Abstract
Legionella pneumophila, the etiological agent of Legionnaires’ disease was first recognized in 1976, during an outbreak of severe pneumonia at the convention of the American Legion in Philadelphia. Since then the genus Legionella has expanded, and to date more than 50 different species are described. However, L. pneumophila remains the major cause of human disease, as it is responsible for over 90 % of legionellosis cases worldwide. This chapter starts with a description of the genus Legionella and the ecology of these bacteria. We then highlight the epidemiology, clinical features, and the different diagnostic assays and methods available. In the second part, we focus on the pathogenesis, the virulence factors, and the immune response of the host. Special emphasis is placed on the implication of the literally hundreds of different effector proteins secreted by the Dot/Icm type IV secretion system. In the third section we discuss recent knowledge acquired on genomics, transcriptomics, and the metabolic features of Legionella, and, particularly, we present new insight on comparative genomics, evolution, horizontal gene transfer, and the regulation of the life cycle of L. pneumophila.
Parts of this chapter were modified from the Legionella chapter of the previous book edition written by Paul H. Edelstein and Nicholas P. Cianciotto.
Access provided by Autonomous University of Puebla. Download reference work entry PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
Introduction, Taxonomy, and Ecology
Introduction and History
Legionnaires’ disease is an acute pneumonia caused by bacteria of the genus Legionella. Pontiac fever is a febrile, nonpneumonic, systemic illness that is associated with, if not caused by, species of Legionella. Legionellosis refers to all diseases caused by the Legionella bacteria. Legionnaires’ disease accounts for >99 % of legionellosis cases (CDC 2011).
Legionnaires’ disease was first recognized as a clinical entity when it caused an epidemic of pneumonia at an American Legion convention in Philadelphia in 1976 (Fraser et al. 1977). In that outbreak, 221 people were affected, and 34 died. There was enough national concern to prompt two congressional investigations of the outbreak. An intense epidemiologic review determined that the disease was likely airborne and focused primarily at one convention hotel. About 6 months later, Joseph McDade and Charles Shepard of the United States Centers for Disease Control and Prevention discovered the etiologic agent, a fastidious Gram-negative bacterium (McDade et al. 1977). Because of the association with the American Legion convention, this disease is now called “Legionnaires’ disease,” and the etiologic agents belong to the family Legionellaceae, with L. pneumophila (type strain Philadelphia 1) being the agent responsible for the 1976 epidemic (Brenner et al. 1979). Retrospective serologic studies indicated that unsolved outbreaks of pneumonia in 1957, 1965, 1973, and 1974 had been Legionnaires’ disease (Brenner 1987; Winn 1988). Unsolved outbreaks of a nonpneumonic illness in 1968 and 1973 were also determined to be due to exposure to Legionella bacteria, and this illness was termed “Pontiac fever,” named after the city in Michigan where the 1968 outbreak had occurred (Brenner 1987; Winn 1988). Additionally, stored isolates from 1940s to 1950s were found to be Legionella bacteria, including L. pneumophila strain OLDA from 1947, L. micdadei strains TATLOCK and HEBA from 1943 to 1959, respectively, L. bozemanae strain WIGA from 1959, and a strain of L. lytica from 1954 (Brenner 1987; Fields et al. 2002; Winn 1988).
Legionella bacteria are Gram-negative rods that occur frequently in natural aquatic habitats, with infection of humans usually occurring via the inhalation of Legionella-contaminated water droplets (Fields et al. 2002). The legionellae are aerobic, non-spore forming, and unencapsulated. All but a few Legionella species are motile by means of flagella. Amino acids are considered their primary energy source. The pH and temperature optima for in vitro growth are 6.8–7.0 and 25–42 °C, respectively, with optimal growth occurring at 35–37 °C. L-cysteine is required for growth, and iron is needed for initial isolation from the environment or clinical specimens. Buffered charcoal yeast extract (BCYE) agar that is supplemented with L-cysteine, iron, and α-ketoglutarate is the preferred growth medium for culturing Legionella bacteria. As will be detailed below, L. pneumophila and probably all Legionella species are facultative intracellular parasites of eukaryotic cells. In the environment, legionellae persist within species of free-living amoebae, and in the human lung, the bacteria primarily parasitize the alveolar macrophages (Newton et al. 2010).
The Legionella Genus
Legionella is almost universally regarded as the sole genus in the family Legionellaceae within the γ-2 subdivision of Proteobacteria (Benson and Fields 1998; Fry et al. 1991; Ludwig and Stackebrandt 1983). The nearest phylogenetic relative to Legionellaceae is the family Coxiellaceae, which includes Coxiella burnetii, the etiologic agent of Q fever (Weisburg et al. 1989; Williams et al. 2010). Currently, there are 56 validly published species of Legionella (Table 9.1 ). Besides the named Legionella species, there are bacteria that resemble Legionella but have not yet been cultured outside of their protozoal host in order to permit further characterization. These organisms are designated as Legionella-like amoebal pathogens (LLAP) (Adeleke et al. 1996; Fields et al. 2002; Hookey et al. 1996). There appear to be at least four Legionella spp. among the uncharacterized LLAPs (Adeleke et al. 2001; Birtles et al. 1996; Newsome et al. 1998). Genomic techniques are the definitive means of identifying recognized Legionella spp. and of differentiating them from novel species. PCR and sequencing of the 16S ribosomal RNA subunit (16S rRNA) gene is the initial step (Fields et al. 2002; Kampfer 2012; Pearce et al. 2012). Strains possessing 16S rRNA sequences that are <97.0 % similar to that of all known species are considered a new species (Tindall et al. 2010). When the level of similarity falls between 97.0 % and 99.9 %, DNA-DNA hybridization is done; strains showing <70 % similarity by DNA-DNA hybridization are deemed novel species (Fields et al. 2002; Pearce et al. 2012; Tindall et al. 2010). To further facilitate identification, additional genes are subjected to PCR and sequence analysis in a method known as multi-locus sequence typing (Kampfer 2012; Tindall et al. 2010). The sequence targets that are used for Legionella studies include the macrophage infectivity potentiator (mip), RNase P (rnpB), DNA gyrase (gyrA), RNA polymerase β-subunit (rpoB), and the intergenic 16S-23S ribosomal spacer (Edelstein et al. 2011; Feddersen et al. 2000; Ko et al. 2002; Lo Presti et al. 2001; Luck et al. 2010; Pearce et al. 2012; Ratcliff et al. 1998; Rubin et al. 2005). The European Working Group for Legionella Infections (EWGLI) has created an on-line database to aid in mip-based identification (Fry et al. 2007). Rapid whole-genome sequencing will likely play a large role in defining species in the future (Kampfer 2012). Thus far, the genome sequence has been determined for five strains of L. pneumophila, two strains of L. longbeachae, and one strain of L. drancourtii (Cazalet et al. 2010, 2004; Chien et al. 2004; D’Auria et al. 2010; Glockner et al. 2008; Kozak et al. 2010; Moliner et al. 2009b; Schroeder et al. 2010). Phenotypic traits, such as growth characteristics, fatty acid composition, and serology, continue to be important in the definition of the species (Fields et al. 2002; Kampfer 2012; Pearce et al. 2012). L. pneumophila contains at least 16 different serogroups (Helbig et al. 2007; 2002; Luck et al. 1995). Eight of the other species contain two serogroups, and the remaining species consist of single serogroups (Benson and Fields 1998; Harrison and Saunders 1994).
Thus far, 37 of the Legionella species have been linked to Legionnaires’ disease, with 26 of them being isolated from patients and the remaining 11 implicated based upon serologic evidence (Table 9.1 ). It is likely that additional species will prove to be etiologic agents of disease; for example, at least one of the unnamed LLAPs appears pathogenic based upon serologic evidence (Adeleke et al. 2001; Lamoth and Greub 2010; Marrie et al. 2001; McNally et al. 2000). Except for L. tucsonensis and the recently described L. cardiaca and L. steelei, all of the Legionella species that have been isolated from clinical sources have also been isolated from the environment (Table 9.1 ) (Buse et al. 2012; Edelstein et al. 2011; Flannery et al. 2006; Graham et al. 2011; Pearce et al. 2012; Thacker et al. 1989). In the United States and Europe, L. pneumophila accounts for approximately 90–95 % of Legionnaires’ disease cases (Benin et al. 2002b; Joseph 2004; Joseph and Ricketts 2010; Yu et al. 2002). The next most common causes tend to be L. anisa, L. bozemanae, L. dumoffii, L. longbeachae, and L. micdadei, accounting for approximately 2–8 % of cases (Aurell et al. 2003; Benin et al. 2002b; Joseph and Ricketts 2010; Svarrer and Uldum 2011; Yu et al. 2002). In Australia, New Zealand, and parts of Asia, however, L. longbeachae is the most commonly isolated species, representing up to 30–53 % of clinical cases (Gobin et al. 2009; Graham et al. 2011; Whiley and Bentham 2011).
L. pneumophila serogroup 1, which caused the 1976 Philadelphia outbreak, is the cause of 80–95 % of all cases of Legionnaires’ disease (Amemura-Maekawa et al. 2010; Aurell et al. 2003; Borchardt et al. 2008; Campese et al. 2011; Doleans et al. 2004; Harrison et al. 2009, 2007; Helbig et al. 2002; Joseph 2004; Joseph and Ricketts 2010; Ricketts et al. 2010; Ricketts and Joseph 2007, 2005; Yu et al. 2002). A single subtype of L. pneumophila serogroup 1 is responsible for 64–92 % of cases of Legionnaires’ disease due to L. pneumophila, and 80–94 % of cases due to L. pneumophila serogroup 1 (Amemura-Maekawa et al. 2010; Edelstein and Metlay 2009; Harrison et al. 2009, 2007; Helbig et al. 2002; Kozak et al. 2009). This subtype is defined by its reactivity with a particular monoclonal antibody and is variously designated as the “Pontiac,” “Joly monoclonal type 2 (MAb2),” or “Dresden monoclonal type 3/1 (MAb 3/1)” monoclonal subtype. A sequence-based typing (SBT) method has also been developed for characterizing L. pneumophila isolates, and once again, a subset of strains (e.g., those belonging to ST1, ST47, ST213, ST222) is responsible for most clinical cases (Amemura-Maekawa et al. 2010; Harrison et al. 2009; Hilbi et al. 2010; Kozak et al. 2009; Vergnes et al. 2011). Interestingly, when environmental isolates are examined, there is not such a predominance of serogroup 1 strains, MAb2- or MAb3/1-positive strains, or those sequence subtypes, implying that these strains have enhanced virulence and/or transmissibility (Cazalet et al. 2008; Doleans et al. 2004; Hilbi et al. 2010; Kozak et al. 2009).
The Legionella species that have been linked to cases of Pontiac fever are L. anisa, L. feeleii, L. longbeachae, L. maceachernii, L. micdadei, and L. pneumophila (Cramp et al. 2010; Huhn et al. 2005; Jones et al. 2003). The most common is L. pneumophila, accounting for approximately >70 % of reported cases.
Environmental Ecology of Legionella
Legionella bacteria are ubiquitous in natural aqueous environments. Shortly after its discovery, planktonic L. pneumophila was detected in virtually all of the 267 freshwater habitats (i.e., lakes, ponds, rivers, creeks, swamps, wet soil) examined in the United States (Fliermans et al. 1981, 1979). The ubiquity of L. pneumophila in freshwater has been confirmed throughout the world (Bercovier et al. 1986a; Carvalho et al. 2008, 2007; Castellani Pastoris et al. 1989; Dutka and Ewan 1983; Joly et al. 1984; Lawrence et al. 1999; Lee et al. 2010; Ortiz-Roque and Hazen 1987; Parthuisot et al. 2010; Sheehan et al. 2005; Tobiansky et al. 1986; Verissimo et al. 1991), and, over the years, the organism has also been found in marine and estuarine environments (Heller et al. 1998; Ortiz-Roque and Hazen 1987; Palmer et al. 1993; Paszko-Kolva et al. 1993). In man-made (engineered) water systems, L. pneumophila is similarly widespread, existing, in some areas, within the plumbing of 60–85 % of large and small public buildings, as well as in private residences (Alary and Joly 1991; Atlas 1999; Lasheras et al. 2006; Lee and West 1991; Mouchtouri et al. 2007; Ragull et al. 2007). The broad distribution of L. pneumophila is partly due to the organism’s capacity to survive at 4–63 °C (Atlas 1999; Fliermans et al. 1981, 1979; Heller et al. 1998; Joly et al. 1984; Wadowsky et al. 1985; Wullings and van der Kooij 2006). However, warm water is more likely to contain the bacterium, especially in hot water tanks and heaters and in water-cooled heat rejection devices such as cooling towers. The ability of L. pneumophila to survive and grow at lower temperatures has been linked to its type II secretion system (see below), a secreted protein foldase, lipid A modifications, RNase R, and an RNA helicase, among other things (Charpentier et al. 2008; Söderberg and Cianciotto 2008; Söderberg and Cianciotto 2010; Söderberg et al. 2004, 2008). Although fewer studies have focused on assessing the distribution of non-pneumophila species, a variety of these bacteria are often easily found in natural and engineered water systems (Buse et al. 2012; Lee et al. 2010; Parthuisot et al. 2010). L. pneumophila, L. longbeachae, and several other Legionella species are also found in soil, potting soil, and compost (Hughes and Steele 1994; Lindsay et al. 2012). Under certain conditions, including low-nutrient environments, oxidative and osmotic stresses, and heat shock, viable but not cultivatable (VBNC) forms of L. pneumophila have been observed (Allegra et al. 2008; Delgado-Viscogliosi et al. 2005, 2009; Dusserre et al. 2008; Edagawa et al. 2008; Hay et al. 1995; Hussong et al. 1987; Paszko-Kolva et al. 1992, 1993; Yamamoto et al. 1996). It is unknown if VBNC legionellae are directly pathogenic for mammals.
It is now accepted by most authorities that L. pneumophila and probably all other Legionella species are facultative parasites of free-living amoebae and that the major replicative form of the organism in the environment is within amoebae (Buse et al. 2012; Fields et al. 2002; Hilbi et al. 2010; Lau and Ashbolt 2009; Pagnier et al. 2009; Taylor et al. 2009; Thomas et al. 2010). Several lines of evidence support this viewpoint. First, waters that contain legionellae, including those sources linked to disease transmission, are always rich in protozoa; indeed, the number of legionellae in a water sample correlates with the number of protozoa (Breiman et al. 1990b; Fields et al. 1989; Lasheras et al. 2006; Moore et al. 2006; Paszko-Kolva et al. 1991; Patterson et al. 1997; Valster et al. 2011; Yamamoto et al. 1992a). Second, amoebae (e.g., hartmannellae, acanthamoebae, naegleriae) isolated from natural and engineered aquatic environments (as well as soil samples) harbor intracellular legionellae, including L. pneumophila (Berk et al. 2006; Declerck et al. 2007a; Gast et al. 2011; Harf and Monteil 1988; Iovieno et al. 2010; Michel et al. 1998; Newsome et al. 1998; Singh and Coogan 2005; Thomas et al. 2006). Third, the capacity of a Legionella-containing water sample to support bacterial growth is dependent upon the presence of the amoebae (Barbaree et al. 1986; Fields et al. 1989; Kuiper et al. 2004; Nahapetian et al. 1991; Steinert et al. 1998; Wadowsky et al. 1988, 1991). Fourth, in coculture experiments done in the laboratory, L. pneumophila replicates in at least 20 different types of amoebae, including representatives from the genus Acanthamoeba (8 species), Balamuthia (1 species), Dictyostelium (1 species), Echinamoeba (1 species), Hartmannella (2 species), Naegleria (6 species), Vahlkampfia (1 species), and Willaertia (1 species) (Anand et al. 1983; Barbaree et al. 1986; Dey et al. 2009; Fields 1996; Fields et al. 1989; Hagele et al. 2000; Harada et al. 2010; Harf and Monteil 1988; Henke and Seidel 1986; Holden et al. 1984; Michel et al. 1998; Miyamoto et al. 2003; Molmeret et al. 2001; Newsome et al. 1985; Rowbotham 1986, 1980; Shadrach et al. 2005; Solomon et al. 2000; Tyndall and Domingue 1982; Wadowsky et al. 1991). Finally, a recent study of engineered water systems suggests that L. pneumophila might also be capable of replicating within Diphylleia and Neoparamoeba (Valster et al. 2010). In addition to parasitizing amoebae, L. pneumophila has the ability to infect and grow in at least 3 species of ciliates belonging to the genus Tetrahymena (Barbaree et al. 1986; Fields 1996; Fields et al. 1986, 1984; Kikuhara et al. 1994). Intra-amoebal growth of L. pneumophila occurs at temperatures ranging from 22 °C to 37 °C (Buse and Ashbolt 2011; Newsome et al. 1985; Söderberg et al. 2008), whereas replication in Tetrahymena occurs at 30–35 °C (Fields et al. 1984; Kikuhara et al. 1994; Steele and McLennan 1996). Other Legionella species that have been shown to infect amoebae and/or tetrahymenae are L. anisa, L. bozemanae, L. drancourtii, L. dresdenensis, L. drozanskii, L. dumoffii, L. fallonii, L. feeleii, L. gormanii, L. hackeliae, L. jamestowniensis, L. jordanis, L. londiniensis, L. longbeachae, L. lytica, L. micdadei, L. oakridgensis, L. quinlivanii, L. rowbothamii, L. rubrilucens, L. steelei, L. steigerwaltii, and L. worsleiensis (Adeleke et al. 2001; Doyle et al. 1998; Edelstein et al. 2011; Fields et al. 1986, 1990; Furuhata et al. 2010; Hsu et al. 2011; Kuroki et al. 2007; La Scola et al. 2003, 2004; Luck et al. 2010; Moffat and Tompkins 1992; Neumeister et al. 1997; Rowbotham 1983, 1986; Steele and McLennan 1996; Wadowsky et al. 1991). Not all legionellae will grow in the same protozoan host, indicating strain-to-strain variation in the selection of the optimal host cell (Buse and Ashbolt 2011; Dey et al. 2009; Fields et al. 1989, 1990; Rowbotham 1986; Steinert et al. 1994; Wadowsky et al. 1991). Among the various protozoa that are permissive for L. pneumophila growth, Hartmannella vermiformis amoebae are most often cited as being the critical host cell within environmental water systems, including those linked to disease (Breiman et al. 1990b; Buse et al. 2012; Fields et al. 1989; Hsu et al. 2011; Kuiper et al. 2004; Taylor et al. 2009; Valster et al. 2010, 2011; Wadowsky et al. 1988). Of the remaining types of protozoan hosts, the acanthamoebae and naegleriae are also considered important natural reservoirs (Taylor et al. 2009).
In vitro studies have shown that L. pneumophila can infect the soil nematode Caenorhabditis elegans (Brassinga et al. 2010; Komura et al. 2010, 2012). However, it is unknown whether legionellae survive and/or grow within nematodes in the natural environment. Based upon antibody-detection methods, L. pneumophila has been observed within various types of insects living in aquatic habitats, suggesting that insects may be another natural reservoir for legionellae (Castellani Pastoris et al. 1989).
In addition to surviving within the planktonic phase and protozoan hosts, Legionella bacteria also exist within multi-organismal biofilms that cover surfaces within natural and engineered water systems (Colbourne et al. 1984; Declerck 2010; Emtiazi et al. 2004; Flannery et al. 2006; Hsu et al. 2011; Lau and Ashbolt 2009; Marrao et al. 1993; Riffard et al. 2001; Sheehan et al. 2005; Tison et al. 1980; Wingender and Flemming 2011). In fact, the interaction between Legionella bacteria and its protozoan hosts likely occurs for the most part within and near complex biofilms. Many in vitro studies have examined the ability of L. pneumophila to exist within model biofilms. Physical parameters that have been found to influence the process include the chemical and physical properties of the surface, the flow rate and turbulence of the liquid over the surface, the ambient temperature, organic-carbon content, metal (e.g., iron) concentrations, and the presence of biocides (Bezanson et al. 1992; Cargill et al. 1992; Donlan et al. 2005; Green and Pirrie 1993; Hindre et al. 2008; Lehtola et al. 2007; Liu et al. 2006; Pang and Liu 2006; Pecastaings et al. 2010; Piao et al. 2006; Rogers et al. 1994; Schoenen et al. 1988; Schofield and Locci 1985; Schofield and Wright 1984; Turetgen and Cotuk 2007; Walker et al. 1993; Wright et al. 1989, 1991). Biological factors that influence L. pneumophila within biofilms include both the presence of amoebal hosts (e.g., H. vermiformis, A. castellanii) and the presence of other types of bacteria, some of which promote or are at least compatible with Legionella (e.g., certain species of Acinetobacter, Aeromonas, Empedobacter, Escherichia, Flavobacterium, Microbacterium, Pseudomonas, Sphingomonas, and Stenotrophomonas) and others which inhibit the legionellae (e.g., Corynebacterium and certain other species of Pseudomonas(Declerck et al. 2007b, 2009; Donlan et al. 2005; Giao et al. 2011; Guerrieri et al. 2008; Kimura et al. 2009; Kuiper et al. 2004; Mampel et al. 2006; Manz et al. 1995; Messi et al. 2011; Moritz et al. 2010; Murga et al. 2001; Rogers and Keevil 1992; Storey et al. 2004; Temmerman et al. 2006; Vervaeren et al. 2006; Williams and Braun-Howland 2003). Substances released from dead bacteria as well as blue-green algae can promote Legionella growth (Temmerman et al. 2006; Tison et al. 1980). Legionella factors that are needed for colonization of biofilms include flagella, type IV pili, and Lcl, a surface protein that has collagen-like domains (Duncan et al. 2011; Lucas et al. 2006; Mampel et al. 2006). Some in vitro studies have concluded that L. pneumophila replication within biofilms can occur in the absence of amoebal hosts (Keevil 2003; Mampel et al. 2006; Rogers and Keevil 1992; Temmerman et al. 2006). However, others have argued that, although persistence can occur in the absence of amoebae, replication requires the presence of protozoan hosts (Declerck et al. 2007b, 2009; Kuiper et al. 2004; Murga et al. 2001). Clearly, the mechanism of Legionella growth within natural biofilms is likely to be variable, depending upon extraneous microbial and environmental factors.
In addition to providing a means for bacterial growth in the environment, Legionella infection of protozoa has great significance for the genesis of disease for several more reasons. First, legionellae in amoebae remain viable for long periods of time, increasing the possibility for disease transmission (Bouyer et al. 2007), and residence within the amoebae may protect the bacterium from the harmful effects of aerosolization. Second, ingestion and growth in amoebae “resuscitate” VBNC legionellae, resulting in viable bacteria that are infective (Allegra et al. 2008; Dusserre et al. 2008; Garcia et al. 2007; Hwang et al. 2006; Steinert et al. 1997). Third, the relative chlorine resistance of environmental L. pneumophila and other legionellae is explained in part by the protection afforded the bacterium growing within an amoebal cyst and the phenotype change of the bacterium resulting from intra-amoebal growth (Barker et al. 1992, 1993; Kilvington and Price 1990; King et al. 1988). Fourth, legionellae grown in amoebae maintain and in some cases display enhanced infectivity for macrophages and experimental animals (Cirillo et al. 1994, 1999; Harf and Monteil 1988; Neumeister et al. 2000; Tyndall and Domingue 1982; Vandenesch et al. 1990). Fifth, Legionella-laden protozoa or protozoan vesicles or cysts containing legionellae might be part of the inoculum that initiates lung infection (Berk et al. 1998; Brieland et al. 1996, 1997a, b; Rowbotham 1986). Finally, it is widely believed that the ability of L. pneumophila to parasitize protozoa engendered it with the capacity to infect macrophages and thereby cause disease (Albert-Weissenberger et al. 2007; Cianciotto and Fields 1992; Fields et al. 2002; Hilbi et al. 2011; Lau and Ashbolt 2009; Molmeret et al. 2005; Newton et al. 2010; Shin and Roy 2008; Swanson and Hammer 2000). Indeed, many of the bacterial genes that promote protozoan infection also promote infection of macrophages (Cianciotto and Fields 1992; Gao et al. 1997; Newton et al. 2007; Pruckler et al. 1995; Segal and Shuman 1999). Moreover, the intracellular infection pathway within amoebae is similar to what occurs within macrophages (Abu Kwaik 1996; Bozue and Johnson 1996; Gao et al. 1999; Liles et al. 1999; Newsome et al. 1985). Thus, understanding how Legionella bacteria grow in protozoa is critical to both understanding the natural history of Legionnaires’ disease and devising novel ways of minimizing the risk of disease transmission. A large body of literature exists regarding the molecular and cellular pathogenesis of L. pneumophila for amoebae and its relationship to pathogenicity for macrophages; this topic is covered in a later section of this chapter.
Epidemiology, Clinical Presentation, Treatment, and Laboratory Diagnosis
Epidemiology of Legionnaires’ Disease
Legionnaires’ disease is usually transmitted from the environment to humans by the inhalation of aerosols of Legionella-contaminated water (Breiman and Butler 1998; Carratala and Garcia-Vidal 2010; Stout and Yu 1997). The waters linked to disease include both potable and non-potable sources (Craun et al. 2010; Fry et al. 2003; Kusnetsov et al. 2010). Microaspiration and direct installation of contaminated water into the lungs are another, albeit less common, mode of spread particularly in nosocomial disease (Blatt et al. 1993; Carratala and Garcia-Vidal 2010; Venezia et al. 1994). Aspiration of large amounts of water during near drowning is a rare but reported mode of disease transmission (Hasselmann et al. 1983; Inoue et al. 2011; Lavocat et al. 1987; Miyamoto et al. 1997; Nozue et al. 2005; Sekla et al. 1982). Person-to-person transmission of legionellosis does not occur (Edelstein and Cianciotto 2010).
The sources of Legionella-containing aerosols are usually water-cooled heat rejection equipment such as air-conditioning cooling towers, whirlpool spas, sink taps, and shower heads (Breiman et al. 1990b; Brulet et al. 2008; Campese et al. 2010; Cordes et al. 1981; Den Boer et al. 2002; Ferre et al. 2009; Fields et al. 2002; Garcia-Fulgueiras et al. 2003; Lam et al. 2011; Nguyen et al. 2006; Nicolay et al. 2010; Pagnier et al. 2009; Ricketts et al. 2011). However, virtually, any device that can create an aerosol of water can be a disease source, including ice machines, mist machines (e.g., vegetable misters in grocery stores), decorative fountains and waterfalls, evaporative condensers, industrial air scrubbers, high-pressure power washers, asphalt paving machines, sump pumps, gardening hoses, windshield wipers, and respiratory therapy equipment, including nebulizers, humidifiers, oxygen humidifiers, and ventilator tubing (Arnow et al. 1982; Barrabeig et al. 2010; Breiman et al. 1990a, b; Castor et al. 2005; Cordes et al. 1981; Coscolla et al. 2010; Haupt et al. 2012; Kool et al. 1998; Mahoney et al. 1992; Marrie et al. 1991; Nygard et al. 2008; O’Loughlin et al. 2007; Piso et al. 2007; Schuetz et al. 2009; Wallensten et al. 2010; Woo et al. 1992). L. longbeachae infections are more commonly associated with exposure to soil and compost as opposed to the aquatic environments typically inhabited by L. pneumophila and the other species (Graham et al. 2011; Lindsay et al. 2012; Whiley and Bentham 2011).
Legionnaires’ disease occurs both within the community and in hospital settings, with >90 % of cases being community-acquired pneumonia and <10 % nosocomial pneumonia (Fields et al. 2002; Joseph and Ricketts 2010; Korvick et al. 1987; Ricketts and Joseph 2005, 2007). In both settings, Legionnaires’ disease manifests sporadically and as outbreaks (Fields et al. 2002). Overall, however, the vast majority of cases are sporadic, for example, 89 % of cases in the USA in the 1980s (Fields et al. 2002), 73–82 % of cases in England and Wales during the 1990s (Joseph et al. 1994a, 1995, 1997, 1998; Newton et al. 1996), and 73–91 % of cases in Europe in the 2000s (Joseph 2004; Joseph and Ricketts 2010; Ricketts and Joseph 2005, 2007). Based upon a prospective study, it has been estimated that 8,000–18,000 sporadic cases of Legionnaires’ disease occur each year among US adults needing hospitalization (Marston et al. 1997). When taking into account an earlier study that focused on community-acquired pneumonia not requiring hospitalization (Foy et al. 1979), the incidence of Legionnaires’ disease is estimated as being 18,000–88,000 US cases per year (Edelstein and Cianciotto 2010). Both in Germany and in Spain, the incidence of sporadic Legionella pneumonia is projected to be at least 15,000–30,000 cases per year (von Baum et al. 2008). In various studies aimed at assessing the overall etiology of community-acquired pneumonia, the percentage due to Legionnaires’ disease has ranged from 0 % to 16 % (Borchardt et al. 2008; Breiman and Butler 1998; Cilloniz et al. 2011; Edelstein and Cianciotto 2010; Fields et al. 2002; Muder et al. 1989; Ruiz et al. 1999; Woodhead 2002; Yu and Stout 2008). When considering only those cases requiring hospitalization, most studies place the percentage due to Legionella at 2 % to 9 % (Bohte et al. 1995; Cilloniz et al. 2011; Fields et al. 2002; Ruiz et al. 1999; von Baum et al. 2008; Woodhead 2002; Yu and Stout 2008). Since 2003, there has been an increase in number of cases of Legionnaires’ disease reported to the CDC, suggesting that there might be an increasing incidence of the disease in the USA (Carratala and Garcia-Vidal 2010; CDC 2011; Neil and Berkelman 2008; Ng et al. 2008a). Increased notifications in the last decade have been documented elsewhere, including in Australia and Canada (Li et al. 2002; Ng et al. 2008b). However, other countries, such as the Netherlands, have reported a recent decline in disease incidence (Euser et al. 2012).
Although most Legionnaires’ disease cases are sporadic, outbreaks continue to occur throughout the world; for example, 51 outbreaks happened in Europe between 1997 and 2001 (Joseph 2002). Some outbreaks have been especially serious in terms of the large number of people involved. Table 9.2 lists 13 outbreaks that have occurred since 1999 and resulted in >50 confirmed cases. These outbreaks serve to emphasize four additional points. First, cooling towers continue to be a notorious source for disease transmission. Second, based upon findings obtained during the outbreaks in France and Norway, long-distance spread of the disease agent can be >6 km from a cooling tower source and >10 km from an air scrubber source. Third, disease prevention through the use of proper engineering and construction and of rapid diagnosis by lab testing is important, but knowledge of the factors responsible for disease outbreaks remains lacking.
Host risk factors for community-acquired Legionnaires’ disease include administration of glucocorticosteroid medications, anti-TNF-α therapy, and other forms of immunosuppression, organ transplantation, smoking, end-stage renal disease, age greater than 50 years, AIDS, hematologic malignancies, lung cancer, chronic heart or lung disease, diabetes, silicosis, and male gender (Beigel et al. 2009; Broome and Fraser 1979; Carratala et al. 1994; Den Boer et al. 2002; Ginevra et al. 2009; Girard and Gregson 2007; Gudiol et al. 2009; Hofmann et al. 2009; Jacobson et al. 2008; Jinno et al. 2009; Marston et al. 1994; Nguyen et al. 2006; Straus et al. 1996; Tubach et al. 2006). Alcohol abuse may or may not be a significant risk factor (Broome and Fraser 1979; Carratala et al. 1994; Ferre et al. 2009; Lettinga et al. 2002; Marston et al. 1994; Straus et al. 1996). The same host risk factors seem to apply for nosocomial acquisition (Carratala et al. 1994; Haley et al. 1979; Joseph et al. 1994b). Recent surgery, or more probably general anesthesia, has been a risk factor in some nosocomial epidemics (Korvick and Yu 1987; Serota et al. 1981). Legionnaires’ disease is rare in children, accounting for ≤1 % of pediatric pneumonias. When it does occur, it usually results from nosocomial infection of immunosuppressed children (Edelstein and Cianciotto 2010).
In addition to the host factors named above, activities that increase the chances of exposure to Legionella bacteria in water heighten the risks of disease. Activities that increase the risk of community-acquired Legionnaires’ disease include recent overnight travel, use of well water, plumbing work in the home, and disruptions in the water supply that result in “brown” water in taps (Alary and Joly 1991; Fields et al. 2002; Joseph et al. 2010; Straus et al. 1996). Additional risk activities include living near or proximity to a cooling tower or other wet cooling systems, using or being nearby whirlpool spas or hot spring baths, being near decorative fountains, working in underground wells, and being a professional (e.g., taxi, bus) driver (Bhopal et al. 1991; Den Boer et al. 2002; Hlady et al. 1993; Jernigan et al. 1996; Miyamoto et al. 1997; Ricci et al. 2010; Ricketts et al. 2011; Sakamoto et al. 2009; Wallensten et al. 2010). Activities that have been more associated with an increased risk of nosocomial disease include the use of respiratory therapy equipment and on rare occasions wound treatments and water birthing (Edelstein and Cianciotto 2010; Lowry et al. 1991; Nagai et al. 2003).
Cases of community-acquired Legionnaires’ disease tend to be most frequent during the summer and early fall (CDC 2011; Joseph and Ricketts 2010; Li et al. 2002; Neil and Berkelman 2008). Nosocomial disease does not show this seasonal variation. Wet, humid weather, rainfall, and low winds can increase the risk for disease (Ferre et al. 2009; Fisman et al. 2005; Hicks et al. 2007). Geographical location is also a factor; for example, within the USA, cases are more frequent in the Northeast and Midwest regions (Neil and Berkelman 2008).
Clinical Presentation and Treatment of Legionnaires’ Disease
Legionnaires’ disease presents clinically as pneumonia, with features indistinguishable from other common forms of bacterial pneumonia, such as pneumococcal pneumonia (Diederen 2008; Edelstein 1993; Edelstein and Cianciotto 2010; Granados et al. 1989; Roig et al. 1991; Sopena et al. 1998; Tan et al. 2000; Tsai et al. 1979). The incubation period of the disease is usually 2–10 days but can be more than 2 weeks (Breiman and Butler 1998; Den Boer et al. 2002). A prodromal illness may occur, lasting for hours to several days, with symptoms such as headache and myalgia. There was some suggestion that a combination of factors such as diarrhea, hyponatremia, and increased serum creatine kinase is more consistent with Legionnaires’ disease than other pneumonic diseases, but no study has shown this unequivocally. Recently, it has been suggested that a useful diagnostic might involve high body temperature, absence of sputum production, low serum sodium, high levels of lactate dehydrogenase and C-reactive protein, and low platelet counts (Carratala and Garcia-Vidal 2010; Fiumefreddo et al. 2009). Thus, the currently accepted clinical presentation generally consists of fever, fatigue, often headache or muscle aches, and nonproductive cough. Chest pain, diarrhea, confusion, shaking chills, and shortness of breath also may be seen. The chest roentgenogram usually demonstrates alveolar filling, focal infiltrates, and lung consolidation with or without pleural effusions. Lung abscesses can occur, but rarely (Yu et al. 2009). Extrapulmonary infection occurs rarely as disseminated infection in patients with pneumonia or very rarely as isolated primary infection (Edelstein 1993; Lowry and Tompkins 1993; Stout and Yu 1997). Pleural empyema, myocarditis, pericarditis, endocarditis, meningitis, encephalitis, vascular shunt infections, arthritis, peritonitis, and colitis have all been documented to very rarely occur during the course of pneumonia (Edelstein and Cianciotto 2010; Fernandez-Cruz et al. 2011; Flendrie et al. 2011; Pearce et al. 2011). Other sites of metastatic infection have been the intestines, spleen, liver, bone marrow, and surgical wounds including prosthetic heart valves and aorta. Isolated infections, without pneumonia, include disease of prosthetic heart valves, respiratory sinuses, open wounds, soft tissue abscesses, and cellulitis (Edelstein and Cianciotto 2010; Han et al. 2010; Loridant et al. 2011).
Fatality rates for Legionnaires’ disease vary greatly, ranging from 1 % to 80 % (Benin et al. 2002b; Graham et al. 2011; Lam et al. 2011). Factors influencing the rate include host risk factors, time to effective therapy, and whether disease is sporadic vs. outbreak and nosocomial vs. community acquired (Edelstein and Cianciotto 2010). Overall, the lowest fatality rates tend to be associated with large community outbreaks, whereas the highest rates occur with untreated nosocomial infections.
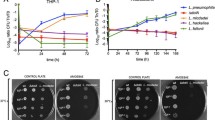
Legionnaires’ disease is treated with macrolide, fluoroquinolone, or tetracycline antimicrobial agents (Carratala and Garcia-Vidal 2010; Cunha 2010; Diederen 2008; Edelstein 1998; Edelstein and Cianciotto 2010; Fields et al. 2002). All of these agents concentrate within macrophages and therefore are able to act on the replicating, intracellular legionellae. The drugs of choice to treat mild disease in community-acquired pneumonia include erythromycin, doxycycline, azithromycin, and levofloxacin. For severely ill patients, or immunocompromised ones, either azithromycin or levofloxacin is the drug of choice (Edelstein 1995, 1998). Antimicrobial agents that are ineffective include all ß-lactam agents and penems, aminoglycosides, glycopeptides, and chloramphenicol. The response to treatment depends on the patient’s age, underlying diseases, degree of pulmonary involvement, the timing of treatment in relation to disease onset, and severity of disease. Untreated disease is fatal in 5–80 % of patients, depending on the above factors; previously, healthy people with minimal disease have the best outcome, and otherwise ill or immunocompromised patients with extensive pneumonia the worst outcome. Prompt-specific therapy reduces the fatality rate by two- to sixfold. The duration of therapy, depending on the agent used and the presence of immunosuppression, ranges from 3 to 21 days; patients with endocarditis or cavitating pneumonia may require longer courses of therapy. Although antibiotic-resistance genes have been identified in Legionella bacteria, including β-lactamases and aminoglycoside kinases (Fu and Neu 1979; Mercuri et al. 2001; Suter et al. 1997; Thompson et al. 1998), clinically relevant resistance has fortunately not emerged.
Epidemiology and Clinical Aspects of Pontiac Fever
Pontiac fever is a self-limited, nonpneumonic illness of short duration (Edelstein 2007; Glick et al. 1978; Goldberg et al. 1989; Luttichau et al. 1998; Mangione et al. 1985; Tossa et al. 2006). Its incubation period is usually 4 h to 6 days (median of 2 days), but it can be as long as 9 days. The attack rate is quite high, with >80 % of those exposed becoming ill. The sources of the Legionella-contaminated aerosols for Pontiac fever are similar to those for Legionnaires’ disease, with a variety of aerosol-generating devices implicated as well as potting soil (Castor et al. 2005; Cramp et al. 2010; Euser et al. 2010; Jones et al. 2003; Nicolay et al. 2010). No host risk factors have been identified for Pontiac fever (Edelstein and Cianciotto 2010). Most reported cases of Pontiac fever have been linked to outbreaks, although there have been cases occurring in a non-epidemic setting (Bauer et al. 2008). Fever, headache, myalgia, and asthenia are the main symptoms of Pontiac fever. Less common symptoms are cough, dyspnea, anorexia, arthralgia, and abdominal discomfort. Most patients are not ill enough to seek medical attention. Recovery usually occurs without any specific treatment, 3–5 days after disease onset. The basis for Pontiac fever remains obscure (Edelstein 2007). The short incubation period, short duration of illness, and full recovery without antibiotics cause most to conclude that the disease is not the result of a Legionella infection. Also, the percentage of persons in outbreaks that have elevated titers of anti-Legionella antibodies is quite variable, ranging from 30 % to 85 %. Thus, the prevailing hypotheses to explain this disease include the inhalation of a bacterial (endo)toxin, an allergic reaction to inhaled live or dead bacteria, or inhalation of amoebae that are also present in the contaminated water (Edelstein 2007). Interestingly, several outbreaks have consisted of both Legionnaires’ disease and Pontiac fever; however, it is unclear why some people developed pneumonia whereas others the nonpneumonic form of disease (Benin et al. 2002a; Euser et al. 2010).
Laboratory Diagnosis of Legionella Infections
Rapid diagnosis of infection due to Legionella spp., in particular L. pneumophila, is important for both patient management and effective public health action. The methods currently available for diagnosis of Legionnaires’ disease are culture, urinary antigen detection, direct fluorescent antibody testing, and detection of nucleic acids or of specific antibodies in serum samples. However, presently, none of the diagnostic tests available offer the desired quality with respect to sensitivity and specificity.
Culture of Legionella spp.
Culture is still the “gold standard” among all diagnostic methods for Legionella infections. The medium used, supplemented charcoal yeast extract medium (BCYE), is easily prepared by any large clinical microbiology laboratory and can be made in a selective form. Use of selective media and specimen decontamination with acid are obligatory for optimal culture yield from normally nonsterile tissues and fluids. To obtain optimal yield, specimens with and without acid pretreatment are plated on three different media (all commercially available): BCYE (nonselective), BMPA (selective, also called “CAP” or “PAC”), and MYEA (selective, also called “PAV” or “VAP”) (Edelstein 1985a, b; Vickers et al. 1987). Use of two different selective media is required as some Legionella spp., and some strains of L. pneumophila serogroup 1, will not grow on BMPA, which is the most selective medium. Use of multiple media also increases the chances of detecting very small numbers of Legionella spp. bacteria present in the specimen. Specimen dilution before plating is also important, as Legionella spp. growth may be inhibited by certain cations, other bacteria, and by tissue factors. The organism has been successfully isolated from sputum, transtracheal aspirates, endotracheal suction specimens, blood, lung biopsy, pleural fluid, bronchial lavage, pericardial fluid, peritoneal fluid, wounds, bowel abscesses, prosthetic heart valves, brain abscesses, myocardium, kidney, liver, vascular grafts, and respiratory sinuses. Cultures generally remain positive for several days after the initiation of antimicrobial therapy and may remain positive for weeks or months from pulmonary abscesses. Broad-spectrum antimicrobial therapy decreases culture yield.
The sensitivity of culture for the diagnosis of Legionnaires’ disease has been estimated to be in the range of 11–65 % by retrospective studies performed in different reference laboratories (Den Boer and Yzerman 2004; Hayden et al. 2001; Lindsay et al. 2004). Legionella colonies usually form within 3–5 days, which are relatively easy to identify due to their specific colony morphology. To ascertain that it is Legionella suspected colonies are subcultured on BCYE agar and on cysteine-free BCYE agar, as Legionella have a growth requirement for L-cysteine. So far, a positive culture is the only method that allows the comparison of patient and environmental Legionella strains, thus confirming or excluding a given environmental reservoir as the source of infection.
Detection of Legionella Antigen in Urine
The most widely used method for laboratory diagnosis of Legionnaires’ disease is the urinary antigen test. This ELISA test is based on the identification of a lipopolysaccharide component of L. pneumophila that is heat stable, resistant to enzymatic cleavage, and about 10kDa of molecular weight. This soluble antigen appears very early after infection, about 2–3 days after the first clinical symptoms, and can be excreted for a long time. In average, this antigen is present for several days up to 2 month and has been detected in one patient for nearly 1 year (Kohler et al. 1984). Compared to culture, urinary antigen tests are much faster, easy to perform, cheaper, and more sensitive. The specificity of these assays that were mainly evaluated by testing urine samples from patients with urinary tract infections or pneumonia caused by other pathogens has been reported to be more than 99 % (Den Boer and Yzerman 2004; Domínguez et al. 1998; Helbig et al. 2001b, 2003b). In contrast, the sensitivity of the assay varies between 56 % and 99 % according to the study (Birtles et al. 1990; Domínguez et al. 1998, 2001; Helbig et al. 2001b, 2003b; Kazandjian et al. 1997; Plouffe et al. 1995; Ruf et al. 1990; Yzerman et al. 2002). There are several commercial enzyme immunoassay kits available (e.g., Binax, Biotest, Bartels); the best-studied one is made by the Binax Company. However, all available urinary antigen tests have the disadvantage that they lack sensitivity for serogroups other than L. pneumophila serogroup 1 (Olsen et al. 2009). Recently, a new kit, XpectTM Legionella test (Oxoid), was introduced, which was designed for the direct, qualitative detection of L. pneumophila serogroup 1 and 6 antigens in human urine samples. However, a recent study reported a sensitivity of 79 % for the Binax EIA test, and only 32 % for the Xpect kit. Furthermore, none of the 10 L. pneumophila serogroup six samples tested were positive by the Xpect test, which claimed to recognize also serogroup 6 (Svarrer et al. 2012). The sensitivity of the urinary antigen-based tests was reported to correlate highly with the severity of the illness (Blázquez et al. 2005; Lück et al. 2006; Yzerman et al. 2002) and the presence of underlying diseases (Sopena et al. 2002).
Besides these tests, a rapid immunochromatographic (ICT) test (BinaxNOW) has been on the markets since several years. It detects urinary antigen very rapidly, and no lab equipment is required. The sensitivities and specificities were estimated to be 89 % and 100 %, respectively, for the Oxoid Xpect Legionella test kit and 86 % and 100 %, respectively, for the BinaxNOW test (Diederen et al. 2009). Similarly, Higa and colleagues reported a sensitivity and specificity of ICT using respiratory samples of 1.0 and 0.99, respectively (Higa et al. 2008). In contrast, a recent study reported a sensitivity of only 47 % for the BinaxNOW test (Svarrer et al. 2012). Recently, other immunochromatographic assays were released, like the SAS Legionella test, the Rapid U test, or the SD Bioline assay.
However, despite the great advantages of the urinary antigen test like easy, rapid, and highly specific, one should keep in mind that a negative urinary antigen test never excludes a Legionella infection.
Direct Fluorescence Antibody Testing
Legionella antibodies can be detected in clinical samples by direct florescence antibody (DEA) testing using commercially available monoclonal antibodies specific for the species L. pneumophila. However, no monoclonal antibodies are available for Legionella species other than L. pneumophila (Edelstein et al. 1985). Results can be obtained in 2–4 h. The principal drawback of this method is its low sensitivity reported to be between 25 % and 70 % (Hayden et al. 2001; Lindsay et al. 2004). The specificity which is 60–70 % is due to cross-reactions with certain Gram-negative bacteria like Pseudomonas aeruginosa, P. fluorescens, Stenotrophomonas maltophilia, Bordetella pertussis, Bacteroides fragilis, and Francisella tularensis (Jarraud and Etienne 2012). Therefore, a negative result does not rule out disease, and a positive result is almost always diagnostic of it (Edelstein et al. 1980). The protein antigen detected by this test is not degraded after fixation with formalin. Thus, this test allows the detection of the etiologic agent in formalin-fixed lung tissue, which is not possible with the other methods available (Lück 2008).
Serology
The first test that identified antibodies directed against L. pneumophila used indirect immunofluorescent microscopy (IFA) and was set up by the CDC (Centers for Disease Control and Prevention) during the Philadelphia outbreak in 1976 (McDade et al. 1977). The vast majority of laboratories use the IFA technique to determine antibody concentrations. Only measurement of antibody to L. pneumophila serogroup 1 by IFA is well standardized and is the “gold standard” test used to diagnose Legionnaires’ disease by serologic means.
There are two widely used reference methods of antigen preparation for the IFA test: heat fixation of plate-grown bacteria and formalin fixation of chicken embryo yolk sac-grown bacteria (Edelstein 1997). The latter method may be more specific, although large head-to-head comparative studies have not been performed (Harrison and Taylor 1982). Many commercial laboratories sell kits containing formalin-fixed plate-grown bacteria, but these kits are not known to provide the same results as either of the reference methods. Use and results interpretation of these commercial IFA kits may not give results similar to those obtained using the reference methods. The specificity of the IFA test in a hospitalized population is not well known; this probably approximates 90 % for a fourfold titer rise, although in an epidemic situation in nonhospitalized patients, the specificity is close to 100 %. Cross-reactions for the IFA test have been reported with Mycobacteria, Leptospira, Chlamydia, Mycoplasma, Citrobacter, Campylobacter, Coxiella burnetii, Pseudomonas, and Bacteroides fragilis (Bornstein et al. 1984; Boswell et al. 1996; Collins et al. 1984; Gray et al. 1991; Klein 1980). Cross-reactions have been also frequently observed among the different Legionella species and serogroups, which makes diagnostic sometimes difficult (Wilkinson et al. 1983). One of the most frequent cross-reactions occurs between L. pneumophila serogroups 1 and 6.
About three-quarters of patients with culture-proven Legionnaires’ disease caused by L. pneumophila serogroup 1 develop a fourfold rise in IFA titer from 1 to 2 weeks after onset of illness. The mean time required for demonstration of seroconversion is about 2 weeks; however, up to 25 % of seroconversions are missed unless serum is collected up to 8 weeks after onset of illness. However, in certain cases, despite the diagnosis of legionellosis was confirmed, seroconversion was never observed. Furthermore, the possibility of cross-reactions and the fact that serologic testing is retrospective in nature (and does not influence choice of therapy), the other major drawback of diagnosing Legionella infections using serologic means is that the test may be negative because the serotype of the infecting organism is not tested for. Thus, serologic testing in the diagnosis of this disease is much more helpful to epidemiologists than to clinicians caring for individual patients.
Detection of Nucleic Acids of Legionella
The detection of DNA of Legionella by PCR in respiratory samples was reported first in 1992 (Jaulhac et al. 1992). By investigating the performance of PCR in bronchoalveolar lavage fluid specimens, the investigators established the principle that PCR is suitable for detection of Legionella DNA in clinical samples. Meanwhile PCR methods have been developed for testing for the presence of Legionella in many different samples like from bronchoalveolar lavage fluid, throat swaps, blood, peripheral leukocytes, serum, and urine (Helbig et al. 1999; Jaulhac et al. 1992; Jonas et al. 1995; Maiwald et al. 1995; Matsiota-Bernard et al. 1994; Murdoch and Chambers 2000; Ramirez et al. 1996; Weir et al. 1998). The majority of the PCR assays target the 5S and 16S rDNA genes or the intergenic region of the 23S-5S rDNA genes to detect specifically the genus Legionella, and the gene mip (macrophage infectivity potentiator) to detect specifically the species L. pneumophila. Recently, a specific real-time PCR for simultaneous detection and identification of L. pneumophila serogroup 1 in water and clinical samples was developed (Merault et al. 2011). As L. pneumophila is associated with 90 % of human disease, and within the 15 serogroups (Sg), L. pneumophila Sg1 causes more than 84 % of Legionnaires’ disease worldwide (Yu et al. 2002), rapid and specific identification of L. pneumophila Sg1 is important for the evaluation of the contamination of collective water systems and the risk posed. This PCR targets wzm, a gene present in the L. pneumophila serogroup 1 lipopolysaccharide gene cluster (Merault et al. 2011).
PCR, Real-Time PCR, and Multiplex PCR
The sensibility of these tests varies depending on the study from 11 % to 100 %, but the majority of the studies report specificity close to 100 %. However, the performance of the PCR tests depends largely on the kind of sample tested (respiratory sample, urine, serum, etc.) and when the study was reported. Clearly, the improvements of the DNA extraction techniques and the development of real-time PCR methods led to a considerable increase in the sensibility of detection. In particular, the high sensitivity for detection of Legionella DNA in respiratory samples demonstrated by several studies suggests that PCR may exceed culture in its ability to detect Legionella in respiratory samples (Cloud et al. 2000; Den Boer and Yzerman 2004; Hayden et al. 2001; Koide et al. 2004; Rantakokko-Jalava and Jalava 2001; Reischl et al. 2002). All studies using real-time PCR for detection of Legionella in pulmonary samples report 100 % specificity (Ballard et al. 2000; Hayden et al. 2001; Herpers et al. 2003), and for classical PCR, the specificity lies between 93 % and 100 %. The specificity of the PCR on serum is also close to 100 %, but it is with 80–100 % a little less on urine samples. However, the sensibility is relatively weak as it varies between 29 % and 100 %. This weak sensibility seems to be due to the fact that bacteremia occurs only rarely during disease and even when a bacteremia occurs only little amounts of DNA can be found in the blood (Matsiota-Bernard et al. 2000). Similarly, the sensibility for PCR on urine samples is quite variable, as values from 7 % to 86 % have been reported (Matsiota-Bernard et al. 2000; Murdoch et al. 1996). Taken together, if legionellosis is suspected, it seems to be important to take samples of different origin (urine, serum, etc.) and to repeat these sampling for several days to improve the sensibility of the test results (Lindsay et al. 2004). Furthermore, although culture is still the golden standard, PCR and real-time PCR should be considered a useful diagnostic method for Legionnaires’ disease (Zarogoulidis et al. 2011).
Recently, multiplex real-time PCR was evaluated as a method for rapid differential detection of five bacterial causes of community-acquired pneumonia (CAP) (Streptococcus pneumoniae, Burkholderia pseudomallei, and atypical bacterial pathogens, namely, Mycoplasma pneumoniae, Chlamydophila pneumoniae, and Legionella pneumophila) in blood and respiratory samples of CAP patients attending a hospital in Malaysia (Mustafa et al. 2011). This study showed that multiplex real-time PCR is a useful tool for identifying CAP causative agents. By supplementing traditional diagnostic methods with real-time PCR, a higher microbial detection rate was achieved for both typical and atypical pneumonia (Mustafa et al. 2011). Cho and colleagues reported a comparison of sputum and nasopharyngeal swab specimens for molecular diagnosis of Mycoplasma pneumoniae, Chlamydophila pneumoniae, and Legionella pneumophila using multiplex PCR using the PneumoBacter assay. To determine the diagnostic performance of this assay, sputum samples were also tested using BD ProbeTec ET Atypical Pneumonia Assay (APA; Becton Dickinson). Sputum testing appeared more sensitive than nasopharyngeal swab specimens testing (P = 0.002) for L. pneumophila diagnosis, but PneumoBacter and APA tests agreed 100 % (Cho et al. 2012). Although culture is still the golden standard, PCR and real-time PCR should be considered a useful diagnostic method for Legionnaires’ disease (Zarogoulidis et al. 2011).
Pathogenesis, Virulence Factors, and Immune Responses
Overview of L. pneumophila Pathogenesis
Given that Legionnaires’ disease is most associated with infection by L. pneumophila, the vast majority of studies on Legionella pathogenesis have focused on that species and, in particular, strains belonging to its first serogroup. Therefore, the following description is an account of L. pneumophila (serogroup 1) pathogenesis. Observations that have been made concerning other Legionella species will be highlighted toward the end of this chapter.
As discussed above, L. pneumophila infects humans following the introduction of contaminated water into the lower respiratory tract. The nature of the infectious particle that triggers disease is still unknown, with the possibilities being planktonic (free, extracellular) bacteria, legionellae contained within amoebae or amoebal cysts, and biofilm-containing legionellae. Within the lung, L. pneumophila invades and proliferates within the resident macrophages that line the alveoli (Cianciotto et al. 1989a; Horwitz 1992; Swanson and Hammer 2000; Winn 1988). Bacterial growth results in the lysis of the macrophage hosts and subsequent rounds of intracellular infection. The release of tissue-destructive substances from the bacteria and the dead and dying host cells lead to local tissue destruction (Cianciotto 2001; Newton et al. 2010; Winn 1988). Chemokines and cytokines released by the infected macrophages trigger the infiltration of polymorphonuclear leukocytes, additional macrophages, and erythrocytes, and capillary leakage results in local edema (Akamine et al. 2005; Archer and Roy 2006; Blanchard et al. 1987; Case et al. 2009; Coers et al. 2007b; Matsunaga et al. 2001, 2002; McHugh et al. 2000; Molofsky et al. 2006; Neumeister et al. 1998b; Park and Skerrett 1996; Shin et al. 2008; Winn 1988; Zamboni et al. 2006). When the host’s innate immune response and/or adaptive, cell-mediated immune response (see below) is functioning normally, further amplification of the L. pneumophila infection is usually limited. However, when host defenses are impaired, as described above as host risk factors, bacterial proliferation and lung pathology are further pronounced, and extrapulmonary dissemination may occur. Ultimately, L. pneumophila infection has the potential to be fatal, particularly if the infected individual is not quickly treated with the proper antibiotics. Animal models have been an invaluable tool for determining the basic course of an L. pneumophila infection, the role of particular bacterial factors in pathogenesis, and aspects of host defense. Legionnaires’ disease is most often reproduced by infection of guinea pigs or susceptible inbred (e.g., A/J) mice, using the aerosol or intratracheal routes of inoculation (Baskerville et al. 1983; Berendt et al. 1980; Brieland et al. 1994; Collins 1986; Davis et al. 1982; Edelstein et al. 1984; Skerrett et al. 1989; Susa et al. 1998; Winn et al. 1982). A number of inbred mice are not susceptible to L. pneumophila, and thus crosses between them and A/J mice have been used to identify a mammalian gene (i.e., Naip5/Birc1e) that influences host susceptibility (Beckers et al. 1995; Dietrich et al. 1995; Diez et al. 2003; Fortier et al. 2005; Wright et al. 2003; Yamamoto et al. 1992b; Yoshida et al. 1991).
