Abstract
Betulin and betulinic acid are natural product with antitumor and anti-HIV-1 activity. Betulin can be got by chemical extraction from a lot of plants. Betulinic acid can be synthesized from betulin. In order to get high biological activity compounds with antitumor and anti-HIV-1 activity from the bark of the white birch, the extraction and purification method of betulin was studied; the optimized extraction and purification condition was obtained. The two-step synthetic routes of betulinic acid from betulin were discussed. Betulinic acid was synthesized by oxidizing betulin with Jones reagent, followed by selective reduction with NaBH4. IR, HPLC–MS, H1-NMR were used to identify the structure of betulin and betulinic acid.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
1 Introduction
Cancer and AIDS have been the most important diseases leading to death nowadays. But most of the commonly used medicines of anti-HIV or anticancer are proved to have many limitations in clinical application, especially toxic side effect and drug-resistance. Therefore, the development of medicine with perfect anticancer and anti-HIV effect is needed.
Betulin exists in various plant resources, especially in the white birch bark. Betulin is a kind of bioactive substance with diverse biological activities, such as anti-inflammatory, antitumor, anti-HIV [1], which can be widely applied in food, cosmetic, and pharmaceutical fields. Betulin, as an important intermediate of medicine, can be obtained by extraction with organic solvent from the white birch bark [2].
Betulinic acid has antitumor, anti-HIV-1 and anti-inflammatory activities [3, 4], especially has an excellent effect on melanoma. As a potential precursor medicine of antitumor or anti-HIV, it has drawn the attention of researchers and investors. Betulin and betulinic acid have become a hotspot because of their excellent biological properties and good selectivity, low toxicity, and less side effects [5–8].
2 Materials and Methods
2.1 Materials, Reagents, Instruments
The white birch bark was collected in summer, Heilongjiang Province (2008–2009), in China. The birch bark was shattered to 60 meshes and immediately dried at 60 °C and stored in a dry and dark place.
Methanol, acetonitrile, ethanol, methanol, chloroform (Chl.), isopropyl alcohol (IPA), petroleum ether (B.P.: 60–90 °C, PE), ethyl acetate (EA), tetrahydrofuran (THF), and NaBH4 are all of analytical grade.
H1-NMR spectra are recorded on Bruker AM-400 NMR spectrometer in deuterated chloroform. HPLC spectra are recorded on Hitachi D–2000 spectrometers with UV detector. And Mass Detector is Waters 3,100; IR spectrometer is PerkinElmer spectrum65.
2.2 Methods
2.2.1 The Extraction and Purification of Betulin
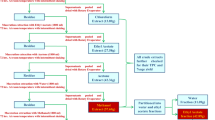
The birch bark (100 g) with ethanol/IPA (10/1, V/V, 1,100 mL) was refluxed for 6 h in a Soxhlet’s extractor; solvent was kept overnight at 0 °C. The precipitate was separated by filtration, washed on the filter with cold ethanol and then dried in vacuum drying oven.
In order to determine the optimum purification conditions for betulin, the solvents (A), solid–liquid ratio (B), and recrystallization times (C) were evaluated. And in every factor, we have chosen 3 levels to take the orthogonal design test (Table 164.1). Record every yield of crystal and determine the purity of betulin. The optimal recrystallization process was determined based on both the yield of crystal (40 %) and the purity of betulin (60 %) as the combined value.
2.2.2 Synthesis and Purification of Betulinic Acid
-
A.
Synthesis and Purification of Betulonic Acid
Freshly prepared Jones solution (2.3 mL) was added to a solution with betulin (1.0 g, 2.26 mmol) in acetone (100 mL) at 0 °C and the mixture were stirred at 0 °C. The course of the reaction was confirmed by TLC. After the reaction, the solvent was quenched with methanol (25 mL), stirred for 5 min, and added 40 mL H2O. All the solid impurities in mixture were filtered out. After removing the organic solvent by reduced pressure distillation, the aqueous residue was extracted twice with ethyl acetate (40 mL). The organic layer was washed (H2O, brine), dried (MgSO4), and filtered. The solvent was removed in vacuum. The residue was column chromatographed over silica gel (200–300 mesh) using PE/EA (8/1) to obtain betulonic acid.
-
B.
Synthesis and Purification of Betulinic Acid
NaBH4 (0.227 g, 6 mmol) was gradually added to a solution with betulonic acid (0.90 g, 2 mmol) in THF (20 mL) at 0 °C and the mixture were stirred at 0 °C. The course of the reaction is confirmed by TLC. The reaction was quenched with HCl solution (3 mL, 2 mol/L). The solution was diluted with ethyl acetate (60 mL). The organic layer was washed (H2O, brine), dried (MgSO4), and filtered. The solvent was removed in vacuum to obtain crude betulinic acid. The crude betulinic acid was column chromatographed over silica gel (200–300 mesh) using PE/EA (6/1) to obtain betulinic acid.
