Abstract
This chapter deals with the pioneering years of superheavy element research, from the mid 1960s to the mid 1980s. The prediction that superheavy nuclides could form an island around element 114 with half-lives long enough to have survived in Nature since nucleosynthesis led to intensive searches—not unlike “gold fever”—for such relic nuclei in all sorts of natural environments. Positive claims were raised from time to time but could not stand up under further scrutiny. Numerous attempts to synthesize superheavy nuclei by large leaps from the mainland of elemental stability to the island of superheavy elements went without success as well. The discovery of three more transactinide elements, 107–109, from 1981 to 1984 encouraged chemists to resume research on the chemistry of transactinide elements with a new approach: automated chemical procedures.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Introduction
In 1955, Wheeler [1] performed a courageous extrapolation of nuclear masses and decay half-lives and concluded that nuclei twice as heavy as the then heaviest known nuclei existed; he subsequently called them superheavy nuclei. Two years later, Scharff-Goldhaber [2] mentioned in a discussion of the nuclear shell model that beyond the well-established proton shell at Z = 82 (lead), the next proton shell should be completed at Z = 126 in analogy to the known N = 126 neutron shell. Together with a new N = 184 shell, these shell closures should lead to a local region of relative stability. These speculations, however, did not impact contemporary research since such extremely heavy nuclei were experimentally beyond reach.
The situation changed in 1966 due to the publication of three theoretical papers. In a study of nuclear masses and deformations, Myers and Swiatecki [3] emphasized the enormous stabilization against fission gained by shell closures. Nuclei at the next proton shell closure beyond Z = 82 should have fission barriers even higher than that of uranium, making them quite stable against spontaneous fission. This was in sharp contrast to the liquid-drop nuclear model, which predicts vanishing fission barriers in the same region, and therefore prompt instability due to disruption by fission. Remarkably, although the discussion in this paper focused on Z = 126 as the next proton shell, a closure at Z = 114 was already mentioned as an alternative, with reference to unpublished calculations by H. Meldner. He presented his results [4] at the symposium “Why and How Should We Investigate Nuclides far off the Stability Line?”, in 1966 in Lysekil, Sweden [5], the seminal event for superheavy element research. Simultaneously, Sobiczewski et al. [6] also derived that 114 should be the next magic proton number. Other groups using different theoretical approaches soon agreed. A fantastic perspective was thus opened—an island of superheavy elements located not too far from the then heaviest known element, 103, and hence perhaps within reach.
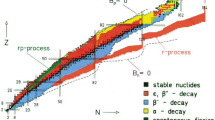
First theoretical estimates [7–10] of decay half-lives around the doubly magic, spherical nucleus Z = 114, N = 184 revealed a topology as depicted in Fig. 1 [10]. Three major decay modes were considered: spontaneous fission, α decay, and β- decay or electron capture. Spontaneous fission half-lives were found to peak sharply at the doubly magic nucleus, descending by orders of magnitude within short distances in the Z–N plane, thus causing the island-like shape. In contrast, half-lives for α decay should decrease rather uniformly with increasing proton number, with some zigzag at the nuclear shell closures. The β-stable nuclei would cross the plane as a diagonal belt. For Z = 114, N = 184 an enormous spontaneous fission half-life of 2 × 1019 y was estimated, but only 10 y were estimated for α decay. In a limited region, long half-lives for both decay modes were expected to meet, most spectacularly at Z = 110, N = 184 where an overall half-life of 2 × 108 y should result—sufficiently long for the occurrence of superheavy elements in Nature! Additional stability was expected for odd elements such as 111 or 113 [8, 11] due to the well-known hindrance of spontaneous fission and α decay for odd proton numbers.
Topology of the island of superheavy nuclei around the shell closures at proton number Z = 114 and neutron number N = 184 as predicted in 1969. Thick solid lines are contours of spontaneous fission half-lives; broken lines refer to α-decay half-lives. Shaded nuclei are stable against β decay. Reproduced from [10]
These predictions of very long half-lives immediately stirred up a gold-rush period of hunting for superheavy elements in natural samples. Everybody was encouraged to participate. Almost nothing was needed to perform these experiments except for only a little money. Almost no equipment was needed, no research group or permission by a laboratory director was required, no accelerator beam time or proposal to funding agencies was needed—not even a garage. Just an intelligent choice of a natural sample and a corner in the kitchen at home could be sufficient to make an outstanding discovery: new and superheavy elements in Nature. The detector could be a simple microscope from school days now used to detect fission fragment tracks, which had accumulated in the sample since geological times. Such tracks (Fig. 2) are caused by radiation damage in the surrounding solid when the energetic fragments are slowed down to rest, and they can be made visible by chemical etching.
Due to the topology of the island, superheavy nuclei should decay by spontaneous fission, either immediately or after a sequence of other decay steps. In a detailed theoretical exploration, [12] of the Z–N plane around the island, the longest lived nuclide again turned out to be Z = 110, N = 184, decaying with a 3 × 109 y half-life via α-particle emission to 290108. From there, two subsequent β− transitions should lead via 290109 to 290110, where the chain should terminate by spontaneous fission with a 140 d half-life. The doubly magic 298114, with a half-life of 790 y, should also decay into 290110 by two α-particle emissions via 294112 as the intermediate step.
Since spontaneous fission is extremely rare in Nature, detection of fission events in natural samples would give a strong hint as to the existence of superheavy elements. α-particle spectra would be less specific, because the energies predicted for superheavy nuclei should fall into the range covered by decay products of uranium and thorium, and elaborate chemical treatment would be required to distinguish them.
The first attempts to synthesize superheavy nuclei in the laboratory were already under way in the late 1960s. Complete fusion of medium-heavy projectiles with very heavy targets—the successful approach in the extension of the Periodic Table—was considered the most promising approach. However, a large gap had to be bridged in a single step from conceivable targets, such as uranium or curium, to the island. The then existing heavy-ion accelerators could not provide the required medium-element projectiles in adequate intensities if at all. These demands had a strong impact on accelerator technology in order to upgrade the existing facilities and build novel ones.
The situation is illustrated in a cartoon, Fig. 3, which entertained the audiences of related conferences in the early 1970s. Several sailors are shown attempting to cross the sea of instability, fighting against hostile forces. Already on the way are the crews of the JINR (Joint Institute for Nuclear Research at Dubna, Soviet Union) with the Heavy-ion U-300 Cyclotron (Xe), and the IPN (Institut de Physique Nucléaire at Orsay, France) with the ALICE cyclotron (Kr); those of the LBL (Lawrence Berkeley Laboratory at Berkeley, USA) are just launching the SuperHILAC linear accelerator (Ge), whereas the UNILAC linear accelerator (U) of the GSI (Gesellschaft für Schwerionenforschung at Darmstadt, Germany) is still under construction.
Allegorical view of heavy-ion accelerator projects launched in the early 1970s for a journey to the island of superheavy elements. The facilities are identified by flags showing their most advanced projectile beam, see text. Newly colored version of a cartoon originally provided by Flerov [13]
Turning now to chemistry, the crucial question was: where are the superheavy elements located in the Periodic Table and how well do they fit into its architecture? The answer had immediate implications for the ongoing “search for” campaigns, for the selection of natural samples as well as for the design of chemical identification procedures. In a naive continuation of the Table, element 110 is located below platinum, 112 below mercury, 114 below lead, and 118 becomes the next noble gas below radon. Quantum–mechanical calculations of ground-state electronic configurations [14, 15] supported this view. The electron configurations should indeed be analogous to that of the homologs; e.g., two 7s valence electrons were predicted for element 112, as there are two 6s electrons in mercury.
Extrapolations within the respective groups of the Periodic Table should thus be an appropriate approach to predict the chemical behavior of superheavy elements [14] even in some detail, such as for the 7p elements 113 and 114, eka-thallium and eka-lead, respectively [16]. Specific chemical properties were needed as the basis for identification, but properties common to several superheavy elements were also of interest for group separations in enrichment procedures; for example, the high volatility expected for elements 112–116 in the metallic states [17] or the strong bromide complexes of elements 108–116 [18].
Deviations from straightforward extrapolations within the Periodic Table could be caused by relativistic effects in the electron shells of superheavy elements. The inner electrons rotate around the nucleus with such a high velocity that they gain substantial mass; the s- and p-orbitals shrink, whereas higher lying orbitals expand. As a consequence [19], the two s electrons in element 112 and also the two p 1/2 electrons in element 114 could form closed electron shells, and thus eka-mercury and eka-lead would be chemically inert gases like element 118, eka-radon. Beyond element 121, eka-actinium, a series of 6f elements may occur, in analogy to the 5f actinide elements following actinium. But the 5g orbital may also be filled in competition to form a series of 32 superactinide elements [20].
Within a few years, many aspects of superheavy nuclei and elements were predicted. A review [21] covering the literature until the end of 1973 was based on 329 references, and status reports [22–27] published from time-to-time illustrated how the field had further developed.
