Abstract
The ability of microbes to reductively transform a variety of metals has wide-reaching implications for controlling the mobility of contaminants in the subsurface, resulting in the degradation of toxic organics or the reductive immobilization of metals. For example, soluble toxic metal contaminants, including Cr(VI), Hg(II), V(V), Co(III), U(VI), Tc(VII) and Np(V) can be reduced directly and removed from solution by enzymatic processes, often being used as terminal electron acceptors during anoxic respiration. In many cases these transformations can also be mediated indirectly via reactive end products of metal reduction, including biogenic Fe(II) or Pd(0). Similar indirect mechanisms for the reductive transformations of organics, such as chlorinated solvents, are also possible, as is the enzymatic oxidation of several organic “xenobiotics”, coupled directly to the reduction of metals such as Fe(III). Many of these processes occur naturally within contaminant systems, and the ability to accelerate them during bioremediation applications has attracted much recent interest. Over the past two decades, studies have sought to understand these processes from the molecular level while applying them at field or industrial scale. This review seeks to summarise these findings and address areas of current and prospective progress in the bioremediation field.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Introduction
Anthropogenic activities have resulted in the widespread contamination of the environment with both inorganic and organic pollutants. In many examples, pollutant behaviour is regulated by complex biogeochemical reactions mediated by extant microbial communities. The harnessing of the inherent ability of microorganisms, to detoxify or precipitate inorganic and degrade organic contaminants, can be key to their bioremediation. Due to the near ubiquitous presence of such microbes in contaminated environments, and their enormous gene pool, the potential for their use in bioremediation applications is significant (Lovley et al. 2004).
Many inorganic contaminants are redox active and can be reduced to less harmful or less mobile forms. Mounting research has highlighted the role of microbes in the reduction of inorganics, including transition metals and radionuclides, and often used as the terminal electron acceptor in anaerobic metabolism (Lloyd 2003). Organic contaminants can also play a direct role in microbial metal reduction as an electron donor for Fe(III) reduction (Lovley et al. 1989a) or can be reduced by resulting biogenic Fe(II) (McCormick et al. 2002).
In order to better understand contaminant processes coupled to microbial metal reduction, a variety of approaches from the molecular to the field scale have been addressed. The subsequent application of microbes with metal-reducing properties for bioremediation has led to many proposed application technologies. These typically encompass “biostimulation” strategies to enhance the activities of indigenous microbes and “bioaugmentation”, via the addition of model metal-reducing microbes. Many bioremediation approaches have been proven at laboratory scale, while others have reached pilot (Wagner-Döbler 2003) and field-scale in situ application (Li et al. 2009).
This review aims to give an overview of a variety of bioremediation processes underpinned by microbial metal reduction. This will include studies concerning the diversity of the microorganisms involved, the mechanisms of contaminant transformation, the environmental fate of the contaminant, strategies for optimising these processes and finally the impact of the latest post-genomic approaches on increasing our knowledge of these complex biogeochemical systems.
2 Mechanisms of Microbial Fe(III) Reduction
The biogeochemical cycling of Fe is an important global process and has influence over the behaviour of many other bulk and trace elements. The cycling between the oxidised (Fe(III)), and reduced (Fe(II)), states is often microbially mediated, where Fe(III) is used as a terminal electron acceptor in anaerobic metabolism and Fe(II) as an electron donor in both aerobic and anaerobic processes (Weber et al. 2006).
The microbial reduction of Fe(III) plays a key role in the oxidation of organics within the anaerobic subsurface, where Fe(III) is often the most abundant electron acceptor (Nealson and Myers 1992). The ability of microbes to reduce Fe(III) as an electron acceptor during metabolism has long been established (see Lovley and Phillips, 1986). The isolation of organisms capable of Fe(III) reduction coupled to growth came later with the isolation of Geobacter metallireducens (formerly GS-15) (Lovley and Phillips 1988) and the realisation that Shewanella oneidensis (formerly Alteromonas putrefaciens) could also respire Fe(III) (Lovley et al. 1989b). Following these findings, many of the strains isolated, capable of Fe(III) reduction, have belonged to the family Geobacteraceae of the δ-Proteobacteria (Lloyd 2003). This is significant as members of the family Geobacteraceae are commonly identified within natural environments, and are capable of coupling Fe(III) reduction to the oxidation of acetate and many other organic substrates (Lovley et al. 2004). Many Fe(III)-reducing bacteria can also utilise other redox active metals and reducible organics as electron acceptors (Lovley 1993). For an extensive review of the diversity of organisms capable of Fe(III) reduction, please Refer to Chap. 6 of this book and to (Lovley et al. 2004). Further studies have extended the range of environments in which Fe(III) reduction can occur, to encompass bacteria adapted to extreme environments including thermophiles (Tor and Lovley 2001) acidophiles (Coupland and Johnson 2008) and alkaliphiles (Roh et al. 2007).
Under near-neutral environments, Fe(III) is typically poorly soluble, occurring as Fe(III) oxides or adsorbed onto mineral surfaces (Weber et al. 2006). The rate of microbial reductive dissolution of the Fe(III) phase is thought to be controlled by the surface area (Roden and Zachara 1996) and the crystallinity of the Fe(III) phase (Hansel et al. 2003b; Cutting et al. 2009). The accumulation of Fe(II) on the Fe(III) oxide surface has also been found to influence the rate and extent of reduction by preventing further reduction of the bulk mineral (Roden 2006).
To deal with the poor solubility of Fe(III) in the environment, microbes have evolved a variety of mechanisms which enable electron transfer to the microbe-mineral interface, resulting in reductive dissolution of extracellular Fe(III) minerals (Lovley et al. 2004). Early studies suggested that outer membrane cytochromes were a key component for electron transfer of Shewanella spp. (Myers and Myers 1992). The cellular electron transfer apparatus, in the model Fe(III) reducers, Geobacter spp. and Shewanella spp., have now been characterised in some detail (Lovley et al. 2004; Hartshorne et al. 2009). This is reflected in the corresponding bacterial genomes, where genes encoding c-type cyctochromes are present in abundance (Richter et al. 2012).
Some Fe(III)-reducing bacteria were found to have the ability to produce soluble compounds, termed “electron shuttles”, which can accelerate electron transfer between a remote cell and Fe(III) mineral (Lloyd et al. 2003). The ability to produce such compounds was first demonstrated in Shewanella (von Canstein et al. 2008; Marsili et al. 2008). The secretion of such “endogenous” electron shuttles appears to be lacking in Geobacter spp. (Nevin and Lovley 2000). However, other naturally occurring organic substances, for example humics (Lovley et al. 1996), can be opportunistically used as electron shuttles by a wide variety of Fe(III)-reducing bacteria. In addition, other organisms such as Geothrix fermentans, are also capable of producing iron chelating compounds in order to promote Fe(III) dissolution and thereby increasing accessibility for reduction (Nevin and Lovley 2002).
The production of pili has also been observed in Geobacter spp., and these structures were hypothesised to aid contact between cells and Fe(III) minerals (Childers et al. 2002). However, more recent studies have noted these pili to be conductive, therefore implicating a role of electron transfer in Geobacter spp. (Reguera et al. 2005; Reguera et al. 2007) and also Shewanella spp. (Gorby et al. 2006).
The resulting biogenic Fe(II) not only adsorbs to the original Fe(III) phase, but can form discrete secondary biominerals (Lloyd et al. 2008). The mineralogy of the resulting Fe(II) phase is determined by a complex interaction of microbial and geochemical controls (Hansel et al. 2003a). Although a variety of biominerals can result from Fe(III) reduction; siderite, green rust and vivianite for example, the common end member biomineral formed is often biogenic magnetite, especially in laboratory experiments utilising ferrihydrite as the Fe(III) mineral phase (Lloyd et al. 2008; Coker et al. 2006).
2.1 Oxidation of Organic Contaminants Coupled to Fe(III) Reduction
Many organic molecules occur as subsurface contaminants due to their release during a variety of anthropogenic activities. These organic molecules often consist of aromatic compounds which, due to the thermodynamic stability of the benzene ring, are persistent in the environment (Carmona and Díaz 2005). Anaerobic conditions are established rapidly within organic contaminant plumes, where oxygen is quickly utilised as the favourable electron acceptor in catabolism of the abundant organic electron donors (Carmona et al. 2009). During the resulting redox sequence, an abundance of Fe(III) oxides within the subsurface can result in much of the plume being located within the Fe(III) reduction zone (Lloyd et al. 2003). In situ monitoring of such environments has confirmed degradation of organic contaminants under Fe(III)-reducing conditions (Lyngkilde and Christensen 1992; Nielsen et al. 1995; Christensen et al. 2001), although the persistence of many aromatics within Fe(III) reducing zones suggests this process in the subsurface can be kinetically slow (Lovley, 1995).
The ability of a single organism to couple Fe(III) reduction to the oxidation of aromatic hydrocarbons was first reported for the bacterium G. metallireducens (Lovley et al. 1989a). This bacterium, isolated from a hydrocarbon-contaminated aquifer, was able to obtain energy from oxidation of benzoate, toluene, phenol or p-cresol using Fe(III) as the sole electron acceptor (Lovley and Lonergan 1990). The bacterium in this study was able to mineralise the aromatic compounds to CO2, with little accumulation of intermediate compounds. For an in-depth genomic view of the aromatic catabolism of G. metallireducens refer to (Wischgoll et al. 2005) and (Carmona et al. 2009). However, few further isolated Geobacter sp. have exhibited the ability to couple Fe(III) reduction to the oxidation of aromatic compounds (Butler et al. 2007). Exceptions include Geobacter hydrogenophilus and Geobacter grbiciae, both able to oxidise benzoate and the latter bacterium toluene (Coates et al. 2001). Outside the Geobacter genus, few axenic cultures of bacteria have demonstrated Fe(III) reduction coupled to aromatic metabolism. Notable examples are the thermophile, Ferroglobus placidus, which could indicate aromatic oxidation coupled to Fe(III) reduction is an important metabolic process in hot subsurface environments (Tor and Lovley 2001). Another recent study described the isolation of two bacteria capable of this metabolic trait, Geobacter toluenoxydans and Desulfitobacterium aromaticivoran, the latter being a member of the Gram-positive Clostridia group (Kunapuli et al. 2010).
Although the ability of bacteria to couple Fe(III) reduction to aromatic oxidation seems to be not expressed widely across known Fe(III)-reducing bacteria outside the family Geobacteraceae, the abundance of Geobacter species in many sedimentary environments where Fe(III)-reducing conditions prevail suggests that these processes are of environmental significance (Coates et al. 1996). Using microbial ecology techniques, such as 16S rDNA profiling, these organisms have been found to contribute a significant proportion of the microbial community in subsurface environments contaminated with aromatics (Rooney-Varga et al. 1999; Roling et al. 2001; Staats et al. 2011; Snoeyenbos-West et al. 2000). Thus, it would seem reasonable that biostimulation of these organisms could offer a viable bioremediation strategy, to enhance biodegradation. Here, a key limiting step in the process could be the poor accessibility of insoluble Fe(III) oxides to microbial Fe(III) reduction (Lovley et al. 1994; Lovley et al. 1996). A study using anoxic aquifer sediments was able to increase aromatic oxidation rates, coupled to Fe(III) reduction, to values approaching those seen in oxic systems, by the addition of ligands which increased Fe(III) oxide solubility (Lovley et al. 1994). The promotion of Fe(III) reduction using electron shuttling compounds was also linked to an increased rate of aromatic oxidation within the subsurface (Borch et al. 2010). Multiple studies have demonstrated this through the use of the humic analogue AQDS (anthraquinone-2,6-disulphonic acid) (Snoeyenbos-West et al. 2000; Jahn et al. 2005). For a more detailed review on the addition of electron shuttles to promote the degradation of contaminants see (Van der Zee and Cervantes 2009). Fe(III) reduction coupled to these processes also leads to the production of reactive Fe(II) species. A recent study demonstrated Fe(II)-mediated reduction of an enzymatically recalcitrant contaminant, a nitroaromatic compound, using Fe(II) generated from the oxidation of BTEX (benzene, toluene, ethylbenzene and xylene) compounds by G. metallireducens (Tobler et al. 2007). Thus, this study demonstrated that microbial Fe(III) reduction coupled to aromatic oxidation, can result in abiotic degradation of co-contaminants that cannot be utilised directly (Tobler et al. 2007).
2.2 Fe(II)-Mediated Contaminant Reduction
As noted above, the microbial reduction of Fe(III) can mediate reductive transformation of contaminants via abiotic electron exchange reactions with biogenic Fe(II), see Fig. 1 (Lloyd 2003). Indeed, ionic Fe(II) is a well-known reductant commonly used in industrial processes and during chemical remediation of contaminants (Charlet et al. 1998).
Biogenic Fe(II) is often associated with Fe(II)-bearing biominerals, either within the structure of the mineral itself or sorbed to the surface (Cutting et al. 2009). The reactivity of various synthetic Fe(II)-bearing minerals towards dehalogenation of hexachloroethane and nitroaryl reduction of 4-chloronitrobenzene has been demonstrated (Elsner et al. 2004). This study found variability within surface area normalised reaction rates, dependent upon the Fe mineral composition, and where Fe sulphides exhibited the highest reaction rates. As magnetite is ubiquitous in the subsurface environment (and also a common product of microbial Fe(III) reduction) it has received much attention regarding reactivity towards contaminants (Gorski and Scherer 2009; Gorski et al. 2010). The latter of these studies demonstrated an increasing rate of reactivity towards nitrobenzene, of synthetic magnetites, with increasing Fe(II) content up to those of stoichiometric magnetite.
Several studies have carried out contaminant interaction experiments using biogenic magnetite (McCormick et al. 2002; McCormick and Adriaens 2004; Williams et al. 2005; Telling et al. 2009; Cutting et al. 2010). These biogenic magnetites were synthesised via reduction of an amorphous Fe(III)-starting mineral (e.g., ferrihydrite) using Fe(III)-reducing bacteria of the genus Geobacter. In one study, the resulting biogenic magnetite was used to transform recalcitrant carbon tetrachloride (McCormick and Adriaens 2004). A variety of daughter compounds formed during exposure, including chloroform and the intermediate dichloromethane. A proportion of this was subsequently hydrolysed to carbon monoxide or was further reduced to methane, although the mechanism of methane formation was not elucidated. The formation of chloroform and dichloromethane is of concern as these products are also toxic; however, a large proportion (~47 %) of carbon tetrachloride was fully dechlorinated to the relatively benign CO and CH4. In a separate study, biogenic magnetite, with minor components of siderite and an unknown Fe(II) phase, was demonstrated to be active for transformation of the toxic groundwater contaminant, hexahydro-1,3,5-trinitro-1,3,5-triazine (RDX), a component of military explosives (Williams et al. 2005). Sequential reaction of a series of nitroso products occurred, eventually leading to the accumulation of 1,3,5-trinitroso-1,3,5-triazacyclohexane, although the products only accounted for a maximum of 30 % of the original RDX. Interestingly, this paper also noted a decrease in reactivity of the biogenic minerals after increased incubation time with the bacterium G. metallireducens during mineral biosynthesis. This effect was hypothesised to be possibly the result of the release of cell lysis products.
Biogenic magnetite was also demonstrated to be effective for the reduction of the common soluble inorganic contaminant Cr(VI) to the insoluble and non-toxic Cr(III), and the reduction of the fission product Tc(VII) to insoluble Tc(IV) (Cutting et al. 2010), see Sects. 3.1 and 4.2 for more details of Cr and Tc contamination, respectively. This study utilised biogenic magnetites produced from different starting Fe(III) phases, which exhibited contrasting Fe(II) yields, and these corresponded to differing activities against Cr(VI). The Cr was shown, using X-ray magnetic circular dichroism (XMCD) analysis, to be converted into Cr(III) and incorporated into the octahedral site of a CrFe2O4 spinel, at the surface of the magnetite. Retention of 99mTc(VII) was investigated using flow through columns of quartz sand with a reactive layer of the biogenic magnetite, and the quantities of 99mTc retained in the column and in the effluent were monitored using a gamma camera imaging system. Efficient retention, 77.9 and 97.8 %, for ferrihydrite- and schwertmannite-derived magnetites, respectively, was observed compared to only 16.2 % accumulation in control columns with no magnetite.
