Abstract
This chapter first discusses the challenges with the tool material selection for machining of high-silicon aluminum-matrix composites. It is shown that the combination of a soft easy-to-adhere Al-matrix and highly abrasive particles limits the use of cemented carbide tools due to high rate of adhesion and abrasion wear. The issue becomes intolerable in high-speed machining applications. As a result, polycrystalline diamond (PCD) is slowly becoming a material of choice for such applications. The chapter presents the major research advances in PCD as a tool material. The wear mechanism of PCD is discussed at macro- and micro levels. A discussion on the need and a report on the progress in the development of thermal stable grades of PCD conclude the chapter.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1.1 Introduction
The selection of a cutting tool material type and its particular grade is an important factor to consider when planning a successful machining operation. A basic knowledge of each cutting tool material and its performance is therefore important so that the correct selection for each application can be made. Considerations include the type and properties of the material to be machined, the part/blank type and shape, machining conditions, and quality requirements of the machined parts for the considered operation. Note that the cost per machined part (the size of production lot, yearly production, existing machine/machining practice, etc.) should also be considered in the selection of the proper (technically and economically) tool material.
The aim of this chapter is to familiarize readers with problems existing in high-speed machining of abrasive work materials and to present the research advances in polycrystalline diamond (PCD) tool material to address these problems.
1.2 Challenges with Work Materials
There are a number of new work materials that have been in extensive use since the beginning of the 21st century. Among them, two distinctive groups of work materials are of prime concern in many modern manufacturing facilities: metal and polymer base composite materials due to the increased volume of parts made of these materials. Although some types of such materials have been known for a long time, a new challenge in their machining was brought by the necessity of wide implementation of real high-speed, and thus high-efficiency, machining under the pressure to reduce the direct manufacturing cost, on one hand, and significant increase in quality requirements to the machined part, including dimensional accuracy and surface integrity. This chapter concentrates on machining of high-silicon aluminum-matrix metal-matrix composites (HSAM MMCs).
1.2.1 Metal-Matrix Composites (MMCs)
Progress in design and manufacturing led to the development of new engineering materials including a wide group of composites. One class of composites being looked at more and more by the aerospace and automotive industries is HSAM MMCs. This group of work materials includes lightweight, and relatively low-strength alloys of aluminum, magnesium, or titanium reinforced by adding second-phase particles, whiskers, fibers, wires, or filaments. For the last 20 years, the focus of interest for the more common automotive and aerospace applications seems to be on aluminum reinforced with particles of silicon carbide or alumina fibers. Applications for these materials are being developed in which heavier steel or iron components are replaced by lighter MMC substitutes. Examples in the automotive industry include engine blocks, transmission cases, brake disks, axels, or, in the leisure industry, items such as tennis racquets. Whether the application is a connecting rod or a tennis racquet, efficiency in use is obtained by reduction in weight, and acceleration is improved by reduction in the inertia of the moving mass.
Progress has been made both in the formulation of alloy compositions and in manufacturing routes. Powdered metallurgy, co-spraying, low-pressure liquid metal infiltration, and die-casting techniques have been developed, and in some of these, near net shape components can be made.
MMCs offer high strength-to-weight ratio, high stiffness, and good damage resistance over a wide range of operating conditions, making them an attractive option in replacing conventional material for many engineering applications. Typically, MMCs are aluminum, titanium, copper, and magnesium alloys, while the reinforcement materials are silicon carbide, aluminum oxide, boron carbide, graphite, etc., in the form of fibers, whiskers, and particles. Probably the single most important difference between fiber-reinforced and particulate composites or conventional metallic materials is the anisotropy or directionality of properties, that is, particulate composites and conventional metallic materials are isotropic, while the fiber-reinforced composites are generally anisotropic. Particulate-reinforced composites offer higher ductility. Their isotropic nature as compared to fiber-reinforced composites makes them an attractive alternative [1]. Although there are a great number of MMCs, HSAM MMCs are of highest usage due to the high volume of automotive manufacturing.
In the context of global competition, manufacturing companies are compelled to improve productivities through the optimizations of their production operations including machining. In the automotive industry, transmission and engine components are made of HSAM MMCs, which have a high strength to weight ratio. Aluminum is cast at a temperature of 650 °C (1,200 °F). It is alloyed with silicon (9 %) and copper (3.5 %) to form the Aluminum Association 380 alloy (UNS A03800) which is widely used in automotive transmissions. Silicon increases the melt fluidity and reduces machinability. Copper increases hardness and reduces ductility. By greatly reducing the amount of copper (less than 0.6 %) the chemical resistance is improved making AA 386 (UNS A03860) well suited for use in marine environments. AA 390 (UNS A03900) with 17 % silicon for high wear resistance is also used in automotive transmissions (for pump covers) and engines (for cylinder castings).
Machining of such alloys presents a great challenge due to their unique properties, namely, a combination of a soft easy-to-adhere Al-matrix and highly abrasive particles including silicon and sludge [2–5]. Figure 1.1a shows a scanning electron microscope (SEM) micrograph of 380 Al—alloy as it is reported to the customer and appears in manufacturing books. In reality, however, real die aluminum castings supplied to automotive plants contain clusters of sludge as shown in Fig. 1.1b. The presence of this sludge and silicon particles make Al-alloys highly abrasive that causes premature tool wear and significant heat generation during machining. The latter causes thermal distortions of the machined parts, resulting in location and diametric errors in machined parts.
1.2.2 Basic Problems with Tools Made of Cemented Carbides
The tool material of choice in modern manufacturing is cemented carbide. The cemented carbides are a range of composite materials, which consist of hard grains of the carbides of transition metals (Ti, V, Cr, Zr, Mo, Nb, Hf, Ta, and/or W) cemented or bound together by a softer metallic binder consisting of Co, Ni, and/or Fe (or alloys of these metals) known as the matrix. The grades of cemented carbide that include only tungsten carbides (WC) in their composition are known as straight grades. Because most of the commercially important cemented carbides contain mostly WC as the hard phase, the terms “cemented carbide” and “tungsten carbide” are often used interchangeably.
Unfortunately, cutting tools made of cemented carbides do not perform well in machining of HSAM MMCs. Low tool life and subpar quality of the machined surfaces became unbearable with introduction of high-speed machining (HSM) in the automotive industry. In the authors’ opinion, abrasion and adhesion are two basic mechanisms of carbide cutting tool wear in machining of HSAM MMCs, although they are not properly addressed in metal cutting studies/tool applications.
Figure 1.2 shows a worn cutting edge and the rounded drill corner as a result of severe abrasion wear. As can be seen, the drill is completely destroyed. Moreover, a significant amount of the tool material should be ground off to restore the drill to working condition. Figure 1.3 shows the typical appearance of abrasive wear. As can be seen, the worn surface contains deep scratches in the direction of sliding left by abrasive particles/solid phases in the work material.
