Abstract
In this chapter the potential of Organic–Organic heteroepitaxy is discussed concerning the ability to provide efficient color tuning of organic nano-fibers deposited on muscovite mica substrates. The first part is focused on the epitaxial growth of rod-like molecules on sheet silicates which has been analyzed by depositing p-6P and 6T using hot-wall epitaxy. It is demonstrated that substituting para-phenylenes by other molecules for efficient color tuning is not trivial leading in the general case to multidirectional nano-fibers. The presented growth model is based on detailed analysis using XRD pole-figure measurements, atomic force microscopy and force-field simulations. In the second part it is demonstrated that organic–organic heteroepitaxy provides a proper method for efficient color tuning of organic nano-fibers. It is shown that using p-6P nano-fiber templates can be used as fundament for the epitaxial growth of 6T crystallites. The formed 6T crystallites adopt the molecular and morphological orientation of the p-6P layer beneath and provide highly polarized emission in the blue, green, and red spectral range.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Introduction
During the last years a clear trend towards organic electronics could be recognized within the scientific community. The huge spectrum of organic molecules available by chemical synthesis [1] and adequate for the implementation of electronic, photonic and optoelectronic devices, combined with cheap and easy processing and the ability to manufacture devices on flexible substrates opens niches not occupied by anorganic semiconductors [2]. Moreover using organic molecules instead of inorganic compounds in order to fabricate nanostructures, coincides with higher luminescence efficiency at the same material density, more flexible spectroscopic properties and easier processing since controlled, self-assembled growth can be implemented [3].
Consequently a lot effort has been undertaken to study the growth of thiophenes and phenylenes on various substrates [3–10], motivated by the discussed advantages and especially due to their high affinity to form highly crystalline organic nano-needles [7, 11, 12]. Nevertheless it turned out that oligo-p-phenylenes in combination with muscovite mica as substrate represent a prominent and outstanding material combination, forming a stable molecular building block [13, 14]. In that sense it has been demonstrated that their ability for generating long, parallel-aligned nano-fibers provides a proper fundament for several applications e.g. waveguiding and lasing [15–17]. Motivated by this promising criterion the growth of para-hexaphenyl (p-6P) on muscovite mica has been investigated intensely by several groups [4, 5, 12, 18] and as a consequence the discussed material system is well understood concerning morphological, structural, and optical properties.
Nevertheless, the variety of physical properties covered by oligo-p-phenylenes is quite narrow and the optical emission spectrum shifts with decreasing number of phenyl rings from the blue to the UV [20]. As a result it becomes difficult to tune the optical properties of p-6P nano-fibers [7] leading to a lot of efforts to substitute oligo-p-phenylenes by other molecules. Among the huge variety of possible organic molecules, thiophenes and phenylenes are most promising building blocks for the nano-fibers because of their photonic and electronic properties as well as their thermal stability [21]. As a consequence, beside the growth of para-phenylene oligomers—thiophenes [7, 21], thiophene/phenylene co-oligomers [21, 22], and functionalized para-phenylene molecules [6, 14, 23, 24] have been deposited on muscovite mica.
Thiophenes represent an important and well-known class of rod-like molecules for fabrication of active layers providing highly efficient electrical, structural and opto-electrical properties [25, 26]. Most prominent representatives within this group of molecules are α-quaterthiophene (4T) and α-sexithiophene (6T), providing intense green and orange luminescence [7]. By depositing 6T and 4T on muscovite mica, needle-like structures can be obtained [7], but it has to be stated that two main differences of thiophene- in comparison to phenylene-needles are observed: On the one hand, beside needle formation flat islands are formed on muscovite mica (001) showing weak fluorescence which gives a first hint for the formation of upright standing molecules [7, 27]. On the other hand, needle formation shows a lower macroscopic anisotropy as compared to p-6P, which is caused by a needle formation in multiple directions [7, 21] and consequently polarized fluorescence of the nano-fibers is significantly hampered.
A similarly complex behavior is found for thiophene/phenylene co-oligomers [21]. In particular, the growth of 5,5′-di-4-biphenylyl-2,2′-bithiophene (PPTTPP)—also known as BP2T [28, 29] and 4,4′-di(2,2′-bithienyl)-biphenyl (TTPPTT) on muscovite mica has already been studied [21, 22].
Another approach to control the morphology and the luminescence of nano-fibers is provided by chemical functionalizing the organic molecules [24]. Nevertheless, it is rather difficult to modify para-phenylenes because of their low solubility, which even decreases with increasing chain length of the molecules. A generic concept has been developed to access a wider range of molecules on the basis of a functionalized para-quaterphenylene (p-4P) unit [30] which represents a compromise between solubility and critical chain length being the key for mutually parallel needle alignment. In particular, functional groups (e.g. methoxy, chloro, cyano, amino, dimethylamino and benzylamino) are attached on the two para-positions of the p-4P unit which allows to synthesize two different molecular classes—symmetrically [14, 24] and non-symmetrically [6, 30, 31] substituted oligomers. It has been demonstrated that functionalized p-4P oligomers form well-defined fiber-like nanostructures upon vapor deposition on muscovite mica [6] and consequently it can be stated that the suggested strategy represents up to now the most promising approach in order to achieve macroscopically highly ordered parallel organic nano-needles (beside the well-known para-phenylenes). This fact has been underlined by the demonstration of optical devices, such as frequency doublers [31]. Furthermore, the integration of non-symmetric molecules within organic nano-fibers represents an interesting, novel aspect for optical applications, as is expected from theory that they feature second-order non-linear optical activity [6]. The degree of freedom with functionalized quaterphenylenes is quite narrow, in particular concerning their optical response. All molecules investigated so far, emit in the blue spectral range (from 383 nm to 452 nm) [6] which restricts the potential for optical applications.
A graphical summary of the present state of research is shown in Fig. 3.1, indicating by arrows the main optical emission range of thiophene/phenylene co-oligomers in the green [22], of thiophenes in the green to orange [7], of functionalized quaterphenylenes in the blue (383–452 nm) [6] and of para-phenylenes in the UV to blue spectral range [20]. The macroscopic degree of order is sketched as graphical model visualizing parallel (phenylenes, functionalized quaterphenylenes), rhombic (thiophene/phenylene co-oligomers) and x-shaped (thiophenes) needle formation.
Observed needle orientations on muscovite mica with respect to the fluorescence emission wavelength [19]. Whereas for oligo-phenylenes and functionalized phenylenes parallel needle formation has been observed, oligothiophenes and thiophene/phenylene oligomers are characterized by x-shaped or rhombical morphology
Based on these observations several criteria can be defined which have to be fulfilled to achieve long nano-fibers providing highly polarized emission:
-
Parallel molecular alignment In order to achieve a maximum degree of anisotropy, rod-like molecules have to be aligned parallel to each other. Only in that way it becomes possible to make use of the single molecular polarized emission/absorption on an macroscopic scale being essential for device applications.
-
Parallel formation of nano-fibers plays an essential role to obtain large single-crystalline nanostructures. As observed for thiophenes and thiophene/phenylene co-oligomers non-parallel growth of nano-fibers inevitable leads to an increased probability that nano-fibers which originate from different nucleation centers cross each other. Consequently effective lateral needle growth is significantly hampered leading to shorter structures.
-
High crystal quality In order to reduce parasitic processes within the organic crystallites high crystal quality represents an inevitable prerequisite for optical applications.
-
Tunable fiber cross section As lasing of organic nano-fibers can only be obtained by efficient waveguiding within the crystallites, the achievable fiber cross section represents a crucial issue in particular for shifting the lasing emission to longer wavelength.
2 Sheet Silicate Substrates
In order to investigate and in particular to understand the epitaxial growth on mica surfaces a detailed knowledge about the substrate surface morphology, geometry and composition seems inevitable. Substrate surfaces for evaporation of organic molecules and force-field simulations in the presented work are all representatives of the phyllosilicate group. The nomenclature of this mineral group is strongly connected to its crystal structure and in further consequence to its physical, mechanical, and structural properties. In particular all phyllosilicates are built up in a sheet like structure which is expressed by the Greek word phyllon which can be translated by leaf [32] rooting in another often used nomenclature, namely sheet silicates. The crystal structure of sheet silicates can be assembled by two elementary units which are represented by tetrahedral and octahedral cation–anion bonds, depicted in Fig. 3.2a.
(a) Elementary units of sheet silicates: tetrahedra (left) and octahedra (right) are built up by Si, Mg or Al atoms surrounded by oxygens. (b) Sheet like structure of 2:1 phyllosilicates defined by octahedral units sandwiched between tetrahedra. The crystal formation of muscovite and phlogopite is depicted beside showing a potassium interlayer holding the single sheets together. Contrary pyrophyllite and talc do not show such an interlayer. (c) Top view onto a {001} surface of a dioctahedral phyllosilicate (muscovite). As indicated by the pm space group beside, surface symmetry is showing mirror axes parallel to the surface grooves, indicated by a solid arrow. A side view along the grooved direction is sketched beside showing the tilted tetrahedral units. (d) Contrary trioctahedral phyllosilicate surfaces provide p31m symmetry, characterized by three mirror axes and three-fold symmetry
In the case of the tetrahedral unit Si or Al atoms form the center of a tetrahedra surrounded by four oxygen atoms (three basal and one apical oxygen). Single tetrahedra are linked together by means of the basal oxygen atoms resulting in the formation of a two dimensional hexagonal mesh. Analogous considerations hold true for the formation of the octahedral sheet.