Other studies have focussed on biogenic Fe(II)-mediated contaminant transformations using direct culturing of Fe(III)-reducing bacteria grown with reducible Fe(III) phases (Wielinga et al. 2001; Hansel et al. 2003b; Borch et al. 2005). The degradation of 2,4,6-trinitrotoluene (TNT), a groundwater contaminant from the explosives industries, was investigated using a Cellulomonas sp. incubated with and without ferrihydrite and the electron shuttle AQDS (Borch et al. 2005). The TNT reduction products, which are inferred to be less mobile than the parent molecule, were formed more rapidly and to a greater extent in the presence of the Fe(III)-bearing substrate ferrihydrite and AQDS. Studies using direct culturing of Shewanella alga in contact with ferrihydrite and a Cr(VI) solution also showed significant Fe(II)-mediated reduction and removal of Cr(VI) (Wielinga et al. 2001; Hansel et al. 2003b). The first of these studies also noted that, under Fe-limiting conditions, cycling of Fe between the two valence states occurred with microbial Fe(III) reduction, and then subsequent abiotic Fe(II) oxidation coupled to Cr(VI) reduction. The latter study characterised the resulting Cr(III) phase by X-ray absorption near-edge structure (XANES) and extended X-ray absorption fine structure (EXAFS), identifying an insoluble mixed Fe(III)-Cr(III) hydroxide.
The capacity for indigenous Fe(III) microbial communities to mediate organic degradation was investigated for TNT degradation by charging a column containing ferrogenic aquifer sediments with acetate (Hofstetter et al. 1999). Rapid and complete reductive degradation, via biogenic Fe(II), to 2,4,6-triaminotoluene was observed in amended columns, while unamended columns exhibited a slower rate of reduction.
A series of studies on alkaline Cr(VI) containing leachates, from chromite processing ore residue waste sites, indicated in situ biogenic immobilisation of Cr(VI) by Fe(II) in adjacent soils (Stewart et al. 2007; Stewart et al. 2010; Whittleston et al. 2011a). When the soils, with alkaline Cr(VI) leachates, were amended with the electron donor acetate, the utilisation of the cascade of electron acceptors occurred, passing from nitrate reduction to sulphate reduction via Fe(III) reduction (Whittleston et al. 2011a). Accumulation of Fe(II) coincided with the removal of Cr(VI) from solution (Whittleston et al. 2011a). Enrichments of a bacterial consortium from the Fe(III)-reducing soils were dominated by Fe(III)-reducing Fermicutes species (Whittleston et al. 2011b). These studies appear to show the natural occurrence of a microbially-mediated Fe(III)-reducing reactive zone which had the potential for reducing and sequestering alkaline Cr(VI) leachates.
Reduction and immobilisation of Tc(VII) is well documented within anoxic sediments (Lloyd et al. 2000a; Wildung et al. 2004; Burke et al. 2005, 2006; McBeth et al. 2007). Reduction typically coincides with microbial Fe(III) reduction, and is thought to be mediated indirectly by the microbial production of the reductant Fe(II) (Lear et al. 2009). This is in part due to low concentrations of Tc(VII) present in most Tc-contaminated environments, and poor recognition of the substrate by the hydrogenases believed to be responsible for enzymatic Tc(VII) reduction in microbes (Lloyd et al. 1999). The microbial reduction of Fe(III) within sediments, from the US Department of Energy’s Hanford and Oak Ridge sites, inoculated with the Fe(III)-reducing bacterium Shewanella oneidensis, was shown to increase the capacity for Tc(VII) reduction and immobilisation (Fredrickson et al. 2004). Alternative studies have also shown the effectiveness of stimulation of indigenous Fe(III)-reducing bacterial consortia for indirect reduction of Tc(VII) (Burke et al. 2005; McBeth et al. 2007; Lear et al. 2009). Upon amendment with an electron donor, the reduction and removal of Tc(VII) from estuarine or aquifer sediment microcosms, coincided with microbial Fe(III) reduction (Burke et al. 2005; McBeth et al. 2007). In the sediments of the latter study, the known Fe(III)-reducing bacteria Geothrix and Geobacter species were confirmed to be present by PCR-based analyses. X-ray absorption spectroscopy (XAS) analysis confirmed reduction to Tc(IV)(as TcO2) within the sediments (Burke et al. 2005). It should be noted that upon exposure to air, reoxidation of immobilised reduced Tc(IV), resulted in the release of Tc(VII) back into solution, of ~50 % (Burke et al. 2006) and ~80 % (McBeth et al. 2007) of total Tc, although much lower release values were noted when nitrate (a common nuclear contaminant) was used to reoxidise the reduced Tc(IV). A recent study used a γ-camera to image retention of the radiotracer 99mTc, in a biostimulated flow-through sediment column, alongside geochemical and molecular ecology techniques (Lear et al. 2009). This was able to spatially correlate areas of Fe(II) accumulation, Tc(VII) reduction and immobilisation and an increase in the proportion of Fe(III)-reducing bacteria.
3 Enzymatic Transition Metal Contaminant Reduction
A variety of transition metals occur as contaminants, and many of these are redox active and amenable to enzymatic reductive remediation, see Fig. 2. Other transition elements do not typically occur as contaminants, for example Pd(II), but may present opportunities for remediation processes by their enzymatic reduction, forming highly active precious metal catalysts, useful for contaminant destruction.
3.1 Reduction of Cr(VI)
The pervasive pollutant, Cr, is associated with a significant proportion of contaminated sites worldwide. This is as a consequence of its widespread industrial use, from its initial mining and processing as a chromite ore through to its use in metallurgy, leather tanning, pigment production and wood preservation (Kamaludeen et al. 2003).
The metal is redox active, forming two valence states which dominate in environmental systems; Cr(VI) and Cr(III). Cr(III) species are dominant under acidic and moderate to reducing conditions while Cr(VI) is dominant in more oxidising and alkaline conditions (Kimbrough et al. 1999). Cr(III), being relatively insoluble in all but highly acidic environments, typically forms chromium (oxyhydr)-oxides, often in association with iron or strongly adsorbed to mineral surfaces (Fendorf 1995). The Cr(VI) species; H2CrO4, HCrO4–, CrO4 2– and C2O4 2–, are by contrast readily soluble and weakly adsorb to mineral surfaces (Kimbrough et al. 1999).
Cr(VI) has a variety of toxic properties behaving as an irritant, carcinogen and allergen (Chen and Hao 1998). In humans, this manifests itself as dermal irritation upon skin contact, lung and respiratory tract damage upon inhalation and liver and kidney damage upon ingestion (Gad 1989). This toxic effect is in part due to the mobile nature of the Cr(VI) which enables diffusion across cell membranes, where intracellular reduction causes oxidative damage to the cell (Dayan and Paine 2001). Cr(III), however, is by contrast regarded as non-toxic and an essential trace nutrient necessary for glucose and lipid metabolism (Wang 2000). The risks involved with exposure to Cr(VI) have led to it being regarded as a priority pollutant by the US Environmental Protection Agency (USEPA 2009), and its use controlled by the European Restriction of Hazardous Substances Directive (RoHS 2003). The World Health Organisation currently sets an upper limit of 0.05 mg L−1 for Cr(VI) in drinking water (WHO 2008).
Conventional treatments for Cr(VI)-containing wastes target chemical reduction and precipitation as poorly soluble Cr(III) (oxyhydr)-oxides. Due to the high Cr(VI)/Cr(III) redox couple, re-oxidation by contact with dissolved oxygen is kinetically very slow (Rai et al. 1989). While more oxidising species found in waters and soils such as Mn oxides are capable of re-oxidation of Cr(III), this process is dissolution controlled and kinetically slow (Rai et al. 1989).
Although reduction can be a consequence of indirect microbial reduction via the formation of intermediary reducing species, such as Fe(II) and sulphides, (see Sect. 2.2) direct enzymatic Cr(VI) reduction can occur (Lovley and Phillips 1994). Since the first recorded isolations of organisms capable of enzymatic microbial Cr(VI) reduction in the late 1970s, Pseudomonas dechromaticen (Romanenko and Koren’kov 1977) and Pseudomonas chromatophila (Lebedeva and Lialikova 1979), a diverse group of Cr(VI)-reducing microbes have been identified (Cervantes et al. 2007). The majority are facultative anaerobes which can be found in natural unpolluted systems (Wang and Shen 1995). Early studies primarily identified bacteria that did not conserve energy through Cr(VI) reduction and was likely due to the secondary activity of enzymes with different physiological functions (Ishibashi et al. 1990). Later studies, however, also identified bacteria capable of coupling Cr(VI) reduction to growth (Tebo and Obraztsova 1998; Francis et al. 2000). Enzymatic Cr(VI) reduction has been demonstrated under aerobic and anaerobic conditions, with some bacteria exhibiting reduction under both conditions; for example Escherichia coli ATCC 33456 (Shen and Wang 1993).
Multiple enzymatic reduction mechanisms are reported within the literature, and appear to be associated with specific locations within the cell (Cervantes et al. 2007). Typically, membrane-bound Cr(VI) reduction is thought to be mediated by the electron transfer chain and related cytochromes, while cytoplasmic Cr(VI) reduction occurs by flavin-dependent reductases (Magnuson et al. 2010). Early studies of Pseudomonas ambigua G-1 and Pseudomonas putida exhibited Cr(VI) reduction via soluble Cr(VI) reductases under aerobic conditions, dependent upon NAD(P)H as the electron donor; (Suzuki et al. 1992) and (Ishibashi et al. 1990; Suzuki et al. 1992) respectively. Since these studies, other soluble Cr(VI)-reducing enzymes have been identified. Some of the more extensively studied include; ChrR (formerly referred to as YieF when expressed in E. coli), ChrR6 and the nitroreductase NfsA (Ackerley et al. 2004, Barak et al. 2006). The reduction mechanism is enzyme specific, for example the NfsA dimer (of E. coli) reduces Cr(VI) via a combination of one and two electron transfer reactions (Ackerley et al. 2004). This mechanism proceeds via the reactive intermediary Cr(V), generating reactive oxygen species (ROS) which are implicated in Cr(VI) toxicity within the cell (Barak et al. 2006). Indeed, a proteomics study correlated Cr(VI) stress in Pseudomonas aeruginosa to overexpressed production of the ROS detoxification protein glutathione (Kilic et al. 2009). Other soluble reductases, such as ChrR of E. coli, which normally transfers four electrons in redox reactions, reduces Cr(VI) to Cr(III) in a one-step three electron transfer, with one electron going to molecular oxygen, thus limiting ROS generation (Ackerley et al. 2004).
Anaerobic Cr(VI) reduction, in which Cr(VI) acts as the terminal electron acceptor, has been demonstrated in several obligate anaerobes. Sulphate-reducing bacteria have received much attention as they are able to couple lactate oxidation to Cr(VI) reduction, due to similarities between the sulphate and chromate anions (Lloyd et al. 2001). In sulphate-reducing bacteria (Desulfovibrio spp.), reduction has been shown to occur via c-type cytochromes operating with hydrogenases, facilitating the use of hydrogen as an electron donor for Cr(VI) (Lovley and Phillips 1994). A recent study of S. oneidensis was able to identify the MtrC and OmcA as the cytochromes acting as the terminal reductases of the electron transfer chain responsible for extracellular Cr(VI) reduction (Belchik et al. 2011). This was confirmed via deletion of the respective genes, mtrC and omcA, resulting in a loss of reduction efficiency and lack of extracellular Cr(III) precipitation with a concurrent increase in intracellular Cr(III) precipitation (Belchik et al. 2011).
The development of microbial Cr(VI) reduction to a feasible bioremediation strategy has been the aim of numerous studies. For example, ex situ and wastewater treatment bioreactors have demonstrated efficient Cr(VI) reduction. These have been composed of simple batch cultures of planktonic cells (Tripathi 2002) or immobilised cultures, forming a biofilm upon a support substrate (Konovalova et al. 2003). A noteworthy study compared Cr(VI) removal within a variety of bioreactors, using the model microbe P. aeruginosa A2Chr (Tripathi 2002). The results indicate that cells entrapped in biofilms are less susceptible to Cr(VI) toxicity than planktonic forms, and thus more applicable to higher Cr(VI) concentrations. Immobilised biofilm beds have also been demonstrated using Bacillus spp. (Chirwa and Wang 1997), mixed cultures of sulphate reducers (Smith 2001) and mixed cultures obtained from Cr(VI) contaminated environments (Nancharaiah et al. 2010). The latter study was effective under both aerobic and anaerobic conditions. Biomass-dependent Cr(VI) removal was observed to be slightly greater in the absence of oxygen, at 0.17 mM day−1g−1 compared to 0.15 mM day−1g−1 for aerobic conditions, inferred to be due to oxygen acting as a competing electron acceptor. A study, using a mixed culture, to investigate optimum parameters for microbial Cr(VI) reduction found that biomass exerted the overall control over the reaction (Jeyasingh and Philip 2005). However, the pH of the medium also exerted influence with loss in reduction efficiency away from circum-neutral values.
The potential in situ treatment of Cr(VI)-contaminated sites has also attracted significant interest. The diversity of microbes capable of Cr(VI) reduction means most soils and sediments have an indigenous population of Cr(VI) reducers (Bader et al. 1999). Stimulation of this metabolic capability is achievable by a variety of amendments. For example, addition of tryptic soy broth and a mix of glucose and mineral salts stimulated CO2 evolution, as a product of respiration, coupled to significant Cr(VI) reduction over 128 days from a contaminated soil from an electroplating facility (Turick et al. 1998). In situ conditions are not easily controlled and thus not often optimum for metabolic activity, for example, most subsurface aquifer conditions are typically relatively low temperature, and limited in electron donor concentrations. However, a study of Cr(VI)-contaminated sediments from the Hanford facility led to culturing of an indigenous psychrophilic bacterium, Arthrobacter aurescens, capable of complete reduction of 60 mg/L Cr(VI) within 120 h at 10 °C (Horton et al. 2006). Extremes of pH are also a key factor for in situ Cr(VI) remediation, and Cr(VI) is particularly associated with highly alkaline environments. Several recent publications have however isolated bacteria from alkaline environments capable of high pH (typically pH 9–10) Cr(VI) reduction; Alkaliphilus metalliredigens (QYMF) (Roh et al. 2007), Burkholderia cepacia MCMB-821 (Wani et al. 2007) and Halomonas sp. (VanEngelen et al. 2008). Bioaugmentation with known Cr(VI) reducers has also been effective in laboratory-based studies where addition of mixed cultures, obtained from contaminated samples, has been undertaken (Jeyasingh and Philip 2005; Jeyasingh et al. 2010). The former study of a contaminated soil found that the addition of a mixed culture of Cr(VI)-reducing bacteria was capable of complete Cr(VI) reduction. The latter study trialled targeted inoculation in a “bio-barrier”, which effectively creates a reactive zone of Cr(VI)-reducing bacteria perpendicular to groundwater flow within a laboratory-based system. Under highly controlled conditions of moderate and uniform groundwater flow, with high biomass, effective removal of Cr(VI) from an artificial groundwater plume was observed.
3.2 Reduction of Hg(II)
The bioremediation of mercury (Hg), another important pollutant, has also received significant attention. Although significant natural emissions occur, anthropogenic emissions (2479 Mg/yr−1 in 2006) (Streets et al. 2009), represent a considerable input to the Hg biogeochemical cycle. These primarily result from fossil fuel combustion, mining, gold and nonferrous metal production and the chloralkali processes (von Canstein et al. 2002).
