Abstract
The chapter is dedicated to a promising method of biomass treatment—plasma gasification. Increased temperatures and energy supply allows significantly increase the range of wastes and other carbonaceous materials which could be efficiently processed. Features of plasma usage in updraft and downdraft biomass gasification are described. Several promising renewable energy sources (wood, energy crops, wastes of livestock, and poultry industry) are examined for the usage in downdraft plasma gasification. The correlation of key parameters of biomass plasma gasification was studied in thermodynamic equilibrium approach along with syngas usage for liquid fuel production. Institute for Electrophysics and Electric Power RAS experimental installation is described. Its primary component is a downdraft plasma gasifier for processing of biomass and wastes. Its technical characteristics and functionality are described. A brief survey of existing pilot and industrial projects is given. Methods of energy supply into plasma chemical reactor are described. The review of powerful plasma torches for industrial application is represented. Experimental procedures and test results on biomass gasification by air-plasma are presented as well as the comparison with the calculated data.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Plasma
- Gasification
- Syngas
- Plasma torch
- Gasifier
- Energy balance
- Efficiency
- Biomass
- Renewable energy
- Alternative energy
1 Introduction
The importance of the decrease of anthropogenic impact on the environment rises dramatically nowadays. In particular, it is a problem of carbon dioxide emissions [1]. CO2 is the basic component of combustion products of widely used kinds of fuel; it possesses high radiative forcing and is one of the main (and the most dangerous) greenhouse gases [2]. In 2010, the global emission of carbon dioxide increases on ~5.9 % and for the first time exceeds 9 Pg per year [3]. The increase in emission is mainly caused by economic growth of developing economics in which even during a global economic crisis the CO2 emission increased [3]. Economic growth and increase in the living standards of the population invariably lead to growth of energy consumption per capita. According to IEA [4], in 2009 about 80.9 % of mankind energy demands were provided by fossil fuel combustion, about 5.8 %—nuclear energy, about 2.3 % hydroenergetics, and about 10.2 %—energy of biofuels and waste, which in total is 509 EJ. The world’s reserve of fossil fuels is about 35,094 EJ (~23.6 %—oil, ~20.4 %—natural gas, ~56.0 %—coal) [5]. By estimations [6] world oil production will peak before 2020, coal before 2030, and natural gas around 2040. Unfortunately, the comprehension of not only the importance, but also the fact of finiteness of fossil fuels occurs extremely slowly among people defining directions of development both on regional and global level. The citation ideally illustrates the situation: “perpetual growth is often held as a pious belief and fundamental assumption for economists” [7]. The only possibility for humanity to prevent impending energy crisis is usage of renewable energy sources: biomass, solar energy, wind, tidal and wave power, hydroenergetics, and thermal power. Electricity generation from solar energy is very expensive that is why construction of large power plants is unlikely. The wind farms have low efficiency and their applicability is limited. Nowadays, the natural resources for development of hydroenergetics are almost exhausted. In spite of biosphere limits on bioresources generation governed by necessity to sustain the ecological balance and growth of mankind food demand the biomass is one of the most promising types of renewable energy sources. According to the forecasts [8] in 2050, the global potential of biomass energy will be about 1,135–1,300 EJ (without the use of seaweed as biomass), while the world consumption will reach ~826 EJ (on average under different scenarios). Energy use of biomass does not increase CO2emission, as far as the whole carbon dioxide, formed after bioenergy use, is absorbed by green plants in the process of biomass formation. Other renewable sources hardly will be widely used; in particular, nuclear fusion energy can be practically used not until the end of the century. Nuclear power cost will rapidly increase due to safety issues. Thus, it is clear that if the cultivation requirements of bioresources are met, then the bio-energetic development is one of the most promising ways to form a sustainable and independent economics for both developed and developing countries.
2 Biomass Sources
All organics substances originating from living nowadays or recently lived organisms are consideredas a biomass. For example, coal and oil are not a biomass as they were formed from organisms living millions years ago. On the other hand, a municipal and industrial waste can be considered as a biomass because an essential part of their organic weight is such kinds of a biomass as wood, rubbers, food waste, and other materials of an organic nature. Clearly, a biomass is one of the most diverse (by quantity of representatives) class of fuels. Therefore, determination of the perspectives of their energy use demands examination of energy characteristics and chemical composition along with the rates of biomass formation and expenditures connected with its production. We will examine four common types of biomass: wood, energy crops, animal waste, and poultry waste.
2.1 Wood Waste
Wood, perhaps, is one of the most common types of biomass. Approximately 40.5 % of annually extracted forest resources are used for obtaining the roundwood, ~6.3 % in paper manufacturing, ~45.1 % as a fuel, and about 8.1 % at charcoal production (total wood consumption ~2.48 Gt) [9]. The average density of wood with moisture of 20 % (~624 kg/m3 [10]) was used to estimate mass of wood by its volume. The wood (moisture ~20 %) consumption during pyrolysis aimed on charcoal production was estimated by proximate analysis results on total yield of ash and fixed carbon (~12.1 % [11]). Wood, compared to fossil fuels, basically does not contain sulfur and other ecologically unfriendly elements. However, its use in the wood industry is more economically sound than its energy application. Therefore, it is more expedient to process lumbering wastes, residues, and municipal waste mostly consisting of woody materials.
2.2 Energy Crops
Development of the biofuel production processes had begun in the nineteenth century; in the beginning of the twentieth century interest to the biofuels had died out because of the rapid growth of cheaper fossil fuels usage; developments in this field have been resumed again due to the oil crisis in 1973 [12]. Today, the development of biofuels production and using technologies is driven by: increase in prices of energy resources, fossil fuels depletion, and also CO2 emission issue.
Energy crops are plants which have been cultivated as a source of energy. Basically they are represented as herbaceous or woody fast-growing plants, for example switchgrass [13] and willow [14]. Algae are one of the most promising biofuels. Fertile lands are not required for their cultivation, and they grow in virtually any kind of water [15].
Brazil was the major producer of biofuels until 2000; however, by 2008 its world output has increased from ~20 billion liters a year to almost 75 billion, basically due to the rapid development of the bio-energy technologies in the USA and Canada [16].
One of the most promising sources of biomass is Panicum virgatum, well-known as switchgrass. Let us examine it as a characteristic representative of energy crops.
2.3 Livestock and Poultry Waste
The cattle and poultry breeding are the vital branches of agriculture which overall production is closely associated with population and its living standards. Local demand on their production grows with the increase of the population density. Partly therefore a shift toward larger farms which do not produce their own forage is observed recently in this sector; it made the manure disposal issue very important. Its mishandling leads to bacteriological contamination of ground waters [17]. Meanwhile manure is a biomass and it can be used as a renewable energy source.
The manure composition can significantly vary depending on disposal technologies, even if it is produced by the same group of animals. Therefore, determination of average composition of waste demands separate investigation. Here, for example, cow and chicken manure will be studied.
2.4 Comparison of their Suitability for Plasma Gasification
Determination of the most promising types of biomass for the plasma gasification is a big challenge. For its solution it is necessary to carry out researches in absolutely different areas: gasification technologies (taking into account special features of plasma usage), wood industry, agriculture, and also other industries producing biomass as end- or by-product. Therefore, in this work the problem of prospectivity is considered not among the vast diversity of biomass types, but among several representatives chosen for the consideration.
Let us discuss plasma gasification features versus autothermal one. Currently gasification utilizes only thermal plasma. Correspondingly, the plasma influence on the process is defined by its contribution to energy balance (the higher share of plasma energy is the grater difference it makes for the process). In the plasma injection zone of a reactor the temperatures grows significantly in comparison with the similar autothermal gasifiers that results in increase of chemical reaction rates. Content and yield of valuable products of gasification (hydrogen and carbon monoxide) increase due to additional energy injected with plasma. Moreover, high efficiency of energy introduction makes it possible to gasify feedstock by pure steam and carbon dioxide.