2 Search for Superheavy Elements in Nature
Since the solar system and the Earth’s crust were formed about 4 × 109 y ago, a half-life of some 108 y for superheavy nuclei would be long enough for their survival until present day. Heavy elements beyond iron are created in gigantic stellar collisions and explosions, the supernovae, which produce free neutrons in tremendous densities and initiate the so-called r-process of nucleosynthesis where r stands for “rapid”. Starting at seed nuclei around iron, several neutrons are captured to form very neutron-rich isotopes which decay quickly by β− transitions to the next heavier element. These daughter products again capture neutrons and undergo β− decay and so forth. In this way, the r-process path proceeds parallel to the belt of stable nuclei from iron to the heaviest elements but shifted to much higher neutron numbers, with discontinuities at magic neutron numbers, as is sketched in Fig. 4.
Nucleosynthesis of superheavy nuclei by the r-process. Shown in the Z–N plane is the r-process path of very neutron-rich nuclei formed during a supernova event by rapid neutron capture alternating with β− decay. After the event, the r-process nuclei decay via long β−-decay chains toward the belt of β-stable nuclei (arrows). Those originating at Z ≈ 104 end up around closed-shell nuclei with Z ≈ 114, N ≈ 184 (dots). From [28]
As soon as the stellar explosion ceases, the very short-lived nuclei decay toward the region of β-stability by a chain of fast β− transitions, thereby further increasing their atomic number. Somewhere at very high atomic numbers, the r-process is terminated by fission. Figure 4 shows these results [28] for a stellar temperature of 1.8 × 109 K (T 9), a neutron density of 1028 cm−3 (ρ n) and a cycle time of only 8 s for the whole process. This study predicted continuation of the process up to Z ≈ 104, sufficient to feed magic nuclei with Z = 114, N = 184 by decay. Other early treatments [29–31] denied the production of superheavy elements in the r-process. The question remained controversial for quite some time [32].
But even if the half-lives of superheavy nuclides did not exceed the 108 y level, there was hope to discover them in Nature. Although now extinct, they may have left detectable traces such as fission tracks or fission products in certain samples. Another possible source could be the cosmic radiation impinging on Earth whose heavy component may be formed by a r-process nucleosynthesis in our galaxy not longer than 107 y ago [33], and may hence contain superheavy nuclei with half-lives down to some 105 years.
In this context, attention was also drawn [11] to quite a number of earlier reports on natural α-particle emitters with energies that did not match any known natural radioactive source but fell into the region predicted for superheavy nuclides. Were the superheavies already there, but unrecognized?
2.1 Terrestrial Samples
Any search for superheavy elements in terrestrial material begins with the choice of a sample. Relevant geochemical aspects are discussed in Refs. [34, 35]. First searches were reported in 1969 by the Berkeley [10] and the Dubna groups [36]. In Berkeley, a search for element 110, eka-platinum, in natural platinum ores with standard analytical techniques remained negative at a concentration limit of 1 ppb, and low-level counting techniques did not reveal any activity above background. In Dubna, however, fission tracks discovered in old lead glasses were tentatively attributed to element 114, eka-lead, present in concentrations of 10−11–10−12 gram per gram of sample with an assumed half-life of 1 × 109 y of the radioactive source. As a convention, 1 × 109 y is the half-life assumed for the conversion of measured specific activities (count rate per gram of sample) into the more instructive concentration (gram of radionuclide per gram of sample). Examinations of the same and of other lead-bearing samples for spontaneous fission events with large proportional counters in Dubna seemed to confirm these findings, but further measurements [37] of thin samples sandwiched between two plastic fission track detectors showed that the events were background caused by cosmic-ray induced reactions of lead.
Other groups [38] found no evidence for spontaneous fission activities in lead and other samples at a lower detection limit of 10−13 g/g achieved with the sandwich technique. Even limits down to 10−17 g/g can be reached by etching fission tracks in suitable minerals where they would have accumulated over millions of years. Such searches in a variety of minerals [35, 39] remained inconclusive, however.
A versatile technique for spontaneous fission detection is counting the neutrons emitted in the fission process. Although neutron detection is less efficient than fission fragment or α-particle counting, it can compete because much larger samples, up to tens of kilograms, can be inspected. With a simple arrangement of six 3He-filled neutron counters, a sensitivity of 10−11 g/g was reached in 2 days of counting [40], allowing a quick survey of a great variety of samples. Activities were found with all heavy metals in the Periodic Table from platinum to bismuth, but with identical decay rates. Furthermore, as Fig. 5 shows, the rates for lighter elements fall on a curve representing cross-sections for high-energy spallation reactions as a function of atomic number. This shows that a background of neutrons is created by cosmic-rays impinging on the samples during counting, which requires additional shielding.
Neutron counting as detection method for spontaneous fission events of superheavy nuclei. The recorded neutron rates (points) were found to follow the relative cross-sections of cosmic-ray induced spallation reactions (curve), and were thus due to background events. The numbers are the rates measured for natural uranium and thorium. From [40]
More advanced versions of neutron counting were based on the expectation that spontaneous fission events of superheavy nuclei should be accompanied by the emission of about 10 neutrons [41, 42], distinctly more than two to four observed for any other spontaneous fission decay. Such neutron bursts can be recognized by recording neutron multiplicities—events with several neutrons in coincidence—with 3He-filled counting tubes [43, 44] or large tanks filled with a liquid scintillator sensitive to neutrons [45].
Figure 6 shows a neutron multiplicity detector [43] with 20 3He counting tubes neutron arranged in two rings around the central sample chamber, which accommodated upto 100 kg of a sample. The tubes were embedded in paraffin for slowing down the neutrons. Bursts of ≥10 neutrons from an emitter source at 10−14 g/g concentration should result in about one event per day with multiplicity of four or larger. A similar sensitivity was reached [45] with a scintillator-based neutron detector. To suppress background by cosmic-ray induced neutron showers, the detectors were operated below ground and with an electronic anticoincidence shielding triggered by incoming high-energy particles. With such an instrument no positive results at the 10−14 g/g level were obtained for lead ores or samples from industrial lead processing. The publication Ref. [45] gives an illustrative example of how researchers can be misled by a contamination of a sample by tiny amounts of the common nuclide 2.5-y 252Cf which decays by spontaneous fission and thus emits neutrons.
Large neutron counter with 3He counting tubes for the recording of neutron multiplicities in the spontaneous fission decay of superheavy nuclei (see text). Reproduced from [43]
A quite unconventional approach to fission-event detection is a device called the “spinner.” The instrument, Fig. 7, consists of a glass cylinder with glass arms filled with about one liter of the sample solution. Upon rotation, a negative pressure develops in the solution through the action of centrifugal forces. The solvent does not evaporate, however, but remains in a metastable state until a strongly ionizing event in the solution destroys this state and produces a bubble which is detected optically. The spinner can be operated with very low background rates, as low as one event per month corresponding to a detection limit of 10−13–10−14 g/g. No fission events were observed [46] with salts from the elements platinum to lead in the Periodic Table, and galena (natural lead sulfide).
The spinner detector. The container is filled with a solution of the sample. Upon rotation, a metastable state develops (at left) which breaks down after an ionizing event, as is indicated by the formation of a central bubble (at right). From [46]
Attempts were made to further improve the detection limits by enrichment of superheavy elements from very large samples. Among the “hottest” natural samples were brines from hot springs at the Cheleken Peninsula in the Caspian Sea which are known to be rich in volatile elements, probably due to material escaping from large depths in the Earth’s mantle. They may contain superheavy elements deposited in deeper layers. After processing some 2,000 m3 of spring water through 850 kg of an anion-exchange resin, a weak spontaneous fission activity appeared in the resin, and fractions eluted from the resin showed rates up to five events per day with neutron multiplicities as depicted in Fig. 8 [47]. Evidently, natural 238U can be ruled out as the source, but not a contamination by 2.5-y 252Cf. Attempts to concentrate the activity further for identification of its atomic number failed [48]. A search for such activities in similar brines, Salton Sea in California and Atlantis II at the floor of the Red Sea, gave no positive evidence [49].
Spontaneous fission activity in hot spring water at the Cheleken Peninsula after concentration by ion exchange. Shown is the measured neutron multiplicity distribution (dots) compared with measured distributions for 238U, 246Cm and 252Cf spontaneous fission and calculated distributions for two sets of ν and σ2, the average number of neutrons per fission and its variance, respectively. From [47]
The extreme case of the search for element 114 is flue dust collected during the industrial processing of lead from galena [50]. Eka-lead should be more volatile than lead, and hence enriched in flue dust. Samples collected from 103 to 104 tons of galena were concentrated further by chemical and mass separations. They were finally exposed to reactor neutrons but no induced fission events were found with fission track detectors. The deduced concentration limit of 10−19–10−23 g/g is by far the lowest achieved in searches for superheavy elements in Nature.