The toxic properties of Hg are dependent upon its valence state, with ionic Hg(II) believed to be the most toxic species in comparison to the less toxic Hg(0) (Clarkson 1997). The toxicity of Hg(II) is the result of its ability to bind to, and thus inactivate, key metabolic enzymes (Barkay et al. 2003). In humans, this is manifest by acute toxic shock at high exposures (Clarkson 1997) and predominantly neuronal disorders at lower doses (Wagner-Döbler 2003). Methylated Hg, MeHg, is considered of greater concern due to its more mobile nature, although its toxicity is a result of the intracellular de-methylation to Hg(II) (Morel et al. 1998).
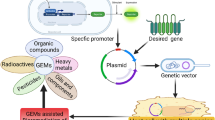
The acute toxicity and natural occurrence of Hg has led to the evolution of a highly conserved bacterial Hg(II) detoxification mechanism (Wagner-Dobler et al. 2000). This consists of uptake, followed by the intracellular reduction of toxic Hg(II) to the far less toxic Hg(0), which is subsequently expelled due to its low solubility and high vapour pressure (Barkay et al. 2005). This phenotype is expressed by a sequence of genes, the mer operon, encoding proteins used in Hg transport and transformation (Barkay et al. 2005). The mer operon is located on transposable elements and plasmids found within a diverse variety of both Gram-positive and Gram-negative bacteria (Wagner-Döbler 2003). Expression is induced by the presence of Hg(II) which binds to the regulatory MerR protein uncoupling it from, and thus activating, the promoter region of the mer operon (Wagner-Döbler 2003).
Unusually, the mer detoxification mechanism (see Fig. 3) actively transports Hg(II) into the cell. This occurs via a succession of specific uptake proteins including periplasmic MerP (in Gram-negative bacteria), and the cytoplasmic membrane-bound proteins MerT, MerC, MerF and Mer E (Barkay et al. 2003). Once inside the cell, the Hg(II) is transferred to the MerA enzyme via redox buffers, such as glutathione or cysteine, or directly from the MerT membrane protein (Barkay et al. 2003). The MerA enzyme is an NAD(P)H dependent mercuric reductase responsible for the reduction of the Hg(II) ion to Hg(0), which passively diffuses out of the cell membrane (Barkay et al. 2003).
A model of the “core” Hg resistance mechanism and associated operon in Gram-negative cells. The alternative transport proteins, MerC, MerE and MerF, may be present in the Gram-negative cell’s inner membrane. For the mer operon diagram below; white boxes indicate regulatory function genes, while grey boxes are transport and transformation genes. The operator/promoter region for the operon is located after the merR gene. The merD gene is present in Gram-negative bacteria and antagonises merR transcription. Diagram adapted from (Barkay et al. 2003, 2005)
The bioremediation of Hg via the microbial mer detoxification mechanism has undergone trials as both a wastewater treatment (von Canstein et al. 1999; Wagner-Döbler 2003) and as an in situ treatment for waters, soils and sediments (Saouter et al. 1995; Nakamura et al. 1999). As a wastewater treatment, a fixed bed bioreactor was employed at technical scale for treating chloralkali electrolysis wastewater (Wagner-Dobler et al. 2000; von Canstein et al. 2002). The aim of the bioreactor was to reduce aqueous Hg(II) from the water and retain the Hg(0) precipitates within the reactor. The bioreactor consisted of an inert carrier matrix, such as pumice, within the main chamber followed by a carbon filter which adsorbed the remaining Hg from the outflow (Wagner-Döbler 2003). The carrier matrix was inoculated with natural isolates of Hg-resistant microbes, predominantly of the Pseudomonas genus, which formed a thick biofilm of cells and exopolysaccharides (EPS) (Wagner-Dobler et al. 2000). The technology was trialled using the wastes of multiple chloralkali plants in Europe alongside synthetic waste analogues (von Canstein et al. 1999). The chloralkali wastewater contained 1.6–7.6 mg/L total Hg, high chloride content of 25 g/L and either acidic or alkaline pH (von Canstein et al. 1999). The wastewater was pre-treated by nutrient amendment, neutralisation of the pH and where necessary dilution, reactor retention times varied between 15 and 60 min. During pilot studies the bioreactor maintained effluent Hg concentrations approaching the theoretical minimum, due to a water solubility of Hg(0) of 50 μg/L, independent of the Hg(II) influent concentrations, which were up to 7 mg/L (von Canstein et al. 1999). The technical-scale bioreactor was able to maintain an Hg removal average of 90 %, including technical failures, retaining 45 kg Hg(0) per 1 m3 bioreactor volume from 10,080 m3 wastewater over 8 months (Wagner-Dobler et al. 2000). Interestingly, microbial community analysis showed a decrease in the abundance of inoculated strains within the biofilm in favour of invading bacteria (von Canstein et al. 2002). The same study also showed that overall diversity was dictated by the selective pressure of mercury contamination, where diversity was lowest at the influent end of the bioreactor and overall diversity lowered during times of higher mercury influent concentrations.
The treatment of Hg-contaminated environments is also a potentially useful in situ bioremediation application. However, due to the complexities of the Hg biogeochemical cycle, fewer studies have used environmental samples. A study of note used an Hg-resistant isolate, Aeromonas hydrophila KT20 obtained from an Hg-contaminated lake, to inoculate microcosms containing contaminated lake water (Saouter et al. 1995). This caused an increase in the flux of Hg(0) gas produced, which was taken as a proxy for microbial Hg(II) reduction. However, this only accounted for the removal of 5 % of the Hg input. The genetic engineering of strains to express or overexpress mer detoxification proteins has also been cited as a possible strategy to improve removal efficiency of microbes (Brim et al. 2000), although environmental applications are limited due to tight regulations on release of genetically engineered microbes (Lloyd et al. 2003). One study in this area focused on genetically engineering the radiation-resistant bacterium, Deinococcus radiodurans, for use in remediation of sites co-contaminated with radionuclides and Hg (Brim et al. 2000). This strain contained the cloned Hg resistance gene merA and was able to reduce Hg(II) to Hg(0) at high concentrations, even when within a highly irradiated environment.
It should also be noted that some metal-reducing bacteria (Kerin et al. 2006) and a wide variety of sulphate-reducing bacteria (King et al. 2000) can also be responsible for production of highly toxic MeHg. Indeed, this is believed to be a key source of MeHg within anoxic sediments (Compeau and Bartha 1985).
3.3 Reduction of V(V)
Vanadium is a relatively abundant transition metal used primarily in the metallurgy industry. Its accumulation to problematic concentrations is commonly associated with mining of titaniferous magnetite deposits (Teng et al. 2006), and its release from the combustion of oil fuels (Costigan and Cary 2001).
The environmental chemistry of vanadium is comparatively complex, existing in three valence states; V(III), V(IV) and V(V), with a variety of complexes, ion pairs and polymers associated with each (Wanty and Goldhaber 1992). Generally, in environmental systems oxic dissolution of vanadium results in the oxidation of V(III) and V(IV) to V(V), which exists primarily as soluble vanadate (Wanty and Goldhaber 1992). Vanadium is an essential trace nutrient in many organisms (French and Jones 1993) and the human health risks of exposure primarily focus upon respiratory tract problems as a result of inhalation (Costigan and Cary 2001). Acute toxicology studies in rats and mice have shown both V(IV) and mobile V(V) compounds to be toxic to mammals when administered intravenously and orally (Llobet and Domingo 1984).
Remediation of mobile, toxic V(V) focuses upon stabilisation via reductive precipitation to the less mobile V(IV). Enzymatic microbial reduction was noted as early as 1962 where the vanadate (V) anion was reduced, with H2 as the electron donor, to the vanadyl (IV) cation by Micrococcus lactilyticus (Woolfolk and Whiteley 1962). More recent studies have identified vanadium-resistant microbes able to reduce vanadate(V), even when exposed to high concentrations of up to 16 mM (Bisconti et al. 1997). Reduction coupled to growth, with V(V) as the sole electron acceptor, has been reported in the anaerobic metal reducers G. metallireducens (Ortiz-Bernad et al. 2004b) and S. oneidensis MR-1 (Carpentier et al. 2005; Carpentier et al. 2003). For the MR-1 strain, reduction was mediated by an electron transfer chain including the outer membrane cytochromes OmcA and OmcB, the periplasmic CymA and the cytoplasmic menaquinone (Myers et al. 2004). It should be noted however that these are also required for the reduction of other transition metals, and are not necessarily specific to V(V) reduction (Carpentier et al. 2005). The study of V(V) reduction by G. metallireducens presented evidence of in situ V(V) reduction at the Rifle, Colorado field site (Ortiz-Bernad et al. 2004b). The electron donor acetate was injected into groundwater containing up to 50 μM soluble V(V). Within 39 days of injection, some downstream monitoring wells recorded V(V) concentrations below detection limits, while others fell to below 6 μM V(V). The injection of acetate caused a dramatic increase in the proportion of microbes of the Geobacteraceae family, inferred to be responsible for either direct enzymatic V(V) reduction or indirect reduction via biogenic Fe(II). Recently, the V(V)-reducing bacterium Enterobacter cloacae EV-SA01 was isolated from a South African deep gold mine, suggesting a widespread occurrence of bacteria capable of V(V) reduction (van Marwijk et al. 2009).
3.4 Reduction of Co(III)
The radioactive isotope 60Co occurs as a contaminant at several US Department of Energy sites, typically, with organic chelates such as EDTA (Means et al. 1978). This can be in the form of the Co(II) or Co(III) valence states, although the Co(III)-EDTA complex is more stable in the environment (Blessing et al. 2001). Despite the short half-life of 60Co (5.27 years), it still causes concern as Co(III)-EDTA is highly mobile and can quickly migrate in the subsurface, outside site boundaries (Hau et al. 2008). Reduction to Co(II)-EDTA can be a favourable remediation reaction as it dissociates, releasing ionic Co(II) which readily sorbs to iron oxides (Gorby et al. 1998).
Dissimilatory reduction of the Co(III)-EDTA complex coupled to growth by the Fe(III)-reducing bacterium G. sulfurreducens was first observed in (Caccavo et al. 1994). Since this study, other bacteria exhibiting Co(III)-EDTA reduction have been studied; S. alga (Gorby et al. 1998), S. oneidensis (Hau et al. 2008) and Desulfovibrio vulgaris (Blessing et al. 2001). Reduction by D. vulgaris was noted not to be related to growth, and was inhibited in the presence of sulphate, although abiotic reduction can take place via the formation of sulphides (Blessing et al. 2001). The presence of Mn(IV) oxides can also prevent effective Co immobilisation, as it was shown to re-oxidise Co(II)-EDTA to Co(III)-EDTA (Gorby et al. 1998). The common occurrence of Mn(IV) oxides within the subsurface could have implications for any in situ bioremediation attempts, although it should be noted that they are readily reduced by many metal-reducing bacteria.
The mechanism of Co(III)-EDTA reduction was investigated for S. oneidensis (Hau et al. 2008). This study demonstrated, using mutants, that the Mtr extracellular respiratory pathway was important for Co(III) reduction. Specific gene deletions showed the greatest loss in reduction when the periplasmic c-type cytochrome MtrA and the outer membrane protein MtrB were not expressed. Interestingly this study also found that Co(II)-EDTA had a profound toxic effect upon S. oneidensis.
3.5 Reduction of Pd(II)
The enzymatic reduction of soluble Pd(II) to nanoparticulate Pd(0) can be used to remove Pd from solution, while making a powerful catalyst for contaminant remediation (Lloyd et al. 2003). Synthetic nano-Pd(0), typically supported on a substrate, has received much attention as a catalyst capable of reducing a variety of organic and inorganic contaminants (Zhang 2003). When supplied with an electron donor, typically H2 gas or formate, nano-Pd(0) can maintain high levels of reductive reactivity (Kopinke et al. 2004).
Conventional Pd(0) synthesis techniques utilise toxic chemicals and can be prohibitively expensive (De Corte et al. 2011). Therefore, biogenic methods provide a more cost effective and environmentally benign alternative (Lloyd et al. 2010). Pd is a highly valuable commodity and its extensive industrial uses, as catalysts, means the market for recovering used Pd is large (Lloyd et al. 2003). Early work focused upon harnessing the metal-reducing potential of sulphate-reducing bacteria to reduce and remove Pd(II) from solution (Lloyd et al. 1998). In this study, enzymatic Pd(II) reduction to nanoparticulate cell surface bound Pd(0) was observed by cells of Desulfovibrio desulfuricans utilising pyruvate, formate or H2 as the electron donor. The use of H2 as an electron donor, the inhibition of activity by Cu(II) and the periplasmic deposition implicated hydrogenase and possibly cytochrome c 3 activity in this reduction.
Later studies, also using sulphate-reducing bacteria, found that in the absence of an added electron donor, an initial Pd(II) biosorption step occurred (Yong et al. 2002b). Subsequent electron donor additions, as formate, initiated reduction and precipitation to Pd(0) at an optimal acidic pH. During these studies, it is believed that the cell acts as a convenient nucleation site for metal precipitation on to the cell (Yong et al. 2002a). Later work expanded upon these findings, using another sulphate reducer, Desulfovibrio fructosivorans (Mikheenko et al. 2008). This study compared nucleation sites of three hydrogenase-deficient mutant strains to a wild-type bacterium, which has both periplasmic hydrogenases and cytoplasmic membrane-bound hydrogenases. In the wild-type strain, Pd(0) nucleation occurred in both the cytoplasmic membrane and the periplasm, whereas in the periplasmic hydrogenase-deficient strain nucleation sites were confined to the cytoplasm. These data implicated both cytoplasmic and periplasmic hydrogenases in Pd(0) reduction and nucleation. The cell-supported nano-Pd(0) formed had varying morphological and catalytic properties, dependent upon the bioreduction/biosynthesis methodology. This was compared for bio-synthesised Pd(0) catalysts by two differing bacterial species; D. desulfuricans and Rhodobacter sphaeroides (Redwood et al. 2007). The D. desulfuricans exhibited a higher frequency of nucleation sites, thus precipitating larger quantities of smaller Pd(0). These smaller Pd(0) particles recorded higher catalytic activity, measuring H2 release, upon reaction with hypophosphite. Both of the cell types supported bio-Pd(0) particles with higher catalytic activity than a synthetic analogue. A study, using the metal reducer S. oneidensis, showed that cell distribution and particle area of the Pd(0) was controlled by the Pd(II) concentration cell ratio (De Windt et al. 2006). Interestingly, this study found that the larger particles were more effective for polychlorinated biphenyls (PCB) degradation, while smaller dispersed particles were optimised for perchlorate degradation.
Alongside direct enzymatic Pd(0) synthesis, a novel two-step biologically mediated synthesis technique was outlined in (Coker et al. 2010), yielding a nano-scale magnetically recoverable catalyst. Initially, a biomagnetite carrier was synthesised via the reduction of ferrihydrite, using cell suspensions of G. sulfurreducens. The resulting reactive bio-magnetite was then added to a solution of NaPd(II)Cl4, where rapid reductive precipitation of Pd(0) occurred on the surface of the biomagnetite nanoparticles. The magnetically recoverable Pd(0) catalyst has potential for industrial synthesis reactions (e.g. Heck coupling) and for contaminant remediation (Table 1).
The resulting biogenic Pd(0) catalysts have been used to demonstrate a variety of reductive, dehalogenation and hydrogenation reactions (Lloyd et al. 2010). These have targeted a variety of organic contaminants including; PCBs (Baxter-Plant et al. 2003; De Windt et al. 2006; Harrad et al. 2007), flame retardant polybrominated diphenyl ether (PBDE) (Harrad et al. 2007; Deplanche et al. 2009), lindane (Mertens et al. 2007), trichloroethylene (TCE) (Hennebel et al. 2009b; Hennebel et al. 2009a; Hennebel et al. 2011) and the inorganic contaminant; Cr(VI) (Beauregard et al. 2010; Chidambaram et al. 2010).