Based on these two units phyllosilicates can be divided into two subclasses namely 1:1 or 2:1 layer types. In the case of 1:1 layers one octahedral sheet is bound to a single tetrahedral one whereas in the 2:1 layer type one octahedral sheet is sandwiched between two tetrahedral units. In both cases sheets are aligned parallel to the {001} netplanes of the bulk crystal and apical oxygens of the tetrahedra are shared with the octahedral sheet. Consequently each tetrahedra points with its apical oxygen to the octahedral layer whereas the basal atoms form a horizontal plane as indicated in Fig. 3.2b for a 2:1 layered structure, which is described below in more detail.
A partial cation substitution of Al3+ instead of Si4+ in the tetrahedral layer crucially influences the crystal stacking in phyllosilicates due to the generation of uncompensated charges within the layers. Pyrophyllite and talc belong to the not substituted phyllosilicates and consequently provide charge neutrality, whereas micas (e.g. muscovite, phlogopite) show an Al:Si ratio of 1:3 which leads to an uncompensated negative charge within the tetrahedral layers. By the addition of a cation interlayer sheet in the crystal structure the charge deficiency has to be balanced. In the case of muscovite and phlogopite this interlayer is provided by K+ ions housed in quasi hexagonal cavities formed by the O2− anions of the opposite tetrahedral sheets. Figure 3.2b sketches the stacking of 2:1 layer phyllosilicates with cation interlayers (e.g. muscovite, phlogopite) binding the single sheets together. Contrary pyrophyllite and talc stack without additional interlayers and consequently the binding between the two neighboring tetrahedral sheets depends exclusively on van der Waals bonds only [32].
Furthermore the presence of a potassium interlayer in micas is finally responsible for their perfect cleavage along {001} net planes as the electrostatic linkage represents the weak point in the crystal structure. The higher stability of cation–anion bonds within the octahedral and tetrahedral layers results in a quasi atomically flat surface being not only suitable for AFM studies [33–35] but also provides a perfect substrate surface for organic crystal growth [12, 36–38] and molecular adsorption [39–41].
It is important that the linkage between tetrahedral and octahedral sheets results in structural deformations (due to changing of bond angles and length) accompanied by a symmetry reduction of the bound tetrahedral–octahedral unit. In particular the hexagonal symmetry of individual tetrahedral and octohedral layers is reduced to at least 3-fold ditrigonal symmetry of the resulting units due to a tilt of the tetrahedra with a rotation axis normal to their basal plane [42]. The octahedral sheet is formed by octahedra which are linked together by sharing their six vertices. This can be provided when either each anion is bound to three cations (trioctahedral, see Fig. 3.2d) or to two cations leaving the third site vacant (dioctahedral, see Fig. 3.2c) [32]. As dioctahedral and trioctahedral sheets do not undergo identical deformations [32], the surface properties strongly differ between these two groups and in particular the symmetry of the cleavage plane crucially depends on the structural configuration of the octahedral layer, therefore we have to distinguish between dioctahedral and trioctahedral phyllosilicates.
2.1 Dioctahedral Phyllosilicates (Muscovite Mica, Pyrophyllite)
Dioctahedral phyllosilicates are characterized by a vacant octahedral site as indicated in Fig. 3.2c, sketching a top view on a dioctahedral phyllosilicate surface (e.g. muscovite mica surface when cleaved at the potassium interlayer). In particular a significant elongation of vacant octahedron edges can be recognized, caused by the absence of the cation in the center reducing attractive forces on the surrounding anions [32]. Thus the linkage between the tetrahedral sheet and a dioctahedral sheet requires an additional rotation of the tetrahedra around axes in the basal planes becoming visible by the shifted apical oxygen atoms (white circles) out of the centered cation (gray circle). The resulting tilt not only reduces the symmetry of the surface unit cell, but additionally causes also a surface corrugation of about 0.2 Å [42]. The situation is indicated in the inset of Fig. 3.2c representing a side view along the groove direction. Solid arrows indicate the rotational tilt of the tetrahedra whereas the dashed arrow marks the resulting groove which are caused by lowered oxygen atoms. At this point it has to be stressed that this corrugation originates from the interlinkage between octahedral and tetrahedral sheets and is not caused by a surface reconstruction due to cleaving. Hence the surface morphology of different dioctahedral phyllosilicates cleaved at the tetrahedral sheet provide analogous behavior and surface symmetry. In particular, the mirror symmetry of the surface unit cell is conserved for dioctahedral phyllosilicates in one axis that coincides with the direction of the present surface grooves and the resulting geometry of the surface unit cell can be described by the 2D-space group pm [43] as indicated in Fig. 3.2c.
As already discussed, muscovite mica and pyrophyllite, both representatives of dioctahedral phyllosilicates group, mainly differ due to their different cation substitution ratios and the resulting stacking sequence (see Fig. 3.2b). The octahedral layers in both materials provide analogous structural properties, 25 % of the Si4+ atoms within the tetrahedral layers of muscovite mica are substituted by Al3+. Consequently the tetrahedral mesh can be formed by (i) Si–O–Si, (ii) Si–O–Al or (iii) Al–O–Al bonds, where the third possibility can be excluded based on electrostatic considerations (Löwenstein’s rule) [44, 45]. As an experimental analysis based on X-ray diffraction exhibits serious difficulties due to the similarity of atomic scattering factors of Al and Si [45], information about two and three dimensional ordering is mainly based on computational models (e.g. Monte Carlo simulations) [44, 46] in combination with experimental data deduced by nuclear magnetic resonance (NMR) [45, 47].
A detailed look at the crystal properties of muscovite mica (Fig. 3.2b) reveals that the bulk unit cell includes two tetrahedral–octahedral–tetrahedral sheet units (compare Table 3.1). Interestingly both sheets provide an angular twist of 120∘ relative to each other leading to an alternating stacking sequence of type αβαβ along (001) crystal orientation. Whereas α planes provide their symmetry axis (and consequently grooved direction) in [110] M direction, the β planes show mirror symmetry along \([1\overline{1}0]_{M}\) [12]. Consequently, the mirror symmetry of the muscovite Mica surface unit cell has been indicated in Fig. 3.2c by two crystallographic directions, [110] α and \([1\overline {1}0]_{\beta}\), respectively. Therefore it becomes evident that the presence of cleavage steps causes the presence of two different surface domains when switching from α to β planes and vice versa [12].
2.2 Trioctahedral Phyllosilicates (Phlogopite Mica, Talc)
Whereas the presence of vacant sites in dioctahedral systems favors deformation effects, such processes do not occur in trioctahedral–tetrahedral sheets [32]. Figure 3.2d depicts a top view of a trioctahedral phyllosilicate surface (phlogopite mica surface when cleaved at the potassium interlayer) providing a detailed model of the tetrahedral and octahedral layers. The hexagonal symmetry of the tetrahedral sheet is lost due to a rotation of the tetrahedra units around the axes perpendicular to the basal plane [32] resulting in a ditrigonal symmetry. Nevertheless the distortion of the octahedral and tetrahedral units in comparison with dioctahedral phyllosilicates (e.g. muscovite mica) is significantly reduced [32] resulting in a highly symmetric surface unit cell which is indicated by solid lines in Fig. 3.2d. A closer look to the atomic positions reveals a 3-fold symmetry with three mirror axes resulting in a p31m 2D-space group [43].
In analogy to dioctahedral phyllosilicates phlogopite mica and talc mainly differ due to their different cation substitution ratios and the resulting stacking sequence. As the surface morphology and symmetry are mainly determined by the formation of the octahedral layer both phyllosilicates show comparable surface morphology and symmetry.
In comparison to muscovite mica the crystal unit cell of phlogopite mica consists of only one tetrahedral–octahedral–tetrahedral sheet and consequently high symmetry directions can be defined uniquely as indicated by dashed–dotted arrows pointing in [110] P , [100] P and \([1\overline{1}0]_{P}\) crystallographic directions, which means that all cleavage planes show the same surface symmetry.
Table 3.1 summarizes the discussed properties for phlogopite, muscovite, talc and pyrophyllite including its bulk unit cell parameters taken from literature [48–52].
2.3 Freshly Cleaved Mica Surfaces
Although the structural and crystallographic properties of phlogopite and muscovite seem conclusive and well understood, a detailed analysis of the morphological and structural properties of a freshly cleaved mica surface poses a demanding challenge. The following paragraph is only focused on the matters of fact playing a major role for the experimental procedures and computer simulations applied within this article.
