Abstract
Primary motor cortex plays an important role in the planning and execution of movement, and motor cortical functions depend on cortical excitability. Here, we review how one can use transcranial magnetic stimulation (TMS) to study the functional changes occurring in M1 during the preparation and selection of actions. Specifically, we emphasise the idea that the brain is organised in a hierarchical way in which the boundaries between perception, cognition and action are weak and these processes occur in parallel. This, in turn, predicts that the motor system should be dynamically influenced by information about forthcoming actions we want to perform; this information is flexible and dynamic, and should be conveyed to the motor system through different routes, depending on the current context in which our actions occur. Using TMS, one can read out dynamic changes in M1 excitability in an effector-specific way, and study how such changes relate to the information that guides our actions. In humans, this provides unique insight into the physiological underpinnings and mechanism of action through which we prepare and select our movements in an ever-changing and uncertain world.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Transcranial Magnetic Stimulation
- Motor System
- Action Selection
- Action Preparation
- Corticospinal Excitability
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
Thinking is easy, acting is difficult, and to put one’s thoughts into action is the most difficult thing in the world. Johann Wolfgang von Goethe
It has now been more than 140 years since Eduard Hitzig and Gustav Fritsch performed their seminal experiments on the effect of electrical stimulation to the cerebrum (Fritsch and Hitzig 1870, 2009), yet we are still puzzled by what function the ‘motor strips’ identified in their experiments yield. For a long time the prevailing idea was that motor cortex and the adjacent premotor cortices are solely concerned with the control of muscles of the body. Little recognition had thus been given to whether the function of these regions may extend beyond connecting the brain to the lower motor neurons via the spinal cord, and to signal which particular muscles to contract. Part of this view originates from classical views of brain organisation that have emphasised that motor control is part of a serially organised system in which sensory information is transformed into neural signals for motor planning and execution through different anatomically distinct stages (Flash and Hogan 1985; Kawato et al. 1990; Bhushan and Shadmehr 1999). This transformation process ultimately culminates in M1, where descending motor commands are generated.
Perception, cognitive processing (e.g. learning, attention and working memory) and action selection have therefore traditionally been considered (and studied) as largely independent processes. The brain, however, is unlikely to adhere to these text book divisions, and while functional specialisation is critical, activity deployment in the brain does not follow these strict boundaries (Cisek 2007b; Pesaran 2010; Ledberg et al. 2007; Bullier and Nowak 1995; Hubbard et al. 2005; Smid et al. 1991; Mesulam 1990). Studies from both human and non-human primates have now, indeed, established that the premotor and motor regions of cortex, rather than being merely concerned with generating muscle commands, are intimately involved in the processing of higher order signals for action selection (Romo et al. 2004; Cisek 2007b; Cisek and Kalaska 2010; Gold and Shadlen 2001, 2007). This body of work has thus led to challenge the view of strictly serial processing as an organisational principle for brain function, and thus, our view on the foundations of action selection. For example, decisions for actions are now assumed to involve the parallel activation of multiple options, with the commitment to a specific action when activity associated to that particular action reaches a given threshold (Ivry and Spencer 2004; Cisek 2007a, b).
Studies in non-human primates, for example, show that responses of single neurons in dorsal premotor cortex (PMd) and primary motor cortex (M1) correlate with a variety of processes, such as prior expectation, time, reward, motivation or uncertainty (Bastian et al. 1998; Roesch and Olson 2003, 2004, 2007; Weinrich et al. 1984; Wise et al. 1983, 1986; Nakamura 2006; Cisek and Kalaska 2004; Crammond and Kalaska 2000, 1994; Rubino et al. 2006; Renoult et al. 2006; Requin et al. 1988; Roux et al. 2003, 2006). These signals do not directly reflect the production of the actual descending signals required for movement. At the same time, brain regions commonly regarded as specialised in, for example, decision making, learning or attention also contain neurons responding to planning and performing movements (Carello and Krauzlis 2004; Cisek and Kalaska 2005; Coe et al. 2002; Gold and Shadlen 2001; Horwitz et al. 2004; Hoshi et al. 2000; Platt and Glimcher 1999; Romo et al. 2004; Schall 2001).
The structures of the motor system are characterised by anatomical connections with a number of areas concerned with higher level computations that could provide routes through which information is transmitted to the motor system. These include regions in parietal, prefrontal and cingulate cortex, and the basal ganglia: for example, connections exist to different parietal areas (Rizzolatti et al. 1998) involved in the representation of probabilistic information (Yang and Shadlen 2007) and reward (Platt and Glimcher 1999), but also in guiding decisions about hand choice (Oliveira et al. 2010); regions of cingulate cortex (Van Hoesen and Solodkin 1993) encoding uncertainty about reward expectation and the value of actions (Rudebeck et al. 2008); and regions of prefrontal cortex (Dum and Strick 2005; Lu et al. 1994) and subcortical basal ganglia-thalamic circuits (Alexander et al. 1990) involved in processing motivational decision variables (Schultz 2006). Within prefrontal cortex, value-based influences from ventromedial prefrontal cortex (Boorman et al. 2009) may reach the motor cortices via the anterior cingulate sulcus.
In this chapter, we address how one can usefully study the functional role and neural underpinnings of signals that might influence and bias the selection of actions in humans. How can we explain the apparent richness of signals observed in the motor system that appear to be not strictly motor related, and how these can studied in the complex and flexible behaviour only humans are capable of?
The advent of techniques for non-invasive stimulation of cortex has opened the possibility to address such issues. These techniques complement direct recordings and microstimulation approaches in animals and their impact on the functional state of M1. In this chapter, we specifically ask how one can use transcranial magnetic stimulation (TMS) to study the functional role of M1 (and PMD) for action preparation and selection, and how activity in these regions might be influenced by cognitive operations that ensure our flexible and accurate movements in an uncertain and ever-changing world. This is of relevance because it not only allows for novel insights into M1/PMd function, but also allows for inferences about the hierarchical processing for action selection.
1 Using Motor-Evoked Potentials to Read-Out the Functional State of M1/PMD During Behaviour
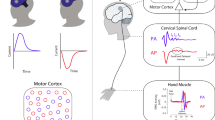
First, we briefly review how we can utilise cortical stimulation techniques such as TMS to investigate the role of the human motor system in cognition. When applied to M1, TMS can evoke descending cortico-spinal volleys that cause contralateral muscle movement. This movement can be quantified using surface electromyography and provides a direct measure of corticospinal excitability. TMS can therefore be used in humans to non-invasively assess the functional state of the corticospinal motor system (Box 10.1). The excellent temporal resolution of TMS allows for measuring the excitability of the corticospinal system at various time points within a task. Any task-specific change in the size of evoked electromyographic responses reflects changes in the functional state or excitability of the motor output system at the time of stimulation. This is a crucial asset of the technique because, in principle, changes in the functional state can now be read out with millisecond precision.