2.2.3 Identification of the Chemical Structure and Purity of Betulin and Betulinic Acid
-
(1)
Identification of the chemical structure
The chemical structure of betulin and betulinic acid was confirmed by IR, HPLC–MS,and H1-NMR.
The HPLC analysis was carried out by VenusilC8 (4.6 × 250 mm). The mobile phase was methanol/acetonitrile/H2O (45/45/10, V/V). The flow rate and injection volume were 1.0 mL/min and 10 μL. Betulin and betulinic acid were quantified, respectively, by UV detector at λ = 210 nm. All chromatographic operations were carried out at 30 °C [9, 10].
The MS conditions: The ion source is ESI (betulinic acid); the ion mode is negative; the scanning cover is 50–1000 u, the scanning mode is full scanning, the flow of desolvation gas is 700 L/h, the temperature of desolvation gas is 350 °C, the temperature of ion source is 150 °C.
-
(2)
Determination of the purity of betulin and betulinic acid
-
A.
The HPLC Conditions
The HPLC analysis was carried out by VenusilC8 (4.6 mm × 250 mm). The mobile phase was methanol/acetonitrile/H2O (45/45/10, V/V). The flow rate and injection volume were 1.0 ml/min and 10 μL. Betulin and betulinic acid were quantified by UV detector at λ = 210 nm, respectively. All chromatographic operations were carried out at ambient temperature.
-
B.
Preparation of Sample Solution
Accurately weighed equivalent to 2.0 mg of betulin or betulinic acid were dissolved with methanol/acetonitrile/H2O (45/45/10, V/V, 1 mL). And then the solution was filtered through a membrane filter (φ = 0.45 μm).
-
C.
The preparation of the blank reference solution
The blank reference solution were methanol/acetonitrile/H2O (45/45/10, V/V) at the same conditions.
3 Results and Discussion
3.1 Results
3.1.1 The optimization process of the extraction and purification of betulin
The white birch bark (60 mesh, 100 g) with ethanol/IPA (10:1, V/V, 1100 mL) was refluxed for 6 h at 78 °C to give the crude betulin (34.7 g).
The orthogonal design test result of betulin purification is shown in Table 164.2.
According to the data (K1, K2, and K3) in Table 164.1, the effect of solvent, solid–liquid ratio, and recrystallization times on betulin quality were shown in Fig. 164.1.
The optimum recrystallization condition is: the solvent is IPA/methanol (3/1, V/V), the solid–liquid ratio is 1/30 (g/mL) and the recrystallization time is 3 times.
3.1.2 The chemical structure and purity of betulin and betulinic acid
-
(1)
The chemical structure of betulin and betulinic acid
The chemical structures of the betulinic acid and the betulin have been determined by H1-NMR (Fig. 164.2a, b), respectively. IR (Fig. 164.3a, b) was used to identify the results. HPLC–MS spectrum of betulinic acid was carried out too (Fig. 164.4).
-
(2)
The purity of betulin and betulinic acid
The HPLC spectrum showed that the purity of crude betulin is 50.56 % with a yield of 34.7 %. By the optimum recrystallization process, the purity of betulin is 95.2 % (Fig. 164.5a); yield of crystal is 20.8 %; and the purity of betulinic acid is 96.2 % (Fig. 164.5b) with a yield of 78.6 %.
3.2 Discussion
Betulin and betulinic acid possess antitumor, anti-HIV, and other biological properties. In the past decade, betulin, betulinic acid, and their derivatives have drawn the attention of researchers because of their excellent biological properties [11]. High quality betulin and betulinic acid have a huge market value.
The commonly used organic-solvents, such as chloroform, methanol [12], methylbenzene [13], ethanol, dichloromethane, and diethyl ether [14], are ineffective for the extraction and purification of betulin. The experimental results showed: mixed-solvent ethanol/IPA is effective extraction solvent, which can improve the extraction yield as well as the purity of betulin. In this study, the orthogonal design test was used to optimize the recrystallization technology.
Betulinic acid can be obtained by extraction from plants and chemical synthesis [15–17]. But it is difficult to obtain betulinic acid by the method of extraction because of its very low content in plants [18]. Several kinds of synthesis methods can be used to obtain betulinic acid [19, 20]. Semi-synthesis method by two-step synthesis with betulin as raw material is the most effective one. To this method, the betulin was oxidized by Jones reagent at first, followed by a reduction with NaBH4 to afford crude betulinic acid of 73.4 % yield. The crude betulinic acid was purified by column chromatographed over silica gel (200–300 mesh) using PE/EA (6/1) to obtain 96.2 % betulinic acid (HPLC data) with a yield of 84.0 %.
