Abstract
This review is a first attempt to systematize data on the potential of transgenic Y. lipolytica to catalyze diverse reactions of steroid transformation. The yeast Y. lipolytica was tested as host for P450-catalyzed biotransformation of steroids, including the mammalian P450scc system (three components) and/or the two-component P450c17 system, being functionally active with yeast NADPH-P450 reductase (CPR). New strategies for the construction of recombinant Y. lipolytica strains containing several expression cassettes containing heterologous cDNA (up to 4, in five vectors) under control of the isocitrate lyase (ICL1) promoter have been developed. Characteristics of recombinant Y. lipolytica strains functionally expressing the P450scc system and/or P450c17, being functionally active with yeast NADPH-P450 reductase (YlCPR), are presented.
Functional expression of P450 systems in yeasts was proved by biotransformation of cholesterol (Cho) or by 17α-hydroxylation of progesterone (Pro) or pregnenolone (Pre). Strains coexpressing the P450scc system and P450c17 exhibited a high biotransformation capacity of Pro into 17α-hydroxyprogesterone (17HPro); the conversion of Cho to Pre and 17α-hydroxypregnenolone occurred rather slowly. For selected P450c17 expressing Y. lipolytica strains, the cultivation conditions (induction, bioconversion) were optimized for high product yield (up to 95 % 17HPro) and reduction of the diol side-product formation from 19–22 % to 1–2 % without gene destruction.
The results obtained could be used for elaboration of new biotechnological approaches with using recombinant yeast strains for synthesis of pharmaceutically active steroids and for screening of compounds which inhibit the P450c17 enzyme activity, playing important roles in the development of hormonal carcinogenesis.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Introduction
Steroid substances, which include steroid alkaloids, glycosides and saponins of plants, steroid hormones, vitamin D, bile acids of animals, as well as various insecticides, fungicides, and plant growth regulators, find increasing use in medicine and agriculture. Steroid compounds are conventionally obtained by extraction from plants and animal tissues, complete chemical synthesis, and combined chemical and enzymatic synthesis. The above approaches have made available a broad spectrum of steroid drugs and hormones. Several microbial bioconversions of steroids and sterols have been reported ever since focusing mainly on steroid hydroxylations, Δ 1-dehydrogenation, and sterol side-chain cleavage (Sedlaczek 1988; Ahmad et al. 1992; Fernandes et al. 2003). These biotransformations, mostly associated with chemical synthesis steps, have provided adequate tools for the large-scale production of natural or modified steroid analogues. Fungal biotransformation of steroids is among the earliest examples of biocatalysis for producing stereo- and site-specific products, including commercially important cytochrome P450-mediated steroid hydroxylations. Such biotransformations of steroids are of applied interest due to the economic importance of stereo- and regiospecific reactions to produce steroidal products and vitamin D (Sedlaczek 1988; Pajic et al. 1999; Fernandes et al. 2003).
Cytochrome P450 (P450 or CYP) enzymes constitute a large, ubiquitous family of heme-thiolate monooxygenases (CYP gene superfamily) that are involved in the oxidative metabolism of a wide variety of endo- and xenobiotic chemicals (Ortiz de Montellano 2005; Bernhardt 2006). Most P450 systems are composed of a P450 monooxygenase and one (class II) or two (class I) additional proteins, constituting an electron transfer chain. These P450 system components are either expressed as individual genes or linked resulting in a single peptide as self-sufficient P450s (class III).
Eleven proteins are directly involved in the steroidogenic pathway from cholesterol to steroid hormones in mammal, among which are six P450s, 17β-hydroxysteroid dehydrogenases (17β-HSD), 3β-hydroxysteroid dehydrogenases/Δ5,4 isomerase (3β-HSD), and three electron transfer proteins (Bernhardt 2006). The P450s are membrane-bound proteins associated with either the mitochondrial inner membranes (P450scc, P450c11, P450c18—type I P450 enzymes, which receive reducing equivalents via electron transfer chains consisting of adrenodoxin, a [2Fe-2S] ferredoxin, Adx, and NADPH-adrenodoxin reductase, a FAD-flavoprotein, AdR) or the endoplasmic reticulum membranes (P450c17, P450c21, P450c19—type II P450 enzymes, receiving reducing equivalents from a single FAD/FMN-flavoprotein, NADPH-cytochrome P450 reductase (CPR)).
By heterologous expression of selected P450s, it is possible to combine the strict regio- and stereoselectivity of steroidogenic enzymes and biotechnological advantages of microorganisms. The use of recombinant microorganisms, synthesising a few enzymes involved in steroidogenesis, can afford an opportunity to realise several consecutive reactions to a single stage that will simplify so essentially the technology of steroid drug synthesis. There are two limitations for widespread using of biotechnological approaches for synthesis of steroids. The first is the fact that natural microorganisms, which are used for synthesis of target steroids, usually have their own enzymatic systems producing unwanted by-products. This basic disadvantage can be overcome taking advantage of heterologous expression of substrate-specific steroidogenic enzymes (including mammalian P450 systems) in selected microbial hosts. The second restriction consists in low solubility of steroids in aqueous environments. Different means are for overcoming of this limitation (liquid two-phase systems, organic additives), which may complicate technological processes. However, one perspective for improvement of mass-transfer parameters of hydrophobic steroids into the cells is to use alkane-utilizing yeasts, like Yarrowia (Y.) lipolytica, which is capable to utilise very efficiently hydrophobic substrates, like lipids, fatty acids or alkanes. This yeast could be used for bioconversion of such hydrophobic compounds into valuable products, like dicarboxylic acid (DCA), for flavour or aroma (lactones), for bioremediation purposes, as well as for degradation of triglycerides into organic acids (citric, isocitric, or 2-ketoglutaric acids; Barth and Gaillardin 1996; Fickers et al. 2005; Bankar et al. 2009; Beopoulos et al. 2009, 2011; Thevenieau et al. 2009, 2010; Coelho et al. 2010). Therefore, there probably exist fundamental advantages of this yeast for synthesis of steroids in comparison with Escherichia (E. coli) and the yeast Saccharomyces (S. сerevisiae).
Alkane-utilising yeasts, like Y. lipolytica or Candida spp., exhibit a high catalytic activity of their alkane- or fatty acid-inducible P450 systems (ALK genes of CYP52 family, alkane or fatty acid ω-hydroxylase activities with turnover numbers of 1–2 μmol/nmol P450 × min (calculated in vivo P450 activity in alkane-utilising cells), catalysing terminal hydroxylations of n-alkanes or fatty acids). This high P450 activity is supported by an efficient subcellular organisation of the substrate and product transport processes (inducible uptake and excretion systems for hydrophobic substrates, ER–peroxisomes interaction for substrate and product transport processes, like fatty acids and DCA), a proliferation of the ER during growth on alkanes, and the efficiency of electron transfer systems (alkane- or fatty-acid-inducible higher content of microsomal electron transfer components, NAD(P)H-dependent P450 reductases, and cytochrome b5 then in S. cerevisiae) to host-own P450s (Mauersberger et al. 1987, 1996; Schunck et al. 1987a, b; Fickers et al. 2005; cf. Mauersberger 2013), which might also support the function of heterologous P450 functionally expressed in these yeasts. Therefore, the putative advantages of a hydrocarbon-assimilating yeast cell as a host for heterologous P450, catalysing biotransformation reactions with hydrophobic substrates, were tested by functional expression of selected P450s in Y. lipolytica (e.g. P450c17, P4501A1, P450scc, P4502D6, and P4503A4; cf. Sect. 2.1, Table 1). Indeed, comparison of functional P450c17 expression in Y. lipolytica and in the commonly used yeast S. cerevisiae revealed significant advantages of alkane-assimilating yeast cells for P450-catalyzed biotransformations of hydrophobic substrates (Juretzek et al. 2000b). In this contribution, the present knowledge on heterologous expression of different P450 forms in the yeast Y. lipolytica will be given, ranging from the first functional expression of bovine P450c17 in middle of 1990 to recent data on evaluation and application of this promising yeast host for P450-catalyzed biotransformation of mainly hydrophobic substrates, including steroids and sterols by different P450 forms.
2 Heterologous Expression of Cytochromes P450 in Yeasts
To make use of the high regio- and stereoselectivity of hydroxylations by P450 systems for biotransformation of hydrophobic substrates, the P450 enzymes need to be functionally expressed in an appropriate host cells. Several recombinant P450 expression systems have been investigated in the past 25 years, including mammalian and Baculovirus-infected insect cell systems and bacterial (mainly Escherichia coli), fungal (mainly Saccharomyces cerevisiae), and plant expression systems (cf. Table 1 for earlier review ref; Dumas et al. 2006; Novikova et al. 2009; Cornelissen et al. 2012). Preferred hosts are E. coli and S. cerevisiae. Other suitable host microorganisms are found also among bacteria (Bacillus, Pseudomonas, Streptomyces species), yeasts (Pichia pastoris, Schizosaccharomyces pombe, Kluyveromyces lactis, Candida spp., Y. lipolytica), and filamentous fungi (Fusarium verticillioides; cf. Sect. 2.1, Table 1).
2.1 Overview on Heterologous Cytochrome P450 Expression in Yeasts and Filamentous Fungi
A short overview on established P450 expression systems in yeasts and filamentous fungi is presented in Table 1. A brief description of P450 expression data in Y. lipolytica will be given at the end of this section. The first functional expression of mammalian P450s was demonstrated in 1980s in baker’s yeast S. cerevisiae (Oeda et al. 1985; Sakaki et al. 1989, 1990, 1991; cf. Table 1). The mutant yeasts combine the ease of handling of single-cell microbial systems with the specific features of eukaryotic cells. Since then numerous mammalian and non-mammalian CYP genes have been expressed in the commonly used yeast S. cerevisiae and more recently also in the non-conventional yeasts P. pastoris, S. pombe, K. lactis, and Y. lipolytica (cf. Table 1 for ref.). In particular, numerous studies have been published on heterologous expression of individual or coexpression of several mammalian steroidogenic P450s, i.e. microsomal P450c17, P450c19, P450c21, mitochondrial P450scc and P450c11 (P450 expressed in their natural or artificial fused forms with respective electron transfer components, including also P450 coexpression with 3β-HSD, Adx, CPR, or cytochrome b5), using besides E. coli mainly the yeast S. cerevisiae (for ref. of reviews cf. Table 1). The first successful expression of microsomal steroidogenic P450 enzymes was based on the fact that yeast CPR can support the activities not only of host-own but also of expressed heterologous P450s, in particular P450c17 and P450c21 (Sakaki et al. 1989, 1991). These recombinant yeast cells expressing heterologous P450s were used for the characterisation of individual CYP forms and the interaction with coexpressed redox partners and were applied for P450-catalyzed biotransformations of predominantly hydrophobic substrates (including steroids) with whole cells or cell fractions. These whole cells (“yeast cell factories”) can be easily used for biotransformations, either in single or in multistep reactions, to deal with inherent stability problems of P450 enzymes and regeneration of NADPH.
After first successful reports in 1990s, application of S. pombe and P. pastoris for heterologous P450 expression developed rapidly in the last decade. In particular, S. pombe was successfully used in heterologous P450 expression studies and applied for P450-catalyzed biotransformations of steroids and other substrates. Otherwise, K. lactis was tested for P450scc, P450c17, and P450c21 expression in first studies only. Heterologous P450 expression in the well-developed host–vector systems for Hansenula polymorpha (Pichia angusta) and Arxula adeninivorans was not yet reported. In addition to the widely used yeast host–vector systems, first reports on heterologous expression of P450 in filamentous fungi appeared. The P450 monooxygenase-encoding TRI1 and TRI4 genes of Fusarium sporotrichioides (FsTRI1), Fusarium graminearum (FgTRI1, FgTRI4), were heterologously expressed in the trichothecene-nonproducing species Fusarium verticillioides under promoter control of the fumonisin biosynthetic gene FUM8 to study their function in the trichothecene mycotoxins (McCormick et al. 2006). The alkane-utilising Candida yeasts (C. maltosa, C. tropicalis) are not very useful for heterologous P450 expression due to a deviation of the universal genetic code observed in these yeasts (Zimmer and Schunck 1995). However, for the yeasts C. maltosa, C. tropicalis, and Y. lipolytica, a gene-dose-dependent overexpression of host-own P450 and NADPH-P450 reductases was performed, in particular to increase the alkane- or fatty acid-hydroxylating P450 activities (CYP52 family) involved in the formation of dicarboxylic acids (DCA) derived from alkanes or fatty acids (for ref. cf. Table 1).
Expression of Heterologous P450 in Yarrowia lipolytica
In contrast to the Candida yeasts, functional heterologous P450 expression was successfully demonstrated for the hydrocarbon-assimilating yeast Y. lipolytica. In this section, a brief description of all P450 expression studies in Y. lipolytica will be given. Details on the expression of P450c17 and the P450scc system will be presented in Sects. 3 and 4. At least eight heterologous P450 (CYP1A1, CYP2D6, CYP3A4, CYP11A1, CYP17A, CYP52A3, CYP53B1, CYP74) and three heterologous electron transfer components (all human NADPH-P450 reductase, hCPR; adrenodoxin, hADX; NADPH-adrenodoxin reductase, hADR; additionally, homologous YlCPR overexpression) were expressed in the yeast Y. lipolytica. In total 12 P450 and related electron transfer proteins were expressed under control of different promoters. Among them are five mammalian P450 proteins (CYP1A1, CYP2D6, CYP3A4, CYP11A1, human and bovine CYP17), one plant P450 (CYP74), two yeast proteins (CYP52A3 of C. maltosa, CYP53B1 of Rhodotorula minuta), and three human electron transfer proteins (hCPR, hADX, hADR), and additionally, the host-own P450 reductase (YlCPR) was overexpressed in several cases (cf. Table 1). The heterologous P450s expressed in Y. lipolytica summarized in Table 1 are the following:
-
1.
The CYP52A3 ( ALK1 ) gene encoding the alkane hydroxylating P450Cm1 of C. maltosa (Schunck et al. 1991) was expressed under pICL1 control in Y. lipolytica using a low-copy ARS-CEN replicative vector, comparable to pIC17α shown in Fig. 1b (Prinz 1995; Mauersberger et al. 1995; unpublished meeting reports; Juretzek et al. 1997). The P450Cm1 protein was detected in Western blots; determination of the P450Cm1 activity was hindered due to the interference with host-own P450 ALK.
-
2.
The functional heterologous expression of bovine steroidogenic P450c17 (CYP17A cDNA) in the yeast Y. lipolytica, which is naturally well adapted to the utilization of hydrophobic substrates, was first studied to test the assumed advantages of an alkane-utilising yeast as host for heterologous P450, catalyzing biotransformation reactions with hydrophobic substrates (cf. Sects. 1 and 3.2, Table 1). For this purpose, the non-conventional oleaginous yeast Y. lipolytica was selected, which is non-pathogenic and phylogenetically very distant from the commonly studied S. cerevisiae, and for which the main genetic engineering tools were available. Bovine P450c17 was the first mammalian P450 expressed in Y. lipolytica (Table 1). In particular, functional expression of the ER-resident and steroid transforming P450c17 under control of the strong and regulated isocitrate lyase promoter pICL1 in Y. lipolytica was established and used for steroid biotransformation with recombinant yeast cells (Juretzek and Mauersberger et al. 1995, unpublished meeting reports; Shkumatov et al. 1998, 2003, 2006; Juretzek 1999; Juretzek et al. 1999, 2000b; Novikova et al. 2009; Table 1). These first studies of P450c17 expression in Y. lipolytica indicated several advantages (hydrophobic substrate uptake and transport, cellular properties supporting P450-catalyzed reactions) of the alkane-assimilating yeast cells for P450-catalyzed biotransformations of hydrophobic steroid substrates in comparison with S. cerevisiae (cf. Sects. 3 and 4 for further details).
-
3.
Subsequently, functional expression of first human (second mammalian) P4501A1 (CYP1A1, involved in drug oxidation and catalysing 7-ethoxyresorufin O-deethylase activity, EROD) in Y. lipolytica under control of pPOX2 promoter, with or without overproduction of host-own NADPH-P450 reductase (YlCPR) expressed under pICL1 or pPOX2 promoters’ control was demonstrated (Nthangeni et al. 2004). Significantly (up to 50-fold) increased P4501A1 activity in whole-cell biotransformation of 7-ethoxyresorufin to resorufin (7-hydroxy-3H-phenoxiazin-3-one) due to CYP1A1 copy number increase and YlCPR coexpression under these promoters was observed.
-
4.
The first heterologous expression of a plant P450 in Y. lipolytica was shown using the green bell pepper HPO lyase gene (CYP74B ) under the control of the pPOX2 using non-homologous LTR zeta-based integration into the genome (Bourel et al. 2004; Santiago-Gómez et al. 2007). The expression of this unusual P450 (170 kDa) with HPO lyase activity (fatty acid hydroperoxide lyase, HPL) in Y. lipolytica resulted in the production of high yields of volatile lipid-derived C6-aldehydes (hexanal and trans-2-hexenal, components of green notes aroma), much higher than using the plant system. The derived volatile products are used industrially to reconstitute the “fresh green odour” of fruits and vegetables lost during processing (Fickers et al. 2005; Santiago-Gómez et al. 2007). Thus, it was demonstrated that Y. lipolytica could be a useful host for the expression of HPO lyase and a simple process that could yield high quantities of C6-aldehydes was established with the recombinant yeast.
-
5.
CYP53B1 (P45053B1 ) from Rhodotorula minuta encoding a benzoate para-hydroxylase, completely absent in the host, was functionally expressed in Y. lipolytica strain E150 after multicopy integration of expression cassettes under control of pPOX2, with and without pICL1-controlled coexpression of the YlCPR (Shiningavamwe et al. 2006). Whole-cell biotransformation of benzoic acid to para-hydroxybenzoic acid (pHBA) was used to analyse the hydroxylase activity of the recombinant Y. lipolytica cells, which was one of the highest hydroxylation activities thus reported for whole-cell biotransformation studies carried out with yeasts expressing foreign CYP450s.
-
6.
The functional expression of the cholesterol side-chain cleavage P450scc system, a three-component, class I P450 system, consisting of CYP11A1 (human or bovine), and the electron transfer proteins NADPH-adrenodoxin reductase (human AdR) and adrenodoxin (human Adx) with coexpression of P450c17 (CYP17A) in Y. lipolytica was established in frame of an INTAS project in our three laboratories (cf. Table 1 for ref.; Yovkova 2006; Novikova et al. 2008, 2009; details will be presented in Sect. 4).
-
7.
More recently coexpression of human CYP2D6 or CYP3A4 genes together with human P450 reductase (hCPR) or the host-own P450 reductase (YlCPR) in Y. lipolytica H222-S4 was shown (Braun et al. 2012; cf. Table 1). With these recombinant whole-cell biocatalysts, the potential of the hydrocarbon-assimilating yeast Y. lipolytica for the bioconversion of poorly soluble hydrophobic steroids (testosterone, 17α-testosterone, progesterone) was tested. Additionally, two-liquid biphasic culture systems (aqueous and organic solvent phases) were evaluated to increase the substrate availability. Best bioconversion results were observed in a bioreactor employing a biphasic system with the organic solvent and Y. lipolytica carbon source ethyl oleate (compared to bis-ethylhexyl phthalate, BEHP, or dibutyl phthalate, DBP) for the whole-cell bioconversion of progesterone. Multicopy transformants showed a 50–70-fold increase of P450 activity as compared to single-copy strains, and coexpression of human CPR gene resulted in a 4–10-fold higher specific P450 activity compared to co-overexpression of the YlCPR gene. These results demonstrated the high potential of P450 expressing Y. lipolytica cells for biotransformations of hydrophobic steroid substrates in two-liquid biphasic systems. Especially organic solvent phases which can be efficiently taken up and metabolised by the cell enable more efficient bioconversion as compared to aqueous systems and even enable high-yield long-time processes.