In particular it has to be stated that the conditions during the cleaving procedure predominate the surface conditions and in further consequence certainly effect further growth experiments. In particular it has been demonstrated by Low Energy Electron Diffraction (LEED) that cleaving mica in ultra high vacuum conditions (UHV) leads to charging effects accompanied by triangular shaped diffraction patterns which are attributed to surface dipole fields [53, 54]. Complementary atomic force microscopy (AFM) investigations found UHV cleaved mica surfaces to be highly charged, effectively prohibiting AFM imaging of any reasonable resolution [55]. It is reported that exposure to air for a couple of minutes significantly reduces the charging effects [55] and in particular it is stressed that triangular shaped LEED pattern are never observed for mica substrates cleaved in air at atmospheric pressure [18, 54]. All these observations are in agreement with experiments providing chemical sensitivity, e.g. secondary-ion mass spectrometry (SIMS) and Auger electron spectroscopy (AES). In particular clear signals originating from carbon compounds have been detected on air cleaved mica by complementary techniques [18, 56, 57]. Interestingly it was found that an additional adsorption component is necessary to bind gaseous carbon compounds which is assumed to be provided by water [56]. In summary all these observations hint that a chemical reaction of residual surface potassium ions with carbonaceous gases and water [34, 55] takes place on air cleaved mica, which significantly forces the neutralization of uncompensated charges (potassium ions) in an irreversible way [54].
Furthermore morphological and symmetrical properties of the mica surfaces are strongly related to the structural formation of the octahedral layers. In particular the vacant sites within dioctahedral systems are mainly responsible for the symmetry breaking and structural deformations [32]. The consistent picture is further substantiated by the comparison between experimentally deduced bulk crystal structures of muscovite [50] and phlogopite [48], showing the presence of parallel surface corrugations only within the dioctahedral muscovite mica. Additionally reported AFM measurements [33] on air-cleaved muscovite clearly reveal the presence of such grooves that break the 3-fold symmetry of the surface unit cell. Contrary AFM images reported from phlogopite mica surfaces show quasi hexagonal symmetry as expected by geometrical considerations (compare Fig. 3.2) [32].
In summary it can be justified that the surface morphology dominates the molecule-surface interactions during the deposition process whereas electrostatic forces due to Al–Si substitution and uncompensated potassium ions play a minor role when cleaving the mica substrate in air.
3 Epitaxial Growth of Rod-Like Molecules on Sheet Silicates
In order to discuss the epitaxial growth on sheet silicate substrates we have selected two representative rod-like molecules, namely p-6P and 6T. Both molecules are already intensively investigated and it has been demonstrated that they can be successfully used for organic device applications [58–60].
3.1 Para-Hexaphenyl
As depicted in Fig. 3.3a each p-6P molecule consists of six phenyl rings linked together in a chain like structure. Organic p-6P crystallites provide a large energy gap (3.1 eV) between highest occupied molecular orbital and lowest unoccupied molecular orbital, excellent optical properties including photoluminescence with high quantum yield and outstanding structural properties. These properties provide a proper fundament for optoelectronic devices which further explains that p-6P based organic light-emitting devices were among the first candidates applied for blue emission [58, 59].
(a) Molecular representation of the p-6P molecule, characterized by six phenyl rings linked together. (b) Primitive monoclinic lattice of p-6P β-structure representing the equilibrium bulk structure (a=8.091 Å, b=5.568 Å, c=26.241 Å and β=98.17∘) [61]
During the last years the epitaxial growth of p-6P has been analyzed extensively on several substrates by optical, crystallographic and morphological methods. In that sense also the crystal formation on muscovite mica has been studied in detail and it has been found that p-6P tends to crystallize in its β-structure [61] which represents the equilibrium bulk structure [12]. Figure 3.3b indicates the β-structure primitive unit cell which consists of two p-6P molecules packed in herringbone fashion. Depending on the chosen substrate temperature it has been shown that p-6P crystallizes either with its \((11\overline{1})\) or \((11\overline{2})\) crystal planes parallel to the muscovite mica substrate surface [12]. As the dominating fraction of p-6P crystallites are characterized by a \((11\overline{1})\) contact plane further discussion is focused only on this orientation.
To analyze the epitaxial growth of p-6P on sheet silicate substrates a series of samples has been prepared by hot-wall epitaxy (HWE). The growth time was chosen to 40 min and the substrate temperature has been kept constant at 90 ∘C during the deposition procedure while the source and wall ovens were kept at 240 ∘C and 260 ∘C, respectively. As substrates two different types of mica have been chosen, namely muscovite mica and phlogopite mica. In order to verify the morphological properties of the grown nanostructures atomic force microscopy (AFM) images have been acquired. In the case of muscovite mica highly parallel needle formation can be observed providing nanostructures which are several micrometers long (see Fig. 3.4a). To analyze the azimuthal alignment and consequently the epitaxial relationships between the organic crystallites and the mica substrates in detailed XRD pole-figure measurements have been performed. The measured pole figure for the (\(11\overline{1}\)) diffraction peaks are shown in Fig. 3.4c. For a direct comparison with the geometry of the muscovite mica substrate surface, the mirror axis for a β-cleavage plane [12] is indicated in the pole figure by a solid line along \([1\overline{1}0]_{M}\) crystallographic direction. Diffraction peaks which can be attributed to organic p-6P crystallites are indicated by arrows. Strikingly organic diffraction peaks observed in the upper and lower hemispheres of the polar plot can be nicely correlated with each other by a mirror operation along the \([1\overline{1}0]_{M}\) direction. So it is demonstrated that the expected mirror symmetry of the substrate surface is nicely adopted by the organic crystallites. Thus, the measurement can be well explained by the presence of four crystal orientations, which have in common the mirror symmetry originating from the substrate and a 2-fold symmetry due to the geometry of the p6P molecule. For each p-6P crystallite a unique long needle axis (LNA) can be defined by the orientation of its \([1\overline{1}0]\) crystallographic direction [12]. The resulting orientations are indicated in the left part of Fig. 3.4b, following the geometric considerations already discussed. As AFM measurements provide direct access to the azimuthal distribution of the LNAs, the calculated FFT pattern of Fig. 3.4a is presented in the right part of Fig. 3.4b providing excellent agreement with crystallographic analysis. As indicated in Fig. 3.3b p-6P molecules are aligned quasi parallel with their long molecular axis (LMA) within the crystal unit cell. Consequently each organic crystallite can be correlated with a unique LMA providing information about the alignment of the p-6P molecules relative to the substrate surface. As indicated in Fig. 3.4d p-6P molecules align nearly normal to the mirror axis of the muscovite mica substrate.
AFM images of para-hexaphenyl (p-6P) nano-needles grown on muscovite (a) and phlogopite (e) mica. Azimuthal alignment of the long needle axis (LNA) deduced by XRD pole-figure measurements (left) and AFM-FFT analysis (right) for p-6P deposited on muscovite (b)/phlogopite (f) substrates. XRD pole-figure measurement performed at a scattering angle of 29.5∘ with an acceptance angle of ±1∘ performed on p-6P crystals on muscovite (c) and phlogopite (g) mica. Azimuthal alignment of the long molecular axis (LMA) deduced by XRD pole-figure measurements (d, h)
In order to analyze the influence of di- and trioctahedral mica substrates on the organic needle formation, analogue analysis has been applied to p-6P films deposited on phlogopite mica. As indicated by the AFM image depicted in Fig. 3.4e the higher substrate surface symmetry of phlogopite mica is directly reflected by the orientation of the LNA. Whereas muscovite mica shows only one dominant LNA, p-6P deposited on phlogopite mica is characterized by triangular structures resulting from the three-fold symmetry of the substrate surface. Due to crossing of the individual p-6P crystallites nano-fibers become much shorter in length as compared with the same growth conditions on muscovite mica substrates. Again FFT analysis of the AFM images has been performed and is depicted in the right part of Fig. 3.4f showing three streaks separated by 60∘. In order to analyze the epitaxial relationship of organic crystallites and the substrate XRD pole-figure measurements have been performed and are reported in Fig. 3.4g. To provide direct comparison with the muscovite mica substrate, XRD pole-figure measurements have been performed with unchanged conditions choosing an scattering angle of 29.5∘ with an acceptance angle of ±1∘. Strikingly the XRD pattern observed on muscovite mica are also reflected for the trioctahedral mica substrate. This observation is consistent with specular XRD diffraction spectra which show analogue configuration concerning the contact plane of p-6P nano-needles on both substrates, namely a dominating formation of p-6P crystallites characterized by a parallel \((11\overline{1})\) crystal plane to the substrate surface. Moreover azimuthal alignment of p-6P crystallites varies only marginally between both substrates due to a similar configuration of the tetrahedral layer determining the interface between substrate and organic crystallites. Nevertheless higher symmetry of the phlogopite mica substrate is clearly reflected by a three-fold symmetry of the pole figure. As expected also the azimuthal alignment of the LNA, which is indicated in the left part of Fig. 3.4, reflects this geometry. Again nice correlation with AFM-FFT analysis being sensitive to morphology can be observed. The orientation of the LMA is depicted in Fig. 3.4h reporting a preferred molecular orientation along the [310] P , \([3\overline{1}0]_{P}\) and [010] P crystallographic directions of phlogopite mica.
