Abstract
Olfactory dysfunction is extremely common (affecting up to 20 % of the population) and impacts negatively the quality of life. Since therapies remain very limited, it is mandatory to perform a complete clinical evaluation of patients suffering from olfactory disorder in order to provide them an optional treatment but also a prognosis of recovery and adapted counseling.
Recently, the developments of new reliable psychophysical, electrophysiological, and imaging techniques have allowed the investigation of smell in humans, not only in research but also in clinical practice.
In this chapter, we will focus on the different techniques related to evaluation of olfactory function in clinical practice. New methods that may be useful in the future will also be discussed.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
FormalPara Core Messages-
Precise clinical workup is mandatory in patients suffering from olfactory dysfunction, in order to (1) accurately assess their olfactory deficit and, hence, provide them appropriate counseling and prognosis, (2) assess recovery from or progression of the olfactory dysfunction, and (3) evaluate a therapeutic success.
-
Self-assessment of olfactory function is not correlated to the results of olfactory testing.
-
In psychophysical evaluation, it is important to evaluate both orthonasal and retronasal olfactory functions since these two pathways have different central processing.
-
Psychophysical testings are semi-objective techniques and might be subject to patient’s bias.
-
Electrophysiological techniques are widely used to provide a relatively unbiased evaluation of olfactory system.
-
MRI is the imaging modality of choice to evaluate the olfactory apparatus.
30.1 Introduction
Evaluation of olfactory function in humans remained poorly explored for a long time. This was mostly due to the difficulty of producing selective and controlled olfactory stimuli (Moncrief 1962). Recently, the development of reliable techniques to investigate olfactory system has led to an increasing interest on the research on this field. Opposite to what has been assumed for many years, our chemosensory systems were shown to be an essential factor in terms of our behavior and well-being (for a review see Stevenson 2010).
In the last years, several authors have reported that olfactory disorders occur at a much higher frequency than previously assumed (Murphy et al. 2002; Bramerson et al. 2004; Landis et al. 2004; Landis and Hummel 2006), and it has been reported that 20 % of the population suffers from olfactory disorders (Landis and Hummel 2006). Hence, the field of research on olfaction is not only on interest for basic scientists but also for clinicians.
It is widely assumed that a precise clinical workup procedure is mandatory in order to assess the olfactory function of patients suffering from smell disorders. Indeed, it is essential to (1) accurately assess their olfactory deficit and, hence, provide them appropriate counseling and prognosis, (2) assess recovery from or progression of the olfactory dysfunction, and (3) evaluate a therapeutic success.
The direct way to monitor olfactory function is self-assessment. Nevertheless, self-assessment is biased by unspecific factors (i.e., nasal airway patency (Landis et al. 2003), mood (Savina et al. 2003)), and it seems to be uncorrelated to results from olfactory testings (Landis et al. 2003).
Several methods have been developed and validated to quantify the olfactory function.
Today, we dispose of reliable psychophysical testing, electrophysiological testing of chemosensory function, and high-performance imaging.
This chapter will focus on the techniques related to evaluation of olfactory function.
30.2 Psychophysical Evaluation
As mentioned in the chapter, “olfaction” odorants might reach the olfactory receptor neurons by two ways: orthonasally or retronasally. It has been demonstrated that these two pathways are different regarding to the perceptual (Hummel et al. 2006) and central nervous processing (Small et al. 2005). Clinically, intact orthonasal and altered retronasal olfaction (or vice versa) have been found in several conditions (Hummel et al. 2007a), although usually orthonasal and retronasal functions are well corrected. Hence, it is important to evaluate both orthonasal and retronasal olfactory function in patients complaining of olfactory disorders. Several tests have been proposed to assess psychophysically the olfactory function (for a review, see Scadding et al. 2011), based on odor identification, odor detection thresholds, odor discrimination, or a combination of two or more of these items. A non-exhaustive list of these tests is proposed in Table 30.1.
30.2.1 Orthonasal Olfactory Function
The evaluation of the orthonasal olfactory function is most often performed using psychophysical test such as Sniffin’ Sticks test (Kobal et al. 1996; Hummel et al. 2007b) or the University of Pennsylvania Smell Identification Test (UPSIT) (Doty et al. 1984). The majority of odor tests are forced choice, meaning that the subject must provide a response even if no odor is perceived. The Sniffin’ Sticks test (Fig. 30.1) consists of pen-like odor-dispensing devices that are presented in front of the nose of the patient. There exist two versions of the Sniffin’ Sticks test: the screening test and the extended version. The screening test version is based on an odor identification test of 12 different odorants (Hummel et al. 2001). The extended version encompasses three different approaches, namely, tests for odor threshold (T), odor discrimination (D), and odor identification (I). The odor thresholds for n-butanol are assessed using a single-staircase, three-alternative forced-choice procedure. The odor discrimination is also assessed using a triple forced-choice procedure. Triplets of pens are presented to the subject, with two containing the same and one containing one different odorant. Finally, odor identification is assessed for 16 common odors using a multiple-choice task identification of individual odors from a list of four descriptors. To judge olfactory function, results from the three subtests are summed up to provide a total TDI score with a maximum of 48 points (Kobal et al. 1996; Hummel et al. 2007b). The UPSIT uses 40 items. It encompasses four “scratch and sniff” booklets. Odorants are embedded in microcapsules placed on strips at the bottom of the page of booklets. The stimuli are released by scratching the strip with a pencil, and subjects have to choose one of the four proposed descriptors that best correspond to the respective odor (Doty et al. 1984; Tourbier and Doty 2007).
Sniffin’ Sticks test. The Sniffin’ Sticks test consists of pen-like odor-dispensing devices that are presented in front of the nose of the patient. The extended version encompasses three different approaches, namely, tests for odor threshold (T), odor discrimination (D), and odor identification (I). To judge olfactory function, results from the 3 subtests, each quoted out of 16, are summed up to provide a total TDI score with a maximum of 48
30.2.2 Retronasal Olfactory Function
Retronasal olfaction is assessed following a standardized method using a row of 20 items. The substances presented to the subjects are grocery store condiments and food items available in powder (e.g., spices, instant soup). Powderized substances are applied using squeezable plastic vials (Fig. 30.2) in the middle of the tongue inside the oral cavity. Before application of the first stimulant and after each trial, subjects rinsed their oral cavity with tap water, in order to minimize the interindividual differences in salivation, which might interfere with the release of odorants. Each substance is identified by means of a 4-verbal-item forced-choice procedure (Heilmann et al. 2002).