Replicative and integrative vectors for the heterologous expression of bovine adrenal cytochrome P450c17 (CYP17 cDNA) in the yeasts Saccharomyces cerevisiae (a) and Yarrowia lipolytica (b, d) and for the overexpression of the yeast NADPH-cytochrome P450 reductase (YlCPR) in Yarrowia lipolytica (c). (a) YEp5117α: high-copy, 2μ-based replicative expression vector for P450c17 in S. cerevisiae; GAL10, strong galactose-inducible promoter; Amp R, ampicillin resistance genes for selection in E. coli; CYP17, cDNA for bovine P450c17; ScLEU2, yeast selection marker gene. (b) pIC17α: low-copy (1–2) ARS18/CEN expression vector; pICL1D, full-length, strong and regulated ICL1 promoter D (induced by alkanes, fatty acids, ethanol or acetate, almost 90–95 % repressed by glucose); ICL1i, intron in the ICL1 gene; ICL1t, ICL1 terminator; YlLEU2, selection marker in Y. lipolytica. (c) p67RYl: multicopy (at least 8–10) LTR zeta-based integrative vector for pICL1-controlled high-level expression of the host-own ER-resident NADPH-P450 reductase (YlCPR gene) in Y. lipolytica; ura3d4, defective, promoter-truncated URA3 gene as multicopy selection marker in Y. lipolytica; zeta, long-terminal repeat LTR zeta of the Y. lipolytica retrotransposon Ylt1 as vector integration targeting sequence after its linearisation by NotI prior transformation. (d) p67IC17: comparable multicopy LTR zeta-based integrative vector for high-level expression of the bovine P450c17 in Y. lipolytica under pICL1 control these and comparable expression vectors and their use for gene-dose dependent high-level heterologous protein expression in S. cerevisiae and Y. lipolytica were described in Juretzek (1999), Juretzek et al. (2000a, b, 2001), and Shkumatov et al. (1998, 2002)
Thus, the stable high-level and functional expression of heterologous P450s together with its NADPH-P450 reductase opens new perspectives for further improvement of the efficiency of biotransformation reactions with recombinant Y. lipolytica cells, a system which seems to be useful especially for bioconversion of hydrophobic substrates.
2.2 Reconstruction of Mammalian Steroid Synthesis in Saccharomyces cerevisiae
In a long-term project (cf. reviews Dumas et al. 2006; Brocard-Masson and Dumas 2006 and ref. therein) first the two initial stages of mammalian steroidogenesis were reconstituted in S. cerevisiae, which were realised by the bovine Р450scc system and human 3β-hydroxysteroid dehydrogenases/isomerase (3β-HSD) coexpressed with a plant sterol Δ7-reductase, leading to self-sufficient biosynthesis of pregnenolone and progesterone during growth of the engineered yeast cells on glucose or ethanol (Duport et al. 1998). This result was achieved using two principally new approaches: First, the major problem proved to be expression of the Р450scc system in yeast mitochondria, due to differences of P450scc topogenesis within baker’s yeast from its topogenesis within mammalian mitochondria (Minenko et al. 2008; for ref. cf. Novikova et al. 2009). Duport et al. (1998) demonstrated that the Р450scc system can be functionally expressed in yeast with non-mitochondrial location using cDNAs encoding mature (m) protein forms without mitochondrial targeting sequences. Despite different localization of these mature form-proteins (mP450scc is mostly plasma membrane and partially ER associated, whereas mAdR and mAdx are ER localized and cytosolic, respectively), this P450scc system was shown to be catalytically active, which indicated on non-obligatory necessity of mitochondrial surrounding for Р450scc (Duport et al. 2003). Second, it is known that сonversion of cholesterol into pregnenolone by recombinant yeast is difficult because cholesterol is not efficiently taken up by aerobically grown baker’s yeast (Ness et al. 1998). To prepare steroid producing transgenic S. cerevisiae strains, Duport et al. (1998) had to reroute the yeast metabolism (disrupting yeast P450 gene CYP61A or ERG5, encoding sterol Δ22-desaturase, P45022DS, and expressing the plant gene encoding sterol Δ7-reductase from Arabidopsis thaliana) in such a way that the yeast produced during growth campesterol (ergosta-5-еnol), a very close analogue of cholesterol, instead of the natural yeast sterol, i.e. ergosterol. It was shown that campesterol, which can support the vital functions of yeasts replacing ergosterol in membranes and simultaneously acts as substrate for mammalian Р450scc, is transformed in vivo into pregnenolone and progesterone, thus realising the first self-sufficient steroid biosynthesis by engineered S. cerevisiae (Duport et al. 1998). Finally, Szczebara et al. (2003) reported the construction of transgenic S. cerevisiae strains that produce hydrocortisone (cortisol) from simple carbon sources (glucose, ethanol) in a single fermentation step. For metabolic engineering of these strains, the same approaches (sterol biosynthesis rerouting to campesterol and brassicasterol, directed changes in protein topogenesis, fine-tuning of gene expression) were used as described by Duport et al. (1998), including additional expression or destruction of 13 genes in the recombinant yeasts cells. Eight heterologous proteins (mature forms of P450scc, Adx, and AdR; mitochondrial forms of Adx and P450c11; cytosolic 3β-HSD; microsomal P450c17 and P450c21) of the mammalian steroidogenic pathway were simultaneously produced in a modified host (yeast genes ATF2, GCY1, YPR1 disrupted, ARH1 overexpressed). The P450c11 system activity in mitochondria was relying on a partially artificial electron transfer chain combining the host-own reductase Arh1p (adrenodoxin reductase homologue 1) and the bovine Adx electron carrier (Duport et al. 2003; Szczebara et al. 2003; Dumas et al. 2006). The ER-located yeast NADPH-P450 reductase CPR (Ncp1p encoded by ScNCP1) supports the heterologously expressed and ER-resident mammalian P450c17 and P450c21 (Szczebara et al. 2003).
Moreover, in these and preceding studies, the major unwanted side reactions in S. cerevisiae were identified, like the esterification of pregnenolone (P450scc steroid product) by alcohol O-acetyltransferase (ATF2) and of campesterol (P450scc sterol substrate) by yeast steryl ester synthases (ACAT and ARE, ACAT-related enzyme, encoded by ARE1 and ARE2), as well as the 20-keto reduction of the P450c17 product 17α-hydroxyprogesterone (Duport et al. 2003; Szczebara et al. 2003). Pregnenolone 3β-acetate formed by Atf2p cannot be converted by P450c17, P450c21, and 3β-HSD. The esterification (Sakaki et al. 1989; Cauet et al. 1999; Vico et al. 2002) and 20-keto reduction of steroids by yeast enzymes (Shkumatov et al. 2002, 2003, 2006) were described also by other authors. Therefore, for optimising the steroid-producing strains, overexpression of gene TGL1, encoding a steryl ester hydrolase, and additionally disruption of the nonessential steryl ester synthases encoding genes ARE1 and ARE2 (although only with minor effects) and the pregnenolone acetyltransferase gene ATF2 (major effect) were accomplished, resulting in increased levels of free campesterol and pregnenolone (Duport et al. 2003; Szczebara et al. 2003).
The 20-keto reduction of 17α-hydroxyprogesterone into the diols 17α-hydroxy-20-dihydroprogesterone, predominantly 17α,20α-dihydroxypregn-4-ene-3-one, as demonstrated by Shkumatov et al. (2002, 2003, 2006), is assumed to be catalyzed by a concerted action of GCY1 (galactose-inducible crystallin-like yeast protein) and YPR1 (aldo–keto reductase) gene products (mainly Gcy1p functions as yeast 20-HSD or 20-steroid reductase), indicated by their sequence homology with bovine 20α-HSD. Disruption of both genes was leading to a loss of NADPH-keto-reductase activity with 17α-hydroxyprogesterone in vitro and of 17α,20α-dihydroxypregn-4-ene-3-one formation in vivo (Szczebara et al. 2003). As a result, S. cerevisiae recombinant strains were capable of self-sufficient production of hydrocortisone (11-deoxycortisol and corticosterone as main by-products) when growing in glucose- or ethanol-containing media. The amount of produced steroids during 72 h cultivation was increased from 1.6 to 16.6 μg/ml (with up to 70 % hydrocortisone of total steroids) by strain engineering.
Thus, Szczebara et al. (2003) summarised the long-term project resulting in the first production of “biohydrocortisone” by engineering of a highly complex mammalian biosynthetic pathway into baker’s yeast as microbial host, which includes several coupled membrane enzymes. This comprehensive “metabolic engineering turned a unicellular microorganism into a drug-synthesizing yeast cell factory” (as titled in review) and can be considered a major breakthrough for using P450-catalyzed bioconversion in an industrial process. Such a process would yield hydrocortisone in a single fermentation step from simple carbon sources, replace a multistep chemical synthesis, and reduce the environmental impact by reducing the consumption of solvents, energy, and catalysts (Dumas et al. 2006; Brocard-Masson and Dumas 2006).
3 Transgenic Yarrowia lipolytica Strains in Steroid 17α-Hydroxylation
In this section, an overview on different aspects of the heterologous expression of bovine steroidogenic P450c17 in Y. lipolytica will be presented, describing the first functional expression of a mammalian P450 in this yeast, obtained in the end of the 1990s by Juretzek and Mauersberger at the MDC in Berlin-Buch (cf. Sect. 2.1, Table 1). Additionally, results on coexpression of P450c17 with the side-chain cleavage P450scc system in Y. lipolytica will be given in Sect. 4.
3.1 Endogenous Yeast Enzymes Involved in Steroid Biotransformations
To predict more precisely the catalytic properties of the designed whole-cell biocatalysts on the basis of transgenic yeast, the ability of yeast-own enzymes to catalyse transformation of substrates and/or products of mammalian steroidogenic Р450 as unwanted side reactions has been investigated. Otherwise, the yeast cells contain enzymes which can directly support the functional activity of heterologously expressed P450 forms.
Yeast Enzymes Catalysing Unwanted Side Reactions of Heterologous Steroidogenic P450
With wild-type or recipient strains of Y. lipolytica (H222, B204-12A-213), C. maltosa (EH15, VSB779), and S. cerevisiae (GRF18), no hydroxylation activities in the C21-, C17-, or C11-positions were found towards progesterone, testosterone, deoxycorticosterone, or deoxycortisol, but different NAD(H)/NADP(H)-dependent hydroxysteroid dehydrogenase activities (oxidating the hydroxyl groups at positions 3β-, 17β-, 20α-, and 20β-HSD, or reducing the carbonyl group at positions C3, C17 and C20) catalyzed by cytosolic yeast enzymes were detected (Shkumatov et al. 1998, 2002, 2003, 2006), as it was described for S. pombe and S. cerevisiae (Pajic et al. 1999; Szczebara et al. 2003). Progesterone was reduced by alkane-growing wild-type strains of Y. lipolytica and C. maltosa at the C3- and C20-keto groups (product yields less than 2 %) and one metabolite identified as 3α-hydroxy-5α-pregnane-20-one. Furthermore, compounds corresponding to references 20α(or 20β)-dihydroprogesterone (0.6 % yield), testosterone (0.4 %), and androstenedione (0.5 %) were detected after 24 h incubation of radioactively labelled progesterone with Y. lipolytica and S. cerevisiae. Obviously, these compounds were formed as a result of 20-reduction of progesterone (20α,20β-HSD) and a Baeyer–Villiger conversion of dihydroprogesterone to testosterone followed by 17β-oxidation to androstenedione (Shkumatov et al. 1998, 2006). A similar conversion was reported for Aspergillus ochraceus (Dutta et al. 1993). Testosterone and 4-androstene-3,17-dione were interconverted by in vivo biotransformations (17β-HSD activity) using alkane-grown cells of Y. lipolytica and C. maltosa (Shkumatov et al. 1998).
Thus, in contrast to several filamentous fungi, like Aspergillus spp., Curvularia lunata, Cochliobolus lunatus, or Rhizopus nigricans, which contain endogenous steroid hydroxylating enzymes, including P450 systems, and are in particular used in industrial microbiological steroid bioconversion steps (Sedlaczek 1988; Pajic et al. 1999), all yeast species tested so far do not perform any steroid hydroxylation reactions. Nevertheless, several yeast-own enzymes may have negative effects on desired P450-catalyzed steroid biotransformation reactions when expressing heterologous P450, because they perform unwanted side reactions of desired products or applied substrates. Some of these undesirable side reactions (e.g. 20-HSD activities, encoded by GCY1 and YPR1, pregnenolone 3β-acetylation, ATF2) were shown to be diminished by corresponding gene deletions in S. cerevisiae (Szczebara et al. 2003; Dumas et al. 2006; cf. Sect. 2.2). Otherwise, in special cases, one can take advantage from the side reactions occurring in yeast and create new steroid biosynthetic paths with high yield of interesting steroid products, combining both highly specific reactions catalyzed by heterologous P450c17 and yeast 20α,β-HSD-like enzyme activities with chemical synthesis (Shkumatov et al. 2003; cf. Sect. 3.5).
Yeast Enzymes Supporting Heterologous P450
The yeasts Y. lipolytica, C. maltosa, S. cerevisiae, and S. pombe and other tested and completely sequenced species (cf. Fukuda and Ohta 2013; Mauersberger 2013) contain one or two own microsomal (ER-resident) NADPH-P450 reductases (encoded by CPR or NCP1), cytochrome b 5 and NADH-b 5 reductases as natural electron transfer partners for endogenous P450 (at least two P450s, 51A1 and 61A1, involved in ergosterol biosynthesis present in all yeast species, additionally up to 15 P450 genes detected species dependent, including members of the CYP52 gene family; cf. Mauersberger 2013). These enzymes can also support the function of heterologous microsomal P450 (P450c17, P450c21; first demonstrated by Sakaki et al. 1989, 1991 in S. cerevisiae, for other yeast species cf. Table 1 and Sect. 3 for Y. lipolytica) and even mitochondrial P450 (P450c27, Sakaki et al. 1996), although with different efficiency dependent on the expressed heterologous P450 (reviews by Urban et al. 1994; Pompon et al. 1996, 1997; Sakaki and Inouye 2000; Szczebara et al. 2003; Dumas et al. 2006).
Furthermore, mitochondrial P450 systems of type I expressed in yeast (e.g. P450c11) are partially supported by the host-own NADPH-adrenodoxin reductase homologues 1 protein Arh1p and the bovine adrenodoxin Adx electron carrier (Duport et al. 2003; Szczebara et al. 2003; Dumas et al. 2006). In S. cerevisiae, both CPR and ARH1 are essential genes. Electron transfer proteins encoding ScARH1 homologous genes were also found in Y. lipolytica and S. pombe genome. In fission yeast both heterologous and homologous redox chains, SpArh1p-etp1fd, SpArh1p-Adx, AdR-etp1fd, and AdR–Adx, can function with the heterologous P450c18, P450c11, or P450scc (Bureik et al. 2002; Schiffler et al. 2004; Ewen et al. 2008). The adrenodoxin homologous yeast gene YAH1 (encoding a mitochondrial matrix iron–sulphur protein, S. cerevisiae ferredoxin Yah1p; Barros and Nobrega 1999) is involved in the biogenesis of iron–sulphur proteins and heme A synthesis (for rev. see Schiffler et al. 2004; Ewen et al. 2008). This Adx homolog Yah1p is highly conserved in fungi, plant, and animals, and it is contained in Y. lipolytica, P. pastoris, and in most yeast and filamentous fungi (cf. Mauersberger 2013). However, ScYah1p could not substitute Adx in reconstitution of steroid hydroxylation systems in vivo (Dumas et al. 1996). Contrarily, a single ferredoxin ScYah1p-like encoding gene was not found in fission yeasts S. pombe and S. japonicus , which contain ETP1, encoding the adrenodoxin-like mitochondrial electron transfer protein 1 (etp1), a fusion protein consisting of the N-terminal COX15 etp1cd (functions in cytochrome oxidase COX complex assembly) and the carboxy-terminal ferredoxin etp1fd (ferredoxin-like [2Fe-2S]-cluster, with high homology to the ferredoxin family) domains (Bureik et al. 2002; Schiffler et al. 2004). In contrast to the S. cerevisiae ferredoxin Yah1, the closely related iron–sulphur protein etp1fd can replace Adx in the interaction with its redox partners AdR and P450. Therefore, etp1fd resembles Adx more than yeast ferredoxin Yah1 in its structural and functional features. SpEtp1p-like fusion proteins were not detected in Y. lipolytica, S. cerevisiae, and other yeast and filamentous fungi. Therefore, the appearance of etp1 fusion proteins containing the adrenodoxin-like etp1fd domain, which is after cleavage from the COX15 etp1cd domain in mitochondria functional in electron transfer, is obviously restricted to fission yeasts.
3.2 Recombinant Yarrowia lipolytica Strains Expressing Cytochrome P450c17 and CPR
Functional expression of the bovine ER-resident and steroid transforming P450c17 (encoded by CYP17A1 cDNA) in Y. lipolytica was performed to test the putative advantages of this alkane-utilising yeast as a host for heterologous P450, catalysing biotransformation reactions with hydrophobic substrates (Shkumatov et al. 1998, 2003, 2006; Juretzek 1999; Juretzek et al. 1999, 2000b; Table 1; cf. Sect. 2.1). For comparison, P450c17 was expressed in the commonly used yeast S. cerevisiae (host-vector system according to Schunck et al. 1991: strain GRF18 and high-copy replicative vector YEp5117α; cf. Fig. 1a) under control of the very strong galactose-inducible and glucose-repressible promoter pGAL10 (Shkumatov et al. 1998, 2002; Juretzek et al. 2000b). This comparison demonstrated the high potential of Y. lipolytica to perform P450-dependent biotransformation of hydrophobic steroid substrates.
First Functional Expression of P450c17 in Y. lipolytica by Low-Copy Replicative Vectors
New host–vector systems (using replicative ARS/CEN low-copy and integrative multicopy or single-copy expression vectors) were developed for heterologous P450 expression in Y. lipolytica under control of the strong and regulated isocitrate lyase promoter (pICL1), which is strongly inducible during growth on ethanol, hydrocarbons, or fatty acids, repressed on glucose to a low basic expression level (5–10 %, in contrast to pGAL10 used for expression in S. cerevisiae) and only slightly derepressed on glycerol (Juretzek et al. 1997, 2000a, 2001; Table 2). The pICL1 is of comparable strengths as other available strong and regulated Y. lipolytica promoters, e.g. pPOX2, pPOT1, pALK1, or pXPR2 (Juretzek et al. 2000a). The pICL1 has the advantage to be inducible by hydrophobic substrates (alkanes, fatty acid, or triglycerides) as well as by the hydrophilic substrates ethanol or acetate.
The first functional expression of heterologous P450c17 in Y. lipolytica was demonstrated using the ARS/CEN low-copy expression plasmid pIC17α (Fig. 1b, Table 2). The expressed P450c17 was found to be functionally active in whole cells and derived microsomal membrane fractions, indicated by the highly sensitive in vivo and in vitro biotransformation assays with (radiolabelled) progesterone into 17α-hydroxyprogesterone as the major product, (Juretzek 1999; Juretzek et al. 1999, 2000b; Shkumatov et al. 1998, 2003; Table 2; cf. Sects. 3.4 and 3.5 for more details). Yeast transformants with the replicative vectors pIC17α or YEp5117α grown in minimal media containing appropriate inducers (ethanol or alkanes for Y. lipolytica, galactose for S. cerevisiae) exhibited steroid biotransformation activities (17α-hydroxylase converting steroids into 17α-hydroxy-derivatives, Table 2), indicating the functional integrity of heterologously expressed P450c17 in yeast ER and its efficient interaction with the host-own NADPH-P450 reductase (Juretzek 1999; Juretzek et al. 1999, 2000b; Shkumatov et al. 1998, 2002, 2003), as repeatedly demonstrated with different P450s (Table 1).
The P450c17-catalyzed progesterone biotransformation activity in the low-copy transformant B204-12A-213/pIC17α was induced by the growth on ethanol or hexadecane and repressed on glucose, although, in contrast to pGAL10 used for expression in S. cerevisiae, not completely, in accordance with the carbon-source-dependent induction of pICL1-controlled gene expression in Y. lipolytica (Juretzek et al. 1997, 2000a, 2001). Interestingly, the biomass-specific progesterone biotransformation activity (q HP) of the ethanol- or alkane-induced Y. lipolytica low-copy transformant B204-12A-213/pIC17α was already in the same range with that of the galactose-induced high-copy transformant S. cerevisiae GRF18/YEp5117α (Table 2).