Moreover, it is often neglected that this technique provides a causal measure in its true meaning—the signal evoked in contralateral muscles is caused by the stimulation, and nothing else. Changes in MEP size can have different origins, including noise, variations in wakefulness and attention, or even small variations in the precise stimulation point and can have both cortical and spinal origins. Critically, however, when carefully conducted, a significant proportion of variance will originate from changes in the functional state of the motor system at the time the TMS pulse is applied.
TMS provides a unique and complimentary measure to other techniques because, for example, the effector-specific changes in corticospinal excitability (CSE) are not easily observed otherwise, e.g. with EEG or fMRI approaches. Moreover, the possibility to assess systematic changes in intracortical inhibition and excitation (Ziemann and Rothwell 2000; Di Lazzaro et al. 1999, 2004; Kujirai et al. 1993; Chen 2004) offers unprecedented non-invasive information about their specific role in action control. Finally, as discussed in more detail elsewhere in this book, double-coil approaches (Civardi et al. 2001; Mochizuki et al. 2004) can be used together with complementary neuroimaging techniques (Bestmann et al. 2008b; Ruff et al. 2009) to highlight the functional interactions among interconnected networks in the brain, and how these may relate to the preparation and selection of action.
3 Assessing the Functional State of M1 During Action Execution/Voluntary Movement
One question TMS allows for addressing is how the functional state of M1 changes during the execution phase of an action. This can be investigated by probing M1 excitability at different times immediately prior to, during and after the overt response during simple or choice RT and stop-signal tasks (Chen et al. 1998; Davey et al. 1998; Soto et al. 2010; Leocani et al. 2000; Hiraoka et al. 2010; Chen and Hallett 1999; Hasbroucq et al. 2000; Romaiguere et al. 1997). Interestingly, as discussed below, the specific changes in M1 during this period may differ from those in the preceding period of action preparation.
One consistently observed finding is that CSE increases immediately before the electromyographic burst in the agonist muscle that precedes the actual voluntary movement (Hoshiyama et al. 1996a, 1997), which often corresponds to the period starting around 100 ms after the appearance of an imperative (‘Go’) stimulus. Moreover, this effect is accompanied by a reduction in intracortical inhibition preceding the voluntary movement by around 100 ms (Reynolds and Ashby 1999). It, therefore, seems that intracortical inhibition is involved in simple and choice RT tasks, even when there is no requirement to stop the movement (Burle et al. 2004). This increase is furthermore mirrored by a (usually much smaller) CSE increase (Hoshiyama et al. 1996b; Duque and Ivry 2009; Koch et al. 2006; Leocani et al. 2000), or even significant decrease (Michelet et al. 2010; Tandonnet et al. 2010; Liepert et al. 2001) in muscles not involved in an action. This latter pattern may depend on whether a muscle is merely not involved, or an antagonistic to the selected muscle. It is important to recall, however, that intracortical facilitation (ICF) and ICI as well as different dynamic behaviours of ICF and pre-movement facilitation may change simultaneously and influence one another. Depending on their relative contribution in a specific task, and the specific time at which we measure CSE, the specific pattern of CSE changes that can be observed thus may vary significantly (see above). The consistent observation is that there are very specific and mostly antagonistic changes in CSE between the selected and unselected effectors during the execution of an action. Taken together, these results show how TMS can provide unique insight into the physiological processes in M1 during the execution of selected actions.
4 Assessing the Functional State of M1 During Action Preparation
It is generally thought that actions are prepared and selected before they are executed. For example, we know from behavioural reaction time experiments that prior information (such as visual cues) can be used to prepare actions (Rosenbaum 1980). One hallmark feature in these experiments is a shortening of reaction times for more predictable stimuli, which has been taken as evidence that the respective action has been ‘mentally’ prepared, and therefore a response can be made with greater speed once the imperative stimulus is presented.
The most successful approach to study preparation experimentally are instructed delay tasks, in which a cue stimulus provides information about the likely action, which can only be executed after a delay when a subsequent imperative stimulus has been presented. This period prior to an overt action is therefore unconfounded by descending motor commands, and thus can provide useful insights into the physiological mechanism that underpin the transformation of perceptual and cognitive signals into action. The point is that under a parallel processing account, we expect to see gradual changes in CSE in the period prior to action, when actions are prepared.
In non-human primates, neurons representing the selected action progressively increase their firing rates during a delay period inserted between presentation of a visual cue and the appearance of a visual stimulus. This increase depends, for example, on the degree of predictability of the forthcoming movement—on average, more predictable sensory information leads to a stronger gradual activity build-up in premotor and motor cortex (Roux et al. 2006; Wise et al. 1983; Tanji and Evarts 1976; Bastian et al. 1998; Cisek and Kalaska 2005; Cisek 2005; Crammond and Kalaska 2000, 1994; Nakamura 2006). This observation is seen as a physiological correlate of action preparation.
In humans, TMS has been used to assess preparatory activity changes non-invasively, using delayed-response or instructed delay tasks. A rich set of studies have now established the general finding that CSE undergoes significant changes during action preparation (Bestmann et al. 2008a; van den et al. 2007; Coxon et al. 2006; van Elswijk et al. 2007, 2008; Mars et al. 2008; Duque and Ivry 2009; Duque et al. 2010; Hasbroucq et al. 1997, 1999a, b; Sinclair and Hammond 2008, 2009; Touge et al. 1998; Mars et al. 2007). These findings demonstrate that in humans, TMS can serve as a non-invasive analogue to invasive direct recordings of delay-period activity in non-human primates. TMS allows for differentiating between different intrinsic muscles, and thus provides sufficient resolution to distinguish the physiological underpinnings of action preparation and selection for different unilateral finger movements (Bestmann et al. 2008a). Moreover, the ability to assess intracortical inhibition and facilitation non-invasively through the use of paired-pulse protocols (Kujirai et al. 1993; Di Lazzaro et al. 2004) provides an additional window into the physiological interplay between intracortical excitation and inhibition during the preparation and selection of different actions.
6 Studying Dynamic, Trial-By-Trial Changes in M1 Excitability During Action Preparation
A fundamental feature of human movement is that anticipatory knowledge of an impending action improves the speed and accuracy of the response. This implies that we learn about the predictability of sensory information, and modify activity at the level of motor output accordingly, while preparing an action. For example, although sensory information provides useful cues for guiding actions, it is also inherently uncertain, and learning about this uncertainty can enable the nervous system to prepare motor output for action prior to an event. There is a good deal of information showing that, on average, predictable sensory information guiding actions leads to a gradual activity build-up in premotor and motor cortex during preparation for action. This may also be reflected in specific excitability changes of corticospinal projections, in line with a burgeoning set of studies demonstrating quantifiable effects of visual information on the motor system, including the spinal cord. Taken together, this implies that the predictability of sensory information is learned and represented explicitly in the brain, and that its representation is directed to the level of motor output for anticipatory action preparation.
