Abstract
The aroma profile of alcoholic beverages is a major factor that distinguishes one product from another, and it is a key attribute that drives consumer preference at points of sale. A longstanding objective has, therefore, been to identify those aromatic compounds that are important to particular olfactory attributes of different styles of wine, beer and cider—whether perceived ortho- or retro-nasally—and to modulate them according to consumer preferences. That this has been achieved only to a relatively small extent to date is partly a reflection on the complexity of the perception of aroma mixtures and also the presence of very low concentrations of potent aroma compounds in these products. It is known, although perhaps not appreciated as widely as it should be, that aroma compounds will interact with each other, with masking or suppressing effects being probably universal for compounds at supra-threshold concentrations, together with additive interactions for compounds at sub-threshold concentrations. Thus it is likely that volatile compounds with marginal aroma impact when isolated, can together provide an influence on aroma. Some of these aroma-active compounds are produced during fermentation. Different yeasts produce differing ranges of aroma-active substances, which may greatly affect the complex flavour of a fermented product such as wine, beer and cider. While these secondary metabolites are often formed only in trace amounts, their concentrations may well determine the distinct aroma of these beverages. This chapter reviews the production of the most important aroma-active compounds produced by yeast at molecular level and seeks to understand how they might be perceived by consumers.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
10.1 The Fundamentals of Aroma and Flavour Perception of Fermented Alcoholic Beverages
The aroma of a given foodstuff is formed by the pool of volatile molecules contained in that product with the ability to impact the olfactory receptors located in the olfactory region in the human nose. Since volatile molecules can reach this olfactory region both via orthonasal and retronasal pathways, the information elicited by those receptors affects two different sensory properties of products, i.e. their smell and their flavour. Smell is primarily information originating from the excited olfactory receptors together with, eventually, information produced by trigeminal nerve terminals located in the nostrils, which are also present in the mouth, pharynx and eyes. The information generated by these terminals falls in the category of chemesthesis and is related to semi-tactile properties such as refreshing/cooling effects (menthol), irritation (acids and alkalis) or pungency (chilies’ capsaicins) (Bandell et al. 2004; Bautista et al. 2007; Caterina et al. 1997; Macpherson et al. 2006). Many aroma chemicals are able to produce some chemesthesis, although in general the intensity of the response is smaller than the purely olfactory (Prescott 1999a). On the other hand, the flavour of a product is formed by the cerebral integration of the different sensory responses elicited during the consumption of a product. These sensory responses stem from three different chemical sensory systems (olfaction, taste and chemesthesis) and in the tactile and thermal sensory systems which give information about the temperature and rheological properties of the food. From a qualitative point of view, the olfactory system is the one carrying the biggest amount of information and that explains the limited amount of sensory information that can be perceived when the nose is blocked.
Flavour is an integrated cerebral response and it, therefore, not always possible to clearly assign the origin of the stimulus causing a particular flavour perception (Delwiche 2004; Prescott 1999b). For instance, whenever vanillin is present together with sweet tastants, the intensity of its smell increases (Green et al. 2012) and vice versa, the presence of vanillin can increase the perception of sweetness (Sakai et al. 2001). These complex phenomena are broadly included into the concept of perceptual interactions that are responsible for some unexpected and important observations in wine flavour chemistry, such as the prominent role played by fruity aromas on the perception of sweetness, bitterness and astringency (Saenz-Navajas et al. 2010). Although these phenomena are well documented in the general scientific literature, they are not yet well understood in the context of wine and other fermented alcoholic beverages. It is therefore important to note that aroma compounds play a sensory role in fermented beverages that it is not limited to the perception of an odour.
To fully understand the role played by individual chemicals, it is equally important to note that fermented beverages are considered by flavour chemists as ‘complex’ products. Although there is no definite border between what products are categorised as ‘simple’ and ‘complex’, the fact that the aroma of any fermented beverage is formed by at least 25 different aroma chemicals—all of which are present at concentrations above their corresponding odour thresholds—classes wine, beer and cider as ‘complex’ products. So, given the fact that the sense of olfaction has to be by nature a ‘synthetic’ rather than ‘analytic’ sense (Wilson and Stevenson 2003), the overall odour of fermented beverages has to be a global perception in which the individual chemicals are just poorly identified. In addition, since the odour of fermented beverages has accompanied humankind since the beginning of time, we can postulate the existence of what psychophysicists define as an ‘odour object’ (Ferreira 2012; Stevenson and Wilson 2007; Yeshurun and Sobel 2010). In other words, the human brain transforms the complex signals produced by the interactions of the chemicals present in all alcoholic beverages (alcohol, fusel alcohols, fatty acids, branched acids, ethyl esters, acetates, etc.) into a single unified concept that would be defined as ‘alcoholic’ or ‘vinous’. This is a highly efficient and ‘economic’ of signal processing and this capability has great practical importance in the understanding the chemical basis of aroma perception.
Therefore, although in the following sections we refer to individual compounds or groups of compounds and mention the specific odour properties of a compound or groups of compounds, it should not be concluded that those odour properties are directly responsible for such odour perception in wine, beer or cider.
10.2 The Basic Hierarchy of Aroma Compounds in Fermented Alcoholic Beverages
The previous notes, together with many experimental observations obtained in different reconstitution studies performed mainly in wine, make it possible to provide a basic rationale for understanding the contribution of the different chemicals to the aroma of a naturally fermented beverage. At the core of this rationale lies the aforementioned complexity of aroma and flavour perception in relation to fermented beverages and the so-called ‘aroma buffering effect’. The ‘buffering aroma effect’ of a product’s base refers to the demonstrated resistance of a particular aroma mixture to change its aroma both upon the elimination of some of its components or upon the addition of some new aroma compounds (Ferreira et al. 2002; Escudero et al. 2004). This does not mean that such a base always bears the same aroma and has the same composition. The composition of the base depends on some basic factors such as, for example, the concentration of sugar in a grape must, the prevalent yeast strain(s) and the degree of anaerobiosis during fermentation. For instance, the latter factor makes white and rosé wines richer in fatty acids and their ethyl esters while containing less alcohols and isoacids than red wines (Ferreira et al. 1996). Another less well-known factor is that the concentrations of fusel alcohols, fusel alcohol acetates, isoacids and their ethyl esters, all of them related to the yeast amino acid metabolism, are related to the varietal origin of the must (Ferreira et al. 2000; Hernández-Orte et al. 2002). Different compositions mean slightly different aromas and slightly different buffering abilities.
Notwithstanding of this, there are some compounds or combinations of compounds, that at the ‘natural’ concentrations at which they usually occur in fermented beverages, can break the buffer and transmit to the mixture their specific aroma or a particular feature of their aroma. There are five different possibilities for this to happen:
-
1.
The aroma buffer will be broken whenever a beverage contains an ‘aromatic vector’ with enough odour intensity.
-
2.
Such an aroma vector can be an individual compound (a so-called ‘impact compound’), a family of aroma compounds belonging to the same chemical series, such as ethyl esters of fatty acids, or even an association of aroma compounds sharing a generic descriptor (such as ‘fruity’ or ‘floral’).
-
3.
The vector will express in the beverage an aromatic nuance whose intensity and vicinity to the innate aroma character of the vector will be proportional to its concentration. For instance, isoamyl acetate bearing a characteristic smell of banana, if present at a low concentration does not transmit to the beverage its characteristic aroma. Rather it just transmits its generic ‘fruity’ character.
-
4.
Hence, concentration modulates the role that the vector actually plays in the mixture. The following intensity categories can be identified:
-
a.
null
-
b.
minor contributor—the elimination of the vector does not bring about any clear aromatic change
-
c.
neat contributor—the elimination of the vector brings about a clear decrease in the intensity of the aroma nuance to which the vector contributes
-
d.
major contributor—in this case the elimination of such vector will cause a dramatic drop in the intensity of the aroma nuance with even possible qualitative changes in the overall aroma profile
-
e.
impact compound—in this case the elimination of the vector will cause a dramatic change on the aroma profile.
-
a.
-
5.
When several aromatic vectors coexist in the same beverage, they will interact at perceptual level following three different potential patterns of interaction:
Whenever it comes to fermentative compounds, they are the basic constituents of the base and some of them can also play the role of contributors to different aroma nuances. To the best of our knowledge, they only seldom play the role of impact compounds. For instance, isoamyl alcohol or β-phenyl alcohols, even if they are present at relatively high concentrations never reach the level at which they would act as real impact compounds. So far, in wine, only isoamyl acetate, ethyl acetate, acetic acid, acetaldehyde and diacetyl can play such a role individually, and leaving aside some specific quirky wine styles, when they are clearly perceived the quality of the wine can be questioned. Fermentative compounds form the following aroma vectors:
-
1.
Alcohols (ethanol, isobutanol, isoamyl alcohol).
-
2.
Methionol.
-
3.
Ethyl esters of fatty acids (ethyl butyrate, hexanoate, octanoate and decanoate).
-
4.
Ethyl acetate.
-
5.
Acetic acid.
-
6.
Fatty acids (butyric, hexanoic, octanoic and decanoic acids).
-
7.
Isoamyl acetate.
-
8.
β-phenyl acetate and β-phenylethanol.
-
9.
Branched fatty acids [isobutyric, isovaleric, 2-methylbutyric and the recently discovered 2-, 3 and 4-methylpentanoic and cyclohexanoic acids (Campo et al. 2007)].
-
10.
Ethyl esters of the previously mentioned branched fatty acids.
-
11.
Diacetyl.
-
12.
Acetaldehyde.
As previously mentioned, these aroma vectors rarely reach the category of impact compounds, but they are important contributors to some key aroma nuances of fermented beverages. The role of two of the vectors, namely fatty acids and branched fatty acids, is quite complex, since apparently they form a kind of ‘creative’ interaction with the ‘fruity’ vectors (ethyl esters of fatty acids, branched acids and isoamyl acetate) to form the aroma of ‘fresh fruit’ (San Juan et al. 2011). Another aspect that must be kept in mind is that compounds of apparently ‘bad’ aroma, such as isovaleric and 2-methylbutyric acids, are in fact precursors for the strawberry-smelling ethyl isovalerate and ethyl 2-methylbutyrate which are formed by slow esterification of the acids with ethanol during ageing.
Bearing these fundamental aspects of aroma perception of fermented alcoholic beverages and the basic hierarchy of aroma compounds involved in mind, the following sections focus on the contribution of yeast to the aroma and overall quality of fermented beverages.
10.3 Wine Yeasts
Winemaking is a complex chemical and biological process in which different genera of yeast and bacteria are involved. During the early stages of spontaneous wine fermentation, different genera of non-Saccharomyces yeasts, such as Candida, Cryptococcus, Debaryomyces, Hanseniaspora and its asexual counterpart Kloeckera, Kluyveromyces, Metschnikowia, Pichia, Rhodotorula, Saccharomycodes, Schizosaccharomyces and Zygosaccharomyces play a role (Pretorius et al. 1999). Yeasts of the genera Kloeckera, Hanseniaspora and Candida predominate in the early stages, followed by several species of Metschnikowia and Pichia in the middle stages when the ethanol concentration rises to 3–4 % (Fleet and Heard 1993). However, some species of Schizosaccharomyces, Zygosaccharomyces, Brettanomyces and its sexual (‘perfect’) equivalent, Dekkera, are more resistant to high concentrations of ethanol and SO2 and, if present under certain conditions, can adversely affect the sensory quality of wine.
On the other hand, the principal species conducting the alcoholic fermentation in grape wine is Saccharomyces cerevisiae, but the closely-related Saccharomyces uvarum (Saccharomyces bayanus var. uvarum) can also participate (Demuyter et al. 2004; Massoutier et al. 1998; Naumov et al. 2000, 2001; Sipiczki 2002, 2008). Both S. cerevisiae and S. uvarum are able to grow on substrates characterised by high sugar and ethanol content, low pH, high sulphur dioxide concentrations and remains of fungicides, demonstrating that they are genetically well adapted to winemaking conditions (Sipiczki 2008). However, S. cerevisiae has higher resistance to high temperature stress (up to 37 °C) and ethanol levels (up to 15 %) than S. uvarum (Belloch et al. 2008). From an oenological point of view, these Saccharomyces species differ in several properties. Comparison between S. uvarum and S. cerevisiae reveals that the former is more cryotolerant, produces less acetic acid, lower levels of amyl alcohols, but higher concentrations of glycerol, succinic acid, malic acid, isobutyl alcohol, isoamyl alcohol and numerous secondary compounds (Sipiczki 2002). Wines produced by S. uvarum strains have a higher aromatic intensity than those produced by S. cerevisiae (Coloretti et al. 2006; Henschke et al. 2000). Specifically, S. uvarum produces more of 2-phenylethanol, 2-phenylethyl acetate and ethyl lactate than S. cerevisiae (Antonelli et al. 1999; Di Stefano et al. 1981; Gangl et al. 2009). On the other hand, S. uvarum is less common and appears mainly in fermentations at low temperatures (Antunovics et al. 2003; Demuyter et al. 2004; Masneuf-Pomarède et al. 2010; Sipiczki et al. 2001).
Other members of the genus Saccharomyces (S. cariocanus, S. kudriavzevii, S. mikatae, S. paradoxus, S. arboricolus, S. pastorianus) are not likely to play important roles in wine fermentation (Sipiczki 2008). Nevertheless, S. paradoxus has been found in grapes in the north-western region of Croatia and it is currently used to ferment wines (Redzepovic et al. 2002). Likewise, S. kudriavzevii has only been isolated in natural environments, like decayed leaves (Naumov et al. 2000) or oak barks (Sampaio and Gonçalves 2008; Lopes et al. 2010). However, there are reports that indicate that S. kudriavzevii may participate in hybrid formation with wine-related S. cerevisiae and S. bayanus species. For example, the genome sequence of a widely used wine yeast strain, VIN7, revealed an allotriploid hybrid genome with S. cerevisiae and S. kudriavzevii origins (Borneman et al. 2012). Physiological characterization of S. kudriavzevii strains has shown that they are able to grow at relatively low (10 °C) and high (up to 30 °C) temperatures; however, they are not able to tolerate more than 5 % of ethanol (Belloch et al. 2008).
