Abstract
Food safety is one of the major issues related to fresh fruit and vegetables. Microbial growth is one of the most important causes of postharvest fruit losses, being fungi the main causal agent associated with the postharvest diseases. The preservation of the ‘freshness’ quality of these products is relevant due to their economical impact. As an alternative to synthetic preservatives, natural antimicrobial agents have attracted the attention of modern consumers and the fresh produce industry. Particularly, natural antimicrobials based on plant essential oils are gaining support. This chapter is a comprehensive review of the use of essential oils from different sources and their constituents on the control of postharvest fungal decay and overall quality preservation of fresh fruit and vegetables. Emphasis has been on the sources of essential oils and their constituents studied up to now, and their effects on controlling postharvest fungal decay, either in vitro or in vivo, and their effect on overall quality and storage life of fresh commodities.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Introduction
Fruit and vegetables are important components of the human diet and their consumption is essential in healthy diets, preventing a wide number of chronic diseases (Wiley 2000). Fresh fruits and vegetables are highly perishable products as a cause of their intrinsic characteristics. Microbial growth, sensorial attributes decay and loss of nutrients are amongst the major causes that compromise quality and safety of fresh produce (Ayala-Zavala et al. 2008a, b). In many cases, decay of commodities is only apparent at the latest steps of the handling process. This latent damage can be the result of physical impact, stress injury or quiescent infections by fungi started at the preharvest period. Chemical synthetic additives can reduce decay rate, but consumers are concerned about chemical residues in the product, which could affect their health and cause environmental pollution (White and McFadden 2008), thereby giving rise to the need of developing alternative methods for controlling fresh fruit and vegetable decay. A new worldwide trend to explore alternatives that control postharvest diseases, giving priority to decay-preventing methods with a minimal impact on human health and environment (Bautista-Banos et al. 2006) has emerged. Indeed, the recent exploitation of natural products to control biological spoilage and extend the storage life of perishables has received more and more attention (Tripathi and Dubey 2004). Many fungi that can be the cause of food decay can be inhibited using natural compounds (Fisher and Phillips 2008). Among these, several essential oils, alcohols, organic acids and aromatic compounds have resulted to be biologically active against microbial growth. Particularly, natural antimicrobials based on plant essential oils are gaining support (Isman 2000). These natural compounds are generally recognised as safe (GRAS) for environment and human health, so interest in their use in the goal for sustainable agriculture has increased and a lot of research has been done (Ayala-Zavala et al. 2008c) proving, in many cases, that plant-essential oils and extracts have a role as food preservatives (Hammer et al. 2001). The main reason for promoting the application of natural products in fresh fruits and vegetables is the consumer’s demand for natural and/or organic methods to preserve foods. There is an increasing portion of consumers choosing convenient and ready-to-use fruits and vegetables with a fresh-like quality, containing only natural ingredients (Roller and Lusengo 1997). In addition, consumers have become more health-conscious regarding safety aspects concerning the handling of fruit and vegetables. Therefore, not only the ‘external aspect’ but also the absence of hazardous substances has become imperious issues for buyers (Drzyzga 2003; Harker et al. 2003; Tripathi and Dubey 2004). Different studies have been focused on improving the efficiency of natural compounds as emerging technologies to preserve fresh fruits safety and quality (Ayala-Zavala et al. 2008a, c, d). However, regulatory actions on the use of natural alternative additives are still being analysed. Demands from increasingly mistrustful consumers have led to numerous legislation reviews, which are expected to result in well-planned laws regarding regulations on natural food additives. The main objective of this chapter is to compile the knowledge concerning the use of essential oils in the postharvest fungal decay control of both fruit and vegetables, their efficiency and safety, and to address critical issues requiring further study. In approaching the subject, related issues, such as food quality and antioxidant properties of essential oils were included in this review.
2 Fungal Decay as a Major Problem During the Postharvest of Fruit and Vegetables
Harvested fresh produce exhibits two lines of defence: a physical (skin or peel) and a chemical barrier (proteins, cell walls modifications, organic acids, phenols and phytoalexins) against microbial growth (Uritani 1999; Wisniewski et al. 2003). Only a small fraction of bacterial and fungal decay agents can enter the tissue either by their natural openings (e.g. stomata, lenticels) or by direct penetration of the intact cuticle (Wiley 2000). Natural fresh produce defences against microorganisms can be weakened and compromised by stress injury and/or physiological disorders arising from either preharvest and/or handling factors. This is because injuries and disorders cause disruption of tissues that compromises the integrity of commodities, providing favourable conditions for the invasion of decay agents (Batu 2003). Postharvest processing operations include unitary units such as peeling, cutting, shredding or slicing greatly increase tissue damage of fresh-cut fruits. These may result in several biochemical deteriorations such as browning, flavours, loss of texture as well as detriment of nutritional value and microbial quality of the products. Increases in microbial populations on minimally processed products are associated with damaged tissues and broken cells, as microbial growth is much greater on fresh-cut products than intact product. Cell disruption leads to the release and intermixing of enzymes and substrates that may be used by native or exogenous microorganisms to grow on the product.
Fungi, the collective term for a rather large group of related eukaryotic organisms, are the most important group of postharvest decay causal agents, leading to loss of quality of fresh commodities and economic loss in the postharvest period. Moulds are probably the most well known and have the greatest impact on the postharvest quality of fruit and vegetables. Their growth in food crops are also responsible for off-flavour formation which lead to quality losses (Nielsen and Rios 2000). Fungi, besides being responsible for biological spoilage of fresh produce, can also cause foodborne illnesses. In fact, many fungi responsible for the decay of horticultural commodities, besides breaking the natural barriers against other microorganisms, such as bacteria and other human pathogens, can also produce toxic metabolites in the affected sites, named mycotoxins (Tournas 2005). These highly toxic compounds produced by fungi in the genera Aspergillus, Penicillium, Alternaria and Fusarium are mainly present in pome and stone fruit, and are often carcinogenic and teratogenic. The severity of the toxicity depends on the type of mycotoxin in question, the respective dose and the person consuming it (Tournas 2005).
