Abstract
Sympathetic reinnervation of the transplanted heart is a unique example of the plasticity and regenerative capacity of the autonomic nervous system. Radionuclide imaging studies have played a key role in demonstrating that cardiac allografts regain catecholamine storage capacity, i.e., functional sympathetic nerve terminals after complete denervation due to transplant surgery. Since its initial demonstration, the regionally heterogeneous pattern of reinnervation, its time course and determinants, as well as its functional effects on the transplanted heart have been described in detail, as summarized in this chapter.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Heart Rate Variability
- Transplant Recipient
- Sympathetic Innervation
- Sympathetic Nerve Fiber
- Sympathetic Nerve Terminal
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
17.1 Physiology of the Transplanted Heart
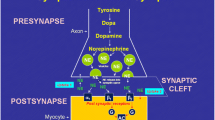
At cardiac transplantation, postganglionic sympathetic nerve fibers of the donor heart are surgically interrupted, causing rapid depletion of norepinephrine within the nerve terminal and thus resulting in complete denervation (Cooper et al. 1962). This state of denervation explains the typical hemodynamic alterations that are encountered early after successful transplantation: Baseline heart rate is increased, there is chronotropic incompetence (i.e., lack of sufficient increase of heart rate) during exercise (Quigg et al. 1989), and diastolic ventricular function is slightly reduced (Paulus et al. 1992). As a consequence, exercise capacity in transplant recipients often remains reduced compared to healthy normal subjects (Kao et al. 1994).
It has also been suggested that presynaptic denervation of the cardiac allograft influences postsynaptic adrenergic signal transduction. Increased catecholamine sensitivity has, e.g., been described, which has mainly been attributed to loss of presynaptic neuronal uptake capacity (von Scheidt et al. 1992). Overall postsynaptic ß-adrenergic receptor density has been found to be normal in allografts (Denniss et al. 1989), but a shift in subtype from ß1- to ß2-adrenoceptors has been discussed, with possible implications for the response to systemic catecholamines (Leenen et al. 1995). Because of the reliance of denervated hearts on circulating catecholamines, concerns have been raised against the therapeutic use of ß-blockers in transplant recipients (Bexton et al. 1983). Some early studies have suggested detrimental effects of ß-blockade on exercise capacity and attributed their observations to cardiac denervation (Verani et al. 1994), but other studies did not confirm negative effects (Gilbert et al. 1989; Bengel et al. 2004).
The occurrence of sympathetic reinnervation after transplantation has first been reported in various animal models (Willman et al. 1964; Norvell and Lower 1973). In humans then, initial evidence was derived from reoccurrence of anginal symptoms (Stark et al. 1991), invasive measurements of tyramine- or handgrip-induced cardiac spillover of norepinephrine (Wilson et al. 1991), electrophysiologic measurements of heart rate variability (Kaye et al. 1993), and non-invasive imaging of the myocardial uptake of radiolabeled norepinephrine analogues (Schwaiger et al. 1991; DeMarco et al. 1995). Among those techniques, non-invasive PET and SPECT imaging have been especially helpful in understanding the phenomenon of sympathetic neuronal regeneration, its pattern, determinants, and physiologic importance.
17.2 The Denervated Transplanted Heart as a Model to Test Specificity of Neuronal Imaging Agents
Before discussing the process of reinnervation in more depth, it should be noted that the denervated heart early after transplantation is a useful model to test the specificity of neuronal imaging agents. Owing to the absence of any neuronal uptake and storage sites in these hearts, any fraction of myocardial retention of a neuronal imaging agent would point towards a nonspecific mechanism. In the initial publication reporting feasibility of C-11 metahydroxyephedrine ([11C]-mHED) for PET imaging of cardiac sympathetic innervation, e.g., Schwaiger et al. used early transplant recipients to show absence of myocardial [11C]-mHED retention when compared to healthy volunteers and to thereby prove usefulness of the agent (Schwaiger et al. 1990). Likewise, the specificity of other clinical sympathetic neuronal imaging agents such as I-123 metaiodobenzylguanidine ([123I]-MIBG) (DeMarco et al. 1995) or [11C]-epinephrine (Munch et al. 2000) has also been supported by the absence of significant myocardial retention in human allografts early after transplantation.
The postsynaptic receptor side, on the other hand, may remain completely unaffected despite the absence of presynaptic nerve terminals after transplantation. This has been suggested for adrenergic receptors using tissue analysis (Denniss et al. 1989), while PET imaging with [11C]-MQNB has been used to study the parasympathetic muscarinic receptors, which also were unaffected by denervation (Le Guludec et al. 1994).