Generally, adhesive wear is thought of as the mechanical transfer of material from one contacting surface to another. It occurs when high loads, temperatures or pressures cause the asperities on two contacting metal surfaces in relative motion to adhere together then immediately tear apart, shearing the metal in small, discrete areas. The surface may be left rough and jagged or relatively smooth due to smearing/deformation of the metal. In general machinery, adhesion occurs in equipment operating in the mixed and boundary lubrication regimes due to insufficient lubricant supply, inadequate viscosity, incorrect internal clearances, incorrect installation or misalignment. This can occur in rings and cylinders, bearings and gears. Normal break-in is a form of mild adhesive wear, as is frosting. Scuffing usually refers to moderate adhesive wear, while galling, smearing and seizing result from severe adhesion. Adhesion can be prevented by lower loads, avoiding shock loading and ensuring that the correct oil viscosity grade is being used for lubrication. If necessary, extreme pressure and anti-wear additives are added to the lubrication oil to reduce the damage due to adhesion.
In metal cutting, adhesion occurs due to mechanical bonding of juvenile freshly-formed surface free of oxides. The harder the contact pressure and the rougher the tool contact surface, the stronger the bonding. Such an adhesion can be referred to as pure adhesion in metal cutting. It takes place at low cutting speeds when the built-up edge on the tool rake face is great. Due to high plastic deformation of the chip and high contact pressures at the tool-chip and tool-workpiece interfaces, extreme pressure and anti-wear additives cannot penetrate into these interfaces [6]. As a result of adhesion of the work material converted into the chip and the tool rake face, the so-called built-up edge (commonly referred to as BUE in the literature on metal machining) is formed. Its appearance is shown in Fig. 1.4. Note the following particularities:
-
1.
BUE forms in machining of the first part.
-
2.
It is well known in steel machining that BUE exists only at low cutting speed. When cutting speed exceeds 60 m/min, BUE disappears [7]. This is not the case in machining of HSAM MMCs where it exists in the whole range of cutting speed being at a maximum at the drill corner (for the drill shown in Fig. 1.4, it is 300 m/min—the maximum speed allowed by the cemented carbide used as the tool material for the application) to the drill center where the cutting speed converges to virtual zero. All attempts to use the most advanced coatings, including CVD microcrystalline diamond coating, did not result in a noticeable reduction of BUE.
The appearance of BUE (Fig. 1.4) is deceptive so that many literature sources in the past and many specialists up to the present believe that BUE can protect the cutting edge from wear. As it appears as a continuation of the cutting edge as shown in Fig. 1.5, it is believed that “stable” BUE can practically eliminate the contact between the tool flank face and the workpiece so that it “protects” the tool flank face reducing its wear. As discussed by Astakhov earlier [8], this “looks-nice” story ends when one realizes that tool life is lowest for the cutting conditions where BUE is greatest. In machining of steel, a reasonable tool life is achieved at high cutting speeds where BUE does not form at all (greater than 60 m/min for common steels).
Figure 1.6 shows the appearance of adhesion wear of a carbide drill in machining of HSAM MMCs. As can be seen, the drill major cutting edges are completely destroyed by “the protector” BUE. This is because BUE is not stable in metal cutting so it changes within each cycle of chip formation [8]. As BUE adheres to the rake face, the adhesion causes mechanical bonding (as glue with a piece of paper). When BUE is periodically removed by the moving chip (as its height becomes sufficient), it brings a small piece of the tool materials with it (as with glue removed from paper). The process repeats itself many, many times so the tool becomes worn by this process. Note that no “welding” (the term often used in the literature) or other physical/chemical processes are involved in adhesion wear.
Abrasion and adhesion are the principle problems encountered in the machining of HSAM MMCs where tool life is significantly shortened causing high tooling cost per unit (a machined part). Moreover, the presence of BUE causes poor surface roughness of machined parts. As a result, tungsten carbide tools are used only for roughing operations because achieving the required diametric accuracy and mirror-shiny surface roughness is impossible particularly in modern HSM. Therefore, PCD is slowly becoming a material of choice for such applications particularly with the wider use of HSM in the automotive industry.
1.3 PCD as the Tool Material
1.3.1 Brief History
The 1797 discovery that diamond was pure carbon catalyzed many attempts to make artificial diamond. If the common carbon of commerce could be turned into diamond, a millionfold increase in the value of the starting material would be obtained. There was, in addition, the interest of the scientific achievement. The earliest claim of success (C. Cagniard de la Tour) was made in the year 1823. The diamond synthesis problem has attracted the interest of thousands. Those pursuing the problem have ranged from charlatans through rank amateurs to the world's greatest scientists. Included among the great are Boyle, Bragg, Bridgman, Crookes, Davey, Despretz, Friedel, Liebig, Ludwig, Moissan, Parsons, Taman, and Wohler.
The literature on diamond synthesis is not very extensive. Most of the work has gone unpublished. Many of the world's large industrial organizations have considered the problem and have spent millions of dollars on research and development. So many years passed without success that those working on the problem felt embarrassed to admit that they were so engaged. Another aspect of the problem was chicanery and fraud. Quite a number have claimed to possess a procedure for converting graphite into diamond, invited the unwary to invest their money, and then vanished.
The earliest successes were reported by James Ballantyne Hannay in 1879 [9] and by Ferdinand Frédéric Henri Moissan in 1893 [10]. Their method involved heating charcoal at up to 3,500 °C with iron inside a carbon crucible in a furnace. Whereas Hannay used a flame-heated tube, Moissan applied his newly developed electric arc furnace, in which an electric arc was struck between carbon rods inside blocks of lime. The molten iron was then rapidly cooled by immersion in water. The contraction generated by the cooling supposedly produced the high pressure required to transform graphite into diamond. Moissan published his work in a series of articles in the 1890s [11].
Despite these early successes, many following scientists experienced difficulty in reproducing such results. Spending nearly 30 years of his life and over £30,000, British scientist Charles Algernon Parsons went through great lengths in order to reproduce a high-pressure high-temperature diamond. While claiming to have produced high-pressure high-temperature diamonds, overtime he reluctantly admitted that no scientist, past or present, could create synthetic diamonds and that the best they could create were spinels, or a simple class of minerals crystallized in an octahedral form [12].
Despite past failures and inspired by the vast industrial uses of diamonds, especially during wartime, General Electric (GE) resumed the synthetic diamond project in 1951. Schenectady Laboratories of GE and a high pressure diamond group formed. In 1954, H. Tracy Hall (General Electric) successfully synthesized the first diamond using a high-pressure high-temperature (HPHT) process. The commercial promise of diamond synthesized by HPHT was fulfilled when GE opened their first production unit in 1956. Since this date, production of HPHT synthetic diamond has increased every year and is currently in excess of 300 tons/year.
In machining where the wear mechanism is mainly abrasion, PCD made using HPHT synthesis has already proved itself to be a superior tool material. Over the last 20 years, PCD has been accepted for volume production of hypereutectic aluminum–silicon alloy components in the automotive industry, the machining of nonferrous alloys of copper, the machining of abrasive plastics and plastic composites such as glass fiber and printed circuit boards, as well as volume machining of wood composites, such as chipboard.