Very unexpected news arrived in the summer of 1976—evidence for element 126, and possibly 124 and 116 in Nature. The evidence was obtained [51] in a study of radioactive halos, a phenomenon known since the early days of radioactivity research. They appear in certain minerals as spherical zones of discoloration around a central mineral grain and are due to radiation damage by α-particles emitted from uranium or thorium present in the grain. Cuts through such halos reveal a well-resolved ring structure reflecting the ranges of α particles in the surrounding mineral. There are, however, ranges which cannot be associated with known natural nuclides. In particular, in biotite from Madagascar, giant halos were observed [52] with ranges equivalent to about 14 MeV α particles, an energy predicted for α transitions at Z ≈ 126. Such halos occur around relatively large inclusions of monazite crystals (a lanthanide-thorium-uranium phosphate), as Fig. 9 shows, together with haloes of the thorium and uranium decay series around large and also small central crystals.
Radioactive halos around large central monazite inclusions in biotite from Madagascar. Top: giant halo, bottom: thorium and uranium halos; at right: well-resolved uranium halo around a small central grain for comparison. All photographs are on the same scale; the outer diameter of the halo at top is 250 μm. From [53]
In order to verify the presence of elements around Z ≈ 126, the monazite inclusions were irradiated with sharply collimated proton beams to excite the X-ray spectra of the elements. As can be seen in Fig. 10, two well-separated groups of strong peaks appear [51], the L X-rays of uranium and thorium (at left), and the K X-rays of the lanthanide elements (at right). In between, with energies between 24 and 29 keV, much weaker peaks were identified and assigned to the L α1 X-rays of the elements 116, 124 and 126. Surprisingly high concentrations, 10–100 ppm in the grain, result from the observed peak intensity. If such concentrations would also hold for bulk monazite, tons of superheavy elements would become easily accessible in some regions of the Earth, e.g., at Indian beaches.
Proton-induced X-ray spectrum of a monazite inclusion in the center of a giant radioactive halo (at top). The region in the gap around channel 400 is shown enlarged at the bottom (dots) together with the spectrum of a U-Th halo (circles) and a smoothed background (line). From [51]
The occurrence of elements 126 and 124 in monazites would perhaps not be unlikely because in a superactinide series of elements they would be homologs of uranium and thorium [14]. Element 116, however, would be a homolog of polonium. Since polonium is known to be strongly enriched by some marine invertebrates, it was suggested [54] to search for element 116 in crustacea such as lobsters, shrimps, and crabs in coastal waters at beaches rich in monazite sand—perhaps a gourmet’s recommendation.
Objections against these findings were soon raised. The strongest peak attributed to element 126 could experimentally be accounted for [55] by a prompt γ ray emitted during the proton bombardment in the (p,n) reaction with 140Ce, cerium is a major component of monazite. The weaker peaks were shown to stem from K X-rays of traces of ordinary elements such as antimony and tellurium [56]. When the X-ray spectra were selectively excited by monochromatic synchrotron radiation tuned to the X-ray absorption edges of the supposed elements, the evidence for superheavy elements vanished [57, 58]. Furthermore, attempts failed [59] to detect them in bulk monazites through isolation of an A > 294 fraction with a mass separator. Chemical enrichments [60] from bulk samples also remained without success. The conclusion is that giant halos are not due to superheavy elements, but a generally accepted explanation of what they are is still lacking.
2.2 Extra-Terrestrial Samples
Lunar rocks showed no indication of spontaneous fission activity in neutron multiplicity counting of a 3 kg sample [45].
Much attention was paid to the evidence for extinct superheavy elements appearing in a class of primitive meteorites, the carbonaceous chondrites. These are low-temperature condensates from a solar gas that have more or less escaped subsequent differentiating processes, and may therefore represent the material from which the solar system was made. They contain a surplus of the neutron-rich xenon isotopes 131–136 [61], at first attributed to the spontaneous fission of the now extinct 244Pu. But when this assignment became questionable, it was suggested [62, 63] that superheavy elements might be the progenitors. Correlations between the concentrations of excess xenon and of volatile elements such as thallium, bismuth, and indium in meteorites pointed to elements 115, 114, or 113 [64, 65]. The strange xenon was found to be strongly enriched [66] in a host phase comprising less than 0.5% of the meteorite, isolated after dissolution of its bulk in strong acids.
Light xenon isotopes from 129 to 124 were also over-abundant in such meteorites [61, 67, 68] and enriched [66] in the tiny host phase although they are not formed in fission. Whether there are two anomalous xenon components of different origin remained controversial for years [69]. Eventually, the fission origin of the anomalous xenon was ruled out [70] because in a host phase containing the excess xenon, no excess was detected for the adjacent, but nonvolatile fission products barium 130–138.
Stimulated by these studies, samples of primitive meteorites were inspected by neutron multiplicity counting. In the Allende meteorite available in large quantities, a weak fission activity at the 10−14 g/g level was reported [71–73] but could not be enriched chemically [74–76].
Another class of “search for” experiments is the measurement of heavy element abundances in the cosmic radiation by exposure to particle track detectors—nuclear emulsions or plastic sheets—in balloon flights at high altitudes with analysis of the recorded tracks for atomic number and abundance. A survey [33] of all data obtained until 1970 showed one single event beyond Z ≈ 100. With the data collected in the Skylab space station, the limit became more stringent: no superheavy nucleus in spite of the 204 recorded tracks with atomic number 74–87 [77]. A similar limit was deduced [78] after exposure in a satellite. In a study of cosmic-ray induced tracks in olivine crystals enclosed in iron-stone meteorites, which were exposed in space over millions of years, unusually long tracks were found and attributed to superheavy elements [79, 80]. However, this conclusion could not be maintained [81, 82] after calibration experiments of track dimensions with energetic 238U beams delivered by accelerators.
The largest collector surface for elements impinging on the Globe is of course the Sea. Heavy elements deposited in seawater are enriched in certain sediments, such as iron-manganese hydroxides called manganese nodules. Fission tracks were found [83] in feldspar inclusions in such nodules, but no evidence was obtained [40, 45] for spontaneous fission activities by counting nodules with neutron detectors.
In summary, there was no evidence beyond a doubt for superheavy elements in Nature. Since improved theoretical calculations of half-lives tended with time to reach much shorter values than those required for occurrence in Nature, the enthusiasm for further searches ceased in the early 1980s. This colorful intermezzo in superheavy element research appeared to be finished, but remarkably, a search for primordial element 108 (hassium) in its homolog osmium was recently undertaken [84].
3 Early Attempts to Synthesize Superheavy Elements
First attempts to synthesize superheavy nuclei in the late 1960s followed the approach that was so successful for actinide elements: complete fusion of a projectile and a target nucleus chosen to attain by amalgamation the desired proton number. As long as elements around 126 were the goal, the perspective looked promising. Fusion cross-sections as large as tens of millibarns were extrapolated [85, 86] from data for heavy actinides, including the 232Th(80Kr, 2n) reaction directly leading to the doubly magic Z = 126, N = 184 nucleus. With element 114 as the focus, the situation is different in that the doubly magic nucleus Z = 114, N = 184 is extremely neutron rich. Its neutron-to-proton ratio cannot be achieved by any realistic projectile-target combination. Close approach to Z = 114 is connected with a neutron number far below N = 184, whereas to meet N = 184 requires an overshooting of Z = 114 by about 10 protons [85, 87].
Evidence for success would be the observation of very energetic α particles or spontaneous fission activities not common among ordinary nuclei. But such evidence would require further examination. Unfortunately, the identification of a new element by means of its radioactive decay into well-known nuclides of a known element—very successful for actinide elements—would not be possible in a region far apart from already explored territory.
The first attempt to synthesize element 114 was made in 1969 in Berkeley [10] by bombarding neutron-rich 248Cm with 40Ar projectiles. The experiment was negative at a cross-section limit of about 10 nanobarns. The compound nucleus 288114 contains only 174 neutrons, and it should evaporate four neutrons to 284114170, which is probably located outside the island. With the large variety of heavy-ion beams soon becoming available, attempts to reach the island by complete fusion reactions were carried out on a broad basis. Within a few years, twenty different reactions were tried [24] covering compound nuclei with proton numbers from 110 to 128 and neutron numbers from 168 to 194.
To give an example, high-energy α particles with energies of 13–15 MeV—as predicted for elements around 126—from short-lived emitters were observed at the ALICE Orsay in bombardments of thorium with krypton. This was taken as evidence for the synthesis of a compound nucleus of element 126 [88]. But attempts in the same laboratory to secure the evidence by a direct mass identification failed [89]. In these control experiments, the magnetic rigidity, kinetic energy and time-of-flight of fragments produced in interactions of 84Kr with 232Th, 208Pb and 238U were measured.