The important point here is that predictability and prior expectations can only be established through learning, and that the functional state of the motor system should, therefore, reflect such learning. This interesting dynamic, however, cannot be revealed by inspecting M1 excitability changes on average alone. Understanding how the brain uses the predictability of events to inform preparation for action requires models of how, for example, the predictability of an event is learnt and represented over time. One solution to this is the use of model-based approaches (Mars et al. 2010; Corrado and Doya 2007; O’Doherty et al. 2007). These can provide trial-by-trial predictions about the possible rules used by the motor system to harness the probability of future events for action decisions.
In a first study investigating whether predictive (in this case, information theoretic) models can explain a substantial amount of CSE changes during action preparation, we previously measured CSE during an instructed delay task, in which an instruction cue provided information about the forthcoming movement with varying degree of certainty (Bestmann et al. 2008a). Thus, participants had to respond to an imperative cue stimulus but critically, in different blocks of trials, a preceding instruction cue predicted the identity of this imperative cue only with 85, 70 or 55% validity, respectively (Fig. 10.1a). This, therefore, varied the uncertainty about the required action, given the instruction cue. The important point here is that these probabilities were unknown to the participants, and thus had to be learned over time in each block.
Influence of uncertainty and surprise on corticospinal excitability during action preparation. a Schematic of the task. On valid trials, a preparatory CS predicted the identity of a subsequent IS, cueing a button press with the right thumb or little finger. On invalid trials, the CS-IS mapping was invalid as the CS was followed by the alternative IS. The validity of the CS varied across blocks of 105 trials between 85:15, 70:30 and 55:45%, respectively, creating blocks with, low, medium and high uncertainty about imperative stimuli. A single TMS pulse was applied during every trial, 200 ms before IS appearance, to read out changes in M1 excitability. b Average behavioural and electrophysiological results. Reaction times (grey/black) are, on average, significantly shorter for more predictable (i.e. more validly cued) actions. Average changes in corticospinal excitability parallel this effect, with CSE being largest for prepared actions in a predictable context. c CSE (top) for validly and invalidly cued trials from all subjects, plotted against the average uncertainty (entropy; left) and trial-by-trial surprise (right). CSE was generally higher when uncertainty (entropy) was low, and trials were preceded by surprising events. Reaction times for validly and invalidly cued trials plotted against average uncertainty (entropy; left) and trial-by-trial surprise (right). Reaction times were generally faster when uncertainty (entropy) and surprise were low. These results suggest that predictions about events based on both the average uncertainty, and trial-by-trial surprise conveyed by visual events can modulate the voluntary motor system on a trial-by-trial basis. Furthermore, these results show how measurements of corticospinal excitability with TMS can provide a window to examine computational processes about how humans implement decisions in real time.
Behavioural data showed that participants reacted, on average, faster on trials in which the instruction cue was more reliable, indicating some sort of learning that enabled more efficient action preparation (Fig. 10.1b). But this does not reveal the dynamics through which this learning may take place, and influence action preparation. We therefore quantified the uncertainty about the forthcoming movement on a trial-by-trial basis for each block basis, using a simple information theoretic model that quantified the uncertainties inherent in trial sequences. We therefore asked whether these quantities might predict subject’s responses and their preparatory state prior to these (as measured through CSE). RTs and muscle-specific CSE changes were indeed influenced by both entropy and surprise: High uncertainty (high entropy) about the upcoming imperative cue was associated with decreases in CSE during the preparatory period (Fig. 10.1c). Moreover, a surprising imperative cue on the preceding trial resulted in a similar decrease in CSE. Thus, delay-period CSE, which provides an index of the preparatory state of a subject was lower when preparatory cues resolved less uncertainty (entropy), and when surprise in the preceding trigger cues was large (Fig. 10.1c). This effect was mirrored in the behavioural reaction time data—subjects were slower to respond when average uncertainty about the forthcoming movement was high, and furthermore, when imperative stimuli were surprising given the preceding cue (Fig. 10.1c).
One important aspect is the use of Bayesian model comparison, to test whether the specific model chosen indeed provides a good explanation of the data, compared to other competing models. This model comparison showed that there was indeed more evidence supporting the specific information theoretic model (given the observed RT and the CSE data), compared to a small group of alternative models that did not, or not fully, account for the contextual uncertainty inherent in the sequence of trials. The novel point made by these data is that human motor cortex is dynamically biased according to (inferred or learned) representations of contextual probabilities inherent in imperative visual events. These representations are likely encoded in the brain upstream of M1, but dynamically influence action preparation and selection. Recent support that this may reflect a general mechanism through which representations for actions are shaped by current contextual requirements comes from work on value-based decisions for actions (Klein-Flügge and Bestmann 2012). If action selection in motor regions emerges from a competitive process that is gradually biased by evidence signals originating in other regions, then biases reflecting the evaluation of more or less valuable choice options should be traceable in the motor system, before the decision process is complete. Using TMS to read-out CSE changes during such value-based decisions, Klein-Flügge and Bestmann (2012) found that excitability for chosen versus unchosen actions indeed distinguishes the forthcoming choice, but critically so could demonstrate that this occurs before the decision process is complete. Importantly, this required a trial-by-trial quantification of the value that participants assigned to their choices. The subjective value that participants assigned to the options offered on each trial was inferred using cumulative prospect theory (cf. Tversky and Kahnemann 1992), which could then be regressed against the dynamic trial-by-trial changes in CSE. Support for the idea that the observed dynamic changes in CSE during the choice process were indeed value-driven comes from the finding that both excitability and reaction times varied as a function of the subjective value difference between chosen and unchosen actions, and that such a relationship does not occur in the absence of a decision. This provides novel evidence in humans, using non-invasive TMS as read-out of the functional state of motor cortex, that internally generated value-based decisions influence the competition between action representations in motor cortex before the decision process is complete. More generally, these results demonstrate the importance of studying the dynamic, trial-by-trial changes that guide the preparation and selection of movements in an ever-changing world, and that such dynamics can now be usefully studied with the combination of TMS and model-based approaches.
7 Frameworks for Action Selection
The studies reviewed above clearly show that the human motor system is dynamically shaped by our prior expectations about forthcoming movements. Such prior expectations can be instilled by variables that are currently relevant for action selection, such as the expected reward that can be obtained following an action, or the likelihood of an event occurring. TMS can reveal the dynamic changes in the functional state of M1 during and prior to actions, and how these relate to our prior expectations.