The gasifier type and the duration of main stages of gasification determine the syngas composition. The gasifier type defines the direction of mass stream of the process. Plasma is used in two general types of gasifiers: counter-current fixed bed (updraft), co-current fixed bed (downdraft). In the former, the gas stream (oxidant and gasification products) is directed upward, whereas in the latter it is downward, but in both cases the feedstock stream is directed downward. Solid gasification products mainly consist of inorganic components and are discharged from the bottom of the reactor
In the updraft process, the organic matter during gasification is first devolatilized and then oxidized. Pyrolysis products (volatiles) are formed and leave the reactor volume at rather cold zones where tars, water, and some permanent gases are not converted to syngas. Sometimes in such cases the separate unit for tar plasma conversion is used. Plasma usage in the updraft gasifier allows liquid slag discharge.
In the downdraft process, the organic matter during gasification is also first devolatilized and then oxidized. However, in this case the pyrolysis products pass through the high-temperature oxidizing zone, where they are converted by plasma. It results in considerable reduction of tar concentration of syngas and allows using plasma energy to increase H2 and CO yield and content.
There are parameters permitting preliminary estimation of the prospects and suitability of raw materials for plasma gasification (see Table 12.1).
Proximate analysis data show that organic matter of chicken manure consists of more than 90 % (moisture + volatile matter) from substances turning into gaseous phase during the devolatilization stage, for other types of biomass this value is more than 80 %. In general, more the content of volatile matter, easier and more effective is its use in the downdraft plasma gasification [31]. In the updraft process, it becomes a disadvantage because volatile matter is not exposed to high-energy plasma flow and the produced syngas is heavily contaminated by tars [32]. Conversion of these tars is an important line of plasma usage development. For example, Europlasma is developing a reactor module comprising an autothermal gasifier (similar to updraft one on organization of mass flows) and plasma system for tar conversion, thus the most complete conversion occurs at energy consumption of ~1.8 MW, while syngas yield possesses ~10.2 MW of chemical energy [33]. Non-equilibrium plasma is used for conversion of synthesis gas with very low tar content (0.7–1.9 g/Nm3). In order to decrease tar concentration by ~20 % it is required that 27–39 % of electricity is produced by a gasifier [34]. The content of tars in the downdraft plasma process is already significantly reduced because they pass through the high-temperature oxidizing zone and almost does not affect the energy balance [35].
Feedstock’s fixed carbon/ash mass ratio is important for downdraft process since feedstock and oxidizer flows are co-current; the gasification rate decreases dramatically downstream. Concentration of an oxidizing component in gas phase and carbon in char-ash residue decrease, and multiplication of these concentrations determines the mass exchange rate. Thus, fuels with high carbon content in a char-ash residue are preferred for downdraft plasma and autothermal gasification processes. Wood waste (~93 % carbon in the char-ash residue) and switchgrass (~64 %) are the best raw materials according to this characteristic.
In the updraft process feedstock and oxidizer flow in counter-current configuration. The plasma flow at the inlet contacts with the extremely carbon depleted char-ash residue. This method versus the downdraft gasification allows achievement of higher carbon conversion level of char-ash residue. Moreover, position of the high temperature zone at the bottom of the reactor simplifies liquid slag removal and slag vitrification [36].
Data on proximate and ultimate analysis allow determination of oxygen consumption required for complete gasification of carbon and for complete combustion of feedstock. These values should be considered simultaneously with the heating value. Jointly they determine adiabatic combustion temperature having higher impact on practical value of a feedstock for energy industry than heating value. The fuel can have a significant heating value, but the higher amount of oxygen is required for its combustion, the larger amount of energy is spent for heating of neutral nitrogen at operation on air. The decrease of adiabatic combustion temperature is mainly caused by three factors: excessive moisture, high content of oxygen in a feedstock, and in a less degree, high ash content. Only moisture can be easily altered, its removal leads to a decrease of oxygen and hydrogen content in the feedstock. Updraft plasma gasification process compared to downdraft one allows decreasing of energy consumption for fuels with high ash and moisture if their removal is impossible or leads to a decline in economic viability of the whole process. However, usage of such types of feedstocks for energy needs usually is not rational.
One of the key parameters for all plasma processes is a relationship of energy and oxygen consumption for stoichiometric carbon gasification conditions. However, it is impossible to determine energy consumption without calculation of the gasification process. At the analysis initial stages we could examine ratios of chemical energy yield to a heating value and oxygen consumptions for combustion and gasification. The higher these values are the harder optimum parameters of plasma gasification are achieved. The most challenging feedstock in the given context is cattle manure (ratios: ~1.31 and ∞, respectively), and the simplest switchgrass (~1.09 and ~9.07).
It should be noted that estimations of chemical energy yield limit are correct if the downdraft plasma gasification or any other method with complete tar conversion is used. It is impossible to determine clearly the most promising feedstock by this value, because it is necessary to spend energy and funds for feedstock acquisition and to take into account transportation expenses. Both these parameters depend on used methods and technologies of feedstock collection and transportation. Assuming that thermal energy of gasification products in all cases amounts to 3 MJ in energy balance on 1 kg (this rough approximation is admissible for stoichiometric gasification of many types of feedstocks with LHV less than ~15 MJ/kg), synthesis gas will be used in the combined cycle with efficiency of ~60 % [37] and electricity cost amounts to 5 ¢/kW h, we define that treatment of chicken manure will be the most profitable. According to the energy balance (per 1 kg), energy consumption will be ~1.38 kW h (~6.9 ¢), electricity yield ~2.3 kW h (~11.7 ¢), and income from treatment of manure ~1.3 ¢. In this case, the income from treatment of 1 kg of feedstock will be ~6.1 ¢.
3 Numerical Simulation of Plasma Gasification
According to Forest Products Laboratory (U.S. Department of Agriculture) data [38], the efficiency of wood-fired power plants is 18–24 %. And wood is used as a fuel by millennia and is one of easiest to use energy resources. The efficiency of electricity generation can be increased up to ~29 % using gasification and combined cycle technologies, and using plasma gasification up to ~35 % [39, 40]. That is why the development of energy industry technologies based on plasma gasification is one of the most promising ways of evolution of energy use. During plasma generation, the molecules disintegrate to electrons, ions, atoms, and radicals [41], which makes it highly reactive. Plasma application leads to an increase in rates of chemical reactions, which in particular enhances the conversion in endothermal gas-phase processes [42]. Plasma is a universal oxidizer using for gasification of virtually any kind of feedstock including wood waste [43, 44], coal [45], RDF [46], etc. Though plasma has not yet widely used, such advantages as reduction of trace contaminants [47], tar conversion [48], high rates of heat exchange [49], and also high throughput at low plasma flow rate [50] attract attention to its use in pyrolysis and gasification. The main deficiency of plasma technologies is low level of their industrialization [51].
3.1 Calculation Methods
It is useful to perform numerical simulation of the process to now the prospects of plasma gasification of chicken manure. Calculation of equilibrium composition allows evaluating the yield limits of valuable gasification products at the set parameters (oxidant/feedstock ratio, temperature, and pressure) [52]. Deficiencies of the method are the restrictions imposed by assumptions: ideal mixing and unlimited residence time [53]. Nevertheless, the given approach accords satisfactory with the experimental data [39, 54–56].
Calculations of the equilibrium composition were implemented by means of the software Chemical WorkBench (Kinetic Technologies Ltd., http://www.kintech.ru/).
Possibility to supply energy with plasma almost completely removes the kinetic restrictions imposed on the process by low temperatures in autothermal modes. Actually, the plasma mode boundary lines are determined by the oxygen consumption required for oxidation of carbon of the feedstock to CO (so-called carbon boundary) and autothermal limit when additional energy is not required for achievement of the set parameters. For chicken manure, the carbon boundary is ~245 g/kg (mode 1, Fig. 12.1) for air and ~63.9 g/kg (mode 3) for steam plasma. Autothermal mode (temperature 1,500 K, pressure 1 atm) air gasification is achieved at oxidizer consumption ~2.16 kg/kg. Accordingly, all regimes between these two air consumptions are allothermal under equal conditions (temperature and pressure).