These orthonasal and retronasal tests have the advantage of being easy to implement, having been validated in multicenter studies (Heilmann et al. 2002, 2007b), and of having high test-retest reliability (Doty et al. 1984; Heilmann et al. 2002; Haehner et al. 2009). There is a correlation between the orthonasal and retronasal score (Rombaux et al. 2009c) (Fig. 30.3). However, these tests have the disadvantage of being semi-objective and of being subjects to the patient’s response bias. This may constitute a major issue when evaluating patients with olfactory disorder, particularly within a medicolegal context.
Correlation between orthonasal and retronasal olfactory function. Figure illustrates the correlation between orthonasal and retronasal olfactory function in cohorts of patients suffering from postinfectious olfactory loss, posttraumatic olfactory loss, and idiopathic olfactory loss (unpublished data)
30.3 Electrophysiological Evaluation
Electrophysiological techniques are widely used to provide a relatively unbiased evaluation of sensory systems. Unlike other sensory modalities (auditory, visual, somatosensory), the use of electrophysiological recordings to assess the chemosensory system in humans was not possible for a long time. This was mostly due to the difficulty to produce selective and controlled olfactory stimulus (Moncrief 1962). Indeed, the major difficulties consist in delivering odorants on the mucosa without producing artifacts such as thermal, tactile, or nociceptive co-activation.
The development of stimulation devices allowing delivering selective stimuli opened new perspectives for exploring noninvasively how the human brain processes chemosensory information. This was interesting not only for basic scientists but also for clinicians, since we know that certain clinical situation, such as examining demented patients or children, or even medicolegal situation requires unbiased tests (Hummel et al. 2000, 2010a; Rombaux et al. 2009a, c).
30.3.1 Olfactometer
Early stimulation techniques relied on the delivery of brief, odorized air pulses. Inevitably, the sudden increase in airflow associated with the presentation of an air puff activates mechano-sensitive trigeminal afferents and will produce brain responses, irrespective whether subjects would perceive the chemical stimulus or not.
In 1978, Kobal and Plattig (1978) introduced a device capable of delivering transient chemosensory stimuli to the olfactory neuroepithelium of the nasal mucosa. This air-dilution olfactometer delivers pulses of odorants embedded within a constant airflow at a constant temperature and humidity, thus avoiding concomitant mechanical or thermal stimulation of the nasal mucosa, making it possible to study brain responses related specifically to the activation of chemosensitive afferents (Fig. 30.4). Furthermore, using specific odorants, the device can be used to activate olfactory and trigeminal chemosensory receptors relatively selectively. For example, 2-phenylethanol is regarded as a relatively specific olfactory stimulant. It can be used to elicit olfactory event-related potentials (ERP). In contrast, gaseous CO2 is regarded to be virtually odorless, but produces irritating sensations. It is used to elicit trigeminal chemosensory ERP (Kobal 1981; Hummel and Kobal 1999; Lotsch and Hummel 2006; Rombaux et al. 2006a, b, c, 2007). Several studies have shown that this technique can be used to explore the cortical processing of olfactory and trigeminal chemosensory input in humans, through the recording of olfactory and trigeminal ERP (Pause and Krauel 2000; Rombaux et al. 2006a, b, c; Laudien et al. 2008). In addition, it has been shown that olfactory and trigeminal ERP exhibit a good test-retest reliability and were thus valuable for the clinical evaluation of patients (Hummel et al. 2000; Thesen and Murphy 2002; Welge-Lussen et al. 2003; Rombaux et al. 2009a, c).
Olfactometer. (a) Electrophysiological assessment of olfactory and trigeminal chemosensory function requires the use of an olfactometer that produces transient chemosensory stimuli to the olfactory neuroepithelium of the nasal mucosa. (b) The air-dilution olfactometer delivers pulses of odorants embedded within a constant airflow at a constant temperature and humidity, avoiding concomitant mechanical or thermal stimulation of the nasal mucosa (Olfactometer OM2S, Burghart Medical Technology, Wedel, Germany)
30.3.2 EEG Data Analyses
30.3.2.1 Time-Domain Averaging
Until now, the electroencephalographic (EEG) responses to chemosensory stimulation have been identified mainly using across-trial averaging in the time domain. This procedure cancels out changes in the EEG signal that are not strictly time locked and phase locked to the stimulus onset and, thereby, enhances the signal-to-noise ratio of time-locked ERP (Hummel et al. 1998; Pause and Krauel 2000; Rombaux et al. 2006a, b, c; Boesveldt et al. 2007; Mouraux and Iannetti 2008). Using such an approach the EEG responses to chemosensory stimulation have been characterized as a negative wave peaking approximately 320–500 ms after stimulus onset (N1), followed by a late positive wave peaking approximately 450–800 ms after stimulus onset (termed as P2 and/or P3) (Kobal 1985; Hummel and Kobal 1992; Pause et al. 1996; Geisler and Murphy 2000; Rombaux et al. 2006a, b, c; Hummel et al. 2010a; Haehner et al. 2011) (Fig. 30.5). All of these responses exhibit largest amplitudes over the midline recording sites. While the centro-parietal maximum for the N1 amplitude is commonly observed for olfactory responses, a more central maximum is observed for trigeminal stimuli (Kobal 1985; Geisler and Murphy 2000; Livermore and Hummel 2004; Olofsson et al. 2006; Hummel et al. 2010a).
Unfortunately, chemosensory ERPs – in particular, olfactory ERPs – usually exhibit a low signal-to-noise ratio (Lotsch and Hummel 2006; Boesveldt et al. 2007; Rombaux et al. 2007). Hence, although the recording of chemosensory ERPs is considered as a technique having great potentials, its clinical usefulness remains limited, particularly in the context of clinical diagnosis.