However, these findings themselves do not yet provide a mechanistic account that can explain the functional role of such modulations and influences, nor how such signals actually reach the motor system. It is now established that M1 exhibits responses to sensory signals in a variety of modalities including vision and somatosensation (Hatsopoulos and Suminski 2011), and thus is likely to integrate such signals for the selection and preparation of appropriate movements. Two recent theories provide architectures and mechanistic accounts on how this may actually happen—the affordance competition hypothesis (Cisek 2006, 2007a, b; Cisek and Kalaska 2010) and active inference (Friston et al. 2009, 2011b). We note that these two accounts are not competing or mutually exclusive—in fact, it is the subject of intense ongoing research to explore their commonalities. We briefly outline the core concepts of these accounts and how they help to explain the findings reviewed above, but any discussion of these accounts in the present chapter can only be brief, and the reader is referred to the original work for in-depth details.
7.1 Affordance Competition
The key point of the affordance competition hypothesis (Cisek and Kalaska 2010) is that sensory information is continuously used to specify potentially available, and competing actions, whilst other kinds of information (such as motivation, reward expectation, new sensory information) will be accumulated and provide evidence that ultimately leads to selecting one action from the available set of actions (Cisek 2006, 2007b; Cisek and Kalaska 2010). Potential actions therefore ‘compete’ with one another, and internal representations influence this competition. As initially introduced by (Gibson 1979), the concept of affordances reflects the idea that these internal representations are opportunities, or affordances, for action defined by the environment (Fig. 10.2).
Graphical illustration of the affordance competition hypothesis. Sensory information (here illustrated for visual information flow) continuously influences the representation of potentially available actions, which ‘compete’ with one another. Internal representations and signals such as motivation, reward expectation, new sensory information provide additional evidence that ultimately leads to selecting one action from the available set of actions. As shown here, starting with the visual cortex, information is passed to the parietal lobe, where visual information is likely transformed into representations of potential actions. These representations are captured by different, and potentially competing neural populations in parietal cortex. This competition is biased by additional input from e.g. the basal ganglia and prefrontal cortical regions that accumulate and process additional information required for action selection (here indicated by the red arrows). Once competition has led to the selection of an action over its potential action alternatives, it is unleashed. This also caused feedback from the induced changes in the environment (dotted blue arrow) and feedback caused by the predictive collateral via the cerebellum. Modified with permission from Cisek and Kalaska (2010)
These affordances for action are based on incoming sensory information and internally represented decision variables (e.g. subjective reward, motivation, wakefulness, hunger), which are continuously transformed into parameters of action (Cisek and Kalaska 2010). This also implies that multiple actions might initially be available, but competition between these alternative options ultimately leads to the commitment to one specific action. This competition is thought to be driven by mutual inhibition among cells with different tuning properties (Cisek 2006), and/or through differential selection in corticostriatal circuits as likely physiological substrates.
Critically, the competition at the level of PMd/M1 is driven by inputs from other regions, such as parietal and prefrontal cortex and the basal ganglia that contain information or evidence about the most appropriate action, given the context. These regions therefore bias the competition among actions until some (unspecified) threshold is reached and a commitment to an action is made.
The TMS work in humans reviewed above indeed provides strong support for this idea. The observation that CSE changes relate to the prior expectation of an event, for example, suggest that our expectation of what will happen (and consequently what we will have to do) ‘biases’ the competition among available actions. In humans, this bias can be quantified using TMS. For example, a recent study by Michelet and co-workers shows that CSE during the reaction time of the Eriksen flanker task increases gradually for the agonist muscle, and decreases for the antagonistic muscle (Michelet et al. 2010). Critically, the opposite is initially observed in an incongruent condition—when information about which action to perform is misleading, the competition among two actions initially favours the erroneous action, and only later reverses as sensory information provides sufficient evidence for the correct action. This is reflected in the observed CSE changes which initially increase for the erroneous action, and then reverse gradually. These findings elegantly show that the dynamic modulation in CSE resembles the competition among alternative actions, which ultimately leads to the selection of one response and the rejection of the other.
7.2 Active Inference and Predictive Coding
Active inference is a corollary of the free-energy principle and the predictive coding account (Friston and Kiebel 2009). In short, this idea states that a self-organising system like the brain should minimise the free energy of sensory states it samples. Here, free energy itself is an upper bound on the surprise (prediction error) associated with sensory signals, such as visual cues. Simply put, free energy is the (Bayesian) evidence for the brain’s model of its world. This means that when the brain minimises free energy, it reduces surprising exchanges with the world. Equivalently, it means that it maximises the evidence for its own internal model of its sensory world (see Fig. 10.3).
Active inference and predictive coding. Active inference is a generalisation of predictive coding that covers motor behaviours and itself is a special instance of the principle of free energy minimisation (cf. Friston et al. 2011b). Free energy is a statistical quantity that bounds the surprise (self-information) associated with sensory signals. This surprise is quantified in relation to a generative model of how those signals were caused. Predictive coding uses prediction error as a proxy for free energy (cf. surprise) and rests on a hierarchical model, in which prediction errors are passed up the hierarchy (red arrows) to optimise high-level representations that provide top-down predictions (black arrows). In this schematic, prediction error units are portrayed in red and units encoding the conditional expectations of the hidden causes of sensory input are shown in blue. During perception, the best explanation for sensory input emerges when the top-down predictions can explain as much of the prediction error (at each hierarchical level) as possible. Active inference takes this one step further and notes that certain sensory modalities can use prediction errors to drive motoneurons to eliminate prediction error directly (through classical motor reflex arcs). This is shown schematically on the lower left, using units in the dorsal and ventral horns of the spinal cord. Under active inference, a movement just fulfils the predictions afforded by percepts that predict both exteroceptive (e.g. visual) and interoceptive (e.g. stretch receptor) consequences. This high-level (sensorimotor) percept is activated by an exteroceptive (sensory) cue, and the ensuing top-down predictions propagate to both sensory cortex (to suppress exteroceptive prediction error) and the motor system. However, in the motor system, the predictions engender a prediction error that is eliminated by movement (adapted from Brown et al. 2011)
More specifically, predictive coding is based on the assumption that the brain makes inferences about the causes of its own sensations and percepts (see Feldman and Friston 2010; Friston 2006, 2009, 2010; Friston et al. 2010; Friston and Stephan 2007). These inferences are driven (or inhibited) by bottom-up or feed-forward sensory information (see Fig. 10.3). This information is conveyed to higher brain areas in the form of prediction errors (Rao and Ballard 1999; Friston et al. 2008). By contrast, top-down or backward connections signal the predictions the brain makes about the information that will be received at the lower level. These predictions aim at suppressing prediction errors. An ideal state would be when predictions are optimal, and prediction errors therefore are minimal, i.e. the brain would perfectly explain (predict) the world which it samples through its own sensations.
