Abstract
Denitrification is the dissimilatory reduction of nitrate to nitrogen gas. This respiratory process requires four enzymes that produce three obligatory intermediates prior to production of the terminal product. Denitrification is found in diverse array of microbes including members of both bacteria and archaea. However, no bacterium has been described that solely depends on denitrification as a form of energy generation. All denitrifiers, with one exception, are aerobes. Genome sequencing has provided a better appreciation of the distribution of denitrification genes among microbes. Complete denitrification, the reduction of nitrate to N2, is less frequent than partial denitrification among sequenced bacteria. Partial denitrification chains of nearly all possible arrangments have been found. This includes chains with only a single enzyme or discontinuous chains of two or more enzymes.
Nitrate reductase catalyzes the reduction of nitrate to nitrite and is used in a number of pathways other than denitrification; therefore, its distribution has not been a focus of this chapter. Nitrite reductase catalyzes the reduction of nitrite to nitric oxide and is the defining reaction of denitrification since it is the first step to produce a gaseous nitrogen oxide. There are two unrelated types of nitrite reductase, one of which has copper cofactors while the other contains heme-bound iron. The copper form has several different subtypes with N- and C-terminal extensions containing metal-binding sites. Some members of the Actinobacteria have a particularly large copper nitrite reductase with a membrane-bound domain of unknown function. Nitric oxide reductase catalyzes the reduction of nitric oxide to nitrous oxide. This enzyme is membrane bound and occurs in two subtypes referred to as cNor and qNor. The former receives electrons from cytochrome c while the latter carries an N-terminal extension allowing it to oxidize quinol. Nitrous oxide reductase is a soluble copper-containing enzyme with one of the copper centers, designated the CuZ center, being unique to this enzyme.
While most model denitrifiers use denitrification to support growth when oxygen is limiting, this may not be the case in all bacteria that contain genes encoding denitrification-associated nitrogen oxide reductases. Bacteria with partial chains consisting of a single enzyme may use that enzyme for alternative functions. For example, some Staphylococcus aureus subspecies aureus strains only contain nitric oxide reductase which is likely used for detoxification of nitric oxide. There are a number of bacteria which only contain nitrite reductase and the function of this enzyme is unclear in these organisms since its turnover will produce nitric oxide, which is toxic due to its reactivity with metal centers and other compounds.
Environmental studies have found denitrification genes are nearly universal in environments that receive some exposure to oxygen. Quantitative studies have found that the genes for nitrous oxide reductase are frequently underrepresented compared to other denitrification genes. While common in soil and aquatic environments, denitrifiers are also found in association with humans. Sequencing of both skin and oral microbiomes has revealed a significant number of denitrifiers, consistent with the occurrence of both nitrate and nitrite in these areas.
Access provided by Autonomous University of Puebla. Download reference work entry PDF
Similar content being viewed by others
Keywords
- Nitrate Reductase
- Horizontal Gene Transfer
- Nitrite Reductase
- Aerobic Denitrification
- Nitrous Oxide Reductase
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
Introduction
One of the hallmarks of bacteria is the ability to use a wide variety of compounds as terminal oxidants for respiration. O2 (O2) respiration provides the most energy and so is the dominant form of respiration among living organisms. However, if O2 becomes limiting, many bacteria will continue respiration by switching the terminal electron acceptor to alternative compounds such as nitrate. Nitrate respiration occurs via two dissimilar pathways that utilize the same initial substrate but produce different end products (Canfield et al. 2010). One of these pathways, termed ammonification (or dissimilatory reduction of nitrate to ammonia [DNRA]), is carried out by bacteria such as Escherichia coli, and is marked by reduction of nitrate to nitrite and then to ammonia. The second pathway of nitrate respiration is denitrification, which is the reduction of nitrate to gaseous nitrogen oxides, and then nitrogen gas (Fig. 10.1 ).
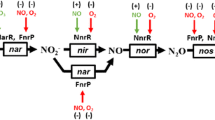
The initial step in both denitrification and DNRA is the reduction of nitrate to nitrite (Richardson et al. 2001). The next step differentiates the two pathways. In DNRA, nitrite undergoes a six electron reduction to ammonia (Simon 2002). In denitrification, nitrite is reduced to nitric oxide (NO), the defining step of this pathway (Zumft 1997). This conversion of a fixed, nongaseous, and biologically preferred form of nitrogen to a gaseous form is the reason this respiratory process is termed “denitrification.” The enzyme carrying out this reaction will be referred to as Nir. There are two types of Nir in denitrifiers. One contains copper and will be referred to as CuNir. The other contains heme and will be referred to as cd 1-Nir. NO is not the terminal product of denitrification since it can be reduced to nitrous oxide (N2O) by a membrane-bound nitric oxide reductase, which will be referred to as Nor. As with Nir, there are two forms of Nor. One accepts electrons from quinol and will be referred to as qNor while the other accepts electrons from c-type cytochromes and will be referred to as cNor. The terminal step in denitrification is the reduction of N2O to nitrogen gas, N2, by nitrous oxide reductase, which will be referred to as Nos. The production of N2 connects denitrification to the nitrogen cycle via nitrogen fixation. While N2 is the terminal product of the pathway, it is important to note that it is not the case that a bacterium either has four enzymes of the denitrification pathway or none. Many bacteria have truncated or partial pathways that sometimes only include a single enzyme.
The biologically mediated nitrogen cycle is shown in Fig. 10.2 . Nitrogen fixation produces ammonia. This ammonia, and the ammonia produced by DNRA, can be converted by nitrifying bacteria to nitrite and nitrate, substrates for denitrification. The anaerobic oxidation of ammonia, anammox, also results in the reduction of nitrogen oxides to N2 but is distinguished from denitrification by using electrons from ammonia (Martinez-Espinosa et al. 2011). Gayon and Dupetit carried out the first systematic study of nitrate conversion to gaseous forms of nitrogen in 1882 (Gayon and Dupetit 1882). Noting the loss of nitrate from decomposing sewage, they called it “denitrification” and were the first to isolate denitrifying bacteria (Gayon and Dupetit 1886), which they dubbed Bacterium denitrificans α and β. In the early stages of the study of denitrification, it was erroneously assumed that nitrate was releasing, and thus supplying, elemental O2 to organisms that subsequently carried out a reaction equivalent to O2 respiration. The observation of denitrification, although biologically significant, was unsettling to agronomists who soon realized that the addition of organic matter to soils could lead to the loss of fixed nitrogen. The agricultural importance of the process provided the impetus for much of the early work on denitrification, and by the end of the nineteenth century, denitrification had been reasonably well defined.
Significant interest in the agricultural impact of denitrification has continued to be an important focus of research efforts. However, with the realization that NO and N2O play important roles in atmospheric and biological chemistry, research emphases have shifted to the environmental consequences of denitrification and the molecular mechanisms of enzymes and gene regulation (Brunekreef and Holgate 2002; Canfield et al. 2010; Ravishankara et al. 2009; Richardson et al. 2009).
Defining the Denitrifiers
Denitrification is most frequently, but not always, used as a respiratory process. That is, reduction of nitrate and other intermediates proceeds through a series of protein complexes which results in the production of a proton motive force that can be used to generate ATP (van Spanning et al. 2007; Zumft 1997). Prokaryotes, mostly Bacteria, and a few Archaea, constitute the vast majority of organisms capable of denitrification. A number of fungal isolates carry out reduction of nitrate to N2O, but the contribution of this reduction to cell growth is variable (Kim et al. 2009; Nakanishi et al. 2010). More recent work has also found denitrification can occur in some multicellular eukaryotes (Risgaard-Petersen et al. 2006). With one or two exceptions, denitrifiers also respire O2 and, because it is usually available at higher concentrations and its reduction provides more energy to the cell, O2 is the preferred electron acceptor. There are no bacteria known in which denitrification is the only means of producing ATP, meaning denitrification is never an essential physiological trait.
The four separate enzymes catalyzing nitrate reduction to N2 produce freely diffusible products and catalyze reactions with a negative ΔG, making each step energetically favorable (Zumft 1997). This means each enzyme can potentially be an autonomous electron-accepting unit and, by extension, no reason a denitrifying bacterium must catalyze the complete reduction of nitrate to N2. While a few partial denitrifiers were found in the pre-genomic era (Denariaz et al. 1989; Greenberg and Becker 1977; Toffanin et al. 1996), genome sequencing has revealed partial denitrification is widespread. The occurrence of partial denitrification pathways makes defining a bacterium as a “denitrifier” somewhat tricky. Are denitrifiers only those bacteria that reduce nitrate to N2 or should the definition be more inclusive? For this chapter, a denitrifier is taken as any bacterium that has Nir, Nor, or Nos. Nitrate reductase is excluded since it is not unique to denitrification. Using this definition, a bacterium such as Escherichia coli, which can reduce nitrate and nitrite, is not considered a denitrifier because it reduces nitrite to ammonia and does not have denitrifying type enzymes for the reduction of NO or N2O (Simon 2002). In contrast, a bacterium such as Wolinella succinogenes is considered a denitrifier because it contains Nos, which can be used to support growth under anoxic conditions (Yoshinari 1980). Like E. coli, W. succinogenes can reduce nitrate to ammonia but this does not impact its categorization as a denitrifier (Bokranz et al. 1983). Because of this delimiting, nitrate reductase will not be a focus of this chapter.
Phylogenetic Distribution of Denitrification
In the pre-genomic era, establishing a bacterium was a denitrifier entailed testing its ability to grow under O2-limiting conditions with nitrate most frequently provided as terminal oxidant (Payne 1981). As a consequence, nearly all denitrifiers characterized were complete denitrifiers that showed robust growth under denitrifying conditions. This perhaps gave a somewhat inaccurate perception that denitrifiers would all grow well under anoxic conditions and produce N2. However, with genome analysis supplanting phenotypic assignment as the principal means of identifying denitrifiers, both complete and partial denitrifiers can be identified, allowing a more accurate assessment of the actual phylogenetic distribution of denitrification genes. The diversity uncovered by this analysis and the often patchy gene distribution makes it unrealistic to provide a complete list of all denitrifiers. Therefore, in the discussion below, only representative examples will be described. Much of this analysis was generated by searches of genomes available through the DOE IMG website (http://img.jgi.doe.gov/cgi-bin/w/main.cgi).
Archaea: Several denitrifying Archaea were characterized in the pre-genomic era, so it has been known for some time that denitrification occurred in this group. Genome sequencing has revealed additional denitrifiers. With one exception, all the known archaeal denitrifiers are also capable of aerobic respiration. One review on denitrification in Archaea is available (Cabello et al. 2004).
Euryarchaeota: A number of halophilic Euryarchaeota are denitrifiers. This includes members of the Haloarcula, Haloferax, and Halomicrobium genera (Inatomi and Hochstein 1996; Mancinelli and Hochstein 1986). Ferroglobus placidus DSM 10642 is another member of this phylum with denitrification genes. This bacterium is particularly unique in that it has been reported to be an obligate anaerobe capable of denitrification (Vorholt et al. 1997). Recent sequencing of this bacterium’s genome has shown this phenotypic characterization is accurate since it lacks genes encoding proteins required for O2 respiration. Despite this, F. placidus does have a respiratory-based physiology since its main mode of energy generation is to use Fe(II) as reductant and either nitrate or thiosulfate as terminal oxidant (Hafenbradl et al. 1996). Analysis of the genome of F. placidus reveals the presence of a nitrate reductase, a qNor and Nos. Unexpectedly, there is no obvious Nir of either the copper- or heme-containing type. Biochemical data suggest the bacterium has nitrite reductase capacity (Vorholt et al. 1997). However, the rates of NO production were slow and initial characterization of this bacterium suggested nitrite was the major product of nitrate reduction (Hafenbradl et al. 1996). It is strange this bacterium, which is a strict anaerobe, would have lost the capacity to reduce nitrite.
Crenarchaeota: Members of the genus Pyrobaculum, which are hyperthermophilic Crenarchaeota, have been found to be robust denitrifiers (Volkl et al. 1993). A qNor has been purified from Pyrobaculum aerophilum (de Vries et al. 2003). Genome analysis indicates this bacterium also has a cd 1-Nir, which has not been purified (Cabello et al. 2004). The genome also encodes the genes for a nitrate reductase but lacks the genes encoding the catalytic subunit of Nos. However, this bacterium has been shown to produce N2 as a major end product during denitrification, suggesting the presence of a novel N2O reductase (Volkl et al. 1993). It might be predicted then that the extremophilic nature of this bacteria might be the reason for a novel Nos. However, one member of this genus, Pyrobaculum calidifontis JCM 11548, has a nos gene cluster, suggesting hyperthermophilic conditions do not require a novel protein for N2O reduction.
Genome sequencing has revealed several Sulfolobus species, which are also Crenarchaeota, are partial denitrifiers. They have extremely truncated denitrification pathways since they only contain qNor. A few other genera within the Crenarchaeota including Vulcanisaeta, Acidilobus, and Caldivirga also contain species that have a qNor as their only obvious nitrogen oxide reductase.
The denitrification components of these Archaea have not been characterized extensively. A copper-containing nitrite reductase has been purified from Haloferax denitrificans and was shown to be spectroscopically similar to related eubacterial nitrite reductases (Inatomi and Hochstein 1996). A CuNir as well as a dissimilatory nitrate reductase have been purified from Haloarcula marismortui (Ichiki et al. 2001; Yoshimatsu et al. 2000). The qNor purified from P. aerophilum has many similarities to orthologs from Eubacterial sources (de Vries et al. 2003). While limited, these studies confirm the nitrogen oxide reductases of the Archaea are orthologous with those found in the Eubacteria.
One other notable archaeon with CuNir is the nitrifying archaeon Nitrosopumilus maritimus. This bacterium has two nirK genes, the designation for genes encoding CuNir, but lacks any obvious Nor or Nos. (Walker et al. 2010). The proteins encoded by these genes have high similarity suggesting they are paralogous. The genome of this bacterium is enriched for genes encoding copper-containing proteins perhaps explaining the CuNir duplication. Nitrite has been proposed as an alternate terminal electron acceptor, but it is not clear how cells would cope with the NO generated by this reaction (Walker et al. 2010).
Eubacteria: Denitrification genes are widely distributed among the eubacteria, and current evidence indicates they are only found in eubacteria capable of aerobic growth. Most of the characterized denitrifiers in the eubacteria belong to the proteobacteria. Due to the relative paucity of understanding of denitrification among non-proteobacterial denitrifiers, they have been placed into one section.
Non-proteobacterial Denitrifiers
Firmicutes: The frequency of denitrification among firmicutes is uncertain. Importantly though, it is relatively uncommon among most of the model organisms used to study this group. In the genus Bacillus, for example, denitrification is not considered important since most strains of Bacillus subtilis are not denitrifiers. However, a recent study of denitrification in a large collection of Bacillus strains suggested that denitrification occurred in nearly half (Verbaendert et al. 2011). No complete denitrifiers are currently found among those Bacillus species whose genomes have been sequenced. This may only be temporary though since some Bacilli are known to be complete denitrifiers. For example, strains of Bacillus azotoformans have been shown to be denitrifiers (Mahne and Tiedje 1995). More recently, a variety of bacilli were tested for gas production under denitrifying conditions and found to be complete denitrifiers (Jones et al. 2011). Genome sequencing has revealed the potential for partial denitrification in some Bacillus species. For example, qNor is present in Bacillus tusciae strain DSM 2912 and some Bacillus licheniformis strains, but these are their only denitrification enzymes.
