7):369–375
Rossi C, Coral W, Barrientos A (2012) Robotic fish to lead the school. In: Palstra AP, Planas JV (eds) Swimming physiology of fish. Springer, New York, pp 407–421
Ward AJW, Sumpter DJT, Couzin ID, Hart PJB, Krause J (2008) Quorum decision-making facilitates information transfer in fish shoals. Proc Natl Acad Sci USA 105(19):6948–6953
Partan SR, Larco CP, Owens MJ (2009) Wild tree squirrels respond with multisensory enhancement to conspecific robot alarm behaviour. Anim Behav 77(5):1127–1135
Halloy J, Sempo G, Caprari G, Rivault C, Asadpour M, Tâche F, Saïd I, Durier V, Canonge S, Amé JM, Detrain C, Correll N, Martinoli A, Mondada F, Siegwart R, Deneubourg JL (2007) Social integration of robots into groups of cockroaches to control self-organized choices. Science 318(5853):1155–1158
Spinello C, Macrì S, Porfiri M (2013) Acute ethanol administration affects zebrafish preference for a biologically-inspired robot. Alcohol 47(5):391–398
Todd DJ (1993) Mobile robots-the lessons from nature. Robot Biol Syst Towards New Bionics 102(1):193–206
Webb B (2002) Can robots make good models of biological behaviour? Behav Brain Sci 24(06):1033–1050
Tokekar P, Branson E, Vander Hook J, Isler V (2013) Tracking aquatic invaders: autonomous robots for monitoring invasive fish. Robot Autom Mag 20(3):33–41
Faria JJ, Dyer JRG, Clément RO, Couzin ID, Holt N, Ward AJW, Waters D, Krause J (2010) A novel method for investigating the collective behaviour of fish: introducing ‘Robofish’. Behav Ecol Sociobiol 64(8):1–8
Marras S, Porfiri M (2012) Fish and robots swimming together: attraction towards the robot demands biomimetic locomotion. J R Soc Interface 9(73):1856–1868
Cianca V, Bartolini T, Porfiri M, Macrì S (2013) A robotics-based behavioral paradigm to measure anxiety-related responses in zebrafish. PLoS ONE 8(7):e69661
Kopman V, Laut J, Polverino G, Porfiri M (2013) Closed-loop control of zebrafish response using a bioinspired robotic-fish in a preference test. J R Soc Interface 10(78):20120540
Butail S, Bartolini T, Porfiri M (2013) Collective response of zebrafish shoals to a free-swimming robotic fish. PLoS ONE 8(10):e76123
Miklósi A, Andrew RJ (2006) The zebrafish as a model for behavioral studies. Zebrafish 3(2):227–234
Gerlai R (2010) Zebrafish antipredatory responses: a future for translational research? Behav Brain Res 207(2):223–231
Kalueff AV, Stewart AM (eds) (2012) Zebrafish protocols for neurobehavioral research. Humana Press, New York
Ladu F, Bartolini T, Panitz S, Chiarotti F, Butail S, Macrì S & Porfiri M (2015) Live predators, robots, and computer-animated images elicit differential avoidance responses in zebrafish, Zebrafish. doi: 10.1089/zeb.2014.1041
Polverino G, Abaid N, Kopman V, Macrì S, Porfiri M (2012) Zebrafish response to robotic fish: preference experiments on isolated individuals and small shoals. Bioinspir Biomim 7(3):036019
Abaid N, Bartolini T, Macrì S, Porfiri M (2012) Zebrafish responds differentially to a robotic fish of varying aspect ratio, tail beat frequency, noise, and color. Behav Brain Res 233(2):545–553
Polverino G, Porfiri M (2013) Mosquitofish (Gambusia affinis) responds differentially to a robotic fish of varying swimming depth and aspect ratio. Behav Brain Res 250(1):133–138
Polverino G, Porfiri M (2013) Zebrafish (Danio rerio) behavioural response to bioinspired robotic fish and mosquitofish (Gambusia affinis). Bioinspir Biomim 8(4):044001
Polverino G, Phamduy P, Porfiri M (2013) Fish and robots swimming together in a water tunnel: robot color and tail-beat frequency influence fish behavior. PLoS ONE 8(10):e77589
Abaid N, Marras S, Fitzgibbons C, Porfiri M (2013) Modulation of risk-taking behaviour in golden shiners (Notemigonus crysoleucas) using robotic fish. Behav Process 100:9–12
Butail S, Ladu F, Spinello D, Porfiri M (2014) Information flow in animal-robot interactions. Entropy (Spec Iss Inf Dyn Syst Complex Syst) 16(3):1315–1330
