Abstract
Peste des petits ruminants virus (PPRV) is a highly contagious and infectious virus of small ruminants and camel and is endemic in several African, Middle Eastern and Asian countries. Recently, intensive efforts have been made to develop and produce potent vaccines and efficient diagnostic kits, and to understand the molecular epidemiology in endemic countries. These efforts, along with success story of rinderpest, have established baseline for the control of PPRV. Efforts for effective control and subsequent eradication include focused vaccinations in high-risk small ruminants followed by carpet vaccination, understanding socio-economic and culture situation of the small ruminants holder, established infrastructure to cope emergence of disease, and co-operation in countries where disease is endemic with essential involvement of international organizations. In this chapter, all these requirements and deliverables are comprehensively discussed.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Vero Cell
- Small Ruminant
- Mass Vaccination
- Recombinase Polymerase Amplification
- Modify Vaccinia Virus Ankara
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
13.1 Introduction
The word “eradicate” is defined as “to pull up by the roots” or “to remove totally” or “to get rid off”. Disease eradication has been defined as permanent reduction of the worldwide incidence of a disease to zero as a result of deliberate efforts, obviating the need for implementation of further control measures. Such practices have led to the eradication of large ruminant disease, rinderpest , around the globe. In contrast, disease elimination is the permanent reduction to zero of the incidence of a specified disease in a defined geographical area. However, continued intervention measures are required. A classical example of disease elimination was the outbreak of foot and mouth disease during 2001 in United Kingdom and rabies in many parts of the world.
13.2 Infection and Disease
The difference between infection and disease is that an infection implies growth of pathogenic microorganisms in the host. As a result, body functions may or may not be affected. The disease is a change from a state of health with impaired body functions. The disease state is associated with the manifestation of symptoms such as fever and pain.
13.3 Incidence and Prevalence
Incidence is the rate of new or newly diagnosed cases of the disease. It is generally reported as the number of new cases occurring within a period of time (e.g., per month, per year). The prevalence is the measure of the total number of cases of disease in a population rather than the rate of occurrence of new cases. Thus, incidence relates to the risk of contracting the disease, whereas prevalence indicates how widespread the disease is.
13.4 Motivations for the Eradication of a Disease
Disease eradication requires complete removal of the disease burden in susceptible host population in given geographical locations. Therefore, the disease eradication programme can be extremely expensive and may require global co-operation and collaborations. The amount of resources’ input is rewarded, once the disease is permanently eradicated, in terms of vaccine utilization, and social and welfare impact. There are several biological indicators that could favour the disease eradication programme:
-
1.
Diseases caused by viruses that are genetically stable and antigenically homologous
-
2.
No animal reservoir hosts
-
3.
Diseases that are not very contagious
-
4.
Availability of a potent vaccine eliciting long-lasting immunity
-
5.
A high proportion of population already being vaccinated
-
6.
Availability of sensitive and specific diagnostic tools
-
7.
A global consensus and collaboration
Several human diseases are considered as potential candidates for disease eradication , such as dracunculiasis (guinea worm disease), poliomyelitis, lymphatic filariasis, leprosy, measles and malaria. World Health Organization (WHO) adopted elimination of dracunculiasis in 1986 and polio in 1988. However, although both diseases appear close to eradication, they are still present even long after the deadlines for global eradication. This highlights the daunting nature of disease eradication programmes. Two diseases, smallpox and rinderpest , have been eradicated from humans and animals, respectively. A parallel comparison of some of biological indicators is shown in Table 13.1. These factors could probably be assessed along with the nature of eradicated viruses for any successful efforts to eradicate PPRV from the globe.
13.5 Factors Supporting PPRV Eradication Campaign
Some indicators are crucial on PPR eradicability and are discussed briefly.
13.5.1 Economic Concerns
Although all diseases are theoretically candidates for disease eradication, the major driver is the cost/benefit ratio between eradication and alternative use of resources. In this context, the decision-making is not easy and is largely determined by the economic status of the country and the perceived advantages. There would be no second opinion that rabies has to be eradicated because of its zoonotic nature and is a hundred per cent fatal disease, which is totally preventable. In contrast, if goat pox needs to be eradicated, its economic importance may preclude it from immediate implementation of disease eradication . Therefore, it is a better candidate for effective control rather than eradication.
A comparison between control of a disease and eradication of a disease is crucial factor not only for motivation but also to build commitments for any organization in initiating alliance on disease eradication . The main challenge is to convince financial administrators to mobilize resources for such programmes. They need convincing based on economical gain accrued following eradication; many of the health effects following absence of disease may not be very tangible. The offshoot benefits of eradication programmes are establishment of laboratories for disease surveillance and develop well-trained and highly motivated para-veterinary staff and a sense of togetherness across disciplines and departments. Some of these benchmarks apply to PPRV since economic importance has been realized owing to its disease impact, global alliance has been initiated and due to the presence of a good eradication model, rinderpest .
13.5.2 Social and Political Concerns
Political support is another success-determining factor in any disease eradication programmes. For this to occur, the disease chosen for eradication should be need-based, of international relevance, technically feasible and should be considered as a worthy goal if achieved. There should be a strong commitment at different levels primarily in the field staff, which should be clearly awarded of the goals and the modalities and are motivated with incentives. The other essential social requirement in disease eradication programmes is perseverance. Currently, both social importance and political importance of PPR have been realized and could further be exploited to favour the PPR eradication programme at an international level.
13.6 Why Peste des Petits Ruminants Virus (PPRV) Can Be Considered as an Ideal Candidate for Eradication of the Next Animal Disease?
The moral or logical standpoint in countries where rinderpest and PPR may have coexisted is that PPR was indirectly under check due to the cross-protection effects of rinderpest vaccines , which was used, even in small ruminants in Southern India. Thus, when rinderpest vaccinations were stopped, there was an upsurge of PPR in small ruminants. Albeit difference between PPRV and rinderpest virus (RPV), eradication of RP left an excellent model for antigenically and genentically related virus, PPRV. There are several points that require discussion before any final conclusions can be made.
13.6.1 Is PPR a Global Disease?
The disease is only prevalent in countries of Asia, Africa and Middle East with inclusion of China, Turkey and Morocco. However, the disease has not been reported in Europe, America and Australia, but there is always threat especially in the context of increasing trend in the spread of the disease in recent times due to globalization and trade.
13.6.2 Is PPR an Economically Important Disease?
The disease imparts both direct and indirect economic losses in small ruminants. Although in adults, it is self-limiting, the kids and lambs succumb to infections. Morbidity is almost 100 %, while mortality ranges from 50 to 80 %. The mortality and morbidity rates varied depending on the species/breeds affected and the strain of virus involved.
There have been reports of virus outbreaks affecting predominantly sheep or goats or both species. When different strains of PPRV were infected into West African dwarf goats experimentally (Couacy-Hymann et al. 2007a, b), some strains of PPRV produced peracute disease; some produced acute disease; and some produced acute to mild disease, while some others caused a mild disease with recovery of infected animals. In another study, when PPRV replication in peripheral blood mononuclear cells was compared across 4 breeds of goats in India, it was seen that virus titres were higher in Barbari and Tellicherry breeds as compared to Kanni and Salem Black (unpublished data). Severe outbreaks of PPR have been reported in newly introduced Barbari goats with mortality rates of 16.67–65.0 % (Rita et al. 2008; Amjad et al. 1996). Recently, a severe outbreak of PPR was reported in Tellicherry breed of goats with 100 % mortality among young and 87.5 % mortality among adults (Parimal Roy et al. 2010). Infection rate was 52.99 % in sheep and 51.47 % in goats. The mortality rate was 13.50 % in sheep and 8.53 % in goats (Thombare and Sinha 2009). Collectively, these studies indicate that mortality and morbidity rates depend on both virus and host factors including age of the host, its population density, nutrition status and innate immunity.
