Abstract
The γ-hexachlorocyclohexane (γ-HCH, lindane) is an organochlorine pesticide used in agriculture and medicine to world level. It has a big tendency to bioaccumulation into the environment so is listed as a priority pollutant by the US EPA. Hence the development of new technologies to remediate these sites using microorganisms is every time more necessary. The actinomycetes are Gram-positive bacteria with great potential to bioremediate xenobiotics. One strain, Streptomyces sp. M7, isolated from organochlorine pesticide contaminated sediment, was selected for its capacity to grow in presence of lindane as only carbon source. This microorganism was cultured in soil extract medium added of lindane 100 μg L−1, obtaining a maximal growth of 0.065 mg mL−1, similar to the control, with a highest lindane remotion of 70.4 % at 30°C and pH 7. When different initial pesticide concentrations (100, 150, 200, and 300 μg L−1) were added in soil medium, an increment of the microbial growth was detected in all the concentrations tested. Also a diminution of the residual lindane concentration was determined in the soil samples in relation to controls without bacteria (29.1, 78.0, 38.8, and 14.4 %, respectively). Besides, it was determined the optimum Streptomyces sp. M7 inoculum when lindane 100 μg kg−1soil was added to the soil sample. The optimum inoculum was 2 g kg−1 soil for obtaining the most efficiently bioremediation process: the lindane removal in these conditions was 67.8 % at 28 days of incubation. Later it was considered necessary to know the pesticide effects on maize plants seeded in lindane-contaminated soil previously inoculated with Streptomyces sp. M7. Lindane concentrations of 100, 200, and 400 mg kg−1 soil did not affect the germination and vigor index of maize plants seeded in contaminated soils without Streptomyces sp. M7. When this microorganism was inoculated at the same conditions, a better vigor index was observed and 68 % of lindane was removed. In this connection, Streptomyces sp. M7 was grown on culture medium in presence of root exudates of maize, spiked with 1.66 mg L−1 of lindane. The highest level of pesticide removal obtained on this condition suggests that root exudates enhanced removal of lindane by the bacterium. On the other hand, little information is available on the ability of biotransformation of organochlorine pesticides by actinomycete strains. It was demonstrated that Streptomyces sp. M7 possesses the LinA enzyme that catalyzes dehydrochlorination of lindane to 1,3,4,6-tetrachloro-1,4-cyclohexadiene (1,4-TCDN) via γ-pentachlorocyclohexene (γ-PCCH). These results confirm that actinomycete strains could be considered one of the most promising bacterial groups for lindane biodegradation in contaminated environment. Particularly, Streptomyces sp. M7 could be used for this purpose.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Introduction
Organochlorine pesticides have been used extensively all over the world for public health and agricultural purposes. Currently, their use is being phased out because of their toxicity, environmental persistence, and accumulation in the food chain. Hexachlorocyclohexane (HCH) is one of the most extensively used organochlorine pesticides for both agriculture and medical purposes. Though the use of technical mixture containing eight stereoisomers was banned in several advanced countries in the 1970s, many developing countries continue to use lindane (γ-HCH) for economic reasons. Thus, new sites are continuously being contaminated by γ-HCH and its stereoisomers (Blais et al. 1998; Kidd et al. 2008; Fuentes et al. 2010). Although only lindane is insecticidal, HCH as a group is toxic and considered as potential carcinogens (Walker et al. 1999).
For the supply of γ isomer, the other stereoisomers are separated from γ-HCH and dumped as waste at different spots on the production sites causing serious soil pollution (Li 1999). HCH continues to pose a serious toxicological problem at industrial sites where post-production of lindane along with unsound disposal practices has led to serious contamination, and HCH contamination continues to be a global issue (Kidd et al. 2008). These compounds have moderate volatility and can be transported by air to remote locations (Galiulin et al. 2002). Therefore, a possible pathway for bioremediation of contaminated soils is the use of indigenous microorganisms. It is known that the microbial degradation of chlorinated pesticides such as HCH is usually carried out by using either pure or mixed culture systems. There have been some reports regarding aerobic degradation of γ-HCH by Gram-negative bacteria like Sphingomonas (Singh et al. 2000; Kidd et al. 2008) and by the white-rot fungi Trametes hirsuta, Phanerochaete chrysosporium, Cyathus bulleri, and Phanerochaete sordida (Mougin et al. 1999; Singh and Kuhad 2000). However, little information is available on the ability of organochlorine pesticide biotransformation by Gram-positive microorganisms and particularly by actinomycete species, the main group of bacteria present in soils and sediments (De Schrijver and De Mot 1999). These Gram-positive microorganisms have a great potential for biodegradation of organic and inorganic toxic compounds and also could remove different organochlorine pesticides when other carbon sources are present in the medium as energy source (Ravel et al. 1998; Amoroso et al. 1998; Benimeli et al. 2003). Therefore, the ability of actinomycetes to transform organochlorine pesticides has not been widely investigated, despite studies demonstrating that actinomycetes, specifically of the genus Streptomyces, have been able to oxidize, partially dechlorinate, and dealkylate aldrin, DDT, and herbicides like metolachlor or atrazine (Ferguson and Korte 1977; Radosevich et al. 1995). In addition to their potential metabolic diversity, strains of Streptomyces may be well suited for soil inoculation as a consequence of their mycelial growth habit, relatively rapid rates of growth, colonization of semi-selective substrates, and their ability to be genetically manipulated (Shelton et al. 1996). One additional advantage is that the vegetative hyphal mass of these microorganisms can differentiate into spores that assist in spread and persistence.