Natural hybrids of S. cerevisiae, S. bayanus and S. kudriavzevii conducting wine fermentations have been recently discovered and characterised by genetic approaches (Belloch et al. 2009; Borneman et al. 2012; Dunn and Sherlock 2008; González et al. 2006, 2008; Horinouchi et al. 2010; Masneuf et al. 1998; Nguyen et al. 2000; Sipiczki 2008). The hybridisation process between Saccharomyces species has been proposed as an adaptation mechanism of yeasts to ferment at low temperatures (de Barros Lopes et al. 2002; Barrio et al. 2006; Sipiczki 2008). Physiological data suggest that Saccharomyces hybrids might have inherited the ability to grow at high temperatures (30–37 °C) and their ethanol tolerance from their S. cerevisiae parent and the ability to grow at low temperatures (10–16 °C) from their S. bayanus and S. kudriavzevii parents. These physiological characteristics point to Saccharomyces hybrids as better adapted to meet the winemakers’ trends, such as conducting wine fermentation at low temperatures, which may cause wine aroma improvement (Lambrechts and Pretorius 2000; Torija et al. 2003; Llauradó et al. 2002, 2005; Novo et al. 2003).
Oenological characterization of S. cerevisiae × S. kudriavzevii hybrid strains has demonstrated that the hybrids are well adapted to ferment at low and intermediate temperatures, producing moderate or higher levels of glycerol and less acetic acid with regard to reference strains of S. cerevisiae and S. kudriavzevii (Gangl et al. 2009; González et al. 2007). Similar comparative studies, which also included S. uvarum and a hybrid between S. cerevisiae and S. uvarum, in wine and cider (Masneuf et al. 1998; Nguyen et al. 2000), indicated that the highest production of glycerol was produced by S. uvarum, S. kudriavzevii and the S. cerevisiae × S. uvarum hybrid (Gamero et al. 2013). Regarding aroma formation, one study indicated that hybrids produced the same quantity of aromatic compounds as S. cerevisiae at high temperatures, and the same aromatic intensity as S. kudriavzevii at low temperatures (González et al. 2007), whereas in another study this trend was only observed in the case of fusel alcohol production (Gamero et al. 2013). In the latter study, S. cerevisiae strains yielded the highest aroma amounts at 28 °C were, whereas S. uvarum and some hybrids excelled at 12 °C. Altogether, these studies pointed to the fact that aroma formation is highly dependent on both yeast strain and fermentation temperature (Gamero et al. 2013).
10.4 Beer Yeasts
In brewing, a distinction is made between ale yeasts (top fermentation) and lager yeasts (bottom fermentation). Ale yeasts are classified as S. cerevisiae and are mostly used for the production of specialty beers where the fermentation temperatures are relatively high (15–25 °C). Lager yeasts are classified as Saccharomyces pastorianus, which include S. carlsbergenis and S. monacensis isolated by EC Hansen in 1908. Lager yeasts are used for the production of pilsner type beers, fermented at lower temperatures than ale yeasts (6–14 °C). The genomes of lager yeasts are complex as they are aneuploid and consist of a hybrid of mixed genetic lines of the Saccharomyces genus (Kodama et al. 2006). DNA/DNA reassociation studies on the type strains of S. bayanus (CBS380), S. carlsbergensis (CBS1513) and S. monacensis (CBS1503) presented S. bayanus as one of the contributors to S. pastorianus genome (Vaughan-Martini and Kurtzman 1985). Thereafter many reports agreed with this fact (Tamai et al. 2000; Rainieri et al. 2006; Dunn and Sherlock 2008; Nakao et al. 2009). Later, some evidences pointed to S. bayanus to be a hybrid between S. uvarum and S. cerevisiae (Nguyen et al. 2000; Nguyen and Gaillardin 2005), which was recently confirmed (Nguyen et al. 2011) and being one of its parents S. uvarum and the other, a new species isolated from Patagonia and named S. eubayanus (Libkind et al. 2011). As S. eubayanus carries a ‘pure’ or monogenome it is very likely to be the common contributor of S. bayanus and S. pastorianus. Lager brewing yeast is now recognised by many authors as S. eubayanus/S. cerevisiae hybrid (Dunn et al. 2012; Piotrowski et al. 2012; Cousseau et al. 2013; Pengelly and Wheals 2012).
10.5 Cider Yeasts
Studies on population dynamics in cider have shown that the composition of yeast flora can vary according to climatic conditions, apple varieties, geographic location and the cider-making technology employed (Cabranes et al. 1990; del Campo et al. 2003; Suárez et al. 2007a). First, an oxidative phase carried out by autochthonous non-Saccharomyces yeasts with a low fermentation capacity and with the predominance of Metschnikowia pulcherrima, Hanseniaspora uvarum, Hanseniaspora valbyensis and Candida yeasts was observed (Michel et al. 1988; Morrissey et al. 2004; Coton et al. 2006; Suárez et al. 2007a). Furthermore, species of the genera Pichia, Torulaspora, Rhodotorula, Cryptococcus, Zygosaccharomyces and Brettanomyces/Dekkera yeasts, originating from apples or the environment have been also related to cider production (Beech 1993; Michel et al. 1988; Morrissey et al. 2004).
Second, strains with a greater tolerance to ethanol (Saccharomyces spp.) completed the cider fermentations. In the aforementioned studies on population dynamics in cider, the Saccharomyces species found to be present were S. cerevisiae and S. bayanus. The data indicated that S. bayanus was the predominant species at the beginning and the middle fermentation phases of the fermentation process, reaching a percentage of isolation between 33 and 41 %, whereas S. cerevisiae took over the process in the final stages of fermentation (Suárez et al. 2007a). A study that was carried out to examine the dynamics and variability of wild Saccharomyces spp. (Suárez et al. 2007b) determined that the number of strains observed was higher than those reported for Saccharomyces populations in some wine-growing regions in other studies (Frezier and Dubourdieu 1992; Querol et al. 1994; Gutiérrez et al. 1999; Torija et al. 2001; Schuller et al. 2005).
Finally and as commented before, natural hybrids between S. bayanus and S. cerevisiae have been described by some authors in wine and cider some years ago (Masneuf et al. 1998; Nguyen et al. 2000).
10.6 Fermentative Aroma
The most important compounds within fermentative aroma are higher alcohols, acetate and ethyl esters, aldehydes (acetaldehyde), ketones (diacetyl), organic acids (acetic acid), volatile phenols (4-vinylphenol, 4-vinilguaiacol) and sulphurous compounds (hydrogen sulphide, mercaptans, volatile thiols). A scheme of the synthesis of the main fermentative aroma compounds is shown in Fig. 10.1, while Table 10.1 presents their aroma descriptors and odour thresholds. Finally, Table 10.2 depicts the most important genes involved in flavour-active compound synthesis identified in S. cerevisiae.
10.6.1 Higher Alcohols
Higher or fusel alcohols are alcohols with two or more carbon atoms with molecular weights and boiling points higher than those of ethanol (Lambrechts and Pretorius 2000). From a quantitative point of view, higher alcohols are the most important group of volatile compounds produced by yeast during wine fermentation. Higher alcohols are classified in aliphatics like isobutanol, hexanol and isoamyl alcohol and aromatics like 2-phenylethanol and benzyl alcohol. Higher alcohols contribute with an intense aroma to the flavour of wine and other alcoholic beverages. According to Rapp and Versini (1991), concentrations of higher alcohols below 300 mg/l add desirable complexity to wine aroma, whereas higher concentrations (400 mg/l) can be detrimental to wine quality by disguising ester-based fruity aromas and imparting a strong, pungent smell and taste. On the other hand, the concentration of each higher alcohol acting positively or negatively on the aroma is variable. In beer, the flavour of the aliphatic alcohols is distinctly alcoholic (e.g. ethanol) and the aromatic alcohols have a rather sweet, alcoholic or bitter taste (Meilgaard 1975). Nevertheless, in spite of having aroma themselves, the main oenological importance of higher alcohols lies in the fact that they are precursors of acetate esters (Soles et al. 1982).
Many factors affect the levels of higher alcohols in alcoholic beverages. For example, in wine, viticultural conditions, yeast strain and species, initial sugar content of the grape must, pH and composition of the juice, fermentation temperature, assimilable nitrogen and aeration have a strong influence (Fleet and Heard 1993; Houtman et al. 1980a, b; Houtman and du Plessis 1981). In beer, the addition of fatty acids and sterols (Taylor et al. 1979), oxygenation (Quain and Duffield 1985) or high temperatures (Landaud et al. 2001), cause an increase in higher alcohol content. Those factors also stimulate yeast growth in the fermenting wort. In addition, the production of 2-phenylethyl alcohol appears to be particularly sensitive to temperature, whereas the synthesis of other higher alcohols is relatively unaffected by this factor.
Several studies have demonstrated that S. bayanus produces higher amounts of several fusel alcohols (2-phenylethanol, isobutyl alcohol and isoamyl alcohol) than S. cerevisiae (Antonelli et al. 1999; Massoutier et al. 1998). Other authors observed that Saccharomyces species generally produce higher concentrations of fusels alcohols than non-Saccharomyces species (Gil et al. 1996; Herraiz et al. 1990).
Higher alcohols are synthesised by the Ehrlich pathway from branched-chain amino acids, leucine, valine and isoleucine; aromatic amino acids, phenylalanine, tyrosine and tryptophan; and the sulphur-containing amino acid methionine. In this metabolic pathway, the amino acids are transaminated to the corresponding α-ketoacid, followed by decarboxylation to aldehydes. Finally, these aldehydes are reduced to higher alcohols and NADH becomes NAD+. These chemical reactions are carried out by amino acid permeases, transaminases, decarboxylases and dehydrogenases. Amino acid permeases are encoded by the genes GAP1, BAP2, BAP3, MMP1 and MUP3 (Didion et al. 1998; Grauslund et al. 1995; Isnard et al. 1996; Jauniaux and Grenson 1990; Mai and Lipp 1994; Rouillon et al. 1999); branched-chain amino acid transaminases by BAT1 and BAT2 and aromatic amino acids transaminases by ARO8 and ARO9 (Dickinson and Norte 1993; Eden et al. 2001; Hazelwood et al. 2008; Kispal et al. 1996; Lilly et al. 2006b; Ugliano and Henschke 2009). In the valine-degradation pathway, any one of the three isozymes of the pyruvate dehydrogenase complex (PDC), encoded by PDC1, PDC5 and PDC6, will decarboxylate α-ketoisovaleric acid (Dickinson et al. 1998); in isoleucine catabolism, any one of the family of decarboxylases encoded by PDC1, PDC5, PDC6, KID1 or ARO10 is sufficient for the decarboxylation reaction (Dickinson et al. 2000); in the leucine-degradation pathway, the major decarboxylase is encoded by KID1 (Dickinson et al. 1997); in the case of aromatic amino acids, PDC1, PDC5, PDC6 or ARO10 are involved (Dickinson et al. 2003). And finally, ethanol dehydrogenases are codified by ADH1, ADH2, ADH3, ADH4, ADH5, ADH6, ADH7 and SFA1 (encoding formaldehyde dehydrogenase) (Delneri et al. 1999; Hazelwood et al. 2008). On the other hand, aryl alcohol dehydrogenases, AAD10 and AAD14, are believed to be responsible for the degradation of aromatic aldehydes into their corresponding higher alcohols (Delneri et al. 1999) and higher alcohols can also be produced de novo through carbohydrate metabolism (Äyräpää 1968, 1971).
Several studies have been carried out in order to understand the complexity of higher alcohol formation and to be able to modulate the aroma of alcoholic beverages by yeasts Recent screenings based on constructing double- and triple-deletion mutants presented AAD6, BAT2, HOM2, PAD1, PRO2, SPE1 and THI3 as the most important genes affecting higher alcohol production, being BAT2 the dominant gene in this respect and suggesting that the initial transaminase step of the Ehrlich pathway is rate-limiting (Styger et al. 2011, 2013). Other studies showed that overexpression of the branched-chain amino acids transaminases BAT1 or BAT2 under the control of the constitutive phosphoglycerate kinase I gene (PGK1) lead to an increase in the levels of isoamyl alcohol, isoamyl acetate and, to a lesser extent, isobutanol and isobutyric acid or an increase in isobutanol, isobutyric acid and propionic acid, respectively. In both cases, wines presenting higher ‘peach’ and ‘apricot’ notes were obtained (Lilly et al. 2006b). In the case of the wort fermentation, BAP2 gene was overexpressed under the glyceraldehyde 3-phosphate dehydrogenase promotor (TDH3) in a brewer’s yeast (Kodama et al. 2001). As a result, accelerated assimilation rates of branched-chain amino acids resulted in an increased production of isoamyl alcohol derived from leucine, while no increases of isobutyl alcohol derived from valine or of active amyl alcohol derived from isoleucine were observed. These results suggest that the mechanisms for the production of each higher alcohol are, although interconnected, not the same. Finally, a recent study has shown that the synthesis of higher alcohols seems to be influenced by the NAD+/NADH availability, having the redox balance an important impact (Jain et al. 2012).
10.6.2 Acetate Esters
Acetate esters such as ethyl acetate (‘solvent’-like aroma), isoamyl acetate (‘banana’ aroma), ethyl caproate and ethyl caprylate (‘sour apple’ aroma) and 2-phenylethyl acetate (‘flowery’, ‘roses’ and ‘honey’ aromas), give desirable ‘fruity’ and ‘floral’ aromas in the alcoholic beverages (Lambrechts and Pretorius 2000; Swiegers et al. 2005).
The concentration of acetate esters in wines is affected by different factors such as maturity and sugar content (Houtman et al. 1980a, b), yeast species, fermentation temperature (Piendl and Geiger 1980), alcoholic and malolactic fermentation, winemaking method (Herraiz and Ough 1993; Gómez et al. 1994) or the presence of non-soluble material in the must (Edwards et al. 1985). Besides, different factors after the fermentative process, such as time and temperature of ageing and storage, affects ester content in wine (Marais and Pool 1980; Ramey and Ough 1980). Regarding yeast species carrying out the fermentation, acetate ester production depends on each strain (Antonelli et al. 1999; Mateo et al. 1992). Some studies have demonstrated that S. cerevisiae produces high amounts of several acetate esters such as isopenthyl acetate, phenylethyl acetate, isoamyl acetate, hexyl acetate (Nykänen and Nykänen 1977; Soles et al. 1982; Suomalainen and Lehtonen 1979), whereas S. bayanus has demonstrated to be a good 2-phenylethyl acetate producer (Soles et al. 1982). Comparison between Saccharomyces and non-Saccharomyces regarding acetate esters production showed species dependence in the production of these aromatic compounds (Gil et al. 1996; Lema et al. 1996; Rojas et al. 2001).