4 Conclusion
Betulin was obtained from the white birch bark with ethanol/IPA as extraction solvent. Betulinic acid was prepared from betulin with two-step method. The optimal process and conditions were determined for the extraction of betulin and synthesis of betulinic acid. The betulin extraction method developed in this report may bring huge potential value for the development of new antitumor and anti-HIV-1 drug.
References
Fan GZ, Zhan YZ (2008) Advances in studies on betulin. Chin Tradit Herbal Drugs 39:1591–1594
Flekhter OB, Nigmatullina LR, Baltina LA et al (2002) Synthesis of betulinic acid from betulin extract and study of the antiviral and antiulcer activity of some related terpenoids. Pharm Chem J 36:484–487
Robert HC, Samir AK (2004) Chemistry, biological activity and chemotherapeutic potential of betulinic acid for the prevention and treatment of cancer and HIV infection. Med Res Rev 24:90–114
Toshihiro F, Yoshiki K, Robert E et al (1994) Anti-AIDS agents betulinic acid and platonic acid as anti-HIV principles from Syzigium Claviflorum, and the anti-HIV activity of structurally related triterpenoids. J Nat Prod 57:243–247
Darrick SHLK, Chen ZD, Nguyen VT et al (1997) A concise semi-synthetic approach to betulinic acid from betulin. Synth Commun 27:1607–1612
Csuk R, Schmuck K, schäfer R (2006) An practical synthesis of betulinic acid. Tetrahedron Lett 47:8769–8770
Pichettea A, Liu HY, Christian R et al (2004) Selective oxidation of betulin for the preparation of betulinic acid, an antitumoral compound. Synth Commun 34:3925–3937
Liu J, Zhang HF, He GQ et al (2009) Research progress and foreground of betulin and betulinic acid. Sci Technol Food Indus 30:360–366
Zhao GL, Yan WD, Dan C (2007) Simultaneous determination of betulin and betulinic acid in white birch using RP-HPLC. J Pharm Biomed 43:956–962
Zhang Z, Sun H (2004) Determination of betulin content from bark of Betula Platyphylla Suk by HPLC. Chem Indus For Prod 24:61–63
Cichewicz RH, Kouzi SA (2004) Chemistry, biological activity and chemotherapeutic potential of betulinic acid for the prevention and treatment of cancer and HIV infection. Med Res Rev 24:90–114
Yi JE, Wen LX, Yuan LY et al (2010) Study on extraction of betulin from white birch and synthesis of betulinic acid. J Hunan Agric Univ (Nat Sci) 36:574–580
Han SY, Fang GZ, Li SS et al (2005) Separation and purification of betulin by tetrahydrofuran-benzene extraction. Chem Indus For Prod 25:129–132
Wang SF, Zhang MG, Li YP (2007) Study on extraction and purification of betulin from birch bark. Chem Indus For Prod 27:22–26
Lai L, Yang X, Yang G et al (2006) Orthogonal test for optimizing extraction technique of betulinic acid in Semen Ziziphi Spinosae. Chin Tradit Herbal Drugs 4:532–534
Prakash Chaturvedula VS, Schilling JK, Johnson RK et al (2006) New cytotoxic lupane triterpenoids from the twigs of coussarea paniculata. J Nat Prod 66:4533–4541
Klaus W, Hartmut S (1995) Dodeca acetyl prodelphinidin B3 from the dried leaves of ziziphus spina-christ. Phytochemistry 38:505–507
Margaret MO, Bentley MD, Campbell CS et al (1988) Betulin and lupeol in bark from four white barked birches. Phytochemistry 27:2175–2176
Gaudet D, Pichette A. Process for preparing natural product derivatives from plants in a single step. US: 6280778
Krasutsky PA, Kolomitsyna O. Methods of manufacturing bioactive 3-esters of betulinic aldehyde and betulinic acid. WO. Patent 2006105356
Acknowledgments
Financial support from the Science and Technology Project of Tianjin (No. 10JCZDJC22500,10ZXCXSY10100) is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer-Verlag Berlin Heidelberg
About this paper
Cite this paper
Zhang, TC., Xu, W., Wang, N., Luo, X., Wang, J., Hu, X. (2014). Studying on Extraction of Betulin from the White Birch and Synthesis of Betulinic Acid. In: Zhang, TC., Ouyang, P., Kaplan, S., Skarnes, B. (eds) Proceedings of the 2012 International Conference on Applied Biotechnology (ICAB 2012). Lecture Notes in Electrical Engineering, vol 251. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-642-37925-3_164
Download citation
DOI: https://doi.org/10.1007/978-3-642-37925-3_164
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-642-37924-6
Online ISBN: 978-3-642-37925-3
eBook Packages: EngineeringEngineering (R0)