Very surprising news appeared in the early 1970s—can superheavy nuclei be made by heavy-ion reactions without using a heavy-ion accelerator? A long-lived spontaneous fission activity was chemically isolated and assigned [90] to element 112, eka-mercury, from tungsten plates bombarded over a long time in the beam dump of the 24 GeV proton beam at the CERN in Geneva. A two-stage production process was postulated: proton-induced spallation of tungsten generates energetic recoil atoms, which fuse with tungsten to produce superheavy nuclei. Attempts to confirm the results in other laboratories failed [46, 91, 92], a conclusion finally shared by most of the original authors [93].
Finally, the efforts focused on experiments with 48Ca projectiles, which are doubly magic (Z = 20, N = 28) and very neutron rich, but also very rare and expensive. After survey experiments with this projectile at Berkeley [94, 95] and Dubna [96, 97], the 48Ca + 248Cm reaction was considered to be the most promising because the compound nucleus Z = 116, N = 180 provides a relatively close approach to the neutron shell with moderate overshooting of the proton shell. Also, the predicted decay chain after evaporation of four neutrons [12] appeared to be suitable for detection. The decay chain should start at the evaporation residue 292116 with α decay having a few seconds half-life, followed within several minutes by two electron captures of 288114 and 288113, and end at 288112 by spontaneous fission with 50 min half-life.
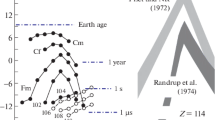
Figure 11 refers to a series of 48Ca + 248Cm experiments by an international collaboration [98] performed at the UNILAC and the SuperHILAC. With a variety of techniques, a half-life range of 14 orders of magnitude, from 1 μs to 10 y, was covered. The upper limits of the production cross-sections achieved in these negative experiments are plotted versus the assumed half-life of the product. Each technique has a specific half-life region of highest sensitivity, but is less sensitive at shorter half-lives because of decay before detection, and at longer ones by a decay rate which is too low for detection.
Search for superheavy nuclides in the reaction of 48Ca with 248Cm. Upper limits of production cross-sections are plotted versus half-life. The curves refer to different separation techniques: recoil-fragment separators (curves 1, 2), fast on-line chemistry (3–5), and off-line chemistry with low-background counting (6–8), see also text. From [98]
The region of very short half-lives (curves 1 and 2) was inspected with two recoil-fragment separators with fragment detection by surface-barrier detectors. For intermediate half-lives (3–5), chemical on-line separations were applied, and off-line chemistry was used in the detection of long-lived products (6–8). The chemical procedures were based on volatilization at high temperature (3 and 8) for elements 112 through 116 [17], and at room temperature (4 and 6) for 112 and 114 [19]. Anion exchange of bromide complexes (5 and 7) was applied for elements 108–116 [18]. The cross-section limit was generally about 200 picobarns, with some extra sensitivity gained for long-lived products by fission fragment-fission neutron coincidence counting [99].
The 48Ca + 254Es reaction with Z = 119 as the compound nucleus was also studied [100] with negative results.
Was there an alternative? Would it help to offer a projectile with far more protons and neutrons than what was required to fill the gap between target and superheavy nuclei? In deep-inelastic reactions, massive projectile and target nuclei stick together in a dumbbell shaped, dinuclear collision complex, but their mutual electrostatic repulsion drives the complex apart before complete amalgamation is reached. During the contact nucleons are exchanged between the partners, and with some probability one part grows considerably at the expense of the other one. Would this also happen if a 238U146 beam interacts with a 238U146 target; could one partner in the dinuclear collision grow to become doubly magic 298114184, whereas the other one would shrink to 17870108, a known neutron-rich isotope of ytterbium?
A radiochemical study [101] of the element distribution in the 238U + 238U reaction at the UNILAC revealed the expected broad distribution of reaction products. Figure 12 shows the production cross-sections for nuclides beyond uranium. They decrease from plutonium to fermium by eight orders of magnitude, indicating severe losses by fission of freshly formed transfer products. Nonetheless, an extrapolation to surviving Z = 114 fragments gives about 10 picobarn cross-sections, not a completely hopeless situation. For the complementary products below uranium where fission decay is not significant, the yields decrease exponentially from Z = 92 down to Z = 73. This trend is well reproduced [102] by a theoretical model treating nucleon transfer in the intermediate collision complex as a diffusion process. Extrapolation of the model to Z = 70 gives about 100 microbarn total production cross-section.
Production cross-sections of transuranium nuclides in the interaction of 238U with 238U (solid lines) plotted versus mass number. Also shown are data for the 136Xe + 238U interaction (dashed lines). From [101]
Direct searches for superheavy elements in the 238U + 238U reaction were also undertaken at the UNILAC by several groups. All these efforts unfortunately did not result in positive evidence of the production of superheavy elements. Figure 13 gives a summary of these searches. The curve labeled CHEM [103] was obtained with off-line chemical separations and inspection for α and spontaneous fission activities; here, the 10 picobarn level was reached for half-lives between several days and years. The curve labeled GAS [104] holds for an on-line search of species, which are volatile at room temperature. WHEEL [105] refers to fission track detection in the unseparated product mixture deposited on a rotating catcher, REC [106] to the inspection of unseparated recoil atoms implanted in a surface barrier detector, and JET [107, 108] after their on-line transport from target to detector by a gas jet system.
Search for superheavy nuclides in the 238U + 238U reaction: upper limits for the production cross-section obtained with various techniques (see text), plotted versus half-life. Reproduced from [105]
Attempts to find superheavy elements in the 238U + 248Cm reaction [109] failed, too, although the production cross-sections for transcurium isotopes increase by three orders of magnitude [110] compared with the 238U + 238U reaction.
Two decades later the reaction of 48Ca with 248Cm was repeated by a Dubna-Livermore collaboration [84, 111], this time successfully with the discovery of element 116. The isotopes 293116 and 292116, α-particle emitters with 61 and 18 ms half-lives, were produced by the 248Cm(48Ca,3n) and (48Ca,4n) reactions with 1.0 and 3 picobarn cross-sections, respectively. This level is two orders of magnitude below the level reached in the earlier experiments shown in Fig. 11 [98]. The previous approach appeared to not be sensitive enough to these low cross-sections.
4 New Elements, New Chemistry
Besides the hunting for “superheavies”, attempts to extend the Periodic Table element-after-element continued also in these years and in 1974 reached element 106 (seaborgium). It was synthesized in Berkeley [112] by the 249Cf(18O,4n)263106 reaction and was identified to be an α-particle emitter with a half-life of only 0.9s. The evaporation of four neutrons after fusion of projectile and target indicates an excitation energy of 40–50 MeV energy, which is typical for actinide-based fusion reactions, thus, called “hot fusions”. But such excitations are unwanted in the synthesis of fragile nuclei at the limit of stability. Fortunately, they can largely be avoided by using the closed-shell nuclei 208Pb and 209Bi as targets [113], which, as an additional advantage, are generally available and inactive. This “cold fusion” approach requires medium-weight, neutron-rich projectiles, which were not generally available at the then existing heavy-ion accelerators but were offered in appropriate energies and intensities at the new UNILAC accelerator at the GSI in Darmstadt.
Element 107 (bohrium) was the first element discovered at the GSI by cold fusion [114]. Its synthesis succeeded in 1981 by the 209Bi(54Cr,n) reaction leading to 262107, which decays with 4.7 ms half-life by α-particle emission into a chain of well-known nuclides; the assignments of proton and mass number of the new element were, therefore, beyond any doubt. Essential for this success was SHIP, the powerful recoil-fragment separator, which separated online the very few wanted nuclei from a huge bulk of waste particles. In addition, it had a sophisticated detection system, which recorded the decay events that followed a suspected event at the spot where it was collected. Two more elements, 108 (hassium) and 109 (meitnerium), were discovered by the SHIP group in the following 3 years. These nuclei also decay by α-particle emission with millisecond half-lives. No evidence was obtained for the onset of spontaneous fission, which was expected in this region due to the decreasing barrier heights against fission, as predicted by the liquid-drop nuclear model. In a comprehensive analysis of the observations, Armbruster concluded that these nuclei are already shell stabilized in the ground state; they “correspond to what superheavy elements are to be” [115]. Thus, the superheavies were already there, but not as spherical nuclei like those supposed to exist around element 114, but as deformed nuclei with elongated shapes. Extra stability around Z = 108 and N = 162, due to shell stabilization of deformed nuclei, was already indicated in theoretical studies [116, 117] and was confirmed later by further work.
The new elements should belong to the 6d transition elements beginning with element 104 (rutherfordium) for which a colorful chemistry is expected, quite different from the monotone chemistry of the preceding heavy actinides. Very little was known, however, about the chemistry of the transactinides. In aqueous solutions, cationic and anionic species of element 104 had been studied with standard column techniques [118, 119], and in the gaseous state, halide compounds of 104 and 105 by their volatilization and deposition on solid surfaces [120–122]. The elements 104 and 105 behaved, in all cases, like homologs of hafnium zirconium (104) and tantalum niobium (105), respectively.