The P450c17 content in B204-12A-213 or PO1d transformants with the ARS/CEN low-copy vector pIC17α (Fig. 1b) was with maximally 4–5 pmol/mg cell protein hardly detectable by CO-difference spectra (COD) of ethanol-grown whole cells and therefore calculated from the P450c17 content of 14 or 10–20 pmol/mg protein, detected by COD or estimated from Western blots using microsomal fractions of these cells. The P450c17-content determination in alkane-grown B204-12A-213/pIC17α cells by COD interfered with the host-own alkane-induced P450s (cf. contribution Mauersberger 2013). The microsomal P450c17 content (about 10–20 pmol/mg microsomal protein) was therefore estimated from Western blots in comparison with microsomal P450c17 content of ethanol-grown cells. Despite low P450c17 content in this low-copy transformant, detection of P450c17 activity was possible with whole cells using the sensitive assay with radioactively labelled progesterone as substrate (Table 2). Based on above estimations of its cellular content P450c17 expressed in Y. lipolytica, B204-12A-213/pIC17α exhibited high specific progesterone 17α-hydroxylase activity, especially in alkane-grown whole cells (124 nmol/nmol P450 × min), compared to ethanol cells (74 nmol/nmol P450 × min) and to galactose-induced cells of S. cerevisiae GRF18/YEP51α (11–19 nmol/nmol P450 × min) with significant higher P450c17 content of 70–80 pmol/mg cell protein (Shkumatov et al. 1998; Juretzek et al. 2000b). The differences may reflect the different molar ratios CPR/P450c17 detected in the microsomal fractions of these cells ranging from 8.1 (hexadecane cells) and 2.6 (ethanol cells) for Y. lipolytica B204-12A-213/pIC17α to 0.12 for galactose-induced cells of S. cerevisiae GRF18/YEP51α (calculation of the CPR/P450 ratio made according to CPR data from C. maltosa: NADPH-P450 reductase purified of 79 kDa, 60 U/mg protein, 1 U = 210 pmol CPR; cf. Förster 2001). Otherwise, the specific P450c17 activities of the microsomal fractions of these cells were not significantly different (9.8, 8.1, and 7.1 nmol/nmol P450 × min) in the same order. Thus, the 1.7-fold higher specific P450c17 activity found in whole-cell progesterone biotransformation assays with hexadecane-grown cells is probably caused by the 3.3-fold increased NADPH-P450 reductase expression (175–580 mU/mg microsomal protein) as well as by the other specific attributes of yeast cells adapted to utilisation of the hydrophobic substrate alkane compared to the hydrophilic substrate ethanol. The comparison of the functional P450c17 expression in Y. lipolytica and S. cerevisiae demonstrates therefore the high potential of Y. lipolytica to perform P450-dependent biotransformation of steroids even when using low-copy replicative vectors (Table 2).
Multicopy Integrative Expression Vectors
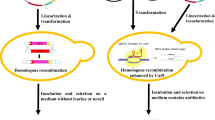
To increase the steroid biotransformation capacity of Y. lipolytica cells, a pICL1-controlled and gene-dose-dependent high-level functional expression of both P450c17 and the homologous NADPH-P450 reductase (YlCPR gene) in haploid multicopy transformants and their coexpression in derived diploid strains was obtained using integrative multicopy vectors (Fig. 1c, d), because high-copy replicative vectors are not available for Y. lipolytica. In order to increase the copy number of expression cassettes in Y. lipolytica, a series of multicopy integrative plasmids, including self-cloning plasmids, with the defective, promoter-truncated ura3d4 gene as multicopy selection marker, rDNA or LTR zeta of Ylt1 as integration targeting sequences, and pICL1/ICL1t-controlled expression cassettes for bovine P450c17 (CYP17 cDNA in p64IC17 or p67IC17, Fig. 1d; Juretzek et al. 2000b) or the host-own NADPH-P450 reductase encoding gene YlCPR (p67RYl, Fig. 1c) was developed and applied according to comparable integrative vectors (p64IP- and p67IP-series and derived expression vectors for lacZ or ICL1) described by Juretzek et al. (2001).
The haploid Y. lipolytica strains E129, E150, PO1d, and CXAU1 were used for integrative transformation (according to Barth and Gaillardin 1996) with the NotI-linearised multicopy plasmids p67IC17 or p67RYl, respectively (Figs. 1 and 2). Transformants with a normal growth rate carry at least 8–12 copies of expression vectors per cell due to the used multicopy selection marker ura3d4. The function of this type of integrative plasmids was evaluated using the lacZ reporter gene of E. coli under pICL1-control in p64IL43 and p67IL43. The expression level of β-galactosidase in Y. lipolytica correlated with the copy number of integrated cassettes and increased up to 13 times in comparison with the low-copy ARS/CEN plasmid pIL43 contained in averaged 1.6 copies per cell (Juretzek et al. 2000b, 2001).
Recombinant haploid and diploid Yarrowia lipolytica strain lines tested in biotransformation of steroids after heterologous expression of bovine cytochrome P450c17 (CYP17) and overexpression of the host-own NADPH-dependent cytochrome P450-reductase (YlCPR) under control of promoter pICL1 Prototrophic diploid strains, like A15T4 and E129A15, were obtained by crossing the haploid CYP17 multicopy transformants PO1d(p67IC17) T4 (MATA leu2-270 xpr2-322) or E129(p67IC17) (MATA leu2-270 lys211-23 xpr2-322)—both resulting from integrative transformation with the ura3d4-based multicopy vector p67IC17 (Fig. 1) of the recipient strains PO1d (MATA leu2-270 ura3-302 xpr2-322) or E129 (MATA leu2-270 lys211-23 ura3-302 xpr2-322), respectively—with the wild-type-derived auxotrophic strain A1-5 (MATB met6). The diploid strain lines DE(RYlCYP17)—strain DE (auxotrophic, due to the presence of leu2-270) and DC(RYlCYP17)—strains DC1 to DC5 (prototrophic) were obtained by crossing of the haploid CYP17 multicopy transformant E150(p67IC17) with the YlCPR multicopy transformants E129(p67RYl) or CXAU1(p67RYl)—resulting from integrative transformations of the recipient strains E150 (MATB leu2-270 his-1 ura3-302 xpr2-322), E129 (MATA leu2-270 lys211-23 ura3-302 xpr2-322), or CXAU1 (MATB ade1 ura3) with one ura3d4-based multicopy vector p67IC17 or p67RYl (Fig. 1), respectively. These recombinant diploids allowed high-level coexpression of bovine P450c17 and the host-own NADPH-P450 reductase during growth on ethanol, alkanes, or fatty acids (induction of pICL1). The diploid strains of the DE line are characterised by a reduced growth in minimal medium M (YNB-like mineral salt medium with ammonium sulphate as nitrogen source) due to the leu2 auxotrophy, in contrast to prototrophic strains. This growth defect could be overcome by using E129L (leu2 marker complemented by transformation with pINA62 containing LEU2) instead of E129 as recipient strain for transformation with the multicopy plasmids and subsequent diploidisation. Additionally, DE strains exhibited a significant delay in alkane utilisation (Alk(+)), characteristic for all strains of the E-line (French inbreeding line, like E150 and E129; Barth and Gaillardin 1996) and strains directly derived from the French wild-type strain W29 (like PO1d), in contrast to the CXAU1 (derived from an American wild-type) and the German wild-type strains A1 and H222 exhibiting normal growth on alkanes (Alk+, Mauersberger et al. 2001). Thus, in diploids of type DC and derived from A15, these growth defects were complemented
High copy numbers of the integrated expression vectors p67IC17 or p67RYl, ranging from 8 to 25 (rarely up to 35), were detected in these multicopy Y. lipolytica transformants as estimated from Southern blots (Table 2) in accordance with Juretzek et al. (2001). These multicopy vectors integrated most probably in one (maximally two) cluster at one site of integration (at least 8–12 copies totally), predominantly in tandem (head to tail) or rarely in inverse tandem (head to head) orientation as described below in Sect. 4.2. Thus, by the same approach, multicopy transformant strains of the opposite mating type expressing high levels of the heterologous bovine P450c17 or the homologous NADPH-P450 reductase (YlCPR gene) under pICL1 control (Fig. 1c, d) were constructed (Fig. 2; Juretzek 1999; Juretzek et al. 1999, 2000b; Gerber 1999; Förster 2001; Shkumatov et al. 1998, 2003).
Subsequently, several diploid Y. lipolytica strain lines (A15T4, E129A15, DE, and DC) were obtained by crossing respective multicopy transformants of the opposite mating type or with the wild-type derived Y. lipolytica strain A1-5. Whereas diploid strains A15T4 and E129A15 contain multiple expression cassettes only for P450c17, the diploid strains of lines DE(RYlCYP17) and DC(RYlCYP17) contain multiple expression cassettes for both P450c17 (CYP17) and NADPH-P450 reductase (CPR, indicated by RYl, for reductase of Y. lipolytica) originating from different haploid multicopy transformants of the recipient strains E129, E150, or CXAU1 (Fig. 2).
The integrative multicopy transformants with vector p67IC17 exhibited compared to low-copy transformants with pIC17α (in average 1.6 copies per cell, Juretzek et al. 2001) significantly increased P450c17 content and specific biotransformation activity (Table 2) in correlation with corresponding copy numbers (Juretzek 1999; Juretzek et al. 1999, 2000b). The P450c17-catalyzed biotransformation activity of progesterone increased 3.3- to 11-fold in multicopy compared to low-copy transformants, and the carbon-source-dependent induction of the pICL1-controlled P450c17 expression (induction by ethanol or alkane, slight derepression by glycerol, no complete repression by glucose) was detectable. The biomass-specific progesterone biotransformation activity (q HP) of the ethanol- or alkane-induced Y. lipolytica multicopy transformant PO1d(p67IC17) T4 or its diploid derivative A15T4 was in the same range or up to two times higher compared with the galactose-induced high-copy transformant S. cerevisiae GRF18/Yep5117α (Table 2; Juretzek 1999; Juretzek et al. 2000b).
P450c17 Content
The increase of the P450c17 expression cassette copy number to 10–25 enabled the determination of the P450c17 content directly in ethanol-induced whole yeast cells when using the modified method for CO-difference spectrum (COD) measurement with cytochrome oxidase masking by the presence of 2 mM KCN and progesterone in the assay (cf. Fig. 1 in Mauersberger 2013). The maximal P450c17 content in selected haploid multicopy transformants of types E129(p67IC17), E150(p67IC17), or PO1d(p67IC17) was 40–100 pmol/mg cell protein (30–90 pmol/108 cells) after 18–40 h growth in minimal medium with 1 % ethanol (pICL1-induction conditions) using two different cultivation regimes, with and without minimal medium (M) change prior to the induction (medium change: from preculture MG with 1 % glucose to ME with 1 % ethanol or from MY with 1 % glycerol to ME; without medium change: from MG 0.5 % to ME or MY 0.5 % to ME; Juretzek et al. 2000b; Förster 2001). In strain PO1d(p67IC17) T4 (copy number 12–14; cf. Fig. 2) a strongly increased P450c17 content of 200 pmol/108 cells was detected after prolonged induction by ethanol (30–40 h and longer) under special culture conditions (repeated feeding with ethanol). Therefore, the P450c17 expression level in multicopy transformants (at least 10–12 copies) significantly increased due to gene-dose effect compared with the low P450c17 content in B204-12A-213 or PO1d transformants with the ARS/CEN low-copy vector pIC17α containing approximately 3–5 pmol/mg cell protein (not detectable by COD of whole cells, calculated from Western blots). The P450c17 expression level in the diploid strains DE(RYlCYP17) or DC(RYlCYP17) was with 50–60 % considerably lower than in the haploid multicopy transformants in accordance with the decreased copy numbers per haploid genome. The maximal P450c17 content detected in DE(RYlCYP17) diploids was 25–30 pmol/mg cell protein (35–40 pmol/108 cells) after 25–45 h growth in minimal medium with 1 % ethanol. Thus, the heterologous P450c17 expression levels in all tested multicopy integrative Y. lipolytica transformants (ethanol-inducible pICL1: 40–100 pmol/mg protein, from 30 up to 200 pmol/108 cells) were in the same range as in the high-copy S. cerevisiae GRF18/Yep5117α (galactose-inducible pGAL10: 50–100 pmol/108 cells; Juretzek et al. 2000b) and were comparable with the content of the host-own CYP52 P450s induced during growth on alkanes (in total 60–130 pmol/mg cell protein; cf. Sect. 2 and Mauersberger 2013).
Different types of ER proliferation in the Yarrowia lipolytica haploid multicopy transformant strains PO1d(p67IC17) T4 (a, b) overexpressing the bovine cytochrome P450c17 under pICL1 control, CXAU1(p67RYl) T39, (c) overexpressing the host-own NADPH-cytochrome P450 reductase YlCPR, and in the derived diploid strain DC3 (RYlCYP17), and (d) coexpressing both cytochrome P450c17 and YlCPR during growth on 1 % ethanol at different cultivation times. Abbreviations: N nucleus, M mitochondria, ER endoplasmic reticulum, MS karmellae-like membrane stacks, V vacuole, E ethanol, the arrow in c indicates the beginning transfer of membrane stacks to the daughter cell
NADPH-P450 Reductase (CPR) Overexpression
Gene-dose-dependent high-level expression of the homologous NADPH-P450 reductase gene YlCPR under pICL1-control was demonstrated using multicopy transformants (10–25 copies of p67RYl) and derived diploid strains of lines DE and DC (Fig. 2; Gerber 1999; Förster 2001). The CPR activity determined as NADPH-cytochrome c reductase (NCCR) activity in the cell-free extract (supernatant S3) of ethanol-, fatty acid (oleic acid)-, or alkane (hexadecane)-grown cells of the haploid multicopy transformant E129(p67RYl) T6 (maximally 6.0, 5.6, and 4.4 U/mg cell protein, respectively) or CXAU1(p67RYl) T39 (3.9, 3.8, and 3.3 U/mg cell protein) increased significantly compared to the recipient strains E129 or CXAU1 (25–53 and 80–120 mU/mg cell protein in glucose, ethanol, or oleic acid cells and in hexadecane cells). The CPR activity of both multicopy transformants reached a maximum between 30 and 50 h of growth on 1 or 2 % of the inducing carbon sources and was even increased 27–30-fold in glucose-grown cells (1.1 and 1.05 U/mg protein for T6 and T39) due to the gene-dose effect (at least 10–14 copies) and the pICL1-controlled CPR expression compared with expression from one genomic YlCPR copy with its own promoter in the recipient strains. The maximal levels of CPR activities were in the multicopy transformants T6 and T39 approximately 70–220 times higher than in the recipient strains, except glucose- and alkane-grown cells with a nearly 30-fold increase, and the pICL1-controlled CPR expression in the multicopy transformants resulted in a 3.6- to 5.3-fold induction on ethanol, oleic acid, and alkane compared to glucose (Förster 2001; Förster and Mauersberger, unpublished).
Comparable results were obtained with selected diploid strains DE(RYlCYP17) or DC(RYlCYP17) constructed for pICL1-controlled coexpression of P450c17 and CPR (Fig. 2). The maximal CPR activities expressed in the diploid strains DE (0.6, 4.0, 3.8, and 3.7 U/mg cell protein on 2 % glucose, 1 % ethanol, 2 % oleic acid, and 2 % hexadecane, respectively) and DC1 (0.7, 2.8, 3.1, and 3.1 U/mg cell protein, on the same substrates) were 10–30 % lower than in respective haploid multicopy transformants due to decreased copy numbers per haploid genome, although the CPR activities were not so strongly reduced as observed for the P450c17 expression. This might be connected with the prevalence of expression cassettes for CPR over P450c17 in the diploid strains DE (10:2, although both parental strains contained almost equal copies of cassettes) and DC (DC1 10:2; DC3 6:4; DC5 8:2) as evidenced in Southern blots (Förster 2001, unpublished).
Thus, gene-dose-dependent high-level functional expression of bovine P450c17 (CYP17 cDNA) and homologous NADPH-P450 reductase (YlCPR) in haploid multicopy transformants of Y. lipolytica and their coexpression in the derived DC or DE diploid strain lines was obtained under pICL1-control (Fig. 2). Heterologous P450c17 expression and coexpression of YlCPR in both diploid strain lines DE and DC were similar. The striking difference of DE and DC strains is the wild-type-like growth of DC strains on all substrates including alkanes (Alk+) supplied by the CXAU1 derivative, which is in contrast considerably delayed (Alk(+)) in DE strains due to a growth defect observed in its both parental strains, multicopy transformants of E150 and E129 (Fig. 2; cf. Mauersberger et al. 2001).
Simultaneous overexpression of P450c17 and CPR in DE and DC diploid strains resulted in only moderately increased steroid bioconversion rates (Gerber 1999; Förster 2001; Mauersberger et al. 2002; Mauersberger et al. unpublished results; Shkumatov et al. 2003, 2006; cf. Sect. 3.5). The gene-dose-dependent very high-level CPR activity (up to 100–150-fold increase compared to CPR in wild-type strains, CPR:P450 molar ratios of 10–30:1) in the diploid cells DE and DC may be too high and therefore resulted in some negative effects (NADPH depletion, uncoupled P450 reaction cycle) on the P450c17 activity. Contrarily, an assumed moderate increase of the CPR activity (single-copy expression cassette under pICL1- or pPOX2-control) in Y. lipolytica expressing P4501A1 (single-copy or multicopy integrated cassettes) significantly stimulated the P4501A1 activity (whole-cell biotransformation of 7-ethoxyresorufin to resorufin) from 2- to 13-fold (Nthangeni et al. 2004). A moderate 2–3-fold increase of the CPR activity in low-level P450c17 expressing Y. lipolytica cells (low-copy pIC17α, CPR:P450 molar ratios of 2.6–8.1:1) after growth on hexadecane compared to ethanol as carbon source also increased the specific P450c17 activity of these cells in progesterone biotransformation 1.5- to 2-fold (Shkumatov et al. 1998; Juretzek 1999; Juretzek et al. 2000b; Table 2). When p67IC17-multicopy transformants or derived diploids with high-level P450c17 expression and only wild-type CPR expression level (A15T4 or E129A15; CPR:P450 molar ratios of 0.1–0.2:1) were tested, the stimulating effect of the CPR increase in hexadecane-grown cells was less evident (Table 2; Fig. 2). Therefore, the probable stimulating effect of a moderately increased CPR expression using a single-copy integrative vector (Nthangeni et al. 2004) together with multicopy P450c17 expression on the steroid biotransformation capacity of Y. lipolytica cells has to be elucidated by further studies. Additionally, as shown in Sect. 3.3, in both types of multicopy transformants for P450c17 or CPR overexpression and in derived diploid strains DE and DC for coexpression, a strong proliferation of different types of ER (subcompartments) was observed (Förster 2001; Mauersberger et al. 2002, unpublished), which might give negative effects on the steroid biotransformation capacity of the recombinant Y. lipolytica cells due to different localisation of CPR and P450c17.
The comparison of the P450c17-catalyzed steroid bioconversion by recombinant Y. lipolytica and S. cerevisiae cells revealed the significant advantages of the alkane-assimilating yeast Y. lipolytica in P450-dependent biotransformation of the hydrophobic steroid substrates (Juretzek 1999; Juretzek et al. 1999, 2000b; Shkumatov et al. 2003, 2006; cf. Sects. 3.4 and 3.5), a process important for industrial application (Szczebara et al. 2003; Dumas et al. 2006). The stable high-level expression of P450 together with its NADPH-P450 reductase opens new perspectives for further improvement of the efficiency of biotransformation reactions with recombinant Y. lipolytica cells, a system which seems to be useful especially for bioconversion of hydrophobic substrates (Fickers et al. 2005; Beopoulos et al. 2009, 2011; Bankar et al. 2009; Coelho et al. 2010).
3.3 Overexpression of Cytochrome P450c17 and YlCPR Induces ER Proliferation in Yarrowia lipolytica
The NADPH-P450 reductase and most P450 forms in fungi are integral membrane proteins co-located in the endoplasmic reticulum (ER). It was repeatedly demonstrated that overexpression of P450 forms, P450 reductase, or other membrane proteins resulted in a strong proliferation of ER in yeasts like S. cerevisiae or C. maltosa, a phenomenon called inducible membranes (Schunck et al. 1991; Wright 1993; Menzel et al. 1997; Sandig et al. 1999). Therefore, it was tested whether overexpression of P450c17 and NADPH-P450 reductase (CPR) is leading also to a strong ER proliferation in Y. lipolytica.