Much evidence indicates that the capacity of the L. pneumophila to grow within alveolar macrophages is central to the pathogenesis of Legionnaires’ disease (Cianciotto 2001; Newton et al. 2010). First, the majority of bacteria observed in lung samples from infected humans and animals are associated with these cells (Chandler et al. 1979; Davis et al. 1983; Glavin et al. 1979; Katz et al. 1979; Rodgers et al. 1978; Surgot et al. 1988; Watson and Sun 1981; White et al. 1979). Second, L. pneumophila readily replicates within alveolar macrophages and monocytes in vitro (Horwitz and Silverstein 1980; Jacobs et al. 1984; Kishimoto et al. 1979; Nash et al. 1984). Third, the susceptibility of an animal species to infection is correlated with the ability of L. pneumophila to grow within macrophages from that species (Yamamoto et al. 1987, 1988; Yoshida and Mizuguchi 1986). Fourth, the resistance of animals to infection requires cytokines that activate macrophages (Brieland et al. 1994; Skerrett and Martin 1994). Fifth, mutants impaired in their ability to infect macrophages exhibit reduced virulence (Cianciotto et al. 1990b; Edelstein et al. 1999; Liles et al. 1999; Marra et al. 1992; Viswanathan et al. 2000). Finally, as noted earlier, therapy requires antibiotics that enter host cells. Despite the early recognition of the Legionella-macrophage interaction, it was not immediately obvious how L. pneumophila, an organism that neither possesses a mammalian reservoir nor has a “natural” route of infection, evolved the facility to parasitize human phagocytes. As noted above, it is now believed that adaptation to intracellular niches within protozoa engendered in L. pneumophila the ability to infect mammalian cells. Hence, a number of virulence factors likely evolved in response to selective pressures within the protozoan environment. Given the central role that macrophage infection has in Legionnaires’ disease, many studies of L. pneumophila pathogenesis have focused on describing that intracellular infection process and characterizing the bacterial and host factors which are involved in promoting macrophage infection (see below). This work has been facilitated by the use of both explanted monocytes/macrophages from human volunteers and experimental animals (e.g., alveolar- and bone-marrow-derived A/J mouse macrophages) and macrophage cell lines such as the human-derived U937, HL-60, THP-1, and Mono Mac 6 lines and the murine-derived MH-S and J774A.1 and RAW264.7 lines (Cirillo et al. 1994; Kura et al. 1994; Marra et al. 1990; McCoy-Simandle et al. 2011; Neumeister et al. 1997; Pearlman et al. 1988; Susa et al. 1996; Yan and Cirillo 2004).
Without diminishing the significance of growth within macrophages, it is likely that other factors also contribute to the survival and virulence of L. pneumophila within humans (Cianciotto 2001). For example, the bacterium may replicate or, at a minimum, must survive within extracellular spaces in the alveoli (Chandler et al. 1979; Rodgers et al. 1978; Surgot et al. 1988; Watson and Sun 1981; White et al. 1979). The fact that many strains of L. pneumophila are inherently serum resistant may be particularly relevant for extracellular survival following the onset of the inflammatory response (Caparon and Johnson 1988; Horwitz and Silverstein 1981; Luneberg et al. 1998; Plouffe et al. 1985; Verbrugh et al. 1985). Similarly, the ability of L. pneumophila to resist cationic peptides and to secrete enzymes that degrade lung surfactant suggests that the bacterium subverts some of the antimicrobial factors released by lung epithelia (Edelstein 1981; Flieger et al. 2000; Robey et al. 2001; Wadowsky and Yee 1981). Moreover, the presence of legionellae within non-macrophage cells in necropsy material indicates that L. pneumophila may also grow within the alveolar epithelium (Maruta et al. 1998; Rodgers 1979; Watson and Sun 1981). In support of this hypothesis, the microbe invades and replicates within alveolar types I and II cells in vitro (Chang et al. 2005; Cianciotto et al. 1995; Edelstein et al. 2003; Gao et al. 1998; Mody et al. 1993; N’Guessan et al. 2007; Newton et al. 2006; Yaradou et al. 2007). The importance of growth and persistence outside of the macrophage host cell is further indicated by several more observations. First, those L. pneumophila strain types that represent the most common type of clinical isolate (i.e., MAb-2 positive strains) are not necessarily more effective at intracellular infection of macrophages (Edelstein and Edelstein 1993). Second, various types of L. pneumophila mutants that are not defective or only moderately defective for macrophage infection in vitro are impaired or more strikingly impaired in animal models of pneumonia (Allard et al. 2009; Chang et al. 2005; DebRoy et al. 2006b; Edelstein et al. 1999; Liles et al. 1999; Rossier et al. 2004). Third, some of the proteins that are secreted by extracellular L. pneumophila such as a zinc metalloprotease can directly mediate lung tissue damage (Conlan et al. 1988; Dowling et al. 1992; Moffat et al. 1994). Thus, another area of Legionella research has been the identification and characterization of virulence factors that act, entirely or primarily, outside of the macrophage host (see below).
Cell Biology of L. pneumophila Intracellular Infection
While protozoa are the natural hosts of Legionellae, the infection of human phagocytic cells is opportunistic. Given the pivotal role that intracellular parasitism plays in the biology of L. pneumophila, a first-line approach toward understanding legionellosis has been to study the cellular basis of macrophage infection (Abu Kwaik 1998; Cianciotto et al. 1989b; Horwitz 1992; Hubber and Roy 2010a; Isberg et al. 2009; Ott 1994; Roy 1999; Shuman et al. 1998; Vogel and Isberg 1999). This effort has been aided enormously by the availability of human macrophage-like cell lines, such as U937, HL-60, Mono Mac, and THP-1 cells (Cirillo et al. 1994; Marra et al. 1990; Neumeister et al. 1997; Pearlman et al. 1988).
Adherence and Entry into Host Cells
Uptake of L. pneumophila by phagocytic cells, such as macrophages and amoebae, can occur through conventional phagocytosis or coiling phagocytosis as shown in Fig. 9.1 (Bozue and Johnson 1996; Elliott and Winn 1986; Hilbi et al. 2001; Horwitz and Maxfield 1984; King et al. 1991; Rechnitzer and Blom 1989). However, coiling phagocytosis may not play an important role in intracellular survival since heat-killed and formalin-killed L. pneumophila are also ingested within coiled phagosomes but are targeted to the lysosome (Horwitz and Maxfield 1984). Conventional phagocytosis can occur through a complement-mediated mechanism via complement receptor CR1 and CR3 (Payne and Horwitz 1987). Complement component CR3 fixes primarily to the major outer membrane protein MOMP, and the MOMP-CR3 interaction seems to be sufficient to mediate L. pneumophila uptake into macrophages (Bellinger-Kawahara and Horwitz 1990). Several bacterial factors have been implicated in non-complement-mediated adherence of L. pneumophila to phagocytic cells like type IV pili (Stone and Abu Kwaik 1998), Hsp60 (Garduno et al. 1998b), and RtxA, but the receptors possibly involved have not been elucidated (Cirillo et al. 2000). Furthermore, LaiA and SidE (Chang et al. 2005), two secreted factors of L. pneumophila, and EnhC and LpnE, two bacterial proteins containing multiple tetratricopeptide repeats (TPR), are required for efficient host entry (Cirillo et al. 2000; Newton et al. 2006).
Uptake of L. pneumophila. (a) Uptake of L. pneumophila by A. castellanii through coiling phagocytosis at 30 min of co-incubation, scale bar 1μm (Bozue and Johnson 1996). (b) Uptake of L. pneumophila by guinea pig alveolar macrophages through conventional phagocytosis, scale bar 1 μm (Elliott and Winn 1986)
Intracellular Replication
Following ingestion by phagocytes L. pneumophila inhibits phagosome-lysosome maturation and is instead found within a single-membrane vacuole with numerous small vesicles on the cytoplasmic face. During the first hour following uptake, mitochondria cluster around the Legionella-containing vacuole (LCV), and by 4 h, vesicles derived from rough endoplasmic reticulum (ER) collect near the LCV membrane (Fig. 9.2 ). Formalin-killed bacteria do not form such vacuoles, indicating that bacterial factors are responsible for this process (Horwitz 1983). Fluorescence microscopy studies in which markers of secretory vesicles of ER membrane (p58, Sec22b, calnexin) and ER luminal proteins (IgG-binding protein BiP, calreticulin, glucose-6-phosphate, etc.) have been labeled allowed understanding the origin and the kinetics of recruitment of the vesicles surrounding the LCV (Derré and Isberg 2004; Kagan and Roy 2002; Kagan et al. 2004; Swanson and Isberg 1995). Maturation of the LCV occurs in two phases: shortly after uptake, the LCV interacts and fuses with secretory vesicles transiting between ER and Golgi; in a second phase, LCV fuses with ER membranes, resulting in ER content being delivered to the lumen of the LCV (Hilbi and Haas 2012; Robinson and Roy 2006). After the formation of this ER-surrounded LCV, bacterial replication is initiated with generation times of approximately 2 h (Fig. 9.3 ). During the late replicative phase (∼18 h postinfection), the phagosome appears to merge with lysosomes as it becomes more acidic and acquires lysosomal markers like LAMP-1. Fusion with the lysosomal compartment seems to lead a nutrient-rich environment that was shown to promote rather than inhibit L. pneumophila replication (Sturgill-Koszycki and Swanson 2000). Recently, intact LCVs have been purified from L. pneumophila-infected D. discoideum amoebae and analyzed by proteomics (Shevchuk et al. 2009; Urwyler et al. 2009). In one study, D. discoideum producing calnexin-GFP (an ER and LCV marker) was infected with red fluorescent L. pneumophila, and after homogenization of infected amoebae, fluorescent LCVs were isolated by a straightforward two-step protocol (Urwyler et al. 2010). To this end, immunomagnetic separation was applied using a primary antibody against an L. pneumophila “effector protein” (see below) specifically decorating the LCV membrane, and a secondary antibody coupled to magnetic beads, followed by density gradient centrifugation. The proteome of purified LCVs was analyzed by tandem mass spectrometry and revealed more than 560 host proteins (Urwyler et al. 2009). These included several small GTPases of the secretory (Arf1, Rab1, Rab8) or endosomal (Rab7, Rab14) vesicle trafficking pathway, indicating that LCVs communicate not only with the early and late secretory pathways but also with the early and late endosomal pathways.
L. pneumophila recruits ER-derived vesicles. Transverse section through a phagosome containing L. pneumophila in U937 cells after 15 min of infection. Vesicles of ER, both studded with ribosomes and without, are attached to the phagosome. The region indicated is shown at higher magnification in (b). The ER membranes are 60 Å thick. (c) Longitudinal section through a phagosome containing L. pneumophila following 2 h of infection. The area boxed in (c) is shown at a higher magnification in (d) (Tilney et al. 2001)
Intracellular replication of L. pneumophila. L. pneumophila replicating in (a) Hartmannella, scale bar 0.5 μm (Source: Photo: Holland/Özel, Robert Koch-Institut), and (b) guinea pig macrophages, Scale bar 1μm (Elliott and Winn 1986)
It has been shown that the LCV may resemble nascent autophagosomes (Swanson and Isberg 1995). Autophagy is an evolutionarily conserved degradative pathway that captures and transfers a variety of microbes to lysosomes. Biogenesis of L. pneumophila-containing vacuoles and autophagosomes shares several features, including ER-derived membranes; contributions by the host GTPases Rab1, Arf1, and Sar1; and a final destiny in lysosomes (Joshi and Swanson 2011). However, there are some differences between LCVs and autophagosomes: (1) autophagosomes lack ribosomes and are probably derived from ribosome-free region of the ER; (2) within 15 min following the infection, a reduction of the membrane thickness of the LCV is observed, and it becomes more similar to that of the ER, a change not observed in autophagy, while the recruitment of rough ER and mitochondria to LCV is achieved within 15 min following uptake, induction of autophagy takes place in 1 h; and (3) a number of ATG genes required for autophagy are not required for intracellular replication of L. pneumophila (Amer et al. 2005; Amer and Swanson 2005; Dubuisson and Swanson 2006; Otto et al. 2004; Swanson and Molofsky 2005; Tilney et al. 2001). It seems that some bacterial pathogens have evolved mechanisms to evade autophagic recognition or even co-opt the autophagy machinery for their own benefit as a replicative niche (Mostowy and Cossart 2012). L. pneumophila might be one of these pathogens.
Exit from the Host Cell and Transmission
Following replication, depletion of nutrients leads to the transition of L. pneumophila from a replicative form to a virulent form. This transition is accompanied by phenotypic changes: while replicative L. pneumophila is nonmotile, noncytotoxic, sodium resistant, and nonflagellated, bacteria in post-replicative, virulent phase are motile, cytotoxic, and flagellated. This phenotypic switch is observed in both broth cultures and intracellular bacteria. It was postulated that the exit of L. pneumophila from the host cell occurs in two stages: firstly, through disruption of the phagosomal membrane and exit into the cytoplasm (Molmeret et al. 2004, 2010) and, secondly, through the lysis of the plasma membrane of the host cell and the exit of bacteria (Molmeret and Abu Kwaik 2002). Both stages could be possibly mediated by a pore-forming activity (Molmeret et al. 2004). However, it has also been proposed that disruption of the host cell can occur through an apoptosis-mediated process. Surprisingly, despite the activation of caspase-3 by L. pneumophila during early stages of infection, macrophages are not immediately destroyed, and apoptosis is triggered only in late stages of the infection (Gao and Abu Kwaik 1999a, b; Molmeret et al. 2004; Santic et al. 2007). Two Dot/Icm effectors, LepA and LepB, were implicated in the egress of L. pneumophila from protozoa, but not mammalian cells, through a non-lytic process (Chen et al. 2004). These two effectors were initially identified based on their weak homology to eukaryotic SNAREs (Chen et al. 2004), but how LepA and LepB promote egress is unknown. LepB is a Rab1-GAP involved in replicative vacuole biogenesis (Ingmundson et al. 2007). However, LepB may contain other functional domains that contribute to egress (Shin and Roy 2008). In contrast to the series of events just outlined, exposure of a macrophage to large numbers of attached (extracellular) L. pneumophila results in immediate pore formation in the host cell plasma membrane and rapid necrotic cell death (Husmann and Johnson 1994; Kirby et al. 1998; Zuckman et al. 1999).
Secreted Virulence Factors of L. pneumophila
L. pneumophila secretes a plethora of factors that promote intracellular infection and virulence. These factors include both proteins and non-proteinaceous molecules. Arguably, it is the secretion of myriad proteins that most dramatically promotes L. pneumophila pathogenesis. In L. pneumophila, as in other Gram-negative bacteria, the secretion of proteins is a complex process that requires transport across the inner membrane, periplasm, and finally the outer membrane. Gram-negative organisms have six, and possibly eight, systems that can mediate protein export from within the bacterial cell to the extracellular milieu and/or into target host cells (i.e., type I, II, III, IV, and so on) (Desvaux et al. 2009). A great deal of research by different laboratories has shown that type IV and type II secretions are functional and critical for L. pneumophila (Cianciotto 2009; Hubber and Roy 2010b). The two next sections will focus on type IV and type II secretions, highlighting the mechanisms of secretion, the nature of the secreted proteins, and the role of these secretion events in the bacterium’s interactions with its hosts. This will be followed by a discussion of a putative type I secretion system, the Tat system, and other secreted factors, including proteins that appear not to be dependent on type II or type IV secretion, siderophore, pigment, and a quorum-sensing molecule. Genome analysis has suggested that a type V protein secretion system exists in L. pneumophila; that is, strain Paris is predicted to encode an autotransporter (Bruggemann et al. 2006). However, more work is needed in order to confirm that this gene functions in protein secretion.
Type IV Protein Secretion
Type IV secretion systems (T4SS) are devices present in a wide range of bacteria, including bacterial pathogens, that deliver macromolecular (proteins and single-strand DNA) across kingdom barriers, as well as between bacteria and into the surroundings. Their components are ancestrally related to the tra-/trb-encoded conjugation machinery required for transfer of plasmids between bacteria (Christie et al. 2005; Christie and Vogel 2000). The T4SS are divided into two subgroups, type IVA and type IVB, accordingly to sequence similarity (Juhas et al. 2008). L. pneumophila and Coxiella burnetii are the only two bacterial species known to date to utilize type IVB secretion system for pathogenesis (Nagai and Kubori 2011; Segal et al. 2005).
The type IVB secretion system in Legionella is the icm (intracellular multiplication; Marra et al. 1992) and/or dot (defective organelle trafficking; Berger and Isberg 1993) system. It is required for intracellular growth in human macrophages as well as in amoebae and for intracellular trafficking. The dot/icm type IV secretion system of L. pneumophila is probably its most important secretion system as it is involved in many different stages of the intracellular life cycle. It is critical for the ability of L. pneumophila to inhibit phagosome-endolysosomal fusions and to establish its unique replicative niche (Andrews et al. 1998; Berger and Isberg 1993; Berger et al. 1994; Brand et al. 1994; Marra et al. 1992; Purcell and Shuman 1998; Sadosky et al. 1993; Segal et al. 1998; Segal and Shuman 1997; Vogel et al. 1998). Several dot/icm loci have also been shown to be essential for virulence in a guinea pig model of disease (Edelstein et al. 1999; Marra et al. 1992). Interestingly, the Dot/Icm secretion system is also structurally and functionally homologous to bacterial conjugation systems, and it was indeed demonstrated to mediate plasmid DNA transfer between two L. pneumophila strains and to E. coli, which indicates that the Dot/Icm system retains an ancestral function and mediates transfer of macromolecules between cells (Segal et al. 1998; Segal and Shuman 1998; Vogel et al. 1998). This macromolecular complex is encoded by 25 genes located on two genomic regions: region I contains seven genes (icm V, W, X, dotA, B, C, D) and region II is composed of 18 genes (icmT, S, R, Q, P, O, N, M, L, K, E, G, C, D, J, B, F, H) as shown in Fig. 9.4a . The dot and icm gene products are thought to assemble into a multiprotein apparatus that function as a type IV secretion system (T4SS) (Fig. 9.4b ) (Segal et al. 1998; Segal and Shuman 1999; Vogel et al. 1998). The L. pneumophila Dot/Icm system is conserved and present in the same chromosomal location in all strains sequenced to date. When analyzed in six L. pneumophila strains (Paris, Lens, Philadelphia, Corby, Lorraine, HL 0604 1035), a very high nucleotide conservation of 98–100 % among orthologs was observed except for dotA, icmX, and for icmC of strain Corby that is shorter and more divergent (84 % nucleotide identity) as compared to icmC of strain Paris. These results indicate that strong negative selection acts on these genes (Table 9.3 ) (Gomez Valero et al. 2011). In contrast, between different Legionella species, the nucleotide sequence similarity is less pronounced (46–79 %) (Gomez-Valero et al. 2011; Morozova et al. 2004). The global organization of the Dot/Icm system in different Legionella species is conserved; however, it has been shown that in region II in L. micdadei, the gene icmR is replaced by two genes, migA and migB, which do not show any homology to icmR. The same gene is replaced in L. longbeachae by a gene called ligB (Feldman and Segal 2004). Further analyses of the region carrying the icmR from 29 Legionella species revealed the presence of a large hypervariable gene family named functional homologues of icmR (fir) gene, located at the icmR genomic position (Feldman et al. 2005). All fir genes were found, together with their corresponding icmQ genes, to function similarly during infection. In addition, all FIR proteins were found to interact with their corresponding IcmQ proteins. Their interaction depends on a variable region located between two conserved domains of IcmQ that probably coevolved with the corresponding FIR protein. A FIR-IcmQ pair was also found in Coxiella burnetii, the only known non-Legionella bacterium that contains a Dot/Icm system, indicating the significance of this protein pair for the function of this type IV secretion system (Feldman et al. 2005). The only other difference observed is present in the DotG/IcmE protein of L. longbeachae (1,525 aa) that is 477 amino acids larger than that of L. pneumophila (1,048 aa) (Cazalet et al. 2010). DotG of L. pneumophila is part of the core transmembrane complex of the secretion system and is composed of three domains: a transmembrane N-terminal domain, a central region composed of 42 repeats of 10 amino acids, and a C-terminal region homologous to VirB10. In contrast, the central region of L. longbeachae DotG is composed of approximately 90 repeats. Among the many VirB10 homologues present in bacteria, the C. burnetii DotG and the Helicobacter pylori Cag7 are the only ones, which also have multiple repeats of 10 amino acids (Segal et al. 2005).
The Dot/Icm type IVB secretion system of L. pneumophila. (a) Schematic representation of the genomic regions encoding the dot/icm type IV secretion system. This macromolecular complex is encoded by 25 genes located on two different genomic regions (Adapted from Gomez Valero et al. 2011). (b) The presumed locations and topological relationships of the various Dot/Icm components based on a study of the stability of individual proteins in the presence of defined deletion mutations (Buscher et al. 2005). Individual letters represent Dot protein names, whereas letters preceded by an “i” indicate Icm protein names (Isberg et al. 2009)
Given the central role of the Dot/Icm system in Legionella pathogenesis, many recent studies have aimed at identifying and characterizing its substrates. The first characterized effector was RalF, required for localization of the host protein ARF-1, a key regulator of vesicle trafficking from the endoplasmic reticulum to the phagosomes (Nagai et al. 2002). RalF is conserved in all strains sequenced to date, like LidA, another substrate involved in recruitment of vesicles during vacuole biogenesis and in maintaining integrity of the Dot/Icm complex (Conover et al. 2003; Derre and Isberg 2005). LidA binds to GDI-free Rab1 and thus combined with SidM to intercept host cell vesicles of the Rab1-regulated early secretory pathway (Machner and Isberg 2006). Similarly, the two Dot/Icm effectors LepA and LepB are conserved in all L. pneumophila strains investigated. These effectors show weak homology to SNAREs and were shown to be functionally involved in the release of Legionella from the vacuole after intracellular multiplication during amoeba infection (Chen et al. 2004). A number of candidate effector proteins named SidA–G were identified in the Philadelphia 1 strain by a two-hybrid screen with IcmG/DotF as bait followed by a screen of proteins transferred interbacterially with a Cre-/loxP-based protein translocation assay (Luo and Isberg 2004). All Sid proteins except SidD contain a coiled-coil domain, a protein motif known to be involved in protein-protein interactions. Most of these effectors have no discernible defects in inhibition of phagolysosome maturation or intracellular growth. Construction of single deletion strains for sidA, sidD, sidF, or sidG in strain Philadelphia did not result in attenuated virulence, pointing to functional redundancy, which probably extends individual substrate families. A quadruple mutant strain lacking sidB and its three paralogs (sdbABC) showed defects in intracellular growth, but this result was not significantly different from a mutant with a single sdbA deletion (Luo and Isberg 2004). One SidE paralog, designated LaiA (=SdeA), was identified in an independent study and characterized as a virulence factor: the integrin-like protein is involved in adhesion to and invasion of human lung alveolar epithelial cells (Chang et al. 2005). Another family of effectors, named PieA-PieE, has been identified on a plasticity zone of the L. pneumophila genome, indicating that they might have been acquired by horizontal gene transfer (Ninio et al. 2009). One of these proteins, PieA, is recruited in a Dot/Icm-dependent manner to the L. pneumophila vacuole and binds to the cytoplasmic face of the vacuole as a result of L. pneumophila-induced modifications to this vacuole. These findings demonstrated the first time that the association of an effector with host vacuoles can be spatially controlled through activities mediated by other effector proteins. Six of these genes were also identified independently and called lirA-F (Zusman et al. 2008). Recently, whole-genome alignment of strains 130b, Lens, Philadelphia 1, Corby, and Paris revealed that this region is part of a larger genomic region that displays considerable divergence among the five sequenced genomes. In strain 130b, this region is constituted of 96 kb, suggesting that the two regions initially described (Ninio et al. 2009; Zusman et al. 2008) constitute the inner core of a much larger 80- to 100-kb region of high genomic plasticity that represents a strain-specific variable effector region (Schroeder et al. 2010).
In more recent years, several other proteins have been shown to be Dot/Icm effectors by either bioinformatics approaches, the adenylate cyclase assay approach, or the β-lactamase reporter system (Burstein et al. 2009; de Felipe et al. 2005, 2008; Zhu et al. 2011). Currently, 278 proteins of L. pneumophila have been described as being translocated by the Dot/Icm T4SS system, a number not equaled by any other bacterial secretion system. Selected substrates and their distribution are listed in Table 9.4 . Analysis of the distribution of the 278 Dot/Icm substrates identified in L. pneumophila strain Philadelphia in six other L. pneumophila and five L. longbeachae genomes sequenced showed that they are highly conserved among different L. pneumophila strains, as over 80 % of the substrates are present in all six L. pneumophila strains (Gomez Valero et al. 2011). In contrast, the search for homologues of these L. pneumophila Dot/Icm substrates in L. longbeachae revealed pronounced differences are present as only 98 of the 278 L. pneumophila Dot/Icm substrates have homologues in the L. longbeachae genomes (Gomez Valero et al. 2011). Many of them are eukaryotic-like proteins and eukaryotic domain proteins, involved in the virulence of the bacterium described afterward (Bruggemann et al. 2006; Cazalet et al. 2004; de Felipe et al. 2005; Franco et al. 2009; Nora et al. 2009).
The first L. pneumophila protein encoding a eukaryotic domain was identified before genome sequencing. The gene ralF encodes a protein with a Sec-7 domain. These domains are found in eukaryotes as components of Arf-specific guanine nucleotide exchange factors (GEFs). GEFs catalyze the nucleotide exchange of Arfs, thereby converting them from an inactive state (GDP-bound) to the active one (GTP-bound). To promote the fusion to ER membranes, L. pneumophila recruits host factors to the surface of the LCVs like Arf-1 and Rab-1, important cell signaling proteins involved in the regulation of the ER-Golgi traffic. This is in part achieved by RalF. Following secretion by T4SS, RalF recruits Arf-1 and then functions like an Arf-1 specific GEF (Nagai et al. 2002). Until recently, the translocation signal for the type IV secretion effectors was not known. Nagai and colleagues, who investigated the mechanism of translocation of RalF (Nagai et al. 2002), identified a 20-amino-acid C-terminal region of the RalF protein as necessary and sufficient for translocation. In particular, a hydrophobic residue at the C-terminal −3 position is critical for secretion of RalF, as a substitution to hydrophilic residues resulted in a severe defect in translocation (Nagai et al. 2005). Comparison with other Dot/Icm substrates identified in most of them a hydrophobic residue or a proline residue at the −3 or −4 position, supporting the idea that these residues are critical for secretion by the type IV system. Additional features have been suggested like the enrichment in alanine, glycine, serine, and threonine at positions −8 to −2, and polar amino acids at positions −13 to +1 (Kubori et al. 2008; Nagai et al. 2005), as well as a region of 6–8 amino acids rich in glutamates, called the E Block motif (Huang et al. 2011).
Interestingly, many effector proteins show no sequence similarity to proteins present in other microorganisms, which indicates the uniqueness of the mechanism by which L. pneumophila subverts eukaryotic host cell functions. Another study identified candidate effector proteins capable of altering endosomal trafficking by screening a L. pneumophila genomic library for genes that induce a VPS (vacuole protein sorting)-negative phenotype in yeast (Shohdy et al. 2005). In Saccharomyces cerevisiae, VPS pathway components control several distinct vesicle trafficking pathways. The identified effectors were designated Vip (VPS inhibitor protein). Three Vip proteins (VipA, VipD, and VipF) are translocated from L. pneumophila into host macrophages via the Dot/Icm apparatus. The L. pneumophila genome encodes in addition three paralogs of VipD (VpdA, VpdB, VpdC), and VpdA and VpdB have also been shown to be translocated by the Dot/Icm T4SS (Shohdy et al. 2005). As VipD is predicted to contain a phospholipase A domain with homology to the type III-secreted protein ExoU from Pseudomonas aeruginosa which is a potent toxin for mammalian cells, VanRheenen and colleagues tested the toxicity of VipD for S. cerevisiae. VipD was shown to be mildly toxic when overproduced in eukaryotic cells (VanRheenen et al. 2006). Recently, VipA was shown to constitute a novel type of actin nucleator that may contribute to the intracellular lifestyle of Legionella by altering cytoskeleton dynamics to target host cell pathways. VipA binds actin in vitro and directly polymerizes microfilaments without the requirement of additional proteins, displaying properties distinct from other bacterial actin nucleators (Franco et al. 2012; Franco and Shuman 2012). Intracellularly, neither the strains bearing deletions of individual genes nor the strain bearing the quadruple mutation was significantly impaired for growth in macrophages (VanRheenen et al. 2006). Thus, the different studies that aimed at characterizing the Dot/Icm substrates point to a probably high functional redundancy of this protein family in Legionella.
Another screen in a different yeast genetic system identified Legionella proteins that conferred a conditional growth defect when overproduced by yeast cultured in the presence of galactose. This screen led to the identification of a new Dot/Icm substrate that was called YlfA, for yeast lethal factor A. The YlfA protein could be observed on the ER-derived replicative vacuole and on punctuate structures throughout the host cell at late stages of the infectious cycle. However, the precise function of YlfA is still under investigation (Campodonico et al. 2005). A systematic analysis of 127 characterized and putative effector proteins for disruption of vesicle trafficking in yeast revealed the disruption of vesicle trafficking by Ceg9, Ceg19, and the novel effector SetA (Heidtman et al. 2009). SetA localized in LAMP-1-positive compartments when expressed in mammalian cells and was proposed to interact with the ubiquitination machinery at late endosomal compartments and to exhibit glycosyltransferase activity (see also below) (Heidtman et al. 2009).
Many of the secreted Dot/Icm substrates mediate posttranslational modifications (PTMs) of host proteins, to promote survival and replication of L. pneumophila inside eukaryotic cells. One of the conserved pathways hijacked by L. pneumophila is the host ubiquitination system. Ubiquitination is one of the best-known PTMs exploited by pathogenic bacteria to interfere with host signaling pathways. Several T3SS and T4SS translocated effectors from bacterial pathogens have been shown to exploit the ubiquitin-proteasome system of the host to their advantage (Rytkonen and Holden 2007). These secreted effectors may function as E3 ubiquitin ligases or deubiquitinating enzymes, leading to their proteasome-dependent degradation (Rohde et al. 2007; Zhang et al. 2006), or they possess deubiquitination (DUB) activity (Le Negrate et al. 2008). L. pneumophila interferes with the host ubiquitination system by translocating different proteins. One is LubX (for L egionella U-box), a protein containing two U-box domains (U-box1 and U-box2) (Kubori et al. 2008). LubX functions as an ubiquitin ligase in conjunction with host UbcH5a or UbcH5c E2 enzymes and mediates polyubiquitination of cellular Clk1 (Cdc2-like kinase 1). The U-box1 domain seems to be critical for E2 binding and the subsequent ubiquitin ligation, whereas the U-box2 domain interacts with the substrate. Interestingly, LubX has a second target within host cells, the L. pneumophila effector protein SidH. LubX directly binds and polyubiquitinates SidH in vitro and mediates its proteasomal degradation in infected cells (Fig. 9.5 ). LubX is the first example of a bacterial “meta-effector” that regulates in space and time the expression level of another effector (Kubori et al. 2010).
Posttranslational modifications induced by L. pneumophila. (a) L. pneumophila exploits AMPylation and deAMPylation. DrrA/SidM possesses three domains: an AMP-transfer domain (AT) in its N-terminal region, a nucleotide exchange factor (GEF) domain in the central part, and a phosphatidylinositol-4-phosphate (PI-4P) binding domain (P4M) in its C-terminal part. After association of DrrA/SidM with the membrane of the LCV (LCV mb) via P4M (1), it recruits Rab1 via the GEF domain and catalyzes the GDP-GTP exchange (2). Rab1 is then adenylylated by the AT domain (3), leading to inhibition of GAP-catalyzed Rab1 deactivation. In step (4), SidD deAMPylates Rab1 and enables LepB to bind Rab1 and promotes its GTP-GDP exchange (5). (b–d) Legionella modulates the ubiquitin-signaling pathway. (b.1) AnkB of strain AA100 and Lp01 ubiquitinates host proteins and exploits the host prenylation machinery to anchor at the cytosolic face of LCV. (b.2) AnkB of L. pneumophila strain Paris that lacks the C-terminal CAAX farnesylation motif binds Skp1 and modulates the ubiquitination level of ParvB, a host protein present in focal adhesions and in lamellipodia. (c) The LegU1 effector interacts with a functional SCF complex through its F-box domain and specifically targets the host protein BAT3, a key regulator of the ER stress response. (d) The effector LubX contains two U-box domains that target cellular Clk1 during infection and promote bacterial replication. Furthermore, LubX functions as an E3 ubiquitin ligase that hijacks the host proteasome to specifically target the bacterial effector protein SidH for degradation to temporally coordinate its function. (e) Effector glycosyltransferases in Legionella. Lgt1, Lgt2, and Lgt3 act as glycosyltransferases that specifically target eEF1A and thereby inhibit the eukaryotic protein translation machinery. (f) Control of host cell phosphorylation. LegK1 and LnaB target the NF-kB pathway by phosphorylation activity. (g) Legionella exploits the cell phosphocholination (PC)/de-PC pathway to modify Rab1 (Adapted from Rolando and Buchrieser 2012)
Other L. pneumophila proteins that interfere with the host ubiquitination pathway are three F-box motifs containing proteins (Cazalet et al. 2004; de Felipe et al. 2005; Gomez Valero et al. 2011). In eukaryotes, F-box proteins have been shown to function as E3 ubiquitin ligases within the modular SCF (Skp1-Cullin-F-box protein) multiprotein complex. Indeed, soon after infection of eukaryotic cells with L. pneumophila, ubiquitinated proteins accumulate on the LCV containing wild-type bacteria, but not on vacuoles containing mutants with a nonfunctional Dot/Icm system (Dorer et al. 2006). The addition of a proteasomal inhibitor during host cell infection led to reduced intracellular replication of L. pneumophila, suggesting that interference with the host ubiquitination pathway is important for the intracellular survival of L. pneumophila and dependent on secreted Dot/Icm substrates (Dorer et al. 2006). Among the three F-box-containing proteins encoded by L. pneumophila strain Philadelphia and Paris, AnkB/LegAU13/Ceg27 (Lpg2144/Lpp2082) is characterized best. Besides its F-box domain, this protein possesses a C-terminal ankyrin (Ank) domain (Cazalet et al. 2004) and a CAAX motif (where C represents cysteine and A an aliphatic amino acid) (Ivanov et al. 2010; Price et al. 2010b). Deletion of this conserved F-box protein-encoding gene (ankB/lpg2144) in strain AA100/130B resulted in a mutant exhibiting a severe replication defect within eukaryotic cells (Al-Khodor et al. 2008). Strain Paris missing AnkB/Lpp2082 is slightly attenuated in infection of protozoan cells, but it is outcompeted during competitive infection of lungs of A/J mice (Lomma et al. 2010). AnkB is a Dot/Icm-translocated effector that is involved in the recruitment of polyubiquitinated proteins around the LCV and interacts with Skp1 during infection (Lomma et al. 2010; Price et al. 2009). Silencing of Skp1 by RNAi blocked intracellular replication of L. pneumophila, indicating that Skp1 recruitment by L. pneumophila is essential for hijacking the host ubiquitination machinery, which is leading to an advantage in intracellular replication (Price et al. 2009) (Fig. 9.5 ). A yeast two-hybrid screen and co-immunoprecipitation analysis identified Parvin-β (ParvB) as one target of the L. pneumophila F-box protein AnkB/Lpp2082 encoded by strain Paris (Lomma et al. 2010). Parvins are known to have important roles in focal adhesion, cell spreading, and motility (Sepulveda and Wu 2006). ParvB is endogenously ubiquitinated and co-immunoprecipitates in vivo with AnkB/Lpp2082 (Lomma et al. 2010). Surprisingly, expression of AnkB/Lpp2082 led to a decrease of ubiquitination of ParvB. Thus, L. pneumophila seems to modulate ubiquitination of ParvB by competing with the eukaryotic E3 ligase for the specific protein-protein interaction site of ParvB, in order to promote caspase-3 activation and apoptosis (Lomma et al. 2010) (Fig. 9.5 ). Another appealing hypothesis is that AnkB/Lpp2082 targets the eukaryotic ubiquitin ligase that ubiquitinates ParvB, which would also lead to a decrease in ubiquitinated ParvB as observed during L. pneumophila infection (Fig. 9.5 ).
Another F-box protein of L. pneumophila protein is LegU1/Lpg0171, which is also a translocated Dot/Icm type IV substrate and can be integrated into a functional SCF complex that confers E3 ubiquitin ligase activity. LegU1 specifically targets the host chaperon protein BAT3, a key regulator of the endoplasmic reticulum (ER) stress response (Fig. 9.5 ). LegU1 associates with BAT3 and mediates its polyubiquitination in vitro (Ensminger and Isberg 2010). Moreover, another translocated L. pneumophila protein, Lpg2160, plays a role in this complex, by binding both the SCF complex and BAT3, suggesting that this multicomplex formation leads to BAT3 ubiquitination, probably to modulate the ER response during infection (Ensminger and Isberg 2010).
Furthermore, L. pneumophila is able to block DALIS (dendritic cell aggresome-like induced structure) formation in infected macrophages and dendritic cells (DC) (Ivanov and Roy 2009). DALIS formation in macrophages in response to L. pneumophila infection occurs downstream of TLR2 activation, but intracellular L. pneumophila blocks this activation in a Dot/Icm-dependent manner to maintain an LCV decorated with ubiquitinated proteins (Ivanov and Roy 2009). Interestingly, L. pneumophila is the only pathogen known to disrupt DALIS formation. A hypothesis is that the ability of L. pneumophila to disrupt DALIS might result in premature or inefficient antigen presentation (Ivanov and Roy 2009). Thus, the exploitation of ubiquitin signaling is a remarkable example of how L. pneumophila exploits conserved eukaryotic pathways and regulations to proliferate.
In addition to hijacking the host ubiquitination machinery, L. pneumophila exploits the host prenylation apparatus (Ivanov and Roy 2009; Price et al. 2010b). Prenylation (farnesylation or geranylgeranylation) is a PTM of eukaryotic proteins that covalently links a lipid moiety at a CAAX tetrapeptide motif in the C-terminal region of proteins (Wright and Philips 2006). It renders proteins hydrophobic to target them to membranes by facilitating their anchoring to the lipid bilayer of membranes or their association with other hydrophobic proteins (Wright and Philips 2006). Interestingly, in silico analysis of the proteins predicted in the L. pneumophila genome identified several proteins encoding a CAAX motif (Ivanov et al. 2010; Price et al. 2010c). One of the secreted effectors of L. pneumophila, AnkB, of strains L. pneumophila AA100 and Philadelphia Lp01 contains such a CAAX motif. Interestingly, this protein can be lipidated at its CAAX motif by the host farnesylation machinery. This is thought to facilitate its anchoring to the membrane of the LCV in vivo (Ivanov et al. 2010; Price et al. 2010c) (Fig. 9.5 ). Lipidation of L. pneumophila effectors by the host is important for intracellular replication, as perturbation of host prenyltransferases during infection adversely affected the remodeling of the LCV (Ivanov et al. 2010). Thus, L. pneumophila utilizes the host prenylation machinery to facilitate targeting of effector proteins to membrane-bound organelles during intracellular infection (Ivanov et al. 2010; Price et al. 2010b; Price et al. 2010c). Most interestingly, in some L. pneumophila strains like strain Paris, AnkB does not contain this CAAX motif, which suggests that other CAAX motif proteins might take over this function. It was further proposed that AnkB functions as platform for the docking of polyubiquitinated proteins to the LCV to enable intravacuolar proliferation in macrophages and amoeba (Al-Quadan et al. 2011; Price et al. 2010b) and that AnkB helps L. pneumophila to exploit the eukaryotic proteasomal degradation pathway of K48-linked polyubiquitinated proteins to generate amino acids for its own replication (Al-Quadan et al. 2012; Price et al. 2011). This is an appealing idea, but as the AnkB homologue of a considerable number of L. pneumophila strains does not contain the CAAX motif, AnkB might not be the key effector of L. pneumophila that is generating nutrients for intracellular growth. However, AnkB is a versatile protein as it can take advantage of two distinct posttranslational mechanisms, one targeting host proteins and leading to modulation of their ubiquitination status and another one where the bacterial effector itself is modified by host proteins to help its intracellular proliferation.
One of the best characterized and most common PMTs is protein phosphorylation. Nearly all cellular processes are regulated by reversible phosphorylation; hence, many pathogens and also L. pneumophila interfere with the host phosphorylation machinery to target major signaling pathways to promote their own survival. L. pneumophila encodes three eukaryotic serine/threonine protein kinases, and one, LegK1, directly phosphorylates the NF-κB inhibitor IκBα, leading to a robust NF-κB activation, independently of the IKK (Iκβ kinase) complex (Ge et al. 2009). NF-κB is a master transcriptional regulator of the mammalian innate immune response, leading to activation of proinflammatory cytokines, chemokines, and cell survival genes (Karin and Lin 2002). The activity of LegK1 appears to be specific toward the Iκβ (inhibitor of κB) family proteins by mimicking the host IKK. However, the authors observed the phosphorylation activity in vitro, but a deletion of legK1 in L. pneumophila strain Philadelphia had no notable effect on intracellular replication (Ge et al. 2009). Many studies investigated NF-κB activation by L. pneumophila. Activation occurs through both a TLR-dependent pathway, shortly after contact, as a robust and a persistent Dot/Icm-dependent pathway (Bartfeld et al. 2009). Even though LegK1 activates the NF-κB pathway, the redundancy of effectors in Legionella and the fact that a legK1 deletion mutant does not impact the intracellular replication suggest that several additional proteins might contribute to the NF-κB response. Indeed, another Dot/Icm substrate, LnaB, has been shown to activate the NF-κB pathway. LnaB is a protein that has no sequence similarity to any known protein; however, it strongly activates NF-κB (Losick et al. 2010) (Fig. 9.5 ). LegK2, another serine-protein kinase encoded by L. pneumophila, does not seem to act in the NF-κB pathway, but it plays a key role in virulence during amoeba infection, and it exhibits protein kinase activity in vitro. It was shown that LegK2 is able to phosphorylate the general eukaryotic protein kinase substrate myelin basic protein (MBP), but its cellular target is still unknown (Hervet et al. 2011). Thus, L. pneumophila is exploiting the host phosphorylation system to perturb key signaling pathways, like the NF-κB pathway, in order to modify and then to block the cellular response against the bacteria (Haenssler and Isberg 2011).
The glycosyltransferases Lgt1, Lgt2, and Lgt3 of L. pneumophila are Dot/Icm-secreted substrates and are able to glycosylate host proteins (Belyi et al. 2011). Glycosyltransferases catalyze the transfer of a sugar residue from an activated sugar donor to various acceptor molecules, which may be proteins, lipids, saccharides, or metabolites. These protein modifications are emerging as regulators of central processes like cell signaling or gene transcription (Lairson et al. 2008). Lgt1 is a glucosyltransferase with sugar-specific enzymatic activity (only UDP-glucose). Its specific cellular target is the host elongation factor 1A (eEF1A) that Lgt1 modifies by mono-O-glucosylation at Ser53 of eEF1A (Belyi et al. 2003; Belyi et al. 2006). As eEF1A represents one of the key players in ribosome-dependent synthesis due to its GTPase activity is necessary for recruitment of aminoacylated tRNA to the A-site of ribosomes charged with translated mRNA, Lgt1 blocks protein synthesis and causes death of target cells (Belyi et al. 2006). The two other glycosyltransferases encoded by L. pneumophila, Lgt2 and Lgt3, also target eEF1A at Ser53 and kill infected cells (Aktories 2011; Belyi et al. 2008) (Fig. 9.5 ). By comparison of uncharged tRNA with two distinct aminoacyl-tRNAs (His-tRNA(His) and Phe-tRNA(Phe)), it could be shown that aminoacylation is crucial for Lgt-catalyzed glucosylation. Aminoacyl-tRNA had no effect on the enzymatic properties of the Lgt proteins and did not enhance the glucosylation rate of eEF1A truncation mutants, consisting of the GTPase domain only or of a 5-kDa peptide covering Ser-53 of eEF1A. Furthermore, binding of aminoacyl-tRNA to eEF1A was not altered by glucosylation. The authors thus suggest that the ternary complex, consisting of eEF1A, aminoacyl-tRNA, and GTP, is the bona fide substrate for Lgt proteins (Tzivelekidis et al. 2011). Inhibition of protein synthesis of the host cell seems to be very important for intracellular replication of L. pneumophila, as not only Lgt1-3 target protein synthesis but also two other effectors of the Dot/Icm secretion system, SidI and SidL. SidI exhibits toxicity for eukaryotic cells, but it has no glycosyltransferase activity. Direct binding in vitro and in vivo between SidI and eEF1A and eEF1Bγ and a role in protein synthesis inhibition have been clearly determined, but no specific enzymatic activity could be defined (Shen et al. 2009). SidL is toxic for mammalian cells and able to inhibit protein translation in vitro via an unknown mechanism, and the concerted action of all five effectors targeting protein synthesis (Lgt1, Lgt2, Lgt3, SidI, SidL) is critical for the induction of the Dot/Icm-dependent transcriptional response of host cells (Fontana et al. 2011; Massis and Zamboni 2011). During infection, these five effectors induce a global decrease of host translation, thereby preventing synthesis of the transcription factor NF-κB inhibitor IκB. An additional putative glycosyltransferase produced by L. pneumophila, SetA (subversion of eukaryotic vesicle trafficking A), has been identified by a large-scale screening of candidate Dot/Icm effectors that modulate host vesicle trafficking pathways (Heidtman et al. 2009). SetA possesses a functional glycosyltransferase domain containing the conserved DxD motif that essential for the activity of bacterial glycosylating enzymes (Heidtman et al. 2009). SetA has a multidomain protein with an N-terminal glucosyltransferase domain and a C-terminal phosphatidylinositol 3-phosphate binding domain. Thus, the catalytic activity is located at the N-terminus of SetA, and the C-terminus (amino acids 401–644) is essential for guidance of SetA to vesicular compartments of host cells. Both the localization and the glucosyltransferase domains of SetA are crucial for cellular functions (Jank et al. 2012).
Phosphoinositide (PI) lipids are phosphorylated products of phosphatidylinositol (PtdIns) that play a key role in the regulation of eukaryotic signal transduction, cytoskeleton architecture, membrane dynamics, and vesicle trafficking pathways (Di Paolo and De Camilli 2006). Accordingly, many bacterial pathogens subvert PI metabolism to promote cell infection and intracellular replication (Weber et al. 2009b). L. pneumophila replicates more efficiently in absence of PI 3-kinases (PI3Ks) (Weber et al. 2006) or PI 5-phosphatases (Dd5P4/OCRL) (Weber et al. 2009a). LCVs are decorated with PtdIns (Abu Kwaik et al. 1993) P, and a number of L. pneumophila effector proteins bind non-covalently to PIs (Hilbi et al. 2011). The Dot/Icm substrate SidC and its paralog SdcA selectively bind to PtdIns(4)P via a novel “P4C” domain (Ragaz et al. 2008; Weber et al. 2006), and LidA preferentially binds monophosphorylated PIs (Brombacher et al. 2009). Moreover, a screen for L. pneumophila PI-binding proteins using different PIs coupled to agarose beads revealed that the Dot/Icm-translocated effector DrrA/SidM specifically binds to PtdIns(4)P via the “P4M” domain and competes with SidC for PI binding (Brombacher et al. 2009). The P4C and P4M PtdIns(4)P binding domains are neither related to one another nor to eukaryotic PI-binding folds. Together, these results indicate that L. pneumophila modulates host cell PI metabolism in a Dot/Icm-dependent manner and exploits monophosphorylated PIs to anchor Dot/Icm-translocated effector proteins to the LCV membrane (Hilbi et al. 2011).
AMPylation or adenylylation consists of adding covalently an adenosine monophosphate (AMP) moiety to a threonine, tyrosine residues, or possibly, serine residue of a protein by using ATP (Ribet and Cossart 2011). Via the Dot/Icm-secreted effector DrrA/SidM, L. pneumophila modulates AMPylation of Rab1b, a small GTPase involved in intracellular vesicular transport (Müller et al. 2010). Interestingly, the catalytic domain of DrrA/SidM is distinct from the Fic domains observed in other bacterial proteins able to AMPylate host proteins (Roy and Mukherjee 2009). DrrA/SidM is a protein with three functional domains that have been defined biochemically and resolved structurally (Müller et al. 2010; Schoebel et al. 2009; Suh et al. 2010; Zhu et al. 2010). In the carboxy-terminal region, it carries a phosphatidylinositol-4-phosphate (PI-4P) binding domain (P4M), responsible for anchoring to the LCV shortly after Dot/Icm translocation (Brombacher et al. 2009) that targets DrrA/SidM to the plasma membrane (Murata et al. 2006). Then DrrA/SidM recruits the vesicular trafficking regulator Rab1 through its guanine nucleotide exchange factor (GEF) domain that is localized in the central region of the protein (Fig. 9.5 ). Rab1 belongs to the Rab proteins, involved in organizing membranes for the formation of vesicular carriers. They interconvert between an active, GTP-bound and an inactive GDP-bound form. GTP activation occurs via enzymes known as GEFs that exchange the bound GDP nucleotide for a GTP, whereas GTPase-activating proteins (GAP) help Rabs to hydrolyze Rab-bound GTP to produce GDP. Moreover, inactive Rabs are maintained in the cytosol by binding to GDP-dissociation inhibitor (GDI). Hence, specialized proteins known as GDI-displacement factors (GDFs) are required to displace GDI from Rabs before Rab activation by GEFs (Sprang 1997). DrrA/SidM functions as both a GDF and a GEF (Ingmundson et al. 2007; Machner and Isberg 2007). It releases Rab1 from GDI, loads it with GTP, and due to its own LCV localization, recruits it to this organelle (Fig. 9.5 ). However, DrrA/SidM is a catalytically highly efficient GEF, and this activity seems sufficient for effective GDI displacement from the Rab1/GDP/GDI complex (Schoebel et al. 2009; Suh et al. 2010; Zhu et al. 2010). Thus, when Rab1/GTP is localized on the LCV, it is AMPylated by the N-terminal domain of DrrA/SidM. This domain has adenylylation activity (AT) toward Rab1, resulting in posttranslational modification of the GTPase on Tyr77 with an adenosine monophosphate (AMP) moiety (Müller et al. 2010) (Fig. 9.5 ). Interestingly, Rab1 AMPylation blocks its binding to another L. pneumophila effector, LepB, which possesses specific GAP activity toward Rab1/GTP (Ingmundson et al. 2007) and loosens this activity toward Rab1/GTP/AMP. Whereas DrrA/SidM and Rab1 appear on the LCV already shortly after infection, the recruitment of LepB is delayed (Ingmundson et al. 2007). During the progression of infection, DrrA/SidM and Rab1 are removed from the LCV, whereas LepB increases. Thus, DrrA mediates the recruitment and activation of Rab1 on the plasma membrane-derived vacuole that harbors Legionella. However, the molecular details of how ER-derived vesicles fuse with the LCV remain unknown. A possible role for Rab1 (Arasaki and Roy 2010) was reported. Furthermore, membrane fusion between the LCV and ER-derived vesicles involves interactions between the v-SNARE Sec22b on the ER-derived vesicles and a plasma membrane t-SNARE complex containing host syntaxins (Arasaki and Roy 2010). Recently, it was shown that the DrrA protein promotes also the tethering of ER-derived vesicles with the plasma membrane-derived organelle, which leads to membrane fusion through Sec22b interactions with PM-localized syntaxins (Arasaki et al. 2012).
AMPylation is a reversible process and may be modulated by proteins exhibiting deAMPylation activity. Indeed, L. pneumophila is able to reverse AMPylation. It was the first bacterial pathogen that was reported to mediate deAMPylation of a host protein (Neunuebel et al. 2011; Tan and Luo 2011). During infection, Rab1 can be removed from the LCV only when it is in a GDP-bound form. The L. pneumophila LepB protein was shown to be a Rab1 GTPase-activating protein (GAP) capable of inactivating Rab1 (Ingmundson et al. 2007). However, LepB can catalyze its GDP binding only on deAMPylated Rab1. This deAMPylation activity is mediated by another effector of the Dot/Icm secretion system, by SidD (Neunuebel et al. 2011; Tan and Luo 2011). SidD mediates the removal of the AMP moiety from modified Rab1 and allows in this way, via the LepB-GAP activity, the displacement of Rab1 from the LCV (Fig. 9.5 ). Importantly, SidD is not present in all L. pneumophila strains. This effector is missing in strain Paris, suggesting that another, not yet identified, secreted effector catalyzes Rab1/GTP deAMPylation. Rigden and colleagues showed that the deAMPylase SidD contains a metal-dependent protein phosphatase (PPM) fold catalytic domain (Rigden 2011).
As mentioned above, AMPylation activity is known to be mediated by proteins containing FIC domains from the Fido domain superfamily (Kinch et al. 2009). Interestingly, L. pneumophila contains a FIC domain protein, AnkX, but it has no AMPylation activity. Instead it prevents microtubule-dependent vesicular transport to disrupt LCV fusion with late endosomes (Pan et al. 2008) and mediates a novel PTM on Rab1, phosphocholination (Mukherjee et al. 2011). Using mass spectrometry, Mukherjee and colleagues identified a covalent addition of a phosphocholine group on Rab1 during L. pneumophila infection (Mukherjee et al. 2011). The serine preceding the tyrosine that is the site of AMP addition was phosphocholinated. Golgi disruption mediated by AnkX is dependent on its phosphocholine transferase activity (Mukherjee et al. 2011). Similar to the capacity to reverse AMPylation, L. pneumophila has also evolved the capacity to reverse phosphocholination. This activity is mediated by the Dot/Icm effector Lem3 that regulates AnkX activity (Tan and Luo 2011). Lem3 (lpg0696) possesses a biochemical activity allowing it to remove the phosphocholine moiety from Rab1 (Fig. 9.5 ). Importantly phosphocholination interferes with the GTPase activity of Rab1, through blocking GTP loading and LepB-induced GTPase activity, suggesting that this enzymatic activity may account for the inhibition of the eukaryotic secretory pathway by AnkX (Tan and Luo 2011).
Interestingly, many genes coding for Dot/Icm substrates form clusters: ankX and lem3 are closely linked genes, as well as lubX and sidH (Kubori and Nagai 2011) and sidM/DrrA and sidD (Neunuebel et al. 2011; Tan and Luo 2011). This observation emphasizes the spatiotemporal regulation of Dot/Icm effectors, each of which is playing a subtle role during infection, and all together modulate multiple cellular pathways at the same time to orchestrate Legionella survival.