Conventional time-domain averaging presents some drawbacks, which could contribute to the low signal-to-noise ratio of chemosensory ERPs. First, temporal jitter could affect the brain responses to chemosensory stimulation. This jitter would result from the variability in timing of the different steps separating the occurrence of the sensory event and the occurrence of a cortical response. If this jitter is significant, the elicited ERPs will be cancelled out by the time-domain averaging procedure, as the responses are no longer stationary across trials. Because of all steps implied in chemosensory transduction, it is reasonable to expect that the responses to chemosensory stimulation are subject to a significant amount of jitter and that this jitter leads to an important distortion of the averaged ERP waveform. Second, time-domain averaging is unable to reveal any transient event-related modulation of the power of ongoing EEG oscillations (i.e., event-related desynchronization and event-related synchronization), which are thought to reflect cortical activation or deactivation, as these oscillations are cancelled out by conventional time-domain averaging procedures.
Taken together, time-domain averaging is thus blind to a significant fraction of the elicited EEG response (ERPs subject to a significant amount of temporal jitter, ERD, and ERS). This could contribute to explain why CSERPs are sometimes difficult to identify even in healthy subjects.
Olfactory chemosensory ERPs (Fz, Cz, Pz vs. A1A2) in one healthy normosmic volunteer. 2-Phenylethanol (50 %v/v) was used to selectively activate olfactory afferents. 30 stimuli of each chemical were presented, lasting 200 ms (20-ms rise time), separated by a 60-s interstimulus interval. Olfactory stimulation elicited an olfactory event-related potential (OERP). Two distinct peaks can be identified, maximal at the scalp vertex: N1 and P2
30.3.2.2 Time-Frequency Analysis
Time-frequency analysis constitutes an alternative approach to reveal activity that is induced by a chemosensory stimulus, but not sufficiently stationary across trials to be revealed by classic averaging in time domain. In this way, it could increase the signal-to-noise ratio of chemosensory EEG responses. Different methods exist to perform a time-frequency decomposition of EEG epochs. These methods rely on techniques to estimate within each EEG epoch the amplitude of the signal as a function of time and frequency, regardless of the phase. The obtained time-varying expressions of oscillation amplitude are then averaged across trials, thereby disclosing both phase-locked and non-phase-locked modulations of signal amplitude, provided that these modulations are relatively well time locked to the onset of the event and consistent in frequency. Identified in the time-frequency domain, EEG responses to sensory stimulation can be characterized by their latency, their frequency, their magnitude (often expressed as percentage relative to baseline), and their scalp distribution (Mouraux and Iannetti 2008). Several approaches have been proposed. At present, the continuous wavelet transform (CWT) is frequently used, as it is particularly well suited for the analysis of EEG signals. Indeed, by adapting the window width as a function of the estimated frequency, the CWT offers an optimal compromise for time-frequency resolution and is thus adequate for the evaluation of event-related modulations of the EEG spectrum within a wide range of frequencies (Mouraux and Iannetti 2008). The CWT can be performed in two ways. First, it can be applied at the level of each single EEG epochs (CWT-SINGLE). Second, it can be applied to the ERP waveforms obtained by averaging signals in the time domain (CWT-AVERAGE). The CWT-SINGLE transform enhances the signal-to-noise ratio of all time-locked EEG responses, regardless of whether they are phase locked to the onset of the stimulus, i.e., ERPs even when subject to a significant amount of temporal jitter, ERS, and ERD. In contrast, the CWT-AVERAGE average transform yields a time-frequency representation of the signals obtained using conventional time-domain averaging and will thus contain only EEG responses that are consistently phase locked to the stimulus. Therefore, EEG responses that are visible in both the CWT-SINGLE and CWT-AVERAGE can be considered as phase locked, whereas activities that are visible only in the CWT-SINGLE can be considered as non-phase locked (ERPs subject to jitter, ERS, and ERD) (Mouraux and Iannetti 2008). We have recently shown that the time-frequency approach markedly improved the signal-to-noise ratio of the EEG responses to chemosensory stimulation (in particular following olfactory stimulation), in comparison to conventional time-domain averaging. In addition, this approach allowed characterizing for the first time non-phase-locked components (ERS and ERD) that could not be identified using conventional time-domain averaging (Huart et al. 2012).
30.3.2.3 Event-Related Source Imaging
It is generally agreed that EEG has a high temporal resolution but a poor spatial resolution, whereas techniques based on hemodynamic measures (e.g., BOLD fMRI) have a poor temporal resolution but a high spatial resolution (Luck 2005). Although source analysis techniques are more appropriate to localize signals originating close to the scalp surface, several recent studies have suggested that EEG responses originating from deep brain structures can also be recorded and localized accurately (Kettenmann et al. 2001; Zumsteg et al. 2005). Source localization methods rely on mathematical models of the bioelectrical generators and the volume conductors within which they lie. The key limitation of these methods is that the inverse problem is highly undetermined and some assumptions have to be made when solving it. Hence, the validity of the obtained source configuration is strongly conditioned by the validity of these assumptions (e.g., assumptions concerning the number of sources or their approximate location). Source analysis of CSERP is probably particularly problematic, not only because of the relatively deep location of the hypothesized sources but also because multiple bilateral sources are thought to be simultaneously active, thus making it difficult to draw significant conclusions. Nevertheless, using high-resolution EEG, some researchers have attempted to localize the cortical structures generating the different components of CSERPs (Miyanari et al. 2006; Lascano et al. 2010). For example, in an attempt to provide information on the spatiotemporal sequence of information processing in the olfactory pathway, Lascano et al. (2010) performed source analysis of CSERPs, in which they suggested that olfactory input is processed first in the medial and lateral temporal cortex of the hemisphere ipsilateral to the stimulated nostril and only subsequently in the corresponding structures of the contralateral hemisphere. Until now, no clinical studies have been performed using such an analysis.
30.4 Imaging Evaluation
Advances in medical imaging allowed better morphological representation of chemosensory pathways and brain structures associated with chemosensory perception. Imaging modalities that are the frequently used in the clinical evaluation of patients include the computer tomography (CT) scan and magnetic resonance imaging (MRI). Recently, functional imaging became available.
30.4.1 Structural Imagery
MRI is the imaging modality of choice to evaluate the olfactory apparatus since it allows examining the olfactory bulb, olfactory tract, olfactory sulcus, and central olfactory projection areas (Fig. 30.6).
