Abstract
The main emphasis of this study is to examine the effects of biodiesel thermo-physical properties on the fuel spray development using CFD modelling. A complete set of 12 thermo-physical properties is estimated for PME, SME and CME. The methods employed for this as reported here are generic as the methods are dependent on the chemical compositions and temperature. Sensitivity analysis is performed by integrating the estimated fuel properties into CFD modelling. Variations in spray development such as mass of fuel evaporated and liquid and vapour axial penetration length of biodiesel fuels are found to be different from fossil diesel due to the difference in thermo-physical properties. A total of five biodiesel properties are identified to have profound impacts on fuel spray development, which are liquid density, liquid viscosity, liquid surface tension, vapour pressure and vapour diffusivity. Nevertheless, only liquid surface tension and vapour pressure are the most sensitive fuel properties to the fuel spray development. The work has provided better representation of biodiesel properties, which improve the in-cylinder CFD simulation of reacting spray jet for the fuel.
F2012-B01-023
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
1 Introduction
Despite the widespread use of biodiesel in conventional diesel engine, there is a need for more comprehensive research work to conclusively determine the benefits and drawbacks of biodiesel [1, 2]. One such effort is the utilisation of computational fluid dynamics (CFD) techniques to better understand and improve biodiesel fuel spray, combustion and emission characteristics in compression ignition (CI) engines.
For accurate in-cylinder CFD simulations of biodiesel spray combustion process, the most important element is the spray and vapour structures of fuel as these structures dictate the fuel vaporisation rate and the subsequent ignition, combustion and pollutant formation processes. Since fuel spray and vapour structures are primarily governed by the fuel thermo-physical properties, it is imperative to understand the effects of these properties of different biodiesel fuels in comparisons to fossil diesel. Nevertheless, there are limited studies on developing and establishing the impacts of thermo-physical properties of biodiesel fuels [1–3]. Moreover, most of the fuel properties were developed based on the mixture compositions of soybean [1–3] or based on approximated single component molecule, for example methyl oleate (C19H36O2) that represents rapeseed methyl ester [4]. Thus, fuel spray modelling using the approximated generic biodiesel fuel properties inherently result in a certain level of inaccuracy in the predictions.
In line with the discussion above, the reported work here is based on palm methyl ester (PME), soybean methyl ester (SME) and coconut methyl ester (CME) to represent biodiesel fuels with low, moderate and high degree of unsaturation, respectively. The fuel properties of PME, SME and CME are first developed due to limited comprehensive validated thermo-physical properties of biodiesel fuels available. Then, a set of numerical experiments are performed to investigate the sensitivity of individual fuel properties under constant volume combustion. Fuel spray characteristics of biodiesel fuels and fossil diesel are the main interest of comparisons for the sensitivity analysis of fuel properties.
2 Development of Biodiesel Thermo-Physical Properties
There is a total of 12 pertinent biodiesel thermo-physical properties excluding critical properties. The evaluation of biodiesel properties is done using empirical methods available in the literature. The selection of appropriate methods of evaluation is assessed through the applicability of these methods over a wide range of temperature. In order to reduce the complexity in the evaluation of fuel properties, the chemical compositions of fatty acid methyl ester (FAME) components for each biodiesel in this study, PME, SME and CME are maintained to five distinct components as listed in Table 1. Fuel properties of fossil diesel, represented by tetradecane (C14H30) are used as the basis of comparisons for the estimated biodiesel properties.
In the estimation of biodiesel thermo-physical properties, the critical properties, critical pressure, critical volume and critical temperature of each FAME component are first evaluated using Lydersen’s method [5] as listed in Table 2. Then, Lee-Kesler mixing rule [5] is imposed according to respective biodiesel chemical compositions to obtain the critical properties of PME, SME and CME. These critical properties are vital as any biodiesel properties beyond these properties will cease to be valid. The estimated critical properties of FAME components in this study are validated against those in literature [3]. Approximately 20 % of error is obtained for critical pressure, whereas less than 0.6 % of error is found for the estimated critical temperature and critical volume. Besides that, Lydersen’s method [5] is proven accurate with reasonable error margin of only 10 % [6]. Therefore, the estimated critical properties for other biodiesel fuels are reasonably accurate as the critical properties are evaluated based on the respective chemical compositions of FAME components.