In general, for each species of fruit or vegetables, it is possible to find a wide range of diseases caused by different agents. Regarding diseases in fruit and vegetables caused by fungi, these are mostly microscopic and include species from all classes: lower fungi (or Phycomycetes), Ascomycetes, Basidiomycetes and Deuteromycetes (Kushalappa and Zulfiqar 2001). Spores and vegetative cells of yeasts and moulds are abundant in the atmosphere and on the surface of fruits and vegetables as they approach maturity in the field. In general, fungi are very sensitive to low temperature and most of them are not able to penetrate the surface of the host. However, once they gain entry through wounds or natural openings, they may cause extensive rotting of mature produce (Dennis 1987). Indeed, they are capable of secreting pectic enzymes that cause the maceration of tissues, producing highly severe soft rots (Kushalappa and Zulfiqar 2001).
It is important to note, that all interactions between the commodity and the respective pathogenic agent are largely influenced by environmental factors, notably temperature, pH, water activity, nutrients availability, atmosphere composition, competition imposed by other organisms and presence of antimicrobial compounds (Kushalappa and Zulfiqar 2001). In particular, concerning abiotic factors, there is an optimum level for each specific microorganism at which it can find the appropriate conditions to grow and reproduce extensively. At these optimal conditions, decay agents cause great infections and have a large impact on the food quality. One should also have in mind that the intrinsic conditions of commodities also change widely during ripening, with the decay microflora changing accordingly, starting with fungal infections and followed by bacterial ones, or vice versa. For instance, horticultural produce pH is a major factor in the prevalence of fungal caused diseases in fruit, due to their higher tolerance to acidic environments. On the other hand, vegetables are more susceptible to bacteria, due to their higher pH. Furthermore, moulds are able to neutralise mildly acidic foods, which can lead to safety problems because acidity is often relied on to prevent the growth of decay or pathogenic bacteria (Wade and Beuchat 2003).
Several postharvest treatments have been developed to preserve the quality of fresh produce, including ultraviolet light, controlled and modified atmospheres, edible coatings, heat treatments and natural compounds, among others. Most postharvest treatments involve the alteration of the natural conditions of the fruit in order to prolong its postharvest life. For example, high O2 atmospheres and irradiation cause damage to some vital molecules of food deteriorative microorganisms, in addition to altering some biochemical processes in the fruit (Charles et al. 2009); heat treatments affect a wide range of fruit ripening processes such as ethylene synthesis, respiration, softening and cell-wall metabolism (Zhang et al. 2009). Gas composition in storage atmospheres might also be more or less favourable to disease spreading, either by fungi or bacteria, although a general trend is not possible to define (Jacques and Morris 1995; DeEll et al. 2003; Gomez and Artes 2004).
To manage postharvest losses caused by fungi, producers usually rely on a release of chemical fungicides (group of benzimidazoles, aromatic hydrocarbons). Currently, there is a strong debate about the safety aspects of chemical preservatives since they are considered responsible for many carcinogenic and teratogenic attributes as well as residual toxicity. For these reasons, consumers tend to be suspicious of chemical additives and thus the demand for natural preservatives has been intensified. The use of synthetic chemicals to control postharvest decay has been restricted to few fruit and vegetables, due to their high and acute residual toxicity, long degradation period, environmental pollution, effects on food and other side-effects on humans ( Lingk 1991; Mari and Guizzardi 1998; Tripathi and Dubey 2004).The increase of fungal resistance to classical drugs, the treatment costs and the fact that most available antifungal drugs have only fungistatic activity, justify the search for new strategies (Rapp 2004). Altogether, this has stimulated intensive research efforts to find alternatives to synthetic chemicals, such as new technologies, substances and practices to be used in postharvest preservation (Jobling 2000; Terry and Joyce 2004; Tripathi and Dubey 2004; Bokshi et al. 2007). Thus, the replacement of synthetic fungicides by natural products (particularly of plant origin), which are non-toxic and specific in their action, is gaining considerable attention (Tripathi and Dubey 2004; Bautista-Banos et al. 2006; Bajpai et al. 2008).
3 Essential Oils with Antifungal Power
Natural antimicrobial compounds are a re-emerging alternative to fresh produce preservation (Corbo et al. 2009). The antimicrobial power of plants and herb extracts has been recognised for centuries, and mainly used as natural medicine. Plant volatiles have been widely used as food flavouring agents, and many are generally GRAS. Essential oils (EOs), also called volatile oils, are aromatic oily liquids obtained from plant materials (flowers, herbs, buds, leaves, fruits, twigs, bark, seeds, wood and roots). EOs can be obtained by extraction, fermentation or expression, but steam distillation is the most commonly used method.
Essential oils are very complex natural mixtures which contain about 20–60 components at quite different concentrations. They are characterised by two or three major components at fairly high concentrations (20–70 %) compared to others components present in trace amounts. For example, carvacrol (30 %) and thymol (27 %) are the major components of the Origanum compactum essential oil, linalol (68 %) of the Coriandrum sativum essential oil, α- and β-thuyone (57 %) and camphor (24 %) of the Artemisia herba-alba essential oil, 1,8-cineole (50 %) of the Cinnamomum camphora essential oil, α-phellandrene (36 %) and limonene (31 %) of leaf and carvone (58 %) and limonene (37 %) of seed Anethum graveolens essential oil, menthol (59 %) and menthone (19 %) of Mentha piperita essential oil. Generally, these major components determine the biological properties of the essential oils. The components include different groups of distinct biosynthetical origin depending on the plant source (Croteau et al. 2000; Betts 2001; Bowles 2003; Pichersky et al. 2006). The main group is composed of terpenes and terpenoids and the other of aromatic and aliphatic constituents, all characterised by low molecular weight.
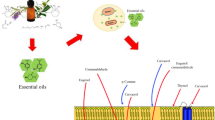
The inherent aroma and antimicrobial activity of EOs are related commonly to the chemical configuration of the components, to the proportions in which they are present and to interactions between them, affecting their bioactive properties (Fisher and Phillips 2008). Considering the complex mixture of EOs constituents is difficult to attribute the antimicrobial mode of action to one specific mechanism, being reported several targets in the microbial cell. It seems that they may cause deterioration of cell wall, damage to cytoplasmic membrane, damage to membrane proteins, leakage of cell contents, coagulation of cytoplasm, depletion of proton motive active sites, inactivation of essential enzymes and disturbance of genetic material functionality (Burt 2004; Ayala-Zavala et al. 2008b; Gutierrez et al. 2008).
