Abstract
Cardiovascular disease is characterized by enhanced oxidative stress leading to low-grade inflammation in the vascular wall, heart, kidney, and brain. Epidemiological cohort studies have suggested that antioxidants such as vitamins C and E and α- and β-carotene may be useful in cardiovascular and cancer prevention. Interventional trials using antioxidants, however, have provided mixed results, with some small trials demonstrating benefit, which could not be confirmed in larger trials, some of which even showed deleterious consequences. It will be necessary to further investigate cellular effects of antioxidants, which can also have prooxidant actions that counterbalance their antioxidant effects. Angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, and mineralocorticoid receptor blockers reduce the generation of reactive oxygen species in experimental models and in humans and have been proven to produce beneficial cardiovascular effects. Polyphenols and other antioxidants present in foods and beverages may as well have cardiovascular protective actions. More studies are therefore necessary in order to develop antioxidants that will indeed be able to buffer the oxidative stress in cardiovascular tissues and accordingly result in improved cardiovascular outcomes.
Access provided by Autonomous University of Puebla. Download reference work entry PDF
Similar content being viewed by others
Keywords
- Antioxidants
- Hydrogen peroxide
- NADPH oxidase
- Nitric oxide synthase
- Oxidative stress
- Oxygen free radicals
- Superoxide dismutase
- Superoxide
Introduction
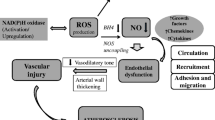
Production of oxygen free radicals or reactive oxygen species (ROS) and resultant oxidative stress is enhanced in cardiovascular tissues in hypertension, atherosclerosis, coronary artery disease (CAD), and other forms of cardiovascular disease. ROS generation plays a role in the mechanisms of cardiovascular injury, in part by triggering low-grade inflammation. ROS involved in induction of oxidative stress in the cardiovascular system include superoxide (O2 •−), hydrogen peroxide (H2O2), hydroxyl anion (OH•−), hypochlorous acid (HOCl) and the reactive nitrogen species (RNS), nitric oxide (NO), and peroxynitrite (ONOO−). ROS and RNS are usually highly regulated and function as part of the intracellular signaling mechanisms of cells (Harrison 1997; Datla and Griendling 2010). In hypertension, atherosclerosis, CAD, heart failure, diabetes, and other conditions associated with vascular disease, increased ROS production leads to endothelial dysfunction, enhanced contractility and growth of vascular smooth muscle cells, lipid peroxidation, inflammation, and increased deposition of extracellular matrix proteins and results in increases in markers of systemic oxidative stress as found in both experimental and human hypertension (Romero and Reckelhoff 1999; Touyz and Schiffrin 2004).
Antioxidant treatment or superoxide dismutase mimetics lower blood pressure (BP) and improve vascular structure and function in experimental hypertension when given parenterally or orally and by oral administration in human hypertension (Chen et al. 2001; Hoagland et al. 2003). If the genes of ROS-generating enzymes (e.g., NOX1 or NOX2) are deleted in mice, BP response to angiotensin II (AngII) infusion is lower (Touyz and Schiffrin 2004). The ability to counter oxidant stress by the antioxidant mechanisms available in tissues is reduced in hypertension in both rodents and humans, resulting in enhanced bioavailability of ROS, and consequently reduced bioavailability of NO, which is converted into ONOO−, leading to protein nitration and dysfunction. Accordingly, antioxidants should benefit patients with cardiovascular disease such as hypertension, atherosclerosis, CAD, or diabetes by reducing oxidative stress and cardiovascular injury, with the resulting improvement in outcomes.
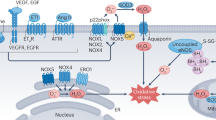
ROS are produced by different enzyme systems, the major one in the vascular wall being NADPH oxidase (Fig. 51.1). However, under some circumstances, other sources of ROS such as uncoupled nitric oxide synthase (NOS), the mitochondrial electron transport chain, xanthine oxidase, cyclooxygenase, lipoxygenase, heme oxygenase, and cytochrome P450 monooxygenase may become very important contributors to oxidative stress in the cardiovascular system, particularly uncoupled NOS and mitochondrial electron transport. Reduction of antioxidant mechanisms with failure to quench ROS generated in the vascular wall will also promote oxidative stress and participates in cardiovascular and renal oxidative injury in hypertension. Among the antioxidant defenses available, reduction of superoxide dismutase, catalase and glutathione (GSH) peroxidase activity have been reported in hypertensive patients (Chrissobolis et al. 2008; Ebrahimian and Touyz 2008; Wilcox and Pearlman 2008). Antioxidants should therefore be able to reduce the oxidative stress present in cardiovascular disease and produce beneficial results with respect to events and outcomes (Park et al. 2002; Welch et al. 2006).