Main parameters of integrated plasma gasification and Fischer–Tropsch system for chicken manure processing: a and b Composition of dry syngas; c Energy consumption per mass unit of feedstock (LHV basis); d Yield of syngas chemical energy per mass unit of feedstock (LHV basis); e Hourly space velocity of Fischer–Tropsch synthesis; f Yield of synthetic fuel per mass unit of feedstock. Data are represented for plasma gasification modes consuming: 1 ~245 g, 2 ~1.20 kg of air and 3 ~63.9 g, 4 ~314 g of steam per 1 kg of feedstock
Let us examine modes of stoichiometric gasification of chicken manure by air and steam, and also a mode with an air consumption ~1.20 kg/kg (mode 2) that is between autothermal and stoichiometric modes, and a mode with the steam consumption ~0.314 kg/kg (mode 4), matching to the previous one by the amount of fed oxygen. Figure 12.1 shows the results of calculations. In calculations, the composition and heating value of a chicken manure specified in Table 12.1 were used and also the following composition of air was used: N2—78.09, O2—20.95, Ar—0.93, CO2—0.03 %mol.
Approximation of synthesis rate was used for calculation of space velocity of Fischer–Tropsch process on Co–Mn/TiO2 catalyst [57] at pressure 10 bar and temperature 523 K. Before Fischer–Tropsch synthesis, the syngas was cleaned from sulfur compounds and nitrogen oxides, part of CO was converted to H2 by water–gas shift reaction to provide stoichiometric relation of synthesis—H2/CO = 2 according to its chemical equation (12.1).
The synthesis continued until CO content in convertible gas decreased to 0.1 %mol. The process rate was calculated according to equation (12.2) [57].
where, r CO is CO conversion rate (mmolCO/min gcat), k P and b CO are kinetic parameters equal to 0.367 and 1.454, respectively, P CO and \(P_{{\rm{H}}_{\rm{2}} } \) – are partial pressures of CO and H2.
3.2 Discussion
Let us discuss presented plasma gasification modes of chicken manure with various consumptions and oxidants—1–4 (according to the data of Fig. 12.1). As it is seen the CO content in syngas and the chemical energy yield increase while the temperature grows at the stoichiometric gasification mode. It is generally caused by the decrease of the graphite yield which becomes less than 0.1 g/kg only at temperatures more than ~1,400 K, and its conversion to carbon monoxide. On modes 3 and 4, change of composition is caused by the shift of equilibrium toward H2 and CO2 formation according to stoichiometry of water–gas shift reaction and insignificant methane content increase (up to ~1.2–1.6 %mol) at temperature decrease. The influence of the process temperature on a chemical energy yield is insignificant, as volumetric heating values of H2 and CO are close, and during water–gas shift reaction one is replaced by another.
Increase of energy consumption with the temperature growth in all modes is mainly caused by the increase of the sensible heat of the system, and also with the graphite gasification on stoichiometric modes. The influence of this effect on energy balance upon reaching 1,200 K decreases to ~2 kJ/kg K.
The syngas composition is a major factor defining rate of the Fischer–Tropsch synthesis. During the water–gas shift reaction, the sum of concentrations of hydrogen and carbon monoxide in syngas decreases (steam during the conversion is replaced by carbon dioxide), therefore the more initial H2/CO ratio close to stoichiometric, the higher is the rate of Fischer–Tropsch synthesiswhile using the converted syngas. It causes lower rates of synthesis for syngas produced by air plasma gasification in comparison with the steam plasma gasification. Rate decreases at the reduction of the plasma energy.
The content of contaminants (such as H2S, COS, NH3, HCN, HCl, soot, tars, BTX, volatile metals, and dust) in syngas should be limited to prevent the accelerated aging of catalyst [58]. The residual content and residence time of these contaminants define catalyst lifetime, therefore the higher space velocity is the more valuable products can be made by the catalyst pellets before its deactivation. Usage of the steam plasma instead of the air plasma allows increasing space velocity by 15–59 % that leads to a decrease of financial expenses for the catalyst pellets in the Fischer–Tropsch process.
Alterations in liquid fuel yield are caused by changes in syngas composition and resemble the dependence of the chemical energy yield; however, they are not proportional as methane is inert to the Fischer–Tropsch synthesis.
The energy consumption (associated with energy used for plasma generation) is about 5.0–6.2 kW h for the steam and 1.9–5.2 kW h for the air oxidant to produce 1 kg of synthetic fuels by plasma gasification.
Carbon dioxide is a byproduct of synthetic fuel production from biomass. CO2 content in the residual gas can be very high (more than 90 %), while using steam plasma that makes it very attractive for innovative applications [59]. For example, CO2 reforming of methane [60] or synthesis of CH3OH by non-equilibrium electrocatalysis plasma reactor [61].
4 Implementation of Plasma Gasification
Nowadays, there are a lot of companies in the world which are engaged in the commercial advancement of plasma gasification technologies. The most successful among them are: AlterNRG [62], Integrated Environmental Technology LLC [63], Advanced Plasma Power [64], Plasco Energy Group [65], Pyrogenesys Canada Inc. [66], etc. These companies have created pilot plants of various sizes. Some of them are implementing the large commercial projects. Generally, these gasification processes are based on bath of molten slag (Integrated Environmental Technology LLC), usage of bath and with the subsequent plasma conversion of the crude syngas (Pyrogenesys), traditional gasification in the updraft process with plasma conversion of crude syngas (Plasco Energy Group, Advanced Plasma Power), or updraft process of plasma gasification (AlterNRG).
Metallurgical furnaces with the Joule heating of molten slag are used in the gasification on bath where waste is supplied. This area heated either by the electric arc ignited on the slag surface, or by the plasma stream from the plasma torch. Sometimes, the plasma torch is installed in the separate chamber at the outlet of the reactor. There the crude syngas mixes with a high-temperature oxidizer causing the conversion of tars. Advantage of this process is the possibility to create large single installations. The second advantage is the possibility to discharge the incombustible components of waste in the liquid phase which allows separating metallic and nonmetallic fractions. An essential disadvantage is the growth of the energy consumption to maintain slag in liquid phase.
In the updraft gasification process of the AlterNRG company, the metallurgical coke and limestone are supplied into the reactor together with waste. Plasma generators are installed in the bottom part of the gasifier and generally used for heating and maintaining the slag in a liquid phase (limestone is fed to reduce melting point). Oxygen is supplied into the gasification zone above the plasma injection zone. The syngas produced in the gasification zone passes through the upper colder layers of waste. There the syngas is polluted by tars, formed in pyrolysis zones. Evaporating water does not participate in the conversion process due to low temperatures. The outlet for syngas is at the top of the gasifier. It is an essential disadvantage of the up-draft process. Produced gas also requires either additional conversion or tar cleaning.
The common essential disadvantage of all these technologies is the usage either free-burning arcs or DC plasma torches. Low efficiency of energy transfer from an arc to gas ~30 % is typical for free-burning arcs. The efficiency for DC plasma torches is about 60 % due to the high losses in power-supply system. In our opinion, the most effective systems generating plasma are AC plasma torches. Plasma torches and power-supply systems developed in Institute for Electrophysics and Electric Power RAS (IEE RAS) provide energy transmission efficiency of the electric energy from the electrical grid into the plasma energy about 90–94 %.
4.1 Methods of Energy Transfer
Now plasma technologies of treatment and gasification of organic substances develop in two directions differed by the way of energy transfer to the plasma-chemical reactor. One of them is based on the electric arcs burn directly in the reactor volume (transferred arc). Arcs close between the electrode injected into the reactor and electroconductive melt (molten slag) in the bottom part of the reactor. Electrodes can be made of graphite or metal. The advantage of this method is possibility to create a large single plasma-chemical reactors with power consumption of about 5–30 MW. Free-burning arcs are used in large-scale metallurgical installations which are almost ready to commercial operation.
The disadvantages are rapid wear of electrodes, considerable quantity of admixtures, and low-energy transfer coefficient of a free-burning arc into the processing substance. The efficiency of such installations, as a rule, does not exceed 30–35 %.