17.3 Transplant Reinnervation: Pattern, Time Course, and Determinants
The first imaging evidence of human allograft sympathetic reinnervation was obtained at >1 year after transplantation using PET with [11C]-mHED, which showed reappearance of significant regional tracer retention in basal anterior left ventricular myocardium (Schwaiger et al. 1991) (Fig. 17.1). Likewise, using [123I]-MIBG and SPECT, visible cardiac catecholamine uptake was shown in approximately 50 % of patients at 1–2 years after transplantation (DeMarco et al. 1995). Further studies have greatly improved the understanding of the reinnervation process:
Polar maps of myocardial retention of C-11 metahydroxyephedrine ([11C]-mHED) in four cardiac transplant recipients at different times after surgery, illustrating time course and regional extent of sympathetic reinnervation (Reprinted with permission by Springer Science and Business Media, from Bengel and Schwaiger (2004))
Serial assessment using two [11C]-mHED PET scans within 3–4 years demonstrated a continuous increase of extent and intensity of reinnervation with time after transplantation (Bengel et al. 1999). Sympathetic nerve terminals first reappeared in the basal parts of the myocardium and then extended further into distal parts, while the apex was occasionally involved late after transplantation. This finding is consistent with growth of sympathetic fibers along arterial structures. If reinnervation occurs, basal parts are reached first. In addition to a gradient from base to apex, anterior and septal walls were reinnervated earlier, while the lateral wall was involved later. These results suggest that sympathetic nerves are first restored in the territory of the left anterior descending artery, while later the left circumflex territory is involved additionally. Complete restoration of sympathetic innervation, however, was not observed until 15 years after transplantation, because inferior myocardium consistently remained denervated (Bengel et al. 1999; Uberfuhr et al. 2000b).
Using regionally heterogeneous reinnervation as a model, several studies have validated the results of catecholamine imaging by comparison with alternative tests of sympathetic innervation. [11C]-mHED PET-derived evidence of ventricular reinnervation, e.g., correlated with invasive measurements of tyramine-induced coronary arteriovenous norepinephrine spillover (Odaka et al. 2001) and with electrophysiologic indexes of reinnervation derived from heart rate variability measurements (Ziegler et al. 1996; Uberfuhr et al. 2000a).
While time after surgery is considered a major determinant of presence and extent of reinnervation, observations of interindividual heterogeneity and the regionally incomplete pattern suggest that additional determinants are involved. This has been studied in a comparably large sample of 77 transplant recipients by a multivariate analysis. In this analysis using [11C]-mHED PET, some patients remained denervated until late after transplantation, and other factors such as donor and recipient age, duration and complexity of transplant surgery, and frequency of allograft rejection were identified as independent determinants of sympathetic reinnervation (Bengel et al. 2002) (Table 17.1). Aging has been suggested to be associated with reduced availability of target-derived neurotrophic factors, which may explain reduced sympathetic reinnervation with increasing age. Reduced availability and synthesizing capacity of neurotrophins in the myocardium may also explain the lower degree of reinnervation in case of more frequent rejection episodes. Also, because surgical dissection results in axonal degeneration, sympathetic nerve fibers need to regrow along arterial structures to reach the allograft as their target organ. Extensive areas of scar tissue or other morphologic alterations along the path of regrowth may thus impair reappearance of nerve terminals in the myocardium. This is confirmed by less extensive reinnervation in patients with aortic complications at transplant surgery and by a significant inverse correlation with aortic cross-clamp time (Bengel et al. 2002). Hence, the surgical procedure appears to be another factor which may influence reinnervation. The observation of more intense reinnervation in patients transplanted for dilated compared to ischemic cardiomyopathy (Bengel et al. 2002) may also be explained in this context, as regrowth along sclerotic aorta and other vessels may be more difficult.
Finally, diabetes mellitus has also been shown to influence sympathetic reinnervation of the transplanted heart (Bengel et al. 2006). The regional extent of reinnervation and the regeneration rate were significantly reduced in diabetic transplant recipients compared to a matched transplant recipient group without diabetes (Fig. 17.2). The regenerative capacity of the sympathetic nervous system of the heart was reduced, but not abolished, by diabetes mellitus.