Type II Protein Secretion
Type II protein secretion (T2S) systems are common, although not universal, among the various types of Gram-negative bacteria (Cianciotto 2005; Evans et al. 2008). T2S is a multistage process (Filloux 2004; Johnson et al. 2006). The proteins that are to be secreted are first translocated across the inner membrane. In the majority of cases, unfolded protein substrates transit across the inner membrane via the Sec pathway. However, sometimes, folded substrates are transported across the inner membrane by the twin-arginine translocation (Tat) (Lee et al. 2006; Ochsner et al. 2002; Sargent et al. 2006). Upon delivery into the periplasm, the unfolded substrates assume their tertiary conformation and in some instances oligomerize. In the final step of secretion, protein substrates are translocated across the outer membrane by a complex of proteins that is specifically dedicated to T2S, namely, the T2S apparatus. The T2S apparatus has 12 “core” proteins, that is, a cytosolic ATPase (T2S E), three inner membrane proteins that make a platform for T2S E (T2S F, L, M), major and minor pseudopilins which create a pilus-like structure that spans the periplasmic space (T2S G, H, I, J, K), an inner membrane peptidase that cleaves pseudopilins before their placement into the apparatus (T2S O), an outer membrane “secretin” that oligomerizes to create the secretion pore (T2S D), and finally a protein that appears to link inner and outer membrane components (T2S C) (Filloux 2004; Forest 2008; Johnson et al. 2006; Korotkov et al. 2011; Yanez et al. 2008). The current model for T2S is that substrates destined for secretion are somehow recognized by the apparatus and then, using energy generated at the inner membrane, the pseudopilus behaves like a piston to push the proteins through the secretin pore. The characteristic that defines a protein as a substrate for T2S is still not known but likely involves the protein’s tertiary structure and initial interactions with T2S C and D (Korotkov et al. 2011).
The first indication that L. pneumophila has a T2S system was the discovery of the pilD gene, encoding the pseudopilin peptidase (T2S O) (Liles et al. 1998). Mutation of pilD in serogroup 1 strain 130b altered secretion, as shown by the loss of proteins in mutant culture supernatants (Liles et al. 1999). Study of serogroup 1 strain Philadelphia 1 revealed the presence of lspFGHIJK, which encodes the T2S F, G, H, I, J, and K proteins (Hales and Shuman 1999a). In further studies of strain 130b, genes encoding homologues of T2S D and E (lspDE), C (lspC), and L and M (lspLM) were reported, with mutation of lspDE affirming the role of the genes in secretion (Rossier and Cianciotto 2001; Rossier et al. 2004). That L. pneumophila has a complete set of T2S-specific genes was confirmed when the genomes of serogroup 1 strains Alcoy, 130b, Corby, Lens, Paris, and Philadelphia 1 were sequenced (Cazalet et al. 2004; Chien et al. 2004; D’Auria et al. 2010; Glockner et al. 2008; Schroeder et al. 2010). Southern blots and PCR analysis have determined the presence of T2S genes in many more strains of L. pneumophila (Costa et al. 2011; Huang et al. 2006; Rossier et al. 2004). Additional analysis has confirmed that L. pneumophila contains genes encoding the Sec and Tat systems (De Buck et al. 2004; Geukens et al. 2006; Lammertyn and Anne 2004; Rossier and Cianciotto 2005) (see below for further discussion of Tat). T2S-specific mutants of L. pneumophila replicate normally in bacteriologic media at 37°C (Rossier and Cianciotto 2001; Rossier et al. 2004), indicating that T2S is not required for optimal extracellular growth at 37°C.
Initially, 12 exoenzymes were shown to be dependent upon the T2S system of strain 130b (Banerji et al. 2005; Cianciotto 2005; Rossier et al. 2004). This conclusion was based upon the loss of activities from the culture supernatants of lsp mutants grown in BYE broth at 37 °C (Aragon et al. 2000; Liles et al. 1999; Rossier and Cianciotto 2001; Rossier et al. 2004). The activities identified were the tartrate-sensitive and tartrate-resistant acid phosphatases; Mip-dependent and Mip-independent phospholipases C; phospholipase A; lysophospholipase A; glycerophospholipid cholesterol acyltransferase (GCAT); mono-, di-, and triacylglycerol lipases; ribonuclease; and protease (Aragon et al. 2000, 2001, 2002; Banerji et al. 2005; DebRoy et al. 2006a; Flieger et al. 2001, 2002; Hales and Shuman 1999a; Liles et al. 1999; Rossier and Cianciotto 2001; Rossier et al. 2004). Recent analysis of strain Paris has identified a starch/glycogen-degrading activity that is T2S dependent (Herrmann et al. 2011), and a similar result has been obtained for strain 130b (J. Schmitt and N. Cianciotto, unpublished results). In some cases, the structural genes (proteins) encoding the T2S-dependent secreted activities have been identified. These include map (Map) for the tartrate-sensitive acid phosphatase (Aragon et al. 2001), plcA (PlcA) for phospholipase C activity (Aragon et al. 2002; Rossier and Cianciotto 2005), plaA (PlaA) for the lysophospholipase A (Flieger et al. 2002), plaC (PlaC) for GCAT (Banerji et al. 2005), lipA (LipA) and lipB (LipB) for mono- and triacylglycerol lipases (Aragon et al. 2002), gamA (GamA) for the starch hydrolase (Herrmann et al. 2011), and proA/msp (ProA/Msp) for a metalloprotease (Hales and Shuman 1999a; Liles et al. 1999). The analysis of supernatants from proA mutants demonstrates that some secreted proteins are cleaved and perhaps activated by the T2S-dependent protease (Banerji et al. 2005; Flieger et al. 2002). Interestingly, one of the first secreted substrates to be described, the Map acid phosphatase, shows striking sequence similarity to eukaryotic enzymes (Aragon et al. 2001), suggesting that L. pneumophila has usurped the strategies of its host cells over the course of its evolution as an intracellular parasite. Many more examples of Legionella eukaryotic-like proteins have emerged from the study of the L. pneumophila type IV secretion system as well as the further study of T2S.
In order to define additional T2S-dependent proteins secreted by L. pneumophila, strain 130b and an lsp mutant were grown in broth at 37 °C, and then supernatants were compared by two-dimensional PAGE. Mass spectrometry (MS) was then utilized to identify the secreted proteins that were present for wild type but absent for the T2S mutant (DebRoy et al. 2006b). Three of the identified proteins had been previously defined as T2S substrates, that is, ProA, Map, and PlaA. A fourth (SrnA) proved to encode the previously found ribonuclease activity (Rossier et al. 2009). Others were predicted, based upon their sequence, to be “new” enzymes, and subsequent cloning and mutational analyses confirmed that CelA encodes an endoglucanase (cellulase); ChiA, a chitinase; and LapA and LapB, two distinct aminopeptidases (DebRoy et al. 2006b; Pearce and Cianciotto 2009; Rossier et al. 2008, 2009). Others showed sequence similarity to eukaryotic proteins, with one having collagen-like repeats, and the other relatedness to astacin-like proteinases (DebRoy et al. 2006b). Subsequent studies showed that the protein with collagen-like repeats (Lcl) has heparin-binding activity and may promote adherence events (Duncan et al. 2011; Vandersmissen et al. 2010). The astacin-like proteinase (LegP) was later found to be translocated by Dot/Icm type IV secretion when the legionellae were growing within macrophages (de Felipe et al. 2008), raising the possibility that some proteins may be secreted or influenced by multiple pathways, with the environmental conditions potentially dictating which secretion pathway(s) is most critical. Other type II-dependent proteins showed weak similarity to bacterial amidases and cysteine proteases, but their true activities are yet to be confirmed (DebRoy et al. 2006b). Interestingly, five others had no similarity to any known protein in the database and thus might encode novel activities (DebRoy et al. 2006b). Results similar to these obtained from using strain 130b have now been reported after proteomic analysis of Philadelphia 1 (Galka et al. 2008). The results of the proteomics combined with assessments of secreted enzymatic activities indicate that the number of proteins secreted by L. pneumophila T2S is at least 25. That the T2S output is >25 is supported by several arguments; for example, (1) low-level expression or degradation is very likely to have impaired detection of some secreted proteins, (2) the comparisons between wild type and mutant utilized bacterial cultures grown under a single condition, (3) mutations eliminating specific genes did not always completely abolish the corresponding enzymatic activity in supernatants, and (4) in silico analysis of L. pneumophila genomes reveals 60 proteins that contain a signal sequence and are predicted to be extracellular by at least one bioinformatics program (DebRoy et al. 2006b). Finally, recent studies have shown that Legionella T2S is required for the secretion of a surfactant that mediates bacterial translocation (i.e., sliding) over surfaces (Stewart et al. 2009, 2011). However, in this case, it appears that T2S has an indirect role, promoting the release of surfactant through a TolC-containing efflux pump (see below) (Stewart et al. 2011).
Although T2S mutants of L. pneumophila replicate normally at 30–37 °C, they are defective for growth in media at 25 °C, 17 °C, and 12 °C (Söderberg et al. 2004). In experiments that mimic aquatic habitats, T2S mutants show reduced survival in tap water incubated at 25, 17, 12, and 4 °C (Söderberg et al. 2008). T2S mutants grow better at low temperatures when plated next to wild type or wild-type supernatants, suggesting that a secreted factor promotes low-temperature growth (Söderberg et al. 2004, 2008). Supporting this idea, when wild-type L. pneumophila is grown at 17 °C or 12 °C, new proteins appear in culture supernatants, including a Sec-dependent protein that is predicted to have PPIase activity (Söderberg and Cianciotto 2008). In another type of study, transcriptional profiling revealed that a number of genes encoding T2S-dependent exoproteins are hyperexpressed when L. pneumophila is grown in a biofilm at 20 °C (Hindre et al. 2008). Finally, the T2S-dependent surfactant of L. pneumophila is antagonistic toward other species of Legionella (Stewart et al. 2011). Overall, these various data implicate T2S as a key factor in the planktonic persistence of L. pneumophila in the environment and as such identify T2S as an important factor in disease transmission.
L. pneumophila T2S mutants are highly impaired for intracellular growth in amoebae, including Hartmannella vermiformis and Acanthamoeba castellanii (Hales and Shuman 1999a; Liles et al. 1999; Polesky et al. 2001; Rossier and Cianciotto 2001; Rossier et al. 2004). Indeed, T2S mutants of strain 130b and Philadelphia 1 show very little, if any, evidence of growth in amoebae. The reduced infectivity of the T2S mutants is complemented (i.e., reversed) when an intact copy of the T2S gene is introduced, confirming that T2S is required for infection. Although the initial assessments of amoebal infection were done at 35–37 °C, more recent studies have shown that the T2S mutant defect is also manifest when amoebae are cultured at 22–25 °C (Söderberg et al. 2008). Additional assays have determined that the T2S mutants are not impaired for entry into the amoebal hosts (Söderberg et al. 2008), indicating that T2S is promoting bacterial resistance to intracellular killing and/or facilitating bacterial replication itself. Among T2S effectors, the ProA protease and SrnA ribonuclease are necessary for optimal infection of H. vermiformis (Rossier et al. 2008, 2009). This implies that the infection defects of T2S mutants are due to the loss of secreted effectors vs. being simply due to changes in the bacterial cell envelope. Double mutants lacking both ProA and SrnA show a defect that is greater than the corresponding single mutants, implying that the role of T2S in amoebal infection is due to the combined effect of multiple secreted proteins (Rossier et al. 2009). The protease and ribonuclease may be facilitating growth by helping legionellae to generate amino acids, nucleotides, or phosphate for nutrient acquisition. Alternatively, ProA and SrnA might be degrading amoebal proteins and RNA that can influence Legionella growth. Interestingly, ProA exhibits differential importance among the amoebae tested, being important for infection of hartmannellae but not acanthamoebae (Rossier et al. 2008). These data were the first to demonstrate that some secreted factors have evolved to target certain protozoan hosts. A similar result occurs for SrnA (J. Schmitt & N. Cianciotto, unpublished results) as well as some type IV-secreted proteins (O’Connor et al. 2011). Given the key role of protozoa in L. pneumophila survival in water, these data further establish T2S as a major factor in Legionella persistence in the environment. Because infected amoebae might be part of the infective dose that initiates lung infection (Brieland et al. 1996; Cirillo et al. 1999), these data also signal the relevance of T2S for disease.
Importantly, T2S mutants of L. pneumophila are also very defective in an animal model of Legionnaires’ disease (McCoy-Simandle et al. 2011; Rossier et al. 2004). Whereas the parental wild-type strain increases at least tenfold in the lungs of A/J mice, a T2S mutant exhibits no increase in number and is cleared much more rapidly. An examination of sera obtained from animals infected with the wild-type strain further revealed that T2S-dependent proteins are expressed in vivo (Rossier et al. 2004). Thus, T2S is an important contributor to L. pneumophila virulence. Among all of the effectors tested thus far, the chitinase stands out as being necessary for bacterial survival in the lungs (DebRoy et al. 2006b; Rossier et al. 2008, 2009). ChiA mutants are impaired fourfold when tested in the mouse model, and immunoblot analysis showed that ChiA is one of the T2S-dependent proteins that is expressed in vivo (DebRoy et al. 2006b). Since the chiA mutant grows normally in macrophages in vitro and since its reduced survival in the lung was only manifest in the later stages of infection, ChiA likely promotes persistence vs. initial replication. Since mammals do not have chitin, these data lead to the hypothesis that there is a chitin-like factor in the lung whose degradation aids bacterial persistence. Alternately, ChiA could be a bifunctional enzyme that has another substrate. That a protein having chitinase activity can promote the survival of a pathogen in a mammalian host had not been previously seen. Thus, factors that are traditionally viewed as only being important in the environment may actually have direct relevance to disease. Although proA mutants have not been shown to clearly exhibit reduced growth or survival in the lungs of experimental animals, ProA is believed to contribute to disease by promoting the destruction of lung tissue (Baskerville et al. 1986; Blander et al. 1990; Conlan et al. 1986, 1988; DebRoy et al. 2006b; Moffat et al. 1994; Williams et al. 1987). It can also degrade transferrin and therefore may contribute to iron acquisition (James et al. 1997).
Finally, L. pneumophila T2S mutants are impaired for intracellular infection of macrophages and lung epithelial cells (Liles et al. 1999; McCoy-Simandle et al. 2011; Polesky et al. 2001; Rossier et al. 2004). Thus, T2S also promotes infection by facilitating bacterial growth in resident lung cells. Besides the chiA mutant, L. pneumophila map, plcA, plaA, plaC, lipA, lipB, proA/msp, lapA, lapB, srnA, celA, and gamA mutants that lack particular T2S effectors have been tested for alterations in infection of macrophages. However, all grow normally, indicating that the proteins encoded by these genes are not required for macrophage infection (Aragon et al. 2001; Aragon et al. 2002; Banerji et al. 2005; DebRoy et al. 2006b; Flieger et al. 2002; Herrmann et al. 2011; Pearce and Cianciotto 2009; Rossier et al. 2008, 2009). These data indicate that the T2S system secretes a yet-to-be-defined factor that is necessary for macrophage infection. On the other hand, there might be redundancy in the effectors such that one secreted factor can compensate for the loss of another. Because T2S mutant numbers do not increase in the lungs, whereas they do, although not optimally, in macrophages and epithelial cells in vitro, it was hypothesized that L. pneumophila T2S also promotes processes that are relevant to disease. Following infection of macrophages, T2S mutants (but not a complemented mutant) elicit significantly higher levels of cytokines and chemokines (McCoy-Simandle et al. 2011). A similar result was obtained with infected lung epithelial cell lines and the lungs of infected A/J mice. Infection with a mutant specifically lacking the T2S-dependent ProA protease (but not a complemented proA mutant) results in a partial elevation of cytokine levels (McCoy-Simandle et al. 2011). These data indicate that the T2S system of L. pneumophila dampens the cytokine/chemokine output of infected host cells. Based on quantitative RT-PCR analysis of infected host cells, a T2S mutant, but not the proA mutant, produced significantly higher levels of cytokine transcripts, implying that some T2S-dependent effector dampens signal transduction and transcription, whereas others, such as ProA, act at a posttranscriptional step in cytokine expression (McCoy-Simandle et al. 2011).
In summary, the role of T2S in Legionnaires’ disease is a combination of at least seven factors, that is, (1) extracellular survival in environmental water samples (which is the source of infection), (2) growth in amoebae (which is the main replicative niche for L. pneumophila in water and which may be part of the infective particle), (3) intracellular infection of lung macrophages (which are the primary host cell in the lung), (4) intracellular infection of lung epithelial cells (which are an alternative host cell in vivo), (5) dampening of the chemokine and cytokine output of infected macrophages and epithelial cells (which is predicted to dampen the inflammatory cell infiltrate into the lung, allowing for prolonged bacterial growth), (6) the elaboration of ChiA (which appears to promote intrapulmonary persistence independent of macrophage infection), and (7) the secretion of ProA (which degrades both host cytokines and lung tissue). Further research will undoubtedly reveal even more roles for this multifactorial secretion system.
Type I Protein Secretion
All sequenced strains of L. pneumophila encode the outer membrane protein TolC and putative inner membrane translocase proteins that would together constitute a type I secretion system (Ferhat et al. 2009; Jacobi and Heuner 2003; Newton et al. 2010; Stewart et al. 2011). Well studied in many other bacteria, TolC is best known as the outer membrane component of multidrug efflux pumps (Blair and Piddock 2009; Koronakis et al. 2004; Nikaido and Takatsuka 2009; Zgurskaya 2009). Indeed, L. pneumophila tolC mutants exhibit increased sensitivity to various drugs (Ferhat et al. 2009; Stewart et al. 2011). More interestingly, in L. pneumophila, TolC mediates the secretion of a surfactant that allows the bacterium to move across surfaces, that is, sliding motility (Stewart et al. 2011). The surfactant also has antimicrobial activity against other Legionella species. Structurally, the surfactant appears to be a lipoprotein or lipopeptide, providing the first experimental indication that L. pneumophila encodes a functional type I secretion system (Stewart et al. 2011). Equally important, L. pneumophila tolC mutants are impaired for in vitro intracellular infection, suggesting that TolC and one or more of its substrates are required for Legionella pathogenesis (Ferhat et al. 2009).
The rtxA gene of L. pneumophila is predicted to encode a very large (approximately 7,000-aa) protein that has sequence similarity to Rtx (“repeats-in-toxin”) toxins that are found in other bacterial pathogens and secreted in those cases via a type I system (Cirillo et al. 2001; D’Auria et al. 2008). A L. pneumophila mutant lacking rtxA is impaired for entry and intracellular growth within macrophages, epithelial cells, and amoebae (Cirillo et al. 2001, 2002). The mutant also shows impaired growth within the lungs of infected mice (Cirillo et al. 2001), indicating that RtxA is a virulence factor for L. pneumophila. The rtxA gene is well conserved among clinical isolates of L. pneumophila (Huang et al. 2006). RtxA has not been studied biochemically, and its location in L. pneumophila has not been determined. But it is reasonable to suspect that the protein is either secreted outside of the bacterial cell via the type I system or present in the outer membrane.
Tat-Dependent Secretion, Other Secreted Proteins, and Outer Membrane Vesicles
In Gram-negative bacteria, the twin-arginine translocation (Tat) pathway mediates translocation of proteins across the inner membrane to the periplasm (De Buck et al. 2008b). The majority of Tat substrates remain cell associated, residing in the periplasm or inner membrane (De Buck et al. 2004, 2007; Rossier and Cianciotto 2005). However, as noted above, some Tat substrates are further secreted out of the cell via the T2S system. In the case of L. pneumophila, a T2S effector that has been shown to be Tat dependent is PlcA phospholipase C (Rossier and Cianciotto 2005). Bioinformatics and subsequent proteomic analysis has determined that additional proteins are secreted into culture supernatants in a Tat-dependent manner, including a 3′, 5′-cyclic nucleotide phosphodiesterase (De Buck et al. 2008a; Rossier and Cianciotto 2005). More work is needed in order to determine whether these other proteins are delivered into the extracellular space via T2S or an alternative secretion system. Phenotypic analysis of tat mutants has determined that an intact Tat pathway is required for a variety of other aspects of L. pneumophila, including virulence-associated traits (De Buck et al. 2005; Rossier and Cianciotto 2005). When tested for infection of macrophages, tat mutants show an approximate 15-fold reduction in growth. Double mutants lacking Tat and T2S are even more defective, indicating that Tat has an intracellular role that is independent of T2S. The mutants are also impaired for cytochrome c oxidase, growth in amoebae cultured in presence of an iron chelator, extracellular growth on low-iron bacteriologic media, and biofilm formation in plastic microtiter plates. All these mutant phenotypes are reversed by reintroduction of intact tat. Thus, the Tat pathway of L. pneumophila has a role in secretion of exoenzymes, formation of a respiratory complex, growth in low-iron conditions, and intracellular infection.
There are several L. pneumophila proteins that are secreted into culture supernatants by an unknown mechanism. Lpg1905 is an ecto-nucleoside triphosphate diphosphohydrolase with ATPase and ADPase activities (Sansom et al. 2007; Vivian et al. 2010). The release of Lpg1905 in supernatants was shown to not be dependent upon a type II or Dot/Icm type IV secretion system. Importantly, this protein is required for intracellular infection of macrophages and alveolar epithelial cells (Sansom et al. 2007; Vivian et al. 2010). PlaD is a secreted phospholipase A that does not contain a signal sequence typical of a T2S substrate (Banerji et al. 2008). Taken together, these data further suggest that more than two protein secretion systems are operative in L. pneumophila.
In addition to secreting various sorts of proteins, L. pneumophila releases outer membrane vesicles (OMVs) when it grows within liquid culture (Fernandez-Moreira et al. 2006; Flesher et al. 1979; Galka et al. 2008). L. pneumophila shares this trait with many other types of Gram-negative bacteria (Ellis and Kuehn 2010). OMVs of L. pneumophila contain LPS and as many as 74 different proteins, including 33 proteins that do not appear to be also secreted via type II or Dot/Icm type IV secretion (Fernandez-Moreira et al. 2006; Galka et al. 2008). The OMVs can also associate with the plasma membrane of human epithelial cell (Galka et al. 2008), suggesting that they may represent an alternate means of delivering toxins and other effectors into host cells. It has also been posited that OMVs produced by intracellular legionellae can fuse with the phagosomal membrane and alter trafficking events (Fernandez-Moreira et al. 2006).
Non-protein Secretion: Siderophore, Pigment, and Quorum-Sensing Molecules
A critical non-protein molecule that is secreted by L. pneumophila is the siderophore legiobactin. The ability of L. pneumophila to replicate in the mammalian host is highly dependent on iron (Cianciotto 2007). For example, iron supplementation increases the susceptibility of animals and macrophages to infection, and legionellae grown under iron-limiting conditions exhibit reduced virulence (Gebran et al. 1994; James et al. 1995). Furthermore, human macrophages treated with iron chelators do not support Legionella growth, and some host cytokines inhibit intracellular growth by limiting iron (Byrd and Horwitz 2000; Viswanathan et al. 2000). The first genetic data on the role of iron in Legionella was the identification of the gene for the transcriptional repressor Fur (Hickey and Cianciotto 1994). The importance of L. pneumophila iron acquisition became evident from the identification of iron- and Fur-regulated genes that are required for optimal intracellular infection (Hickey and Cianciotto 1997). The principal means of L. pneumophila Fe3+ assimilation is now known to be secreted legiobactin. When L. pneumophila is grown in a low-iron, chemically defined medium, it secretes this low-molecular-weight siderophore that is most readily detected by the chrome azurol S assay (Allard et al. 2006; Liles et al. 2000). Legiobactin is also recognized by its ability to stimulate the growth of iron-starved legionellae (Allard et al. 2006, 2009). Legiobactin contains 13 aliphatic carbons (three carbonyls) and no aromatic carbons, and spectra further indicate that it is a polycarboxylate (Allard et al. 2009). Two linked genes, lbtA and lbtB, are required for the expression of legiobactin; that is, supernatants from mutants inactivated for lbtA or lbtB lack CAS reactivity and show an inability to stimulate the growth of iron-starved legionellae (Allard et al. 2006, 2009). Cytoplasmic LbtA is homologous to siderophore synthetases and is undoubtedly involved in the synthesis of legiobactin. LbtB, a member of the major facilitator superfamily (MFS) of transporters, is akin to inner membrane siderophore exporters and most likely promotes the transport of legiobactin across the inner membrane prior to its final export. A third gene that has been implicated in the secretion or maturation of legiobactin is cyc4, which encodes a periplasmic c-type cytochrome (Yip et al. 2011). Importantly, lbtA mutants, but not their complemented derivatives, are defective for infection of the murine lung, demonstrating a role for legiobactin in L. pneumophila virulence (Allard et al. 2009). Because legiobactin mutants are not impaired for intracellular infection in vitro, the pathogenic role of legiobactin may derive from extra-macrophage events. Given the in vivo relevance of legiobactin, recent studies have characterized its outer membrane receptor, LbtU (Chatfield et al. 2011). Interestingly, LbtU has a 16-stranded transmembrane β-barrel, multiple extracellular domains, and short periplasmic tails. The sequence and structure of LbtU are distinct from known siderophore receptors, which generally have a 22-stranded β-barrel and an N-terminus that binds TonB in order to transduce energy from the inner membrane. This observation coupled with the fact that L. pneumophila does not encode TonB suggests that L. pneumophila uses a mode of siderophore uptake that is mechanistically distinct from existing paradigms (Chatfield et al. 2011). In further support of this hypothesis, it has recently been shown that the inner membrane protein (LbtC) required for utilization of legiobactin is unlike known permeases in the MFS. In addition to its ferric iron-legiobactin pathway, L. pneumophila can utilize ferrous iron through the action of an inner membrane Fe2+ transporter known as FeoB. Mutants lacking feoB are impaired for both intracellular infection and intrapulmonary growth (Robey and Cianciotto 2002), demonstrating the importance of ferrous iron transport.
For a long time, it has been known that L. pneumophila secretes a brown pigment when it is grown in bacteriologic media (Baine and Rasheed 1979; Baine et al. 1978; Feeley et al. 1978; Orrison et al. 1981; Pine et al. 1979; Ristroph et al. 1981; Vickers and Yu 1984; Warren and Miller 1979). Early reports showed that the production of the pigment is dependent upon L-tyrosine in the growth medium and most apparent in bacteria experiencing slowed growth (Baine and Rasheed 1979; Baine et al. 1978; Berg et al. 1985). It was later established that the pigment results from the spontaneous and oxidative polymerization of homogentisic acid (HGA) which is secreted into the supernatant (Steinert et al. 2001). HGA, in turn, is made through the action of Lly, a p-hydroxyphenylpyruvate dioxygenase (Gillespie et al. 2002; Steinert et al. 2001; Wintermeyer et al. 1991, 1994). Thus, the pigment is a pyomelanin or HGA-melanin, a type of molecule that is produced by a variety of other environmental bacteria as well as some pathogens (Plonka and Grabacka 2006; Turick et al. 2002; Weiner 1997). Based upon the behavior of lly mutants, it is believed that the pigment is not a required for intracellular infection (Steinert et al. 1995; Wintermeyer et al. 1994). However, a recent study has determined that the pyomelanin has intrinsic ferric reductase activity, converting Fe3+ to Fe2+ (Chatfield and Cianciotto 2007). Compatible with the nature of HGA-melanin, the secreted ferric reductase activity was positively influenced by the amount of tyrosine in growth media, resistant to protease, acid precipitable, and heterogeneous in size. Thus, L. pneumophila secretes a ferric reductase activity that likely facilitates the acquisition of ferrous iron. Since the virulence of L. pneumophila is dependent upon its capacity to acquire ferrous iron, it is possible that secreted pyomelanin has a nutritional role within the infected lung. The mechanism of pyomelanin secretion is unknown.
L. pneumophila secretes a quorum-sensing molecule known as LAI-1 (Legionella autoinducer-1) (Spirig et al. 2008). LAI-1 is further identified as 3-hydroxy-pentadecan-4-one. Signaling through this quorum-sensing system promotes a number of processes pertinent to intracellular infection and virulence, including phagocytosis, formation of the replicative phagosome, and intracellular replication (Tiaden et al. 2007). This is explained at least in part by the fact that the LAI-1 system modulates the expression of Dot/Icm type IV effectors (Tiaden et al. 2008). However, Legionella 3-hydroxy-pentadecan-4-one influences a variety of other processes including motility, the formation of extracellular filaments, and the regulation of a wide array of genes that goes well beyond type IV secretion (Tiaden et al. 2010a, b). The production of LAI-1 is controlled by RpoS and LetA (Newton et al. 2010; Tiaden et al. 2007, 2010a); however, the mechanism of LAI-1 secretion has not been defined.
Other Surface Structures, Outer Membrane Proteins, and LPS
In addition to its secretion systems and vast array of secreted factors, L. pneumophila expresses several surface structures that are important in infection. The first prominent surface feature of L. pneumophila is its flagella (Chandler et al. 1980; Rodgers et al. 1980). The gene encoding the flagellin subunit has been cloned, sequenced, and found to be regulated by temperature, growth phase, amino acids, viscosity, and osmolarity (Albert-Weissenberger et al. 2010; Hammer and Swanson 1999; Heuner et al. 1995, 1999; Ott et al. 1991). Although Legionella flagella are not required for intracellular replication per se, they promote bacterial entry into host cells, presumably by hastening the approach of legionellae to the host cell (Dietrich et al. 2001; Merriam et al. 1997; Pruckler et al. 1995). L. pneumophila flagellin that is released into the cytosol of infected macrophages is recognized by Naip5/Birc1e which helps trigger the innate immune response (Molofsky et al. 2006; Ren et al. 2006; Whitfield et al. 2010). A second prominent surface feature of L. pneumophila is its pili. The bacterium has at least two types of pili, including temperature-regulated, bundle-forming type IV pili that mediate twitching motility (Chandler et al. 1980; Coil and Anne 2009, 2010; Liles et al. 1998; Rodgers et al. 1979, 1980; Stone and Abu Kwaik 1998). Although type IV pili modestly facilitate bacterial attachment to host cells in vitro (Stone and Abu Kwaik 1998), they appear not to be required for lung infection in the A/J mouse model (Rossier et al. 2004).
Besides its multiprotein structures, the surface of L. pneumophila contains a variety of outer membrane proteins that play different roles in disease. The first to mention is the genus-wide, peptidoglycan-linked, outer membrane porin (Gabay et al. 1985; Hoffman et al. 1992a, b). This protein, which is also known as the major outer membrane protein (MOMP), binds complement components C3 and C1q and thus can mediate phagocytosis by macrophages (Bellinger-Kawahara and Horwitz 1990; Mintz et al. 1995). A cloned copy of a MOMP gene can confer increased adherence upon E. coli (High et al. 1993; Krinos et al. 1999). Since MOMP mutants cannot be made, the importance of this surface protein in lung infection is presumed but not proven. The second surface protein that has received attention is a 19-kDa outer membrane lipoprotein. The gene encoding this genus-wide antigen, known as Pal, has been cloned and sequenced (Engleberg et al. 1991; Hindahl and Iglewski 1987; Kim et al. 2003; Ludwig et al. 1991). Although the inability to mutate the gene has precluded an assessment of the importance of the protein in an infection model, Pal does trigger cytokine production by binding to TLR2 on macrophages (Shim et al. 2009). A third surface-exposed protein is the 60-kDa heat shock protein known as Hsp60 or HtpB (Garduno et al. 1998a; Hindahl and Iglewski 1987; Hoffman et al. 1990; Sampson et al. 1990). Upon intracellular infection, HtpB appears, based upon studies done using HeLa cells, to promote epithelial cell invasion (Abu Kwaik et al. 1993; Fernandez et al. 1996; Garduno et al. 1998b). When introduced into a host cytoplasm using protein-coated beads or ectopic expression, HtpB alters mitochondrial trafficking and microfilament organization (Chong et al. 2009). Within host cells, HtpB may also function to ensure a supply of polyamines, which are required for intracellular growth of L. pneumophila (Nasrallah et al. 2011). Finally, purified HtpB elicits proinflammatory cytokine (IL-1) expression by macrophages (Retzlaff et al. 1994, 1996). A fourth protein associated with the outer membrane is the PlaB (lyso) phospholipase A (Bender et al. 2009; Lang and Flieger 2011). PlaB has contact-dependent hemolytic activity and is important in a guinea pig model of disease (Schunder et al. 2010).
Arguably, the most studied surface protein is the now-crystallized 24-kDa Mip, a genus-wide protein that exists as a homodimer and possesses peptidyl-proline isomerase (PPIase) activity (Cianciotto et al. 1990a; Engleberg et al. 1989; Fischer et al. 1992; Helbig et al. 1995b, 2001a; Ludwig et al. 1994; Riboldi-Tunnicliffe et al. 2001; Riffard et al. 1996; Schmidt et al. 1994). The protein is notable for sharing sequence similarity with eukaryotic proteins belonging to the FK506-binding protein family (Cianciotto and Fields 1992; Fischer et al. 1992). The first virulence factor to be defined in Legionella, Mip, is required for the early (postentry) stages of intracellular infection of macrophages, protozoa, and lung epithelia and for virulence following intratracheal inoculation of guinea pigs (Cianciotto et al. 1989b, 1990b, 1995; Cianciotto and Fields 1992; Helbig et al. 2001a, 2003a; Kohler et al. 2000; Susa et al. 1996; Wieland et al. 2002; Wintermeyer et al. 1995). The target of Mip action within the infected host cell is still unknown; however, it has recently been reported that Mip is required for efficient secretion of a novel T2S-dependent phospholipase C-like enzyme (DebRoy et al. 2006a), suggesting that Mip may facilitate expression of multiple other infectivity determinants. More recent studies indicate that Mip promotes pathogenesis by binding collagen IV and enabling L. pneumophila to transmigrate through a lung epithelial barrier (Unal et al. 2011; Wagner et al. 2007).
Because L. pneumophila is Gram-negative, another one of its critical surface molecules is lipopolysaccharide. L. pneumophila LPS is the serogroup-specific O antigen (Ciesielski et al. 1986; Conlan and Ashworth 1986; Knirel et al. 1997; Nolte et al. 1986; Otten et al. 1986; Zahringer et al. 1995). A particular LPS epitope of serogroup 1 that is recognized by the typing monoclonal antibodies MAb 2 and MAb3/1 is more frequently expressed on clinical vs. environmental isolates (Dournon et al. 1988; Helbig et al. 1995a). However, the loss of the epitope itself does not diminish the ability of L. pneumophila to infect macrophages (Mintz and Zou 1992; Zou et al. 1999), indicating that the increased prevalence of MAb2-positive strains is due to other factors, including physiochemical surface properties (Gosselin et al. 2011). On the other hand, other sorts of antigenic changes in Legionella LPS expression do result in reductions in serum resistance, intracellular growth, and virulence (Luneberg et al. 1998; Rogers et al. 1992). Additionally, recent studies indicate that LPS is shed during intracellular growth and delivered into the cytoplasm of the host cell where it may play a role in bacterial evasion of lysosomes (Reichardt et al. 2010; Seeger et al. 2010). The lipid A component of L. pneumophila LPS has relatively weak endotoxin activity, and this appears to be due to its unusual long-chain and branched fatty acids as well as its low affinity for the CD14 receptor on macrophages (Highsmith et al. 1978; Moll et al. 1992; Neumeister et al. 1998a; Schramek et al. 1982; Wong et al. 1979; Zahringer et al. 1995). A more significant role for lipid A in L. pneumophila pathogenesis was documented when a gene (rcp) involved in the palmitoylation of lipid A was inactivated and there was a simultaneous reduction in resistance to cationic antimicrobial peptides, intracellular infectivity, and virulence in an animal model of disease (Robey et al. 2001). As is the case for the LPS of other pathogens, the LPS of Legionella is recognized by the host innate immune system. However, unlike most LPS molecules, which are recognized by the host TLR4 receptor, the LPS of L. pneumophila is recognized by TLR2 (Braedel-Ruoff et al. 2005; Girard et al. 2003). L. pneumophila LPS is also bound by lung surfactant proteins that can suppress the growth of the bacterium (Sawada et al. 2010).
Periplasmic and Cytoplasmic Virulence Factors
Several important infectivity determinants have been localized to the L. pneumophila periplasm or cytoplasm. The first group of these is proteins involved in detoxification and stress responses. A copper-zinc superoxide dismutase (SOD) resides in the periplasm, affording resistance to toxic superoxide anions (by converting them to H2O2) and promoting survival during stationary phase (John and Steinman 1996). Mutational analysis indicates, however, that the enzyme is not required for infection of macrophages. A second SOD, which bears iron as its cofactor and exists within the Legionella cytoplasm, is essential for bacterial viability, and thus its role in pathogenesis cannot be assessed by the genetic approach (Sadosky et al. 1994). L. pneumophila has two catalase-peroxidase enzymes that convert H2O2 to water and oxygen. The KatA catalase-peroxidase is located in the periplasm, induced during stationary phase, and required for optimal intracellular infection of macrophages (Amemura-Maekawa et al. 1999; Bandyopadhyay et al. 2003; Bandyopadhyay and Steinman 2000). The KatB enzyme is cytoplasmic, and mutational analysis has identified a role for the katB gene in macrophage infection (Bandyopadhyay et al. 2003; Bandyopadhyay and Steinman 1998). Though not individually required for infection, AhpC1 and AhpC2 are expressed intracellularly and are important detoxifiers of H2O2 and organic peroxides (LeBlanc et al. 2006, 2008; Rankin et al. 2002). HtrA, a periplasmic chaperone-protease that facilitates the refolding or degradation of defective outer membrane proteins, is required for L. pneumophila infection of macrophages (Pedersen et al. 2001; Wrase et al. 2011). L. pneumophila has two periplasmic oxidoreductases (DsbA1, DsbA2) that catalyze the formation of disulphide bonds in extracytoplasmic proteins; important targets for DsbA2 are components of the type IV secretion system (Jameson-Lee et al. 2011). Although not required for intracellular infection, McoL, a multicopper oxidase that is associated with the inner membrane, acts to prevent toxic effects of ferrous iron during aerobic growth (Huston et al. 2008). NudA is a nucleoside diphosphate pyrophosphatase (Nudix hydrolase) that degrades toxic intracellular compounds, and based upon mutational analysis, the nudA gene is required for infection of macrophages and the lungs (Edelstein et al. 2005). The inner membrane protein LadC is a putative adenylate cyclase that is required for adherence to macrophages and growth in the lungs (Newton et al. 2008).
Other infectivity determinants are involved in intracellular metabolism and growth. As for respiration considerations, the cytochrome c maturation system is critical for both intracellular and intrapulmonary growth, with the c1 and c5 cytochromes being most important for intracellular infection (Yip et al. 2011). Finally, as mentioned above, another important aspect of intracellular infection and virulence is iron acquisition. In addition to legiobactin, pyomelanin, and FeoB-mediated ferrous iron transport, factors that are involved in iron acquisition include periplasmic and cytoplasmic ferric reductases, a methyltransferase (iraA) and membrane (iron-)peptide transporter (iraB), the inner membrane cytochrome c biogenesis system (ccm), an LbtA-like synthetase (frgA), and a hemin-binding protein (hbp) (Cianciotto et al. 2005; Hickey and Cianciotto 1997; James et al. 1997; Johnson et al. 1991; Naylor and Cianciotto 2004; O’Connell et al. 1996b; Poch and Johnson 1993; Pope et al. 1996; Robey and Cianciotto 2002; Viswanathan et al. 2000; Viswanathan et al. 2002). Other genes encoding cytosolic proteins that have been shown to be required for optimal intracellular infection of macrophages include bdhA-patD (polyhydroxybutyrate metabolism) (Aurass et al. 2009), oad (oxaloacetate decarboxylase) (Jain et al. 1996), asd (aspartate-β-semialdehyde) (Harb and Abu Kwaik 1998), pts (phosphoenolpyruvate phosphotransferase) (Edelstein et al. 1999; Higa and Edelstein 2001), pmi (phosphomannose isomerase) (Gao et al. 1997), prp (propionate catabolism) (Stone et al. 1999), and ssrS (regulatory 6S RNA) (Faucher et al. 2010). Finally, it has recently been determined that cyclic diguanylate signaling proteins modulate intracellular growth of L. pneumophila (Levet-Paulo et al. 2011; Levi et al. 2011).
Pathogenesis of Other Legionella Species
Besides L. pneumophila, 37 of the other Legionella species have been associated with human disease (Table 9.1 ). Thus, many of these species have been tested for their ability to grow within macrophages, as an initial attempt to explain their pathogenicity. Twenty-two of these species are known to replicate in one or more types of macrophages, including L. anisa, L. brunensis, L. birminghamensis, L. bozemanae, L. cardiaca, L. cherrii, L. dumoffii, L. feeleii, L. gormanii, L. hackeliae, L. jordanis, L. lansingensis, L. longbeachae, L. maceachernii, L. micdadei, L. oakridgensis, L. parisiensis, L. sainthelensi, L. santicrucis, L. spiritensis, L. tusconensis, and L. wadsworthii (Alli et al. 2003; Buse et al. 2011; Doyle et al. 2001; Izu et al. 1999; Levi et al. 1987; Miyamoto et al. 1996; Neumeister et al. 1997; O’Connell et al. 1996a; Pearce and Cianciotto 2012; Pearce et al. 2012; Weinbaum et al. 1984; Whitfield et al. 2010). Thus, the pneumonia caused by these 22 species likely derives from the ability of the legionellae to infect and grow within alveolar macrophages, as is the case for L. pneumophila. In contrast to these results, six other species that have been linked to disease, that is, L. cincinnatiensis, L. erythra, L. gratiana, L. londiniensis, L. quinlivanii, and L. rubrilucens, do not show evidence of intracellular replication (Alli et al. 2003; Izu et al. 1999; O’Connell et al. 1996a). Although these data might suggest that some Legionella species do not cause disease by being an intracellular parasite, it is also quite possible that the studies on these bacteria were limited by their in vitro infection assay and/or employed a bacterial strain that had been attenuated by laboratory passage. The remaining nine species that have been linked to disease, that is, L. drancourtii, L. drozanskii, L. fallonii, L. lytica, L. nagasakiensis, L. nautarum, L. rowbothamii, L. waltersii, and L. worsleiensis, have not been tested. Five of the 19 Legionella species that have not been linked to human disease have been tested for in vitro infection of macrophages. Interestingly, among these, L. jamestowniensis and L. steigerwaltii show an ability to grow intracellularly (Neumeister et al. 1997; O’Connell et al. 1996a). These data support the hypothesis that these two species, and perhaps other environmental legionellae, have pathogenic potential. A similar hypothesis had initially been put forward for L. parisiensis (O’Connell et al. 1996a), and soon thereafter pneumonia caused by L. parisiensis was reported (Igel et al. 2004).
A limited number of studies have sought to further define the intracellular infection process of the non-pneumophila species. In some cases, the non-pneumophila species exhibit traits that are similar to those of L. pneumophila. For example, mip and dot/icm genes promote intracellular infection by L. longbeachae and L. micdadei, as they do for L. pneumophila (Doyle et al. 1998; Feldman and Segal 2004; O’Connell et al. 1995). However, there are instances where their mode of macrophage infection is notably different from that of L. pneumophila. Strains of L. micdadei, for the most part, do not reside within phagosomes that evade lysosomes and recruit rough endoplasmic reticulum (Gao et al. 1999; Gerhardt et al. 2000; Joshi and Swanson 1999; Rechnitzer and Blom 1989; Weinbaum et al. 1984). L. parisiensis and L. tucsonensis do not effectively evade LAMP-1 (Whitfield et al. 2010). L. longbeachae colocalizes with early endosomal (EEA1) and late endosomal (LAMP-2) markers (Asare and Abu Kwaik 2007). L. dumoffii and L. oakridgensis multiply free within the cytoplasm of non-macrophage hosts (Maruta et al. 1998; Takekawa et al. 2012). Other sorts of studies have found that secreted activities can vary significantly between the Legionella species, including type II protein effectors, type IV protein effectors, siderophore, and surfactant (Cazalet et al. 2010; Kozak et al. 2010; Nagai et al. 2002; Newton et al. 2006; Pearce and Cianciotto 2009; Pearce et al. 2012; Söderberg et al. 2008; Starkenburg et al. 2004; Stewart et al. 2009). Thus, as various legionellae become associated with human disease, it will become increasingly important for researchers to examine them carefully and not assume that they are simple equivalents of L. pneumophila.
Immune Response and Host Susceptibility to Legionella Infections
Upon inhalation of contaminated aerosols, L. pneumophila reaches the lung and triggers an acute inflammation. In line with a higher virulence of protozoa-grown L. pneumophila, coinfection of the bacteria with Hartmannella vermiformis amoeba significantly enhances intrapulmonary bacterial growth and aggravates inflammation (Brieland et al. 1996, 1997a, b). A prerequisite for L. pneumophila to cause an inflammatory disease is the Dot/Icm-dependent ability to resist but not necessarily replicate in macrophages (Spörri et al. 2006). Growth of L. pneumophila is restricted in macrophages from most laboratory mouse strains including BALB/c or C57BL/6, yet macrophages from the A/J mouse strain support replication of the bacteria in vitro (Yamamoto et al. 1988), and A/J mice are a suitable model for Legionnaires’ disease (Brieland et al. 1994). In A/J mice, L. pneumophila elicits an acute inflammatory reaction and strong innate immune responses (Blanchard et al. 1988a, b; Brieland et al. 1995, 1998; Tateda et al. 2001a, b). Early after infection, inflammatory cytokines such as tumor necrosis factor (TNF)-α (Blanchard et al. 1987, 1988a; Brieland et al. 1995), interferon (IFN)-γ (Blanchard et al. 1988b, 1989), interleukin (IL)-12 (Brieland et al. 1998), and IL-18 (Brieland et al. 2000) are released and restrict the replication of the pathogen. In particular, IFN-γ is crucial to resolve an infection with L. pneumophila and also restricts growth of the bacteria in monocytes and alveolar macrophages, thus further contributing to limiting the infection (Bhardwaj et al. 1986; Byrd and Horwitz 1989; Nash et al. 1988).
Inflammatory cytokines act in concert with the activation and recruitment of polymorphonuclear neutrophil granulocytes (PMN) (Blanchard et al. 1988a, 1989; Tateda et al. 2001b). PMN are important innate effector cells that resolve Legionella infection and also function as cytokine producers. A/J mice depleted of PMN or natural killer (NK) cells, or mice lacking the type II IFN receptor, are unable to clear L. pneumophila due to a lack of the critical cytokine IFN-γ or its receptor (Spörri et al. 2006, 2008). In response to L. pneumophila infection, PMN activate caspase-1, leading to the production of mature IL-18. This cytokine then activates NK cells, which in turn produce IFN-γ (Spörri et al. 2008). In a feedback loop, IFN-γ stimulates dendritic (DC) cells, which produce IL-12 to activate NK cells, and thus, DC cells are also essential to control L. pneumophila infection (Ang et al. 2010). Interestingly, DCs restrict the intracellular growth of L. pneumophila, despite the fact that the bacteria reside in an ER-derived compartment (Neild and Roy 2003).
The innate immune response toward pathogens is directed against specific prokaryotic or viral “pathogen-associated molecular patterns” (PAMPs), which are recognized by eukaryotic “pattern recognition receptors” (PRRs) (Janeway and Medzhitov 2002). The PRRs include transmembrane toll-like receptors (TLRs), and the cytoplasmic nod-like receptors (NLRs) or retinoic acid inducible gene-I (RIG-I)-like receptors (RLRs) (Creagh and O’Neill 2006). Legionella spp. evolved as parasites of free-living protozoa, and the human host represents a dead end for the “accidental” pathogen. Thus, L. pneumophila likely has not been exposed to a rigorous evolutionary selection to avoid recognition by mammalian PRRs, and accordingly, the bacteria trigger activation of all families of PRRs (Massis and Zamboni 2011).
The activation of TLRs proceeds through dimerization and recruitment of adaptor proteins such as MyD88 (myeloid differentiation primary response gene 88) and culminates in the activation of the transcription factor NF-κB and the expression of inflammatory genes. TLR2 recognizes bacterial lipopeptides and lipoproteins. Mice lacking TLR2 are impaired for PMN migration to the site of L. pneumophila infection and, consequently, cannot efficiently clear the bacteria (Archer and Roy 2006; Fuse et al. 2007; Hawn et al. 2006). TLR4 responds to bacterial LPS. In contrast to TLR2, the absence of TLR4 does not affect the susceptibility of mice toward L. pneumophila (Archer and Roy 2006; Fuse et al. 2007; Lettinga et al. 2002). This lack of response might be due to the structure of L. pneumophila LPS, which contains a lipid A moiety with very long acyl chains that triggers TLR4 less potently than enterobacterial LPS. Yet, the situation is more complicated, as TLR4 polymorphisms are associated with resistance to Legionnaires’ disease in humans (Hawn et al. 2005). TLR5 senses bacterial flagellin, including the flagellum of L. pneumophila. TLR5 plays an important role in L. pneumophila infection, as a common dominant TLR5 polymorphism that abolishes flagellin signaling is associated with an increased susceptibility to Legionnaires’ disease (Hawn et al. 2003). A role for TLR5 in Legionnaires’ disease is corroborated by mice lacking the gene, which show altered leukocyte recruitment and inflammatory responses upon infection with L. pneumophila (Hawn et al. 2007). Taken together, mice lacking individual TLR genes are not substantially more susceptible to L. pneumophila. In contrast, deletion of MyD88 generates mice that fail to produce cytokines such as NK cell-derived IFN-γ and are highly susceptible to L. pneumophila infection (Archer et al. 2009, 2010; Archer and Roy 2006; Hawn et al. 2006; Neild et al. 2005; Spörri et al. 2006).
The NLRs represent a large family of PRRs that promote the expression of inflammatory genes and the activation of caspase-1 in multiprotein complexes termed inflammasomes (Schroeder et al. 2010). Three groups of NLRs can be distinguished: (1) nucleotide-binding oligomerization domain (NOD) proteins, (2) NALP (NRLP) receptors that require the adaptor protein ASC to trigger inflammasomes, and (3) NAIP (IPAF/NLRC) proteins that do not require ASC. The NOD-1 and NOD-2 receptors, which detect bacterial cell wall molecules, modulate the in vivo pulmonary immune response against L. pneumophila, albeit in reciprocal ways (Berrington et al. 2010; Shin et al. 2008). NOD-dependent signaling proceeds through the RIP-2 kinase and, in concert with MyD88- and NAIP5/NLRC4-dependent signaling (see below), cooperatively contributes to protection from L. pneumophila (Archer et al. 2010). ASC-dependent inflammasome activation leads to the secretion of mature IL-1β and restricts L. pneumophila replication in human macrophages (Abdelaziz et al. 2011), but neither activates the NALP3 (NLRP3) inflammasome nor contributes to controlling L. pneumophila infection in mice (Case and Roy 2011; Case et al. 2009; Molofsky et al. 2006; Ren et al. 2006; Zamboni et al. 2006). In contrast, ASC-independent recognition of L. pneumophila flagellin through the NAIP5 (Birc1e)/ NLRC4 (Ipaf) inflammasome triggers caspase-1 activation, pore formation, and pyroptosis (Amer et al. 2006; Case et al. 2009; Coers et al. 2007a; Derré and Isberg 2004; Fortier et al. 2007; Molofsky et al. 2006; Ren et al. 2006; Silveira and Zamboni 2010; Vinzing et al. 2008; Whitfield et al. 2010; Zamboni et al. 2006). Recognition of flagellin through the NAIP5/NLRC4 inflammasome is arguably the most important mechanism of L. pneumophila restriction. The elucidation of this intricate pathway finally provided a molecular and mechanistic rational for the long-standing fact that A/J mice fail to restrict L. pneumophila, a feature that previously was mapped to the NAIP5 gene (Diez et al. 2003; Wright et al. 2003) within the Lgn1 locus (Dietrich et al. 1995). In agreement with this concept, macrophages as well as DC restrict L. pneumophila replication through a cell death pathway mediated by NAIP5, caspase-1, and also caspase-3 (Nogueira et al. 2009). Moreover, mice lacking NAIP5 fail to activate caspase-1 and restrict flagellated L. pneumophila (Lightfield et al. 2008).
Finally, RLRs are nucleic acid-sensing PRRs, which upon activation lead to the production of type I IFNs, such as IFN-α and IFN-β. During L. pneumophila infection, type I IFNs are produced dependent on the bacterial Dot/Icm T4SS and the eukaryotic regulators IFN-β promoter stimulator-1 (IPS-1) as well as IFN regulatory factor (IRF-3) (Lippmann et al. 2008; Opitz et al. 2006; Stetson and Medzhitov 2006). A role of RIG-1 for L. pneumophila DNA-dependent type I IFN production was debated; yet, recent data suggest that L. pneumophila RNA triggers the RIG-I-dependent production of type I IFNs (Monroe et al. 2009). Moreover, in this study, a Dot/Icm-secreted effector protein, SdhA, was identified as a key suppressor of the IFN response to L. pneumophila. Similar to IFN-γ, but through a different pathway, the addition of type I IFNs to macrophages abolishes intracellular growth of L. pneumophila in vitro (Plumlee et al. 2009; Schiavoni et al. 2004). In contrast, the role of type I IFNs in murine infection is less prominent (Ang et al. 2010; Monroe et al. 2009).
Genomics, Transcriptomics, and Metabolomics
Genetics and Genomics
Since the publication of the first bacterial genome sequence in 1995 (Fleischmann et al. 1995), a tremendous increase in genomic information has substantially altered our view on bacterial pathogenesis and has led to the application of many different genomics and post-genomics approaches in microbial research. In Legionella research, the genomics era started only in 2004 with the completion, analysis, and publication of the genome sequence of three clinical L. pneumophila isolates (Cazalet et al. 2004; Chien et al. 2004). The sequenced strains are the endemic strain Paris and the epidemic strain Lens, responsible for two major outbreaks in France in 2001 and 2004 (Cazalet et al. 2004), and strain Philadelphia 1, isolated from the first recognized outbreak of the disease in 1976 (Chien et al. 2004). Recently, the genomes of five additional isolates were determined: L. pneumophila strain Corby, a virulent strain isolated from a human legionellosis case (Jepras et al. 1985; Steinert et al. 2007); L. pneumophila strain Alcoy, a particularly persistent and recurrent strain in the region of Alcoy, Spain, that was isolated during one of the most significant outbreaks between the years 1999 and 2000 in Alcoy (D’Auria et al. 2010); strain L. pneumophila 130b, a clinical isolate from the Wadsworth Veterans Administration Hospital, Los Angeles, CA (Edelstein 1986; Engleberg et al. 1984; Schroeder et al. 2010); and two stains isolated in France. L. pneumophila strain Lorraine is rarely isolated from the environment, but its prevalence in human disease is increasing considerably in the last years (Ginevra et al. 2008), and L. pneumophila strain HL 0604 1035 has been frequently isolated from a hospital water system since over 10 years but never caused disease (Gomez-Valero et al. 2011). Knowledge of these sequences is now the basis for major breakthroughs in understanding the biology of L. pneumophila, as it gives new insight into the bacterium’s lifestyle and its way of adapting to the host. As described below in detail, the analysis of these genome sequences revealed several specific features of L. pneumophila, some of which are undoubtedly related to its intracellular life, and allowed generation of hypothesis on how L. pneumophila subverts host functions to its advantage. Furthermore, the availability of the genome sequence of L. pneumophila provided the basis for the application of new powerful approaches like bioinformatics analyses, transcriptomics, and proteomics studies to better understand the biology of this organism.
General Features of the L. pneumophila Genomes
Legionella pneumophila has a single, circular chromosome of in average 3.4 Mb, with an average GC content of 38 % (Table 9.5 ). Strains Paris, Lens, and Lorraine each contain a plasmid, 131.9 kb, 59.8 kb, and 150.4 kb in size, respectively. In the other sequenced strains, no plasmid was identified. The genomes contain each ∼3,000 genes distributed fairly evenly between the two strands (∼57 % on the leading strand) and accounting for ∼88 % of the potential coding capacity. No function can be predicted for about 40 % of the L. pneumophila genes, and about 20% of the predicted genes are unique to the genus Legionella. As seen in Table 9.5 , the main features of the sequenced L. pneumophila genomes (e.g., genome size, GC content, and coding density) are highly conserved. The core genome of the L. pneumophila genomes comprises about 2,200 genes, which represents roughly 80 % of the predicted genes in each genome. Comparative analysis of the genome structure of the L. pneumophila genomes showed high colinearity, with only few translocations, duplications, deletions, or inversions. Principally, the genomes contain four large plasticity zones, where the synteny is disrupted: a 260-kb inversion in strain Lens with respect to all other strains, a 130-kb fragment which is partly similar partly different and which is inserted in different genomic location in the different strains, the genomic island-like region carrying the Pie effectors, and the chromosomal region carrying the Lvh T4SS.