Due to the confusing clinical presentation of the disease, it is not differentially diagnosed with other infections, and thereby, the economic impacts of PPR are under estimated. However, it has been realized well in time that PPR is one of the major constraints of small ruminant farming in the tropics (Taylor 1984).
The complete and comprehensive economic impact of PPR on the small ruminants is currently not known; however, few recent studies have estimated economic losses. Some of these are summarized below:
-
PPR causes annual economic losses of about US$342.15 million (Hussain et al. 2008)
-
Direct economic losses of US$3.6 million with the rate 5 % mortality
-
PPR causes economic losses of 1,800 million Indian rupees (Rs.) (US$39 million) every year in India
-
Direct economic losses of US$13 millions with the rate 17 % mortality in sheep and 29 % mortality in goats in India (Singh et al. 2009)
-
The total losses are estimated as Rs. 918 in sheep to Rs. 945 in goats. Reduction in the market value of animals leads to a loss of Rs. 404 (44 %) in sheep and Rs. 408 (43 %) in goats. Indirect losses include production and yield reduction, expenditure on medicine, veterinary and labour services. Major loss is also due to infertility (Thombare and Sinha 2009).
-
The average annual economic loss due to seven diseases in goats over a 15-year period (1991–2005) was Rs. 264.8 lakh (26.48 million). The average annual economic loss was highest due to PPR (Rs. 91.4 lakh or 9.14 million) (Singh and Shivprasad 2008).
-
Opasina and Putt (1985) estimated an annual sum ranging from 2.47£ per goat at high loss and 0.36£ per goat at lowest.
-
The losses due to PPR in Nigeria were estimated to be 1.5 million dollars annually (Hamdy et al. 1976).
-
The economic benefits of vaccination against PPR in Niger showed that the anticipated net present value return in 5 years of 24 millions USD following an investment of two millions USD.
In most of these cost-benefit analyses, indirect losses have not been considered which occur in the form of biosecurity measures, trade restrictions, and curtailing animal movements and consequent local trading.
13.6.3 What Is the Nature of Spread of PPR Disease?
There is no vector-mediated transmission in PPRV, and the spread is only by close contact or through fomites. However, the disease is highly contagious. In Morocco, the first outbreak of PPR was confirmed on 18 July 2008 although the first case is believed to be detected on 12 June 2008. More than 92 outbreaks were identified by 4 August 2008 in which more than 2833 animals were identified with 50 % case fatalities (Defra 2008).
Infected animals shed the virus in secretions and excretions (from the mouth, eye and nose, faeces, semen and urine) for 10 days after the onset of fever. Sneezing and coughing by the infected animal can spread infection through droplets. Transmission of virus can occur through close contact of infected and non-infected animals, inhalation (over a distance of 10 m), ingestion and conjunctival penetration. It is unlikely to occur through fomites due to its rapid inactivation in external dry conditions. The estimated half-life of PPRV is 2.2 min at 560 °C and 3.3 h at 370 °C (Rossiter and Taylor 1994).
The recovered animals show strong immunity. Although there is no carrier state, infection is likely to be spread during the incubation period before the onset of clinical signs (Couacy-Hymann et al. 2007a, b). Ezeibe et al. (2008a, b) have shown that goats infected with PPRV can shed viral haemagglutinin antigens in faeces for 11 weeks after recovery.
Epidemiological studies have indicated that the outbreaks of PPR are normally associated with the following:
-
1.
Recent introduction of new animals
-
2.
Housing of animals from different origins and ages
-
3.
Sharing of food, water sources or shelters with “foreign” animals or returning animals that were not sold in the market
-
4.
Stress related to changes in diet, habitat, rains, climate change, intensification and most predominantly transport.
13.6.4 Is There Any Carrier Status of the Disease?
PPR is an acute contagious disease with no persistent infections reported. Carrier status of the disease has also not been reported. Although cattle get infected and show seroconversion, they do not excrete the virus and may not show clinical manifestations. In fact, the major difference between rinderpest and PPR was the host specificity. Initial observations of the disease in Ivory Coast in 1942 indicated that the disease was not transmissible from small ruminants to cattle. This led them to believe that the identified virus was different from RPV (Gargadennec and Lalanne 1942).
13.6.5 What Is the Role of Wildlife in the Spread of PPR?
PPR has been reported from a number of wild animals such as camels, gazelle, water buck, Impala, Afghan goats, Bubal hartebeests and several others. Mortality has also been observed in some outbreaks in wild animals. Although these species are susceptible to virus infection, whether they shed the virus that can in turn infect small ruminants needs more extensive studies. Whether they serve as dead-end hosts or act as a focus of infection for small ruminants needs to be more systematically explored.
Peste des petits ruminants infection has been reported in many species of wildlife such as Bharal, Dorcas gazelle, Black tailed gazelle, Thomson’s gazelle, Rheem gazelle, Lanstan sheep, Nubian Ibex, African buffalo, Water buck, Spring buck, Kob, Bushbuck, Impala and white tailed deer (Munir 2013) either through serology, RT-PCR or clinical assessments. However, virus isolations have been done in few cases only. In many cases, it appears that PPR disease outbreaks in small ruminants resulted in virus spread to wild animals and not vice versa (Abubakar et al. 2011a, b).
Another interesting feature is that all PPRV in wild animals belong to lineage IV only and no other lineage has been identified so far. Whether this is a reflection of the massive spread of this lineage virus recently or whether other lineages are not capable of infecting wild animals also needs detailed future analysis.
The advantages of wildlife in PPRV epidemiology is that they can serve as an indicator for the presence of infection in small ruminants since, in the absence of wildlife vaccination, all the antibodies would be from infection. A comparison of the advantages and disadvantages has comprehensively been shown in Table 13.2.
13.6.6 Genotypes of PPRV—Relevance to Disease Control?
The PPRV is genetically grouped into four distinct lineages (I, II, III and IV) based on the sequences of a 372-bp product covering position 777–1148 nucleotides of the fusion protein (F) gene (Forsyth and Barrett 1995). Lineage I includes isolates from Western Africa, lineage II includes isolates from West African countries, the Ivory Coast, Guinea and Burkina Faso, lineage III represents strains from Eastern Africa, the Sudan, Yemen and Oman, and lineage IV includes isolates from the Arabian Peninsula, the Middle East and South Asia and recently from the Africa (Dhar et al. 2002; Shaila et al. 1996).
Classification of PPRV is also based on the sequence analysis of N gene. It is also suggested that N gene being more divergent is more appropriate for molecular characterization of closely related isolates. Despite four distinct lineages, only a single serotype of PPRV is reported.
The classification of PPRV into lineages has broadened our understanding of the molecular epidemiology and spread of PPR viruses across the world. For example, phylogenetic analysis of the PPRV strain involved in the Aboumi outbreak belonged to lineage IV (Maganga et al. 2013) similar to the Cameroon strain. It has been predicted that the Asian lineage IV was introduced in Cameroon and later spread to Gabon through importation of living animals. However it’s spread from the Republic of Congo could have also occurred. The way lineage IV PPRV is spreading it may encompass all PPRV endemic countries in near future.