Diagram shows the sources of vascular reactive oxygen species (ROS), of which the most important is NADPH oxidase. Four different Noxes have been identified in the vasculature, of which Nox5 is present only in humans. Superoxide (O2 •−) is dismutated to hydrogen peroxide by superoxide dismutase (SOD) and, in turn under the action of catalase or glutathione peroxidase, produces water and oxygen. NADPH reduced nicotinamide adenine dinucleotide, eNOS endothelial nitric oxide synthase
What Are the Molecular Targets of Reactive Oxygen Species in Vascular Cells?
Physiologically, ROS are mediators in many signaling pathways together with redox-sensitive molecules such as transcription factors such as nuclear factor (NF)-κB, activator protein (AP)-1 and hypoxia-inducible factor (HIF)-1, protein tyrosine phosphatases, protein tyrosine kinases, mitogen-activated protein (MAP) kinases, and ion channels (Muller et al. 2000; Tabet et al. 2008). Pathophysiological levels of ROS in cardiovascular diseases such as in hypertension will be associated with inflammation, fibrosis, and alterations in matrix metalloproteinases (MMP). These pathophysiological changes will lead to remodeling of blood vessels and endothelial dysfunction, the latter resulting in part from uncoupling of NOS which leads to production of ROS rather than NO by eNOS. Elevated ROS also decrease the bioavailability of any NO still being generated, as reaction with superoxide anion produces ONOO−.
Oxidative Stress and Clinical Hypertension
Oxidative stress has been reported to be increased in some but not all studies in patients with primary or essential hypertension, in renovascular and in malignant hypertension, salt-sensitive hypertension, cyclosporine-induced hypertension, and preeclampsia (Higashi et al. 2002; Lee et al. 2003; Fortuno et al. 2004). It has been mostly through measurement of plasma thiobarbituric acid-reactive substances (TBARS) and plasma and urinary 8-epi-isoprostanes, biomarkers of lipid peroxidation and oxidative stress, that has made it possible to support the hypothesis that there is increased oxidative stress in hypertension. Plasma H2O2 has also been found to be higher in hypertensive than in normotensive subjects (Lacy et al. 2000), and in normotensive subjects, a family history of hypertension has been associated with elevated H2O2 plasma levels, which indicates that this finding may have a genetic basis (Lacy et al. 1998). Increases in superoxide anion generation by polymorphonuclear leukocytes and platelets have also been reported in hypertensive patients.
Asymmetric dimethylarginine (ADMA), which is an inhibitor of eNOS, and 13-hydroxyoctadecadienoic acid, a marker of ROS production, are also increased in hypertension, and microvessels from patients with the highest levels of these agents are least responsive to NO-mediated relaxation (Wang et al. 2009). ROS production is also increased in vascular smooth muscle cells from resistance arteries of hypertensive patients, associated to enhanced activity of vascular NADPH oxidase (Touyz and Schiffrin 2001). A number of polymorphisms occur in the gene of NADPH oxidase in atherosclerosis and hypertension (Kokubo et al. 2005). Interestingly, patients with chronic granulomatous disease, who are deficient in the NOX2 isoform, do not have lower BP.
A reduction in superoxide dismutase, glutathione peroxidase, or catalase, which function as antioxidant defenses, has been found in hypertension (Chen et al. 2002), and superoxide dismutase activity correlates inversely with BP in hypertension (Chen et al. 2002; Mullan et al. 2002). In patients with diabetes, a reduction of superoxide dismutase activity also correlates inversely with BP and stiffness of the aorta have not been found in either in healthy subjects, preeclampsia, or in postmenopausal women (Mullan et al. 2002; Zureik et al. 2004; Xu et al. 2010).
Atherosclerosis
Oxidative stress promotes the oxidation of lipids and proteins in the vascular wall and the proliferation and migration of vascular smooth muscle cells from the media of large vessels to the intima (Ross 1999; Griendling and Harrison 1999). These effects contribute to the pathogenesis of atherosclerosis. Endothelial dysfunction, which is linked to excessive ROS generation, is a precursor of atherosclerosis. Most cardiovascular risk factors such as aging, smoking, hypertension, hypercholesterolemia, and diabetes mellitus increase ROS generation and lead to progression of atherosclerosis.