Diversity, Mobility, and Plasticity Characterize the L. pneumophila Genomes
When determining the non-orthologous genes specific of each completely sequenced L. pneumophila genome, about 130 and 300 strain-specific genes mainly encoded on mobile genetic elements are identified. Thus, the L. pneumophila genomes have a highly dynamic accessory genome of up to 300 genes each, mainly formed by mobile genetic elements, genomic islands, and genes of unknown function. One of these mobile genetic elements is the above-mentioned Lvh carrying region. Lvh is a genomic island-like region that encodes a T4ASS implicated in conjugation and in virulence-related phenotypes under conditions mimicking the spread of Legionnaires’ disease from environmental niches (Bandyopadhyay et al. 2007; Ridenour et al. 2003). This region can be integrated in the chromosome but can also excise in a site-specific manner and exist as a low copy plasmid (Doleans-Jordheim et al. 2006). It is present in all sequenced L. pneumophila strains except Lorraine, Corby, and Alcoy. In L. pneumophila Corby, there are instead two similar large genomic islands present called Trb-1 and Trb-2 (Steinert et al. 2007). Both islands encode all genes necessary for a functional T4SS with a trb/tra gene organization similar to the tra/trb region of plasmid R751 (IncP) of Enterobacter aerogenes (Thorsted et al. 1998) and contain an oriT-like site. Besides the trb/tra genes, the gene content of each island is specific. Trb-1 is integrated in a Pro tRNA gene, while Trb-2 is inserted in a tmRNA. The ladder integration site is identical to that of the pathogenicity island containing the lvh region in strain Paris and strain Lens. Both Trb-1 and Trb-2 can exist as an integrated and an excised form (Glockner et al. 2008; Steinert et al. 2007). It is interesting to note that in the Legionella strains where the Lvh T4SS is not present, another Tra system, a P-type T4SS that codes for short and rigid pili that allow surface mating for conjugation, is present in the same chromosomal position (Gomez-Valero et al. 2011). Recently, several other regions coding proteins homologous to Tra proteins that might code for a conjugative machinery and/or T4ASS were identified in all Legionella plasmids. They are similar to F-type T4SS that allow the synthesis of a long and flexible pilus for conjugation in liquid and solid media (Lawley et al. 2003). However, such a system is also found in a chromosomal localization in the L. pneumophila strain Philadelphia (Gomez-Valero et al. 2011). These regions are inserted in a tRNA gene next to an integrase and are bordered by flanking repeats. The presence of these elements is suggesting that these T4SSs are mobile and that their heterogeneous distribution is the result of the lateral movement of these plasmids. Furthermore, these different T4SS or conjugative elements can be found in different plasmids or can be completely or partially present in the chromosome, indicating that these regions might have the capacity to integrate and excise from the Legionella genomes as shown for the Lvh carrying region (Doleans-Jordheim et al. 2006; Gomez-Valero et al. 2011). Interestingly, a feature shared by most of the mobile elements (plasmids, lvh gene cluster, 65-kb putative pathogenicity island originally identified in strain Philadelphia 1 (Brassinga et al. 2003), the 130-kb fragment encoding several multidrug efflux pumps, and the putative mobile genetic elements coding P-type and F-type T4SSs) is that they encode a paralog of CsrA, a protein described in L. pneumophila as a repressor of transmission traits and an activator of replication traits (Fettes et al. 2001; Molofsky and Swanson 2004). It is tempting to assume that the paralogs of CsrA encoded on the mobile elements control their expression and/or also regulate the switch between integrated and circular forms of these regions (Cazalet et al. 2008; Gomez-Valero et al. 2011).
Thus, plasticity and genomic diversity are specific features of the L. pneumophila genomes due to the presence of integrative plasmids, putative conjugation elements, and genomic islands. In addition to DNA interchange between different bacterial genera, horizontal gene transfer within the genus Legionella and within the species L. pneumophila has been reported. Several studies suggested that recombination events may also play a role in the evolution of the species L. pneumophila (Bumbaugh et al. 2002; Coscolla et al. 2011; Coscolla and Gonzalez-Candelas 2007; Ko et al. 2003, 2006). Indeed, recently, it was shown that recombination and horizontal gene transfer are frequent in L. pneumophila (Gomez-Valero et al. 2011). In particular, the analyses of the distribution of nucleotide polymorphisms suggested that large chromosomal fragments of over 200kbs can be exchanged between L. pneumophila strains contributing to the genome dynamics in the natural population (Gomez-Valero et al. 2011). L. pneumophila has all necessary features for incorporating foreign DNA, as these bacteria are naturally competent and possess an intact recombination machinery (Mintz 1999; Sexton and Vogel 2004; Stone and Kwaik 1999). Taken together, the L. pneumophila genomes are highly dynamic, a feature allowing different clones to evolve into predominant disease clones and others to replace them subsequently within relatively short periods of time but also allowing L. pneumophila to adapt to the many diverse conditions and environments it encounters.
Coevolution with Protozoa Is Reflected in the L. pneumophila Genome Sequence
Analysis and comparison of the first sequenced genomes revealed an intriguing feature of Legionella, which is the presence of an extended array of eukaryotic-like proteins (Cazalet et al. 2004). A systematic search in the genome sequence identified about 100 genes having their best hit against eukaryotic genomes including both housekeeping genes and genes that may play a role in the virulence of L. pneumophila (Cazalet et al. 2004). Examples for eukaryotic-like proteins are two secreted apyrases, a sphingosine-phosphate lyase and sphingosine kinase, eukaryotic-like glucoamylase, cytokinin oxidase, zinc metalloprotease, or an RNA binding precursor (Bruggemann et al. 2006; Cazalet et al. 2004; de Felipe et al. 2005). Further analyses of additional genomes showed that eukaryotic-like proteins are present in all sequenced L. pneumophila genomes, and most of them are even highly conserved, suggesting their importance for L. pneumophila virulence and survival (Amaro et al. 2012; Cazalet et al. 2004; D’Auria et al. 2010; de Felipe et al. 2005; Gomez Valero et al. 2011; Gomez-Valero et al. 2011; Schroeder et al. 2010). This high conservation was also confirmed by hybridization analyses of over 200 L. pneumophila strains (Cazalet et al. 2008). A search against Pfam and Prosite databases identified over 30 genes coding proteins containing motifs that are present mostly in eukaryotes like ankyrin repeats, Sel-1, Sec7, serine threonine kinase domains (STPK), F-box, or U-box motifs. The identification of these so-called eukaryotic-like proteins (Cazalet et al. 2004) or Legionella eukaryotic genes (leg) (de Felipe et al. 2005) led to the hypothesis that the eukaryotic proteins of L. pneumophila might help to mimic the function of host proteins to manipulate the host physiology and certain cellular functions for the pathogens benefit (Bruggemann et al. 2006; Cazalet et al. 2004). Indeed, many of these eukaryotic-like proteins are substrates of the Dot/Icm T4SS (de Felipe et al. 2008; de Felipe et al. 2005), and for several, it has been shown meanwhile that they are indeed modulating different host cell pathways (Hubber and Roy 2010a; Nora et al. 2009).
A particular example is the large family of ankyrin repeat proteins identified in the L. pneumophila genomes. Different studies showed that many of the L. pneumophila ankyrin proteins, namely, AnkB, AnkC, AnkF, AnkK, AnkQ, AnkW/AnkH, AnkX/AnkN, AnkY, and AnkZ/AnkG, are substrates of the Dot/Icm secretion system and that AnkB, AnkK, AnkQ, AnkX, and AnkY induce a growth defect in Saccharomyces cerevisiae (Al-Khodor et al. 2008; de Felipe et al. 2008; Heidtman et al. 2009; Pan et al. 2008). However, the determination of whether proteins are secreted by the T4SS is not always clear, as, for example, AnkY and AnkZ are not translocated Dot/Icm effectors according to the assay used by de Felipe and colleagues (de Felipe et al. 2008). Recent functional analysis of certain of these ankyrin proteins showed that they are multifunctional and are involved in many cellular pathways. AnkX/AnkN was shown to be an effector of membrane transport that promotes fragmentation of the Golgi apparatus when expressed in mammalian cells (Pan et al. 2008). Golgi fragmentation is presumably the result of inhibition of ER-to-Golgi vesicle transport by interference with microtubule-dependent transport of vesicles (Pan et al. 2008). Furthermore, AnkW, AnkX, AnkY, and AnkZ showed different patterns of subcellular localization in mammalian cells (Pan et al. 2008). AnkB plays an important role in intracellular growth and in exploiting the ubiquitin system and the farnesylation machinery of the host (see above, paragraph type IV secretion) (Al-Khodor et al. 2008; Al-Quadan and Kwaik 2011; Al-Quadan et al. 2012, 2011; Habyarimana et al. 2008; Ivanov et al. 2010; Price et al. 2009, 2010a, b, c, 2011; Price and Kwaik 2010). AnkH and AnkJ show a significant defect in intracellular replication in amoebae, human macrophages, and protozoa (Habyarimana et al. 2009). Furthermore, when expressed in Saccharomyces cerevisiae, AnkB, AnkF, AnkQ, AnkX, and AnkY caused severe growth defects, indicating that these proteins impact essential host cell pathways (Heidtman et al. 2009). Thus, ankyrin proteins are translocated effectors of distinct secretion systems that may have many different functions in the eukaryotic hosts by aiding intracellular bacteria to modulate host functions to their advantage.
Another example is the apyrase coding genes. Apyrases or ecto-nucleoside triphosphate diphosphohydrolases (ecto-NTPDases, apyrases) (gene family ENTPD) of the CD39 family are important ectonucleotidases that are characterized by the presence of five “apyrase-conserved regions” (ACR1 to ACR5) and by the ability to hydrolyze nucleotide tri- and diphosphatases to the monophosphate form. Nucleoside monophosphates may then be catalyzed to nucleosides such as adenosine by the action of ecto-5-nucleotidases (e.g., mammalian CD73) (Sansom et al. 2008). While CD39/NTPDase 1 family members are present in all higher eukaryotes, the first prokaryotic ecto-nucleoside triphosphate diphosphohydrolase was identified and characterized in L. pneumophila (Cazalet et al. 2004; Sansom et al. 2007). L. pneumophila encodes two secreted ecto-NTPDases Lpp1880/Lpg1905 and Lpp1033/Lpg0971. Lpp1880/Lpg1905 shares similarities with human CD39 and other eukaryotic ecto-NTPDases, and it has been shown to play a role during uptake of L. pneumophila into the host cell. In humans, CD39 is located on the surface of endothelial cells, and it controls extracellular levels of ATP by converting it in its diphosphate and monophosphate forms. In this way, it plays a major role in maintaining vascular fluidity by regulating platelet aggregation (Marcus et al. 2005). CD39/NTPDases are found in a wide range of pathogens such as in protozoan parasites, but their role in infection is poorly understood. One of the two predicted ecto-NTPDases in L. pneumophila is secreted into the host cell, and its activity is required for successful infection. This defect was not correlated with the ability to recruit the ER or avoiding phagolysosomal fusion but mainly to a less efficient entry (Sansom et al. 2007). Recently, it was shown that the enzyme catalyzed the hydrolysis of ATP and ADP and also of GTP and GDP but had only limited activity against CTP, CDP, UTP, and UDP. Furthermore, mutational analysis revealed that all five apyrase domains are necessary for infection following intratracheal inoculation of A/J mice (Sansom et al. 2008). Interestingly, Lpp1880/Lpg1905 is the first example in which a bacterial ecto-NTPDase is implicated in virulence. In contrast, Lpp1033/Lpg0971, the second ecto-NTPDase encoded by L. pneumophila, is not necessary for entry and replication within amoebae, alveolar epithelial cells, and macrophages (Sansom et al. 2007), but contributes to virulence in a mouse lung infection model (Sansom et al. 2008).
Another intriguing protein identified in the L. pneumophila genomes (Cazalet et al. 2004) is a eukaryote-like sphingosine-1-phosphate lyase. Up to now, SPL-encoding genes have been found in very few prokaryotes only. In eukaryotes, SPL is an enzyme that uses pyridoxal 5’-phosphate as a cofactor for catalyzing the irreversible cleavage of sphingosine-1-phosphate (S1P). S1P, a sphingolipid, like ceramide or sphingosine, is implicated in various physiological processes like cell survival, apoptosis, proliferation, migration, differentiation, platelet aggregation, angiogenesis, lymphocyte trafficking, and development (Alvarez et al. 2007; Bandhuvula and Saba 2007). Despite the fact that the function of the L. pneumophila sphingosine-1-phosphate lyase remains actually unclear, the hypothesis is that it plays a role in autophagy and/or apoptosis. Recently, it has been shown that L. pneumophila SPL is translocated into host cells using a C-terminal translocation domain absent in its eukaryotic homologues and that it is able to complement the sphingosine-sensitive phenotype of Saccharomyces cerevisiae (Degtyar et al. 2009). Unlike the eukaryotic SPL that localizes to the endoplasmic reticulum, L. pneumophila SPL was found to be targeted mainly to host cell mitochondria (Degtyar et al. 2009). Eukaryotic-like proteins are clearly helping L. pneumophila to modulate host functions to its advantage. Thus, molecular mimicry seems to be a main virulence strategy of this environmental pathogen.
The Evolution of Virulence in L. pneumophila: The Evolution of the Eukaryotic-Like Proteins
Eukaryotic-like proteins have also been identified in other bacterial pathogens like Salmonella typhimurium and Pseudomonas aeruginosa (Stebbins and Galan 2001; Vance et al. 2004). Furthermore, a particular high number of serine protein kinases has been identified in Mycobacterium tuberculosis (Cole et al. 1998), or large families of ankyrin repeat proteins are present in Coxiella burnetii (Luhrmann et al. 2010; Pan et al. 2008; Seshadri et al. 2003; Voth et al. 2009), Rickettsia felis (Ogata et al. 2005), or Wolbachia pipientis (Wu et al. 2004). However, L. pneumophila ranks as one of the pathogens that encodes the most and the widest variety of eukaryotic-like proteins or proteins with eukaryotic domains. Thus, the question arises with respect to the origin of these eukaryotic-like proteins. Two hypotheses can be proposed: (1) either they have been acquired by horizontal gene transfer (2) or they evolved by convergent evolution. Most of the Legionella eukaryotic-like genes have a GC content, ranging from 32 % to 48 %; thus, it differs significantly from that of the rest of the genome (38 %), supporting the hypothesis that these genes have been acquired through horizontal gene transfer. Another line of evidence supporting this hypothesis is the presence of an 11.3-kb gene cluster in strain Paris containing 11 genes with similarity to eukaryotic genes that is bordered by a Lys-tRNA which might be the integration site as in many genomic and pathogenicity islands (Hacker and Kaper 2000). ralF was the first gene suggested to have been acquired by L. pneumophila from eukaryotes by HGT, as RalF carries a eukaryotic Sec 7 domain (Nagai et al. 2002). Recently, it has been reported that Legionella drancourtii, a relative of L. pneumophila, has acquired a sterol reductase gene from the Acanthamoeba polyphaga Mimivirus genome, a virus that grows in amoeba (Moliner et al. 2009b). Thus, the acquisition of some of the eukaryotic-like genes of L. pneumophila by HGT from protozoa is plausible and has thus been suggested by different groups (Bruggemann et al. 2006; Cazalet et al. 2008; de Felipe et al. 2005; Hubber and Roy 2010a; Moliner et al. 2010; Moliner et al. 2009a). In order to study their evolutionary origin, a systematic phylogenetic analysis has been undertaken (Lurie-Weinberger et al. 2010). It demonstrated that both lateral gene transfer from eukaryotic hosts and bacterial genes that became eukaryotic like by gradual adaptation to the intracellular milieu or gene fragment acquisition contributed to the existing repertoire of ELPs, which comprise over 3 % of the putative proteome of L. pneumophila (Lurie-Weinberger et al. 2010). A clear example is the eukaryote-like sphingosine-1-phosphate lyase of L. pneumophila described earlier. The phylogenetic analysis shown in Fig. 9.6 revealed that it was most likely acquired from a eukaryotic organism early during Legionella evolution (Degtyar et al. 2009; Gomez Valero et al. 2011; Nora et al. 2009) as the protein sequence of L. pneumophila clearly falls into the eukaryotic clade of SPL sequences. Similarly, as shown in Fig. 9.7 , phylogenetic analyses of an atypical member of the arylamine N-acetyltransferase family encoded in the L. pneumophila genomes, which allows this bacterium to detoxify aromatic amine chemicals and thus to grow in their presence, indicated that this gene has been acquired by horizontal gene transfer (Kubiak et al. 2012).
Phylogenetic tree of a multiple sequence comparison of sphingosine-phosphate lyase proteins present in eukaryotic and prokaryotic genomes. Phylogenetic reconstruction was done with MEGA using the neighbor-joining method. Numbers indicate bootstrap values after 1,000 bootstrap replicates. The red lines indicate the L. pneumophila sequences that are embedded in the eukaryotic clade. The bar at the bottom represents the estimated evolutionary distance (Gomez Valero et al. 2011; Nora et al. 2009)
Phylogenetic tree inferred from NAT amino acid sequences. The tree was constructed by Bayesian analysis. Numbers besides nodes are posterior probabilities recovered by the Bayesian analysis. Numbers in parentheses are bootstrap values based on 1,000 replicates in maximum likelihood analyses. Values below 50 % are not shown. The scale bar represents the estimated evolutionary distance (Kubiak et al. 2012)
Interaction between L. pneumophila and amoeba or more generally freshwater protozoa is central to the ecology and the pathogenesis of L. pneumophila. Thus, it is very likely that L. pneumophila has acquired some of its eukaryotic-like genes from amoeba. Interestingly, L. pneumophila is not the only prokaryote whose genome shows an enrichment of proteins with eukaryotic domains, but it seems to be a common feature of amoeba-associated bacteria. For example, Rickettsia bellii and Protochlamydia amoebophila, both bacteria that live inside amoeba, contain many eukaryotic-like genes, again arguing for acquisition of some of these genes from amoeba. Another example is the genome of “Ca. Amoebophilus asiaticus,” a Gram-negative, obligate intracellular amoeba symbiont whose genome encodes a large number of proteins with eukaryotic domains (Schmitz-Esser et al. 2010). To further investigate the distribution of these protein domains in other bacteria, an enrichment analysis comparing the fraction of all functional protein domains among 514 bacterial proteomes (Schmitz-Esser et al. 2010) has been undertaken. It revealed that the genomes of bacteria for which replication in amoeba has been demonstrated were enriched in protein domains that are predominantly found in eukaryotic proteins. Due to the phylogenetic diversity of these bacteria containing eukaryotic domains and proteins, it is most likely that these traits were acquired independently during evolutionary early interaction with ancient protozoa. Furthermore, comparison of the genome of R. bellii, an obligate intracellular pathogen of amoeba, revealed that many R. bellii ORFs (about 8 %) exhibit a high level of sequence similarity to homologues found in L. pneumophila and the amoeba-endosymbiont P. amoebophila (Ogata et al. 2005). This percentage is significantly higher than that seen for alphaproteobacteria that do not live within amoeba like Mesorhizobium loti (less than 1 %) and Pelagibacter ubique (about 2 %), suggesting that horizontal gene transfers among these different bacterial species may take place within amoeba and that amoebae constitute a gene melting pot, allowing diverse microorganisms to evolve by the same pathway characterized by gene acquisition, and then either adapt to the intra-amoebal lifestyle or create new pathogens (Moliner et al. 2009a, 2010).
Transcriptomics
L. pneumophila has developed a variety of strategies by which it adapts its genetic expression to meet the challenges of the ever-changing surrounding environment. These include specific sigma factors, two-component systems, quorum-sensing systems, repressors, positive regulators, as well as small regulatory RNAs. Alone or in combination, these mechanisms enable L. pneumophila to communicate with its environment, its hosts, and with each other, to adopt specific responses, express specific proteins, or develop specialized structures such as biofilms to ensure survival, colonization of their ecological niches, and dissemination. In the last years, microarray technology has been the method of choice for large-scale gene expression studies. It provides an efficient and rapid method to investigate the entire transcriptome of a cell. In vivo time course transcriptome analysis of L. pneumophila upon infection of amoeba and macrophages as well as in vitro analysis of different regulatory networks has been undertaken. Very recently, the advent of new generation sequencing allowed the application of RNA-seq to L. pneumophila, leading to new and exciting information about small noncoding RNAs and allowing to establish the precise transcriptional map.
Regulatory Repertoire of L. pneumophila
Consistent with the intracellular lifestyle, the regulatory repertoire of L. pneumophila is rather small as compared to other free-living bacteria, for example, Pseudomonas aeruginosa (Stover et al. 2000). Genome analysis identified in average 90 transcriptional regulators per sequenced genome, which represents only 3.0 % of the predicted genes. L. pneumophila encodes six putative sigma factors, the homologues of rpoD (the major sigma factor), rpoH, rpoS, rpoN, fliA, and the ECF-type sigma factor rpoE. With about 14 two-component systems (TCS) encoded in its genome, the number is also lower than that of free-living bacteria like E. coli that encodes 35. The most abundant class of regulators identified in the L. pneumophila genome belongs to the GGDEF/EAL family (over 20 per strain). Analyses of the regulatory proteins of L. pneumophila that contain domains related to cyclic diguanylate synthesis, hydrolysis, and recognition indicated that components of the cyclic diguanylate signaling pathway play an important role in regulating the ability of L. pneumophila to grow in host cells (Levi et al. 2011). Furthermore, one of these proteins was recently shown to belong to a new histidine kinase subfamily based on the conservation of an original H box that we named HGN H box and to be the first example of a bifunctional enzyme that modulates synthesis and turnover of c-di-GMP in response to phosphorylation of its receiver domain (Levet-Paulo et al. 2011). Key regulatory networks of L. pneumophila known to date include that of the two-component systems PmrA/PmrB (Zusman et al. 2007), CpxR/CpxA (Altman and Segal 2008), and LetA/LetS (Hammer et al. 2002), that of the sigma factor RpoS (σS) (Hovel-Miner et al. 2009) and the RNA-binding protein CsrA (Fettes et al. 2001; Molofsky and Swanson 2003), and probably that under the control of Hfq (McNealy et al. 2005).
Regulation of the Intracellular Life Cycle of L. pneumophila
L. pneumophila that reaches the alveolar space of the lungs is engulfed by macrophages. The pathogen inhibits phagosome acidification and fusion with lysosomes. L. pneumophila-containing phagosomes are completely isolated from the endosomal pathway and become surrounded by endoplasmic reticulum, and within these protected vacuoles, L. pneumophila converts to a replicative form. Once the vacuole is no longer favorable for replication, a regulatory cascade coordinates entry into the stationary phase with expression of traits that promote transmission to a new host cell (for a review, see Molofsky and Swanson 2004). Thus, it has been proposed that L. pneumophila cycles between an infectious, non-replicating form thought to promote transmission to a new host and an intracellular, replicative form, which does not express transmission traits (Byrne and Swanson 1998; Rowbotham 1986). This biphasic life cycle can be mimicked in broth culture with exponential (replicative) and postexponential (transmissive) grown bacteria (Molofsky and Swanson 2004). Adaptation of L. pneumophila is governed by a complex regulatory system. Current knowledge of these regulatory networks and the transcriptome responses of L. pneumophila are mainly based on microarray analysis (Brüggemann et al. 2006; Dalebroux et al. 2009, 2010; Faucher et al. 2011; Hovel-Miner et al. 2009, 2010; Sahr et al. 2009).
Genome-wide analyses of the transcriptional response of L. pneumophila grown in broth as well as inside Acanthamoeba castellanii showed that in vitro and inside the host cell L. pneumophila ensures a precise timing of its life cycle reflected by a major shift in gene expression from replicative to transmissive phase, concerning nearly half of the genes predicted in the genome (Brüggemann et al. 2006). Furthermore, in three different L. pneumophila strains – Paris, Philadelphia, and Lens – similar gene expression patterns were found, indicating that the Legionella life cycle is based on common regulatory mechanisms (Brüggemann et al. 2006). During the replicative phase, most parts of the carbohydrate, amino acid, fatty acid, and nucleotide uptake and/or metabolism were strongly expressed as well as genes encoding proteins of the respiratory chain, ATP synthesis, and ribosome biogenesis. This suggests the consumption of host nutrients by L. pneumophila to gain energy and components for DNA replication, protein biosynthesis, and lipid biosynthesis necessary for efficient multiplication. Traits necessary for bacterial entry and lysosome evasion (EnhC, RalF, LidA, VipA, etc.) or motility – in particular the most abundant flagellar protein FlaA – are not activated. These genes are expressed during transmissive phase together with many other substrates of the Dot/Icm secretion system, proteins related to UV, heat or osmotic stress response, the flagellar regulon, or numerous Legionella-specific yet unknown proteins (Brüggemann et al. 2006). Similarly, the analyses of the gene expression profile of L. pneumophila during infection of macrophage-like cells derived from the human THP-1 monocyte cell line found that genes involved in the metabolism of amino acids, lipids, carbohydrates, nucleotides, cofactors, and vitamins were induced inside cells; however, the authors report that this induction was independent of the time postinfection (Faucher et al. 2011). Furthermore, many transport systems involved in amino acid and iron uptake and genes involved in catabolism of glycerol were also induced during intracellular growth, suggesting that glycerol could be used as a carbon source. Like during amoeba infection, the genes encoding several translocated effectors were strongly induced (Faucher et al. 2011). Recently, Weissenmayer and colleagues used the RNA-seq technique to study the intracellular response of L. pneumophila at different time points. Their results largely confirmed the microarray results but also identified small ncRNAs induced during infection (Weissenmayer et al. 2011). How these different traits are activated and deactivated in Legionella is beginning to be examined in molecular detail, and the study of exponential and postexponential phase forms has provided valuable data about the regulatory networks that control life cycle-related phenotypic changes of L. pneumophila. Central in the regulation of the biphasic life cycle is the CsrA system of L. pneumophila that includes the two-component system LetA/LetS and two small noncoding RNAs (ncRNA) RsmY and RsmZ and in some strains also RsmX (Edwards et al. 2010; Fettes et al. 2001; Hammer et al. 2002; Molofsky and Swanson 2004; Rasis and Segal 2009; Sahr et al. 2009, 2012). Furthermore, the sigma factor RpoS, the RNA chaperone Hfq, the sigma 28 factor FliA, and the Lqs system have been shown to be part of this regulatory network (Bachman and Swanson 2004; Brüggemann et al. 2006; Hovel-Miner et al. 2009; McNealy et al. 2005; Tiaden et al. 2007, 2010b).
The LetA/LetS System and Posttranscriptional Regulation by CsrA
One of the best-studied TCS in Legionella is the LetA/LetS system. LetA/LetS has orthologous systems in many other Gram-negative bacteria, such as Salmonella enterica BarA/SirA, Erwinia carotovora ExpA/ExpS, Vibrio cholerae VarA/VarS, Pseudomonas spp. GacA/GacS, or E. coli UvrY/BarA (Babitzke and Romeo 2007; Cui et al. 2001; Kay et al. 2005; Lenz et al. 2005; Suzuki et al. 2002). letA and letS mutants are nonmotile, noncytotoxic, sodium sensitive, and less proficient in infecting macrophages; however, letA mutants still multiply in macrophage host cells (Gal-Mor and Segal 2003; Hammer et al. 2002). Furthermore, letA mutants are more sensitive to oxidative and acid stress than the wild type (Lynch et al. 2003), and infectivity of A. castellanii is reduced (Hammer et al. 2002; Lynch et al. 2003; Molofsky and Swanson 2004). The LetA/LetS TCS belongs to a family of signal-transducing proteins that employ a four-step phosphorelay to regulate gene expression. Histidine 307 of the LetS protein is the primary site of phosphorylation required to activate LetA (Edwards et al. 2010). Additionally, a threonine substitution at position 311 of LetS generated a L. pneumophila mutant with an intermediate phenotype (Edwards et al. 2010), in which gene expression of the flagellar regulon and numerous other loci was delayed when compared to wild-type bacteria (Edwards et al. 2010). A common feature of this family of TCS is that they regulate the expression of small noncoding RNAs (ncRNAs) that subsequently interact with proteins of the CsrA/RsmA family. Indeed, like in other bacteria where homologues of CsrA and LetA/LetS exist, two small ncRNAs (RsmY and RsmZ) are induced by the activation of LetA, and they link LetA and CsrA (Rasis and Segal 2009; Sahr et al. 2009). RsmY and RsmZ bind CsrA and antagonize its activity by sequestering CsrA. CsrA-mediated repression involves the binding of CsrA to the ribosome binding site of target transcripts, thereby blocking ribosome access to the mRNA. In contrast, activation by CsrA seems to be due to mRNA stabilization, similar to what is described for Escherichia coli. Thus, after detection of a yet unknown activating stimuli that trigger the sensor kinase LetS autophosphorylation, LetS activates LetA, leading to an increased transcription of RsmY and RsmZ. Subsequently, CsrA interacts with the specific loop structure of the two ncRNA containing a GGA binding motif and sequestering CsrA, thereby releasing it from its targets. Analysis of the transcriptional programs of the letA, letS, and rsmYZ double mutants in different growth phases revealed that the mutants are both blocked in the replicative phase, while the switch to the transmissive phase is partially blocked as judged by the downregulation of many transmission factors, such as Dot/Icm-secreted substrates. One major difference between the letA, letS, and rsmYZ double mutants was that the latter synthesizes flagella (Sahr et al. 2009). A regulatory link between the two RNA-binding proteins CsrA and Hfq has been proposed (McNealy et al. 2005), and both CsrA and Hfq are under the control of LetA and RpoS. Thus, it can be speculated that in Legionella – as it is described for E. coli – CsrA and Hfq might bind to the same ncRNA in a competitive or concomitant way. Also involved in the regulatory network governed by the TCS LetA/LetS is LetE, a small, Legionella-specific protein (Bachman and Swanson 2004; Hammer et al. 2002). L. pneumophila possesses also a homologue of CsrD of E. coli, which accordingly might be required for the decay of the regulatory RNAs sequestering CsrA.
The Regulatory Network Controlled by ppGpp and RpoS
The LetA/LetS two-component system of L. pneumophila probably responds to ppGpp (Bachman and Swanson 2004; Hammer et al. 2002; Molofsky and Swanson 2003). In accordance, the entry in the transmissive phase is initiated by a mechanism called “stringent response” (Jain et al. 2006). It was proposed that under conditions of nutrient starvation, signaled probably by low amino acid levels (Hammer and Swanson 1999), RelA synthesizes the alarmone molecule ppGpp (Hammer and Swanson 1999; Hammer et al. 2002; Zusman et al. 2002). In addition to RelA, Legionella encodes SpoT, a bifunctional enzyme with ppGpp hydrolysis and weak synthesis activity. SpoT senses perturbations in the fatty acid synthesis by binding to the acyl carrier protein ACP (Dalebroux et al. 2009). Thus, RelA, which senses amino acid starvation, and SpoT that monitors fatty acid biosynthesis together control the biphasic life cycle of L. pneumophila and promote expression of traits dedicated to the transmissive phase. relA mutants are unable to accumulate (p)ppGpp and transcribe several phase-dependent traits like motility or pigmentation only poorly even when reaching transmissive phase, but they survive and replicate efficiently in host cells (Zusman et al. 2002). In contrast, a relAspoT double mutant is strongly diminished in infectivity, indicating that a complete loss of (p)ppGpp has a severe effect on virulence and cytotoxicity and that transmissive phase bacteria require SpoT to reenter the replicative phase (Dalebroux et al. 2009). Many of the physiological effects of ppGpp are mediated through interactions with RNA polymerase (RNAP) in cooperation with the RNAP secondary channel interacting protein DksA (Potrykus and Cashel 2008). Transcriptional profiling of a L. pneumophila dksA mutant revealed that during transmission, alarmone accumulation increases the mRNA for flagellar and type IV secretion components, secreted host effectors and regulators, and decreases transcripts for translation, membrane modification, and the ATP synthesis machinery. DksA is critical for the life cycle switch, since mutants are defective for stationary phase survival, flagellar gene activation, lysosome avoidance, and macrophage cytotoxicity. Thus, DksA is thought to respond to the level of ppGpp and other stress signals to coordinate L. pneumophila differentiation (Dalebroux et al. 2010). Interestingly, LetA and RpoS are described to be regulated by (p)ppGpp (Abu-Zant et al. 2006; Hammer and Swanson 1999), connecting the second messenger and regulation of the mRNA-binding protein CsrA to a complex regulatory network.
RpoS (σS) is playing a dominant role during the life cycle of L. pneumophila as it has been shown to regulate a number of known virulence factors including many Icm/Dot effectors. RpoS is required for intracellular multiplication in amoeba and primary macrophages but not in macrophage-like cell lines, probably because of their reduced antimicrobial capacity (Abu-Zant et al. 2006; Hales and Shuman 1999b). Transcriptional profiling during exponential and postexponential growth of an rpoS mutant compared to the wild-type strain showed that RpoS affects distinct groups of genes that contribute to intracellular multiplication of L. pneumophila (Hovel-Miner et al. 2009). In particular, RpoS affects the expression of many genes encoding Icm/Dot substrates as well as genes encoding regulators required for intracellular multiplication. Furthermore, these analyses revealed that the arginine repressor ArgR is required for efficient intracellular multiplication (Hovel-Miner et al. 2009). Subsequent characterization of the L. pneumophila ArgR regulon by global gene expression analysis showed that ArgR repression is dependent upon the presence of L-arginine and demonstrated that ArgR-regulated genes are derepressed during intracellular growth. In addition to amino acid metabolism, the categories transport and binding, Icm/Dot-translocated substrates, nucleotide metabolism, and detoxification and stress adaptation were affected. These results suggest that L-arginine availability functions as a regulatory signal during intracellular growth (Hovel-Miner et al. 2010).
Most importantly, RpoS regulates the transcription of several regulators important for intracellular multiplication. First, expression of the two ncRNAs RsmY and RsmZ in L. pneumophila that regulate CsrA activity is RpoS dependent (Hovel-Miner et al. 2009; Sahr et al. 2009). Furthermore, RpoS affects the expression of FleQ and FliA (sigma 28) that are together with the sigma factor RpoN the major regulators of flagellum biosynthesis (Albert-Weissenberger et al. 2010). In a hierarchal and possibly temporal ordered mechanism, these two factors coordinate the synthesis and assembly of the different components indispensible for motility like FliA that is regulating the transcription of the most abundant flagellar protein, FlaA (Albert-Weissenberger et al. 2010). Additional important regulators under the control of RpoS are the TCS Cpx and Pmr. CpxA/CpxR directly control the regulation of dot/icm virulence genes (Altman and Segal 2008), and PmrA/PmrS is a global regulation system implicated in the regulation of the Dot/Icm type IV secretion system and intracellular growth of L. pneumophila (Al-Khodor et al. 2009; Zusman et al. 2007). Finally, it was shown that RpoS (and to a lesser extent also LetA) control LqsR (Tiaden et al. 2007). LqsR is part of a gene cluster homologous to the Vibrio cholera CqsAS quorum-sensing system. DNA microarray experiments revealed that LqsR regulates the expression of genes involved in virulence, motility, and cell division, consistent with a role for LqsR in the transition from the replicative to the transmissive phase. lqsR mutants are deficient in pathogen-host interactions and entry into the replicative growth phase (Tiaden and Hilbi 2012; Tiaden et al. 2007, 2008, 2010a). Taken together, RpoS is controlling regulatory elements for motility and virulence to express them both in a concerted manner. Thus, the ppGpp-RpoS-LetA network comprises the regulatory systems CsrA, LetE, PmrA, CpxR, or LqsR, which are all together of major importance for L. pneumophila to achieve optimal gene expression at each step of its life cycle.
The L. pneumophila Flagellum and the FliA (σ28) Sigma Factor
Transcriptomic analyses of L. pneumophila wild type showed that in in vitro and in vivo conditions, the flagellin-encoding gene flaA is upregulated up to 100 times in transmissive phase cells as compared to replicative cells. Similarly, several genes encoding proteins implicated in flagellum biosynthesis (e.g., fliS, fliD, fliN, flgBCDEFGHIJKL, fhF, fleN) and the fliA gene, encoding the sigma factor FliA (σ28) that regulates flaA gene expression, are strongly upregulated in the late phases of growth (Heuner et al. 1997). FliA is thought to be a main regulator of flagellar genes. Indeed, further transcriptional analysis of a fliA mutant as compared to the wt identified several FliA targets implicated in flagellum biosynthesis or motility (flaA, fliD, fliS, motY) (Brüggemann et al. 2006). Upstream of these genes, a FliA-binding consensus sequence was identified, suggesting direct regulation of these genes by FliA. From the expression profiling results, it appears that FliA controls only few targets. However, FliA also controls the expression of genes that were predicted to affect the first steps of cell invasion such as EnhA homologues or a GGDEF/EAL regulator, which may explain lower invasiveness and cytotoxicity of fliA mutants and points to an implication of this sigma factor in the infection process (Brüggemann et al. 2006). Further analyses of FleQ and RpoN mutants identified the enhancer-binding protein FleQ as the master regulator of the flagellar regulon (Albert-Weissenberger et al. 2010). Expression of FleQ is probably transcriptionally controlled by the σ70 factor and posttranscriptionally controlled by an unknown factor. Together with the σ54 factor RpoN, FleQ enhances flagellar class II gene transcription, and FleR and RpoN seem to couple protein biosynthesis and metabolism to the requirements of flagellar biosynthesis. Transcription of flagellar class III genes in L. pneumophila is solely enhanced by FleQ. As last step in flagellar biosynthesis, expression of the σ28 factor FliA (encoded by fliA) induces expression of flagellar class IV genes coding, for example, for flagellin which leads to the completion of the flagellum (Albert-Weissenberger et al. 2010; Brüggemann et al. 2006; Heuner et al. 1995, 1997, 2002; Heuner and Steinert 2003). FliA in L. pneumophila seems also to be responsible for a negative feedback loop on flagellar class II and III genes (Brüggemann et al. 2006). This negative control, as a response to the completion of the flagellum, may be an important mechanism used by the cell to turn off flagellar gene expression once the gene products are no longer needed.
The Transcriptional Map of L. pneumophila and Identification of Noncoding RNAs
The important implication of noncoding RNAs (ncRNAs) in regulatory processes in bacteria is becoming more and more evident as it has been shown in the last years that ncRNAs control adaptive responses and pathogenesis by regulation of gene expression via transcription interference and termination, translational interference, effects on the stability of target RNA, and interaction with RNA-binding proteins (for a recent review, see Storz et al. 2011). Noncoding RNAs are also important for L. pneumophila virulence as shown with the crucial role of RsmY and RsmZ in virulence and life cycle regulation and the implication of 6S RNA (Faucher et al. 2010; Faucher and Shuman 2011; Rasis and Segal 2009; Sahr et al. 2009). First studies attempting to identify noncoding RNAs in L. pneumophila used bioinformatics tools and microarray analyses (Faucher et al. 2010). This led to the prediction of 143 putative small RNAs, of which 22 were shown to be expressed as revealed by microarray analyses and six were confirmed by Northern blot and RACE (Faucher et al. 2010). One of these ncRNAs is the widely distributed 6S RNA, which was shown in E. coli to bind to RNA polymerase holoenzyme σ70 to inhibit transcription, leading to altered cell survival, perhaps by redirecting resource utilization under nutrient-limiting conditions (Wassarman 2007). The 6S RNA of L. pneumophila has also an important role, as it is necessary to optimize intracellular replication in regulating positively genes encoding type IV secretion system effectors (Faucher et al. 2010).
Recently, deep sequencing of cDNAs has emerged as a powerful tool to explore these ncRNAs and also to establish the complete transcriptional landscape of different organisms in a genome-wide manner (Wang et al. 2009). Weissenmayer and colleagues were the first to apply deep sequencing technologies to L. pneumophila, which allowed identifying 48 new ncRNAs (Weissenmayer et al. 2011). Thirty-three of these ncRNAs were at least partially complementary to genes encoding proteins, some being known virulence factors like Lpr0020 that is encoded antisense to a gene encoding a homologue of RtxA involved in intracellular survival and modification of trafficking (Cirillo et al. 2001, 2002). However, in addition to cis-encoded sRNAs, also trans-encoded sRNAs have been identified, and the next step will be now to identify the targets of these sRNAs.
A differential RNA sequencing approach was applied to establish the complete transcriptional map of L. pneumophila. The L. pneumophila operon map contains 623 transcriptional units comprising 1,791 genes organized in two to 32 genes, with an average operon size of 2.8 genes. 2561 primary transcriptional start sites (TSS) were identified. Interestingly, 187 of the 1,805 TSS of protein-coding genes contained tandem promoters, of which 93 show alternative usage dependent on the growth phase. Furthermore, 713 ncRNAs were identified, of which over 60 % are phase dependently regulated (Sahr et al. 2012). This study also identified a new ncRNA regulated by LetA that is part of the LetA-CsrA regulatory cascade, which was named RsmX (Sahr et al. 2012). In the near future, it will be important to characterize the specific roles of sRNA in L. pneumophila biology and to identify the sRNA targets and the phenotypes of the mutants that are defective in the expression of these sRNAs.
Metabolomics
L. pneumophila utilizes amino acids as carbon and preferred energy sources (George et al. 1980; Ristroph et al. 1981; Tesh and Miller 1981; Tesh et al. 1983). Underscoring the importance of amino acid metabolism for L. pneumophila, bacterial or host cell amino acid transporters are required for intracellular growth (Sauer et al. 2005; Wieland et al. 2005). However, L. pneumophila also utilizes carbohydrates as carbon sources. In agreement with this notion, glucose was found to have no effect on growth of L. pneumophila in defined media (Pine et al. 1979; Warren and Miller 1979), yet early radiorespirometry studies already suggested that [14C]glucose is consumed and metabolized to pyruvate primarily by the Entner-Doudoroff (ED) and/or the pentose phosphate (PP) pathway, rather than by the Embden-Meyerhof-Parnas (EMP) glycolytic pathway (Tesh et al. 1983). Recent data indicate that intracellular L. pneumophila indeed partakes in glucose utilization (Eylert et al. 2010; Harada et al. 2010). Finally, fatty acids are another important determinant of intracellular growth (Edwards et al. 2009).
The completed genome sequences of Legionella spp. provided insights into how these bacteria metabolize carbohydrates (Cazalet et al. 2004, 2010; Chien et al. 2004; D’Auria et al. 2010; Glockner et al. 2008; Gomez-Valero et al. 2011; Kozak et al. 2010; Moliner et al. 2009b; Schroeder et al. 2010). The genomes of L. pneumophila as well as L. longbeachae encode complete pathways required for metabolism of carbohydrates, including the EMP, the ED, and the PP pathway. Moreover, transcriptome studies revealed that during the replicative phase in infected Acanthamoeba castellanii, L. pneumophila upregulated genes of the ED pathway in addition to components of the respiratory chain and amino acid (Brüggemann et al. 2006). Thus, intracellular bacteria apparently utilize not only proteins and peptides as nutrients but also host carbohydrates.
The catabolism of glucose by L. pneumophila grown in broth was studied by isotopologue profiling (Eylert et al. 2010). Using [U-13C6]glucose, these metabolomic experiments revealed that the carbohydrate is indeed used as a carbon source, and the label accumulated in different amino acids, as well as in the storage compound poly-3-hydroxybutyrate (PHB). Moreover, the distinct labeling patterns obtained with [1,2-13C2]glucose identified the ED pathway as the predominant route for glucose amino acid utilization. Accordingly, L. pneumophila lacking key components of the ED pathway failed to metabolize glucose. An L. pneumophila strain deleted for glucose-6-phosphate dehydrogenase encoded by zwf, the first gene of an operon comprising the genes of the ED pathway (zwf-pgl-edd-glk-eda-ywtG), did not incorporate label from glucose and was outcompeted by the wild-type strain in coinfection experiments using A. castellanii (Eylert et al. 2010). In line with these observations, L. pneumophila lacking other components of the ED pathway, either glucokinase (glk), phosphogluconate dehydratase (edd), 2-keto-3-deoxy-phosphogluconate (KDPG) aldolase (eda), or a putative sugar transporter (ywtG), was no longer able to metabolize glucose and was defective for growth in Acanthamoeba culbertsoni or mammalian cells (Harada et al. 2010). Therefore, the ED pathway is essential for glucose metabolism and intracellular growth of L. pneumophila. The results also implicate that the conditions prevailing in host cells within LCVs do not allow L. pneumophila to solely grow on amino acids as carbon and energy sources.
Legionella spp. also degrade more complex carbohydrates. L. longbeachae harbors a number of genes likely involved in cellulose degradation (Cazalet et al. 2010), and L. pneumophila contains genes putatively involved in the degradation of cellulose, chitin, starch, and glycogen (Brüggemann et al. 2006). Moreover, as outlined above, L. pneumophila secretes via the T2SS a chitinase, as well as an endoglucanase, which metabolizes carboxymethyl cellulose (DebRoy et al. 2006b). An endoglucanase (CelA) was indeed found to degrade cellulose (Pearce and Cianciotto 2009, p. 48), and a eukaryotic-like glucoamylase (GamA) degraded carboxymethyl cellulose, glycogen, or starch (Herrmann et al. 2011). Yet, neither CelA nor GamA was required for growth of L. pneumophila in amoebae. In summary, insights from metabolomics, genomics, transcriptomics, as well as biochemical experiments indicate that L. pneumophila utilizes simple and complex carbohydrates as important sources of carbon and energy during extra- and intracellular growth.