Recent studies have focussed upon the development of remediation applications for bio-Pd(0), and several novel technologies have been proposed. Two studies, (Mertens et al. 2007, Hennebel et al. 2009a), utilised S. oneidensis-derived, cell-supported Pd(0) in membrane reactors to, respectively, dechlorinate lindane and dehalogenate TCE. The bio-Pd(0) was encapsulated within dialysis membranes and the contaminant stream cycled through the reactor as a treatment for wastewaters. Both studies observed efficient degradation with both formate and H2 gas as the hydrogen donor, with H2 exhibiting stronger catalytic activity for TCE degradation.
An alternative TCE wastewater treatment process incorporated a fixed bed reactor which encapsulated bio-Pd(0), also from S. oneidensis, within a variety of polymer and silica beads or supported on zeolites (Hennebel et al. 2009b). Reaction rates varied dependent upon porosity of the support, with the zeolite recording highest degradation rates. All however were substantially lower than those recorded for bio-Pd(0) suspensions and approximately 50 % of the aforementioned TCE membrane reactor, although the fixed bed reactor is thought to be a more cost-effective method. The fixed bed reactor technique was also used, in a modified form, as a support for a biofilm supported Pd(0) (Beauregard et al. 2010). A matrix of polyurethane foam disks were colonised with Serratia sp. bacteria in an air-lift fermenter and subsequently fitted into glass columns along with un-colonised controls. The column was then charged with Pd(II) solution and supplied with H2 gas as the electron donor to promote reductive precipitation to Pd(0). After charging the bio-Pd(0) films with formate, Cr(VI) reduction was monitored in batch and continuous flow experiments. This was then monitored by UV–visible spectroscopy to quantify Cr(VI) concentration in solution and magnetic resonance imaging (MRI) to visualise the reduced Cr(III) in the columns. Both flow methods observed efficient reduction of Cr(VI) to Cr(III), although significant spatial heterogeneity was observed due to uneven bio-Pd(0) covering.
Few in situ remediation methods using BioPd have been reported, this is likely as a result of the high cost of Pd, which would be difficult to retrieve, and the difficulty of effective hydrogen supply to the subsurface (although formate could be a viable alternative). A notable exception provided a novel solution to hydrogen supply problems in the subsurface for the treatment of Cr(VI) detailed in (Chidambaram et al. 2010). Using the anaerobic metal-reducing and fermenting bacterium, Clostridium pasteurianum BC1, quartz sand was inoculated allowing a biofilm to coat the grains surface and precipitate cell supported Pd(0). Fermentation of a glucose medium by the bacterium subsequently produced an in situ hydrogen supply system. Reduction of Cr(VI) and immobilisation of reduced Cr(III) was confirmed by XANES and μX-ray Fluorescence (μXRF) analysis and effluent conditions showed efficient Cr(VI) removal compared to controls.
3.6 Reduction of Se(VI), Se(IV) and Se(0)
The metalloid, Se, can occur naturally in soils and waters at varying concentrations, ranging from “selenium deficient” to “seleniferous” (Garbisu et al. 1996). Alongside naturally occurring problematic high concentrations of Se, in seleniferous soils, anthropogenic activities can concentrate Se through processes such as agricultural irrigation, combustion and processing of fossil fuels (Haygarth 1994). Well-known examples are in the agricultural regions of California, where irrigation waters mobilize Se and transfer elevated concentrations to surface waters (Presser and Ohlendorf 1987).
Under environmental conditions, Se occurs in the following valence states; Se(VI), Se (IV) and Se(0) (Dungan and Frankenberger 1999). The former two species predominantly form the soluble and toxic anions; Se(VI)O4 2− and Se(IV)O3 2−, while Se(0) is insoluble (Masscheleyn et al. 1990), thus making reductive stabilization to Se(0) and further to Se(-II) (selenide) a desirable remediation reaction (Lenz and Lens 2009). It should be noted however that re-oxidation and re-solubilization of Se(0) has been recorded indicating reductive precipitation is not a long-term solution (Losi and Frankenberger 1998).
Se uptake concentrations in animals are important, due to its requirement as an essential element; deficiency can cause a variety of severe health problems (Lenz and Lens 2009). An excess of Se, however, can also cause severe health problems, including dermal or neurological effects. Like other toxic oxyanions this is believed to be related to oxidative stress upon the cell (Lenz and Lens 2009). Toxicity is particularly a problem in livestock where bioaccumulation in feedstock can cause severe Se poisoning symptoms (Spallholz 1994). The effect of elevated Se levels has also been widely reported at the ecosystem level, in particular the bioaccumulation and poisoning of wildlife in the Kesterson area of California, resulting from highly seleniferous geology (Presser 1994).
The reduction of Se(VI) and Se(IV) oxyanions to particulate Se(0) and Se(-II) by bacteria has long been established in both pure cultures and environmental samples (Oremland et al. 1989). Reduction can be due to a detoxification strategy or coupled to growth. The dissimilatory reduction of Se oxyanions is expressed in a variety of bacteria (Dungan and Frankenberger 1999). A study using a facultative isolate from seleniferous agricultural wastewater, identified as E. cloacae, was able to reduce SeO4 2− via the intermediary SeO3 2− to nanoparticulate Se(0) (Losi and Frankenberger 1997). This study indicates intracellular reduction, alongside NO3 − reduction, by membrane bound reductases followed by expulsion of the Se(0) nanoparticle. The intensively studied organisms S. oneidensis and G. sulfurreducens have been shown to exhibit the capacity for Se(IV) reductions (Pearce et al. 2009). This study demonstrated different mechanisms for reduction between the two metal reducers, resulting in different end products of metabolism, whereby G. sulfurreducens was able to reduce Se(IV) to Se(0), and then further to Se(-II) while S. oneidensis could only reduce Se(IV) to nanoparticulate Se(0) phases. This study also implicated the involvement of c-type cytochromes and ferredoxin in the formation of Se(0) nanoparticles. Further reduction of Se(0) to Se(-II) is noted in other bacterial strains such as; Bacillus selenitireducens (Herbel et al. 2003) and Veillonella atypical (Pearce et al. 2008). The former of these studies found when incubated with seleniferous sediments the end product of bacterial reduction was as solid precipitate of FeSe as opposed to soluble HSe−, indicating a possibly in situ treatment application for the organism. The latter study showed that biogenic selenide, after reaction with transition metals, could be used to synthesize quantum dots for photonic applications. Ex situ bioreactor treatment using the Se-reducing organism Bacillus sp. SF-1 has also been explored (Fujita et al. 2002). This study demonstrated reduction of Se(VI), via Se(IV), to Se(0) in a synthetic waste-stream by the organism, using lactate as an electron donor.
4 Enzymatic Reduction of Actinides and Fission Products
Human exposure to radiation is principally from naturally occurring radionuclides. There is, however, public concern regarding exposure as a result of anthropogenic activities such as nuclear weapons testing and nuclear power generation (MacKenzie 2000). Currently, nuclear power activities are the principal anthropogenic release of radionuclides (Lloyd et al. 2002). Several processes in the nuclear fuel cycle result in localised radionuclide release alongside concerns over the resulting radioactive waste generated (Macaskie 1991). Much public concern regarding nuclear power generating processes is as a result of high profile and large-scale accidents such as the 1986 Chernobyl and 2011 Fukushima disasters.
The bioremediation of radioactive waste has also received much attention. In common with many transition metal, several key radionuclides including both actinides and fission products such a technetium are redox active, and become less soluble in their reduced state (Lloyd 2003); see Fig. 4 for a graphical representation of key oxidation states of key priority radionuclides. Microbial reduction has therefore been the focus of several bioremediation strategies (Lloyd 2003).
Oxidation states of key contaminant actinides and fission products addressed within this chapter, presented as standard reduction potentials at pH 7. A redox tower of common metabolic processes is located on the right for comparison. Adapted from (Silva and Nitsche 1995; Lloyd et al. 2002; Stumm 1996; Banaszak et al. 1999)
4.1 Reduction of U(VI)
Uranium is a common radionuclide contaminant occurring at sites associated with various aspects of the nuclear fuel cycle. Under environmental conditions it most commonly occurs as the U(IV) and U(VI) oxidation states. The former occurring primarily as poorly soluble U(IV) oxides and the latter as widely soluble U(VI) carbonate complexes, for example UO2(CO3) 2−2 and UO2(CO3) 4−3 (Clark et al. 1995). Reduction can therefore lead to immobilisation of uranium and could offer a useful remediation mechanism, preventing migration of U(VI) to groundwater resources.
The accumulation of uranium within anaerobic environments had previously been explained by indirect reduction mechanisms, for example linked to the microbial production of reducing agents such as Fe(II) or sulphide (Lovley et al. 1991). Direct enzymatic microbial U(VI) reduction coupled to growth was first demonstrated for the dissimilatory metal reducers G. metallireducens and S. oneidensis, with acetate and H2 as the respective electron donors (Lovley et al. 1991). This work was soon followed by the demonstration of U(VI) reduction using the sulphate-reducing bacteria, D. desulfuricans, (Lovley and Phillips 1992) and D. vulgaris (Lovley et al. 1993). Although these sulphate reducers were unable to conserve energy for growth in these experiments, U(VI) did rapidly transform to U(IV), forming poorly soluble uraninite (UO2). Importantly enzymatic reduction occurred faster than abiological mechanisms, suggesting an environmentally relevant role for enzymatic U(VI) reduction (Lovley et al. 1991). The mechanism of enzymatic U(VI) reduction in D. vulgaris was shown to be mediated by cytochrome c 3 (which is also a Cr(VI) reductase), as reductive capability was lost when this electron transfer protein was removed from the soluble fraction of cells via a cationic exchange column (Lovley et al. 1993). The mechanism of U(VI) reduction by G. sulfurreducens seems to be more complicated, with several of the many cytochromes in this organism implicated, including those in the periplasm (Lloyd et al. 2002) and outer membrane (Shelobolina et al. 2007), while a recent report has suggested that extracellular nanowires could also be involved (Cologgi et al. 2011). Whatever the precise mechanism of electron transfer to U(VI) in this important model organism, reduction most likely proceeds via a single electron transfer to form an unstable U(V) intermediate, which then disproportionates to give U(IV) (Renshaw et al. 2005).
Several studies have addressed the complex behaviour of U(VI) within environmentally relevant substrates. A study using a variety of synthetic and natural Fe(III) oxides and the U(VI) reducer G. sulfurreducens found that U(VI) reduction was slower in the presence of natural Fe(III) oxide phases (Jeon et al. 2004), suggesting that at circumneutral pH, aqueous U(VI) can be removed by reduction, while the adsorbed fraction can remain and potentially desorb over time if under non-reducing conditions (Jeon et al. 2004). These findings were supported by experiments with U(VI) contaminated sediments, where the rate of microbial reduction of aqueous U(VI) was far greater than that of adsorbed U(VI) (Ortiz-Bernad et al. 2004a). Studies using sediments and waters collected from near a uranium mill tailings pile, located in Shiprock, New Mexico, also found that under anaerobic conditions addition of acetate or glucose stimulated reduction from 10 μM soluble U(VI) to <1 μM U(VI) within 15 days (Finneran et al. 2002). This reduction was considered enzymatic, as addition of the abiotic reductants; Fe(II), sulphide and reduced AQDS, did not reduce U(VI) in the sediments. Using the same sediments, 16S rDNA analysis found that acetate amendment, and also Fe(III) and U(VI) reduction, corresponded to an increase in the proportion of sequences belonging to the family Geobacteraceae, to ca. 40 % (in comparison to 5 % in un-amended controls; (Holmes et al. 2002)). These studies support the hypothesis that bacteria of the family Geobacteraceae can, via electron donor addition, enzymatically reduce and remove aqueous U(VI) from contaminated soils and sediments.
A significant number of studies have looked at the possible bioremediation of U(VI) via in situ enzymatic reduction at U.S. Department of Energy (U.S. DOE) sites (Roh et al. 2000). One such site, at Oak Ridge, Tennessee, has extensive U(VI) contamination with levels up to 60 mg/L U(VI) in groundwater and 800 mg/kg in sediments (Cardenas et al. 2008). The groundwater conditions in some parts of the site present challenges to in situ bioremediation due to an acidic pH, ~3.4, high concentrations of clogging agents such as Al and Ca, and U(VI) reduction inhibitors (Wu et al. 2006a). Therefore, prior to bioremediation tests, a pre-treatment groundwater recirculation system was established consisting of a series of pumps and wells, detailed in (Wu et al. 2006a). This enabled pumping and ex situ treatment of groundwater to remove the problematic components and buffer the pH to a circumneutral value. It should be noted during groundwater pre-treatment, the U(VI) concentration fell from ~300 to ~5 μM linked to neutralisation and subsequent uranium precipitation. Ethanol was chosen as the electron donor for in situ biostimulation, as bench experiments indicated faster U(VI) reduction than with other electron donors (Wu et al. 2006b). The results of this trial, presented in (Wu et al. 2006b), show that periodic additions of ethanol and low level carbonate to desorb U(VI), over an approximately 400 day period, first resulted in a denitrification phase followed by U(VI) reduction. Groundwater U(VI) concentrations fell from 5 to ~1 μM. The proportion of uranium as reduced U(IV), determined by XANES analysis of sediment samples, increased from below detection limit prior to treatment to 51 %, 35 % and 28 % after treatment for the injection, monitoring and extraction wells, respectively. Further studies of this treatment area note that after 2 years of operation the uranium levels fell below the U.S. EPA drinking water maximum of 0.126 μM (Cardenas et al. 2008, Xu et al. 2010). Treated wells showed an increase in denitrifiers, sulphate and iron-reducing bacteria compared to untreated wells from 16S rRNA analysis (Cardenas et al. 2008). Specific U(VI)-reducing bacteria were identified including Geobacter spp., Anaeromyxobacter spp., Desulfovibrio spp., Desulfosporosinus spp. and Acidovorax spp. A subsequent study carried out functional gene array (GeoChip) analysis of sediment from the electron donor treated and untreated wells (Xu et al. 2010). Treated wells recorded higher total signal intensities of functional gene categories, including those associated with U(VI) reduction; e.g. cytochrome c and metal resistance genes. The sum of these studies at the Oak Ridge site have given a deeper insight into the complexities of in situ U(VI) bioremediation projects.
A further extensive field study focuses on a U(VI)-contaminated aquifer at the former uranium ore processing facility in Rifle, Colorado (Anderson et al. 2003). In situ biostimulation treatment of the U(VI) groundwater plume, described in detail in (Anderson et al. 2003, Williams et al. 2011), was assessed by a series of 15 monitoring wells downgradient and untreated control wells upgradient of the injection wells. To stimulate microbial activity the electron donor acetate (1–3 mM) was injected into the groundwater plume. Within 9 days of injection, aqueous U(VI) concentrations had fallen from 0.6–1.2 μM to below the target limit of 0.18 μM in some of the wells, coinciding with an increase in Fe(II). However, it was noted that after 50 days U(VI) concentrations began to increase again, and Fe(II) levels decreased. This initial study also correlated U(VI) removal and Fe(II) production with an increase in bulk biomass and Geobacter species abundance. The authors inferred that Geobacter species were predominantly responsible for enzymatic U(VI) and Fe(III) reduction. It should be noted the later increase in U(VI) correlates with a period of sulphate reduction where sulphate reducers dominate the microbial ecology of the study site, suggesting these are not able to reduce U(VI) in these sediments. Interestingly lactate (but not acetate)-dependent sulphate reducers are known to reduce U(VI). A further study used a more detailed sampling arrangement, including depth profiles, of the same field sampling array (Vrionis et al. 2005). This study reported greater heterogeneity of the geochemistry and microbial ecology than previously demonstrated with areas of high U(VI) reduction occurring at disparate areas of the array. Again, increased representation of Geobacter 16S rRNA gene sequences correlated with areas of maximal U(VI) removal and Fe(II) production. A more recent study has applied proteomic monitoring of the bacterial species at this site (Wilkins et al. 2009). Peptide analyses of planktonic organisms in the areas, where Geobacter species dominated, were compared to those expected from those encoded by seven Geobacter genome sequences. Closest matches were to Geobacter bemidjiensis proteins, while data suggested that other species closely related to Geobacter lovleyi became dominant during later stages of the field trial. The proteome of the samples, throughout acetate addition, were dominated by enzymes related to acetate metabolism and energy generation and thus inferred to represent microbial proliferation and growth linked with acetate-driven U(VI) and Fe(III) reduction. The series of studies at this site again depict the complexities encountered during in situ biostimulation remediation of U(VI), and have enabled greater knowledge of how to approach such projects over prolonged periods of U(IV) bioreduction.
