Abstract
Vessels, vascular networks, and the endothelium are dynamic structures, which continuously adapt to varying conditions in response to local hemodynamic and metabolic stimuli. This adaptation entails changes in smooth muscle tone, vessel diameter, vessel wall thickness, and vessel density or number. This angioadaptation which in principle takes place in vessels of all sizes but is most expressed in the smaller (below about 300 μm) vessels of the microcirculation can contribute to hypertension in at least two ways. First, an initial increase in blood pressure, e.g., elicited by an increase in cardiac output, would lead to vasoconstriction and in a long run to reduction in vessel diameters via wall remodeling; second, a reduction of vessel density by rarefaction of microvessels and capillaries causes further reduction of the total peripheral vascular cross section. Based on recent evidence it appears that beside this vicious cycle of “structural autoregulation,” primary changes in vascular adaptation to pressure or of endothelial function play a role in hypertension.
The importance of angioadaptation for the development of hypertension pertains to the effectiveness of different pharmacological strategies to lower blood pressure with respect to the normalization of endothelial function and structure of terminal vascular beds of the microcirculation. Better knowledge of the underlying mechanisms is a prerequisite for future development of therapeutic approaches aiming at recovery of normal vascular structure and function.
Access provided by Autonomous University of Puebla. Download reference work entry PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
Angioadaptation
During the last decades, it became increasingly obvious that vascular adaptation of vessels in terminal vascular beds appears to be a central factor in pathophysiology of hypertension (Levy et al. 2001). Microvascular networks and their constituent vessels are dynamic structures which exhibit continuous adaptation to local stimuli. Such stimuli include the hemodynamic effects of blood flow (wall shear stress) and blood pressure (circumferential wall stress) as well as metabolic factors, e.g., oxygen partial pressure or related metabolic signals (Reglin et al. 1997). In addition, the transfer of information about the local metabolic situation along arterial vessels via electrical conduction and along venous vessels via convection of metabolic signal substances seems to be relevant (Pries et al. 2003, 2005, 2010; Fig. 1).
Mechanisms of adaptation of microvessels and microvascular networks (“angioadaptation”) (Zakrzewicz et al. 2002). On the left side, changes in vessel diameter and wall thickness are shown, while the right side addresses changes of vessel number. Left: hypertension generally leads to an increase in vessel tone (upper left) and inward eutrophic remodeling (lower left), i.e., a decrease in vessel diameter at constant wall mass – with the consequence of thicker vessel walls and increase wall/lumen ratio (red arrow). Upper right: new vessels may be generated by sprouts emerging from existing vessels or by the separation of one existing vessel in two branches (splitting). Lower right: vessels, which are not needed for tissue supply or do not exhibit relevant blood flow (like the small vascular “ring” on the left picture), are eliminated by a process termed “pruning”
It is of note that vascular adaptation occurs on different time scales and concerns vascular smooth muscle tone (especially in arterioles), structural components of the vessel wall, and the vessel density. The fastest responses are mediated by modulation of vascular tone and may elicit changes of vessel diameter within seconds. Persistent changes of local conditions and vascular tone (Bakker et al. 2008) lead to adaptation of the vascular wall structure, which are generally termed “remodeling” (van den Akker et al. 2010). They can be characterized according to the observed changes in vessel diameter and wall thickness (Fig. 1) and/or in wall mass. Diameter increase and decrease is addressed as “outward” and “inward” remodeling (Mulvany 1999). This can be further refined by recognizing the change of the wall mass in the process. If wall mass increases, remodeling is classified as “hypertrophic” (e.g., by medial hypertrophy); if it decreases, as “hypotrophic”; and if no changes occur, as “eutrophic.” A number of experimental investigations in hypertension both experimental and in humans have shown that in idiopathic, essential hypertension, the typical vascular reaction is represented by reduction of vessel diameter at constant wall mass, i.e.‚ “inward eutrophic remodeling” (Mulvany 2012; Rizzoni et al. 2003). This corresponds to a maintained number of vascular smooth muscle cells which are arranged in a different way to form a smaller vessel with a thicker media.
In addition to changes affecting the existing vessels, microvascular networks also exhibit changes in the number of vessels and vascular density in response to hemodynamic and metabolic stimuli. The generation of new vessels in adults is effected by capillary sprouting and by splitting or “intussusception” (Risau 1997; Secomb et al. 2013; Styp-Rekowska et al. 2011). Very relevant in the context of hypertension is the opposite reaction, i.e. the reduction of vascular density by elimination of microvessels, the so-called pruning (Antonios 2006; Cheng et al. 2008). The exact mechanisms of pruning are not yet understood but it might be interpreted as an extreme form of hypotrophic inward remodeling which leads to destruction of the vessel.