Recent studies demonstrate significantly enhanced dissipation and/or mineralization of persistent organic pollutants (such as organochlorine pesticides; Kidd et al. 2008) at the root–soil interface or rhizosphere (rhizodegradation) (Kuiper et al. 2004; Chaudhry et al. 2005; Krutz et al. 2005). This rhizosphere effect is generally attributed to an increase in microbial density, diversity and/or metabolic activity due to the release of plant root exudates, mucigel, and root lysates (Curl and Truelove 1986). A summary of potential root zone carbon sources is given in Table 10.1. Rhizodeposits not only provide a nutrient-rich habitat for microorganisms but can potentially enhance biodegradation in different ways: they may facilitate the co-metabolic transformation of pollutants with similar structures, induce genes encoding enzymes involved in the degradation process, increase contaminant bioavailability, and/or selectively increase the number and activity of pollutant degraders in the rhizosphere (Schnoor et al. 1995; Burken and Schnoor 1998; Miya and Firestone 2001; Shaw and Burns 2003).
The objective of this chapter is to study the bioremediation capacity of indigenous actinomycete strain and the effect of root exudates of Zea mays on this process.
2 Lindane Removal by Streptomyces sp. M7 in a Soil Extract Medium
Benimeli et al. (2007a) studied the growth of Streptomyces sp. M7 in a soil extract medium (SE) with and without lindane addition. Carbon and nitrogen composition of SE were 0.5 and 0.01 g L−1, respectively. Nevertheless and despite the poor organic matter in SE, Streptomyces sp. M7 was able to grow in this medium for limited time.
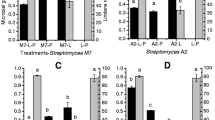
When the effect of the temperature (25, 30, and 35 °C) on the growth of Streptomyces sp. M7 in SE was analyzed, it was observed that 25 °C was the optimal temperature of microbial growth. When Streptomyces sp. M7 was cultured in SE supplemented with lindane 100 μg L−1, at different incubation temperatures (Fig. 10.1), a maximum growth of 0.11 mg mL−1 was observed at 25 °C. Significant differences in the biomass were not observed at 30 and 35 °C. These results would indicate that the optimal temperature for the growth of Streptomyces sp. M7 in SE, in presence as well as in absence of the pesticide, is 25 °C.
It is important to observe that the presence of lindane in culture medium did not inhibit the growth of Streptomyces sp. M7, since significant differences in bacterial growth in SE with and without the pesticide were not observed (p > 0.05). Similar results were obtained previously (Benimeli et al. 2007b), when Streptomyces sp. M7 was cultured in minimal medium supplemented with lindane 100 μg L−1, suggesting that the pesticide could not be toxic for this microorganism and that would not either be accumulated toxic intermediary metabolites that had an inhibiting effect on the growth.
Streptomyces sp. M7 was able to grow in SE over a relatively wide range of initial pH. No significant differences were observed when the microorganism was cultured at initial pH of 5, 7, or 9 (p > 0.05). When Streptomyces sp. M7 was cultured in SE added with lindane 100 μg L−1, at different initial pH, a maximum growth of 0.06 mg mL−1 was observed at pH 7; nevertheless, the microorganism was not able to grow at pH 5 and 9 (Fig. 10.2). The obtained results would indicate that the optimal initial pH for the growth of Streptomyces sp. M7 in SE with lindane is 7.
Figure 10.3 shows the impact of the incubation temperature on the lindane removal by Streptomyces sp. M7. The maximum lindane removal was 70.4 % when the microorganism was incubated in SE at 30 °C. Although the optimum temperature for Streptomyces sp. M7 growth, with and without lindane, was 25 °C, the optimal temperature for the pesticide removal was 30 °C.