References
Abdelaziz DH, Gavrilin MA, Akhter A, Caution K, Kotrange S, Khweek AA, Abdulrahman BA, Grandhi J, Hassan ZA, Marsh C, Wewers MD, Amer AO (2011) Apoptosis-associated speck-like protein (ASC) controls Legionella pneumophila infection in human monocytes. J Biol Chem 286:3203–3208
Abu Kwaik Y (1998) Fatal attraction of mammalian cells to Legionella pneumophila. Mol Microbiol 30:689–695
Abu Kwaik Y (1996) The phagosome containing Legionella pneumophila within the protozoan Hartmannella vermiformis is surrounded by the rough endoplasmic reticulum. Appl Environ Microbiol 62:2022–2028
Abu Kwaik Y, Eisenstein BI, Engleberg NC (1993) Phenotypic modulation by Legionella pneumophila upon infection of macrophages. Infect Immun 61:1320–1329
Abu-Zant A, Asare R, Graham JE, Abu Kwaik Y (2006) Role for RpoS but not RelA of Legionella pneumophila in modulation of phagosome biogenesis and adaptation to the phagosomal microenvironment. Infect Immun 74:3021–3026
Adeleke A, Pruckler J, Benson R, Rowbotham T, Halablab M, Fields B (1996) Legionella-like amebal pathogens–phylogenetic status and possible role in respiratory disease. Emerg Infect Dis 2:225–230
Adeleke AA, Fields BS, Benson RF, Daneshaver MI, Pruckler JM, Ratcliff RM, Harrison TG, Weyant RS, Birtles RJ, Raoult D, Halablab MA (2001) Legionella drozanskii sp. nov., Legionella rowbathamii sp. nov. and Legionella fallonii sp. nov.: three unusual new Legionella species. Int J Syst Evol Microbiol 51:1151–1160
Akamine M, Higa F, Arakaki N, Kawakami K, Takeda K, Akira S, Saito A (2005) Differential roles of Toll-like receptors 2 and 4 in in vitro responses of macrophages to Legionella pneumophila. Infect Immun 73:352–361
Aktories K (2011) Bacterial protein toxins that modify host regulatory GTPases. Nat Rev Microbiol 9:487–498
Al-Khodor S, Kalachikov S, Morozova I, Price CT, Abu Kwaik Y (2009) The PmrA/PmrB two-component system of Legionella pneumophila is a global regulator required for intracellular replication within macrophages and protozoa. Infect Immun 77:374–386
Al-Khodor S, Price CT, Habyarimana F, Kalia A, Abu Kwaik Y (2008) A Dot/Icm-translocated ankyrin protein of Legionella pneumophila is required for intracellular proliferation within human macrophages and protozoa. Mol Microbiol 70:908–923
Al-Quadan T, Kwaik YA (2011) Molecular characterization of exploitation of the polyubiquitination and farnesylation machineries of Dictyostelium Discoideum by the AnkB F-Box effector of Legionella Pneumophila. Front Microbiol 2:23
Al-Quadan T, Price CT, Abu Kwaik Y (2012) Exploitation of evolutionarily conserved amoeba and mammalian processes by Legionella. Trends Microbiol 20:299–306
Al-Quadan T, Price CT, London N, Schueler-Furman O, Abu Kwaik Y (2011) Anchoring of bacterial effectors to host membranes through host-mediated lipidation by prenylation: a common paradigm. Trends Microbiol 19:573–579
Alary M, Joly JR (1991) Risk factors for contamination of domestic hot water systems by legionellae. Appl Environ Microbiol 57:2360–2367
Albert-Weissenberger C, Cazalet C, Buchrieser C (2007) Legionella pneumophila – a human pathogen that co-evolved with fresh water protozoa. Cell Mol Life Sci 64:432–448
Albert-Weissenberger C, Sahr T, Sismeiro O, Hacker J, Heuner K, Buchrieser C (2010) Control of flagellar gene regulation in Legionella pneumophila and its relation to growth phase. J Bacteriol 192:446–455
Allard KA, Dao J, Sanjeevaiah P, McCoy-Simandle K, Chatfield CH, Crumrine DS, Castignetti D, Cianciotto NP (2009) Purification of legiobactin and the importance of this siderophore in lung infection by Legionella pneumophila. Infect Immun 77:2887–2895
Allard KA, Viswanathan VK, Cianciotto NP (2006) lbtA and lbtB are required for production of the Legionella pneumophila siderophore legiobactin. J Bacteriol 188:1351–1363
Allegra S, Berger F, Berthelot P, Grattard F, Pozzetto B, Riffard S (2008) Use of flow cytometry to monitor Legionella viability. Appl Environ Microbiol 74:7813–7816
Alli OA, Zink S, von Lackum NK, Abu-Kwaik Y (2003) Comparative assessment of virulence traits in Legionella spp. Microbiology 149:631–641
Altman E, Segal G (2008) The response regulator CpxR directly regulates expression of several Legionella pneumophila icm/dot components as well as new translocated substrates. J Bacteriol 90:1985–1996
Alvarez SE, Milstien S, Spiegel S (2007) Autocrine and paracrine roles of sphingosine-1-phosphate. Trends Endocrinol Metab 18:300–307
Amaro F, Gilbert JA, Owens S, Trimble W, Shuman HA (2012) Whole-genome sequence of the human pathogen Legionella pneumophila serogroup 12 strain 570-CO-H. J Bacteriol 194:1613–1614
Amemura-Maekawa J, Kura F, Helbig JH, Chang B, Kaneko A, Watanabe Y, Isobe J, Nukina M, Nakajima H, Kawano K, Tada Y, Watanabe H (2010) Characterization of Legionella pneumophila isolates from patients in Japan according to serogroups, monoclonal antibody subgroups and sequence types. J Med Microbiol 59:653–659
Amemura-Maekawa J, Mishima-Abe S, Kura F, Takahashi T, Watanabe H (1999) Identification of a novel periplasmic catalase-peroxidase KatA of Legionella pneumophila. FEMS Microbiol Lett 176:339–344
Amer A, Franchi L, Kanneganti TD, Body-Malapel M, Ozoren N, Brady G, Meshinchi S, Jagirdar R, Gewirtz A, Akira S, Nunez G (2006) Regulation of Legionella phagosome maturation and infection through flagellin and host Ipaf. J Biol Chem 281:35217–35223
Amer AO, Byrne BG, Swanson MS (2005) Macrophages rapidly transfer pathogens from lipid raft vacuoles to autophagosomes. Autophagy 1:53–58
Amer AO, Swanson MS (2005) Autophagy is an immediate macrophage response to Legionella pneumophila. Cell Microbiol 7:765–778
Anand CM, Skinner AR, Malic A, Kurtz JB (1983) Interaction of L. pneumophila and a free living amoeba (Acanthamoeba palestinensis). J Hyg (Cambridge) 91:167–178
Andrews HL, Vogel JP, Isberg RR (1998) Identification of linked Legionella pneumophila genes essential for intracellular growth and evasion of the endocytic pathway. Infect Immun 66:950–958
Ang DK, Oates CV, Schuelein R, Kelly M, Sansom FM, Bourges D, Boon L, Hertzog PJ, Hartland EL, van Driel IR (2010) Cutting edge: pulmonary Legionella pneumophila is controlled by plasmacytoid dendritic cells but not type I IFN. J Immunol 184:5429–5433
Aragon V, Kurtz S, Cianciotto NP (2001) Legionella pneumophila major acid phosphatase and its role in intracellular infection. Infect Immun 69:177–185
Aragon V, Kurtz S, Flieger A, Neumeister B, Cianciotto NP (2000) Secreted enzymatic activities of wild-type and pilD-deficient Legionella pneumophila. Infect Immun 68:1855–1863
Aragon V, Rossier O, Cianciotto NP (2002) Legionella pneumophila genes that encode lipase and phospholipase C activities. Microbiology 148:2223–2231
Arasaki K, Roy CR (2010) Legionella pneumophila promotes functional interactions between plasma membrane syntaxins and Sec22b. Traffic 11:587–600
Arasaki K, Toomre DK, Roy CR (2012) The Legionella pneumophila effector DrrA is sufficient to stimulate SNARE-dependent membrane fusion. Cell Host Microbe 11:46–57
Archer KA, Ader F, Kobayashi KS, Flavell RA, Roy CR (2010) Cooperation between multiple microbial pattern recognition systems is important for host protection against the intracellular pathogen Legionella pneumophila. Infect Immun 78:2477–2487
Archer KA, Alexopoulou L, Flavell RA, Roy CR (2009) Multiple MyD88-dependent responses contribute to pulmonary clearance of Legionella pneumophila. Cell Microbiol 11:21–36
Archer KA, Roy CR (2006) MyD88-dependent responses involving toll-like receptor 2 are important for protection and clearance of Legionella pneumophila in a mouse model of Legionnaires’ disease. Infect Immun 74:3325–3333
Arnow PM, Chou T, Weil D, Shapiro EN, Kretzschmar C (1982) Nosocomial Legionnaires disease caused by aerosolized tap water from respiratory devices. J Infect Dis 146:460–467
Asare R, Abu Kwaik Y (2007) Early trafficking and intracellular replication of Legionella longbeachae within an ER-derived late endosome-like phagosome. Cell Microbiol 9:1571–1587
Atlas RM (1999) Legionella: from environmental habitats to disease pathology, detection and control. Environ Microbiol 1:283–293
Aurass P, Pless B, Rydzewski K, Holland G, Bannert N, Flieger A (2009) bdhA-patD operon as a virulence determinant, revealed by a novel large-scale approach for identification of Legionella pneumophila mutants defective for amoeba infection. Appl Environ Microbiol 75:4506–4515
Aurell H, Etienne J, Forey F, Reyrolle M, Girardo P, Farge P, Decludt B, Campese C, Vandenesch F, Jarraud S (2003) Legionella pneumophila serogroup 1 strain Paris: endemic distribution throughout France. J Clin Microbiol 41:3320–3322
Babitzke P, Romeo T (2007) CsrB sRNA family: sequestration of RNA-binding regulatory proteins. Curr Opin Microbiol 10:156–163
Bachman MA, Swanson MS (2004) Genetic evidence that Legionella pneumophila RpoS modulates expression of the transmission phenotype in both the exponential phase and the stationary phase. Infect Immun 72:2468–2476
Baine WB, Rasheed JK (1979) Aromatic substrate specificity of browning by cultures of the Legionnaires’ disease bacterium. Ann Int Med 90:619–620
Baine WB, Rasheed JK, Feeley JC, Gorman GW, Casida LE (1978) Effect of supplemental L-tyrosine on pigment production in cultures of the Legionnaires’ disease bacterium. Curr Microbiol 1:93–94
Ballard AL, Fry NK, Chan L, Surman SB, Lee JV, Harrison TG, Towner KJ (2000) Detection of Legionella pneumophila using a real-time PCR hybridization assay. J Clin Microbiol 38:4215–4218
Bandhuvula P, Saba JD (2007) Sphingosine-1-phosphate lyase in immunity and cancer: silencing the siren. Trends Mol Med 13:210–217
Bandyopadhyay P, Byrne B, Chan Y, Swanson MS, Steinman HM (2003) Legionella pneumophila catalase-peroxidases are required for proper trafficking and growth in primary macrophages. Infect Immun 71:4526–4535
Bandyopadhyay P, Liu S, Gabbai CB, Venitelli Z, Steinman HM (2007) Environmental mimics and the Lvh Type IVA secretion system contribute to virulence-related phenotypes of Legionella pneumophila. Infect Immun 75:723–735
Bandyopadhyay P, Steinman HM (2000) Catalase-peroxidases of Legionella pneumophila: cloning of the katA gene and studies of KatA function. J Bacteriol 182:6679–6686
Bandyopadhyay P, Steinman HM (1998) Legionella pneumophila catalase-peroxidase: cloning of the katB gene and studies of KatB function. J Bacteriol 180:5369–5374
Banerji S, Aurass P, Flieger A (2008) The manifold phospholipases A of Legionella pneumophila - identification, export, regulation, and their link to bacterial virulence. Int J Med Microbiol 298:169–181
Banerji S, Bewersdorff M, Hermes B, Cianciotto NP, Flieger A (2005) Characterization of the major secreted zinc metalloprotease-dependent glycerophospholipid:cholesterol acyltransferase, PlaC, of Legionella pneumophila. Infect Immun 73:2899–2909
Barbaree JM, Fields BS, Feeley JC, Gorman GW, Martin WT (1986) Isolation of protozoa from water associated with a legionellosis outbreak and demonstration of intracellular multiplication of Legionella pneumophila. Appl Environ Microbiol 51:422–424
Barker J, Brown MR, Collier PJ, Farrell I, Gilbert P (1992) Relationship between Legionella pneumophila and Acanthamoeba polyphaga: physiological status and susceptibility to chemical inactivation. Appl Environ Microbiol 58:2420–2425
Barker J, Lambert PA, Brown MR (1993) Influence of intra-amoebic and other growth conditions on the surface properties of Legionella pneumophila. Infect Immun 61:3503–3510
Barrabeig I, Rovira A, Garcia M, Oliva JM, Vilamala A, Ferrer MD, Sabria M, Dominguez A (2010) Outbreak of Legionnaires’ disease associated with a supermarket mist machine. Epidemiol Infect 138:1823–1828
Bartfeld S, Engels C, Bauer B, Aurass P, Flieger A, Bruggemann H, Meyer TF (2009) Temporal resolution of two-tracked NF-kappaB activation by Legionella pneumophila. Cell Microbiol 11:1638–1651
Baskerville A, Conlan JW, Ashworth LA, Dowsett AB (1986) Pulmonary damage caused by a protease from Legionella pneumophila. Br J Exp Pathol 67:527–536
Baskerville A, Fitzgeorge RB, Broster M, Hambleton P (1983) Histopathology of experimental Legionnaires’ disease in guinea pigs, rhesus monkeys and marmosets. J Pathol 139:349–362
Bauer M, Mathieu L, Deloge-Abarkan M, Remen T, Tossa P, Hartemann P, Zmirou-Navier D (2008) Legionella bacteria in shower aerosols increase the risk of Pontiac fever among older people in retirement homes. J. Epidemiol. Commun. Health 62:913–920
Beckers MC, Yoshida S, Morgan K, Skamene E, Gros P (1995) Natural resistance to infection with Legionella pneumophila: chromosomal localization of the Lgn1 susceptibility gene. Mamm Genome 6:540–545
Beigel F, Jurgens M, Filik L, Bader L, Luck C, Goke B, Ochsenkuhn T, Brand S, Seiderer J (2009) Severe Legionella pneumophila pneumonia following infliximab therapy in a patient with Crohn’s disease. Inflamm Bowel Dis 15:1240–1244
Bellinger-Kawahara C, Horwitz MA (1990) Complement component C3 fixes selectively to the major outer membrane protein (MOMP) of Legionella pneumophila and mediates phagocytosis of liposome-MOMP complexes by human monocytes. J Exp Med 172:1201–1210
Belyi I, Popoff MR, Cianciotto NP (2003) Purification and characterization of a UDP-glucosyltransferase produced by Legionella pneumophila. Infect Immun 71:181–186
Belyi Y, Jank T, Aktories K (2011) Effector glycosyltransferases in Legionella. Front Microbiol 2:76
Belyi Y, Niggeweg R, Opitz B, Vogelsgesang M, Hippenstiel S, Wilm M, Aktories K (2006) Legionella pneumophila glucosyltransferase inhibits host elongation factor 1A. Proc Natl Acad Sci USA 103:16953–16958
Belyi Y, Tabakova I, Stahl M, Aktories K (2008) Lgt: a family of cytotoxic glucosyltransferases produced by Legionella pneumophila. J Bacteriol 190:3026–3035
Bender J, Rydzewski K, Broich M, Schunder E, Heuner K, Flieger A (2009) Phospholipase PlaB of Legionella pneumophila represents a novel lipase family: protein residues essential for lipolytic activity, substrate specificity, and hemolysis. J Biol Chem 284:27185–27194
Benin AL, Benson RF, Arnold KE, Fiore AE, Cook PG, Williams LK, Fields B, Besser RE (2002a) An outbreak of travel-associated Legionnaires disease and Pontiac fever: the need for enhanced surveillance of travel-associated legionellosis in the United States. J Infect Dis 185:237–243
Benin AL, Benson RF, Besser RE (2002b) Trends in Legionnaires disease, 1980-1998: declining mortality and new patterns of diagnosis. Clin Infect Dis 35:1039–1046
Benson RF, Fields BS (1998) Classification of the genus Legionella. Semin Respir Infect 13:90–99
Benson RF, Thacker WL, Daneshvar MI, Brenner DJ (1996) Legionella waltersii sp. nov. and an unnamed Legionella genomospecies isolated from water in Australia. Int J Syst Bacteriol 46:631–634
Benson RF, Thacker WL, Fang FC, Kanter B, Mayberry WR, Brenner DJ (1990) Legionella sainthelensi serogroup 2 isolated from patients with pneumonia. Res Microbiol 141:453–463
Benson RF, Thacker WL, Lanser JA, Sangster N, Mayberry WR, Brenner DJ (1991) Legionella adelaidensis, a new species isolated from cooling tower water. J Clin Microbiol 29:1004–1006
Benson RF, Thacker WL, Waters RP, Quinlivan PA, Mayberry WR, Brenner DJ, Wilkinson HW (1989) Legionella quinlivanii sp. nov. isolated from water. Curr Microbiol 18:195–197
Bercovier H, Fattal B, Shuval H (1986a) Seasonal distribution of legionellae isolated from various types of water in Israel. Isr J Med Sci 22:644–646
Bercovier H, Steigerwalt AG, Derhi-Cochin M, Moss CW, Wilkinson HW, Benson RF, Brenner DJ (1986b) Isolation of legionellae from oxidation ponds and fishponds in Israel and description of Legionella israelensis sp. nov. Int J Syst Bacteriol 36:368–371
Berendt RF, Young HW, Allen RG, Knutsen GL (1980) Dose-response of guinea pigs experimentally infected with aerosols of Legionella pneumophila. J Infect Dis 141:186–192
Berg JD, Hoff JC, Roberts PV, Matin A (1985) Growth of Legionella pneumophila in continuous culture. Appl Environ Microbiol 49:1534–1537
Berger KH, Isberg RR (1993) Two distinct defects in intracellular growth complemented by a single genetic locus in Legionella pneumophila. Mol Microbiol 7:7–19
Berger KH, Merriam JJ, Isberg RR (1994) Altered intracellular targeting properties associated with mutations in the Legionella pneumophila dotA gene. Mol Microbiol 14:809–822
Berger P, Papazian L, Drancourt M, La Scola B, Auffray JP, Raoult D (2006) Ameba-associated microorganisms and diagnosis of nosocomial pneumonia. Emerg Infect Dis 12:248–255
Berk SG, Gunderson JH, Newsome AL, Farone AL, Hayes BJ, Redding KS, Uddin N, Williams EL, Johnson RA, Farsian M, Reid A, Skimmyhorn J, Farone MB (2006) Occurrence of infected amoebae in cooling towers compared with natural aquatic environments: implications for emerging pathogens. Environ Sci Technol 40:7440–7444
Berk SG, Ting RS, Turner GW, Ashburn RJ (1998) Production of respirable vesicles containing live Legionella pneumophila cells by two Acanthamoeba spp. Appl Environ Microbiol 64:279–286
Berrington WR, Iyer R, Wells RD, Smith KD, Skerrett SJ, Hawn TR (2010) NOD1 and NOD2 regulation of pulmonary innate immunity to Legionella pneumophila. Eur J Immunol 40:3519–3527
Bezanson G, Burbridge S, Haldane D, Marrie T (1992) In situ colonization of polyvinyl chloride, brass, and copper by Legionella pneumophila. Can J Microbiol 38:328–330
Bhardwaj N, Nash TW, Horwitz MA (1986) Interferon-gamma-activated human monocytes inhibit the intracellular multiplication of Legionella pneumophila. J Immunol 137:2662–2669
Bhopal RS, Fallon RJ, Buist EC, Black RJ, Urquhart JD (1991) Proximity of the home to a cooling tower and risk of non-outbreak Legionnaires’ disease. Brit Med J 302:378–383
Birtles RJ, Harrison TG, Samuel D, Taylor AG (1990) Evaluation of urinary antigen ELISA for diagnosing Legionella pneumophila serogroup 1 infection. J Clin Pathol 43:685–690
Birtles RJ, Rowbotham TJ, Raoult D, Harrison TG (1996) Phylogenetic diversity of intra-amoebal legionellae as revealed by 16S rRNA gene sequence comparison. Microbiology 142:3525–3530
Blair JM, Piddock LJ (2009) Structure, function and inhibition of RND efflux pumps in Gram-negative bacteria: an update. Curr Opin Microbiol 12:512–519
Blanchard DK, Friedman H, Stewart WE, 2nd, Klein TW, Djeu JY (1988b) Role of gamma interferon in induction of natural killer activity by Legionella pneumophila in vitro and in an experimental murine infection model. Infect Immun 56:1187–1193
Blanchard DK, Djeu JY, Klein TW, Friedman H, Stewart WE, 2nd (1988a) Protective effects of tumor necrosis factor in experimental Legionella pneumophila infections of mice via activation of PMN function. J Leukoc Biol 43:429–435
Blanchard DK, Djeu JY, Klein TW, Friedman H, Stewart WE, II (1987) Induction of tumor necrosis factor by Legionella pneumophila. Infect Immun 55:433–437
Blanchard DK, Friedman H, Klein TW, Djeu JY (1989) Induction of interferon-gamma and tumor necrosis factor by Legionella pneumophila: augmentation of human neutrophil bactericidal activity. J Leukoc Biol 45:538–545
Blander SJ, Szeto L, Shuman HA, Horwitz MA (1990) An immunoprotective molecule, the major secretory protein of Legionella pneumophila, is not a virulence factor in a guinea pig model of Legionnaires’ disease. J Clin Invest 86:817–824
Blatt SP, Parkinson MD, Pace E, Hoffman P, Dolan D, Lauderdale P, Zajac RA, Melcher GP (1993) Nosocomial Legionnaires’ disease: aspiration as a primary mode of disease acquisition. Am J Med 95:16–22
Blázquez RM, Espinosa FJ, Martínez-Toldos CM, Alemany L, García-Orenes MC, Segovia M (2005) Sensitivity of urinary antigen test in relation to clinical severity in a large outbreak of Legionella pneumonia in Spain. Eur J Clin Microbiol Infect Dis 24:488–491
Bohte R, van Furth R, van den Broek PJ (1995) Aetiology of community-acquired pneumonia: a prospective study among adults requiring admission to hospital. Thorax 50:543–547
Borchardt J, Helbig JH, Luck PC (2008) Occurrence and distribution of sequence types among Legionella pneumophila strains isolated from patients in Germany: common features and differences to other regions of the world. Eur J Clin Microbiol Infect Dis 27:29–36
Bornstein N, Fleurette J, Bosshard S, Bouvet C, Thouvenot D, Aymard M (1984) Evaluation of the incidence of serological cross reactions between Legionella and Mycoplasma or Chlamydia. Pathol Biol (Paris) 32:165–168
Bornstein N, Marmet D, Surgot M, Nowicki M, Meugnier H, Fleurette J, Ageron E, Grimont F, Grimont PA, Thacker WL, Benson RF, Brenner DJ (1989) Legionella gratiana sp. nov. isolated from French spa water. Res Microbiol 140:541–552
Boswell TC, Marshall LE, Kudesia G (1996) False-positive Legionella titres in routine clinical serology testing detected by absorption with Campylobacter: implications for the serological diagnosis of legionnaires' disease. J Infect 32:23–26
Bouyer S, Imbert C, Rodier MH, Hechard Y (2007) Long-term survival of Legionella pneumophila associated with Acanthamoeba castellanii vesicles. Environ Microbiol 9:1341–1344
Bozue JA, Johnson W (1996) Interaction of Legionella pneumophila with Acanthamoeba castellanii: uptake by coiling phagocytosis and inhibition of phagosome-lysosome fusion. Infect Immun 64:668–673
Braedel-Ruoff S, Faigle M, Hilf N, Neumeister B, Schild H (2005) Legionella pneumophila mediated activation of dendritic cells involves CD14 and TLR2. J Endotoxin Res 11:89–96
Brand BC, Sadosky AB, Shuman HA (1994) The Legionella pneumophila icm locus: a set of genes required for intracellular multiplication in human macrophages. Mol Microbiol 14:797–808
Brassinga AK, Hiltz MF, Sisson GR, Morash MG, Hill N, Garduno E, Edelstein PH, Garduno RA, Hoffman PS (2003) A 65-kilobase pathogenicity island is unique to philadelphia-1 strains of Legionella pneumophila. J Bacteriol 185:4630–3637
Brassinga AK, Kinchen JM, Cupp ME, Day SR, Hoffman PS, Sifri CD (2010) Caenorhabditis is a metazoan host for Legionella. Cell Microbiol 12:343–361
Breiman RF, Butler JC (1998) Legionnaires’ disease: clinical, epidemiological, and public health perspectives. Semin Respir Infect 13:84–89
Breiman RF, Cozen W, Fields BS, Mastro TD, Carr SJ, Spika JS, Mascola L (1990a) Role of air sampling in investigation of an outbreak of legionnaires’ disease associated with exposure to aerosols from an evaporative condenser. J Infect Dis 161:1257–1261
Breiman RF, Fields BS, Sanden GN, Volmer L, Meier A, Spika JS (1990b) Association of shower use with Legionnaires’ disease. Possible role of amoebae. JAMA 263:2924–2926
Brenner DJ (1987) Classification of the Legionellae. Sem Resp Infect 2:190–205
Brenner DJ, Steigerwalt AG, Gorman GW, Weaver RE, Feeley JC, Cordes LG, Wilkinson HW, Patton C, Thomason BM, Lewallen-Sasseville KR (1980) Legionella bozemanii sp. nov. and Legionella dumoffii sp. nov.: classification of two additional species of Legionella associated with human pneumonia. Curr Microbiol 4:111–116
Brenner DJ, Steigerwalt AG, Gorman GW, Wilkinson HW, Bibb WF, Hackel M, Tyndall RL, Campbell J, Feeley JC, Thacker WL, Skaliy P, Martin WT, Brake BJ, Fields BS, McEachern HV, Corcoran LK (1985) Ten new species of Legionella. Int J Syst Bacteriol 35:50–59
Brenner DJ, Steigerwalt AG, McDade JE (1979) Classification of the Legionnaires’ disease bacterium: Legionella pneumophila, genus novum, species nova, of the family Legionellaceae, familia nova. Ann Intern Med 90:656–658
Brieland J, Freeman P, Kunkel R, Chrisp C, Hurley M, Fantone J, Engleberg C (1994) Replicative Legionella pneumophila lung infection in intratracheally inoculated A/J mice. A murine model of human Legionnaires’ disease. Am J Pathol 145:1537–1546
Brieland J, McClain M, Heath L, Chrisp C, Huffnagle G, LeGendre M, Hurley M, Fantone J, Engleberg C (1996) Coinoculation with Hartmannella vermiformis enhances replicative Legionella pneumophila lung infection in a murine model of Legionnaires’ disease. Infect Immun 64:2449–2456
Brieland J, McClain M, LeGendre M, Engleberg C (1997a) Intrapulmonary Hartmannella vermiformis: a potential niche for Legionella pneumophila replication in a murine model of legionellosis. Infect Immun 65:4892–4896
Brieland JK, Fantone JC, Remick DG, LeGendre M, McClain M, Engleberg NC (1997b) The role of Legionella pneumophila-infected Hartmannella vermiformis as an infectious particle in a murine model of Legionnaire’s disease. Infect Immun 65:5330–5333
Brieland JK, Jackson C, Hurst S, Loebenberg D, Muchamuel T, Debets R, Kastelein R, Churakova T, Abrams J, Hare R, O'Garra A (2000) Immunomodulatory role of endogenous interleukin-18 in gamma interferon-mediated resolution of replicative Legionella pneumophila lung infection. Infect Immun 68:6567–6573
Brieland JK, Remick DG, Freeman PT, Hurley MC, Fantone JC, Engleberg NC (1995) In vivo regulation of replicative Legionella pneumophila lung infection by endogenous tumor necrosis factor alpha and nitric oxide. Infect Immun 63:3253–3258
Brieland JK, Remick DG, LeGendre ML, Engleberg NC, Fantone JC (1998) In vivo regulation of replicative Legionella pneumophila lung infection by endogenous interleukin-12. Infect Immun 66:65–69
Brombacher E, Urwyler S, Ragaz C, Weber SS, Kami K, Overduin M, Hilbi H (2009) Rab1 guanine nucleotide exchange factor SidM is a major phosphatidylinositol 4-phosphate-binding effector protein of Legionella pneumophila. J Biol Chem 284:4846–4856
Broome CV, Fraser DW (1979) Epidemiologic aspects of legionellosis. Epidemiol Rev 1:1–16
Bruggemann H, Cazalet C, Buchrieser C (2006) Adaptation of Legionella pneumophila to the host environment: role of protein secretion, effectors and eukaryotic-like proteins. Curr Opin Microbiol 9:86–94
Brüggemann H, Hagman A, Jules M, Sismeiro O, Dillies MA, Gouyette C, Kunst F, Steinert M, Heuner K, Coppee JY, Buchrieser C (2006) Virulence strategies for infecting phagocytes deduced from the in vivo transcriptional program of Legionella pneumophila. Cell Microbiol 8:1228–1240
Brulet A, Nicolle MC, Giard M, Nicolini FE, Michallet M, Jarraud S, Etienne J, Vanhems P (2008) Fatal nosocomial Legionella pneumophila infection due to exposure to contaminated water from a washbasin in a hematology unit. Infect Control Hosp Epidemiol 29:1091–1093
Bumbaugh AC, McGraw EA, Page KL, Selander RK, Whittam TS (2002) Sequence polymorphism of dotA and mip alleles mediating invasion and intracellular replication of Legionella pneumophila. Curr Microbiol 44:314–322
Burstein D, Zusman T, Degtyar E, Viner R, Segal G, Pupko T (2009) Genome-scale identification of Legionella pneumophila effectors using a machine learning approach. PLoS Pathog 5:e1000508
Buscher BA, Conover GM, Miller JL, Vogel SA, Meyers SN, Isberg RR, Vogel JP (2005) The DotL protein, a member of the TraG-coupling protein family, is essential for viability of Legionella pneumophila strain Lp02. J Bacteriol 187:2927–2938
Buse HY, Ashbolt NJ (2011) Differential growth of Legionella pneumophila strains within a range of amoebae at various temperatures associated with in-premise plumbing. Lett Appl Microbiol 53:217–224
Buse HY, Brehm A, Santo Domingo JW, Ashbolt NJ (2011) Screening-level assays for potentially human-infectious environmental Legionella spp. J Microbiol 49:200–207
Buse HY, Schoen ME, Ashbolt NJ (2012) Legionellae in engineered systems and use of quantitative microbial risk assessment to predict exposure. Water Res. doi:10:1016/j.watres.2011.12.022
Byrd TF, Horwitz MA (1989) Interferon gamma-activated human monocytes downregulate transferrin receptors and inhibit the intracellular multiplication of Legionella pneumophila by limiting the availability of iron. J Clin Invest 83:1457–1465
Byrd TF, Horwitz MA (2000) Aberrantly low transferrin receptor expression on human monocytes is associated with nonpermissiveness for Legionella pneumophila growth. J Infect Dis 181:1394–1400
Byrne B, Swanson MS (1998) Expression of Legionella pneumophila virulence traits in response to growth conditions. Infect Immun 66:3029–3034
Campbell J, Bibb WF, Lambert MA, Eng S, Steigerwalt AG, Allard J, Moss CW, Brenner DJ (1984) Legionella sainthelensi: A new species of Legionella isolated from water near Mt. St. Helens. Appl Environ Microbiol 47:369–373
Campese C, Bitar D, Jarraud S, Maine C, Forey F, Etienne J, Desenclos JC, Saura C, Che D (2011) Progress in the surveillance and control of Legionella infection in France, 1998–2008. Int J Infect Dis 15:e30–e37
Campese C, Roche D, Clement C, Fierobe F, Jarraud S, de Waelle P, Perrin H, Che D (2010) Cluster of Legionnaires’ disease associated with a public whirlpool spa. Euro Surveill 15
Campodonico EM, Chesnel L, Roy CR (2005) A yeast genetic system for the identification and characterization of substrate proteins transferred into host cells by the Legionella pneumophila Dot/Icm system. Mol Microbiol 56:918–933
Caparon M, Johnson W (1988) Macrophage toxicity and complement sensitivity of virulent and avirulent strains of Legionella pneumophila. Rev Infect Dis 10:S377–S381
Cargill KL, Pyle BH, Sauer RL, McFeters GA (1992) Effects of culture conditions and biofilm formation on the iodine susceptibility of Legionella pneumophila. Can J Microbiol 38:423–429
Carratala J, Garcia-Vidal C (2010) An update on Legionella. Curr Opin Infect Dis 23:152–157
Carratala J, Gudiol F, Pallares R, Dorca J, Verdaguer R, Ariza J, Manresa F (1994) Risk factors for nosocomial Legionella pneumophila pneumonia. Am J Respir Crit Care Med 149:625–629
Carvalho FR, Nastasi FR, Gamba RC, Foronda AS, Pellizari VH (2008) Occurrence and diversity of legionellaceae in polar lakes of the antarctic peninsula. Curr Microbiol 57:294–300
Carvalho FR, Vazoller RF, Foronda AS, Pellizari VH (2007) Phylogenetic study of Legionella species in pristine and polluted aquatic samples from a tropical atlantic forest ecosystem. Curr Microbiol 55:288–293
Case CL, Roy CR (2011) Asc modulates the function of NLRC4 in response to infection of macrophages by Legionella pneumophila. MBio 2
Case CL, Shin S, Roy CR (2009) Asc and Ipaf Inflammasomes direct distinct pathways for caspase-1 activation in response to Legionella pneumophila. Infect Immun 77:1981–1991
Castellani Pastoris M, Passi C, Maroli M (1989) Evidence of Legionella pneumophila in some arthropods and related natural aquatic habitats. FEMS Microbiol Ecol 62:259–264
Castilla J, Barricarte A, Aldaz J, Garcia Cenoz M, Ferrer T, Pelaz C, Pineda S, Baladron B, Martin I, Goni B, Aratajo P, Chamorro J, Lameiro F, Torroba L, Dorronsoro I, Martinez-Artola V, Esparza MJ, Gastaminza MA, Fraile P, Aldaz P (2008) A large Legionnaires’ disease outbreak in Pamplona, Spain: early detection, rapid control and no case fatality. Epidemiol Infect 136:823–832
Castor ML, Wagstrom EA, Danila RN, Smith KE, Naimi TS, Besser JM, Peacock KA, Juni BA, Hunt JM, Bartkus JM, Kirkhorn SR, Lynfield R (2005) An outbreak of Pontiac fever with respiratory distress among workers performing high-pressure cleaning at a sugar-beet processing plant. J Infect Dis 191:1530–1537
Cazalet C, Gomez-Valero L, Rusniok C, Lomma M, Dervins-Ravault D, Newton HJ, Sansom FM, Jarraud S, Zidane N, Ma L, Bouchier C, Etienne J, Hartland EL, Buchrieser C (2010) Analysis of the Legionella longbeachae genome and transcriptome uncovers unique strategies to cause Legionnaires’ disease. PLoS Genet 6:e1000851
Cazalet C, Jarraud S, Ghavi-Helm Y, Kunst F, Glaser P, Etienne J, Buchrieser C (2008) Multigenome analysis identifies a worldwide distributed epidemic Legionella pneumophila clone that emerged within a highly diverse species. Genome Res 18:431–441
Cazalet C, Rusniok C, Bruggemann H, Zidane N, Magnier A, Ma L, Tichit M, Jarraud S, Bouchier C, Vandenesch F, Kunst F, Etienne J, Glaser P, Buchrieser C (2004) Evidence in the Legionella pneumophila genome for exploitation of host cell functions and high genome plasticity. Nat Genet 36:1165–1173
CDC (2011) Legionellosis – United States, 2000–2009. MMWR 60:1083–1086
Chandler FW, Blackmon JA, Hicklin MD, Cole RM, Callaway CS (1979) Ultrastructure of the agent of Legionnaires’ disease in the human lung. Am J Clin Pathol 71:43–50
Chandler FW, Roth IL, Callaway CS, Bump JL, Thomason BM, Weaver RE (1980) Flagella on Legionnaires’ disease bacteria: ultastructural observations. Ann Int Med 93:711–714
Chang B, Kura F, Amemura-Maekawa J, Koizumi N, Watanabe H (2005) Identification of a novel adhesion molecule involved in the virulence of Legionella pneumophila. Infect Immun 73:4272–4280
Charpentier X, Faucher SP, Kalachikov S, Shuman HA (2008) Loss of RNase R induces competence development in Legionella pneumophila. J Bacteriol 190:8126–8136
Chatfield CH, Cianciotto NP (2007) The secreted pyomelanin pigment of Legionella pneumophila confers ferric reductase activity. Infect Immun 75:4062–4070
Chatfield CH, Mulhern BJ, Burnside DM, Cianciotto NP (2011) Legionella pneumophila LbtU acts as a novel, TonB-independent receptor for the Legiobactin siderophore. J Bacteriol 193:1563–1575
Chee CE, Baddour LM (2007) Legionella maceachernii soft tissue infection. Am J Med Sci 334:410–413
Chen J, de Felipe KS, Clarke M, Lu H, Anderson OR, Segal G, Shuman HA (2004) Legionella effectors that promote nonlytic release from protozoa. Science 303:1358–1361
Cherry WB, Gorman GW, Orrison LH, Moss CW, Steigerwalt AG, Wilkinson HW, Johnson SE, McKinney RM, Brenner DJ (1982) Legionella jordanis: a new species of Legionella isolated from water and sewage. J Clin Microbiol 15:290–297
Chien M, Morozova I, Shi S, Sheng H, Chen J, Gomez SM, Asamani G, Hill K, Nuara J, Feder M, Rineer J, Greenberg JJ, Steshenko V, Park SH, Zhao B, Teplitskaya E, Edwards JR, Pampou S, Georghiou A, Chou IC, Iannuccilli W, Ulz ME, Kim DH, Geringer-Sameth A, Goldsberry C, Morozov P, Fischer SG, Segal G, Qu X, Rzhetsky A, Zhang P, Cayanis E, De Jong PJ, Ju J, Kalachikov S, Shuman HA, Russo JJ (2004) The genomic sequence of the accidental pathogen Legionella pneumophila. Science 305:1966–1968
Cho MC, Kim H, An D, Lee M, Noh SA, Kim MN, Chong YP, Woo JH (2012) Comparison of sputum and nasopharyngeal swab specimens for molecular diagnosis of Mycoplasma pneumoniae, Chlamydophila pneumoniae, and Legionella pneumophila. Ann Lab Med 32:133–138
Chong A, Lima CA, Allan DS, Nasrallah GK, Garduno RA (2009) The purified and recombinant Legionella pneumophila chaperonin alters mitochondrial trafficking and microfilament organization. Infect Immun 77:4724–4739
Christie PJ, Atmakuri K, Krishnamoorthy V, Jakubowski S, Cascales E (2005) Biogenesis, architecture, and function of bacterial type IV secretion systems. Annu Rev Microbiol 59:451–485
Christie PJ, Vogel JP (2000) Bacterial type IV secretion: conjugation systems adapted to deliver effector molecules to host cells. Trends Microbiol 8:354–360
Cianciotto N, Eisenstein BI, Engleberg NC, Shuman H (1989a) Genetics and molecular pathogenesis of Legionella pneumophila, an intracellular parasite of macrophages. Molec Biol Med 6:490–424
Cianciotto NP, Eisenstein BI, Mody CH, Toews GB, Engleberg NC (1989b) A Legionella pneumophila gene encoding a species-specific surface protein potentiates initiation of intracellular infection. Infect Immun 57:1255–1262
Cianciotto NP, Bangsborg JM, Eisenstein BI, Engleberg NC (1990a) Identification of mip-like genes in the genus Legionella. Infect Immun 58:2912–2918
Cianciotto NP, Eisenstein BI, Mody CH, Engleberg NC (1990b) A mutation in the mip gene results in an attenuation of Legionella pneumophila virulence. J Infect Dis 162:121–126
Cianciotto NP, Fields BS (1992) Legionella pneumophila mip gene potentiates intracellular infection of protozoa and human macrophages. Proc Natl Acad Sci USA 89:5188–5191
Cianciotto NP, Kim Stamos J, Kamp DW (1995) Infectivity of Legionella pneumophila mip mutant for alveolar epithelial cells. Curr Microbiol 30:247–250
Cianciotto NP (2001) Pathogenicity of Legionella pneumophila. Int J Med Microbiol 291:331–343
Cianciotto NP (2005) Type II secretion: a protein secretion system for all seasons. Trends Microbiol 13:581–588
Cianciotto NP, Cornelis P, Baysse C (2005) Impact of the bacterial type I cytochrome c maturation system on different biological processes. Mol Microbiol 56:1408–1415
Cianciotto NP (2007) Iron acquisition by Legionella pneumophila. Biometals 20:323–331
Cianciotto NP (2009) Many substrates and functions of type II protein secretion: lessons learned from Legionella pneumophila. Future Microbiol 4:797–805
Ciesielski CA, Blaser MJ, Wang W-LL (1986) Serogroup specificity of Legionella pneumophila is related to lipopolysaccharide characteristics. Infect Immun 51:397–404
Cilloniz C, Ewig S, Polverino E, Marcos MA, Esquinas C, Gabarrus A, Mensa J, Torres A (2011) Microbial aetiology of community-acquired pneumonia and its relation to severity. Thorax 66:340–346
Cirillo JD, Cirillo SLG, Yan L, Bermudez LE, Falkow S, Tompkins LS (1999) Intracellular growth in Acanthamoeba castellani affects monocyte entry mechanism and enhances virulence of Legionella pneumophila. Infect Immun 67:4427–4434
Cirillo JD, Falkow S, Tompkins LS (1994) Growth of Legionella pneumophila in Acanthamoeba castellanii enhances invasion. Infect Immun 62:3254–3261
Cirillo SL, Bermudez LE, El-Etr SH, Duhamel GE, Cirillo JD (2001) Legionella pneumophila entry gene rtxA is involved in virulence. Infect Immun 69:508–517
Cirillo SL, Lum J, Cirillo JD (2000) Identification of novel loci involved in entry by Legionella pneumophila. Microbiology 146:1345–1359
Cirillo SL, Yan L, Littman M, Samrakandi MM, Cirillo JD (2002) Role of the Legionella pneumophila rtxA gene in amoebae. Microbiology 148:1667–1677
Cloud JL, Carroll KC, Pixton P, Erali M, Hillyard DR (2000) Detection of Legionella species in respiratory specimens using PCR with sequencing confirmation. J Clin Microbiol 38:1709–1712
Coers J, Vance RE, Fontana MF, Dietrich WF (2007a) Restriction of Legionella pneumophila growth in macrophages requires the concerted action of cytokine and Naip5/Ipaf signalling pathways. Cell Microbiol 9:2344–2357
Coers J, Vance RE, Fontana MF, Dietrich WF (2007b) Restriction of Legionella pneumophila growth in macrophages requires the concerted action of cytokine and Naip5/Ipaf signalling pathways. Cell Microbiol 9:2344–2357
Coil DA, Anne J (2010) The role of fimV and the importance of its tandem repeat copy number in twitching motility, pigment production, and morphology in Legionella pneumophila. Arch Microbiol 192:625–631
Coil DA, Anne J (2009) Twitching motility in Legionella pneumophila. FEMS Microbiol Lett 293:271–277
Colbourne JS, Pratt DJ, Smith MG, Fisher-Hoch SP, Harper D (1984) Water fittings as sources of Legionella pneumophila in a hospital plumbing system. Lancet 1:210–213
Cole ST, Brosch R, Parkhill J, Garnier T, Churcher C, Harris D, Gordon SV, Eiglmeier K, Gas S, Barry CE, 3rd, Tekaia F, Badcock K, Basham D, Brown D, Chillingworth T, Connor R, Davies R, Devlin K, Feltwell T, Gentles S, Hamlin N, Holroyd S, Hornsby T, Jagels K, Barrell BG, et al. (1998) Deciphering the biology of Mycobacterium tuberculosis from the complete genome sequence. Nature 393:537–544
Collins MT (1986) Legionella infections in animals. Isr J Med Sci 22:662–673
Collins MT, McDonald J, Høiby N, Aalund O (1984) Agglutinating antibody titers to members of the family Legionellaceae in cystic fibrosis patients as a result of cross-reacting antibodies to Pseudomonas aeruginosa. J Clin Microbiol 19:757–762
Conlan JW, Ashworth LA (1986) The relationship between the serogroup antigen and lipopolysaccharide of Legionella pneumophila. J Hyg 96:39–48
Conlan JW, Baskerville A, Ashworth LAE (1986) Separation of Legionella pneumophila proteases and purification of a protease which produces lesions like those of Legionnaires’ disease in guinea pig lung. J Gen Microbiol 132:1565–1574
Conlan JW, Williams A, Ashworth LA (1988) In vivo production of a tissue-destructive protease by Legionella pneumophila in the lungs of experimentally infected guinea-pigs. J Gen Microbiol 134:143–149
Conover GM, Derre I, Vogel JP, Isberg RR (2003) The Legionella pneumophila LidA protein: a translocated substrate of the Dot/Icm system associated with maintenance of bacterial integrity. Mol Microbiol 48:305–321
Cordes LG, Wiesenthal AM, Gorman GW, Phair JP, Sommers HM, Brown A, Yu VL, Magnussen MH, Meyer RD, Wolf JS, Shands KN, Fraser DW (1981) Isolation of Legionella pneumophila from hospital shower heads. Ann Intern Med 94:195–197
Coscolla M, Comas I, Gonzalez-Candelas F (2011) Quantifying nonvertical inheritance in the evolution of Legionella pneumophila. Mol Biol Evol 28:985–1001
Coscolla M, Fenollar J, Escribano I, Gonzalez-Candelas F (2010) Legionellosis outbreak associated with asphalt paving machine, Spain, 2009. Emerg Infect Dis 16:1381–1387
Coscolla M, Gonzalez-Candelas F (2007) Population structure and recombination in environmental isolates of Legionella pneumophila. Environ Microbiol 9:643–656
Costa J, d’Avo AF, da Costa MS, Verissimo A (2011) Molecular evolution of key genes for type II secretion in Legionella pneumophila. Environ Microbiol doi: 10.1111/j.1462-2920.2011.02646.x
Cramp GJ, Harte D, Douglas NM, Graham F, Schousboe M, Sykes K (2010) An outbreak of Pontiac fever due to Legionella longbeachae serogroup 2 found in potting mix in a horticultural nursery in New Zealand. Epidemiol Infect 138:15–20
Craun GF, Brunkard JM, Yoder JS, Roberts VA, Carpenter J, Wade T, Calderon RL, Roberts JM, Beach MJ, Roy SL (2010) Causes of outbreaks associated with drinking water in the United States from 1971 to 2006. Clin Microbiol Rev 23:507–528
Creagh EM, O'Neill LA (2006) TLRs, NLRs and RLRs: a trinity of pathogen sensors that co-operate in innate immunity. Trends Immunol 27:352–357
Cui Y, Chatterjee A, Chatterjee AK (2001) Effects of the two-component system comprising GacA and GacS of Erwinia carotovora subsp. carotovora on the production of global regulatory rsmB RNA, extracellular enzymes, and harpinEcc. Mol Plant Microbe Interact 14:516–526
Cunha BA (2010) Legionnaires’ disease: clinical differentiation from typical and other atypical pneumonias. Infect Dis Clin North Am 24:73–105
D'Auria G, Jimenez N, Peris-Bondia F, Pelaz C, Latorre A, Moya A (2008) Virulence factor rtx in Legionella pneumophila, evidence suggesting it is a modular multifunctional protein. BMC Genomics 9:14
D'Auria G, Jimenez-Hernandez N, Peris-Bondia F, Moya A, Latorre A (2010) Legionella pneumophila pangenome reveals strain-specific virulence factors. BMC Genomics 11:181
Dalebroux ZD, Edwards RL, Swanson MS (2009) SpoT governs Legionella pneumophila differentiation in host macrophages. Mol Microbiol 71:640–658
Dalebroux ZD, Yagi BF, Sahr T, Buchrieser C, Swanson MS (2010) Distinct roles of ppGpp and DksA in Legionella pneumophila differentiation. Mol Microbiol 76:200–219
Davis GS, Winn WC, Jr, Gump DW, Beaty HN (1983) The kinetics of early inflammatory events during experimental pneumonia due to Legionella pneumophila in guinea pigs. J Infect Dis 148:823–835
Davis GS, Winn WC, Jr, Gump DW, Craighead JE, Beaty HN (1982) Legionnaires’ pneumonia after aerosol exposure in guinea pigs and rats. Am Rev Respir Dis 126:1050–1057
De Buck E, Hoper D, Lammertyn E, Hecker M, Anne J (2008a) Differential 2-D protein gel electrophoresis analysis of Legionella pneumophila wild type and Tat secretion mutants. Int J Med Microbiol 298:449–461
De Buck E, Lammertyn E, Anne J (2008b) The importance of the twin-arginine translocation pathway for bacterial virulence. Trends Microbiol 16:442–453
De Buck E, Lebeau I, Maes L, Geukens N, Meyen E, Van Mellaert L, Anne J, Lammertyn E (2004) A putative twin-arginine translocation pathway in Legionella pneumophila. Biochem Biophys Res Commun 317:654–661
De Buck E, Maes L, Meyen E, Van Mellaert L, Geukens N, Anne J, Lammertyn E (2005) Legionella pneumophila Philadelphia-1 tatB and tatC affect intracellular replication and biofilm formation. Biochem Biophys Res Commun 331:1413–1420
De Buck E, Vranckx L, Meyen E, Maes L, Vandersmissen L, Anne J, Lammertyn E (2007) The twin-arginine translocation pathway is necessary for correct membrane insertion of the Rieske Fe/S protein in Legionella pneumophila. FEBS Lett 581:259–264
de Felipe KS, Glover RT, Charpentier X, Anderson OR, Reyes M, Pericone CD, Shuman HA (2008) Legionella eukaryotic-like type IV substrates interfere with organelle trafficking. PLoS Pathog 4:e1000117
de Felipe KS, Pampou S, Jovanovic OS, Pericone CD, Ye SF, Kalachikov S, Shuman HA (2005) Evidence for acquisition of Legionella type IV secretion substrates via interdomain horizontal gene transfer. J Bacteriol 187:7716–7726
DebRoy S, Aragon V, Kurtz S, Cianciotto NP (2006a) Legionella pneumophila Mip, a surface-exposed peptidylproline cis-trans-isomerase, promotes the presence of phospholipase C-like activity in culture supernatants. Infect Immun 74:5152–5160
DebRoy S, Dao J, Soderberg M, Rossier O, Cianciotto NP (2006b) Legionella pneumophila type II secretome reveals unique exoproteins and a chitinase that promotes bacterial persistence in the lung. Proc Natl Acad Sci USA 103:19146–19151
Declerck P (2010) Biofilms: the environmental playground of Legionella pneumophila. Environ Microbiol 12:557–566
Declerck P, Behets J, Margineanu A, van Hoef V, De Keersmaecker B, Ollevier F (2009) Replication of Legionella pneumophila in biofilms of water distribution pipes. Microbiol Res 164:593–603
Declerck P, Behets J, van Hoef V, Ollevier F (2007a) Detection of Legionella spp. and some of their amoeba hosts in floating biofilms from anthropogenic and natural aquatic environments. Water Res 41:3159–3167
Declerck P, Behets J, van Hoef V, Ollevier F (2007b) Replication of Legionella pneumophila in floating biofilms. Curr Microbiol 55:435–440
Degtyar E, Zusman T, Ehrlich M, Segal G (2009) A Legionella effector acquired from protozoa is involved in sphingolipids metabolism and is targeted to the host cell mitochondria. Cell Microbiol 11:1219–1235
Delgado-Viscogliosi P, Simonart T, Parent V, Marchand G, Dobbelaere M, Pierlot E, Pierzo V, Menard-Szczebara F, Gaudard-Ferveur E, Delabre K, Delattre JM (2005) Rapid method for enumeration of viable Legionella pneumophila and other Legionella spp. in water. Appl Environ Microbiol 71:4086–4096
Delgado-Viscogliosi P, Solignac L, Delattre JM (2009) Viability PCR, a culture-independent method for rapid and selective quantification of viable Legionella pneumophila cells in environmental water samples. Appl Environ Microbiol 75:3502–3512
Den Boer JW, Yzerman EP (2004) Diagnosis of Legionella infection in Legionnaires’ disease. Eur J Clin Microbiol Infect Dis 23:871–878
Den Boer JW, Yzerman EP, Schellekens J, Lettinga KD, Boshuizen HC, Van Steenbergen JE, Bosman A, Van den Hof S, Van Vliet HA, Peeters MF, Van Ketel RJ, Speelman P, Kool JL, Conyn-Van Spaendonck MA (2002) A large outbreak of Legionnaires’ disease at a flower show, the Netherlands, 1999. Emerg Infect Dis 8:37–43
Dennis PJ, Brenner DJ, Thacker WL, Wait R, Vesey G, Steigerwalt AG, Benson RF (1993) Five new Legionella species isolated from water. Int J Syst Bacteriol 43:329–337
Derre I, Isberg RR (2005) LidA, a translocated substrate of the Legionella pneumophila type IV secretion system, interferes with the early secretory pathway. Infect Immun 73:4370–4380
Derré I, Isberg RR (2004) Legionella pneumophila replication vacuole formation involves rapid recruitment of proteins of the early secretory system. Infect Immun 72:3048–3053
Desvaux M, Hebraud M, Talon R, Henderson IR (2009) Secretion and subcellular localizations of bacterial proteins: a semantic awareness issue. Trends Microbiol 17:139–145
Dey R, Bodennec J, Mameri MO, Pernin P (2009) Free-living freshwater amoebae differ in their susceptibility to the pathogenic bacterium Legionella pneumophila. FEMS Microbiol Lett 290:10–17
Di Paolo G, De Camilli P (2006) Phosphoinositides in cell regulation and membrane dynamics. Nature 443:651–657
Diederen BM (2008) Legionella spp. and Legionnaires’ disease. J Infect 56:1–12
Diederen BM, Bruin JP, Scopes E, Peeters MF, Ijzerman EP (2009) Evaluation of the Oxoid Xpect Legionella test kit for detection of Legionella pneumophila serogroup 1 antigen in urine. J Clin Microbiol 47:2272–2274
Dietrich C, Heuner K, Brand BC, Hacker J, Steinert M (2001) Flagellum of Legionella pneumophila positively affects the early phase of infection of eukaryotic host cells. Infect Immun 69:2116–2122
Dietrich WF, Damron DM, Isberg RR, Lander ES, Swanson MS (1995) Lgn1, a gene that determines susceptibility to Legionella pneumophila, maps to mouse chromosome 13. Genomics 26:443–450
Diez E, Lee SH, Gauthier S, Yaraghi Z, Tremblay M, Vidal S, Gros P (2003) Birc1e is the gene within the Lgn1 locus associated with resistance to Legionella pneumophila. Nat Genet 33:55–60
Doleans A, Aurell H, Reyrolle M, Lina G, Freney J, Vandenesch F, Etienne J, Jarraud S (2004) Clinical and environmental distributions of Legionella strains in France are different. J Clin Microbiol 42:458–460
Doleans-Jordheim A, Akermi M, Ginevra C, Cazalet C, Kay E, Schneider D, Buchrieser C, Atlan D, Vandenesch F, Etienne J, Jarraud S (2006) Growth-phase-dependent mobility of the lvh-encoding region in Legionella pneumophila strain Paris. Microbiology 152:3561–3568
Domínguez J, Galí N, Blanco S, Pedroso P, Prat C, Matas L, Ausina V (2001) Assessment of a new test to detect Legionella urinary antigen for the diagnosis of Legionnaires' disease. Diagn Microbiol Infect Dis 41:199–203
Domínguez JA, Galí N, Pedroso P, Fargas A, Padilla E, Manterola JM, Matas L (1998) Comparison of the Binax Legionella urinary antigen enzyme immunoassay (EIA) with the Biotest Legionella Urin antigen EIA for detection of Legionella antigen in both concentrated and nonconcentrated urine samples. J Clin Microbiol 36:2718–2722
Donlan RM, Forster T, Murga R, Brown E, Lucas C, Carpenter J, Fields B (2005) Legionella pneumophila associated with the protozoan Hartmannella vermiformis in a model multi-species biofilm has reduced susceptibility to disinfectants. Biofouling 21:1–7
Dorer MS, Kirton D, Bader JS, Isberg RR (2006) RNA interference analysis of Legionella in Drosophila cells: exploitation of early secretory apparatus dynamics. PLoS Pathog 2:e34
Dournon E, Bibb WF, Rajagopalan P, Desplaces N, McKinney RM (1988) Monoclonal antibody reactivity as a virulence marker for Legionella pneumophila serogroup 1 strains. J Infect Dis 157:496–501
Dowling JN, Saha AK, Glew RH (1992) Virulence factors of the family Legionellaceae. Microbiol Rev 56:32–60
Doyle RM, Cianciotto NP, Banvi S, Manning PA, Heuzenroeder MW (2001) Comparison of virulence of Legionella longbeachae strains in guinea pigs and U937 macrophage-like cells. Infect Immun 69:5335–5344
Doyle RM, Steele TW, McLennan AM, Parkinson IH, Manning PA, Heuzenroeder MW (1998) Sequence analysis of the mip gene of the soilborne pathogen Legionella longbeachae. Infect Immun 66:1492–1499
Dubuisson JF, Swanson MS (2006) Mouse infection by Legionella, a model to analyze autophagy. Autophagy 2:179–182
Duncan C, Prashar A, So J, Tang P, Low DE, Terebiznik M, Guyard C (2011) Lcl of Legionella pneumophila is an immunogenic GAG binding adhesin that promotes interactions with lung epithelial cells and plays a crucial role in biofilm formation. Infect Immun 79:2168–2181
Dusserre E, Ginevra C, Hallier-Soulier S, Vandenesch F, Festoc G, Etienne J, Jarraud S, Molmeret M (2008) A PCR-based method for monitoring Legionella pneumophila in water samples detects viable but noncultivable legionellae that can recover their cultivability. Appl Environ Microbiol 74:4817–4824