For that purpose, pICL1-controlled and gene-dose-dependent high-level functional expression of bovine P450c17 and homologous NADPH-P450 reductase (YlCPR gene) in haploid multicopy transformants and their coexpression in derived DC- or DE-type diploid strains of Y. lipolytica were obtained as shown above in Sect. 3.2 (cf. Fig. 2). Indeed, the high-level expression of P450c17 and NADPH-P450 reductase in ethanol-growing cells resulted in a strong proliferation of different ER types in Y. lipolytica (Fig. 3). Overexpression of P450c17 only induced the formation of a mostly tubular network of ER membranes in various parts of cytoplasm and of plasma membrane associated ER, but no karmellae-like membrane stacks were observed in these cells (Fig. 3a, b). Contrary, overexpression of the homologous NADPH-P450 reductase was leading to a special type of ER proliferation, forming karmellae-like membrane stacks (MS) of up to 25 membrane layers, mainly in close vicinity to the nucleus, but partially also extending into cytoplasm (Fig. 3c). In non-overexpressing cells of the Y. lipolytica recipient strains, ER membranes appeared normal (not shown). In P450c17 and CPR coexpressing diploid cells DE, both forms of proliferated ER were evident (Fig. 3d, e). Thus, for first time, a strong proliferation of ER in Y. lipolytica was shown after high-level expression of the integral membrane proteins P450c17 and NADPH-P450 reductase. Like S. cerevisiae, Y. lipolytica also exhibited different types of proliferated ER membranes depending on the expressed protein. Expression of P450c17 resulted in proliferation of a mainly tubular membrane network or plasma membrane associated ER, and during high-level expression of YlCPR mainly karmellae-like membrane stacks were formed.
Interestingly, there are culture time differences concerning the formation of P450c17- and CPR-depending ER proliferation types. Mostly a tubular network of ER membranes proliferated during P450c17 overexpression and in the early phase of coexpression in diploid strains (Fig. 3a, b, d), the CPR-dependent karmellae-like membrane stacks were observed almost in the later phase (after 40 h growth on ethanol) of high-level P450-reductase expression or in coexpressing diploid cells (Fig. 3e). The observed strong proliferation of different ER subcompartments, depending on which protein was overexpressed, can be used as a model to study ER proliferation and its regulation in yeast cells. The question whether these subcompartments contain predominantly the yeast P450-reductase or the heterologous P450 has to be elucidated. On the other site, if a very high-level expression of the P450 reductase and/or P450 is leading to a different localisation of both P450 system components, this might be a putative drawback for the efficiency of the P450-catalyzed biotransformation capacity of the recombinant yeast cell factory and should be investigated by further studies.
3.4 Functional Activity of Genetically Modified Yarrowia lipolytica in Steroid 17α-Hydroxylation
As shown in Sect. 3.2, recombinant haploid and diploid Y. lipolytica strains capable to catalyse 17α-hydroxylation of progesterone or pregnenolone upon different levels of functional expression of bovine P450c17 (CYP17) and host-own NADPH-P450 reductase (YlCPR) have been constructed and tested for P450c17-catalyzed steroid biotransformation (Figs. 2 and 4, Table 2; Shkumatov et al. 1998, 2003, 2006; Juretzek 1999; Juretzek et al. 1999, 2000b). The expressed P450c17 was found to be functionally highly active in ethanol-grown and especially in alkane-grown cells, catalysing the biotransformation of progesterone into 17α-hydroxyprogesterone as main product (Table 2, Fig. 4).
Biotransformation of progesterone to 17α-hydroxyprogesterone in a 1 L bioreactor culture by hexadecane-growing cells of Yarrowia lipolytica A15T4 expressing the ER-resident bovine cytochrome P450c17. (a) Reaction scheme of progesterone biotransformation, catalyzed by bovine P450c17 (P450c17, CYP17A) expressed in Y. lipolytica; electron transfer from NADPH to heterologous P450c17 by functional interaction with the host-own microsomal NADPH-P450 reductase (YlCPR). (b) P450c17-catalyzed progesterone biotransformation by alkane-induced A15T4 cells during growth on hexadecane (Juretzek 1999; Juretzek et al. 2000b). Recombinant Y. lipolytica diploid strain A15T4, derived by crossing of PO1d(p67IC17) T4 x A1-5, containing multiple copies of the expression cassette pICL1-CYP17A cDNA-ICL1t (cf. Fig. 2); preculture on glucose; 1-l-bioreactor culture on 1 % hexadecane (C16, induction of pICL1); after 14 h cultivation 0.5 g progesterone (P) in 10 ml dimethylformamide and 2 % C16 were added. Samples were taken and extracted and product formation (17α-HP, 17α-hydroxyprogesterone as main product) from progesterone was analysed by HPLC
HPLC separation of steroid products formed upon progesterone biotransformation with ethanol-induced cells of the recombinant diploid Yarrowia lipolytica strain E129A15 expressing cytochrome P450c17 under pICL1 control. Cultivation and steroid biotransformation were carried out in 250-ml Erlenmeyer flasks (culture volume of 50 ml) at 28–29 °C, pH 5–6, under aeration conditions (200 rpm). The strain Y. lipolytica E129A15 (cf. Fig. 2 for its construction) was cultivated in YPD medium (Difco) containing 1 % yeast extract, 2 % peptone and 2 % glucose. The inducing substrate ethanol (up to 1 % final concentration) was added to the flasks at 24 h, after complete consumption of D-glucose by the culture. After 6 h, 1 % ethanol was once more added, and after 18 h cultivation, the cells were centrifuged and transferred to YPE medium (YP-ethanol medium containing 1 % ethanol) and 100 μM progesterone was added (medium change to biotransformation medium). From the biotransformation assay 1 ml samples were taken at 3 and 18 h after addition of steroid substrates, the cells were separated by centrifugation, the supernatant was extracted twice with 2 ml ethyl acetate and the combined organic phase was evaporated on a rotary evaporator. The dry residue was dissolved in 500 μl methanol and analysed by HPLC. Column Kromasil 100-C18, 5 μm, 125 × 4 mm; elution with a gradient of solution B (acetonitrile) in a solution A (H2O): 5 min, 20 % B; 5–10 min, 20 → 80 % B; 10–17 min, 80 % B; 17–20 min, 80 → 20 % B; flow rate 1 ml/min; spectrophotometric registration in the range of 220–340 nm; ordinate axis: absorption A at 240 nm; Peak I, starting substrate progesterone; peak II, 17α-hydroxyprogesterone (the major product); peaks III and IV, steroid by-products, detected after 3 h (a) and 18 h (b) incubation of induced cells with progesterone. Figure was taken and modified from Shkumatov et al. (2003)
Steroid Bioconversion Capacity of P450-Expressing Yeast Cells
As shown in Sect. 3.2 (Table 2), the values for the biomass-specific progesterone biotransformation activity (q HP, 17α-HP formation rate as nmol product/OD600 × h), determined externally in 1 ml assays with 100 nmol labelled progesterone (100 μM) of the ethanol- or alkane-induced Y. lipolytica low-copy replicative and high-copy integrative transformants (like B204-12A-213/pIC17α and PO1d(p67IC17) T4) and derived diploids strains (A15T4), were in the same range with that of the galactose-induced high-copy transformant S. cerevisiae GRF18/YEp5117α. Using these external determined q HP rates, one can calculate the volumetric productivities (Q HP, 17α-HP formation rate as μM/h or mg product/l × h) of these bioreactor or shaking flask cultures. For galactose-induced S. cerevisiae GRF18/YEp5117α cultures, Q HP of 44.0 μM/h (14.5 mg 17α-HP/l × h, cells of a 5-l-bioreactor culture, 13.3 final OD600) was calculated, being in good agreement with the product formation rate of 42.5 μM/h determined in 50-ml shaking flask cultures (Shkumatov et al. 2006) and exceeding significantly the Q HP rate of 6.3–11.0 μM/h reported by Sakaki et al. (1989) [11.0 μM/h for 7 × 107 cells/ml, extrapolated from 1.1 μM/h for 7 × 106 cells/ml] and Ness et al. (1998) [6.3–6.7 μM/h for approximately 10 × 107 cells/ml] for progesterone biotransformation with S. cerevisiae expressing bovine P450c17. For ethanol- or hexadecane-induced cultures of the Y. lipolytica low-copy replicative transformant B204-12A-213/pIC17α, Q HP of 17.8 or 34.9–39.6 μM/h (cells of 5-l-bioreactor cultures, 13.4 or 15.5 final OD600) were calculated, respectively (Juretzek 1999). According to Table 2, the Q HP values of the ethanol- or alkane-induced multicopy transformant and derived diploid strain cultures (like Y. lipolytica PO1d(p67IC17) T4 or A15T4) might be even up to threefold higher, provided optimal biotransformation conditions in bioreactors without oxygen limitations occurring in shaking flask cultures, although some growth limitations were observed with E129- and PO1d-based multicopy transformants and derived diploid DE strains (cf. Fig. 2).
The bioconversion capacity of Y. lipolytica expressing bovine P450c17 was experimentally demonstrated with selected diploid strains, like A15T4 (normal growth on alkanes, high-level expression of P450c17 due to 5–6 vector copies per haploid genome, alkane-induced CPR level; Fig. 2, Table 2), using a 1-l-bioreactor culture with hexadecane as carbon source and pICL1-inducing substrate, permitting sufficient oxygen supply (pO2 > 40 %) and high biomass concentrations (OD600 approximately 100, 5–10 × 108 cells/ml) of up to 150 g/l yeast wet weight (Fig. 4; Juretzek 1999; Juretzek et al. 2000b). After adding progesterone (0.5 g in 10 ml dimethylforamide, i.e. 1,590 μM compared to 100 μM used in usual external biotransformation assays; cf. Table 2) to alkane-growing (P450c17-induced) cells, at 14 h of cultivation, the biotransformation to 17α-hydroxyprogesterone (17α-HP) as main product started immediately and continued in the stationary growth phase reached due to hexadecane exhaustion. The main part of substrate and product were found in the cell-free culture medium. The overall biotransformation rate Q HP was 23.3 μM/h (maximal rate Q HPmax 26.8 μM/h) under these conditions (Fig. 4). Comparable results were obtained with ethanol-induced cells of diploid strain DE13 (coexpression of P450scc system and P450c17; cf. Sect. 4) exhibiting a productivity Q HP of 26.7 μM/h in shaking flasks when using 100 μM progesteron.
Compared with other recombinant yeasts, these results for biotransformation of progesterone to 17α-hydroxyprogesterone with Y. lipolytica and S. cerevisiae are the highest values published to date for P450-catalyzed steroid biotransformations with yeasts. For the shark P450c17 expressed in Pichia pastoris, a progesterone biotransformation rate of 1.3 μM/h (109 cells/ml) was described (Trant 1996). For Schizosaccharomyces pombe expressing human P450c11 (CYP11B1, 11β-hydroxylase), conversion of 11-deoxycortisol to cortisol with a rate of 8.4–9.4 μM/h (4–5 × 107cells/ml) was reported (Drăgan et al. 2005), whereas expression of human P450c18 (CYP11B2, aldosterone synthase) resulted in maximally 0.03 μM/h corticosterone formation from 11-deoxycorticosterone (Bureik et al. 2002, 2004). The conversion of progesterone to 11-deoxycorticosterone and that of 17α-hydroxyprogesterone to 11-deoxycortisol occurred in S. pombe expressing P450c21 (CYP21, 21-hydroxylase) with maximal rates of 0.9 and 10.8 μM/h, respectively (Drăgan et al. 2006). Biotransformations with recombinant baker’s yeast expressing bovine CYP21 had comparable or lower steroid conversion rates of 17α-HP to 11-deoxycortisol (Sakaki et al. 1990, 1991; 5.1 μM/h with 1 × 107 cells/ml, extrapolated to 25.5 μM/h with 5 × 107 cells/ml, Wu et al. 1991; 4.8–5.7 μM/h, cell density not given, Szczebara et al. 2003), but the experimental setup (cell density, substrate concentration) was not entirely comparable.
These values were calculated from literature data to compare the steroid bioconversion capacity of the recombinant yeasts, although different cell densities were applied for bioconversion assays, e.g. P. pastoris, 109 cells/ml; S . pombe 107 cells/ml (Bureik et al. 2002, 2004; Drăgan et al. 2005, 2006); and Y. lipolytica 107–108 cells/ml (external biotransformation assay; cf. Table 2; bioreactor culture; cf. Fig. 4). For comparison, with recombinant S. cerevisiae strains, self-sufficient production of hydrocortisone (70 %, with 11-deoxycortisol and corticosterone as by-products) from glucose or ethanol with an overall steroid product formation rate of 0.27 μM/h (with 0.18 μM/h for hydrocortisone) was reported (Szczebara et al. 2003; cf. Sect. 2.2), whereas for progesterone biosynthesis, the product formation rate Q P was 1.91 μM/h (Duport et al. 1998).
Substrate Specificity of P450c17 Expressed in Yeast
The first activity of mammalian Р450c17 is to catalyse hydroxylation of pregnenolone and progesterone at C17 position (17α-hydroxylase) to generate 17α-hydroxypregnenolone and 17α-hydroxyprogesterone. The second enzymatic activity follows in cleavage of the C17–C20 bond (17,20-lyase) of either 17α-hydroxypregnenolone or 17α-hydroxyprogesterone to form dehydroepiandrosterone (DHEA) and androstenedione, respectively (Lieberman and Warne 2001). The ratio of 17,20-lyase to 17α-hydroxylase activities in mammalia is regulated by the CPR/P450c17 ratio, presence of cytochrome b5, or serine/threonine phosphorylation of P450c17.
Biotransformation of the steroids progesterone, pregnenolone and related derivatives was demonstrated with recombinant yeast cells of Y. lipolytica and S. cerevisiae expressing bovine P450c17 (Shkumatov et al. 1998, 2002, 2003, 2006; Juretzek 1999; Juretzek et al. 2000b; Novikova et al. 2009). Among several substrates tested, progesterone was hydroxylated by P450c17 expressed in S. cerevisiae GRF18/Yep5117α with the highest activity (Shkumatov et al. 2002, 2006). The 17α-hydroxylase activity of P450c17 towards tested substrates decreased in the sequence progesterone >> 11α- > 11β- >> 19- or 21-hydroxyprogesterone (11-deoxycorticosterone, no activity detected for the two last compounds), whereas the 20α-ketoreduction (yeast 20α-HSD) was observed for 17α- > 21- >> 19-hydroxyprogesterone (no activity) and a minor 20α-HSD activity was also detected for progesterone resulting in the formation of 20α-dihydroprogesterone (Shkumatov et al. 2002, 2003, 2006). Although besides progesterone and pregnenolone the steroid derivatives 11β-, 11α-, 21-, or 19-hydroxyprogesterone were not tested for P450c17 expressed in Y. lipolytica, one can assume a comparable substrate specificity in this case. These in vivo experiments in yeasts did not reveal other P450c17 activity towards progesterone, in particular, 16α-hydroxylase activity (Shkumatov et al. 2006). Because no significant androstenedione (the product of the 17,20-lyase reaction catalyzed by P450c17) formation was detected, bovine P450c17 expressed in both yeast S. cerevisiae or Y. lipolytica exhibited only the 17α-hydroxylase activity and lacked the 17,20-lyase activity (Shkumatov et al. 2002, 2006), as it was previously described for P450c17 expressed in S. cerevisiae (Sakaki et al. 1989).
Side-Product Formation
With the recombinant diploid Y. lipolytica E129A15 (multiple copies of cassette pICL1-CYP17-ICL1t for P450c17 expression; see Fig. 2 for its construction), the conversion of progesterone (I) into the target main product 17α-hydroxyprogesterone (II) was found already at the initial stage (2–6 h) of biotransformation, whereas steroid side products III and IV (17α,20(α or β)-diols) were accumulated later (12–30 h) after nearly complete conversion of I to II (Fig. 5, see also Sect. 4 for strain DE13; Shkumatov et al. 2003, 2006). For identification, side products were prepared in large quantities after prolonged progesterone biotransformation with Y. lipolytica E129A15 and purified by a combination of TLC and HPLC. The data of 1H NMR spectroscopy, mass spectrometry, and HPLC for the steroid side products III and IV and their comparison with the corresponding data for chemically synthesised 17α,20β-dihydroxypregn-4-ene-3-one (Kovganko et al. 2001) and with those for the isolated progesterone side metabolites of S. cerevisiae GRF18/YEp5117α expressing P450c17 (Shkumatov et al. 2002) allowed to ascribe them the structures of 17α,20β-dihydroxypregn-4-ene-3-one and 17α,20α-dihydroxypregn-4-ene-3-one, respectively.
The reduction of 17α-hydroxyprogesterone (II) to 17α,20β- (III) and 17α,20α-dihydroxypregn-4-ene-3-one (IV) is due to the functioning of the corresponding yeast 20β- and 20α-dihydroxysteroid dehydrogenase (20-steroid reductase) activities (20β- and 20α-HSD). It is worth mentioning some distinctive features of progesterone bioconversion by recombinant yeasts expressing bovine P450c17, indicating potential advantages of Y. lipolytica compared to S. cerevisiae. In case of S. cerevisiae GRF18/YEp5117α transformants, the major side product was the diol IV (III:IV was about 1:50), whereas in case of Y. lipolytica E129A15, the ratio was close to 1:1 with a small prevalence of diol III (Fig. 5). In addition, these both side compounds did not exceed 40–45 % in the latter case even after 48–96 h biotransformation in the YP-ethanol medium (Shkumatov et al. 2003, 2006). At the same time, in the case of S. cerevisiae GRF18/YEp5117α, the yield of steroid IV can exceed 60–80 % of the starting progesterone after 24–96 h biotransformation in the nonselective YP-galactose medium (Shkumatov et al. 2002, 2003, 2006). This was due to D-galactose induction of biosynthesis of both bovine P450c17 and yeast Gcy1p protein (crystallin-like protein), an ortholog of mammalian 20α-HSD (Szczebara et al. 2003).
Interestingly, when 20α- or 20β-dihydro derivatives of progesterone (pregn-4-ene-(20α,β)-ol-3-ones, V and VI) were used as starting substrates for bioconversion to study the conjugation between the activities of 17α-hydoxylase and 20-dehydrogenase of recombinant Y. lipolytica and S. cerevisiae cells, the following sequence of reactions was observed: first oxidation of 20-dihydroprogesterones (by the yeast 20-HSD back reaction or by an oxidase activity of P450c17 towards V and VI) to progesterone (I) followed by its 17α-hydoxylation to II (by P450c17) and final stereoselective 20α- or 20β-reduction (yeast 20-HSDs) to III and IV, indicated by the appearance of I, II, III, and IV as intermediates and products of V or VI bioconversion (Shkumatov et al. 2003, 2006; Novikova et al. 2009).
Side-Product Reduction by Chemical Oxidation
The formation of the reduced derivatives III and IV (17α,20(α,β)diols by HSD-like yeast enzymes) as side products along with the main product 17α-hydroxyprogesterone (II) during progesterone biotransformation by P450c17 expressed in recombinant strains makes the yield of the target product II lower and hampers its isolation from the mixture. Therefore, the Jones oxidation with chromic acid (H2Cr2O7) in acetone of steroid mixtures II to IV formed during progesterone biotransformation by Y. lipolytica E129A15 or S. cerevisiae GRF18/YEp5117α cultured on nonselective media with the addition of the corresponding inducers (ethanol or hexadecane or galactose, respectively) has been studied (Shkumatov et al. 2003). In all the cases, 17α-hydroxyprogesterone (II) was not oxidised. At the same time, 17α,20β-diol (III) and 17α,20α-diol (IV) were almost completely converted into androst-4-ene-3,17-dione, the second natural product of P450c17 (due to 17,20-lyase activity), which was although almost not formed by bovine P450c17 expressed in yeasts. The yield of 17α-hydroxyprogesterone was only slightly increased due to partial conversion of the side product IV (17α,20α-diol) into II, whereas the main part of III and IV was oxidized to androstenedione (Shkumatov et al. 2003). These results extend the possibilities of chemical and enzymatic steroid synthesis. A combination of biotechnological methods using various recombinant microorganisms producing mammalian P450c17 and chemical oxidation methods allows the preparation of the target steroid set (II to IV and androstenedione) from progesterone as the only starting compound, which can be useful as raw materials for the production of steroid medicines and other applications (Shkumatov et al. 2003).