The relevance of lineages in disease eradication is unclear. It may have a role in diagnosis of PPR if nucleic acid-based detection methods such as PCR or real-time PCR are be used. While designing primers and probes, it has to be ensured that the sequences are conserved across all of the 4 lineages of PPRV to ensure detection of at least all known lineages of PPRV. A one-step real-time RT-PCR assay for PPRV has been developed to detect all four lineages of PPRV by targeting the nucleoprotein (N) gene of the virus (Kwiatek et al. 2010). The sensitivity of this assay for detection of lineage II PPRV was higher than the earlier method developed by Bao et al. (2008).
It is supposed that cross-protection should occur across PPRV lineages since PPRV belongs to a single serotype . In fact, we even propose a “debatable” hypothesis that if a vaccine belonging to a lineage other than one occurring in a particular country is used, then it becomes easy to differentiate vaccine from field viruses at least till a time when other lineage viruses are not reported in that particular country. Even DIVA may be possible if some epitopes in the sequentially different regions of F or N gene are identified and peptide ELISA could be developed and validated.
13.6.7 Genotypes Versus Serotypes Versus Protectotypes
Although genotypic studies have no relevance in assessing the protection in vivo, it has become a easy and effective tool for virus epidemiology studies. The serotypic classification is based on in vitro virus neutralization tests using homologous and heterologous serum. If two viruses are serotypically different, then neutralization by heterologous serum would be very low or non-existent. Even when two viruses are serotypically different, it is possible that they may show some degree of cross-protection in vivo wherein stimulation of cross-reactive immune responses may trigger it (cross-protection). In this case, they are said to belong to the same protectotype, even if they are serotypically different.
In the case of PPR, there is only one serotype of virus known. PPRV and RPV are serotypically different in terms of neutralization titres although there exists cross-protection between PPRV and RPV, probably triggered by the existence of conserved proteins between the two viruses. Three genetically distinct lineages (1–3) of RPV have been recognized. However, a single vaccine was used throughout the world, the RBOK strain (rinderpest bovine Kabete “O” strain), as that of PPRV. Some of these characteristics are summarized in Table 13.3.
13.7 How Is PPRV Eradication Different from RPV?
Despite having fundamental antigenic and immunological similarity, there are differences in these related viruses that require consideration in the context of any PPR controlling strategies (Table 13.4).
13.8 Diagnosis of PPR
Diagnosis of a viral disease is conventionally based on the following:
-
Direct examination of the virus by electron microscopy
-
Virus isolation and identification
-
Detection of antigen
-
Detection and/or quantification of the genome
-
Rise in specific antibody titres
Each assay has its own features of specificity, sensitivity, cost, availability of reagents, quality and cross-reactivity of the reagents, varying interpretation of results based on the context in which it is applied, etc. For disease diagnosis in general, a tier system of diagnosis is proposed. For example, in the case of human immunodeficiency virus (HIV) infections, the first tier of mass screening would be based on strip tests, a second tier based on ELISA and the confirmation based on Western blotting assays.
The choice of the assays is governed by the ease of performance vis-a-vis the specificity of the results generated. Although ELISA is amenable to mass screening, the chances for false positive is present that needs to be ruled out by Western blotting which is cumbersome to perform and time consuming, although confirmatory. For any endemic disease diagnosis , a test with higher specificity could be preferred under conventional conditions of diagnosis. For any emerging infections, a test with higher sensitivity should be the test of choice, so that any focus of infections is not missed out. During a disease eradication strategy, the same would be applicable. When vaccinations have been stopped, a test with greater sensitivity should be used. During mass vaccinations, a test with higher specificity may be preferred!
During the phase when RP and PPR coexisted, the context of diagnosis should always be linked to differential diagnosis of these diseases. But since RP has been declared eradicated, now PPR diagnosis could be a “stand-alone” diagnostic situation!
The following section lists the tests that have been applied for PPR diagnosis in the literature. The antigen detection tests used in PPR diagnosis include agar gel immunodiffusion (AGID), counterimmunoelectrophoresis (CIEP), haemagglutination (HA) test and immunocapture ELISA (ICE).
The AGID test is the most commonly used, simple, cheap technique, which gives result in 1 day and useful as an initial test. However, AGID is not sensitive enough to detect low quantities of excreted virus, as may be the case with milder forms of PPR. Counterimmunoelectrophoresis is a more rapid test for detecting PPR viral antigen giving results in an hour. CIEP has been shown to be more sensitive than AGID (80.3 % vs. 42.6 %) (Obi and Patrick 1984).
Unlike rinderpest virus, PPRV have been shown to possess HA property (Wosu 1985). In addition to HA, neuraminidase activity has also been demonstrated (Seth and Shaila 2001). The HA property of PPRV has been used by different authors for the detection of PPRV antigen and antibody detection (Dhinakar Raj et al. 2000; Manoharan et al. 2005). However, HA is not detected in all the PPRV isolates. Indian PPRV vaccine strains Arasur 87 and Sungri 97 show HA property, while the Sungri 96 vaccine strain lacks HA property (Hegde et al. 2009). Hence, this aspect has to be taken into account in applying HA and HI tests in PPR diagnosis.
The immunocapture (sandwich) ELISA is suitable for routine diagnosis of RPV and PPRV in field samples such as ocular and nasal swabs. The limit of virus detection using this test is 100.6TCID50/well for PPRV, but only 102.2TCID50/well for RPV. The main advantages of this assay are rapidity (it can be performed in precoated plate in less than 2 h) and specificity (Libeau et al. 1994).
Detection of viral nucleic acid is an alternative approach used in the diagnosis of viral disease. In the case of PPR, first method used for nucleic acid detection was DNA hybridization. This technique was used as early as 1989 to differentiate PPR and RPV infections using cDNA clones corresponding to their respective N genes as probes (Diallo et al. 1989). The technique is not suitable for routine diagnosis due to the short half-life of the 32P isotope, health hazard due to radioactivity and the need for special equipment to protect the users.
Polymerase chain reaction is the most popular and highly sensitive tool available so far for diagnosis of PPR. Since the genome of PPRV consists of a single strand of RNA, it must be first copied into DNA using reverse transcriptase, in a two-step or a single-step reaction known as reverse transcription–polymerase chain reaction. Several reverse RT-PCR methods have been developed for the rapid and specific detection of PPRV genome (Table 13.5).
However, these conventional RT-PCR assays are labour intensive, as they require gel analysis for the detection of PCR products with a consequent high risk of contamination, and they are not suitable for high-throughput testing. To overcome these drawbacks, real-time RT-PCR, which completes amplification and analysis in a closed system, has been recently developed by few researchers.
The real-time PCR assays (qRT-PCR) developed for PPR has been shown to be 10–100 times more sensitive than conventional PCR assays.
Another improvement over the regular PCR assay is the loop-mediated isothermal amplification (LAMP) assay. In this assay, the target amplification occurs at single temperature and the developed product can be detected by naked eye. The sensitivity of the assay was similar to that of qRT-PCR and tenfold higher than that of conventional RT-PCR (Li et al. 2010)
One of the common problems of using RT-PCR , qRT-PCR or LAMP assays in a routine diagnostic laboratory is its potential to generate aerosol contamination leading to false-positive results in the absence of good laboratory practices (GLP) adherence. Various methods of detecting those using appropriate controls such as no-template controls to rule out reagent contamination and controls using RNA in place of cDNA to rule out genomic DNA controls have been recommended. Of the assays, qRT-PCR may be the test of choice especially using TaqMan probe chemistry since it avoids potential aerosol contamination using the UNG enzyme, reads result in real time without the need for opening the assay tubes and use of probes avoids non-specific amplification. Although the quantification of genome of PPRV is not essential in a diagnostic set-up, the above advantages drive towards the use of this assay for PPR diagnosis at least during the later stages of disease eradication.