The renin-angiotensin system (RAS) plays an important role in the redox-sensitive pathogenesis of atherosclerosis. The type 1 receptor of angiotensin (AT1) is upregulated in atheromatous plaques (Nickenig and Harrison 2002). AngII via the AT1 receptor activates superoxide generation by NADPH oxidase and inflammation. Enhanced ROS leads to upregulation of LOX-1, the human endothelial receptor for oxidized low-density lipoprotein (LDL) (Morawietz et al. 1999); uptake of oxidized LDL by macrophages (Keidar and Attias 1997); and progression of atherosclerosis in apoE-deficient mice (Weiss et al. 2001). Matrix metalloproteinases (MMPs) are stimulated by AngII (Brassard et al. 2005), contributing to the progression of plaque formation, making as well the plaques vulnerable and unstable (Rajagopalan et al. 1996). ROS promotes angiogenesis of atheroma (Khatri et al. 2004), which has been demonstrated to lead to progression of plaques. Accordingly, blockade of the RAS with angiotensin I-converting enzyme inhibitors (ACEIs) or AT1 angiotensin receptor blockers (ARBs) has been touted to exert vascular protective effects not only on smaller vessels but also on large vessel disease and atherosclerosis (Yusuf et al. 2000b), in part by inhibiting ROS generation. However, ACEIs may be less effective if patients are already treated with statins, acetylsalicylic acid, and antioxidants, and thus oxidant systems are already controlled, which could be a reason for the lack of efficacy compared to control subjects, for example, in the Prevention of Events with Angiotensin-Converting Enzyme Inhibition (PEACE) trial, a randomized, double-blind, placebo-controlled study in which 8,290 patients were assigned to receive either trandolapril 4 mg/day or placebo (PEACE Trial Investigators, 2004). The study was designed to test the hypothesis that patients with stable coronary artery disease but normal or slightly reduced left ventricular function could derive benefit from the addition of ACEIs to conventional therapy. After 4.8 years, the incidence of a composite endpoint of cardiovascular mortality, nonfatal myocardial infarction, and coronary revascularization was nearly identical in the two study groups.
Enhanced expression of the p22phox subunit of NADPH oxidase in mice is associated with progression of vascular injury (Datla and Griendling 2010). Increased H2O2 and vascular endothelial growth factor (VEGF) are found when NADPH oxidase activity is enhanced, likely reflecting increased expression of HIF-1α induced by oxidative stress. Neovascularization occurs in atheroma and plays a role in atherosclerosis progression and plaque instability (Tenaglia et al. 1998). Indeed, the p47phox subunit of NADPH oxidase and H2O2 appear to participate critically in the development and progression of atherosclerotic plaques in hypercholesterolemic mice through the induction of this angiogenic switch (Barry-Lane et al. 2001).
ROS generation by NADPH oxidase has been demonstrated in human atherectomy specimens (Azumi and Inoue 2002). Particular catalytic subunits of NADPH oxidase isoforms (i.e., Nox2 or gp91phox, Nox1, and Nox4) participate in a cell-specific manner in the increased oxidative stress that is found in human coronary atherosclerosis (Sorescu et al. 2002). ROS formation can be detected throughout the intima, media, and adventitia of segments of nonatherosclerotic coronary arteries from explanted human hearts. In atherosclerotic arteries, ROS generation is particularly increased in the plaque shoulder, which is a region rich in macrophages and α-actin-positive smooth muscle cells. Whereas the p22phox subunit of NADPH oxidase colocalizes with gp91phox mainly in macrophages, Nox4 is found in nonphagocytic vascular (α-actin-positive) smooth muscle cells (Sorescu et al. 2002). Levels of measurable mRNAs of gp91phox- and p22phox correlate with the severity of atherosclerosis.
As in the case of hypertension, NADPH oxidase is not the only source of ROS that exerts an influence on atherosclerosis progression. Xanthine oxidase, myeloperoxidase, and eNOS also play a role (McMillen et al. 2005). Mitochondrial dysfunction, which can be triggered by AngII, is important in the development of endothelial dysfunction and atherosclerosis. Mitochondria generate ROS and participate in the formation of vascular lesions (Puddu et al. 2009).
In contrast to mechanisms of ROS formation, protection from atherosclerosis depends on enzymes involved in quenching ROS such as superoxide dismutase and catalase. Aldose reductase is an enzyme present in macrophage-rich regions of atherosclerotic lesions of apoE-null mice proportionately to lesion progression (Srivastava et al. 2009). It exerts antioxidant actions as demonstrated by inhibition or gene deletion of the enzyme, which promotes lesion progression. Heme oxygenase (HO)-1, the rate-limiting enzyme in the catabolism of heme, also appears to exert anti-atherosclerotic and anti-inflammatory actions via generation of the antioxidant biliverdin, iron, and carbon monoxide. HO-1-deficient macrophages have higher levels of ROS and pro-inflammatory cytokines such as MCP-1 and IL-6 (Orozco et al. 2007) and exhibit enhanced foam cell formation when after exposure to oxidized LDL.
The lectin-like LOX-1 receptor binds oxidized LDL and mediates its uptake by endothelial cells. It is increased in diabetes, hypertension, and dyslipidemia, all conditions contributing to progression of atherosclerosis. Aortic atherosclerosis can be blunted if LOX-1 activity is knocked down in LDL receptor-deficient mice, which leads to upregulation of anti-inflammatory signals (Mehta et al. 2007).