1.3.2 Blanks
The vast majority of PCD tools are actually PCD-tipped tools made from PCD discs manufactured using HPHT. Figure 1.7 shows a PCD disk (blank) and its geometrical parameters. Such a blank consists of a layer of fine diamond powder sintered together into a dense uniform mass, approximately 0.5–2.0 mm thick, supported on a substrate of cemented carbide. The expensive and technically difficult HPHT process used to produce the basic disc has been refined over the years and now PCD blanks are made as discs of up to 74 mm in diameter. By using different grain sizes of diamond and by changing the composition of the diamond and carbide layers, it has been possible to optimize the properties of PCD for specific applications. As a result, various grades are now synthesized by a number of companies around the world. The differences are in stability of sizes (H db and t dl ), quality including consistency and residual stresses, actual composition, and many others which are not specified by PCD blanks manufacturers.
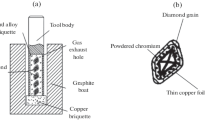
In manufacturing PCD discs, diamond powder and WC-Co substrate are wrapped up in a tantalum (Ta) shell as shown in Fig. 1.8. Such a wrapping is placed is the assembly composed of a solid pressure salt (NaCl) surrounded by the crucible and graphite heater and then placed in the press for sintering. First, pressure is raised to its nominal level with little or no heating. During this stage, all the crystals are being pushed against each other with increasing force. Many diamond particles are sliding relative to each other and many are cracking into two or more fragments with the overall effect of increasing powder density [13]. A coarser powder presents a higher degree of crushing than a fine one as the former includes a much smaller average number of contact points and thus much higher contact stresses (compared to fine powders) that cause the described crushing.
After the crushed and compacted powder is under full pressure, the temperature is raised at a heating rate of 60 °C/min to its nominal value (approx. 1,600 °C). The pressure-temperature condition is always kept in the thermodynamically stable region of diamond above the eutectic temperature of carbon and Co. As the diamond powder is packed against the WC-Co substrate, cobalt is the source for the catalyst metal that promotes the sintering process. When the cobalt reaches its melting temperature of 1,435 °C at 5.8 GPa, it is instantaneously squeezed into the open porosity left in the layer of compacted diamond powder. At this point, the sintering process takes place through a mechanism of carbon dissolution and precipitation. Technically, this process is defined as a pressure-assisted liquid-phase sintering. The driving force for the densification under an extreme pressure is determined by the pressure itself and also by the contact area relative to the cross-sectional area of the particles.
The result of the process is a disc (consisting of the WC-Co substrate and diamond layer of certain thickness strongly bonded with this substrate). In this disc PCD composite is a fully dense mass of randomly oriented, intergrown micron-size diamond-particles that are sintered together in the presence of a metallic catalyst phase, usually cobalt. Small pockets of the catalyst phase, which promotes the necessary intergrowth between the diamond particles, are left behind within the composite material.
Coarse PCD grades are normally used for interrupted and/or high-impact cutting conditions, e.g. in milling where PCDs with 30 μ or even higher grain sizes are recommended by tool manufacturers. When machining under harsh conditions, the differences in PCD wear resistance become more evident, therefore coarse-grained PCD tends to be used in such conditions. When machining under moderately harsh conditions, the PCD wear resistance is of less importance and factors such as edge quality/surface finish must be considered, therefore medium (10–15 μ) and fine-grained (2–8 μ) PCD grades tend to be used. While an ultra-coarse PCD has the theoretical abrasion resistance required for increased performance, the coarseness of the particles results in a substantially rougher cutting edge, which has a significant negative influence in contributing to overall tool performance.
In PCD tool manufacturing, a PCD disc is cut into inserts using wire electro-discharge machining (known as EDMing) or laser cutting in the manner shown in Fig. 1.9. Then the tips are brazed into the pockets made in tool bodies, trimmed and finished using EDMing, laser cutting, or grinding (polishing). Figure 1.10 shows typical cutting tools with PCD inserts.
1.4 Research Advances
Although PCD is a relatively new tool material , its application is expanding in an unparalleled pace in the automotive and airspace industries due to the wide use of challenging work materials combined with machining efficiency requirements imposed by high-speed machining. As a result, a number of problems in the implementation of PCDs are piling up rapidly, and thus require solutions using research advances in the field of machining.
1.4.1 Challenges in Tool Life Testing of PCD Tools
The general mechanisms that govern tool wear of carbide tools are well-known [14]: (1) abrasion, (2) diffusion, (3) oxidation, (4) fatigue, (5) adhesion. The essence of these tool wear mechanisms are well-defined by fundamental research and well documented (for example, Shaw [15], Trent and Wright [16], Astakhov [6]). It is established that one or another mechanism normally prevails in a given machining operation, and thus well-known measures can be taken to reduce tool wear and thus increase tool life [6]. It also allows for the proper selection of the carbide grade and tool geometry, and thus the design of application-specific tools [17]. Unfortunately, no one study has revealed the mechanisms of tool wear of PCD tools . It is pointed out [18] that micro-chipping of the cutting edge due to low toughness of PCD is the prime wear mode [19]. This is particularly true in interrupted cutting of HSAM MMCs, where cutting edges chip readily, and tool consumption is unacceptably high.
Chipping of the cutting edge commonly occurs during the initial stage of an interrupted machining process (common in powertrain veined components such as valve bodies, transmission cases, pump covers, etc.). As these chips accumulate, surface smoothness of the workpiece degrades and burring increases. The composition of conventional PCDs depends on the sintering process by which they are manufactured. Because this process yields unbalanced distributions of diamond particles and a metal catalyst (primarily cobalt), an inverse relationship exists between wear resistance and strength in the tool. Therefore, only very vague recommendations for the selection of PCD grades are available. For example, coarse grades of PCD tools (containing larger diamond particles of 20–30 μ) are the most wear-resistant types, but they also have the least strength and toughness. Conversely, fine grades of PCD (normally 5–10 μ) tools are very tough, but wear resistance is poor. Because of this performance trade-off, medium grades of conventional PCD tools are typically used to machine aluminum parts. As a result, there are no specific recommendations for the selection of a particular PCD grade for a given application. In the automotive industry, a 300–500 % scatter in tool life and unexplained breakage of PCD tools used for similar applications are common. This greatly affects reliability of unattended production lines and manufacturing cells as each premature tool failure results in the scrapping of 10–15 semi-finished parts and up to 30 min line downtime.
In the authors’ opinion, which is based on many-years experience in the field, the lack of information on PCD wear mechanisms can be explained by difficulties of conducting experimental studies at universities and R&D facilities. Tool life of carbide tools used in steel machining is measured in minutes. To carry out tool life tests and thus to obtain information on the wear pattern to reveal the wear mechanism, conventional machines, fixtures and inexpensive standard tools are used. A dozen workpieces (normally standard bars) are typically sufficient to complete the study. This is not nearly the case in machining of automotive aluminum alloys with PCD tools where tool life is measured by tens of thousands of parts. A special, expensive high-speed machine with precision bearings (having spindle runout no more than 0.5 μ at full radial and axial loads with active control and vibration suspensions), special coolants (concentration, filtration, pH, anti-foam and anti-rust additives, etc.), and special non-regrindable cutting tools (cost of a common PCD tool varies from $1,000 to $10,000 depending on tool diameters and number of stages) are needed. Each test point requires a new tool. The listed particularities make PCD testing difficult, expensive, time consuming, and thus not feasible in university labs and small R&D facilities.