Denitrification is more common among sequenced Geobacillus than Bacillus species. Some species in this genus are complete denitrifiers, including Geobacillus sp. G11MC16 and Geobacillus thermodenitrificans NG80-2. These are among only a few gram-positive bacteria in which the nos gene cluster has been found. Many others strains including Geobacillus kaustophilus HTA426 lack nos. The G. kaustophilus strain is also notable because it lacks a respiratory nitrate reductase while the others have the genes for this enzyme.
Abbreviated denitrification pathways are found in strains of both Lactobacillus and Staphylococcus. Genome sequencing has found strains in both genera have qNor but lack Nir and Nos. qNor distribution among the staphylococci is somewhat limited with a number of the S. aureus subsp. aureus strains having the gene encoding this protein, while it is totally absent in the S. aureus genomes. The region of the genome containing the gene encoding qNor in these staphylococci is syntenic, suggesting it has not been a recent acquisition. qNor function in these bacteria is likely to detoxify NO, although this has not been demonstrated. Many of these same strains also have a membrane-bound dissimilatory nitrate reductase. Nitrate reductase appears to be coupled to a cytoplasmic NADH-dependent nitrite reductase which reduces nitrite to ammonia (Schlag et al. 2008). Several strains of Lactobacillus fermentans have a Nor but are the only lactobacilli to have this gene. These Lactobacillus species possess a nitrate reductase but lack any type of nitrite reductase. Some Lactobacillus such as Lactobacillus plantarum WCFS1 and JDM1 have been found to have nitrate reductase but lack Nir or Nor. In WCFS1, nitrate reductase expression is repressed by glucose and its activity requires exogenous heme and ubiquinol (Brooijmans et al. 2009). Nar activity in these strains can be used to support growth.
Another interesting Firmicute with the capacity for nitrogen oxide reduction is Symbiobacterium thermophilum. This bacterium is obligately syntrophic with its growth being dependent on the presence of Geobacillus stearothermophilus (Ueda et al. 2004). Genome analysis has shown that S. thermophilum contains a CuNir and a periplasmic nitrate reductase. Unexpectedly, it lacks Nor. It is uncertain if its Geobacillus partner contains an NO reductase since its genome has not been sequenced. Another Symbiobacterium species, Symbiobacterium toebii, has been shown to be able to grow under anoxic conditions by reducing nitrate (Rhee et al. 2002). Under these conditions, nitrite accumulated stoichiometrically, indicating it lacks a nitrite reductase. Other Firmicutes with Nir while lacking Nor can be found in the genus Thermaerobacter. Thermaerobacter subterraneus and marianensis both have a CuNirK but lack any detectable Nor. They also both lack a nitrate reductase.
Actinobacteria: The majority of the actinobacteria capable of denitrification have a severely truncated denitrification pathway. A good example of this is provided by several Actinomyces species which only have only a nitrate and nitrite reductase. For example, genome analysis suggests Actinomyces coleocanis DSM 15436 and Actinomyces odontolyticus ATCC 17982 can reduce nitrate to NO but apparently lack the Nor necessary to reduce this toxic compound to nontoxic nitrous oxide. A number of members of the genus Corynebacterium also have truncated chains. Corynebacterium pseudogenitalium ATCC 33035 has a CuNir but appears to lack all the other nitrogen oxide reductases, including nitrate reductase. Several other strains, including Corynebacterium efficiens YS-314, have both a nitrate and nitrite reductase but lack Nor. A few other strains, including Corynebacterium diphtheriae NCTC 13129, have Nar, CuNir, and a qNor, indicating they could reduce nitrate to N2O but lack an obvious nos ortholog.
Another member of this group with a truncated denitrification pathway is Jonesia denitrificans. This bacterium’s genome has been sequenced and it has been found to have a membrane-bound Nar and a CuNir (Pukall et al. 2009). There are no obvious Nor- or Nos-encoding genes. The Bergey’s description of this bacterium states that it is facultatively anaerobic and can reduce nitrate to nitrite. There is no mention of gas from nitrate in the description, consistent with genome predictions. This indicates that despites its name, Jonesia denitrificans is only a very limited denitrifier. It is puzzling that this bacterium can grow as a denitrifier since the product of its denitrification chain, NO, is toxic.
Denitrification seems to be an important trait in Propionibacterium acnes since all sequenced strains contain denitrification genes. All of the strains have a membrane-bound nitrate reductase, a unique CuNir and a qNor but lack Nos. As expected, isolates of this strain have been found to obtain a growth benefit from the presence of nitrate and that nitrous oxide is the final product of nitrate reduction (Allison and Macfarlane 1989). Even though the physiology of Propionibacterium is traditionally associated with fermentation, they are aerotolerant and encode genes whose products are associated with O2 respiration, indicating respiration is an important part of their bioenergetic capacity. Sequence of the skin microbiome has found Corynebacterium, Propionibacterium, and Staphylococcus species are present in high numbers (Kong 2011). Since denitrification is common in these genera, it suggests nitrate or other nitrogen oxides are frequently present in this environment. This has been borne out by the finding that both nitrate and nitrite are present in sweat although the source of these compounds is uncertain (Mowbray et al. 2009).
Mycobacterium is another medically important genus in the Actinobacteria whose members contain denitrification genes. Strains including Mycobacterium avium 104, Mycobacterium intracellulare ATCC 13950, and Mycobacterium parascrofulaceum ATCC BAA-614 contain a qNor but no other denitrification genes. It is likely this protein is used for mitigating NO toxicity. However, in this context, it is perhaps unexpected that none of the Mycobacterium tuberculosis strains, which will encounter host-produced NO during infection, contain an NO reductase or other denitrification genes (Voskuil et al. 2011).
Assorted others: A variety of other non-proteobacteria species have bits and pieces of the denitrification pathway. Various strains in the family Flavobacteriaceae, which are members of the Bacteroidetes phylum, are partial denitrifiers. Some, like Capnocytophaga ochracea strain ATCC 27872, have only one gene, in this case a qNor. In contrast, the related Capnocytophaga gingivalis JCVIHMP016 lacks Nor but has a CuNir and a Nos. Another pattern is exhibited by Capnocytophaga sputigena Capno which has a CuNir and a qNor. These bacteria are additional examples of bacteria associated with human surfaces since all three were isolated from the oral cavity (http://www.homd.org/index.php#).
Nonhuman associated members of the family Flavobacteriaceae also contain denitrification genes. Robiginitalea biformata HTCC2501, which was isolated from the Sargasso Sea, encodes a Nos but no other denitrification genes. The same is true for Gramella forsetii KT0803, which was isolated from the North Sea. In contrast, Maribacter sp. HTCC2170 which was isolated off the Oregon coast has the complete set of denitrification genes including cNor. This bacterium, along with Marivirga tractuosa DSM 4126, is the only member of the phylum Bacteroidetes that has a cNor. The latter also has a CuNir and a Nos. Flavobacterium johnsoniae UW101, a soil isolate, has CuNir and qNor but lacks Nos.
A few cyanobacteria contain denitrification genes. Analysis of the genome sequence of Synechocystis sp. PCC 6803 showed that it encodes a qNor and a transcriptional regulator that may regulate expression of this enzyme (Kaneko et al. 1996). The same is true for Arthrospira sp. PCC 8005. Acaryochloris marina MBIC11017 has two related copies of genes encoding a quinol-oxidizing Nor. It lacks all other denitrification genes. It is not obvious why any of these free-living bacteria would require the activity of a NO-reducing enzyme.
Several members of the genus Chloroflexus, including Chloroflexus aurantiacus J-10-fl and Chloroflexus sp. Y-400-fl, have a CuNir. Unexpectedly, this is the only denitrification gene they possess. Chthoniobacter flavus Ellin428, a member of the phylum Verrucomicrobia, also only has a CuNir. Opitutus terrae PB90-1, another member of the Verrucomicrobia, has a CuNir and Nos but lacks genes for Nor.
Two members of the Leptospira, which is within the phylum Spirochaetes, are denitrifiers. Both strains of Leptospira biflexa Patoc 1, Ames and Paris, are complete denitrifiers. They possess cNor and a CuNir. These are nonpathogenic, saprophytic members of this genus (Picardeau et al. 2008). None of the pathogenic Leptospira have denitrification genes.
One of the most interesting denitrifiers isolated recently is Candidatus “Methylomirabilis oxyfera.” This is a novel methylotroph capable of coupling methane oxidation to denitrification (Ettwig et al. 2010). Metagenome analysis has revealed the presence of a cd 1-Nir and three copies of genes encoding qNor. The genome lacks the nos gene cluster which was unexpected since a culture highly enriched with this bacterium produced N2 from nitrite, which is the preferred nitrogen oxide substrate. Recent studies suggest N2O is produced by a novel mechanism in which two molecules of NO are dismutated to produce N2 and O2 with the liberated O2 being used for methane oxidation (Wu et al. 2011).
Proteobacteria: The majority of denitrifiers are found in the phylum Proteobacteria. The proteobacteria have been subdivided into five classes: α, β, γ, δ, and ε. Denitrifiers have been found in all of these subdivisions and so each will be discussed separately.
δ Proteobacteria: While this subdivision contains a number of strict anaerobes some members do possess denitrification genes. A number of these are members of the genus Geobacter, including Geobacter metallireducens strain GS-15, Geobacter sp. M18, and Geobacter sp. FRC-32 which have only the qNor (Fig. 10.3 ). G. metallireducens strain GS-15 can grow anaerobically with nitrate but it reduces it to ammonia (Lovley and Phillips 1988). Geobacter bemidjiensis strain Bem and Geobacter sp. strain M21, also have qNor. Unexpectedly, these two strains also have cNor as well (Fig. 10.3 ). No strain has the cNor alone. The qNor in G. metallireducens strain GS-15 is more closely related to the qNor in Desulfovibrio sp. FW1012B, another δ proteobacterium, than other Geobacter strains. Several species in the genus Anaeromyxobacter, such as Anaeromyxobacter sp. Fw109-5, have qNor and Nos but lack nitrite reductase. The Fw109-5 strain also contains a cNor but is the only member of this genus to do so. Anaeromyxobacter sp. strain K is noteworthy because it has three copies of genes encoding qNor. Identity between the three copies is low, about 40 %, making it difficult to determine if the genes are paralogous. These bacteria also can reduce nitrate to ammonia (Sanford et al. 2002).
Another interesting member of the δ subdivision with a partial denitrifying pathway is Bdellovibrio bacteriovorus HD100, which has a CuNir and a cNor but no Nos (Rendulic et al. 2004) (Fig. 10.3 ). The nirK gene product forms a clade with Nir from γ proteobacteria including Ralstonia and Burkholderia. The nor gene cluster of this bacterium is most closely related to putative orthologs from Leptospira, which are members of the phylum Spirochaetes. Since Bdellovibrio are bacteriovorus, it is possible these genes were acquired via some type of horizontal gene transfer (HGT). The G + C content of the Bdellovibrio genome is about 51 %. If HGT was the source of these genes in Bdellovibrio, this might be detected by anomalous G + C content. The bacteria with the most closely related CuNir have a higher G + C than Bdellovibrio while the Leptospira species have a lower G + C content than Bdellovibrio. However, the genes encoding the CuNir and cNor in Bdellovibrio have near backbone average G + C content, so their exact source remains unclear.
One other interesting member of the δ subdivision is Syntrophobacter fumaroxidans MPOBT. This bacterium was isolated from anaerobic sludge and is a propionate oxidizer in the presence of hydrogen- and formate-utilizing bacteria (Harmsen et al. 1998). It is described as a strict anaerobe but its genome revealed the presence of a bd-type oxidase that could be used for O2 respiration as well as the presence of a qNor but no other denitrification genes.
ε Proteobacteria: A number of strains of Campylobacter, which, in general, are important human pathogens, have truncated denitrification chains. For example, Campylobacter concisus (strain 13826) and Campylobacter curvus (strain 525.92) both have a quinol-oxidizing Nor and Nos. Campylobacter rectus RM3267 and Campylobacter fetus subsp. fetus strain 82-40 lack the Nor but retain Nos. Other members of the order Campylobacterales such as Wolinella succinogenes also only have a Nos present in the genome. This bacterium along with several of the Campylobacter have been shown to be able to grow using N2O as a terminal electron acceptor (Payne et al. 1982; Yoshinari 1980). It is interesting though that none of these bacteria have a NO-producing Nir, requiring that either the NO or N2O needed for denitrification be produced by an exogenous source.
Sulfurimonas (Thiomicrospira) denitrificans ATCC 33889, another member of the order Campylobacterales, is a complete denitrifier with a heme type nitrite reductase and a cNor. It also has two copies of the gene encoding the substrate-binding subunit of Nos in its genome but only one set of the accessory genes required for assembly of the copper-containing cofactors. This bacterium was isolated from a hydrothermal vent (Sievert et al. 2008). Other ε proteobacterial denitrifiers have been isolated from hydrothermal vents such as, Nitratiruptor sp. SB155-2 which is a complete denitrifier with a cd 1-Nir and a cNor (Nakagawa et al. 2007). Nitratifractor salsuginis DSM 16511 was isolated from the same hydrothermal vent system as the Nitratiruptor sp. and also has a cd 1-Nir, cNor, and Nos (Nakagawa et al. 2005). These latter two bacteria are not members of the Sulfurimonas.
α Proteobacteria: Denitrification is a very common trait among the α subdivision of the proteobacteria. Probably the best studied denitrifier, Paracoccus denitrificans, is a member of this group (Baker et al. 1998). This bacterium is a complete denitrifier with a cd 1-Nir and a cNor. While complete denitrification is common among members of this subdivision, there is also significant variation in pathway length, reflecting the varied roles of the N-oxide reductases and the evolutionary histories of the various strains. A good example of this is provided by the photosynthetic α-proteobacterium Rhodobacter sphaeroides. Rhodobacter sphaeroides 2.4.3 has the genes for all four nitrogen oxide reductases; however, a transposase IS4 family protein has disrupted the nos operon, resulting in N2O being the terminal pathway product (Hartsock and Shapleigh 2010) (and unpublished). R. sphaeroides KD131 has the genes for CuNir, cNor, and Nos but lacks a dissimilatory nitrate reductase (Lim et al. 2009). The type strain, R. sphaeroides 2.4.1, only has Nap and cNor (Mackenzie et al. 2001). The remaining sequenced strain, 2.4.9, only has cNor. So the only N-oxide reductase structural genes they have in common are the ones encoding Nor, which are located in the same location on chromosome I in all the strains. The gene for CuNir is also located in a syntenic region of chromosome I in both 2.4.3 and KD131. Both 2.4.1 and 2.4.9 appear to have had nirK but have lost it by deletion. The nitrate reductase is on a plasmid in 2.4.1 that is not present in 2.4.9, which otherwise is highly similar to the type strain (Choudhary et al. 2007). The nitrate reductase common to 2.4.1 and 2.4.3 is also on a plasmid but not in a syntenic region. The 2.4.3 strain encodes a second nitrate reductase that is located on chromosome II. This second nitrate reductase is of a type more commonly found in members of the γ-proteobacteria, suggesting acquisition via HGT. The nos gene cluster is found on chromosome II in 2.4.3 but on a plasmid in KD131 and there is no conservation of flanking genes. All the denitrification genes in these strains have G + C content and codon usage bias that is indistinguishable from backbone norms.