Identification of 20α-, 20β-, and 17β-Steroid Reductases in Y. lipolytica
The main side products of progesterone biotransformation by recombinant yeast cells expressing P450c17 were 17α,20α- and 17α,20β-dihydroxypregn-4-ene-3-ones (Fig. 5, Table 3; Shkumatov et al. 2003, 2006), indicating the existence of yeast enzymes acting as mammalian 20α -, 20β-, and 17β-hydroxysteroid dehydrogenases (HSDs). The proteins Gcy1p and Ypr1p belonging to the aldo–keto reductase superfamily AKR may be analogues of 20α-HSD in S. cerevisiae (Szczebara et al. 2003; cf. Sect. 2.2), whereas analogues of 17β-HSD in S. cerevisiae may be Ayr1p (1-acylhydroxyacetone-phosphate reductase) for reduction and Fox2p (hydroxyacyl-CoA-dehydrogenase) for oxidation (Vico et al. 2002). A search for steroid side product generating 20α/β- and 17β-steroid reductase analogues (HSD) performed in the Y. lipolytica genome database (Dujon et al. 2004) revealed at least six proteins with statistically significant similarity to the 20α-/17β-HSD members of the AKR superfamily (Shkumatov et al. 2006). The function of these proteins in relation to the formation of side products occurring during 17α-hydroxyprogesterone conversion remains to be elucidated by gene disruption studies. The 20α-HSD was purified from Y. lipolytica E129A15 cells. The substrate specificity (reduction of 17α-hydroxyprogesterone and progesterone, oxidation of 17α,20α-dihydroxypregn-4-ene-3-one, and 20α-dihydroprogesterone), the cofactor dependence and molecular weight of 39 kDa relate the Y. lipolytica enzyme to 20α-HSD isoenzyme I of mammalia (Shkumatov et al. unpublished results).
Liquidation of Side Products by Selective Inhibition
In S. cerevisiae ATF2 encoded O-acetyltransferase catalyses acetylation of 3β-OH-groups of pregnenolone, 17α-hydroxypregnenolone, or DHEA. Because pregnenolone 3β-acetate cannot be converted by P450c17, Atf2p activity decreases significantly the P450c17 biotransformation efficiency for pregnenolone, and ATF2 gene disruption was successfully used to avoid undesirable 3β-O-acetylation of pregnenolone (Cauet et al. 1999; Vico et al. 2002; Szczebara et al. 2003; cf. Sect. 2.2). It was also shown that Atf2p can catalyse formation of acetyl esters of isoamyl alcohol and some other aliphatic alcohols (Vadali et al. 2004) opening an alternative approach to diminish the side-product pregnenolone 3β-acetate formation. Addition of such aliphatic alcohols to cultural medium may decrease pregnenolone acetylation in S. cerevisiae due to competition of steroid with non-steroid alcohols for interaction with Atf2p. Cells of transgenic strain S. cerevisiae GRF18/YEp5117α expressing P450c17 were used to carry out pregnenolone biotransformation in rich YP-galactose medium. Formation of 17α-hydroxypregnenolone, pregnenolone 3β-acetate, and 17α-hydroxypregnenolone 3β-acetate was detected. Concentration ratio of 17α-hydroxypregnenolone to pregnenolone 3β-acetate was increased up to 5.3 (0.8 in the control) in the case of addition of 0.5 % vol. isoamyl alcohol (Faletrov et al. 2008a).
Interestingly, genome analysis of Y. lipolytica revealed the absence of S. cerevisiae Atf2p homologues although other O-acetyltransferases are present. Indeed, during pregnenolone biotransformation using Y. lipolytica transgenic strain DE54 (DE5-type; see Sect. 4.2) expressing P450c17 under pICL1-control, the steroid was quantitatively converted to 17α-hydroxypregnenolone, and formation of 3β-O-acetates of 17α-hydroxypregnenolone or pregnenolone was not detected (Faletrov et al. 2008a, b).
Construction of recombinant Yarrowia lipolytica strains of types DE1 to DE6 for pICL1-controlled expression of the cytochrome P450scc system components and of cytochrome P450c17 by diploidisation using haploid multicopy transformants (a), schematic diagram of vectors organisation in the multicopy transformants (b), and Southern blot of DNA from selected haploid parental and derived diploid strains for testing the presence of multiple copies of different expression cassettes (c). (a) Description of multicopy transformants of strains Y. lipolytica E150 and E129L, selected as parental haploid strains for diploidisation, e.g. E150(AdRAxP) T8 (shortly E150 T8), for transformant T8 of strain E150 with vector pair p64AdR and p64AxP simultaneously (both vectors integrated), or E129L(AxAxPP17) T2, E129L(AxPP17) T10 (shortly E129L T2, E129L T10), and E129L(Ax) T4, for transformants T2, T10, and T4 of strain E129L with vectors p67Ax, p67AxP, and p67IC17 simultaneously, in which three, two, or only one vector was found integrated in multiple copies (in total at least 10–12), respectively. Designation of other transformants followed the same rules. For the parental transformants selected for the creation of DE1- and DE4-type diploids, evidence for integration of two (E150 T8, E129L T10) or three (E129L T2) expression vectors is provided in Southern blot (c). Almost all tested individual diploid strains of types DE1 to DE6 (except one out of three DE5-type strains tested, not shown) obtained by crossing the indicated haploid parental strains contained the expected three to four different expression cassettes (in three to five integrated vectors; DE1- and DE2-type diploids contained AxP twice delivered by each parental strain containing p64AxP or p67AxP) originating from both parental haploid transformants as summarised in principle (a) and evidenced by Southern blotting (c). (b) Principles of integrated vector organisation in clusters, containing multiple copies of one, two or three vectors used simultaneously for transformation, evidenced by additional Southern blot experiments of multicopy transformants (not shown). The multicopy vectors integrated in one or two clusters (most probably at one site of integration) of at least 10–12 copies totally, predominantly in tandem (head to tail) or rarely in inverse tandem (head to head) orientation. Therefore, two or three simultaneously transformed vectors of the same p64- or p67-type but containing different expression cassettes were found in the same cluster of multiple vector copies, as illustrated in b. (c) Southern blot for individual diploid strains of type DE1 and DE4 in comparison with the respective parental strains. All tested diploids contained the expected four expression cassettes reflecting the content provided by both parental strains with slight differences in copy number ratios. Chromosomal DNA of all strains was digested by EcoRV. The selected probe pICL1-AxP (SalI fragment from vector p67AxP) detected simultaneously the ICL1 gene (1.9 kb band, one genomic copy per haploid genome, as internal standard for comparison with the multiple copies of integrated vectors) and, due to the presence of pICL1, all four different expression cassettes (multiple copies, visible as fragments of different size from 2.40 to 3.65 kb, with significantly higher signal intensities compared to the 1.90 kb ICL1 fragment). Abbreviations: Alk(+), delay in growth on alkanes, compared wild-type strains like H222 or CXAU1 (Mauersberger et al. 2001; cf. Fig. 2); P17, P450c17; M, molecular weight standard λ-DNA EcoRI-HindIII digested; 11–18, DE1-type diploids DE11 to DE18; 40–44, DE4-type diploids DE40 to DE44; g, band from the one genomic copy of ICL1; v, multicopy bands from the expression cassettes of integrated vectors
3.5 Optimisation of Progesterone to 17α-Hydroxyprogesterone Bioconversion
It was established that chemical Jones oxidation of the purified side products 17α,20(α,β)diols (III and IV) (cf. Sect. 3.5) is not the only effective way to decrease the number and change the nature of side products formed during progesterone biotransformation with transgenic Y. lipolytica strains. The following representative members of the recombinant diploid strain lines E129A15, DC, and DE of Y. lipolytica (cf. Fig. 2 for their construction) were investigated to compare their abilities as catalysts of progesterone 17α-hydroxylation and 20-oxidation/reduction: E129A15 (pICL1-controlled Р450c17 overexpression from multiple expression cassettes, basic YlCPR expression from one genomic copy, CPR: P450c17 ratio nearly 0.33:1), DC3, DC5 (both strains of DC line), and DE (one strain of DE line; DC and DE strains with pICL1-controlled coexpression of Р450c17 and YlCPR from multiple expression cassettes, CPR:P450c17 ratio 10–15:1). With these diploid strains, the catalytic characteristics of P450c17 expressed in Y. lipolytica with and without overexpression of the host-own NADPH-P450 reductase (YlCPR) and the product yield of progesterone biotransformations were compared. Ethanol-induced cells (18–24 h induction in YP-ethanol after pre-growth in YPD as described in Fig. 5, Shkumatov et al. 2006) were used for short- (for kinetic data) and long-term (for the influence of induction and biotransformation conditions on the main- and side-product yield, Table 3) progesterone biotransformation assays and the expression levels of P450c17 (3.3–4.2 × 104 P450c17 molecules/cell, determined by CO-difference spectra with whole cells) and of CPR (24- to 36-fold increased CPR expression in strains DC5 and DE compared to E129A15, measured as NADPH-cytochrome c reductase activities) were determined in these cells. The general activity (multiplication of P450c17 molecule number expressed per cell with the Vmax values of the biotransformation reaction) was somewhat higher for strain E129A15 (47) compared to DE (39) and DC5 (43). Concerning their catalytic efficiency (kcat/Km, min−1 × 106 M−1), the recombinant diploid strains can be ranked as DC5 (14.3) > DE (13.1) > E129A15 (10.0). Thus, the very high-level coexpression of CPR in DE and DC strains did not lead to a significant increase in the catalytic efficiency of the expressed P450c17. This might be connected with the observed strong proliferation of different ER membrane subcompartments in P450c17 and CPR overexpressing Y. lipolytica cells (cf. Sect. 3.3).
During exhaustive progesterone biotransformation with the Y. lipolytica diploid strains E129A15, DE, DC3, and DC5, a strain- and culture-condition-dependent formation of main and side products was observed (Table 3). Long-term progesterone biotransformation over 24 h by Y. lipolytica strain E129A15 grown in rich medium YP-ethanol (cells pre-grown 24 h in YPD or till glucose exhaustion, followed by 18–24 h ethanol-induced P450c17 expression and subsequent biotransformation in YP-ethanol) with medium change prior to biotransformation (as indicated in Fig. 5; Shkumatov et al. 2006) was associated with relatively high diols (III and IV, 17α,20β- and 17α,20α-dihydroxypregn-4-ene-3-one) formation as side products (21.0 % and 18.7 %, respectively) with a moderate main product II (17α-hydroxyprogesterone) yield of 58.2 % (Table 3). Significant higher main product II yields were achieved in YP-ethanol or YNB-ethanol medium without medium change prior to biotransformation (80.4 % and 86.0 % for E129A15), and this was associated with lower side-product III and IV formation. Comparable results were obtained with diploid strain DC3 (pICL1-controlled overexpression of P450c17 and YlCPR), although with lower main product yield. With strain DC3, the formation of small amounts (5.6 and 6.3 %) of the minor products V and VI (20β-and 20α-dihydroprogesterone) was observed when medium change prior biotransformation was applied, obviously arising from low activities of the yeast 20α- and 20β-HSD enzymes towards progesterone (Shkumatov et al. 2006), although these side products were almost not found with the strains E129A15, DE or DC5.
The diploid strain DE exhibited a main steroid product II yield of 80.7 % in selective minimal medium YNB with low side product III and IV levels of 2.5–3 % even when using medium change to fresh YNB-ethanol prior to biotransformation (Table 3). The maximal main product II yield of up to 94.7 % was achieved by using strain DC5 in YP-ethanol medium (with and without medium change), which also exhibited low residual substrate and side-product III and VI (2.0 % and lower) levels (Table 3). This practically full side-product absence (total <4.5 %) is the major advantage of the Y. lipolytica diploid strain DC5 because it allowed not destroying 20α- and 20β-HSD analogues genes for gaining efficient biotransformation of progesterone (Rudaya et al. 2006; Rudaya 2007; Shkumatov et al. 2007a; Novikova et al. 2008). Contrarily, long-term progesterone biotransformation over 24 h with S. cerevisiae GRF18/YEp5117α in YP-galactose medium led to the appearance of yeast 20α-hydroxysteroid dehydrogenase activity (20α-HSD) resulting in considerable formation of mainly 17α,20α-dihydroxypregn-4-ene-3-one (IV) of 60 % up to 86 %. Otherwise, side-product III to VI formation with Y. lipolytica strains E129A15 and DC3 did not exceed 35–52 % in YP-ethanol medium with medium change (Table 3; Shkumatov et al. 2002, 2003, 2006), and this amount was finally reduced below 4.5 % by strain selection and optimisation of the culture, induction and biotransformation conditions.
Additionally, for strains Y. lipolytica E129A15, DE, and DC5, the repressive effect of initial glucose concentration in the growth media on the pICL1-dependent P450c17 expression was studied. The decrease of initial glucose concentration in YPD medium for growth from 2 to 0.4 % caused an increased specific rate of progesterone 17α-hydroxylation due to earlier carbon source shift from pICL1-repressing glucose to pICL1-inducing ethanol or hexadecane (according to Juretzek et al. 2000a, b, 2001) and the complete absence of residual glucose in the induction medium. In particular, this and simultaneous inducer and progesterone addition (shown below) resulted in decrease of time for 50 % progesterone to 17α-hydroxyprogesterone bioconversion from approximately 10–2 h.
Furthermore, simultaneous addition of the steroid substrate (progesterone) and the P450c17-inducer (ethanol or hexadecane) decreased the time needed for more than 90 % bioconversion of progesterone to 17α-hydroxyprogesterone to 4–8 h (Rudaya 2007; Shkumatov et al. 2007a; Novikova et al. 2008). Absence of unproductive oxygen redox cycles catalyzed by P450c17 without substrate(s) leading to its inactivation can be a reason for increased 17α-hydroxyprogesterone yield. Besides, during long-time cultivation in rich YPD medium, induction of 20-HSD orthologues (like the proteins Gcy1p and Ypr1p in S. cerevisiae) is possible, especially at high sugar concentration (Chang et al. 2007).
Cholesterol and progesterone biotransformation by the recombinant diploid Yarrowia lipolytica strain DE13 after ethanol-induced coexpression of the cholesterol side-chain cleavage cytochrome P450scc system and cytochrome P450c17. The DE1-type diploid strain Y. lipolytica DE13(AdR Ax AxP P17) was derived from the parental haploid strains E150(p64AdR p64AxP) T8 and E129L(p67Ax p67AxP p67IC17) T3 and contains multiple copies of five expression cassettes for mature forms of human AdR, Ax, and AxP (from both vectors p64AxP and p67AxP) and bovine P450c17 (P17) under control of the isocitrate lyase promoter pICL1 (Fig. 6). Western blot results demonstrated the ethanol-induced heterologous expression of the P450scc system proteins AdR, Ax, and AxP (human Ax-P450scc fusion protein). Functional expression of P450c17 was demonstrated by its enzymatic activity in progesterone biotransformation using whole cells. Cultivation and induction of heterologous protein expression was performed in shaking flasks in YPD with 0.4 % glucose for 24 h (to exhaustion of glucose). At 24 h and second time at 30 h, 1 % ethanol was added as inducing carbon sources to start the pICL1-controlled heterologous protein expression of the cholesterol side-chain cleavage system proteins AdR, Ax, and AxP and of P450c17. After glucose exhaustion at 24 h to glucose pre-grown cells, 50 μM cholesterol or 100 μM progesterone was added together with the inducing substrate 1 % ethanol to test their biotransformation; thus, induction and biotransformation were performed simultaneously. Culture samples were taken 2, 3, 6, 24, and 48 h after biotransformation substrate addition, and organic extracts were analysed by GC or HPLC
Thus, Y. lipolytica strains and conditions were selected with very low side-product content allowing efficient progesterone biotransformation into 17α-hydroxyprogesterone without prior destroying 20α- and 20β-HSD analogue genes. The highly selective production of 17α-hydroxyprogesterone with Y. lipolytica strains like DC5 was achieved by the selected conditions for pICL1-controlled P450c17 expression and biotransformation on ethanol or n-alkanes (absence of galactose-induced protein Gcy1p or Ypr1p, which in case of pGAL10-controlled P450c17 expression in S. cerevisiae on galactose led to strongly increased formation of 17α,20α-dihydroxypregn-4-ene-3-one as the main side-product IV), as well as by the rapid excretion of the desired product 17α-hydroxyprogesterone by Y. lipolytica cells. This was indicated by a lower rate of reconsumption of 17α-hydroxyprogesterone (which is more hydrophilic than progesterone and obviously does not induce yeast 20α,β-HSD) and the observed faster consumption of 20α- or 20β-dihydroprogesterone by ethanol-induced Y. lipolytica cells compared to galactose-induced S. cerevisiae cells expressing Р450с17 (Shkumatov et al. 2006, 2007a).
Thus, as shown in Sects. 3.4 and 3.5, distinctive features of Р450с17-expressing Y. lipolytica strains are the easily selectable conditions for low activity of 20(α,β)-hydroxysteroid dehydrogenases and the absence of side reactions of 3-О-acetylation for C21-3β-OH-Δ5-steroids (pregnenolone or 17α-hydroxypregnenolone). This was leading to a significant reduced extent of side products of progesterone or pregnenolone bioconversion obtained by Y. lipolytica strain selection and phenotypical optimisation without the necessity for 20α,20β-HSD analogue gene disruption and indicates several advantages of Y. lipolytica as alternative host–vector systems for heterologous expression of steroidogenic P450s compared to S. cerevisiae and other yeasts.
4 Transgenic Yarrowia lipolytica Strains for Sterol Side-Chain Cleavage and 17α-Hydroxylation
After successful functional expression of bovine P450c17, the mammalian sterol (cholesterol) side-chain cleavage P450scc (CYP11A1) system was selected for coexpression with P450c17 in Y. lipolytica to generate a multistep steroid bioconversion chain in this yeast. Whereas the expressed bovine P450c17 protein was shown to be functionally active with the host-own NADPH-P450 reductase (CPR) for functional expression of P450scc, additional coexpression of the two electron transfer proteins NADPH-adrenodoxin reductase (AdR) and adrenodoxin ([2FE-2S]ferredoxin, Adx) will be necessary, as indicated by previous in vitro reconstitution experiments with selected purified P450s (mitochondrial P450scc and P450c11, microsomal P450c21 and P450Coh) using purified or partially purified yeast NADPH-P450 reductases from C. maltosa or Y. lipolytica, respectively, showing only minor activity with P450scc or P450c11 (0.2–1.4 %), but significant activities with ER-resident P450c21 or coumarin hydroxylating P450Coh (42–85 %) in comparison with their natural electron transfer components (Shkumatov and Smettan 1991; Novikova et al. 2009; Shkumatov et al. unpublished results).
4.1 Substrate Specificity of Cytochrome P450scc
The first reaction leading to the biosynthesis of all the steroid hormones in mammalian is generally considered to be that concerned with the conversion of the sterol precursor, cholesterol, to the C21-steroid, pregnenolone. The widely accepted pathway for side-chain cleavage can be summarized as follows: cholesterol → (22R)-22-hydroxycholesterol → (20R,22R),20,22-dihydroxy-cholesterol → pregnenolone and aldehyde. The process requires 3 mol of NADPH, 3 mol of molecular oxygen, and three proteins, P450scc (CYP11A1), and electron-transferring proteins, ferredoxin reductase (in the adrenal, adrenodoxin reductase—AdR) and ferredoxin (in the adrenal, adrenodoxin—Adx). It was considered earlier that Р450scc has narrow substrate specificity. Some modifications, especially 22,23-double bond in structures of ergosterol or some phytosterols, play critical role for the ability of sterols to be substrates for P450scc. Specificity of P450scc towards cholesterol being the only physiological substrate established is considered to be rather strict (Pikuleva 2006).
Phytosterols as Substrates for P450scc
The interaction of P450scc with cholesterol and plant oil phytosterols (β-sitosterol, campesterol, brassicasterol) was studied in vitro using a spectrophotometric titration approach and the reconstituted P450scc system from purified AdR, Adx, and P450scc. Evidence was given that selected plant oil phytosterols can serve as substrates for the side-chain cleavage P450scc system. Both cholesterol and β-sitosterol caused a type I spectral change typical for sterol substrate-P450 interaction, and β-sitosterol was converted by a reconstituted P450scc system into pregnenolone with approximately 40 % of the rate of cholesterol (Shkumatov and Smettan 1991; Novikova et al. 2009; Shkumatov et al. unpublished). With a phytosterol mixture from the sterol fraction of rape oil distillate (containing 40 % β-sitosterol, 31 % campesterol, 29 % brassicasterol, as well as tocopherol and a fatty acids mixture) as substrate, it was shown that besides cholesterol also β-sitosterol and campesterol (but not brassicasterol) were converted to pregnenolone by the reconstituted P450scc system, confirming that additional ethyl or methyl groups at C24 of a potential substrate do not alter significantly the P450scc substrate specificity. It was also established that brassicasterol (campesterol analogue with Δ23-24 bond) can act as competitive inhibitor of P450scc. Intracellularly produced campesterol was used as substrate by the P450scc expressed in S. cerevisiae (Duport et al. 1998, 2003; Szczebara et al. 2003; cf. Sect. 2.2).