Dutka BJ, Ewan P (1983) First isolation of Legionella pneumophila from the Canadian Great Lakes. J Great Lakes Res 9:430–432
Edagawa A, Kimura A, Doi H, Tanaka H, Tomioka K, Sakabe K, Nakajima C, Suzuki Y (2008) Detection of culturable and nonculturable Legionella species from hot water systems of public buildings in Japan. J Appl Microbiol 105:2104–2114
Edelstein PH (1981) Improved semiselective medium for isolation of Legionella pneumophila from contaminated clinical and environmental specimens. J Clin Microbiol 14:298–303
Edelstein PH (1985b) Legionnaires’ disease. Laboratory manual, vol. 3. National Technical Information Service, Springfield, pp 1–146
Edelstein PH (1985a) Legionella. In: Lennette EH, Balows A, Hausler WJ, Jr, Shadomy HJ (ed) Manual of clinical microbiology. American Society for Microbiology, Washington, DC, pp 373–381
Edelstein PH (1986) Control of Legionella in hospitals. J Hosp Infect 8:109–115
Edelstein PH (1995) Antimicrobial chemotherapy for legionnaires' disease: a review. Clin Infect Dis 21:S265–S276
Edelstein PH (1993) Legionnaires’ disease. Clin Infect Dis 16:741–747
Edelstein PH (1997) Detection of antibodies to Legionella spp. In: Rose NR, de Macario EC, Folds JD, Lane HC, Nakamura RM (ed) Manual of clinical laboratory immunology. American society for microbiology, Washington, DC, pp 502–509
Edelstein PH (1998) Antimicrobial chemotherapy for Legionnaires disease: time for a change. Ann Intern Med 129:328–330
Edelstein PH (2007) Urine antigen tests positive for Pontiac fever: implications for diagnosis and pathogenesis. Clin Infect Dis 44:229–231
Edelstein PH, Beer KB, Sturge JC, Watson AJ, Goldstein LC (1985) Clinical utility of a monoclonal direct fluorescent reagent specific for Legionella pneumophila: comparative study with other reagents. J Clin Microbiol 22:419–421
Edelstein PH, Brenner DJ, Moss CW, Steigerwalt AG, Francis EM, George WL (1982) Legionella wadsworthii species nova: a cause of human pneumonia. Ann Intern Med 97:809–813
Edelstein PH, Calarco K, Yasui VK (1984) Antimicrobial therapy of experimentally induced Legionnaires’ disease in guinea pigs. Am Rev Respir Dis 130:849–856
Edelstein PH, Cianciotto NP (2010) Legionella. In: Mandell GL, Bennett JE, Dolin R (ed) Principles and practice of infectious diseases, 7th edition, vol 2. Elsevier, Churchill Livingstone, pp 2969–2984
Edelstein PH, Edelstein MA (1993) Intracellular growth of Legionella pneumophila serogroup 1 monoclonal antibody type 2 positive and negative bacteria. Epidemiol Infect 111:499–502
Edelstein PH, Edelstein MA, Shephard LJ, Ward KW, Ratcliff RM (2011) Legionella steelei sp. nov. isolated from human respiratory specimens in California and South Australia. Int J Syst Evol Microbiol 62:1766–1771
Edelstein PH, Edelstein MAC, Higa F, Falkow S (1999) Discovery of virulence genes of Legionella pneumophila by using signature tagged mutagenesis in a guinea pig pneumonia model. Proc Natl Acad Sci USA 96:8190–8195
Edelstein PH, Hu B, Higa F, Edelstein MA (2003) lvgA, a novel Legionella pneumophila virulence factor. Infect Immun 71:2394–2403
Edelstein PH, Hu B, Shinzato T, Edelstein MA, Xu W, Bessman MJ (2005) Legionella pneumophila NudA Is a Nudix hydrolase and virulence factor. Infect Immun 73:6567–6576
Edelstein PH, Metlay JP (2009) Legionella pneumophila goes clonal–Paris and Lorraine strain-specific risk factors. Clin Infect Dis 49:192–194
Edelstein PH, Meyer RD, Finegold SM (1980) Laboratory diagnosis of Legionnaires’ disease. Am Rev Respir Dis 121:317–327
Edwards RL, Dalebroux ZD, Swanson MS (2009) Legionella pneumophila couples fatty acid flux to microbial differentiation and virulence. Mol Microbiol 71:1190–1204
Edwards RL, Jules M, Sahr T, Buchrieser C, Swanson MS (2010) The Legionella pneumophila LetA/LetS two-component system exhibits rheostat-like behavior. Infect Immun 78:2571–2583
Elliott JA, Winn WCJ (1986) Treatment of alveolar macrophages with cytochalasin D inhibits uptake and subsequent growth of Legionella pneumophila. Infect Immun 51:31–36
Ellis TN, Kuehn MJ (2010) Virulence and immunomodulatory roles of bacterial outer membrane vesicles. Microbiol Mol Biol Rev 74:81–94
Emtiazi F, Schwartz T, Marten SM, Krolla-Sidenstein P, Obst U (2004) Investigation of natural biofilms formed during the production of drinking water from surface water embankment filtration. Water Res 38:1197–1206
Engleberg NC, Carter C, Weber DR, Cianciotto NP, Eisenstein BI (1989) DNA sequence of mip, a Legionella pneumophila gene associated with macrophage infectivity. Infect Immun 57:1263–1270
Engleberg NC, Drutz DJ, Eisenstein BI (1984) Cloning and expression of Legionella pneumophila antigens in Escherichia coli. Infect Immun 44:222–227
Engleberg NC, Howe DC, Rogers JE, Arroyo J, Eisenstein BI (1991) Characterization of a Legionella pneumophila gene encoding a lipoprotein antigen. Mol Microbiol 5:2021–2029
Ensminger AW, Isberg RR (2010) E3 ubiquitin ligase activity and targeting of BAT3 by multiple Legionella pneumophila translocated substrates. Infect Immun 78:3905–3919
Euser SM, Bruin JP, Mooi-Kokenberg EA, Peeters M, Verbakel H, Yzerman EP, Den Boer JW (2012) Diagnostic testing for Legionnaires’ disease in the Netherlands between 2007 and 2009: a possible cause for the decline in reported Legionnaires’ disease patients. Eur J Clin Microbiol Infect Dis. doi: 10.1007/s10096-011-1528-z
Euser SM, Pelgrim M, den Boer JW (2010) Legionnaires’ disease and Pontiac fever after using a private outdoor whirlpool spa. Scand J Infect Dis 42:910–916
Evans FF, Egan S, Kjelleberg S (2008) Ecology of type II secretion in marine gammaproteobacteria. Environ Microbiol 10:1101–1107
Eylert E, Herrmann V, Jules M, Gillmaier N, Lautner M, Buchrieser C, Eisenreich W, Heuner K (2010) Isotopologue profiling of Legionella pneumophila: role of serine and glucose as carbon substrates. J Biol Chem 285:22232–22243
Fallon RJ, Stack BH (1990) Legionnaires’ disease due to Legionella anisa. J Infect 20:227–229
Fang GD, Yu VL, Vickers RM (1989) Disease due to the Legionellaceae (other than Legionella pneumophila): historical, microbiological, clinical, and epidemiological review. Medicine (Baltimore) 68:116–132
Faucher SP, Friedlander G, Livny J, Margalit H, Shuman HA (2010) Legionella pneumophila 6S RNA optimizes intracellular multiplication. Proc Natl Acad Sci USA 107:7533–7538
Faucher SP, Mueller CA, Shuman HA (2011) Legionella pneumophila transcriptome during intracellular multiplication in human macrophages. Front Microbiol 2:60
Faucher SP, Shuman HA (2011) Small regulatory RNA and Legionella pneumophila. Front Microbiol 2:98
Feddersen A, Meyer HG, Matthes P, Bhakdi S, Husmann M (2000) GyrA sequence-based typing of Legionella. Med Microbiol Immunol 189:7–11
Feeley JC, Gorman GW, Weaver RE, Mackel DC, Smith HW (1978) Primary isolation media for Legionnaires disease bacterium. J Clin Microbiol 8:320–325
Feldman M, Segal G (2004) A specific genomic location within the icm/dot pathogenesis region of different Legionella species encodes functionally similar but nonhomologous virulence proteins. Infect Immun 72:4503–4511
Feldman M, Zusman T, Hagag S, Segal G (2005) Coevolution between nonhomologous but functionally similar proteins and their conserved partners in the Legionella pathogenesis system. Proc Natl Acad Sci USA 102:12206–12211
Ferhat M, Atlan D, Vianney A, Lazzaroni JC, Doublet P, Gilbert C (2009) The TolC protein of Legionella pneumophila plays a major role in multi-drug resistance and the early steps of host invasion. PLoS One 4:e7732
Fernandez RC, Logan SM, Lee SH, Hoffman PS (1996) Elevated levels of Legionella pneumophila stress protein Hsp60 early in infection of human monocytes and L929 cells correlate with virulence. Infect Immun 64:1968–1976
Fernandez-Cruz A, Marin M, Castelo L, Usubillaga R, Martin-Rabadan P, Bouza E (2011) Legionella micdadei, a new cause of prosthetic joint infection. J Clin Microbiol 49:3409–3410
Fernandez-Moreira E, Helbig JH, Swanson MS (2006) Membrane vesicles shed by Legionella pneumophila inhibit fusion of phagosomes with lysosomes. Infect Immun 74:3285–3295
Ferre MR, Arias C, Oliva JM, Pedrol A, Garcia M, Pellicer T, Roura P, Dominguez A (2009) A community outbreak of Legionnaires’ disease associated with a cooling tower in Vic and Gurb, Catalonia (Spain) in 2005. Eur J Clin Microbiol Infect Dis 28:153–159
Fettes PS, Forsbach-Birk V, Lynch D, Marre R (2001) Overexpression of a Legionella pneumophila homologue of the E. coli regulator csrA affects cell size, flagellation, and pigmentation. Int J Med Microbiol 291:353–360
Fields BS (1996) The molecular ecology of legionellae. Trends Microbiol 4:286–290
Fields BS, Barbaree JM, Sanden GN, Morrill WE (1990) Virulence of a Legionella anisa strain associated with Pontiac fever: an evaluation using protozoan, cell culture, and guinea pig models. Infect Immun 58:3139–3142
Fields BS, Barbaree JM, Shotts EB, Jr, Feeley JC, Morrill WE, Sanden GN, Dykstra MJ (1986) Comparison of guinea pig and protozoan models for determining virulence of Legionella species. Infect Immun 53:553–559
Fields BS, Benson RF, Besser RE (2002) Legionella and Legionnaires’ disease: 25 years of investigation. Clin Microbiol Rev 15:506–526
Fields BS, Sanden GN, Barbaree JM, Morrill WE, Wadowsky RM, White EH, Feeley JC (1989) Intracellular multiplication of Legionella pneumophila in amoebae isolated from hospital hot water tanks. Curr Microbiol 18:131–137
Fields BS, Shotts EBJR, Feeley JC, Gorman GW, Martin WT (1984) Proliferation of Legionella pneumophila as an intracellular parasite of the ciliated protozoan Tetrahymena pyriformis. Appl Environ Microbiol 47:467–471
Filloux A (2004) The underlying mechanisms of type II protein secretion. Biochim Biophys Acta-Mol Cell Res 1694:163–179
Fischer G, Bang H, Ludwig B, Mann K, Hacker J (1992) Mip protein of Legionella pneumophila exhibits peptidyl-prolyl-cis/trans isomerase (PPlase) activity. Mol Microbiol 6:1375–1383
Fisman DN, Lim S, Wellenius GA, Johnson C, Britz P, Gaskins M, Maher J, Mittleman MA, Spain CV, Haas CN, Newbern C (2005) It's not the heat, it’s the humidity: wet weather increases legionellosis risk in the greater Philadelphia metropolitan area. J Infect Dis 192:2066–2073
Fiumefreddo R, Zaborsky R, Haeuptle J, Christ-Crain M, Trampuz A, Steffen I, Frei R, Muller B, Schuetz P (2009) Clinical predictors for Legionella in patients presenting with community-acquired pneumonia to the emergency department. BMC Pulm Med 9:4
Flannery B, Gelling LB, Vugia DJ, Weintraub JM, Salerno JJ, Conroy MJ, Stevens VA, Rose CE, Moore MR, Fields BS, Besser RE (2006) Reducing Legionella colonization in water systems with monochloramine. Emerg Infect Dis 12:588–596
Fleischmann RD, Adams MD, White O, Clayton RA, Kirkness EF, Kerlavage AR, Bult CJ, Tomb JF, Dougherty BA, Merrick JM, et al. (1995) Whole-genome random sequencing and assembly of Haemophilus influenzae Rd. Science 269:496–512
Flendrie M, Jeurissen M, Franssen M, Kwa D, Klaassen C, Vos F (2011) Septic arthritis caused by Legionella dumoffii in a patient with systemic lupus erythematosus-like disease. J Clin Microbiol 49:746–749
Flesher AR, Ito S, Mansheim BJ, Kasper DL (1979) The cell envelope of the Legionnaires' disease bacterium. Morphologic and biochemical characteristics. Ann Intern Med 90:628–630
Flieger A, Gong S, Faigle M, Mayer HA, Kehrer U, Mubotter J, Bartmann P, Neumeister B (2000) Phospholipase A secreted by Legionella pneumophila destroys alveolar surfactant phospholipids. FEMS Microbiol Lett 188:129–133
Flieger A, Gong S, Faigle M, Stevanovic S, Cianciotto NP, Neumeister B (2001) Novel lysophospholipase A secreted by Legionella pneumophila. J Bacteriol 183:2121–2124
Flieger A, Neumeister B, Cianciotto NP (2002) Characterization of the gene encoding the major secreted lysophospholipase A of Legionella pneumophila and its role in detoxification of lysophosphatidylcholine. Infect Immun 70:6094–6106
Fliermans CB, Cherry WB, Orrison LH, Smith SJ, Tison DL, Pope DH (1981) Ecological distribution of Legionella pneumophila. Appl Environ Microbiol 41:9–16
Fliermans CB, Cherry WB, Orrison LH, Thacker L (1979) Isolation of Legionella pneumophila from nonepidemic-related aquatic habitats. Appl Environ Microbiol 37:1239–1242
Fontana MF, Banga S, Barry KC, Shen X, Tan Y, Luo ZQ, Vance RE (2011) Secreted bacterial effectors that inhibit host protein synthesis are critical for induction of the innate immune response to virulent Legionella pneumophila. PLoS Pathog 7:e1001289
Forest KT (2008) The type II secretion arrowhead: the structure of GspI-GspJ-GspK. Nat Struct Mol Biol 15:428–430
Fortier A, de Chastellier C, Balor S, Gros P (2007) Birc1e/Naip5 rapidly antagonizes modulation of phagosome maturation by Legionella pneumophila. Cell Microbiol 9:910–923
Fortier A, Diez E, Gros P (2005) Naip5/Birc1e and susceptibility to Legionella pneumophila. Trends Microbiol 13:328–335
Foy HM, Broome CV, Hayes PS, Allan I, Cooney MK, Tobe R (1979) Legionnaires’ disease in a prepaid medical-care group in Seattle 1963–1975. Lancet 1:767–770
Franco IS, Shohdy N, Shuman HA (2012) The Legionella pneumophila effector VipA is an actin nucleator that alters host cell organelle trafficking. PLoS Pathog 8:e1002546
Franco IS, Shuman HA (2012) A pathogen’s journey in the host cell: bridges between actin and traffic. Bioarchitecture 2:38–42
Franco IS, Shuman HA, Charpentier X (2009) The perplexing functions and surprising origins of Legionella pneumophila type IV secretion effectors. Cell Microbiol 11:1435–1443
Fraser DW, Tsai TR, Orenstein W, Parkin WE, Beecham HJ, Sharrar RG, Harris J, Mallison GF, Martin SM, McDade JE, Shepard CC, Brachman PS (1977) Legionnaires’ disease: description of an epidemic of pneumonia. N Engl J Med 297:1189–1197
Freudenmann M, Kurz S, von Baum H, Reick D, Schreff AM, Essig A, Luck C, Gonser T, Brockmann SO, Harter G, Eberhardt B, Embacher A, Holler C (2011) Interdisciplinary management of a large Legionella outbreak in Germany. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz 54:1161–1169
Fry AM, Rutman M, Allan T, Scaife H, Salehi E, Benson R, Fields B, Nowicki S, Parrish MK, Carpenter J, Brown E, Lucas C, Horgan T, Koch E, Besser RE (2003) Legionnaires’ disease outbreak in an automobile engine manufacturing plant. J Infect Dis 187:1015–1018
Fry NK, Afshar B, Bellamy W, Underwood AP, Ratcliff RM, Harrison TG (2007) Identification of Legionella spp. by 19 European reference laboratories: results of the European Working Group for Legionella Infections External Quality Assessment Scheme using DNA sequencing of the macrophage infectivity potentiator gene and dedicated online tools. Clin Microbiol Infect 13:1119–1124
Fry NK, Warwick S, Saunders NA, Embley TM (1991) The use of 16S ribosomal RNA analyses to investigate the phylogeny of the family Legionellaceae. J Gen Microbiol 137:1215–1222
Fu KP, Neu HC (1979) Inactivation of beta-lactam antibiotics by Legionella pneumophila. Antimicrob Agents Chemother 16:561–564
Furuhata K, Ogihara K, Ishizaki N, Oonaka K, Yoshida Y, Goto K, Hara M, Miyamoto H, Yoshida S, Fukuyama M (2010) Identification of Legionella londiniensis isolated from hot spring water samples in Shizuoka, Japan, and cytotoxicity of isolates. J Infect Chemother 16:367–371
Fuse ET, Tateda K, Kikuchi Y, Matsumoto T, Gondaira F, Azuma A, Kudoh S, Standiford TJ, Yamaguchi K (2007) Role of toll-like receptor 2 in recognition of Legionella pneumophila in a murine pneumonia model. J Med Microbiol 56:305–312
Gabay JE, Blake M, Niles WD, Horwitz MA (1985) Purification of Legionella pneumophila major outer membrane protein and demonstration that it is a porin. J Bacteriol 162:85–91
Gal-Mor O, Segal G (2003) The Legionella pneumophila GacA homolog (LetA) is involved in the regulation of icm virulence genes and is required for intracellular multiplication in Acanthamoeba castellanii. Microb Pathog 34:187–194
Galka F, Wai SN, Kusch H, Engelmann S, Hecker M, Schmeck B, Hippenstiel S, Uhlin BE, Steinert M (2008) Proteomic characterization of the whole secretome of Legionella pneumophila and functional analysis of outer membrane vesicles. Infect Immun 76:1825–1836
Gao L-Y, Susa M, Ticac B, Abu Kwaik Y (1999) Heterogeneity in intracellular replication and cytopathogenicity of Legionella pneumophila and Legionella micdadei in mammalian and protozoan cells. Microb Pathogen 27:273–287
Gao LY, Abu Kwaik Y (1999a) Activation of caspase 3 during Legionella pneumophila-induced apoptosis. Infect Immun 67:4886–4894
Gao LY, Abu Kwaik Y (1999b) Apoptosis in macrophages and alveolar epithelial cells during early stages of infection by Legionella pneumophila and its role in cytopathogenicity. Infect Immun 67:862–870
Gao LY, Harb OS, Abu Kwaik Y (1997) Utilization of similar mechanisms by Legionella pneumophila to parasitize two evolutionarily distant host cells, mammalian macrophages and protozoa. Infect Immun 65:4738–4746
Gao LY, Stone BJ, Brieland JK, Abu Kwaik Y (1998) Different fates of Legionella pneumophila pmi and mil mutants within macrophages and alveolar epithelial cells. Microb Pathog 25:291–306
Garcia MT, Jones S, Pelaz C, Millar RD, Abu Kwaik Y (2007) Acanthamoeba polyphaga resuscitates viable non-culturable Legionella pneumophila after disinfection. Environ Microbiol 9:1267–1277
Garcia-Fulgueiras A, Navarro C, Fenoll D, Garcia J, Gonzales-Diego P, Jimenez-Bunuelas T, Rodriguez M, Lopez R, Pacheco F, Ruiz J, Segovia M, Balandron B, Pelaz C (2003) Legionnaires’ disease outbreak in Murcia Spain. Emerg Infect Dis 9:915–921
Garduno RA, Faulkner G, Trevors MA, Vats N, Hoffman PS (1998a) Immunolocalization of Hsp60 in Legionella pneumophila. J Bacteriol 180:505–513
Garduno RA, Garduno E, Hoffman PS (1998b) Surface-associated Hsp60 chaperonin of Legionella pneumophila mediates invasion in a HeLa cell model. Infect Immun 66:4602–4610
Gast RJ, Moran DM, Dennett MR, Wurtsbaugh WA, Amaral-Zettler LA (2011) Amoebae and Legionella pneumophila in saline environments. J Water Health 9:37–52
Ge J, Xu H, Li T, Zhou Y, Zhang Z, Li S, Liu L, Shao F (2009) A Legionella type IV effector activates the NF-kappaB pathway by phosphorylating the IkappaB family of inhibitors. Proc Natl Acad Sci USA 106:13725–13730
Gebran SJ, Newton C, Yamamoto Y, Widen R, Klein TW, Friedman H (1994) Macrophage permissiveness for Legionella pneumophila growth modulated by iron. Infect Immun 62:564–568
George JR, Pine L, Reeves MW, Harrell WK (1980) Amino acid requirements of Legionella pneumophila. J Clin Microbiol 11:286–291
Gerhardt H, Walz MJ, Faigle M, Northoff H, Wolburg H, Neumeister B (2000) Localization of Legionella bacteria within ribosome-studded phagosomes is not restricted to Legionella pneumophila. FEMS Microbiol Lett 192:145–152
Geukens N, De Buck E, Meyen E, Maes L, Vranckx L, Van Mellaert L, Anne J, Lammertyn E (2006) The type II signal peptidase of Legionella pneumophila. Res Microbiol 157:836–841
Giao MS, Azevedo NF, Wilks SA, Vieira MJ, Keevil CW (2011) Interaction of Legionella pneumophila and Helicobacter pylori with bacterial species isolated from drinking water biofilms. BMC Microbiol 11:57
Gillespie DE, Brady SF, Bettermann AD, Cianciotto NP, Liles MR, Rondon MR, Clardy J, Goodman RM, Handelsman J (2002) Isolation of antibiotics turbomycin A and B from a metagenomic library of soil microbial DNA. Appl Environ Microbiol 68:4301–4306
Gilmour MW, Bernard K, Tracz DM, Olson AB, Corbett CR, Burdz T, Ng B, Wiebe D, Broukhanski G, Boleszczuk P, Tang P, Jamieson F, Van Domselaar G, Plummer FA, Berry JD (2007) Molecular typing of a Legionella pneumophila outbreak in Ontario Canada. J Med Microbiol 56:336–341
Ginevra C, Duclos A, Vanhems P, Campese C, Forey F, Lina G, Che D, Etienne J, Jarraud S (2009) Host-related risk factors and clinical features of community-acquired Legionnaires disease due to the Paris and Lorraine endemic strains, 1998-2007, France. Clin Infect Dis 49:184–191
Ginevra C, Forey F, Campese C, Reyrolle M, Che D, Etienne J, Jarraud S (2008) Lorraine strain of Legionella pneumophila serogroup 1, France. Emerg Infect Dis 14:673–675
Girard LP, Gregson DB (2007) Community-acquired lung abscess caused by Legionella micdadei in a myeloma patient receiving thalidomide treatment. J Clin Microbiol 45:3135–3137
Girard R, Pedron T, Uematsu S, Balloy V, Chignard M, Akira S, Chaby R (2003) Lipopolysaccharides from Legionella and Rhizobium stimulate mouse bone marrow granulocytes via Toll-like receptor 2. J Cell Sci 116:293–302
Glavin FL, Winn WC, Jr, Craighead JE (1979) Ultrastructure of lung in Legionnaires’ disease. Observations of three biopsies done during the Vermont epidemic. Ann. Int. Med. 90:555–559
Glick TH, Gregg MB, Berman B, Mallison G, Rhodes WW, Jr, Kassanoff I (1978) Pontiac fever. An epidemic of unknown etiology in a health department: I. Clinical and epidemiologic aspects. Am J Epidemiol 107:149–160
Glockner G, Albert-Weissenberger C, Weinmann E, Jacobi S, Schunder E, Steinert M, Hacker J, Heuner K (2008) Identification and characterization of a new conjugation/type IVA secretion system (trb/tra) of Legionella pneumophila Corby localized on two mobile genomic islands. Int J Med Microbiol 298:411–428
Gobin I, Newton PR, Hartland EL, Newton HJ (2009) Infections caused by nonpneumophila species of Legionella. Rev Med Microbiol 20:1–11
Goldberg DJ, Wrench JG, Collier PW, Emslie JA, Fallon RJ, Forbes GI, McKay TM, Macpherson AC, Markwick TA, Reid D (1989) Lochgoilhead fever: outbreak of non-pneumonic legionellosis due to Legionella micdadei. Lancet 1:316–318
Gomez Valero L, Runsiok C, Cazalet C, Buchrieser C (2011) Comparative and functional genomics of Legionella identified eukaryotic like proteins as key players in host-pathogen interactions. Front Microbiol 2:208
Gomez-Valero L, Rusniok C, Jarraud S, Vacherie B, Rouy Z, Barbe V, Medigue C, Etienne J, Buchrieser C (2011) Extensive recombination events and horizontal gene transfer shaped the Legionella pneumophila genomes. BMC Genomics 12:536
Gorman GW, Feeley JC, Steigerwalt A, Edelstein PH, Moss CW, Brenner DJ (1985) Legionella anisa: a new species of Legionella isolated from potable waters and a cooling tower. Appl Environ Microbiol 49:305–309
Gosselin F, Duval JF, Simonet J, Ginevra C, Gaboriaud F, Jarraud S, Mathieu L (2011) Impact of the virulence-associated MAb3/1 epitope on the physicochemical surface properties of Legionella pneumophila sg1: an issue to explain infection potential? Colloids Surf B Biointerfaces 82:283–290
Graham FF, White PS, Harte DJ, Kingham SP (2011) Changing epidemiological trends of legionellosis in New Zealand, 1979–2009. Epidemiol Infect 140:1–16
Granados A, Podzamczer D, Gudiol F, Manresa F (1989) Pneumonia due to Legionella pneumophila and pneumococcal pneumonia: similarities and differences on presentation. Eur Respir J 2:130–134
Gray JJ, Ward KN, Warren RE, Farrington M (1991) Serological cross-reaction between Legionella pneumophila and Citrobacter freundii in indirect immunofluorescence and rapid microagglutination tests. J Clin Microbiol 29:200–201
Green PN, Pirrie RS (1993) A laboratory apparatus for the generation and biocide efficacy testing of Legionella biofilms. J Appl Bacteriol 74:388–393
Greig JE, Carnie JA, Tallis GF, Ryan NJ, Tan AG, Gordon IR, Zwolak B, Leydon JA, Guest CS, Hart WG (2004) An outbreak of Legionnaires' disease at the Melbourne Aquarium, April 2000: investigation and case-control studies. Med J Aust 180:566–572
Gubler JG, Schorr M, Gaia V, Zbinden R, Altwegg M (2001) Recurrent soft tissue abscesses caused by Legionella cincinnatiensis. J Clin Microbiol 39:4568–4570
Gudiol C, Garcia-Vidal C, Fernandez-Sabe N, Verdaguer R, Llado L, Roca J, Gil-Vernet S, Carratala J (2009) Clinical features and outcomes of Legionnaires’ disease in solid organ transplant recipients. Transpl Infect Dis 11:78–82
Guerrieri E, Bondi M, Sabia C, de Niederhausern S, Borella P, Messi P (2008) Effect of bacterial interference on biofilm development by Legionella pneumophila. Curr Microbiol 57:532–536
Habyarimana F, Al-Khodor S, Kalia A, Graham JE, Price CT, Garcia MT, Kwaik YA (2008) Role for the Ankyrin eukaryotic-like genes of Legionella pneumophila in parasitism of protozoan hosts and human macrophages. Environ Microbiol 10:1460–1474
Habyarimana F, Price CT, Santic M, Al-Khodor S, Kwaik YA (2009) Molecular characterization of the Dot/Icm-translocated AnkH and AnkJ eukaryotic-like effectors of Legionella pneumophila. Infect Immun 78:1123–1134
Hacker J, Kaper JB (2000) Pathogenicity islands and the evolution of microbes. Annu Rev Microbiol 54:641–679
Haenssler E, Isberg RR (2011) Control of host cell phosphorylation by Legionella pneumophila. Front Microbio 2:doi: 10.3389/fmicb.2011.00064
Hagele S, Kohler R, Merkert H, Schleicher M, Hacker J, Steinert M (2000) Dictyostelium discoideum: a new host model system for intracellular pathogens of the genus Legionella. Cell Microbiol 2:165–171
Hales LM, Shuman HA (1999a) Legionella pneumophila contains a type II general secretion pathway required for growth in amoebae as well as for secretion of the Msp protease. Infect Immun 67:3662–3666
Hales LM, Shuman HA (1999b) The Legionella pneumophila rpoS gene is required for growth within Acanthamoeba castellanii. J Bacteriol 181:4879–4889
Haley CE, Cohen ML, Halter J, Meyer RD (1979) Nosocomial Legionnaires’ disease: a continuing common-source epidemic at Wadsworth Medical Center. Ann Intern Med 90:583–586
Hammer BK, Swanson MS (1999) Co-ordination of Legionella pneumophila virulence with entry into stationary phase by ppGpp. Mol Microbiol 33:721–731
Hammer BK, Tateda ES, Swanson MS (2002) A two-component regulator induces the transmission phenotype of stationary-phase Legionella pneumophila. Mol Microbiol 44:107–118
Han JH, Nguyen JC, Harada S, Baddour LM, Edelstein PH (2010) Relapsing Legionella pneumophila cellulitis: a case report and review of the literature. J Infect Chemother 16:439–442
Harada E, Iida K, Shiota S, Nakayama H, Yoshida S (2010) Glucose metabolism in Legionella pneumophila: dependence on the Entner-Doudoroff pathway and connection with intracellular bacterial growth. J Bacteriol 192:2892–2899
Harb OS, Abu Kwaik Y (1998) Identification of the aspartate-beta-semialdehyde dehydrogenase gene of Legionella pneumophila and characterization of a null mutant. Infect Immun 66:1898–1903
Harf C, Monteil H (1988) Interactions between free-living amoebae and Legionella in the environment. Wat Sci Tech 20:235–239
Harris A, Lally M, Albrecht M (1998) Legionella bozemanii pneumonia in three patients with AIDS. Clin Infect Dis 27:97–99
Harrison TG, Afshar B, Doshi N, Fry NK, Lee JV (2009) Distribution of Legionella pneumophila serogroups, monoclonal antibody subgroups and DNA sequence types in recent clinical and environmental isolates from England and Wales (2000-2008). Eur J Clin Microbiol Infect Dis 28:781–791
Harrison TG, Doshi N, Fry NK, Joseph CA (2007) Comparison of clinical and environmental isolates of Legionella pneumophila obtained in the UK over 19 years. Clin Microbiol Infect 13:78–85
Harrison TG, Saunders NA (1994) Taxonomy and typing of legionellae. Rev Med Microbiol 5:79–90
Harrison TG, Taylor AG (1982) Diagnosis of Legionella pneumophila infections by means of formolised yolk sac antigens. J Clin Pathol 35:211–214
Hasselmann M, Vuillemin E, Maurier F, Lutun P, Montiel H, Tempe JD (1983) Formes graves de la maladie des legionnaires: a propos de huit observations. J Med Strasbourg 14:705–708
Haupt TE, Heffernan RT, Kazmierczak JJ, Nehls-Lowe H, Rheineck B, Powell C, Leonhardt KK, Chitnis AS, Davis JP (2012) An outbreak of legionnaires disease associated with a decorative water wall fountain in a hospital. Infect Control Hosp Epidemiol 33:185–191
Hawn TR, Berrington WR, Smith IA, Uematsu S, Akira S, Aderem A, Smith KD, Skerrett SJ (2007) Altered inflammatory responses in TLR5-deficient mice infected with Legionella pneumophila. J Immunol 179:6981–6987
Hawn TR, Smith KD, Aderem A, Skerrett SJ (2006) Myeloid differentiation primary response gene (88)- and toll-like receptor 2-deficient mice are susceptible to infection with aerosolized Legionella pneumophila. J Infect Dis 193:1693–1702
Hawn TR, Verbon A, Janer M, Zhao LP, Beutler B, Aderem A (2005) Toll-like receptor 4 polymorphisms are associated with resistance to Legionnaires' disease. Proc Natl Acad Sci USA 102:2487–2489
Hawn TR, Verbon A, Lettinga KD, Zhao LP, Li SS, Laws RJ, Skerrett SJ, Beutler B, Schroeder L, Nachman A, Ozinsky A, Smith KD, Aderem A (2003) A common dominant TLR5 stop codon polymorphism abolishes flagellin signaling and is associated with susceptibility to legionnaires' disease. J Exp Med 198:1563–1572
Hay J, Seal DV, Billcliffe B, Freer JH (1995) Non-culturable Legionella pneumophila associated with Acanthamoeba castellanii: detection of the bacterium using DNA amplification and hybridization. J Appl Bacteriol 78:61–65
Hayden RT, Uhl JR, Qian X, Hopkins MK, Aubry MC, Limper AH, Lloyd RV, Cockerill FR (2001) Direct detection of Legionella species from bronchoalveolar lavage and open lung biopsy specimens: comparison of LightCycler PCR, in situ hybridization, direct fluorescence antigen detection, and culture. J Clin Microbiol 39:2618–2626
Hebert GA, Steigerwalt AG, Brenner DJ (1980) Legionella micdadei species nova: classification of a third species of Legionella associated with human pneumonia. Curr Microbiol 3:255–257
Heidtman M, Chen EJ, Moy MY, Isberg RR (2009) Large-scale identification of Legionella pneumophila Dot/Icm substrates that modulate host cell vesicle trafficking pathways. Cell Microbiol 11:230–248
Helbig JH, Benson RF, Pelaz C, Jacobs E, Luck PC (2007) Identification and serotyping of atypical Legionella pneumophila strains isolated from human and environmental sources. J Appl Microbiol 102:100–105
Helbig JH, Bernander S, Castellani Pastoris M, Etienne J, Gaia V, Lauwers S, Lindsay D, Luck PC, Marques T, Mentula S, Peeters MF, Pelaz C, Struelens M, Uldum SA, Wewalka G, Harrison TG (2002) Pan-European study on culture-proven Legionnaires’ disease: distribution of Legionella pneumophila serogroups and monoclonal subgroups. Eur J Clin Microbiol Infect Dis 21:710–716
Helbig JH, Engelstadter T, Maiwald M, Uldum SA, Witzleb W, Luck PC (1999) Diagnostic relevance of the detection of Legionella DNA in urine samples by the polymerase chain reaction. Eur J Clin Microbiol Infect Dis 18:716–722
Helbig JH, Konig B, Knospe H, Bubert B, Yu C, Luck CP, Riboldi-Tunnicliffe A, Hilgenfeld R, Jacobs E, Hacker J, Fischer G (2003a) The PPIase active site of Legionella pneumophila Mip protein is involved in the infection of eukaryotic host cells. Biol Chem 384:125–137
Helbig JH, Luck PC, Knirel YA, Witzleb W, Zahringer U (1995a) Molecular characterization of a virulence-associated epitope on the lipopolysaccharide of Legionella pneumophila serogroup 1. Epidemiol Infect 115:71–78
Helbig JH, Luck PC, Steinert M, Jacobs E, Witt M (2001a) Immunolocalization of the Mip protein of intracellularly and extracellularly grown Legionella pneumophila. Lett Appl Microbiol 32:83–88
Helbig JH, Ludwig B, Luck PC, Groh A, Witzleb W, Hacker J (1995b) Monoclonal antibodies to Legionella Mip proteins recognize genus-and species-specific epitopes. Clin Diagn Lab Immunol 2:160–165
Helbig JH, Uldum SA, Bernander S, Luck PC, Wewalka G, Abraham B, Gaia V, Harrison TG (2003b) Clinical utility of urinary antigen detection for diagnosis of community-acquired, travel-associated, and nosocomial legionnaires’ disease. J Clin Microbiol 41:838–840
Helbig JH, Uldum SA, Luck PC, Harrison TG (2001b) Detection of Legionella pneumophila antigen in urine samples by the BinaxNOW immunochromatographic assay and comparison with both Binax Legionella Urinary Enzyme Immunoassay (EIA) and Biotest Legionella Urin Antigen EIA. J Med Microbiol 50:509–516
Heller R, Holler C, Sussmuth R, Gundermann KO (1998) Effect of salt concentration and temperature on survival of Legionella pneumophila. Letts Appl Microbiol 26:64–68
Henke M, Seidel KM (1986) Association between Legionella pneumophila and amoebae in water. Isr J Med Sci 22:690–695
Herpers BL, de Jongh BM, van der Zwaluw K, van Hannen EJ (2003) Real-time PCR assay targets the 23S-5S spacer for direct detection and differentiation of Legionella spp. and Legionella pneumophila. J Clin Microbiol 41:4815–4816
Herrmann V, Eidner A, Rydzewski K, Bladel I, Jules M, Buchrieser C, Eisenreich W, Heuner K (2011) GamA is a eukaryotic-like glucoamylase responsible for glycogen- and starch-degrading activity of Legionella pneumophila. Int J Med Microbiol 301:133–139
Hervet E, Charpentier X, Vianney A, Lazzaroni JC, Gilbert C, Atlan D, Doublet P (2011) Protein kinase LegK2 is a type IV secretion system effector involved in endoplasmic reticulum recruitment and intracellular replication of Legionella pneumophila. Infect Immun 79:1936–1950
Herwaldt LA, Gorman GW, McGrath T, Toma S, Brake B, Hightower AW, Jones J, Reingold AL, Boxer PA, Tang PW, Moss CW, Wilkinson H, Brenner DJ, Steigerwalt AG, Broome CV (1984) A new Legionella species, Legionella feeleii species nova, causes Pontiac fever in an automobile plant. Ann Inter Med 100:333–338
Heuner K, Bender-Beck L, Brand BC, Luck PC, Mann KH, Marre R, Ott M, Hacker J (1995) Cloning and genetic characterization of the flagellum subunit gene (flaA) of Legionella pneumophila serogroup 1. Infect Immun 63:2499–2507
Heuner K, Brand BC, Hacker J (1999) The expression of the flagellum of Legionella pneumophila is modulated by different environmental factors. FEMS Microbiol Lett 175:69–77
Heuner K, Dietrich C, Skriwan C, Steinert M, Hacker J (2002) Influence of the alternative sigma(28) factor on virulence and flagellum expression of Legionella pneumophila. Infect Immun 70:1604–1608
Heuner K, Hacker J, Brand BC (1997) The alternative sigma factor sigma28 of Legionella pneumophila restores flagellation and motility to an Escherichia coli fliA mutant. J Bacteriol 179:17–23
Heuner K, Steinert M (2003) The flagellum of Legionella pneumophila and its link to the expression of the virulent phenotype. Int J Med Microbiol 293:133–143
Hickey EK, Cianciotto NP (1994) Cloning and sequencing of the Legionella pneumophila fur gene. Gene 143:117–121
Hickey EK, Cianciotto NP (1997) An iron- and fur-repressed Legionella pneumophila gene that promotes intracellular infection and encodes a protein with similarity to the Escherichia coli aerobactin synthetases. Infect Immun 65:133–143
Hicks LA, Rose CE, Jr, Fields BS, Drees ML, Engel JP, Jenkins PR, Rouse BS, Blythe D, Khalifah AP, Feikin DR, Whitney CG (2007) Increased rainfall is associated with increased risk for legionellosis. Epidemiol Infect 135:811–817
Higa F, Edelstein PH (2001) Potential virulence role of the Legionella pneumophila ptsP ortholog. Infect Immun 69:4782–4789
Higa F, Koide M, Furugen M, Akamine M, Hibiya K, Haranaga S, Yara S, Tateyama M, Yamane N, Fujita J (2008) Detection of Legionella pneumophila serogroup 1 antigen in respiratory samples using an immunochromatographic membrane test. J Microbiol Methods 74:121–122
High AS, Torosian SD, Rodgers FG (1993) Cloning, nucleotide sequence and expression in Escherichia coli of a gene (ompM) encoding a 25 kDa major outer-membrane protein (MOMP) of Legionella pneumophila. J Gen Microbiol 139:1715–1721
Highsmith AK, Mackel DC, Baine WB, Anderson RL, Fraser DW (1978) Observations of endotoxin-like activity associated with the Legionnaires’ disease bacterium. Curr Microbiol 1:315–317
Hilbi H, Haas A (2012) Secretive bacterial pathogens and the secretory pathway. Traffic 13(9):1187–1197
Hilbi H, Hoffmann C, Harrison CF (2011) Legionella spp. outdoors: colonization, communication, and persistence. Environ Microbiol Reports 3:286–296
Hilbi H, Jarraud S, Hartland E, Buchrieser C (2010) Update on Legionnaires’ disease: pathogenesis, epidemiology, detection and control. Mol Microbiol 76:1–11
Hilbi H, Segal G, Shuman HA (2001) Icm/Dot-dependent upregulation of phagocytosis by Legionella pneumophila. Mol Microbiol 42:603–617
Hindahl MS, Iglewski BH (1987) Cloning and expression of a common Legionella outer membrane antigen in Escherichia coli. Microb Pathogen 2:91–99
Hindre T, Bruggemann H, Buchrieser C, Hechard Y (2008) Transcriptional profiling of Legionella pneumophila biofilm cells and the influence of iron on biofilm formation. Microbiology 154:30–41
Hlady WG, Mullen RC, Mintz CS, Shelton BG, Hopkins RS, Daikos GL (1993) Outbreak of Legionnaire’s disease linked to a decorative fountain by molecular epidemiology. Am J Epidemiol 138:555–562
Hoffman PS, Houston L, Butler CA (1990) Legionella pneumophila htpAB heat shock operon: nucleotide sequence and expression of the 60-kilodalton antigen in L. pneumophila-infected HeLa cells. Infect Immun 58:3380–3387
Hoffman PS, Ripley M, Weeratna R (1992a) Cloning and nucleotide sequence of a gene (ompS) encoding the major outer membrane protein of Legionella pneumophila. J Bacteriol 174:914–920
Hoffman PS, Seyer JH, Butler CA (1992b) Molecular characterization of the 28- and 31-kilodalton subunits of the Legionella pneumophila major outer membrane protein. J Bacteriol 174:908–913
Hofmann A, Beaulieu Y, Bernard F, Rico P (2009) Fulminant legionellosis in two patients treated with infliximab for Crohn’s disease: case series and literature review. Can J Gastroenterol 23:829–833
Holden EP, Winkler HH, Wood DO, Leinbach ED (1984) Intracellular growth of Legionella pneumophila within Acanthamoeba castellanii Neff. Infect Immun 45:18–24
Hookey JV, Saunders NA, Fry NK, Birtles RJ, Harrison TG (1996) Phylogeny of Legionellaceae based on small-subunit ribosomal DNA sequences and proposal of Legionella lytica comb. nov. for Legionella-like amoebal pathogens. Int J Syst Bacteriol 46:526–531
Horwitz MA (1983) Formation of a novel phagosome by the Legionnaires’ disease bacterium (Legionella pneumophila) in human monocytes. J Exp Med 158:1319–1331
Horwitz MA (1992) Interactions between macrophages and Legionella pneumophila. Curr Topics Microbiol Immunol 181:265–282
Horwitz MA, Maxfield FR (1984) Legionella pneumophila inhibits acidification of its phagosome in human monocytes. J Cell Biol 99:1936–1943
Horwitz MA, Silverstein SC (1980) Legionnaire’ disease bacterium (Legionella pneumophila) multiples intracellularly in human monocytes. J Clin Invest 66:441–450
Horwitz MA, Silverstein SC (1981) Interaction of the Legionnaires' disease bacterium (Legionella pneumophila) with human phagocytes. I. L. pneumophila resists killing by polymorphonuclear leukocytes, antibody, and complement. J Exp Med 153:386–397
Hovel-Miner G, Faucher SP, Charpentier X, Shuman HA (2010) ArgR-regulated genes are derepressed in the Legionella-containing vacuole. J Bacteriol 192:4504–4516
Hovel-Miner G, Pampou S, Faucher SP, Clarke M, Morozova I, Morozov P, Russo JJ, Shuman HA, Kalachikov S (2009) SigmaS controls multiple pathways associated with intracellular multiplication of Legionella pneumophila. J Bacteriol 191:2461–2473
Hsu BM, Huang CC, Chen JS, Chen NH, Huang JT (2011) Comparison of potentially pathogenic free-living amoeba hosts by Legionella spp. in substrate-associated biofilms and floating biofilms from spring environments. Water Res 45:5171–5183
Huang B, Yuan Z, Heron BA, Gray BR, Eglezos S, Bates JR, Savill J (2006) Distribution of 19 major virulence genes in Legionella pneumophila serogroup 1 isolates from patients and water in Queensland. (Australia) J Med Microbiol 55:993–997
Huang L, Boyd D, Amyot WM, Hempstead AD, Luo ZQ, O'Connor TJ, Chen C, Machner M, Montminy T, Isberg RR (2011) The E Block motif is associated with Legionella pneumophila translocated substrates. Cell Microbiol 13:227–245
Hubber A, Roy CR (2010a) Modulation of host cell function by Legionella pneumophila type IV effectors. Annu Rev Cell Dev Biol 26:261–283
Hubber A, Roy CR (2010b) Modulation of host cell function by Legionella pneumophila type IV effectors. Annu Rev Cell Dev Biol 26:261–283
Hughes MS, Steele TW (1994) Occurrence and distribution of Legionella species in composted plant materials. Appl Environ Microbiol 60:2003–2005
Huhn GD, Adam B, Ruden R, Hilliard L, Kirkpatrick P, Todd J, Crafts W, Passaro D, Dworkin MS (2005) Outbreak of travel-related Pontiac fever among hotel guests illustrating the need for better diagnostic tests. J Travel Med 12:173–179
Husmann LK, Johnson W (1994) Cytotoxicity of extracellular Legionella pneumophila. Infect Immun 62:2111–2114
Hussong D, Colwell RR, O'Brien M, Weiss E, Pearson AD, Weiner RM, Burge WD (1987) Viable Legionella pneumophila not detectable by culture on agar media. Biotechnology 5:947–950
Huston WM, Naylor J, Cianciotto NP, Jennings MP, McEwan AG (2008) Functional analysis of the multi-copper oxidase from Legionella pneumophila. Microbes Infect 10:497–503
Hwang MG, Katayama H, Ohgaki S (2006) Effect of intracellular resuscitation of Legionella pneumophila in Acanthamoeba polyphage cells on the antimicrobial properties of silver and copper. Environ Sci Technol 40:7434–7439
Igel L, Helbig JH, Luck PC (2004) Isolation and characterization of a nonfluorescent strain of Legionella parisiensis. J Clin Microbiol 42:2877–2878
Ingmundson A, Delprato A, Lambright DG, Roy CR (2007) Legionella pneumophila proteins that regulate Rab1 membrane cycling. Nature 450:365–369
Inoue Y, Fujino Y, Onodera M, Kikuchi S, Shozushima T, Ogino N, Mori K, Oikawa H, Koeda Y, Ueda H, Takahashi T, Terui K, Nakadate T, Aoki H, Endo S (2011) Tsunami lung. J Anesth doi 10.1007/s00540-011-1273-6
Iovieno A, Ledee DR, Miller D, Alfonso EC (2010) Detection of bacterial endosymbionts in clinical Acanthamoeba isolates. Ophthalmology 117:445–452
Isberg RR, O'Connor TJ, Heidtman M (2009) The Legionella pneumophila replication vacuole: making a cosy niche inside host cells. Nat Rev Microbiol 7:13–24
Ivanov SS, Charron G, Hang HC, Roy CR (2010) Lipidation by the host prenyltransferase machinery facilitates membrane localization of Legionella pneumophila effector proteins. J Biol Chem 285:34686–34698
Ivanov SS, Roy CR (2009) Modulation of ubiquitin dynamics and suppression of DALIS formation by the Legionella pneumophila Dot/Icm system. Cell Microbiol 11:261–278
Izu K, Yoshida S, Miyamoto H, Chang B, Ogawa M, Yamamoto H, Goto Y, Taniguchi H (1999) Grouping of 20 reference strains of Legionella species by the growth ability within mouse and guinea pig macrophages. FEMS Immunol Med Microbiol 26:61–68
Jacobi S, Heuner K (2003) Description of a putative type I secretion system in Legionella pneumophila. Int J Med Microbiol 293:349–358
Jacobs RF, Locksley RM, Wilson CB, Haas JE, Klebanoff SJ (1984) Interaction of primate alveolar macrophages and Legionella pneumophila. J Clin Invest 73:1515–1523
Jacobson KL, Miceli MH, Tarrand JJ, Kontoyiannis DP (2008) Legionella pneumonia in cancer patients. Medicine (Baltimore) 87:152–159
Jain B, Brand BC, Luck PC, Di Berardino M, Dimroth P, Hacker J (1996) An oxaloacetate decarboxylase homologue protein influences the intracellular survival of Legionella pneumophila. FEMS Microbiol Lett 145:273–279
Jain V, Kumar M, Chatterji D (2006) ppGpp stringent response and survival. J Microbiol 44:1–10
James BW, Mauchline WS, Dennis PJ, Keevil CW (1997) A study of iron acquisition mechanisms of Legionella pneumophila grown in chemostat culture. Curr Microbiol 34:238–243
James BW, Mauchline WS, Fitzgeorge RB, Dennis PJ, Keevil CW (1995) Influence of iron-limited continuous culture on physiology and virulence of Legionella pneumophila. Infect Immun 63:4224–4230
Jameson-Lee M, Garduno RA, Hoffman PS (2011) DsbA2 (27 kDa Com1-like protein) of Legionella pneumophila catalyses extracytoplasmic disulphide-bond formation in proteins including the Dot/Icm type IV secretion system. Mol Microbiol 80:835–852
Janeway CA, Jr, Medzhitov R (2002) Innate immune recognition. Annu Rev Immunol 20:197–216
Jank T, Böhmer KE, Tzivelekidis T, Schwan C, Belyi Y, Aktories K (2012) Domain organization of Legionella effector SetA. Cell Microbiol 14:852–868
Jarraud S, Etienne J (2012) Caractéistiques clinique et diagnostic des cas de légionellose. In: Jarraud S, Freney J (ed) Legionella, vol 2. Lavoisier, Paris, pp 147–177
Jaulhac B, Nowicki M, Bornstein N, Meunier O, Prevost G, Piemont Y, Fleurette J, Monteil H (1992) Detection of Legionella spp. in bronchoalveolar lavage fluids by DNA amplification. J Clin Microbiol 30:920–924
Jepras RI, Fitzgeorge RB, Baskerville A (1985) A comparison of virulence of two strains of Legionella pneumophila based on experimental aerosol infection of guinea pigs. J Hyg 95:29–38
Jernigan DB, Hofmann J, Cetron MS, Genese CA, Nuorti JP, Fields BS, Benson RF, Carter RJ, Edelstein PH, Guerrero IC, Paul SM, Lipman HB, Breiman R (1996) Outbreak of Legionnaires’ disease among cruise ship passengers exposed to a contaminated whirlpool spa. Lancet 347:494–499
Jinno S, Pulido S, Pien BC (2009) First reported United States case of Legionella pneumophila serogroup 1 pneumonia in a patient receiving anti-tumor necrosis factor-a therapy. Hawaii Med J 68:109–112
Johnson TL, Abendroth J, Hol WG, Sandkvist M (2006) Type II secretion: from structure to function. FEMS Microbiol Lett 255:175–186
Johnson W, Varner L, Poch M (1991) Acquisition of iron by Legionella pneumophila: role of iron reductase. Infect Immun 59:2376–2381
Joly JR, Boissinot M, Duchaine J, Duval M, Rafrafi J, Ramsay D, Letarte R (1984) Ecological distribution of Legionellaceae in the Quebec City area. Can J Microbiol 30:63–67
Jonas D, Rosenbaum A, Weyrich S, Bhakdi S (1995) Enzyme-linked immunoassay for detection of PCR-amplified DNA of legionellae in bronchoalveolar fluid. J Clin Microbiol 33:1247–1252
Jones TF, Benson RF, Brown EW, Rowland JR, Crosier SC, Schaffner W (2003) Epidemiologic investigation of a restaurant-associated outbreak of Pontiac fever. Clin Infect Dis 37:1292–1297
Joseph C (2002) New outbreak of Legionnaires’ disease in the United Kingdom. BMJ 325:347–348
Joseph CA (2004) Legionnaires’ disease in Europe 2000-2002. Epidemiol Infect 132:417–424
Joseph CA, Dedman D, Birtles R, Watson JM, Bartlett CL (1994a) Legionnaires’ disease surveillance: England and Wales, 1993. Commun Dis Rep CDR Rev 4:R109–R111
Joseph CA, Watson JM, Harrison TG, Bartlett CL (1994b) Nosocomial Legionnaires’ disease in England and Wales, 1980-92. Epidemiol Infect 112:329–345
Joseph CA, Harrison TG, Ilijic-Car D, Bartlett CL (1997) Legionnaires’ disease in residents of England and Wales: 1996. Commun Dis Rep CDR Rev 7:R153–R159
Joseph CA, Harrison TG, Ilijic-Car D, Bartlett CL (1998) Legionnaires’ disease in residents of England and Wales: 1997. Commun Dis Public Health 1:252–258
Joseph CA, Hutchinson EJ, Dedman D, Birtles RJ, Watson JM, Bartlett CL (1995) Legionnaires’ disease surveillance: England and Wales 1994. Commun Dis Rep CDR Rev 5:R180–R183
Joseph CA, Ricketts KD (2010) Legionnaires disease in Europe 2007-2008. Euro Surveill 15:19493
Joseph CA, Ricketts KD, Yadav R, Patel S (2010) Travel-associated Legionnaires disease in Europe in 2009. Euro Surveill 15:19683
Joshi AD, Swanson MS (1999) Comparative analysis of Legionella pneumophila and Legionella micdadei virulence traits. Infect Immun 67:4134–4142
Joshi AD, Swanson MS (2011) Secrets of a successful pathogen: Legionella resistance to progression along the autophagic pathway. Front Microbiol 2:138
Juhas M, Crook DW, Hood DW (2008) Type IV secretion systems: tools of bacterial horizontal gene transfer and virulence. Cell Microbiol 10:2377–2386
Kagan JC, Roy CR (2002) Legionella phagosomes intercept vesicular traffic from endoplasmic reticulum exit sites. Nat Cell Biol 4:945–954
Kagan JC, Stein MP, Pypaert M, Roy CR (2004) Legionella subvert the functions of Rab1 and Sec22b to create a replicative organelle. J Exp Med 199:1201–1211
Kampfer P (2012) Systematics of prokaryotes: the state of the art. Anton Van Leeuwenhoek 101:3–11
Karin M, Lin A (2002) NF-kappaB at the crossroads of life and death. Nat Immunol 3:221–227
Katz SM, Brodsky I, Kahn SB (1979) Legionnaires’ disease: ultrastructural appearance of the agent in a lung biopsy specimen. Arch Pathol Lab Med 103:261–264
Kay E, Dubuis C, Haas D (2005) Three small RNAs jointly ensure secondary metabolism and biocontrol in Pseudomonas fluorescens CHA0. Proc Natl Acad Sci USA 102:17136–17141
Kazandjian D, Chiew R, Gilbert GL (1997) Rapid diagnosis of Legionella pneumophila serogroup 1 infection with the Binax enzyme immunoassay urinary antigen test. J Clin Microbiol 35:954–956
Keevil CW (2003) Rapid detection of biofilms and adherent pathogens using scanning confocal laser microscopy and episcopic differential interference contrast microscopy. Water Sci Technol 47:105–116
Kikuhara H, Ogawa M, Miyamoto H, Nikaido Y, Yoshida S (1994) Intracellular multiplication of Legionella pneumophila in Tetrahymena thermophila. Sangyo Ika Daigaku Zasshi 16:263–275
Kilvington S, Price J (1990) Survival of Legionella pneumophila within cysts of Acanthamoeba polyphaga following chlorine exposure. J Appl Bacteriol 68:519–525
Kim MJ, Sohn JW, Park DW, Park SC, Chun BC (2003) Characterization of a lipoprotein common to Legionella species as a urinary broad-spectrum antigen for diagnosis of Legionnaires’ disease. J Clin Microbiol 41:2974–2979
Kimura S, Tateda K, Ishii Y, Horikawa M, Miyairi S, Gotoh N, Ishiguro M, Yamaguchi K (2009) Pseudomonas aeruginosa Las quorum sensing autoinducer suppresses growth and biofilm production in Legionella species. Microbiology 155:1934–1939
Kinch LN, Yarbrough ML, Orth K, Grishin NV (2009) Fido, a novel AMPylation domain common to fic, doc, and AvrB. PLoS One 4:e5818
King CH, Fields BS, Shotts EB, Jr, White EH (1991) Effects of cytochalasin D and methylamine on intracellular growth of Legionella pneumophila in amoebae and human monocyte-like cells. Infect Immun 59:758–763
King CH, Shotts EB, Wooley RE, Porter KG (1988) Survival of coliforms and bacterial pathogens within protozoa during chlorination. Appl Environ Microbiol 54:3023–3033
Kirby JE, Vogel JP, Andrews HL, Isberg RR (1998) Evidence for pore-forming ability by Legionella pneumophila. Mol Microbiol 27:323–336
Kishimoto RA, Kastello MD, White JD, Shirey FG, McGann VG, Larson EW, Hedlund KW (1979) In vitro interaction between normal cynolmolgus monkey alveolar macrophages and Legionnaires disease bacteria. Infect Immun 25:761–763
Klein GW (1980) Cross-reaction to Legionella pneumophila antigen in sera with elevated titers to Pseudomonas pseudomallei. J Clin Microbiol 11:27–29
Knirel YA, Moll H, Helbig JH, Zahringer U (1997) Chemical characterization of a new 5,7-diamino-3,5,7,9-tetradeoxynonulosonic acid released by mild acid hydrolysis of the Legionella pneumophila serogroup 1 lipopolysaccharide. Carbohydr Res 304:77–79
Ko KS, Hong SK, Lee HK, Park MY, Kook YH (2003) Molecular evolution of the dotA gene in Legionella pneumophila. J Bacteriol 185:6269–6277
Ko KS, Lee HK, Park MY, Lee KH, Yun YJ, Woo SY, Miyamoto H, Kook YH (2002) Application of RNA polymerase beta-subunit gene (rpoB) sequences for the molecular differentiation of Legionella species. J Clin Microbiol 40:2653–2658
Ko KS, Miyamoto H, Lee HK, Park MY, Fukuda K, Park BJ, Kook YH (2006) Genetic diversity of Legionella pneumophila inferred from rpoB and dotA sequences. Clin Microbiol Infect 12:254–261
Kohler R, Bubert A, Goebel W, Steinert M, Hacker J, Bubert B (2000) Expression and use of the green fluorescent protein as a reporter system in Legionella pneumophila. Mol Gen Genet 262:1060–1069