Several EOs such as oils of garlic, cinnamon, thyme, oregano, clove, basil, coriander, citrus peel, laurel, ginger, rosemary and peppermint, among others, have been studied as antimicrobial natural products against both bacteria and moulds (Burt 2004; Burt et al. 2005; Ayala-Zavala et al. 2008c, d; Corbo et al. 2009). The biological activity of essential oils and/or their constituents can act as fungistatic and/or fungicidal agents, this depending, for instance, on the concentrations used. Indeed, cinnamon (Cinnamomum zeylanicum L.) and clove (Syzygium aromaticum L.), essential oils tested against anthracnose (Colletotrichum musae) and crown rot pathogens (Lasiodiplodia theobromae, C. musae and Fusarium proliferatum) isolated from banana, showed, in vitro, fungistatic and fungicidal activity against these decay agents within the range 0.3–1.1 mg/L. (Jobling 2000; Ranasinghe et al. 2002) The essential oil components carvone, cuminaldehyde, perillaldehyde, cinnamaldehyde, salicylaldehyde and benzaldehyde were found to be the most potent inhibitors of in vitro growth of Penicillium hirsutum (Smid et al. 1995). Fungal growth inhibition by carvone was found to be reversible, but exposure to cuminaldehyde, perillaldehyde, cinnamaldehyde and salicylaldehyde caused irreversible inhibition of fungal growth. Specifically, cinnamaldehyde has been shown to be a very potent fungicidal agent. Smid et al. (1995) found a 40-fold reduction of the fungal population when dipping tulip bulbs in an aqueous solution of 515 mg/L cinnamaldehyde.
The same essential oil and/or respective compounds can be active against a wide spectrum of microorganism species, although the minimum inhibitory concentration (MIC) used can be very changeable, according to the microbial species and/or the commodity. A varying degree of growth inhibition by the essential oils of several plants against some decay agents, such as Fusarium, Botrytis and Aspergillus spp., due to their different chemical composition, has been reported (Singh et al. 2002; Bouchra et al. 2003). Bajpai et al. (2008) found that the essential oil isolated from the floral parts of Silene armenia L. had a remarkable antifungal effect, against not only B. cinerea growth in vitro but also other critical decay agents. Cassia oil completely inhibited the in vitro growth of Alternaria alternata at 300 or 500 mg/L exposure for 6 and 3 days, respectively. When applied to tomatoes, cassia oil at 500 mg/L reduced the percentage of decay by 40–50 % (Feng and Zheng 2007).
Interestingly, the antifungal kinetics of S. armenia essential oil tested against B. cinerea correlated positively with increased exposure time and oil concentration. At concentrations of 62.5 and 125 mg/L, the fungicidal activity was very rapid (120 and 150 min, respectively), due to the presence of 2-butene, caryophyllene oxide, methylcyclopropane and α-butylene components in the oil (Bajpai et al. 2008). However, the fungus Sclerotinia sclerotiorum was found to be slightly resistant to the same oil, showing a MIC of 1000 mg/L.
Otherwise, different essential oils and/or compounds obtained from different plant species can exhibit different MICs for the same microbial agent. Bouchra et al. (2003) found that the essential oils of Origanum compactum Benth and Thymus glandulosus Req., consisting mainly of carvacrol and thymol, were the most efficient in the control of B. cinerea, by completely inhibiting mycelial growth in vitro at 100 mg/L. In contrast, essential oils of species such as Chenopodium ambrosioides L., Eucalyptus citriodora Hook, Eupatorium cannabinum L., Lawsonia inermis L., Ocimum canum Sim., Ocimum Gratissimum L., Ocimum Sanctum L., Prunus persica (L.) Batsch, Zingiber cassumunar Roxb and Zingiber officinale Rosc were found to exhibit in vitro fungitoxic activity against B. cinerea at 500 mg/L (Tripathi et al. 2008). When used to control grey mould in grapes caused by B. cinerea during storage, essential oils from O. sanctum, P. persica and Z. officinale showed MIC values of 200, 100 and 100 mg/L, respectively, and promoted the enhancement of storage life up to 4–6 days (Tripathi et al. 2008). Thyme oil exhibited a higher degree of inhibition of A. alternata (62.0 % at 500 mg/l) than cassia oil (40–50 % at 500 mg/l) in tomato (Feng and Zheng 2007).
The mechanism underlying the action of essential oil enrichment on the switch between vegetative and reproductive phases of fungal development remains to be fully understood. The negative impact of essential oils on fungi sporulation may reflect the effect of the volatiles emitted by oils on surface mycelia development and/or the perception/transduction of signals involved in the switch from vegetative to reproductive development (Tzortzakis 2007).
Nevertheless, suppression of spore production by essential oils could play a major role in limiting the spread of the pathogen, by lowering the spore load in the storage atmosphere and on surfaces (Tzortzakis 2007). Tzortzakis and Economakis (2007) found that in Colletotrichum coccodes, Botrytis cinerea, Cladosporium herbarum and Rhizopus stolonifer, spore production grown in vitro was reduced up to 70 % when exposed to 25 mg/L lemongrass (Cympopogon citratus L.) essential oil, and completely inhibited at 500 mg/L. In contrast, the same authors found that lemongrass oil (up to 100 mg/L) accelerated spore germination in Aspergillus niger. This, however, was reversed at 500 mg/L, when the process was fully inhibited, as for the other pathogens, due to failure of spore production.