Virus isolation is the most confirmatory technique in diagnosis. PPR virus can be isolated during the acute stage of the disease when clinical signs are still apparent. Virus is present for approximately 10 days after the onset of fever. Swabs of the eye (conjunctival sac), nasal secretions, mouth and rectal linings and whole blood (with EDTA anticoagulant), may be used for isolation. At post-mortem fresh samples of spleen, lymph nodes and affected sections of alimentary tract mucosa may be collected for virus isolation. The most widely used cell culture systems are primary lamb kidney, Vero cells, B95a, MDBK and BHK-21. However, it should be noted that virus isolation can only be attempted in well-equipped laboratories and on freshly collected samples.
When undertaking PPR eradication programme, highly sensitive and rapid tests are required. Rapid field-level tests would be preferable as the diagnosis can be made at the site itself facilitating immediate control measures at the locality. Laboratory assays such as ICE ELISA and qRT–PCR will be useful as confirmatory tests. Sensitive and rapid antibody detection assays suitable for large-scale sample processing are required to assess vaccination efficacy. Validated competitive ELISA techniques having good correlation with neutralization test will be useful. The tests developed for PPRV detection and antibody assessment are tabulated below.
13.9 Vaccines Against PPR
During the time when RP and PPR coexisted, there existed a cross-protective effect of RPV vaccination against PPRV especially in places where RP vaccines were used in sheep and goats. This was also evident from the fact that PPR infections increased dramatically when the RP vaccination totally ceased. Not to forget that PPR diagnosis and differential diagnosis also evolved during this period with the advent of molecular techniques.
The first homologous PPR vaccine was developed by attenuation of PPRV Nigeria 75/1 in Vero cells (Diallo et al. 1989). It is used widely in many countries. In India, three live attenuated vaccines, namely Arasur 87, Coimbatore 97 and Sungri 97, were developed from local isolates by attenuation in Vero cells. The Arasur 87 (sheep isolate) and Coimbatore 97 (goat isolate) vaccine viruses were developed by attenuation of the viruses by 75 serial passages in Vero cells. The Sungri 97 (goat) vaccine strain was developed by attenuation of the virus by 60 serial passages in Vero cells. All the three vaccines have been shown to be potent and suitable for commercial production and use (Saravanan et al. 2010) (Table 13.6).
In the eradication context, attenuated live vaccines are available for PPRV and production strategies need to be geared up to meet the demand of vaccines. Developing vaccines in fermenters using either Vero cells in microcarrier systems or in suspension cultures such as BHK21 cells may also be considered if sufficient doses may not be produced in stationery cultures or roller bottles.
13.9.1 Quality Assurance
When large doses of vaccines are required for such disease eradication programmes, the quality of vaccines produced needs to be strictly monitored. One way to facilitate strict compliance is to have an independent evaluation and certification of vaccine quality assurance. The quality control of vaccines also needs to be ensured, if possible by a “third-party” evaluator distinct from the vaccine manufacturers. Similar international standards for the laboratory diagnosis of PPR with harmonisation of test protocols and availability of reference reagents to be used in these tests are also a prerequisite for effective diagnostic surveillance .
13.9.2 New Generation Vaccines
Different strategies are used for the development of new generation PPR vaccines. These studies aim at developing thermostable vaccines, which can also act as marker vaccines so that it is possible to differentiate infected from vaccinated animals (DIVA ) (Table 13.7).
Capripox virus has been used as vector for the development of dual vaccines to protect against PPRV and capripox. PPRV F protein expressed in capripox virus was effective at a low dose of 0.1 PFU (Berhe et al. 2003). Recombinant capripox virus expressing PPRV H or F proteins elicited long-lasting immunity against PPR (Chen et al. 2010).
Vaccinia virus has also been used as vector for developing recombinant PPRV vaccines. Recombinant attenuated modified vaccinia virus Ankara viruses (MVA) expressing the PPRV fusion (F) and hemagglutinin (H) glycoproteins, induced protective immune response in goats. The vaccinated goats were completely protected against virulent virus challenge 4 months post-vaccination (Chandran et al. 2010).
PPRV expressing green fluorescent protein (GFP) was generated by reverse genetics approach. This virus was found suitable for high-throughput assessment of neutralization test (Hu et al. 2012).
Buczkowski et al. (2012) have developed a new strategy by incorporating mutation in viral protein by reverse genetics method for the production of morbillivirus DIVA vaccines . This is an alternative to expression of PPRV proteins in other viruses. In this method, the epitope in the vaccine virus is altered so that the vaccine immune response lacks antibodies against the epitope.
Recombinant canine adenonovirus type 2 (CAV-2) expressing PPRV H protein was generated and shown to induce neutralizing antibody response in goats (Qin et al. 2012). Recombinant adenovirus expressing PPRV H, F or H–F fusion proteins was shown to induce neutralizing antibodies, which lasted for 21 weeks post-vaccination (Wang et al. 2013).
Production of edible vaccines by expression of viral antigens in plants is a cost-effective method for vaccine manufacture. The hemagglutinin (H) protein of PPRV was expressed in peanut plants (Arachis hypogea). The expressed protein was in sheep by oral immunization. Virus neutralizing antibodies and cell-mediated immune responses were detected in the immunized sheep (Khandelwal et al. 2011).
All these new generation vaccines are in various stages of development and validation. The live attenuated conventional vaccines available for PPR can be used to initiate disease eradication programmes. However, the DIVA vaccines may have a role in later stages of disease eradication.
13.9.3 Thermostable Vaccines for PPR
The conventional live attenuated PPR vaccines require maintenance of proper cold chain as PPRV is thermolabile. Hence, thermostabilization of existing vaccine strains or evolving thermostable vaccines would be very useful for PPR eradication, as the cost of cold chain maintenance will be saved.
PPRV freeze-dried in the trehalose containing stabilizer has been shown to become more thermotolerant. This vaccine remained stable for 14 days at 45 °C with minimal loss of potency (Worrall et al. 2001).
PPRV freeze-dried with lactalbumin hydrolysate–sucrose and trehalose stabilizers were more stable than Weybridge medium and buffered gelatine–sorbital stabilizers. The vaccine diluent 1 M magnesium sulphate was shown to maintain required vaccine titre for a longer time when compared to water and 85 % saline (Sarkar et al. 2003). Deuterated vaccine preparation reconstituted with heavy water maintained higher titres when compared with conventional vaccine (Sen et al. 2009).
Stabilizer containing Tris with trehalose was found to stabilize PPRV vaccine virus better when compared to Weybridge medium. The freeze-dried vaccine prepared with Tris trehalose stabilizer maintained the viral titre above 104 TCID50/ml for 21 months at 4 °C and 144 h at 37 °C (Silva et al. 2011).
PPRV AR 87 was exposed to higher temperatures in succession to evolve a thermo-adapted vaccine virus. The titre and potency of the thermo-adapted vaccine remained constant at 105.5 TCID50/100 μl level up to 1 month at room temperature (Palaniswami et al. 2005). Sen et al. (2010) developed two thermostable PPR vaccines . The shelf life of these vaccines was 7.62 and 3.68 days at 37 and 40 °C, respectively.