Kohler RB, Winn WCJ, Wheat LJ (1984) Onset and duration of urinary antigen excretion in Legionnaires disease. J Clin Microbiol 20:605–607
Koide M, Higa F, Tateyama M, Sakugawa H, Saito A (2004) Comparison of polymerase chain reaction and two urinary antigen detection kits for detecting Legionella in clinical samples. Eur J Clin Microbiol Infect Dis 23:221–223
Komura T, Ikeda T, Hoshino K, Shibamura A, Nishikawa Y (2012) Caenorhabditis elegans as an alternative model to study senescence of host defense and the prevention by immunonutrition. Adv Exp Med Biol 710:19–27
Komura T, Yasui C, Miyamoto H, Nishikawa Y (2010) Caenorhabditis elegans as an alternative model host for Legionella pneumophila, and protective effects of Bifidobacterium infantis. Appl Environ Microbiol 76:4105–4108
Konig C, Hebestreit H, Valenza G, Abele-Horn M, Speer CP (2005) Legionella waltersii–a novel cause of pneumonia? Acta Paediatr 94:1505–1507
Kool JL, Warwick MC, Pruckler JM, Brown EW, Butler JC (1998) Outbreak of Legionnaires' disease at a bar after basement flooding. Lancet 351:1030
Koronakis V, Eswaran J, Hughes C (2004) Structure and function of TolC: the bacterial exit duct for proteins and drugs. Annu Rev Biochem 73:467–489
Korotkov KV, Gonen T, Hol WG (2011) Secretins: dynamic channels for protein transport across membranes. Trends Biochem Sci 36:433–443
Korvick JA, Yu VL (1987) Legionnaire’ disease: an emerging surgical problem. Ann Thorac Surg 43:341–347
Korvick JA, Yu VL, Fang GD (1987) Legionella species as hospital-acquired respiratory pathogens. Semin Respir Infect 2:34–47
Kozak NA, Benson RF, Brown E, Alexander NT, Taylor TH, Jr, Shelton BG, Fields BS (2009) Distribution of lag-1 alleles and sequence-based types among Legionella pneumophila serogroup 1 clinical and environmental isolates in the United States. J Clin Microbiol 47:2525–2535
Kozak NA, Buss M, Lucas CE, Frace M, Govil D, Travis T, Olsen-Rasmussen M, Benson RF, Fields BS (2010) Virulence factors encoded by Legionella longbeachae identified on the basis of the genome sequence analysis of clinical isolate D-4968. J Bacteriol 192:1030–1044
Krinos C, High AS, Rodgers FG (1999) Role of the 25 kDa major outer membrane protein of Legionella pneumophila in attachment to U-937 cells and its potential as a virulence factor for chick embryos. J Appl Microbiol 86:237–244
Kubiak X, Dervins-Ravault D, Pluvinage B, Chaffotte AF, Gomez-Valero L, Dairou J, Busi F, Dupret JM, Buchrieser C, Rodrigues-Lima F (2012) Characterization of an acetyltransferase that detoxifies aromatic chemicals in Legionella pneumophila. Biochem J 445:219–229
Kubori T, Hyakutake A, Nagai H (2008) Legionella translocates an E3 ubiquitin ligase that has multiple U-boxes with distinct functions. Mol Microbiol 67:1307–1319
Kubori T, Nagai A (2011) Bacterial effector-involved temporal and spatial regulation by hijack of the host ubiquitin pathway. Front Microbiol 2:145
Kubori T, Shinzawa N, Kanuka H, Nagai H (2010) Legionella metaeffector exploits host proteasome to temporally regulate cognate effector. PLoS Pathog 6:e1001216
Kuiper MW, Wullings BA, Akkermans AD, Beumer RR, van der Kooij D (2004) Intracellular proliferation of Legionella pneumophila in Hartmannella vermiformis in aquatic biofilms grown on plasticized polyvinyl chloride. Appl Environ Microbiol 70:6826–6833
Kura F, Suzuki K, Watanabe H, Akamatsu Y, Amano F (1994) Difference in Legionella pneumophila growth permissiveness between J774.1 murine macrophage-like JA-4 cells and lipopolysaccharide (LPS)-resistant mutant cells, LPS1916, after stimulation with LPS. Infect Immun 62:5419–5423
Kuroki H, Miyamoto H, Fukuda K, Iihara H, Kawamura Y, Ogawa M, Wang Y, Ezaki T, Taniguchi H (2007) Legionella impletisoli sp. nov. and Legionella yabuuchiae sp. nov., isolated from soils contaminated with industrial wastes in Japan. Syst Appl Microbiol 30:273–279
Kusnetsov J, Neuvonen LK, Korpio T, Uldum SA, Mentula S, Putus T, Tran Minh NN, Martimo KP (2010) Two Legionnaires' disease cases associated with industrial waste water treatment plants: a case report. BMC Infect Dis 10:343
La Scola B, Birtles RJ, Greub G, Harrison TJ, Ratcliff RM, Raoult D (2004) Legionella drancourtii sp. nov., a strictly intracellular amoebal pathogen. Int J Syst Evol Microbiol 54:699–703
La Scola B, Boyadjiev I, Greub G, Khamis A, Martin C, Raoult D (2003) Amoeba-resisting bacteria and ventilator-associated pneumonia. Emerg Infect Dis 9:815–821
Lairson LL, Henrissat B, Davies GJ, Withers SG (2008) Glycosyltransferases: structures, functions, and mechanisms. Annu Rev Biochem 77:521–555
Lam MC, Ang LW, Tan AL, James L, Goh KT (2011) Epidemiology and control of legionellosis. (Singapore) Emerg Infect Dis 17:1209–1215
Lammertyn E, Anne J (2004) Protein secretion in Legionella pneumophila and its relation to virulence. FEMS Microbiol Lett 238:273–279
Lamoth F, Greub G (2010) Amoebal pathogens as emerging causal agents of pneumonia. FEMS Microbiol Rev 34:260–280
Lang C, Flieger A (2011) Characterisation of Legionella pneumophila phospholipases and their impact on host cells. Eur J Cell Biol 90:903–912
Lasheras A, Boulestreau H, Rogues AM, Ohayon-Courtes C, Labadie JC, Gachie JP (2006) Influence of amoebae and physical and chemical characteristics of water on presence and proliferation of Legionella species in hospital water systems. Am J Infect Control 34:520–525
Lau HY, Ashbolt NJ (2009) The role of biofilms and protozoa in Legionella pathogenesis: implications for drinking water. J Appl Microbiol 107:368–378
Lavocat MP, Berthier JC, Rousson A, Bornstein N, Hartemann E (1987) Pulmonary legionnaires’ disease in a child following drowning in fresh water. Presse Med 16:780
Lawley TD, Klimke WA, Gubbins MJ, Frost LS (2003) F factor conjugation is a true type IV secretion system. FEMS Microbiol Lett 224:1–15
Lawrence C, Reyrolle M, Dubrou S, Forey F, Decludt B, Goulvestre C, Matsiota-Bernard P, Etienne J, Nauciel C (1999) Single clonal origin of a high proportion of Legionella pneumophila serogroup 1 isolates from patients and the environment in the area of Paris, France, over a 10-year period. J Clin Microbiol 37:2652–2655
Le Negrate G, Faustin B, Welsh K, Loeffler M, Krajewska M, Hasegawa P, Mukherjee S, Orth K, Krajewskin S, Godzik A, Guiney DG, Reed JC (2008) Salmonella secreted factor L deubiquitinase of Salmonella typhimurium inhibits NF-kappaB, suppresses IkappaBalpha ubiquitination and modulates innate immune responses. J Immunol 80:5045–5056
LeBlanc JJ, Brassinga AK, Ewann F, Davidson RJ, Hoffman PS (2008) An ortholog of OxyR in Legionella pneumophila is expressed postexponentially and negatively regulates the alkyl hydroperoxide reductase (ahpC2D) operon. J Bacteriol 190:3444–3455
LeBlanc JJ, Davidson RJ, Hoffman PS (2006) Compensatory functions of two alkyl hydroperoxide reductases in the oxidative defense system of Legionella pneumophila. J Bacteriol 188:6235–6244
Lee HK, Shim JI, Kim HE, Yu JY, Kang YH (2010) Distribution of Legionella species from environmental water sources of public facilities and genetic diversity of L. pneumophila serogroup 1 in South Korea. Appl Environ Microbiol 76:6547–6554
Lee J, Caplivski D, Wu M, Huprikar S (2009) Pneumonia due to Legionella feeleii: case report and review of the literature. Transpl Infect Dis 11:337–340
Lee JV, West AA (1991) Survival and growth of Legionella species in the environment. J Appl Bacteriol (Suppl) 70:121S–129S
Lee PA, Tullman-Ercek D, Georgiou G (2006) The bacterial twin-arginine translocation pathway. Annu Rev Microbiol 60:373–395
Lehtola MJ, Torvinen E, Kusnetsov J, Pitkanen T, Maunula L, von Bonsdorff CH, Martikainen PJ, Wilks SA, Keevil CW, Miettinen IT (2007) Survival of Mycobacterium avium, Legionella pneumophila, Escherichia coli, and caliciviruses in drinking water-associated biofilms grown under high-shear turbulent flow. Appl Environ Microbiol 73:2854–2859
Lenz DH, Miller MB, Zhu J, Kulkarni RV, Bassler BL (2005) CsrA and three redundant small RNAs regulate quorum sensing in Vibrio cholerae. Mol Microbiol 58:1186–1202
Lettinga KD, Verbon A, Weverling GJ, Schellekens JF, Den Boer JW, Yzerman EP, Prins J, Boersma WG, van Ketel RJ, Prins JM, Speelman P (2002) Legionnaires’ disease at a Dutch flower show: prognostic factors and impact of therapy. Emerg Infect Dis 8:1448–1454
Levet-Paulo M, Lazzaroni JC, Gilbert C, Atlan D, Doublet P, Vianney A (2011) The atypical two-component sensor kinase Lpl0330 from Legionella pneumophila controls the bifunctional diguanylate cyclase-phosphodiesterase Lpl0329 to modulate bis-(3'-5')-cyclic dimeric GMP synthesis. J Biol Chem 286:31136–31144
Levi A, Folcher M, Jenal U, Shuman HA (2011) Cyclic diguanylate signaling proteins control intracellular growth of Legionella pneumophila. MBio 2:e00316–10
Levi MH, Pasculle AW, Dowling JN (1987) Role of the alveolar macrophage in host defense and immunity to Legionella micdadei pneumonia in the guinea pig. Microb Pathogen 2:269–282
Li JS, O'Brien ED, Guest C (2002) A review of national legionellosis surveillance in Australia, 1991 to 2000. Commun Dis Intell 26:461–468
Lieberman D, Shmarkov O, Gelfer Y, Ben-Yaakov M, Lazarovich Z, Boldur I (2002) Serological evidence of Legionella species infection in acute exacerbation of COPD. Eur Respir J 19:392–397
Lightfield KL, Persson J, Brubaker SW, Witte CE, von Moltke J, Dunipace EA, Henry T, Sun YH, Cado D, Dietrich WF, Monack DM, Tsolis RM, Vance RE (2008) Critical function for Naip5 in inflammasome activation by a conserved carboxy-terminal domain of flagellin. Nat Immunol 9:1171–1178
Liles MR, Aber Scheel T, Cianciotto NP (2000) Discovery of a nonclassical siderophore, legiobactin, produced by strains of Legionella pneumophila. J Bacteriol 182:749–757
Liles MR, Edelstein PH, Cianciotto NP (1999) The prepilin peptidase is required for protein secretion by and the virulence of the intracellular pathogen Legionella pneumophila. Mol Microbiol 31:959–970
Liles MR, Viswanathan VK, Cianciotto NP (1998) Identification and temperature regulation of Legionella pneumophila genes involved in type IV pilus biogenesis and type II protein secretion. Infect Immun 66:1776–1782
Lindsay DS, Abraham WH, Findlay W, Christie P, Johnston F, Edwards GF (2004) Laboratory diagnosis of legionnaires' disease due to Legionella pneumophila serogroup 1: comparison of phenotypic and genotypic methods. J Med Microbiol 53:183–187
Lindsay DS, Brown AW, Brown DJ, Pravinkumar J, Anderson E, Edwards GF (2012) Legionella longbeachae serogroup 1 infections linked to Potting Compost. J Med Microbiol 61:218–222
Lippmann J, Rothenburg S, Deigendesch N, Eitel J, Meixenberger K, van Laak V, Slevogt H, N’Guessan PD, Hippenstiel S, Chakraborty T, Flieger A, Suttorp N, Opitz B (2008) IFNbeta responses induced by intracellular bacteria or cytosolic DNA in different human cells do not require ZBP1 (DLM-1/DAI). Cell Microbiol 10:2579–2588
Liu Z, Lin YE, Stout JE, Hwang CC, Vidic RD, Yu VL (2006) Effect of flow regimes on the presence of Legionella within the biofilm of a model plumbing system. J Appl Microbiol 101:437–442
Lo Presti F, Riffard S, Jarraud S, Le Gallou F, Richet H, Vandenesch F, Etienne J (2000) Isolation of Legionella oakridgensis from two patients with pleural effusion living in the same geographical area. J Clin Microbiol 38:3128–3130
Lo Presti F, Riffard S, Meugnier H, Reyrolle M, Lasne Y, Grimont PA, Grimont F, Benson RF, Brenner DJ, Steigerwalt AG, Etienne J, Freney J (2001) Legionella gresilensis sp. nov. and Legionella beliardensis sp. nov., isolated from water in France. Int J Syst Evol Microbiol 51:1949–1957
Lo Presti F, Riffard S, Meugnier H, Reyrolle M, Lasne Y, Grimont PA, Grimont F, Vandenesch F, Etienne J, Fleurette J, Freney J (1999) Legionella taurinensis sp. nov., a new species antigenically similar to Legionella spiritensis. Int J Syst Bacteriol 49:397–403
Lo Presti F, Riffard S, Vandenesch F, Reyrolle M, Ronco E, Ichai P, Etienne J (1997) The first clinical isolate of Legionella parisiensis, from a liver transplant patient with pneumonia. J Clin Microbiol 35:1706–1709
Loeb M, Simor AE, Mandell L, Krueger P, McArthur M, James M, Walter S, Richardson E, Lingley M, Stout J, Stronach D, McGeer A (1999) Two nursing home outbreaks of respiratory infection with Legionella sainthelensi. J Am Geriatr Soc 47:547–552
Lomma M, Dervins-Ravault D, Rolando M, Nora T, Newton HJ, Sansom FM, Sahr T, Gomez-Valero L, Jules M, Hartland EL, Buchrieser C (2010) The Legionella pneumophila F-box protein Lpp 2082 (AnkB) modulates ubiquitination of the host protein parvin B and promotes intracellular replication. Cell Microbiol 12:1272–91
Loridant S, Lagier JC, La Scola B (2011) Identification of Legionella feeleii cellulitis. Emerg Infect Dis 17:145–146
Losick VP, Haenssler E, Moy MY, Isberg RR (2010) LnaB: a Legionella pneumophila activator of NF-kappaB. Cell Microbiol 12:1087–1097
Lowry PW, Blankenship RJ, Gridley W, Troup NJ, Tompkins LS (1991) A cluster of Legionella sternal-wound infections due to postoperative topical exposure to contaminated tap water. N Engl J Med 324:109–113
Lowry PW, Tompkins LS (1993) Nosocomial legionellosis: a review of pulmonary and extrapulmonary syndromes. Am J Infect Control 21:21–27
Lucas CE, Brown E, Fields BS (2006) Type IV pili and type II secretion play a limited role in Legionella pneumophila biofilm colonization and retention. Microbiology 152:3569–3573
Lück CP (2008) Diagnostics and clinical disease treatment. In: Heuner KJ, Swanson MS (ed) Legionella: molecular microbiology. Academic, Caister, pp 19–34
Luck PC, Helbig JH, Ehret W, Ott M (1995) Isolation of a Legionella pneumophila strain serologically distinguishable from all known serogroups. Zbl Bakt 282:35–39
Lück PC, Helbig JH, von Baum H, Marre R (2006) Diagnostics and clinical disease treatment: usefulness of microbiological diagnostic methods for detection of Legionella infections. In: Cianciotto NP, Abu Kwaik Y, Edelstein PH, Fields BS, Geary DF, Harrison TG, Joseph CA, Ratcliff RM, Stout JE, Swanson MS (ed) Legionella: state of the Art 30 years after its recognition, vol 2. ASM Press, Washington, DC
Luck PC, Jacobs E, Roske I, Schroter-Bobsin U, Dumke R, Gronow S (2010) Legionella dresdenensis sp. nov., isolated from river water. Int J Syst Evol Microbiol 60:2557–2562
Ludwig B, Rahfeld J, Schmidt B, Mann K, Wintermeyer E, Fischer G, Hacker J (1994) Characterization of Mip proteins of Legionella pneumophila. FEMS Microbiol Lett 118:23–30
Ludwig B, Schmid A, Marre R, Hacker J (1991) Cloning, genetic analysis, and nucleotide sequence of a determinant coding for a 19-kilodalton peptidoglycan-associated protein (Ppl) of Legionella pneumophila. Infect Immun 59:2515–2521
Ludwig W, Stackebrandt E (1983) A phylogenetic analysis of Legionella. Arch Microbiol 135:45–50
Luhrmann A, Nogueira CV, Carey KL, Roy CR (2010) Inhibition of pathogen-induced apoptosis by a Coxiella burnetii type IV effector protein. Proc Natl Acad Sci USA 107:18997–9001
Luneberg E, Zahringer U, Knirel YA, Steinmann D, Hartmann M, Steinmetz I, Rohde M, Kohl J, Frosch M (1998) Phase-variable expression of lipopolysaccharide contributes to the virulence of Legionella pneumophila. J Exp Med 188:49–60
Luo ZQ, Isberg RR (2004) Multiple substrates of the Legionella pneumophila Dot/Icm system identified by interbacterial protein transfer. Proc Natl Acad Sci USA 101:841–6
Lurie-Weinberger MN, Gomez-Valero L, Merault N, Glockner G, Buchrieser C, Gophna U (2010) The origins of eukaryotic-like proteins in Legionella pneumophila. Int J Med Microbiol 300:470–81
Luttichau HR, Vinther C, Uldum SA, Moller J, Faber M, Jensen JS (1998) An outbreak of Pontiac fever among children following use of a whirlpool. Clin Infect Dis 26:1374–1378
Lynch D, Fieser N, Gloggler K, Forsbach-Birk V, Marre R (2003) The response regulator LetA regulates the stationary-phase stress response in Legionella pneumophila and is required for efficient infection of Acanthamoeba castellanii. FEMS Microbiol Lett 219:241–8
Machner MP, Isberg RR (2007) A bifunctional bacterial protein links GDI displacement to Rab1 activation. Science 318:974–977
Machner MP, Isberg RR (2006) Targeting of host Rab GTPase function by the intravacuolar pathogen Legionella pneumophila. Dev Cell 11:47–56
Mahoney FJ, Hoge CW, Farley TA, Barbaree JM, Breiman RF, Benson RF, McFarland LM (1992) Communitywide outbreak of Legionnaires’ disease associated with a grocery store mist machine. J Infect Dis 165:736–739
Maiwald M, Schill M, Stockinger C, Helbig JH, Luck PC, Witzleb W, Sonntag HG (1995) Detection of Legionella DNA in human and guinea pig urine samples by the polymerase chain reaction. Eur J Clin Microbiol Infect Dis 14:25–33
Mampel J, Spirig T, Weber SS, Haagensen JA, Molin S, Hilbi H (2006) Planktonic replication is essential for biofilm formation by Legionella pneumophila in a complex medium under static and dynamic flow conditions. Appl Environ Microbiol 72:2885–2895
Mangione EJ, Remis RS, Tait KA, McGee HB, Gorman GW, Wentworth BB, Baron PA, Hightower AW, Barbaree JM, Broome CV (1985) An outbreak of Pontiac fever related to whirlpool use, Michigan 1982. JAMA 253:535–539
Manz W, Amann R, Szewzyk R, Szewzyk U, Stenstrom TA, Hutzler P, Schleifer KH (1995) In situ identification of Legionellaceae using 16S rRNA-targeted oligonucleotide probes and confocal laser scanning microscopy. Microbiology 141:29–39
Marcus AJ, Broekman MJ, Drosopoulos JH, Olson KE, Islam N, Pinsky DJ, Levi R (2005) Role of CD39 (NTPDase-1) in thromboregulation, cerebroprotection, and cardioprotection. Semin Thromb Hemost 31:234–246
Marra A, Blander SJ, Horwitz MA, Shuman HA (1992) Identification of a Legionella pneumophila locus required for intracellular multiplication in human macrophages. Proc Natl Acad Sci USA 89:9607–9611
Marra A, Horwitz MA, Shuman HA (1990) The HL-60 model for the interaction of human macrophages with the Legionnaires' disease bacterium. J Immunol 144:2738–2744
Marrao G, Verissimo A, Bowker RG, daCosta MS (1993) Biofilms as major sources of Legionella spp. in hydrothermal areas and their disperion into stream water. FEMS Microbiol Ecol 12:25–33
Marrie TJ, Haldane D, MacDonald S, Clarke K, Fanning C, Le Fort-Jost S, Bezanson G, Joly J (1991) Control of endemic nosocomial Legionnaires' disease by using sterile potable water for high risk patients. Epidemiol Infect 107:591–605
Marrie TJ, Raoult D, La Scola B, Birtles RJ, de Carolis E (2001) Legionella-like and other amoebal pathogens as agents of community-acquired pneumonia. Emerg Infect Dis 7:1026–1029
Marston BJ, Lipman HB, Breiman RF (1994) Surveillance for Legionnaires’ disease: risk factors for morbidity and mortality. Arch Intern Med 154:2417–2422
Marston BJ, Plouffe JF, File TM, Jr, Hackman BA, Salstrom SJ, Lipman HB, Kolczak MS, Breiman RF (1997) Incidence of community-acquired pneumonia requiring hospitalization. Results of a population-based active surveillance Study in Ohio. The Community-Based Pneumonia Incidence Study Group. Arch Intern Med 157:1709–1718
Maruta K, Miyamoto H, Hamada T, Ogawa M, Taniguchi H, Yoshida S (1998) Entry and intracellular growth of Legionella dumoffii in alveolar epithelial cells. Am J Respir Crit Care Med 157:1967–1974
Massis LM, Zamboni DS (2011) Innate immunity to Legionella pneumophila. Front Microbiol 2:109
Matsiota-Bernard P, Pitsouni E, Legakis N, Nauciel C (1994) Evaluation of commercial amplification kit for detection of Legionella pneumophila in clinical specimens. J Clin Microbiol 32:1503–1505
Matsiota-Bernard P, Waser S, Vrioni G (2000) Detection of Legionella pneumophila DNA in urine and serum samples from patients with pneumonia. Clin Microbiol Infect 6:223–225
Matsui M, Fujii S, Shiroiwa R, Amemura-Maekawa J, Chang B, Kura F, Yamauchi K (2010) Isolation of Legionella rubrilucens from a pneumonia patient co-infected with Legionella pneumophila. J Med Microbiol 59:1242–1246
Matsunaga K, Klein TW, Friedman H, Yamamoto Y (2002) In vitro therapeutic effect of epigallocatechin gallate on nicotine-induced impairment of resistance to Legionella pneumophila infection of established MH-S alveolar macrophages. J Infect Dis 185:229–236
Matsunaga K, Klein TW, Newton C, Friedman H, Yamamoto Y (2001) Legionella pneumophila suppresses interleukin-12 production by macrophages. Infect Immun 69:1929–1933
McCoy-Simandle K, Stewart CR, Dao J, Debroy S, Rossier O, Bryce PJ, Cianciotto NP (2011) Legionella pneumophila type II secretion dampens the cytokine response of infected macrophages and epithelia. Infect Immun 79:1984–1997
McDade JE, Shepard CC, Fraser DW, Tsai TR, Redus MA, Dowdle WR (1977) Legionnaires’ disease: isolation of a bacterium and demonstration of its role in other respiratory disease. N Eng J Med 297:1197–1203
McHugh SL, Yamamoto Y, Klein TW, Friedman H (2000) Murine macrophages differentially produce proinflammatory cytokines after infection with virulent vs. avirulent Legionella pneumophila. J Leukoc Biol 67:863–868
McKinney RM, Porschen RK, Edelstein PH, Bissett ML, Harris PP, Bondell SP, Steigerwalt AG, Weaver RE, Ein ME, Lindquist DS, Kops RS, Brenner DJ (1981) Legionella longbeachae species nova, another etiologic agent of human pneumonia. Ann Intern Med 94:739–743
McNally C, Hackman B, Fields BS, Plouffe JF (2000) Potential importance of Legionella species as etiologies in community acquired pneumonia (CAP). Diagn Microbiol Infect Dis 38:79–82
McNealy TL, Forsbach-Birk V, Shi C, Marre R (2005) The Hfq homolog in Legionella pneumophila demonstrates regulation by LetA and RpoS and interacts with the global regulator CsrA. J Bacteriol 187:1527–1532
Merault N, Rusniok C, Jarraud S, Gomez-Valero L, Cazalet C, Marin M, Brachet E, Aegerter P, Gaillard JL, Etienne J, Herrmann JL, Lawrence C, Buchrieser C (2011) A specific real-time PCR for simultaneous detection and identification of Legionella pneumophila serogroup 1 in water and clinical samples. Appl Environ Microbiol 77:1705–1717
Mercuri PS, Bouillenne F, Boschi L, Lamotte-Brasseur J, Amicosante G, Devreese B, van Beeumen J, Frere JM, Rossolini GM, Galleni M (2001) Biochemical characterization of the FEZ-1 metallo-beta-lactamase of Legionella gormanii ATCC 33297 T produced in Escherichia coli. Antimicrob Agents Chemother 45:1254–1262
Merriam JJ, Mathur R, Maxfield-Boumil R, Isberg RR (1997) Analysis of the Legionella pneumophila fliI gene: intracellular growth of a defined mutant defective for flagellum biosynthesis. Infect Immun 65:2497–2501
Messi P, Anacarso I, Bargellini A, Bondi M, Marchesi I, de Niederhausern S, Borella P (2011) Ecological behaviour of three serogroups of Legionella pneumophila within a model plumbing system. Biofouling 27:165–172
Meyer R, Rappo U, Glickman M, Seo SK, Sepkowitz K, Eagan J, Small TN (2011) Legionella jordanis in hematopoietic SCT patients radiographically mimicking invasive mold infection. Bone Marrow Transplant 46:1099–1103
Michel R, Muller KD, Amann R, Schmid EN (1998) Legionella-like slender rods multiplying within a strain of Acanthamoeba sp. isolated from drinking water. Parasitol Res 84:84–88
Mintz CS (1999) Gene transfer in Legionella pneumophila. Microbes Infect 1:1203–9
Mintz CS, Arnold PI, Johnson W, Schultz DR (1995) Antibody-independent binding of complement component C1q by Legionella pneumophila. Infect Immun 63:4939–4943
Mintz CS, Zou CH (1992) Isolation and characterization of a lipopolysaccharide mutant of Legionella pneumophila. FEMS Microbiol Lett 72:249–253
Miyamoto H, Jitsurong S, Shiota R, Maruta K, Yoshida S, Yabuuchi E (1997) Molecular determination of infection source of a sporadic Legionella pneumonia case associated with a hot spring bath. Microbiol Immunol 41:197–202
Miyamoto H, Maruta K, Ogawa M, Beckers MC, Gros P, Yoshida S (1996) Spectrum of Legionella species whose intracellular multiplication in murine macrophages is genetically controlled by Lgn1. Infect Immun 64:1842–1845
Miyamoto H, Taniguchi H, Yoshida S (2003) A simple qualitative assay for intracellular growth of Legionella pneumophila within Acanthamoeba culbertsoni. Kansenshogaku Zasshi 77:343–345
Mody CH, 3rd, Paine R, Shahrabadi MS, Simon RH, Pearlman E, Eisenstein BI, Toews GB (1993) Legionella pneumophila replicates within rat alveolar epithelial cells. J Infect Dis 167:1138–1145
Moffat JF, Edelstein PH, Regula DP, Jr, Cirillo JD, Tompkins LS (1994) Effects of an isogenic Zn-metalloprotease-deficient mutant of Legionella pneumophila in a guinea-pig pneumonia model. Mol Microbiol 12:693–705
Moffat JF, Tompkins LS (1992) A quantitative model of intracellular growth of Legionella pneumophila in Acanthamoeba castellanii. Infect Immun 60:296–301
Moliner C, Fournier PE, Raoult D (2010) Genome analysis of microorganisms living in amoebae reveals a melting pot of evolution. FEMS Microbiol Rev 34:281–294
Moliner C, Raoult D, Fournier PE (2009a) Evidence of horizontal gene transfer between amoeba and bacteria. Clin Microbiol Infect 15:178–180
Moliner C, Raoult D, Fournier PE (2009b) Evidence that the intra-amoebal Legionella drancourtii acquired a sterol reductase gene from eukaryotes. BMC Res Notes 2:51
Moll H, Sonesson A, Jantzen E, Marre R, Zahringer U (1992) Identification of 27-oxo-octacosanoic acid and heptacosane-1,27-dioic acid in Legionella pneumophila. FEMS Microbiol Lett 76:1–6
Molmeret M, Abu Kwaik Y (2002) How does Legionella pneumophila exit the host cell? Trends Microbiol 10:258–60
Molmeret M, Bitar DM, Han L, Kwaik YA (2004) Disruption of the phagosomal membrane and egress of Legionella pneumophila into the cytoplasm during the last stages of intracellular infection of macrophages and Acanthamoeba polyphaga. Infect Immun 72:4040–51
Molmeret M, Horn M, Wagner M, Santic M, Abu Kwaik Y (2005) Amoebae as training grounds for intracellular bacterial pathogens. Appl Environ Microbiol 71:20–28
Molmeret M, Jarraud S, Mori JP, Pernin P, Forey F, Reyrolle M, Vandenesch F, Etienne J, Farge P (2001) Different growth rates in amoeba of genotypically related environmental and clinical Legionella pneumophila strains isolated from a thermal spa. Epidemiol Infect 126:231–239
Molmeret M, Jones S, Santic M, Habyarimana F, Garcia Esteban MT, Abu Kwaik Y (2010) Temporal and spatial trigger of post-exponential virulence-associated regulatory cascades by Legionella pneumophila after bacterial escape into the host cell cytosol. Environ Microbiol 12:704–715
Molofsky AB, Byrne BG, Whitfield NN, Madigan CA, Fuse ET, Tateda K, Swanson MS (2006) Cytosolic recognition of flagellin by mouse macrophages restricts Legionella pneumophila infection. J Exp Med 203:1093–1104
Molofsky AB, Swanson MS (2004) Differentiate to thrive: lessons from the Legionella pneumophila life cycle. Mol Microbiol 53:29–40
Molofsky AB, Swanson MS (2003) Legionella pneumophila CsrA is a pivotal repressor of transmission traits and activator of replication. Mol Microbiol 50:445–61
Monroe KM, McWhirter SM, Vance RE (2009) Identification of host cytosolic sensors and bacterial factors regulating the type I interferon response to Legionella pneumophila. PLoS Pathog 5:e1000665
Moore MR, Pryor M, Fields B, Lucas C, Phelan M, Besser RE (2006) Introduction of monochloramine into a municipal water system: impact on colonization of buildings by Legionella spp. Appl Environ Microbiol 72:378–383
Moritz MM, Flemming HC, Wingender J (2010) Integration of Pseudomonas aeruginosa and Legionella pneumophila in drinking water biofilms grown on domestic plumbing materials. Int J Hyg Environ Health 213:190–197
Morozova I, Qu X, Shi S, Asamani G, Greenberg JE, Shuman HA, Russo JJ (2004) Comparative sequence analysis of the icm/dot genes in Legionella. Plasmid 51:127–147
Morris GK, Steigerwalt A, Feeley JC, Wong ES, Martin WT, Patton CM, Brenner DJ (1980) Legionella gormanii sp. nov. J Clin Microbiol 12:718–721
Mostowy S, Cossart P (2012) Bacterial autophagy: restriction or promotion of bacterial replication? Trends Cell Biol 22(6):283–291
Mouchtouri V, Velonakis E, Tsakalof A, Kapoula C, Goutziana G, Vatopoulos A, Kremastinou J, Hadjichristodoulou C (2007) Risk factors for contamination of hotel water distribution systems by Legionella species. Appl Environ Microbiol 73:1489–1492
Muder RR, Yu VL, Fang GD (1989) Community-acquired Legionnaires’ disease. Semin Respir Infect 4:32–39
Mukherjee S, Liu X, Arasaki K, McDonough J, Galán JE, Roy CR (2011) Modulation of Rab GTPase function by a protein phosphocholine transferase. Nature 477:103–106
Müller MP, Peters H, Blümer J, Blankenfeldt W, Goody RS, Itzen A (2010) The Legionella effector protein DrrA AMPylates the membrane traffic regulator Rab1b. Science 329:946–9
Murata T, Delprato A, Ingmundson A, Toomre DK, Lambright DG, Roy CR (2006) The Legionella pneumophila effector protein DrrA is a Rab1 guanine nucleotide-exchange factor. Nat Cell Biol 8:971–977
Murdoch DR, Chambers ST (2000) Detection of Legionella DNA in peripheral leukocytes, serum, and urine from a patient with pneumonia caused by Legionella dumoffii. Clin Infect Dis 30:382–383
Murdoch DR, Walford EJ, Jennings LC, Light GJ, Schousboe MI, Chereshsky AY, Chambers ST, Town GI (1996) Use of the polymerase chain reaction to detect Legionella DNA in urine and serum samples from patients with pneumonia. Clin Infect Dis 23:475–480
Murga R, Forster TS, Brown E, Pruckler JM, Fields BS, Donlan RM (2001) Role of biofilms in the survival of Legionella pneumophila in a model potable-water system. Microbiology 147:3121–3126
Mustafa MI, Al-Marzooq F, How SH, Kuan YC, Ng TH (2011) The use of multiplex real-time PCR improves the detection of the bacterial etiology of community acquired pneumonia. Trop Biomed 28:531–544
N'Guessan PD, Etouem MO, Schmeck B, Hocke AC, Scharf S, Vardarova K, Opitz B, Flieger A, Suttorp N, Hippenstiel S (2007) Legionella pneumophila-induced PKCa-, MAPK-, and NF-kB-dependent COX-2 expression in human lung epithelium. Am J Physiol Lung Cell Mol Physiol 292:L267–L277
Nagai H, Cambronne ED, Kagan JC, Amor JC, Kahn RA, Roy CR (2005) A C-terminal translocation signal required for Dot/Icm-dependent delivery of the Legionella RalF protein to host cells. Proc Natl Acad Sci USA 102:826–31
Nagai H, Kagan JC, Zhu X, Kahn RA, Roy CR (2002) A bacterial guanine nucleotide exchange factor activates ARF on Legionella phagosomes. Science 295:679–682
Nagai H, Kubori T (2011) Type IVB secretion systems of Legionella and other Gram-negative bacteria. Front Microbiol 2:136
Nagai T, Sobajima H, Iwasa M, Tsuzuki T, Kura F, Amemura-Maekawa J, Watanabe H (2003) Neonatal sudden death due to Legionella pneumonia associated with water birth in a domestic spa bath. J Clin Microbiol 41:2227–2229
Nahapetian K, Challemel O, Beurtin D, Dubrou S, Gounon P, Squinazi F (1991) The intracellular multiplication of Legionella pneumophila in protozoa from hospital plumbing systems. Res Microbiol 142:677–685
Nash TW, Libby DM, Horwitz MA (1988) IFN-gamma-activated human alveolar macrophages inhibit the intracellular multiplication of Legionella pneumophila. J Immunol 140:3978–3981
Nash TW, Libby DM, Horwitz MA (1984) Interaction between the legionnaires' disease bacterium (Legionella pneumophila) and human alveolar macrophages. Influence of antibody, lymphokines, and hydrocortisone. J Clin Invest 74:771–782
Nasrallah GK, Riveroll AL, Chong A, Murray LE, Lewis PJ, Garduno RA (2011) Legionella pneumophila requires polyamines for optimal intracellular growth. J Bacteriol 193:4346–4360
Naylor J, Cianciotto NP (2004) Cytochrome c maturation proteins are critical for in vivo growth of Legionella pneumophila. FEMS Microbiol Lett 241:249–256
Neil K, Berkelman R (2008) Increasing incidence of legionellosis in the United States, 1990-2005: changing epidemiologic trends. Clin Infect Dis 47:591–599
Neild AL, Roy CR (2003) Legionella reveal dendritic cell functions that facilitate selection of antigens for MHC class II presentation. Immunity 18:813–23
Neild AL, Shin S, Roy CR (2005) Activated macrophages infected with Legionella inhibit T cells by means of MyD88-dependent production of prostaglandins. J Immunol 175:8181–90
Neumeister B, Faigle M, Sommer M, Zahringer U, Stelter F, Menzel R, Schutt C, Northoff H (1998a) Low endotoxic potential of Legionella pneumophila lipopolysaccharide due to failure of interaction with the monocyte lipopolysaccharide receptor CD14. Infect Immun 66:4151–4157
Neumeister B, Kleihauer A, Rossmann V, Fehrenbach E, Faigle M, Baumbach S, Northoff H (1998b) Induction of cytokines and expression of surface receptors in Mono Mac 6 cells after infection with different Legionella species. APMIS 106:319–333
Neumeister B, Reiff G, Faigle M, Dietz K, Northoff H, Lang F (2000) Influence of Acanthamoeba castellanii on intracellular growth of different Legionella species in human monocytes. Appl Environ Microbiol 66:914–919
Neumeister B, Schoniger S, Faigle M, Eichner M, Dietz K (1997) Multiplication of different Legionella species in Mono Mac 6 cells and in Acanthamoeba castellanii. Appl Environ Microbiol 63:1219–1224
Neunuebel MR, Chen Y, Gaspar AH, Backlund PS, Jr, Yergey A, Machner MP (2011) De-AMPylation of the Small GTPase Rab1 by the pathogen Legionella pneumophila. Science 333:453–456
Newsome AL, Baker RL, Miller RD, Arnold RR (1985) Interactions between Naegleria fowleri and Legionella pneumophila. Infect Immun 50:449–452
Newsome AL, Scott TM, Benson RF, Fields BS (1998) Isolation of an amoeba naturally harboring a distinctive Legionella species. Appl Environ Microbiol 64:1688–1693
Newton HJ, Ang DK, van Driel IR, Hartland EL (2010) Molecular pathogenesis of infections caused by Legionella pneumophila. Clin Microbiol Rev 23:274–298
Newton HJ, Sansom FM, Bennett-Wood V, Hartland EL (2006) Identification of Legionella pneumophila-specific genes by genomic subtractive hybridization with Legionella micdadei and identification of lpnE, a gene required for efficient host cell entry. Infect Immun 74:1683–1691
Newton HJ, Sansom FM, Dao J, Cazalet C, Bruggemann H, Albert-Weissenberger C, Buchrieser C, Cianciotto NP, Hartland EL (2008) Significant role for ladC in initiation of Legionella pneumophila infection. Infect Immun 76:3075–3085
Newton HJ, Sansom FM, Dao J, McAlister AD, Sloan J, Cianciotto NP, Hartland EL (2007) Sel1 repeat protein LpnE is a Legionella pneumophila virulence determinant that influences vacuolar trafficking. Infect Immun 75:5575–5585
Newton LH, Joseph CA, Hutchinson EJ, Harrison TG, Watson JM, Bartlett CL (1996) Legionnaires' disease surveillance: England and Wales, 1995. Commun Dis Rep CDR Rev 6:R151–5
Ng V, Tang P, Fisman DN (2008a) Our evolving understanding of legionellosis epidemiology: learning to count. Clin Infect Dis 47:600–602
Ng V, Tang P, Jamieson F, Drews SJ, Brown S, Low DE, Johnson CC, Fisman DN (2008b) Going with the flow: legionellosis risk in Toronto, Canada is strongly associated with local watershed hydrology. Ecohealth 5:482–490
Nguyen TM, Ilef D, Jarraud S, Rouil L, Campese C, Che D, Haeghebaert S, Ganiayre F, Marcel F, Etienne J, Desenclos JC (2006) A community-wide outbreak of legionnaires disease linked to industrial cooling towers–how far can contaminated aerosols spread? J Infect Dis 193:102–111
Nicolay N, Boland M, Ward M, Hickey L, Collins C, Lynch M, McCarthy M, O'Donnell J (2010) Investigation of Pontiac-like illness in office workers during an outbreak of Legionnaires' disease, 2008. Epidemiol Infect 138:1667–1673
Nikaido H, Takatsuka Y (2009) Mechanisms of RND multidrug efflux pumps. Biochim Biophys Acta 1794:769–781
Ninio S, Celli J, Roy CR (2009) A Legionella pneumophila effector protein encoded in a region of genomic plasticity binds to Dot/Icm-modified vacuoles. PLoS Pathog 5:e1000278
Ninio S, Roy CR (2007) Effector proteins translocated by Legionella pneumophila: strength in numbers. Trends Microbiol 15:372–380
Nogueira CV, Lindsten T, Jamieson AM, Case CL, Shin S, Thompson CB, Roy CR (2009) Rapid pathogen-induced apoptosis: a mechanism used by dendritic cells to limit intracellular replication of Legionella pneumophila. PLoS Pathog 5:e1000478
Nolte FS, Conlin CA, Motley MA (1986) Electrophoretic and serological characterization of the lipopolysaccharides of Legionella pneumophila. Infect Immun 52:676–681
Nora T, Lomma M, Gomez-Valero L, Buchrieser C (2009) Molecular mimicry: an important virulence strategy employed by Legionella pneumophila to subvert host functions. Future Microbiol 4:691–701
Nozue T, Chikazawa H, Miyanishi S, Shimazaki T, Oka R, Shimazaki S, Miyamoto S (2005) Legionella pneumonia associated with adult respiratory distress syndrome caused by Legionella pneumophila serogroup 3. Intern Med 44:73–78
Nygard K, Werner-Johansen O, Ronsen S, Caugant DA, Simonsen O, Kanestrom A, Ask E, Ringstad J, Odegard R, Jensen T, Krogh T, Hoiby EA, Ragnhildstveit E, Aaberge IS, Aavitsland P (2008) An outbreak of Legionnaires disease caused by long-distance spread from an industrial air scrubber in Sarpsborg, Norway. Clin Infect Dis 46:61–69
O'Connell WA, Bangsborg JM, Cianciotto NP (1995) Characterization of a Legionella micdadei mip mutant. Infect Immun 63:2840–2845
O'Connell WA, Dhand L, Cianciotto NP (1996a) Infection of macrophage-like cells by Legionella species that have not been associated with disease. Infect Immun 64:4381–4384
O'Connell WA, Hickey EK, Cianciotto NP (1996b) A Legionella pneumophila gene that promotes hemin binding. Infect Immun 64:842–848
O'Connor TJ, Adepoju Y, Boyd D, Isberg RR (2011) Minimization of the Legionella pneumophila genome reveals chromosomal regions involved in host range expansion. Proc Natl Acad Sci USA 108:14733–14740
O'Loughlin RE, Kightlinger L, Werpy MC, Brown E, Stevens V, Hepper C, Keane T, Benson RF, Fields BS, Moore MR (2007) Restaurant outbreak of Legionnaires’ disease associated with a decorative fountain: an environmental and case-control study. BMC Infect Dis 7:93–101
Ochsner UA, Snyder A, Vasil AI, Vasil ML (2002) Effects of the twin-arginine translocase on secretion of virulence factors, stress response, and pathogenesis. Proc Natl Acad Sci USA 99:8312–8317
Ogata H, Renesto P, Audic S, Robert C, Blanc G, Fournier PE, Parinello H, Claverie JM, Raoult D, Galvao MA, Mafra C, Chamone CB, Calic SB, Zavala-Velazquez JE, Walker DH, Kelly PJ, Meads N, Theobald A, Fang R, Houhamdi L, Azad AF, Parola P, Sanogo OY, Lerdthusnee K, Zeaiter Z, Chauvancy G, Gonzalez JP, Miller RS, Telford SR, 3rd, Wongsrichanalai C, McDaniel P, Rolain JM, Franc M, Davoust B, La Scola B, Meconi S, Fenollar F, Roux V, Stuhl L, Maurin M, Richter J, Petridou J, Haussinger D, Enea M, de Lamballerie X (2005) The genome sequence of Rickettsia felis identifies the first putative conjugative plasmid in an obligate intracellular parasite. PLoS Biol 3:e248. Epub 2005 Jul 5
Okada M, Kawano K, Kura F, Amemura-Maekawa J, Watanabe H, Yagita K, Endo T, Suzuki S (2005) The largest outbreak of legionellosis in Japan associated with spa baths: epidemic curve and environmental investigation. Kansenshogaku Zasshi 79:365–374
Olsen CW, Elverdal P, Jørgensen CS, Uldum SA (2009) Comparison of the sensitivity of the Legionella urinary antigen EIA kits from Binax and Biotest with urine from patients with infections caused by less common serogroups and subgroups of Legionella. Eur J Clin Microbiol Infect Dis 28:817–820
Opitz B, Vinzing M, van Laak V, Schmeck B, Heine G, Gunther S, Preissner R, Slevogt H, N'Guessan PD, Eitel J, Goldmann T, Flieger A, Suttorp N, Hippenstiel S (2006) Legionella pneumophila induces IFNbeta in lung epithelial cells via IPS-1 and IRF3, which also control bacterial replication. J Biol Chem 281:36173–9
Orrison LH, Cherry WB, Fliermans CB, Dees SB, McDougal LK, Dodd DJ (1981) Characteristics of environmental isolates of Legionella pneumophila. Appl Environ Microbiol 42:109–115
Orrison LH, Cherry WB, Tyndall RL, Fliermans CB, Gough SB, Lambert MA, McDougal LK, Bibb WF, Brenner DJ (1983) Legionella oakridgensis: unusual new species isolated from cooling tower water. Appl Environ Microbiol 45:536–545
Ortiz-Roque CM, Hazen TC (1987) Abundance and distribution of Legionellaceae in Puerto Rican waters. Appl Environ Microbiol 53:2231–2236
Ott M (1994) Genetic approaches to study Legionella pneumophila pathogenicity. FEMS Microbiol Rev 14:161–176
Ott M, Messner P, Heesemann J, Marre R, Hacker J (1991) Temperature-dependent expression of flagella in Legionella. J Gen Microbiol 137:1955–1961
Otten S, Iyer S, Johnson W, Montgomery R (1986) Serospecific antigens of Legionella pneumophila. J Bacteriol 167:893–904
Otto GP, Wu MY, Clarke M, Lu H, Anderson OR, Hilbi H, Shuman HA, Kessin RH (2004) Macroautophagy is dispensable for intracellular replication of Legionella pneumophila in Dictyostelium discoideum. Mol Microbiol 51:63–72
Pagnier I, Merchat M, La Scola B (2009) Potentially pathogenic amoeba-associated microorganisms in cooling towers and their control. Future Microbiol 4:615–629
Palmer CJ, Tsai YL, Paszko-Kolva C, Mayer C, Sangermano LR (1993) Detection of Legionella species in sewage and ocean water by polymerase chain reaction, direct fluorescent-antibody, and plate culture methods. Appl Environ Microbiol 59:3618–3624
Pan X, Lührmann A, Satoh A, Laskowski-Arce MA, Roy CR (2008) Ankyrin repeat proteins comprise a diverse family of bacterial type IV effectors. Science 320:1651–1654
Pang CM, Liu WT (2006) Biological filtration limits carbon availability and affects downstream biofilm formation and community structure. Appl Environ Microbiol 72:5702–5712
Park DR, Skerrett SJ (1996) IL-10 enhances the growth of Legionella pneumophila in human mononuclear phagocytes and reverses the protective effect of IFN-g. J Immunol 157:2528–2538
Park MY, Ko KS, Lee HK, Park MS, Kook YH (2003) Legionella busanensis sp. nov., isolated from cooling tower water in Korea. Int J Syst Evol Microbiol 53:77–80
Parthuisot N, West NJ, Lebaron P, Baudart J (2010) High diversity and abundance of Legionella spp. in a pristine river and impact of seasonal and anthropogenic effects. Appl Environ Microbiol 76:8201–8210
Pasculle AW, Feeley JC, Gibson RJ, Cordes LG, Myerowitz RL, Patton CM, Gorman GW, Carmack CL, Ezzell JW, Dowling JN (1980) Pittsburgh pneumonia agent: direct isolation from human lung tissue. J Infect Dis 141:727–732
Paszko-Kolva C, Shahamat M, Colwell RR (1993) Effect of temperature on survival of Legionella pneumophila in the aquatic environment. Microb Releases 2:73–79
Paszko-Kolva C, Shahamat M, Colwell RR (1992) Long-term survival of Legionella pneumophila serogroup 1 under low-nutrient conditions and associated morphological changes. FEMS Microbiol Ecol 102:45–55
Paszko-Kolva C, Yamamoto H, Shahamat M, Sawyer TK, Morris G, Colwell RR (1991) Isolation of amoebae and Pseudomonas and Legionella spp. from eyewash stations. Appl Environ Microbiol 57:163–167
Patterson WJ, Hay J, Seal DV, McLuckie JC (1997) Colonization of transplant unit water supplies with Legionella and protozoa: precautions required to reduce the risk of legionellosis. J Hosp Infect 37:7–17
Payne NR, Horwitz MA (1987) Phagocytosis of Legionella pneumophila is mediated by human monocyte complement receptors. J Exp Med 166:1377–89
Pearce MM, Cianciotto NP (2009) Legionella pneumophila secretes an endoglucanase that belongs to the family-5 of glycosyl hydrolases and is dependent upon type II secretion. FEMS Microbiol Lett 300:256–264
Pearce MM, Cianciotto NP (2012) Epub ahead of print doi:10.1099/ijs.0.039248-0
Pearce MM, Theodoropoulos N, Mandel MJ, Brown E, Reed KD, Cianciotto NP (2012) Legionella cardiaca sp. nov., isolated from a case of native valve endocarditis in a human heart. Int J Syst Evol Microbiol
Pearce MM, Theodoropoulos N, Noskin GA, Flaherty JP, Stemper ME, Aspeslet T, Cianciotto NP, Reed KD (2011) Native valve endocarditis due to a novel strain of Legionella. J Clin Microbiol 49:3340–3342
Pearlman E, Jiwa AH, Engleberg NC, Eisenstein BI (1988) Growth of Legionella pneumophila in a human macrophage-like (U937) cell line. Microb Pathogen 5:87–95
Pecastaings S, Berge M, Dubourg KM, Roques C (2010) Sessile Legionella pneumophila is able to grow on surfaces and generate structured monospecies biofilms. Biofouling 26:809–819
Pedersen LL, Radulic M, Doric M, Abu Kwaik Y (2001) HtrA homologue of Legionella pneumophila: an indispensable element for intracellular infection of mammalian but not protozoan cells. Infect Immun 69:2569–2579
Piao Z, Sze CC, Barysheva O, Iida K, Yoshida S (2006) Temperature-regulated formation of mycelial mat-like biofilms by Legionella pneumophila. Appl Environ Microbiol 72:1613–1622
Pine L, George JR, Reeves MW, Harrell WK (1979) Development of a chemically defined liquid medium for growth of Legionella pneumophila. J Clin Microbiol 9:615–626
Piso RJ, Caruso A, Nebiker M (2007) Hose as a source of Legionella pneumonia. A new risk factor for gardeners? J Hosp Infect 67:396–397
Plonka PM, Grabacka M (2006) Melanin synthesis in microorganisms–biotechnological and medical aspects. Acta Biochim Pol 53:429–443
Plouffe JF, File TM, Jr, Breiman RF, Hackman BA, Salstrom SJ, Marston BJ, Fields BS (1995) Reevaluation of the definition of Legionnaires’ disease: use of the urinary antigen assay. Community Based Pneumonia Incidence Study Group. Clin Infect Dis 20:1286–91
Plouffe JF, Para MF, Fuller KA (1985) Serum bactericidal activity against Legionella pneumophila. J Clin Microbiol 22:863–864
Plumlee CR, Lee C, Beg AA, Decker T, Shuman HA, Schindler C (2009) Interferons direct an effective innate response to Legionella pneumophila infection. J Biol Chem 284:30058–66
Poch MT, Johnson W (1993) Ferric reductases of Legionella pneumophila. Biometals 6:107–114
Polesky AH, Ross JT, Falkow S, Tompkins LS (2001) Identification of Legionella pneumophila genes important for infection of amoebas by signature-tagged mutagenesis. Infect Immun 69:977–987
Pope CD, O'Connell W, Cianciotto NP (1996) Legionella pneumophila mutants that are defective for iron acquisition and assimilation and intracellular infection. Infect Immun 64:629–636
Potrykus K, Cashel M (2008) (p)ppGpp still magical? Annu Rev Microbiol 62:35–51
Price CT, Al-Khodor S, Al-Quadan T, Santic M, Habyarimana F, Kalia A, Kwaik YA (2009) Molecular Mimicry by an F-Box effector of Legionella pneumophila Hijacks a conserved polyubiquitination machinery within macrophages and protozoa. PLoS Pathog 5:e1000704
Price CT, Kwaik YA (2010) Exploitation of host polyubiquitination machinery through molecular mimicry by eukaryotic-like bacterial F-Box effectors. Front Microbiol 1:122
Price CT, Al-Khodor S, Al-Quadan T, Abu Kwaik Y (2010a) Indispensable role for the eukaryotic-like ankyrin domains of the ankyrin B effector of Legionella pneumophila within macrophages and amoebae. Infect Immun 78:2079–88
Price CT, Al-Quadan T, Santic M, Jones SC, Abu Kwaik Y (2010b) Exploitation of conserved eukaryotic host cell farnesylation machinery by an F-box effector of Legionella pneumophila. J Exp Med 207:1713–26
Price CT, Jones SC, Amundson KE, Kwaik YA (2010c) Host-mediated post-translational prenylation of novel dot/icm-translocated effectors of Legionella pneumophila. Front Microbiol 1:131
Price CT, Al-Quadan T, Santic M, Rosenshine I, Abu Kwaik Y (2011) Host proteasomal degradation generates amino acids essential for intracellular bacterial growth. Science 334(6062):1553–1557
Pruckler JM, Benson RF, Moyenuddin M, Martin WT, Fields BS (1995) Association of flagellum expression and intracellular growth of Legionella pneumophila. Infect Immun 63:4928–4932
Purcell M, Shuman HA (1998) The Legionella pneumophila icmGCDJBF genes are required for killing of human macrophages. Infect Immun 66:2245–55
Ragaz C, Pietsch H, Urwyler S, Tiaden A, Weber SS, Hilbi H (2008) The Legionella pneumophila phosphatidylinositol-4 phosphate-binding type IV substrate SidC recruits endoplasmic reticulum vesicles to a replication-permissive vacuole. Cell Microbiol 10:2416–33
Ragull S, Garcia-Nunez M, Pedro-Botet ML, Sopena N, Esteve M, Montenegro R, Sabria M (2007) Legionella pneumophila in cooling towers: fluctuations in counts, determination of genetic variability by pulsed-field gel electrophoresis (PFGE), and persistence of PFGE patterns. Appl Environ Microbiol 73:5382–5384
Ramirez JA, Ahkee S, Tolentino A, Miller RD, Summersgill JT (1996) Diagnosis of Legionella pneumophila, Mycoplasma pneumoniae, or Chlamydia pneumoniae lower respiratory infection using the polymerase chain reaction on a single throat swab specimen. Diagn Microbiol Infect Dis 24:7–14
Rankin S, Li Z, Isberg RR (2002) Macrophage-induced genes of Legionella pneumophila: protection from reactive intermediates and solute imbalance during intracellular growth. Infect Immun 70:3637–3648
Rantakokko-Jalava K, Jalava J (2001) Development of conventional and real-time PCR assays for detection of Legionella DNA in respiratory specimens. J Clin Microbiol 39:2904–2910
Rasis M, Segal G (2009) The LetA-RsmYZ-CsrA regulatory cascade, together with RpoS and PmrA, post-transcriptionally regulates stationary phase activation of Legionella pneumophila Icm/Dot effectors. Mol Microbiol 72:995–1010
Ratcliff RM, Lanser JA, Manning PA, Heuzenroeder MW (1998) Sequence-based classification scheme for the genus Legionella targeting the mip gene. J Clin Microbiol 36:1560–1567
Rechnitzer C, Blom J (1989) Engulfment of the Philadelphia strain of Legionella pneumophila within pseudopod coils in human phagocytes. Comparison with other Legionella strains and species. APMIS 97:105–114
Reichardt K, Jacobs E, Roske I, Helbig JH (2010) Legionella pneumophila carrying the virulence-associated lipopolysaccharide epitope possesses two functionally different LPS components. Microbiology 156:2953–2961
Reischl U, Linde HJ, Lehn N, Landt O, Barratt K, Wellinghausen N (2002) Direct detection and differentiation of Legionella spp. and Legionella pneumophila in clinical specimens by dual-color real-time PCR and melting curve analysis. J Clin Microbiol 40:3814–3817
Ren T, Zamboni DS, Roy CR, Dietrich WF, Vance RE (2006) Flagellin-deficient Legionella mutants evade caspase-1- and Naip5-mediated macrophage immunity. PLoS Pathog 2:e18
Retzlaff C, Yamamoto Y, Hoffman PS, Friedman H, Klein TW (1994) Bacterial heat shock proteins directly induce cytokine mRNA and interleukin-1 secretion in macrophage cultures. Infect Immun 62:5689–5693
Retzlaff C, Yamamoto Y, Okubo S, Hoffman PS, Friedman H, Klein TW (1996) Legionella pneumophila heat-shock protein-induced increase of interleukin-1 beta mRNA involves protein kinase C signalling in macrophages. Immunology 89:281–288
Ribet D, Cossart P (2011) Pathogen-mediated posttranslational modifications: a re-emerging field. Cell 143:694–702
Riboldi-Tunnicliffe A, Konig B, Jessen S, Weiss MS, Rahfeld J, Hacker J, Fischer G, Hilgenfeld R (2001) Crystal structure of Mip, a prolylisomerase from Legionella pneumophila. Nat Struct Biol 8:779–783
Ricci ML, Fontana S, Bella A, Gaggioli A, Cascella R, Cassone A, Scaturro M (2010) A preliminary assessment of the occupational risk of acquiring Legionnaire’' disease for people working in telephone manholes, a new workplace environment for Legionella growth. Am J Infect Control 38:540–545
Ricketts K, Joseph CA, Yadav R (2010) Travel-associated Legionnaires disease in Europe in 2008. Euro Surveill 15:19578
Ricketts KD, Joseph CA (2007) Legionnaires disease in Europe: 2005-2006. Euro Surveill 12:E7–8
Ricketts KD, Joseph CA (2005) Legionnaires’ disease in Europe 2003-2004. Euro Surveill 10:256–259
Ricketts KD, Joseph CA, Lee JV, Wilkinson P (2011) Wet cooling systems as a source of sporadic Legionnaires' disease: a geographical analysis of data for England and Wales, 1996–2006. J Epidemiol Commun Health. doi: 10.1136/jech.2010.117952