4.2 Reduction of Tc(VII)
Technetium is a product of nuclear fission with appreciable amounts produced during nuclear weapon tests and as a by-product of the nuclear fuel cycle (Schulte and Scoppa 1987). It occurs predominantly as the β-emitting 99Tc isotope which due to its long half-life, 2.15 × 105 years, is an important component of high and medium level wastes (Schulte and Scoppa 1987). Within most oxic environmental systems, technetium occurs in the Tc(VII) valence state as the pertechnetate anion (TcO4 −). Pertechnetate is highly soluble in water and exhibits poor adsorption to mineral surfaces, making it highly mobile in the subsurface where its migration can contaminate groundwater resources (Icenhower et al. 2008). However, technetium under reducing conditions usually exists as relatively insoluble Tc(IV), as less soluble hydrated TcO2 or mineral surface adsorbed Tc(IV) (Lear et al. 2009).
Early studies recorded a removal of Tc(VII) from solution under anaerobic conditions in the presence of mixed bacterial cultures (Henrot 1989) or sediments (Pignolet et al. 1989). These were, however, unable to identify the mechanism of removal. Direct enzymatic microbial reduction of Tc(VII) was later confirmed in cultures of G. metallireducens and S. oneidensis (Lloyd and Macaskie 1996). In this study G. metallireducens was able to remove 70 % Tc(VII) from a ~0.27 mM solution within 22 h. S. oneidensis was able to reduce 60 % of the Tc(VII), but the reduced form in cultures of this organism were largely soluble under the conditions tested. Both S. oneidensis (Wildung et al. 2000) and another Geobacter spp.; G. sulfurreducens (Lloyd et al. 2000a) respectively, exhibited optimal Tc(VII) reduction capacity when H2, (rather than lactate or acetate), was used as the electron donor. The fate of the reduced Tc(IV) in the former study consisted of a dark Tc(IV) oxide associated with the periplasm and cell surface, although when in bicarbonate medium, extracellular particulates and possible carbonate complexes formed. The latter study used transmission electron microscopy (TEM) and XAS to confirm Tc(VII) reduction to Tc(IV) as TcO2, see Fig. 5. In this latter study, very rapid and efficient reduction of Tc(VII) mediated by biogenic Fe(II) e.g. associated with magnetite, was also demonstrated. Given the very efficient scavenging of Tc(VII) by biogenic Fe(II), and the less efficient enzymatic (hydrogenase mediated) mechanism (Lloyd et al. 1997a, b, 1999, 2000a), many subsequent studies have focused on indirect mechanisms of Tc(VII) reduction. These include studies on aquifer sediments, collected from the Oak Ridge Field Research Centre (McBeth et al. 2007), estuarine sediments (Burke et al. 2005) and model Fe(II) biomineral phases (McBeth et al. 2011). The clear involvement of Fe(III)-reducing bacteria in Oak Ridge sediments has also been confirmed using novel nuclear imaging techniques, in combination with molecular ecology and mineralogical approaches (Lear et al. 2009).
TEM images of thin sections of G. sulfurreducens with Tc-containing precipitates. a An electron dense periplasmic Tc precipitate formed via direct enzymatic reduction. b EDX spectrum associated with the periplasmic precipitate in a. c TEM image of air dried whole-cell preparations with extracellular Tc-containing magnetite crystals via indirect Fe(II) mediated Tc(VII) reduction. d EDX spectrum of the annotated area in C, showing Tc presence on the magnetite. Bars 0.5 μm. Diagram adapted from (Lloyd et al. 2000a)
4.3 Reduction of Np(V)
Neptunium (Np), like Tc, has a long half-life (2.14 × 106 years) and is considered an important radioactive component of high and intermediate level wastes over geological timespans (Sasaki et al. 2001). This is primarily due to the isotope 237Np which increases in proportion within wastes due to the radioactive decay of the short lived isotope 241Am (half-life = 423.7 years) (Kaszuba and Runde 1999). 237Np is a key α-emitting isotope and is regarded as chemically and radiologically toxic to life (Ruggiero et al. 2005). Under most environmental conditions Np is stable as the Np(IV) and Np(V) valence states (Kaszuba and Runde 1999). Np(V) occurs primarily in oxic conditions, as the soluble NpO2 + species which is highly mobile in the subsurface as it sorbs poorly to mineral phases (Kaszuba and Runde 1999). By contrast Np(IV) species, which dominate in reducing conditions, are less soluble and can form complexes which are easily adsorbed to mineral phases (Law et al. 2010). Thus, a reduction from Np(V) to Np(IV) potentially leads to immobilisation of Np within the subsurface.
Direct enzymatic reduction of soluble Np(V) species has been demonstrated for the metal-reducing bacterium S. oneidensis (Lloyd et al. 2000b; Icopini et al. 2007). The former study, using paper chromatography with phosphorimaging techniques for Np oxidation state separation, suggested Np(V) to Np(IV) bioreduction after inoculation with S. oneidensis, with H2 as the electron donor. The majority of the resulting Np(IV) remained in solution, but in combination with an extracellular phosphate producing Citrobacter sp., 95 % of Np(IV) was removed, presumably as an Np(IV) phosphate. Then in the latter study (Icopini et al. 2007), however, S. oneidensis was able to simultaneously reduce and precipitate Np(IV) from a Np(V) solution, using lactate as the electron donor. The same study failed to replicate this reduction with the bacterium G. metallireducens, and it has been shown previously that G. sulfurreducens is also unable to reduce Np(V) (Renshaw et al. 2005). G. metallireducens did show Np reduction when Np(V) was present as a citrate complex, which was inferred to reduce its toxic effects upon the cell. A further study was able to reduce and precipitate Np(V) to Np(IV), utilising either pyruvate or H2 as electron donors (Rittmann et al. 2002). This was achieved using a consortium of bacteria, containing several sulphate reducers of the Desulfovibrio genus, isolated from creek sediments. When pyruvate was used as the electron donor, complexation of Np(IV) with fermentation intermediates was suggested to prevent complete precipitation. The contribution of enzymatic Np(V) reduction to total reduction within complex environmental media, such as sediments, is difficult to determine. A recent study tackled this challenge and attributed reduction in sediment microcosms to abiotic reduction by Fe(II) coupled to microbial Fe(III) reduction (Law et al. 2010).
4.4 Reduction of Pu(V) and Pu(VI)
The release of Pu into the environment is of particular concern due to its long half-life (2.4 × 104 years) and its high toxicity (Boukhalfa et al. 2007). Its biogeochemical behaviour is more complex than the other actinides as it can occur in the environment as four valence states; Pu(III), Pu(IV), Pu(V) and Pu(VI) (Panak and Nitsche 2001). The oxidised forms, Pu(V) and Pu(VI), generally occur as relatively more soluble species than the Pu(IV) species (Neu et al. 2006). The solubility of Pu(III) however is often controlled by the presence of complexing ligands which increase its solubility (Icopini et al. 2009). The action of humics in natural systems can also reduce Pu(V) and Pu(VI) to Pu(IV) thus effectively decreasing the solubility of Pu (Choppin et al. 2001). The Pu(IV) valence state is easily hydrolysed, causing adsorption to particles and generally maintains its low solubility at circum-neutral pH (Choppin et al. 2001).
Due to the complexities of Pu chemistry only a few studies have approached microbial Pu reduction (Neu et al. 2006). These have focussed upon the intensely studied metal-reducing bacteria of S. oneidensis (Boukhalfa et al. 2007; Icopini et al. 2009; Renshaw et al. 2009), S. alga (Reed et al. 2007; Deo et al. 2011), G. metallireducens (Boukhalfa et al. 2007; Icopini et al. 2009) and G. sulfurreducens (Renshaw et al. 2009). Enzymatic reduction of higher valence Pu(V) and Pu(VI) to Pu(IV) was observed for S. oneidensis and G. metallireducens (Icopini et al. 2009). The former exhibiting the highest reduction rates with near complete reduction of ~0.4 mM within 5 h using a cell density of 5 × 108 cells/ml. The resulting Pu(IV) formed a crystalline nanoparticulate phase on the surface or within the cell walls of the bacteria thus immobilising Pu from solution. Importantly, no further reduction to the more soluble Pu(III) was noted. A further study demonstrated reduction of Pu(V) (PuO2 +) via a Pu(IV) intermediate to Pu(III) as a PuPO4 precipitate, using the bacterium S. alga (Deo et al. 2011). This was carried out in the presence and absence of Fe(III) and a nitrilotriacetic acid (NTA) ligand, although at higher NTA concentrations, some soluble Pu(III) complexes were observed. Reduction of Pu(VI) to Pu(IV) by G. sulfurreducens concurrent with a decrease of aqueous Pu has also been recorded (Renshaw et al. 2009). This study also noted limited further reduction to Pu(III), by G. sulfurreducens and S. oneidensis. This however did not cause an increase in the soluble Pu fraction, theorised to be the result of adsorption to the cell surface. Other studies have observed an increase in Pu solubility from the reduction of Pu(IV) to Pu(III) when in the presence of an EDTA ligand (Boukhalfa et al. 2007). This study however recorded no reduction of the amorphous Pu(IV)(OH)4 starting material in the absence of complexing agents.
Despite the potential to mobilise Pu(IV), few studies have addressed the impact of stimulated bioreduction strategies on Pu solubility in environmental systems. However, a recent study using Pu contaminated soils from the UK Atomic Weapons Establishment (AWE) site, Pu occurring as Pu(IV) proved highly refractory during the bioreducing conditions (Kimber et al. 2012). Thus in sediments contaminated with a broad-range of redox active radionuclides, reductive immobilisation of uranium, neptunium and technetium would be expected, without the mobilisation of any Pu(IV) present.
5 Conclusions
The role of microbial metal reduction in the bioremediation of environments contaminated with metals and organics is now well established. Underpinning investigations range from those addressing a molecular-scale understanding of microbial reduction to the complexities associated with field application. The emerging “omics” approaches to environmental microbiology, alongside other advanced analytical techniques, are increasingly being utilised to give greater insights into bioremediation processes. While many of these studies are currently at a “proof of concept” laboratory-scale, some notable exceptions have been implemented for field or industrial-scale problems. These can lead to the complete oxidation of toxic organics, to the irreversible reduction of toxic metals and radionuclides; see (Law et al. 2010; McBeth et al. 2007) for recent examples of reduced, insoluble radionuclide phases that are resistant to re-oxidation and re-mobilisation. It can be anticipated that further field-scale applications utilising microbial metal reduction as a bioremediation tool will emerge.
References
Ackerley DF, Gonzalez CF, Keyhan M, Blake R, Matin A (2004) Mechanism of chromate reduction by the Escherichia coli protein, NfsA, and the role of different chromate reductases in minimizing oxidative stress during chromate reduction. Environ Microbiol 6:851–860
Anderson RT, Vrionis HA, Ortiz-Bernad I, Resch CT, Long PE, Dayvault R, Karp K, Marutzky S, Metzler DR, Peacock A, White DC, Lowe M, Lovley DR (2003) Stimulating the in situ activity of Geobacter species to remove uranium from the groundwater of a uranium-contaminated aquifer. Appl Environ Microbiol 69:5884–5891
Bader JL, Gonzalez G, Goodell PC, Ali A-MS, Pillai SD (1999) Aerobic reduction of hexavalent chromium in soil by indigenous microorganisms. Bioremediation J 3:201–211
Banaszak J, Rittmann B, Reed D (1999) Subsurface interactions of actinide species and microorganisms: implications for the bioremediation of actinide-organic mixtures. J Radioanal Nucl Chem 241:385–435
Barak Y, Ackerley DF, Dodge CJ, Banwari L, Alex C, Francis AJ, Matin A (2006) Analysis of novel soluble chromate and uranyl reductases and generation of an improved enzyme by directed evolution. Appl Environ Microbiol 72:7074–7082
Barkay T, Miller SM, Summers AO (2003) Bacterial mercury resistance from atoms to ecosystems. FEMS Microbiol Rev 27:355–384
Barkay T, Wagner-Dobler I, Allen I, Laskin JWB, Geoffrey MG (2005) Microbial transformations of mercury: potentials, challenges, and achievements in controlling mercury toxicity in the environment. Adv Appl Microbiol 57. Academic Press
Baxter-Plant VS, Mikheenko IP, Macaskie LE (2003) Sulphate-reducing bacteria, palladium and the reductive dehalogenation of chlorinated aromatic compounds. Biodegradation 14:83–90
Beauregard DA, Yong P, Macaskie LE, Johns ML (2010) Using non-invasive magnetic resonance imaging (MRI) to assess the reduction of Cr(VI) using a biofilm–palladium catalyst. Biotechnol Bioeng 107:11–20
Belchik SM, Kennedy DW, Dohnalkova AC, Wang Y, Sevinc PC, Wu H, Lin Y, Lu HP, Fredrickson JK, Shi L (2011) Extracellular reduction of hexavalent chromium by cytochromes MtrC and OmcA of Shewanella oneidensis MR-1. Appl Environ Microbiol 77:4035–4041
Bisconti L, Pepi M, Mangani S, Baldi F (1997) Reduction of vanadate to vanadyl by a strain of Saccharomyces cerevisiae. BioMetals 10:239–246
Blessing TC, Wielinga BW, Morra MJ, Fendorf S (2001) CoIIIEDTA- reduction by Desulfovibrio vulgaris and propagation of reactions involving dissolved sulfide and polysulfides. Environ Sci Technol 35:1599–1603
Borch T, Inskeep WP, Harwood JA, Gerlach R (2005) Impact of ferrihydrite and anthraquinone-2,6-disulfonate on the reductive transformation of 2,4,6-trinitrotoluene by a gram-positive fermenting bacterium. Environ Sci Technol 39:7126–7133
Borch T, Kretzschmar R, Kappler A, Cappellen PV, Ginder-Vogel M, Voegelin A, Campbell K (2010) Biogeochemical redox processes and their impact on contaminant dynamics. Environ Sci Technol 44:15–23