13.10 Strategies for Control of PPR
Disease eradication has to be undertaken in two phases:
-
Present no susceptible hosts to virus and abolish transmission cycle
-
Prove the above process/attempt is successful
For any eradication strategy, there is a need for an integrated approach combining the following:
-
Vaccination
-
Biosecurity
-
Epidemiological understanding of the disease
When deciding to vaccinate, decision should be taken on either “targeted vaccination” or “mass vaccination”. For rinderpest eradication , while India initially followed the mass vaccination model, the South Asia Rinderpest Eradication Campaign used vaccination only in identified places where virus transmission was occurring.
The disadvantages of mass vaccination are as follows:
-
Very expensive
-
Vaccinating animals that are not perceived to be at risk
-
Non-compliance since risk not perceived
Challenges of mass vaccinations include:
-
Limited human resources
-
Limited stakeholder involvement
-
Financial constraints
-
Inadequate cold chain
-
Civil unrest
-
Poor infrastructure
-
Poor communication
-
Uncontrolled movement across borders
The only country, which reduced its rinderpest incidence to zero, without recourse to a mass vaccination campaign, was Pakistan where the last detected outbreaks occurred in Karachi in 2001. Although mass vaccinations led to decrease in rinderpest disease incidence, it was no way near eradication . Hence, a targeted pulsed, 2-year-long intensive vaccination strategy in the enzootically infected states of southern was adopted that was highly successful and led to the eradication of rinderpest .
13.11 Disease Surveillance
Before disease surveillance is embarked upon, the country should publicly cease vaccination in a declaration of provisional freedom from disease.
This freedom should be proven through the following:
-
Clinical disease surveillance
-
Serosurveillance and
-
Wildlife surveillance
Disease surveillance needs to be undertaken, through veterinary searches within the community that had previously experienced disease/infection and provide negative incidence reports. The required number of searches should be based on statistical methods.
Then, unvaccinated animals must be sampled for PPRV antibodies using an OIE validated assay to assess the trend of antibodies in its population. Statistically significant numbers of samples need to be collected to obtain evidence of freedom from disease as well as freedom from infection status. With PPRV antibodies present in several wildlife species, both clinical surveillance and serological surveillance in wildlife populations are a prerequisite of global eradication.
Now, to embark on a vaccination strategy for PPR, the epidemiology of the disease in each country needs to be ascertained and a decision made.
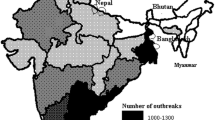
PPR is widely endemic in India (unlike rinderpest which was more common in Southern India). However, it appears that goats are more severely affected in Northern India and sheep in Southern India. There is unrestricted movement of sheep and goats across several states. Small ruminants are more prone to mixing during purchase fairs, grazing, etc.
Keeping the above factors, it may be needed to use a mass vaccination strategy initially for 3–5 years, vaccinating the newly born kids and lambs every year. Although costly and a huge challenge in terms of personnel and infrastructure costs, the disease epidemiology (unlike large ruminant disease, rinderpest) probably requires this approach.
The second stage could be a more focussed targeted vaccination where still disease/infection has been reported or for high-risk groups of animals. This needs to be coupled with disease diagnosis strategies with the development of infrastructure, assays, training, reporting, documentation, etc. Once freedom from infection is achieved, then the OIE pathway for disease/infection surveillance may be followed, following stoppage of vaccination.
Briefly, PPR eradication should not only rely on the use of vaccines but also on concomitant biosecurity measures. First, small ruminants should be immunized in sufficient “depth” that fresh transmission chains are not be established and, secondly, to “stamp out” the infection in enzootic areas by high-intensity, pulsed vaccination.
During devising the strategy for PPR eradication, the following distinct epidemiological aspects should also be borne in mind (that may be different from rinderpest ):
PPR clinical picture is more “subdued” especially in adults where the disease may be self-limiting. Thus, it may be more easily misdiagnosed or undiagnosed leading to persistence of the focus of infection. This could also lead to slaughter of infected animals again favouring disease spread. There seems to be a wide variation in the genetic susceptibility of the breeds and species.
The movement and intermixing of small ruminants are higher in the form of migration of flocks and local markets, fairs and common grazing. It is well documented and observed that PPRV infection occurs especially when new animals are introduced into a flock or when animals that were not sold were brought back from the local animal fairs.
Sheep and goats are higher in population density than cattle/buffaloes. Higher density would increase disease transmission rates providing effective contact between infected and susceptible animals.
Providing vaccine coverage among small ruminant animals may be more difficult. Even if 100 % animals are vaccinated, only 80–85 % seroconversion is seen due to practical immunization methods, time taken for animal restraint, environmental temperature, cold storage and transport facilities.
The birth of young ones is more in small ruminants, and thereby, the availability of naive susceptible animals that could serve as a focus of new infections is higher.
Disease spread could be much higher due to large distances of movement among small ruminants. Further social requirements could also be a deterrent for eradication. In Tamil Nadu, India, there are no camels, but during festive seasons, camels are transported from states such as Rajasthan that can facilitate disease transmission through distances of more than 2,000 km. Disease introduction from across borders may be easier through straying of small ruminant animals.
13.12 What Is the Role of Cattle or Buffaloes in Disease Control?
Though PPRV multiplication and seroconversion occur in cattle and buffaloes, they do not suffer the disease. In cattle and buffaloes, an overall PPRV antibody prevalence of 4.58 % has been reported in a study conducted in India (Balamurugan et al. 2012). This indicates that under field conditions, natural PPRV infection occurs in cattle and buffaloes. These species could be used as additional indicators of infection foci during later stages of PPR eradication .
13.13 Pulse PPRV Vaccination Strategy ?
Pulse vaccination is the repeated application of a vaccine over a defined age range. This method has been applied successfully as Pulse Polio campaigns in India. Unlike constant vaccination, where high vaccine coverage is essential (more than 95 %), pulse vaccination requires only low vaccination coverage to prevent epidemic outbreaks. The “inter-pulse” intervals should be decided based on the population dynamics.
However, a mixed strategy may be considered for PPR. Initially, the constant vaccination may be applied to reduce the number of susceptible animals by means of a high percentage of vaccination coverage, and the second (pulse) vaccination with relatively low coverage with very long inter-pulse intervals, for kids and lambs between 4 and 12 months that would create a “infection-free” condition for preventing the PPRV focus of infection.
13.14 Strategies for Disease Control in Borders
When PPR occurs in a particular country and eradication efforts are on, the neighbouring countries should also place the same emphasis. This requires political will and a “non-political” medium for communication between countries. Across borders, governments should favour joint rather than unilateral action. Regional bodies can play a pivotal role through coordinating and ensuring that governments act together.
The disease preparedness should also be high at these borders. This may be complemented with diagnostic methods, which can provide a result in minutes at the field level. Although several assays such as lateral flow devices are available, the sensitivity of these methods is questionable. The more recent technique called recombinase polymerase amplification (RPA) assay appears to have a great potential since results are obtained in 4–10 min and also can be done without the need for many equipment at the field. This assay has been tested for many biological warfare agents (Wang et al. 2013).
13.15 Differentiation of Infected from Vaccinated Animals (DIVA)
The term “differentiation of infected from vaccinated animals (DIVA )” was coined in 1999 by Jan T van Oirschot. Marker vaccines were deletion mutants of wild-type pathogens, used along with a companion diagnostic test (CDT). The underlying principle is based on a DIVA vaccine producing an antibody response that is different from the response produced by the wild-type pathogen.