The application mode of essential oils/their compounds has shown to have different results. For instance, spraying with basil oil (Ocimum basilicum L.) emulsion of 160 mg/L controlled crown rot and anthracnose, prolonging the storage life of ‘Embul’ bananas (Anthony et al. 2003). Eucalyptus (Eucaliptus globules L.) and cinnamon (Cinnamomum zeylanicum, Blume) are essential oil vapours applied at 50 mg/L concentrations for 8 h at 20 °C reduced fruit decay and improved the quality of tomatoes and strawberries during late storage life (Tzortzakis 2007). It is noteworthy that, when applied at 500 mg/L, the results obtained for the same compounds were similar. Tsao and Zhou (2000) found that thymol and carvacrol were effective in controlling brown rot caused by Monilinia fructicola in sweet cherries (Prumus avium L.), in either dipping or fumigation.
B. cinerea and Alternaria arborescens, isolated from tomatoes, showed complete in vitro growth inhibition when exposed to oregano (Oreganum vulgare L.), thyme (Thymus vulgaris L.) and lemongrass (Cymbopogon citrates L.) vapours at 50 mg/L for up to 12 h (Plotto et al. 2003). Geotrichum candidum was more sensitive to lemongrass oil (citral) vapours than to thyme or oregano oils. The same authors reported that only vapours of thyme and oregano oils (thymol and carvacrol) inhibited R. stolonifer. Interestingly, when incorporated into the growth medium, thyme and oregano oils showed a fungicidal or fungistatic activity for all four fungi at 500 mg/L, while lemongrass oil has the same effect at only 1,000 mg/L for all species except Rhizopus, for which no inhibition was reported. Cilantro (Coriandrum sativum L.) oil (trans-2-decenal) was fungicidal to Botrytis, Alternaria and Geotrichum as vapour, but lost its activity when incorporated into the growth medium (Plotto et al. 2003). However, none of the essential oils used by Plotto et al. (2003) in vitro succeeded in controlling disease development in tomato fruits inoculated with B. cinerea, A. arborescens or R. stolonifer, when applied as vapours. Moreover, phytotoxicity has been observed in fruits after 24 h exposure, either in tomatoes fumigated with the essential oils or with their respective major constituents alone. When dip treatments were done at 5,000 and 10,000 mg/L, it was found that thyme and oregano oils reduced disease development in tomatoes inoculated with B. cinerea or A. arborescens but also caused some phytotoxicity at those concentrations if the emulsion was not complete (Plotto et al. 2003). Lemongrass was more phytotoxic at 10,000 mg/L than thyme or oregano oils, probably due to some compounds present in this oil. Neither thyme nor oregano oils could control Rhizopus spp. inoculated in tomato wounds. On the contrary, the higher the concentration applied, the more the disease developed, probably due to a local phytotoxic effect of the essential oils in the wound, making the tissue more susceptible to this pathogen (Plotto et al. 2003).
Methyl jasmonate (MJ) is a natural compound widely distributed in plants. It was first detected as a sweet fragrant compound in Jasminum essential oil and other plant species (González-Aguilar et al. 2006). MJ is known to regulate plant development and response to environmental stress (Demo et al. 2005; Yao and Tian 2005), affecting many biochemical and physiological reactions in the tissue of whole and fresh-cut fruits and vegetables and extending shelf-life of whole and fresh-cut tomatoes, mangoes, guavas and strawberries (González-Aguilar et al. 2006). Ayala-Zavala et al. (2005, 2008c) reported that MJ alone or in conjunction with ethanol treatment increased antioxidant capacity, volatile compounds and post-harvest life of strawberry fruit, as well as extending shelf-life of fresh-cut tomatoes, suppressing fungal growth. Methyl jasmonate (MJ) either as a vapour or as an emulsion has shown to suppress green mould growth on grapefruit (Droby et al. 1999) and inhibit grey mould infection on strawberries alone or as a co-fumigant with ethanol (Ayala-Zavala et al. 2005).
3.1 Oils Rich in Terpenes
Terpenes form structurally and functionally different classes. The main terpenes are the monoterpenes (C10) and sesquiterpenes (C15), but hemiterpenes (C5), diterpenes (C20), triterpenes (C30) and tetraterpenes (C40) also exist. A terpene containing oxygen is called a terpenoid. The monoterpenes are formed from the coupling of two isoprene units (C10). They are the most representative molecules constituting 90 % of the essential oils and allow a great variety of structures. They consist of several functions, which are displayed in Table 15.1.
The sesquiterpenes are formed from the assembly of three isoprene units (C15). The extension of the chain increases the number of cyclisations which allows a great variety of structures. The structure and function of the sesquiterpenes are similar to those of the monoterpenes.
Examples of plants containing these compounds are angelica, bergamot, caraway, celery, citronella, coriander, eucalyptus, geranium, juniper, lavandin, lavander, lemon, lemongrass, mandarin, mint, orange, peppermint, petitgrain, pine, rosemary, sage, thyme.
3.2 Oils Rich in Phenolic Compounds
Derived from phenylpropane, the phenolic compounds occur less frequently than the terpenes. The biosynthetic pathways concerning terpenes and phenylpropanic derivatives generally are separated in plants but may coexist in some, with one major pathway taking over (see, cinnamon oil with cinnamaldehyde as major and eugenol as minor constituents, also clove oil, fennel, etc.). The phenolic compounds are depicted in Table 15.1.
The phenolic compounds found in essential oils normally have a carbon side chain and here we can look at compounds such as thymol, eugenol and carvacrol, that are classified as monoterpenic phenolic compounds. These components have great antiseptic, anti-bacterial and disinfectant qualities and also have greatly stimulating therapeutic properties. Evidence suggests that phenol induces progressive loss of intracellular constituents from treated bacteria and produces generalised membrane damage with intracellular coagulation occurring at higher concentrations. The plasma membrane of fungi is also damaged. The mechanisms thought to be responsible for the phenolic toxicity to microorganisms include enzyme inhibition by the oxidised compounds, possibly through reaction with sulphydryl groups or through more non-specific interactions with the proteins. Phenols are always present in conjugated form, usually with glucosidic attachment. They may be released in the free form during the fungal infection through enzymatic or other hydrolysis mechanisms. The site(s) and number of hydroxyl groups on the phenol group are thought to be related with the relative toxicity to microorganisms, with evidence that increased hydroxylation results in increased toxicity (Troncoso-Rojas and Tiznado-Hernández 2007). Due to the nature of phenols, essential oils that are high in them should be used in low concentrations and for short periods of time, since they can lead to toxicity if used over long periods of time. The principal plant sources for these compounds are anise, cinnamon, clove, fennel, nutmeg, parsley, sassafras, star anise, tarragon and some botanical families (Apiaceae, Lamiaceae, Myrtaceae, Rutaceae).