As mentioned above, the ability to grow under denitrifying conditions was the main test for determining if a bacterium was a denitrifier in the pre-genomic era. It can be seen from the example with R. sphaeroides that only the 2.4.3 strain would likely show up in a phenotypic survey. However, even this is somewhat in doubt since 2.4.3 grows quite poorly as a denitrifier (Michalski and Nicholas 1988). A similar result was observed with Agrobacterium tumefaciens, which has genes for Nap, CuNir, and a cNor but lacks genes for Nos. This bacterium is frequently referred to as aerobic because its denitrification growth rate is quite slow, particularly compared to robust denitrifiers such as P. denitrificans. This poor growth trait is probably common among natural isolates and would lead to an underestimation of denitrifiers in the environment if culture techniques were the primary method of enumeration.
Other interesting denitrifying members of this subdivision include a number of strains which have Nir but lack Nor and Nos. For example, there are currently six members of the family Phyllobacteriaceae with sequenced genomes. Two of these, Mesorhizobium opportunistum WSM2075 and Chelativorans (formerly Mesorhizobium) sp. BNC1, have a denitrification pathway consisting only of a CuNir. The latter strain actually has two copies of the nitrite reductase gene. A third member of this family, Parvibaculum lavamentivorans DS-1, has a CuNir and a qNor. The CuNir of the latter does not cluster with the CuNir of the other two Phyllobacteriaceae. Instead, it clusters with a group of orthologs found in bacteria such as Azospirillum and Hyphomicrobium in a larger group that also includes Polaromonas and Nitrospira, which are β-proteobacteria (Fig. 10.4 ). The qNor in P. lavamentivorans DS-1 is also not closely related to proteins from other α proteobacteria but is more closely related to orthologs from Rhodanobacter. These data suggest the denitrification genes in these Phyllobacteriaceae were acquired by HGT, but as with the other examples, examination of DNA sequence does not provide evidence in support of this conclusion. The Nir-only pathway variation is not restricted to the Phyllobacteriaceae since Octadecabacter antarcticus 307, a member of the family Rhodobacteraceae, also only contains a pathway consisting of only CuNir.
Some members of this subdivision also contain multiple Nir-encoding genes which seem unlikely to be a result of gene duplication but reflect independent origins. For example, Afipia sp. 1NLS2 has three copies. One of these is significantly longer than the other two due to presence of an additional cytochrome c–binding domain. The other two proteins are dissimilar from each other, with one grouping with CuNir from Nitrosomonas and Nitrobacter while the other clusters with orthologs from members of the genus Rhodopseudomonas (Fig. 10.4 ). The Afipia strain is a complete denitrifier and it is uncertain if the three nirK are functionally redundant or serve distinct physiological purposes. Oligotropha carboxidovorans strain Om5 also has two distinct nirK genes with one having a c-type cytochrome domain while the other does not. This bacterium lacks Nor but has Nos. The region of the genome containing the nos gene cluster as well as the genes encoding Nar is not present in the nearly identical Om4 strain (Volland et al. 2011). Evidence suggests Om5 is the ancestor and the genes have been lost from Om4. Neither nirK is located in this unconserved region.
The most heavily sequenced α-proteobacterial genus with denitrifying members is Brucella. Brucella species are zoonotic pathogens that are no longer free-living (Roop et al. 2009). Many strains from a variety of mammalian hosts have been sequenced. Nearly all are complete denitrifiers. This is not too surprising since this genus is in the order Rhizobiales, which includes many other denitrifiers. Since Brucella are intracellular pathogens, it is likely they are undergoing genome reduction (Wattam et al. 2009). Surprisingly, there is not much evidence of an enhanced rate of loss of denitrification genes. All sequenced strains seem to have an intact cNor-encoding gene cluster. Only one sequenced strain, B. neotomae, lacks nirK and has been shown to lack Nir activity (Baek et al. 2004). The presence of nos is more variable. Most strains have an intact gene cluster but in a number there has been an accumulation of stop codons. For example, in B. melitensis ATCC 23457, the nosZ gene, which encodes the catalytic subunit, contains a single stop codon at about the middle of the orf. All of the other nos subunits, required for assembly of the copper-containing prosthetic groups, remain intact. However, the inactivation of nos also occurs in free-living denitrifiers. For example, two strains of Ochrobactrum, free-living close relatives of Brucella, have been sequenced. One, Ochrobactrum anthropi ATCC 49188, is a complete denitrifier, while the other, Ochrobactrum intermedium LMG 3301, lacks the nos cluster (Chain et al. 2011). The O. anthropi strain is another example of an α-proteobacterium with two nirK genes. One has the nirV gene that has been shown to form an operon with nirK in many α-proteobacteria (Jain and Shapleigh 2001) and is in a region of the genome with other denitrification genes. The second copy lacks nirV and is not proximal to other denitrification genes, suggesting it may not be required for denitrification. The two genes are not closely related (Fig. 10.4 ).
In general, most α-proteobacterial denitrifiers possess cNor. However, a few bacteria such as Sphingomonas wittichii RW1 have qNor. Another α-proteobacteria with a qNor is Pseudovibrio sp. JE062, which is a complete denitrifier, and its Nor is related to the one found in S. wittichii. Interestingly, Pseudovibrio is another bacterium with both qNor and cNor. The genes encoding the latter are found in a cluster with other denitrification genes, suggesting it may be used during denitrification growth. The function of the qNor is uncertain. The occurrence of Nir is not so biased, with both CuNir and cd 1-Nir denitrifiers being common among α-proteobacteria. Some closely related denitrifiers, such as P. denitrificans and R. sphaeroides, both members of the family Rhodobacteraceae, have different types of nitrite reductases, with the former having a cd 1-Nir and the latter a CuNir.
Another interesting group of α-proteobacteria with denitrification genes are nitrifiers of the genus Nitrobacter. The three sequenced representatives of this genus all contain a CuNir. These bacteria lack Nor as well as Nos. The Nir in these bacteria is particularly short, only 300 residues versus the 365–370 residue long proteins other Bradyrhizobiaceae, of which Nitrobacter is a member. This shorter version is also found in Sphingomonas wittichii RW1 and some other α-proteobacteria that are not nitrifiers. The function of the nitrite reductase in nitrifiers is not clear (Beaumont et al. 2004; Cua and Stein 2011; Kampschreur et al. 2007).
β Proteobacteria: The β proteobacterial class also includes many organisms with denitrification genes. The most heavily represented in genome databases are members of the genus Burkholderia. Burkholderia mallei is the causative agent of glanders and is derived from Burkholderia pseudomallei, which can be isolated from tropical soils (Galyov et al. 2010). B. mallei has undergone genome reduction and has lost the ability to survive in the environment. All of the B. mallei strains have CuNir and a qNor but some have lost Nos. All of the sequenced strains of B. pseudomallei appear to be complete denitrifiers. Despite the frequent occurrence of denitrification, there has been very little research on this genus’ denitrification capacity. Genome analysis indicates these bacteria have a number of unique denitrification-related traits. Most strains have two CuNir-encoding genes. One of these is adjacent to and may form an operon with a gene encoding a qNor. The other gene does not cluster with other denitrification genes. While each CuNir is highly conserved when aligned against orthologs from other Burkholderia, paralogs have only about 40 % identity (Fig. 10.4 ). Interestingly, one of the two forms is lacking a histidine residue known to be a ligand for one of the copper cofactors in CuNir. This indicates this protein would be inactive. This putatively inactive Nir is encoded by the nirK adjacent to nor. However, this is not the only gene encoding a Nor ortholog in Burkholderia. As with nirK, there is a second nor not clustered with other denitrification genes. Also, as with nirK, the nor orthologs are highly conserved but the paralogs less so. Unlike with nirK, both copies of the nor genes possess the His residues required for ligand binding in nearly all the strains. In a few cases, the nor gene adjacent to nirK has lost conserved His required for cofactor binding or has undergone frame shifts. This suggests the clustered nirK-nor region may have been supplanted by other copies of these genes located elsewhere in the genome and is being lost through site-specific mutation and orf inactivation.
Two other β proteobacterial genera, Ralstonia and Thauera, have many denitrifying members. These genera are common soil isolates, particularly from environments contaminated with aromatic carbon compounds (Parales et al. 2008). The former is member of the family Burkholderiaceae while the latter is a member of the Rhodocyclaceae. Not all members of the Ralstonia are complete denitrifiers. All sequenced strains have a qNor but some lack the CuNir that is found in others. The nos-encoding gene cluster is also not present in all strains. Cupriavidus necator, which was originally designated Ralstonia eutropha H16, has a cd 1-type Nir. This strain also has two copies of the gene encoding qNor. Only one strain of Thauera, Thauera sp. MZ1T, has a completed genome at this time and it is a complete denitrifier. It has a cNor and a cd 1-type nitrite reductase. Like many Ralstonia and Thauera strains, Polaromonas naphthalenivorans CJ2 was isolated because of its ability to degrade aromatic pollutants (Jeon et al. 2003). Subsequent genome analysis indicates this bacterium is a denitrifier with a CuNir and a qNor (Yagi et al. 2009). Attempts to grow this bacterium as a denitrifier have failed (Madsen 2011, personal communication).
Denitrification is a common characteristic of most species in the genus Neisseria, a well-studied member of the β subdivision. All of the Neisseria gonorrhoeae sequenced to date contain genes for CuNir and qNor. The two genes are adjacent but divergently transcribed. All of these strains also contain the nos gene cluster. Even though they have the cluster, they lack Nos activity since the Nos structural gene encodes a truncated protein lacking residues critical for binding one of the copper cofactors (Barth et al. 2009). None of these strains seems to have a nitrate reductase, suggesting that nitrite is the principal nitrogen oxide encountered in their natural environment. Most strains of Neisseria meningitidis also have a nirK and an adjacent qNor-encoding gene (Barth et al. 2009). However, in a couple of strains, these genes are inactivated. In Neisseria meningitidis C, 8013 both nirK and nor are pseudogenes. Interestingly, in Neisseria meningitidis 053442 ST-4821, the nirK has been displaced by a putative transposon. None of the Neisseria meningitidis strains have the entire nos gene cluster. However, there are remnant genes of the nos cluster present, suggesting these strains had nos capacity at some point (Barth et al. 2009). The N. meningitidis strains also lack a nitrate reductase. All of the other Neisseria species sequenced to date also have a nirK and qNor gene, indicating denitrification is more conserved in this genus than in most other genera characterized to any level of detail. However, with the exception of Neisseria mucosa, all lack a nitrate reductase.
The β proteobacteria also have several nitrifiers that have denitrification genes. This includes Nitrosospira multiformis ATCC 25196 which has CuNir and a cNor. Another member of the Nitrosomonadaceae family, Nitrosomonas sp. AL212, also has a CuNir and a cNor.
γ Proteobacteria: This group contains a large number of denitrifiers including the important model denitrifiers Pseudomonas stutzeri and Pseudomonas aeruginosa (Zumft 1997). While denitrification is found in many pseudomonads, many important branches of this genus lack this capacity. For example, all P. aeruginosa strains in the genome database used in this analysis have a cd 1-Nir, a cNor, and Nos gene cluster. In contrast, other well-characterized species such as Pseudomonas syringae do not contain any denitrifying strains. Partial denitrifiers can also be found among the pseudomonads. For example, both Pseudomonas entomophila strain L48 and Pseudomonas fluorescens strain Pf-5 have a CuNir but lack Nor- and Nos-encoding genes. In general though, partial denitrification seems less prevalent among sequenced pseudomonads.
Other γ proteobacteria capable of denitrification include Legionella. Most strains sequenced to date contain a qNor but no other denitrification genes. Allochromatium vinosum strain ATCC 17899, a member of the order Chromatiales, also only has a pathway consisting of qNor. Single step pathways are not restricted to qNor-containing bacteria since Methylococcus capsulatus Bath has cNor but no other denitrification genes. While some of the groups discussed above have thermophilic denitrifiers, the γ proteobacteria has at least one psychrophilic denitrifier. Pseudoalteromonas haloplanktis TAC125, whose denitrification pathway consists of CuNir and qNor, was isolated from Antarctic coastal waters and is a model bacterium for the study of adaptation to a psychrophilic environment (Medigue et al. 2005). Genome analysis has shown this bacterium has completely lost the capacity for molybdopterin metabolism, a cofactor essential for nitrate reductase (Medigue et al. 2005). One of the more unexpected truncated denitrification pathways is found in Vibrio orientalis CIP 102891. This strain of V. orientalis has a nos cluster that appears to be capable of producing an active Nos. No other member of the enteric bacteria has any denitrification capacity. Phylogenetic analysis indicates this gene cluster has close relatives in other γ proteobacteria including Colwellia and Photobacterium.
Another genus in this group which contains a large number of denitrifiers is Shewanella. This is not too surprising given this genus’ respiratory versatility (Fredrickson et al. 2008). Denitrification is not universally conserved in this genus though since Shewanella baltica seem to lack the capacity for denitrification completely, but on the whole, most species seem to have at least some denitrification genes. Some are complete denitrifiers such as the appropriately named Shewanella denitrificans (Brettar et al. 2002). Many are more similar to the Shewanella putrefaciens strains that have a qNor and no other denitrification genes. It is interesting that S. denitrificans has a cNor instead of the qNor found in other Shewanella. The cNor of S. denitrificans is related to the protein found in other members of the γ subdivision such as the pseudomonads or Marinobacter. In contrast, the nirK found in S. denitrificans is similar to the nirK found in other Shewanella such as S. amazonensis SB2B, although there is no synteny in the nirK region of their genomes.
Horizontal Gene Transfer
Given the seemingly disjointed scattering of denitrification genes described above, it is not unreasonable to suggest that horizontal gene transfer (HGT) has played an important role in the exchange of genes between strains. However, this cannot be demonstrated definitively since, in most cases, denitrification genes do not have anomalous G + C content or codon usage, nor are they located within obvious mobile elements. One exception is provided by the occurrence of a nirK in Tn6061, a transposon (Tn) found in P. aeruginosa (Coyne et al. 2010). This Tn is 26.5 kb in length and is notable because it encodes resistance to a number of antibiotics. The nirK within Tn6061 is not closely related to nirK in other proteobacteria and is rather divergent from most other nirK orfs (Fig. 10.4 ). The most closely related proteins are found in Rhodanobacter, which is a γ proteobacteria but with a divergent CuNir, and Opitutus terrae strain DSM 11246, which is a member of the phylum Verrucomicrobia. Many of the bacteria with closely related nirK genes also lack a Nor, suggesting the function of the Nir is something other than anaerobic respiration of nitrogen oxides. This may explain why it is present on a Tn that has no other denitrification genes. Recently, HGT of the nir-nor cluster of Thermus thermophilus has been demonstrated (Alvarez et al. 2011). Transfer of the nir-nor region and an adjacent nar region allowed nitrate-dependent anoxic growth of a recipient that had been obligately aerobic. The nir-nor region may be on a megaplasmid that can be transferred between strains via conjugation.
Jones et al. (Hallin et al. 2009) have carried out a thorough phylogenetic analysis of Nir, Nor, and Nos. They note a number of examples where the phylogenies based on 16S and denitrification genes are incongruous. Similar analyses done with sequences available at the time this chapter was written have uncovered additional examples of which a few will be mentioned. Others examples include Methylocella silvestris strain BL2, which is a member of the family Rhizobiales of the α proteobacteria. This strain contains a CuNir that does not group with other family members but clusters with bacteria from the β and γ subdivisions (Fig. 10.4 ). In contrast, the cNor from this bacterium is found within a clade with many other α proteobacteria. A few other examples include the CuNir of Moraxella catarrhalis strain RH4, a γ proteobacteria, which clusters with orthologs from a variety of β-proteobacteria; the CuNir from Methylacidiphilum infernorum V4, a member of the Verrucomicrobia phylum, which clusters with CuNir from the proteobacteria; and the qNor of Arcobacter butzleri strain RM4018, an ε proteobacterium, which forms a clade with several members of the Aquificae phylum.