Boukhalfa H, Icopini GA, Reilly SD, Neu MP (2007) Plutonium(IV) reduction by the metal-reducing bacteria Geobacter metallireducens GS15 and Shewanella oneidensis MR1. Appl Environ Microbiol 73:5897–5903
Brim H, McFarlan SC, Fredrickson JK, Minton KW, Zhai M, Wackett LP, Daly MJ (2000) Engineering Deinococcus radiodurans for metal remediation in radioactive mixed waste environments. Nat Biotechnol 18:85–90
Bunge M, Søbjerg LS, Rotaru A-E, Gauthier D, Lindhardt AT, Hause G, Finster K, Kingshott P, Skrydstrup T, Meyer RL (2010) Formation of palladium(0) nanoparticles at microbial surfaces. Biotechnol Bioengineering 107:206–215
Burke IT, Boothman C, Lloyd JR, Livens FR, Charnock JM, McBeth JM, Mortimer RJG, Morris K (2006) Reoxidation behavior of technetium, iron, and sulfur in estuarine sediments. Environ Sci Technol 40:3529–3535
Burke IT, Boothman C, Lloyd JR, Mortimer RJG, Livens FR, Morris K (2005) Effects of progressive anoxia on the solubility of technetium in sediments. Environ Sci Technol 39:4109–4116
Butler J, He Q, Nevin K, He Z, Zhou J, Lovley D (2007) Genomic and microarray analysis of aromatics degradation in Geobacter metallireducens and comparison to a Geobacter isolate from a contaminated field site. BMC Genomics 8:180
Caccavo F, Lonergan DJ, Lovley DR, Davis M, Stolz JF, McInerney MJ (1994) Geobacter sulfurreducens sp. nov., a hydrogen- and acetate-oxidizing dissimilatory metal-reducing microorganism. Appl Environ Microbiol 60:3752–3759
Cardenas E, Wu W-M, Leigh MB, Carley J, Carroll S, Gentry T, Luo J, Watson D, Gu B, Ginder-Vogel M, Kitanidis PK, Jardine PM, Zhou J, Criddle CS, Marsh TL, Tiedje JM (2008) Microbial communities in contaminated sediments, associated with bioremediation of uranium to submicromolar levels. Appl Environ Microbiol 74:3718–3729
Carmona M, Díaz E (2005) Iron-reducing bacteria unravel novel strategies for the anaerobic catabolism of aromatic compounds. Mol Microbiol 58:1210–1215
Carmona M, Zamarro MAT, Blãzquez B, Durante-Rodrã-Guez G, Juãrez JF, Valderrama JAS, Barragãn MAJL, Garcia JL, Diaz E (2009) Anaerobic catabolism of aromatic compounds: a genetic and genomic view. Microbiol Mol Biol Rev 73:71–133
Carpentier W, de Smet L, van Beeumen J, Briga A (2005) Respiration and growth of Shewanella oneidensis MR-1 using vanadate as the sole electron acceptor. J Bacteriol 187:3293–3301
Carpentier W, Sandra K, de Smet I, Briga A, de Smet L, van Beeumen J (2003) Microbial reduction and precipitation of vanadium by Shewanella oneidensis. Appl Environ Microbiol 69:3636–3639
Cervantes C, Campos-García J, Nies D, Silver S (2007) Reduction and efflux of chromate by bacteria molecular microbiology of heavy metals. Springer, Berlin/Heidelberg
Charlet L, Silvester E, Liger E (1998) N-compound reduction and actinide immobilisation in surficial fluids by Fe(II): the surface FeIIIOFeIIOH species, as major reductant. Chem Geol 151:85–93
Chen JM, Hao OJ (1998) Microbial chromium (VI) reduction. Crit Rev Environ Sci Technol 28:219–251
Chidambaram D, Hennebel T, Taghavi S, Mast J, Boon N, Verstraete W, van der Lelie D, Fitts JP (2010) Concomitant microbial generation of palladium nanoparticles and hydrogen to immobilize chromate. Environ Sci Technol 44:7635–7640
Childers SE, Ciufo S, Lovley DR (2002) Geobacter metallireducens accesses insoluble Fe(III) oxide by chemotaxis. Nature 416:767–769
Chirwa EMN, Wang Y-T (1997) Hexavalent chromium reduction by Bacillus sp. in a packed-bed bioreactor. Environ Sci Technol 31:1446–1451
Choppin GR, Morgenstern A, Kudo A (2001) Distribution and movement of environmental plutonium. In: Radioactivity in the Environment. Elsevier, Amsterdam
Christensen TH, Kjeldsen P, Bjerg PL, Jensen DL, Christensen JB, Baun A, Albrechtsen H-JR, Heron G (2001) Biogeochemistry of landfill leachate plumes. Appl Geochem 16:659–718
Clark DL, Hobart DE, Neu MP (1995) Actinide carbonate complexes and their importance in actinide environmental chemistry. Chem Rev 95:25–48
Clarkson TW (1997) The toxicology of mercury. Crit Rev Clin Lab Sci 34:369–403
Coates JD, Bhupathiraju VK, Achenbach LA, McLnerney MJ, Lovley DR (2001) Geobacter hydrogenophilus, Geobacter chapellei and Geobacter grbiciae, three new, strictly anaerobic, dissimilatory Fe(III)-reducers. Int J Syst Evol Microbiol 51:581–588
Coates JD, Phillips EJ, Lonergan DJ, Jenter H, Lovley DR (1996) Isolation of Geobacter species from diverse sedimentary environments. Appl Environ Microbiol 62:1531–1536
Coker V, Pattrick RAD, Van der Laan G, Lloyd JR (2006) Formation of magnetic minerals by non-magnetotactic prokaryotes. In: Steinbuchel A (ed) Magnetoreception and magnetosomes in bacteria, ed dirk shuler series; microbiology monographs, Springer, pp 275–300
Coker VS, Bennett JA, Telling ND, Henkel T, Charnock JM, van der Laan G, Pattrick RAD, Pearce CI, Cutting RS, Shannon IJ, Wood J, Arenholz E, Lyon IC, Lloyd JR (2010) Microbial engineering of nanoheterostructures: biological synthesis of a magnetically recoverable palladium nanocatalyst. ACS Nano 4:2577–2584
Cologgi DL, Lampa-Pastirk S, Speers AM, Kelly SD, Reguera G (2011) Extracellular reduction of uranium via Geobacter conductive pili as a protective cellular mechanism. Proc Natl Acad Sci 108:15248–15252
Compeau GC, Bartha R (1985) Sulfate-reducing bacteria: principal methylators of mercury in anoxic estuarine sediment. Appl Environ Microbiol 50:498–502
Costigan M, Cary R (2001) Vanadium pentoxide and other inorganic vanadium compounds. World Health Organization, Geneva, Switzerland
Coupland K, Johnson DB (2008) Evidence that the potential for dissimilatory ferric iron reduction is widespread among acidophilic heterotrophic bacteria. FEMS Microbiol Lett 279:30–35
Cutting RS, Coker VS, Fellowes JW, Lloyd JR, Vaughan DJ (2009) Mineralogical and morphological constraints on the reduction of Fe(III) minerals by Geobacter sulfurreducens. Geochim Cosmochim Acta 73:4004–4022
Cutting RS, Coker VS, Telling ND, Kimber RL, Pearce CI, Ellis BL, Lawson RS, van der Laan G, Pattrick RAD, Vaughan DJ, Arenholz E, Lloyd JR (2010) Optimizing Cr(VI) and Tc(VII) remediation through nanoscale biomineral engineering. Environ Sci Technol 44:2577–2584
Creamer NJ, Deplanche K, Snape TJ, Mikheenko IP, Yong P, Samyahumbi D, Wood J, Pollmann K, Selenska-Pobell S, Macaskie LE (2008) A biogenic catalyst for hydrogenation, reduction and selective dehalogenation in non-aqueous solvents. Hydrometallurgy 94:138–143
Dayan AD, Paine AJ (2001) Mechanisms of chromium toxicity, carcinogenicity and allergenicity: review of the literature from 1985 to 2000. Hum Exp Toxicol 20:439–451
de Corte S, Hennebel T, de Gusseme B, Verstraete W, Boon N (2011) Bio-palladium: from metal recovery to catalytic applications. Microbiol Biotechnol 5:5–17
de Windt W, Boon N, van den Bulcke J, Rubberecht L, Prata F, Mast J, Hennebel T, Verstraete W (2006) Biological control of the size and reactivity of catalytic Pd(0) produced by Shewanella oneidensis. Antonie Van Leeuwenhoek 90:377–389
Deo R, Rittmann B, Reed D (2011) Bacterial Pu(V) reduction in the absence and presence of Fe(III)–NTA: modeling and experimental approach. Biodegradation 22:921–929
Deplanche K, Snape TJ, Hazrati S, Harrad S, Macaskie LE (2009) Versatility of a new bioinorganic catalyst: palladized cells of Desulfovibrio desulfuricans and application to dehalogenation of flame retardant materials. Environ Technol 30:681–692
Dungan RS, Frankenberger WT (1999) Microbial transformations of selenium and the bioremediation of seleniferous environments. Bioremediation J 3:171–188
Elsner M, Schwarzenbach RP, Haderlein SB (2004) Reactivity of Fe(II)-bearing minerals toward reductive transformation of organic contaminants. Environ Sci Technol 38:799–807
Fendorf SE (1995) Surface reactions of chromium in soils and waters. Geoderma 67:55–71
Finneran KT, Anderson RT, Nevin KP, Lovley DR (2002) Potential for bioremediation of uranium-contaminated aquifers with microbial U(VI) reduction. Soil Sediment Contam Int J 11:339–357
Francis CA, Obraztsova AY, Tebo BM (2000) Dissimilatory metal reduction by the facultative anaerobe Pantoea agglomerans SP1. Appl Environ Microbiol 66:543–548
Fredrickson JK, Zachara JM, Kennedy DW, Kukkadapu RK, McKinley JP, Heald SM, Liu C, Plymale AE (2004) Reduction of TcO4 − by sediment-associated biogenic Fe(II). Geochim Cosmochim Acta 68:3171–3187
French RJ, Jones PJH (1993) Role of vanadium in nutrition: metabolism, essentiality and dietary considerations. Life Sci 52:339–346
Fujita M, Ike M, Kashiwa M, Hashimoto R, Soda S (2002) Laboratory-scale continuous reactor for soluble selenium removal using selenate-reducing bacterium, Bacillus sp. SF-1. Biotechnol Bioeng 80:755–761
Gad SC (1989) Acute and chronic systemic chromium toxicity. Sci Total Environ 86:149–157
Garbisu C, Ishii T, Leighton T, Buchanan BB (1996) Bacterial reduction of selenite to elemental selenium. Chem Geol 132:199–204
Gorby YA, Caccavo F, Bolton H (1998) Microbial reduction of cobalt III EDTA- in the presence and absence of manganese(IV) oxide. Environ Sci Technol 32:244–250
Gorby YA, Yanina S, McLean JS, Rosso KM, Moyles D, Dohnalkova A, Beveridge TJ, Chang IS, Kim BH, Kim KS, Culley DE, Reed SB, Romine MF, Saffarini DA, Hill EA, Shi L, Elias DA, Kennedy DW, Pinchuk G, Watanabe K, Ishii SI, Logan B, Nealson KH, Fredrickson JK (2006) Electrically conductive bacterial nanowires produced by Shewanella oneidensis strain MR-1 and other microorganisms. Proc Natl Acad Sci 103:11358–11363
Gorski CA, Nurmi JT, Tratnyek PG, Hofstetter TB, Scherer MM (2010) Redox behavior of magnetite: implications for contaminant reduction. Environ Sci Technol 44:55–60
Gorski CA, Scherer MM (2009) Influence of magnetite stoichiometry on FeII uptake and nitrobenzene reduction. Environ Sci Technol 43:3675–3680
Hansel CM, Benner SG, Neiss J, Dohnalkova A, Kukkadapu RK, Fendorf S (2003a) Secondary mineralization pathways induced by dissimilatory iron reduction of ferrihydrite under advective flow. Geochim Cosmochim Acta 67:2977–2992
Hansel CM, Wielinga BW, Fendorf S (2003b) Structural and compositional evolution of Cr/Fe solids after indirect chromate reduction by dissimilatory iron-reducing bacteria. Geochim Cosmochim Acta 67:401–412
Harrad S, Robson M, Hazrati S, Baxter-Plant VS, Deplanche K, Redwood MD, Macaskie LE (2007) Dehalogenation of polychlorinated biphenyls and polybrominated diphenyl ethers using a hybrid bioinorganic catalyst. J Environ Monit 9:314–318
Hartshorne RS, Reardon CL, Ross D, Nuester J, Clarke TA, Gates AJ, Mills PC, Fredrickson JK, Zachara JM, Shi L, Beliaev AS, Marshall MJ, Tien M, Brantley S, Butt JN, Richardson DJ (2009) Characterization of an electron conduit between bacteria and the extracellular environment. Proc Natl Acad Sci 106:22169–22174
Hau HH, Gilbert A, Coursolle D, Gralnick JA (2008) Mechanism and consequences of anaerobic respiration of cobalt by Shewanella oneidensis strain MR-1. Appl Environ Microbiol 74:6880–6886
Haygarth PM (1994) Global importance of global cycling of selenium. In: Frankenberger WT Jr, Benson S (eds) Selenium in the Environment. Marcel Dekker Inc., New York, pp 1–28
Hennebel T, Benner J, Clauwaert P, Vanhaecke L, Aelterman P, Callebaut R, Boon N, Verstraete W (2011) Dehalogenation of environmental pollutants in microbial electrolysis cells with biogenic palladium nanoparticles. Biotechnol Lett 33:89–95
Hennebel T, Simoen H, de Windt W, Verloo M, Boon N, Verstraete W (2009a) Biocatalytic dechlorination of trichloroethylene with bio-palladium in a pilot-scale membrane reactor. Biotechnol Bioeng 102:995–1002
Hennebel T, Verhagen P, Simoen H, Gusseme BD, Vlaeminck SE, Boon N, Verstraete W (2009b) Remediation of trichloroethylene by bio-precipitated and encapsulated palladium nanoparticles in a fixed bed reactor. Chemosphere 76:1221–1225
Henrot J (1989) Bioaccumulation and chemical modification of Tc by soil bacteria. Health Phys 57:239–245
Herbel MJ, Blum JS, Oremland RS, Borglin SE (2003) Reduction of elemental selenium to selenide: experiments with anoxic sediments and bacteria that respire Se-Oxyanions. Geomicrobiol J 20:587–602
Hofstetter TB, Heijman CG, Haderlein SB, Holliger C, Schwarzenbach RP (1999) Complete reduction of TNT and other (poly)nitroaromatic compounds under iron-reducing subsurface conditions. Environ Sci Technol 33:1479–1487
Holmes DE, Finneran KT, O’Neil RA, Lovley DR (2002) Enrichment of members of the family Geobacteraceae associated with stimulation of dissimilatory metal reduction in uranium-contaminated aquifer sediments. Appl Environ Microbiol 68:2300–2306
Horton R, Apel W, Thompson V, Sheridan P (2006) Low temperature reduction of hexavalent chromium by a microbial enrichment consortium and a novel strain of Arthrobacter aurescens. BMC Microbiol 6:1–8
Icenhower JP, Qafoku NP, Martin WJ, Zachara JM (2008) The geochemistry of technetium: A summary of the behaviour of an artificial element in the natural environment. PNNL-18139, U.S. Department of Energy
Icopini GA, Boukhalfa H, Neu MP (2007) Biological reduction of Np(V) and Np(V) citrate by metal-reducing bacteria. Environ Sci Technol 41:2764–2769
Icopini GA, Lack JG, Hersman LE, Neu MP, Boukhalfa H (2009) Plutonium(V/VI) reduction by the metal-reducing bacteria Geobacter metallireducens GS-15 and Shewanella oneidensis MR-1. Appl Environ Microbiol 75:3641–3647
Ishibashi Y, Cervantes C, Silver S (1990) Chromium reduction in Pseudomonas putida. Appl Environ Microbiol 56:2268–2270
Jahn MK, Haderlein SB, Meckenstock RU (2005) Anaerobic degradation of benzene, toluene, ethylbenzene, and o-xylene in sediment-free iron-reducing enrichment cultures. Appl Environ Microbiol 71:3355–3358
Jeon B-H, Kelly SD, Kemner KM, Barnett MO, Burgos WD, Dempsey BA, Roden EE (2004) Microbial reduction of U(VI) at the solid water interface. Environ Sci Technol 38:5649–5655
Jeyasingh J, Philip L (2005) Bioremediation of chromium contaminated soil: optimization of operating parameters under laboratory conditions. J Hazard Mater 118:113–120