3.2 Sexithiophene
α-Sexithiophene, a conjugated oligomer consisting of six thiophene units linked at alpha position (see Fig. 3.5a), is one of the most promising materials for organic-based electronic devices, primarily, organic field-effect transistors (OFETs) [60, 62, 63]. Concerning its crystallization the appearance of polymorphism is observed leading to two different crystal configurations [64]. Whereas 6T crystallites grown from the vapor phase pack with four molecules (low-temperature—LT—phase, sketched in Fig. 3.5b) [65] those grown from the melt are characterized by two molecules per unit cell (high temperature—HT phase) [66]. Whereas p-6P emits in the blue spectral range, the emission of 6T is significantly red shifted leading to orange-red fluorescence when optically excited [62, 63].
All these properties imply that 6T represents a promising candidate to study the epitaxial growth on sheet silicates. Again, two different kind of mica substrates have been used to study the epitaxial growth of 6T by HWE. After cleaving the mica substrates in air, they have been transferred to the HWE chamber working at 9⋅10−6 mbar. After a thermal treatment of the substrate at 90 ∘C, the sample has been transferred in vacuum conditions to the 6T HWE oven. Subsequently 6T has been deposited for 90 min, keeping a substrate temperature of 90 ∘C. The source material has been evaporated at 190 ∘C and the wall was heated to 220 ∘C.
To analyze sample morphology optical microscope images have been taken and are reported in Fig. 3.6a. Comparable to p-6P organic nano-needles become visible on the muscovite mica substrate. Nevertheless, anisotropy is significantly lowered which becomes visible by multiple azimuthal orientations. In order to analyze the angular distribution of the organic nano-needles in more detail, FFT analysis has been applied to the optical microscope image and is reported in the right part of Fig. 3.6b. A closer look to the depicted pattern reveals four sharp streaks, each representing a distinct needle orientation. The obtained crystal orientation has been analyzed in more detail by XRD pole-figure measurements which are reported in Fig. 3.6c. In particular, the orientations of 6T (\(\overline{2}11\)) netplanes were probed to determine the azimuthal alignment of the organic crystallites. As indicated in Fig. 3.6c by black arrows, XRD pattern reveal eight diffraction spots which underline a defined azimuthal order of the 6T crystallites. This pattern can be explained by a packing of 6T molecules in the so-called low-temperature phase [65] and a parallel orientation of the \(\{ \overline{4}11\}\) planes to the muscovite mica (001) M substrate surface. The diffraction spots in Fig. 3.6c again clearly reflect the geometry of the muscovite mica surface unit cell. Analogous to the analysis reported for p-6P the orientation of the LNA and LMA has been deduced from the experimental data. Whereas the LNA of the 6T crystallites can be deduced by their \([0\overline{1}1]\) crystallographic orientation, the angular orientation of the LMA is concluded by the molecular alignment within the crystal unit cell. As indicated in the left part of Fig. 3.6b XRD experiments provide excellent agreement with morphological investigations, indicating four distinct needle orientations. Contrary the LMA, as reported in Fig. 3.6d, are aligned along two orientations which are defined by the [110] M and [100] M crystallographic directions of the muscovite mica substrate. Moreover, the diffraction spots in Fig. 3.6c clearly reflect the geometry of the muscovite mica surface unit cell. This becomes evident by the presence of a mirror symmetry along the [110] M direction, implying crystal growth on a α terminated muscovite mica substrate [12].
Optical microscope image of 6T nano-needles deposited on muscovite (a) and phlogopite mica (e). The angular configuration of the long needle axes (LNA) deduced by XRD (left) and AFM-FFT (right) analysis for 6T crystallites deposited on muscovite (b)/phlogopite (f) mica. XRD pole-figure measurements of the \(\{\overline{2}11\}\) diffraction peaks, arrows indicate azimuthal position of the \(\{\overline{2}11\}\) poles. Results for sexithiophene crystals deposited on (c) muscovite mica and (g) phlogopite mica are presented. (c)–(h) Orientation of the 6T long molecular axis (LMA) deduced from the corresponding XRD pole-figure measurements depicted above
To compare the crystal growth on tri- and dioctahedral mica substrates, comparable investigations have been applied to nano-needles which have been deposited on phlogopite mica. As expected film morphology is dominated by nano-needles which are oriented along multiple directions (Fig. 3.6a). Whereas the microscope image indicates quasi random azimuthal order, the calculated FFT pattern, which is reported in the right part of Fig. 3.6b clearly reveals distinct azimuthal orientations. As already demonstrated for p-6P crystallites the deduced FFT pattern nicely reflects the symmetry of the substrate surface unit cell. In particular three-fold and mirror symmetry along [100] P , \([1\overline{1}0]_{P}\) and \([\overline{1}10]_{P}\) crystallographic orientations of phlogopite mica becomes visible. As expected also XRD pole-figure measurements, which are reported in Fig. 3.6b reflect these observations. Again, black arrows indicate the azimuthal angle where \(\overline{2}11\) diffraction spots are observed. The XRD pattern can be explained by a packing of 6T molecules in the low-temperature phase [65] and a parallel orientation of the \(\{\overline{4}11\}\) planes to the phlogopite mica (001) P substrate surface. As observed for p-6P the crystal orientation on phlogopite mica nearly follows the trend observed for muscovite mica substrates. In particular, the orientations of LMA and LNA observed for muscovite mica are quasi conserved and are accompanied by further possible configurations due to three-fold symmetry of the substrate surface unit cell. As indicated in Fig. 3.6b crystallographic analysis nicely correlates with morphological investigations provided by optical microscopy. The angular orientation of the LMA is depicted in Fig. 3.6h showing an alignment along the high symmetry orientations of the phlogopite mica substrate.
For the sake of completeness it has to be mentioned that beside the discussed needle formation additionally island like structures are observed on both kind of mica substrates. These entities can be related to 6T crystallites which are characterized by a (100) contact plane and are consequently formed by quasi standing 6T molecules on the substrate surface. Interestingly, also these crystallites provide a distinct azimuthal orientation which follows substrate surface symmetry and can be explained by a distinct relationship to 6T crystallites forming the organic nano-needles. More details concerning the epitaxial growth of 6T on mica substrates can be found elsewhere [27].
3.3 Growth Model of Rod-Like Molecules on Sheet Silicates
In order to understand the discussed experimental observations in more detail Fig. 3.7 sketches a growth model which can explain the epitaxial growth on sheet silicates.
-
1.
Molecular Adsorption In the initial phase of organic crystal growth single rod-like molecules align flat lying on the substrate surface. It can be expected that for each molecule type there exists an energetically preferred azimuthal orientation which leads to distinct azimuthal orientation of the LMA. In order to verify this statement force-field simulations have been performed on tri- and dioctahedral sheet silicate substrates, namely talc and pyrophyllite [19]. As discussed extensively pyrophyllite and muscovite mica mainly differ concerning the Al–Si substitution of the tetrahedral layer, whereas their substrate surface geometry is comparable. Consequently the influence of electric fields originating from the mica substrate can be excluded whereas just the effect of surface corrugation is considered by the force-field calculations. By minimizing the adsorption energy for each angle ϕ the optimal adsorption position of an isolated organic molecule on top of such a substrate can be determined. In that way each azimuthal molecular orientation can be correlated with an adsorption energy which is defined as the difference between the energies of the isolated subsystems and the energy of the combined system, i.e., the molecule and the substrate. Therefore, maxima in the E ad vs. ϕ curve evidence the favorable adsorption geometries. Figure 3.7(1) presents the obtained results as a polar diagram for 6T (left) and p-6P (right) molecules. As both molecules provide two-fold symmetry full polar plots can be deduced by a 180∘ rotation. As expected also simulations nicely reflect the symmetry of the di-octahedral substrate. To compare simulations with experimental results gray areas indicate the orientation of the LMA deduced by XRD pole-figure measurements. As indicated by the red curve in the left part of Fig. 3.7(1) simulations for 6T molecules reveal three local maxima (90∘, 120∘, 180∘) which represent energetically preferable molecular adsorption geometries. Out of these configurations, the intermediate, but not the strongest, peak is in accordance with experiment. This small discrepancy can be explained by the usage of empirical potentials which in some cases may yield the wrong energetic ordering of competing structure solutions [67]. In the case of p-6P which is presented on the right side of Fig. 3.7(1) simulations provide perfect agreement with experimental data. Strikingly, the global maxima located at ϕ=90∘ perfectly overlaps with experimental findings. The corresponding molecular adsorption site on the pyrophyllite surface is indicated in the center of Fig. 3.7(1) showing two different adsorption angles for 6T and p-6P molecules.
Fig. 3.7 Graphical overview demonstrating the epitaxial growth of rod-like molecules on sheet silicates. In the initial stage (1) the interaction between single molecules and the substrate surface plays a major role. Depending on the molecules different angular alignment can be expected representing an energetically favorable configuration. In a further step (2) substrate surface geometry and in particular mirror symmetry leads to multiple orientations which are energetically equivalent. Molecular configuration within the unit cell of the organic crystallite leads to the formation of multiple needle orientations (3) nucleating at the molecule adsorption sites. With growing crystal size molecule–molecule interactions within the organic crystallite become dominant and can further lead to slight angular realignment (4) on the substrate surface
-
2.