The proteasome contributes to cell protection against oxidative stress, and chronic proteasome inhibition exacerbates coronary artery oxidative stress and early atherosclerosis in pigs (Herrmann et al. 2007). Senescence-associated α-galactosidase (SAαG) and p16 and p21 cyclin-dependent kinase inhibitors (CDKIs) are not found in healthy vessels (Matthews et al. 2006). Plaques and fibrous cap smooth muscle cells have shorter telomeres, which correlates with the severity and extension of atherosclerosis. Vascular senescence results from changes in cyclins D/E, p16, p21, and retinoblastoma protein (pRB) in the presence of low telomerase activity. Overexpressing telomerase will rescue plaque vascular cell senescence, despite telomere shortening, with normalization of CDKI and pRB. Oxidants induce premature senescence in vitro, with accelerated telomere shortening and reduced telomerase activity. Thus aging, telomerase deficiency, short telomeres, and ROS contribute to progression of atherosclerosis via oxidative stress-induced DNA damage.
Antioxidant Therapy and Cardiovascular Disease
Based on the data referred to above, there has been interest in targeting oxidative stress to prevent cardiovascular disease, including atherosclerosis and hypertension. Increasing antioxidant bioavailability (with diet or supplements) and reducing ROS generation (by inhibiting superoxide-generating enzymes) are two strategies that have been used to achieve this objective. Experimental, observational, and epidemiological studies in humans provide support for the idea that antioxidants have the ability to prevent or treat conditions associated with oxidative stress (Rimm et al. 1993; Stampfer et al. 1993; Stephens et al. 1996; Duffy et al. 1999; GISSI-Prevenzione Investigators 1999; Fotheby et al. 2000; Yusuf et al. 2000a; Brown and Hu 2001; Khaw et al. 2001; Boshtam et al. 2002; Chen et al. 2002; Heart Protection Study Collaborative Group 2002; Mullan et al. 2002; Shihabi et al. 2002; Salvemini and Cuzzocrea 2003; Vivekananthan et al. 2003; Roberts et al. 2010). However, there has been significant inconsistency between different studies. In fact, most large trials of antioxidants have been negative in relation to cardiovascular outcomes. For example, a recent study investigating the effects of vitamins C and E on the development of hypertension in pregnancy failed to show any benefit of antioxidant vitamins in hypertension (Roberts et al. 2010), in contrast to previous well-controlled smaller clinical studies (Duffy et al. 1999; Fotheby et al. 2000; Boshtam et al. 2002; Mullan et al. 2002). The current state of knowledge only allows us to recommend that the general population should consume a balanced diet (e.g., the DASH diet) with emphasis on antioxidant-rich fruits, vegetables, and whole grains (Carr and Frei 2000; Sacks et al. 2001; John et al. 2002).
Vitamin E
Two prospective studies evaluated effects of vitamin E: Nurses’ Health Study which recruited 87,245 female nurses for 8 years (Stampfer et al. 1993) and the Male Health Professionals’ Study with 39,910 male health professionals for 4 years (Rimm et al. 1993). Intake of more than 100 IU of vitamin E per day for more than 2 years was associated in both studies with reduced CAD risk. A Finnish study of 5,000 men and women demonstrated significant cardiovascular benefits with vitamin E supplementation for women but not for men (Knekt et al. 1994). In the Women’s Health Study (Lee et al. 2005), 39,876 apparently healthy US women over 45 years of age randomly assigned to receive natural-source vitamin E at a dose of 600 IU on alternate days or placebo and aspirin or placebo were followed for an average of 10 years. However, there was no benefit for major cardiovascular events or cancer and no effect on total mortality, but there was a decrease in cardiovascular mortality in healthy women.
Beta-Carotene
The Nurses’ Health Study could not demonstrate any benefit from intake of beta-carotene after taking into account intake of vitamins C and E (Stampfer et al. 1993). The follow-up Male Health Professionals’ Study reported in smokers beneficial effects of beta-carotene after adjusting for intake of vitamins C and E. However, nonsmokers experienced no benefit from beta-carotene (Virtamo et al. 1998). The Finnish study mentioned earlier did not find any reduction in cardiovascular risk that could be attributed to beta-carotene (Knekt et al. 1994).
Primary Prevention Randomized Clinical Trials
In the Cambridge Heart Antioxidant Study (CHAOS), a randomized, placebo-controlled, double-blind trial of 2,002 patients with CAD (proven by coronary angiography) who received up to 800 IU of vitamin E versus placebo for less than 3 years (Stephens et al. 1996), there was a highly significant reduction in fatal myocardial infarction, raising hopes for antioxidant therapy for prevention of cardiovascular events. However, there were problems of design and randomization, and many patients died in the vitamin E group. In another double-blind, placebo-controlled, randomized clinical trial of primary prevention, in this case directed to lung and other cancers and carried out in Finland on male smokers for up to 8 years, the Alpha-Tocopherol, Beta-Carotene Cancer (ATBC) Prevention Study (Knekt et al. 1994), there was no effect on cancer and slight benefits on incident angina, but no reduction of cardiovascular deaths and, in fact, an increase in hemorrhagic stroke. The dose of vitamin E given was 50 mg per day, which may have been too small. A meta-analysis of interventional trials that suggests low doses of vitamin E may be ineffective, whereas higher doses could actually be harmful (Miller et al. 2005).