Effect of diabetes mellitus on transplant reinnervation. Shown are representative left ventricular short- and long-axis tomographic images (a) and polar maps (b) of cardiac transplant recipient without evidence of diabetes mellitus (top) and another recipient with history of diabetes (bottom). Gray-scale images show homogeneous myocardial perfusion, determined by [13N]NH3. Color-scale images show regional uptake of the neurotransmitter [11C]-epinephrine, indicating reinnervation in basal anterior wall. Extent of reinnervation was 42 % in nondiabetic recipient and 13 % in diabetic recipient. (c) Group results (mean ± SE) for neuronal regeneration rate. EPI [11C]-epinephrine, HTx heart transplantation, LA left atrium, LV left ventricle, RA right atrium, RV right ventricle. *P < 0.05 (Reprinted with permission from Bengel et al. (2006). This research was originally published in JNM. © by the Society of Nuclear Medicine and Molecular Imaging, Inc)
17.4 Transplant Reinnervation: Functional Effects
The pattern of regionally heterogeneous reinnervation on the one hand makes the transplanted heart a good model to determine physiologic effects of sympathetic innervation in vivo, by an intraindividual comparison of innervated and denervated myocardium. On the other hand, it also raises the general question whether this regenerative process, which remains incomplete, has general beneficial functional effects for the transplant recipients. Both issues have been studied in various elegant multi-tracer radionuclide imaging studies.
PET was used to determine myocardial blood flow, flow response to the cold pressor test as an index of endothelial-dependent vasodilatation, and flow response to adenosine as a composite index of endothelial-dependent and endothelial-independent vasodilatation in non-rejecting, otherwise healthy reinnervated transplant recipients. They observed a significant improvement of flow response to cold pressor in innervated compared to denervated vascular territories, while there was no difference for the response to adenosine. These results demonstrated the importance of sympathetic innervation for regulation of endothelial-dependent vascular reactivity in general, and they also supported the physiologic relevance of reinnervation for transplant recipients (Di Carli et al. 1997). Other studies focused on the effect of innervation on myocardial substrate utilization: Higher utilization of glucose were found at equal rates of overall oxidative metabolism in denervated compared to reinnervated myocardium of allografts, suggesting a metabolic switch from free fatty acids to glucose under conditions of denervation (Bengel et al. 2000). In another study, non-invasively determined allograft efficiency was shown to be improved in transplant recipients compared with failing hearts and was comparable to normal hearts. Differences between denervated and reinnervated allografts were not surveyed, and the dependency on loading conditions and contractility was preserved. These data suggested that normal regulatory interactions for efficiency are intact and that sympathetic tone does not play a role under resting conditions (Bengel et al. 2001b).
Finally, the effect of reinnervation on exercise performance was determined in a group of 29 transplant recipients by [11C]-mHED PET and standardized exercise radionuclide angiography. Restoration of sympathetic innervation was associated with improved responses of heart rate and global as well as regional contractile function to exercise (Fig. 17.3). These results support the functional importance of reinnervation in transplanted hearts and suggest a clinical benefit for the transplant recipient through enhanced exercise capacity (Bengel et al. 2001a). A subsequent study tested the effect of acute β-adrenergic blockade on the response to exercise in denervated and reinnervated transplant recipients and showed that differences of chronotropic and inotropic response between groups were no longer present following beta-blockade. While beta-blockade was well tolerated, these results confirmed that reappearance of sympathetic nerve terminals is associated with reestablishment of intact pre-/postsynaptic interaction (Bengel et al. 2004).
Effect of sympathetic reinnervation on cardiac allograft performance. Left ventricular retention of the catecholamine analogue [11C]-mHED (hydroxyephedrine) in transplant recipients with denervation and with reinnervation (left). Global left ventricular ejection fraction as determined by gated radionuclide angiography (right). During exercise, the ejection fraction was lower in patients with denervation than in those with reinnervation or normal subjects (P < 0.01 for both comparisons); the ejection fraction did not differ significantly between the patients with reinnervation and the normal subjects (Reprinted with permission by Massachusetts Medical Society, from Bengel et al. (2001a). © by Massachusetts Medical Society)
17.5 Summary and Conclusions
In summary, cardiac neuronal imaging has provided unique insights into the biology of the autonomic nervous system after cardiac transplantation. Based on imaging studies, it is now well known that sympathetic reinnervation may occur later after transplantation; that presence and extent of reinnervation are determined not only by time after transplantation but also by other factors related to age, surgery, and rejection; and that reinnervation remains regionally heterogeneous but nevertheless has physiologic effects on myocardial flow regulation, metabolism, and exercise performance.
While physiologic effects of reinnervation have been consistently reported, the relevance of this phenomenon for outcome of patients after transplantation remains uncertain. To date, sample sizes in reinnervation studies have been too small, and the influence of critical issues such as rejection, allograft vasculopathy, and dysfunction as well as immunosuppression-related side effects on patient outcome is too dominant to demonstrate a survival benefit of reinnervation (Bengel et al. 2002). Given the regionally heterogeneous pattern of transplant innervation, it is notable on the other hand that coexistence of denervated and innervated viable myocardium, unlike in ischemic heart disease or cardiomyopathy (Sasano et al. 2008), is not associated with an increased arrhythmogenic risk.