MRI coronal T2-weighted 2-mm-thick views using fast spin-echo (FSE) sequence. Figure shows comparative pictures between control subject with normal olfactory function (a) and patients suffering from postinfectious olfactory loss (b), posttraumatic olfactory loss (c), and congenital anosmia (d). The control subject has normal olfactory bulbs (white arrow) and olfactory sulcus (black arrows). In contrast, the patient with postinfectious olfactory loss (b) has decreased olfactory bulb volume (white arrow), and the patient suffering from posttraumatic (c) olfactory loss exhibits fragmented olfactory bulb (white arrow) and basifrontal contusion, principally in the right gyrus rectus (black asterisk). Finally, the patient with congenital anosmia (d) has no identifiable olfactory bulbs and olfactory sulcus
The olfactory bulb is often considered as the most important relay station in odor processing, and the olfactory bulb volume, assessed with MRI-based volumetric analyses, seems to be connected to the functional state of the olfactory system. Indeed, it was established that there was a good correlation between the olfactory bulb and the olfactory function not only in adults but also in children (Buschhuter et al. 2008; Hummel et al. 2011). Therefore, the assessment of the olfactory bulb volume is useful in the clinical evaluation of patients suffering from olfactory disorders. Several studies have shown that olfactory bulb volume was decreased in patients with postinfectious olfactory loss (Mueller et al. 2005; Rombaux et al. 2006a, b, c), posttraumatic olfactory loss (Yousem et al. 1996, 1999; Mueller et al. 2005; Rombaux et al. 2006a, b, c), idiopathic olfactory loss (Rombaux et al. 2010), congenital anosmia (Abolmaali et al. 2002), neurodegenerative disorder (Thomann et al. 2009), and psychiatric disease (Turetsky et al. 2000). Interestingly, a recent study conducted by Gudziol et al. (2009) showed that the olfactory bulb had a plasticity, since its volume can increase after treatment for chronic rhinosinusitis.
The olfactory sulcus is linked to the development of the olfactory system since it receives projections from the olfactory bulb and tract. Hummel et al. showed that the depth of the olfactory sulcus in the plane of the posterior tangent through the eyeball (PPTE) was related to the overall olfactory function in healthy subjects (Hummel et al. 2003). It was also demonstrated that the depth of the OS in the PPTE was significantly smaller in patients with congenital anosmia (Abolmaali et al. 2002; Huart et al. 2011). The assessment of the OS in the PPTE is easy and quick to perform (Rombaux et al. 2009b) and should be considered as a standard in the evaluation of congenital anosmia (Huart et al. 2011). Nevertheless, it is still unknown if an acquired modification of sensory input may lead to morphological changes of OS. A recent study based on voxel-based morphometry has shown that in cases of acquired anosmia, there was a significant volume decrease in grey matter in primary as well as in secondary olfactory cortex (Bitter et al. 2011) (see below).
Central olfactory projection areas can also reveal abnormalities in pathologic situations. For example, in posttraumatic olfactory loss, contusions in basofrontal and temporal areas can be noted (Collet et al. 2009). In patients with multiple sclerosis, there seems to exist a correlation between smell loss and the lesions load in brain olfactory areas (Doty et al. 1998; Zorzon et al. 2000). In patients suffering from Alzheimer’s disease, neurodegeneration in olfactory bulb and tract and mediotemporal lobe seem to be linked (Thomann et al. 2009). In addition, MRI can also reveal tumoral process in the brain, being responsible for the olfactory disorder (Choi et al. 2009; Mahdavi et al. 2009; Darie et al. 2010). Although MRI is the imaging modality of choice, CT scan can also be useful in the assessment of patients with olfactory dysfunction, mostly when associated with a rhinologic disease (i.e., chronic rhinosinusitis). CT is particularly useful for the diagnosis of olfactory cleft disease (Biacabe et al. 2004; Jankowski et al. 2007).
Recently, new automated whole-brain techniques have been developed, aiming to segment brain structures into grey and white matter and cerebrospinal fluid. These techniques, such as voxel-based morphometry and cortical thickness metric, allowed measuring the cortical thickness. In healthy subjects, it has been demonstrated that there is a link between the cortical thickness of some neuroanatomical structures (insula, medial orbitofrontal cortex, piriform cortex) and olfactory function, in that a thicker cortex is typically associated with better olfactory performance (Frasnelli et al. 2010). In hyposmic and anosmic patients, studies have also demonstrated a cortical atrophy in brain regions related to olfactory processing (Pardini et al. 2009; Wattendorf et al. 2009; Bitter et al. 2010, 2011). In addition, this cortical atrophy seems to correlate with the degree of olfactory dysfunction and the duration of the disease (Wattendorf et al. 2009; Bitter et al. 2010). However, the assessment of cortical thickness is usually not performed in the routine clinical evaluation of patients suffering of olfactory disorder.
To our knowledge, there are no studies investigating the neuroanatomical correlate of gustatory performances in humans.
30.4.2 Functional Imagery
The functional magnetic resonance imaging (fMRI) is a technique that measures the hemodynamic response in brain areas, related to the activity of a neuronal population. Numerous imaging studies using fMRI have provided considerable information regarding the processing of chemosensory information. Nevertheless, this technique is still mainly used in basic research and only few clinical conditions have been investigated.
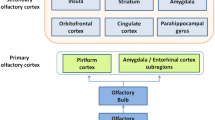
Since the first fMRI study about olfactory system by Zatorre et al. (1992), numerous imaging studies have investigated the processing of olfactory information. Brain structures involved in olfactory processing include primary olfactory cortex (=piriform cortex) (Zatorre et al. 1992; Sobel et al. 2000; Gottfried et al. 2002; Cerf-Ducastel and Murphy 2003), orbitofrontal cortex (O’Doherty et al. 2000; Anderson et al. 2003; Gottfried and Dolan 2003; Gottfried et al. 2004), amygdala (Anderson et al. 2003; Gottfried et al. 2003; Herz et al. 2004), insular cortex (Royet et al. 2003; Wicker et al. 2003), cerebellum (Sobel et al. 1998), thalamus, and hypothalamus (Sobel et al. 1999; Zatorre et al. 2000). Importantly, fMRI not only allows the structural identification of brain structures involved in olfactory processing but it also allows the identification of the functional role of certain brain areas. For example, the posterior piriform cortex seems to be involved in odor quality coding (Howard et al. 2009).