Increases in mortality in the subjects taking beta-carotene were the result of the ATBC trial, with trends to increased lung cancer and ischemic heart disease (Knekt et al. 1994). In the beta-Carotene and Retinol Efficacy Trial (CARET), 18,314 smokers, former smokers, and asbestos workers were treated with beta-carotene and retinol or placebo for up to 4 years (Omenn et al. 1994). The trial had to be stopped early because of increased incident of lung cancer in the beta-carotene and retinol group. The Physicians’ Health Study I (PHS I) included 22,071 male physicians for a mean of 12 years (Hennekens et al. 1996). It was unable to find either a beneficial or negative effect of 50 mg per day of beta-carotene on cancer incidence, CAD, or mortality. The Physicians’ Health Study II, performed on 7,641 volunteers (41 %) from the PHS I who continued on the original beta-carotene treatment and 7,000 new physicians randomized in a 2 × 2 × 2 × 2 factorial design with mean follow-up of 8 years (Sesso et al. 2008), again showed no benefit on major cardiovascular events, total myocardial infarction, total stroke, and cardiovascular mortality.
Secondary Prevention Randomized Clinical Trials
The Heart Protection Trial in the UK (20,536 subjects) was a secondary prevention trial in which subjects received vitamin E (600 mg), vitamin C (250 mg), and beta-carotene (20 mg). It was unable to demonstrate any cardiovascular benefit (Heart Protection Study Collaborative Group 2002). In HOPE-TOO (The HOPE and HOPE-TOO Trial Investigators 2005), 4,732 of the initial HOPE participants agreed to extended observation and were evaluated every 6 months, and of these, 3,994 (i.e., 65.3 % of those surviving) were followed for a median of 7 years. Vitamin E supplementation for this long period did not prevent cancer or major cardiovascular events and paradoxically may have increased the risk of heart failure. The Women’s Antioxidant Cardiovascular Study (WACS) in the US recruited 8,171 female health professionals who were 40 years or older with a prior history of cardiovascular disease or three or more cardiovascular risk factors (Cook et al. 2007). The trial had a 2 × 2 × 2 factorial design, and participants were followed for an average of 9.4 years. No beneficial cardiovascular effect was found.
Why Negative Results in Antioxidant Trials?
The dose of antioxidants and duration of treatment may have been insufficient. Antioxidants may not reach the right cell compartments or targets. Vitamin E concentrates in lipoproteins and may not reach cytoplasmic ROS. Vitamins E and C may become prooxidant in tissues. Some of the trials, particularly vitamin E and beta-carotene studies, were observational, and thus the evidence that they provide is less compelling. Patients recruited to these trials may have had advanced cardiovascular disease, whether or not resulting from oxidant damage, and may not have been amenable to the antioxidant effects of the vitamins. Trial subjects in these studies were never proven to have increased oxidative stress in the cardiovascular system, however likely this may be. In none of the trials discussed here was BP a primary endpoint. Finally, patients were often taking aspirin, which has antioxidant properties (El Midaoui et al. 2002), and thus no further benefit from antioxidant therapy could be obtained.
Strategies to Reduce ROS Generation
New agents may have to be developed, such as ones that target the mitochondrion (e.g., mitoTEMPO), an important source of ROS, which may thus buffer mitochondrial superoxide generation (Dikalova et al. 2010). Beneficial effects of antihypertensive agents, such as some ß-adrenergic blockers like carvedilol, which has antioxidant properties, ACEIs, ARBs, mineralocorticoid receptor blockers, and some Ca2+ channel blockers like nifedipine, may be partially mediated by decreasing vascular oxidative stress (Oliveira et al. 2005; Cifuentes and Pagano 2006; Berk 2007; Chen et al. 2008; Sugiura et al. 2008) either via direct inhibition of NADPH oxidase activity or intrinsic antioxidant properties. HMG-CoA reductase inhibitors (statins), allopurinol, PPAR gamma activators, and aspirin are some other drugs that have antioxidant effects. Lifestyle modification approaches including diet (Mediterranean, DASH) and regular exercise play important roles. In experimental models of hypertension and in humans with CAD, exercise indeed reduced vascular NADPH oxidase activity and ROS production, ameliorated vascular injury, and reduced blood pressure (Adams et al. 2005).
Agents that reduce oxidant formation could be more efficacious than antioxidant vitamin ROS scavengers based on the demonstration that inhibition of NADPH oxidase-mediated superoxide generation achieved either through pharmacological or gene-deletion approaches leads to regression of vascular remodeling, improved endothelial function, lowering of blood pressure, and prevention of atherosclerosis. Increasing antioxidant capacity could also be achieved by using drugs that selectively inhibit MRP1 to prevent cell glutathione loss, protecting from oxidative damage, endothelial dysfunction, and hypertension (Widder et al. 2007). Another possible approach is to target glucose-6-phosphate dehydrogenase (G6PD), source of NADPH, the substrate for Noxes (Gupte 2008). Inhibition of G6PD can improve pulmonary hypertension, possibly through decreased oxidative stress. Only investigational G6PD inhibitors are available to date.