Accordingly, imaging for reinnervation after transplantation has not achieved recognition in clinical practice to date. The transplanted heart, however, remains a unique and important model in cardiac neuronal imaging sciences: On the one hand, the denervated heart early after transplantation can be used to study the specificity of neuronal imaging agents. On the other hand, the reinnervating heart later after transplantation can be used to study the unique plasticity and regenerative capacity of the cardiac autonomic nervous system.
References
Bengel FM, Schwaiger M (2004) Assessment of cardiac sympathetic neuronal function using PET imaging. J Nucl Cardiol 11:603–616
Bengel FM, Ueberfuhr P, Hesse T, Schiepel N, Ziegler SI, Scholz S et al (2002) Clinical determinants of ventricular sympathetic reinnervation after orthotopic heart transplantation. Circulation 106:831–835
Bengel FM, Ueberfuhr P, Karja J, Schreiber K, Nekolla SG, Reichart B et al (2004) Sympathetic reinnervation, exercise performance and effects of beta-adrenergic blockade in cardiac transplant recipients. Eur Heart J 25:1726–1733
Bengel FM, Ueberfuhr P, Schafer D, Nekolla SG, Reichart B, Schwaiger M (2006) Effect of diabetes mellitus on sympathetic neuronal regeneration studied in the model of transplant reinnervation. J Nucl Med 47:1413–1419
Bengel FM, Ueberfuhr P, Schiepel N, Nekolla SG, Reichart B, Schwaiger M (2001a) Effect of sympathetic reinnervation on cardiac performance after heart transplantation. N Engl J Med 345:731–738
Bengel FM, Ueberfuhr P, Schiepel N, Nekolla SG, Reichart B, Schwaiger M (2001b) Myocardial efficiency and sympathetic reinnervation after orthotopic heart transplantation: a noninvasive study with positron emission tomography. Circulation 103:1881–1886
Bengel FM, Ueberfuhr P, Ziegler SI, Nekolla S, Reichart B, Schwaiger M (1999) Serial assessment of sympathetic reinnervation after orthotopic heart transplantation. A longitudinal study using PET and C-11 hydroxyephedrine. Circulation 99:1866–1871
Bengel FM, Ueberfuhr P, Ziegler SI, Nekolla SG, Odaka K, Reichart B et al (2000) Non-invasive assessment of the effect of cardiac sympathetic innervation on metabolism of the human heart. Eur J Nucl Med 27:1650–1657
Bexton RS, Milne JR, Cory-Pearce R, English TA, Camm AJ (1983) Effect of beta blockade on exercise response after cardiac transplantation. Br Heart J 49:584–588
Cooper T, Willman VL, Jellinek M, Hanlon CR (1962) Heart autotransplantation: effect on myocardial catecholamine and histamine. Science 138:40–41
DeMarco T, Dae M, Yuen GM, Kumar S, Sudhir K, Keith F et al (1995) Iodine-123 metaiodobenzylguanidine scintigraphic assessment of the transplanted human heart: evidence for late reinnervation. J Am Coll Cardiol 25:927–931
Denniss AR, Marsh JD, Quigg RJ, Gordon JB, Colucci WS (1989) Beta-adrenergic receptor number and adenylate cyclase function in denervated transplanted and cardiomyopathic human hearts. Circulation 79:1028–1034
Di Carli MF, Tobes MC, Mangner T, Levine AB, Muzik O, Chakroborty P et al (1997) Effects of cardiac sympathetic innervation on coronary blood flow. N Engl J Med 336:1208–1215
Gilbert EM, Eiswirth CC, Mealey PC, Larrabee P, Herick CM, Bristow MR (1989) ß-adrenergic supersensitivity of the transplanted human heart is presynaptic in origin. Circulation 79:344–349
Kao AC, Van TP 3rd, Shaeffer MG, Shaw JP, Kuzil BB, Page RD et al (1994) Central and peripheral limitations to upright exercise in untrained cardiac transplant recipients. Circulation 89:2605–2615
Kaye DM, Esler M, Kingwell B, McPherson G, Esmore D, Jennings G (1993) Functional and neurochemical evidence for partial cardiac sympathetic reinnervation after cardiac transplantation in humans. Circulation 88:1110–1118
Le Guludec D, Delforge J, Syrota A, Desruennes M, Valette H, Gandjbakhch I et al (1994) In vivo quantification of myocardial muscarinic receptors in heart transplant patients. Circulation 90:172–178
Leenen FH, Davies RA, Fourney A (1995) Role of cardiac beta 2-adrenergic responses to exercise in cardiac transplant patients. Circulation 91:685–690