Among “pathologic populations” the most studied are patients with Alzheimer’s and Parkinson’s disease. It has been demonstrated that patients with Alzheimer’s disease have a perceptual impairment of odor quality discrimination, which occurs in conjunction with a disruption of odor quality coding, for example, in the posterior piriform cortex (Li et al. 2010). It has also been demonstrated that patients with Alzheimer’s disease show lower activation in the primary olfactory cortex than control subjects (Wang et al. 2010). In patients with Parkinson’s disease, neuronal activities in the amygdale and hippocampus are reduced compared to controls (Westermann et al. 2008; Hummel et al. 2010b). In addition, activity of brain areas relevant for olfactory processing seems to be well correlated with the presence or absence of ERP. Indeed, patients having ERP have a higher activation than patients having no ERP (Welge-Lussen et al. 2009). Despite of these advances, as of today, fMRI is not performed in the basic clinical evaluation of individual patients suffering from olfactory disorder.
30.4.3 Diffusion Imagery
Diffusion tensor imaging (DTI) is an application of diffusion MRI technique. Diffusion MRI examines the local microstructural characteristics of water diffusion in tissues. DTI is based on the fact that the diffusion of water molecules in organic tissues is often anisotropic (Tanner 1979), and the diffusion coefficient of water may vary depending on the orientation along which the diffusion-weighted measurements are taken: water diffuses more rapidly in the direction aligned with the examined structure and more slowly in the perpendicular direction. In this way, this technique allows the tractography of nervous structures and can also, by evaluating the fractional anisotropy of brain regions, detect changes in white matter integrity. Although there are numerous studies reporting the use of tractography for the visualization of various cranial nerves (Hodaie et al. 2010; Chen et al. 2011; Kolbe et al. 2012; Smith et al. 2011), we found only one study about the diffusion tensor fiber tractography of the olfactory tract (Skorpil et al. 2011). Some authors have used the diffusion tensor parameters to study the relationship between olfactory impairment in Parkinson’s disease and white matter abnormalities in central olfactory areas and showed that there was microstructural white matter reduction in central olfactory system of patients with early stage Parkinson’s disease; these reductions seemed to be associated with the olfactory loss (Ibarretxe-Bilbao et al. 2010; Rolheiser et al. 2011; Zhang et al. 2011).
Since studies about this technique in the evaluation of olfaction are lacking, further investigations should be necessary to evaluate the usefulness of DTI techniques in the clinical, individual assessment of olfactory disorders.
30.5 Future Perspectives
Although progress has been made in the clinical evaluation of olfactory function, much is left unclear. This is particularly true in the matter of prognosis and differential diagnosis of patients suffering from olfactory disorders. Indeed, while for some patients the cause of the olfactory disorder is clear (e.g., posttraumatic olfactory loss, postinfectious loss), there is still a large population of patients for whom no clear etiology can be found. Also, we still miss a reliable tool to evaluate the prognosis of patients. We hope that future advances in electrophysiological techniques, such as time-frequency analysis, or in imaging techniques, such as fMRI or cortical thickness metric, will provide us with at least partial responses to these questions.
30.6 Conclusion
In the last years, the field of olfaction has known a considerable development. Nowadays, it is relatively easy to diagnose or confirm an olfactory dysfunction in clinical practice owing to reliable and validated psychophysical and electrophysiological testing, and imaging techniques allowed to have a precise morphological representation of structures implicated in smell perception. In research, new electrophysiological (i.e., source analysis, time-frequency analysis) and new functional and morphological imaging techniques are being evaluated and seem very promising for the evaluation of patients suffering from smell or taste disorder; further studies are necessary to evaluate the usefulness of these techniques in clinical practice. Particular attention should be paid to assessment of differential diagnosis or evaluation of patient’s prognosis, since these points remain unclear.
References
Abolmaali ND, Hietschold V, et al. MR evaluation in patients with isolated anosmia since birth or early childhood. AJNR Am J Neuroradiol. 2002;23(1):157–64.
Ahlskog JE, Waring SC, et al. Olfactory dysfunction in Guamanian ALS, parkinsonism, and dementia. Neurology. 1998;51(6):1672–7.
Anderson AK, Christoff K, et al. Dissociated neural representations of intensity and valence in human olfaction. Nat Neurosci. 2003;6(2):196–202.
Biacabe B, Faulcon P, et al. Olfactory cleft disease: an analysis of 13 cases. Otolaryngol Head Neck Surg. 2004;130(2):202–8.
Bitter T, Bruderle J, et al. Gray and white matter reduction in hyposmic subjects – a voxel-based morphometry study. Brain Res. 2010;1347:42–7.
Bitter T, Gudziol H, et al. Volume alterations in the gray matter of anosmic subjects. Lessons we can learn from voxel-based morphometry. HNO. 2011;59(3):248–54.
Boesveldt S, Haehner A, et al. Signal-to-noise ratio of chemosensory event-related potentials. Clin Neurophysiol. 2007;118(3):690–5.
Bramerson A, Johansson L, et al. Prevalence of olfactory dysfunction: the skovde population-based study. Laryngoscope. 2004;114(4):733–7.
Briner HR, Simmen D. Smell diskettes as screening test of olfaction. Rhinology. 1999;37(4):145–8.
Buschhuter D, Smitka M, et al. Correlation between olfactory bulb volume and olfactory function. Neuroimage. 2008;42(2):498–502.
Cain WS, Gent J, et al. Clinical evaluation of olfaction. Am J Otolaryngol. 1983;4(4):252–6.
Cerf-Ducastel B, Murphy C. FMRI brain activation in response to odors is reduced in primary olfactory areas of elderly subjects. Brain Res. 2003;986(1–2):39–53.
Chen DQ, Quan J, et al. Three-dimensional in vivo modeling of vestibular schwannomas and surrounding cranial nerves with diffusion imaging tractography. Neurosurgery. 2011;68(4):1077–83.
Choi YS, Sung KS, et al. Olfactory schwannoma-case report. J Korean Neurosurg Soc. 2009;45(2):103–6.
Collet S, Grulois V, et al. Post-traumatic olfactory dysfunction: a cohort study and update. B-ENT. 2009;5 Suppl 13:97–107.
Corwin J. Olfactory identification in hemodialysis: acute and chronic effects on discrimination and response bias. Neuropsychologia. 1989;27(4):513–22.
Darie I, Riffaud L, et al. Olfactory ensheathing cell tumour: case report and literature review. J Neurooncol. 2010;100(2):285–9.
Davidson TM, Murphy C. Rapid clinical evaluation of anosmia. The alcohol sniff test. Arch Otolaryngol Head Neck Surg. 1997;123(6):591–4.