Other cases of suspected HGT can also sometimes be inferred if a strain within a genus in which denitrification is common contains denitrification genes distinct from other species within its own genus. For example, the nirK of Shewanella woodyi is quite dissimilar to the nirK of other Shewanella but more similar to those of a nitrifier, Nitrosococcus halophilus (Fig. 10.4 ). Another example within the same genus is provided by Shewanella denitrificans which contains a cNor while all other strains with NO reductase use a qNor. HGT may also explain why some bacteria have multiple copies of genes which do not seem to be paralogous in origin. For example, Afipia, a member of the genus Bradyrhizobiaceae, has three nirK. One of these is a member of the Group I CuNir that can be identified with the sequence motif TRPHL, while a second has the sequence SSFH(V/I/P) at the same location, indicating it is a member of the Group II family of CuNir (Hallin et al. 2009). The third nirK encodes a protein which has neither motif but is obviously a CuNir. It seems unlikely that evolution of paralogs gave rise to multiple genes representing well-known subsets of CuNir (Fig. 10.4 ). Another example of a bacterium with multiple, non-paralogous copies of a denitrification gene is provided by Anaeromyxobacterium strain 109-5. This strain has three copies of a gene that encodes Nor. Two encode qNor and may be paralogous but the third encodes a cNor. Other examples of a single strain with both qNor and cNor were described above. Sphingomonas wittichii strain RW1, an α proteobacterium, has two qNor, which are only about 50 % identical. One of these clusters with qNor from other α proteobacteria while the other is part of a large clade of enzymes from β or γ proteobacteria.
The occurrence of a denitrification pathway consisting of one enzyme may also indicate acquisition via HGT. For example, the occurrence of qNor in some of the Staphylococcus aureus subsp. aureus strains but not at all in the Staphylococcus aureus strains or most other Staphylococcus species suggests HGT into one branch of the Staphylococcus group. A similar explanation likely accounts for the occurrence of nirK in only a few members of the Chloroflexi. CuNir from these bacteria clusters weakly with CuNir from a variety of bacteria from the β subdivision of the proteobacteria. A somewhat related example is provided by Pyrobaculum calidifontis strain JCM 11548, which is the only member of the Crenarchaeota with a nos gene cluster. As discussed above, the other Pyrobaculum species may have a novel Nos, suggesting that the nos cluster has been recently acquired by JCM 11548 (Zumft and Kroneck 2006).
Enzymology of Denitrification
As shown in Fig. 10.1 , complete denitrification is a multistep process, requiring four separate enzymes for the reduction of nitrate and three intermediate nitrogen oxides to nitrogen gas. The basic arrangement of the nitrogen oxide reductases is shown in Figs. 10.5 , 10.6 and 10.7 . The periplasmic location of several of the N-oxide reductases as well as the fact that none of these enzymes are proton pumps makes denitrification a rather inefficient form of respiration (Strohm et al. 2007). A summary description of the nature of these proteins is presented here. Additional information can be obtained from several recent reviews (MacPherson and Murphy 2007; Tavares et al. 2006; Zumft and Kroneck 2006).
Nitrate Reductase: Even though nitrate reductase is not a major focus of this chapter, it is useful to present a brief description of the various types of nitrate reductase (Fig. 10.5 ). Nitrate reductase catalyzes the two-electron reduction of nitrate to nitrite. Early studies of nitrate reductase activity in cells demonstrated the existence of at least two types (Zumft 1997). One is a soluble assimilatory enzyme, termed Nas, used when nitrate is the nitrogen source. The other is a membrane-associated respiratory enzyme, termed Nar. More recently, a third type of enzyme was found when a dissimilatory nitrate reductase activity was detected in the periplasm of R. sphaeroides IL 106 (Sawada and Satoh 1980). Further work has demonstrated that this periplasmic enzyme, termed Nap, occurs in a wide variety of bacteria, including both denitrifiers and non-denitrifiers such as E. coli and related enteric bacteria (Richardson et al. 2001). Many denitrifiers contain more than one type of nitrate reductase.
The membrane-associated Nar enzymes that have been biochemically characterized consist of a three-subunit complex (Richardson et al. 2001; Tavares et al. 2006). Two of the subunits, NarG (α) and NarH (β), form a cytoplasmically located heterodimer. They are anchored to the membrane by NarI (γ) which is sometimes lost during purification. NarG contains molybdenum, bound by the cofactor molybdopterin guanine dinucleotide and a [4Fe-4S] center (Schwarz et al. 2009). NarH contains several [4Fe-4S] centers and a [3Fe-4S] center. The membrane-anchoring subunit NarI typically contains b-type heme. The direct electron donor to the respiratory nitrate reductase is quinol. The electrons from quinol are thought to be transferred through the heme in the membrane-anchoring subunit to the [Fe-S] centers in NarH and then to the molybdenum center in NarG where nitrate reduction occurs.
Nap enzymes are typically heterodimers with prosthetic groups similar to those found in the membrane-associated nitrate reductase (Arnoux et al. 2003). In the heterodimeric form, the largest subunit (NapA) binds molybdopterin guanine dinucleotide and a [4Fe-4S] center. The smaller subunit (NapB) binds c-type heme that is required for transfer of electrons to the active site. In many denitrifiers, electrons are transferred from the membrane-associated quinol pool to the Nap complex by a membrane-bound tetra-heme c-type cytochrome, NapC. Some Nap proteins have been found to have two additional subunits known as NapG and NapH (Brondijk et al. 2004). NapH is an integral membrane protein with multiple [Fe-S] centers while NapG is found in the periplasm but also contains [Fe-S] centers. NapG and NapH likely form a dimer involved in transferring electrons from some form of quinol to the reaction center. In some bacteria such as W. succinogenes, the NapC subunit is missing and its function has been suggested to be taken over by NapGH (Kern and Simon 2008).
The periplasmic and membrane-bound enzymes can be distinguished in several ways. First, the membrane-bound enzyme is sensitive to micromolar levels of azide, whereas the periplasmic form is not (Bell et al. 1990). Second, the membrane-bound enzyme can reduce chlorate but the periplasmic enzyme is limited to nitrate, a result that led to the development of a useful method to select nitrate reductase mutants (Bell et al. 1990; McEwan et al. 1984). Third, because the active sites of the two enzymes are on different sides of the inner membrane, the differential membrane solubilities of methyl viologen and benzyl viologen can be used to differentiate activities (Carter et al. 1995). Methyl viologen, which is membrane permeant, can be used as an electron source for both enzymes in whole cell assays. Benzyl viologen, which is membrane impermeant, will act as an electron source only for the periplasmic enzyme in whole cell assays. By comparing the nitrate reductase activity determined with each viologen, the relative levels of activity of each form of nitrate reductase can be estimated.
While the function of the respiratory nitrate reductase in denitrification is almost always associated with respiration, the physiological role of the periplasmic enzyme is more variable. If Nap is the sole nitrate reductase in denitrifiers that reduce nitrate to N2O or N2, the enzyme is used for denitrification even though it cannot directly contribute to formation of a proton motive force. However, in denitrifiers with both Nar and Nap, the function of the latter is often not respiration but instead redox homeostasis. This explains why the Nap enzyme is often expressed under oxic conditions and is consistent with the observation that the Nap of Paracoccus pantotrophus is expressed at a higher level in medium containing electron-rich substrates than in medium with more oxidized substrates (Gavira et al. 2002; Sears et al. 2000; Tabata et al. 2005).
Nitrite Reductase: Nitrite reductase catalyzes the one-electron reduction of nitrite to NO, the step that differentiates denitrification from other forms of nitrate metabolism (Fig. 10.6 ). There are two types of nitrite reductases that are not structurally related and contain different prosthetic groups (Zumft 1997). One contains copper as a redox active metal (CuNir), and the other utilizes heme-bound iron (cd 1). They are both located in the periplasm and appear to be functionally redundant (Glockner et al. 1993). In gram-negative bacteria, both types of Nir receive electrons from the bc 1 complex via small, mobile electron shuttles (Hartsock and Shapleigh 2010; van Spanning et al. 2007). Either Nir can be found within species within the same genus but have never been found to occur together in a single bacterium. While there are ecophysiological patterns seen in the phylogenetic distribution of the two enzymes in most cases, the reasons underlying the distribution within a genus or higher taxonomic grouping are not obvious.
CuNir has been studied extensively, and much is known about its structure and the nature of the copper centers. Enzymes from several different denitrifiers have been crystallized under different conditions and their high-resolution structures determined (Godden et al. 1991; Jacobson et al. 2005; Nojiri et al. 2009a; Tocheva et al. 2004). These studies have revealed that the enzyme is a homotrimer with each monomer containing two copper atoms. One copper is bound by Cys, Met, and two His residues and is referred to as a type-1 copper center. In multi-copper enzymes, including CuNir, the type-1 copper is involved in electron transfer to the active site. The other copper atom in CuNir is bound by three His residues making it a type-2 copper center. Type-2 centers are found in many multi-copper enzymes and are frequently sites of substrate binding. In CuNir, the type-2 center has been shown to be the site of nitrite binding (Tocheva et al. 2004).
While there is a common functional design shared by all CuNir, there is significant variation in the primary structure of the CuNir family. The first recognition of this diversity was from studies of Neisseria gonorrhoeae which reported that a highly expressed protein known as AniA was in fact a CuNir (Mellies et al. 1997). This was somewhat unexpected since this enzyme had low sequence identity, ∼30 %, with previously characterized CuNir. Determination of a high-resolution structure of the AniA from Neisseria gonorrhoeae showed that despite the limited sequence conservation, the overall structure was similar to previously determined CuNir structures and contained both type-1 and type-2 centers and protein folds to this family of enzymes (Boulanger and Murphy 2002).
More recently, additional CuNir variants have been described. The CuNir from Hypomicrobium denitrificans is notable because of the presence of an ∼15 kDa N-terminal extension. This extension contains a second type-1 copper center (Nojiri et al. 2007). Another variant of this type has been described in a number of denitrifiers including members of the genus Ralstonia. However, this variant has an extension at its C-terminus and it contains a sequence motif consistent with the binding of a c-type heme (Ellis et al. 2007). This extension also adds ∼15 kDa to the protein. An even larger variant has been found in the genome of P. acnes (Nojiri et al. 2009b). This protein is about 900 residues long, in comparison to the ∼350 residues of the prototypical CuNir from bacteria such as Achromobacter cycloclastes or the ∼500 residues of the aforementioned extended C- and N-terminus variants. The P. acnes protein contains a classic CuNir type enzyme at its C-terminus, but this is preceded by two additional domains. One of these is a copper-binding domain and the other a predicted transmembrane domain of about 400 residues. The function of the transmembrane domain is uncertain. A number of other Actinobacteria, such as members of the genus Rothia as well as J. denitrificans, have a CuNir with similar domain organization.
The cd 1-type nitrite reductase is a homodimer that contains a single c-type heme and d 1 heme molecule per monomer. The d 1 heme is a modified tetrapyrrole ring that is partly reduced and has oxo, methyl, and acrylate side groups (Chang and Wu 1986). The high-resolution structures of the cd 1-type enzymes from P. pantotrophus (Fulop et al. 1995) and P. aeruginosa (Nurizzo et al. 1997) have been determined. The protein consists of two domains with the c-type heme located in the smaller domain and the d 1-heme, which is also the site of nitrite reduction, located in the larger domain. The reason underlying the usage of the novel d 1 heme is uncertain. It has been suggested the d 1 heme helps foster a catalytic site with a low affinity for NO and higher affinity for nitrite (Rinaldo et al. 2011). NO has a high affinity for reduced metal centers, so ensuring product release is critical for efficient Nir function.
Interestingly, both the CuNir and cd 1 enzymes also can reduce O2. Early studies often designated the cd 1 enzyme a cytochrome oxidase (Wharton and Gibson 1976). The product of O2 reduction by the cd 1 enzyme is water (Lam and Nicholas 1969) although it is not clear how four electrons are passed to the O2 in this process, inasmuch as a one-electron reduction is normally carried out.
Nitric Oxide Reductase: In a continuing theme, and as mentioned above, there are multiple forms of Nor (Fig. 10.7 ). The first form discovered was cNor. The isolation of the genes encoding cNor in P. stutzeri provided significant insight into the structural organization of the enzyme and confirmed that NO is an obligatory intermediate in denitrification (Zumft et al. 1994). Examination of the deduced primary sequence indicated that cNor is related to the heme-copper family of cytochrome oxidases (HCO) (Saraste and Castresana 1994; van der Oost et al. 1994). Although the overall identity of cNor and HCO members is low, a set of six His residues is conserved in pairwise alignments of subunit I of cytochrome oxidase and the equivalent NorB subunit of Nor. In the HCO family, these residues bind a six-coordinate heme, five-coordinate heme, and copper, the latter two metal centers constituting a binuclear center that is the site of O2 binding and reduction (Iwata et al. 1995). In NorB, the equivalent His residues ligate a six-coordinate heme, a five-coordinate heme, and a non-heme iron which replaces the copper at the active site binuclear center.
Typically, cNor is purified as a heterodimer with its two subunits, NorB and NorC, being integral membrane proteins. NorC is a c-type cytochrome, proposed to accept electron from the respiratory chain and then transfer them to a b-type heme in NorB. This electron is next passed to the binuclear center which is where NO is bound and reduced to N2O. A recent crystal structure of cNor from P. aeruginosa has confirmed the relatedness of the Nor and HCO families (Hino et al. 2010). One important difference between the two structures though is that the Nor structure lacks residues that form channels or hydrogen bond networks used for proton pumping in some HCO. This is consistent with previous work indicating Nor turnover does not directly lead to formation of a proton motive force (Zumft 1997). While the structure provided significant insight into the organization of Nor, the reaction chemistry by which two molecules of NO are bound and converted to one molecule of N2O remains to be elucidated.
A second Nor type was first identified in the bacterium Ralstonia eutropha (Alcaligenes eutrophus H16) (Cramm et al. 1997). This bacterium contains two Nor, one designated NorZ, a product of a gene located on the chromosome, and the other designated NorB, a product of a gene located on a plasmid. The products of these genes have significant identity (>90 %) and are functionally redundant. These enzymes also have significant similarity with the NorB subunit of the cNor, with the exception of an N-terminal extension of about 300 residues. This N-terminal extension binds quinol, which serves as the immediate electron donor for this family of enzymes (Cramm et al. 1999). As with cNor, qNor is related to the HCO family. No high-resolution structure of a qNor is currently available. Genome sequencing has revealed that many denitrifiers utilize qNor. As with the two types of Nir, there is no clear phylogenetic distribution of cNor and qNor but qNor seems to be the preferred Nor among many β and γ proteobacteria.
A third type of Nor has been found in B. azotoformans and is the only example of a Nor purified from a gram-positive bacterium (Suharti et al. 2001). This enzyme is a dimer like NorCB but lacks c-type heme. Another distinguishing feature is the presence of a CuA-type binuclear copper center. This metal center is also found in HCO and Nos (see below). This enzyme has been designated qCuANor since it will accept electrons from quinol (Suharti and Heering, and S. de Vries. 2004). Unlike qNor though, it will also accept electrons from a membrane-bound c-type cytochrome.