One important concept is that of top-down first-order and contextual second-order predictions. The former drive (or inhibit) neurons reporting prediction errors, whereas the latter reflect the precision (or reliability) of these predictions errors. Put simply, the precision can be regarded as representing the reliability, ambiguity, or uncertainty about sensory signals, such as visual cues. As seen previously, there is a rich body of work that shows that the motor system is highly sensitive to such second-order effects, e.g. changes in the reliability of visual cues instructing movement (Bestmann et al. 2008a; Brown et al. 2011; Mars et al. 2007). Top-down predictions can therefore have a direct (first-order) or a modulatory (second-order) effect on the responses of prediction error units that make the ensuing predictions as efficient as possible.
Active inference (Brown et al. 2011; Friston et al. 2011b) extends this architecture, suggesting that exactly the same recursive message passing also applies to the motor system. The only difference here is that prediction errors at the lowest level (i.e. the cranial nerve nuclei and spinal cord) are suppressed by movement, through classical reflex arcs. In this view, descending (corticospinal) signals are not motor commands in the traditional sense per se, but predictions of the proprioceptive signals that arise from movement. The peripheral motor system, through movement, therefore tries to fulfil its predictions about proprioceptive signals (see Friston (2009, 2010) for an in-depth treatment). In this view, a sensory cued movement is generated by a high-level (sensorimotor) representation that predicts a particular pattern of proprioceptive and exteroceptive sensory signals. This representation arises to explain prediction errors caused by, e.g., a visual cue, while motor reflexes suppress the ensuing prediction errors in the proprioceptive domain. This framework has been used to explain several features of the motor system and a series of behaviours, from visual tracking (Friston 2009; Friston and Kiebel 2009), motor preparation (Brown et al. 2011), to action observation (Friston et al. 2010). An obvious appeal of this idea is that the same architecture and principle about hierarchical message passing and integration can now be applied to both sensory and motor systems. This has intuitive appeal because it assumes that the brain does not use different approaches for dealing with similar problems.
In other words, this framework provides a unifying account for the organizational principles underpinning sensory perception and action: if ascending sensory signals are prediction errors and descending motor commands are predictions, then the optimisation of predictions (and the resulting movements) should depend on optimising precision (i.e. reliability) in exactly the same way as in sensory processing. Initial modelling work and behavioural experiments (Brown et al. 2011) support this view. These suggest that motor preparation (and selection) is ultimately directed towards proprioceptive sensations, i.e., the predicted sensory feedback that will be elicited from the anticipated motor response (Brown et al. 2011).
Importantly, both concepts can be brought together when considering that high-level sensorimotor representations are often dynamic in nature. Time variant neural dynamics represent prior beliefs or expectations about, for example, the sequence of sensorimotor events or trajectories that will arise in the near future (Friston et al. 2011a). One way of viewing these is as attractors that provide proprioceptive and sensory predictions for sensorimotor integration. These, in other words, are the representations of affordance. The selection of an action relies on accurate bottom-up prediction errors conveying salient sensory information that has yet to be explained. Or, phrased differently, the brain aims to select those representations with an affordance that best explains sensory input, which is equivalent to affordance competition. Put simply, bottom-up prediction errors bias competition amongst high(er) level sensorimotor representations (attractors).
The key point here is that both accounts introduced here predict that our motor system will be influenced by the predictions our brain makes about forthcoming movements, and that they make specific statements on how such influences originate. For example, the affordance competition model provides testable predictions about the likely routes through which specific types of information will be conveyed to the motor system; this depends on the functional specialisation of regions in parietal or frontal cortex, and the basal ganglia. Recent double-coil TMS studies have indeed addressed how, for example, premotor and parietal regions influence the functional state of M1 during different types of movement tasks, and at rest (Koch et al. 2006, 2007, 2008). Active inference, and the hierarchical predictive coding account it is resting on, makes specific predictions about the type of connections that convey the information that allows for an action to be chosen and executed. Bottom-up sensory information (prediction errors) that has yet to be explained by top-down predictions is generally associated with the activity of superficial pyramidal cells (Mumford 1992; Friston et al. 2010; Brown et al. 2011). With regards to action selection, descending (cortico-spinal) signals are not motor commands in the traditional sense per se, but predictions of the proprioceptive signals that arise from movement. In the future, it will therefore be of interest to record from the different descending and ascending pathways, and to determine whether information in these may indeed reflect movement-related predictions and prediction errors, respectively. Both accounts vary in their specific aims, complexity and architecture. However, they provide frameworks in which to address how information influences our motor system to ensure that our actions remain flexible and accurate in an uncertain and ever-changing world.
8 Summary
Transcranial magnetic stimulation (TMS) provides a window to examine computational processes that the brain may use to implement actions in real time, and their influence on output stage. In humans, TMS can thus be used to read-out the functional state of motor system during action preparation and selection, and thus provide insights into their physiological underpinnings in an unprecedented way.