Butail S, Polverino G, Phamduy P, Del Sette F, Porfiri M (2014) Influence of robotic shoal size, configuration, and activity on zebrafish behavior in a free-swimming environment. Behav Brain Res 275:269–280
Phamduy P, Polverino G, Fuller RC, Porfiri M (2014) Fish and robot dancing together: bluefin killifish females respond differently to the courtship of a robot with varying color morphs. Bioinspir Biomim 9(3):036021
Aureli M, Porfiri M (2010) Coordination of self-propelled particles through external leadership. Europhys Lett 92(4):40004
Aureli M, Fiorilli F, Porfiri M (2012) Portraits of self-organization in fish schools interacting with robots. Phys D Nonlinear Phenom 241(9):908–920
Kopman V, Laut J, Acquaviva F, Rizzo A, Porfiri M (2014) Dynamic modeling of a robotic fish propelled by a compliant tail. IEEE J Ocean Eng PP(99):1–13
Strefling PC, Helium AM, Mukherjee R (2012) Modeling, simulation, and performance of a synergistically propelled ichthyoid. IEEE/ASME Trans Mechatron 17(1):36–45
Low KH, Chong CW (2010) Parametric study of the swimming performance of a fish robot propelled by a flexible caudal fin. Bioinspir Biomim 5(4):046002
Barrett DS, Triantafyllou MS, Yue DKP, Grosenbaugh MA, Wolfgang MJ (1999) Drag reduction in fish-like locomotion. J Fluid Mech 392:183–212
Shao J, Wang L, Yu J (2008) Development of an artificial fish-like robot and its application in cooperative transportation. Control Eng Pract 16(5):569–584
Malec M, Morawski M, Zajac J (2010) Fish-like swimming prototype of mobile underwater robot. J Autom Mobile Robot Intell Syst 4:25–30
Liu J, Hu H (2010) Biological inspiration: from carangiform fish to multi-joint robotic fish. J Bionic Eng 7(1):35–48
Morgansen KA, Triplett BI, Klein DJ (2007) Geometric methods for modeling and control of free-swimming fin-actuated underwater vehicles. IEEE Trans Robot 23(6):1184–1199
Guo J (2006) A waypoint-tracking controller for a biomimetic autonomous underwater vehicle. Ocean Eng 33(17):2369–2380
Alvarado PV y, Youcef-Toumi K (2006) Design of machines with compliant bodies for biomimetic locomotion in liquid environments. J Dyn Syst Meas Control 128(1):3–13
Kopman V, Porfiri M (2012) Design, modeling, and characterization of a miniature robotic-fish for research and education in biomimetics and bioinspiration. IEEE/ASME Trans Mechatron 18(2):471–483
Abaid N, Kopman V, Porfiri M (2013) An attraction toward engineering careers: the story of a Brooklyn outreach program on biomimetics, underwater robotics, and marine science for K-12 students. IEEE Robot Autom Mag 20(2):31–39
Laut J, Bartolini T, Porfiri M (2014) Bioinspiring an interest in STEM. IEEE Trans Educ 58(1):48–55
Abaid N, Bernhardt J, Frank JA, Kapila V, Kimani D, Porfiri M (2013) Controlling a robotic fish with a smart phone. Mechatronics 23(5):491–496
Abaid N, Yuvienco C, Kapil V, Iskander M (2011) Mechatronics mania at the inaugural USA science and engineering festival. IEEE Control Syst Mag 31(5):105–124
Blaser R, Gerlai R (2006) Behavioral phenotyping in zebrafish: comparison of three behavioral quantification methods. Behav Res Methods 38(3):456–469
Pérez-Escudero A, Vicente-Page J, Hinz RC, Arganda S, de Polavieja GG (2014) idTracker: tracking individuals in a group by automatic identification of unmarked animals. Nat Methods 11:743–748
Delcourt J, Denoël M, Ylieff M, Poncin P (2013) Video multitracking of fish behaviour: a synthesis and future perspectives. Fish Fish 14:186–204
Dell AI, Bender JA, Branson K, Couzin ID, de Polavieja GG, Noldus LP, et al. (2014) Automated image-based tracking and its application in ecology. Trends Ecol Evol 29:417–428
Kuhn HW (1955) The Hungarian method for the assignment problem. Nav Res Logist Q 2:83–97
Ladu F, Butail S, Macrì S, Porfiri M (2014) Sociality modulates the effects of ethanol in zebrafish. Alcohol Clin Exp Res 38(7):2096–2104
Carson C, Belongie S (2002) Blobworld: image segmentation using expectation-maximization and its application to image querying. IEEE Trans Pattern Anal Mach Intell 24(8):1026–1038
Lieschke GJ, Currie PD (2007) Animal models of human disease: zebrafish swim into view. Nat Rev Genet 8(5):353–367