Four more transactinides, 106–109, were added and the possibility to proceed to still heavier elements was positive; a strong motivation to initiate extended chemical studies. Furthermore, with increasing atomic number, a new and unique chemical aspect should become more and more visible: chemical consequences of increasingly strong relativistic effects in the electron shells. How will they change the architecture of the Periodic Table; by minor irregularities or by drastic breaks? Will, for example, eka-lead (element 114) at ambient temperature behave like a metallic element or an inert gas?
Research on the chemistry of transactinide elements was resumed in the mid 1980s at Berkeley by the first study of element 105 in aqueous solution [123]. The α-particle emitter, 35-s 262Db, produced by the 249Bk(18O,5n) reaction, served as a probe. The investigated chemical topic was the adsorption on glass in very strong nitric acid, a characteristic property of tantalum and niobium. Dubnium was found to share this property. Due to the very low production rate of 262Db, some 800 manually performed experiments were required to obtain a statistically satisfying result based on 24 α-decay events altogether. This example showed that automated, computer-controlled online procedures were needed for a broad exploration of the open territory.
Automated procedures had already been developed and were applied in studies of short-lived fission products [124], but additional and stringent conditions have to be met for their applications to the heaviest elements. This work at the one-atom-at-a-time level with short-lived nuclei sets extremely strong constraints on the choice of chemical procedures. The chemistry and also the α-particle spectroscopy required for identification have to be fast in terms of the half-life of the radionuclide. In addition, the procedures should be robust enough for running them over days or weeks in order to catch and study the few produced atoms. Developments in this direction were mainly pushed by groups at GSI/Darmstadt—University of Mainz (Germany) and Paul Scherrer Institut/Villigen—University of Bern (Switzerland), with groups at the Lawrence Berkeley Laboratory—University of California joining for experiments with the heaviest elements. First results were obtained with the last actinide element, 103 (lawrencium). The isotope 3-min 260Lr was used for investigations in aqueous solution [125] and in the gas phase [126]. Improved versions of the key instruments for experiments in aqueous solution [127] and with gases [128] were published. The first applications to a transactinide element—halide complexes of element 105 in aqueous solution [129] and halide species in the gas phase [130]—showed clearly the potential of automated procedures. What followed? See this book.
References
Wheeler, J.A.: Fission physics and nuclear theory. In: Proceedings of International Conference Peaceful Uses of Atomic Energy, Geneva 1955, vol. 2, pp. 155–163, 220–226. United Nations, New York (1956)
Scharff-Goldhaber, G.: Nuclear physics. Nucleonics 15(9), 122–124 (1957)
Myers, W.D., Swiatecki, W.J.: Nuclear masses and deformations. Nucl. Phys. 81, 1–60 (1966)
Meldner, H.: Predictions of new magic regions and masses for super-heavy nuclei from calculations with realistic shell model single particle Hamiltonians. In Ref. [5], pp. 593–598
Proceedings International Symposium. Why and How Should We Investigate Nuclides far off the Stability Line, Lysekil 1966. Arkiv Fysik 36 (1967)
Sobiczewski, A., Gareev, F.A., Kalinkin, B.N.: Closed shells for Z > 82 and N > 126 in a diffuse potential well. Phys. Lett. 22, 500–502 (1966)
Nilsson, S.G., Nix, J.R., Sobiczewski, A., Szymański, Z., Wycech, S., Gustafson, C., Möller, P.: On the spontaneous fission of nuclei with Z near 114 and N near 184. Nucl. Phys. A115, 545–562 (1968)
Grumann, J., Mosel, U., Fink, B., Greiner, W.: Investigation of the stability of superheavy nuclei around Z = 114 and Z = 164. Z. Phys. 228, 371–386 (1969)
Nilsson, S.G., Tsang, C.F., Sobiczewski, A., Szymański, Z., Wycech, S., Gustafson, C., Lamm, I.-L., Möller, P., Nilsson, B.: On the nuclear structure and stability of heavy and superheavy elements. Nucl. Phys. A131, 1–66 (1969)
Nilsson, S.G., Thompson, S.G., Tsang, C.F.: Stability of superheavy nuclei and their possible occurence in nature. Phys. Lett. 28B, 458–461 (1969)
Meldner, H., Herrmann, G.: Superheavy elements in nature? Z. Naturforschung 24a, 1429–1430 (1969)
Fiset, E.O., Nix, J.R.: Calculation of half-lives for superheavy nuclei. Nucl. Phys. A193, 647–671 (1972)
Flerov, G.N.: Proceedings of Nobel Symposium 27, Super-Heavy Elements—Theoretical Predictions and Experimental Generation, Ronneby 1974, Phys. Scr. 10A, 1 (1974)
Fricke, B., Greiner, W., Waber, J.T.: The continuation of the periodic table up to Z = 172. The chemistry of superheavy elements. Theoret. Chim. Acta 21, 235–260 (1971)
Fricke, B.: Superheavy elements. Struct. Bond. 21, 89–144 (1975)
Keller, O.L., Burnett, J.L., Carlson, T.A., Nestor, C.W.: Predicted properties of the super heavy elements. I. Elements 116 and 114, eka-thallium and eka-lead. J. Phys. Chem. 74, 1127–1134 (1970)
Eichler, B.: Das Flüchtigkeitsverhalten von Transactiniden im Bereich um Z = 114 (Voraussage). Kernenergie 19, 307–311 (1976)
Kratz, J.V., Liljenzin, J.O., Seaborg, G.T.: A chemical group separation procedure for superheavy elements and various other reaction products from heavy-ion bombarded uranium targets. Inorg. Nucl. Chem. Lett. 10, 951–957 (1974)
Pitzer, K.S.: Are elements 112, 114, and 118 relatively inert gases? J. Chem. Phys. 63, 1032–1033 (1975)
Waber, J.T., Cromer, D.T., Liberman, D.: SCF Dirac-Slater calculations of the trans-lawrencium elements. J. Chem. Phys. 51, 664–668 (1969)
Herrmann, G.: Superheavy elements. In: Maddock, A.G. (ed.) International Review of Science, Inorganic Chemistry, Radiochemistry, vol. 8, pp. 221–272. Butterworths, London (1975)
Thompson, S.G., Tsang, C.F.: Superheavy elements. Science 178, 1047–1055 (1972)
Seaborg, G.T., Loveland, W., Morrissey, D.J.: Superheavy elements—a crossroads. Science 203, 711–717 (1979)
Herrmann, G.: Superheavy-element research. Nature 280, 543–549 (1979)
Kratz, J.V.: The search for superheavy elements. Radiochim. Acta 32, 25–41 (1983)
Flerov, G.N., Ter-Akopyan, G.M.: Superheavy nuclei. Rep. Prog. Phys. 46, 817–875 (1983)
Herrmann, G.: Synthesis of the heaviest chemical elements—results and perspectives. Angew. Chem. Int. Ed. Engl. 27, 1417–1436 (1988); transl. from Angew. Chem. 100, 1471–1491 (1988)
Schramm, D.N., Fowler, W.A.: Synthesis of superheavy elements in the r-process. Nature 231, 103–106 (1971)
Viola, V.E.: On the production of nuclides with A > 250 in stellar nucleosynthesis. Nucl. Phys. A139, 188–202 (1969)
Schramm, D.N., Fiset, E.O.: Superheavy elements and the r-process. Astrophys. J. 180, 551–570 (1973)
Howard, W.M., Nix, J.R.: Production of superheavy nuclei by multiple capture of neutrons. Nature 247, 17–20 (1974)