Unfortunately, the above-listed known mechanisms of wear cannot be modified correspondingly (accounting for the machining conditions and properties of PCD tool material) to reveal wear behavior of PCD. In the author's opinion, the existing theory of wear developed for general machinery cannot be applied in the considered case due to a number of reasons supported by observations of wear behavior of PCDs in machining of HSAM MMCs. Among them, the following two reasons are outstanding:
-
1.
Wear resistance of a PCD insert improves with increasing grain size. This is the opposite of behavior exhibited by any other material used in tribological joints where the wear-resistance improves with decreasing grain sizes of materials in contact. A number of theories of wear are based on this known fact which holds even in metal cutting for carbide and high speed steel tool materials.
-
2.
Tool life of a PCD tool increases when the tool is allowed a 10 min “rest” between drilling successive holes compared to a PCD tool used in an uninterrupted (continuous) operation (5 s for loading/unloading) as it follows from Fig. 1.11. Not one of the known wear mechanisms is able to explain this phenomenon.
1.4.2 Mechanisms of Tool Wear
1.4.2.1 Macro-Level
A classification of wear patterns of PCD tools is attempted in this chapter in order to help researchers, tool/manufacturing/process engineers and professionals in the field in their assessments of PCD tool quality, wear and operational performance. The face milling tool shown in Fig. 1.12 with PCD-tipped cartridges similar to that shown in Fig. 1.10c is used to represent classification of PCD wear patterns.
Figure 1.13 shows the normal wear pattern of a PCD cartage after completing tool life. Contrary to common perceptions, a detailed microscopic study of this wear pattern shows that when the components of the machining system are coherent and the cutting regime is optimal, chipping does not occur. Rather, a small, pure abrasive-type wear land is the case. This was confirmed with a microscopic study using a scanning-electron microscope (SME).
Figure 1.14 shows SME images of the worn surface at two different magnifications. As can be seen, the wear pattern resembles pure abrasion wear with scratches in the direction of the cutting speed.
Figure 1.15 shows the proper wear pattern of a PCD insert obtained when the tool was run at the optimal cutting conditions and tool life of about 200,000 holes in high-silicon aluminum alloy A380. As can be seen, there is no micro-chipping. Rather, a porosity-like structure of tightly-bonded diamond crystals is developed as the catalysis (cobalt) is taken away by aluminum in the sliding chip. In the authors’ opinion, achieving similar wear patterns is the goal in optimization of a machining operation with a PCD tool.
However, the discussed “ideal” wear of PCD tools occurs rather rarely. Problems occur in PCD tool implementations. Such problems originate in their manufacturing and are enhanced by inherent problems of PCD tool material. This chapter discusses some of these problems which are most common in the practice of PCD tool testing and implementation.
1.4.2.2 Microlevel
The scatter in tool life observed in the practice of testing of tool materials of the same composition and bulk properties from different tool material suppliers may reach 300–400 % which is at least an order greater than that for common engineering materials. The existing tool material classifications (within the same type, e.g. cemented carbide and PCD) are too vague, and thus cannot explain this scatter even in principle. For many years, this fact was not noticed in industry as the machining systems were of subpar quality so the tool performance was determined by other components of the machining system rather than the properties of tool materials. The time has changed and so has the quality of machining systems. Many common excuses for poor tool performance were eliminated. As a result, the difference in the tool material of the same grade but from different tool material manufacturers has becoming more and more noticeable, particularly under challenging applications where tool performance is closely monitored.
If one would attempt to find the difference in the mechanical, physical, chemical, etc. properties of a particular tool material, e.g. a PCD grade, to understand the discussed scatter in tool performance than he or she finds that:
-
Only very few, mostly irrelevant to the cutting performance, properties of a particular grade of the tool material are actually available in colorful catalogs of various tool companies.
-
Contacting the tool material supplier does not help to answer the questions and/or to resolve the issue with the performance of a particular tool material because: (a) The contactor is not sure what to ask for, and (b) The supplier may not have, or may not willing to reveal, the requested property or properties as they are considered “proprietary information”.
A great number of manufacturing processes and alternatives exist in tool materials manufacturing that may result in the wide range of their performance results although the ingredients of a particular tool material can be absolutely the same. The difference in the cost of apparently same tool material from different tool material suppliers can be significant. Moreover, often the bulk mechanical and physical properties of this material are practically the same as reported by various suppliers, so the difference in performance cannot be explained using the known properties and characteristics. As a result, it is rather difficult to justify the higher cost of a tool material of presumable better quality if the reported composition and bulk properties of this material are the same as those of a cheaper one. Unfortunately, many companies do not have time, funding, and sufficiently trained personnel to carry out the full tool life investigation to distinguish the difference in the performance between these two materials so the cheaper tool material is normally selected.
In the authors’ opinion, this problem should be solved particularly because the tool materials and cutting tools have become more and more expensive, and because unattended manufacturing is in wider and wider use. To do this, a fresh look at the physics of wear of tool materials should be taken with no blinds imposed by the known mechanisms of wear used in general machinery. This section describes the foundation of such an approach presenting/explaining some known facts and setting the stage for the further research and development on the matter.
Modern tool materials are composite materials so their structure includes at least two distinctive solid phases: extremely hard non-metallic particles (WC, diamond, boron nitride, Al2O3, etc.) are held together by the matrix material (for example, cobalt). As these materials are sintered/shaped, great deformation and temperatures are applied, interfacial defects as nano- and microcracks, residual stress and even defects of the atomic structure occur. Microcracking can be attributed to a mismatch of the thermal expansion coefficients between the phases, as well as from thermal expansion anisotropy of the phases.
These defects evolve upon cooling, starting from a stress-free high-temperature state, and specifically in areas with large grain-to-grain misorientation [20]. These defects (examples at different magnifications are shown in Fig. 1.16) lower the strength, wear resistance and other useful mechanical and physical properties of tool materials. Moreover, as the population of these defects is of random nature, the great scatter in the performance data is observed in practice. This scatter presents a serious problem in tool materials.
Although inter-granular wear, grain cleavage, peeling and spalling of grains have been suggested as possible wear mechanisms of PCD [21–23], only qualitative descriptions of suggestive nature are normally provided to explain these mechanisms. Moreover, these defects are considered to be formed due to high temperature and stresses in machining while the microdefects due to PCD sintering were not considered. No quantitative characterization of microcracks and their effect on the wear patterns were attempted.
The equation of physical state of solids [20] can be used as the basis to reveal the wear mechanism of PCDs that deals with diamond/diamond interfacial defects (stresses and microcracks) closely coupled in the processes of the generation and propagation of many small cracks occurred at the diamond/cobalt interfaces. However, the role of micro cracking is complex and not well understood on a quantitative basis. Better understanding requires an ability to quantify the crack morphology and how that structure evolves due to stress and temperature fields occurring in metal cutting of high-silicon aluminum alloys.