Resveratrol and Other Polyphenols
Evidence from epidemiological studies suggests that polyphenols from green and black tea, vegetables, fruits, and some wines can decrease cardiovascular risk. Green tea polyphenols, for example, improve endothelial function and insulin sensitivity, reduce blood pressure, and protect against myocardial ischemia/reperfusion injury (Potenza et al. 2007). Interesting polyphenols include resveratrol, naringenin, quercetin, and catechins. Resveratrol is probably the best known. It exerts its effects in part by stimulating endothelial SIRT1, which regulates endothelium-dependent vasodilation (Mattagajasingh et al. 2007), although it may also modulate the activity of nuclear factor-E2-related factor-2 (Nrf2) (Ungvari et al. 2010).
Dark Chocolate
Flavonoid-rich dark chocolate appears to have advantages over flavonoid-free white chocolate with respect to blood pressure and insulin sensitivity in hypertensive patients (Grassi et al. 2008). A population-based prospective study of middle-aged and elderly women reported that regular and moderate consumption of chocolate (i.e., less than one serving per day) was associated with a lower incidence of heart failure, hospitalization, and death (Mostofsky et al. 2010).
Conclusions
Oxidative stress contributes to vascular injury in hypertension and other cardiovascular conditions by promoting inflammation, increased vascular tone, matrix metalloproteinase activation, vascular smooth muscle cell growth, and endothelial dysfunction. These processes lead to enhanced vascular contractility and remodeling as found in blood vessels in hypertension. Dark chocolate, flavonoids in red wine and vegetables and fruits, as well as inhibitors of the renin-angiotensin-aldosterone system suggest that although major trials of vitamins have failed to prove it, antioxidant treatments may protect the cardiovascular system. Novel concepts on mechanisms involved in generation of oxidative stress in cardiovascular tissues, as well as better understanding of the fate of antioxidants after their administration may allow progress in the field, refined therapies and improved outcomes for hypertension and other forms of cardiovascular disease with the use of new antioxidant therapies.
References
Adams V, Linke A, Krankel N, Erbs S, Gielen S, Mobius-Winkler S, Gummert JF, Mohr FW, Schuler G, Hambrecht R (2005) Impact of regular physical activity on the NAD(P)H oxidase and angiotensin receptor system in patients with coronary artery disease. Circulation 111:555–562
Azumi N, Inoue Y (2002) Superoxide generation in directional coronary atherectomy specimens of patients with angina pectoris: important role of NAD(P)H oxidase. Arterioscler Thromb Vasc Biol 22:1838–1844
Barry-Lane PA, Patterson C, van der Merwe M, Hu Z, Holland SM, Yeh ETH, Runge MS (2001) p47phox is required for atherosclerotic lesion progression in ApoE(−/−) mice. J Clin Invest 108:1513–1522
Berk BC (2007) Novel approaches to treat oxidative stress and cardiovascular diseases. Trans Am Clin Climatol Assoc 118:209–214
Boshtam M, Rafiei M, Sadeghi K, Sarraf-Zadegan N (2002) Vitamin E can reduce blood pressure in mild hypertensives. Int J Vitam Nutr Res 72:309–314
Brassard P, Amiri F, Schiffrin EL (2005) Combined angiotensin II type 1 and type 2 receptor blockade on vascular remodeling and matrix metalloproteinases in resistance arteries. Hypertension 46:598–606
Brown AA, Hu FB (2001) Dietary modulation of endothelial function: implications for cardiovascular disease. Am J Clin Nutr 73:673–686
Carr A, Frei B (2000) The role of natural antioxidants in preserving the biological activity of endothelium-derived nitric oxide. Free Radic Biol Med 28:1806–1814
Chen RM, Touyz RM, Park JB, Schiffrin EL (2001) Antioxidant effects of vitamins C and E are associated with altered activation of vascular NADPH oxidase and superoxidase dismutase in stroke-prone SHR. Hypertension 38:606–611
Chen J, He J, Hamm L, Batuman, Whelton PK (2002) Serum antioxidant vitamins and blood pressure in the United States population. Hypertension 40:810–816
Chen S, Ge Y, Si J, Rifai A, Dworkin LD, Gong R (2008) Candesartan suppresses chronic renal inflammation by a novel antioxidant action independent of AT1R blockade. Kidney Int 74:1128–1138