Bachmann et al. (1998) reported that temperature of 30 °C was most favorable for the biodegradation of lindane in soil slurry by the mixed native microbial population of the soil. Arisoy and Kolankaya (1997) observed that the suitable incubation temperature for maximum growth and degradation activity of lindane by the fungus Pleurotus sajor-caju was 30 °C. Manonmani et al. (2000) also observed the degradation of the γ-HCH isomer by a microbial consortium under a wide range of temperatures (4–40 °C) in a liquid culture medium, and 30 °C was the optimum for γ-HCH degradation. Siddique et al. (2002) obtained similar results studying the effect of incubation temperature in the biodegradation of lindane by Pandoraea sp.; an incubation temperature of 30 °C was optimum for degradation of γ-HCH (57.7 %) in liquid culture and soil slurry (51.9 %).
The effect on lindane removal by Streptomyces sp. M7 in SE at initial pH of 5, 7, and 9 is presented in Fig. 10.4. Removal of pesticide (47.2 and 38.0 %) was observed at initial pH of 5 and 9, respectively, at 28 days of incubation. The highest removal ability of Streptomyces sp. M7 (70.4 %) was noted at an initial pH = 7 at 28 days of incubation. The fate of organic pollutants in the environment is influenced by environmental factors, such as pH and temperature, affecting the activity of microorganisms. Streptomyces sp. M7 was able to remove lindane over a wide range of pH in SE.
Arisoy and Kolankaya (1997) reported that medium pH = 5 was the optimum for both growth and degradation activity of lindane by the fungus Pleurotus sajor-caju. Manonmani et al. (2000) examined the influence of pH on the degradation of the γ-HCH isomer in a basal mineral medium by an acclimated consortium of microorganisms. They found that a pH range of 6–8 was most favorable for growth and degradation of the pesticide. Siddique et al. (2002) reported that Pandoraea species showed the highest degradation of α- and γ-HCH at an initial pH of 8 in broth culture.
3 Lindane Removal by Streptomyces sp. M7 in Sterile Soil Samples
Benimeli et al. (2008) studied the growth of Streptomyces sp. M7 in sterile soil samples by adding different lindane concentrations in 4 weeks. Simultaneously, lindane removal by Streptomyces sp. M7 was determined by gas chromatography; similar experiments were carried out without lindane as controls. As shown in Fig. 10.5, the cell concentration increased during incubation and significant differences in the growth were not observed at different lindane concentrations added, as in the control without lindane. These results would indicate that the growth of Streptomyces sp. M7 in soil was not affected by the lindane concentrations assayed, suggesting that the microorganism could tolerate or may degrade the pesticide by producing the necessary dehalogenase enzymes as was demonstrated by Nagata et al. (1999) for Sphingomonas paucimobilis UT26.
Influence of different initial concentrations of lindane on pesticide removal was evaluated by determining residual lindane in the soil samples. At initial lindane concentrations of 100, 150, 200, and 300 μg kg−1, removal of pesticide after 4-week incubation was 29.1, 78.0, 38.8, and 14.4 %, respectively. Lindane concentrations in uninoculated control soils were unchanged after 4 weeks of incubation. This inhibition in the removal ability of Streptomyces sp. M7 could be due to the toxicity of metabolites, which might have former but not detected in this study by the methods employed. For demonstrating that, it could be necessary to measure the intermediary metabolites of lindane degradation as was determined by Nagata et al. (1999) for Sphingomonas paucimobilis UT26.
Awasthi et al. (2000) found that at low initial concentrations of endosulfan in soil (50 and 100 μg g−1 soil), the degradation was very rapid in inoculated soils; at higher concentrations, the degradation rates were slower, leading to a total inhibition of the degradation activity at the initial concentration of 10 mg g−1. Similar findings were reported by Okeke et al. (2002), who studied the ability of a Pandoraea sp. strain to remove lindane in liquid and soil slurry cultures. Their results indicated that the rates and extent of lindane removal increased with increasing concentrations up to 150 mg L−1 but declined at 200 mg L−1, after 4–6 weeks of incubation. However, in a similar study, Liu et al. (1991) found opposite results when they inoculated a Streptomyces sp. strain in sterile soil samples spiked with metolachlor and observed that the amount of residual metolachlor was higher at lower herbicide concentrations, after 1-week incubation.
This result indicates that Streptomyces sp. M7 could remove lindane from soil but at limited pesticide concentration and the viable bacterial count of the soil culture indicated growth and survival of Streptomyces sp. M7. This is not surprising because the soil contains available organic nutrients that the bacteria may prefer for growing. Then, lindane could be used as a secondary substrate source as it was demonstrated previously by Benimeli et al. (2007b) in culture medium where glucose at limited concentration was added.
To arrive at the optimal number of bacterial cells for effective removal of lindane, the influence of different inoculum sizes (0.5–4.0 g kg−1 ww soil) was studied in sterile soil samples with lindane 100 μg kg−1 ww soil (Fig. 10.6). 28 days after inoculation with different Streptomyces sp. M7 concentrations into lindane-amended autoclaved soil, the cell population increased rapidly in 2 weeks and was followed by a stationary phase from 2 to 4 weeks. This growth profile was observed for all assayed bacterial concentrations and was similar in contaminated as well as non-contaminated soil samples. These results reinforce the hypothesis according to which lindane concentrations present in soil were not toxic for Streptomyces sp. M7. The inoculum 2 g cells kg−1 soil (ww) significantly led the maximum bacterial count (9.0 × 1012 CFU kg−1 ww of soil).