Figures 1a–l displays the estimated thermo-physical properties of PME, SME and CME, as well as the fossil diesel properties. The liquid densities of PME, SME and CME are predicted using Rackett equation modified by Spencer and Danner [5]. Less than 10 % of deviation is obtained when the estimated liquid densities of SME across the interested temperature range are compared to the estimated properties of SME by Yuan et al. [3]. Since the trends and range of estimated liquid densities of PME and CME are similar to SME, the estimated liquid densities of PME and CME are proven accurate. Based on Fig. 1a, the liquid densities of PME, SME and CME are 20 % higher than diesel at lower temperatures and linearly decrease with increasing temperatures. Thus, the vaporisation rates of biodiesel fuels are lower than fossil diesel. Liquid surface tensions of PME, SME and CME are predicted using equation proposed by Allen et al. [7]. Liquid surface tensions of PME, SME, CME and fossil diesel are presented in Fig. 1b. The validation of liquid surface tensions is done by comparing the estimated liquid surface tension of SME against predicted value by Allen et al. [7] at 40 °C. The estimated surface tension value at 40 °C for SME in this study is 30.2 mN/m, is approximately 7 % higher than measured value of 28.2 mN/m by Allen et al. [7]. Comparatively, the liquid surface tensions of the biodiesel fuels are 14 % higher than diesel. Thus, the fuel spray break-up and vaporisation rates of biodiesel fuels are expected to be lower than fossil diesel.
Thermo-physical and transport properties of PME, SME and CME as compared to C14H30 for a liquid density, b liquid surface tension, c liquid viscosity, d liquid heat capacity, e liquid thermal conductivity, f vapour pressure, g latent heat of vaporisation, h vapour viscosity, i vapour thermal conductivity, j vapour diffusivity, k vapour heat capacity, and l second virial coefficient
Low temperature liquid viscosities of FAME components of PME, SME and CME are computed using Orrick and Erbar method [5] up to reduced temperature (ratio of temperature to critical temperature) of 0.7. Grunberg and Nissan method [5], a mixing rule specifically for liquid viscosity is then used to compute the liquid viscosities of PME, SME and CME. For liquid viscosity at reduced temperature higher than 0.7, Letsou and Stiel method [5] is utilised. The estimated values of SME seen in Fig. 1c are validated against those measured by Tat and van Gerpen [5] with the largest relative error of 18 % at 0 °C and the least error of 7 % at 100 °C as seen in Table 3. The error is observed to be reduced with increasing temperatures. Therefore, the estimated liquid viscosity values for SME seen in Fig. 1c are relatively accurate as do the estimated liquid viscosity values of PME and CME as the correlations used are dependent on temperature and chemical compositions. It is expected that the break-up processes of fuel droplets will be affected by liquid viscosities since the estimated liquid viscosities of biodiesel fuels are higher than fossil diesel especially at lower temperatures.
The liquid heat capacities of PME, SME and CME are estimated using correlation by van Bommel et al. [8] and simple mixing rules [5]. The trends of estimated liquid heat capacities of PME, SME and CME are compared to fossil diesel. Here, similar trend is found where the estimated liquid heat capacities of biodiesel fuels displayed in Fig. 1d are 25 % lower than fossil diesel at higher temperatures. This implies that fuel droplets of biodiesel fuels are heated up faster than fossil diesel and consequently the vaporisation rate of fuel droplets is enhanced. The liquid thermal conductivities of individual FAME components are predicted using the method of Robbins and Kingrea [5]. After that, Li’s equation [5] is employed to determine the liquid thermal conductivities of PME, SME and CME. Based on Fig. 1e, the estimated biodiesel liquid thermal conductivities are compared to fossil diesel as a result of the limited validation data. Liquid thermal conductivity is required to compute heat transfer across the fuel droplets, where a transient temperature distribution is assumed [9].
Vapour pressure is one of the key thermo-physical properties as it affects the vaporisation process of fuel spray. Vapour pressure of each FAME component is evaluated using Antoine equation [9] and then simple mixing rule [5] is applied to obtain the vapour pressure of PME, SME and CME. Based on Fig. 1f, the estimated vapour pressures for PME, SME and CME remained low from 280 up to 580 K. But the vapour pressures of biodiesel fuels increase to maximum value at their respective critical temperature right after 580 K. The validation of estimated vapour pressures is done by comparing the trends of estimated vapour pressures of PME, SME and CME seen in Fig. 1f against the predicted and measured vapour pressure values of SME [3]. Satisfactory agreement of less than 10 % is found. Latent heat of vaporisation at normal boiling point is estimated using Pitzer acentric factor correlation [5]. Compared to fossil diesel, the latent heat of vaporisations of the biodiesel fuels are 13 % lower along the temperature range as seen in Fig. 1g. Thus, the fuel droplets of biodiesel fuels will be heated up quickly during the vaporisation process.