Ridenour DA, Cirillo SL, Feng S, Samrakandi MM, Cirillo JD (2003) Identification of a gene that affects the efficiency of host cell infection by Legionella pneumophila in a temperature-dependent fashion. Infect Immun 71:6256–63
Riffard S, Douglass S, Brooks T, Springthorpe S, Filion LG, Sattar SA (2001) Occurrence of Legionella in groundwater: an ecological study. Water Sci Technol 43:99–102
Riffard S, Vandenesch F, Reyrolle M, Etienne J (1996) Distribution of mip-related sequences in 39 species (48 serogroups) of Legionellaceae. Epidemiol Infect 117:501–506
Rigden DJ (2011) Identification and modelling of a PPM protein phosphatase fold in the Legionella pneumophila deAMPylase SidD. FEBS Lett 585:2749–2754
Ristroph JD, Hedlund KW, Gowda S (1981) Chemically defined medium for Legionella pneumophila growth. J Clin Microbiol 13:115–119
Robey M, Cianciotto NP (2002) Legionella pneumophila feoAB promotes ferrous iron uptake and intracellular infection. Infect Immun 70:5659–5669
Robey M, O'Connell W, Cianciotto NP (2001) Identification of Legionella pneumophila rcp, a pagP-like gene that confers resistance to cationic antimicrobial peptides and promotes intracellular infection. Infect Immun 69:4276–4286
Robinson CG, Roy CR (2006) Attachment and fusion of endoplasmic reticulum with vacuoles containing Legionella pneumophila. Cell Microbiol 8:793–805
Rodgers FG (1979) Ultrastructure of Legionella pneumophila. J Clin Pathol 32:1195–1202
Rodgers FG, Greaves PW, Macrae AD (1979) Flagella and fimbriae on Legionella organisms. Lancet 2:753–754
Rodgers FG, Greaves PW, Macrae AD, Lewis MJ (1980) Electron microscopic evidence of flagella and pili on Legionella pneumophila. J Clin Pathol 33:1184–1188
Rodgers FG, Macrae AD, Lewis MJ (1978) Electron microscopy of the organism of Legionnaires’ disease. Nature 272:825–826
Rogers J, Dowsett AB, Dennis PJ, Lee JV, Keevil CW (1994) Influence of temperature and plumbing material selection on biofilm formation and growth of Legionella pneumophila in a model potable water system containing complex microbial flora. Appl Environ Microbiol 60:1585–1592
Rogers J, Keevil CW (1992) Immunogold and fluorescein immunolabelling of Legionella pneumophila within an aquatic biofilm visualized by using episcopic differential interference contrast microscopy. Appl Environ Microbiol 58:2326–2330
Rogers JE, Eisenstein BI, Engleberg NC (1992) Spontaneous changes in lipopolysaccharide and monoclonal antibody binding in a single line of Legionella pneumophila serogroup 1 cells. In: Barbaree JM, Breiman RF, Dufour AP (ed) Legionella: current status and emerging perspectives. ASM Press, Washington, DC, pp 73–74
Rohde JR, Breitkreutz A, Chenal A, Sansonetti PJ, Parsot C (2007) Type III secretion effectors of the IpaH family are E3 ubiquitin ligases. Cell Host Microbe 1:77–83
Roig J, Aguilar X, Ruiz J, Domingo C, Mesalles E, Manterola J, Morera J (1991) Comparative study of Legionella pneumophila and other nosocomial-acquired pneumonias. Chest 99:344–350
Rolando M, Buchrieser C (2012) Post-translational modifications of host proteins by Legionella pneumophila: a sophisticated survival strategy. Future Microbiol 7:369–381
Rossier O, Cianciotto NP (2005) The Legionella pneumophila tatB gene facilitates secretion of phospholipase C, growth under iron-limiting conditions, and intracellular infection. Infect Immun 73:2020–2032
Rossier O, Cianciotto NP (2001) Type II protein secretion is a subset of the PilD-dependent processes that facilitate intracellular infection by Legionella pneumophila. Infect Immun 69:2092–2098
Rossier O, Dao J, Cianciotto NP (2008) The type II secretion system of Legionella pneumophila elaborates two aminopeptidases as well as a metalloprotease that contributes to differential infection among protozoan hosts. Appl Environ Microbiol 74:753–761
Rossier O, Dao J, Cianciotto NP (2009) A type II-secreted ribonuclease of Legionella pneumophila facilitates optimal intracellular infection of Hartmannella vermiformis. Microbiology 155:882–890
Rossier O, Starkenburg S, Cianciotto NP (2004) Legionella pneumophila type II protein secretion promotes virulence in the A/J mouse model of Legionnaires' disease pneumonia. Infect Immun 72:310–321
Rowbotham TJ (1986) Current views on the relationships between amoebae, legionellae and man. Isr J Med Sci 22:678–689
Rowbotham TJ (1983) Isolation of Legionella pneumophila from clinical specimens via amoebae, and the interaction of those and other isolates with amoebae. J Clin Pathol 36:978–986
Rowbotham TJ (1980) Preliminary report on the pathogenicity of Legionella pneumophila for freshwater and soil amoebae. J Clin Pathol 33:1179–1183
Roy CR (1999) Trafficking of the Legionella pneumophila phagosome. ASM News 65
Roy CR, Mukherjee S (2009) Bacterial FIC Proteins AMP Up Infection. Sci Signal 2:pe14
Rubin CJ, Thollesson M, Kirsebom LA, Herrmann B (2005) Phylogenetic relationships and species differentiation of 39 Legionella species by sequence determination of the RNase P RNA gene rnpB. Int J Syst Evol Microbiol 55:2039–2049
Ruf B, Schürmann D, Horbach I, Fehrenbach FJ, Pohle HD (1990) Prevalence and diagnosis of Legionella pneumonia: a 3-year prospective study with emphasis on application of urinary antigen detection. J Infect Dis 162:1341–1348
Ruiz M, Ewig S, Marcos MA, Martinez JA, Arancibia F, Mensa J, Torres A (1999) Etiology of community-acquired pneumonia: impact of age, comorbidity, and severity. Am J Respir Crit Care Med 160:397–405
Rytkonen A, Holden DW (2007) Bacterial interference of ubiquitination and deubiquitination. Cell Host Microbe 1:13–22
Sabria M, Alvarez J, Dominguez A, Pedrol A, Sauca G, Salleras L, Lopez A, Garcia-Nunez MA, Parron I, Barrufet MP (2006) A community outbreak of Legionnaires’ disease: evidence of a cooling tower as the source. Clin Microbiol Infect 12:642–647
Sadosky AB, Wiater LA, Shuman HA (1993) Identification of Legionella pneumophila genes required for growth within and killing of human macrophages. Infect Immun 61:5361–73
Sadosky AB, Wilson JW, Steinman HM, Shuman HA (1994) The iron superoxide dismutase of Legionella pneumophila is essential for viability. J Bacteriol 176:3790–3799
Sahr T, Bruggemann H, Jules M, Lomma M, Albert-Weissenberger C, Cazalet C, Buchrieser C (2009) Two small ncRNAs jointly govern virulence and transmission in Legionella pneumophila. Mol Microbiol 72:741–762
Sahr T, Rusniok C, Dervins-Ravault D, Sismeiro O, Coppee JY, Buchrieser C (2012) Deep sequencing defines the transcriptional map of L. pneumophila and identifies growth phase-dependent regulated ncRNAs implicated in virulence. RNA Biol 9(4):503–519
Sakamoto R, Ohno A, Nakahara T, Satomura K, Iwanaga S, Kouyama Y, Kura F, Noami M, Kusaka K, Funato T, Takeda M, Matsubayashi K, Okumiya K, Kato N, Yamaguchi K (2009) Is driving a car a risk for Legionnaires’ disease? Epidemiol Infect 137:1615–1622
Sampson JS, O'Connor SP, Holloway BP, Plikaytis BB, Carlone GM, Mayer LW (1990) Nucleotide sequence of htpB, the Legionella pneumophila gene encoding the 58-kilodalton (kDa) common antigen, formerly designated the 60-kDa common antigen. Infect Immun 58:3154–3157
Sansom FM, Newton HJ, Crikis S, Cianciotto NP, Cowan PJ, D’ Apice AJ, Hartland EL (2007) A bacterial ecto-triphosphate diphosphohydrolase similar to human CD39 is essential for intracellular multiplication of Legionella pneumophila. Cell Microbiol 9:1922–1935
Sansom FM, Robson SC, Hartland EL (2008) Possible effects of microbial ecto-nucleoside triphosphate diphosphohydrolases on host-pathogen interactions. Microbiol Mol Biol Rev 72:765–781
Santic M, Asare R, Doric M, Abu Kwaik Y (2007) Host-dependent trigger of caspases and apoptosis by Legionella pneumophila. Infect Immun 75:2903–2913
Sargent F, Berks BC, Palmer T (2006) Pathfinders and trailblazers: a prokaryotic targeting system for transport of folded proteins. FEMS Microbiol Lett 254:198–207
Sauer JD, Bachman MA, Swanson MS (2005) The phagosomal transporter A couples threonine acquisition to differentiation and replication of Legionella pneumophila in macrophages. Proc Natl Acad Sci USA 102:9924–9
Sawada K, Ariki S, Kojima T, Saito A, Yamazoe M, Nishitani C, Shimizu T, Takahashi M, Mitsuzawa H, Yokota S, Sawada N, Fujii N, Takahashi H, Kuroki Y (2010) Pulmonary collectins protect macrophages against pore-forming activity of Legionella pneumophila and suppress its intracellular growth. J Biol Chem 285:8434–8443
Schiavoni G, Mauri C, Carlei D, Belardelli F, Pastoris MC, Proietti E (2004) Type I IFN protects permissive macrophages from Legionella pneumophila infection through an IFN-gamma-independent pathway. J Immunol 173:1266–75
Schmidt B, Rahfeld J, Schierhorn A, Ludwig B, Hacker J, Fischer G (1994) A homodimer represents an active species of the peptidyl-prolyl cis/trans isomerase FKBP25mem from Legionella pneumophila. FEBS Lett 352:185–190
Schmitz-Esser S, Tischler P, Arnold R, Montanaro J, Wagner M, Rattei T, Horn M (2010) The genome of the amoeba symbiont “Candidatus Amoebophilus asiaticus” reveals common mechanisms for host cell interaction among amoeba-associated bacteria. J Bacteriol 192:1045–57
Schoebel S, Oesterlin LK, Blankenfeldt W, Goody RS, Itzen A (2009) RabGDI displacement by DrrA from Legionella is a consequence of its guanine nucleotide exchange activity. Mol Cell 36:1060–1072
Schoenen D, Schulze-Robbecke R, Schirdewahn N (1988) Microbial contamination of water by pipe and tubing material. 2. Growth of Legionella pneumophila. Zentralbl Bakteriol Mikrobiol Und Hyg B 186:326–332
Schofield GM, Locci R (1985) Colonization of components of a model hot water system by Legionella pneumophila. J Appl Bacteriol 58:151–162
Schofield GM, Wright AE (1984) Survival of Legionella pneumophila in a model hot water distribution system. J Gen Microbiol 130:1751–1756
Schramek S, Kazar J, Bazovska S (1982) Lipid A in Legionella pneumophila. Zbl Bakt Hyg I Abt Orig A 252:401–404
Schroeder GN, Petty NK, Mousnier A, Harding CR, Vogrin AJ, Wee B, Fry NK, Harrison TG, Newton HJ, Thomson NR, Beatson SA, Dougan G, Hartland E, Frankel G (2010) The genome of Legionella pneumophila strain 130b contains a unique combination of type IV secretion systems and encodes novel Dot/Icm secretion system effector proteins. J Bacteriol 192:6001–6016
Schuetz AN, Hughes RL, Howard RM, Williams TC, Nolte FS, Jackson D, Ribner BS (2009) Pseudo-outbreak of Legionella pneumophila serogroup 8 infection associated with a contaminated ice machine in a bronchoscopy suite. Infect Control Hosp Epidemiol 30:461–466
Schunder E, Adam P, Higa F, Remer KA, Lorenz U, Bender J, Schulz T, Flieger A, Steinert M, Heuner K (2010) Phospholipase PlaB is a new virulence factor of Legionella pneumophila. Int J Med Microbiol 300:313–323
Seeger EM, Thuma M, Fernandez-Moreira E, Jacobs E, Schmitz M, Helbig JH (2010) Lipopolysaccharide of Legionella pneumophila shed in a liquid culture as a nonvesicular fraction arrests phagosome maturation in amoeba and monocytic host cells. FEMS Microbiol Lett 307:113–119
Segal G, Feldman M, Zusman T (2005) The Icm/Dot type-IV secretion systems of Legionella pneumophila and Coxiella burnetii. FEMS Microbiol Rev 29:65–81
Segal G, Purcell M, Shuman HA (1998) Host cell killing and bacterial conjugation require overlapping sets of genes within a 22-kb region of the Legionella pneumophila genome. Proc Natl Acad Sci USA 95:1669–74
Segal G, Shuman HA (1997) Characterization of a new region required for macrophage killing by Legionella pneumophila. Infect Immun 65:5057–66
Segal G, Shuman HA (1998) Intracellular multiplication and human macrophage killing by Legionella pneumophila are inhibited by conjugal components of IncQ plasmid RSF1010. Mol Microbiol 30:197–208
Segal G, Shuman HA (1999) Legionella pneumophila utilizes the same genes to multiply within Acanthamoeba castellanii and human macrophages. Infect Immun 67:2117–2124
Sekla LH, Buchanan AG, Parker SE (1982) Legionella pneumophila pneumonia. Can Med Assoc J 126:116–118
Sepulveda JL, Wu C (2006) The parvins. Cell Mol Life Sci 63:25–35
Serota AI, Meyer RD, Wilson SE, Edelstein PH, Finegold SM (1981) Legionnaires’ disease in the postoperative patient. J Surg Res 30:417–427
Seshadri R, Paulsen IT, Eisen JA, Read TD, Nelson KE, Nelson WC, Ward NL, Tettelin H, Davidsen TM, Beanan MJ, Deboy RT, Daugherty SC, Brinkac LM, Madupu R, Dodson RJ, Khouri HM, Lee KH, Carty HA, Scanlan D, Heinzen RA, Thompson HA, Samuel JE, Fraser CM, Heidelberg JF (2003) Complete genome sequence of the Q-fever pathogen Coxiella burnetii. Proc Natl Acad Sci USA 100:5455–5460
Sexton JA, Vogel JP (2004) Regulation of hypercompetence in Legionella pneumophila. J Bacteriol 186:3814–3825
Shadrach WS, Rydzewski K, Laube U, Holland G, Ozel M, Kiderlen AF, Flieger A (2005) Balamuthia mandrillaris, free-living ameba and opportunistic agent of encephalitis, is a potential host for Legionella pneumophila bacteria. Appl Environ Microbiol 71:2244–2249
Sheehan KB, Henson JM, Ferris MJ (2005) Legionella species diversity in an acidic biofilm community in Yellowstone National Park. Appl Environ Microbiol 71:507–511
Shen X, Banga S, Liu Y, Xu L, Gao P, Shamovsky I, Nudler E, Luo ZQ (2009) Targeting eEF1A by a Legionella pneumophila effector leads to inhibition of protein synthesis and induction of host stress response. Cell Microbiol 11:911–926
Shevchuk O, Batzilla C, Hagele S, Kusch H, Engelmann S, Hecker M, Haas A, Heuner K, Glockner G, Steinert M (2009) Proteomic analysis of Legionella-containing phagosomes isolated from Dictyostelium. Int J Med Microbiol 299:489–508
Shim HK, Kim JY, Kim MJ, Sim HS, Park DW, Sohn JW, Kim MJ (2009) Legionella lipoprotein activates toll-like receptor 2 and induces cytokine production and expression of costimulatory molecules in peritoneal macrophages. Exp Mol Med 41:687–694
Shin S, Case CL, Archer KA, Nogueira CV, Kobayashi KS, Flavell RA, Roy CR, Zamboni DS (2008) Type IV secretion-dependent activation of host MAP kinases induces an increased proinflammatory cytokine response to Legionella pneumophila. PLoS Pathog 4:e1000220
Shin S, Roy CR (2008) Host cell processes that influence the intracellular survival of Legionella pneumophila. Cell Microbiol 10:1209–1220
Shohdy N, Efe JA, Emr SD, Shuman HA (2005) Pathogen effector protein screening in yeast identifies Legionella factors that interfere with membrane trafficking. Proc Natl Acad Sci USA 102:4866–4871
Shuman HA, Purcell M, Segal G, Hales L, Wiater LA (1998) Intracellular multiplication of Legionella pneumophila: human pathogen or accidental tourist? Curr Top Microbiol Immunol 225:99–112
Silveira TN, Zamboni DS (2010) Pore formation triggered by Legionella spp. is an Nlrc4 inflammasome-dependent host cell response that precedes pyroptosis. Infect Immun 78:1403–13
Singh T, Coogan MM (2005) Isolation of pathogenic Legionella species and Legionella-laden amoebae in dental unit waterlines. J Hosp Infect 61:257–262
Skerrett SJ, Martin TR (1994) Intratracheal interferon-gamma augments pulmonary defenses in experimental legionellosis. Am J Respir Crit Care Med 149:50–58
Skerrett SJ, Schmidt RA, Martin TR (1989) Impaired clearance of aerosolized Legionella pneumophila in corticosteroid-treated rats: a model of Legionnaires' disease in the compromised host. J Infect Dis 160:261–273
Söderberg MA, Cianciotto NP (2008) A Legionella pneumophila peptidyl-prolyl cis-trans isomerase present in culture supernatants is necessary for optimal growth at low temperatures. Appl Environ Microbiol 74:1634–1638
Söderberg MA, Cianciotto NP (2010) Mediators of lipid A modification, RNA degradation, and central intermediary metabolism facilitate the growth of Legionella pneumophila at low temperatures. Curr Microbiol 60:59–65
Söderberg MA, Dao J, Starkenburg S, Cianciotto NP (2008) Importance of type II secretion for Legionella pneumophila survival in tap water and amoebae at low temperature. Appl Environ Microbiol 74:5583–5588
Söderberg MA, Rossier O, Cianciotto NP (2004) The type II protein secretion system of Legionella pneumophila promotes growth at low temperatures. J Bacteriol 186:3712–3720
Solomon JM, Rupper A, Cardelli JA, Isberg RR (2000) Intracellular growth of Legionella pneumophila in Dictyostelium discoideum, a system for genetic analysis of host-pathogen interactions. Infect Immun 68:2939–2947
Sopena N, Sabrià M, Pedro-Botet ML, Reynaga E, García-Núñez M, Domínguez J, Matas L (2002) Factors related to persistence of Legionella urinary antigen excretion in patients with legionnaires' disease. Eur J Clin Microbiol Infect Dis 21:845–848
Sopena N, Sabria-Leal M, Pedro-Botet ML, Padilla E, Dominguez J, Morera J, Tudela P (1998) Comparative study of the clinical presentation of Legionella pneumonia and other community-acquired pneumonias. Chest 113:1195–1200
Spirig T, Tiaden A, Kiefer P, Buchrieser C, Vorholt JA, Hilbi H (2008) The Legionella autoinducer synthase LqsA produces an alpha-hydroxyketone signaling molecule. J Biol Chem 283:18113–18123
Spörri R, Joller N, Albers U, Hilbi H, Oxenius A (2006) MyD88-dependent IFN-gamma production by NK cells is key for control of Legionella pneumophila infection. J Immunol 176:6162–71
Spörri R, Joller N, Hilbi H, Oxenius A (2008) A novel role for neutrophils as critical activators of NK cells. J Immunol 181:7121–30
Sprang SR (1997) G protein mechanisms: insights from structural analysis. Annu Rev Biochem 66:639–678
St. John G, Steinman HM (1996) Periplasmic copper-zinc superoxide dismutase of Legionella pneumophila: role in stationary-phase survival. J Bacteriol 178:1578–1584
Starkenburg SR, Casey JM, Cianciotto NP (2004) Siderophore activity among members of the Legionella genus. Curr Microbiol 49:203–207
Stebbins CE, Galan JE (2001) Structural mimicry in bacterial virulence. Nature 412:701–705
Steele TW, McLennan AM (1996) Infection of Tetrahymena pyriformis by Legionella longbeachae and other Legionella species found in potting mixes. Appl Environ Microbiol 62:1081–1083
Steinert M, Emody L, Amann R, Hacker J (1997) Resuscitation of viable but nonculturable Legionella pneumophila Philadelphia JR32 by Acanthamoeba castellanii. Appl Environ Microbiol 63:2047–53
Steinert M, Engelhard H, Flugel M, Wintermeyer E, Hacker J (1995) The Lly protein protects Legionella pneumophila from light but does not directly influence its intracellular survival in Hartmannella vermiformis. Appl Environ Microbiol 61:2428–2430
Steinert M, Flugel M, Schuppler M, Helbig JH, Supriyono A, Proksch P, Luck PC (2001) The Lly protein is essential for p-hydroxyphenylpyruvate dioxygenase activity in Legionella pneumophila. FEMS Microbiol Lett 203:41–47
Steinert M, Heuner K, Buchrieser C, Albert-Weissenberger C, Glockner G (2007) Legionella pathogenicity: genome structure, regulatory networks and the host cell response. Int J Med Microbiol 297:577–87
Steinert M, Ockert G, Luck C, Hacker J (1998) Regrowth of Legionella pneumophila in a heat-disinfected plumbing system. Zentralbl Bakteriol 288:331–342
Steinert M, Ott M, Christian Luck P, Tannich E, Hacker J (1994) Studies on the uptake and intracellular replication of Legionella pneumophila in protozoa and in macrophage-like cells. FEMS Microbiol Ecol 15:299–308
Stetson DB, Medzhitov R (2006) Recognition of cytosolic DNA activates an IRF3-dependent innate immune response. Immunity 24:93–103
Stewart CR, Burnside DM, Cianciotto NP (2011) The surfactant of Legionella pneumophila is secreted in a TolC-dependent manner and is antagonistic toward other Legionella species. J Bacteriol 193:5971–5984
Stewart CR, Rossier O, Cianciotto NP (2009) Surface translocation by Legionella pneumophila: a form of sliding motility that is dependent upon type II protein secretion. J Bacteriol 191:1537–1546
Stone BJ, Abu Kwaik Y (1998) Expression of multiple pili by Legionella pneumophila: identification and characterization of a type IV pilin gene and its role in adherence to mammalian and protozoan cells. Infect Immun 66:1768–1775
Stone BJ, Brier A, Abu Kwaik Y (1999) The Legionella pneumophila prp locus; required during infection of macrophages and amoebae. Microb Pathogen 27:369–376
Stone BJ, Kwaik YA (1999) Natural competence for DNA transformation by Legionella pneumophila and its association with expression of type IV pili. J Bacteriol 181:1395–402
Storey MV, Langmark J, Ashbolt NJ, Stenstrom TA (2004) The fate of legionellae within distribution pipe biofilms: measurement of their persistence, inactivation and detachment. Water Sci Technol 49:269–275
Storz G, Vogel J, Wassarman KM (2011) Regulation by small RNAs in bacteria: expanding frontiers. Mol Cell 43:880–891
Stout JE, Yu VL (1997) Legionellosis. N Engl J Med 337:682–687
Stover CK, Pham XQ, Erwin AL, Mizoguchi SD, Warrener P (2000) Complete genome sequence of Pseudomonas aeruginosa PA01, an opportunistic pathogen. Nature 406:959–964
Straus WL, Plouffe JF, File TM, Jr, Lipman HB, Hackman BH, Salstrom SJ, Benson RF, Breiman RF (1996) Risk factors for domestic acquisition of Legionnaires disease. Arch Intern Med 156:1685–1692
Sturgill-Koszycki S, Swanson MS (2000) Legionella pneumophila replication vacuoles mature into acidic, endocytic organelles. J Exp Med 192:1261–72
Suh HY, Lee DW, Lee KH, Ku B, Choi SJ, Woo JS, Kim YG, Oh BH (2010) Structural insights into the dual nucleotide exchange and GDI displacement activity of SidM/DrrA. EMBO J 29:496–504
Surgot M, Barioz CM, Nowicki M, Bornstein N, Fleurette J (1988) An electron microscopy study of Legionella pneumophila after in vitro and in vivo culture. Zbl Bakteriol 269:26–33
Susa M, Hacker J, Marre R (1996) De novo synthesis of Legionella pneumophila antigens during intracellular growth in phagocytic cells. Infect Immun 64:1679–1684
Susa M, Ticac B, Rukavina T, Doric M, Marre R (1998) Legionella pneumophila infection in intratracheally inoculated T cell-depleted or -nondepleted A/J mice. J Immunol 160:316–321
Suter TM, Viswanathan VK, Cianciotto NP (1997) Isolation of a gene encoding a novel spectinomycin phosphotransferase from Legionella pneumophila. Antimicrob Agts Chemother 41:1385–1388
Suzuki K, Wang X, Weilbacher T, Pernestig AK, Melefors O, Georgellis D, Babitzke P, Romeo T (2002) Regulatory circuitry of the CsrA/CsrB and BarA/UvrY systems of Escherichia coli. J Bacteriol 184:5130–5140
Svarrer CW, Lück C, Elverdal PL, Uldum SA (2012) Immunochromatic kits Xpect Legionella and BinaxNOW Legionella for detection of Legionella pneumophila urinary antigen have low sensitivities for the diagnosis of Legionnaires’ disease. J Med Microbiol 61:213–217
Svarrer CW, Uldum SA (2011) The occurrence of Legionella species other than Legionella pneumophila in clinical and environmental samples in Denmark identified by mip gene sequencing and matrix-assisted laser desorption ionization time-of-flight mass spectrometry. Clin Microbiol Infect. doi: 10.1111/j.1469-0691.2011.03698.x.
Swanson MS, Hammer BK (2000) Legionella pneumophila pathogenesis: a fateful journey from amoebae to macrophages. Annu Rev Microbiol 54:567–613
Swanson MS, Isberg RR (1995) Association of Legionella pneumophila with the macrophage endoplasmic reticulum. Infect Immun 63:3609–3620
Swanson MS, Molofsky AB (2005) Autophagy and inflammatory cell death, partners of innate immunity. Autophagy 1:174–176
Takekawa Y, Saito M, Wang C, Qin T, Ogawa M, Kanemaru T, Yoshida SI (2012) Characteristic morphology of intracellular microcolonies of Legionella oakridgensis OR-10. Can J Microbiol. doi:10.1139/w11-126
Tan MJ, Tan JS, Hamor RH, File TM, Jr, Breiman RF (2000) The radiologic manifestations of Legionnaire’s disease. The Ohio Community-Based Pneumonia Incidence Study Group. Chest 117:398–403
Tan Y, Luo ZQ (2011) Legionella pneumophila SidD is a deAMPylase that modifies Rab1. Nature 475:506–509
Tang P, Krishnan C (1993) Legionellosis in Ontario, Canada: laboratory aspects. In: Barbaree JM, Breiman RF, Dufour AP (ed) Legionella: current status and emerging perspectives. ASM Press, Washington, DC, pp 16–17
Tateda K, Moore TA, Deng JC, Newstead MW, Zeng X, Matsukawa A, Swanson MS, Yamaguchi K, Standiford TJ (2001a) Early recruitment of neutrophils determines subsequent T1/T2 host responses in a murine model of Legionella pneumophila pneumonia. J Immunol 166:3355–61
Tateda K, Moore TA, Newstead MW, Tsai WC, Zeng X, Deng JC, Chen G, Reddy R, Yamaguchi K, Standiford TJ (2001b) Chemokine-dependent neutrophil recruitment in a murine model of Legionella pneumonia: potential role of neutrophils as immunoregulatory cells. Infect Immun 69:2017–24
Taylor M, Ross K, Bentham R (2009) Legionella, protozoa, and biofilms: interactions within complex microbial systems. Microb Ecol 58:538–547
Telford D, Partridge S, Cumming I, Smith A, Calvert N (2006) The legionnaires' outbreak in Barrow-in-Furness, summer 2002. J Epidemiol Community Health 60:464–466
Temmerman R, Vervaeren H, Noseda B, Boon N, Verstraete W (2006) Necrotrophic growth of Legionella pneumophila. Appl Environ Microbiol 72:4323–4328
Tesh MJ, Miller RD (1981) Amino acid requirements for Legionella pneumophila growth. J Clin Microbiol 13:865–869
Tesh MJ, Morse SA, Miller RD (1983) Intermediary metabolism in Legionella pneumophila: utilization of amino acids and other compounds as energy sources. J Bacteriol 154:1104–1109
Thacker WL, Benson RF, Hawes L, Gidding H, Dwyer B, Mayberry WR, Brenner DJ (1991) Legionella fairfieldensis sp. nov. isolated from cooling tower waters in Australia. J Clin Microbiol 29:475–478
Thacker WL, Benson RF, Schifman RB, Pugh E, Steigerwalt AG, Mayberry WR, Brenner DJ, Wilkinson HW (1989) Legionella tucsonensis sp. nov. isolated from a renal transplant recipient. J Clin Microbiol 27:1831–1834
Thacker WL, Benson RF, Staneck JL, Vincent SR, Mayberry WR, Brenner DJ, Wilkinson HW (1988) Legionella cincinnatiensis sp. nov. isolated from a patient with pneumonia. J Clin Microbiol 26:418–420
Thacker WL, Dyke JW, Benson RF, Havlichek DH, Jr, Robinson-Dunn B, Stiefel H, Schneider W, Moss CW, Mayberry WR, Brenner DJ (1992) Legionella lansingensis sp. nov. isolated from a patient with pneumonia and underlying chronic lymphocytic leukemia. J Clin Microbiol 30:2398–2401
Thomas V, Herrera-Rimann K, Blanc DS, Greub G (2006) Biodiversity of amoebae and amoeba-resisting bacteria in a hospital water network. Appl Environ Microbiol 72:2428–2438
Thomas V, McDonnell G, Denyer SP, Maillard JY (2010) Free-living amoebae and their intracellular pathogenic microorganisms: risks for water quality. FEMS Microbiol Rev 34:231–259
Thompson PR, Hughes DW, Cianciotto NP, Wright GD (1998) Spectinomycin kinase from Legionella pneumophila. Characterization of substrate specificity and identification of catalytically important residues. J Biol Chem 273:14788–14795
Thorsted PB, Macartney DP, Akhtar P, Haines AS, Ali N, Davidson P, Stafford T, Pocklington MJ, Pansegrau W, Wilkins BM, Lanka E, Thomas CM (1998) Complete sequence of the IncPbeta plasmid R751: implications for evolution and organisation of the IncP backbone. J Mol Biol 282:969–990
Tiaden A, Hilbi H (2012) a-Hydroxyketone synthesis and sensing by Legionella and Vibrio. Sensors 12:2899–2919
Tiaden A, Spirig T, Carranza P, Bruggemann H, Riedel K, Eberl L, Buchrieser C, Hilbi H (2008) Synergistic contribution of the Legionella pneumophila lqs genes to pathogen-host interactions. J Bacteriol 190:7532–47
Tiaden A, Spirig T, Hilbi H (2010a) Bacterial gene regulation by alpha-hydroxyketone signaling. Trends Microbiol 18:288–97
Tiaden A, Spirig T, Sahr T, Walti MA, Boucke K, Buchrieser C, Hilbi H (2010b) The autoinducer synthase LqsA and putative sensor kinase LqsS regulate phagocyte interactions, extracellular filaments and a genomic island of Legionella pneumophila. Environ Microbiol 12:1243–59
Tiaden A, Spirig T, Weber SS, Bruggemann H, Bosshard R, Buchrieser C, Hilbi H (2007) The Legionella pneumophila response regulator LqsR promotes host cell interactions as an element of the virulence regulatory network controlled by RpoS and LetA. Cell Microbiol 9:2903–20
Tilney LG, Harb OS, Connelly PS, Robinson CG, Roy CR (2001) How the parasitic bacterium Legionella pneumophila modifies its phagosome and transforms it into rough ER: implications for conversion of plasma membrane to the ER membrane. J Cell Sci 114:4637–50
Tindall BJ, Rossello-Mora R, Busse HJ, Ludwig W, Kampfer P (2010) Notes on the characterization of prokaryote strains for taxonomic purposes. Int J Syst Evol Microbiol 60:249–266
Tison DL, Pope DH, Cherry WB, Fliermans CB (1980) Growth of Legionella pneumophila in association with blue-green algae (cyanobacteria). Appl Environ Microbiol 39:456–459
Tobiansky L, Drath A, Dubery B, Koornhof HJ (1986) Seasonality of Legionella isolates from environmental sources. Isr J Med Sci 22:640–643
Tossa P, Deloge-Abarkan M, Zmirou-Navier D, Hartemann P, Mathieu L (2006) Pontiac fever: an operational definition for epidemiological studies. BMC Public Health 6:112
Towns ML, Fisher D, Moore J (1994) Community-acquired pneumonia due to Legionella gormanii. Clin Infect Dis 18:265–266
Tsai TF, Finn DR, Plikaytis BD, McCauley W, Martin SM, Fraser DW (1979) Legionnaires’ disease: clinical features of the epidemic in Philadelphia. Ann Intern Med 90:509–517
Tubach F, Ravaud P, Salmon-Ceron D, Petitpain N, Brocq O, Grados F, Guillaume JC, Leport J, Roudaut A, Solau-Gervais E, Lemann M, Mariette X, Lortholary O (2006) Emergence of Legionella pneumophila pneumonia in patients receiving tumor necrosis factor-alpha antagonists. Clin Infect Dis 43:e95–100
Turetgen I, Cotuk A (2007) Monitoring of biofilm-associated Legionella pneumophila on different substrata in model cooling tower system. Environ Monit Assess 125:271–279
Turick CE, Tisa LS, Caccavo F, Jr (2002) Melanin production and use as a soluble electron shuttle for Fe(III) oxide reduction and as a terminal electron acceptor by Shewanella algae BrY. Appl Environ Microbiol 68:2436–2444
Tyndall RL, Domingue EL (1982) Cocultivation of Legionella pneumophila and free-living amoebae. Appl Environ Microbiol 44:954–959
Tzivelekidis T, Jank T, Pohl C, Schlosser A, Rospert S, Knudsen CR, Rodnina MV, Belyi Y, Aktories K (2011) Aminoacyl-tRNA-charged eukaryotic elongation factor 1A is the bona fide substrate for Legionella pneumophila effector glucosyltransferases. PLoS One 6:e29525
Unal C, Schwedhelm KF, Thiele A, Weiwad M, Schweimer K, Frese F, Fischer G, Hacker J, Faber C, Steinert M (2011) Collagen IV-derived peptide binds hydrophobic cavity of Legionella pneumophila Mip and interferes with bacterial epithelial transmigration. Cell Microbiol 13:1558–1572
Urwyler S, Finsel I, Ragaz C, Hilbi H (2010) Isolation of Legionella-containing vacuoles by immuno-magnetic separation. Curr Protoc Cell Biol Chapter 3:Unit 3:34
Urwyler S, Nyfeler Y, Ragaz C, Lee H, Mueller LN, Aebersold R, Hilbi H (2009) Proteome analysis of Legionella vacuoles purified by magnetic immunoseparation reveals secretory and endosomal GTPases. Traffic 10:76–87
Valster RM, Wullings BA, van den Berg R, van der Kooij D (2011) Relationships between free-living protozoa, cultivable Legionella spp., and water quality characteristics in three drinking water supplies in the Caribbean. Appl Environ Microbiol 77:7321–7328
Valster RM, Wullings BA, van der Kooij D (2010) Detection of protozoan hosts for Legionella pneumophila in engineered water systems by using a biofilm batch test. Appl Environ Microbiol 76:7144–7153
Vance RE, Hong S, Gronert K, Serhan CN, Mekalanos JJ (2004) The opportunistic pathogen Pseudomonas aeruginosa carries a secretable arachidonate 15-lipoxygenase. Proc Natl Acad Sci USA 101:2135–2139
Vandenesch F, Surgot M, Bornstein N, Paucod JC, Marmet D, Isoard P, Fleurette J (1990) Relationship between free amoeba and Legionella: studies in vitro and in vivo. Zbl Bakt 272:265–275
Vandersmissen L, De Buck E, Saels V, Coil DA, Anne J (2010) A Legionella pneumophila collagen-like protein encoded by a gene with a variable number of tandem repeats is involved in the adherence and invasion of host cells. FEMS Microbiol Lett 306:168–176
VanRheenen SM, Luo ZQ, O'Connor T, Isberg RR (2006) Members of a Legionella pneumophila family of proteins with ExoU (phospholipase A) active sites are translocated to target cells. Infect Immun 74:3597–3606
Venezia RA, Agresta MD, Hanley EM, Urquhart K, Schoonmaker D (1994) Nosocomial legionellosis associated with aspiration of nasogastric feedings diluted in tap water. Infect Control Hosp Epidemiol 15:529–533
Verbrugh HA, Lee DA, Elliott GR, Keane WF, Hoidal JR, Peterson PK (1985) Opsonization of Legionella pneumophila in human serum: key roles for specific antibodies and the classical complement pathway. Immunology 54:643–653
Vergnes M, Ginevra C, Kay E, Normand P, Thioulouse J, Jarraud S, Maurin M, Schneider D (2011) Insertion sequences as highly resolutive genomic markers for sequence type 1 Legionella pneumophila Paris. J Clin Microbiol 49:315–324
Verissimo A, Marrao G, Gomes da Silva F, Da Costa MS (1991) Distribution of Legionella spp. in hydrothermal areas in continental Portugal and the island of Sao Miguel. Azores Appl Environ Microbiol 57:2921–2927
Verma UK, Brenner DJ, Thacker WL, Benson RF, Vesey G, Kurtz JB, Dennis PJ, Steigerwalt AG, Robinson JS, Moss CW (1992) Legionella shakespearei sp. nov., isolated from cooling tower water. Int J Syst Bacteriol 42:404–407
Vervaeren H, Temmerman R, Devos L, Boon N, Verstraete W (2006) Introduction of a boost of Legionella pneumophila into a stagnant-water model by heat treatment. FEMS Microbiol Ecol 58:583–592
Vickers RM, Stout JE, Yu VL, Rihs JD (1987) Manual of culture methodology for Legionella. Semin Respir Infect 2:274–9
Vickers RM, Yu VL (1984) Clinical laboratory differentiation of Legionellaceae family members with pigment production and fluorescence on media supplemented with aromatic substrates. J Clin Microbiol 19:583–587
Vinzing M, Eitel J, Lippmann J, Hocke AC, Zahlten J, Slevogt H, N’Guessan PD, Gunther S, Schmeck B, Hippenstiel S, Flieger A, Suttorp N, Opitz B (2008) NAIP and Ipaf control Legionella pneumophila replication in human cells. J Immunol 180:6808–6815
Viswanathan VK, Edelstein PH, Pope CD, Cianciotto NP (2000) The Legionella pneumophila iraAB locus is required for iron assimilation, intracellular infection, and virulence. Infect Immun 68:1069–1079
Viswanathan VK, Kurtz S, Pedersen LL, Abu-Kwaik Y, Krcmarik K, Mody S, Cianciotto NP (2002) The cytochrome c maturation locus of Legionella pneumophila promotes iron assimilation and intracellular infection and contains a strain-specific insertion sequence element. Infect Immun 70:1842–1852
Vivian JP, Riedmaier P, Ge H, Le Nours J, Sansom FM, Wilce MC, Byres E, Dias M, Schmidberger JW, Cowan PJ, D’ Apice AJ, Hartland EL, Rossjohn J, Beddoe T (2010) Crystal structure of a Legionella pneumophila ecto -triphosphate diphosphohydrolase, a structural and functional homolog of the eukaryotic NTPDases. Structure 18:228–238
Vogel JP, Andrews HL, Wong SK, Isberg RR (1998) Conjugative transfer by the virulence system of Legionella pneumophila. Science 279:873–6
Vogel JP, Isberg RR (1999) Cell biology of Legionella pneumophila. Curr Opin Microbiol 2:30–4
von Baum H, Ewig S, Marre R, Suttorp N, Gonschior S, Welte T, Luck C (2008) Community-acquired Legionella pneumonia: new insights from the German competence network for community acquired pneumonia. Clin Infect Dis 46:1356–1364
Voth DE, Howe D, Beare PA, Vogel JP, Unsworth N, Samuel JE, Heinzen RA (2009) The Coxiella burnetii ankyrin repeat domain-containing protein family is heterogeneous, with C-terminal truncations that influence Dot/Icm-mediated secretion. J Bacteriol 191:4232–42
Wadowsky RM, Butler LJ, Cook MK, Verma SM, Paul MA, Fields BS, Keleti G, Sykora JL, Yee RB (1988) Growth-supporting activity for Legionella pneumophila in tap water cultures and implication of hartmannellid amoebae as growth factors. Appl Environ Microbiol 54:2677–2682
Wadowsky RM, Wilson TM, Kapp NJ, West AJ, Kuchta JM, States SJ, Dowling JN, Yee RB (1991) Multiplication of Legionella spp. in tap water containing Hartmannella vermiformis. Appl Environ Microbiol 57:1950–1955
Wadowsky RM, Wolford R, McNamara AM, Yee RB (1985) Effect of temperature, pH, and oxygen level on the multiplication of naturally occurring Legionella pneumophila in potable water. Appl Environ Microbiol 49:1197–1205
Wadowsky RM, Yee RB (1981) Glycine-containing selective medium for isolation of Legionellaceae from environmental specimens. Appl Environ Microbiol 42:768–772
Wagner C, Khan AS, Kamphausen T, Schmausser B, Unal C, Lorenz U, Fischer G, Hacker J, Steinert M (2007) Collagen binding protein Mip enables Legionella pneumophila to transmigrate through a barrier of NCI-H292 lung epithelial cells and extracellular matrix. Cell Microbiol 9:450–462
Walker JT, Sonesson A, Keevil CW, White DC (1993) Detection of Legionella pneumophila in biofilms containing a complex microbial consortium by gas chromatography-mass spectrometry analysis of genus-specific hydroxy fatty acids. FEMS Microbiol Lett 113:139–144
Wallensten A, Oliver I, Ricketts K, Kafatos G, Stuart JM, Joseph C (2010) Windscreen wiper fluid without added screenwash in motor vehicles: a newly identified risk factor for Legionnaires' disease. Eur J Epidemiol 25:661–665
Wang Z, Gerstein M, Snyder M (2009) RNA-Seq: a revolutionary tool for transcriptomics. Nat Rev Microbiol 10:57–63
Warren WJ, Miller RD (1979) Growth of Legionnaires disease bacterium (Legionella pneumophila) in chemically defined medium. J Clin Microbiol 10:50–55
Wassarman KM (2007) 6S RNA: a small RNA regulator of transcription. Curr Opin Microbiol 10:164–8
Watson JH, Sun CN (1981) Scanning electron microscopy of Legionella pneumophila in clinical tissue. Scanning Elect Microscr 3:55–64
Weber SS, Ragaz C, Hilbi H (2009a) The inositol polyphosphate 5-phosphatase OCRL1 restricts intracellular growth of Legionella, localizes to the replicative vacuole and binds to the bacterial effector LpnE. Cell Microbiol 11:442–60
Weber SS, Ragaz C, Hilbi H (2009b) Pathogen trafficking pathways and host phosphoinositide metabolism. Mol Microbiol 71:1341–52
Weber SS, Ragaz C, Reus K, Nyfeler Y, Hilbi H (2006) Legionella pneumophila exploits PI(4)P to anchor secreted effector proteins to the replicative vacuole. PLoS Pathog 2:e46
Weinbaum DL, Benner RR, Dowling JN, Alpern A, Pasculle AW, Donowitz GR (1984) Interaction of Legionella micdadei with human monocytes. Infect Immun 46:68–73
Weiner RM (1997) Biopolymers from marine prokaryotes. Trends Biotechnol 15:390–394
Weir SC, Fischer SH, Stock F, Gill VJ (1998) Detection of Legionella by PCR in respiratory specimens using a commercially available kit. Am J Clin Pathol 110:295–300
Weisburg WG, Dobson ME, Samuel JE, Dasch GA, Mallavia LP, Baca O, Mandelco L, Sechrest JE, Weiss E, Woese CR (1989) Phylogenetic diversity of the Rickettsiae. J Bacteriol 171:4202–4206
Weissenmayer BA, Prendergast JG, Lohan AJ, Loftus BJ (2011) Sequencing illustrates the transcriptional response of Legionella pneumophila during infection and identifies seventy novel small non-coding RNAs. PLoS One 6:e17570
Whiley H, Bentham R (2011) Legionella longbeachae and legionellosis. Emerg Infect Dis 17:579–583
White HJ, Sun CN, Hui AN (1979) An ultrastructural demonstration of the agent of Legionnaires' disease in the human lung. Hum Pathol 10:96–99
Whitfield NN, Byrne BG, Swanson MS (2010) Mouse macrophages are permissive to motile Legionella species that fail to trigger pyroptosis. Infect Immun 78:423–432
Wieland H, Faigle M, Lang F, Northoff H, Neumeister B (2002) Regulation of the Legionella mip-promotor during infection of human monocytes. FEMS Microbiol Lett 212:127–132
Wieland H, Ullrich S, Lang F, Neumeister B (2005) Intracellular multiplication of Legionella pneumophila depends on host cell amino acid transporter SLC1A5. Mol Microbiol 55:1528–37
Wilkinson HW, Drasar V, Thacker WL, Benson RF, Schindler J, Potuznikova B, Mayberry WR, Brenner DJ (1988) Legionella moravica sp. nov. and Legionella brunensis sp. nov. isolated from cooling-tower water. Ann Inst Pasteur Microbiol 139:393–402
Wilkinson HW, Reingold AL, Brake BJ, McGiboney DL, Gorman GW, Broome CV (1983) Reactivity of serum from patients with suspected legionellosis against 29 antigens of legionellaceae and Legionella-like organisms by indirect immunofluorescence assay. J Infect Dis 147:23–31
Wilkinson HW, Thacker WL, Benson RF, Polt SS, Brookings E, Mayberry WR, Brenner DJ, Gilley RG, Kirklin JK (1987) Legionella birminghamensis sp. nov. isolated from a cardiac transplant recipient. J Clin Microbiol 25:2120–2122
Wilkinson HW, Thacker WL, Steigerwalt AG, Brenner DJ, Ampel NM, Wing EJ (1985) Second serogroup of Legionella hackeliae isolated from a patient with pneumonia. J Clin Microbiol 22:488–489
Williams A, Baskerville A, Dowsett AB, Conlan JW (1987) Immunocytochemical demonstration of the association between Legionella pneumophila, its tissue-destructive protease, and pulmonary lesions in experimental Legionnaires' disease. J Pathol 153:257–264
Williams KP, Gillespie JJ, Sobral BW, Nordberg EK, Snyder EE, Shallom JM, Dickerman AW (2010) Phylogeny of gammaproteobacteria. J Bacteriol 192:2305–2314
Williams MM, Braun-Howland EB (2003) Growth of Escherichia coli in model distribution system biofilms exposed to hypochlorous acid or monochloramine. Appl Environ Microbiol 69:5463–5471
Wingender J, Flemming HC (2011) Biofilms in drinking water and their role as reservoir for pathogens. Int J Hyg Environ Health 214:417–423
Winn WC, Jr, Davis GS, Gump DW, Craighead JE, Beaty HN (1982) Legionnaires' pneumonia after intratracheal inoculation of guinea pigs and rats. Lab Invest 47:568–578
Winn WCJ (1988) Legionnaires disease: historical perspective. Clin Microbiol Rev 1:60–81
Wintermeyer E, Flugel M, Ott M, Steinert M, Rdest U, Mann KH, Hacker J (1994) Sequence determination and mutational analysis of the lly locus of Legionella pneumophila. Infect Immun 62:1109–1117
Wintermeyer E, Ludwig B, Steinert M, Schmidt B, Fischer G, Hacker J (1995) Influence of site specifically altered Mip proteins on intracellular survival of Legionella pneumophila in eukaryotic cells. Infect Immun 63:4576–4583
Wintermeyer E, Rdest U, Ludwig B, Debes A, Hacker J (1991) Characterization of legiolysin (lly), responsible for haemolytic activity, colour production and fluorescence of Legionella pneumophila. Mol Microbiol 5:1135–1143
Wong KH, Moss CW, Hochstein DH, Arko RJ, Schalla WO (1979) “Endotoxicity” of the Legionnaires' disease bacterium. Ann Int Med 90:624–627
Woo AH, Goetz A, Yu VL (1992) Transmission of Legionella by respiratory equipment and aerosol generating devices. Chest 102:1586–1590
Woodhead M (2002) Community-acquired pneumonia in Europe: causative pathogens and resistance patterns. Eur Respir J Suppl 36:20s–27s
Wrase R, Scott H, Hilgenfeld R, Hansen G (2011) The Legionella HtrA homologue DegQ is a self-compartmentizing protease that forms large 12-meric assemblies. Proc Natl Acad Sci USA 108:10490–10495
Wright EK, Goodart SA, Growney JD, Hadinoto V, Endrizzi MG, Long EM, Sadigh K, Abney AL, Bernstein-Hanley I, Dietrich WF (2003) Naip5 affects host susceptibility to the intracellular pathogen Legionella pneumophila. Curr Biol 13:27–36
Wright JB, Ruseska I, Athar MA, Corbett S, Costerton JW (1989) Legionella pneumophila grows adherent to surfaces in vitro and in situ. Infect Control Hosp Epidemiol 10:408–415
Wright JB, Ruseska I, Costerton JW (1991) Decreased biocide susceptibility of adherent Legionella pneumophila. J Appl Bacteriol 71:531–538
Wright LP, Philips MR (2006) Thematic review series: lipid posttranslational modifications. CAAX modification and membrane targeting of Ras. J Lipid Res 47:883–91
Wu M, Sun LV, Vamathevan J, Riegler M, Deboy R, Brownlie JC, McGraw EA, Martin W, Esser C, Ahmadinejad N, Wiegand C, Madupu R, Beanan MJ, Brinkac LM, Daugherty SC, Durkin AS, Kolonay JF, Nelson WC, Mohamoud Y, Lee P, Berry K, Young MB, Utterback T, Weidman J, Nierman WC, Paulsen IT, Nelson KE, Tettelin H, O'Neill SL, Eisen JA (2004) Phylogenomics of the reproductive parasite Wolbachia pipientis wMel: a streamlined genome overrun by mobile genetic elements. PLoS Biol 2:E69
Wullings BA, van der Kooij D (2006) Occurrence and genetic diversity of uncultured Legionella spp. in drinking water treated at temperatures below 15 degrees C. Appl Environ Microbiol 72:157–166
Yamamoto H, Hashimoto Y, Ezaki T (1996) Study of nonculturable Legionella pneumophila cells during multiple-nutrient starvation. FEMS Microbiol Ecol 20:149–154
Yamamoto H, Sugiura M, Kusunoki S, Ezaki T, Ikedo M, Yabuuchi E (1992a) Factors stimulating propagation of Legionellae in cooling tower water. Appl Environ Microbiol 58:1394–1397
Yamamoto Y, Klein TW, Friedman H (1992b) Genetic control of macrophage susceptibility to infection by Legionella pneumophila. FEMS Microbiol Immunol 89:137–146
Yamamoto Y, Klein TW, Newton CA, Widen R, Friedman H (1987) Differential growth of Legionella pneumophila in guinea pig versus mouse macrophage cultures. Infect Immun 55:1369–1374
Yamamoto Y, Klein TW, Newton CA, Widen R, Friedman H (1988) Growth of Legionella pneumophila in thioglycolate-elicited peritoneal macrophages from A/J mice. Infect Immun 56:370–375
Yan L, Cirillo JD (2004) Infection of murine macrophage cell lines by Legionella pneumophila. FEMS Microbiol Lett 230:147–152
Yanez ME, Korotkov KV, Abendroth J, Hol WG (2008) Structure of the minor pseudopilin EpsH from the Type 2 secretion system of Vibrio cholerae. J Mol Biol 377:91–103
Yang G, Benson RF, Ratcliff R, Brown EW, Steigerwalt AG, Thacker LW, Daneshvar M, Morey RE, Saito A, Fields BS (2011) Legionella nagasakiensis sp. nov., isolated from water samples in Japan and Australia and from a patient with pneumonia in the United States. Int J Syst Evol Microbiol 62:248–288
Yaradou DF, Raze D, Ginevra C, Ader F, Doleans-Jordheim A, Vandenesch F, Menozzi FD, Etienne J, Jarraud S (2007) Zinc-dependent cytoadherence of Legionella pneumophila to human alveolar epithelial cells in vitro. Microb Pathog 43:234–242
Yip ES, Burnside DM, Cianciotto NP (2011) Cytochrome c4 is required for siderophore expression by Legionella pneumophila, whereas cytochromes c1 and c5 promote intracellular infection. Microbiology 157:868–878
Yoshida S, Goto Y, Mizuguchi Y, Nomoto K, Skamene E (1991) Genetic control of natural resistance in mouse macrophages regulating intracellular Legionella pneumophila multiplication in vitro. Infect Immun 59:428–432
Yoshida S, Mizuguchi Y (1986) Multiplication of Legionella pneumophila Philadelphia-1 in cultured peritoneal macrophages and its correlation to susceptibility of animals. Can J Microbiol 32:438–442
Yu H, Higa F, Koide M, Haranaga S, Yara S, Tateyama M, Li H, Fujita J (2009) Lung abscess caused by Legionella species: implication of the immune status of hosts. Intern Med 48:1997–2002
Yu VL, Plouffe JF, Pastoris MC, Stout JE, Schousboe M, Widmer A, Summersgill J, File T, Heath CM, Paterson DL, Chereshsky A (2002) Distribution of Legionella species and serogroups isolated by culture in patients with sporadic community-acquired legionellosis: an international collaborative survey. J Infect Dis 186:127–128
Yu VL, Stout JE (2008) Community-acquired legionnaires disease: implications for underdiagnosis and laboratory testing. Clin Infect Dis 46:1365–1367
Yzerman EP, den Boer JW, Lettinga KD, Schellekens J, Dankert J, Peeters MF (2002) Sensitivity of three urinary antigen tests associated with clinical severity in a large outbreak of Legionnaires' disease in The Netherlands. J Clin Microbiol 40:3232–3236
Zahringer U, Knirel YA, Lindner B, Helbig JH, Sonesson A, Marre R, Rietschel ET (1995) The lipopolysaccharide of Legionella pneumophila serogroup 1 (strain Philadelphia 1): chemical structure and biological significance. Prog Clin Biol Res 392:113–139
Zamboni DS, Kobayashi KS, Kohlsdorf T, Ogura Y, Long EM, Vance RE, Kuida K, Mariathasan S, Dixit VM, Flavell RA, Dietrich WF, Roy CR (2006) The Birc1e cytosolic pattern-recognition receptor contributes to the detection and control of Legionella pneumophila infection. Nat Immunol 7:318–325
Zarogoulidis P, Alexandropoulou I, Romanidou G, Konstasntinidis TG, Terzi E, Saridou S, Stefanis A, Zarogoulidis K, Constantinidis TC (2011) Community-acquired pneumonia due to Legionella pneumophila, the utility of PCR, and a review of the antibiotics used. Int J Gen Med 4:15–19
Zgurskaya HI (2009) Multicomponent drug efflux complexes: architecture and mechanism of assembly. Future Microbiol 4:919–932
Zhang Y, Higashide WM, McCormick BA, Chen J, Zhou D (2006) The inflammation-associated Salmonella SopA is a HECT-like E3 ubiquitin ligase. Mol Microbiol 62:786–93
Zhu W, Banga S, Tan Y, Zheng C, Stephenson R, Gately J, Luo ZQ (2011) Comprehensive identification of protein substrates of the Dot/Icm type iv transporter of Legionella pneumophila. PLoS One 6:e17638
Zhu Y, Hu L, Zhou Y, Yao Q, Liu L, Shao F (2010) Structural mechanism of host Rab1 activation by the bifunctional Legionella type IV effector SidM/DrrA. Proc Natl Acad Sci USA 107:4699–704
Zou CH, Knirel YA, Helbig JH, Zahringer U, Mintz CS (1999) Molecular cloning and characterization of a locus responsible for O acetylation of the O polysaccharide of Legionella pneumophila serogroup 1 lipopolysaccharide. J Bacteriol 181:4137–4141
Zuckman DM, Hung JB, Roy CR (1999) Pore-forming activity is not sufficient for Legionella pneumophila phagosome trafficking and intracellular growth. Mol Microbiol 32:990–1001
Zusman T, Aloni G, Halperin E, Kotzer H, Degtyar E, Feldman M, Segal G (2007) The response regulator PmrA is a major regulator of the icm/dot type IV secretion system in Legionella pneumophila and Coxiella burnetii. Mol Microbiol 63:1508–1523
Zusman T, Degtyar E, Segal G (2008) Identification of a hypervariable region containing new Legionella pneumophila Icm/Dot translocated substrates by using the conserved icmQ regulatory signature. Infect Immun 76:4581–4591
Zusman T, Gal-Mor O, Segal G (2002) Characterization of a Legionella pneumophila relA insertion mutant and toles of RelA and RpoS in virulence gene expression. J Bacteriol 184:67–75
Acknowledgments
Research in CB’s group received financial support from the Institut Pasteur, the Centre National de la Recherche (CNRS) the Institut Carnot-Pasteur MI the Fondation pour la Recherche Médicale (FRM), the grant n°ANR-10-LABX-62-IBEID and the ANR-10-PATH-004 project MobilGenomics, in the frame of ERA-Net PathoGenoMics. Research in HH’s group was funded by the Max von Pettenkofer Institute, LMU Munich, the DFG (HI 1511/1-1, HI 1511/1-2, SPP1316, SPP1580, SPP1617, SFB914), the BMBF (“Medical Infection Genomics” initiative, grant 0315834C) and the SNF (31003A-125369; Sinergia CRSI33_130016). Research in NC’s group was funded, in part, by NIH grants AI034937, AI043987 and AI076693.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2013 Springer-Verlag Berlin Heidelberg
About this entry
Cite this entry
Cianciotto, N.P., Hilbi, H., Buchrieser, C. (2013). Legionnaires’ Disease. In: Rosenberg, E., DeLong, E.F., Lory, S., Stackebrandt, E., Thompson, F. (eds) The Prokaryotes. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-642-30144-5_94
Download citation
DOI: https://doi.org/10.1007/978-3-642-30144-5_94
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-642-30143-8
Online ISBN: 978-3-642-30144-5
eBook Packages: Biomedical and Life SciencesReference Module Biomedical and Life Sciences