A substantial decline of the residual lindane at different inoculum concentrations was observed within 0–2 weeks of incubation, whereas the compound did not disappear from the uninoculated sterile control. However, the percentage of lindane removal was not entirely proportional to the amount of Streptomyces sp. M7 initially added to the soils, indicating that the two parameters are not in direct proportion. Maximal pesticide depletion (56.0 %) was observed at 2 g cells kg−1 soil (ww) and thereafter decreased at 4 g cells kg−1 soil (Table 10.2).
In a similar work, Liu et al. (1991) found that approximately 80 % of the pesticide metolachlor was transformed in a sterile soil inoculated with a Streptomyces sp. strain after 1 week of incubation; however, the rate of metolachlor transformation was not proportional to the inoculum size. Kumar-Ajit et al. (1998) studied the inoculation of 3-chloro and 4-chlorobenzoate-treated sterile soil with a chlorobenzoate-degrading Pseudomonas aeruginosa 3mT; they probed three inoculum sizes (1, 2, and 4 mg cells kg−1 soil), and these were effective in degrading the chemical in the soil, but the degradation was faster with a larger inoculum. On the other hand, Johri et al. (2000) found that an increase in the inoculum size of Sphingomonas paucimobilis resulted in an increase in the degradation rate of the HCH isomers in the culture medium, indicating that the two parameters are in direct proportion.
4 Bioremediation of Lindane-Contaminated Soil Samples by Streptomyces sp. M7 and Effect of Pesticide on Maize Plants
Nonsterile soil spiked with lindane 100 μg kg−1 was inoculated with 2 g kg−1 Streptomyces sp. M7, and after 14 days incubation, Zea mays seeds were grown in this soil. Concentrations of residual lindane in soil were monitored periodically to assess the effectiveness of the inoculated strain in the bioremediation of the soil.
A strong decrease in the residual concentration of added lindane was observed in Streptomyces sp. M7-inoculated soil. After 14 days of incubation, 68 % of lindane was removed (Fig. 10.7). There were no evident changes in the concentration of the pesticide in the uninoculated soil, indicating that the native microorganisms were not involved into the pesticide removal.
In soil bioremediated with Streptomyces sp. M7, normal germination (100 %) and an increased in the seedling vigor were observed, compared to the control maize seeds (Table 10.3). It is possible that the pesticide was removed by the inoculated microorganism before seed germination began and the involvement of indigenous microflora capable of metabolizing lindane was not observed.
In a similar study by Krueger et al. (1991), soybean and pea seedlings susceptible to the herbicide dicamba were protected from its deleterious effect by inoculating soils with dicamba-degrading microorganisms. Kumar-Ajit et al. (1998) demonstrated that chlorobenzoates adversely affect the seed germination and seedling vigor of tomato. However, the bioremediation of the soil with Pseudomonas aeruginosa 3mT protected the tomato seeds, resulting in the normal germination and seedling vigor. Bidlan et al. (2004) observed that the effect of tech-HCH on germinating radish and green gram seeds was nullified by treatment of contaminated soil with a HCH-degrading microbial consortium.
Although no maize plant growth promotion was observed when Streptomyces sp. M7 was inoculated into the contaminated lindane soil samples, the fact that this pesticide can be removed and therefore its uptake by the plant into biomass could be lowered, avoiding the contamination through the food chain. The low water solubility and high hydrophobicity of lindane make their uptake and translocation within the plant unlikely (Kidd et al. 2008).
5 Lindane Removal by Streptomyces sp. M7 Cultured on Medium Amended with Root Exudates of Maize
Root exudates are products released in the root zone of plants with significant levels of carbon (and possibly nitrogen). The exudates are likely to favor fast-growing microbes, provided they have the corresponding metabolic abilities. In this context, Alvarez et al. (2010; Alvarez et al. 2012) studied the growth of Streptomyces sp. M7 in minimal medium supplemented with root exudates of maize and/or glucose (0.8 g L−1) and spiked with lindane (1.66 mg L−1). Cultures were incubated at 30 °C at 150 rpm for 7 days. Similar experiments were carried out without lindane as controls. Root exudates were collected from solution cultures of maize according to Luo et al. (2006). Exudates were lyophilised and stored at 4 °C until sample processing.
Regarding composition, carbohydrate and organic carbon of root exudates were 41.6 mg g−1 and 40 mg g−1, respectively. The highest level of pesticide removal was obtained when Streptomyces sp. M7 was cultured in presence of root exudates (Table 10.4). In contrast, although biomass production was highest in the glucose treatment, the percentage of lindane removal under this condition was lower.