4 Sulphur-Riched Compounds
These compounds are another kind of natural volatiles that had been shown a strong antifungal activity. They are present in several plants like onion, garlic and others. The active constituents of garlic and onion are sulphur-riched compounds that are rapidly absorbed and metabolised. Allicin is considered to be the most important biologically active compound in garlic; however, during processing of garlic this compound is transformed to other sulfur compounds. Chemical analysis of garlic showed that 54.5 % of the total sulphides were the sum of diallyl monosulphide, diallyl disulphide, diallyl trisulphide and diallyl tetrasulphide (Troncoso-Rojas and Tiznado-Hernández 2007). Most of the reports in the literature regarding the antimicrobial effect of garlic oil are referent to antibacterial activity; while a little information about the antifungal activity is reported.
5 Collateral Effects of the Use of Essential Oils as Treatment of Fruit and Vegetables
The postharvest antifungal activity of essential oils is well and positively documented from a wide number of in vitro and in vivo experiments. The concentrations of essential oils and the respective compounds necessary to inhibit microbial growth are usually higher in foods than in culture media, which might be the result of interactions between EOs compounds and the food matrix (Nuchas and Tassou 2000) and should be taken into account in commercial applications (Tzortzakis 2007). Normally, direct application of antimicrobials to food must be done at high concentrations to achieve good antimicrobial activity against target microorganisms in food produce meant to be stored for an extended period of time. The ways in which EOs are applied and the concentrations at which they are used are important factors related to their effectiveness, and in some circumstances. EOs could be the cause of changes in flavour, odour and other characteristics of food products due to the strong odour-flavour that can be transmitted from the oil to the vegetable product. The chemical reactivity of EOs with the food and package matrix could significantly affect the sensorial properties of the produce (Ayala-Zavala et al. 2008d). The intense sensory attributes of some of these compounds may also be an impediment for their use in fresh commodities and therefore, in the application of natural antimicrobial and flavouring compounds such as fresh-cut fruits and vegetables preservatives, the sensorial impact should be considered (Ayala-Zavala et al. 2008a). A residual taste from thymol on fumigated cherries made this alternative treatment not commercially applicable. However, the same authors have found that when tomatoes were treated at the ‘breaker’ or ‘turning’ stages, enough time passed from initial application to eating maturity to allow volatilization, since no residual taste appeared on the tomatoes 10 days after treatment. Encapsulation in β-cyclodextrin (β-CD) is one method to control the odour and reactivity of active compounds throughout the release of natural antimicrobial compounds (Ayala-Zavala et al. 2008d). Microencapsulation can be a solution to solve this problem, because during the microencapsulation process, the active antimicrobial compounds will be trapped, masking odour and flavour until release to the atmosphere in constant low doses (Del Toro-Sánchez et al. 2010). This can protect the product from microbial growth without affecting its sensory acceptability.
Essential oils, as natural sources of phenolic components, attract investigators to evaluate their activity as antioxidants or free radical scavengers. The essential oils of basil, cinnamon, clove, nutmeg, oregano and thyme have proven radical-scavenging and antioxidant properties in the DPPH radical assay at room temperature (Tomaino et al. 2005). The order of effectiveness was found to be: clove ≫ cinnamon > nutmeg > basil ≥ oregano ≫ thyme. The essential oil of Thymus serpyllum showed a free radical scavenging activity close to that of the synthetic butylated hydroxytoluene (BHT) in a β-carotene/linoleic acid system (Tepe et al. 2005). The antioxidant activity was attributed to the high content of the phenolics thymol and carvacrol (20.5 and 58.1 %, respectively). Thymus spathulifolius essential oil also possessed an antioxidant activity due to the high thymol and carvacrol content (36.5, 29.8 %, respectively; Sokmen et al. 2004). The antioxidant activity of oregano (Origanum vulgare L., ssp. hirtum) essential oil was comparable to that of α-tocopherol and BHT, but less effective than ascorbic acid (Kulisic et al. 2004). The activity is again attributed to the content of thymol and carvacrol (35.0, 32.0 %, respectively).
The essential oils of Salvia cryptantha and Salvia multicaulis have the capacity to scavenge free radicals. The activity of these oils was higher than that of curcumin, ascorbic acid or BHT (Tepe et al. 2004). In addition, Curcuma zedoaria essential oil was found to be an excellent scavenger for DPPH radical (Mau et al. 2003).The antioxidant activity of essential oils cannot be attributed only to the presence of phenolic constituents; monoterpene alcohols, ketones, aldehydes, hydrocarbons and ethers also contribute to the free radical scavenging activity of some essential oils. For instance, the essential oil of Thymus caespititius, Thymus camphorates and Thymus mastichina showed antioxidant activity which in some cases was equal to that of α-tocopherol (Miguel et al. 2004). Surprisingly, the three species are characterised by high contents of linalool and 1,8-cineole, while thymol or carvacrol are almost absent. The essential oil of lemon balm (Melissa officinalis L.) shows an antioxidant and free radical scavenging activity (Mimica-Dukic et al. 2004) with the most powerful scavenging constituents comprising neral/geranial, citronellal, isomenthone and menthone. Tea tree (Melaleuca alternifolia) oil has been suggested as a natural antioxidant alternative for BHT (Kim et al. 2004) with the inherent antioxidant activity attributed mainly to the α-terpinene, γ-terpinene and α-terpinolene content. Essential oils isolated from Mentha aquatica L., Mentha longifolia L. and Mentha piperita L., were able to reduce DPPH radicals into the neutral DPPH-H form (Mimica-Dukic et al. 2003). The most powerful scavenging constituents were found to be 1,8-cineole for the oil of M. aquatica while menthone and isomenthone were the active principles of M. longifolia and M. piperita. It is clear that essential oils may be considered as potential natural antioxidants and could perhaps be formulated as a part of daily supplements or additives to prevent oxidative stress that contributes too many degenerative diseases. And its addition to fruit and vegetables can cause the increment of the antioxidant activity of the treated produce.