Nitrous Oxide Reductase: In general, Nos is located in the periplasm and receives electrons from the bc 1 complex via electron shuttles (Fig. 10.7 ). Nitrous oxide reductases from several complete denitrifiers have been extensively characterized and several high-resolution structures have been obtained (Brown et al. 2000; Haltia et al. 2003; Paraskevopoulos et al. 2006). Excellent reviews on the genetics and biochemistry of Nos are available (Tavares et al. 2006; Zumft and Kroneck 2006). Purification and characterization of Nos was difficult because its activity is lost in cell extracts. Nutritional studies had identified copper as an essential nutrient for Nos activity (Matsubara and Zumft 1982) and further work led to the isolation of a soluble copper protein which, under the proper conditions, had Nos activity (Zumft and Matsubara 1982). Nos is homodimeric in most preparations, with four coppers per subunit. Additional studies have demonstrated that when the enzyme is purified anaerobically and assayed using reduced viologens as the electron donor, it has the highest specific activity.
The high-resolution structures have defined the nature of the copper-containing sites in Nos (Brown et al. 2000; Haltia et al. 2003; Paraskevopoulos et al. 2006). One of the copper centers has been structurally defined as a CuA site. The CuA center was described originally in the HCO family of proteins (Iwata et al. 1995). CuA is a binuclear site liganded by His, Cys, and Trp residues that is used to transfer electrons from an external donor to the active site, CuZ. CuZ is a tetranuclear site only found in Nos. Histidines are the amino acid ligands to the coppers in the site, but a single sulfide is also an important structural component (Haltia et al. 2003). The high-resolution structure has revealed that the actual number of Cu per dimer is 12 not 8 as suggested from earlier work. The reason for the discrepancy is unclear. It should be noted that crystals were made from protein that had been exposed to O2 which is known to impact function in the enzyme. Therefore, it is uncertain if the metal centers in the published structures are identical to those in the active enzyme.
As with the other N-oxide reductases, there is variation among Nos proteins. Campylobacter species and a few other related ε proteobacteria contain a C-terminal extension that is a c-type heme-binding domain. W. succinogenes has a Nos modified in this way which is known to be functional since this bacterium will grow with N2O as sole terminal oxidant (Simon et al. 2004; Yoshinari 1980). Unexpectedly though, the genome sequence indicates that the nosZ, which encodes Nos, in W. succinogenes is disrupted by an insertion element. Further studies revealed that some portion of the population carry an intact nosZ gene, likely as a result of the resolving of the insertion element (Simon et al. 2004).
Quantitative studies have shown that nos is the least prevalent of the denitrification N-oxide reductases in many environments (Bru et al. 2011; Henry et al. 2006; Keil et al. 2011). This is consistent with genome analyses which find the nos cluster being the least common of all the N-oxide reductases. Genome comparison of closely related bacteria provides frequent examples of nos cluster genes undergoing frame shifts or deletion. For example, a number of Brucella strains show a loss of nosZ or one of the other genes in the nos cluster. Moreover, as mentioned above, Neisseria species express a truncated, inactive Nos (Barth et al. 2009). While Nos seems to be lost at a high rate, it is obvious it is beneficial to some bacteria. For example, almost all of the Burkholderia mallei and pseudomallei strains that have been sequenced have an intact nos gene cluster. Moreover, it is notable that W. succinogenes, Capnocytophaga gingivalis JCVIHMP016, and Gramella forsetii KT0803, isolated from bovine rumen fluid, human oral cavity, and seawater, respectively, all contain a nos gene cluster but lack Nor, indicating that exogenous N2O is an environmentally relevant terminal electron acceptor.
Regulation of Genes Required for Denitrification
This section will focus primarily on the regulation of those genes encoding Nir, Nor, and Nos. In most denitrifiers, the expression of the genes encoding these proteins depends on the presence of nitrogen oxides. Therefore, it is natural to describe the denitrification genes as being part of a stimulon, a term that refers to operons responding together to a particular environmental stimulus (Neidhardt et al. 1990). In general, Nir and Nor are part of a NO stimulon and Nos is part of a N2O stimulon. However, it is important to note that denitrification is a secondary process and so its regulation occurs within organism-specific hierarchies of stimulons and regulons required for aerobic and anaerobic growth. This inevitably will lead to diverse regulation and, in fact, not all of the regulatory strategies described here explain all known regulatory patterns and it is certain that as additional research on regulation of the N-oxide reductases is carried out, additional regulatory patterns will be encountered. Nevertheless, the general patterns described here are consistent with regulatory models developed for many model denitrifiers.
One important consideration in the regulation of denitrification is the role of O2 in controlling expression of denitrification genes. While denitrification is traditionally considered as an anaerobic form of respiration, there are many reports in the literature of aerobic denitrification (Kim et al. 2008; Wan et al. 2011; Xie et al. 2003). Studies with model organisms have consistently found that reduction of nitrate or nitrite to gaseous products occurs at low O2 (Shapleigh 2011; Zumft 1997). In particular, it seems that the regulation of nir and nor genes is particularly sensitive to O2 and it is their expression that sets the upper limit of O2 at which denitrification can occur (Bergaust et al. 2011, 2008; Korner and Zumft 1989). None of the reported aerobic denitrifiers have been characterized in sufficient detail to determine if they expresses nir and nor in an O2-independent manner or at O2 levels higher than observed in current studies of model denitrifiers. Until this occurs, aerobic denitrification will remain unverified. When considering aerobic denitrification, it is important to note that the regulation of nitrate reductase is not so tightly controlled by O2. There are examples of Nap-type nitrate reductases that have been demonstrated to be expressed under fully aerobic conditions (Gavira et al. 2002; Hartsock and Shapleigh 2011; Tabata et al. 2005). Another important consideration in evaluating reports of aerobic denitrification is that, with the exception of Nos, none of the N-oxide reductases are inhibited by O2. So, unless care is taken to ensure that cells used in aerobic denitrification experiments have been maintained under aerobic conditions, it is possible that proteome carryover may lead to N-oxide reductase activity that is misattributed to aerobic denitrification.
Nir and Nor Regulation: A number of early studies demonstrated that expression of both Nir and Nor in some model denitrifiers depended on Nir activity (de Boer et al. 1996; Tosques et al. 1997; Ye et al. 1992; Zumft et al. 1994). The observation that it was Nir activity and not nitrite reductase per se that was required for the expression of genes encoding Nir and Nor suggested that a product of nitrite reduction was required for gene expression (Fig. 10.8 ). An obvious candidate for the likely effector is NO, a possibility consistent with the observation that addition of NO generators to nitrite reductase-deficient strains results in expression of both Nir and Nor genes (Kwiatkowski and Shapleigh 1996; Van Spanning et al. 1999). Moreover, trapping of NO by hemoglobin decreases expression of nir and nor genes (Kwiatkowski and Shapleigh 1996). The accumulated evidence strongly indicates that it is the production of NO that stimulates expression, but activation by a derivative of NO has not been excluded.
Representation of the regulation of the nitrogen oxide reductases in Rhodobacter sphaeroides 2.4.3. This bacterium has two Nap enzymes, designated oNap and rNap, with the former being expressed aerobically and the latter being expressed under microoxic conditions (Hartsock). Lines in the cytoplasm represent genes and transcripts. oNap is green to indicate it is expressed under oxic conditions. Lines from PrrB and NnrR ending in arrows indicate positive regulation while the one ending in a line indicates negative regulation. Transcriptional regulators of rNap are unknown, so the only known controlling factor, lack of O2, is indicated. See text for description of regulatory factors
The primary regulator of the genes that are part of the denitrification-related NO stimulon has been identified in a number of denitrifiers and in most cases is a member of the FNR/CRP family of transcriptional regulators (Korner et al. 2003). This protein has been variously designated NnrR, NNR, or DNR. An excellent review on this family of proteins has recently been published (Korner et al. 2003). Recent work has suggested this protein binds heme, explaining its ability to bind NO with high affinity (Castiglione et al. 2009; Giardina et al. 2008).
Computational studies as well as mutagenesis studies have determined the binding sites targeted by some members of this family (Rodionov et al. 2005; Hartsock and Shapleigh 2010). Recent work suggests the regulon controlled by the NNR/DNR family is small. In R. sphaeroides, NnrR likely only has eight binding sites which affect a total of 13 genes (Hartsock and Shapleigh 2010). The regulon of the primary NO-dependent regulator controlling expression of Nir and Nor expression in Neisseria meningitidis is similar in size (Heurlier et al. 2008). This regulator, named NsrR, is not a member of the FNR/CRP family. It acts as a repressor while the members of the DNR/NNR family are activators.
Not all denitrifiers control nir expression via an NO stimulon. In some Rhizobium strains, members of the NO-responsive NNR family have been found to be important for expression of the nor genes (Mesa et al. 2002). nirK expression appears to be outside the NNR regulon and NO stimulon because it is regulated by a gene termed FixK2, a member of the FixK family shown to be critical for regulation of many processes related to nodulation in these bacteria (Torres et al. 2011). FixK2 itself is under control of FixJ, a global regulator of low O2 gene expression in rhizobia.
Some denitrifiers use additional layers of regulation to link expression of nir and nor to O2 availability. Agrobacterium tumefaciens uses the two component sensor regulator pair ActRS to regulate nirK expression (Baek et al. 2008) (Fig. 10.8 ). These regulators are a member of the Reg/Prr family of proteins used by many photosynthetic bacteria to regulate expression of genes whose products are required for photosynthesis. R. sphaeroides 2.4.3 has been shown to use a similar regulatory strategy (Laratta et al. 2002). Members of the Reg/Prr family of regulatory proteins are apparently responsive to changes in the flow of electrons through the electron transport chain (Kim et al. 2007; Swem et al. 2001). As O2 decreases, electron flow through the chain decreases, leading to phosphorylation of the response regulators which are necessary for nirK expression. nor expression is not directly regulated by Reg/Prr but since nirK is, this places nor under indirect control of Reg/Prr since nor is part of the NO stimulon (Baek et al. 2008).
Nos Regulation: nos expression is likely controlled by a number of factors, leading to diverse control mechanisms. Evidence for a N2O stimulon was initially provided by the observation that growth on N2O stimulates Nos expression, modestly stimulates expression of the nitrate reductase, and does not stimulate expression of Nir or Nor in P. stutzeri (Korner and Zumft 1989). Additional support for NO-independent expression of nos has been provided by studies of P. denitrificans, where it was shown that expression of nos preceded that of nirS and nor (Bergaust et al. 2011). Use of N2O as an effector is not universal though since P. aeruginosa grown on N2O alone will not express Nos. Nos is expressed when the cells are grown on nitrate and nos is likely within the NO stimulon (Arai et al. 2003). In P. stutzeri, while N2O alone can activate expression of nos, growth on nitrate and subsequent production of NO leads to even higher expression of the nos gene cluster (Vollack and Zumft 2001). This suggests nos is within both the NO and N2O stimulons in this bacterium, which is probably the case in many complete denitrifiers.
Little is known about specific regulators required for nos expression outside of the NNR/DNR family. One protein that appears important for nos expression in nearly all denitrifiers is NosR. NosR is an integral membrane protein which contains [Fe-S] clusters as well as flavin (Wunsch and Zumft 2005). The gene for nosR is almost always part of the nos gene cluster. How NosR regulates nos expression and Nos activity is uncertain. Insertional inactivation of nosR leads to a decrease in nosZ transcription (Cuypers et al. 1992; Velasco et al. 2004). However, nosR does not have any obvious DNA-binding motifs and its membrane localization is seemingly inconsistent with it being a DNA-binding protein. NosR may play a role in Nos assembly since removal of regions with conserved motifs led to overexpression of an inactive Nos in P. stutzeri (Wunsch and Zumft 2005). While the vast majority of denitrifiers contain NosR-encoding genes, W. succinogenes lacks any obvious nosR orthologs (Simon et al. 2004).
Density-Dependent Regulation: In addition to nitrogen oxide and O2-dependent control, some of the denitrification genes in P. aeruginosa show evidence of density-dependent regulation. Expression of nirS, nor, and nos in this bacterium is regulated by members of the DNR family (Arai et al. 1997, 2003). However, it was observed that inactivation of genes responsible for density-dependent expression led to an increase in the rate of N2 production from nitrate (Toyofuku et al. 2007). As expected, expression of the genes encoding Nir, Nor, and Nos showed decreased expression in the presence of an effector. This indicates that under conditions of high cell density, expression of these genes will be repressed.
The regulatory mechanisms used by denitrifiers to control Nir, Nor, and Nos expression are consistent with denitrification being nonessential and secondary to O2 respiration. These regulatory strategies also provide evidence of how cells use regulation to ensure denitrification is used to support other more critical physiological processes. For example, in rhizobia, the Fix system of regulators that controls Nir expression is critical for regulating a variety of genes required for nodule formation and diazotrophy (Mesa et al. 2008). Similarly, photosynthesis is a major mode of energy generation in R. sphaeroides and so it is not unexpected to find the denitrification genes are regulated by global regulators involved in adapting cell physiology for photosynthetic growth, also a low O2 process (Kaplan et al. 2005). Density-dependent regulation in P. aeruginosa provides another example. This bacterium uses biofilms as a key means of survival and growth, and biofilm formation is controlled in part by density-dependent regulatory systems (Harmsen et al. 2011). Biofilms often have low O2 microenvironments due to O2 gradients established by respiration. Therefore, it is not surprising to find denitrification genes are controlled by the regulators also required for the formation of biofilms. NO has been shown to convert sessile cells to motile cells which may explain why denitrification genes are repressed by the density-dependent regulators (Barraud et al. 2006).
Environmental Studies
There have been many efforts to characterize denitrifier communities in the environment. As might be expected from the broad phylogenetic distribution of denitrification genes, nearly all environments have denitrification genes (Bru et al. 2011; Keil et al. 2011; Philippot et al. 2009). Quantitative assessment of the frequency of occurrence of individual genes has found that genes for nitrate reductase occur at the highest frequency, likely representing its use in both denitrification and DNRA. Genes encoding Nir occur at a lower frequency than Nar and Nap genes. Genes encoding Nos occur at the lowest frequency. Nos activity also appears to be inhibited at low pH (Liu et al. 2010; Simek and Cooper 2002; Thomsen et al. 1994). Low pH environments are often found to produce large amounts of N2O under denitrification conditions (Simek and Cooper 2002).
Analysis of the distribution patterns of particular gene types has identified some large-scale trends. A thorough analysis of the distribution of nirK and nirS, the structural gene for the cd 1-Nir, has been carried out by Jones and Hallin (Jones and Hallin 2010). They found that nirS sequences were more enriched in saline environments. However, nearly all of these studies are reliant on the use of oligonucleotide primers that may not work with divergent or novel genes. For example, sequencing of the genome of a denitrifying Rhodanobacter strain showed that standard PCR primers would be unlikely to amplify the nirK from this bacterium (Green et al. 2010). This is significant because members of the genus Rhodanobacter are common denitrifiers in low pH environments. It is well established that low pH has been shown to impact denitrifier populations in general, so it is possible that diversity is being underestimated by the use of standard primer sets (Deiglmayr et al. 2004; Green et al. 2010; van den Heuvel et al. 2010).