Recently, it has been demonstrated that pure and/or mixed cultures of several non-Saccharomyces strains are able to increase ester levels in wine: Hanseniaspora guillermondii (2-phenyl ethyl acetate) and H. uvarum (isoamyl acetate) (Moreira et al. 2008); Hanseniaspora osmophila, H.vinae and H. anomala (2-phenyl ethyl acetate) (Viana et al. 2008, 2009, 2011; Izquierdo-Canas et al. 2011); Pichia membranifaciens and Pichia klyuveri (Viana et al. 2009; Swiegers et al. 2011); Williopsis saturnus and T. delbrueckii (ethyl and isoamyl acetate) (Erten and Tanguler 2010; Swiegers et al. 2011; Izquierdo-Canas et al. 2011; Tanguler 2012; Azzolini et al. 2012). Some of the strains of the genera Hanseniaspora spp., Torulaspora spp., Kluyveromyces spp., Pichia spp., and Williopsis spp. have been commercialised.
In lager beers, the only acetate ester that can be sensorially perceived is isoamyl acetate (Dufour and Malcorps 1995). However, the presence of multiple esters can have a synergistic effect, having an impact on the overall flavour (Meilgaard 1975). In addition, it has been demonstrated that small changes in ester concentration can have a significant impact on beer flavour (Hammond 1995). Several fermentation conditions have an important impact on ester formation during brewery fermentations (Verstrepen et al. 2003a): fatty acids (Saerens et al. 2008a), temperature (Saerens et al. 2008a), wort gravity (Saerens et al. 2008b; Piddocke et al. 2009; Lei et al. 2012), pitching rate (Verbelen et al. 2009a) and oxygen (Verbelen et al. 2009b).
Acetate esters are synthesised by a condensation reaction between higher alcohols and acetyl-CoA. This reaction is mediated by acetyltransferases codified by genes ATF1, Lg-ATF1 and ATF2 (Fujii et al. 1994, 1996; Fujiwara et al. 1999; Lilly et al. 2006a; Saerens et al. 2008b, 2010; Verstrepen et al. 2003c). ATF1 and ATF2 are present in both ale and lager strains, but Lg-ATF1 is found only in lager strains (Yoshimoto et al. 1998). During fermentation, acetate ester production rates are dependent on alcohol acetyltransferases activity (Malcorps et al. 1991). Besides, the effect of esterases encoded by IAH1 and TIP1 is also important for the final concentration of acetate esters (Horsted et al. 1998; Lilly et al. 2006a; Saerens et al. 2008b, 2010).
Deletion/overexpression studies indicated that the ATF2-encoded enzyme of S. cerevisiae plays a minor role as compared with its ATF1-encoded enzyme (Lilly et al. 2000, 2006a; Verstrepen et al. 2003b). Additionally, the fact that the double-deletion strain produced considerable amounts of certain esters suggests the existence of additional, as yet unknown, ester synthases in the yeast proteome (Verstrepen et al. 2003b). Interestingly, overexpression of different alleles of ATF1 and ATF2 led to different ester-production rates, indicating that differences in the aroma profiles of yeast strains may be partially due to mutations in their ATF genes (Verstrepen et al. 2003b).
In addition, it has been recently postulated that the ratio acetyl-CoA/CoA could affect acetate ester synthesis (Cordente et al. 2007). The carnitine acetyltransferases catalyse the reversible reaction between carnitine and acetyl-CoA to form acetylcarnitine and CoA. Overexpression of CAT2-encoded mitochondrial and cytosol carnitine acetyltransferases resulted in lower levels of acetate esters in the fermentation since less acetyl-CoA is available for acetate ester synthesis (Cordente et al. 2007).
10.6.3 Ethyl Esters
Ethyl esters such as ethyl propanoate, ethyl butanoate, ethyl hexanoate (ethyl caprylate), ethyl octanoate (ethyl caproate), ethyl decanoate (ethyl caprate) and ethyl lactate give desirable fruity and flowery aroma to the wine. They are produced by condensation between ethanol and acyl-CoA, reaction mediated by acyltransferases. These acyltransferases are encoded by the genes EHT1 (ethanol hexanoyl transferase 1) and EEB1 (ethanol hexanoyl transferase) (Rossouw et al. 2008; Saerens et al. 2006, 2008a, 2010), the latter being responsible for the majority of ethyl ester production in S. cerevisiae as shown in deletion studies (Saerens et al. 2006). The final concentration of ethyl esters in wine will therefore be influenced by the esterase activity of EHT1 and EEB1 encoded-transferases (Saerens et al. 2006), as well as the effect of esterases encoded by IAH1 and TIP1 (Horsted et al. 1998; Lilly et al. 2006a; Saerens et al. 2008b, 2010).
Ethyl ester concentrations in alcoholic beverages are affected by the same factors mentioned for acetate esters in the previous section. Regarding yeast species carrying out the fermentation, ester production depends on each strain (Mateo et al. 1992). Several studies have demonstrated that S. cerevisiae produced high amounts of several ethyl esters such as ethyl caproate, ethyl caprylate and ethyl caprate (Antonelli et al. 1999; Nykänen and Nykänen 1977; Soles et al. 1982; Suomalainen and Lehtonen 1979), whereas S. bayanus has demonstrated to be a good ethyl caprate and ethyl lactate producer (Antonelli et al. 1999; Soles et al. 1982). Comparison between Saccharomyces and non-Saccharomyces ethyl ester production showed Saccharomyces species produced equal or higher ethyl esters amounts (Gil et al. 1996; Lema et al. 1996).
10.6.4 Aldehydes
Acetaldehyde is the most important aldehyde present in alcoholic beverages from a quantitative point of view. In beer, acetaldehyde is normally present at close to its flavour threshold (Engan 1981), whereas different levels can be found in wines. The average values are about 80 mg/l for white wine, 30 mg/l for red wine and 300 mg/l for sherries (McCloskey and Mahaney 1981). At low levels, it gives a pleasant, fruity aroma, but at high concentrations it possesses a pungent irritating odour (Miyake and Shibamoto 1993). Excess acetaldehyde produces a ‘green’, ‘grassy’ or ‘apple-like’ off-flavour in beer (Margalith 1981; Adams and Moss 2000), cider (Williams 1974) and wine (Henschke and Jiranek 1993), with the exception of sherry-type wines, where high acetaldehyde content is a characteristic feature (Sponholz 1993; Cortes et al. 1998).
Acetaldehyde, also called ethanal, is an intermediary of alcoholic fermentation obtained by the decarboxylation of pyruvate. Pyruvate decarboxylase enzymes encoded by PDC1, PDC2 and PDC3 participate in this process. Later on, acetaldehyde is reduced to ethanol by alcohol dehydrogenase enzymes, primarily the enzyme encoded by the ADH1 gene (Pronk et al. 1996), although a little quantity always remains in the wine. The conversion of acetaldehyde to ethanol is required for the maintenance of the redox balance of the cell, since it re-oxidises NADH to NAD+, which will be available for glycolysis. In this way, sugar is the primary substrate for acetaldehyde formation, but metabolism of amino acids such as alanine also contributes to the synthesis of this compound (Henschke and Jiranek 1993; Boulton et al. 1998).
In alcoholic beverages, acetaldehyde is mainly produced in the first stages of fermentation and its concentration drops at the end of the fermentation and during maturation due to yeast activity. The levels of acetaldehyde in vinification can be considerably affected, from 0.5 to 286 mg/l, depending on the yeast strain (Liu and Pilone 2000), but other factors can affect acetaldehyde level in wines, such as low quantity of zinc, presence of oxygen late in the fermentation, the nature of insoluble material used to clarify the must, increasing fermentation temperature or the excessive use of SO2 in grape must (Delfini and Costa 1993; Romano et al. 1994; Liu and Pilone 2000). Excessive acetaldehyde levels contribute to a perception of oxidation, although in some Jerez wines such as Fino and Manzanilla high concentrations of this compound are desirable (Zamora 2009). In the case of the beer, high acetaldehyde concentrations reflect premature flocculation or a decrease in yeast viability.
10.6.5 Ketones
Vicinal diketones appear normally in beer fermentation and are undesirable compounds affecting lager beer flavour (Inoue 1992; Wainwright 1973), whereas it is a characteristic flavour of some ale beers. The two most important vicinal diketones are diacetyl (2,3-butanedione) and 2,3-pentanedione. Diacetyl confers a ‘butterscotch’-like aroma and pentanedione, a ‘honey’-like aroma. In the case of the wine, diacetyl can contribute to wine aroma complexity in low concentrations, giving ‘nutty’ or ‘toasty’ nuances, but it becomes undesirable at levels between 1 and 4 mg/l (Sponholz 1993).
Diacetyl is synthesised from α-acetolactate, an intermediate in the valine and leucine biosynthesis pathway, by spontaneous oxidative decarboxylation. Yeasts are also able to reduce diacetyl to acetoin, which may then be further reduced to 2,3-butanediol. Acetoin has a much higher flavour threshold (50 mg/l) than diacetyl, exhibits ‘fruity’, ‘mouldy’ and ‘woody’ flavours (Meilgaard 1975) and does not cause any off-flavours in the beer. The production of diacetyl in beer is increased by low pH, high temperature, oxygen and the presence of metal ions (Haukeli and Lie 1978) and can be regulated in wort by nitrogen content, valine addition (Krogerus and Gibson 2013) and the enzyme α-acetolactate decarboxylase (Godtfredsen and Ottesen 1982).
The ILV-encoded enzyme forms α-acetolactate from pyruvate. This enzyme is subject to general amino acid control and very strong feedback inhibition by valine. In the case of lager yeasts, 90–95 % of the diacetyl reductase activity is accounted for alcohol dehydrogenases (Bamforth and Kanauchi 2004), whereas in the case of ale yeasts, enzymes other than alcohol dehydrogenases appear to be more important, these enzymes being only responsible for the 60 % of the reductase activity.
There have been several attempts to try to reduce diacetyl formation in brewing, such as the disruption of the gene ILV2. Mutants lacking this gene did not produce diacetyl but because of their inability to synthesise valine and leucine, such yeasts fermented poorly (Ryder and Masschelein 1983). Changing the upstream regulatory sequence of ILV2 could reduce the level of this enzyme rather than to eliminate it completely (Petersen et al. 1983). An alternative approach was to increase the flux to amino acid synthesis, which has been achieved transforming yeasts with multiple copies of the ILV5 gene (Villanueva et al. 1990; Goossens et al. 1991). Conversely, transformations with the ILV3 gene had no effect on diacetyl concentration (Goossens et al. 1987).
10.6.6 Organic Acids
Acetic acid is the main responsible for volatile acidity of wines. Other contributors to volatile acidity are propionic acid and hexanoic acid. The optimal concentration in wine is 0.2–0.7 g/l (Corison et al. 1979; Dubois 1983). At high concentrations (0.7–1.1 g/l), acetic acid imparts a ‘vinegar’ flavour to the wine. S. cerevisiae wine strains can produce from 100 mg/l to 2 g/l of acetic acid depending on the conditions during fermentation and the type of strain (Radler 1993). S. bayanus and S. uvarum usually produce less acetic acid than S. cerevisiae (Giudici et al. 1995; Eglinton et al. 2000). Furthermore, certain strains of T. delbrueckii have been shown to reduce acetic acid production in wine (Bely et al. 2008; Van Breda et al. 2013).
Acetate is produced through acetaldehyde oxidation in a reaction catalysed by acetaldehyde dehydrogenases encoded by ALD4 and ALD5 (mitochondrial isoforms) and ALD6, ALD2 and ALD3 (cytosolic isoforms) (Navarro-Aviño et al. 1999). During winemaking, Ald6p, Ald5p and Ald4p are the main enzymes responsible for acetate formation (Saint-Prix et al. 2004). Deletion of both alleles of ALD6 in a wine yeast caused a 2-fold reduction in the amount of acetate produced during fermentation, but as a consequence of the redox imbalance generated, glycerol, succinate and 2,3-butanedediol production was slightly increased (Remize et al. 2000).
On the other hand, mutations in the stress response gene YAP1 (Cordente et al. 2013) or use of non-Saccharomyces yeasts such as T. delbrueckii (Bely et al. 2008; Van Breda et al. 2013) constitute succesful examples of non-GMO approaches to decrease acetic acid formation during fermentation.
In high-gravity brewing, yeast cells are stressed because of high sugar and ethanol concentrations, which can lead to higher production of acetic acid, which can be a problem to beer quality (Mizuno et al. 2003). An alternative to solve this problem could be to employ a mutant overexpressing ALD4 (Mizuno et al. 2006), which produced half the amount of acetic acid and 1.1 % more ethanol than beer brewed using the wild-type.
10.6.7 Volatile Phenols
Volatile phenols can appear in wine as a consequence of a non-oxidative decarboxylation of hydroxycinnamic acids p-coumaric and ferulic carried out by yeasts (Chatonnet et al. 1993; Grando et al. 1993) or through decarboxylation of phenolic acids, usually first into 4-vinyl derivatives that are then reduced to 4-ethyl derivatives through enzymes called phenolic acid decarboxylases (Cavin et al. 1993). The genes encoding phenolic acid decarboxylases include PAD1 (also known as POF1). However, phenolic acid decarboxylase activity is very low in most S. cerevisiae strains (Barthelmebs et al. 2000a, b) and several attemps have been carried out to develop mutant strains to modulate volatile phenol production. Strains overexpressing the Bacillus subtilis phenolic acid decarboxylase gene (padc), the Lactobacillus plantarum p-coumaric acid decarboxylase gene (pdc) and strains in which PAD1/POF1 gene was disrupted, are examples of succesful volatile phenol modulation (Smit et al. 2003). Contrarily, constructed strains overexpressing S. cerevisiae phenylacrylic acid decarboxylase gene (PAD1/POF1) has no significant effect in volatile phenol synthesis (Smit et al. 2003).
Volatile phenols possess low sensory thresholds and, in spite of the fact that they can be desirable in certain wines, normally they appear as off-flavours (‘stable’, ‘barnyard’, ‘pharmaceutical’) (Dubois 1983). Ethyl phenols (4-ethyl guaiacol and 4-ethyl phenol) present a special negative contribution and are derived from the reduction of vinyl phenols (4-vinyl guaiacol and 4-vinyl phenol). Vinyl reductase activity is typically associated with Brettanomyces and Dekkera spp.