An increase in degradation of a wide range of organic pollutants was observed as result of modified microbial activity in the rhizosphere of several plants (Pandya et al. 1999; White 2001; Chekol et al. 2002; Corgié et al. 2003; Muratova et al. 2003; Shaw and Burns 2005; Phillips et al. 2005). Enhanced transformation of the explosive trinitrotoluene (TNT) by the forage grasses Phalaris arundinacea and Panicum virgatum was shown by Chekol et al. (2002). Concentrations of the organochlorine p,p′ DDE (2,2-bis (p-chlorophenyl) 1,1-dichloroethylene), a metabolite of DDT, were significantly reduced in the rhizosphere of field-grown zucchini, pumpkin, and spinach compared to bulk soil (White 2001). In contrast, phenanthrene-degrading activity of Pseudomonas putida ATCC 17484 was repressed after incubation with plant root extracts (Rentz et al. 2004, 2005). However, these authors suggested that the enhanced microbial growth on rhizodeposits is likely to compensate for this partial repression since a larger microbial population leads to a faster degradation rate.
Little is known about the mechanisms involved in interaction of root exudates with bacteria. In this respect, Chen and Aitken (1999) and Hegde and Fletcher (1996) found that terpenes (such as cymene, α-pyrene, and α-terpinene) and phenolics (such as salicylate) released by roots of certain plants induced biphenyl dioxygenase in polychlorinated biphenyls-degrading bacteria. It could be possible that root exudates of maize enhanced removal of lindane by Streptomyces sp. M7 by means of a similar mechanism.
6 Lindane and Metabolite Determination in Cell-Free Extract by GC-Mass Spectrometry Analysis
The first signs of the aerobic lindane degradation were determined by Nagata et al. (1999). It was demonstrated that Sphingobium japonicum UT26 possesses a dechlorinase enzyme, LinA (γ-hexachlorocyclohexane dehydrochlorinase, EC 4.5.1), encoded by the linA gene that catalyzes two dehydrochlorination steps: γ-HCH to 1,3,4,6-tetrachloro-1,4-cyclohexadiene (1,4-TCDN) via γ-pentachlorocyclohexene (γ-PCCH). In addition to γ-HCH and γ-PCCH, α- and δ-isomers of HCH were also dehydrochlorinated by LinA, whereas β-HCH was not (Nagata et al. 1993). Furthermore, it was experimentally confirmed that dehydrochlorination of γ-HCH proceeds by a 1,2-ante dehydrochlorination reaction (Nagata et al. 2007).
Regarding the environmental problems caused by lindane and the current lack of information about the presence of dechlorinase activity in Streptomyces, the aim of this point was to demonstrate, for the first time, a specific dechlorinase activity in Streptomyces using lindane as substrate. In order to determine lindane and metabolites in cell-free extract of Streptomyces sp. M7, the strain was grown in flasks with 250 mL of MM containing γ-HCH 100 μg mL−1 and incubated at 30 °C at 100 rpm for 48 and 96 h. At the beginning of the experiment, the inoculum was 150 μl of concentrated spore suspension (109 CFU mL−1). Supernatants were extracted by solid phase extraction (SPE) using C18 columns, evaporated to dryness under reduced pressure, and the residue was resuspended in hexane. Routine quantitative determinations of lindane (γ-HCH) γ-pentachlorocyclohexene (γ-PCCH) and 1,3,4,6-tetrachloro-1,4-cyclohexadiene (1,4-TCDN) were carried out with gas chromatograph-micro-electron capture detection (GC-μECD) (Cuozzo et al. 2009).
The gas chromatography results of the cell-free extracts obtained at 48 and 96 h of growth of Streptomyces sp. M7 revealed the appearance of γ-PCCH (Rt 6.26 min) and 1,4-TCDN,(Rt 5.29 min), the first and second product of the lindane catabolism by the specific dechlorinase in the catabolic way proposed by Nagata et al. (2007). The relative abundance of γ-PCCH and the 1,4-TCDN increased one and half times, at 96 h compared to 48 h of growth (Table 10.5).
However, these results indirectly demonstrated the presence of one specific enzyme in the lindane degradation way from Streptomyces sp. M7. It is the first report on dehalogenase activity in actinomycetes with lindane as specific substrate. This has only been reported in Sphingomonas (Nagata et al. 2007; Normand et al. 2007) detected a putative 2,5-dichloro-2,5cyclohexadiene-1,4-diol dehydrogenase (2,5-DDOL dehydrogenase) in Frankia. Genetic studies of this strain are necessary for a proper understanding of the principle of its ability to degrade different chlorinated hydrocarbon compounds.