The complexity and strong coupling of micro cracking to thermal and mechanical properties has been widely recognized [24–27] but is still not well understood and thus correlated with tool wear . Improved understanding is hampered by (a) an inability to quantify micro cracking, (b) limited observations of microcrack array responses to loads, and (c) limited evaluations of the mechanics models which propose to explain the role of microcracks and their role in tool wear mechanisms [28–30]. In the author's opinion, the first and foremost stage in further PCD development should be concerned with the quantification of microcrack structure [31]. Special techniques to classify, count and measure the length, spacing and orientation of microcracks should be developed for PCDs and other tool materials using the available methodologies and test equipment (e.g., the electron channeling contrast methodology using CamScan 44 FE SEM equipped with a Schottky thermal field emission gun [32]). Digital image processing should be applied to take the measurements, thereby providing time effective, reproducible, statistically significant estimates of crack morphology. A key feature of the image processing strategy is to abstract the crack structure to a set of medial axis lines.
As discussed above, abrasive wear is predominant in the practice of PCD applications. Abrasive wear is a phenomenon familiar to all and, like so much which relates to real-life, it is controlled by a subtle interplay of many effects. This makes the task of constructing a generalized model of the process from scratch extremely ambitious. In the author's opinion, this can now be attempted thanks to decades of painstaking research by many workers into different aspects of abrasion and provides the foundation on which a generalized model may be built.
The focus of a new model should be on the interatomic bonds of a solid and their consequences for wear behavior. The theory of the non-equilibrium thermodynamics of solids [20] can be used to understand the response of PCD to forces, heat, and other energy fields in metal cutting. The internal pressure or stress in PCD is defined as the vector representing the resistance to volume change, P = d F/ds, where F is the force of atomic interaction and s is the surface area enclosing a volume. The thermodynamic equation of state [20] PV = s(N,V,T,P)T, where s is the entropy vector and P is the magnitude of P, should be used at the most general level of wear studies. The state of PCD is defined to be the shape of the rotos resulting from the diamond (not PCD) sintering process. A rotos is a closed dynamic cell of solids. The equation of state relates the temperature of the solid structure to its ability to generate resistance forces. Compressions are those atoms located on the decreasing portion of a bond force minimum and provide resistance under heat absorption, and dilatons are those located on the increasing portion to a force maximum and offer resistance in heat radiation. The compression-dilaton pattern of the bonds in a structure determines its response to loads, temperature, and other environmental conditions. Influence on the size effect, stresses, and aging in the wear-resistance response of PCD should be studied accounting for the number of interfacial defects (microcracks and voids) in PCD and the possibility of crack growth and crack coalescence that lead to micro and then to macro chipping of PCD.
The description of dynamic loading and wear due to this loading can be developed from the equation of state to explain the physical nature of the time-dependent response. The increased resistance influences the initiation and propagation of microcracks that, according to this equation, are related to entropy. The thermodynamic potentials of PCDs with varying number of interatomic bonds can then be derived to develop parameters of state using the periodic law of variations in state including mechanical hysteresis and its effects on the dilatons/compressions transformations. The theory of strength in such a development is a generalization of the kinetic theory of strength which postulates that thermal fluctuations are a key in breaking atomic bonds that can be directly correlated with wear. Wear, chipping and fracture are attributed to what is called the Maxwell-Boltzmann factor (from the distribution of energy states), which describes the concentration density and energy of particles in a given region of the solid and which introduces stress-concentrations. Breaking of bonds releases internal energy. Fracture and wear, however, are considered to be thermally dependent processes governed by the intensity of the potential field caused by stress and temperature anisotropy in the presence of dilaton traps.
The first level of the development of such a model uses the equation of physical state of solids [20]. Diffusion self-healing can be briefly explained as follows.
Centuries-old buildings have been said to have survived these centuries because of the inherent self-healing capacity of the binders used for cementing building blocks together. This self-healing capacity has never been termed like that. This is a well-known fact that was seen as the forgiveness of nature rather than self-healing of inherently smart building materials. Today, the field of self-healing is considered a new area of materials research. It was in 2001 that White et al. [33] published their results on self-healing in polymer-based systems by microencapsulated healing agents. This and related research in other fields of materials science was the result of an initiative by NASA launched amongst selected top institutes in the USA in 1996. Since then the field has been developing rapidly. Because healing presupposes the presence of a defect and a defect generally emerges at a very small scale, probably at the nanoscale, it is not surprising that self-healing is one of the promising application fields of nanotechnology.
Komarovsky and Astakhov [20] presented the complete theory of diffusion healing of composite materials showing that practically all of these materials can be considered as self-healing materials. A number of advanced methods of self-healing are suggested in this publication. Today our understanding of self-healing materials is as follows. Self-healing materials are man-made materials, which have the built-in capability to repair structural damage autonomously or with some help of an external stimulus (energy fields [20]). The initial stage of failure in materials is often caused by the occurrence of small microcracks/microdefects (even at atomic-molecular scale) throughout the material. The proper characterization of such microdefects can help explain the ranging of tool materials quality formed in their manufacturing so that the cost of better tool materials can be easily and quantitatively justified. The proper application of the external energy fields to enhance diffusion healing of such microdefects in composite tool materials in order to improve their quality should be the goal of the first stage of the development of better tool materials. The next stage can be development of truly self-healing tool materials. In such materials, the occurrence of these microcracks is 'recognized' in some way. Subsequently, mobile species, e.g. atoms, have to be triggered to move to these places and perform their self-healing capacity. These processes are ideally triggered by the occurrence of damage itself, in which case it is called an autonomous self-healing event. In practice one could well imagine self-healing that is triggered by an external stimulus greatly enhances the reliability and durability of tool materials.
The self-healing takes place in machining. For example, tool life of a PCD tool increases when the tool is allowed a 10 min 'rest' between drilling successive holes compared to a PCD tool used in an uninterrupted (continuous) operation (5 s for loading/unloading) as it follows from Fig. 1.11. Not one of the known wear mechanisms is able to explain this phenomenon.
Diffusion healing of tool materials has been known since the 1950s although no one study or other literature source refer to these known treatments as such. This is because such treatments are covered by the generic name “cryogenic treatment”. Such a name is misleading as much more complicated thermomechanical processes that involve a combination of deep freezing and tempering cycles. Generally, they can be described as a controlled lowering of temperature from room temperature to cryogenic temperature, maintenance of the temperature for hours, followed by a controlled rising of the temperature. As an example, Fig. 1.17 shows the process diagram of modern cryogenic treatment used to enhance wear resistance of cemented carbides suggested by Gill et al. [34].
The Diffusion© process was developed by Diffusion Ltd. (Windsor ON, Canada) primarily for use in the automotive industry for enhancing and stabilizing properties of the tool materials. This is because severe working conditions and the absence of the so-called safety factor used in the part design can easily contrast the difference in the performance of the original tools and those treated by diffusion. The preliminary testing showed 50–200 % improvement in tool life of various tool materials. Diffusion© process has also been used for improving the performance of structural components of the engines, transmissions, body parts etc. including Canadian NASCAR engines. Preliminary test results showed that the tougher working conditions of a structural component, the greater performance results are achieved due to the treatment.