Stationary plasma torches are used for plasma generation in another method. The plasma-forming gas gains energy from the electric arcs burning in the discharge chamber of the plasma torch (non-transferred arc), and then arrives into the reactor volume.
The efficiency of heat exchange between the generated plasma and processed substance is significantly higher in comparison with the first method. The efficiency of plasma torches optimized for industrial applications exceeds 90 % and energy of a plasma jet almost completely transfers to the processed substance. That rather simplifies control over the chemical composition of syngas. Now, the application of this method is restrained by absence of sufficiently powerful plasma torches capable of generating plasma from oxidizing media (air, steam, etc.) in prolonged modes with high efficiency and lifetime of electrodes.
Powerful stationary electric arc plasma torches, meeting the requirements of plasma-chemical technologies, can be classified by current type: alternating current (AC) plasma torches [67–81] and direct current (DC) plasma torches [82–86]. They also can be divided by a type of plasma forming gas: neutral, reducing, or oxidizing. Devices differ in design and other features: type of discharge chambers, material and form of electrodes, a way of working gas supply, a principle of the arc stabilization, etc. Most plasma torch designs utilize a combination of methods of arc discharge stabilization comprising gas stream organization, arc contraction by the insulated inserts, and magnetic field. Electrode designs of plasma torches are rod, toroidal, ring, and tubular; in some cases the electric arc chamber is one of the electrodes. We will present only basic types due to enormous variety of designs.
4.1.1 Direct Current Plasma Torches
Until now, most gasification and pyrolysis systems use DC plasma torches. Figure 12.2a shows the typical schematic diagram of power-supply circuit. The ballast resistor is used to stabilize burning of DC arc that causes considerable real power losses.
DC plasma torches: a Typical power-supply schematic diagram of DC plasma torch (P plasma torch, D rectifier, L–C filter, R ballast resistor, SF automatic circuit breaker); b After [87] schematic representation of Westinghouse Plasma Corporation plasma torch (1 plasma column, 2 electrode, 3 entering process gas, 4 heated process gas); c Photo of 300 kW Europlasma’s plasma torch. (Reprinted from [89], with permission from the International Plasma Chemistry Society)
The most widespread DC plasma torches are Westinghouse Plasma Corp plasma torches with cylindrical electrodes. Sketch of this type of plasma torch is presented in Fig. 12.2b.
Power ranges for plasma torches MARC-3A and MARC-11L are 130–300 kW and 300–800 kW [87, 88], respectively, which are most attractive for industry. These are the most advanced designs. Their efficiency of energy transfer from the arc to plasma (thermal efficiency) is 70–85 %. The total efficiency of the system including ohmic resistance losses in power-supply system is significantly lower. The lifetimes of electrodes for these models are 600 and 1,000 h, respectively.
EUROPLASMA designed a series of plasma torches of the same type which could be fed by CO2, CO, CH4, H2, N2, and their mixtures. Figure 12.2c shows a 300 kW plasma torch [89].
Series of “Linde” plasma torches are described in [83–85]. These devices are designed for short-time operation at high pressures and power up to 20 MW. These plasma torches use cylindrical copper electrodes. Some models have the working gas enthalpy up to 10 MJ/kg. Stabilized rectifiers power these devices. Voltage of power sources is up to 10 kV. Typically, the flow rates of cooling agents for ballast resistor and plasma torch are virtually equal. Pressure in the cooling system is about 4 MPa. Plasma torches MDC-200 and MDC-300 [86] use air as a working gas and operate in relatively short-time mode, they are intended for special experiments. Maximum working pressure of gas in the plasma torch chamber is 25 MPa, a range of operating currents 100–1,200 A, voltage 1.2–14 kV, thus power changes from 0.7 to 10.5 MW.
4.1.2 Alternating Current Plasma Torches
AC plasma torches are more promising for industrial applications and technologies requiring relatively high powers (e.g., waste treatment). Figure 12.3a represents the typical schematic diagram of a power-supply circuit. The power-supply system in this case is essentially cheaper and more reliable, rather than power-supply systems of DC plasma torches. Their maintenance is easier. The thermal efficiency of AC plasma torches is high. In AC power-supply systems, the losses do not exceed several percent as the reactive ballasts stabilizing an arc are used. Reactive power losses are minimized using standard capacitor compensators.
AC plasma torches: a Schematic diagram of power-supply circuit of AC plasma torch (P plasma torch, K contactor, T standard step-up transformer, L1–L3 current limiting inductances, C1–C3 capacitor compensators, SF automatic circuit breaker); b Multi-phase electric arc Westinghouse heating system (schematic of the installation) [73]; c NOL electric arc heater (1 ring electrodes, 2 current leads, 3 nozzle unit)
Existing AC plasma torches can be divided into three basic groups: the single-phase [72, 73], the three-phase single-chamber, and the three-phase multi-chamber plasma torches. In some cases, a DC plasma torch with linear circuit and cylindrical electrodes is taken as a basis for single-phase AC plasma torches, thus AC power source is used. Working gas is usually supplied tangentially into such system. Another option of a single-phase plasma torch is a construction with the central rod electrode and ring or toroidal electrode. Usually, the arc is stabilized by the magnetic field rotating the arc in the interelectrode gap. Such type plasma torches use axial supply of the working gas.
Multi-phase multi-chamber AC plasma torches comprise various combinations of several single-phase plasma torches using multi-phase AC electrical grid. There are constructions consisting of three separate single-phase plasma torches. It is possible to connect three single-phase plasma torches sharing one mixing chamber, while the connection configuration can be different. There are systems designed by this principle, for example, a multi-phase electric arc heating system (see Fig. 12.3b) described in [73].
Multi-phase single-chamber AC plasma torches are described in [70]. Their feature is installation of an electrode system of the plasma torch in a single-electric arc chamber. The electrode systems of multiphase single-chamber plasma torches can have the form of rings, toruses, or rods. In case of using toroidal or ring electrodes (Fig. 12.3c), generally the first and the last electrode are connected to the same phase. Electrodes are usually separated from each other by heat-resistant insulating pads. Stabilization and arc twirl are supported either by a magnetic field by means of the solenoid mounted on the plasma torch case, or by the creation of the tangential gas vortex setting the arc column on an axis of the electric arc chamber and moving the attachment points of the arc along the electrode surface.
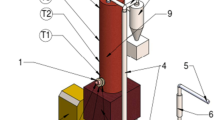
Another widely used group of single-chamber multiphase plasma torches are plasma torches with rod electrodes. Several types of plasma torches, including the ones with rod electrodes have been developed, produced, and tested in IEE RAS. Research and development, and design experience are described in [67, 80, 90]. Figure 12.4a shows a single-chamber plasma torch with rod electrodes.
Powerful single-chamber AC plasma torches: a Three-phase plasma torch of EDP type (1 electrode tip, 2 insulator, 3 current lead, 4 gas supply loop); b Single-chamber three-phase plasma torch with rail electrodes (1 electrode tip, 2 insulator, 3 current lead, 4 gas supply, 5 injector); c Photo of operating plasma torch with rail electrodes, power 500 kW
Ignition of several simultaneously burning AC arcs in a single chamber allowed creation of simple and reliable plasma torches transforming the electric current energy into plasma energy with high efficiency of 80–90 %.
Several different designs were developed. Tungsten or tungsten-containing rod electrodes were used for operation on inert gases, nitrogen, and hydrogen. Water-cooled copper tubular electrodes were used for operation on oxidizing media. Plasma torches with rod electrodes can be divided into three groups: with power up to 200 kW and 2 MW, and also working at short-time modes with power 4–50 MW [90]. Both types have the similar design comprising three main parts: case, arc chamber (nozzle), and electrode unit. Multi-phase mode of arc burning in the discharge chamber allows using low voltage of re-ignition due to the preliminary ionization of the discharge gap. Tungsten with additives of rear earth metals and compounds having low work function were used as an electrode material. The advantages of single-phase plasma torches with rod electrodes are: simple design, high efficiency providing by optimal relation of volume and surface area of the arc chamber, and also the possibility of electrode operation in the thermo-emission mode. In these systems, it is easier to stabilize burning of AC arcs.