7 Conclusions and Perspectives
The review clearly suggests that Streptomyces sp. M7 has the capacity to grow in a soil extract broth, a nutritionally poor medium, in the presence of lindane, and to remove the pesticide. Since streptomycetes are metabolically diverse and relatively resistant to adverse conditions that may occur in the soil environment, Streptomyces sp. M7 could be considered as attractive targets for lindane degradation in situ. In addition, the development of bioremediation processes using indigenous microorganisms is advantageous, as strains isolated are already adapted to the substrate, and to local soil and climatic conditions, and regulatory and legislation issues are simpler, compared to the introduction and release of exogenous microorganisms and genetically modified organisms into the environment. On the other hand, Streptomyces sp. M7 bioremediation activity is not inhibited by the natural soil microbial flora. Moreover, Streptomyces sp. M7 growth was not inhibited by 300 μg kg−1 of lindane.
Regarding the use of plants (or their products) for the treatment of contaminated sites, preliminary results suggest that Streptomyces sp. M7 was stimulated by the root exudates of maize amended to the culture. In this context, phytostimulation may be a promising strategy for the remediation of HCH-contaminated soils. It is important to continue these studies not only at the laboratory but also in field trails in order to evaluate the potential of Streptomyces sp. M7 in the bioremediation of natural habitats. Further studies evaluating the soil–plant–microbe system and its influence on HCH biodegradation are necessary so as to better explore and exploit an undoubtedly huge potential.
References
Alvarez A, Benimeli CS, Sesto Cabral ME, Amoroso MJ (2010) Efecto de los exudados radiculares de plantas de maíz en la remoción de lindano por la cepa de Actinomycetes nativa Streptomyces sp. M7. In: Revista Argentina de Microbiología 2010: abstracts of the annual meeting of the Argentinean Society of Microbiology. Argentinean Society of Microbiology, Bs As, p 24
Alvarez A, Yañez LM, Benimeli CS, Amoroso MJ (2012). Maize plants (Zea mays) root exudates enhance lindane removal by native Streptomyces strains. Int Biodeterior Biodegrad 66:14–18.
Amoroso MJ, Castro G, Carlino F, Romero NC, Hill R, Oliver G (1998) Screening of actinomycetes isolated from Salí river tolerant to heavy metal. J Gen Appl Microbiol 44:29–32
Arisoy M, Kolankaya N (1997) Biodegradation of lindane by Pleurotus sajor-caju and toxic effects of lindane and its metabolites on mice. Environ Contam Toxicol 59:352–359
Awasthi N, Ahuja R, Kumar A (2000) Factors influencing the degradation of soil applied endosulfan isomers. Soil Biol Biochem 32:1697–1705
Bachmann A, Wijnen P, DeBruin W, Huntjens JL, Roelofsen W, Zehnder AJ (1998) Biodegradation of alpha- and beta-hexachlorocyclohexane in a soil slurry and under different redox conditions. Appl Environ Microbiol 54:143–149
Benimeli CS, Amoroso MJ, Chaile AP, Castro GR (2003) Isolation of four aquatic streptomycetes strains capable of growth on organochlorine pesticides. Bioresour Technol 89:133–138
Benimeli CS, Castro GR, Chaile AP, Amoroso MJ (2007a) Lindane uptake and degradation by aquatic Streptomyces sp. M7 Strain. Int Biodeter Biodegr 59:148–155
Benimeli CS, Gonzalez AJ, Chaile AP, Amoroso MJ (2007b) Temperature and pH effect on lindane removal by Streptomyces sp. M7 in soil extract. J Basic Microbiol 47:468–473
Benimeli CS, Fuentes MS, Abate CM, Amoroso MJ (2008) Bioremediation of lindane contaminated soil by Streptomyces sp M7 and its effects on Zea mays growth. Int Biodeter Biodegr 61:233–239
Bidlan R, Afsar M, Manonmani HK (2004) Bioremediation of HCH-contaminated soil: elimination of inhibitory effects of the insecticide on radish and green gram seed germination. Chemosphere 56:803–811
Blais JM, Schindler DW, Mair DC, Kimpe LE, Donald DB, Rosenberg B (1998) Acclimation of persistent organochlorine compounds in mountains of Western Canada. Nature 395:585–588
Burken JG, Schnoor JL (1998) Predictive relationships for uptake of organic contaminants by hybrid poplar trees. Environ Sci Technol 32:3379–3385