References
Alexander GE, Crutcher MD, DeLong MR (1990) Basal ganglia-thalamocortical circuits: parallel substrates for motor, oculomotor, “prefrontal” and “limbic” functions. Prog Brain Res 85:119–146
Bastian A, Riehle A, Erlhagen W, Schoner G (1998) Prior information preshapes the population representation of movement direction in motor cortex. NeuroReport 9:315–319
Bestmann S, Harrison LM, Blankenburg F, Mars RB, Haggard P, Friston KJ, Rothwell JC (2008a) Influence of uncertainty and surprise on human corticospinal excitability during preparation for action. Curr Biol 18:775–780
Bestmann S, Ruff CC, Blankenburg F, Weiskopf N, Driver J, Rothwell JC (2008b) Mapping causal interregional influences with concurrent TMS-fMRI. Exp Brain Res 191:383–402
Bhushan N, Shadmehr R (1999) Computational nature of human adaptive control during learning of reaching movements in force fields. Biol Cybern 81:39–60
Boorman ED, Behrens TE, Woolrich MW, Rushworth MF (2009) How green is the grass on the other side? frontopolar cortex and the evidence in favor of alternative courses of action. Neuron 62:733–743
Brown H, Friston K, Bestmann S (2011) Active inference, attention, and motor preparation. Front Hum Neurosci 2:A218
Bullier J, Nowak LG (1995) Parallel versus serial processing: new vistas on the distributed organization of the visual system. Curr Opin Neurobiol 5:497–503
Burle B, Vidal F, Tandonnet C, Hasbroucq T (2004) Physiological evidence for response inhibition in choice reaction time tasks. Brain Cogn 56:153–164
Carello CD, Krauzlis RJ (2004) Manipulating intent: evidence for a causal role of the superior colliculus in target selection. Neuron 43:575–583
Coe B, Tomihara K, Matsuzawa M, Hikosaka O (2002) Visual and anticipatory bias in three cortical eye fields of the monkey during an adaptive decision-making task. J Neurosci 22:5081–5090
Chen R (2004) Interactions between inhibitory and excitatory circuits in the human motor cortex. Exp Brain Res 154:1–10
Chen R, Hallett M (1999) The time course of changes in motor cortex excitability associated with voluntary movement. Can J Neurol Sci 26:163–169
Chen R, Yaseen Z, Cohen LG, Hallett M (1998) Time course of corticospinal excitability in reaction time and self-paced movements. Ann Neurol 44:317–325
Cisek P (2005) Neural representations of motor plans, desired trajectories, and controlled objects. Cogn Process 6:15–24
Cisek P (2006) Integrated neural processes for defining potential actions and deciding between them: a computational model. J Neurosci 26:9761–9770
Cisek P (2007a) A parallel framework for interactive behavior. Prog Brain Res 165:475–492
Cisek P (2007b) Cortical mechanisms of action selection: the affordance competition hypothesis. Philos Trans R Soc Lond B Biol Sci 362:1585–1599
Cisek P, Kalaska JF (2004) Neural correlates of mental rehearsal in dorsal premotor cortex. Nature 431:993–996
Cisek P, Kalaska JF (2005) Neural correlates of reaching decisions in dorsal premotor cortex: specification of multiple direction choices and final selection of action. Neuron 45:801–814
Cisek P, Kalaska JF (2010) Neural mechanisms for interacting with a world full of action choices. Annu Rev Neurosci 33:269–298
Civardi C, Cantello R, Asselman P, Rothwell JC (2001) Transcranial magnetic stimulation can be used to test connections to primary motor areas from frontal and medial cortex in humans. Neuroimage 14:1444–1453
Corrado G, Doya K (2007) Understanding neural coding through the model-based analysis of decision making. J Neurosci 27:8178–8180
Coxon JP, Stinear CM, Byblow WD (2006) Intracortical inhibition during volitional inhibition of prepared action. J Neurophysiol 95:3371–3383
Crammond DJ, Kalaska JF (1994) Modulation of preparatory neuronal activity in dorsal premotor cortex due to stimulus-response compatibility. J Neurophysiol 71:1281–1284
Crammond DJ, Kalaska JF (2000) Prior information in motor and premotor cortex: activity during the delay period and effect on pre-movement activity. J Neurophysiol 84:986–1005
Davey NJ, Rawlinson SR, Maskill DW, Ellaway PH (1998) Facilitation of a hand muscle response to stimulation of the motor cortex preceding a simple reaction task. Mot Control 2:241–250
Di Lazzaro, V, Oliviero A, Profice P, Insola A, Mazzone P, Tonali P, Rothwell JC (1999) Direct demonstration of interhemispheric inhibition of the human motor cortex produced by transcranial magnetic stimulation. Exp Brain Res 124:520–524
Di Lazzaro V, Oliviero A, Pilato F, Saturno E, Dileone M, Mazzone P, Insola A, Tonali PA, Rothwell JC (2004) The physiological basis of transcranial motor cortex stimulation in conscious humans. Clin Neurophysiol 115:255–266
Dum RP, Strick PL (2005) Frontal lobe inputs to the digit representations of the motor areas on the lateral surface of the hemisphere. J Neurosci 25:1375–1386
Duque J, Ivry RB (2009) Role of corticospinal suppression during motor preparation. Cereb Cortex 19:2013–2024
Duque J, Lew D, Mazzocchio R, Olivier E, Ivry RB (2010) Evidence for two concurrent inhibitory mechanisms during response preparation. J Neurosci 30:3793–3802
Feldman H, Friston KJ (2010) Attention, uncertainty, and free-energy. Front Hum Neurosci 4:215
Flash T, Hogan N (1985) The coordination of arm movements: an experimentally confirmed mathematical model. J Neurosci 5:1688–1703
Friston K (2009) The free-energy principle: a rough guide to the brain? Trends Cogn Sci 13:293–301
Friston K (2008) Hierarchical models in the brain. PLoS Comput Biol 4:e1000211
Friston K (2010) The free-energy principle: a unified brain theory? Nat Rev Neurosci 11:127–138
Friston K, Kiebel S (2009) Predictive coding under the free-energy principle. Philos Trans R Soc Lond B Biol Sci 364:1211–1221
Friston KJ, Stephan KE (2007) Free-energy and the brain. Synthese 159:417–458
Friston K, Kilner J, Harrison L (2006) A free energy principle for the brain. J Physiol Paris 100:70–87
Friston KJ, Daunizeau J, Kiebel SJ (2009) Reinforcement learning or active inference? PLoS One 4:e6421
Friston KJ, Daunizeau J, Kilner J, Kiebel SJ (2010) Action and behavior: a free-energy formulation. Biol Cybern 102:227–260
Friston K, Shiner T, Fitzgerald T, Galea J, Adams R, Brown H, Dolan R, Moran R, Stephan KE, Bestmann S (2011a) Dopamine, precision and affordance in active inference. Plos Comp Biol 8:e1002327
Friston K, Mattout J, Kilner J (2011b) Action understanding and active inference. Biol Cybern 104:137–160
Fritsch G, Hitzig E (1870) Über die elektrische Erregbarkeit des Grosshirns. Arch Anat Physiol Wissen 37:300–332
Fritsch G, Hitzig E (2009) Electric excitability of the cerebrum (Uber die elektrische Erregbarkeit des Grosshirns). Epilepsy Behav 15:123–130
Gibson JJ (1979) The ecological approach to visual perception. Houghton Mifflin, Boston