Substrate Surface Symmetry Due to substrate surface geometry, each adsorption site has a mirrored energetically equivalent adsorption site on the surface as indicated in Fig. 3.7(2). This energetically equivalence results statistically in an equally appearance of these adsorption sites and furthermore in nucleation on these sites. This expectation can be nicely confirmed by XRD pole-figure measurements which directly proof mirror symmetry of the organic crystallites. As a consequence of this finding it has to be stated that maximized anisotropy of organic crystallites can only be provided when molecules align parallel or normal to the substrate surface mirror axis. Exactly this situation holds true for p-6P whereas 6T molecules are accompanied by a twin configuration leading to X-shaped needle formation.
-
3.
Organic Crystal Nucleation As the density of molecules on the surface increases, needles start to nucleate from a single molecule. Due to clustering of molecules the crystal structure of the respective bulk phase is adopted. There are, in principle, two possible growth directions. This is visualized in Fig. 3.7(3) by the solid and dashed ovals representing the rod-like molecules. The molecules in one needle are turned upside down (mirrored) with respect to the molecules in the other needle. Consequently each molecular adsorption site leads to the formation of two needle directions. In general, this will not lead to two energetically equivalent geometries. As these two orientations are further doubled due to the substrate surface mirror symmetry the existence of four needle orientations can be expected in the general case.
-
4.
Molecular/Crystal Re-adjustment During needle growth molecule–molecule interactions will become more and more important. This will cause a slight readjustment of the LMA in the order of a few degrees to obtain a better lattice match with the substrate [12, 68]. This adjustment can be assumed to be different for the two needle-growth directions. As indicated in Fig. 3.6d and Fig. 3.6h XRD pole-figure measurements indicate a slight splitting of the LMA which has been verified also by force-field calculations [19].
Summarizing the discussed growth model it has to be stated that parallel needle formation, as in the case of p-6P and functionalized quaterphenylenes, can be only achieved in outstanding molecular systems. In particular two main criteria have to be fulfilled:
-
The long molecular axis of the chosen molecule has to be aligned parallel or normal to the mirror axis of the muscovite mica substrate. Only in these configurations the mirrored adsorption site coincides with its twin position.
-
The molecular configuration within the unit cell of the organic crystallite crucially influences the expected splitting of organic nano-needles. An optimized configuration can be expected if the surface unit cell of the organic crystallite provides an rectangular shape which would theoretically lead to only one needle orientation per molecular adsorption site.
Based on these findings it has to be stated that substitution of p-6P molecules by other species in order to achieve an efficient color shift of organic nano-fibers grown on muscovite mica is not trivial and it can be expected that only a small group of molecules can fulfill the inevitable conditions. In further consequence other solutions have to be found to achieve parallel molecular alignment being an essential requisite for polarized emission.
4 Organic Hetero-epitaxy of Nano-fibers
In recent years, heteroepitaxy of organic–organic nanostructures has been demonstrated as a valuable technique to explore the full potential of organic semiconductors for optoelectronic applications. Crystalline and highly ordered heterostructures with different morphology and molecular orientations can be realized by heteroepitaxy starting from conjugated oligomers, aimed at tailoring their optical properties and transport characteristics [69–73].
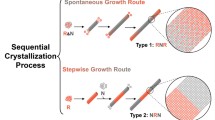
Whereas the deposition of organic molecules directly on muscovite mica seems not very promising in order to obtain highly anisotropic nano-fibers, organic heteroepitaxy could provide a proper tool to achieve the desired goal. The principal idea is sketched in Fig. 3.8 and proposes the organic crystal growth on top of p-6P fiber templates. As material couple for heteroepitaxy p-6P and 6T seem compatible due to their rod-like configuration and comparable chain length. Moreover it has already been demonstrated that molecular orientation can be influenced by organic heteroepitaxy of p-6P/6T [72, 73].
Deep-blue-emitting highly oriented p-6P nano-fibers (left) and red-orange 6T fibers (right) grown on muscovite mica. Schematic representations of multilayer fibers are provided above, with crystalline p-6P represented by blue sticks, and crystalline 6T as red sticks. The schematic below sketches the desired behavior when using organic–organic heteroepitaxy. The usage of p-6P template needles should force a realignment of 6T crystallites
In order to verify if organic–organic heteroepitaxy can be successfully implemented on organic nano-fibers, samples have been fabricated on muscovite mica (001) substrates by using HWE. Immediately after cleaving, the mica substrates were transferred via a load lock to the growth chamber, providing two separated HWE reactors equipped with p-6P and 6T source material. The optimized evaporation temperature for p-6P is given at 240 ∘C (190 ∘C), leading to a nominal growth rate of 3 nm/min (4.5 nm/min). In order to avoid temperature gradients during growth and to reduce adsorbed species on the surface, the substrate has been preheated at 120 ∘C for 30 min. The chosen temperature is kept constant during the whole growth procedure. After depositing p-6P for 40 min (≈120 nm fiber height), the sample is automatically transferred in high vacuum conditions to the 6T source oven. Subsequently, 6T has been deposited for 1 s up to 90 min (≈405 nm). The nominal layer thickness is defined as average fiber height.
In a first step heterostructures grown with nominally 120 nm p-6P and 400 nm 6T layer thickness have been investigated by AFM. As indicated in Fig. 3.9a film morphology of the pure p-6P needle template seems to be conserved becoming visible by nicely parallel oriented nano-fibers. A closer look to the AFM image indicates the presence of two different needle morphologies which becomes obvious by excerpting a cross-sectional view as indicated in Fig. 3.9b. Interestingly, needles with two characteristic height levels are formed on the muscovite mica substrate. Moreover, the height histogram of the AFM image (Fig. 3.9b) underlines this observation showing three peaks which can be attributed to the bare muscovite mica substrate (A), the p-6P template needles (B) and p-6P needles which have been overgrown by 6T crystallites. Corresponding regions are indicated also in the AFM image and in the depicted cross section. Moreover needle shape of both needle structures seem to differ significantly as indicated in Fig. 3.9b. Whereas lower needles provide a nearly rectangular shape of their cross section, which is expected for p-6P crystallites [30], organic–organic heterostructures are characterized by a triangular rounded silhouette already hinting a different growth morphology.
(a) AFM image of the heteroepitaxial sample structure showing parallel aligned nano-fibers. (b) Height histogram of AFM image showing three peaks originating from the bare mica substrate, visible between the fiber structures (A), the p-6P fibers (B), and the overgrown 6T/p-6P fibers (C). Corresponding regions are marked in (a). (c) XRD pole-figure measurement (q=1.34 Å) providing information about the azimuthal orientation of the p-6P and 6T crystals relative to the muscovite mica substrate. The inset depicts a zoom of the pole figure, indicating the presence of p-6P and 6T crystallites
For a more detailed analysis XRD pole-figure measurements have been performed providing information about crystal phase, contact plane and azimuthal orientation. In particular, the azimuthal orientation of \((\overline{2}11)\) of 6T (q=1.34 Å) has been analyzed in detail and is reported in Fig. 3.9c. Experiments reveal two significant spots located symmetrically aligned around the [110] direction of muscovite mica and are consequently characteristic for a single fiber domain deposited on an α-cleavage plane [12]. A more detailed analysis of the pole figure (see the inset) allows to resolve additional distinct diffraction peaks originating from the \(\{\overline{2}11\}\) netplanes of 6T as well as from the \(\overline{1}11\) netplanes of p-6P. The observed diffraction pattern can be described by four crystallites of each molecular species representing a two-fold symmetry with an additional mirror axis around the [110] direction of muscovite mica. Whereas p-6P crystallites provide a \((\overline{1}11)\) contact plane 6T crystallites are oriented parallel with their \(\{\overline {4}11\}\) netplanes to the muscovite mica surface. Interestingly an analogous configuration concerning the contact plane has also been observed for 6T nano-fibers directly deposited on bare muscovite mica substrates. Nevertheless their pole-figure pattern significantly differ underlining a changed azimuthal orientation due to organic–organic heteroepitaxy.
Based on pole-figure measurements it is possible to deduce a real space model of the crystal and molecular orientations (Fig. 3.10a). The crystal stack on the left side demonstrates the molecular orientation of p-6P (gray) molecules in one crystallite, and the white arrow points in the \([1\overline{1}0]\) direction of the p-6P stack, defining the resulting LNA [12]. On top of the organic template, 6T molecules (yellow atoms represent sulfur) are indicated, packed in the low-temperature herringbone structure and characterized by a (\(\overline{4}11\)) contact plane parallel to the substrate surface. A more detailed inspection of the p-6P/6T interface (shown in the inset of Fig. 3.10a) resolves the expected herringbone package providing nearly perfect adoption of 6T to p-6P layers. To aid the reader, the mirrored crystallite is reduced to two single molecules and the corresponding LNA that has an angle of 6∘ with respect to the [110] direction of muscovite mica. As a consequence of these orientations, both LMAs are parallel to each other and have an angle of 83∘ with respect to the high symmetry axis of muscovite mica. A splitting of the LNA around the [110] direction of muscovite mica due to mirror symmetry can be further outlined by the morphological arrangement of two single nano-fibers seen in the center of the plot (Fig. 3.10a). The magnification is taken from a 10×10 μm AFM image, and its origin is indicated by a white polygon in Fig. 3.9a.