Uptake of Exogenously Added Sterols and Their Intracellular Modifications
Accessible sterols (sitosterol, campesterol, cholesterol) can represent itself substrates for production of steroid hormones. Transgenic microorganisms, which express the mammalian P450scc-dependent sterol side-chain cleavage system, can be used as biocatalysts for one-stage microbiological synthesis of pregnenolone and other pregnanes (C21-steroids, including mineralo- and glucocorticoids). This could be useful to avoid advanced chemical stage(s) for addition of the C20–C21 fragment to 17-carbon of androgens used in the classical technology.
Some principal features of yeasts restrict their potential as the basis of such biocatalysts. These are (1) the inability of wild-type yeast strains, like S. cerevisiae, to uptake exogenously supplied sterols from media under aerobic conditions, the so-called phenomenon of aerobic sterol exclusion (Crowley et al. 1998; Ness et al. 1998; Wilcox et al. 2002; Duport et al. 2003; Szczebara et al. 2003; Raychaudhuri and Prinz 2006), and (2) the formation of steryl esters inside yeast cells of S. cerevisiae or Y. lipolytica (Czabany et al. 2007; Athenstaedt et al. 2006; Beopoulos et al. 2009, 2011). The aerobic sterol exclusion phenomenon has been thoroughly studied with S. cerevisiae in connection with ergosterol biosynthesis (inhibitors, mutants, or gene disruption on squalene synthase or HMG-CoA reductase) to improve uptake of exogenously added sterols, but comparable studies with the strictly aerobic yeast Y. lipolytica are required. The established steryl ester (SE) formation with fatty acids from sterols in yeast endoplasmic reticulum can compete with their conversion by expressed P450scc. Otherwise, SE can be hydrolyzed to maintain intracellular pool of free sterols, which is under strict control. Interestingly, for sterols without Δ7- or Δ22-bond or the 24β-methyl smaller uptake rate and larger SE formation degree were observed (Taylor and Parks 1981). In S. cerevisiae, SE formation is catalyzed by the steryl ester synthase (acyl-CoA sterol acyltransferase) enzymes Are1p and Are2p (ER-located), and their hydrolysis by the steryl ester hydrolases (sterol esterases) Yeh1p, Tgl1p (both in lipid particles), and plasma membrane located Yeh2p (Czabany et al. 2007). Slight differences (YlARE1—ScARE1/ARE2-like, YlARE2—different from ScARE1/ARE2, YlTGL1, YlYEH genes involved in SE metabolism) were found for Y. lipolytica. Interestingly, only small amounts (2–5 %) of SE and mainly triacylglycerols (TAG) are contained in Y. lipolytica, whereas the SE and TAG fractions account each for 50 % of the storage lipids in S. cerevisiae, located mainly in lipid particles (Czabany et al. 2007; Athenstaedt et al. 2006; Beopoulos et al. 2009, 2011). The problems connected with the undesirable 3β-O-acetylation (acetyltransferase Atf2p encoded by ATF2 gene) of sterol-derived steroid products like pregnenolone in the yeast S. cerevisiae were discussed above (Sects. 2.2 and 3.1), although they were obviously not significant in Y. lipolytica due to the absence of Atf2p-like acetyltransferase in this yeast (cf. Sect. 3.5; Faletrov et al. 2008a, b).
4.2 Strain Construction for Coexpression of the Cytochrome P450scc System and Cytochrome P450c17
For testing the functional expression of the cholesterol side-chain cleavage P450scc system, the construction of recombinant Y. lipolytica strains has been performed containing pICL1-controlled expression cassettes with cDNAs for the mature (m) forms of all three individual protein components of this enzyme system, namely, NADPH-adrenodoxin reductase (human mAdR, shortly AdR), adrenodoxin (human mAdx, shortly Ax), and P450scc (mP450scc, CYP11A1, human or bovine, short names Ph or Pb, respectively), or alternatively the fusion protein mAdx-mP450scc (human, shortly AxP). The decision to express mature protein forms of the P450scc system in Y. lipolytica was based on previous studies demonstrating a catalytically active P450scc system when mature forms of P450scc, Adx, and AdR were expressed in S. cerevisiae (Duport et al. 1998, 2003; Szczebara et al. 2003) and overcame serious problems for the expression of mitochondrial localised P450scc in yeasts (cf. Sect. 2.2).
Several new Y. lipolytica expression vectors were constructed on the basis of the multicopy integrative plasmids p64PT and p67PT, which are comparable to p64IP and p67IP (only part of pICL1D to BamHI site in front of the ICL1 intron, ICL1i, contained), described by Juretzek et al. (2001), except they contain the complete pICL1-SphI-ICL1t unit for insertion of a cDNA or gene to be expressed into the SphI site (cf. p67RYl and p67IC17 in Fig. 1 and Sect. 3.2). Respectively, integrative expression vectors of the p64-series (p64AdR, p64AxP, p64Pb, p64Ph) and of the p67-series (p67AdR, p67Ax, p67AxP, p67Pb, p67Ph) for components of the cholesterol side-chain cleavage system (cDNAs for mature forms) have been constructed and used for integrative transformation into selected Y. lipolytica strains (Yovkova 2006; Yovkova et al. 2007; Novikova et al. 2008, 2009; Fig. 6; cf. Fig. 1). To combine expression cassettes for all three components of the P450scc system and P450c17 in one recombinant yeast, integrative multicopy transformants of the haploid Y. lipolytica strains E129L (MATA LEU2 lys11-23 ura3-302 xpr2-322) and E150 (MATB leu2-270 his-1 ura3-302 xpr2-322) were obtained by simultaneous integrative transformation of mixtures of two to four ura3d4-based multicopy expression vectors of the same p64- or p67-series, respectively, flanked by rDNA- or LTR- zeta-sequences after linearisation prior to transformation but containing different expression cassettes, e.g. p64AdR and p64AxP or p67AdR, p67Ax, p67Pb and p67IC17 for strain E150, and p67Ax, p67AxP, p67IC17 or p67AdR, p67Ax, p67Pb and p67IC17 for strain E129L.
By Southern blotting (Fig. 6c), evidence was given for simultaneous integration in total of at least 10–12 copies per haploid genome of up to three different pICL1-controlled expression cassettes containing vectors into one multicopy transformant, such as E129L(p67Ax p67AxP p67IC17), shortly E129L(Ax Axp P17) T2 or T3, representing two strains with three integrated vectors out of 10 transformants tested, which was first time demonstrated for Y. lipolytica. In other E129L transformants of this assay, at least 10–12 copies of one (T4) or two vectors (seven transformants: T1, T5-T15) were integrated (Fig. 6a, c). Other transformants with two integrated vectors, such as E150(AdR AxP) T8 (E150 T8, shortly for E150(p64AdR p64AxP) T8), transformed with two p64-series vectors (p64AdR, p64AxP) and E129L(Ax P17) T10 (E129L T10, shortly for E129L(p67AxP p67IC17) T10), transformed with three p67-series vectors (p67Ax, p67AxP, p67IC17), both used as parental strains for subsequent diploidisation are shown together with E129L(Ax Axp P17) T2 as examples in Fig. 6c.
The copy number ratios of two or three integrated multicopy vectors varied in individual transformants, from rare dominance of one vector (E150 T8) to predominantly nearly equal distribution of two or three vectors (E129L T2 and T10, Fig. 6c). The initially determined total vector copy numbers of these multicopy transformants were from 6 to 32 with a majority from 8 to 13. Therefore, three vectors were found integrated in at least three to four copies each, considering that most probably in total 10–12 vector copies integrated and stabilised due to the ura3d4 multicopy selection marker used, although initially lower and higher copy numbers up to 30–40 were found less frequent with these vector types, and upon further cultivation, it stabilised at 10–12 copies in average (amplification and deamplification, observed by Juretzek et al. 2001).
Thus, individual transformants contained one, two, or three of the vectors p67Ax, p67AxP, and p67IC17 used for E129L transformation, p64AdR and p64AxP used for E150 transformation, and p67AdR, p67Ax, p67Pb, or p67IC17 used for E150 and E129L transformations, respectively (Fig. 6a, c). Although simultaneous integrative transformations of mixtures of up to four vectors (p67AdR, p67Ax, p67Pb, and p67IC17) were also tested, maximally three vectors were found integrated in one transformant up till now.
As evidenced in additional Southern blots, these vectors integrated in multiple copies in tandem (head to tail, dominant) or inverse tandem (head to head, rare) orientation mostly at one site of integration in the genome. Vectors of the same type (p67- or p64-type) with up to three different expression cassettes were found integrated in the same cluster of multiple vector copies as shown in the schematic diagram in Fig. 6b. This simultaneous integration in clusters of up to three expression vectors (each in at least three to four copies per haploid genome) of the p64 or p67 series was first time demonstrated for Y. lipolytica during these studies.
Subsequently, by diploidisation, new recombinant strains of Y. lipolytica were obtained from selected multicopy transformants of the opposite mating types MATA or MATB of the haploid strains E150 and E129L (containing one to three expression vectors), as shown in principle in Fig. 6 for the DE1 to DE6 types of recombinant diploid strains (Yovkova 2006; Yovkova et al. 2007; Novikova et al. 2008, 2009). These diploid strains and the additionally constructed control strains of type DE7 to DE10 contain several combinations of up to four expression cassettes (or up to five different vectors) for the P450scc system and P450c17 under control of the inducible promoter pICL1. On the one hand, diploid strains of types DE1 to DE4 all contain the AxP fusion as the P450scc component (vectors p64AxP, p67AxP), whereas the DE5-, DE6-, DE9-, and DE10-type strains contain single Pb (p67Pb) instead of the AxP fusion (Fig. 6). Otherwise, two series of recombinant diploid strains were constructed containing up to four different expression cassettes (each in at least two to five copies per haploid genome) for several cDNAs encoding either all three protein components of the cholesterol side-chain cleavage P450scc system (AdR, Ax, and Pb in DE6-type or fusion protein AxP in DE2- and DE3-type strains) or cDNAs encoding the three P450scc system components as well as P450c17 for coexpression (DE5 with Pb, or DE1 and DE4 with AxP, respectively, Fig. 6). Additionally, control strains without cDNAs for some of these four proteins (DE7 lacking P450scc—Pb, DE8 containing P450c17 and lacking Pb, DE9 lacking Adx, DE10 lacking AdR) were constructed to test the possible contribution of host-own proteins in the formation of the functional P450scc enzyme system, such as the presence of a functional adrenodoxin reductase-like or adrenodoxin-like proteins in Y. lipolytica.
For selected recombinant diploid Y. lipolytica strains of types DE1 to DE10 the ethanol- or alkane-induced heterologous expression of all three components of the P450scc system (human AdR, Adx, fusion protein AxP or bovine Pb) was demonstrated by Western blotting with anti-AdR, -Adx, or -P450scc antibodies (Agalarov 2008; Novikova et al. 2008). Heterologous protein expression was detected from 6 to 72 h after addition of the pICL1-inducing carbon sources ethanol or hexadecane to glucose-grown cells. These Western blot results were in good agreement with the results concerning the expression cassettes present in these strains shown by Southern blotting (Fig. 6). Additionally, in strains of type DE1, DE4 and DE5 functional coexpression of bovine P450c17 with the human P450scc system proteins was shown under comparable cultivation and ethanol-induction conditions, evidenced by the nearly complete P450c17-catalyzed biotransformation of progesterone into 17α-hydroxyprogesterone during 6–10 h (cf. Fig. 7 for DE13).
4.3 Sterol and Steroid Bioconversion with Recombinant Yarrowia lipolytica Coexpressing AdR, Adx, and Cytochromes P450scc and P450c17
For selected recombinant diploid Y. lipolytica strains of types DE1 to DE10, heterologous expression of the mammalian P450scc system and its coexpression with bovine P450c17 were demonstrated by Western blotting (cf. Sect. 4.2), and functional expression of human AdR, Adx, and the fusion protein AxP as well as of bovine P450scc (Pb) and P450c17 (P17) was tested by steroid or sterol biotransformation studies (Fig. 7). To confirm the functional coexpression of the P450scc system and of P450c17 in recombinant Y. lipolytica DE strains, the bioconversion of cholesterol or β-sitosterol into pregnenolone (P450scc system) and further to 17α-pregnenolone (P450c17) or of progesterone into 17α-hydroxyprogesterone (P450c17) was studied using selected DE1 to DE6-type diploid strains. The diploid strains D13 (containing expression cassettes for AdR, Adx, AxP twice, P17), D31 (AdR, Adx, AxP), D40 (AdR, Adx, AxP, P17), DE51 or DE54 (AdR, Adx, Pb, P17), and DE61(AdR, Adx, Pb) were used (cf. Figs. 6 and 7 for detailed strain descriptions and results with DE13).
Commercially available pure cholesterol or β-sitosterol was used as substrates for P450scc to be transformed into pregnenolone. Heterologous protein expression was induced by adding to glucose-grown Y. lipolytica cells (24-h shaking flask cultivation in YPD with 0.4 % glucose to reach glucose exhaustion, because glucose should be completely consumed at the moment of inducer addition; cf. Sect. 3.5) either 1 % (v/v) of ethanol (Fig. 7) or 1 % hexadecane at 24 h of cultivation (beginning of induction phase) and second time at 30 h of cultivation. Higher glucose content in the YPD preculture (1–2 %) will significantly delay heterologous protein expression. These cultivation and expression conditions were confirmed by Western blotting and tested with strains coexpressing P450c17 (DE13, DE40, DE51, DE54), for which the P450-catalyzed progesterone or pregnenolone biotransformation activity was easily detectable (see the cultivation and biotransformation conditions in Figs. 5 and 7). The bioconversion substrate (50 μM cholesterol, 50 μM β-sitosterol, or up 100 μM progesterone) was added together with the inducing carbon source at 24 h of cultivation. Biotransformation substrate addition made first 24 h after the start of induction (48 h cultivation) was less efficient, as discussed in Sect. 3.5 for progesterone biotransformation.
With ethanol-induced cells of strain DE13 (coexpression of P450c17 and P450scc system, i.e. AxP, Adx, and AdR) a time-dependent formation of small amounts of pregnenolone (up to 8 % in 24 h) and 17α-hydroxypregnenolone (4 %) relatively to faster decreasing quantities of cholesterol was detected (Fig. 7). The P450scc activity of these cells was rather low compared with their high P450c17-catalyzed biotransformation capacity of progesterone into 17α-hydroxyprogesterone (Fig. 7) or of pregnenolone into 17α-hydroxypregnenolone (without formation of 3β-O-acetylated side products, due to the absence of ScATF2 homologues encoded O-acetyltransferase activity in Y. lipolytica) with strain DE54 (Faletrov et al. 2008 b; cf. Sects. 3.5 and 4.1). Thus, under these conditions, strain DE13 was catalysing at least partial biotransformation of cholesterol into pregnenolone, which was subsequently converted to 17α-hydroxypregnenolone by coexpressed P450c17, whereas with β-sitosterol no significant product formation was found. Contrarily, β-sitosterol and campesterol were shown to be converted in vitro by a reconstituted P450scc system to pregnenolone (cf. Sect. 4.1). Further conversion of 17α-hydroxypregnenolone into dihydroepiandrosterone could not be detected, obviously due to the low rate of sterol conversion by P450scc and the very low 17,20-lyase activity of bovine P450c17 expressed in yeast.
The results exemplified for strain DE13 in Fig. 7 provided evidence for efficient biotransformation of progesterone (strains DE13, DE40, DE51) or of pregnenolone (strain DE54, Faletrov et al. 2008a, b) into their 17α-hydroxy products, whereas under these conditions, the bioconversion of cholesterol (or β-sitosterol) to pregnenolone by the same recombinant yeast cells was less efficient. The reasons for the low cholesterol and very low β-sitosterol bioconversion rates of the tested DE-type strains (DE13 and others) are currently under investigation and perspectives for their elimination should be elucidated (as discussed in Sect. 4.1). The low sterol bioconversion abilities of these Y. lipolytica DE strains are probably mainly due to (1) the very low sterol uptake of Y. lipolytica cells under the applied cultivation conditions (possibly due to its potential toxicity and sterols planar structure) versus long-chain n-alkanes serving as very good growth substrates and/or (2) the occurring sterol or product modifications by the yeast cells, i.e. conversion of intracellular pool of the sterol and/or its P450scc product(s) to substances, which are not able to be exported from the cells. Contrarily, under the same culture conditions DE1, DE3, or DE4-type strains are able to perform efficiently P450c17-catalyzed biotransformation of the steroids progesterone and pregnenolone and to excrete the steroid products into the medium.
Interestingly, there is a striking decrease of the cholesterol content not correlating with the observed slow product formation rate (Fig. 7). Additionally, when analysing cell-free supernatant fractions, it was found that about 50 % of cholesterol or β-sitosterol were taken up by the cells and/or absorbed on cell surface 6 h after addition and then stayed practically constant in cases strains exhibited no bioconversion. Whether cholesterol uptake by yeast cells can be stimulated using addition of atorvastatin (known to block partially the in situ ergosterol biosynthesis) or by moderate ultrasound exposure is recently under investigation.
Otherwise, whereas, in contrast to S. cerevisiae, 3-O-acetylation of at least pregnenolone and derivates as undesirable metabolic way may be not significant in Y. lipolytica due to the absence of acetyltransferase activity (no ScATF2 homologues gene) in this yeast, the formation of steryl esters (SE) from sterols and fatty acids by acyl-CoA sterol acyltransferases (YlARE1, YlARE2) and their deposit in lipid particles (Beopoulos et al. 2009, 2011) may contribute to the observed low activity of the expressed P450scc system in the DE strains of Y. lipolytica. This has to be investigated by further studies, including disruption of YlARE1/2 (or application of inhibitors) and overexpression of steryl ester hydrolases (YlTGL1 and YlYEH1/2).
Thus, despite all difficulties in detection of cholesterol or β-sitosterol bioconversion to pregnenolone, which was the expected crucial point of these studies (cf. Sects. 3.1 and 4.1), bioconversion activity of both P450scc and P450c17 enzymes was demonstrated in principle with ethanol-induced cells of the strain DE13 coexpressing AdR, Ax, and AxP as well as P450c17, evidenced by the conversion of cholesterol into pregnenolone and subsequent formation 17α-hydroxypregnenolone. Additionally, these cells of DE13 as well as other strains, like DE40, DE51, or DE54, exhibited a high P450c17-catalyzed biotransformation capacity of progesterone or pregnenolone into 17α-hydroxy products (Fig. 7). These promising results clearly demonstrated first time the coexpression of a functionally active cholesterol side-chain cleavage P450scc system and of P450c17 in Y. lipolytica cells, thus catalysing the coupled bioconversion from cholesterol (and probably also from β-sitosterol) into pregnenolone followed by subsequent 17α-hydroxylation of pregnenolone to 17α-hydroxypregnenolone. Because P450c17 expressed in yeast showed no or very low 17,20-lyase activity towards 17α-hydroxy products (17α-hydroxyprogesterone and 17α-hydroxypregnenolone), formation of dihydroepiandrosterone was not detected.
5 Conclusions
The growing demand for steroid pharmaceutical preparations generates a need for increasing level of their production. Present-day steroid industry couples the chemical and biological approaches taking advantage of the best aspects of each. New technologies of steroid drug synthesis are based on the use of transgenic microorganisms capable of reproducing some processes in steroidogenic organs or tissues of mammals.
Prospects for increasing the productivity of transgenic microorganisms are the genetic manipulations, directed to alteration of intracellular metabolism with the rise of target steroids and decrease of producing unwanted by-products which are result of host-own enzymatic systems functioning («switching off», activation or additional expression of definite genes). Besides, genetic manipulations can be directed to the improvement of expression level and/or activity of mammalian proteins (especially P450 systems) in the microorganisms (e.g. mutagenesis of native cDNAs resulting in more effective transcription or/and translation; modification, substitution, or removal of the inherent N-terminal addressing amino acid presequences, which ensure their targeting into subcellular compartments; construction of new vectors including more effective promoters; increasing of heterologous genes copy number in the host cell; and coexpression of heterologous proteins with their own native partners, involved in certain pathway).