Gold JI, Shadlen MN (2001) Neural computations that underlie decisions about sensory stimuli. Trends Cogn Sci 5:10–16
Gold JI, Shadlen MN (2007) The neural basis of decision making. Annu Rev Neurosci 30:535–574
Groppa S, Schlaak BH, Munchau A, Werner-Petroll N, Dunnweber J, Baumer T, van Nuenen BF, Siebner HR (2011) The human dorsal premotor cortex facilitates the excitability of ipsilateral primary motor cortex via a short latency cortico-cortical route. Hum Brain Mapp 33:419–430
Hasbroucq T, Kaneko H, Akamatsu M, Possamai CA (1997) Preparatory inhibition of cortico-spinal excitability: a transcranial magnetic stimulation study in man. Brain Res Cogn Brain Res 5:185–192
Hasbroucq T, Kaneko H, Akamatsu M, Possamai CA (1999a) The time-course of preparatory spinal and cortico-spinal inhibition: an H-reflex and transcranial magnetic stimulation study in man. Exp Brain Res 124:33–41
Hasbroucq T, Osman A, Possamai CA, Burle B, Carron S, Depy D, Latour S, Mouret I (1999b) Cortico-spinal inhibition reflects time but not event preparation: neural mechanisms of preparation dissociated by transcranial magnetic stimulation. Acta Psychol (Amst) 101:243–266
Hasbroucq T, Akamatsu M, Burle B, Bonnet M, Possamai CA (2000) Changes in spinal excitability during choice reaction time: the H reflex as a probe of information transmission. Psychophysiology 37:385–393
Hatsopoulos NG, Suminski AJ (2011) Sensing with the motor cortex. Neuron 72:477–487
Hiraoka K, Kamata N, Matsugi A, Iwata A (2010) Premovement facilitation of corticospinal excitability before simple and sequential movement. Percept Mot Skills 111:129–140
Hoshiyama M, Kitamura Y, Koyama S, Watanabe S, Shimojo M, Kakigi R (1996a) Reciprocal change of motor evoked potentials preceding voluntary movement in humans. Muscle Nerve 19:125–131
Hoshi E, Shima K, Tanji J (2000) Neuronal activity in the primate prefrontal cortex in the process of motor selection based on two behavioral rules. J. Neurophysiol 83:2355–2373
Horwitz GD, Batista AP, Newsome WT (2004) Representation of an abstract perceptual decision in macaque superior colliculus. J. Neurophysiol 91:2281–2296
Hoshiyama M, Kitamura Y, Koyama S, Watanabe S, Shimojo M, Kakigi R (1996b) Reciprocal change of motor evoked potentials preceding voluntary movement in humans. Muscle Nerve 19:125–131
Hoshiyama M, Kakigi R, Koyama S, Takeshima Y, Watanabe S, Shimojo M (1997) Temporal changes of pyramidal tract activities after decision of movement: a study using transcranial magnetic stimulation of the motor cortex in humans. Electroencephalogr Clin Neurophysiol 105:255–261
Hubbard EM, Piazza M, Pinel P, Dehaene S (2005) Interactions between number and space in parietal cortex. Nat Rev Neurosci 6:435–448
Hummel FC, Steven B, Hoppe J, Heise K, Thomalla G, Cohen LG, Gerloff C (2009) Deficient intracortical inhibition (SICI) during movement preparation after chronic stroke. Neurology 72:1766–1772
Ivry RB, Spencer RM (2004) The neural representation of time. Curr Opin Neurobiol 14:225–232
Kawato M, Maeda Y, Uno Y, Suzuki R (1990) Trajectory formation of arm movement by cascade neural network model based on minimum torque-change criterion. Biol Cybern 62:275–288
Koch G, Franca M, Del Olmo MF, Cheeran B, Milton R, Alvarez SM, Rothwell JC (2006) Time course of functional connectivity between dorsal premotor and contralateral motor cortex during movement selection. J Neurosci 26:7452–7459
Koch G, Franca M, Mochizuki H, Marconi B, Caltagirone C, Rothwell JC (2007) Interactions between pairs of transcranial magnetic stimuli over the human left dorsal premotor cortex differ from those seen in primary motor cortex. J Physiol 578:551–562
Koch G, Fernandez DO, Cheeran B, Schippling S, Caltagirone C, Driver J, Rothwell JC (2008) Functional interplay between posterior parietal and ipsilateral motor cortex revealed by twin-coil transcranial magnetic stimulation during reach planning toward contralateral space. J Neurosci 28:5944–5953
Klein-Flügge MC, Bestmann S (2012) Time-dependent changes in human corticospinal excitability reveal value-based competition for action during decision processing. J Neurosci 32:8373–8382
Kujirai T, Caramia MD, Rothwell JC, Day BL, Thompson PD, Ferbert A, Wroe S, Asselman P, Marsden CD (1993) Corticocortical inhibition in human motor cortex. J Physiol 471:501–519
Ledberg A, Bressler SL, Ding M, Coppola R, Nakamura R (2007) Large-scale visuomotor integration in the cerebral cortex. Cereb Cortex 17:44–62
Leocani L, Cohen LG, Wassermann EM, Ikoma K, Hallett M (2000) Human corticospinal excitability evaluated with transcranial magnetic stimulation during different reaction time paradigms. Brain 123(Pt 6):1161–1173
Liepert J, Dettmers C, Terborg C, Weiller C (2001) Inhibition of ipsilateral motor cortex during phasic generation of low force. Clin Neurophysiol 112:114–121
Liuzzi G, Horniss V, Hoppe J, Heise K, Zimerman M, Gerloff C, Hummel FC (2010) Distinct temporospatial interhemispheric interactions in the human primary and premotor cortex during movement preparation. Cereb Cortex 20:1323–1331
Lu MT, Preston JB, Strick PL (1994) Interconnections between the prefrontal cortex and the premotor areas in the frontal lobe. J Comp Neurol 341:375–392
Mars RB, Bestmann S, Rothwell JC, Haggard P (2007) Effects of motor preparation and spatial attention on corticospinal excitability in a delayed-response paradigm. Exp Brain Res 182:125–129
Mars RB, Hulstijn W, Toni I (2008) Selection, preparation, and monitoring: current approaches to studying the neural control of action. Cortex 44:479–481
Mars RB, Shea NJ, Kolling N, Rushworth MF (2010) Model-based analyses: promises, pitfalls, and example applications to the study of cognitive control. Q J Exp Psychol (Colchester) 29:1–16
Mesulam MM (1990) Large-scale neurocognitive networks and distributed processing for attention, language, and memory. Ann Neurol 28:597–613
Michelet T, Duncan GH, Cisek P (2010) Response competition in the primary motor cortex: corticospinal excitability reflects response replacement during simple decisions. J Neurophysiol 104:119–127
Mochizuki H, Huang YZ, Rothwell JC (2004) Interhemispheric interaction between human dorsal premotor and contralateral primary motor cortex. J Physiol 561:331–338
Mumford D (1992) On the computational architecture of the neocortex. II. The role of cortico-cortical loops. Biol Cybern 66:241–251
Nakamura K (2006) Neural representation of information measure in the primate premotor cortex. J Neurophysiol 96:478–485
O’Doherty JP, Hampton A, Kim H (2007) Model-based fMRI and its application to reward learning and decision making. Ann N Y Acad Sci 1104:35–53