Vapour viscosities and vapour thermal conductivities are calculated by employing the Chapman-Enskog kinetic theory [5] as proposed by Chung et al. [10, 11]. Both vapour viscosities and thermal conductivities of PME, SME and CME are fairly accurate when vapour viscosity and thermal conductivity of fossil diesel are taken as the baseline case for validation. Vapour viscosities of the PME, SME and CME are comparatively lower than fossil diesel as seen in Fig. 1h. From Fig. 1i, vapour thermal conductivities of biodiesel fuels are also lower than fossil diesel. Hence, it is important to investigate the rate of break-ups of vapour fuel droplets and the heat transfers across the combustion chamber as these two phenomena are affected by vapour viscosity and thermal conductivity.
Vapour diffusivity defines the speed of movements of fuel vapours in the combustion chamber. The estimated vapour diffusivities of PME, SME and CME are presented in Fig. 1j and are evaluated using Wilke and Lee method [12]. Good agreement is obtained when the trends of estimated vapour diffusivities of PME, SME, CME and fossil diesel are compared. Meanwhile, values of vapour heat capacity are predicted using the method of Rihani and Doraiswamy [5] as presented in Fig. 1k. The transient heat transfer of surrounding gas to the fuel droplet surface depends on the vapour heat capacity of fuel. Vapour heat capacity is also required to predict the vapour viscosities and vapour thermal conductivities of the PME, SME and CME. On the other hand, second virial coefficient is a coefficient used in gas equation expansion. The second virial coefficients of FAME components are computed using the method of Tsonopoulos [5]. The Lee-Kesler mixing rule [5] is then applied to determine the second virial coefficient of PME, SME and CME, as presented in Fig. 1l.
3 Numerical and Experimental Operating Conditions
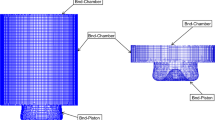
In-cylinder constant volume combustion is simulated using OpenFOAM to examine the sensitivity of individual biodiesel properties to the fuel spray development. The constant volume combustion chamber utilised displayed in Fig. 2 with a total volume of 2 L is built based on Chalmer’s high-pressure, high-temperature spray rig [13]. The operating conditions for the constant volume combustion are listed in Table 4, where a vertically aligned and single-hole injector operating at 313.15 K temperature and 1200 bar pressure is used [13]. In order to examine the effects of individual properties on the fuel spray characteristics, the properties of PME are set as base properties. Then, each individual fuel property is varied to that of fossil diesel. The main results of interest here are the development of fuel spray, liquid and vapour axial penetration length, mass of fuel evaporated and Sauter Mean Diameter (SMD). Due to limited experimental data available for SME and CME, the sensitivity analysis in this study is only conducted for PME.
4 Sensitivity Analysis of Biodiesel Thermo-Physical Properties Under Constant Volume Combustion
Only 5 out of the 12 thermo-physical properties of PME have been identified as significant properties since profound changes on the biodiesel spray analysis are found as shown in Fig. 3. The five significant properties include liquid density, liquid surface tension, liquid viscosity, vapour pressure, and vapour diffusivity. Among the identified five significant properties, vapour pressure has the largest impact based on the changes on fuel spray structures as seen in Figs. 3a–d. The reason for this is due to the notable difference in biodiesel properties as illustrated in Fig. 1f, where vapour pressure of PME is lower than fossil diesel. Hence, the vaporisation rate of fuel droplets is higher. In Fig. 3b, vapour pressure has the largest influence to the vaporisation rate amongst other fuel properties. Besides that, the liquid axial penetration for vapour pressure is also one the lowest, which implies that the vaporisation rate of the fuel droplet is the fastest.
Apart from vapour pressure, liquid surface tension is also observed to have noticeable effects on the spray structure. Larger liquid surface tension value of PME than fossil diesel is found as displayed in Fig. 1b proves that the break-ups and atomisation rates of fuel droplets into smaller particles are slower. Thus, the liquid axial penetration length of liquid surface tension is comparatively longer than other fuel properties due to larger fuel droplets formed with high momentum to penetrate across the combustion chamber as seen in Fig. 3d. Liquid viscosity, liquid density and vapour diffusivity have marginal effects on the spray structure such as the mass of fuel evaporated and SMD as compared to vapour pressure and liquid surface tension. This is presented in Fig. 3b and c. Based on Fig. 3a–d, liquid heat capacity, liquid thermal conductivity, latent heat of vaporisation, vapour viscosity, vapour thermal conductivity, vapour heat capacity and second virial coefficient are deemed to be less important since only marginal effects are observed.