On the other hand, volatile phenols can contribute positively or negatively depending on the beer product. The presence of excessive amounts of vinyl phenols is considered undesirable in bottom-fermented pilsners. Hence the term ‘phenolic off-flavour’ (POF) is attributed to beers with a strong aroma described as ‘pharmaceutical’, ‘medicinal’, ‘solvent’, ‘spicy’, ‘clove-like’, ‘smokey’ or ‘barbeque’. However, these compounds are crucial for the characteristic aroma of Belgian white beers (made with unmalted wheat), German rauch beers and Weizen beers (made with malted wheat) and in many top-fermented blond and dark specialty beers.
10.6.8 Sulphur Compounds
Hydrogen sulphide imparts a ‘rotten egg’ aroma and has a very low odour threshold of 10–80 μg/l (Swiegers et al. 2005). The concentration of H2S produced during wine fermentation depends on the presence of sulphur compounds, wine yeast strain, fermentation conditions, and the nutritional status of the grape juice (Henschke and Jiranek 1991; Rauhut 1993; Spiropoulos and Bisson 2000). However, some strains produce H2S constitutively without being affected by environmental conditions (Jiranek et al. 1995; Spiropoulos and Bisson 2000; Mendes-Ferreira et al. 2002).
During wine fermentation, yeast can synthesise hydrogen sulphide from either inorganic sulphur compounds (sulphate and sulfite) or from organic sulphur compounds (cysteine and glutathionine) (Henschke and Jiranek 1993; Rauhut 1993; Hallinan et al. 1999; Spiropoulos and Bisson 2000).
The sulphate reduction sequence (SRS) is activated in response to the necessity to produce cysteine and methionine, usually insufficient in wine must (Henschke and Jiranek 1993). The firs step involves the transportation of suphate from the medium into the yeast cell by sulphate permease. Several steps follow to reduce sulphate to sulphide using the enzymes ATP-sulfurylase and sulfite reductase. Subsequently, O-acetylserine (from the amino acid serine) combines with sulphide to form cysteine, and O-acetylhomoserine (from the amino acid aspartate) combines with sulphide to form homocysteine, which can then be converted to methionine (Thornton and Bunker 1989; Yamagata 1989; Henschke and Jiranek 1993; Rauhut 1993; Jiranek et al. 1995; Spiropoulos and Bisson 2000). Nitrogen limitation leads to insufficient of these precursors and sulphide is accumulated and released to the medium as hydrogen sulphide (Henschke and Jiranek 1993; Rauhut 1993; Jiranek et al. 1995; Spiropoulos and Bisson 2000). Additionally, significant amounts of H2S can be produced when the fermentation medium is rich in sulphite since it can diffuse into the cell.
Several attempts have been made to modulate H2S production by using certain wine and brewing yeasts that are commercially available. The consequences of overexpression of the MET17 gene, which encodes O-acetylserine and O-acetylhomoserine sulfhydrylase in S. cerevisiae, seemed to be strain dependent (Omura et al. 1995; Spiropoulos and Bisson 2000). Conversely, the deletion of the MET14 gene (encoding an adenosylphosphosulfate kinase) or the MRX1 gene (encoding a methionine sulfoxide reductase), might be the most effective way to prevent wine yeast from producing H2S in fermentations (Pretorius 2000, 2003, 2004; Pretorius and Høj 2005). Another attempt to prevent H2S formation was carried out through modifying the activity of the sulfite reductase enzyme by engineering one of the enzyme subunits codified by MET10 (Sutherland et al. 2003). This strategy has been succesfully applied in beer (Hansen and Kielland-Brandt 1996). On the other hand, classical mutagenesis which lead to mutants presenting mutations in MET5 and MET10 genes produced 50–99 % less H2S than the parental strain (Cordente et al. 2009). Some of these wine strains are now in commercial use. Finally, increased expression of CYS4 in brewing yeast, encoding the cystathionine β-synthase, has been shown to suppress the formation of H2S (Tezuka et al. 1992).
Another sulphur compound that can be detrimental for the flavour of alcoholic beverages is ethanethiol (‘onion’ aroma), synthesised through the reaction of hydrogen sulphide and ethanol or acetaldehyde (Rauhut 1993). On the other hand, dimethyl sulphide (DMS), which present ‘asparagus’, ‘corn’ and ‘molasses’ notes, might be produced in wine via cleavage of S-methyl-L-methionine to homoserine and DMS. In beer production, heat decomposition during malting of S-methylmethionine produces dimethyl sulfoxide (DMSO), which can be reduced to DMS, during storage (Rauhut 1993) or fermentation by yeasts. In S. cerevisiae, the MXR1 gene has been shown to encode a methionine sulfoxide reductase and its disruption prevents DMS production (Hansen 1999). Finally, DMS formation during fermentation has also been linked to cysteine, cystine or glutathione metabolism in yeast (Rauhut 1993; Ribéreau-Gayon et al. 2000).
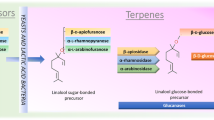
10.7 Yeasts and Its Role in the Development of Varietal Aroma in Wine
In addition to the aroma-active compounds synthesised by yeasts during alcoholic fermentations, some yeasts play a relevant role in the development of the primary or varietal aroma of wines (Gamero et al. 2011a, b). Wine’s primary aroma consists of lactones, benzenes, volatile phenols, vanillins, norisoprenoids, terpenes and some polyfunctional mercaptans present at low concentrations in the ng/l–μg/l range (Loscos et al. 2007; Mateo-Vivaracho et al. 2010; Tominaga et al. 1998b). Most of these aromas appear in grapes as odourless precursors (glycosides, polyhydroxylated molecules or cysteinyl-derivatives). It has been demonstrated that some yeasts are able to release those aroma compounds by cleavage of the precursor molecules or are even able to synthesise new aroma molecules similar to the ones present in grapes (Darriet et al. 1988; Delcroix et al. 1994; Delfini et al. 2001; Fernández-González et al. 2003; Fernández-González and Di Stefano 2004; Gamero et al. 2011a, b; Hernández et al. 2003; Hernández-Orte et al. 2008; Loscos et al. 2007; Mateo and Di Stefano 1997; Spagna et al. 2002; Ugliano et al. 2006; Ugliano and Moio 2008). In this way, yeast can enhance wine varietal aroma. For instance, Saccharomyces species and hybrids are able to release and synthesise de novo vanillins, terpenes, lipid derivatives, volatile phenols and norisoprenoids (Gamero et al. 2011a, b).
In certain wines, varietal aroma compounds play a crucial role. This is the case of some polyfunctional mercaptans in certain white wines (Tominaga et al. 1998b; Mateo-Vivaracho et al. 2010), of linalool and other terpenols in Muscat wines (Ribéreau-Gayon et al. 2000) or of cis-rose oxide in Gewürztraminer (Guth 1997). On the other hand, in most wines, varietal aroma is formed by combinations of many grape- and yeast-derived compounds, none of which play a predominant aroma role, and it is the overall aroma profile the responsible for varietal and origin related difference (Escudero et al. 2007; Loscos et al. 2007, 2010).
10.7.1 Monoterpenes
Among the most important key odorants in the so-called ‘aromatic’ grape varieties (e.g. Muscat) are monoterpenes such as linalool, geraniol, nerol, citronellol and α-terpineol. (Gunata et al. 1985; Loscos et al. 2007; Maicas and Mateo 2005; Strauss et al. 1986; Ugliano and Henschke 2009). The common precursor of all the monoterpenoids is isopentyl pyrophosphate.
During must fermentation the grape-derived glycosidic precursors are hydrolysed by the action of glycosidases and the aromatic volatile compounds released into the wine. Among the most important glycosidases are β-glucosidases, α-l-arabinofuranosidases, α-l-rhamnosidases and β-d-xylosidases (Maicas and Mateo 2005; Van Rensburg and Pretorius 2000; Sarry and Gunata 2004). Several research groups the world over have investigated various wine-related Saccharomyces and non-Saccharomyces (Brettanomyces/Dekkera, Candida, Debaryomyces, Hanseniaspora and Pichia) yeasts for their ability to produce suitable glycosidases and other enzymes that can release varietal aromas (Charoenchai et al. 1997; Esteve-Zarzoso et al. 1998; Fernández et al. 2000; Fleet 2008; McMahon et al. 1999; Strauss et al. 2001; Ugliano et al. 2006; Zoecklein et al. 1997).
In addition to the search for yeasts that naturally produce aroma-enhancing enzymes, several mutants have been constructed with the aim of enhancing monoterpene-based varietal flavours during wine fermentation. These mutants include a yeast expressing the β-1,4-glucanase gene from Trichoderma longibratum (Villanueva et al. 2000) and a wine yeast expressing the BGL1 and BGL2 β-glucosidase genes of Saccharomycopsis fibuligera, the ABF2 α-l-arabinofuranosidase gene of Aspergillus niger and a glucanase-encoding gene cassette consisting of several glucanase genes (BEG1, END1 and EXG1) (Pretorius 2000, 2003, 2004; Van Rensburg and Pretorius 2000; Pretorius and Bauer 2002; de Barros Lopes et al. 2006).
In addition to studies focused on the release of monoterpenes by yeasts, there were also several studies undertaken concerning the biotransformation of terpenes by Saccharomyces species and hybrids, such as the reduction of geraniol to citronellol, translocation of geraniol to linalool, isomerisation of nerol to geraniol and cyclicizations of linalool to α-terpineol (Gamero et al. 2011b; Gramatica et al. 1982; King and Dickinson 2000; Zea et al. 1995; Zoecklein et al. 1997).
In the case of brewing, it has also recently been shown that different hop varieties have different concentrations of monoterpenoids (Takoi et al. 2010).
10.7.2 Volatile Thiols
Volatile thiols are sulphur compounds that can appear in wines in very low concentrations, but they can have a profound impact on the aroma of certain wine varieties, such as Sauvignon Blanc, Colombard, Riesling, Semillon, Merlot and Cabernet Sauvignon, since they present very low sensory thresholds (ng/l level) (Tominaga et al. 1995, 1998a, b; Murat et al. 2001b). These compounds are responsible for the ‘fruity’ or ‘tropical’ organoleptic flavours. Some examples of volatile thiols are 4-mercapto-4-methylpentan-2-one (4MMP), reminiscent of ‘box tree’, ‘passion-fruit’, ‘broom’ and ‘black current’ bud; 3-mercaptohexan-1-ol (3MH) and 3-mercaptohexyl acetate (3MHA), responsible for ‘passion-fruit’, ‘grapefruit’ and ‘citrus’ aromas; 4-mercapto-4-methylpentan-2-ol (4MMPOH) that can also contribute to the characters of ‘citrus’, ‘passion-fruit’ and ‘grapefruit’, although its organoleptic role is more limited, due to its concentration in wines seldom exceeding its olfactory threshold of 55 ng/l and 2-furfurylthiol, which can contribute roast coffee aroma to the bouquet of wines aged in oak barrels (Darriet et al. 1995; Tominaga et al. 1996, 1998b, 2000; Tominaga and Dubourdieu 2006).
Most of the thiols that are present in grapes appear as non-volatile, cysteine-bound conjugates and can be released by the action of carbon–sulphur lyases of certain yeasts (Darriet et al. 1995; Tominaga et al. 1995). Deletion and overexpression of the genes encoding these enzymes resulted in a decrease and increase, respectively, in the levels of the corresponding thiols (Howell et al. 2005; Swiegers et al. 2007). The release of thiols occurs during fermentation in a low percentage since it has been detected that only a small fraction of cysteine-bound conjugates (1.6–3.2 %) is released as 3MH (Dubourdieu et al. 2006; Murat et al. 2001b). The efficiency of thiol release is strain dependent (Dubourdieu et al. 2006; Howell et al. 2004); however, some studies reported that S. bayanus and S. bayanus/S. cerevisiae hybrid strains have stronger abilities than S. cerevisiae in this sense (Murat et al. 2001a; Swiegers et al. 2006a). In addition, certain non-Saccharomyces yeasts can have a significant impact on volatile thiol concentration. Co-inoculation of Pichia kluyveri, isolated from a spontaneous fermentation of Chardonnay must, with specific commercial wine strains of S. cerevisiae resulted in an increase of the concentration of 3MHA in Sauvignon Blanc wines (Anfang et al. 2009). Recent work also showed that some strains of Metschnikowia pulcherrima, T. delbrueckii and K. thermotolerans have relatively high capacities to release 3MH (Zott et al. 2011).
Thiols can also be synthesised by yeasts. For instance, it has been proposed that cysteine desulfhydrase enzyme catalyses the formation of furfurylthiol from furfural (Tominaga et al. 2000) and the formation of H2S enhances this process. In this way, the production of furfurylthiol is linked to the production of the HS− anion, which is not produced when sufficient ammonium sulphate is present in the medium (Tominaga et al. 2000). During fermentation, 3MH can be converted to 3MHA by the action of alcohol acetyltransferase, encoded by the ATF1 gene (Swiegers et al. 2006b). There is significant variation in the conversion rates present by the different yeast strains, which is not correlated with the ability to release 4MMP (Swiegers et al. 2006b).
In addition to the specific yeast strain conducting the fermentation, temperature is also a relevant factor in determining volatile thiol concentration. Concentrations of 4MMP, 3MH and 3MHA were higher when the alcoholic fermentation was conducted at 20 °C compared to 13 °C or a 18 °C compared to 23 °C and 28 °C (Masneuf-Pomarède et al. 2006; Swiegers et al. 2006a). So around 18–20 °C seems to be the optimum.
Regarding beer, there is not much knowledge about the occurrence of volatile thiols. However, Vermeulen et al. (2006) detected more than ten of these compounds in fresh lager beer. Thiols do not appear in wort. The most powerful thiol in beer is 3-methyl-2-buten-1-thiol, and this thiol together with 2-mercapto-3-methylbutanol and 3-mercapto-3-methylbutanol are thought to be derived from hop allylic alcohols (Vermeulen et al. 2006). On the other hand, it is hypothesised that the origin of 2-mercaptoethanol and 3-mercaptopropanol and their corresponding acetates could be Ehrlich degradation of sulphur amino acids, whereas 2-methyl-3-furanthiol could be produced through Maillard reactions (Vermeulen et al. 2006).