Meyer, B.S., Möller, P., Howard, W.M., Mathews, G.J.: Fission barriers for r-process nuclei and implications for astrophysics. In: Behrens, J.W., Carlson, A.D. (eds.) Proceedings of Conference 50 Years with Nuclear Fission, Gaithersburg 1989, pp. 587–591. Amer. Nucl. Soc., La Grange Park (1989)
Price, B.P., Fowler, P.H., Kidd, J.M., Kobetich, E.J., Fleischer, R.L., Nichols, G.E.: Study of the charge spectrum of extremely heavy cosmic rays using combined plastic detectors and nuclear emulsions. Phys. Rev. D3, 815–823 (1971)
Vdovenko, V.M., Sobotovich, M.: The problem of the possible existence of super-heavy elements in nature. Sov. Phys. Doklady 14, 1179–1182 (1969); transl. from Dokl. Akad. Nauk SSSR Fiz. Ser. 189, 980–983 (1969)
Haack, U.: Suche nach überschweren Transuranelementen. Untersuchung irdischer Minerale mit Hilfe der Spaltspurmethode. Naturwissenschaften 60, 65–70 (1973)
Flerov, G.N., Perelygin, V.P.: Spontaneous fission of lead and the search for transuranium elements. Sov. J. Atom. Energy 26, 603–605 (1969); transl. from Atomnaya Energiya 26, 520–522 (1969)
Flerov, G.N., Perelygin, V.P., Otgonsurén, O.: Origin of tracks of fission products in lead glasses. Sov. J. Atom. Energy 33, 1144–1149 (1972); transl. from Atomnaya Energiya 33, 979–984 (1972)
Geisler, F.H., Phillips, P.R., Walker, R.M.: Search for superheavy elements in natural and proton-irradiated materials. Nature 244, 428–429 (1973)
Price, P.B., Fleischer, R.L., Woods, R.T.: Search for spontaneously fissioning elements in nature. Phys. Rev. C1, 1819–1821 (1970)
Grimm, W., Herrmann, G., Schüssler, H.-D.: Search for superheavy elements in terrestrial matter. Phys. Rev. Lett. 26, 1040–1043, err. 1408 (1971)
Nix, J.R.: Predicted properties of the fission of super-heavy nuclei. Phys. Lett. 30B, 1–4 (1969)
Schmitt, H.W., Mosel, U.: Fission properties of heavy and superheavy nuclei. Nucl. Phys. A186, 1–14 (1972)
Macklin, R.L., Glass, F.M., Halperin, J., Roseberry, R.T., Schmitt, H.W., Stoughton, R.W., Tobias, M.: Neutron multiplicity counter. Nucl. Instr. Meth. 102, 181–187 (1972)
Ter-Akopyan, G.M., Popeko, A.G., Sokol, E.A., Chelnokov, L.P., Smirnov, V.I., Gorshkov, V.A.: A neutron multiplicity counter for rare spontaneous fission events. Nucl. Instr. Meth. Phys. Res. 190, 119–124 (1981)
Cheifetz, E., Jared, R.C., Giusti, E.R., Thompson, S.G.: Search for superheavy elements in nature. Phys. Rev. C6, 1348–1361 (1972)
Behringer, K., Grütter, A., von Gunten, H.R., Schmid, A., Wyttenbach, A., Hahn, B., Moser, U., Reist, H.W.: Search for superheavy elements. Phys. Rev. C9, 48–55 (1974)
Flerov, G.N., Korotkin, Yu.S., Ter-Akopyan, G.M., Zvara, I., Oganessian, Yu.Ts., Popeko, A.G., Chuburkov, Yu.T., Chelnokov, L.P., Maslov, O.D., Smirnov, V.I., Gerstenberger, R.: Results of the searches for superheavy nuclei in the Cheleken penninsula geothermal waters. Z. Phys. A292, 43–48 (1979)
Chuburkov, Yu.T., Popeko, A.G., Skobelev, N.K.: Concentrating a new natural spontaneously fissile nuclide from Cheleken geothermal brine. Sov. Radiochem. 30, 108–117 (1988); transl. from Radiokhimiya 30, 112–121 (1988)
Ter-Akopyan, G.M., Sokol, E.A., Fam Ngoc Chuong, Ivanov, M.P., Popeko, G.S., Molzahn, D., Lund, T., Feige, G., Brandt, R.: Search for spontaneous fission activity in Salton sea and Atlantis II hot brines. Z. Phys. A316, 213–215 (1984)
McMinn, J., Ihle, H.R., Wagner, R.: Use of an electromagnetic isotope separator in the search for element 114 in nature. Nucl. Instr. Meth. 139, 175–180 (1976)
Gentry, R.V., Cahill, T.A., Fletcher, N.R., Kaufmann, H.C., Medsker, L.R., Nelson, J.W., Flocchini, R.G.: Evidence for primordial superheavy elements. Phys. Rev. Lett. 37, 11–15 (1976)
Gentry, R.V.: Radiohalos in a radiochronological and cosmological perspective. Science 184, 62–66 (1970)
Gentry, R.V.: Radioactive halos. Ann. Rev. Nucl. Sci. 23, 347–362 (1973)
Wolke, R.L.: Proposed experiment to corroborate the existence of superheavy element 116 in nature. Phys. Rev. Lett. 37, 1098–1100 (1976)
Bosch, F., El Goresy, A., Krätschmer, W., Martin, B., Povh, B., Nobiling, R., Traxel, K., Schwalm, D.: Comment on the reported evidence for primordial superheavy elements. Phys. Rev. Lett. 37, 1515–1517 (1976)
Wölfli, W., Lang, J., Bonani, G., Suter, M., Stoller, Ch., Nissen, H.-U.: Evidence for primordial superheavy elements? J. Phys. G3, L33–L37 (1977)
Sparks, C.J., Raman, S., Yakel, H.L., Gentry, R.V., Krause, M.O.: Search with synchrotron radiation for superheavy elements in giant-halo inclusions. Phys. Rev. Lett. 38, 205–208 (1977)
Sparks, C.J., Raman, S., Ricci, E., Gentry, R.V., Krause, M.O.: Evidence against superheavy elements in giant-halo inclusions re-examined with synchrotron radiation. Phys. Rev. Lett. 40, 507–511, err. 1112 (1978)
Stéphan, C., Epherre, M., Cieślak, E., Sowiński, M., Tys, J.: Search for superheavy elements in monazite ore from madagascar. Phys. Rev. Lett. 37, 1534–1536 (1976)
Stakemann, R., Heimann, R., Herrmann, G., Tittel, G., Trautmann, N.: Search for superheavy elements in monazites using chemical enrichment. Nature 297, 136–137 (1982)
Reynolds, J.H., Turner, G.: Rare gases in the chondrite Renazzo. J. Geophys. Res. 69, 3263–3281 (1964)
Anders, E., Heymann, D.: Elements 112–119: were they present in meteorites? Science 164, 821–823 (1969)
Dakowski, M.: The possibility of extinct superheavy elements occuring in meteorites. Earth Planet. Sci. Lett. 6, 152–154 (1969)
Anders, E., Larimer, J.W.: Extinct superheavy element in meteorites: attempted characterization. Science 175, 981–983 (1972)
Anders, E., Higuchi, H., Gros, J., Takahashi, H., Morgan, J.W.: Extinct superheavy element in the allende meteorite. Science 190, 1262–1271 (1975)
Lewis, R.S., Srinivasan, B., Anders, E.: Host phase of a strange xenon component in Allende. Science 190, 1251–1262 (1975)
Manuel, O.K., Hennecke, E.W., Sabu, D.D.: Xenon in carbonaceous chondrites. Nature Phys. Sci. 240, 99–101 (1972)
Manuel, O.K., Sabu, D.D.: Strange xenon, extinct superheavy elements, and the solar neutrino puzzle. Science 195, 208–209 (1977)
Begemann, F.: Isotopic anomalies in meteorites. Rep. Prog. Phys. 43, 1309–1356 (1980)
Lewis, R.S., Anders, E., Shimamura, T., Lugmair, G.W.: Barium isotopes in Allende meteorite: evidence against an extinct superheavy element. Science 222, 1013–1015 (1983)
Popeko, A.G., Skobelev, N.K., Ter-Akopyan, G.M., Goncharov, G.N.: Search for superheavy elements in meteorites. Phys. Lett. 52B, 417–420 (1974)
Flerov, G.N., Ter-Akopyan, G.M., Popeko, A.G., Fefilov, B.V., Subbotin, V.G.: Observation of a new spontaneously fissile nuclide in certain meterorites. Sov. J. Nucl. Phys. 26, 237–240 (1977); transl. from Yadern. Fiz. 26, 449–454 (1977)
Amirbekyan, A.V., Davtyan, L.S., Markaryan, D.V., Khudaverdyan, A.G.: Once again on the new spontaneously fissile nuclide in the Allende meteorite. Sov. J. Nucl. Phys. 36, 786–787 (1982); transl. from Yadern. Fiz. 36, 1356–1359 (1982)