Munch G, Nguyen NT, Nekolla S, Ziegler S, Muzik O, Chakraborty P et al (2000) Evaluation of sympathetic nerve terminals with [(11)C]epinephrine and [(11)C]hydroxyephedrine and positron emission tomography. Circulation 101:516–523
Norvell JE, Lower RR (1973) Degeneration and regeneration of the nerves of the heart after transplantation. Transplantation 15:337–344
Odaka K, von Scheidt W, Ziegler SI, Ueberfuhr P, Nekolla SG, Reichart B et al (2001) Reappearance of cardiac presynaptic sympathetic nerve terminals in the transplanted heart: correlation between PET using (11)C-hydroxyephedrine and invasively measured norepinephrine release. J Nucl Med 42:1011–1016
Paulus WJ, Bronzwaer JGF, Felice H, Kishan N, Wellens F (1992) Deficient acceleration of left ventricular relaxation during exercise after heart transplantation. Circulation 86:1175–1185
Quigg RJ, Rocco MB, Gauthier DF, Creager MA, Hartley H, Colucci WS (1989) Mechanism of the attenuated peak heart rate response to exercise after orthotopic cardiac transplantation. J Am Coll Cardiol 14:338–344
Sasano T, Abraham MR, Chang KC, Ashikaga H, Mills KJ, Holt DP et al (2008) Abnormal sympathetic innervation of viable myocardium and the substrate of ventricular tachycardia after myocardial infarction. J Am Coll Cardiol 51:2266–2275
Schwaiger M, Hutchins GD, Kalff V, Rosenspire K, Haka MS, Mallette S et al (1991) Evidence for regional catecholamine uptake and storage sites in the transplanted human heart by positron emission tomography. J Clin Invest 87:1681–1690
Schwaiger M, Kalff V, Rosenspire K, Haka MS, Molina E, Hutchins GD et al (1990) Noninvasive evaluation of sympathetic nervous system in human heart by positron emission tomography. Circulation 82:457–464
Stark RP, McGinn AL, Wilson RF (1991) Chest pain in cardiac-transplant recipients. Evidence of sensory reinnervation after cardiac transplantation. N Engl J Med 324:1791–1794
Uberfuhr P, Frey AW, Ziegler S, Reichart B, Schwaiger M (2000a) Sympathetic reinnervation of sinus node and left ventricle after heart transplantation in humans: regional differences assessed by heart rate variability and positron emission tomography. J Heart Lung Transplant 19:317–323
Uberfuhr P, Ziegler S, Schwaiblmair M, Reichart B, Schwaiger M (2000b) Incomplete sympathic reinnervation of the orthotopically transplanted human heart: observation up to 13 years after heart transplantation. Eur J Cardiothorac Surg 17:161–168
Verani MS, Nishimura S, Mahmarian JJ, Hays JT, Young JB (1994) Cardiac function after orthotopic heart transplantation: response to postural changes, exercise, and beta-adrenergic blockade. J Heart Lung Transplant 13:181–193
von Scheidt W, Böhm M, Schneider B, Reichart B, Erdmann E, Autenrieth G (1992) Isolated presynaptic inotropic ß-adrenergic supersensitivity of the transplanted denervated human heart in vivo. Circulation 85:1056–1063
Willman VL, Cooper T, Hanlon CR (1964) Return of neural responses after autotransplantation of the heart. Am J Physiol 207:187–189
Wilson RF, Christensen BV, Olivari MT, Simon A, White CW, Laxson DD (1991) Evidence for structural sympathetic reinnervation after orthotopic cardiac transplantation in humans. Circulation 83:1210–1220
Ziegler SI, Frey AW, Uberfuhr P, Dambacher M, Watzlowik P, Nekolla S et al (1996) Assessment of myocardial reinnervation in cardiac transplants by positron emission tomography: functional significance tested by heart rate variability. Clin Sci (Lond) 91:126–128
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
Bengel, F.M. (2015). Autonomic Imaging in Heart Transplantation. In: Slart, R., Tio, R., Elsinga, P., Schwaiger, M. (eds) Autonomic Innervation of the Heart. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-662-45074-1_17
Download citation
DOI: https://doi.org/10.1007/978-3-662-45074-1_17
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-662-45073-4
Online ISBN: 978-3-662-45074-1
eBook Packages: MedicineMedicine (R0)