10.8 Concluding Remarks
The aroma profile of wine, beer and cider is a defining component of the value proposition to consumers. Producers are therefore keen to understand what the optimal ‘absolute’ and ‘relative’ concentrations of the most important aroma-active compounds are and how they can adapt their practices to gain control over the composition of their products. It is widely accepted that one way to adjust the aroma profile of certain styles of fermented beverages is choice of yeast strain(s) with which the fermentation is conducted. However, further research is required into the range of ‘aroma phenotypes’ that wine yeast exhibit, and how this knowledge can be applied to develop novel aroma-enhancing yeast strains or combinations of yeast strains or mixtures of different yeast species.
References
Adams MR, Moss MO (2000) Food microbiology. Royal Society of Chemistry, Cambridge
Anfang N, Brajkovich M, Goddard M (2009) Co-fermentation with Pichia kluyveri increases varietal thiol concentrations in Sauvignon Blanc. Austr J Grape Wine Res 15:1–8
Antonelli A, Castellari L, Zambonelli C et al (1999) Yeast influence on volatile composition of wines. J Agric Food Chem 47:1139–1144
Antunovics Z, Csoma H, Sipiczki M (2003) Molecular and genetic analysis of the yeast flora of botrytized Tokaj wines. Bull de l’OIV 76:380–397
Äyräpää T (1968) Formation of higher alcohols by various yeasts. J Inst Brew 74:169–179
Äyräpää T (1971) Biosynthetic formation of higher alcohols by yeast. Dependence on the nitrogenous nutrient level of the medium. J Inst Brew 77:266–276
Azzolini M, Fedrizzi B, Tosi E et al (2012) Effects of Torulaspora delbrueckii and Saccharomyces cerevisiae mixed cultures on fermentation and aroma of Amarone wine. Eur Food Res Tech 235:303–313
Bamforth CW, Kanauchi M (2004) Enzymology of vicinal diketone reduction in brewer’s yeast. J Inst Brew 110:83–93
Bandell M, Story GM, Hwang SW et al (2004) Noxious cold ion channel TRPA1 is activated by pungent compounds and bradykinin. Neuron 41(6):849–857
Barrio E, González SS, Arias A et al (2006) Molecular mechanisms involved in the adaptive evolution of industrial yeasts. In: Querol A, Fleet GH (eds) Yeasts in food and beverages. The yeast handbook. Springer, Berlin, pp 153–174
Barthelmebs L, Divies C, Cavin JF (2000a) Knockout of the p-coumarate decarboxylase gene from Lactobacillus plantarum reveals the existence of two other inducible enzymatic activities involved in phenolic acid metabolism. Appl Environ Microbiol 66:3368–3375
Barthelmebs L, Lecomte B, Divies C et al (2000b) Inducible metabolism of phenolic acids in Pediococcus pentosaceus is encoded by an autoregulated operon which involves a new class of negative transcriptional regulator. J Bacteriol 182:6724–6731
Bautista DM, Siemens J, Glazer JM et al (2007) The menthol receptor TRPM8 is the principal detector of environmental cold. Nature 448(7150):204–208
Beech FW (1993) Yeasts in cider-making. In: Rose AH, Harrison JS (eds) The yeasts. Yeast technology, vol. 5, 2nd edn. Academic Press Limited, London, pp 169–213
Bely M, Stoeckle P, Masneuf-Pomarède I et al (2008) Impact of mixed Torulaspora delbrueckii–Saccharomyces cerevisiae culture on high-sugar fermentation. Int J Food Microbiol 122:312–320
Belloch C, Orlic S, Barrio E et al (2008) Fermentative stress adaptation of hybrids within the Saccharomyces sensu stricto complex. Int J Food Microbiol 122(1–2):188–195
Belloch C, Pérez-Torrado R, Gonzalez SS et al (2009) Chimeric genomes of natural hybrids of Saccharomyces cerevisiae and Saccharomyces kudriavzevii. Appl Environ Microbiol 75:2534–2544
Borneman AR, Desany BA, Riches D et al (2012) The genome sequence of the wine yeast VIN7 reveals an allotriploid hybrid genome with Saccharomyces cerevisiae and Saccharomyces kudriavzevii. FEMS Yeast Res 12:88–96
Boulton RB, Singleton VL, Bisson LF et al (1998) Principles and practices of winemaking. Chapman & Hall, New York
Cabranes C, Moreno J, Mangas JJ (1990) Dynamics of yeast population during cider fermentation in the Asturian Region of Spain. Appl Environ Microbiol 56:381–384
Campo E, Cacho J, Ferreira V (2007) Solid phase extraction, multidimensional gas chromatography mass spectrometry determination of four novel aroma powerful ethyl esters: assessment of their occurrence and importance in wine and other alcoholic beverages. J Chromatogr A 1140(1–2):180–188
Campo E, Ferreira V, Escudero A et al (2005) Prediction of the wine sensory properties related to grape variety from dynamic-headspace gas chromatography-olfactometry data. J Agric Food Chem 53(14):5682–5690
Caterina MJ, Schumacher MA, Tominaga M et al (1997) The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature 389(6653):816–824
Cavin JF, Andioc V, Etievant PX et al (1993) Ability of wine lactic acid bacteria to metabolize phenol carboxylic acids. Am J Enol Vitic 44:76–80
Charoenchai C, Fleet GH, Henschke PA et al (1997) Screening of non-Saccharomyces wine yeasts for the presence of extracellular hydrolytic enzymes. Austr J Grape Wine Res 3:2–8
Chatonnet P, Dubourdieu D, Boidron JN et al (1993) Synthesis of volatile phenols by Saccharomyces cerevisiae in wines. J Sci Food Agric 62:191–202
Coloretti F, Zambonelli C, Tini V (2006) Characterization of flocculent Saccharomyces interspecific hybrids for the production of sparkling wines. Food Microbiol 23:672–676
Cordente AG, Cordero-Bueso G, Pretorius IS et al (2013) Novel wine yeast with mutations in YAP1 that produce less acetic acid during fermentation. FEMS Yeast Res 13(1):62–73
Cordente AG, Heinrich A, Pretorius IS et al (2009) Isolation of sulfite reductase variants of a commercial wine yeast with significantly reduced hydrogen sulfide production. FEMS Yeast Res 9(3):446–459
Cordente AG, Swiegers JH, Hegardt FG et al (2007) Modulating aroma compounds during wine fermentation by manipulating carnitine acetyltransferases in Saccharomyces cerevisiae. FEMS Microbiol Lett 267:159–166
Corison CA, Ough CS, Berg HW et al (1979) Must acetic acid and ethyl acetate as mold and rot indicators in grapes. Am J Enol Vitic 30:130–134
Cortes MB, Moreno J, Zea L et al (1998) Changes in aroma compounds of sherry wines during their biological aging carried out by Saccharomyces cerevisiae races bayanus and capensis. J Agric Food Chem 46:2389–2394
Coton E, Coton M, Levert D et al (2006) Yeast ecology in French cider and black olive natural fermentations. Int J Food Microbiol 108:130–135
Cousseau FE, Alves SL Jr, Trichez D et al (2013) Characterization of maltotriose transporters from the Saccharomyces eubayanus sub-genome of the hybrid Saccharomyces pastorianus lager brewing yeast strain Weihenstephan 34/70. Lett Appl Microbiol 56(1):21–29
Darriet P, Boidron JN, Dubourdieu D (1988) Hydrolysis of the terpene heterosides of small-seed Muscat grapes by periplasmic enzymes of Saccharomyces cerevisiae. Connaissance de la Vigne et du Vin 22:189–195
Darriet P, Tominaga T, Lavigne V et al (1995) Identification of a powerful aromatic compound of Vitis vinifera L. var. Sauvignon wines: 4-mercapto-4-methylpentan-2-one. Flav Fragr J 10:385–392
De Barros Lopes MA, Bartowsky EJ, Pretorius IS (2006) The application of gene technology in the wine industry. In: Hui HY, Castell-Pérez E, Cunha LM et al (eds) Handbook of food science, technology, and engineering. CRC Taylor & Francis, New York, pp 1–21
de Barros Lopes M, Bellon JR, Shirly NJ et al (2002) Evidence for multiple interspecific hybridization in Saccharomyces sensu stricto species. FEMS Yeast Res 1:323–331
del Campo G, Santos JL, Berregi I et al (2003) Ciders produced by two types of presses and fermented in stainless steel and wooden vats. J Inst Brew 109:342–348
Delcroix A, Gunata Z, Sapis JC et al (1994) Glycoside activities of three enological yeast strains during winemaking: effects of the terpenol content of Muscat wine. Am J Enol Vitic 45:291–296
Delfini C, Cocito C, Bonino M et al (2001) Definitive evidence for the actual contribution of yeast in the transformation of neutral precursors of grape aromas. J Agric Food Chem 49(11):5397–5408
Delfini C, Costa A (1993) Effects of the grape must lees and insoluble materials on the alcoholic fermentation rate and the production of acetic acid, pyruvic acid, and acetaldehyde. Am J Enol Vitic 44:86–92
Delneri D, Gardner DCJ, Bruschi CV et al (1999) Disruption of seven hypothetical aryl alcohol dehydrogenase genes from Saccharomyces cerevisiae and construction of a multiple knock-out strain. Yeast 15:1681–1689
Delwiche J (2004) The impact of perceptual interactions on perceived flavor. Food Qual Pref 15(2):137–146
Demuyter C, Lollier M, Legras JL et al (2004) Predominance of Saccharomyces uvarum during spontaneous alcoholic fermentation, for three consecutive years, in an Alsatian winery. J Appl Microbiol 97:1140–1148
Di Stefano R, Ciolfi G, Delfini C (1981) Composti volatili prodotti dei lieviti. Rivista di Viticoltura e di Enologia 34:342–355
Dickinson JR, Harrison SJ, Dickinson JA et al (2000) An investigation of the metabolism of isoleucine to active amyl alcohol in Saccharomyces cerevisiae. J Biol Chem 275:10937–10942
Dickinson JR, Harrison SJ, Hewlins MJE (1998) An investigation of the metabolism of valine to isobutyl alcohol in Saccharomyces cerevisiae. J Biol Chem 273:25751–25756
Dickinson JR, Lanterman MM, Danner DJ et al (1997) A 13C nuclear magnetic resonance investigation of the metabolism of leucine to isoamyl alcohol in Saccharomyces cerevisiae. J Biol Chem 272:26871–26878
Dickinson JR, Norte V (1993) A study of branched-chain amino acid aminotransferase and isolation of mutations affecting the catabolism of branched-chain amino acids in Saccharomyces cerevisiae. FEBS Lett 326:29–32
Dickinson JR, Salgado LE, Hewlins MJE (2003) The catabolism of amino acids to long chain and complex alcohols in Saccharomyces cerevisiae. J Biol Chem 278:8028–8034
Didion T, Regenberg B, Jorgensen MU et al (1998) The permease homologue Ssy1p controls the expression of amino acid and peptide transporter genes in Saccharomyces cerevisiae. Mol Microbiol 27:643–650
Dubois P (1983) Volatile phenols in wine. In: Piggott JR (ed) Flavours of distilled beverages. Ellis Horwood, Chichester, pp 110–119
Dubourdieu D, Tominaga T, Masneuf I et al (2006) The role of yeasts in grape flavor development during fermentation: the example of Sauvignon Blanc. Am J Enol Vitic 57:81–88
Dufour JP, Malcorps P (1995) Ester synthesis during fermentation: enzymes characterization and modulation mechanisms. In: Campbell I, Priest FG (eds) 4th Aviemore conference on malting, brewing and distilling. The Institute of Brewing, London, pp 137–151
Dunn B, Richter C, Kvitek DJ et al (2012) Analysis of the Saccharomyces cerevisiae pan-genome reveals a pool of copy number variants distributed in diverse yeast strains from differing industrial environments. Genome Res 22(5):908–924
Dunn B, Sherlock G (2008) Reconstruction of the genome origins and evolution of the hybrid lager yeast Saccharomyces pastorianus. Genome Res 18(10):1610–1623
Eden A, Van Nedervelde L, Drukker M et al (2001) Involvement of branched-chain amino acid aminotransferases in the production of fusel alcohols during fermentation in yeast. Appl Microbiol Biotechnol 55:296–300
Edwards TL, Singleton VL, Boulton R (1985) Formation of ethyl esters of tartaric acid during wine aging: chemical and sensory effects. Am J Enol Vitic 36:118–124
Eglinton JM, McWilliam SJ, Fogarty MW et al (2000) The effect of Saccharomyces bayanus-mediated fermentation on the chemical composition and aroma profile of Chardonnay wine. Austr J Grape Wine Res 6:190–196
Engan S (1981) Beer composition: Volatile substances. In: Pollock JRA (ed) Brewing science, vol 2. Academic Press, London, pp 93–165
Erten H, Tanguler H (2010) Influence of Williopsis saturnus yeasts in combination with Saccharomyces cerevisiae on wine fermentation. Lett Appl Microbiol 50:447–449
Escudero A, Campo E, Farina L et al (2007) Analytical characterization of the aroma of five premium red wines. Insights into the role of odor families and the concept of fruitiness of wines. J Agric Food Chem 55(11):4501–4510
Escudero AB, Gogorza MA, Melús N et al (2004) Characterization of the aroma of a wine from Maccabeo. Key role played by compounds with low odor activity value. J Agric Food Chem 52:3516–3524
Esteve-Zarzoso B, Manzanares P, Ramón D et al (1998) The role of non-Saccharomyces yeasts in industrial winemaking. Int Microbiol 1(2):143–148
Etiévant PX (1991) Wine. In: Maarse H (ed) Volatile compounds of food and beverages. Marcel Dekker, New York, pp 483–546
Fernández M, Úbeda JF, Briones AI (2000) Typing of non-Saccharomyces yeasts with enzymatic activities of interest in wine-making. Int J Food Microbiol 59:29–36
Fernández-González M, Di Stefano R (2004) Fractionation of glycoside aroma precursors in neutral grapes. Hydrolysis and conversion by Saccharomyces cerevisiae. Food Sci Technol 37(4):467–473
Fernández-González M, Di Stefano R, Briones A (2003) Hydrolysis and transformation of terpene glycosides from Muscat must by different yeast species. Food Microbiol 20:35–41