Zvara, I., Flerov, G.N., Zhuĭkov, B.L., Reetz, T., Shalaevskiĭ, M.R., Skobelev, N.K.: Experiments on chemical concentration of a new spontaneously fissile nuclide from material of the Allende meteorite. Sov. J. Nucl. Phys. 26, 240–243 (1977); transl. from Yadern. Fiz. 26, 455–460 (1977)
Lund, T., Becker, H.-J., Jungclas, H., Molzahn, D., Vater, P., Brandt, R.: Are the superheavy elements in the meteorite Allende? Inorg. Nucl. Chem. Lett. 15, 413–416 (1979)
Lund, T., Tress, G., Khan, E.U., Molzahn, D., Vater, P., Brandt, R.: Further attempts to isolate superheavy elements in the meteorite Allende. J. Radioanal. Nucl. Chem. Lett. 93, 363–370 (1985)
Shirk, E.K., Price, P.B.: Charge and energy spectra of cosmic rays with Z > 60: the skylab experiment. Astrophys. J. 220, 719–733 (1978)
Fowler, P.H., Walter, R.N.F., Masheder, M.R.W., Moses, R.T., Worley, A., Gay, A.M.: Ariel 6 measurements of the flux of ultraheavy cosmic rays. Astrophys. J. 314, 739–747 (1987)
Otgonsurén, O., Perelygin, V.P., Stetsenko, S.G., Gavrilova, N.N., Fiéni, C., Pellas, P.: Abundances of Z > 52 nuclei in galactic cosmic rays: long-term averages based on studies of pallasites. Astrophys. J. 210, 258–266 (1976)
Perelygin, V.P., Stetsenko, S.G.: Search for the tracks of galactic cosmic nuclei with Z > 110 in meteorite olivins. JETP Lett. 32, 608–610 (1980); transl. from Pisma Zh. Eksp. Teor. Fiz. 32, 622–625 (1980)
Perron, C., Bourot-Denise, M., Perelygin, V.P., Birkholz, W., Stetsenko, S.G., Dersch, R., Zhu, T.C., Vater, P., Brandt, R.: Reveletion of heavy ion tracks in olivine: orientation dependent annealing or etching? Nucl. Tracks Radiat. Meas. 15, 231–234 (1988)
Perelygin, V.P., Stetsenko, S.G.: Results of a calibration of olivines from meteorites by means of 238U nuclei at the Bevalac accelerator. JETP Lett. 49, 292–296 (1989); transl. from Pisma Zh. Eksp. Teor. Fiz. 49, 257–260 (1989)
Otgonsurén, O., Perelygin, V.P., Flerov, G.N.: Search for far transuranic elements in ferromanganese nodules. Sov. Phys. Doklady 14, 1194–1197 (1970); transl. from Dokl. Akad. Nauk SSSR Fiz. Ser. 189, 1200–1203 (1969)
Oganessian, Yu.: Heaviest nuclei from 48Ca-induced reactions. J. Phys. G34, R165–R242 (2007)
Sikkeland, T.: Synthesis of nuclei in the region of Z = 126 and N = 184. In: Ref. [5], pp. 539–552
Wong, C.Y.: On the production of the superheavy nucleus 310126. Nucl. Phys. A103, 625–643 (1967)
Lefort, M.: Recherche des éléments superlourds par synthèse nucléaire. Ann. Physique 5, 355–376 (1970)
Bimbot, R., Deprun, C., Gardès, D., Gauvin, H., Le Beyec, Y., Lefort, M., Pèter, J., Tamain, B.: Complete fusion induced by krypton ions: Indications for synthesis of superheavy nuclei. Nature 234, 215–216 (1971)
Colombani, P., Gatty, B., Jacmart, J.C., Lefort, M., Peter, J., Riou, M., Stéphan, C., Tarrago, X.: Superheavy compound nuclei investigated with in-flight mass spectroscopy. Phys. Lett. 42B, 208–210 (1972)
Marinov, A., Batty, C.J., Kilvingston, A.I., Newton, G.W.A., Robinson, V.J., Hemingway, J.D.: Evidence for the possible existence of a superheavy element with atomic number 112. Nature 229, 464–467 (1971)
Unik, J.P., Horwitz, E.P., Wolf, K.L., Ahmad, I., Fried, S., Cohen, D., Fields, P.R., Bloomquist, C.A.A., Henderson, D.J.: Production of actinides and the search for super-heavy elements using secondary reactions induced by GeV protons. Nucl. Phys. A191, 233–244 (1972)
Westgaard, L., Erdal, B.R., Hansen, P.G., Kugler, E., Sletten, G., Sundell, S., Fritsch, T., Henrich, E., Theis, W., Wolf, G.K., Camplan, J., Klapisch, R., Meunier, R., Poszkanzer, A.M., Stéphan, C., Tys, J.: Search for super-heavy elements produced by secondary reactions in uranium. Nucl. Phys. A192, 519–523 (1972)
Batty, C.J., Kilvingston, A.I., Weil, J.L., Newton, G.A.W., Skarestad, M., Hemingway, J.D.: Search for superheavy elements and actinides produced by secondary reactions in a tungsten target. Nature 244, 429–430 (1973)
Hulet, E.K., Lougheed, R.W., Wild, J.F., Landrum, J.H., Stevenson, P.C., Ghiorso, A., Nitschke, J.M., Otto, R.J., Morrissey, D.J., Baisden, P.A., Gavin, B.F., Lee, D., Silva, R.J., Fowler, M.M., Seaborg, G.T.: Search for superheavy elements in the bombardment of 248Cm with 48Ca. Phys. Rev. Lett. 39, 385–389 (1977)
Illige, J.D., Hulet, E.K., Nitschke, J.M., Dougan, R.J., Lougheed, R.W., Ghiorso, A., Landrum, J.H.: Search for volatile superheavy elements from the reaction 248Cm + 48Ca. Phys. Lett. 78B, 209–212 (1978)
Oganessian, Yu.Ts., Bruchertseifer, H., Buklanov, G.V., Chepigin, V.I., Choy Val Sek, Eichler, B., Gavrilov, K.A., Gäggeler, H., Korotkin, Yu.S., Orlova, O.A., Reetz, T., Seidel, W., Ter-Akopyan, G.M., Tretyakova, S.P., Zvara, I.: Experiments to pro-duce isotopes of superheavy elements with atomic numbers 114–116 in 48Ca ion reactions. Nucl. Phys. A294, 213–224 (1978)
Ter-Akopyan, G.M., Bruchertseifer, H., Buklanov, G.V., Orlova, O.A., Pleve, A.A., Cherpigin, V.I., Choy Val Sek: Experiments on synthesis of odd neutron deficient isotopes of superheavy elements in reactions induced by 48Ca ions. Sov. J. Nucl. Phys. 29, 312–316 (1979); transl. from Yad. Fiz. 29, 608–614 (1979)
Armbruster, P., Agarwal, Y.K., Brüchle, W., Brügger, M., Dufour, J.P., Gäggeler, H., Hessberger, F.P., Hofmann, S., Lemmertz, P., Münzenberg, G., Poppensieker, K., Reisdorf, W., Schädel, M., Schmidt, K.-H., Schneider, J.H.R., Schneider, W.F.W., Sümmerer, K., Vermeulen, D., Wirth, G., Ghiorso, A., Gregorich, K.E., Lee, D., Leino, M., Moody, K.J., Seaborg, G.T., Welch, R.B., Wilmarth, P., Yashita, S., Frink, C., Greulich, N., Herrmann, G., Hickmann, U., Hildebrand, N., Kratz, J.V., Trautmann, N., Fowler, M.M., Hoffman, D.C., Daniels, W.R., von Gunten, H.R., Dornhöfer, H.: Attempts to produce superheavy elements by fusion of 48Ca with 248Cm in the bombarding energy range of 4.5–5.2 MeV/u. Phys. Rev. Lett. 54, 406–409 (1985)
Peuser, P., Tharun, U., Keim, H.-J., Trautmann, N., Herrmann, G., Wirth, G.: A low background detection system for fragments and neutrons from spontaneous fission sources. Nucl. Instr. Meth. Phys. Res. A239, 529–535 (1985)
Lougheed, R.W., Landrum, J.H., Hulet, E.K., Wild, J.F., Dougan, R.J., Dougan, A.D., Gäggeler, H., Schädel, M., Moody, K.J., Gregorich, K.E., Seaborg, G.T.: Search for superheavy elements using the 48Ca + 254Esg reaction. Phys. Rev. C32, 1760–1763 (1985)
Schädel, M., Kratz, J.V., Ahrens, H., Brüchle, W., Franz, G., Gäggeler, H., Warnecke, I., Wirth, G., Herrmann, G., Trautmann, N., Weis, M.: Isotope distribution in the reaction of 238U with 238U. Phys. Rev. Lett. 41, 469–472 (1978)
Riedel, C., Nörenberg, W.: Theoretical estimates for the production of transuranium elements in heavy-ion collisions. Z. Phys. A290, 385–391 (1979)
Herrmann, G.: Search for superheavy elements in damped collisions of 238U with 238U. Pure Appl. Chem. 53, 949–964 (1981)
Hildebrand, N., Frink, C., Greulich, N., Hickmann, U., Kratz, J.V., Trautmann, N., Herrmann, G., Brügger, M., Gäggeler, H., Sümmerer, K., Wirth, G.: A cryosystem for the detection of alpha and spontaneous-fission activities in volatile species. Nucl. Instr. Meth. Phys. Res. A260, 407–412 (1987)