6 Physiological Effects
Many authors mentioned beneficial or no detrimental effects on horticultural product quality parameters when essential oils are used after harvest. Tzortzakis (2007) reported that decay was reduced in strawberries and tomatoes by the use of essential oils from eucalyptus and cinnamon, with no effects on fruit firmness. Similar results were observed for cherries and grapes when treated with vapours of eugenol, thymol or menthol (Martinez-Romero et al. 2005). Chinese pears (Pyrus bertschneideri Reld cvs. Laiyang Chili and Ya Li) treated with emulsions (3–9 %) of commercial or refined (reduced a-tocopherol) plant oils (soybean, corn, olive, peanut, linseed and cottonseed) at harvest and stored for 6 months at 0 °C maintained firmness in a concentration-dependent manner during storage (Ju et al. 2000). In the same way, quality attributes such as colour, soluble solids content and titratable acidity were preserved through storage. Pears showed no off-flavours when compared to controls and internal ethanol was not affected by oil treatment. The higher concentrations reduced internal browning of Chinese pears and scald incidence in ‘Delicious’ apples (Ju et al. 2000). Oil vapours increased the levels of total soluble solids during exposure in strawberries and tomatoes but the effect persisted following exposure only in ‘cherry’ tomatoes (Tzortzakis 2007). The same authors reported that fruit samples treated with oil vapours did not differ in percentage weight loss, organic acid content, sweetness and total phenolic content during or following vapour exposure, compared with untreated fruit. Yet table grapes impregnated with 0.5 mL thymol or menthol showed significantly lower weight loss and soluble solids/titratable acidity ratio than controls, as well as reduced firmness and colour changes during storage (Martinez-Romero et al. 2005).
Tsao and Zhou (2000) found that thymol and carvacrol were effective in controlling brown rot caused by the Monilinia fructicola in sweet cherries (Prumus avium L.), but caused stem browning of cherry fruits in the fumigation experiment. This side-effect was reduced by 69 and 73 %, respectively, when methyl jasmonate was used as a co-fumigant.
7 Conclusion
In the last years many studies have been carried out concerning the antifungal activity of EOs. As this review reveals many EOs possess strong antifungal activity, however, some collateral responses in the treated fresh produce should be evaluated. In the application of natural antimicrobial and flavouring compounds, the sensorial impact should be considered. Therefore, sensorial impact, limited stability and high volatility represent drawbacks of EOs which complicate the in vitro tests as well as the storage and application. In addition, the effect of the treatments on the antioxidant and health-related benefits of the treated fruit and vegetables must be contemplated considering the bioactive properties of EOs. Therefore, more research has to be done to develop formulations that maintain the fungicidal activity while not inducing undesirable effects.
References
Anthony S, Abeywickrama K, Wijeratnam SW (2003) The effect of spraying essential oils Cymbopogon nardus, Cymbopogon flexuosus and Ocimum basilicum on postharvest diseases and storage life of Embul banana. J Hortic Sci Biotechnol 78:780–785
Ayala-Zavala JF, Wang SY, Wang CY, Gonzalez-Aguilar GA (2005) Methyl jasmonate in conjunction with ethanol treatment increases antioxidant capacity, volatile compounds and postharvest life of strawberry fruit. Eur Food Res Technol 221:731–738
Ayala-Zavala JF, del Toro-Sánchez L, Alvarez-Parrilla E, Soto-Valdez H, Martín-Belloso O, Ruiz-Cruz S, González-Aguilar GA (2008a) Natural antimicrobial agents incorporated in active packaging to preserve the quality of fresh fruits and vegetables. Stewart Postharvest Rev 4:1–9
Ayala-Zavala JF, Del-Toro-Sánchez L, Alvarez-Parrilla E, González-Aguilar GA (2008b) High relative humidity in-package of fresh-cut fruits and vegetables: advantage or disadvantage considering microbiological problems and antimicrobial delivering systems? J Food Sci 73:R41–R47
Ayala-Zavala JF, Oms-Oliu G, Odriozola-Serrano I, Gonzalez-Aguilar GA, Alvarez-Parrilla E, Martin-Belloso O (2008c) Bio-preservation of fresh-cut tomatoes using natural antimicrobials. Eur Food Res Technol 226:1047–1055
Ayala-Zavala JF, Soto-Valdez H, González-León A, Alvarez-Parrilla E, Martín-Belloso O, González-Aguilar GA (2008d) Microencapsulation of cinnamon leaf (Cinnamomum zeylanicum) and garlic (Allium sativum) oils in betacyclodextrin. J Incl Phenom Macrocycl Chem 60:359–368
Bajpai VK, Shukla S, Kang SC (2008) Chemical composition and antifungal activity of essential oil and various extract of Silene armeria L. Bioresource Technol 99(18):8903–8908
Batu A (2003) Temperature effects on fruit quality of mature green tomatoes during controlled atmosphere storage. Int J Food Sci Nutr 54:201–208
Bautista-Banos S, Hernandez-Lauzardo AN, Velázquez-del Valle MG, Hernandez-Lopez M, Ait-Barka E, Bosquez-Molina E, Wilson CL (2006) Chitosan as a potential natural compound to control pre and postharvest diseases of horticultural commodities. Crop Prot 25:108–118
Betts TJ (2001) Chemical characterization of the different types of volatile oil constituents by various solute retention ratios with the use of conventional and novel commercial gas chromatographic stationary phases. J Chromatogr A 936:33–46
Bokshi AI, Morris SC, McConchie R (2007) Environmentally-safe control of postharvest diseases of melons (Cucumis melo) by integrating heat treatment, safe chemicals and systemic acquired resistance. N Z J Crop Hort 35:179–186