Ferreira V (2012) Revisiting psychophysical work on the quantitative and qualitative odour properties of simple odour mixtures: a flavour chemistry view. Part 2: qualitative aspects. A review. Flav Fragr J 27(3):201–215
Ferreira V, Fernandez P, Cacho JF (1996) A study of factors affecting wine volatile composition and its application in discriminant analysis. Food Sci Technol 29(3):251–259
Ferreira V, Lopez R, Cacho JF (2000) Quantitative determination of the odorants of young red wines from different grape varieties. J Sci Food Agric 80(11):1659–1667
Ferreira V, Ortín N, Escudero A et al (2002) Chemical characterization of the aroma of Grenache rosé wines. Aroma extract dilution analysis, quantitative determination and sensory reconstitution studies. J Agric Food Chem 50:4048–4054
Fleet GH (2008) Wine yeasts for the future. FEMS Yeast Res 8(7):979–995
Fleet GH, Heard GM (1993) Yeasts—Growth during fermentation. In: Fleet GH (ed) Wine microbiology and biotechnology. Harwood Academic Publishers, Chur, pp 27–54
Frezier V, Dubourdieu D (1992) Ecology of yeast strain Saccharomyces cerevisiae during spontaneous fermentation in a Bordeaux winery. Am J Enol Vitic 43:375–380
Fujii T, Nagasawa N, Iwamatsu A et al (1994) Molecular cloning, sequence analysis, and expression of the yeast alcohol acetyltransferase gene. Appl Environ Microbiol 60(8):2786–2792
Fujii T, Yoshimoto H, Tamai Y (1996) Acetate ester production by Saccharomyces cerevisiae lacking the ATF1 gene encoding the alcohol acetyltransferase. J Ferm Bioeng 81(6):538–542
Fujiwara D, Kobayashi O, Yoshimoto H et al (1999) Molecular mechanism of the multiple regulation of the Saccharomyces cerevisiae ATF1 gene encoding alcohol acetyltransferase. Yeast 15:1183–1197
Gamero A, Hernández-Orte P, Ferreira V et al (2011a) Effect of aromatic precursor addition to wine fermentations carried out with different Saccharomyces species and their hybrids. Int J Food Microbiol 147(1):33–44
Gamero A, Manzanares P, Querol A et al (2011b) Monoterpene alcohols release and bioconversion by Saccharomyces species and hybrids. Int J Food Microbiol 145(1):92–97
Gamero A, Tronchoni J, Belloch C et al (2013) Production of aroma compounds by cryotolerant Saccharomyces species and hybrids at low and moderate fermentation temperatures. J Appl Microbiol 114:1405–1414
Gangl H, Batusic M, Tscheik G et al (2009) Exceptional fermentation characteristics of natural hybrids from Saccharomyces cerevisiae and S. kudriavzevii. N Biotechnol 25(4):244–251
Gil JV, Mateo JJ, Jiménez M (1996) Aroma compounds in wine as influenced by apiculate yeasts. J Food Sci 61:1247–1250
Giudici P, Zambonelli C, Passarelli P et al (1995) Improvement of wine composition with cryotolerant Saccharomyces strains. Am J Enol Vitic 46:143–147
Godtfredsen SE, Ottesen M (1982) Maturation of beer with α-acetolactate decarboxylase. Carlsberg Res Commun 47:93–102
Gómez E, Laencina J, Martínez A (1994) Vinification effects on changes in volatile compounds of wine. J Food Sci 59:406–409
González SS, Barrio E, Gafner J et al (2006) Natural hybrids from Saccharomyces bayanus, S. cerevisiae and S. kudriavzevii in wine fermentations. FEMS Yeast Res 6(8):1221–1234
González SS, Barrio E, Querol A (2008) Molecular characterization of new natural hybrids of Saccharomyces cerevisiae and S. kudriavzevii in Brewing. Appl Environ Microbiol 74(8):2314–2320
González SS, Gallo L, Climent MD et al (2007) Enological characterization of natural hybrids between Saccharomyces cerevisiae and S. kudriavzevii. Int J Food Microbiol 116(1):11–18
Goossens E, Debourg A, Villanueva K et al (1991) Decreased diacetyl production by site directed integration of the ILV5 gene into chromosome XIII of Saccharomyces cerevisiae. In: Proceedings of the congress of the 23rd European Brewery Convention, Lisbon, 13–15 May 1991
Goossens E, Dillemans M, Debourg A et al (1987) Control of diacetyl formation by the intensification of the anabolic flux of acetohydroxy acid intermediates. In: Proceedings of the 21st congress of the European Brewery Convention, Madrid, 10–14 May 1987
Gramatica P, Manitto P, Ranzi BM et al (1982) Stereospecific reduction of geraniol to R-(+)-citronellol by Saccharomyces cerevisiae. Experimentia 38:775–776
Grando MS, Versini G, Nicolini G et al (1993) Selective use of wine yeast strains having different volatile phenols production. Vitis 32(43):50
Grauslund M, Didion T, Kielland-Brandt MC et al (1995) BAP2, a gene encoding a permease for branched-chain amino acids in Saccharomyces cerevisiae. Biochim Biophys Acta 1269(3):275–280
Green BG, Nachtigal D, Hammond S et al (2012) Enhancement of retronasal odors by taste. Chem Senses 37(1):77–86
Gunata YZ, Bayonove CL, Baumes RL et al (1985) The aroma of grapes. Extraction and determination of free and glycosidically bound fractions of some grape aroma components. J Chromatogr 331:83–90
Guth H (1997) Identification of character impact odorants of different white wine varieties. J Agric Food Chem 45(8):3022–3026
Gutiérrez AR, Santamaría P, Epifanio S et al (1999) Ecology of spontaneous fermentation in one winery during 5 consecutive years. Lett Appl Microbiol 29:411–415
Hallinan CP, Saul DJ, Jiranek V (1999) Differential utilisation of sulfur compounds for H2S liberation by nitrogen-starved wine yeasts. Austr J Grape Wine Res 5:82–90
Hammond JRM (1995) Genetically-modified brewing yeasts for the 21st century. Progress to date. Yeast 11:1613–1627
Hansen J (1999) Inactivation of MXR1 abolishes formation of dimethyl sulfide from dimethyl sulfoxide in Saccharomyces cerevisiae. Appl Environ Microbiol 65:3915–3919
Hansen J, Kielland-Brandt MC (1996) Inactivation of MET2 in brewer’s yeast increases the level of sulfite in beer. J Biotechnol 50:75–87
Haukeli AD, Lie S (1978) Conversion of α-acetolactate and removal of diacetyl: a kinetic study. J Inst Brew 84:85–89
Hazelwood LA, Daran JM, van Maris AJA et al (2008) The Ehrlich pathway for fusel alcohol production: a century of research on Saccharomyces cerevisiae. Metabol Appl Environ Microbiol 74(8):2259–2266
Henschke PA, Jiranek V (1991) Hydrogen sulfide formation during fermentation: effect of nitrogen composition in model grape must. In: Proceedings of the international symposium on nitrogen in grapes and wine. American Society for Enology and Viticulture, Seattle, Washington, 18–19 June 1991
Henschke PA, Jiranek V (1993) Yeast—Metabolism of nitrogen compounds. In: Fleet GH (ed) Wine microbiology and biotechnology. Harwood Academic Publishers, Chur, pp 77–164
Henschke PA, Kwiatkowski MJ, Fogarty MW et al (2000) The effect of Saccharomyces bayanus-mediated fermentation on the chemical composition and aroma profile of Chardonnay wine. Austr J Grape Wine Res 6:190–196
Hernández LF, Espinosa JC, Fernández-González M et al (2003) β-glucosidase activity in a Saccharomyces cerevisiae wine strain. Int J Food Microbiol 80:171–176
Hernández-Orte P, Cacho J, Ferreira V (2002) Relationship between the varietal amino acid profile of grapes and the wine aromatic composition. Experiments with model solutions and chemometric study. J Agric Food Chem 50:2891–2899
Hernández-Orte P, Cersosimo M, Loscos N et al (2008) The development of varietal aroma from non-floral grapes by yeasts of different genera. Food Chem 107:1064–1077
Herraiz T, Ough CS (1993) Formation of ethyl esters of amino acids by yeasts during the alcoholic fermentation of grape Juice. Am J Enol Vitic 44:41–48
Herraiz T, Reglero G, Herraiz M et al (1990) The influence of the Yeast and type of culture on the volatile composition of wines fermented without sulfur dioxide. Am J Enol Vitic 41:313–318
Horinouchi T, Yoshikawa K, Kawaide R et al (2010) Genome-wide expression analysis of Saccharomyces pastorianus orthologous genes using oligonucleotide microarrays. J Biosci Bioeng 110(5):602–607
Horsted MW, Dey ES, Holmberg S et al (1998) A novel esterase from Saccharomyces carlsbergensis, a possible function for the yeast TIP1 gene. Yeast 14(9):793–803
Houtman AC, Du Plessis CS (1981) The effect of juice clarity and several conditions promoting yeast growth on fermentation rate, the production of aroma components and wine quality. S Afr J Enol Vitic 2:71–81
Houtman AC, Marais J, Du Plessis CS (1980a) Factors affecting the reproducibility of fermentation of grape juice and of the aroma composition of wines. Grape maturity, sugar, inoculum concentration, aeration, juice turbidity and ergosterol. Vitis 19:37–54
Houtman AC, Marais J, Du Plessis CS (1980b) The possibilities of applying present-day knowledge of wine aroma components: Influence of several juice factors on fermentation rate and ester production during fermentation. S Afr J Enol Vitic 1:27–33
Howell KS, Swiegers JH, Elsey GM et al (2004) Variation in 4-mercapto-4-methyl-pentan-2-one release by Saccharomyces cerevisiae commercial wine strains. FEMS Microbiol Lett 240:125–129
Howell KS, Klein M, Swiegers JH et al (2005) Genetic determinants of volatile-thiol release by Saccharomyces cerevisiae during wine fermentation. Appl Environ Microbiol 71:5420–5426
Inoue T (1992) A review of diacetyl control technology. In: Proceedings of the 22nd Institute of Brewing Convention, Melbourne, 1–6 March 1992
Isnard AD, Thomas D, Surdin-Kerjan Y (1996) The study of methionine uptake in Saccharomyces cerevisiae reveals a new family of amino acid permeases. J Mol Biol 262(4):473–484
Izquierdo-Canas PM, Palacios-Garcia AT, Garcia-Romero E (2011) Enhancement of flavour properties in wines using sequential inoculations of non-Saccharomyces (Hansenula and Torulaspora) and Saccharomyces yeast starters. Vitis 50:177–182
Jain VK, Divol B, Prior BA et al (2012) Effect of NAD+-regenerating pathways on the formation of primary and secondary aroma compounds in a Saccharomyces cerevisiae glycerol-defective mutant. Appl Microbiol Biotechnol 93:131–141
Jauniaux JC, Grenson M (1990) GAP1, the general amino acid permease gene of Saccharomyces cerevisiae. Nucleotide sequence, protein similarity with the other bakers yeast amino acid permeases, and nitrogen catabolite repression. Eur J Biochem 190(1):39–44
Jiranek V, Langridge P, Henschke PA (1995) Validation of bismuth-containing indicator media for predicting H2S-producing potential of Saccharomyces cerevisiae wine yeasts under enological conditions. Am J Enol Vitic 46:269–273
King A, Dickinson JR (2000) Biotransformation of monoterpene alcohols by Saccharomyces cerevisiae, Torulaspora delbrueckii and Kluyveromyces lactis. Yeast 16:499–506
Kispal G, Steiner H, Court DA et al (1996) Mitochondrial and cytosolic branched-chain amino acid transaminases from yeast, homologs of the myc oncogene-regulated Eca39 protein. J Biol Chem 271:24458–24464
Kodama YK, Kielland-Brandt MC, Hansen J (2006) Lager brewing yeast. In: Sunnerhagen P, Piskur J (eds) Comparative genomics. Springer, Berlin, pp 145–164
Kodama Y, Omura F, Miyajima K et al (2001) Control of higher alcohol production by manipulation of the BAP2 gene in brewing yeast. J Am Soc Brew Chem 59:157–162
Krogerus K, Gibson BR (2013) Influence of valine and other amino acids on total diacetyl and 2,3-pentanedione levels during fermentation of brewer’s wort. Appl Microbiol Biotechnol. doi:10.1007/s00253-013-4955-1
Lambrechts MG, Pretorius IS (2000) Yeasts and its importance to wine aroma: a review. S Afr J Enol Vitic 21:97–129
Landaud S, Latrille E, Corrieu G (2001) Top pressure and temperature control the fusel alcohol/ester ratio through yeast growth in beer fermentation. J Inst Brew 107:107–117
Lei H, Zhao H, Yu Z et al (2012) Effects of wort gravity and nitrogen level on fermentation performance of brewer’s yeast and the formation of flavor volatiles. Appl Biochem Biotechnol 166:1562–1574
Lema C, García-Jares C, Orriols I et al (1996) Contribution of Saccharomyces and non-Saccharomyces populations to the production of some components of Albariño wine aroma. Am J Enol Vitic 47:206–216
Libkind D, Hittinger CT, Valério E et al (2011) Microbe domestication and the identification of the wild genetic stock of lager-brewing yeast. Proc Natl Acad Sci USA 108(35):14539–14544
Lilly M, Bauer FF, Lambrechts MG et al (2006a) The effect of increased yeast alcohol acetyltransferase and esterase activity on the flavour profiles of wine and distillates. Yeast 23:641–659
Lilly M, Bauer FF, Styger G et al (2006b) The effect of increased branched-chain amino acid transaminase activity in yeast on the production of higher alcohols and on the flavour profiles of wine and distillates. FEMS Yeast Res 6:726–743
Lilly M, Lambrechts MG, Pretorius IS (2000) Effect of increased yeast alcohol acetyltransferase activity on flavour profiles of wine and distillates. Appl Environ Microbiol 66:744–753
Liu SQ, Pilone GJ (2000) An overview of formation and roles of acetaldehyde in winemaking with emphasis on microbiological implications. Int J Food Sci Technol 35:49–61