Hypertension and Vascular Remodeling
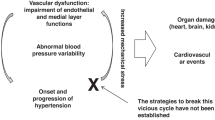
Figure 2 explores the relation between microvascular angioadaptation and the development of hypertension. If the cardiac output is permanently increased (upper left) by whatever mechanism, an increase of vascular tone but also inward remodeling is observed (Mulvany 2002; van den Akker et al. 2010) as well as pruning, evident from vascular rarefaction (Antonios et al. 1999; Greene et al. 1992). This leads to a reduced blood supply and, relevant for the chronic manifestation of hypertension, to an increase in peripheral flow resistance which further increases blood pressure via positive feedback. This phenomenon has been called structural autoregulation (Folkow 1990; Pries et al. 2005). Structural autoregulation leads to a vicious cycle between increase in blood pressure and peripheral resistance which amplifies the hypertension above the level generated by the initial increase of cardiac output. The increased peripheral resistance and its constituents, i.e., tone of vascular smooth muscle cells, inward remodeling, and rarefaction, likely lead to deficits in supply of oxygen and nutrients and consequently to end-organ damage.
The initialization of the vicious cycle of hypertension may be also triggered by primary defects in microvascular adaptation characteristics, i.e., the vascular reaction to pressure or generation of vasoactive factors by the endothelium (Fig. 2; Levy et al. 2001). In line with this concept, abnormalities of microvascular structure and reagibility preceding the onset of hypertension have been reported (Ding et al. 2013). This finding highlights the importance of vascular and endothelial function for the development of hypertension and may explain the effect of antihypertensive drugs which target endothelial function or vascular smooth muscle tone (Levy et al. 2001).
Relevance for Drug Treatment
It has been concluded from a number of investigations and trials that antihypertensive drugs which result in comparable reductions of blood pressure may have quite different capacity to revert the altered vascular structure in hypertension (re-remodeling) – mostly as eutrophic outward remodeling (Antonios 2006; Eftekhari et al. 2011; Levy et al. 2001; Mulvany 2012; Penna et al. 2008). It seems that β-blockers have a very limited effect with respect to re-remodeling and restoration of normal vascular networks (Fig. 3). In the case of diuretics, the observed vascular structure and thus re-remodeling appear to parallel the amount of blood pressure reduction, i.e., the level of wall/lumen ratio under antihypertensive treatment is similar to the respective level in an untreated person with the same blood pressure. In contrast, in treatment with vasoactive substances (such as vasodilators), the re-remodeling seems to exceed the blood pressure reduction achieved. This notion has been supported in in vivo studies of flow-mediated dilatation where vasoactive drugs showed the largest effects in restoring endothelial function to normal levels. Some substances may even exert significant re-remodeling despite relatively low impact on blood pressure per se (Ghiadoni et al. 2012).
Left: in hypertension, the increase of blood pressure leads to vascular adaptation mostly as “inward eutrophic” remodeling, with an increased wall to lumen ratio and reduced lumen diameter. Right: pharmacological treatment with different classes of substances results in different amounts of normalization of vascular structure (re-remodeling) (Antonios 2006; Eftekhari et al. 2011; Levy et al. 2001; Mulvany 2012; Penna et al. 2008). While diuretics show a re-remodeling commensurate to the reduction in blood pressure, it is less prominent in β-blockers and stronger for vasodilating agents
Summary
The presented findings underline the potential relevance of vascular adaptation (angioadaptation) for the development and treatment of hypertension. However, the available clinical and experimental data do not yet allow definite conclusions regarding the impact and role of angioadaptation in hypertension, and more data will be needed to develop and to optimize future antihypertensive therapy.
Abbreviations
- Angioadaptation:
-
Comprises adaptive reactions by vessels and vascular networks in vessel tone, number and structure.
- Inward eutrophic remodeling:
-
Diameter increase and decrease is addressed as “outward” and “inward” remodeling. This can be further refined by recognizing the change of the wall mass in the process. If wall mass increases, remodeling is classified as “hypertrophic”; if it fit decreases, as “hypotrophic”; and if no changes occur, as “eutrophic.”
- Structural adaptation:
-
Structural changes include changes in vessel wall composition and arrangement. They lead to changes in the maximally dilated diameter.