Chaudhry Q, Blom-Zandstra M, Gupta S, Joner EJ (2005) Utilising the synergy between plants and rhizosphere microorganisms to enhance breakdown of organic pollutants in the environment. Environ Sci Pollut Res 12:34–48
Chekol T, Vough LR, Chaney RL (2002) Plant-soil-contaminant specificity affects phytoremediation of organic contaminants. Int J Phytoremediation 4:17–26
Chen SH, Aitken MD (1999) Salicylate stimulates the degradation of high molecular weight polycyclic aromatic hydrocarbons by Pseudomonas saccharophila P15. Environ Sci Technol 33:435–439
Corgié SC, Joner EJ, Leyval C (2003) Rhizospheric degradation of phenanthrene is a function of proximity to roots. Plant Soil 257:143–150
Cuozzo SA, Rollán GC, Abate CM, Amoroso MJ (2009) Specific dechlorinase activity in lindane degradation by Streptomyces sp. M7. World J Microbiol Biotechnol 25:1539–1546
Curl EA, Truelove B (1986) The rhizosphere. Springer, Heidelberg
De Schrijver A, De Mot R (1999) Degradation of pesticides by actinomycetes. Crit Rev Microbiol 25:85–119
Ferguson JA, Korte F (1977) Epoxidation of aldrin to exo-dieldrin by soil bacteria. Appl Environ Microbiol 34:7–13
Fuentes MS, Benimeli CS, Cuozzo SA, Amoroso MJ (2010) Isolation of pesticide-degrading actinomycetes from a contaminated site: bacterial growth, removal and dechlorination of organochlorine pesticides. Int Biodeter Biodegr. doi:10.1016/j.biod.2010.05.001
Galiulin RV, Bashkin VN, Galiulina RA (2002) Behaviour of persistent organic pollutants in the air–plant–soil system. Water Air Soil Pollut 137:179–191
Hegde RS, Fletcher JS (1996) Influence of plant growth stage and season on the release of root phenolics by mulberry as related to development of phytoremediation technology. Chemosphere 32:2471–2479
Johri AK, Dua M, Saxena DM, Sethunathan N (2000) Enhanced degradation of hexachlorocyclohexane isomers by Sphingomonas paucimobilis. Curr Microbiol 41:309–311
Kidd PS, Prieto-Fernández A, Monterroso C, Acea MJ (2008) Rhizosphere microbial community and hexachlorocyclohexane degradative potential in contrasting plant species. Plant Soil 302:233–247
Krueger JP, Butz RG, Cork DJ (1991) Use of dicamba-degradating microorganisms to protect dicamba susceptible plant species. J Agric Food Chem 39:1000–1003
Krutz LJ, Beyrouty CA, Gentry TJ, Wolf DC, Reynolds CM (2005) Selective enrichment of a pyrene degrader population and enhanced pyrene degradation in Bermuda grass rhizosphere. Biol Fertil Soils 41:359–364
Kuiper I, Lagendijk EL, Bloemberg GV, Lugtenberg BJ (2004) Rhizoremediation: a beneficial plant-microbe interaction. Mol Plant Microbe Interact 17:6–15
Kumar-Ajit PV, Gangadhara KP, Manilal P, Kunhi AAM (1998) Soil inoculation with Pseudomonas aeruginosa 3mT eliminates the inhibitory effect of 3-chloro- and 4-chlorobenzoate on tomato seed germination. Soil Biol Biochem 30:1053–1059
Li YF (1999) Global technical hexachlorocyclohexane usage and its contamination consequences in the environment from: 1948–1997. Sci Total Environ 232:121–158
Liu SY, Freyer AJ, Bollag JM (1991) Microbial dechlorination of the herbicide metolachlor. J Agric Food Chem 39:631–636
Luo L, Zhang S, Shan X, Zhu Y (2006) Oxalate and root exudates enhance the desorption of p,p′-DDT from soils. Chemosphere 63:1273–1279
Manonmani HK, Chandrashekariah DH, Sreedhar Reddy N, Elecy CD, Kunhi AA (2000) Isolation and acclimation of a microbial consortium for improved aerobic degradation of α-hexachlorocyclohexane. J Agric Food Chem 48:4341–4351
Miya KR, Firestone MK (2001) Enhanced phenanthrene biodegradation in soil by slender oat root exudates and root debris. J Environ Qual 30:1911–1918
Mougin C, Pericaud C, Malosse C, Laugero C, Ashter M (1999) Biotransformation of the insecticide lindane by the white-rot basidiomycete Phanerochaete chrysosporium. Pept Sci 47:51–59
Muratova A, Thorsten H, Narula N, Wand H, Turkovskaya O, Kuschk P, Jahn R, Merbach W (2003) Rhizosphere microflora of plants used for the phytoremediation of bitumen-contaminated soil. Microbiol Res 158:151–161
Nagata Y, Nariya T, Ohtomo R, Fukuda M, Yano K, Takagi M (1993) Cloning and sequencing of a dehalogenase gene encoding an enzyme with hydrolase activity involved in the degradation of gamma-hexachlorocyclohexane (γ-HCH) in Pseudomonas paucimobilis. J Bacteriol 175:6403–6410