1.4.3 Casting Defects and PCD Tool Wear/ Failure
Casting defects are principal “enemies” of PCD tools as such tools are very sensitive to these defects. This section attempts to classify the known casting defects and their effects on PCD tool failure.
The casting defects affecting drill performance can be classified as follows:
-
Porosity and microinclusions
-
Casting cavities
-
Macroinclusions
Because the nature of porosity and content of inclusions depends on a particular material, method of casting and many other metallurgical particularities, the further consideration is restricted to HSAM MMCs because they are the materials of choice for many automotive and aerospace parts as well as domestic food and pump part castings. Other applications include components for food handling and marine castings.
Hydrogen is the only gas that is appreciably soluble in aluminum and its alloys. Porosity in aluminum is caused by the precipitation of hydrogen from the melt or by shrinkage during solidification, and most often by a combination of these defects [35]. Dissolution of hydrogen in large amounts in aluminum castings results in hydrogen gas porosity. For castings that are a few kilograms in weight the pores are usually 0.01–0.5 mm (0.00039–0.020 in) in size [36]. In larger casting they can be up to a millimeter (0.040 in) in diameter. Standard ISO 10049:1992 “Aluminium alloy castings—Visual method for assessing the porosity” specifies a method of inspection and describes the acceptance conditions, tabulates the severity levels, i.e. number and size of pores, and the interpretation of the results.
Pores that form in the matrix of aluminum alloy castings lead to significant deterioration in casting quality. In brief, porosity is formed and distributed in the matrix of the casting. The pore count of aluminum-silicon alloys can be affected by the solidification mode of the alloy, the amount of oxide film and/or particle inclusions, the cooling rate, the atmospheric pressure and the hydrogen level in the melt.
Porosity and porosity-size inclusions present a serious challenge in high-speed machining of HSAM MMCs as a myriad of pores should be thought of as countless number of small sharp razor-type edges enhanced by hard SiC microinclusions. These literally shave the tool when their amount is excessive. It takes its toll heavily on carbide tools. Figure 1.18a shows an example of an excessively worn carbide drill and Fig. 1.18b shows the appearance of severe porosity in the sectioned machined hole. PCD tools, however, can withstand small porosity much better although tool wear is increased. Figure 1.19 shows excessive abrasive wear of a PCD cartage in the presence of casting porosity.
Unfortunately, there is no standard/procedure/methodology for microporosity and microinclusions assessment in terms of their influence on tool wear. As a result, the problem should be dealt with on a case-by-case basis.
Figure 1.20 shows the appearance of casting cavities and hard inclusions on a milled surface. Casting cavities are large shrinkage cavities. Nonmetallic inclusions are a particular concern in cast aluminum. Because of its reactivity, aluminum oxidizes readily in liquid and solid states. Oxidation rate is greater at molten metal temperatures and increases with temperature and time of exposure. Magnesium in aluminum alloys oxidizes and with time and temperature reacts with oxygen and aluminum oxide to form spinel. Many oxide forms display densities similar to that of molten aluminum and sizes that reduce the effectiveness of gravimetric separation. Also, most oxides are wet by molten aluminum, reducing the effectiveness of mechanical separation methods. Inclusions occur as varying types with differing sizes and shapes. Aluminum oxides are of different crystallographic or amorphous forms as films, flakes, and agglomerated particles. Magnesium oxide is typically present as a fine particulate. Spinels can be small, hard nodules or large complex shapes.
Surprisingly, PCD tools can handle casting cavities and inclusions much better than carbide tools. However, it is true only for relatively small cavities and inclusions. When the size of these defects increases, PCD inserts are chipped. Figure 1.21a shows chipping of the PCD insert within the PCD layer that happens when the size of defects just exceed what the PCD tool material can handle. Further increases in the size of the discussed casting defects lead to a bulk fracture of PCD inserts through the carbide substrate (Fig. 1.21b).
1.4.4 Thermal Stability as the Weakest Link of Conventional PCDs
The structure and the origin of strength/hardness of PCD is principally different compared to other tool materials as, for example, cemented carbide. On HPHT sintering of PCD, cobalt acts as a catalyst. It is recognized that this catalytic effect promotes diamond-to-diamond bonds and unitized the diamond layer to the sintered carbide substrate. The result is an abrasion-resistant tool material suitable for machining use. This explains why coarse-grained PCD grades have greater strength and abrasion resistance as such grades have larger areas of diamond-to-diamond interfaces. Formed on HPHT sintering of PCD, the integrity of diamond-to-diamond interfaces should be preserved during PCD tool manufacturing and use.
The major, though not fully recognized and thus not accounted for, problem is that PCD has limited thermal resistance. At atmospheric pressure, a surface of diamond turns to graphite at 900 °C. In a vacuum or inert gas, diamond does not graphitize easily, even at 1,400 °C. But during use, PCD cutters experience a sharp decline in tool performance when they experience temperatures around 700 °C in their manufacturing and/or machining.
The presence of cobalt is the major reason that PCD converts to graphite at a lower temperature than simple diamond. Thermal degradation occurs due to the presence of cobalt in the interstitial regions and the adherence of cobalt to the diamond crystals. Specifically, cobalt is known to cause an undesired catalyzed phase transformation in diamond (converting it to carbon monoxide, carbon dioxide, or graphite) with increasing temperature [37–40]. As temperature increases, graphitization of the diamond in the presence of cobalt becomes a dominant effect. PCD wear is then due to allotropic transformation into graphite or amorphous carbon under influence of cutting force and cutting temperatures. This transformation is accelerated in the presence of cobalt through a combination of mechanical and chemical effects. Figure 1.22 shows a schematic representation of this process.
The second significant cause of thermal degradation of PCDs is a significant difference between the thermal expansion coefficients of cobalt and diamond. Thermal degradation due to differential thermal expansion characteristics between the interstitial catalyst material and the intercrystalline bonded diamond. Such differential thermal expansion is known to occur at temperatures of about 400 °C, causing ruptures to occur in the diamond-to-diamond bonding, and resulting in the formation of cracks and chips in the PCD structure. Chipping and micro-cracking of cutting edges occur as a result [41–43].
These issues limit practical use of conventional PCD tool materials to about 700 °C. This fact presents a significant yet not fully understood, and thus recognized problem, in PCD tool manufacturing and implementation practices. While high temperatures may occur in cutting at high cutting speeds and feeds, it is not likely that it occurs in many cases of machining of high thermal conductivity work material (e.g., aluminum alloys) with high-flow rate water-based coolant supplied through tools. If this was the case, all PCD tools would fail. This is not the case as most of the tools complete tool life.
Therefore, the thermal damage to PCD inserts likely occurs during PCD tool manufacturing. The critical manufacturing operations in terms of thermal damage to PCD are brazing, EDMing and grinding because temperatures generated during these operations can be well above 700°. Figure 1.23 shows some results of tool overheating in its manufacturing.