Bouchra C, Achouri M, Hassani LMI, Hmamouchi M (2003) Chemical composition and antifungal activity of essential oils of seven Moroccan Labiatae against Botrytis cinerea Pers. J Ethnopharmacol 89:165–169
Bowles EJ (2003) The Chemistry of Aromatherapeutic Oils. Allen & Unwin, ISBN 174114051X
Burt SA (2004) Essential oils: their antibacterial properties and potential applications in foods—a review. Int J Food Microbiol 94:223–253
Burt SA, Vlielander R, Haagsman HP, Veldhuizen EJA (2005) Increase in activity of essential oil components carvacrol and thymol against Escherichia coli O157:H7 by addition of food stabilizers. J Food Prot 68:919–926
Charles MT, Tano K, Asselin A, Arul J (2009) Physiological basis of UV-C induced resistance to Botrytis cinerea in tomato fruit. Constitutive defense enzymes and inducible pathogenesis-related proteins. Postharvest Biol Technol 51:414–424
Corbo MR, Bevilacqua A, Campaniello D, d’Amato D, Speranza B, Sinigaglia M (2009) Prolonging microbial shelf life of foods through the use of natural compounds and nonthermal approaches—a review. Int J Food Sci Technol 44:223–241
Croteau R, Kutchan TM, Lewis NG (2000) Natural products (secondary metabolites). In: Buchanan B, Gruissem W, Jones R (eds) Biochemistry and molecular biology of plants. American Society of Plant Physiologists, Rockville, pp 1250–1318
DeEll JR, Toivonen PMA, Doussineau J, Roger C, Vigneault C (2003) Effect of different methods for application of an antifog shrink film to maintain cauliflower quality during storage. J Food Qual 26:211–218
Del Toro-Sánchez CL, Ayala-Zavala JF, Machi L, Santacruz H, Villegas-Ochoa MA, Alvarez-Parrilla E, González-Aguilar GA (2010) Controlled release of antifungal volatiles of thyme essential oil from b-cyclodextrin capsules. J Incl Phenom Macrocycl Chem 67:431–441
Demo M, de Las M, Olivia M, Lopez ML, Zunino MP, Zygadlo JA (2005) Antimicrobial activity of essential oils obtained from aromatic plants of Argentina. Pharm Biol 43:129–134
Droby S, Porat R, Cohen L, Weiss B, Shapiro B, Philosoph-Hada S, Meir S (1999) Suppressing green mold decay in grapefruit with postharvest jasmonate application. J Am Soc Hort Sci 124:184–188
Drzyzga O (2003) Diphenylamine and derivatives in the environment: a review. Chemosphere 53:809–818
Feng W, Zheng X (2007) Essential oils to control Alternaria alternata in vitro and in vivo. Food Control 18:1126–1130
Fisher K, Phillips C (2008) Potential antimicrobial uses of essential oils in food: is citrus the answer? Trends Food Sci Technol 19:156–164
Gomez PA, Artes F (2004) Controlled atmospheres enhance postharvest green celery quality. Postharvest Biol Technol 34:203–209
González-Aguilar GA, Tiznado-Hernandez M, Wang C-Y (2006) Physiological and biochemical responses of horticultural products to methyl jasmonate. Stewart Postharvest Rev 2:1–9
Gutierrez J, Barry-Ryan C, Bourke P (2008) The antimicrobial efficacy of plant essential oil combinations and interactions with food ingredients. Int J Food Microbiol 124:91–97
Hammer KA, Carson CF, Riley TV (2001) Antimicrobial activity of essential oils and other plant extracts. J Appl Microbiol 86:985–990
Harker FR, Gunson FA, Jaeger SR (2003) The case for fruit quality: an interpretative review of consumer attitudes, and preferences for apples. Postharvest Biol Technol 28:333–347
Isman MB (2000) Plant essential oils for pest and disease management. Crop Prot 19:603–608
Jobling J (2000) Essential oils: a new idea for postharvest disease control. Good Fruit Vegetables Mag 11:50
Ju Z, Duan Y, Ju Z (2000) Plant oil emulsion modifies internal atmosphere, delays fruit ripening, and inhibits internal browning in Chinese pears. Postharvest Biol Technol 20:243–250
Kim H, Chen F, Wu C, Wang X, Chung H, Jin Z (2004) Evaluation of antioxidant activity of Australian tea tree (Melaleuca alternifolia) oil and its components. J Agric Food Chem 52:2849–2854
Kulisic T, Radonic A, Katalinic V, Milos M (2004) Analytical, nutritional and clinical methods use of different methods for testing antioxidative activity of oregano essential oil. Food Chem 85:633–640
Kushalappa AC, Zulfiqar M (2001) Effect of wet incubation time and temperature on infection, and of storage time and temperature on soft lesion expansion in potatoes inoculated with Erwinia Carotovora ssp. Carotovora. Potato Res 44:233–242
Lingk W (1991) Health risk evaluation of pesticide contaminations in drinking water. Gesunde Pflanz 43:21–25
Mari M, Guizzardi M (1998) The postharvest phase: emerging technologies for the control of fungal diseases. Phytoparasitica 26:59–66
Martinez-Romero D, Castillo S, Valverde JM, Guillen F, Valero D, Serrano M (2005) The use of natural aromatic essential oils helps to maintain postharvest quality of ‘Crimson’ table grapes. Acta Hort 682:1723–1729
Mau J, Laib E, Wang N, Chen C, Chang C, Chyau C (2003) Composition and antioxidant activity of the essential oil from Curcuma zedoaria. Food Chem 82:583–591
Miguel G, Simones M, Figueiredo A, Barroso J, Pedro L, Carvalho L (2004) Composition and antioxidant activities of the essential oils of Thymus caespititius, Thymus camphorates and Thymus mastichina. Food Chem 86:183–188
Mimica-Dukic N, Bozin B, Sokovic M, Mihajlovic B, Matavulj M (2003) Antimicrobial and antioxidant activities of three Mentha species essential oils. Planta Med 69:413–419
Mimica-Dukic N, Bozin B, Sokovic M, Simin N (2004) Antimicrobial and antioxidant activities of Melissa officinalis L. (Lamiaceae) essential oil. J Agric Food Chem 52:2485–2489