(a) Simulation of the molecular alignment within the nano-fibers concluded from the XRD pole-figure measurements. The angle between the long needle axis (LNA), the long molecular axis (LMA), and the mica [110] direction is indicated in the mirrored crystal model on the right. The inset indicates the herringbone structure of p-6P and 6T at the crystal interface. (b) TEM cross section of p-6P/6T heterostructure, indicating the deposited aluminum layer (1), muscovite mica substrate (2), p-6P template region (3), and 6T crystallite (4). (c) Simulated view along LNA of p-6P templates showing the expected angular alignment of 6T low energy (100) plane
In order to further clarify the epitaxial overgrowth of p-6P nano-fibers by 6T crystallites, transmission electron microscopy (TEM) analysis has been conducted, providing a nanoscopic view on the structural properties. Before preparing TEM slides, samples have been covered with a 25 nm thick aluminum (Al) layer for protection purposes, becoming visible by an enclosing cover layer, indicated in Fig. 3.10b by region 1. Furthermore, the muscovite mica substrate can be clearly determined by a sharp contrast and is indicated by region 2. Structures characteristic for the two different fiber types are labeled B and C. In particular, rectangular appearance of fiber-type B in combination with a correlation in height justifies the direct comparison with AFM analysis. Strikingly, fiber structures of type C are characterized by a tilted entity on top of a rectangular fiber basement, emphasizing the difference in shape already observed by AFM analysis. In order to understand the observed behavior, molecular stacking has been modeled based on structural investigations determined by XRD pole-figure measurements. The obtained result is indicated in Fig. 3.10c, providing a cross-sectional view along the fiber directions and consequently comparable with TEM analysis. The lower part of the image indicates a single p-6P crystallite characterized by a horizontal alignment of its \(\{\overline{1}11\}\) netplanes. On the contrary, \(\{\overline{4}11\}\) netplanes of the overgrown 6T crystallite are aligned horizontally, fulfilling structural alignment deduced by XRD. The presented model stack directly reveals a different stacking of 6T and p-6P crystallites along the growth direction, which can be explained by the different tilt of low index planes, namely, (001)p−6P and (100)6T , providing the lowest surface energy. Whereas p-6P low index plane is more or less oriented vertically, the low energy facet of 6T is remarkably tilted and astonishingly provides excellent agreement with experimental data (demonstrated in Fig. 3.10b).
In summary structural and morphological investigations have demonstrated that organic–organic heteroepitaxy can be successfully implemented on nano-fibers. Whereas 6T crystallites deposited directly on muscovite mica substrates are characterized by X-shaped morphology, organic–organic heteroepitaxy provides a proper method to force a realignment of the crystallites. Moreover, TEM analysis has demonstrated that a sharp organic–organic interface can be achieved underlining a well-defined epitaxial relationship between both organic crystallites. As shown by XRD pole-figure measurements the LMA of 6T molecules adopt the highly parallel ordered arrangement of p-6P molecules in the template crystallite which promises highly polarized emission of p-6P as well as 6T crystallites.
For the optical characterization of the p-6P/6T heterostructures in a first step fluorescence microscopy has been applied on various samples providing a first hint concerning the emission properties. Interestingly emission properties strongly depend on the deposited thickness of 6T layers and consequently Fig. 3.11 summarizes the obtained results for the three representative configurations. In a first step p-6P template structures have been investigated under UV excitation providing deep-blue emission (top). In a next step 6T has been deposited on top of a p-6P needle template with nominally submonolayer coverages. As indicated in Fig. 3.11 (center), green-emitting fibers are obtained. The emission spectrum is dominated by that of the 6T molecules deposited on top of the p-6P fibers while blue fluorescence of p-6P is significantly quenched. For increasing 6T coverage, the red-orange fluorescence band typical of 6T polycrystalline films appears, suggesting that crystal nucleation occurs on top of the fibers. The emission intensity of the new band is much weaker than that of a submonolayer film grown on p-6P fibers, whereas it is comparable to that of a pure 6T/muscovite mica film of polyoriented crystalline fibers. Scanning probe fluorescence images collected in different spectral bands are indicated in Fig. 3.11 (bottom) and make it possible to map the distributions of green-emitting 6T molecules and 6T crystals along the 6P template fibers. Fluorescence intensity measured through a green band-pass filter shows quite a homogeneous distribution of green-emitting molecules, whereas fluorescence imaging through a red-transmitting filter proves that 6T crystals are superimposed to the templating fibers and form fibers themselves. Consequently optics underlines the drawn picture based on structural and morphological investigations.
Fluorescence microscopy images of p-6P/6T heterostructures at different 6T layer thickness. Whereas pure p-6P fibers which are used as templates provide deep-blue emission (top) the evaporation of 6T with nominally sub monolayer coverage provides green emission under UV excitation (center). With increasing 6T deposition time 6T crystallites are formed on top of the needle structures which provide red emission
For a deeper understanding concerning the optical anisotropy, molecular orientation and long-range order of the p-6P/6T heteroepitaxial films, samples have been investigated by combined fluorescence spectroscopy and fluorescence polarization averaged over sample areas as large as 500 μm2. The emission spectrum of the films can be decomposed in its three components arising from crystalline p-6P, 6T interfacial layers and crystalline 6T, which emit in the blue, green and red, respectively. The three components are singled out from the emission spectrum by measuring pure p-6P films, p-6P/6T films with short 6T deposition times, and pure 6T films on muscovite mica (dashed lines in Fig. 3.12a). It is noteworthy that the spectrum of green-emitting, submonolayer 6T aggregates (Fig. 3.12a) is considerably blueshifted with respect to that of 6T crystals and only slightly redshifted from that of noninteracting molecules [74]. This is reminiscent of what has been reported for submonolayer films of lying 6T molecules deposited on silicon dioxide, which could be understood as an effect of J-type aggregation [75]. In our case, J-type aggregation would in fact explain the very intense emission of the interfacial 6T material. A linear superposition of the three reference spectra fits well to the emission spectrum, hinting to the potential ability of tuning fiber chromaticity across the entire visible spectrum by selecting proper p-6P and 6T layer thicknesses. Polarization-resolved measurements can then be used to determine molecular orientation of each material phase within the fibers. The polar plot (Fig. 3.12b) of fitted spectral weights versus polarization angle clearly shows that emission dipoles of crystalline p-6P, interfacial 6T and crystalline 6T are fairly well aligned along the same direction, i.e., perpendicular to the fibers’ axis (at 0∘ angle). Strong polarization anisotropy is demonstrated by the high (up to 9 dB) ratios obtained between fluorescence intensity maxima and minima vs. angle, thereby revealing a high orientational order of the fibers’ constituent material phases.
(a) Fluorescence spectra of a film with nominally 400 nm 6T fiber thickness, acquired at 0∘ (long fiber axis) and 90∘ polarization angle. The blue, green, and red dashed lines represent the fitted contribution of blue (p-6P), green (interfacial 6T submonolayer), and red (crystalline 6T) components. (b) Polar plot of the p-6P and 6T material phases’ fluorescence intensity versus polarization angle. (c) Contribution of crystalline p-6P to the single spectra, representing a magnification of section (b) for better visibility (solid lines represent a cos2 fit to the experimental data)
Overall, structural, morphological and optical investigations provide a consistent picture concerning the epitaxial growth of p-6P/6T heterostructures. It is demonstrated that organic–organic heteroepitaxy provides the ability to drive the growth of highly oriented 6T fibers on p-6P fiber templates. In particular it is demonstrated that organic–organic heteroepitaxy can force the molecules to lie parallel to each other. Moreover structural investigations underline a sharp organic–organic interface determined by parallel orientated 6T molecules on top of the p-6P needle template. Optical spectroscopy underlines that at low 6T coverage the interface exhibits uniformly distributed submonolayer aggregates made of co-oriented molecules that are determinant for the green fiber emission color. With increasing 6T layer thickness red emission of nucleated 6T crystals contributes to the emission spectrum which is also observed by fluorescence microscopy investigations. It is found that green-emitting 6T phase can be efficiently sensitized by p-6P molecules via energy transfer. Moreover, the green emission band provides a more intense emission as compared to the red crystalline 6T phase.
5 Summary
In order to deduce a model explaining the epitaxial growth of rod-like molecules on sheet silicate substrates detailed crystallographic and morphological analysis has been conducted. As model system 6T and p-6P molecules have been chosen. Whereas both molecules crystallize in nano-fibers, p-6P provides much higher anisotropy due to a nearly perfect parallel alignment of its crystallites. Based on experimental data a growth model has been presented which is further underlined by force-field simulations. It is shown that in the general case the formation of polyoriented nano-fibers can be expected when choosing muscovite mica as substrate surface. Parallel needles can only be obtained when the rod-like molecules align parallel or perpendicular to the mirror axis-direction of the mica substrate surface unit cell in combination with a rectangular surface unit cell of the molecules. In all other configurations an energetically equivalent adsorption site will lead to non-parallel molecular alignment and multiple needle directions. Consequently para-phenylenes in combination with muscovite mica are characterized as an outstanding material system fulfilling all conditions for optimized optical and morphological anisotropy. In further consequence it has to be stated that efficient color tuning of organic nano aggregates grown on muscovite mica seems strongly limited to a small group of molecules.