References
Antonios TF (2006) Microvascular rarefaction in hypertension–reversal or over-correction by treatment? Am J Hypertens 19:484–485
Antonios TF, Singer DR, Markandu ND, Mortimer PS, MacGregor GA (1999) Structural skin capillary rarefaction in essential hypertension. Hypertension 33:998–1001
Bakker EN, Matlung HL, Bonta P, de Vries CJ, van Rooijen N, VanBavel E (2008) Blood flow-dependent arterial remodelling is facilitated by inflammation but directed by vascular tone. Cardiovasc Res 78:341–348
Cheng C, Diamond JJ, Falkner B (2008) Functional capillary rarefaction in mild blood pressure elevation. Clin Transl Sci 1:75–79
Ding J, Wai KL, McGeechan K, Ikram MK, Kawasaki R, Xie J, Klein R, Klein BB, Cotch MF, Wang JJ, Mitchell P, Shaw JE, Takamasa K, Sharrett AR, Wong TY (2013) Retinal vascular caliber and the development of hypertension: a meta-analysis of individual participant data. J Hypertens 32(2):207–215. doi:10.1097/HJH.0b013e32836586f4
Eftekhari A, Mathiassen ON, Buus NH, Gotzsche O, Mulvany MJ, Christensen KL (2011) Disproportionally impaired microvascular structure in essential hypertension. J Hypertens 29:896–905
Folkow B (1990) “Structural factor” in primary and secondary hypertension. Hypertension 16:89–101
Ghiadoni L, Taddei S, Virdis A (2012) Hypertension and endothelial dysfunction: therapeutic approach. Curr Vasc Pharmacol 10:42–60
Greene AS, Tonellato PJ, Zhang Z, Lombard JH, Cowley AW Jr (1992) Effect of microvascular rarefaction on tissue oxygen delivery in hypertension. Am J Physiol 262:H1486–H1493
Levy BI, Ambrosio G, Pries AR, Struijker-Boudier HA (2001) Microcirculation in hypertension: a new target for treatment? Circulation 104:735–740
Mulvany MJ (1999) Vascular remodelling of resistance vessels: can we define this? Cardiovasc Res 41:9–13
Mulvany MJ (2002) Small artery remodeling and significance in the development of hypertension. News Physiol Sci 17:105–109
Mulvany MJ (2012) Small artery remodelling in hypertension. Basic Clin Pharmacol Toxicol 110:49–55
Penna GL, Garbero RF, Neves MF, Oigman W, Bottino DA, Bouskela E (2008) Treatment of essential hypertension does not normalize capillary rarefaction. Clinics (Sao Paulo) 63:613–618
Pries AR, Reglin B, Secomb TW (2003) Structural response of microcirculatory networks to changes in demand: information transfer by shear stress. Am J Physiol 284:H2204–H2212
Pries AR, Reglin B, Secomb TW (2005) Remodeling of blood vessels: responses of diameter and wall thickness to hemodynamic and metabolic stimuli. Hypertension 46:726–731
Pries AR, Hopfner M, Le Noble F, Dewhirst MW, Secomb TW (2010) The shunt problem: control of functional shunting in normal and tumour vasculature. Nat Rev Cancer 10:587–593
Reglin B, Secomb TW, Pries AR (1997) Structural adaptation of microvessel diameters in response to metabolic stimuli: where are the oxygen sensors? Am J Physiol Heart Circ Physiol 297:H2206–H2219
Risau W (1997) Mechanisms of angiogenesis. Nature 386:671–674
Rizzoni D, Porteri E, Boari GE, De CC, Sleiman I, Muiesan ML, Castellano M, Miclini M, Gabiti-Rosei E (2003) Prognostic significance of small-artery structure in hypertension. Circulation 108:2230–2235
Secomb TW, Alberding JP, Hsu R, Dewhirst MW, Pries AR (2013) Angiogenesis: an adaptive dynamic biological patterning problem. PLoS Comput Biol 9:e1002983
Styp-Rekowska B, Hlushchuk R, Pries AR, Djonov V (2011) Intussusceptive angiogenesis: pillars against the blood flow. Acta Physiol (Oxf) 202:213–223
van den Akker J, Schoorl MJ, Bakker EN, Van Bavel E (2010) Small artery remodeling: current concepts and questions. J Vasc Res 47:183–202
Zakrzewicz A, Secomb TW, Pries AR (2002) Angioadaptation: keeping the vascular system in shape. News Physiol Sci 17:197–201
Further Reading
Homeister JW, Willis M (eds) (2012) Molecular and translational vascular medicine. Humana Press, New York
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer-Verlag Berlin Heidelberg
About this entry
Cite this entry
Pries, A.R. (2015). Vascular Adaptation in Hypertension. In: Lanzer, P. (eds) PanVascular Medicine. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-642-37078-6_48
Download citation
DOI: https://doi.org/10.1007/978-3-642-37078-6_48
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-642-37077-9
Online ISBN: 978-3-642-37078-6
eBook Packages: MedicineReference Module Medicine