Jeyasingh J, Somasundaram V, Philip L, Bhallamudi SM (2010) Pilot scale studies on the remediation of chromium contaminated aquifer using bio-barrier and reactive zone technologies. Chem Eng J 167:206–214
Kamaludeen S, Megharaj M, Juhasz A, Sethunathan N, Naidu R (2003) Chromium-microorganism interactions in soils: remediation implications. In: Albert LA, Gerba CP, Giesy J, Hutzinger O, Knaak JB, Stevens JT, Tjeerdema RS, Voogt P, Ware GW (eds) Reviews of Environmental Contamination and Toxicology. Springer, New York
Kaszuba JP, Runde WH (1999) The aqueous geochemistry of neptunium: dynamic control of soluble concentrations with applications to nuclear waste disposal. Environ Sci Technol 33:4427–4433
Kerin EJ, Gilmour CC, Roden E, Suzuki MT, Coates JD, Mason RP (2006) Mercury methylation by dissimilatory iron-reducing bacteria. Appl Environ Microbiol 72:7919–7921
Kilic NK, Stensballe A, Otzen DE, Donmez (2009) Proteomic changes in response to chromium(VI) toxicity in Pseudomonas aeruginosa. Bioresour Technol 101:2134–2140
Kimber R, Boothman C, Purdie P, Livens FR, Lloyd JR (2012) Biogeochemical behaviour of plutonium during anoxic biostimulation of contaminated sediments. Miner Mag 76(3):567–578
Kimbrough DE, Cohen Y, Winer AM, Creelman L, Mabuni C (1999) A critical assessment of chromium in the environment. Crit Rev Environ Sci Technol 29:1–46
King JK, Kostka JE, Frischer ME, Saunders FM (2000) Sulfate-reducing bacteria methylate mercury at variable rates in pure culture and in marine sediments. Appl Environ Microbiol 66:2430–2437
Konovalova VV, Dmytrenko GM, Nigmatullin RR, Bryk MT, Gvozdyak PI (2003) Chromium(VI) reduction in a membrane bioreactor with immobilized Pseudomonas cells. Enzyme Microbial Technol 33:899–907
Kopinke F-D, Mackenzie K, Koehler R, Georgi A (2004) Alternative sources of hydrogen for hydrodechlorination of chlorinated organic compounds in water on Pd catalysts. Appl Catal A 271:119–128
Kunapuli U, Jahn MK, Lueders T, Geyer R, Heipieper HJ, Meckenstock RU (2010) Desulfitobacterium aromaticivorans sp. nov. and Geobacter toluenoxydans sp. nov., iron-reducing bacteria capable of anaerobic degradation of monoaromatic hydrocarbons. Int J Syst Evol Microbiol 60:686–695
Law GTW, Geissler A, Lloyd JR, Livens FR, Boothman C, Begg JDC, Denecke MA, Rothe JR, Dardenne K, Burke IT, Charnock JM, Morris K (2010) Geomicrobiological redox cycling of the transuranic element neptunium. Environ Sci Technol 44:8924–8929
Lear G, McBeth JM, Boothman C, Gunning DJ, Ellis BL, Lawson RS, Morris K, Burke IT, Bryan ND, Brown AP, Livens FR, Lloyd JR (2009) Probing the biogeochemical behavior of technetium using a novel nuclear imaging approach. Environ Sci Technol 44:156–162
Lebedeva, Lialikova (1979) Crocite reduction by a culture of Pseudomonas chromatophila sp. nov. Mikrobiologiia 48:517–522
Lenz M, Lens PNL (2009) The essential toxin: the changing perception of selenium in environmental sciences. Sci Total Environ 407:3620–3633
Li L, Steefel CI, Williams KH, Wilkins MJ, Hubbard SS (2009) Mineral transformation and biomass accumulation associated with uranium bioremediation at Rifle, Colorado. Environ Sci Technol 43:5429–5435
Llobet JM, Domingo JL (1984) Acute toxicity of vanadium compounds in rats and mice. Toxicol Lett 23:227–231
Lloyd JR (2003) Microbial reduction of metals and radionuclides. FEMS Microbiol Rev 27:411–425
Lloyd JR, Byrne JM, Coker VS (2010) Biotechnological synthesis of functional nanomaterials. Curr Opin Biotechnol 22:509–515
Lloyd JR, Chesnes J, Glasauer S, Bunker DJ, Livens FR, Lovley DR (2002) Reduction of actinides and fission products by Fe(III)-reducing bacteria. Geomicrobiol J 19:103–120
Lloyd JR, Cole JA, Macaskie LE (1997a) Reduction and removal of heptavalent technetium from solution by Escherichia coli. J Bacteriol 179:2014–2021
Lloyd JR, Harding CL, Macaskie LE (1997b) Tc(VII) reduction and accumulation by immobilized cells of Escherichia coli. Biotechnol Bioeng 55:505–510
Lloyd JR, Lovley DR, Macaskie LE, Allen I, Laskin JWB, Geoffrey MG (2003) Biotechnological application of metal-reducing microorganisms. Adv Appl Microbiol 53:85–128. Academic Press
Lloyd JR, Mabbett AN, Williams DR, Macaskie LE (2001) Metal reduction by sulphate-reducing bacteria: physiological diversity and metal specificity. Hydrometallurgy 59:327–337
Lloyd JR, Macaskie LE (1996) A novel phosphorimager-based technique for monitoring the microbial reduction of technetium. Appl Environ Microbiol 62:578–582
Lloyd JR, Pearce CI, Coker VS, Pattrick RAD, van der Laan G, Cutting R, Vaughan DJ, Paterson-Beedle M, Mikheenko IP, Yong P, Macaskie LE (2008) Biomineralization: linking the fossil record to the production of high value functional materials. Geobiology 6:285–297
Lloyd JR, Sole VA, van Praagh CVG, Lovley DR (2000a) Direct and Fe(II)-mediated reduction of technetium by Fe(III)-reducing bacteria. Appl Environ Microbiol 66:3743–3749
Lloyd JR, Thomas GH, Finlay JA, Cole JA, Macaskie LE (1999) Microbial reduction of technetium by Escherichia coli and Desulfovibrio desulfuricans: enhancement via the use of high-activity strains and effect of process parameters. Biotechnol Bioeng 66:122–130
Lloyd JR, Yong P, Macaskie LE (1998) Enzymatic recovery of elemental palladium by using sulfate-reducing bacteria. Appl Environ Microbiol 64:4607–4609
Lloyd JR, Yong P, Macaskie LE (2000b) Biological reduction and removal of Np(V) by two microorganisms. Environ Sci Technol 34:1297–1301
Losi ME, Frankenberger WT (1997) Reduction of selenium oxyanions by Enterobacter cloacae SLD1a-1: isolation and growth of the bacterium and its expulsion of selenium particles. Appl Environ Microbiol 63:3079–3084
Losi ME, Frankenberger WT (1998) Microbial oxidation and solubilization of precipitated elemental selenium in soil. J Environ Qual 27:836–843
Lovley DR (1993) Dissimilatory metal reduction. Annu Rev Microbiol 47:263–290
Lovley DR (1995) Bioremediation of organic and metal contaminants with dissimilatory metal reduction. J Ind Microbiol Biotechnol 14:85–93
Lovley DR, Baedecker MJ, Lonergan DJ, Cozzarelli IM, Phillips EJP, Siegel DI (1989a) Oxidation of aromatic contaminants coupled to microbial iron reduction. Nature 339:297–300
Lovley DR, Coates JD, Blunt-Harris EL, Phillips EJP, Woodward JC (1996) Humic substances as electron acceptors for microbial respiration. Nature 382:445–448
Lovley DR, Holmes DE, Nevin KP, Poole (2004) Dissimilatory Fe(III) and Mn(IV) reduction. Adv Microbial Physiol 49:219–286. Academic Press
Lovley DR, Lonergan DJ (1990) Anaerobic oxidation of toluene, phenol, and p-cresol by the dissimilatory iron-reducing organism, GS-15. Appl Environ Microbiol 56:1858–1864
Lovley DR, Phillips EJ (1992) Reduction of uranium by Desulfovibrio desulfuricans. Appl Environ Microbiol 58:850–856
Lovley DR, Phillips EJP (1986) Availability of ferric iron for microbial reduction in bottom sediments of the freshwater tidal Potomac River. Appl Environ Microbiol 52:751–757
Lovley DR, Phillips EJP (1988) Novel mode of microbial energy metabolism: organic carbon oxidation coupled to dissimilatory reduction of iron or manganese. Appl Environ Microbiol 54:1472–1480
Lovley DR, Phillips EJP (1994) Reduction of chromate by Desulfovibrio vulgaris and its c3 cytochrome. Appl Environ Microbiol 60:726–728
Lovley DR, Phillips EJP, Gorby YA, Landa ER (1991) Microbial reduction of uranium. Nature 350:413–416
Lovley DR, Phillips EJP, Lonergan DJ (1989b) Hydrogen and formate oxidation coupled to dissimilatory reduction of iron or manganese by Alteromonas putrefaciens. Appl Environ Microbiol 55:700–706
Lovley DR, Widman PK, Woodward JC, Phillips EJ (1993) Reduction of uranium by cytochrome c3 of Desulfovibrio vulgaris. Appl Environ Microbiol 59:3572–3576
Lovley DR, Woodward JC, Chapelle FH (1994) Stimulated anoxic biodegradation of aromatic hydrocarbons using Fe(III) ligands. Nature 370:128–131
Lyngkilde J, Christensen TH (1992) Fate of organic contaminants in the redox zones of a landfill leachate pollution plume (Vejen, Denmark). J Contam Hydrol 10:291–307
Mabbett AN Macaskie LE (2002) A new bioinorganic process for the remediation of Cr(VI). J Chem Tech Biotechnol 77:1169–1175
Macaskie LE (1991) The application of biotechnology to the treatment of wastes produced from the nuclear fuel cycle: biodegradation and bioaccumulation as a means of treating radionuclide-containing streams. Crit Rev Biotechnol 11:41–112
Mackenzie AB (2000) Environmental radioactivity: experience from the 20th century trends and issues for the 21st century. Sci Total Environ 249:313–329
Magnuson T, Swenson M, Paszczynski A, Deobald L, Kerk D, Cummings D (2010) Proteogenomic and functional analysis of chromate reduction in; Acidiphilium cryptum JF-5, an Fe(III)-respiring acidophile. Biometals 23:1129–1138
Marsili E, Baron DB, Shikhare ID, Coursolle D, Gralnick JA, Bond DR (2008) Shewanella secretes flavins that mediate extracellular electron transfer. Proc NatL Acad Sci 105:3968–3973
Masscheleyn PH, Delaune RD, Patrick WH (1990) Transformations of selenium as affected by sediment oxidation-reduction potential and pH. Environ Sci Technol 24:91–96
McBeth JM, Lear G, Lloyd JR, Livens FR, Morris K, Burke IT (2007) Technetium reduction and reoxidation in aquifer sediments. Geomicrobiol J 24:189–197
McBeth JM, Lloyd JR, Law GTW, Livens FR, Burke IT, Morris K (2011) Redox interactions of technetium with iron-bearing minerals. Mineral Mag 75:2419–2430
McCormick ML, Adriaens P (2004) Carbon tetrachloride transformation on the surface of nanoscale biogenic magnetite particles. Environ Sci Technol 38:1045–1053
McCormick ML, Bouwer EJ, Adriaens P (2002) Carbon tetrachloride transformation in a model iron-reducing culture: relative kinetics of biotic and abiotic reactions. Environ Sci Technol 36:403–410
Means JL, Crerar DA, Borcsik MP, Duguid JO (1978) Adsorption of Co and selected actinides by Mn and Fe oxides in soils and sediments. Geochim Cosmochim Acta 42:1763–1773
Mertens B, Blothe C, Windey K, de Windt W, Verstraete W (2007) Biocatalytic dechlorination of lindane by nano-scale particles of Pd(0) deposited on Shewanella oneidensis. Chemosphere 66:99–105
Mikheenko IP, Rousset M, Dementin S, Macaskie LE (2008) Bioaccumulation of palladium by Desulfovibrio fructosivorans wild-type and hydrogenase-deficient strains. Appl Environ Microbiol 74:6144–6146
Morel FOMM, Kraepiel AML, Amyot M (1998) The chemical cycle and bioaccumulation of mercury. Annu Rev Ecol Syst 29:543–566
Myers CR, Myers JM (1992) Localization of cytochromes to the outer membrane of anaerobically grown Shewanella putrefaciens MR-1. J Bacteriol 174:3429–3438
Myers JM, Antholine WE, Myers CR (2004) Vanadium(V) reduction by Shewanella oneidensis MR-1 requires menaquinone and cytochromes from the cytoplasmic and outer membranes. Appl Environ Microbiol 70:1405–1412
Nakamura K, Hagimine M, Sakai M, Furukawa K (1999) Removal of mercury from mercury-contaminated sediments using a combined method of chemical leaching and volatilization of mercury by bacteria. Biodegradation 10:443–447
Nancharaiah YV, Dodge C, Venugopalan VP, Narasimhan SV, Francis AJ (2010) Immobilization of Cr(VI) and its reduction to Cr(III) phosphate by granular biofilms comprising a mixture of microbes. Appl Environ Microbiol 76:2433–2438
Nealson KH, Myers CR (1992) Microbial reduction of manganese and iron: new approaches to carbon cycling. Appl Environ Microbiol 58:439–443
Neu MP, Icopini GA, Boukhalfa H (2006) Plutonium speciation affected by environmental bacteria. ChemInform, 37 no–no
Nevin KP, Lovley DR (2000) Lack of production of electron-shuttling compounds or solubilization of Fe(III) during reduction of insoluble Fe(III) oxide by Geobacter metallireducens. Appl Environ Microbiol 66:2248–2251
Nevin KP, Lovley DR (2002) Mechanisms for accessing insoluble Fe(III) oxide during dissimilatory Fe(III) reduction by Geothrix fermentans. Appl Environ Microbiol 68:2294–2299
Newman DK, Kolter R (2000) A role for excreted quinones in extracellular electron transfer. Nature 405:94–97
Nielsen PH, Albrechtsen H-JR, Heron G, Christensen TH (1995) In situ and laboratory studies on the fate of specific organic compounds in an anaerobic landfill leachate plume, 1. Experimental conditions and fate of phenolic compounds. J Contam Hydrol 20:27–50
Oremland RS, Hollibaugh JT, Maest AS, Presser TS, Miller LG, Culbertson CW (1989) Selenate reduction to elemental selenium by anaerobic bacteria in sediments and culture: biogeochemical significance of a novel, sulfate-independent respiration. Appl Environ Microbiol 55:2333–2343
Ortiz-Bernad I, Anderson RT, Vrionis HA, Lovley DR (2004a) Resistance of solid-phase U(VI) to microbial reduction during in situ bioremediation of uranium-contaminated groundwater. Appl Environ Microbiol 70:7558–7560
Ortiz-Bernad I, Anderson RT, Vrionis HA, Lovley DR (2004b) Vanadium respiration by Geobacter metallireducens: novel strategy for in situ removal of vanadium from groundwater. Appl Environ Microbiol 70:3091–3095
Panak P, Nitsche H (2001) Interaction of aerobic soil bacteria with plutonium(VI). Radiochim Acta 89:499
Pearce CI, Coker VS, Charnock JM, Pattrick RAD, Mosselmans JFW, Law N, Beveridge TJ, Lloyd J (2008) Microbial manufacture of chalcogenide-based nanoparticles via the reduction of selenite using Veillonella atypica: an in situ EXAFS study. Nanotechnology 19:155603
Pearce CI, Pattrick RAD, Law N, Charnock JM, Coker VS, Fellowes JW, Oremland RS, Lloyd JR (2009) Investigating different mechanisms for biogenic selenite transformations: Geobacter sulfurreducens, Shewanella oneidensis and Veillonella atypica. Environ Technol 30:1313–1326
Pignolet L, Auvray F, Fonsny K, Capot F, Moureau Z (1989) Role of various microorganisms on Tc behavior in sediments. Health Phys 57:791–800
Presser TS (1994) The Kesterson effect. Environ Manag 18:437–454
Presser TS, Ohlendorf HM (1987) Biogeochemical cycling of selenium in the San Joaquin Valley, California, USA. Environ Manag 11:805–821