Doty RL, Shaman P, et al. University of Pennsylvania smell identification test: a rapid quantitative olfactory function test for the clinic. Laryngoscope. 1984;94(2 Pt 1):176–8.
Doty RL, McKeown DA, et al. A study of the test-retest reliability of ten olfactory tests. Chem Senses. 1995;20(6):645–56.
Doty RL, Li C, et al. Olfactory dysfunction in multiple sclerosis. Relation to plaque load in inferior frontal and temporal lobes. Ann N Y Acad Sci. 1998;855:781–6.
Duff K, McCaffrey RJ, et al. The Pocket Smell Test: successfully discriminating probable Alzheimer’s dementia from vascular dementia and major depression. J Neuropsychiatry Clin Neurosci. 2002;14(2):197–201.
Frasnelli J, Lundstrom JN, et al. Neuroanatomical correlates of olfactory performance. Exp Brain Res. 2010;201(1):1–11.
Geisler MW, Murphy C. Event-related brain potentials to attended and ignored olfactory and trigeminal stimuli. Int J Psychophysiol. 2000;37(3):309–15.
Gottfried JA, Dolan RJ. The nose smells what the eye sees: crossmodal visual facilitation of human olfactory perception. Neuron. 2003;39(2):375–86.
Gottfried JA, Deichmann R, et al. Functional heterogeneity in human olfactory cortex: an event-related functional magnetic resonance imaging study. J Neurosci. 2002;22(24):10819–28.
Gottfried JA, O’Doherty J, et al. Encoding predictive reward value in human amygdala and orbitofrontal cortex. Science. 2003;301(5636):1104–7.
Gottfried JA, Smith AP, et al. Remembrance of odors past: human olfactory cortex in cross-modal recognition memory. Neuron. 2004;42(4):687–95.
Gudziol V, Buschhuter D, et al. Increasing olfactory bulb volume due to treatment of chronic rhinosinusitis–a longitudinal study. Brain. 2009;132(Pt 11):3096–101.
Guilemany JM, Garcia-Pinero A, et al. Persistent allergic rhinitis has a moderate impact on the sense of smell, depending on both nasal congestion and inflammation. Laryngoscope. 2009;119(2):233–8.
Haehner A, Mayer AM, et al. High test-retest reliability of the extended version of the “Sniffin’ Sticks” test. Chem Senses. 2009;34(8):705–11.
Haehner A, Gruenewald G, et al. Responses to olfactory and intranasal trigeminal stimuli: relation to the respiratory cycle. Neuroscience. 2011;175:178–83.
Heilmann S, Strehle G, et al. Clinical assessment of retronasal olfactory function. Arch Otolaryngol Head Neck Surg. 2002;128(4):414–8.
Hendriks AP. Olfactory dysfunction. Rhinology. 1988;26(4):229–51.
Herz RS, Eliassen J, et al. Neuroimaging evidence for the emotional potency of odor-evoked memory. Neuropsychologia. 2004;42(3):371–8.
Hodaie M, Quan J, et al. In vivo visualization of cranial nerve pathways in humans using diffusion-based tractography. Neurosurgery. 2010;66(4):788–95. discussion 795–786.
Howard JD, Plailly J, et al. Odor quality coding and categorization in human posterior piriform cortex. Nat Neurosci. 2009;12(7):932–8.
Huart C, Meusel T, et al. The depth of the olfactory sulcus is an indicator of congenital anosmia. AJNR Am J Neuroradiol. 2011;32(10):1911–4.
Huart C, Legrain V, et al. Time-frequency analysis of chemosensory event-related potentials to characterize the cortical representation of odors in humans. PLoS One. 2012;7(3):e33221.
Hummel T, Kobal G. Differences in human evoked potentials related to olfactory or trigeminal chemosensory activation. Electroencephalogr Clin Neurophysiol. 1992;84(1):84–9.
Hummel T, Kobal G. Chemosensory event-related potentials to trigeminal stimuli change in relation to the interval between repetitive stimulation of the nasal mucosa. Eur Arch Otorhinolaryngol. 1999;256(1):16–21.
Hummel T, Sekinger B, et al. ‘Sniffin’ Sticks’: olfactory performance assessed by the combined testing of odor identification, odor discrimination and olfactory threshold. Chem Senses. 1997;22(1):39–52.
Hummel T, Barz S, et al. Chemosensory event-related potentials change with age. Electroencephalogr Clin Neurophysiol. 1998;108(2):208–17.
Hummel T, Klimek L, et al. Chemosensory evoked potentials for clinical diagnosis of olfactory disorders. HNO. 2000;48(6):481–5.
Hummel T, Konnerth CG, et al. Screening of olfactory function with a four-minute odor identification test: reliability, normative data, and investigations in patients with olfactory loss. Ann Otol Rhinol Laryngol. 2001;110(10):976–81.
Hummel T, Damm M, et al. Depth of olfactory sulcus and olfactory function. Brain Res. 2003;975(1–2):85–9.
Hummel T, Heilmann S, et al. Perceptual differences between chemical stimuli presented through the ortho- or retronasal route. Flavor Fragr J. 2006;21(1):42–7.
Hummel T, Hahner A, et al. Examination of the sense of smell. HNO. 2007a;55(10):827–37. quiz 838.
Hummel T, Kobal G, et al. Normative data for the “Sniffin’ Sticks” including tests of odor identification, odor discrimination, and olfactory thresholds: an upgrade based on a group of more than 3,000 subjects. Eur Arch Otorhinolaryngol. 2007b;264(3):237–43.
Hummel T, Genow A, et al. Clinical assessment of human gustatory function using event related potentials. J Neurol Neurosurg Psychiatry. 2010a;81(4):459–64.
Hummel T, Witt M, et al. Immunohistochemical, volumetric, and functional neuroimaging studies in patients with idiopathic Parkinson’s disease. J Neurol Sci. 2010b;289(1–2):119–22.
Hummel T, Smitka M, et al. Correlation between olfactory bulb volume and olfactory function in children and adolescents. Exp Brain Res. 2011;214(2):285–91.
Ibarretxe-Bilbao N, Junque C, et al. Olfactory impairment in Parkinson’s disease and white matter abnormalities in central olfactory areas: a voxel-based diffusion tensor imaging study. Mov Disord. 2010;25(12):1888–94.