Chrissobolis S, Didion S, Kinzenbaw DA, Schrader LI, Dayal S, Lentz SR, Faraci FM (2008) Glutathione peroxidase-1 plays a major role in protecting against angiotensin II-induced vascular dysfunction. Hypertension 51:872–877
Cifuentes ME, Pagano PJ (2006) Targeting reactive oxygen species in hypertension. Curr Opin Nephrol Hypertens 15:179–186
Cook NR, Albert CM, Gaziano JM, Zaharris E, MacFadyen J, Danielson E, Buring JE, Manson JE (2007) A randomized factorial trial of vitamins C and E and beta carotene in the secondary prevention of cardiovascular events in women: results from the women’s antioxidant cardiovascular study. Arch Intern Med 167:1610–1618
Datla SR, Griendling KK (2010) Reactive oxygen species, NADPH oxidases, and hypertension. Hypertension 56:325–330
Dikalova AE, Bikineyeva AT, Budzyn K, Nazarewicz RR, McCann L, Lewis W, Harrison DG, Dikalov SI (2010) Therapeutic targeting of mitochondrial superoxide in hypertension. Circ Res 107:106–116
Duffy SJ, Gokce N, Holbrook M, Huang A, Frei B, Keaney JF, Vita JA (1999) Treatment of hypertension with ascorbic acid. Lancet 354:2048–2049
Ebrahimian T, Touyz RM (2008) Thioredoxin in vascular biology: role in hypertension. Antioxid Redox Signal 10:1127–1136
El Midaoui A, Wu R, de Champlain J (2002) Prevention of hypertension, hyperglycemia and vascular oxidative stress by aspirin treatment in chronically glucose-fed rats. J Hypertens 20:1407–1412
Fortuno A, Olivan S, Beloqui O, San Jose G, Moreno MU, Diez J (2004) Association of increased phagocytic NAD(P)H oxidase-dependent superoxide production with diminished nitric oxide generation in essential hypertension. J Hypertens 22:2169–2175
Fotheby MD, Williams JC, Forster LA, Craner P, Ferns GA (2000) Effect of vitamin C on ambulatory blood pressure and plasma lipids in older patients. J Hypertens 18:411–415
GISSI-Prevenzione Investigators (1999) Dietary supplementation with n-3 polyunsaturated fatty acids and vitamin E after myocardial infarction: results of the GISSI-Prevenzione trial, Gruppo Italiano per lo Studio della Sopravvivenza nell’Infarto miocardico. Lancet 354:447–455
Grassi D, Desideri G, Necozione S, Lippi C, Casale R, Properzi G, Blumberg JB, Ferri C (2008) Blood pressure is reduced and insulin sensitivity increased in glucose-intolerant, hypertensive subjects after 15 days of consuming high-polyphenol dark chocolate. J Nutr 138:1671–1676
Griendling KK, Harrison DG (1999) Dual role of reactive oxygen species in vascular growth. Circ Res 85:562–563
Gupte SA (2008) Glucose-6-phosphate dehydrogenase: a novel therapeutic target in cardiovascular diseases. Curr Opin Investig Drugs 9:993–1000
Harrison DG (1997) Cellular and molecular mechanisms of endothelial cell dysfunction. J Clin Invest 100:2153–2157
Heart Protection Study Collaborative Group (2002) MRC/BHF Heart protection study of antioxidant vitamin supplementation in 20,536 high-risk individuals: a randomized placebo-controlled trial. Lancet 360:23–33
Hennekens CH, Buring JE, Manson JA, Stampfer M, Rosner B, Cook NR, Belanger C, LaMotte F, Gaziano JM, Ridker PM et al (1996) Lack of effect of long-term supplementation with beta carotene on the incidence of malignant neoplasms and cardiovascular disease. N Engl J Med 334:1145–1149
Herrmann AM, Saguner D, Versari TE, Peterson TE, Chade A, Olson M, Lerman LO, Lerman A (2007) Chronic proteasome inhibition contributes to coronary atherosclerosis. Circ Res 101:865–874
Higashi Y, Sasaki S, Nakagawa K, Matsuura H, Oshima T, Chayama K (2002) Endothelial function and oxidative stress in renovascular hypertension. N Engl J Med 346:1954–1962
Hoagland KM, Maier KG, Roman RJ (2003) Contributions of 20-HETE to the antihypertensive effects of Tempol in Dahl salt-sensitive rats. Hypertension 41:697–702
John JH, Ziebland S, Yudkin P, Roe LS, Neil HAW (2002) Effects of fruit and vegetable consumption on plasma antioxidant concentrations and blood pressure: a randomized controlled trial. Lancet 359:1969–1973
Keidar S, Attias J (1997) Angiotensin II injection into mice increases the uptake of oxidized LDL by their macrophages via a proteoglycan-mediated pathway. Biochem Biophys Res Commun 239:63–67
Khatri JJ, Johnson C, Magid R, Lessner SM, Laude KM, Dikalov SL, Harrison DG, Sung HJ, Rong Y, Galis ZS (2004) Vascular oxidant stress enhances progression and angiogenesis of experimental atheroma. Circulation 109:520–525