13.15.1 DIVA Strategy
When undertaking disease control by vaccination, it will be useful to identify infected animals from vaccinated animals. This is possible in certain diseases such as FMD where inactivated vaccine is used. Hence, vaccination antibodies are mainly against structural proteins, while post-infection, there is antibody response against non-structural proteins also. However, it may not be possible to identify and differentiate infection induced antibodies from vaccination antibodies when conventional live or inactivated viral vaccines are used. If the vaccine organism lacks an antigen or contains an additional antigen (marker vaccine), it is possible to differentiate infected animals from vaccinated animals by using suitable companion diagnostic tests.
The advantages of application of the DIVA strategy are as follows:
-
Facilitation of identification of residual focus of infections
-
Identification of remerging infections in “disease-free” or “vaccination-ceased” zones
This can be used to initiate “stamping out” of infected animals to remove these foci and hasten eradication
The disadvantages of DIVA vaccine are as follows:
-
If DIVA is made through reverse genetics approaches, regulatory compliance in all countries may not be easy
-
The developed vaccine need to be tested all over again in multitudes of animals for its safety and potency
-
The CDT also need to be validated
-
Lag time before it is available across the world
-
Technology transfer/licensing issues
13.15.2 Is DIVA Needed for PPR Control?
In the case of PPR, presently, vaccines are live attenuated vaccines which induce immune response against all viral proteins not distinguishable from the immune responsible induced by natural infection. Hence, it is not possible to conduct sero-epidemiosurveillance of the disease in areas where vaccination is carried out.
Rinderpest was eradicated without the availability of DIVA vaccine. Scientifically, DIVA would be needed during the later phases of PPR eradication and not initially.
13.16 Progressive Control Programme (PCP) as Envisaged for FMD—Lessons for PPR
The PCP-FMD is a tool that has been developed jointly by FAO and OIE to assist endemic countries to progressively control the disease and reduce its impact on rural livelihoods.
It is a set of 5 FMD control activity stages:
Stage 1: To understand the epidemiology of FMD and develop a comprehensive approach to reduce its impact
Stage 2: To implement control measures such that the impact of FMD is reduced in one or more livestock sectors and/or in one or more zones
Stage 3: Progressive reduction in disease incidence , followed by elimination of FMD virus circulation in domestic animals in at least one zone of the country
Stage 4: Eventual freedom with vaccination
Stage 5: Freedom without vaccination—disease eradication
Whether a PCP as envisaged for FMD would also apply to PPR is debatable.
Implementation of disease control strategies in one zone of the country may be fraught with danger especially for sheep and goats since they are highly migratory and controlling their migration is a bigger task. Further, this approach may be valuable for diseases such as avian influenza where in slaughter of infected poultry is practised, but not in other cases where slaughter is not possible.
13.17 Risk Analysis
A risk-based approach of controlling diseases tends to be more effective. Hence, during an eradication programme, the efforts can be concentrated on critical points of the disease transmission cycle.
With respect to PPR, the following predisposing factors have been shown to be associated with disease onset or progression:
-
Susceptible population of sheep and goats and susceptible breeds
-
New animal introduction and animal movement
-
Poor biosecurity measures
-
Trade and migratory routes driven by seasons
-
Livestock markets
-
Cultural practices and production systems, water sharing or grazing land sharing
-
Geographical and environmental factors—high temperature and low humidity reduce virus survival and decrease risk
-
Presence and interaction of domestic/wildlife
-
Quality of veterinary services—access to quick diagnosis and preventing spread
-
Quality of ante-mortem examinations
-
Disease information from other parts of the country and world
-
Porous borders
-
Poor roads and inaccessible terrains
These factors may vary from place to place and needs to be determined. The identification of risks enables for contingency planning and surveillance .
13.17.1 Risk Management
New outbreaks of PPR can be prevented using enhanced surveillance systems and early detection methods. When the reports of unusual mortalities in sheep/goat, especially kids, are received, immediate diagnosis and relevant activities to manage the disease should be initiated. The disease reporting system should be incentivized and rewarded. Surveillance will be strengthened in areas, which have had infection using participatory disease search methods.
13.18 Whether Vaccine Can Be Given During Outbreaks?
PPR occurs throughout the year. In some countries such as Nigeria, it peaks in April increasing from December. Hence, a vaccination around November is suggested. In countries such as India where temperatures are high during summer (March–June), it is also recommended to vaccinate during the October–November months due to lower possibility of inactivation of vaccine viruses.
Abubakar et al. (2012) have reported excretion of PPRV in faeces. During a clinical outbreak of PPR in goats in Pakistan, some infected animals were vaccinated in the face of outbreak and some were left unvaccinated. They report that animals that were vaccinated excreted antigen in faecal matter for 1 month following vaccination, while unvaccinated animals continued to shed virus antigen for 2 months. The virus excretion in faeces adds another dimension to PPR epidemiology and needs a thorough examination.
13.19 Conclusions
To sum the detailed discussion, following conclusions can be drawn:
-
The nature of the PPR disease, the availability of tools and its economic importance lead us to believe that this could be the next animal disease to be targeted for eradication .
-
Some of the epidemiological features of the disease such as role of wildlife and excretion in faeces need to be studied more thoroughly
-
Approaches to thermostabilize the available PPRV vaccines may be strengthened as also the field-based diagnosis of PPR
-
DIVA vaccines may be developed and validated for use in later phases of PPR eradication
-
Lessons learnt from rinderpest and FMD control or eradication programmes must be utilized, and a unique PPRV eradication programme must be evolved that could vary based on geographical regions
-
All the countries must join hands in this fight to protect the small ruminants—the ‘poor man’s cows’ for the ultimate goal of poverty alleviation and economic inclusion.