In order to overcome this natural limitation the organic–organic epitaxial growth of 6T on top of p-6P needle templates has been investigated. It is demonstrated by morphological, structural and optical investigations that organic heteroepitaxy can be successfully applied to achieve a higher molecular and morphological order. In particular it is demonstrated by XRD pole-figure measurements and polarization dependent optics that 6T molecules adopt the azimuthal order of the p-6P template. It is shown that the p-6P/6T material couple provides a sharp organic–organic interface and that 6T crystallization takes place dominantly on top of the p-6P template needles. Moreover, optical spectroscopy reveals two different 6T phases emitting in the green and orange-red spectral range. The intense green light emission originates from a thin 6T interface layer and the red emission can be attributed to 6T crystallites which have nucleated on top of the p-6P nano-fibers.
In summary all these observations underline that organic–organic heteroepitaxy seems to be the key to overcome the limitations of muscovite mica substrate surface geometry. It has been demonstrated that the spectral emission of organic nano-fibers can easily be tuned from the blue via the green to the red spectral range simply by choosing a second molecular species. Moreover, it can be expected that organic–organic heteroepitaxy can force highly molecular ordering in other material systems further enlarging the emission properties of organic nano-fibers being essential to become more flexible concerning the fabrication of optoelectronic devices based on organic nano-fibers.
References
Y. Shirota, H. Kageyama, Charge carrier transporting molecular materials and their applications in devices. Chem. Rev. 107, 953 (2007)
S.R. Forrest, The path to ubiquitous and low-cost organic electronic appliances on plastic. Nature 428, 911 (2004)
F. Balzer, H.-G. Rubahn, Growth control and optics of organic nanoaggregates. Adv. Funct. Mater. 15(1), 17 (2005)
F. Balzer, H.-G. Rubahn, Dipole-assisted self-assembly of light-emitting p-np needles on mica. Appl. Phys. Lett. 79, 3860–3862 (2001)
A. Andreev, G. Matt, C. Brabec, H. Sitter, D. Badt, N.S. Sariciftci, Highly anisotropically self-assembled structures of para-sexiphenyl grown by hot-wall epitaxy. Adv. Mater. 12, 629–633 (2000)
M. Schiek, F. Balzer, K. Al-Shamery, A. Lützen, H.-G. Rubahn, Light-emitting organic nanoaggregates from functionalized p-quaterphenylenes. Soft Matter 4, 277–285 (2008)
L. Kankate, F. Balzer, H. Niehus, H.-G. Rubahn, Organic nanofibers from thiophene oligomers. Thin Solid Films 518, 130–137 (2009)
F. Balzer, H.-G. Rubahn, Chain-length dependent para-phenylene film- and needle-growth on dielectrics. Surf. Sci. 548, 170–182 (2004)
H. Yanagi, T. Ohara, T. Morikawa, Self-waveguided gain-narrowing of blue light emission from epitaxially oriented p-sexiphenyl crystals. Adv. Mater. 13(19), 1452 (2001)
G. Koller, S. Berkebile, J.R. Krenn, G. Tzvetkov, G. Hlawacek, O. Lengyel, F.P. Netzer, C. Teichert, R. Resel, M.G. Ramsey, Oriented sexiphenyl single crystal nanoneedles on TiO2(110). Adv. Mater. 16, 2159 (2004)
R. Resel, Crystallographic studies on hexaphenyl thin films—a review. Thin Solid Films 433, 1–11 (2003)
R. Resel, T. Haber, O. Lengyel, H. Sitter, F. Balzer, H.-G. Rubahn, Origins for epitaxial order of sexiphenyl crystals on muscovite (001). Surf. Interface Anal. 41, 764–770 (2009)
M. Schiek, F. Balzer, K. Al-Shamery, A. Lützen, H.-G. Rubahn, Light-emitting organic nanoaggregates from functionalized p-quaterphenylenes. Soft Matter 4, 277–285 (2007)
M. Schiek, A. Lützen, K. Al-Shamery, F. Balzer, H.-G. Rubahn, Nanofibers from methoxy functionalized para-phenylene molecules. Surf. Sci. 600, 4030 (2006)
F. Quochi, Random lasers based on organic epitaxial nanofibers. J. Opt. 12(2), 024003 (2010)
F. Quochi, A. Andreev, F. Cordella, R. Orru, A. Mura, G. Bongiovanni, H. Hoppe, H. Sitter, N.S. Saricifci, Low-threshold blue lasing in epitaxially grown para-sexiphenyl nanofibers. J. Lumin. 112(1), 321 (2005)
F. Balzer, V. Bordo, A. Simonsen, H.-G. Rubahn, Optical waveguiding in individual nanometer-scale organic fibers. Phys. Rev. B 67, 115408 (2003)
P. Frank, G. Hlawacek, O. Lengyel, A. Satka, C. Teichert, R. Resel, A. Winkler, Influence of surface temperature and surface modifications on the initial layer growth of para-hexaphenyl on mica (001). Surf. Sci. 601, 2152 (2007)
C. Simbrunner, D. Nabok, G. Hernandez-Sosa, M. Oehzelt, T. Djuric, R. Resel, L. Romaner, P. Puschnig, C. Ambrosch-Draxl, I. Salzmann, G. Schwabegger, I. Watzinger, H. Sitter, Epitaxy of organic nano-fibers on sheet silicates—a growth model based on experiments and simulations. J. Am. Chem. Soc. 133, 3056–3062 (2011)
C. Ambrosch-Draxl, P. Puschnig, R. Resel, G. Leising, Electronic properties of ppp-oligomers investigated from first-principles. Synth. Met. 101, 673 (1999)
F. Balzer, M. Schiek, H.-G. Rubahn, K. Al-Shamery, A. Lützen, Surface bound organic nanowires. J. Vac. Sci. Technol. B 26(4), 1619–1623 (2008)
M. Schiek, F. Balzer, K. Al-Shamery, A. Lützen, H.-G. Rubahn, Nanoaggregates from thiophene/phenylene co-oligomers. J. Phys. Chem. 113, 9601–9608 (2009)
M. Schiek, A. Lützen, R. Koch, K. Al-Shamery, F. Balzer, R. Frese, H.-G. Rubahn, Nanofibers from functionalized para-phenylene molecules. Appl. Phys. Lett. 86, 153107 (2005)
M. Schiek, A. Lützen, K. Al-Shamery, F. Balzer, H.-G. Rubahn, Organic nanofibers from chloride functionalized p-quaterphenylenes. Cryst. Growth Des. 7, 229–233 (2007)
T. Mori, Molecular materials for organic field-effect transistors. J. Phys. Condens. Matter 20, 184010 (2008)
J. Ivanco, T. Haber, J.R. Krenn, F.P. Netzer, R. Resel, M.G. Ramsey, Sexithiophene films on ordered and disordered TiO2(110) surfaces: electronic, structural and morphological properties. Surf. Sci. 601, 178–187 (2007)
C. Simbrunner, G. Hernandez-Sosa, M. Oehzelt, T. Djuric, I. Salzmann, M. Brinkmann, G. Schwabegger, I. Watzinger, H. Sitter, R. Resel, Epitaxial growth of sexithiophene on mica surfaces. Phys. Rev. B 83, 115443 (2011)
H. Yanagi, T. Morikawa, S. Hotta, K. Yase, Epitaxial growth of thiophene/p-phenylene co-oligomers for highly polarized light-emitting crystals. Adv. Mater. 13(5), 313 (2001)
Y. Yoshida, N. Tanigaki, K. Yase, S. Hotta, Color-tunable highly polarized emission from uniaxially aligned thin films of thiophene/phenylene co-oligomers. Adv. Mater. 12(21), 1587 (2000)
M. Schiek, F. Balzer, K. Al-Shamery, J.R. Brewer, A. Lützen, H.-G. Rubahn, Organic molecular nanotechnology. Small 4, 176–181 (2008)
J. Brewer, M. Schiek, A. Ltzen, K. Al-Shamery, H.-G. Rubahn, Nanofiber frequency doublers. Nano Lett. 6(12), 2656–2659 (2006)
A. Meunier, Clays (Springer, Berlin, 2005)
Y. Kuwahara, Comparison of the surface structure of the tetrahedral sheets of muscovite and phlogopite by AFM. Phys. Chem. Miner. 28, 1–8 (2001)
F. Ostendorf, C. Schmitz, S. Hirth, A. Kühnle, J.J. Kolodziej, M. Reichling, Evidence for potassium carbonate crystallites on air-cleaved mica surfaces. Langmuir 25(18), 10764 (2009)