Gäggeler, H., Trautmann, N., Brüchle, W., Herrmann, G., Kratz, J.V., Peuser, P., Schädel, M., Tittel, G., Wirth, G., Ahrens, H., Folger, H., Franz, G., Sümmerer, K., Zendel, M.: Search for superheavy elements in the 238U + 238U reaction. Phys. Rev. Lett. 45, 1824–1827 (1980)
Hildenbrand, K.D., Freiesleben, H., Pühlhofer, F., Schneider, W.F.W., Bock, R., v. Harrach, D., Specht, H.J.: Reaction between 238U and 238U at 7.42 MeV/nucleon. Phys. Rev. Lett. 39, 1065–1068 (1977)
Jungclas, H., Hirdes, D., Brandt, R., Lemmertz, P., Georg, E., Wollnik, H.: Search for superheavy elements in the interaction of 136Xe and 238U with natU using the gas jet transport technique. Phys. Lett. 79B, 58–60 (1978)
Aumann, D.C., Faleschini, H., Friedmann, L., Weismann, D.: Search for volatile superheavy elements in heavy-ion reactions. Phys. Lett. 82B, 361–364 (1979)
Kratz, J.V., Brüchle, W., Folger, H., Gäggeler, H., Schädel, M., Sümmerer, K., Wirth, G., Greulich, N., Herrmann, G., Hickmann, U., Peuser, P., Trautmann, N., Hulet, E.K., Lougheed, R.W., Nitschke, J.M., Ferguson, R.L., Hahn, R.L.: Search for superheavy elements in damped collisions between 238U and 248Cm. Phys. Rev. C33, 504–508 (1986)
Schädel, M., Brüchle, W., Gäggeler, H., Kratz, J.V., Sümmerer, K., Wirth, G., Herr-mann, G., Stakemann, R., Tittel, G., Trautmann, N., Nitschke, J.M., Hulet, E.K., Lougheed, R.W., Hahn, R.L., Ferguson, R.L.: Actinide production in collisions of 238U with 248Cm. Phys. Rev. Lett. 48, 852–855 (1982)
Oganessian, Yu.Ts., Utyonkov, V.K., Lobanov, Yu.V., Abdullin, F.Sh., Polyakov, A.N., Shirokovsky, I.V., Tsyganov, Yu.S., Gulbekian, G.G., Bogomolov, S.L., Gikal, B.N., Mezentsev, A.N., Iliev, S., Subbotin, V.G., Sukhov, A.M., Voinov, A.A., Buklanov, G.V., Subotic, K., Zagrebaev, V.I., Itkis, M.G., Patin, J.B., Moody, K.J., Wild, J.F., Stoyer, M.A., Stoyer, N.J., Shaughnessy, D.A., Kenneally, J.M., Wilk, P.A., Lougheed, R.W., Ilkaev, R.I., Vesnovskii, S.P.: Measurements of cross sections and decay properties of the isotopes of elements 112, 114, and 116 produced in the fusion reactions of 233,238U, 242Pu, and 248Cm +48Ca. Phys. Rev. C70, 064609–1/064609–14 (2004)
Ghiorso, A., Nitschke, J.M., Alonso, J.R., Alonso, C.T., Nurmia, M., Seaborg, G.T., Hulet, E.K., Lougheed, R.W.: Element 106. Phys. Rev. Lett. 33, 1490–1493 (1974)
Oganessian, Yu.Ts., Iljinov, A.S., Demin, A.G., Tretyakova, S.P.: Experiments on the production of fermium neutron-deficient isotopes and new possibilities of synthesizing elements with Z > 100. Nucl. Phys. A239, 353–364 (1975)
Münzenberg, G., Hofmann, S., Heßberger, F.P., Reisdorf, W., Schmidt, K.H., Schneider, J.H.R., Armbruster, P., Sahm, C.C., Thuma, B.: Identification of element 107 by α correlation chains. Z. Phys. A300, 107–108 (1981)
Armbruster, P.: On the production of heavy elements by cold fusion: the elements 106–109. Ann. Rev. Nucl. Part. Sci. 35, 135–194 (1985)
Čwiok, S., Pashkevich, V.V., Dudek, J., Nazarevicz, W.: Fission barriers of trans-fermium elements. Nucl. Phys. A410, 254–270 (1983)
Möller, P., Leander, G.A., Nix, J.R.: On the stability of transeinsteinium elements. Z. Phys. A323, 41–45 (1986)
Silva, R., Harris, J., Nurmia, M., Eskola, K., Ghiorso, A.: Chemical separation of rutherfordium. Inorg. Nucl. Chem., Lett. 6, 871–877 (1970)
Hulet, E.K., Lougheed, R.W., Wild, J.F., Landrum, J.H., Nitschke, J.M., Ghiorso, A.: Chloride complexation of element 104. J. Inorg. Nucl. Chem. 42, 79–82 (1980)
Zvara, I., Belov, V.Z., Domanov, V.P., Korotkin, Yu.S., Chelnokov, L.P., Shalaevs-kii, M.R., Shegolev, V.A., Hussonnois, M.: Chemical isolation of kurchatovium. Sov. Radiochem. 14, 115–118 (1972); transl. from Radiokhimiya 14, 119–122 (1972)
Zvara, I., Eichler, B., Belov, V.Z., Zvarova, T.S., Korotkin, Yu.S., Shalaevskii, M.R., Shegolev, V.A., Hussonnois, M.: Gas chromatography and thermochromatography in the study of transuranic elements. Sov. Radiochem. 16, 709–715 (1974); transl. from Radiokhimiya 16, 720–727 (1974)
Zvara, I., Belov, V.Z., Domanov, V.P., Shalaevskii, M.R.,: Chemical isolation of nilsbohrium as ekatantalum in the form of the anhydrous bromide. II: Experiments with a spontaneously fissioning isotope of nilsbohrium. Sov. Radiochem. 18, 328–334 (1976); transl. from Radiokhimiya 18, 371–377 (1976)
Gregorich, K.E., Henderson, R.A., Lee, D.M., Nurmia, M.J., Chasteler, R.M., Hall, H.L., Bennett, D.A., Gannett, C.M., Chadwick, R.B., Leyba, J.D., Hoffman, D.C., Herrmann, G.: Aqueous chemistry of element 105. Radiochim. Acta 43, 223–231 (1988)
Herrmann, G., Trautmann, N.: Rapid chemical methods for the identification and study of short-lived nuclides. Ann. Rev. Nucl. Part. Sci. 32, 117–147 (1982)
Brüchle, W., Schädel, M., Scherer, U.W., Kratz, J.V., Gregorich, K.E., Lee, D., Nurmia, M., Chasteler, R.M., Hall, H.L., Henderson, R.A., Hoffman, D.C.: The hydration enthalpies of Md3+ and Lr3+. Inorg. Chim. Acta 146, 267–276 (1988)
Jost, D.T., Gäggeler, H.W., Vogel, Ch., Schädel, M., Jäger, E., Eichler, B., Gregorich, K.E., Hoffman, D.C.: Search for lawrencium as a p-element using gas chromatography techniques. Inorg. Chim. Acta 146, 255–259 (1988)
Schädel, M., Brüchle, W., Jäger, E., Schimpf, E., Kratz, J.V., Scherer, U.W., Zimmermann, H.P.: ARCA II, a new apparatus for fast, repetitive HPLC separations. Radio-chimica Acta 48, 171–176 (1989)
Gäggeler, H.W., Jost, D.T., Baltensperger, U., Weber, A., Kovacs, A., Vermeulen, D., Türler, A.: OLGA II, an on-line gas chemistry apparatus for applications in heavy element research. Nucl. Instr. Meth. Phys. Res. A309, 201–208 (1991)
Kratz, J.V., Zimmermann, H.P., Scherer, U.W., Schädel, M., Brüchle, W., Gregorich, K.E., Gannett, C.M., Hall, H.L., Henderson, R.A., Lee, D.M., Leyba, J.D., Nurmia, M.J., Hoffman, D.C., Gäggeler, H., Jost, D., Baltensperger, U., Nai-Qi, Ya., Türler, A., Lienert, Ch.: Chemical properties of element 105 in aquaeous solution. Halide complex formation and anion exchange into triisooctylamine. Radiochim. Acta 48, 121–133 (1989)
Gäggeler, H.W., Jost, D.T., Kovacs, J., Scherer, U.W., Weber, A., Vermeulen, D., Türler, A., Gregorich, K.E., Henderson, R.A., Czerwinski, K.R., Kadkhodayan, B., Lee, D.M., Nurmia, M.J., Hoffman, D.C., Kratz, J.V., Gober, M.K., Zimmermann, H.P., Schädel, M., Brüchle, W., Schimpf, E., Zvara, I.: Gas phase chromatography experiments with bromides of tantalum and element 105. Radiochim. Acta 57, 93–100 (1992)
Acknowledgment
The author is grateful to Brigitta Schausten, Dawn Shaughnessy and Matthias Schädel for their editing of the article.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
Herrmann, G. (2014). Historical Reminiscences: The Pioneering Years of Superheavy Element Research. In: Schädel, M., Shaughnessy, D. (eds) The Chemistry of Superheavy Elements. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-642-37466-1_9
Download citation
DOI: https://doi.org/10.1007/978-3-642-37466-1_9
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-642-37465-4
Online ISBN: 978-3-642-37466-1
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)