While denitrification genes may be common in most environments, in soils, there is a growing appreciation of the fact that denitrification is “spotty.” Certain sites are “hot spots” relative to other closely located sites (McClain et al. 2003; Vidon et al. 2010). Even within this hotspot framework, it has become obvious that sites are also subject to “hot moments.” Molecular studies have supported the idea of hot spots (Enwall et al. 2010). The occurrence of hot spots and hot moments makes it difficult to accurately quantitate denitrification rates in soils.
While denitrification is common in soil and aqueous environments, it is important to note that human-associated communities have the capacity for denitrification. Human saliva is enriched for nitrate, so it is not surprising that denitrification has been found to occur in human dental plaque (Lundberg and Govoni 2004; Lundberg et al. 2004). A number of partial denitrifiers have also been identified during efforts to sequence the human oral microbiome (http://www.homd.org/modules.php?op=modload&name=HOMD&file=index&taxonomy=1). Also, as mentioned above, a number of pathogens within the Neisseria, Brucella, and Pseudomonas genera are complete denitrifiers. Denitrification genes in these bacteria have been found to be pathogenicity determinants (Baek et al. 2004; Haine et al. 2006; Hassett et al. 2002; Laver et al. 2010; Stevanin et al. 2007; Tunbridge et al. 2006).
References
Allison C, Macfarlane GT (1989) Dissimilatory nitrate reduction by Propionibacterium acnes. Appl Environ Microbiol 55:2899–2903
Alvarez L, Bricio C, Gomez MJ, Berenguer J (2011) Lateral transfer of the denitrification pathway genes among Thermus thermophilus strains. Appl Environ Microbiol 77:1352–1358
Arai H, Kodama T, Igarashi Y (1997) Cascade regulation of the two CRP/FNR-related transcriptional regulators (ANR and DNR) and the denitrification enzymes in Pseudomonas aeruginosa. Mol Microbiol 25:1141–1148
Arai H, Mizutani M, Igarashi Y (2003) Transcriptional regulation of the nos genes for nitrous oxide reductase in Pseudomonas aeruginosa. Microbiology 149:29–36
Arnoux P, Sabaty M, Alric J, Frangioni B, Guigliarelli B, Adriano JM, Pignol D (2003) Structural and redox plasticity in the heterodimeric periplasmic nitrate reductase. Nat Struct Biol 10:928–934
Baek SH, Rajashekara G, Splitter GA, Shapleigh JP (2004) Denitrification genes regulate Brucella virulence in mice. J Bacteriol 186:6025–6031
Baek SH, Hartsock A, Shapleigh JP (2008) Agrobacterium tumefaciens C58 uses ActR and FnrN to control nirK and nor expression. J Bacteriol 190:78–86
Baker SC, Ferguson SJ, Ludwig B, Page MD, Richter OMH, van Spanning RJM (1998) Molecular genetics of the genus Paracoccus: metabolically versatile bacteria with bioenergetic flexibility. Microbiol Mol Biol Rev 62:1046–1078
Barraud N, Hassett DJ, Hwang SH, Rice SA, Kjelleberg S, Webb JS (2006) Involvement of nitric oxide in biofilm dispersal of Pseudomonas aeruginosa. J Bacteriol 188:7344–7353
Barth KR, Isabella VM, Clark VL (2009) Biochemical and genomic analysis of the denitrification pathway within the genus Neisseria. Microbiology 155:4093–4103
Beaumont HJ, Lens SI, Reijnders WN, Westerhoff HV, van Spanning RJ (2004) Expression of nitrite reductase in Nitrosomonas europaea involves NsrR, a novel nitrite-sensitive transcription repressor. Mol Microbiol 54:148–158
Bell LC, Richardson DJ, Ferguson SJ (1990) Periplasmic and membrane-bound respiratory nitrate reductases in Thiosphaera pantotropha. The periplasmic enzyme catalyzes the first step in aerobic denitrification. FEBS Lett 265:85–87
Bergaust L, Shapleigh J, Frostegård Å, Bakken L (2008) Transcription and activities of NOx reductases in Agrobacterium tumefaciens: the influence of nitrate, nitrite and oxygen availability. Environ Microbiol 10:3070–3081
Bergaust L, Mao Y, Bakken LR, Frostegård Å (2011) Denitrification response patterns during the transition to anoxic respiration and posttranscriptional effects of suboptimal pH on nitrous oxide reductase in Paracoccus denitrificans. Appl Environ Microbiol 76:6387–6396
Bokranz M, Katz J, Schroder I, Roberton AM, Kroger A (1983) Energy metabolism and biosynthesis of Vibrio succinogenes growing with nitrate or nitrite as terminal electron acceptor. Arch Microbiol 135:36–41
Boulanger MJ, Murphy ME (2002) Crystal structure of the soluble domain of the major anaerobically induced outer membrane protein (AniA) from pathogenic Neisseria: a new class of copper-containing nitrite reductases. J Mol Biol 315:1111–1127
Brettar I, Christen R, Hofle MG (2002) Shewanella denitrificans sp. nov., a vigorously denitrifying bacterium isolated from the oxic-anoxic interface of the Gotland Deep in the central Baltic Sea. Int J Syst Evol Microbiol 52:2211–2217
Brondijk TH, Nilavongse A, Filenko N, Richardson DJ, Cole JA (2004) NapGH components of the periplasmic nitrate reductase of Escherichia coli K-12: location, topology and physiological roles in quinol oxidation and redox balancing. Biochem J 379:47–55
Brooijmans RJ, de Vos WM, Hugenholtz J (2009) Lactobacillus plantarum WCFS1 electron transport chains. Appl Environ Microbiol 75:3580–3585
Brown K, Prudencio M, Pereira AS, Besson S, Moura JJG, Moura I, Tegoni M, Cambillau C (2000) A novel type of catalytic copper cluster in nitrous oxide reductase. Nat Struct Biol 7:191–195
Bru D, Ramette A, Saby NP, Dequiedt S, Ranjard L, Jolivet C, Arrouays D, Philippot L (2011) Determinants of the distribution of nitrogen-cycling microbial communities at the landscape scale. ISME J 5:532–542
Brunekreef B, Holgate ST (2002) Air pollution and health. Lancet 360:1233–1242
Cabello P, Roldan MD, Moreno-Vivian C (2004) Nitrate reduction and the nitrogen cycle in archaea. Microbiology 150:3527–3546
Canfield DE, Glazer AN, Falkowski PG (2010) The evolution and future of Earth’s nitrogen cycle. Science 330:192–196
Carter JP, Hsiao YH, Spiro S, Richardson DJ (1995) Soil and sediment bacteria capable of aerobic nitrate respiration. Appl Environ Microbiol 61:2852–2858
Castiglione N, Rinaldo S, Giardina G, Cutruzzola F (2009) The transcription factor DNR from Pseudomonas aeruginosa specifically requires nitric oxide and haem for the activation of a target promoter in Escherichia coli. Microbiology 155:2838–2844
Chain PS, Lang DM, Comerci DJ, Malfatti SA, Vergez LM, Shin M, Ugalde RA, Garcia E, Tolmasky ME (2011) Genome of Ochrobactrum anthropi ATCC 49188T, a versatile opportunistic pathogen and symbiont of several eukaryotic hosts. J Bacteriol 193:4274–4275
Chang CK, Wu W (1986) The porphinedione structure of heme d 1: synthesis and spectral properties of model compounds of the prosthetic group of dissimilatory nitrite reductase. J Biol Chem 261:8593–8596
Choudhary M, Zanhua X, Fu YX, Kaplan S (2007) Genome analyses of three strains of Rhodobacter sphaeroides: evidence of rapid evolution of chromosome II. J Bacteriol 189:1914–1921
Coyne S, Courvalin P, Galimand M (2010) Acquisition of multidrug resistance transposon Tn6061 and IS6100-mediated large chromosomal inversions in Pseudomonas aeruginosa clinical isolates. Microbiology 156:1448–1458
Cramm R, Siddiqui RA, Friedrich B (1997) Two isofunctional nitric oxide reductases in Alcaligenes eutrophus H16. J Bacteriol 179:6769–6777
Cramm R, Pohlmann A, Friedrich B (1999) Purification and characterization of the single-component nitric oxide reductase from Ralstonia eutropha H16. FEBS Lett 460:6–10
Cua LS, Stein LY (2011) Effects of nitrite on ammonia-oxidizing activity and gene regulation in three ammonia-oxidizing bacteria. FEMS Microbiol Lett 319:169–175
Cuypers H, Viebrock-Sambale A, Zumft WG (1992) NosR, a membrane-bound regulatory component necessary for expression of nitrous oxide reductase in denitrifying Pseudomonas stutzeri. J Bacteriol 174:5332–5339
de Boer AP, van der Oost J, Reijnders WN, Westerhoff HV, Stouthamer AH, van Spanning RJ (1996) Mutational analysis of the nor gene cluster which encodes nitric-oxide reductase from Paracoccus denitrificans. Eur J Biochem 242:592–600
de Vries S, Strampraad MJ, Lu S, Moenne-Loccoz P, Schroder I (2003) Purification and characterization of the MQH2:NO oxidoreductase from the hyperthermophilic archaeon Pyrobaculum aerophilum. J Biol Chem 278:35861–35868
Deiglmayr K, Philippot L, Hartwig UA, Kandeler E (2004) Structure and activity of the nitrate-reducing community in the rhizosphere of Lolium perenne and Trifolium repens under long-term elevated atmospheric pCO. FEMS Microbiol Ecol 49:445–454
Denariaz G, Payne WJ, Legall J (1989) A halophilic denitrifier, Bacillus halodenitrificans sp. nov. Int J Syst Bacteriol 39:145–151
Ellis MJ, Grossmann JG, Eady RR, Hasnain SS (2007) Genomic analysis reveals widespread occurrence of new classes of copper nitrite reductases. J Biol Inorg Chem 12:1119–1127
Enwall K, Throback IN, Stenberg M, Soderstrom M, Hallin S (2010) Soil resources influence spatial patterns of denitrifying communities at scales compatible with land management. Appl Environ Microbiol 76:2243–2250
Ettwig KF, Butler MK, Le Paslier D, Pelletier E, Mangenot S, Kuypers MM, Schreiber F, Dutilh BE, Zedelius J, de Beer D, Gloerich J, Wessels HJ, van Alen T, Luesken F, Wu ML, van de Pas-Schoonen KT, Op den Camp HJ, Janssen-Megens EM, Francoijs KJ, Stunnenberg H, Weissenbach J, Jetten MS, Strous M (2010) Nitrite-driven anaerobic methane oxidation by oxygenic bacteria. Nature 464:543–548
Fredrickson JK, Romine MF, Beliaev AS, Auchtung JM, Driscoll ME, Gardner TS, Nealson KH, Osterman AL, Pinchuk G, Reed JL, Rodionov DA, Rodrigues JL, Saffarini DA, Serres MH, Spormann AM, Zhulin IB, Tiedje JM (2008) Towards environmental systems biology of Shewanella. Nat Rev Microbiol 6:592–603
Fulop V, Moir JWB, Ferguson SJ, Hajdu J (1995) The anatomy of a bifunctional enzyme: structural basis for reduction of oxygen to water and synthesis of nitric oxide by cytochrome cd 1. Cell 81:369–377
Galyov EE, Brett PJ, DeShazer D (2010) Molecular insights into Burkholderia pseudomallei and Burkholderia mallei pathogenesis. Annu Rev Microbiol 64:495–517
Gavira M, Roldan MD, Castillo F, Moreno-Vivian C (2002) Regulation of nap gene expression and periplasmic nitrate reductase activity in the phototrophic bacterium Rhodobacter sphaeroides DSM158. J Bacteriol 184:1693–1702
Gayon U, Dupetit G (1882) Sur la fermentation des nitrates. C R Acad Sci 95:644–646
Gayon U, Dupetit G (1886) Recherches sur la reduction des nitrates par les infinement petits. Mem Soc Sci Phys Nat Bordeaux Ser 3. 95:201–307
Giardina G, Rinaldo S, Johnson KA, Di Matteo A, Brunori M, Cutruzzola F (2008) NO sensing in Pseudomonas aeruginosa: structure of the transcriptional regulator DNR. J Mol Biol 378:1002–1015
Glockner AB, Juengst A, Zumft WG (1993) Copper containing nitrite reductase from Pseudomonas aureofaciens is functional in a mutationally cytochrome cd 1 -free background (nirs-) negative of Pseudomonas stutzeri. Arch Microbiol 160:18–26
Godden JW, Turley S, Teller DC, Adman ET, Liu MY, Payne WJ, Legall J (1991) The 2.3 angstrom X-ray structure of nitrite reductase from Achromobacter cycloclastes. Science 253:438–442
Green SJ, Prakash O, Gihring TM, Akob DM, Jasrotia P, Jardine PM, Watson DB, Brown SD, Palumbo AV, Kostka JE (2010) Denitrifying bacteria isolated from terrestrial subsurface sediments exposed to mixed-waste contamination. Appl Environ Microbiol 76:3244–3254
Greenberg EP, Becker GE (1977) Nitrous oxide as end product of denitrification by strains of fluorescent pseudomonads. Can J Microbiol 23:903–907
Hafenbradl D, Keller M, Dirmeier R, Rachel R, Rossnagel P, Burggraf S, Huber H, Stetter KO (1996) Ferroglobus placidus gen. nov., sp. nov., A novel hyperthermophilic archaeum that oxidizes Fe +2 at neutral pH under anoxic conditions. Arch Microbiol 166:308–314
Haine V, Dozot M, Dornand J, Letesson JJ, De Bolle X (2006) NnrA is required for full virulence and regulates several Brucella melitensis denitrification genes. J Bacteriol 188:1615–1619
Hallin S, Jones CM, Schloter M, Philippot L (2009) Relationship between N-cycling communities and ecosystem functioning in a 50-year-old fertilization experiment. ISME J 3:597–605
Haltia T, Brown K, Tegoni M, Cambillau C, Saraste M, Mattila K, Djinovic-Carugo K (2003) Crystal structure of nitrous oxide reductase from Paracoccus denitrificans at 1.6 Å resolution. Biochem J 369:77–88
Harmsen HJ, Van Kuijk BL, Plugge CM, Akkermans AD, De Vos WM, Stams AJ (1998) Syntrophobacter fumaroxidans sp. nov., a syntrophic propionate-degrading sulfate-reducing bacterium. Int J Syst Bacteriol 48(Pt4):1383–1387
Harmsen M, Yang L, Pamp SJ, Tolker-Nielsen T (2011) An update on Pseudomonas aeruginosa biofilm formation, tolerance, and dispersal. FEMS Immunol Med Microbiol 59:253–268
Hartsock A, Shapleigh JP (2010) Identification, functional studies, and genomic comparisons of new members of the NnrR regulon in Rhodobacter sphaeroides. J Bacteriol 192:903–911