1.4.5 Developing Thermal Stable Grades
The technologies to manufacture a two-layer composite of diamond-containing/cemented tungsten carbide substrate which has a combination of high wear resistance and high thermostability are under active development. Up to date, attempts to improve thermal stability of PCD and thus to manufacture thermally stable PCD (TSPCD), can be generally classified as:
-
1.
Removal of the catalyst/solvent phase from the PCD material, either in the bulk of the PCD layer or in a volume adjacent to the working surface of the PCD tool (where the working surface typically sees the highest temperatures in the application because of friction events). This is commonly known as cobalt leaching.
-
2.
Two-step sintering.
-
3.
Use of a ceramic matrix material such as SiC as the catalysis.
1.4.5.1 Cobalt Leaching
As a means of improving PCD thermal resistance, polycrystalline diamonds have been created that are not bound to metallic base material, i.e. the matrix metal is completely removed by post-process treatments. Although the resultant material can withstand temperatures up to 1,200 °C, it lacks sufficient hardness and impact strength to be used as a cutting tool material. This is due to cavities that remain in the PCD after the catalyst (matrix) metal is removed.
As an alternative, another technology called cobalt leaching has been developed. The essence of cobalt leaching is as follows (U.S. Pat. Nos. 4,224,380 and 4,288,248). After finishing the PCD conventionally, the metallic phase can be removed from the compact by acid treatment, liquid zinc extraction, electrolytic depleting or similar processes, leaving a compact of substantially 100 % abrasive particles. Cobalt is removed up to 200 μ deep into the PCD layer. As the cobalt phase remains inside the PCD layer, the loss of overall strength is not as significant as with other methods of TSPCD manufactured using other known methods.
Leaching a thin layer at the working surface dramatically reduces diamond degradation and improves the tool's thermal resistance. First, with no cobalt, the diamond-to-diamond bonds remain strong as little graphitization and cracking due to a mismatch of thermal expansion properties occurs at high processing and cutting temperatures. Second, the heat conduction of the contact diamond surface increases, thus the heat is transmitted faster from the tool-chip and tool-workpiece that lowers the maximum cutting temperature.
Figure 1.24 shows wear curves for leached and a non-leached PCD cartridges. As can be seen, after about 12,000 cycles, the wear rate of the unleashed cutter increased dramatically. The leached PCD cutter, in contrast, maintained a relatively constant wear rate for about 15,000 cycles.
Wear curve for: (1) conventional PCD, and (2) leached PCD. Cutting conditions: face milling with the tool shown in Fig. 1.12, work material A380, cutting speed—930 m/min, cartridge tool-in-holder rake angle—0°, normal clearance angle—10°, 20-μ PCD grade
There are several important issues associated with this approach (cobalt leaching) to achieving improved thermal stability . The prime concern is that a continuous network of empty pores result from the leaching, possessing a substantially increased surface area, which can result in increased vulnerability to oxidation (particularly at higher temperatures). This can then result in reduced strength of the PCD cutter at high temperatures. The second concern is the time needed for leaching, and thus its cost. For example, according to US Patent No. 4,288,248, between 8 and 12 days and strong chemicals are used, e.g., concentrated hot acid solutions.
1.4.5.2 Two-Step Sintering
Two-step sintering results in the formation of a bi-layered sintered PCD disc which includes a thermally stable top layer. According to US Patent No. 4,944,772, a leached PCD compact and a cemented carbide support are separately formed. An interlayer of unsintered diamond crystals (having a largest dimension of 30–500 μm) is placed between the carbide and TSPCD layer. A source of catalyst/sintering aid material is also provided in association with this layer of interposed crystals. This assembly is then subjected to the HPHT process, sintering the interlayer and bonding the whole into a bi-layered supported compact. In this application, appreciable re-infiltration of the TSPCD layer is not seen as advantageous, but the requirement for some small degree of re-infiltration is recognized in order to achieve good bonding.
The method according to US Patent No. 5,127,923 is an improvement to this approach, where a porous TSPCD layer is reattached to a carbide substrate during a second HPHT cycle, with the provision of a second “inert” infiltrant source adjacent to the surface of the TSPCD compact removed from the substrate. Infiltration of the TSPCD body with this second infiltrant prevents significant re-infiltration by the catalyst metal of the carbide substrate. When carefully chosen, it does not compromise the thermal stability of the previously leached body. A suitable infiltrant, such as silicon for example, must have a melting point lower than that of the substrate binder.
The disadvantage of the bi-layer sintered PCD is the formation of high internal stresses because of the significant differences in properties between the leached/porous layer, the underlying sintered PCD, and carbide substrate. This is exacerbated by the mono-lithic nature of the leached compact and often causes cracking at the PCD-substrate interface or through the PCD layer itself during the second HPHT cycle. Furthermore, the reattachment process itself can be difficult to control such that appreciable re-infiltration of the TSPCD layer does not occur during the second HPHT cycle. Moreover, multi-step sintering processes are both time consuming and labor intensive. It is, therefore, desired that a thermally stable PCD material be developed in a relatively simple single-step manufacturing process.
This ambitious goal can be achieved if two catalyst metals are used simultaneously in the HPHT process. Figure 1.25 shows the model of the HPHT sintering method with double-sided infiltration of diamond micropowder by molten silicon and Co-WC-C melt from cemented tungsten carbide substrate. By changing the pressure and heating rate, this method allows a change in the size of intermediate polycrystalline diamond-Co layer [44].
Starting and finishing stages of structure formation of thermally stable PCD blanks in HPHT-sintering: a start of infiltration of the diamond layer from one side by liquid Si and from other side by Co-WC-C melt, b finished three-layered composite—top cutting layer is a thermally stable diamond-SiC composite
The SME and X-ray diffraction microanalysis of the structure of the produced samples have shown that a thin transition zone containing 8–12 % cobalt forms between the carbide substrate and the diamond layer during the sintering as shown in Fig. 1.26. The thickness of the transition zone approximately corresponds to the particle size of the initial diamond powder, i.e. the cobalt melt penetrates into the diamond layer to a depth of no more than 40 μm. The amount of cobalt at the carbide-transition zone interface is essentially higher than in the substrate. At a distance of above 40 μm from the substrate the diamond layer exhibits no cobalt. Free silicon is also not observed and this is supported by X-ray diffraction analysis.
The test results showed considerable increases in tool life (up to 2 times), allowable penetration rate (by 30 %) and thermal stability (up to 1,100 °C) of the cutters including the thermal stable inserts manufactured using this method.