Nielsen PV, Rios R (2000) Inhibition of fungal growth on bread by volatile components from species and herbs, and the possible application in active packaging, with special emphasis on mustard essential oil. Int J Food Microbiol 60:219–229
Nuchas GE, Tassou CC (2000) Traditional preservatives: oils and spices. In: Robinson RK, Batt CA, Patel PD (eds) Encyclopedia of food microbiology. Academic Press, London, pp 1717–1722
Pichersky E, Noel JP, Dudareva N (2006) Biosynthesis of plant volatiles: nature’s diversity and ingenuity. Science 311:808–811
Plotto A, Roberts DD, Roberts RG (2003) Evaluation of plant essential oils as natural postharvest disease control of tomato (Lycopersicon esculentum L.). Acta Hort 628:737–745
Ranasinghe L, Jayawardena B, Abeywickrama K (2002) Fungicidal activity of essential oils of Cinnamomum zeylanicum (L.) and Syzygium aromaticum (L.) Merr et L.M. Perry against crown rot and anthracnose pathogens isolated from banana. Lett Appl Microbiol 35:208–211
Rapp RP (2004) Changing strategies for the management of invasive fungal infections. Pharmacotherapy 24:4–28
Roller S, Lusengo J (1997) Developments in natural food preservatives. Agro Food Industry Hi-Tech 8:22–25
Singh G, Singh OP, Maurya S (2002) Chemical and biocidal investigations on essential oils on some Indian Curcuma species. Prog Cryst Growth Charact 45:75–81
Smid EJ, Witte Y, Gorris LGM (1995) Secondary plant metabolites as control agents of postharvest Penicillium rot on tulip bulbs. Postharvest Biol Technol 6:303–312
Sokmen A, Gulluce M, Akpulat A (2004) The in vitro antimicrobial and antioxidant activities of the essential oils and methanol extracts of endemic Thymus spathulifolius. Food Control 15:627–634
Tepe B, Donmez E, Unlub M (2004) Antimicrobial and antioxidative activities of the essential oils and methanol extracts of Salvia cryptantha (Montbret et Aucher ex Benth.) and Salvia multicaulis (Vahl). Food Chem 84:519–525
Tepe B, Sokmen M, Akpulat A, Daferera D, Polissiou M, Sokmen A (2005) Antioxidative activity of the essential oils of Thymus sipyleus subsp. sipyleus var. sipyleus and Thymus sipyleus subsp. sipyleus var. rosulans. J Food Eng 66:447–454
Terry LA, Joyce DC (2004) Elicitors of induced disease resistance in postharvest horticultural crops: a brief review. Postharvest Biol Technol 32:1–19
Tomaino A, Cimino F, Zimbalatti V (2005) Influence of heating on antioxidant activity and the chemical composition of some spice essential oils. Food Chem 89:549–554
Tournas VH (2005) Spoilage of vegetable crops by bacteria and fungi and related health hazards. Crit Rev Microbiol 31:33–44
Tripathi P, Dubey NK (2004) Exploitation of natural products as an alternative strategy to control post harvest fungal rotting of fruit and vegetables. Postharvest Biol Technol 32:235–245
Tripathi P, Dubey NK, Shukla AK (2008) Use of some essential oils as post-harvest botanical fungicides inthemanagement of greymould of grapes caused by Botrytis cinerea. World J Microbiol Biotech 24:39–46
Troncoso-Rojas R and Tiznado-Hernández ME (2007) Natural compounds to control fungal postharvest diseases. In: Troncoso-Rojas R, Tiznado-Hernández M, González-León A (eds) Recent advances in alternative postharvest technologies to control fungal diseases in fruit and vegetables. Research Signpost, Trivandrum-695 023, Kerala, India pp 127–156
Tsao R, Zhou T (2000) Interaction of monoterpenoids, methyl jasmonate, and Ca2+ in controlling postharvest brown rot of sweet cherry. HortScience 35:1304–1307
Tzortzakis NG (2007) Maintaining postharvest quality of fresh produce with volatile compounds. Innovative Food Sci Emerg 8:111–116
Tzortzakis NG, Economakis CM (2007) Antifungal activity of lemongrass (Cympopogon citratus L.) essential oil against key postharvest pathogens. Innovative Food Sci Emerg 8:253–258
Uritani I (1999) Biochemistry on postharvest metabolism and deterioration of some tropical tuberous crops. Bot Bull Acad Sinica 40:177–183
Wade WN, Beuchat LR (2003) Proteolytic fungi isolated from decayed and damaged tomatoes and implications associated with changes in pericarp pH favorable for survival and growth of foodborne pathogens. J Food Prot 66:911–917
White JML, McFadden JP (2008) Contact allergens in food ingredients and additives: atopy and the hapten-atopy hypothesis. Contact Dermatitis 58:245–246
Wiley RC (2000) Minimally processed refrigerated fruits and vegetables. Chapman & Hall, New York
Wisniewski ME, Basset CL, Artlip TS, Webb RP, Janisiewickz WJ, Norelli JL, Goldway M, Droby S (2003) Characterization of a defensin in bark and fruit tissues of peach and antimicrobial activity of a recombinant defensin in the yeast, Pichia pastoris. Physiol Plant 119:563–572
Yao HJ, Tian SP (2005) Effects of biocontrol agent and methyl jasmonate on postharvest diseases of peach fruit and the possible mechanisms involved. J Appl Microbiol 98:941–950
Zhang Z, Nakano K, Maezawa S (2009) Comparison of the antioxidant enzymes of broccoli after cold or heat shock treatment at different storage temperatures. Postharvest Biol Technol 54:101–105
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2013 Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
González-Aguilar, G.A., Ansorena, M.R., Viacava, G.E., Roura, S.I., Ayala-Zavala, J.F. (2013). Plant Essential Oils as Antifungal Treatments on the Postharvest of Fruit and Vegetables. In: Razzaghi-Abyaneh, M., Rai, M. (eds) Antifungal Metabolites from Plants. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-642-38076-1_15
Download citation
DOI: https://doi.org/10.1007/978-3-642-38076-1_15
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-642-38075-4
Online ISBN: 978-3-642-38076-1
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)