Hartsock A, Shapleigh JP (2011) Physiological roles for two periplasmic nitrate reductases in Rhodobacter sphaeroides 2.4.3 (ATCC 17025). J Bacteriol 193:6483–6489
Hassett DJ, Cuppoletti J, Trapnell B, Lymar SV, Rowe JJ, Yoon SS, Hilliard GM, Parvatiyar K, Kamani MC, Wozniak DJ, Hwang SH, McDermott TR, Ochsner UA (2002) Anaerobic metabolism and quorum sensing by Pseudomonas aeruginosa biofilms in chronically infected cystic fibrosis airways: rethinking antibiotic treatment strategies and drug targets. Adv Drug Deliv Rev 54:1425–1443
Henry S, Bru D, Stres B, Hallet S, Philippot L (2006) Quantitative detection of the nosZ gene, encoding nitrous oxide reductase, and comparison of the abundances of 16S rRNA, narG, nirK, and nosZ genes in soils. Appl Environ Microbiol 72:5181–5189
Heurlier K, Thomson MJ, Aziz N, Moir JW (2008) The nitric oxide (NO)-sensing repressor NsrR of Neisseria meningitidis has a compact regulon of genes involved in NO synthesis and detoxification. J Bacteriol 190:2488–2495
Hino T, Matsumoto Y, Nagano S, Sugimoto H, Fukumori Y, Murata T, Iwata S, Shiro Y (2010) Structural basis of biological N2O generation by bacterial nitric oxide reductase. Science 330:1666–1670
Ichiki H, Tanaka Y, Mochizuki K, Yoshimatsu K, Sakurai T, Fujiwara T (2001) Purification, characterization, and genetic analysis of Cu-containing dissimilatory nitrite reductase from a denitrifying halophilic archaeon, Haloarcula marismortui. J Bacteriol 183:4149–4156
Inatomi KI, Hochstein LI (1996) The purification and properties of a copper nitrite reductase from Haloferax denitrificans. Curr Microbiol 32:72–76
Iwata S, Ostermeier C, Ludwig B, Michel H (1995) Structure at 2.8 Å resolution of cytochrome c oxidase from Paracoccus denitrificans. Nature 376:660–669
Jacobson F, Guo H, Olesen K, Okvist M, Neutze R, Sjolin L (2005) Structures of the oxidized and reduced forms of nitrite reductase from Rhodobacter sphaeroides 2.4.3 at high pH: changes in the interactions of the type 2 copper. Acta Crystallogr D Biol Crystallogr 61:1190–1198
Jain R, Shapleigh JP (2001) Characterization of nirV and a gene encoding a novel pseudoazurin in Rhodobacter sphaeroides 2.4.3. Microbiology 147:2505–2515
Jeon CO, Park W, Padmanabhan P, DeRito C, Snape JR, Madsen EL (2003) Discovery of a bacterium, with distinctive dioxygenase, that is responsible for in situ biodegradation in contaminated sediment. Proc Natl Acad Sci USA 100:13591–13596
Jones CM, Hallin S (2010) Ecological and evolutionary factors underlying global and local assembly of denitrifier communities. ISME J 4:633–641
Jones CM, Welsh A, Throback IN, Dorsch P, Bakken LR, Hallin S (2011) Phenotypic and genotypic heterogeneity among closely related soil-borne N2 and N2O-producing Bacillus isolates harboring the nosZ gene. FEMS Microbiol Ecol 76:541–552
Kampschreur MJ, Picioreanu C, Tan N, Kleerebezem R, Jetten MS, van Loosdrecht MC (2007) Unraveling the source of nitric oxide emission during nitrification. Water Environ Res 79:2499–2509
Kaneko T, Sato S, Kotani H, Tanaka A, Asamizu E, Nakamura Y, Miyajima N, Hirosawa M, Sugiura M, Sasamoto S, Kimura T, Hosouchi T, Matsuno A, Muraki A, Nakazaki N, Naruo K, Okumura S, Shimpo S, Takeuchi C, Wada T, Watanabe A, Yamada M, Yasuda M, Tabata S (1996) Sequence analysis of the genome of the unicellular cyanobacterium Synechocystis sp. strain PCC6803. II. Sequence determination of the entire genome and assignment of potential protein-coding regions (supplement). DNA Res 3:185–209
Kaplan S, Eraso J, Roh JH (2005) Interacting regulatory networks in the facultative photosynthetic bacterium, Rhodobacter sphaeroides 2.4.1. Biochem Soc Trans 33:51–55
Keil D, Meyer A, Berner D, Poll C, Schutzenmeister A, Piepho HP, Vlasenko A, Philippot L, Schloter M, Kandeler E, Marhan S (2011) Influence of land-use intensity on the spatial distribution of N-cycling microorganisms in grassland soils. FEMS Microbiol Ecol 77:95–106
Kern M, Simon J (2008) Characterization of the NapGH quinol dehydrogenase complex involved in Wolinella succinogenes nitrate respiration. Mol Microbiol 69:1137–1152
Kim YJ, Ko IJ, Lee JM, Kang HY, Kim YM, Kaplan S, Oh JI (2007) Dominant role of the cbb 3 oxidase in regulation of photosynthesis gene expression through the PrrBA system in Rhodobacter sphaeroides 2.4.1. J Bacteriol 189:5617–5625
Kim M, Jeong SY, Yoon SJ, Cho SJ, Kim YH, Kim MJ, Ryu EY, Lee SJ (2008) Aerobic denitrification of Pseudomonas putida AD-21 at different C/N ratios. J Biosci Bioeng 106:498–502
Kim SW, Fushinobu S, Zhou S, Wakagi T, Shoun H (2009) Eukaryotic nirK genes encoding copper-containing nitrite reductase: originating from the protomitochondrion? Appl Environ Microbiol 75:2652–2658
Kong HH (2011) Skin microbiome: genomics-based insights into the diversity and role of skin microbes. Trends Mol Med 17:320–328
Korner H, Zumft WG (1989) Expression of denitrification enzymes in response to the dissolved oxygen and respiratory substrate in continuous culture of Pseudomonas stutzeri. Appl Environ Microbiol 55:1670–1676
Korner H, Sofia HJ, Zumft WG (2003) Phylogeny of the bacterial superfamily of Crp-Fnr transcription regulators: exploiting the metabolic spectrum by controlling alternative gene programs. FEMS Microbiol Rev 27:559–592
Kwiatkowski A, Shapleigh JP (1996) Requirement of nitric oxide for induction of genes whose products are involved in nitric oxide metabolism in Rhodobacter sphaeroides 2.4.3. J Biol Chem 271:24382–24388
Lam Y, Nicholas DJD (1969) A nitrite reductase with cytochrome oxidase activity from Paracoccus denitrificans. Biochim Biophys Acta 180:459–472
Laratta WP, Choi PS, Tosques IE, Shapleigh JP (2002) Involvement of the PrrB/PrrA two-component system in nitrite respiration in Rhodobacter sphaeroides 2.4.3: evidence for transcriptional regulation. J Bacteriol 184:3521–3529
Laver JR, Stevanin TM, Messenger SL, Lunn AD, Lee ME, Moir JW, Poole RK, Read RC (2010) Bacterial nitric oxide detoxification prevents host cell S-nitrosothiol formation: a novel mechanism of bacterial pathogenesis. FASEB J 24:286–295
Lim SK, Kim SJ, Cha SH, Oh YK, Rhee HJ, Kim MS, Lee JK (2009) Complete genome sequence of Rhodobacter sphaeroides KD131. J Bacteriol 191:1118–1119
Liu B, Morkved PT, Frostegård Å, Bakken LR (2010) Denitrification gene pools, transcription and kinetics of NO, N2O and N2 production as affected by soil pH. FEMS Microbiol Ecol 72:407–417
Lovley DR, Phillips EJ (1988) Novel mode of microbial energy metabolism: organic carbon oxidation coupled to dissimilatory reduction of iron or manganese. Appl Environ Microbiol 54:1472–1480
Lundberg JO, Govoni M (2004) Inorganic nitrate is a possible source for systemic generation of nitric oxide. Free Radic Biol Med 37:395–400
Lundberg JO, Weitzberg E, Cole JA, Benjamin N (2004) Nitrate, bacteria and human health. Nat Rev Microbiol 2:593–602
Mackenzie C, Choudhary M, Larimer FW, Predki PF, Stilwagen S, Armitage JP, Barber RD, Donohue TJ, Hosler JP, Newman JE, Shapleigh JP, Sockett RE, Zeilstra-Ryalls J, Kaplan S (2001) The home stretch, a first analysis of the nearly completed genome of Rhodobacter sphaeroides 2.4.1. Photosyn Res 70:19–41
MacPherson IS, Murphy ME (2007) Type-2 copper-containing enzymes. Cell Mol Life Sci 64:2887–2899
Mahne I, Tiedje JM (1995) Criteria and methodology for identifying respiratory denitrifiers. Appl Environ Microbiol 61:1110–1115
Mancinelli RL, Hochstein LI (1986) The occurrence of denitrification in extremely halophilic bacteria. FEMS Microbiol Lett 35:55–58
Martinez-Espinosa RM, Cole JA, Richardson DJ, Watmough NJ (2011) Enzymology and ecology of the nitrogen cycle. Biochem Soc Trans 39:175–178
Matsubara T, Zumft WG (1982) Identification of a copper protein as part of the nitrous oxide-reducing system in nitrite respiring (denitrifying) pseudomonads. Arch Microbiol 132:322–328
McClain ME, Boyer EW, Dent CL, Gergel SE, Grimm NB, Groffman PM, Hart SC, Harvey JW, Johnston CA, Mayorga E, McDowell WH, Pinay G (2003) Biogeochemical hot spots and hot moments at the interface of terrestrial and aquatic ecosystems. Ecosystems 6:301–312
McEwan AG, Jackson JB, Ferguson SJ (1984) Rationalisation of properties of nitrate reductases in Rhodopseudomonas capsulata. Arch Microbiol 137:344–349
Medigue C, Krin E, Pascal G, Barbe V, Bernsel A, Bertin PN, Cheung F, Cruveiller S, D'Amico S, Duilio A, Fang G, Feller G, Ho C, Mangenot S, Marino G, Nilsson J, Parrilli E, Rocha EP, Rouy Z, Sekowska A, Tutino ML, Vallenet D, von Heijne G, Danchin A (2005) Coping with cold: the genome of the versatile marine Antarctica bacterium Pseudoalteromonas haloplanktis TAC125. Genome Res 15:1325–1335
Mellies J, Jose J, Meyer TF (1997) The Neisseria gonorrhoeae gene aniA encodes an inducible nitrite reductase. Mol Gen Genet 256:525–532
Mesa S, Velasco L, Manzanera ME, Delgado MJ, Bedmar EJ (2002) Characterization of the norCBQD genes, encoding nitric oxide reductase, in the nitrogen fixing bacterium Bradyrhizobium japonicum. Microbiology 148:3553–3560
Mesa S, Hauser F, Friberg M, Malaguti E, Fischer HM, Hennecke H (2008) Comprehensive assessment of the regulons controlled by the FixLJ-FixK2-FixK1 cascade in Bradyrhizobium japonicum. J Bacteriol 190:6568–6579
Michalski W, Nicholas DJD (1988) Identification of two new denitrifying strains of Rhodobacter sphaeroides. FEMS Microbiol Lett 52:239–244
Mowbray M, McLintock S, Weerakoon R, Lomatschinsky N, Jones S, Rossi AG, Weller RB (2009) Enzyme-independent NO stores in human skin: quantification and influence of UV radiation. J Invest Dermatol 129:834–842
Nakagawa S, Takai K, Inagaki F, Horikoshi K, Sako Y (2005) Nitratiruptor tergarcus gen. nov., sp. nov. and Nitratifractor salsuginis gen. nov., sp. nov., nitrate-reducing chemolithoautotrophs of the epsilon-Proteobacteria isolated from a deep-sea hydrothermal system in the Mid-Okinawa trough. Int J Syst Evol Microbiol 55:925–933
Nakagawa S, Takaki Y, Shimamura S, Reysenbach AL, Takai K, Horikoshi K (2007) Deep-sea vent epsilon-proteobacterial genomes provide insights into emergence of pathogens. Proc Natl Acad Sci USA 104:12146–12150
Nakanishi Y, Zhou S, Kim SW, Fushinobu S, Maruyama J, Kitamoto K, Wakagi T, Shoun H (2010) A eukaryotic copper-containing nitrite reductase derived from a NirK homolog gene of Aspergillus oryzae. Biosci Biotechnol Biochem 74:984–991
Neidhardt FC, Ingraham JL, Schaechter M (1990) Physiology of the bacterial cell: a molecular approach. Sinauer Associates, Sunderland
Nojiri M, Xie Y, Inoue T, Yamamoto T, Matsumura H, Kataoka K, Deligeer K, Yamaguchi YK, Suzuki S (2007) Structure and function of a hexameric copper-containing nitrite reductase. Proc Natl Acad Sci USA 104:4315–4320
Nojiri M, Koteishi H, Nakagami T, Kobayashi K, Inoue T, Yamaguchi K, Suzuki S (2009a) Structural basis of inter-protein electron transfer for nitrite reduction in denitrification. Nature 462:117–120
Nojiri M, Shirota F, Hira D, Suzuki S (2009b) Expression, purification, crystallization and preliminary X-ray diffraction analysis of the soluble domain of PPA0092, a putative nitrite reductase from Propionibacterium acnes. Acta Crystallogr Sect F Struct Biol Cryst Commun 65:123–127
Nurizzo D, Silvestrini MC, Mathieu M, Cutruzzola F, Bourgeois D, Fulop V, Hajdu J, Brunori M, Tegoni M, Cambillau C (1997) N-terminal arm exchange is observed in the 2.15 Å crystal structure of oxidized nitrite reductase from Pseudomonas aeruginosa. Structure 15:1157–1171
Parales RE, Parales JV, Pelletier DA, Ditty JL (2008) Diversity of microbial toluene degradation pathways. Adv Appl Microbiol 64:1–73
Paraskevopoulos K, Antonyuk SV, Sawers RG, Eady RR, Hasnain SS (2006) Insight into catalysis of nitrous oxide reductase from high-resolution structures of resting and inhibitor-bound enzyme from Achromobacter cycloclastes. J Mol Biol 362:55–65
Payne WJ (1981) Denitrification. Wiley, New York
Payne WJ, Grant MA, Shapleigh JP, Hoffman P (1982) Nitrogen oxide reduction in Wolinella succinogenes and Campylobacter species. J Bacteriol 152:915–918
Philippot L, Cuhel J, Saby NP, Cheneby D, Chronakova A, Bru D, Arrouays D, Martin-Laurent F, Simek M (2009) Mapping field-scale spatial patterns of size and activity of the denitrifier community. Environ Microbiol 11:1518–1526
Picardeau M, Bulach DM, Bouchier C, Zuerner RL, Zidane N, Wilson PJ, Creno S, Kuczek ES, Bommezzadri S, Davis JC, McGrath A, Johnson MJ, Boursaux-Eude C, Seemann T, Rouy Z, Coppel RL, Rood JI, Lajus A, Davies JK, Medigue C, Adler B (2008) Genome sequence of the saprophyte Leptospira biflexa provides insights into the evolution of Leptospira and the pathogenesis of leptospirosis. PLoS One 3:e1607
Pukall R, Gehrich-Schroter G, Lapidus A, Nolan M, Glavina Del Rio T, Lucas S, Chen F, Tice H, Pitluck S, Cheng JF, Copeland A, Saunders E, Brettin T, Detter JC, Bruce D, Goodwin L, Pati A, Ivanova N, Mavromatis K, Ovchinnikova G, Chen A, Palaniappan K, Land M, Hauser L, Chang YJ, Jeffries CD, Chain P, Goker M, Bristow J, Eisen JA, Markowitz V, Hugenholtz P, Kyrpides NC, Klenk HP, Han C (2009) Complete genome sequence of Jonesia denitrificans type strain (Prevot 55134). Stand Genomic Sci 1:262–269