The yields and activity of heterologous P450s can be improved owing to optimisation of cultivation parameters (e.g. the use of cultural media able to provide for necessary level of cofactor synthesis and heme availability in the cell, using small- or large-scale cultivation conditions of recombinant microorganisms, selection of optimal inducers for P450 protein expression). Moreover, studies on structural–functional features of heterologous P450 proteins, which are expressed in different microorganisms (exhibiting distinct peculiarities and advantages), can generate useful information for selection of suitable host organisms.
The productivity of transgenic microorganisms can be improved, also owing to application of various approaches to increase the cell permeability for hydrophobic P450 substrates, because limited solubility of substrates in water (or cultural media) imposes a limit on the extent of steroid hydroxylated. For example, use of organic solvents, two-phase systems, detergents, cyclodextrins, inhibitors of ergosterol synthesis and action of ultrasound are among these approaches (Fernandes et al. 2003; Lu et al. 2007; Manosroi et al. 2008). In this respect promising results were recently obtained for application of two-phase systems for steroid biotransformation with Y. lipolytica strains expressing heterologous P450s (Braun et al. 2012).
The results presented here on heterologous P450 expression in the yeast Y. lipolytica provide evidence the high potential of this yeast as host for biotransformation of hydrophobic substrates. Comparison of functional P450c17 expression in the alkane-utilising yeast Y. lipolytica and in the commonly used yeast S. cerevisiae showed significant advantages of alkane-assimilating yeast cells for P450 catalyze biotransformations of hydrophobic substrates. New integrative multicopy plasmids for Y. lipolytica were used to increase the copy number (at least 8–12) of P450 expression cassettes, to optimise the electron transfer to P450 by coexpression of heterologous or host-own NADPH-P450 reductases (CPR) and to increase the biotransformation capacity of the recombinant “yeast cell factory”. Yarrowia lipolytica strains and conditions were selected with very low side-product content (total <4.5 %) allowing to perform efficient progesterone bioconversion without prior destroying 20α- and 20β-HSDs analogues genes. In contrast to S. cerevisiae, there is no 3-O-acetyltransferase in Y. lipolytica. Multicopy integration vectors and subsequent diploidisation were used for strain construction expressing the three components of the P450scc system (AdR, AdX, P450scc) and of P450c17 in Y. lipolytica. Improvement of bioconversion of cholesterol (and β-sitosterol) to pregnenolone is under investigation.
In a recent study, functional coexpression of human CYP2D6 or CYP3A4 with hCPR in Y. lipolytica was demonstrated, and the potential of these recombinant yeast cells for bioconversion of hardly soluble hydrophobic steroids (testosterone, 17α-testosterone, progesterone) was tested two-liquid biphasic culture systems. Especially organic solvent phases which can be efficiently taken up and metabolised by the cell (like ethyl oleate) enable a more efficient bioconversion as compared to aqueous systems (Braun et al. 2012).
The data described in this review are not comprehensive, but they demonstrate the wide possibilities of yeast for biotransformation of different steroid substrates. The construction of suitable new strains of Y. lipolytica and the study of their biocatalytic potential can open perspectives for creating of new technologies in this field. The results on steroidogenic P450 expression in the yeasts Y. lipolytica and S. cerevisiae could be used in the following directions: (1) elaboration of new biotechnological approaches for synthesis of pharmaceutically active steroid hormones by using the recombinant yeasts Y. lipolytica and S. cerevisiae (impact on technology and on public health; Faletrov et al. 2008b) and (2) application of new recombinant Y. lipolytica and S. cerevisiae yeast cells as test systems for primary screening of potentially active compounds inhibiting the P450c17 enzyme system, which is involved in steroidogenesis and the development of hormonal carcinogenesis (impact on the science in this field and on public health; Shkumatov et al. 2007a–c; Faletrov et al. 2008a, b; Novikova et al. 2009).
Abbreviations
- 3β-HSD:
-
3β-hydroxysteroid dehydrogenase/Δ5,4 isomerase (3β-hydroxy-5-ene steroid dehydrogenase, EC 1.1.1.145)
- AdR:
-
FAD-containing NADPH-adrenodoxin reductase (EC 1.18.1.2)
- Adx or shortly Ax:
-
Adrenodoxin, [2Fe-2S]-ferredoxin from adrenal cortex
- AxP:
-
Fusion of human Adx and P450scc
- C. :
-
Candida
- CPR:
-
NADPH-cytochrome P450-reductase
- DHEA:
-
Dehydroepiandrosterone
- E. :
-
Escherichia
- HSD:
-
Hydroxysteroid dehydrogenase
- P450 or CYP:
-
Cytochrome P450
- P450c11 (CYP11B1, P45011β):
-
Cytochrome P450 11β-hydroxylase
- P450c17 (CYP17A1):
-
Shortly P17 (CYP17, P45017α), cytochrome P450 17α-hydroxylase/17,20-lyase (EC 1.14.99.9)
- P450c18 (CYP11B2):
-
Cytochrome P450 aldosterone synthase
- P450c19 (CYP19, P450arom):
-
Cytochrome P450 aromatase
- P450c21 (CYP21):
-
Cytochrome P450 21-hydroxylase
- P450scc (CYP11A1):
-
Shortly Pb (bovine) or Ph (human), cytochrome P450 cholesterol hydroxylase/20,22-lyase (EC 1.14.15.6)
- S. :
-
Saccharomyces
- Y.:
-
Yarrowia
References
Agalarov JA (2008) Coexpression of the cholesterol hydroxylase/lyase system components in Yarrowia lipolytica cells. Diploma thesis, Biochemistry Department, Biological Faculty, Lomonosov Moscow State University
Ahmad S, Garg SK, Johri BN (1992) Biotransformation of sterols: selective cleavage of the side chain. Biotechnol Adv 10:1–67
Andersen MD, Møller BL (2002) Use of methylotrophic yeast Pichia pastoris for expression of cytochromes P450. Methods Enzymol 357:333–342
Andersen MD, Busk PK, Svendsen I, Møller BL (2000) Cytochromes P-450 from cassava (Manihot esculenta Crantz) catalyzing the first steps in the biosynthesis of the cyanogenic glucosides linamarin and lotaustralin. Cloning, functional expression in Pichia pastoris, and substrate specificity of the isolated recombinant enzymes. J Biol Chem 275:1966–1975
Athenstaedt K, Jolivet P, Boulard C, Zivy M, Negroni L, Nicaud J-M, Chardot T (2006) Lipid particle composition of the yeast Yarrowia lipolytica depends on the carbon source. Proteomics 6:1450–1459
Bankar AV, Kumar AR, Zinjarde SS (2009) Environmental and industrial application of Yarrowia lipolytica. Minireview. Appl Microbiol Biotechnol 84:847–865
Barros MH, Nobrega FG (1999) YAH1 of Saccharomyces cerevisiae: a new essential gene that codes for a protein homologous to human adrenodoxin. Gene 233:197–203
Barth G, Gaillardin C (1996) Yarrowia lipolytica. In: Wolf K (ed) Nonconventional yeasts in biotechnology. Springer, Heidelberg, pp 313–388
Beopoulos A, Cescut J, Haddouche R, Uribelarrea JL, Molina-Jouve C, Nicaud JM (2009) Yarrowia lipolytica as a model for bio-oil production. Prog Lipid Res 48:375–387
Beopoulos A, Nicaud JM, Gaillardin C (2011) An overview of lipid metabolism in yeasts and its impact on biotechnological processes. Appl Microbiol Biotechnol 90:1193–1206
Bernhardt R (2006) Cytochrome P450 as versatile biocatalysts. J Biotechnol 124:128–145
Bourel G, Nicaud J-M, Nthangeni B, Santiago-Gomez P, Belin J-M, Husson F (2004) Fatty acid hydroperoxide lyase of green bell pepper: cloning in Yarrowia lipolytica and biogenesis of volatile aldehydes. Enzyme Microb Technol 35:293–299
Boyle SM, Popp MP, Smith WC, Greenberg RM, James MO (1998) Expression of CYP2L1 in the yeast Pichia pastoris, and determination of catalytic activity with progesterone and testosterone. Mar Environ Res 46:25–28
Braun A, Geier M, Buehler B, Schmid A, Mauersberger S, Glieder A (2012) Steroid biotransformation in biphasic systems with Yarrowia lipolytica expressing human liver cytochrome P450 genes. Microb Cell Fact 11:106. doi:10.1186/1475-2859-11-106
Brocard-Masson C, Dumas B (2006) The fascinating world of steroids: S. cerevisiae as a model organism for the study of hydrocortisone biosynthesis. Biotechnol Genet Eng Rev 22:213–252
Bureik M, Schiffler B, Hiraoka Y, Vogel F, Bernhardt R (2002) Functional expression of human mitochondrial CYP11B2 in fission yeast and identification of a new internal electron transfer protein, etp1. Biochemistry 41:2311–2321
Bureik M, Hübel K, Drăgan CA, Scher J, Becker H, Lenz N, Bernhardt R (2004) Development of test systems for the discovery of selective human aldosterone synthase (CYP11B2) and 11ß-hydroxylase (CYP11B1) inhibitors. Discovery of a new lead compound for the therapy of congestive heart failure, myocardial fibrosis and hypertension. Mol Cell Endocrinol 217:249–254
Cauet G, Degryse E, Ledoux C, Spagnoli R, Achstetter T (1999) Pregnenolone esterification in Saccharomyces cerevisiae. A potential detoxification mechanism. Eur J Biochem 261:317–324
Chang Q, Griest TA, Harter TM, Petrash JM (2007) Functional studies of aldo-keto reductases in Saccharomyces cerevisiae. Biochim Biophys Acta 1773:321–329
Coelho MAZ, Amaral PFF, Belo I (2010) Yarrowia lipolytica: an industrial workhorse. In: Méndez-Vilas A (ed) Current research, technology and education topics in applied microbiology and microbial biotechnology, vol 2. Formatex, Badajoz, pp 930–944
Cornelissen S, Julsing MK, Schmid A, Bühler B (2012) Comparison of microbial hosts and expression systems for mammalian CYP1A1 catalysis. J Ind Microbiol Biotechnol 39:275–287
Crowley JH, Leak FW, Shianna KV, Tove S, Parks LW (1998) A mutation in a purported regulatory gene affects control of sterol uptake in Saccharomyces cerevisiae. J Bacteriol 180:4177–4183
Czabany T, Athenstaedt K, Daum G (2007) Synthesis, storage and degradation of neutral lipids in yeast. Biochim Biophys Acta 1771:299–309
Dietrich M, Grundmann L, Kurr K, Valinotto L, Saussele T, Schmid RD, Lange S (2005) Recombinant production of human microsomal cytochrome P450 2D6 in the methylotrophic yeast Pichia pastoris. Chembiochem 6:2014–2022
Drăgan CA, Zearo S, Hannemann F, Bernhardt R, Bureik M (2005) Efficient conversion of 11-deoxycortisol to cortisol (hydrocortisone) by recombinant fission yeast Schizosaccharomyces pombe. FEMS Yeast Res 5:621–625
Drăgan CA, Hartmann RW, Bureik M (2006) A fission yeast-based test system for the determination of IC50 values of anti-prostate tumor drugs acting on CYP21. J Enzyme Inhib Med Chem 21:547–556
Dujon B, Sherman D, Fischer G et al (2004) Genome evolution in yeasts. Nature 430:35–44
Dumas B, Cauet G, Lacour T, Degryse E, Laruelle L, Ledoux C, Spagnoli R, Achstetter T (1996) 11 beta-hydroxylase activity in recombinant yeast mitochondria. In vivo conversion of 11-deoxycortisol to hydrocortisone. Eur J Biochem 238:495–504
Dumas B, Brocard-Masson C, Assemat-Lebrun K, Achstetter T (2006) Hydrocortisone made in yeast: metabolic engineering turns a unicellular microorganism into a drug-synthesizing factory. Biotechnol J 1:299–307
Duport C, Spagnoli R, Degryse E, Pompon D (1998) Self-sufficient biosynthesis of pregnenolone and progesterone in engineered yeast. Nat Biotechnol 16:186–189
Duport C, Schoepp B, Chatelain E, Spagnoli R, Dumas B, Pompon D (2003) Critical role of the plasma membrane for expression of mammalian mitochondrial side chain cleavage activity in yeast. Eur J Biochem 270:1502–1514
Dutta TK, Datta J, Samanta TB (1993) Onset of new catalytic activity in immobilized spores of Aspergillus ochraceus TS due to in situ germination: C17-C20 lysis accompanies 11 alpha-hydroxylation of steroid. Biochem Biophys Res Commun 192:119–123
Ehmer PB, Bureik M, Bernhardt R, Müller U, Hartmann RW (2002) Development of a test system for inhibitors of human aldosterone synthase (CYP11B2): screening in fission yeast and evaluation of selectivity in V79 cells. J Steroid Biochem Mol Biol 81:173–179
Ewen KM, Schiffler B, Uhlmann-Schiffler H, Bernhardt R, Hannemann F (2008) The endogenous adrenodoxin reductase-like flavoprotein arh1 supports heterologous cytochrome P450-dependent substrate conversions in Schizosaccharomyces pombe. FEMS Yeast Res 8:432–441
Faletrov YV, Frolova NS, Rudaya EV, Radyuk VG, Shkumatov VM (2008a) Inhibition of pregnenolone and related steroids 3β-acetylation in recombinant yeasts Saccharomyces cerevisiae GRF18/YEp5117α by aliphatic alcohols. In: Kilchevskii AV (ed) Molecular and applied genetics: Proceedings, vol 7, Minsk, pp 45–48
Faletrov YV, Frolenkov KA, Novikova LA, Luzikov VN, Mauersberger S, Barth G, Shkumatov VM (2008b) Transgenic yeasts for pharmacological investigations. In: 9th International symposium on cytochrome P450 biodiversity and biotechnology, Nice, France, 08–12 June 2008, Abstracts
Fallon RD, Payne MS, Picataggio SK, Wu S (1999) Transformed yeast strains and their use for the production of monoterminal and diterminal aliphatic carboxylates. WO9904014
Fallon RD, Payne MS, Picataggio SK, Wu S (2004) Transformed yeast strains and their use for the production of monoterminal and diterminal aliphatic carboxylates. US2004146999
Fernandes P, Cruz A, Angelova B, Pinheiro HM, Cabral JMS (2003) Microbial conversion of steroid compounds: recent developments. Enzyme Microb Technol 32:688–705
Fickers P, Benetti PH, Wache Y, Marty A, Mauersberger S, Smit MS, Nicaud JM (2005) Hydrophobic substrate utilisation by the yeast Yarrowia lipolytica, and its potential applications. FEMS Yeast Res 5:527–543
Förster A (2001) Untersuchungen zur heterologen Expression von steroidwandelnden Cytochrom P450 Systemen in der Hefe Yarrowia lipolytica. Diploma thesis, Technische Universität Dresden, Germany
Fukuda R, Ohta A (2013) Utilization of hydrophobic substrates by Yarrowia lipolytica. In: Barth G (ed) Yarrowia lipolytica, vol 1. Springer, Heidelberg
Gerber J (1999) Untersuchungen zur Optimierung des Elektronentransportsystems für die Cytochrom P450 katalysierte Biotransformation von Steroiden in Yarrowia lipolytica. Diploma thesis, Technische Universität Dresden
Gonzalez FJ, Korzekwa KR (1995) Cytochromes P450 expression systems. Annu Rev Pharmacol Toxicol 35:369–390
Hakki T, Zearo S, Drăgan CA, Bureik M, Bernhardt R (2008) Coexpression of redox partners increases the hydrocortisone (cortisol) production efficiency in CYP11B1 expressing fission yeast Schizosaccharomyces pombe. J Biotechnol 133:351–359
Juretzek T (1999) Entwicklung von Wirts-Vektor-Systemen zur heterologen Expression von Proteinen in der nichtkonventionellen Hefe Yarrowia lipolytica und ihre Anwendung für die Cytochrom P450-katalysierte Stoffumwandlung. PhD thesis, Technische Universität Dresden
Juretzek T, Prinz A, Schunck W-H, Barth G, Mauersberger S (1997) Expressionskassetten zur heterologen Expression von Proteinen in der Hefe Yarrowia lipolytica unter Kontrolle des regulierbaren Promotors der Isocitratlyase. Patent DE19525282
Juretzek T, Mauersberger S, Schunck W-H, Nicaud J-M, Barth G (1999) Functional expression of heterologous cytochrome P450 in the yeast Yarrowia lipolytica and its use in biotransformation of steroids. Curr Genet 35(3–4):307
Juretzek T, Wang H-J, Nicaud J-M, Mauersberger S, Barth G (2000a) Comparison of promoters suitable for regulated overexpression of heterologous genes in the alkane-utilizing yeast Yarrowia lipolytica. Biotechnol Bioprocess Eng 5:320–326
Juretzek T, Mauersberger S, Barth G (2000b) Recombinant haploid or diploid Yarrowia lipolytica cells for the functional heterologous expression of cytochrome P450 systems. Patent WO0003008, DE19932811
Juretzek T, Le Dall MT, Mauersberger S, Gaillardin C, Barth G, Nicaud J-M (2001) Vectors for gene expression and amplification in the yeast Yarrowia lipolytica. Yeast 18:97–113
Katsumata T, Hasegawa A, Fujiwara T, Komatsu T, Notomi M, Abe H, Natsume M, Kawaide H (2008) Arabidopsis CYP85A2 catalyzes lactonization reactions in the biosynthesis of 2-deoxy-7-oxalactone brassinosteroids. Biosci Biotechnol Biochem 72:2110–2117
Ko KW, Lin F, Katsumata T, Sugai Y, Miyazaki S, Kawaide H, Okada K, Nojiri H, Yamane H (2008) Functional identification of a rice ent-kaurene oxidase, OsKO2, using the Pichia pastoris expression system. Biosci Biotechnol Biochem 72:3285–3288
Kolar NW, Swart AC, Mason JI, Swart P (2007) Functional expression and characterisation of human cytochrome P45017α in Pichia pastoris. J Biotechnol 129:635–644
Kovganko NV, Kashkan ZN, Shkumatov VM (2001) Simple synthesis of 17α,20β-dihydroxypregn-4-en-3-one. Chem Nat Comp 37:55–56
Lieberman S, Warne PA (2001) 17-Hydroxylase: an evaluation of the present view of its catalytic role in steroidogenesis. J Steroid Biochem Mol Biol 78:299–312
Lu W, Du L, Wang M, Guo Y, Lu F, Sun B, Wen J, Jia X (2007) A novel substrate addition method in the 11ß-hydroxylation of steroids by Curvularia lunata. Food Bioprod Process 85:63–72
Manosroi J, Saowakhon S, Manosroi A (2008) Enhancement of 17α-hydroxyprogesterone production from progesterone by biotransformation using hydroxypropyl-β-cyclodextrin complexation technique. J Steroid Biochem Mol Biol 112:201–204
Masuda Y, Park SM, Ohkuma M, Ohta A, Takagi M (1994) Expression of an endogenous and a heterologous gene in Candida maltosa by using a promoter of a newly-isolated phosphoglycerate kinase (PGK) gene. Curr Genet 25:412–417
Matsuzaki F, Wariishi H (2005) Molecular characterization of cytochrome P450 catalyzing hydroxylation of benzoates from the white-rot fungus Phanerochaete chrysosporium. Biochem Biophys Res Commun 334:1184–1190
Mauersberger S (2013) Cytochrom P450 of the alkane-utilizing yeast Yarrowia lipolytica. In: Barth G (ed) Yarrowia lipolytica. Springer, Heidelberg
Mauersberger S, Kärgel E, Matyashova RN, Müller H-G (1987) Subcellular organization of alkane oxidation in Candida maltosa. J Basic Microbiol 27:565–582
Mauersberger S, Ohkuma M, Schunck W-H, Takagi M (1996) Chapter 12. Candida maltosa. In: Wolf K (ed) Non-conventional yeasts in biotechnology. A handbook. Springer, Berlin, pp 411–580
Mauersberger S, Wang H-J, Gaillardin C, Barth G, Nicaud J-M (2001) Insertional mutagenesis in the n-alkane-assimilating yeast Yarrowia lipolytica: generation of tagged mutants in genes involved in hydrophobic substrates utilization. J Bacteriol 183:5102–5109