Grunwald DJ, Eisen JS (2002) Headwaters of the zebrafish—emergence of a new model vertebrate. Nat Rev Genet 3(9):717–724
Mathur P, Guo S (2010) Use of zebrafish as a model to understand mechanisms of addiction and complex neurobehavioral phenotypes. Neurobiol Dis 40(1):66–72
Gerlai R (2012) Using zebrafish to unravel the genetics of complex brain disorders. In: Current topics in behavioral neurosciences, behavioral neurogenetic, vol 12. Springer, Berlin, pp 3–24
Goldsmith JR, Jobin C (2012) Think small: zebrafish as a model system of human pathology. J Biomed Biotechnol 2012:817341
Kalueff AV, Steward AM, Gerlai R (2014) Zebrafish as an emerging model for studying complex brain disorders. Trends Pharmacol Sci 35(2):63–75
Stewart AM, Nguyen M, Wong K, Poudel MK, Kalueff AV (2014) Developing zebrafish models of autism spectrum disorder (ASD). Prog Neuro-Psychopharmacol Biol Psychiatry 50:27–36
Laale HW (1977) The biology and use of zebrafish, Brachydanio rerio in fisheries research. A literature review. J Fish Biol 10(2):121–173
Buske C, Gerlai R (2011) Shoaling develops with age in Zebrafish (Danio rerio). Prog. Neuro-Psychopharmacol Biol Psychiatry 35(6):1409–1415
Saverino C, Gerlai R (2008) The social zebrafish: behavioral responses to conspecific, heterospecific, and computer animated fish. Behav Brain Res 191(1):77–87
Pritchard VL, Lawrence J, Butlin RK, Krause J (2001) Shoal choice in zebrafish, Danio rerio: the influence of shoal size and activity. Anim Behav 62(6):1085–1088
Bass SLS, Gerlai R (2008) Zebrafish (Danio rerio) responds differentially to stimulus fish: the effects of sympatric and allopatric predators and harmless fish. Behav Brain Res 186(1):107–117
Snekser JL, Ruhl N (2010) The influence of sex and phenotype on shoaling decisions in zebrafish. Int J Comp Psychol 23(1):70–81
Cahill G (2002) Clock mechanisms in zebrafish. Cell Tissue Res 309(1):27–34
McDonald JH (2009) Handbook of biological statistics. Sparky House Publishing, Baltimore
Wong K, Elegante M, Bartels B, Elkhayat S, Tien D, Roy S, Goodspeed J, Suciu C, Tan J, Grimes C, Chung A, Rosenberg M, Gaikwad S, Denmark A, Jackson A, Kadri F, Chung KM, Stewart A, Gilder T, Beeson E, Zapolsky I, Wu N, Cachat J, Kalueff AV (2010) Analyzing habituation responses to novelty in zebrafish (Danio rerio). Behav Brain Res 208(2):450–457
Guo S, Fukuda T, Asaka K (2003) A new type of fish-like underwater microrobot. IEEE/ASME Trans Mechatron 8(1):136–141
Aureli M, Kopman V, Porfiri M (2010) Free-locomotion of underwater vehicles actuated by ionic polymer metal composites. IEEE/ASME Trans Mechatron 15(4):603–614
Chen Z, Shatara S, Tan X (2010) Modeling of biomimetic robotic fish propelled by an ionic polymer–metal composite caudal fin. IEEE/ASME Trans Mechatron 15(3):448–459
Chen Z, Um TI, Bart-Smith H (2011) A novel fabrication of ionic polymer–metal composite membrane actuator capable of 3-dimensional kinematic motions. Sens Actuators A Phys 168(1):131–139
Cen L, Erturk A (2013) Bio-inspired aquatic robotics by untethered piezohydroelastic actuation. Bioinspir Biomim 8(1):016006
Acknowledgments
This research was supported by National Science Foundation under grants CMMI- 0745753, DGE-0741714, CMMI-1129820, and CMMI-1433670. The authors would also like to thank Giovanni Polverino, Vladislav Kopman, and Tiziana Bartolini who have contributed to the research efforts summarized in this chapter.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
Butail, S., Abaid, N., Macrì, S., Porfiri, M. (2015). Fish–Robot Interactions: Robot Fish in Animal Behavioral Studies. In: Du, R., Li, Z., Youcef-Toumi, K., Valdivia y Alvarado, P. (eds) Robot Fish. Springer Tracts in Mechanical Engineering. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-662-46870-8_12
Download citation
DOI: https://doi.org/10.1007/978-3-662-46870-8_12
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-662-46869-2
Online ISBN: 978-3-662-46870-8
eBook Packages: EngineeringEngineering (R0)