A series of plasma torches with rail electrodes have been developed (Fig. 12.4b, c) [81]. A plasma torch with rail electrodes can provide stable operation with oxidizing (air) and neutral media (nitrogen, inert gases). The range of air flow rates varies from 15 to 70 g/s. Power input into the arcs varies from 100 to 700 kW. Thermal efficiency almost does not differ from the system (plasma torch and power supply) efficiency and is 70–95 %.
The basic principle of plasma torch operation is the rail-gun effect (arcs move along the electrodes in the field of their own current). The movement of arc attachment point along the electrode allows uniform distribution of thermal load, which gives the opportunity to use the water-cooling electrodes made of a fusible material with high thermal conductivity (copper tubes). The multi-phase single-chamber AC plasma torch with rail electrodes uses an integrated single-phase high-voltage plasma torch of low power as an injector. It creates a plasma stream providing sufficient electron concentration in a zone of the minimal interelectrode gap for ignition of the basic arcs.
It allows stable ignition of arcs between the electrodes mounted with a gap up to 20 mm powering from the industrial grid with voltage about 380–500 V. Arcs fill the major part of the discharge chamber, moving in the longitudinal and transverse directions. The insulating layer is formed near the wall where cold gas moves, where concentration of charged particles dramatically decreases, and arcs extinguish. The above-described process repeats continually forming a low-temperature plasma jet with average mass temperature of about 1,500–6,500 K at the plasma torch nozzle.
High-voltage plasma torches with high thermal efficiency 80–95 % have been developed for operation with power up to 100 kW. These plasma torches have rod electrodes in cylindrical channels. Figure 12.5a represents their general view and design. High supply voltage 4–10 kV provides stable ignition and burning of the long arc.
Prolonged lifetime high-voltage AC plasma torches: a Photo of high-voltage AC plasma torches with rod electrodes in cylindrical channels; b Schematic representation of single-phase high-voltage plasma torch with rod electrodes; c Photo of operating high-voltage plasma torch with power 600 kW (IEE RAS), plasma forming gas–air
Currently, AC plasma torches with cylindrical electrodes operating with high arc voltage drop (up to 5 kV) are the most promising ones. Figure 12.5b shows a plasma torch of this type.
The plasma torch except high efficiency has the following advantages: long lifetime of electrodes (more than 1,000 h) and possibility to change the plasma heat content over a wide range, providing at this a range of average mass temperature for air plasma from 1,500 to 7,500 K. Of special note is the ability to provide plasma temperature less than 2,000 K, which is claimed for some technological processes. Moreover, we have developed 6 MW AC plasma torches, but their electrode lifetime did not exceed 100 h [90].
4.2 Large-Scale Experimental Installation
In the late 1990s of the 20th century, interest to gasification technologies of solid fuels has renewed against the background of fossil resources price rising. IEE RAS started researching in this field as it is one of perspective implementations of low-temperature plasma systems. The experimental installation for investigation of plasma gasification process (Fig. 12.6) has been created [91].
General view and schematic diagram of the experimental installation IEE RAS for plasma waste gasification: 1 reactor-gasifier, 2 main plasma torch, 3 auxiliary plasma torch (H2O, CO2), 4 auxiliary plasma torch for initial heating, 5 loading device, 6 device for slag discharge and cooling, 7 branch pipe for syngas removal, 8 afterburner, 9 ignition plasma torch, 10 cyclone, 11 gas-analysis system, 12 spray scrubber, 13 packed bed scrubber, 14 stack, 15 exhaust fan. Zones of: I accumulation, II evaporation, III pyrolysis, IV oxidation, V reduction, VI weak reaction rates, VII slag discharge
The reactor is the key element of this installation. This is a fixed bed downdraft apparatus gasifier. Solid fuel is loaded into the reactor from above (Fig. 12.6, zone I). After that it is gradually moved downwards to a zone of slag discharge (VII) by gravity and due to fuel gasification in the lower layers of the reactor. The raw materials consistently pass a zone of evaporation (II), pyrolysis (III), oxidation (IV), and reduction (V); as a result, the organic mass and water are converted into the syngas. These processes are initiated and supported in the reactor by plasma streams generated by plasma torches. Gasifier has the loading device which allows portion loading of feedstock during the experiment (Fig. 12.6, pos. 5).
The reactor has three oxidant injection points along the shaft length. The first (top) point is meant for supplying the oxidizer of the moderate temperature (no more than ~400–500 °C), the second and the third points are used to supply the low-temperature plasma. Plasma can be supplied in each these two points from two plasma torches simultaneously. High-voltage AC air plasma torches with power up to 50 kW or a combination of air and steam (or CO2) plasma torches are used on the installation.
Syngas is removed from the bottom part of the reactor. In addition, the possibility to supply plasma or other oxidant for the accelerated heating at the first stage of experiment is provided in this zone.
The created gasifier is designed for working under a pressure close to the atmospheric. The gas flow in the gasifier shaft is induced by pressure 0.3 ± 0.2 kPa below an atmospheric pressure created in the outlet branch (Fig. 12.6, pos. 7) by the exhaust fan positioned at the end of the processing chain (Fig. 12.6, pos. 15).
The bottom part of the reactor is equipped with a revolving grate (Fig. 12.6, pos. 6) for the slag removal. Below the revolving grate, the water valve is arranged which has two functions: the reaction chamber closure and explosive valve function.
The installation is designed to investigate the composition of syngas and process characteristics. Gas samples for the analysis are taken at the gasifier outlet (Fig. 12.6, pos. 7).
There are two sampling systems. The first one utilizes two sampling lines in automatic mode. Gas, pumped out from gas duct by the vacuum pump, passes the hot filter, the cyclone, the cooler, the fine gas cleaning filter, and then is analyzed by a time-of-flight mass spectrometer EMG-20–1 (Mettek, Russia).
The second system is designed for separation and measuring of water and tars of syngas. The first element in the system after sampling probe is the hot filter. Two methods of water content measurements based on condensation and absorption are realized. The volume of liquid condensed from the syngas stream during its cooling is measured along with the gas-flow rate and its temperature in the condensation method. In the absorption method, water and steam are completely absorbed from the syngas stream at constant flow rate and measured by the milligram scale. The dried gas sample is pumped to the quadrupole mass-spectrometer MKS Cirrus-300 (MKS Instruments, USA) for composition analysis. Condensed liquid is investigated on tar content.
Mass-spectrometers allow performing the continuous analysis of synthesis gas composition for both the macro- and micro-concentrations. The plasma gasification mode is corrected using these data along with the information about temperatures in the reactor and plasma flow rates. These data are recorded and subsequently analyzed to determine the mass and energy streams of processes and other parameters.
Control over the mass streams, temperatures, and pressures in the reactor and in other elements of the experimental installation allows the installation operation mode regulating. These parameters are continuously measured and recorded. The changing of oxidant flow rate and plasma torch power are the basic leverages of the process.
The produced syngas after samples are taken in branch pipe is subjected to combustion. The afterburner serves for this purpose. In the afterburner, the syngas is mixed with the required amount of air and burns out. The device for forced ignition is installed for flame stabilization and prevention of explosion risk. A single-phase high-voltage plasma torch of low power is used as such a device.
The exhaust gases from the afterburner pass through the gas treatment system to the stack and into the atmosphere. Wet method of cleaning is used. It comprises two consecutive devices: spray and packed bed scrubbers.
4.2.1 Experimental Procedure
The important stage of experiment is determination of moisture and composition of investigated fuel. The fuel moisture is determined by the method of long-time drying at air ambient of 105 °C. The total carbon and hydrogen content in a fuel is determined by ISO 625–96 technique.
Before the experiment, the gasifier shaft is completely filled with char coal. This kind of solid fuel is the most suitable for a stage of preliminary heating up as it possesses the low content of volatile matter.