Ravishankara AR, Daniel JS, Portmann RW (2009) Nitrous oxide (N2O): the dominant ozone-depleting substance emitted in the 21st century. Science 326:123–125
Rendulic S, Jagtap P, Rosinus A, Eppinger M, Baar C, Lanz C, Keller H, Lambert C, Evans KJ, Goesmann A, Meyer F, Sockett RE, Schuster SC (2004) A predator unmasked: life cycle of Bdellovibrio bacteriovorus from a genomic perspective. Science 303:689–692
Rhee SK, Jeon CO, Bae JW, Kim K, Song JJ, Kim JJ, Lee SG, Kim HI, Hong SP, Choi YH, Kim SM, Sung MH (2002) Characterization of Symbiobacterium toebii, an obligate commensal thermophile isolated from compost. Extremophiles 6:57–64
Richardson DJ, Berks BC, Russell DA, Spiro S, Taylor CJ (2001) Functional, biochemical and genetic diversity of prokaryotic nitrate reductases. Cell Mol Life Sci 58:165–178
Richardson D, Felgate H, Watmough N, Thomson A, Baggs E (2009) Mitigating release of the potent greenhouse gas N2O from the nitrogen cycle – could enzymic regulation hold the key? Trends Biotechnol 27:388–397
Rinaldo S, Giardina G, Castiglione N, Stelitano V, Cutruzzola F (2011) The catalytic mechanism of Pseudomonas aeruginosa cd 1 nitrite reductase. Biochem Soc Trans 39:195–200
Risgaard-Petersen N, Langezaal AM, Ingvardsen S, Schmid MC, Jetten MS, Op den Camp HJ, Derksen JW, Pina-Ochoa E, Eriksson SP, Nielsen LP, Revsbech NP, Cedhagen T, van der Zwaan GJ (2006) Evidence for complete denitrification in a benthic foraminifer. Nature 443:93–96
Rodionov DA, Dubchak IL, Arkin AP, Alm EJ, Gelfand MS (2005) Dissimilatory metabolism of nitrogen oxides in bacteria: comparative reconstruction of transcriptional networks. PLoS Comput Biol 1:e55
Roop RM 2nd, Gaines JM, Anderson ES, Caswell CC, Martin DW (2009) Survival of the fittest: how Brucella strains adapt to their intracellular niche in the host. Med Microbiol Immunol 198:221–238
Sanford RA, Cole JR, Tiedje JM (2002) Characterization and description of Anaeromyxobacter dehalogenans gen. nov., sp. nov., an aryl-halorespiring facultative anaerobic myxobacterium. Appl Environ Microbiol 68:893–900
Saraste M, Castresana J (1994) Cytochrome oxidase evolved by tinkering with denitrification enzymes. FEBS Lett 341:1–4
Sawada E, Satoh T (1980) Periplasmic location of dissimilatory nitrate and nitrite reductases in a denitrifying phototrophic bacterium, Rhodopseudomonas sphaeroides forma sp. denitrificans. Plant Cell Physiol 21:205–210
Schlag S, Fuchs S, Nerz C, Gaupp R, Engelmann S, Liebeke M, Lalk M, Hecker M, Gotz F (2008) Characterization of the oxygen-responsive NreABC regulon of Staphylococcus aureus. J Bacteriol 190:7847–7858
Schwarz G, Mendel RR, Ribbe MW (2009) Molybdenum cofactors, enzymes and pathways. Nature 460:839–847
Sears HJ, Sawers G, Berks BC, Ferguson SJ, Richardson DJ (2000) Control of periplasmic nitrate reductase gene expression (napEDABC) from Paracoccus pantotrophus in response to oxygen and carbon substrates. Microbiology 146:2977–2985
Shapleigh JP (2011) Oxygen control of nitrogen oxide respiration, focusing on alpha-proteobacteria. Biochem Soc Trans 39:179–183
Sievert SM, Scott KM, Klotz MG, Chain PS, Hauser LJ, Hemp J, Hugler M, Land M, Lapidus A, Larimer FW, Lucas S, Malfatti SA, Meyer F, Paulsen IT, Ren Q, Simon J (2008) Genome of the epsilonproteobacterial chemolithoautotroph Sulfurimonas denitrificans. Appl Environ Microbiol 74:1145–1156
Simek M, Cooper JE (2002) The influence of soil pH on denitrification: progress towards the understanding of this interaction over the last 50 years. Eur J Soil Sci 53:345–354
Simon J (2002) Enzymology and bioenergetics of respiratory nitrite ammonification. FEMS Microbiol Rev 26:285–309
Simon J, Einsle O, Kroneck PM, Zumft WG (2004) The unprecedented nos gene cluster of Wolinella succinogenes encodes a novel respiratory electron transfer pathway to cytochrome c nitrous oxide reductase. FEBS Lett 569:7–12
Stevanin TM, Laver JR, Poole RK, Moir JW, Read RC (2007) Metabolism of nitric oxide by Neisseria meningitidis modifies release of NO-regulated cytokines and chemokines by human macrophages. Microbes Infect 9:981–987
Strohm TO, Griffin B, Zumft WG, Schink B (2007) Growth yields in bacterial denitrification and nitrate ammonification. Appl Environ Microbiol 73:1420–1424
Suharti, Heering HA, de Vries S (2004) NO reductase from Bacillus azotoformans is a bifunctional enzyme accepting electrons from menaquinol and a specific endogenous membrane-bound cytochrome c 551. Biochemistry 43:13487–13495
Suharti, Strampraad MJF, Schroder I, de VS (2001) A novel copper A containing menaquinol NO reductase from Bacillus azotoformans. Biochemistry 40:2632–2639
Swem LR, Elsen S, Bird TH, Swem DL, Koch HG, Myllykallio H, Daldal F, Bauer CE (2001) The RegB/RegA two-component regulatory system controls synthesis of photosynthesis and respiratory electron transfer components in Rhodobacter capsulatus. J Mol Biol 309:121–138
Tabata A, Yamamoto I, Matsuzaki M, Satoh T (2005) Differential regulation of periplasmic nitrate reductase gene (napKEFDABC) expression between aerobiosis and anaerobiosis with nitrate in a denitrifying phototroph Rhodobacter sphaeroides f. sp. denitrificans. Arch Microbiol 184:108–116
Tavares P, Pereira AS, Moura JJ, Moura I (2006) Metalloenzymes of the denitrification pathway. J Inorg Biochem 100:2087–2100
Thomsen JK, Geest T, Cox RP (1994) Mass-spectrometric studies of the effect of pH on the accumulation of intermediates in denitrification by Paracoccus denitrificans. Appl Environ Microbiol 60:536–541
Tocheva EI, Rosell FI, Mauk AG, Murphy ME (2004) Side-on copper-nitrosyl coordination by nitrite reductase. Science 304:867–870
Toffanin A, Wu Q, Maskus M, Casella S, Abruña HD, Shapleigh JP (1996) Characterization of the gene encoding nitrite reductase and the physiological consequences of its expression in the nondenitrifying Rhizobium “hedysari” strain HCNT1. Appl Environ Microbiol 62:4019–4025
Torres MJ, Bueno E, Mesa S, Bedmar EJ, Delgado MJ (2011) Emerging complexity in the denitrification regulatory network of Bradyrhizobium japonicum. Biochem Soc Trans 39:284–288
Tosques IE, Kwiatkowski AV, Shi J, Shapleigh JP (1997) Characterization and regulation of the gene encoding nitrite reductase in Rhodobacter sphaeroides 2.4.3. J Bacteriol 179:1090–1095
Toyofuku M, Nomura N, Fujii T, Takaya N, Maseda H, Sawada I, Nakajima T, Uchiyama H (2007) Quorum sensing regulates denitrification in Pseudomonas aeruginosa PAO1. J Bacteriol 189:4969–4972
Tunbridge AJ, Stevanin TM, Lee M, Marriott HM, Moir JW, Read RC, Dockrell DH (2006) Inhibition of macrophage apoptosis by Neisseria meningitidis requires nitric oxide detoxification mechanisms. Infect Immun 74:729–733
Ueda K, Yamashita A, Ishikawa J, Shimada M, Watsuji TO, Morimura K, Ikeda H, Hattori M, Beppu T (2004) Genome sequence of Symbiobacterium thermophilum, an uncultivable bacterium that depends on microbial commensalism. Nucleic Acids Res 32:4937–4944
van den Heuvel RN, van der Biezen E, Jetten MS, Hefting MM, Kartal B (2010) Denitrification at pH 4 by a soil-derived Rhodanobacter-dominated community. Environ Microbiol 12:3264–3271
van der Oost J, de Boer APN, De Gier J-WL, Zumft WG, Stouthamer AH, van Spanning RJM (1994) The heme-copper oxidase family consists of three distinct types of oxidases and is related to nitric oxide reductase. FEMS Microbiol Lett 121:1–9
Van Spanning RJ, Houben E, Reijnders WN, Spiro S, Westerhoff HV, Saunders N (1999) Nitric oxide is a signal for NNR-mediated transcription activation in Paracoccus denitrificans. J Bacteriol 181:4129–4132
van Spanning R, Richardson D, Ferguson S (2007) Introduction to the biochemistry and molecular biology of denitrification. In: Bothe H, Ferguson S, Newton W (eds) Biology of the nitrogen cycle. Elsevier, Amsterdam, pp 3–21
Velasco L, Mesa S, Xu CA, Delgado MJ, Bedmar EJ (2004) Molecular characterization of nosRZDFYLX genes coding for denitrifying nitrous oxide reductase of Bradyrhizobium japonicum. Antonie Van Leeuwenhoek 85:229–235
Verbaendert I, Boon N, De Vos P, Heylen K (2011) Denitrification is a common feature among members of the genus Bacillus. Syst Appl Microbiol 34:385–391
Vidon P, Allan C, Burns D, Duval TP, Gurwick N, Inamdar S, Lowrance R, Okay J, Scott D, Sebestyen S (2010) Hot spots and hot moments in riparian zones: potential for improved water quality management. J Am Water Resour Assoc 46:278–298
Volkl P, Huber R, Drobner E, Rachel R, Burggraf S, Trincone A, Stetter KO (1993) Pyrobaculum aerophilum sp. nov., a novel nitrate-reducing hyperthermophilic archaeum. Appl Environ Microbiol 59:2918–2926
Vollack K, Zumft W (2001) Nitric oxide signaling and transcriptional control of denitrification genes in Pseudomonas stutzeri. J Bacteriol 183:2516–2526
Volland S, Rachinger M, Strittmatter A, Daniel R, Gottschalk G, Meyer O (2011) Complete genome sequences of the chemolithoautotrophic strains Oligotropha carboxidovorans OM4 and OM5. J Bacteriol 193:5043
Vorholt JA, Hafenbradl D, Stetter KO, Thauer RK (1997) Pathways of autotrophic CO2 fixation and of dissimilatory nitrate reduction to N2O in Ferroglobus placidus. Arch Microbiol 167:19–23
Voskuil MI, Bartek IL, Visconti K, Schoolnik GK (2011) The response of Mycobacterium tuberculosis to reactive oxygen and nitrogen species. Front Microbiol 2:105
Walker CB, de la Torre JR, Klotz MG, Urakawa H, Pinel N, Arp DJ, Brochier-Armanet C, Chain PS, Chan PP, Gollabgir A, Hemp J, Hugler M, Karr EA, Konneke M, Shin M, Lawton TJ, Lowe T, Martens-Habbena W, Sayavedra-Soto LA, Lang D, Sievert SM, Rosenzweig AC, Manning G, Stahl DA (2010) Nitrosopumilus maritimus genome reveals unique mechanisms for nitrification and autotrophy in globally distributed marine crenarchaea. Proc Natl Acad Sci USA 107:8818–8823
Wan C, Yang X, Lee DJ, Du M, Wan F, Chen C (2011) Aerobic denitrification by novel isolated strain using NO2–N as nitrogen source. Bioresour Technol 102:7244–7248
Wattam AR, Williams KP, Snyder EE, Almeida NF Jr, Shukla M, Dickerman AW, Crasta OR, Kenyon R, Lu J, Shallom JM, Yoo H, Ficht TA, Tsolis RM, Munk C, Tapia R, Han CS, Detter JC, Bruce D, Brettin TS, Sobral BW, Boyle SM, Setubal JC (2009) Analysis of ten Brucella genomes reveals evidence for horizontal gene transfer despite a preferred intracellular lifestyle. J Bacteriol 191:3569–3579
Wharton DC, Gibson QC (1976) Cytochrome oxidase from Pseudomonas aeruginosa IV. Reaction with oxygen and carbon monoxide. Biochim Biophys Acta 292:611–620
Wu ML, Ettwig KF, Jetten MS, Strous M, Keltjens JT, van Niftrik L (2011) A new intra-aerobic metabolism in the nitrite-dependent anaerobic methane-oxidizing bacterium Candidatus “Methylomirabilis oxyfera”. Biochem Soc Trans 39:243–248
Wunsch P, Zumft WG (2005) Functional domains of NosR, a novel transmembrane iron-sulfur flavoprotein necessary for nitrous oxide respiration. J Bacteriol 187:1992–2001
Xie SG, Zhang XJ, Wang ZS (2003) Temperature effect on aerobic denitrification and nitrification. J Environ Sci (China) 15:669–673
Yagi JM, Sims D, Brettin T, Bruce D, Madsen EL (2009) The genome of Polaromonas naphthalenivorans strain CJ2, isolated from coal tar-contaminated sediment, reveals physiological and metabolic versatility and evolution through extensive horizontal gene transfer. Environ Microbiol 11:2253–2270
Ye RW, Arunakumari A, Averill BA, Tiedje JM (1992) Mutants of Pseudomonas fluorescens deficient in dissimilatory nitrite reduction are also altered in nitric oxide reduction. J Bacteriol 174:2560–2564
Yoshimatsu K, Sakurai T, Fujiwara T (2000) Purification and characterization of dissimilatory nitrate reductase from a denitrifying halophilic archaeon, Haloarcula marismortui. FEBS Lett 470:216–220
Yoshinari T (1980) N2O reduction by Vibrio succinogenes. Appl Environ Microbiol 39:81–84
Zumft WG (1997) Cell biology and molecular basis of denitrification. Microbiol Mol Biol Rev 61:533–616
Zumft WG, Kroneck PM (2006) Respiratory transformation of nitrous oxide (N2O) to dinitrogen by bacteria and archaea. Adv Microb Physiol 52:107–227
Zumft WG, Matsubara T (1982) A novel kind of multi-copper protein as terminal oxidoreductase of nitrous oxide respiration in Pseudomonas perfectomarinus. FEBS Lett 148:107–112
Zumft WG, Braun C, Cuypers H (1994) Nitric oxide reductase from Pseudomonas stutzeri: primary structure and gene organization of a novel bacterial cytochrome bc complex. Eur J Biochem 219:481–490
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2013 Springer-Verlag Berlin Heidelberg
About this entry
Cite this entry
Shapleigh, J.P. (2013). Denitrifying Prokaryotes. In: Rosenberg, E., DeLong, E.F., Lory, S., Stackebrandt, E., Thompson, F. (eds) The Prokaryotes. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-642-30141-4_71
Download citation
DOI: https://doi.org/10.1007/978-3-642-30141-4_71
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-642-30140-7
Online ISBN: 978-3-642-30141-4
eBook Packages: Biomedical and Life SciencesReference Module Biomedical and Life Sciences