Llauradó JM, Rozès N, Bobet R et al (2002) Low temperature alcoholic fermentation in high sugar concentration grape must. J Food Sci 67:268–273
Llauradó JM, Rozès N, Constantí M et al (2005) Study of some Saccharomyces cerevisiae strains for winemaking after preadaptation at low temperatures. J Agric Food Chem 53(4):1003–1011
Lopes C, Barrio E, Querol A (2010) Natural hybrids of S. cerevisiae × S. kudriavzevii share alleles with European wild populations of Saccharomyces kudriavzevii. FEMS Yeast Res 10(4):412–421
Loscos N, Hernández-Orte P, Cacho J et al (2007) Release and formation of varietal aroma compounds during alcoholic fermentation from nonfloral grape odorless flavor precursors fractions. J Agric Food Chem 55(16):6674–6684
Loscos N, Hernández-Orte P, Cacho J et al (2010) Evolution of the aroma composition of wines supplemented with grape flavour precursors from different varietals during accelerated wine ageing. Food Chem 120:205–216
Macpherson LJ, Hwang SW, Miyamoto T et al (2006) More than cool: promiscuous relationships of menthol and other sensory compounds. Mol Cell Neurosci 32(4):335–343
Mai B, Lipp M (1994) Cloning and chromosomal organization of a gene encoding a putative amino-acid permease from Saccharomyces cerevisiae. Gene 143:129–133
Maicas S, Mateo JJ (2005) Hydrolysis of terpenyl glycosides in grape juice and other fruit juices: a review. Appl Microbiol Biotechnol 67:322–335
Malcorps P, Cheval JM, Jamil S et al (1991) A new model for the regulation of ester synthesis by alcohol acetyl transferase in Saccharomyces cerevisiae during fermentation. J Am Soc Brew Chem 49:47–53
Marais J, Pool H (1980) Effect of storage time and temperature on the volatile composition and quality of dry white table wines. Vitis 19:151–164
Margalith PZ (1981) Flavour microbiology. Charles C. Thomas Publishers, Springfield
Masneuf I, Hansen J, Groth C et al (1998) New hybrids between Saccharomyces sensu stricto yeast species found among wine and cider production strains. Appl Environ Microbiol 64:3887–3892
Masneuf-Pomarède I, Bely M, Marullo P et al (2010) Reassessment of phenotypic traits for Saccharomyces bayanus var. uvarum wine yeast strains. Int J Food Microbiol 139:79–86
Masneuf-Pomarède I, Mansour C, Murat ML et al (2006) Influence of fermentation temperature on volatile thiols concentrations in Sauvignon blanc wines. Int J Food Microbiol 108:385–390
Massoutier C, Alexandre H, Feulliat M, Charpentier C (1998) Isolation and characterization of cryotolerant Saccharomyces strains. Vitis 37:55–59
Mateo JJ, Di Stefano R (1997) Description of the β-glucosidase activity of wine yeasts. Food Microbiol 14:583–591
Mateo JJ, Jiménez M, Huerta T et al (1992) Comparison of volatiles produced by four Saccharomyces cerevisiae strains isolated from Monastrell musts. Am J Enol Vitic 43:206–209
Mateo-Vivaracho L, Zapata J, Cacho J et al (2010) Analysis, occurrence and potential sensory significance of five polyfunctional mercaptans in white wines. J Agric Food Chem 58:10184–10194
McCloskey LP, Mahaney P (1981) An enzymatic assay for acetaldehyde in grape juice and wine. Am J Enol Vitic 32:159–162
McMahon H, Zoecklein BW, Fugelsang K et al (1999) Quantification of glycosidase activities in selected yeasts and lactic acid bacteria. J Ind Microbiol Biotechnol 23:198–203
Meilgaard MC (1975) Aroma volatiles in beer: Purification, flavour, threshold and interaction. In: Drawert F (ed) Geruch und Geschmackstoffe. Verlag Hans Carl, Nürnberg, pp 211–254
Mendes-Ferreira A, Mendes-Faia A, Leao C (2002) Survey of hydrogen sulphide production by wine yeasts. J Food Prot 65:1033–1037
Michel A, Bizeau C, Drilleau JF (1988) Flore levurienne présente dans les cidreries de l’ouest de la France. Sciences des Aliments 8(3):359–368
Miyake T, Shibamoto T (1993) Quantitative analysis of acetaldehyde in foods and beverages. J Agric Food Chem 41:1968–1970
Mizuno A, Amano H, Mukai N et al (2003) High gravity brewing utilizing an alpha-glucosidase. J Brew Soc Japan 98:376–385
Mizuno A, Tabei H, Iwahuti M (2006) Characterization of low-acetic-acid-producing yeast isolated from 2-deoxyglucose-resistant mutants and its application to high-gravity brewing. J Biosci Bioeng 101:31–37
Moreira N, Mendes F, Guedes de Pinho P et al (2008) Heavy sulphur compounds, higher alcohols and esters production profile of Hanseniaspora uvarum and Hanseniaspora guilliermondii grown as pure and mixed cultures in grape must. Int J Food Microbiol 124:231–238
Morrissey WF, Davenport B, Querol A et al (2004) The role of indigenous yeasts in traditional Irish cider fermentations. J Appl Microbiol 97(3):647–655
Murat ML, Masneuf I, Darriet P et al (2001a) Effect of Saccharomyces cerevisiae yeast strains on the liberation of volatile thiols in Sauvignon blanc wine. Am J Enol Vitic 52:136–139
Murat ML, Tominaga T, Dubourdieu D (2001b) Assessing the aromatic potential of Cabernet Sauvignon and Merlot musts used to produce rose wine by assaying the cysteinylated precurser of 3-mercaptohexan-1-ol. J Agric Food Chem 49:5412–5417
Nakao Y, Kanamori T, Itoh T et al (2009) Genome sequence of the lager brewing yeast, an interspecies hybrid. DNA Res 16(2):115–129
Naumov GI, Masneuf I, Naumova ES et al (2000) Association of Saccharomyces bayanus var. uvarum with some French wines: genetic analysis of yeast populations. Res Microbiol 151:683–691
Naumov GI, Naumova ES, Aigle M et al (2001) Genetic reidentification of the pectinolytic yeast strain SCPP as Saccharomyces bayanus var. uvarum. Appl Microbiol Biotechnol 55:108–111
Navarro-Aviño JP, Prasad R, Miralles VJ et al (1999) A proposal for nomenclature of aldehyde dehydrogenases in Saccharomyces cerevisiae and characterization of the stress-inducible ALD2 and ALD3 genes. Yeast 15:829–842
Nguyen HV, Gaillardin C (2005) Evolutionary relationships between the former species Saccharomyces uvarum and the hybrids Saccharomyces bayanus and Saccharomyces pastorianus; reinstatement of Saccharomyces uvarum (Beijerinck) as a distinct species. FEMS Yeast Res 5:471–483
Nguyen HV, Legras JL, Neuvéglise C et al (2011) Deciphering the hybridisation history leading to the Lager lineage based on the mosaic genomes of Saccharomyces bayanus strains NBRC1948 and CBS380. PLoS ONE 6(10):e25821
Nguyen HV, Lepingle A, Gaillardin C (2000) Molecular typing demonstrates homogeneity of Saccharomyces uvarum strains and reveals the existence of hybrids between S. uvarum and S. cerevisiae including the S. bayanus type strain CBS 380. Syst Appl Microbiol 23:71–85
Novo MT, Beltrán G, Torija MJ et al (2003) Fermentaciones a bajas temperaturas: análisis químico y sensorial. Tecnol Vino 9:51–55
Nykänen L, Nykänen I (1977) Production of esters by different yeast strains in sugar fermentations. J Inst Brew 83:30–31
Omura F, Shibanoy Y, Fukui N et al (1995) Reduction of hydrogen sulfide production in brewing yeast by constitutive expression of MET25 gene. J Am Soc Brew Chem 53:58–62
Pengelly RJ, Wheals AE (2012) Rapid identification of Saccharomyces eubayanus and its hybrids. FEMS Yeast Res 13(2):156–161
Perestrelo R, Fernandes A, Albuquerque FF et al (2006) Analytical characterization of the aroma of Tinta Negra Mole red wine: Identification of the main odorants compounds. Anal Chim Acta 563:154–164
Petersen JGL, Kielland-Brandt MC, Holmberg S et al (1983) Mutational analysis of isoleucine-valine biosynthesis in Saccharomyces cerevisiae. Mapping of ILV2 and ILV5. Carlsberg Res Comm 48:21–34
Piddocke MP, Kreisz S, Heldt-Hansen HP et al (2009) Physiological characterization of brewer’s yeast in high-gravity beer fermentations with glucose or maltose syrups as adjuncts. Appl Microbiol Biotechnol 84:453–464
Piendl A, Geiger E (1980) Technological factors in the formation of esters during fermentation. The Brewer’s Digest 55:26–35
Piotrowski JS, Nagarajan S, Kroll E et al (2012) Different selective pressures lead to different genomic outcomes as newly-formed hybrid yeasts evolve. BMC Evol Biol 12(1):46
Prescott J (1999a) Flavour as a psychological construct: implications for perceiving and measuring the sensory qualities of foods. Food Qual Pref 10(4/5):349–356
Prescott J (1999b) Introduction to the trigeminal sense: The role of pungency in food flavours. In: Bell GA, Watson AJ (eds) Tastes and aromas. The chemical senses in science and industry. UNSW Press, Sydney, pp 38–49
Pretorius IS (2000) Tailoring wine yeast for the new millennium: Novel approaches to the ancient art of winemaking. Yeast 16:675–729
Pretorius IS (2003) The genetic analysis and tailoring of wine yeasts. In: Hohmann S (ed) Topics in current genetics. Springer, Heidelberg, pp 99–142
Pretorius IS (2004) The genetic improvement of wine yeasts. In: Arora DK, Bridge PD, Bhatnagar D (eds) Handbook of fungal biotechnology. Marcel Dekker, New York, pp 209–232
Pretorius IS, Bauer FF (2002) Meeting the consumer challenge through genetically customized wine-yeast strains. Trends Biotechnol 20:426–432
Pretorius IS, Høj PB (2005) Grape and wine biotechnology: challenges, opportunities and potential benefits. Austr J Grape Wine Res 11:83–108
Pretorius IS, Van der Westhuizen TJ, Augustyn OPH (1999) Yeast biodiversity in vineyards and wineries and its importance to the South African wine industry. S Afr J Enol Vitic 20:61–74
Pronk JT, Steensma HY, van Dijken JP (1996) Pyruvate metabolism in Saccharomyces cerevisiae. Yeast 12:1607–1633
Quain DE, Duffield ML (1985) A metabolic function for higher alcohol production by yeast. In: Proceedings of the 20th congress of the European Brewery Convention, Helsinki, 2–7 June 1985
Querol A, Barrio E, Ramón D (1994) Population dynamics of natural Saccharomyces strains during wine fermentation. Int J Food Microbiol 21:315–323
Radler F (1993) Yeast: Metabolism of organic acids. In: Fleet GH (ed) Wine microbiology and biotechnology. Harwood Academic Publishers, Chur, pp 165–182
Rainieri S, Kodama Y, Kaneko Y et al (2006) Pure and mixed genetic lines of Saccharomyces bayanus and Saccharomyces pastorianus and their contribution to the lager brewing strain genome. Appl Environ Microbiol 72:3968–3974
Ramey DD, Ough CS (1980) Volatile ester hydrolysis or formation during storage of model solutions and wines. J Agric Food Chem 28:928–934
Rapp A, Versini G (1991) Influence of nitrogen compounds in grapes on aroma compounds of wine. In: Rantz J (ed) Proceedings of the international symposium on nitrogen in grapes and wines. Davis American Society For Enology and Viticulture, California
Rauhut D (1993) Yeasts: Production of sulfur compounds. In: Fleet GH (ed) Wine microbiology and biotechnology. Harwood Academic Publishers, Chur, pp 183–223
Redzepovic S, Orlic S, Majdak A et al (2002) Identification and characterisation of Saccharomyces cerevisiae and Saccharomyces paradoxus strains isolated from Croatian vineyards. Lett Appl Microbiol 35:305–310
Remize F, Andrieu E, Dequin S (2000) Engineering of the pyruvate dehydrogenase bypass in Saccharomyces cerevisiae: Role of the cytosolic Mg2+ and mitochondrial K+ acetaldehyde dehydrogenases Ald6p and Ald4p in acetate formation during alcoholic fermentation. Appl Environ Microbiol 66:3151–3159
Ribéreau-Gayon P, Dubourdieu D, Doneche B et al (2000) Handbook of enology volume 1: the microbiology of wine and vinification. John Wiley & Sons Ltd., Chichester
Rojas V, Gil JV, Piñaga F et al (2001) Studies on acetate ester production by non-Saccharomyces wine yeasts. Int J Food Microbiol 70:283–289
Romano P, Suzzi G, Turbanti L et al (1994) Acetaldehyde production in Saccharomyces cerevisiae wine yeasts. FEMS Microbiol Lett 118:213–218
Rossouw D, Næs T, Bauer FF (2008) Linking gene regulation and the exo-metabolome: a comparative transcriptomics approach to identify genes that impact on the production of volatile aroma compounds in yeast. BMC Genom 9(1):530–547
Rouillon A, Surdin-Kerjan Y, Thomas D (1999) Transport of sulfonium compounds. Characterization of the s-adenosylmethionine and s-methylmethionine permeases from the yeast Saccharomyces cerevisiae. J Biol Chem 274(40):28096–28105
Ryder DS, Masschelein CA (1983) Aspects of metabolic regulatory systems and physiological limitations with a view to the improvement of brewing yeast performance. In: Proceedings of the 19th European Brewery Convention symposium, London
Saenz-Navajas MP, Campo E, Fernandez-Zurbano P et al (2010) An assessment of the effects of wine volatiles on the perception of taste and astringency in wine. Food Chem 121(4):1139–1149
Saerens SMG, Delvaux FR, Verstrepen KJ et al (2010) Production and biological function of volatile esters in Saccharomyces cerevisiae. Microbial Biotechnol 3:165–177
Saerens SMG, Delvaux F, Verstrepen KJ et al (2008a) Parameters affecting ethyl ester production by Saccharomyces cerevisiae during fermentation. Appl Environ Microbiol 74:454–461
Saerens SMG, Verbelen PJ, Vanbeneden N et al (2008b) Monitoring the influence of high-gravity brewing and fermentation temperature on flavour formation by analysis of gene expression levels in brewing yeast. Appl Microbiol Biotechnol 80:1039–1051