Jankowski R, Georgel T, et al. Endoscopic surgery reveals that woodworkers’ adenocarcinomas originate in the olfactory cleft. Rhinology. 2007;45(4):308–14.
Kettenmann B, Hummel T, et al. Functional imaging of olfactory activation in the human brain. In: Simon SA, Nicolelis MAL, editors. Methods in chemosensory research. Boca Raton: CRC Press; 2001. p. 477–506.
Kobal G. Elektrophysiologische Untersuchungen des menschlichen Geruchssinns. Stuttgart: Georg Thieme; 1981.
Kobal G. Gustatory evoked potentials in man. Electroencephalogr Clin Neurophysiol. 1985;62(6):449–54.
Kobal G, Plattig KH. Objective olfactometry: methodological annotations for recording olfactory EEG-responses from the awake human. EEG EMG Z Elektroenzephalogr Elektromyogr Verwandte Geb. 1978;9(3):135–45.
Kobal G, Hummel T, et al. “Sniffin’ Sticks”: screening of olfactory performance. Rhinology. 1996;34(4):222–6.
Kobal G, Palisch K, et al. A threshold-like measure for the assessment of olfactory sensitivity: the “random” procedure. Eur Arch Otorhinolaryngol. 2001;258(4):168–72.
Kolbe S, Bajraszewski C, et al. Diffusion tensor imaging of the optic radiations after optic neuritis. Hum Brain Mapp. 2012;33(9):2047–61.
Kremer B, Klimek L, et al. Clinical validation of a new olfactory test. Eur Arch Otorhinolaryngol. 1998;255(7):355–8.
Landis BN, Hummel T. New evidence for high occurrence of olfactory dysfunctions within the population. Am J Med. 2006;119(1):91–2.
Landis BN, Hummel T, et al. Ratings of overall olfactory function. Chem Senses. 2003;28(8):691–4.
Landis BN, Konnerth CG, et al. A study on the frequency of olfactory dysfunction. Laryngoscope. 2004;114(10):1764–9.
Lascano AM, Hummel T, et al. Spatio-temporal dynamics of olfactory processing in the human brain: an event-related source imaging study. Neuroscience. 2010;167(3):700–8.
Laudien JH, Wencker S, et al. Context effects on odor processing: an event-related potential study. Neuroimage. 2008;41(4):1426–36.
Li W, Howard JD, et al. Disruption of odour quality coding in piriform cortex mediates olfactory deficits in Alzheimer’s disease. Brain. 2010;133(9):2714–26.
Livermore A, Hummel T. The influence of training on chemosensory event-related potentials and interactions between the olfactory and trigeminal systems. Chem Senses. 2004;29(1):41–51.
Lotsch J, Hummel T. The clinical significance of electrophysiological measures of olfactory function. Behav Brain Res. 2006;170(1):78–83.
Luck SJ. An introduction to the event-related potential technique. Cambridge: MIT Press; 2005.
Mahdavi A, Ahmadi H, et al. Arachnoid cyst of the middle cranial fossae associated with hemianosmia and unilateral paranasal sinus hypoplasia. J Otolaryngol Head Neck Surg. 2009;38(1):E6–8.
McMahon C, Scadding GK. Le Nez du Vin–a quick test of olfaction. Clin Otolaryngol Allied Sci. 1996;21(3):278–80.
Miyanari A, Kaneoke Y, et al. Neuromagnetic changes of brain rhythm evoked by intravenous olfactory stimulation in humans. Brain Topogr. 2006;18(3):189–99.
Moncrief RW. Effect of odours on EEG records. Perfumery essential oil Rec. 1962;53:727–60.
Mouraux A, Iannetti GD. Across-trial averaging of event-related EEG responses and beyond. Magn Reson Imaging. 2008;26(7):1041–54.
Mueller A, Rodewald A, et al. Reduced olfactory bulb volume in post-traumatic and post-infectious olfactory dysfunction. Neuroreport. 2005;16(5):475–8.
Murphy C, Schubert CR, et al. Prevalence of olfactory impairment in older adults. JAMA. 2002;288(18):2307–12.
Nordin S, Bramerson A, et al. The Scandinavian Odor-Identification Test: development, reliability, validity and normative data. Acta Otolaryngol. 1998;118(2):226–34.
O’Doherty J, Rolls ET, et al. Sensory-specific satiety-related olfactory activation of the human orbitofrontal cortex. Neuroreport. 2000;11(2):399–403.
Olofsson JK, Broman DA, et al. Laterality of the olfactory event-related potential response. Chem Senses. 2006;31(7):699–704.
Pardini M, Huey ED, et al. Olfactory function in corticobasal syndrome and frontotemporal dementia. Arch Neurol. 2009;66(1):92–6.
Pause BM, Krauel K. Chemosensory event-related potentials (CSERP) as a key to the psychology of odors. Int J Psychophysiol. 2000;36(2):105–22.
Pause BM, Sojka B, et al. The nature of the late positive complex within the olfactory event-related potential (OERP). Psychophysiology. 1996;33(4):376–84.
Renner B, Mueller CA, et al. The candy smell test: a new test for retronasal olfactory performance. Laryngoscope. 2009;119(3):487–95.
Robson AK, Woollons AC, et al. Validation of the combined olfactory test. Clin Otolaryngol Allied Sci. 1996;21(6):512–8.
Rolheiser TM, Fulton HG, et al. Diffusion tensor imaging and olfactory identification testing in early-stage Parkinson’s disease. J Neurol. 2011;258(7):1254–60.
Rombaux P, Mouraux A, et al. Assessment of olfactory and trigeminal function using chemosensory event-related potentials. Neurophysiol Clin. 2006a;36(2):53–62.
Rombaux P, Mouraux A, et al. Olfactory function and olfactory bulb volume in patients with postinfectious olfactory loss. Laryngoscope. 2006b;116(3):436–9.
Rombaux P, Mouraux A, et al. Retronasal and orthonasal olfactory function in relation to olfactory bulb volume in patients with posttraumatic loss of smell. Laryngoscope. 2006c;116(6):901–5.
Rombaux P, Bertrand B, et al. Clinical significance of olfactory event-related potentials related to orthonasal and retronasal olfactory testing. Laryngoscope. 2007;117(6):1096–101.