Ignition of one of the gasifier’s plasma torches is considered as the beginning of the experiment (timing is counted from this point). Supply of air plasma improves heating and firing of fuel. Heating up the reactor shaft takes at least 8 h and comprises several stages differing by oxidant flow rate. Gasifier lining has very high thermal inertia that is why practically all experiments are carried out at quasi-stationary mode.
The analysis of syngas composition begins at the final stage of preheating and the charcoal feeding is replaced by the investigated material feeding. The transient process from the charcoal gasification to the investigated fuel gasification starts when the investigated material reaches drying and pyrolysis zone. The transient process could be considered completely finished when the whole gasifier shaft is filled by this fuel and the products of its gasification. When the experimental program is completed the fuel feeding into the reactor stops and starts after-burning of fed materials and their remnants, and when they are burned out the installation cools.
Experiments on plasma gasification have been carried out for such fuels as charcoal, wood (of various types: woodchips, pressed sawdust, and chocks), coal, lignite, Refuse Derived Fuel (RDF), and others.
4.2.2 Experimental Results
Figure 12.7 and Table 12.2 represent the experimental and calculated data of investigation of the plasma gasification of biomass using wood waste as the example.
Time dependence of main experimental parameters: a Temperature (1 wall in pyrolysis zone, 2 wall in oxidizing zone, 3 wall in reduction zone, 4 syngas at the outlet); b Dry syngas composition (5 H2, 6 CO, 7 N2, 8 CO2, 9 the others); c Mass flows (10 fuel, 11 air, 12 syngas, 13 steam); d Energy flows. (14 energy losses, 15 fuel LHV, 16 plasma, 17 syngas LHV, 18 sensible heat)
Feedstock consumption was determined by differences of mass streams of major elements (carbon, hydrogen, oxygen, and nitrogen) assuming that ash content and the nitrogen content in wood are negligibly small. The fuel element composition was determined by mean values of flow rates of carbon, hydrogen, and oxygen of fuel for two intervals. The lower heating value (LHV) was estimated by this composition (by formula for an estimation of heating value of biomass [92]).
The mode of wood plasma gasification was observed on the interval 9:43–16:30. About 606.34 kg of wood was processed during this period according to the estimates, and actually about 609.98 kg of wood was loaded into the reactor in the course of the experiment. That validates the technique of experimental data processing.
The experimental results were compared with calculations for two regimes with constant air plasma flow rate and powers of plasma torches (see Table 12.2). The comparison showed that the data agreed well for the chemical energy yields and are satisfactory for syngas composition.
Calculations were carried out assuming adiabatic process and thermodynamic equilibrium of gasification products composition. On these modes, the composition of raw materials which matches to wood with moisture of 8–10 % also had been determined. Well data agreement on the chemical energy yields was explained by the fact that the air plasma flow rate was more than stoichiometric in 2.8–3.0 times. It led to a considerable shortening of a reduction zone, therefore heat losses did not significantly affect syngas composition. More considerable difference in composition is caused by high methane stability and water–gas shift reaction at weak reaction rates and branch pipe sampling zones. As a whole, comparison results confirm usability of equilibrium approach for the estimation of plasma gasification key parameters.
5 Summary
The basic advantage of low-temperature plasma usage in biomass gasification is substantial growth of the hydrogen content and carbon monoxide content in syngas composition. At treatment of a chicken manure, the content of H2 + CO in syngas can be raised to ~97 % that allows an increase in the efficiency of its use in the Fisher–Tropsh process by 15–59 % and reach a specific yield of synthetic fuels ~240–260 g/kg, thus power inputs on the organization of the allothermal process will make only 4–5 MJ/kg. These parameters can be reached in downdraft plasma gasification. Plasma energy is used for liquid slag removal in updraft gasification, and it has rather less influence on syngas composition. The most effective way of plasma generation is by systems on a basis of AC plasma torches thanks to a long lifetime of electrodes (more than 1,000 h), an effective energy transfer of the discharge to the plasma forming gas (to 80–95 %), high power at work in long-time modes (to 2 MW), and low losses in a power-supply system (no more than ~1–5 %). New AC plasma torches have arc voltage drops of about 1–10 kV and discharge currents of about 10–100 A. However, DC plasma torches are used more often. They typically have high currents (0.1–1 kA) and low voltage drops in the discharge (10–1,000 V) which are necessary for DC arc stabilization. Their main advantages are long-time operating experience and hence well-developed plasma torch models. Reliability of the executed estimations proves to be true by a good coordination with the experimental data which are obtained on the large-scale plasma gasifier. Total difference between mass balances was less than 1 %, and for energy balance less than 2 %. A series of long-time experiments on plasma gasification of wood proves that plasma gasification of biomass with use of AC plasma torches is ready for industrial implementation.
References
Juanjuan D, Wenping Z (2011) The analysis to the influence of carbon dioxide emissions in different countries. Energy Procedia 5:2426–2431. doi:10.1016/j.egypro.2011.03.417
Myhre G, Highwood EJ, Shine KP, Stordal F (1998) New estimates of radiative forcing due to well mixed greenhouse gases. Geophys Res Lett 25:2715–2718
Peters GP, Marland G, Le Quéré C, Boden T, Canadell JG, Raupach MR (2012) Rapid growth in CO2 emissions after the 2008–2009 global financial crisis. Nat Clim Change 2:2–4. doi:10.1038/nclimate1332
IEA (2011) Key World Energy Statistics 2011. http://www.iea.org/textbase/nppdf/free/2011/key_world_energy_stats.pdf. Accessed 14 Feb 2012
Whitney G, Behrens CE, Glover C (2011) U.S. fossil fuel resources: terminology, reporting, and summary. CRS report for Congress. http://assets.opencrs.com/rpts/R40872_20110325.pdf. Accessed 14 Feb 2012
Li M (2011) Peak energy and the limits to global economic growth. University of Utah. Annual Report 2011. July 2011. http://www.econ.utah.edu/~mli/Annual%20Reports/Annual%20Report%202011.pdf. Accessed 14 Feb 2012
Hook M, Sivertsson A, Aleklett K (2010) Validity of the fossil fuel production outlooks in the IPCC Emission scenarios. Nat Resour Res 19:63–81. doi:.1007/s11053-010-9113-1
Ladanai S, Vinterbäck J (2009) Global potential of sustainable biomass for energy. Swedish University of Agricultural Sciences, Uppsala
Hassan R, Scholes R, Ash N (2005) Ecosystems and human well-being: current state and trends. Island press, Washington DC
Dietenberger MA, Green DW, Kretschmann DE et al (1999) Wood handbook—wood as an engineering material. General Technical Report FPL–GTR–113. U.S. Department of Agriculture, Madison, WI