Mauersberger S, Förster A, Gerber J, Juretzek T, Barth G (2002) Functional expression of heterologous cytochrome P450 in Yarrowia lipolytica and its use in biotransformation of steroids. In: Third Yarrowia lipolytica international meeting (TYLIM2002), Dresden, Germany, 17–20 July 2002, Abstracts p 114 (Poster 41)
McCormick SP, Alexander NJ, Proctor RH (2006) Heterologous expression of two trichothecene P450 genes in Fusarium verticillioides. Can J Microbiol 52:220–226
Menke HH, Nan LM, Selten GCM, Smaal EB, Slijkhuis H (1990) Cloning and co-expression of bovine adrenal cytochrome P450scc and adrenodoxin in Kluyveromyces lactis. In: 20th FEBS-Meeting, Budapest, Hungary, 19–24 August 1990, program p 215, poster P-TH-381
Menzel R, Vogel F, Kärgel E, Schunck W-H (1997) Inducible membranes in yeast: relation to the unfolded-protein-response pathway. Yeast 13:1211–1229
Minenko AN, Novikova LA, Luzikov VN, Kovaleva IE (2008) Import of hybrid forms of CYP11A1 into yeast mitochondria. Biochim Biophys Acta 1780:1121–1130
Ness F, Achstetter T, Duport C, Karst F, Spagnoli R, Degryse E (1998) Sterol uptake in Saccharomyces cerevisiae heme auxotrophic mutants is affected by ergosterol and oleate but not by palmitoleate or by sterol esterification. J Bacteriol 180:1913–1919
Novikova LA, Yovkova V, Faletrov YV, Agalarov JA, Luzikov VN, Shkumatov VM, Barth G, Mauersberger S (2008) Functional expression of the cholesterol side-chain cleavage cytochrome P450scc system and of P450c17 in the yeast Yarrowia lipolytica. In: 9th International symposium on cytochrome P450 biodiversity and biotechnology, Nice, France, 08–12 June 2008, Abstracts p 68
Novikova LA, Faletrov YV, Kovaleva IE, Mauersberger S, Luzikov VN, Shkumatov VM (2009) From structure and functions of steroidogenic enzymes to new technologies of gene engineering. Review. Biochemistry (Moscow) 74:1482–1504
Nthangeni MB, Urban P, Pompon D, Smit M, Nicaud J-M (2004) The use Yarrowia lipolytica for the expression of human cytochrome P450 CYP1A1. Yeast 21:583–592
Oeda K, Sakaki T, Ohkawa H (1985) Expression of rat liver cytochrome P-450MC cDNA in Saccharomyces cerevisiae. DNA 4:203–210
Ohkuma M, Kobayashi K, Kawai S, Hwang CW, Ohta A, Takagi M (1995a) Identification of a centromeric activity in the autonomously replicating TRA region allows improvement of the host-vector system of Candida maltosa. Mol Gen Genet 249:447–455
Ohkuma M, Park S-M, Zimmer T, Menzel R, Vogel F, Schunck W-H, Ohta A, Takagi M (1995b) Proliferation of intracellular membrane structures upon homologous overproduction of cytochrome P-450 in Candida maltosa. Biochim Biophys Acta (Biomembranes) 1236:163–169
Ortiz de Montellano PR (ed) (2005) Cytochrome P450: structure, mechanism and biochemistry, 3rd edn. Plenum, New York
Pajic T, Vitas M, Zigon D, Pavko A, Kelly S, Komel R (1999) Biotransformation of steroids by the fission yeast Schizosaccharomyces pombe. Yeast 15:639–645
Park SM, Ohkuma M, Masuda Y, Ohta A, Takagi M (1997) Galactose-inducible expression systems in Candida maltosa using promoters of newly-isolated GAL1 and GAL10 genes. Yeast 13:21–29
Peters FT, Drăgan CA, Wilde DR, Meyer MR, Zapp J, Bureik M, Maurer HH (2007) Biotechnological synthesis of drug metabolites using human cytochrome P450 2D6 heterologously expressed in fission yeast exemplified for the designer drug metabolite 4′-hydroxymethyl-alpha-pyrrolidinobutyrophenone. Biochem Pharmacol 74:511–520
Peters FT, Drăgan CA, Schwaninger AE, Sauer C, Zapp J, Bureik M, Maurer HH (2009a) Use of fission yeast heterologously expressing human cytochrome P450 2B6 in biotechnological synthesis of the designer drug metabolite N-(1-phenylcyclohexyl)-2-hydroxyethanamine. Forensic Sci Int 184:69–73
Peters FT, Drăgan CA, Kauffels A, Schwaninger AE, Zapp J, Bureik M, Maurer HH (2009b) Biotechnological synthesis of the designer drug metabolite. 4′-hydroxymethyl-alpha-pyrrolidinohexanophenone in fission yeast heterologously expressing human cytochrome P450 2D6 – a versatile alternative to multistep chemical synthesis. J Anal Toxicol 33:190–197
Picataggio S, Rohrer T, Deanda K, Lanning D, Reynolds R, Mielenz J, Eirich LD (1992) Metabolic engineering of Candida tropicalis for the production of long-chain dicarboxylic acids. Biotechnology (NY) 10:894–898
Pikuleva IA (2006) Cytochrome P450s and cholesterol homeostasis. Pharmacol Ther 112:761–773
Pompon D, Louerat B, Bronine A, Urban P (1996) Yeast expression of animal and plant P450s in optimized redox environments. Methods Enzymol 272:51–64
Pompon D, Gautier JC, Perret A, Truan G, Urban P (1997) Simulation of human xenobiotic metabolism in microorganisms. Yeast a good compromise between E. coli and human cells. Review. J Hepatol 26(Suppl 2):81–85
Prinz A (1995) Testung des ICL1-Promoters zur funktionellen heterologen Expression von Cytochrom P450 in der Hefe Yarrowia lipolytica. Universität Gesamthochschule Kassel, Fachbereich 19 (Biologie/Chemie)
Raychaudhuri S, Prinz WA (2006) Uptake and trafficking of exogenous sterols in Saccharomyces cerevisiae. Biochem Soc Trans 34:359–362
Renaud JP, Peyronneau MA, Urban P, Truan G, Cullin C, Pompon D, Beaune P, Mansuy D (1993) Recombinant yeast in drug metabolism. Review. Toxicology 82:39–52
Rudaya EV (2007) Catalytic efficiency and substrate specificity of recombinant yeast expressing cytochrome Р450с17. PhD Thesis, September 2007, Byelorussian State University
Rudaya EV, Frolova NS, Mauersberger S, Shkumatov VM (2006) Catalytic efficiency of progesterone biotransformation by recombinant Yarrowia lipolytica yeasts. In: International conference on molecular, membrane and basic cellular principles of functions of biosystems (VII. congress of BOOFiB = BSSPhB), 21–23 June 2006, Minsk, Belarus, Poster I, pp 129–131
Sakaki T, Inouye K (2000) Practical application of mammalian cytochrome P450. Review. J Biosci Bioeng 90:583–590
Sakaki T, Shibata M, Yabusaki Y, Murakami H, Ohkawa H (1989) Expression of bovine cytochrome P450c17 cDNA in Saccharomyces cerevisiae. DNA 8:409–418
Sakaki T, Shibata M, Yabusaki Y, Murakami H, Ohkawa H (1990) Expression of bovine cytochrome P450c21 and its fused enzymes with yeast NADPH-cytochrome P450 reductase in Saccharomyces cerevisiae. DNA Cell Biol 9:603–614
Sakaki T, Akiyoshi-Shibata M, Yabusaki Y, Manabe K, Murakami H, Ohkawa H (1991) Progesterone metabolism in recombinant yeast simultaneously expressing bovine cytochromes P450c17 (CYP17A1) and P450c21 (CYP21B1) and yeast NADPH-P450 oxidoreductase. Pharmacogenetics 1:86–93
Sakaki T, Kominami S, Hayashi K, Akiyoshi-Shibata M, Yabusaki Y (1996) Molecular engineering study on electron transfer from NADPH-P450 reductase to rat mitochondrial P450c27 in yeast microsomes. J Biol Chem 271:2609–26213
Sandig G, Kärgel E, Menzel R, Vogel F, Zimmer T, Schunck WH (1999) Regulation of endoplasmic reticulum biogenesis in response to cytochrome P450 overproduction. Drug Metab Rev 31:393–410
Santiago-Gómez MP, Vergely C, Policar C, Nicaud J-M, Belin J-M, Rochette L, Husson F (2007) Characterization of purified green bell pepper hydroperoxide lyase expressed by Yarrowia lipolytica: radicals detection during catalysis. Enzyme Microb Technol 41:13–18
Schiffler B, Bureik M, Reinle W, Müller EC, Hannemann F, Bernhardt R (2004) The adrenodoxin-like ferredoxin of Schizosaccharomyces pombe mitochondria. J Inorg Biochem 98:1229–1237
Schunck W-H, Mauersberger S, Huth J, Riege P, Müller H-G (1987a) Function and regulation of cytochrome P-450 in alkane-assimilating yeast I. Selective inhibition with carbon monoxide in growing cells. Arch Microbiol 147:240–244
Schunck W-H, Mauersberger S, Kärgel E, Huth J, Müller H-G (1987b) Function and regulation of cytochrome P-450 in alkane-assimilating yeast II. Effect of oxygen-limitation. Arch Microbiol 147:245–248
Schunck W-H, Vogel F, Gross B, Kärgel E, Mauersberger S, Köpke K, Gengnagel C, Müller H-G (1991) Comparison of two cytochromes P-450 from Candida maltosa: primary structures, substrate specificities, and effects of their expression in Saccharomyces cerevisiae on the proliferation of the endoplasmic reticulum. Eur J Cell Biol 55:336–345
Sedlaczek L (1988) Biotransformation of steroids. Crit Rev Biotechnol 7:187–236
Shiningavamwe A, Obiero G, Albertyn J, Nicaud J-M, Smit M (2006) Heterologous expression of the benzoate para-hydroxylase encoding gene (CYP53B1) from Rhodotorula minuta by Yarrowia lipolytica. Appl Microbiol Biotechnol 72:323–329
Shkumatov VM, Smettan G (1991) Heterologous reconstruction of monooxygenase systems: “Substrate specific cytochrome P-450-microorganism” (Russian). In: Finogenova TV, Sharyshev AA (eds) Alkane metabolism and oversynthesis of metabolites by microorganisms. Center for Biological Research, USSR Academy of Sciences, Pushchino, Russia, pp 78–86
Shkumatov VM, Radyuk VG, Usova EV, Schunck W-H, Mauersberger S (1998) Transgenic strains of microorganisms in the biosynthesis of steroids (Russian). In: Sviridov VV (ed) Chemical problems in the creation of new materials and technologies. Minsk University Press, Minsk, pp 559–566
Shkumatov VM, Usova EV, Poljakov YS, Frolova NS, Radyuk VG, Mauersberger S, Chernogolov AA, Honeck H, Schunck W-H (2002) Biotransformation of steroids by a recombinant yeast strain expressing bovine cytochrome P-45017α. Biochemistry (Moscow) 67:456–467
Shkumatov VM, Usova EV, Radyuk VG, Kashkan ZN, Kovganko NV, Juretzek T, Mauersberger S (2003) Oxidation of 17α,20ß- and 17α,20α-dihydroxypregn-4-en-3-ones, side products of progesterone biotransformation with recombinant microorganisms, expressing cytochrome P-45017α. Russ J Bioorgan Chem 29:581–587
Shkumatov VM, Frolova NS, Rudaya EV, Faletrov YV, Mauersberger S, Barth G (2006) Range of substrates and steroid bioconversion reactions performed by recombinant microorganisms Saccharomyces cerevisiae and Yarrowia lipolytica expressing cytochrome P450c17. Appl Biochem Microbiol (Moscow) 42:472–478
Shkumatov VM, Falertov YV, Mauersberger S, Barth G, Novikova LA, Luzikov VN (2007a) Recombinant microorganisms for the synthesis of steroids and testing of medicines. EMBO Workshop The chemistry and biochemistry of catalysis by biological systems, 20–22 June 2007, EMBL Hamburg, Abstracts p 20 (invited oral presentation) and p46
Shkumatov VM, Faletrov YV, Mauersberger S, Barth G, Novikova LA, Luzikov VN (2007b) New approaches to the synthesis of steroids and testing of medical compounds. In: XVIIIth Mendeleev congress on general and applied chemistry, 23–28 Sept 2007, Moscow, Russia (invited oral presentation)
Shkumatov VM, Usova EV, Frolova NS, Barth G, Mauersberger S (2007c) Effect of steroid biosynthesis modifiers on the progesterone biotransformation by recombinant yeasts expressing cytochrome P450c17. Biochem (Moscow) Suppl Ser B Biomed Chem 1:87–94
Slijkhuis H, Selten GCM Smaal EB (1989) Process for the biochemical oxidation of steroids and genetically engineered cells used therefor. Patent EP0340878
Slijkhuis H, Smaal EB, Selten GCM (1990–2004) Process for multiple oxidation of steroids and genetically engineered cells used therein. Patent series NL88202080, EP0360361, WO8910963, WO9003428, US20040067579
Storbeck KH, Kolar NW, Stander M, Swart AC, Prevoo D, Swart P (2008) The development of an ultra performance liquid chromatography-coupled atmospheric pressure chemical ionization mass spectrometry assay for seven adrenal steroids. Anal Biochem 372:11–20
Szczebara FM, Chandelier C, Villeret C, Masurel A, Bourot S, Duport C, Blanchard S, Groisillier A, Testet E, Costaglioli P, Cauet G, Degryse E, Balbuena D, Winter J, Achstetter T, Spagnoli R, Pompon D, Dumas B (2003) Total biosynthesis of hydrocortisone from a simple carbon source in yeast. Nat Biotechnol 21:143–149
Taylor FR, Parks LW (1981) An assessment of the specificity of sterol uptake and esterification in Saccharomyces cerevisiae. J Biol Chem 256:13048–13054
Theron CW (2007) Heterologous expression of cytochrome P450 monooxygenases by the yeast Yarrowia lipolytica. M.S. thesis, University of the Free State, Bloemfontein, South Africa
Thevenieau (2006) Ingenierie metabolique de la levure Yarrowia lipolytica pour la production d’acides dicarboxyliques a partier d’huiles vegetales. PhD thesis, INRA, Paris-Grignon
Thevenieau F, Nicaud J-M, Gaillardin C (2009) Chapter 26. Applications of the non-conventional yeast Yarrowia lipolytica. In: Satyanarayana T, Kunze G (eds) Yeast biotechnology: diversity and applications. Springer, Dordrecht, pp 589–613. doi:10.1007/978-1-4020-8292-4_26
Thevenieau F, Beopoulos F, Desfougeres T, Sabirova J, Albertin K, Zinjarde S, Nicaud J-M (2010) Uptake and assimilation of hydrophobic substrates by the oleaginous yeast Yarrowia lipolytica. In: Timmis KN (ed) Handbook of hydrocarbon and lipid microbiology, vol 2, part 7, chap 48. Springer, Berlin, pp 1513–1527
Trant JM (1996) Functional expression of recombinant spiny dogfish shark (Squalus acanthias) cytochrome P450c17 (17α-hydroxylase/C17,20-lyase) in yeast (Pichia pastoris). Arch Biochem Biophys 326:8–14
Urban P, Truan G, Bellamine A, Laine R, Gautier JC, Pompon D (1994) Engineered yeasts simulating P450-dependent metabolisms: tricks, myths and reality. Review. Drug Metabol Drug Interact 11:169–200
Vadali RV, Horton CE, Rudolph FB, Bennett GN, San KY (2004) Production of isoamyl acetate in ackA-pta and/or ldh mutants of Escherichia coli with overexpression of yeast ATF2. Appl Microbiol Biotechnol 63:698–704
Vico P, Cauet G, Rose K, Lathe R, Degryse E (2002) Dehydroepiandrosterone (DHEA) metabolism in Saccharomyces cerevisiae expressing mammalian steroid hydroxylase CYP7B: Ayr1p and Fox2p display 17β-hydroxysteroid dehydrogenase activity. Yeast 19:873–886
Wilcox LJ, Balderes DA, Wharton B, Tinkelenberg AH, Rao G, Sturley SL (2002) Transcriptional profiling identifies two members of the ATP-binding cassette transporter superfamily required for sterol uptake in yeast. J Biol Chem 277:32466–32472
Wilson CR, Craft DL, Eirich LD, Eshoo M, Maduri KK, Cornett CA, Brenner AA, Tang M, Loper JC, Gleeson M (2001) Cytochrome P450 monooxygenase and NADPH cytochrome P450 oxidoreductase genes and proteins related to the omega-hydroxylase complex of Candida tropicalis and methods relating thereto. Patent US6331420
Wright R (1993) Insights from inducible membranes. Curr Biol 3:870–873
Wu DA, Hu MC, Chung BC (1991) Expression and functional study of wild-type and mutant human cytochrome P450c21 in Saccharomyces cerevisiae. DNA Cell Biol 10:201–209
Yamazaki S, Sato K, Suhara K, Sakaguchi M, Mihara K, Omura T (1993) Importance of the proline-rich region following signal-anchor sequence in the formation of correct conformation of microsomal cytochrome P-450s. J Biochem 114:652–657
Yasumori T (1997) Expression of a human cytochrome P450 in Schizosaccharomyces pombe: comparison with the expression in Saccharomyces cerevisiae. In: Giga-Hama Y, Kumagai H (eds) Foreign gene expression in fission yeast Schizosaccharomyces pombe. Springer, Berlin, pp 111–121
Yasumori T, Chen LS, Li QH, Ueda M, Tsuzuki T, Goldstein JA, Kato R, Yamazoe Y (1999) Human CYP2C-mediated stereoselective phenytoin hydroxylation in Japanese: difference in chiral preference of CYP2C9 and CYP2C19. Biochem Pharmacol 57:1297–1303
Yovkova V (2006) Construction of recombinant Yarrowia lipolytica strains for the heterologous expression of the cholesterol-site chain cleaving cytochrome P450 system (German). Diploma Thesis, May 2006, Institute of Microbiology, Dresden University of Technology
Yovkova V, Novikova LA, Luzikov VN, Barth G, Mauersberger S (2007) Expression of the cholesterol side-chain cleavage cytochrome P450scc system in Yarrowia lipolytica. In: IV International symposium “intracellular protein catabolism”, VAAM (Association for General and Applied Microbiology) Annual meeting, Osnabrueck, Germany, 1–4 April 2007, Abstracts PB078, pp 103–104, Biospektrum Sonderausgabe Tagungsband 2007
Zimmer T, Schunck W-H (1995) A deviation from the universal genetic code in Candida maltosa and consequences for heterologous expression of cytochromes P450 52A4 and 52A5 in Saccharomyces cerevisiae. Yeast 11:33–41
Zöllner A, Drăgan CA, Pistorius D, Müller R, Bode HB, Peters FT, Maurer HH, Bureik M (2009) Human CYP4Z1 catalyzes the in-chain hydroxylation of lauric acid and myristic acid. Biol Chem 390:313–317
Zöllner A, Parr MK, Drăgan CA, Dräs S, Schlörer N, Peters FT, Maurer HH, Schänzer W, Bureik M (2010) CYP21-catalyzed production of the long-term urinary metandienone metabolite 17ß-hydroxymethyl-17α-methyl-18-norandrosta-1,4,13-trien-3-one: a contribution to the fight against doping. Biol Chem 391:119–127
Acknowledgements
Parts of this work were supported by the Russian Foundation for Basic Research (projects 05-04-48049a and 08-04-00842a), the Belorussian Foundation for Basic Research (project BO4MC-030), the Bundesministerium für Bildung und Forschung of Germany (BMBF, former Bundesministerium für Forschung und Technologie, BMFT, projects 0310257 and 0310654), and the INTAS project CA 03-51-4366. The authors would like to thank the PhD and diploma students which have been involved in theses studies (since the middle of the 1990s). We thank Wolf-Hagen Schunck (Berlin) for his support and fruitful discussions during the first steps of this research, and we gratefully acknowledge the permanent interest and support of the late (deceased) Valentin N. Luzikov (Moscow), Jean-Marc Nicaud (Thiverval-Grignon) and of Gerold Barth (Dresden) to this research.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2013 Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
Mauersberger, S., Novikova, L.A., Shkumatov, V.M. (2013). Cytochrome P450 Expression in Yarrowia lipolytica and Its Use in Steroid Biotransformation. In: Barth, G. (eds) Yarrowia lipolytica. Microbiology Monographs, vol 25. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-642-38583-4_7
Download citation
DOI: https://doi.org/10.1007/978-3-642-38583-4_7
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-642-38582-7
Online ISBN: 978-3-642-38583-4
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)