Y. Kuwahara, Muscovite surface structure imaged by fluid contact mode AFM. Phys. Chem. Miner. 26, 198 (1999)
L. Kankate, F. Balzer, H. Niehus, H.-G. Rubahn, From clusters to fibers: parameters for discontinuous para-hexaphenylene thin film growth. J. Chem. Phys. 128, 084709 (2008)
G. Hernandez-Sosa, C. Simbrunner, H. Sitter, Growth and optical properties of α-sexithiopene doped para-sexiphenyl nanofibers. Appl. Phys. Lett. 95, 013306 (2009)
F. Balzer, M. Schiek, A. Lützen, H.-G. Rubahn, Self-organized growth of organic thiophene-phenylene nanowires on silicate surfaces. Chem. Mater. 21, 4759–4767 (2009)
C. Xia, X. Fan, J. Locklin, R.C. Advincula, A. Gies, W. Nonidez, Characterization, supermolecular assembly and nanostructures of thiophene dendrimers. J. Am. Chem. Soc. 126(28), 8735 (2004)
I. Díez-Peŕez, M. Luna, F. Teherán, D.F. Ogletree, F. Sanz, M. Salmeron, Interaction of water with self-assembled monolayers of alkylsilanes on mica. Langmuir 20, 1284 (2004)
N. Gunari, M. Schmidt, A. Janshoff, Persistence length of cylindrical brush molecules measured by atomic force microscopy. Macromolecules 39, 2219 (2006)
W.F. Bleam, Electrostatic potential at the basal (001) surface of talc and pyrophyllite as related to tetrahedral sheet distortions. Clays Clay Miner. 38(5), 522 (1990)
H. Wondratschek, U. Müller, International Tables for Crystallography (Springer, Berlin, 2009)
E.J. Palin, M.T. Dove, S.A.T. Redfern, A. Bosenick, C.I. Sainz-Diaz, M.C. Warren, Computational study of tetrahedral Al–Si ordering in muscovite. Phys. Chem. Miner. 28, 534 (2001)
C.P. Herrero, J. Sanz, J.M. Serratosa, Si, Al distribution in micas: analysis by high-resolution 29Si NMR spectroscopy. J. Phys. C 18, 13 (1985)
M.C. Warren, M.T. Dove, E.R. Myers, A. Bosenick, E.J. Palin, C.I. Sainz-Diaz, B.S. Guiton, S.A.T. Redfern, Monte Carlo methods for the study of cation ordering in minerals. Mineral. Mag. 65(2), 221 (2001)
A.R. González-Elipe, J.P. Espinós, G. Munuera, J. Sanz, J.M. Serratosa, Bonding state characterization of constituent elements in phyllosilicate minerals by XPS and NMR. J. Phys. Chem. 92, 3471 (1988)
G.J. Redhammer, G. Roth, Single-crystal structure refinements and crystal chemistry of synthetic trioctahedral micas KM3(Al,Si)4O10(OH)2, where M=Ni, Mg, Co, Fe, Al. Am. Mineral. 87, 1464 (2002)
J.W. Gruner, The crystal structures of talc and pyrophyllite. Z. Kristallogr. 55, 412 (1934)
S.M. Richardson, J.W. Richardson, Crystal structure of a pink muscovite from Archer’s post, Kenya. Am. Mineral. 67, 69 (1982)
B. Perdikatsis, H. Burzlaff, Strukturverfeinerung am talk. Z. Kristallogr. 156, 177 (1981)
J.H. Lee, S. Guggenheim, Single crystal X-ray refinement of pyrophyllite-1tc. Am. Mineral. 66, 350 (1981)
K. Müller, C.C. Chang, Low energy electron diffraction observations of electric dipoles on mica surfaces. Surf. Sci. 8, 455 (1968)
K. Müller, C.C. Chang, Electric dipoles on clean mica surfaces. Surf. Sci. 14, 39 (1969)
F. Ostendorf, C. Schmitz, S. Hirth, A. Kühnle, J.J. Kolodziej, M. Reichling, How flat is an air-cleaved mica surface. Nanotechnology 19, 305705 (2008)
H. Poppa, A.G. Elliot, The surface composition of mica substrates. Surf. Sci. 24, 149 (1971)
M.G. Dowsett, R.M. King, E.H.C. Parker, Evaluation of impurity and contamination levels on mica surfaces using ssims. J. Vac. Sci. Technol. 14(2), 711 (1977)
G. Kranzelbinder, F. Meghdadi, S. Tasch, G. Leising, L. Fasoli, M. Sampietro, Transient electroluminescence in para-hexaphenyl based multilayer device. Synth. Met. 102, 1073 (1999)
S. Tasch, C. Brandstätter, F. Meghdadi, G. Leising, G. Froyer, L. Athouel, Red-green-blue light emission from a thin film electroluminescence device based on parahexaphenyl. Adv. Mater. 9(7), 33 (1997)
G. Horowitz, F. Garnier, A. Yassar, R. Hajlaoui, F. Kouki, Fet with a sexithiophene single crystal. Adv. Mater. 8(1), 52 (1996)
K.N. Baker, A.V. Fratini, T. Resch, H.C. Knachel, W.W. Adams, E.P. Socci, B.L. Farmer Polymer 34, 1571 (1993)
G. Horowitz, F. Kouki, A.E. Kassmi, P. Valat, V. Wintgens, F. Garnier, Structure-dependent fluorescence in sexithiophene single crystals. Adv. Mater. 11, 234 (1999)
M.A. Loi, E.D. Como, F. Dinelli, M. Murgia, R. Zamboni, F. Biscarini, M. Muccini, Supramolecular organization in ultra-thin films of α-sexithiophene on silicon dioxide. Nat. Mater. 4, 81 (2005)
L. Antolini, G. Horowitz, F. Kouki, F. Garnier, Polymorphism in oligothiophenes with an even number of thiophene subunits. Adv. Mater. 10, 382 (1998)
G. Horowitz, B. Bachet, A. Yassar, P. Lang, F. Demanze, J.-L. Fave, F. Garnier, Growth and characterization of sexithiophene single crystals. Chem. Mater. 7, 1337–1341 (1995)
T. Siegrist, R.M. Fleming, R.C. Haddon, R.A. Laudise, A.J. Lovinger, H.E. Katz, P. Bridenbaugh, D.D. Davis, The crystal structure of the high-temperature polymorph of a-hexathienyl (a-6t/ht). J. Mater. Res. 10, 2170–2173 (1995)
R.G.D. Valle, E. Venuti, A. Brillante, A. Girlando, Do computed crystal structures of nonpolar molecules depend on the electrostatic interactions? The case of tetracene. J. Phys. Chem. A 112, 1085–1089 (2008)
T. Haber, S. Muellegger, A. Winkler, R. Resel, Temperature-induced epitaxial growth modes of para-sexiphenyl on Au(111). Phys. Rev. B 74, 045419 (2006)
C. Simbrunner, F. Quochi, G. Hernandez-Sosa, M. Oehzelt, R. Resel, G. Hesser, M. Arndt, M. Saba, A. Mura, G. Bongiovanni, H. Sitter, Organic–organic heteroepitaxy of red-, green-, and blue-emitting nanofibers. ACS Nano 4, 6244 (2010)
S.C.B. Mannsfeld, K. Leo, T. Fritz, Phys. Rev. Lett. 94, 561041 (2005)
S. Timpanaro, A. Sassella, A. Borghesi, W. Porzio, P. Fontaine, M. Goldmann, Adv. Mater. 13, 127 (2001)
M. Oehzelt, G. Koller, J. Ivanco, S. Berkebile, T. Haber, R. Resel, F.P. Netzer, M.G. Ramsey, Organic heteroepitaxy: p-sexiphenyl on uniaxially oriented α-sexithiophene. Adv. Mater. 18, 2466 (2006)
G. Koller, S. Berkebile, J.R. Krenn, F.P. Netzer, M. Oehzelt, T. Haber, R. Resel, M.G. Ramsey, Heteroepitaxy of organic–organic nanostructures. Nano Lett. 6(6), 1207 (2006)
R.S. Becker, J.S. de Melo, A.L. Macuanita, F. Elisei, Comprehensive evaluation of the absorption, photophysical, energy transfer, structural, and theoretical properties of α-oligothiophenes with one to seven rings. J. Phys. Chem. 100, 18683 (1996)
E.D. Como, M.A. Loi, M. Murgia, R. Zamboni, M. Muccini, J-aggregation in α-sexithiophene submonolayer films on silicon dioxide. J. Am. Chem. Soc. 128, 4277 (2006)
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2013 Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
Simbrunner, C. et al. (2013). Organic–Organic Heteroepitaxy—The Method of Choice to Tune Optical Emission of Organic Nano-fibers?. In: Sitter, H., Draxl, C., Ramsey, M. (eds) Small Organic Molecules on Surfaces. Springer Series in Materials Science, vol 173. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-642-33848-9_3
Download citation
DOI: https://doi.org/10.1007/978-3-642-33848-9_3
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-642-33847-2
Online ISBN: 978-3-642-33848-9
eBook Packages: Physics and AstronomyPhysics and Astronomy (R0)