Green DW, Perry RH (2007) Perry’s chemical engineers’ handbook, 8th edn. McGraw-Hill, USA
History of Biofuels (2010) http://biofuel.org.uk/history-of-biofuels.html. Accessed 16 Feb 2012
Barney JN, DiTomaso JM (2010) Bioclimatic predictions of habitat suitability for the biofuel switchgrass in North America under current and future climate scenarios. Biomass Bioenergy 34:124–133. doi:10.1016/j.biombioe.2009.10.009
Keoleian GA, Volk TA (2005) Renewable energy from Willow Biomass Crops: life cycle energy, environmental and economic performance. Crit Rev Plant Sci 24:385–406. doi:10.1080/07352680500316334
Demirbas MF (2011) Biofuels from algae for sustainable development. Appl Energy 88:3473–3480. doi:10.1016/j.apenergy.2011.01.059
Bacovsky D, Mabee W, Worgetter M (2010) How close are second-generation biofuels? Biofuels, Bioprod Biorefin 4:249–252. doi:10.1002/bbb.222
Ribaudo M, Gollehon N, Aillery M (2003) Manure management for water quality: costs to animal feeding operations of applying manure nutrients to land. Agricultural Economic Report 824. U.S. Department of Agriculture. http://www.ers.usda.gov/publications/aer824/aer824.pdf. Accessed 14 Feb 2012
Kärkkäinen L, Matala J, Härkönen K, Kellomäki S, Nuutinen T (2008) Potential recovery of industrial wood and energy wood raw material in different cutting and climate scenarios for Finland. Biomass Bioenergy 32:934–943. doi:10.1016/j.biombioe. Accessed 01 Jan 2008
Yoshioka T (2011) Study on the feasibility of a harvesting, transporting, and chipping system for forest biomass resources in Japan. Agri-Biosci Monographs 1:1–60. doi:10.5047/agbm.2011.00101.0001
Reijnders L (2010) Transport biofuel yields from food and lignocellulosic C4 crops. Biomass Bioenergy 34:152–155. doi:10.1016/j.biombioe.2009.10.004
Duffy MD, Nanhou VY (2001) Costs of producing switchgrass for biomass in Southern Iowa. Iowa State University Extension, Pm 1866. http://iowaswitchgrassdocs/pdf/Costs%20of%20Switchgrass.pdf. Accessed 14 Feb 2012
Weiland NT, Means NC, Morreale BD (2012) Product distributions from isothermal co-pyrolysis of coal and biomass. Fuel 94:563–570. doi:10.1016/j.fuel.2011.10.046
Ghaly AE, Al hattab M (2012) An innovative farm scale biogas/composting facility for a sustainable medium size dairy farm. Am J AgricBiol Sci 7:1–14. doi:10.3844/ajabssp.2012.1.16
MacDonald JM, O’Donoghue EJ, McBride WD, Nehring RF, Sandretto CL, Mosheim R (2007) Profits, costs, and the changing structure of dairy farming. U.S. Department of Agriculture. http://www.ers.usda.gov/publications/err47/err47.pdf. Accessed 14 Feb 2012
Santoianni DA, Bingham MF, Woodard DM, Kinnell JC (2008) Power from animal waste—economic, technical, and regulatory landscape in the United States. Journal of Energy and Environment Conference 2:Paper #01
Hadrich JC, Wolf CA, Black JR, Harsh SB (2008) Incorporating environmentally compliant manure nutrient disposal costs into least-cost livestock ration formulation. J AgricAppl Econ 40:287–300
WA Government (2004) Environmental code of practice for poultry farms in western Australia. The Department of Environment, Australia
Roeper H, Khan S, Koerner I, Stegmann R (2005) Low-tech options for chicken manure treatment and application possibilities in agriculture. Proceedings Sardinia 2005, 10th International Waste Management and Landfill Symposium. Environmental Sanitary Engineering Centre, Italy
Giuntoli J, de Jong W, Arvelakis S, Spliethoff H, Verkooijen AHM (2009) Quantitative and kinetic TG-FTIR study of biomass residue pyrolysis: dry distiller’s grains with solubles (DDGS) and chicken manure. J Anal Appl Pyrolysis 85:301–312. doi:10.1016/j.jaap.2008.12.007
Mkhabela TS (2004) Substitution of fertiliser with poultry manure: is this economically viable? Agrekon 43:347–356. doi:10.1080/03031853.2004.9523654
Popov VE, Bratsev AN, Kuznetsov VA, Shtengel SV, Ufimtsev AA (2011) Plasma gasification of waste as a method of energy saving. J Phys: Conf Ser 275:012015. doi:10.1088/1742–6596/275/1/012015
Zhang Q, Dor L, Fenigshtein D, Yang W, Blasiak W (2012) Gasification of municipal solid waste in the plasma gasification melting process. Appl Energy 90:106–112. doi:10.1016/j.apenergy.2011.01.041
Fourcault A, Marias F, Michon U (2010) Modelling of thermal removal of tars in a high temperature stage fed by a plasma torch. Biomass Bioenergy 34:1363–1374. doi:10.1016/j.biombioe.2010.04.018
Nair SA, Pemen AJM, Yana K et al (2003) Tar removal from biomass-derived fuel gas by pulsed corona discharges. Fuel Process Technol 84:161–173. doi:10.1016/S0378-3820(03)00053-5
Brattsev AN, Kuznetsov VA, Popov VE, Ufimtsev AA (2011) Arc gasification of biomass: example of wood residue. High Temp 49:244–248. doi:10.1134/S0018151X11010020
Moustakas K, Xydis G, Malamis S, Haralambous K-J, Loizidou M (2008) Analysis of results from the operation of a pilot plasma gasification/vitrification unit for optimizing its performance. J Hazard Mater 151:473–480. doi:10.1016/j.jhazmat.2007.06.006
Bassily AM (2008) Enhancing the efficiency and power of the triple-pressure reheat combined cycle by means of gas reheat, gas recuperation, and reduction of the irreversibility in the heat recovery steam generator. Appl Energy 85:1141–1162. doi:10.1016/j.apenergy.2008.02.017
U.S. Department of Agriculture (2004) Wood biomass for energy. http://www.fpl.fs.fed.us/documnts/techline/wood-biomass-for-energy.pdf. Accessed 16 Feb 2012
Rutberg PG, Bratsev AN, Kuznetsov VA, Popov VE, Ufimtsev AA, Shtengel’ SV (2011) On efficiency of plasma gasification of wood residues. Biomass Bioenergy 35:495–504. doi:10.1016/j.biombioe.2010.09.010
Kobayashi Y, Ando Y, Kabata T, Nishiura M, Tomida K, Matake N (2011) Extremely high-efficiency thermal power system-solid oxide fuel cell (SOFC) Triple combined-cycle system. Mitsubishi Heavy Ind Tech Rev 48:9–15
Rutberg PG, Kuznetsov VA, Bratsev AN, Popov VE, Shtengel’ SV, Ufimtsev AA (2011) Use of carbon dioxide in the chemical synthesis technologies, plasma gasification and carbon production. IOP Conference Series: Mater Sci Eng19:012003. doi:10.1088/1757–899X/19/1/012003
Bratsev AN, Kuznetsov VA, Popov VE, Ufimtsev AA, Shtengel SV (2009) Estimation of perspectivity of steam-plasma methane conversion. High Temp Mater Processes: Int J 13:241–246. doi:10.1615/HighTempMatProc.v13.i2.120
Bratsev AN, Glezin IL, Kovshechnikov VB, Kumkova II, Kuznetsov VA, Popov VE, Shtengel SV, Ufimtsev AA (2007) Experimental research of air gasification of waste. The first results. Proceedings of 28th International Conference on Phenomena in Ionized Gases. Institute of Plasma Physics AS CR, Prague. pp 1848–1851
Kuznetsov VA, Bratsev AN, Kovshechnikov VB, Kumkova II, Popov VE, Shtengel SV, Ufimtsev AA (2007) Distinctive features of biomass gasification using ac plasma generators working on air. IEEE Pulsed Power Conference. Digest of technical papers 1976–2007. Omnipress, Madison, WI, USA, pp 1223–1226
Bratsev AN, Kuznetsov VA, Popov VE, Rutberg AP, Ufimtsev AA, Shtengel SV (2009) Experimental development of methods on plasma gasification of coal as the basis for creation of liquid fuel technology. High Temp Mater Processes: Int J 13:147–154. doi:10.1615/HighTempMatProc.v13.i2.30
Bratsev AN, Kumkova II, Kuznetsov VA, Popov VE, Shtengel’ SV, Ufimtsev AA (2011) Air plasma gasification of RDF as a prospective method for reduction of carbon dioxide emission. IOP Conf Ser: Mater Sci Eng19:012004. doi:10.1088/1757–899X/19/1/012004