In short, biodiesel thermo-physical properties are shown to affect the fuel spray development. In particular, liquid density, liquid viscosity, liquid surface tension, vapour pressure and vapour diffusivity are distinguished as determined as the most influential fuel properties based on the observation of fuel spray development and structures. It is imperative to accurately determine all the required fuel properties for different biodiesel fuels for in-cylinder CFD simulation in order to accurately describe the fuel spray characteristics.
5 Conclusion
A set of thermo-physical properties for biodiesel using generic methods is developed here, where the methods employed is suitable for biodiesel produced from various feedstocks. From the sensitivity analysis, liquid density, liquid surface tension, liquid viscosity, vapour pressure and vapour diffusivity exert the most significant influences on biodiesel fuel spray development. Larger fuel droplet and longer fuel spray axial penetration are found as a result of the higher values in liquid density, liquid viscosity and lower vapour pressure for biodiesel. Subsequently, poorer vaporisation rate of the biodiesel fuel spray results, which affect the air-fuel mixture preparation process. The key conclusion drawn from this study is that the thermo-physical properties of biodiesel play an important role in defining the fuel spray development, which subsequently gives rise to its distinct combustion and emission behaviours from fossil diesel combustion. For accurate in-cylinder CFD simulation for biodiesel spray combustion, the thermo-physical properties of biodiesel must be determined appropriately.
References
Chakravarthy K, McFarlane J, Daw SC, Ra Y, Reitz RD (2007) Physical properties of soy bio-diesel and implications for use of bio-diesel in diesel engines. SAE paper
Ra Y, Reizt RD, McFarlane J, Daw SC (2008) Effects of fuel physical properties on diesel engine combustion using diesel and bio-diesel fuels. SAE paper
Yuan W, Hansen AC, Zhang Q (2003) Predicting the physical properties of biodiesel for combustion modelling. Trans ASAE 6(46):1487–1493
Junfeng Y, Golovitchev VI (2009) Construction of combustion models for rapeseed methyl ester bio-diesel fuel for internal combustion engine applications. Biotechnology Advances 27:641–655
Reid RC, Prausnitz JM, Sherwood TK (1987) The properties of gases and liquids. McGraw Hill, New York
Anand K, Sharma RP, Mehta PS (2011) A comprehensive approach for estimating thermo-physical properties of biodiesel fuels. Appl Therm Eng 31:235–242
Allen CW, Watts KC, Ackman RG (1999) Predicting the surface tension of biodiesel fuels from their fatty acid composition. J Am Oil Chem Soc 76(3):317–323
Van Bommel MJ, Oonk HAJ, Van Miltenberg JC (2004) Heat capacity measurements of 13 methyl esters of n-carboxylic acids from methyl octonoate to methyl eicosanoate between 5 and 350 K. J Chem Eng Data 49:1036–1042
Ceriani R, Meirelles AJA (2004) Predicting vapour-liquid equilibria of fatty systems. Fluid Phase Equilib 215(2):227–236
Chung TH, Lee LL, Startling KE (1984) Applications of kinetic gas theory and multiparameter correlation for prediction of dilute gas viscosity and thermal conductivity. Ind Eng Chem Fundam 23:8–13
Chung TH, Ajlan M, Lee LL, Startling KE (1988) Generalized multiparameter correlation for nonpolar and polar fluid transport properties. Ind Eng Chem Res 27:671–679
Poling BE, Prausnitz JM, O’Connel JP (2001) The properties of gases and liquids. McGraw Hill, New York
Ochoterena R, Larsson M, Andersson S, Denbratt I (2008) Optical studies of spray development and combustion characterization of oxygenated and Fischer-Tropsch fuels. SAE paper
Tat ME, Van Gerpen JH (2000) The specific gravity of biodiesel and its blends with diesel fuel. J Am Oil Chem Soc 77(2):115–119
Tat ME, Van Gerpen JH (1999) The kinematic viscosity of biodiesel and its blends with diesel fuel. J Am Oil Chem Soc 76(12):1511–1513
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2013 Springer-Verlag Berlin Heidelberg
About this paper
Cite this paper
Cheng, X., M. Ismail, H., Ng, K., Gan, S., Lucchini, T. (2013). Effects of Fuel Thermo-Physical Properties on Spray Characteristics of Biodiesel Fuels. In: Proceedings of the FISITA 2012 World Automotive Congress. Lecture Notes in Electrical Engineering, vol 191. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-642-33777-2_9
Download citation
DOI: https://doi.org/10.1007/978-3-642-33777-2_9
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-642-33776-5
Online ISBN: 978-3-642-33777-2
eBook Packages: EngineeringEngineering (R0)