References
Shin YC, Dandekar C (2012) Mechanics and modeling of chip formation in machining of MMC. In: Davim JP (ed) Machining of metal matrix composits. Springer, London, pp 1–50
Hung NP, Boey FYC, Khor KA, Phua YS, Lee HF (1996) Machinability of aluminum alloys reinforced with silicon carbide particulates. J Mater Process Technol 56(1–4):966–977
Andrewesa CJE, Feng H-Y, Laub WM (2000) Machining of an aluminum/SiC composite using diamond inserts. J Mater Process Technol 102(1–3):25–29
El-Gallab M, Sklad M (1998) Machining of Al/SiC particulate metal matrix composites. Part I. Tool performance. J Mater Process Technol 83(2):151–158
Tomac N, Tonnessen K (1992) Machinability of particulate aluminum matrix composites. CIRP Ann Manuf Technol 41:55–58
Astakhov VP (2006) Tribology of metal cutting. Elsevier, London
Zorev NN (1966) Metal cutting mechanics. Pergamon Press, Oxford
Astakhov VP (1998/1999) Metal cutting mechanics. CRC Press, Boca Raton
Hannay JB (1879) On the artificial formation of the diamond. Proc R Soc Lond 30(200–205):450–461
Royère C (1999) The electric furnace of Henri Moissan at one hundred years: connection with the electric furnace, the solar furnace, the plasma furnace? Ann Pharm Fr 57(2):116–130
Moissan H (1994) Nouvelles expériences sur la reproduction du diamant. C R Acad Sci Paris 118:320–341
Davis RF, Films Diamond (1993) Diamond films and coating. Noyes Publications, Park Ridge
Uehara K, Yamaya S (1988) High pressure sintering of diamond by cobalt infiltration. Int J Refract Met H 7(4):219–223
Astakhov VP, Davim PJ (2008) Tools (geometry and material) and tool wear. In: Davim PJ (ed) Machining: fundamentals and recent advances. Springer, London, pp 29–58
Shaw MC (2004) Metal cutting principles, 2nd edn. Oxford University Press, Oxford
Trent EM, Wright PK (2000) Metal cutting, 4th edn. Butterworth-Heineman, Woburn
Astakhov VP (2010) Geometry of single-point turning tools and drills. Fundamentals and practical applications. Springer, London
Stenphenson DA, Agapiou JS (1996) Metal cutting theory and practice. Marcel Dekker, New York
El-Gallab M, Sklad M (2000) Machining of Al/SiC particulate metal matrix composites. Part III. Comprehensive tool wear models. J Mater Process Technol 101:10–20
Komarovsky AA, Astakhov VP (2002) Physics of strength and fracture control: fundamentals of the adaptation of engineering materials and structures. CRC Press, Boca Raton
Miklaszewski S, Zurek M, Beer P, Sokolowska A (2000) Micromechanism of polycrystalline cemented diamond tool wear during milling of wood-based materials. Diam Relat Mater 9(3–6):1125–1128
Bai QS, Yao YX, Bex P, Zhang D (2004) Study on wear mechanisms and grain effects of PCD tool in machining laminated flooring. Int J Refract Met Hard Mater 2–3:111–115
Philbin P, Gordon S (2005) Characterisation of the wear behaviour of polycrystalline diamond (PCD) tools when machining wood-based composites. J Mater Process Technol 162–163:665–672
Ortiz M, Suresh S (1993) Statistical properties of residual stresses and inter-granular fracture in ceramic materials. J Appl Mech 60:77–84
Evans AG (1978) Microfracture from thermal expansion anisotropy–I. Acta Metall 26:1845–1853
Evans AG, Fu Y (1985) Some effects of microcracks on the mechanical properties of brittle solids—II. Microcrack toughening. Acta Metall 33(8):1525–1531
Laws N, Lee JC (1989) Microcracking in polycrystalline ceramics: elastic isotropy and thermal anisotropy. J Mech Phys Solids 37(5):603–618
Yousef SG, Rodel J, Fuller ER Jr, Zimmermannz A, El-Dasher BS (2005) Microcrack evolution in alumina ceramics: experiment and simulation. Am Ceram Soc 88(10):2809–2816
Kim BN, Naitoh H, Wakayama S, Kawahara M (1996) Simulation of microfracture process and fracture strength in 2-dimensional polycrystallinematerials. JSME Int J 39(4):548–554
Kim BN, Wakayama S (1997) Simulation of microfracture process of brittle polycrystals: microcracking and crack propagation. Comput Mater Sci 8:327–334
Yurgartis SW, MacGibbon BS, Mulvaney P (1992) Quantification of microcracking in brittle-matrix composites. J Mater Sci 27:6679–6686
Crimp MA (2006) Scanning electron microscope imaging of dislocations in bulk materials, using electron channeling contrast. Microsc Res Tech 69:374–381
White SR, Sottos NR, Moore J, Geubelle P, Kessler M, Brown E, Suresh S, Viswanathan S (2001) Autonomic healing of polymer composites. Nature 409:794–797
Gill SS, Singh R, Singh H, Singh J (2009) Wear behavior of cryogenically treated tungsten carbide inserts under dry and wet turning conditions. Int J Mach Tools Manuf 49:256–260
Zolotorevsky VS, Belov NA, Glazoff MV (2007) Casting aluminum alloys. Elsevier, Oxford
Chen Y-J (2009) Relationship between ultrasonic characteristics and relative porosity in Al and Al-XSi alloys. Mater Trans 50(9):2308–2313
Cook MW, Bossom PK (2000) Trends and recent developments in the material manufacture and cutting tool application of polycrystalline diamond and polycrystalline cubic boron nitride. Int J Refract Metal Hard Mater 18:147–152
Coelho RT, Yamada S, Aspinwall DK, Wise MLH (1995) The application of polycrystalline diamond tool materials when drilling and reaming aluminium based alloys including MMC. Int J Mach Tool Manuf 35:761–774
Fedoseev DV, Vnukov SP, Bukhovets VL, Anikin BA (1986) Surface graphitization of diamond at high temperatures. Surf Coat Technol 28:207–214
Shimada S, Tanaka H, Higuchi M, Yamaguchi T, Honda S, Obata K (2004) Thermo-chemical wear mechanism of diamond tool in machining of ferrous metals. CIRP Ann Manuf Technol 53:57–60
Miess D, Rai G (1996) Fracture toughness and thermal resistance of polycrystalline diamond compacts. Mater Sci Eng A 209:270–276
Vandenbulcke L, De Barros MI (2001) Deposition, structure, mechanical properties and tribological behavior of polycrystalline to smooth fine-grained diamond coatings. Surf Coat Technol 146–147:417–424
Chen Y, Zhang LC, Arsecularatne JA (2007) Polishing of polycrystalline diamond by the technique of dynamic friction. Part 2: material removal mechanism. Int J Mach Tool Manuf 47:1615–1624
Osipov AS, Bondarenko NA, Petrusha IA, Mechnik VA (2010) Drill bits with thermostable PCD inserts. Diam Tooling J 3:31–34
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
Astakhov, V.P., Stanley, A. (2015). Polycrystalline Diamond (PCD) Tool Material: Emerging Applications, Problems, and Possible Solutions. In: Davim, J. (eds) Traditional Machining Processes. Materials Forming, Machining and Tribology. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-662-45088-8_1
Download citation
DOI: https://doi.org/10.1007/978-3-662-45088-8_1
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-662-45087-1
Online ISBN: 978-3-662-45088-8
eBook Packages: EngineeringEngineering (R0)