Rombaux P, Collet S, et al. Olfactory testing in clinical practice. B-ENT. 2009a;5 Suppl 13:39–51.
Rombaux P, Grandin C, et al. How to measure olfactory bulb volume and olfactory sulcus depth? B-ENT. 2009b;5 Suppl 13:53–60.
Rombaux P, Mouraux A, et al. Usefulness and feasibility of psychophysical and electrophysiological olfactory testing in the rhinology clinic. Rhinology. 2009c;47(1):28–35.
Rombaux P, Potier H, et al. Olfactory bulb volume and depth of olfactory sulcus in patients with idiopathic olfactory loss. Eur Arch Otorhinolaryngol. 2010;267(10):1551–6.
Royet JP, Plailly J, et al. fMRI of emotional responses to odors: influence of hedonic valence and judgment, handedness, and gender. Neuroimage. 2003;20(2):713–28.
Savina C, Donini LM, et al. Administering the “AHSP Questionnaire” (appetite, hunger, sensory perception) in a geriatric rehabilitation care. J Nutr Health Aging. 2003;7(6):385–9.
Scadding G, Hellings P, et al. Diagnostic tools in Rhinology EAACI position paper. Clin Transl Allergy. 2011;1(1):2.
Skorpil M, Rolheiser T, et al. Diffusion tensor fiber tractography of the olfactory tract. Magn Reson Imaging. 2011;29(2):289–92.
Small DM, Gerber JC, et al. Differential neural responses evoked by orthonasal versus retronasal odorant perception in humans. Neuron. 2005;47(4):593–605.
Smith SA, Williams ZR, et al. Diffusion tensor imaging of the optic nerve in multiple sclerosis: association with retinal damage and visual disability. AJNR Am J Neuroradiol. 2011;32(9):1662–8.
Sobel N, Prabhakaran V, et al. Odorant-induced and sniff-induced activation in the cerebellum of the human. J Neurosci. 1998;18(21):8990–9001.
Sobel N, Prabhakaran V, et al. Blind smell: brain activation induced by an undetected air-borne chemical. Brain. 1999;122(Pt 2):209–17.
Sobel N, Prabhakaran V, et al. Time course of odorant-induced activation in the human primary olfactory cortex. J Neurophysiol. 2000;83(1):537–51.
Stevenson RJ. An initial evaluation of the functions of human olfaction. Chem Senses. 2010;35(1):3–20.
Takagi SF. A standardized olfactometer in Japan. A review over ten years. Ann N Y Acad Sci. 1987;510:113–8.
Tanner JE. Self diffusion of water in frog muscle. Biophys J. 1979;28(1):107–16.
Thesen T, Murphy C. Reliability analysis of event-related brain potentials to olfactory stimuli. Psychophysiology. 2002;39(6):733–8.
Thomann PA, Dos Santos V, et al. MRI-derived atrophy of the olfactory bulb and tract in mild cognitive impairment and Alzheimer’s disease. J Alzheimers Dis. 2009;17(1):213–21.
Tourbier IA, Doty RL. Sniff magnitude test: relationship to odor identification, detection, and memory tests in a clinic population. Chem Senses. 2007;32(6):515–23.
Trotier D, Bensimon JL, et al. Inflammatory obstruction of the olfactory clefts and olfactory loss in humans: a new syndrome? Chem Senses. 2007;32(3):285–92.
Turetsky BI, Moberg PJ, et al. Reduced olfactory bulb volume in patients with schizophrenia. Am J Psychiatry. 2000;157(5):828–30.
Wang J, Eslinger PJ, et al. Olfactory deficit detected by fMRI in early Alzheimer’s disease. Brain Res. 2010;1357:184–94.
Wattendorf E, Welge-Lussen A, et al. Olfactory impairment predicts brain atrophy in Parkinson’s disease. J Neurosci. 2009;29(49):15410–3.
Welge-Lussen A, Wille C, et al. Test-retest reliability of chemosensory evoked potentials. J Clin Neurophysiol. 2003;20(2):135–42.
Welge-Lussen A, Wattendorf E, et al. Olfactory-induced brain activity in Parkinson’s disease relates to the expression of event-related potentials: a functional magnetic resonance imaging study. Neuroscience. 2009;162(2):537–43.
Westermann B, Wattendorf E, et al. Functional imaging of the cerebral olfactory system in patients with Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2008;79(1):19–24.
Wicker B, Keysers C, et al. Both of us disgusted in My insula: the common neural basis of seeing and feeling disgust. Neuron. 2003;40(3):655–64.
Wright HN. Characterization of olfactory dysfunction. Arch Otolaryngol Head Neck Surg. 1987;113(2):163–8.
Yousem DM, Geckle RJ, et al. Posttraumatic olfactory dysfunction: MR and clinical evaluation. AJNR Am J Neuroradiol. 1996;17(6):1171–9.
Yousem DM, Geckle RJ, et al. Posttraumatic smell loss: relationship of psychophysical tests and volumes of the olfactory bulbs and tracts and the temporal lobes. Acad Radiol. 1999;6(5):264–72.
Zatorre RJ, Jones-Gotman M, et al. Functional localization and lateralization of human olfactory cortex. Nature. 1992;360(6402):339–40.
Zatorre RJ, Jones-Gotman M, et al. Neural mechanisms involved in odor pleasantness and intensity judgments. Neuroreport. 2000;11(12):2711–6.
Zhang K, Yu C, et al. Voxel-based analysis of diffusion tensor indices in the brain in patients with Parkinson’s disease. Eur J Radiol. 2011;77(2):269–73.
Zorzon M, Ukmar M, et al. Olfactory dysfunction and extent of white matter abnormalities in multiple sclerosis: a clinical and MR study. Mult Scler. 2000;6(6):386–90.
Zumsteg D, Friedman A, et al. Source localization of mesial temporal interictal epileptiform discharges: correlation with intracranial foramen ovale electrode recordings. Clin Neurophysiol. 2005;116(12):2810–8.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2013 Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
Rombaux, P., Collet, S., Huart, C. (2013). Assessment of Olfactory Function. In: Önerci, T. (eds) Nasal Physiology and Pathophysiology of Nasal Disorders. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-642-37250-6_30
Download citation
DOI: https://doi.org/10.1007/978-3-642-37250-6_30
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-642-37249-0
Online ISBN: 978-3-642-37250-6
eBook Packages: MedicineMedicine (R0)