References
Abubakar M, Khanb AH, Arsheda MJ, Hussain M, Ali Q (2011a) Peste des petits ruminants (PPR): disease appraisal with global and Pakistan perspective. Small Ruminant Res 96:1–10
Abubakar M, Rajput ZI, Arshed MJ, Sarwar G, Ali Q (2011b) Evidence of peste des petits ruminants virus (PPRV) infection in Sindh Ibex (Capra aegagrus blythi) in Pakistan as confirmed by detection of antigen and antibody. Trop Anim Health Prod 43:745–747
Abubakar M, Arshed MJ, Zahur AB, Ali Q, Banyard AC (2012) Natural infection with Peste des petits ruminants virus: a pre and post vaccinal assessment following an outbreak scenario. Virus Res 167:43–47
Amjad H, Islam QU, Forsyth M, Barrett T, Rossiter PB (1996) Peste des petits ruminants in goat in Pakistan. Vet Rec 139:118–119
Anderson J, Corteyn M, Libeau G (2006) Diagnosis of rinderpest and peste des petits ruminants virus. In: Barrett T, Pastoret PP, Taylor WP (eds) Rinderpest and peste des petits ruminants. Virus plagues of large and small ruminants. Academic Press/Elsevier, London, pp 31–67
OIE (2008) Peste des petits ruminants. In: Manual of diagnostic tests and vaccines for terrestrial animal health, 6th edn, vols I and II. Office International des Epizooties (OIE), Paris, pp 1046–1366 (Chap. 2.7.11)
Balamurugan V, Krishnamoorthy P, Veeregowda BM, Sen A, Rajak KK, Bhanuprakash V, Gajendragad MR, Prabhudas K (2012) Seroprevalence of Peste des petits ruminants in cattle and buffaloes from Southern Peninsular India. Trop Anim Health Pro 44:301–306
Balamurugan V, Sen A, Venkatesan G, Yadav V, Bhanot V, Bhanuprakash V, Singh RK (2010) Application of semi-quantitative M gene-based hydrolysis probe (TaqMan) real-time RT-PCR assay for the detection of peste des petits ruminants virus in the clinical samples for investigation into clinical prevalence of disease. Transbound Emerg Dis 10:1682–1865
Banyard AC, Parida S, Batten C, Oura C, Kwiatek O, Libeau G (2010) Global distribution of peste des petits ruminants virus and prospects for improved diagnosis and control. J Gen Virol 91:2885–2897
Bao J, Li L, Wang Z, Barrett T, Suo L, Zhao W, Liu Y, Liu C, Li J (2008) Development of one-step realtime RT-PCR assay for detection and quantitation of peste des petits ruminants virus. J Virol Methods 148:232–236
Berhe G, Minet C, Le Goff C, Barrett T, Ngangnou A, Grillet C, Libeau G, Fleming M, Black DN, Diallo A (2003) Development of a dual recombinant vaccine to protect small ruminants against peste-des-petits-ruminants virus and capripoxvirus infections. J Virol 77:1571–1577
Brindha K, Raj GD, Ganesan PI, Thiagarajan V, Nainar AM, Nachimuthu K (2001) Comparison of virus isolation and polymerase chain reaction for diagnosis of peste des petits ruminants. Acta Virol 45:169–172
Buczkowski H, Parida S, Bailey D, Barrett T, Banyard AC (2012) A novel approach to generating morbillivirus vaccines: negatively marking the rinderpest vaccine. Vaccine 30:1927–1935
Chandran D, Reddy KB, Vijayan SP, Sugumar P, Rani GS, Kumar PS, Rajendra L, Srinivasan VA (2010) MVA recombinants expressing the fusion and hemagglutinin genes of PPRV protects goats against virulent challenge. Indian J Microbiol 50:266–274
Chen W, Hu S, Qu L, Hu Q, Zhang Q, Zhi H, Huang K, Bu Z (2010) A goat poxvirus-vectored peste-des-petits-ruminants vaccine induces long-lasting neutralization antibody to high levels in goats and sheep. Vaccine 28:4642–4750
Couacy-Hymann E, Bodjo SC, Danho T, Koffi MY, Libeau G, Diallo A (2007a) Early detection of viral excretion from experimentally infected goats with pets des petits ruminants virus. Prev Vet Med 78:85–88
Couacy-Hymann E, Bodjo SC, Danho T, Libeau G, Diallo A (2007b) Evaluation of the virulence of some strains of peste des petits ruminants virus (PPRV) in experimentally infected west African dwarf goats. Vet J 173(1):178–183
Defra (2008) http://archive.defra.gov.uk/foodfarm/farmanimal/diseases/monitoring/documents/ppr-morocco.pdf
Dhar P, Sreenivasa BP, Barrett T, Corteyn M, Singh RP, Bandyopadhyay SK (2002) Recent epidemiology of peste des petits ruminants virus (PPRV). Vet Microbiol 88(2):153–159
Dhinakar Raj G, Nachimuthu K, Nainar AM (2000) A simplified objective method for quantification of peste des petits ruminants virus or neutralizing antibody. J Virol Methods 89:89–95
Dhinakar Raj G, Rajanathan TMC, Kumar CS, Ramathilagam G, Hiremath G, Shaila MS (2008) Detection of peste des petits ruminants virus antigen using immunofiltration and antigen-competition ELISA methods. Vet Microbiol 129:246–251
Diallo A, Barrett T, Barbron M, Subbarao SM, Taylor WP (1989) Differentiation of rinderpest and peste des petits ruminants viruses using specific cDNA clones. J Virol Methods 23:127–136
Diallo A, Libeau G, Couacy-Hymaun E, Barbron M (1995) Recent developments in the diagnosis of rinderpest and peste des petits ruminants. Vet Microbiol 44:307–317
Ezeibe MCO, Okoroafor ON, Ngene AA, Eze JI, Eze IC, Ugonabo JAC (2008a) Persistent detection of peste de petits ruminants antigen in the faeces of recovered goats. Trop Anim Health Prod 40:517–519
Ezeibe MCO, Okoroafor ON, Ngene AA, Eze JI, Eze IC, Ugonabo JAC (2008b) Persistent detection of peste de petits ruminants antigen in the faeces of recovered goats. Trop Anim Health Prod 40:517–519
Forsyth MA, Barrett T (1995) Evaluation of polymerase chain reaction for the detection and characterisation of rinderpest and peste des petits ruminants viruses for epidemiological studies. Virus Res 39:151–163
Gargadennec L, Lalanne A (1942) La peste des petits ruminants. Bull Serv Zootechnol Epizoot Afr Occid 5:16–21
Hamdy FM, Dardiri AH, Nduaka O, Breese SS, Ihemelandu EC (1976) Etiology of the stomatitis pneumoenteritis complex in Nigerian dwarf goats. Can J Comp Med 40:276–284
Hegde R, Gomes AR, Byre Gowda SM, Santhosh AK and Renukaprasad C (2009) Cytopathic effect of PPR vaccine virus strains in Vero cells. Vet World 2:93–94
Hussain M, Irshad H, Khan MQ (2008) Laboratory diagnosis of transboundary animal diseases in Pakistan. Trans Emerg Dis 55:190–195
Hu Q, Chen W, Huang K, Baron MD, Bu Z (2012) Rescue of recombinant peste des petits ruminants virus: creation of a GFP-expressing virus and application in rapid virus neutralization test. Vet Res 43:48
Khandelwal A, Renukaradhya GJ, Rajasekhar M, LakshmiSita G, Shaila MS (2011) Immune responses to hemagglutinin-neuraminidase protein of peste des petits ruminants virus expressed in transgenic peanut plants in sheep. Vet Immunol Immunop 140:291–296
Kwiatek O, Keita D, Gil P, Fernandez-Pinero J, Jimenez Clavero MA, Albina E, Libeau G (2010) Quantitative one-step real-time RT-PCR for the fast detection of the four genotypes of PPRV. J Virol Methods 165:168–177
Libeau G, Diallo A, Colas F, Guerre L (1994) Rapid differential diagnosis of RP and PPR using an immunocapture ELISA. Vet Rec 134:300–304
Libeau G, Prehaud C, Lancelot R, Colas F, Guerre L, Bishop DH, Diallo A (1995) Development of a competitive ELISA for detecting antibodies to the peste des petits ruminants virus using a recombinant nucleoprotein. Res Vet Sci 58:50–55
Li L, Bao J, Wu X, Wang Z, Wang J, Gong M, Liu C and Li J (2010) Rapid detection of peste des petits ruminants virus by a reverse transcription loop-mediated isothermal amplification assay. J Virol Methods 170:37–41
Maganga GD, Verrier D, Zerbinati RM, Drosten C, Drexler JF, Leroy EM (2013) Molecular typing of PPRV strains detected during an outbreak in sheep and goats in south-eastern Gabon in 2011. Virol J 2013(10):82