Nagata Y, Futamura A, Miyauchi K, Takagi M (1999) Two different types of dehalogenases, Lin A and Lin B, involved in γ-hexachlorocyclohexane degradation in Sphingomonas paucimobilis UT26 are localized in the periplasmic space without molecular processing. J Bacteriol 181:5409–5413
Nagata Y, Endo R, Ito M, Ohtsubo Y, Tsuda M (2007) Aerobic degradation of lindane (γ-hexachlorocyclohexane) in bacteria and its biochemical and molecular basis. Appl Microbiol Biotechnol 76:741–752
Normand P, Queiroux C, Tisa LS, Benson DR, Rouy Z, Cruvellier S, Médigue C (2007) Exploring the genomes of Frankia. Physiol Plant 130:331–343
Okeke BC, Siddique T, Arbestain MC, Frankenberger WT (2002) Biodegradation of γ-Hexachlorocyclohexane (lindane) and α-Hexachlorocyclohexane in water and a soil slurry by a Pandoraea species. J Agric Food Chem 50:2548–2555
Pandya S, Iyer P, Gaitonde V, Parekh T, Desai A (1999) Chemotaxis of Rhizobium sp. S2 towards Cajanus cajan root exudates and its major components. Curr Microbiol 38:205–209
Phillips TM, Seech AG, Lee H, Trevors JT (2005) Biodegradation of hexachlorocyclohexane (HCH) by microorganisms. Biodegradation 16:363–392
Radosevich M, Traina SJ, Hao YL, Tuovinen OH (1995) Degradation and mineralization of atrazine by a soil bacterial isolate. Appl Environ Microbiol 61:297–302
Ravel J, Amoroso MJ, Colwell RR, Hill RT (1998) Mercury-resistant actinomycetes from Chesapeake Bay. FEMS Microbiol Lett 162:177–184
Rentz JA, Alvarez PJ, Schnoor JL (2004) Repression of Pseudomonas putida phenanthrene-degrading activity by plant root extracts and exudates. Environ Microbiol 6:574–583
Rentz JA, Alvarez PJ, Schnoor JL (2005) Benzo[a]pyrene co-metabolism in the presence of plant root extracts and exudates: implications for phytoremediation. Environ Pollut 136:477–484
Schnoor JL, Licht LA, McCutcheon SA, Wolfe NL, Carreira LH (1995) Phytoremediation of organic and nutrient contaminants. Environ Sci Technol 29:318–323
Shaw LJ, Burns RG (2003) Biodegradation of organic pollutants in the rhizosphere. Adv Appl Microbiol 53:1–60
Shaw LJ, Burns RG (2005) Rhizodeposition and the enhanced mineralization of 2,4 dichlorophenoxyacetic acid in soil from the Trifolium pratense rhizosphere. Environ Microbiol 7:191–202
Shelton DR, Khader S, Karns JS, Pogell BM (1996) Metabolism of twelve herbicides by Streptomyces. Biodegradation 7:129–136
Siddique T, Okeke BC, Arshad M, Frankerberger WT Jr (2002) Temperature and pH effects on biodegradation of hexachlorocyclohexane isomers in water and soil slurry. J Agric Food Chem 50:5070–5076
Singh BK, Kuhad RC (2000) Degradation of the insecticide lindane (γ-HCH) by white-rot fungi Cyathus bulleri and Phanerochaete sordida. Pest Manag Sci 56:142–146
Singh BK, Kuhad RC, Singh A, Tripathi KK, Ghosh PK (2000) Microbial degradation of the pesticide lindane (gamma-hexachlorocyclohexane). Adv Appl Microbiol 47:269–298
Tang CS, Young C (1982) Collection and identification of allelopathic compounds from the undisturbed root system of Bigalta limpograss (Hemarthria altissima). Plant Physiol 69:155–160
Walker K, Vallero DA, Lewis RG (1999) Factors influencing the distribution of lindane and other hexachlorocyclohexanes in the environment. Environ Sci Technol 33:4373–4378
White JC (2001) Plant-facilitated mobilization and translocation of weathered 2,2-bis(p chlorophenyl)-1,1-dichloroethylene (p, p′-DDE) from an agricultural soil. Environ Toxicol Chem 20:2047–2052
Acknowledgments
This work was supported by Consejo de Investigaciones de la Universidad Nacional de Tucumán (CIUNT), Agencia Nacional de Promoción CientÚfica y Tecnológica (ANPyCT), Consejo Nacional de Investigaciones CientÚficas y Técnicas (CONICET) and Fundación Bunge y Born.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2013 Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
Alvarez, A., Fuentes, M.S., Benimeli, C.S., Cuozzo, S.A., Saez, J.M., Amoroso, M.J. (2013). Pesticides Removal Using Actinomycetes and Plants. In: Goltapeh, E., Danesh, Y., Varma, A. (eds) Fungi as Bioremediators. Soil Biology, vol 32. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-642-33811-3_10
Download citation
DOI: https://doi.org/10.1007/978-3-642-33811-3_10
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-642-33810-6
Online ISBN: 978-3-642-33811-3
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)