Rai D, Eary LE, Zachara JM (1989) Environmental chemistry of chromium. Sci Total Environ 86:15–23
Redwood MD, Deplanche K, Baxter-Plant VS, Macaskie LE (2007) Biomass-supported palladium catalysts on Desulfovibrio desulfuricans and Rhodobacter sphaeroides. Biotechnol Bioeng 99:1045–1054
Reed DT, Pepper SE, Richmann MK, Smith G, Deo R, Rittmann BE (2007) Subsurface bio-mediated reduction of higher-valent uranium and plutonium. J Alloys Compd 444–445:376–382
Reguera G, McCarthy KD, Mehta T, Nicoll JS, Tuominen MT, Lovley DR (2005) Extracellular electron transfer via microbial nanowires. Nature 435:1098–1101
Reguera G, Pollina RB, Nicoll JS, Lovley DR (2007) Possible nonconductive role of Geobacter sulfurreducens pilus nanowires in biofilm formation. J Bacteriol 189:2125–2127
Renshaw J, Law N, Geissler A, Livens F, Lloyd J (2009) Impact of the Fe(III)-reducing bacteria Geobacter sulfurreducens and Shewanella oneidensis on the speciation of plutonium. Biogeochemistry 94:191–196
Renshaw JC, Butchins LJC, Livens FR, May I, Charnock JM, Lloyd JR (2005) Bioreduction of uranium: environmental implications of a pentavalent intermediate. Environ Sci Technol 39:5657–5660
Richter K, Schicklberger M, Gescher J (2012) Dissimilatory reduction of extracellular electron acceptors in anaerobic respiration. Appl Environ Microbiol 78:913–921
Rittmann BE, Banaszak JE, Reed DT (2002) Reduction of Np(V) and precipitation of Np(IV) by an anaerobic microbial consortium. Biodegradation 13:329–342
Roden EE (2006) Geochemical and microbiological controls on dissimilatory iron reduction. CR Geosci 338:456–467
Roden EE, Zachara JM (1996) Microbial reduction of crystalline iron(III) oxides: influence of oxide surface area and potential for cell growth. Environ Sci Technol 30:1618–1628
Roh Y, Chon C-M, Moon J-W (2007) Metal reduction and biomineralization by an alkaliphilic metal-reducing bacterium, Alkaliphilus metalliredigens (QYMF). Geosci J 11:415–423
Roh Y, Lee SR, Choi SK, Elless MP, Lee SY (2000) Physicochemical and mineralogical characterization of uranium-contaminated soils. J Soil Contam 9:463–486
Rohs (2003) Directive 2002/95/EC of the European Parliament and of the council on the restriction of the use of certain hazardous substances in electrical and electronic equipment. Off J Eur Union L 19–23
Roling WFM, van Breukelen BM, Braster M, Lin B, van Verseveld HW (2001) Relationships between microbial community structure and hydrochemistry in a landfill leachate-polluted aquifer. Appl Environ Microbiol 67:4619–4629
Romanenko, Koren’kov (1977) Pure culture of bacteria using chromates and bichromates as hydrogen acceptors during development under anaerobic conditions. Mikrobiologiia 46:414–417
Rooney-Varga JN, Anderson RT, Fraga JL, Ringelberg D, Lovley DR (1999) Microbial communities associated with anaerobic benzene degradation in a petroleum-contaminated aquifer. Appl Environ Microbiol 65:3056–3063
Ruggiero CE, Boukhalfa H, Forsythe JH, Lack JG, Hersman LE, Neu MP (2005) Actinide and metal toxicity to prospective bioremediation bacteria. Environ Microbiol 7:88–97
Saouter E, Gillman M, Barkay T (1995) An evaluation of mer-specified reduction of ionic mercury as a remedial tool of a mercury-contaminated freshwater pond. J Ind Microbiol Biotechnol 14:343–348
Sasaki T, Zheng J, Asano H, Kudo A, Kudo A (2001) Interaction of Pu, Np and Pa with anaerobic microorganisms at geologic repositories. In: Radioactivity in the Environment. Elsevier, Amsterdam
Schulte EH, Scoppa P (1987) Sources and behavior of technetium in the environment. Sci Total Environ 64:163–179
Shelobolina E, Coppi M, Korenevsky A, Didonato L, Sullivan S, Konishi H, Xu H, Leang C, Butler J, Kim B-C, Lovley D (2007) Importance of c-type cytochromes for U(VI) reduction by Geobacter sulfurreducens. BMC Microbiol 7:16
Shen H, Wang YT (1993) Characterization of enzymatic reduction of hexavalent chromium by Escherichia coli ATCC 33456. Appl Environ Microbiol 59:3771–3777
Silva R, Nitsche H (1995) Radiochim Acta 70/71:377
Smith W-L (2001) Hexavalent chromium reduction and precipitation by sulphate-reducing bacterial biofilms. Environ Geochem Health 23:297–300
Snoeyenbos-West OL, Nevin KP, Anderson RT, Lovley DR (2000) Enrichment of Geobacter species in response to stimulation of Fe(III) reduction in sandy aquifer sediments. Microbiol Ecol 39:153–167
Spallholz JE (1994) On the nature of selenium toxicity and carcinostatic activity. Free Radical Biol Med 17:45–64
Staats M, Braster M, Röling WFM (2011) Molecular diversity and distribution of aromatic hydrocarbon-degrading anaerobes across a landfill leachate plume. Environ Microbiol 13:1216–1227
Stewart DI, Burke IT, Hughes-Berry DV, Whittleston RA (2010) Microbially mediated chromate reduction in soil contaminated by highly alkaline leachate from chromium containing waste. Ecol Eng 36:211–221
Stewart DI, Burke IT, Mortimer RJG (2007) Stimulation of microbially mediated chromate reduction in alkaline soil-water systems. Geomicrobiol J 24:655–669
Streets DG, Zhang Q, Wu Y (2009) Projections of global mercury emissions in 2050. Environ Sci Technol 43:2983–2988
Stumm JJM (1996) Aquatic chemistry. Wiley, New York
Suzuki T, Miyata N, Horitsu H, Kawai K, Takamizawa K, Tai Y, Okazaki M (1992) NAD(P)H-dependent chromium (VI) reductase of Pseudomonas ambigua G-1: a Cr(V) intermediate is formed during the reduction of Cr(VI) to Cr(III). J Bacteriol 174:5340–5345
Tebo BM, Obraztsova AY (1998) Sulfate-reducing bacterium grows with Cr(VI), U(VI), Mn(IV), and Fe(III) as electron acceptors. FEMS Microbiol Lett 162:193–198
Telling ND, Coker VS, Cutting RS, Laan GVD, Pearce CI, Pattrick RAD, Arenholz E, Lloyd JR (2009) Remediation of Cr(VI) by biogenic magnetic nanoparticles: An x-ray magnetic circular dichroism study. Appl Phys Lett 95:163–701
Teng Y, Ni S, Zhang C, Wang J, Lin X, Huang Y (2006) Environmental geochemistry and ecological risk of vanadium pollution in Panzhihua mining and smelting area, Sichuan, China. Chin J Geochem 25:379–385
Tobler NB, Hofstetter TB, Straub KL, Fontana D, Schwarzenbach RP (2007) Iron-mediated microbial oxidation and abiotic reduction of organic contaminants under anoxic conditions. Environ Sci Technol 41:7765–7772
Tor JM, Lovley DR (2001) Anaerobic degradation of aromatic compounds coupled to Fe(III) reduction by Ferroglobus placidus. Environ Microbiol 3:281–287
Tripathi AGA (2002) Bioremediation of toxic chromium from electroplating effluent by chromate-reducing Pseudomonas aeruginosa A 2 Chr in two bioreactors. Appl Microbiol Biotechnol 58:416–420
Turick CE, Graves C, Apel WA (1998) Bioremediation Potential of Cr(VI)-Contaminated Soil Using Indigenous Microorganisms. Bioremediation J 2:1–6
USEPA (2009) Identification and listing of hazardous waste 40 CFR 261.4(b): exclusions: solid wastes which are not hazardous wastes. 40 CFR 261.4(b)
van der Zee FP, Cervantes FJ (2009) Impact and application of electron shuttles on the redox (bio)transformation of contaminants: a review. Biotechnol Adv 27:256–277
van Marwijk J, Opperman D, Piater L, van Heerden E (2009) Reduction of vanadium(V) by Enterobacter cloacae EV-SA01 isolated from a South African deep gold mine. Biotechnol Lett 31:845–849
Vanengelen M, Peyton B, Mormile M, Pinkart H (2008) Fe(III), Cr(VI), and Fe(III) mediated Cr(VI) reduction in alkaline media using a Halomonas isolate from Soap Lake, Washington. Biodegradation 19:841–850
von Canstein H, Li Y, Leonhauser J, Haase E, Felske A, Deckwer W-D, Wagner-Dobler I (2002) Spatially oscillating activity and microbial succession of mercury-reducing biofilms in a technical-scale bioremediation system. Appl Environ Microbiol 68:1938–1946
von Canstein H, Li Y, Timmis KN, Deckwer WD, Wagner-Dobler I (1999) Removal of mercury from chloralkali electrolysis wastewater by a mercury-resistant Pseudomonas putida strain. Appl Environ Microbiol 65:5279–5284
von Canstein H, Ogawa J, Shimizu S, Lloyd JR (2008) Secretion of flavins by Shewanella species and their role in extracellular electron transfer. Appl Environ Microbiol 74:615–623
Vrionis HA, Anderson RT, Ortiz-Bernad I, O’Neill KR, Resch CT, Peacock AD, Dayvault R, White DC, Long PE, Lovley DR (2005) Microbiological and geochemical heterogeneity in an in situ uranium bioremediation field site. Appl Environ Microbiol 71:6308–6318
Wagner-Döbler I (2003) Pilot plant for bioremediation of mercury-containing industrial wastewater. Appl Microbiol Biotechnol 62:124–133
Wagner-Dobler I, von Canstein H, Li Y, Timmis KN, Deckwer W-D (2000) Removal of mercury from chemical wastewater by microoganisms in technical scale. Environ Sci Technol 34:4628–4634
Wang Y-T (2000) Microbial reduction of Cr(VI). In: Loveley DR (ed.) Environmental microbe-metal interactions pp 225–235
Wang Y-T, Shen H (1995) Bacterial reduction of hexavalent chromium. J Ind Microbiol Biotechnol 14:159–163
Wani R, Kodam K, Gawai K, Dhakephalkar P (2007) Chromate reduction by Burkholderia cepacia MCMB-821, isolated from the pristine habitat of alkaline crater lake. Appl Microbiol Biotechnol 75:627–632
Wanty RB, Goldhaber MB (1992) Thermodynamics and kinetics of reactions involving vanadium in natural systems: Accumulation of vanadium in sedimentary rocks. Geochim Cosmochim Acta 56:1471–1483
Weber KA, Achenbach LA, Coates JD (2006) Microorganisms pumping iron: anaerobic microbial iron oxidation and reduction. Nat Rev Microbiol 4:752–764
Whittleston RA, Stewart DI, Mortimer RJG, Ashley DJ, Burke IT (2011a) Effect of microbially induced anoxia on Cr(VI) mobility at a site contaminated with hyperalkaline residue from chromite ore processing. Geomicrobiol J 28:68–82
Whittleston RA, Stewart DI, Mortimer RJG, Tilt ZC, Brown AP, Geraki K, Burke IT (2011b) Chromate reduction in Fe(II)-containing soil affected by hyperalkaline leachate from chromite ore processing residue. J Hazard Mater 194:15–23
WHO (2008) Guidelines for drinking water quality
Wielinga B, Mizuba MM, Hansel CM, Fendorf S (2001) Iron promoted reduction of chromate by dissimilatory iron-reducing bacteria. Environ Sci Technol 35:522–527
Wildung RE, Gorby YA, Krupka KM, Hess NJ, Li SW, Plymale AE, McKinley JP, Fredrickson JK (2000) Effect of electron donor and solution chemistry on products of dissimilatory reduction of technetium by Shewanella putrefaciens. Appl Environ Microbiol 66:2451–2460
Wildung RE, Li SW, Murray CJ, Krupka KM, Xie Y, Hess NJ, Roden EE (2004) Technetium reduction in sediments of a shallow aquifer exhibiting dissimilatory iron reduction potential. FEMS Microbiol Ecol 49:151–162
Wilkins MJ, Verberkmoes NC, Williams KH, Callister SJ, Mouser PJ, Elifantz H, Naguessan AL, Thomas BC, Nicora CD, Shah MB, Abraham P, Lipton MS, Lovley DR, Hettich RL, Long PE, Banfield JF (2009) Proteogenomic monitoring of Geobacter physiology during stimulated uranium bioremediation. Appl Environ Microbiol 75:6591–6599
Williams AGB, Gregory KB, Parkin GF, Scherer MM (2005) Hexahydro-1,3,5-trinitro-1,3,5-triazine transformation by biologically reduced ferrihydrite : evolution of fe mineralogy, surface area, and reaction rates. Environ Sci Technol 39:5183–5189
Williams KH, Long PE, Davis JA, Wilkins MJ, N’Guessan AL, Steefel CI, Yang L, Newcomer D, Spane FA, Kerkhof LJ, McGuinness L, Dayvault R, Lovley DR (2011) Acetate availability and its influence on sustainable bioremediation of uranium-contaminated groundwater. Geomicrobiol J 28:519–539
Wischgoll S, Heintz D, Peters F, Erxleben A, Sarnighausen E, Reski R, van Dorsselaer A, Boll M (2005) Gene clusters involved in anaerobic benzoate degradation of Geobacter metallireducens. Mol Microbiol 58:1238–1252
Woolfolk CA, Whiteley HR (1962) Reduction of inorganic compounds with molecular hydrogen by Micrococcus Lactilyticus I. J Bacteriol 84:647–658
Wu W-M, Carley J, Fienen M, Mehlhorn T, Lowe K, Nyman J, Luo J, Gentile ME, Rajan R, Wagner D, Hickey RF, Gu B, Watson D, Cirpka OA, Kitanidis PK, Jardine PM, Criddle CS (2006a) Pilot-scale in situ bioremediation of uranium in a highly contaminated aquifer. 1. conditioning of a treatment Zone. Environ Sci Technol 40:3978–3985
Wu W-M, Carley J, Gentry T, Ginder-Vogel MA, Fienen M, Mehlhorn T, Yan H, Caroll S, Pace MN, Nyman J, Luo J, Gentile ME, Fields MW, Hickey RF, Gu B, Watson D, Cirpka OA, Zhou J, Fendorf S, Kitanidis PK, Jardine PM, Criddle CS (2006b) Pilot-scale in situ bioremedation of uranium in a highly contaminated aquifer. 2. reduction of U(VI) and geochemical control of U(VI) bioavailability. Environ Sci Technol 40:3986–3995
Xu M, Wu W-M, Wu L, He Z, van Nostrand JD, Deng Y, Luo J, Carley J, Ginder-Vogel M, Gentry TJ, Gu B, Watson D, Jardine PM, Marsh TL, Tiedje JM, Hazen T, Criddle CS, Zhou J (2010) Responses of microbial community functional structures to pilot-scale uranium in situ bioremediation. ISME J 4:1060–1070
Yong P, Rowson NA, Farr JP, Harris IR, Macaskie LE (2002a) Bioreduction and biocrystallization of palladium by Desulfovibrio desulfuricans NCIMB 8307. Biotechnol Bioeng 80:369–379
Yong P, Rowson NA, Farr JPG, Harris IR, Macaskie LE (2002b) Bioaccumulation of palladium by Desulfovibrio desulfuricans. J Chem Technol Biotechnol 77:593–601
Zhang W-X (2003) Nanoscale iron particles for environmental remediation: an overview. J Nanopart Res 5:323–332
Acknowledgments
MW acknowledges funding from BBSRC and CASE support from Parsons Brinckerhoff. JRL acknowledges the financial support of the Royal Society, NERC and the EU.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2013 Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
Watts, M.P., Lloyd, J.R. (2013). Bioremediation via Microbial Metal Reduction. In: Gescher, J., Kappler, A. (eds) Microbial Metal Respiration. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-642-32867-1_7
Download citation
DOI: https://doi.org/10.1007/978-3-642-32867-1_7
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-642-32866-4
Online ISBN: 978-3-642-32867-1
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)