Mondal P, Dang GS, Garg MO (2011) Syngas production through gasification and cleanup for downstream applications—recent developments. Fuel Process Technol 92:1395–1410. doi:10.1016/j.fuproc.2011.03.021
Anis S, Zainal ZA (2011) Tar reduction in biomass producer gas via mechanical, catalytic and thermal methods: Rev Renew Sust Energ Rev 15:2355–2377. doi:10.1016/j.rser.2011.02.018
Bahng M-K, Mukarakate C, Robichaud DJ, Nimlos MR (2009) Current technologies for analysis of biomass thermochemical processing: Rev Anal Chim Acta 651:117–138. doi:10.1016/j.aca.2009.08.016
Gomez E, Rani DA, Cheeseman CR, Deegan D, Wise M, Boccaccini AR (2009) Thermal plasma technology for the treatment of wastes: A critical review. J Hazard Mater 161:614–626. doi:10.1016/j.jhazmat.2008.04.017
Yang L, Wang H, Wang H, Wang D, Wang Y (2011) Solid waste plasma disposal plant. J Electrostat 69:411–413. doi:10.1016/j.elstat.2011.05.007
Puig-Arnavat M, Bruno JC, Coronas A (2010) Review and analysis of biomass gasification models. Renew Sust Energ Rev 14:2841–2851. doi:10.1016/j.rser.2010.07.030
Morrin S, Lettieri P, Chapman C, Mazzei L (2012) Two stage fluid bed-plasma gasification process for solid waste valorisation: Technical review and preliminary thermodynamic modelling of sulphur emissions. Waste Management 32:676–684. doi:10.1016/j.wasman.2011.08.020
Loha C, Chatterjee PK, Chattopadhyay H (2011) Performance of fluidized bed steam gasification of biomass – Modeling and experiment. Energ Convers Manage 52:1583–1588. doi:10.1016/j.enconman.2010.11.003
Minutillo M, Perna A, Di Bona D (2009) Modelling and performance analysis of an integrated plasma gasification combined cycle (IPGCC) power plant. Energ Convers Manage 50:2837–2842. doi:10.1016/j.enconman.2009.07.002
Karamarkovic R, Karamarkovic V (2010) Energy and exergy analysis of biomass gasification at different temperatures. Energy 35:537–549. doi:10.1016/j.energy.2009.10.022
Atashi H, Siami F, Mirzaei AA, Sarkari M (2010) Kinetic study of Fischer--Tropsch process on titania-supported cobalt--manganese catalyst. J Ind Eng Chem 16:952–961. doi:10.1016/j.jiec.2010.04.005
van Steen E, Claeys M (2008) Fischer-Tropsch catalysts for the biomass-to-liquid process. Chem Eng Technol 2008:655–666. doi: 10.1002/ceat.200800067
Amouroux J, Siffert P (2011) Carbon dioxide: a raw material and a future chemical fuel for a sustainable energy industry. IOP Conference Series: Mater Sci Eng 19:012001. doi:10.1088/1757–899X/19/1/012001
Machrafi H, Cavadias S, Amouroux J (2011) CO2 reforming of methane: valorizing CO2 by means of dielectric barrier discharge. IOP Conference Series: Mater Sci Eng 19:012006. doi:10.1088/1757–899X/19/1/012006
Amouroux J, Cavadias S, Doubla A (2011) Carbon dioxide reduction by non-equilibrium electrocatalysis plasma reactor. IOP Conference Series: Mater Sci Eng 19:012005. doi:10.1088/1757–899X/19/1/012005
Alter NRG. http://www.alternrg.com/. Accessed 21 Feb 2012
InEnTec. http://www.inentec.com/. Accessed 21 Feb 2012
Advanced Plasma Power. http://www.advancedplasmapower.com/. Accessed 21 Feb 2012
Plasco Energy Group. http://www.plascoenergygroup.com/. Accessed 21 Feb 2012
PyroGenesis Canada Inc. http://www.pyrogenesis.com/. Accessed 21 Feb 2012
Rutberg PG (2003) Plasma pyrolysis of toxic waste. Plasma physics and controlled fusion 45:957–969. doi:10.1088/0741–3335/45/6/309
Rutberg PG, Safronov AA, Goryachev VL (1998) Strong-current arc discharge of alternating current. IEEE T Plasma Sci 26:1297–1306. doi:10.1109/27.725162
Electric arc heater develops very high temperatures (1960) Iron Steel Eng 37(11):149–150
Maniero DA, Kienast PF, Hirayama C (1966) Electric arc heaters for high-temperature chemical processing. Westinghouse engineer 26(3):66–72
Harry JE (1970) A power frequency plasma torch for industrial process heating. IEEE T Ind Gen Appl IGA-6:36–42. doi:10.1109/TIGA.1970.4181126
Iwata M, Shibuya M (1999) Effect on transferred ac arc plasma stability of increasing ambient temperature and superimposing pulse at current zero point. J Phys D: Appl Phys 32:2410–2415. doi:10.1088/0022–3727/32/18/312
Fey MG (1977) U.S. Patent 4013867
Wolf CB, Fey MG (1972) U.S. Patent 3705975
Reed JF, Peterson CW, Curry WH (1975) Electric heater development and performance data for a Mach 14 wind tunnel. J Spacecraft Rockets 12:308–313
Spongberg RM (1964) U.S. Patent 3140421
Reid JW (1960) U.S. Patent 2964678
Feldmeyer E, Schallus E (1960) U.S. Patent 2923811
Roots WK, Kadhim MA (1969) Measuring the electrothermal efficiency of a 50-Hz plasma torch. IEEE T Instrum Meas 18:150–156. doi:10.1109/TIM.1969.4313791
Rutberg PG (1970) Three-phase plasma torch. Some problems of investigation of gas-discharge plasma and creation of strong-current magnetic fields. Nauka, Leningrad (in Russian)
Rutberg PG, Safronov AV, Popov SD, Surov AV, Nakonechny GV (2005) Multiphase stutionary plasma generators working on oxidizing media. Plasma Phys Contr F 47:1681–1696. doi:10.1088/0741-3335/47/10/006
Jenšta J, Takana H, Nishiyama H et al (2011) Integrated parametric study of a hybrid-stabilized argon--water arc under subsonic, transonic and supersonic plasma flow regimes. J Phys D: Appl Phys 44:435204. doi:10.1088/0022-3727/44/43/435204
Eschenbach RC, Bryson DA, Sargent HB, Sarlitto RJ, Troue HH (1964) Characteristics of high voltage vortex-stabilized arc heaters. IEEE T Nucl Sci 11:41–46. doi:10.1109/TNS.1964.4323330
Paintes JH, Saeffer JF (1976) Performance and scaling characteristics of a Huels-type arc heater operating on hydrogen, helium or air. AIAA-14
Boatright WB, Sabol AP, Sebacher DI, Pinckney SZ, Guy RW (1976) Langley facility for tests at Mach 7 of subscale, hydrogen-burning, airframe-integratable, scramjet models. AIAA-11
Painter JH (1977) Hybrid arc air heater performance. AIAA-111
Westinghouse Plasma – Plasma torches. http://www.westinghouse-plasma.com/technology/plasma-torches. Accessed 5 Mar 2012
NRG FOCUS – October 2011 issue. http://alternrg.com/sites/default/files/content/all/NRG%20Focus_Sept%202011_FN_LR_LK.pdf?phpMyAdmin=1,25SWTwdk48LH, ZtfJ1P24LAkc. Accessed 5 Mar 2012
Hacala A, Michon U (2009) Innovative industrial plasma torch for converting biomass into high purity syngas. International Plasma Chemistry Society 19. Conference proceedings. Bochum, Germany. 39. P2.14.04
Rutberg P (2009) Physics and technology of high-current discharges in dense gas media and flows. Nova Science Publishers, New York
Bratsev AN, Popov VE, Rutberg AF, Shtengel’ SV (2006) A facility for plasma gasification of waste of various types. High Temp 44:823–828. doi:.1007/s10740-006-0099-7
Reed TB, Das A (1998) Handbook of biomass downdraft gasifier engine systems. Solar Research Institute, Golden CO
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2013 Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
Rutberg, P.G., Kuznetsov, V.A., Popov, V.E., Bratsev, A.N., Popov, S.D., Surov, A.V. (2013). Improvements of Biomass Gasification Process by Plasma Technologies. In: Fang, Z. (eds) Pretreatment Techniques for Biofuels and Biorefineries. Green Energy and Technology. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-642-32735-3_12
Download citation
DOI: https://doi.org/10.1007/978-3-642-32735-3_12
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-642-32734-6
Online ISBN: 978-3-642-32735-3
eBook Packages: EnergyEnergy (R0)