Manoharan S, Jayakumar R, Govindarajan R, Koteeswaran A (2005) Haemagglutination as a confirmatory test for peste des petits ruminants diagnosis. Small Rum Res 59:75–78
Mariner JC, van den Ende MC, House JA, Mebus CA, Sam S, Stem C (1990) The serological response to a thermostable Vero cell adapted rinderpest vaccine field conditions in Niger. Vet Microbiol 22:119
Meena K, Sarma BJ, Reddy YN (2009) Development and application of latex agglutination test for detection of PPR virus. Indian Vet J 86:234–237
Munir M (2013) Role of wild small ruminants in the epidemiology of peste des petits ruminants. doi:10.1111/tbed.12052
Narayanan R, Gopu P, Baegan S, Barathidasan (2008) Clinical management in an outbreak of peste des petits ruminants in Barbari goats. Vet World 13:81–82
Obi TU, Patrick D (1984) The detection of peste des petits ruminants (PPR) virus antigen by agar gel precipitation test and counterimmunoelectrophoresis. J Hyg 93:579–586
Obi TU, Ojeh CK (1989) Dot enzyme immunoassay for visual detection of peste-des-petits-ruminants virus antigen from infected caprine tissues. J Clin Microbiol 27(9):2096–2099
Opasina BA, Putt SNH (1985) Outbreaks of peste des petits ruminants in village goat flocks in Nigeria. Trop Anim Health Prod 17:219–224
Osman NA, ME AR, Ali AS, Fadol MA (2008) Rapid detection of Peste des Petits Ruminants (PPR) virus antigen in Sudan by agar gel precipitation (AGPT) and haemagglutination (HA) tests. Trop Anim Health Prod 40(5):363–368
Palaniswami KS, Thangavelu A, Velmurugan R (2005) Development of thermostable Peste Des Petits Ruminants (PPR) virus vaccine and assessment of molecular changes in the F Gene. Applications of gene-based technologies for improving animal production and health in developing countries, pp 673–678
Qin J, Huang H, Ruan Y, Hou X, Yang S et al (2012) A novel recombinant peste des petits ruminants-canine adenovirus vaccine elicits long-lasting neutralizing antibody response against PPR in goats. PLoS ONE 7(5):e37170. doi: 10.1371/journal.pone.0037170
Rossiter PB and Taylor WP (1994) Peste des petits ruminants. In: Coezter JAW (ed.) Infectious diseases of livestock, vol II, pp 758
Roy P, Vairamuthu S, Thangavelu A, Chitradevi S, Purushothaman V, Koteeswaran A (2010) An outbreak of peste des petits ruminants among Thelichery breed of goats. J Appl Res Vet Med 8:155–160
Saravanan P, Singh RP, Balamuragan V, Dhar P, Sreenivasa BP, Muthuchelvan D, Sen A, Aleyas AG, Singh RK, Bandyopadhyay SK (2004) Development of a N gene based PCR-ELISA for detection of peste des petits ruminants virus in clinical samples. Acta Virol 48:249–255
Saravanan P, Balamurugan V, Sen A, Bikash B, Singh RK (2006) Development of dot ELISA for diagnosis of peste des petits ruminants (PPR) in small ruminants. J Appl Anim Res 30:121–124
Saravanan P, Sen A, Balamurugan V, Bandyopadhyay SK, Singh RK (2008) Rapid quality control of a live attenuated Peste des petits ruminants (PPR) vaccine by monoclonal antibody based sandwich ELISA. Biologicals 36:1–6
Saravanan P, Sen A, Balamurugan V, Rajak KK, Bhanuprakash V, Palaniswami KS, Nachimuthu K, Thangavelu A, Dhinakar Raj G, Hegde R, Singh RK (2010) Comparative efficacy of peste des petits ruminants (PPR) vaccines. Biologicals 38:479–485
Sarkar J, Sreenivasa BP, Singh RP, Dhar P, Bandyopadhyay SK (2003) Comparative efficacy of various chemical stabilizers on the thermostability of a live-attenuated peste des petits ruminants (PPR) vaccine. Vaccine 21:4728–4735
Sen A, Balamurugan V, Rajak KK, Chakraborty S, Bhanuprakash V, Singh RK (2009) Role of heavy water in biological sciences with an emphasis on thermostabilization of vaccines. Expert Rev Vaccines 8:1587–1602
Sen A, Saravanan P, Balamurugan V, Rajak KK, Sudhakar SB, Bhanuprakash V, Parida S, Singh RK (2010) Vaccines against peste des petits ruminants virus. Expert Rev Vaccines 9:785–796
Senthil Kumar C, Dhinakar Raj G, Thangavelu A, Shaila MS (2007) Performance of RT-PCR-ELISA for the detection of peste des petits ruminants virus. Small Rum Res 72:200–208
Seth S, Shaila MS (2001) The hemagglutinin-neuraminidase protein of peste des petits ruminants virus is biologically active when transiently expressed in mammalian cells. Virus Res 75:169–177
Shaila MS, Shamaki D, Forsyth MA, Diallo A, Goatley L, Kitching RP, Barrett T (1996) Geographic distribution and epidemiology of peste des petits ruminants virus. Virus Res 43:149–153
Silva AC, Manuel JT, Carrondo, Alves PM (2011) Strategies for improved stability of Peste des Petits Ruminants Vaccine. Vaccine 29:4983–4991
Singh RP, Saravanan BP, Dhar P, Shah LC, Bandyopadhyay SK (2004a) Development of a monoclonal antibody based competitive-ELISA for detection and titration of antibodies to peste des petites ruminants (PPR) virus. Vet Microbiol 98:3–15
Singh RP, Srinivasa BP, Dhar P, Badyopadhyay (2004b) A sandwich-ELISA for the diagnosis of Peste des petits ruminants (PPR) infection in small ruminants using anti-nucleocapsid protein monoclonal antibody. Acta Virol 149:2155–2170
Singh RK, Balamurugan V, Bhanuprakash V, Sen A, Saravanan P, Yadav MP (2009) Possible control and eradication of peste des petits ruminants from India: technical aspects. Vet Ital 45:449–462
Singh RP, De UK, Pandey KD (2010) Virological and antigenic characterization of two Peste des Petits Ruminants (PPR) vaccine viruses of Indian origin. Comp Immunol Microbiol Infect Dis 33:343–353
Singh B, Shivprasad (2008) Modelling of economic losses due to some important diseases in goats in India. Agr Econ Res Rev 21:297–302
Sreenivasa BP, Singh RP, Mondal B, Dhar P, Bandyopadhyay SK (2006) Marmoset B95a cells: a sensitive system for cultivation of peste des petits ruminants (PPR) virus. Vet Res Comm 30:103–108
Taylor WP (1984) The distribution and epidemiology of PPR. Prev Vet Med 2:157–166
Thombare NN, Sinha MK (2009) Economic Implications of peste des petits ruminants (PPR) Disease in Sheep and Goats: a sample analysis of district Pune, Maharastra. Agric Econ Res Rev 22:319–322
Wang Y, Euler M, Heidenreich D, Patel P, Strohmeier O, Hakenberg S, Niedrig M, Hufert FT, Weidmann M (2013) Development of a panel of recombinase polymerase amplification assays for detection of biothreat agents. J Clin Microbiol 51:1110–1117
Wei L, Gang L, XiaoJuan F, Kun Z, Qin F, LiJun S, Unger H (2009) Establishment of a rapid method for detection of peste des petits ruminants virus by a reverse transcription loop-mediated isothermal amplification. Chi J Prev Vet Med 31:374–378
Worrwall EE, Litamoi JK, Seck BM, Ayelet G (2001) Xerovac: an ultra rapid method for the dehydration and preservation of live attenuated rinderpest and peste des petits ruminants vaccines. Vaccine 19:834–839
Wosu LO (1985) Agglutination of red blood cells by peste des petite ruminants (PPR) virus. Nigerian Vet J 14:56–58
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
Dhinakar Raj, G., Thangavelu, A., Munir, M. (2015). Strategies and Future of Global Eradication of Peste des Petits Ruminants Virus. In: Munir, M. (eds) Peste des Petits Ruminants Virus. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-662-45165-6_13
Download citation
DOI: https://doi.org/10.1007/978-3-662-45165-6_13
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-662-45164-9
Online ISBN: 978-3-662-45165-6
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)