Oliveira FT, Diedrichsen J, Verstynen T, Duque J, Ivry RB (2010) Transcranial magnetic stimulation of posterior parietal cortex affects decisions of hand choice. Proc Natl Acad Sci U S A 107:17751–17756
Pesaran B (2010) Neural correlations, decisions, and actions. Curr Opin Neurobiol 20:166–171
Platt ML, Glimcher PW (1999) Neural correlates of decision variables in parietal cortex. Nature 400:233–238
Rao RP, Ballard DH (1999) Predictive coding in the visual cortex: a functional interpretation of some extra-classical receptive-field effects. Nat Neurosci 2:79–87
Renoult L, Roux S, Riehle A (2006) Time is a rubberband: neuronal activity in monkey motor cortex in relation to time estimation. Eur J Neurosci 23:3098–3108
Requin J, Riehle A, Seal J (1988) Neuronal activity and information processing in motor control: from stages to continuous flow. Biol Psychol 26:179–198
Reynolds C, Ashby P (1999) Inhibition in the human motor cortex is reduced just before a voluntary contraction. Neurology 53:730–735
Rizzolatti G, Luppino G, Matelli M (1998) The organization of the cortical motor system: new concepts. Electroencephalogr Clin Neurophysiol 106:283–296
Roesch MR, Olson CR (2003) Impact of expected reward on neuronal activity in prefrontal cortex, frontal and supplementary eye fields and premotor cortex. J Neurophysiol 90:1766–1789
Roesch MR, Olson CR (2004) Neuronal activity related to reward value and motivation in primate frontal cortex. Science 304:307–310
Roesch MR, Olson CR (2007) Neuronal activity related to anticipated reward in frontal cortex: does it represent value or reflect motivation? Ann N Y Acad Sci 1121:431–446
Romaiguere P, Possamai CA, Hasbroucq T (1997) Motor cortex involvement during choice reaction time: a transcranial magnetic stimulation study in man. Brain Res 755:181–192
Romo R, Hernandez A, Zainos A (2004) Neuronal correlates of a perceptual decision in ventral premotor cortex. Neuron 41:165–173
Rosenbaum DA (1980) Human movement initiation: specification of arm, direction, and extent. J Exp Psychol Gen 109:444–474
Roux S, Coulmance M, Riehle A (2003) Context-related representation of timing processes in monkey motor cortex. Eur J Neurosci 18:1011–1016
Roux S, MacKay WA, Riehle A (2006) The pre-movement component of motor cortical local field potentials reflects the level of expectancy. Behav Brain Res 169:335–351
Rubino D, Robbins KA, Hatsopoulos NG (2006) Propagating waves mediate information transfer in the motor cortex. Nat Neurosci 9:1549–1557
Rudebeck PH, Bannerman DM, Rushworth MF (2008) The contribution of distinct subregions of the ventromedial frontal cortex to emotion, social behavior, and decision making. Cogn Affect Behav Neurosci 8:485–497
Ruff CC, Driver J, Bestmann S (2009) Combining TMS and fMRI: from ‘virtual lesions’ to functional-network accounts of cognition. Cortex 45:1043–1049
Sakai K, Ugawa Y, Terao Y, Hanajima R, Furubayashi T, Kanazawa I (1997) Preferential activation of different I waves by transcranial magnetic stimulation with a figure-of-eight-shaped coil. Exp Brain Res 113:24–32
Schall JD (2001) Neural basis of deciding, choosing and acting. Nat Rev Neurosci 2:33–42
Schultz W (2006) Behavioral theories and the neurophysiology of reward. Annu Rev Psychol 57:87–115
Sinclair C, Hammond GR (2008) Reduced intracortical inhibition during the foreperiod of a warned reaction time task. Exp Brain Res 186:385–392
Sinclair C, Hammond GR (2009) Excitatory and inhibitory processes in primary motor cortex during the foreperiod of a warned reaction time task are unrelated to response expectancy. Exp Brain Res 194:103–113
Smid HG, Lamain W, Hogeboom MM, Mulder G, Mulder LJ (1991) Psychophysiological evidence for continuous information transmission between visual search and response processes. J Exp Psychol Hum Percept Perform 17:696–714
Soto O, Valls-Sole J, Kumru H (2010) Paired-pulse transcranial magnetic stimulation during preparation for simple and choice reaction time tasks. J Neurophysiol 104:1392–1400
Tandonnet C, Garry MI, Summers JJ (2010) Cortical activation during temporal preparation assessed by transcranial magnetic stimulation. Biol Psychol 85:481–486
Tanji J, Evarts EV (1976) Anticipatory activity of motor cortex neurons in relation to direction of an intended movement. J Neurophysiol 39:1062–1068
Terao Y, Furubayashi T, Okabe S, Mochizuki H, Arai N, Kobayashi S, Ugawa Y (2007) Modifying the cortical processing for motor preparation by repetitive transcranial magnetic stimulation. J Cogn Neurosci 19:1556–1573
Touge T, Taylor JL, Rothwell JC (1998) Reduced excitability of the cortico-spinal system during the warning period of a reaction time task. Electroencephalogr Clin Neurophysiol 109:489–495
Tversky A , Kahneman D (1992) Advances in prospect theory: cumulative representation of uncertainty. J Risk Uncertainty 5:297–323
van den HP, Mars RB, van Elswijk G, Hegeman J, Pasman JW, Bloem BR, Toni I (2007) Online maintenance of sensory and motor representations: effects on corticospinal excitability. J Neurophysiol 97:1642–1648
van Elswijk G, Kleine BU, Overeem S, Stegeman DF (2007) Expectancy induces dynamic modulation of corticospinal excitability. J Cogn Neurosci 19:121–131
van Elswijk G, Schot WD, Stegeman DF, Overeem S (2008) Changes in corticospinal excitability and the direction of evoked movements during motor preparation: a TMS study. BMC Neurosci 9:51
Van Hoesen GW, Solodkin A (1993) Some modular features of temporal cortex in humans as revealed by pathological changes in Alzheimer’s disease. Cereb Cortex 3:465–475
Weinrich M, Wise SP, Mauritz KH (1984) A neurophysiological study of the premotor cortex in the rhesus monkey. Brain 107(Pt 2):385–414
Wise SP, Weinrich M, Mauritz KH (1983) Motor aspects of cue-related neuronal activity in premotor cortex of the rhesus monkey. Brain Res 260:301–305
Wise SP, Weinrich M, Mauritz KH (1986) Movement-related activity in the premotor cortex of rhesus macaques. Prog Brain Res 64:117–131
Yang T, Shadlen MN (2007) Probabilistic reasoning by neurons. Nature 447:1075–1080
Ziemann U, Rothwell JC (2000) I-waves in motor cortex. J Clin Neurophysiol 17:397–405
Acknowledgments
The author is supported by the Biotechnology and Biological Sciences Research Council (BBSRC) and the European Research Council (ERC).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2012 Springer Berlin Heidelberg
About this chapter
Cite this chapter
Bestmann, S. (2012). Functional Modulation of Primary Motor Cortex During Action Selection. In: Chen, R., Rothwell, J. (eds) Cortical Connectivity. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-642-32767-4_10
Download citation
DOI: https://doi.org/10.1007/978-3-642-32767-4_10
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-642-32766-7
Online ISBN: 978-3-642-32767-4
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)