Saerens SMG, Verstrepen KJ, van Laere SDM et al (2006) The Saccharomyces cerevisiae EHT1 and EEB1 genes encode novel enzymes with medium-chain fatty acid ethyl ester synthesis and hydrolysis capacity. J Biol Chem 281:4446–4456
Saint-Prix F, Bonquist L, Dequin S (2004) Functional analysis of the ALD gene family of Saccharomyces cerevisiae during anaerobic growth on glucose: the NADP(+)-dependent Ald6p and Ald5p isoforms play a major role in acetate formation. Microbiol 150:2209–2220
Sakai N, Kobayakawa T, Gotow N et al (2001) Enhancement of sweetness ratings of aspartame by a vanilla odor presented either by orthonasal or retronasal routes. Percept Mot Skills 92(3):1002–1008
Sampaio JP, Gonçalves P (2008) Natural populations of Saccharomyces kudriavzevii in Portugal are associated with oak bark and are sympatric with S. cerevisiae and S. paradoxus. Appl Environ Microbiol 74(7):2144–2152
San Juan F, Escudero A, Cacho J et al (2011) Quality and main aromatic sensory descriptors (mainly fresh and dry fruit character) of Spanish red wines can be predicted from their aroma-active chemical composition. J Agric Food Chem 59. doi:10.1021/jf1048657 (in press)
Sarry JE, Gunata Z (2004) Plant and microbial glycoside hydrolases: volatile release from glycosidic aroma precursors. Food Chem 87:509–521
Schuller D, Alves H, Dequin S (2005) Ecological survey of Saccharomyces cerevisiae strains from vineyards in the Vinho Verde region of Portugal. FEMS Microbiol Ecol 51:167–177
Sipiczki M (2002) Taxonomic and physiological diversity of Saccharomyces bayanus. In: Ciani M (ed) Biodiversity and biotechnology of wine yeasts. Research Signpost, Kerala, pp 53–69
Sipiczki M (2008) Interspecies hybridization and recombination in Saccharomyces wine yeasts. FEMS Yeast Res 8:996–1007
Sipiczki M, Romano P, Lipani G et al (2001) Analysis of yeasts derived from natural fermentation in a Tokaj winery. Antonie Van Leeuwenhoek 79:97–105
Smit A, Cordero Otero RR, Lambrechts MG et al (2003) Enhancing volatile phenol concentrations in wine by expressing various phenolic acid decarboxylase genes in Saccharomyces cerevisiae. J Agric Food Chem 51:4909–4915
Soles RM, Ough CS, Kunkee RE (1982) Ester concentration differences in wine fermented by various species and strains of yeasts. Am J Enol Vitic 33:94–98
Spagna G, Barbagallo RN, Palmeri R et al (2002) Properties of endogenous β-glucosidase of a Pichia anomala strain isolated from Sicilian musts and wines. Enzyme Microb Technol 31(7):1036–1041
Spiropoulos A, Bisson LF (2000) MET17 and hydrogen sulfide formation in Saccharomyces cerevisiae. Appl Environ Microbiol 66:4421–4426
Sponholz WR (1993) Wine spoilage by microorganisms. In: Fleet GH (ed) Wine microbiology and biotechnology. Harwood Academic Publishing, Chur, pp 395–420
Stevenson RJ, Wilson DA (2007) Odour perception: an object-recognition approach. Perception 36(12):1821–1833
Strauss MLA, Jolly NP, Lambretchs MG et al (2001) Screening for the production of extracellular hydrolytic enzymes by non-Saccharomyces wine yeasts. J Appl Microbiol 91:182–190
Strauss MLA, Wilson B, Gooley PR et al (1986) Role of monoterpenes in grape wine flavor. ACS Symp Ser 317:222–242
Styger G, Jacobson D, Bernard P et al (2011) Identifying genes that impact on aroma profiles produced by Saccharomyces cerevisiae and the production of higher alcohols. Appl Microbiol Biotechnol 91:713–730
Styger G, Jacobson D, Bernard P et al (2013) Genetic analysis of the metabolic pathways responsible for aroma metabolite production by Saccharomyces cerevisiae. Appl Microbiol Biotechnol 97:4429–4442
Suárez B, Pando R, Fernández N et al (2007a) Yeast species associated with the spontaneous fermentation of cider. Food Microbiol 24:25–31
Suárez B, Pando R, González A et al (2007b) A molecular genetic study of natural strains of Saccharomyces isolated from Asturian cider fermentations. J Appl Microbiol 103:778–786
Suomalainen H, Lehtonen M (1979) The production of aroma compounds by yeast. J Inst Brew 85:149–156
Sutherland CM, Henschke PA, Langridge P et al (2003) Subunit and cofactor binding of Saccharomyces cerevisiae sulfite reductase-towards developing wine yeast with lowered ability to produce hydrogen sulfide. Austr J Grape Wine Res 9:186–193
Swiegers JH, Bartowsky EJ, Henschke PA et al (2005) Yeast and bacterial modulation of wine aroma and flavour. Austr J Grape Wine Res 11:139–173
Swiegers JH, Bjerre K, Corfitzen LB et al (2011) Winemaking returning back to nature. Austr New Zealand Grapegrower Winemaker 564:46–48
Swiegers JH, Capone DL, Pardon KH et al (2007) Engineering volatile thiol release in Saccharomyces cerevisiae for improved wine aroma. Yeast 24:561–574
Swiegers JH, Francis IL, Herderich MJ et al (2006a) Meeting consumer expectations through management in vineyard and winery: the choice of yeast for fermentation offers great potential to adjust the aroma of Sauvignon Blanc wine. Austr New Zealand Wine Ind J 21:34–42
Swiegers JH, Willmott RL, Hill-Ling A et al (2006b) Modulation of volatile thiol and ester aromas in wine by modified wine yeast. In: Bredie W, Petersen M (eds) Developments in food science vol 43: flavour science recent advances and trends. Elsevier, Amsterdam
Takoi K, Koie K, Itoga Y et al (2010) Biotransformation of hop-derived monoterpene alcohols by lager yeast and their contribution to the flavor of hopped beer. J Agric Food Chem 58:5050–5058
Tamai Y, Tanaka K, Umemoto N et al (2000) Diversity of the HO gene encoding an endonuclease for mating-type conversion in the bottom fermenting yeast Saccharomyces pastorianus. Yeast 16(14):1335–1343
Tanguler H (2012) Evaluation of Williopsis saturnus inoculum level on fermentation and flavor compounds of white wines made from Emir (Vitis vinifera L.) grown in Anatolia. Food Biotechnol 26:351–368
Taylor GT, Thurston PA, Kirsop BH (1979) Influence of lipids derived from malt spent grains on yeast metabolism and fermentation. J Inst Brew 85:219–227
Tezuka H, Mori T, Okumura Y et al (1992) Cloning of a gene suppressing hydrogen sulfide production by Saccharomyces cerevisiae and its expression in a brewing yeast. J Am Soc Brew Chem 50:130–133
Thornton RJ, Bunker A (1989) Characterisation of wine yeasts for genetically modifiable properties. J Inst Brew 95:181–184
Tominaga T, Baltenweck-Guyot R, Peyrot de Gachons C et al (2000) Contribution of volatile thiols to the aromas of white wines made from several Vitis vinifera grape varieties. Am J Enol Vitic 51:178–181
Tominaga T, Darriet P, Dubourdieu D (1996) Identification of 3-mercaptohexyl acetate in Sauvignon wine, a powerful aromatic compound exhibiting box-tree odor. Vitis 35:207–210
Tominaga T, Dubourdieu D (2006) A novel method for quantification of 2-methyl-3-furanthiol and 2-furanmethanethiol in winesmade from Vitis vinifera grape varieties. J Agric Food Chem 54:29–33
Tominaga T, Furrer A, Henry R et al (1998a) Identification of new volatile thiols in the aroma of Vitis vinifera L. var. Sauvignon blanc wines. Flav Fragr J 13:159–162
Tominaga T, Masneuf I, Dubourdieu D (1995) A S-cysteine conjugate, precursor of aroma of white sauvignon. J Int des Sci de la Vigne et du Vin 29:227–232
Tominaga T, Peyrot des Gachons C, Dubourdieu D (1998b) A new type of flavour precursors in Vitis vinifera L. cv. Sauvignon Blanc: S-cysteine conjugates. J Agric Food Chem 46:5215–5219
Torija MJ, Beltrán G, Novo M et al (2003) Effects of fermentation temperature and Saccharomyces species on the cell fatty acid composition and presence of volatile compounds in wine. Int J Food Microbiol 85:127–136
Torija MJ, Rozés N, Poblet M et al (2001) Yeast population dynamics in spontaneous fermentations, comparison between two different wine-producing areas over a period of three years. Antonie Van Leeuwenhoek 79:345–352
Ugliano M, Bartowsky EJ, McCarthy J et al (2006) Hydrolysis and transformation of grape glycosidically bound volatile compounds during fermentation with three Saccharomyces yeast strains. J Agric Food Chem 54(17):6322–6331
Ugliano M, Henschke PA (2009) Yeasts and wine flavour. In: Moreno-Arribas MV, Polo MC (eds) Wine chemistry and biochemistry. Springer, New York, pp 251–274
Ugliano M, Moio L (2008) Free and hydrolytically released volatile compounds of Vitis vinifera L. cv. Fiano grapes as odour-active constituents of Fiano wine. Anal Chim Acta 21(1):79–85
Van Breda V, Jolly N, Van Wyk J (2013) Characterisation of commercial and natural Torulaspora delbrueckii wine yeast strains. Int J Food Microbiol 163:80–88
Van Rensburg P, Pretorius IS (2000) Enzymes in winemaking: harnessing natural catalysts for efficient biotransformations: a review. S Afr J Enol Vitic 21:52–73
Vaughan-Martini A, Kurtzman CP (1985) Deoxyribonucleic acid relatedness among species of Saccharomyces sensu stricto. Int J Syst Bact 35:508–511
Verbelen PJ, Dekoninck TM, Saerens SM et al (2009a) Impact of pitching rate on yeast fermentation performance and beer flavour. Appl Microbiol Biotechnol 82:155–167
Verbelen PJ, Saerens SM, Van Mulders SE et al (2009b) The role of oxygen in yeast metabolism during high cell density brewery fermentations. Appl Microbiol Biotechnol 82:1143–1156
Vermeulen C, Lejeune I, Tran TTH et al (2006) Occurrence of polyfunctional thiols in fresh lager beers. J Agric Food Chem 54:5061–5068
Verstrepen KJ, Derdelinckx G, Dufour JP et al (2003a) The Saccharomyces cerevisiae alcohol acetyl transferase gene ATF1 is a target of the cAMP/PKA and FGM nutrient-signalling pathways. FEMS Yeast Res 4:285–296
Verstrepen KJ, Derdelinckx G, Dufour JP et al (2003b) Flavor-active esters: adding fruitiness to beer. J Biosci Bioeng 96:110–118
Verstrepen KJ, van Laere SDM, Vanderhaegen BMP et al (2003c) Expression levels of the yeast alcohol acetyltransferase genes ATF1, Lg-ATF1, and ATF2 control the formation of a broad range of volatile esters. Appl Environ Microbiol 69:5228–5237
Viana F, Belloch C, Valles S et al (2011) Monitoring a mixed starter of Hanseniaspora vineae–Saccharomyces cerevisiae in natural must: Impact on 2-phenylethyl acetate production. Int J Food Microbiol 151:235–240
Viana F, Gil JV, Genoves S et al (2008) Rational selection of non-Saccharomyces wine yeasts for mixed starters based on ester formation and enological traits. Food Microbiol 25:778–785
Viana F, Gil JV, Valles S et al (2009) Increasing the levels of 2-phenylethyl acetate in wine through the use of a mixed culture of Hanseniaspora osmophila and Saccharomyces cerevisiae. Int J Food Microbiol 135:68–74
Vilanova M, Genisheva Z, Masa A et al (2010) Correlation between volatile composition and sensory properties in Spanish Albariño wines. Microchem J 95:240–246
Villanueva KD, Goossens E, Masschelein CA (1990) Subthreshold vicinal diketone levels in lager brewing yeast fermentations by means of ILV5 gene amplification. J Am Soc Brew Chem 48:111–114
Villanueva A, Ramón D, Valles S et al (2000) Heterologous expression in Aspergillus nidulans of a Trichoderma longibrachiatum endoglucanase of enological relevance. J Agric Food Chem 48:951–957
Wainwright T (1973) Diacetyl: a review. J Inst Brew 79:451–470
Williams AA (1974) Flavour research and the cider industry. J Inst Brew 80:455–470
Wilson DA, Stevenson RJ (2003) The fundamental role of memory in olfactory perception. Trends Neurosc 26(5):243–247
Yamagata S (1989) Roles of O-acetyl-l-homoserine sulfhydrylases in microorganisms. Biochimie 71:1125–1143
Yeshurun Y, Sobel N (2010) An odor is not worth a thousand words: from multidimensional odors to unidimensional odor objects. Annual Rev Psychol 61:219–241
Yoshimoto H, Fujiwara D, Momma T et al (1998) Characterization of the ATF1 and Lg-ATF1 genes encoding alcohol acetyltransferases in the bottom fermenting yeast Saccharomyces pastorianus. J Ferment Bioeng 86:15–20
Zamora F (2009) Biochemistry of alcoholic fermentation. In: Moreno-Arribas MV, Polo MC (eds) Wine chemistry and biochemistry. Springer, New York, pp 3–26
Zea L, Moreno J, Ortega JM et al (1995) Content of free terpenic compounds in cells and musts during vinification with three Saccharomyces cerevisiae races. J Agric Food Chem 43:1110–1114
Zoecklein BW, Marcy JE, Williams JM et al (1997) Effect of native yeasts and selected strains of Saccharomyces cerevisiae on glycosyl glucose, potential volatile terpenes and selected aglycones of white Riesling (Vitis vinifera L.) wines. J Food Compos Anal 10:55–65
Zott K, Thibon C, Bely M et al (2011) The grape must non-Saccharomyces microbial community: impact on volatile thiol release. Int J Food Microbiol 151:210–215
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
Gamero, A., Ferreira, V., Pretorius, I.S., Querol, A. (2014). Wine, Beer and Cider: Unravelling the Aroma Profile. In: Piškur, J., Compagno, C. (eds) Molecular Mechanisms in Yeast Carbon Metabolism. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-642-55013-3_10
Download citation
DOI: https://doi.org/10.1007/978-3-642-55013-3_10
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-642-55012-6
Online ISBN: 978-3-642-55013-3
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)