Khaw KT, Bingham S, Welch A, Luben R, Wareham N, Oakes S, Day N (2001) Relation between plasma ascorbic acid and mortality in men and women in EPIC-Norfolk prospective study: a prospective population study. Lancet 357:657–663
Knekt P, Reunanen A, Jävinen R, Seppänen R, Heliövaara M, Aromaa A (1994) Antioxidant vitamin intake and coronary mortality in a longitudinal population study. Am J Epidemiol 139:1180–1189
Kokubo Y, Iwai N, Tago N, Inamoto N, Okayama A, Yamawaki H, Naraba H, Tomoike H (2005) Association analysis between hypertension and CYBA, CLCNKB, and KCNMB1 functional polymorphisms in the Japanese population – the Suita Study. Circ J 69:138–142
Lacy F, O’Connor DT, Schmid-Schönbein GW (1998) Plasma hydrogen peroxide production in hypertensives and normotensive subjects at genetic risk of hypertension. J Hypertens 16:291–303
Lacy F, Kailasam MT, O’Connor DT, Schmid-Schonbein GW, Parmer RJ (2000) Plasma hydrogen peroxide production in human essential hypertension: role of heredity, gender, and ethnicity. Hypertension 36:878–884
Lee VM, Quinn PA, Jennings SC, Ng LL (2003) Neutrophil activation and production of reactive oxygen species in pre-eclampsia. J Hypertens 21:395–402
Lee IM, Cook NR, Gaziano JM, Gordon D, Ridker PM, Manson JE, Hennekens CH, Buring JE (2005) Vitamin E in the primary prevention of cardiovascular disease and cancer: the women’s health study: a randomized controlled trial. J Am Med Assoc 294:56–66
Mattagajasingh I, Kim CS, Naqvi A, Yamamori T, Hoffman TA, Jung SB, DeRicco J, Kasuno K, Irani K (2007) SIRT1 promotes endothelium-dependent vascular relaxation by activating endothelial nitric oxide. Proc Natl Acad Sci USA 104:14855–14860
Matthews I, Gorenne S, Scott N, Figg P, Kirkpatrick A, Ritchie M, Goddard M, Bennett M (2006) Vascular smooth muscle cells undergo telomere-based senescence in human atherosclerosis effects of telomerase and oxidative stress. Circ Res 99:156–164
McMillen T, Heinecke JW, LeBoeuf RC (2005) Expression of human myeloperoxidase by macrophages promotes atherosclerosis in mice. Circulation 111:2798–2804
Mehta JL, Sanada N, Hu CP, Chen J, Dandapat A, Sugawara F, Satoh H, Inoue K, Kawase Y, Jishage K et al (2007) Deletion of LOX-1 reduces atherogenesis I LDLR knockout mice fed high cholesterol diet. Circ Res 100:1634–1642
Miller ER, Pastor-Barriuso R, Dalal D, Riemersma RA, Appel LJ, Guallar E (2005) Meta-analysis: high-dosage vitamin E supplementation may increase all-cause mortality. Ann Intern Med 142:37–46
Morawietz H, Rueckschloss U, Niemann B (1999) Angiotensin II induces LOX-1, the human endothelial receptor for oxidized low-density lipoprotein. Circulation 100:899–902
Mostofsky E, Levitan EB, Wolk A, Mittleman MA (2010) Chocolate intake and incidence of heart failure. Circ Heart Fail 3:612–616
Mullan BA, Young IS, Fee H, McCance DR (2002) Ascorbic acid reduces blood pressure and arterial stiffness in type 2 diabetes. Hypertension 40:804–809
Muller DN, Dechend R, Mervaala EMA, Park JK, Schmidt F, Fiebeler A (2000) NFκB inhibition ameliorates angiotensin II-induced inflammatory damage in rats. Hypertension 35:193–201
Nickenig G, Harrison DG (2002) The AT1-type angiotensin receptor in oxidative stress and atherogenesis. part I: oxidative stress and atherogenesis. Circulation 105:393–396
Oliveira PJ, Goncalves L, Monteiro P, Providencia LA, Moreno AJ (2005) Are the antioxidant properties of carvedilol important for the protection of cardiac mitochondria? Curr Vasc Pharmacol 3:147–158
Omenn GS, Goodman G, Thornquist M, Grizzle J, Rosenstock L, Barnhart S, Balmes J, Cherniack MG, Cullen MR, Glass A et al (1994) The β-carotene and retinol efficacy trial (CARET) for chemoprevention of lung cancer in high-risk populations: smokers and asbestos-exposed workers. Cancer Res 54:2038–2043
Orozco LD, Kapturczak MH, Barajas B, Wang X, Weinstein MM, Wong J, Deshane J, Bolisetty S, Shaposhnik Z, Shih DM et al (2007) Atherosclerosis. Circ Res 100:1703–1711
Park JB, Touyz RM, Chen X, Schiffrin EL (2002) Chronic treatment with superoxide dismutase mimetic prevents vascular remodeling and progression of hypertension in salt-loaded stroke-prone spontaneously hypertensive rats. Am J Hypertens 15:78–84
PEACE Trial Investigators (2004) Angiotensin-converting-enzyme inhibition in stable coronary artery disease. N Engl J Med 351:2058–2068
Potenza MA, Marasciulo FL, Tarquinio M, Tiravanti E, Colantuono G, Federici A, Kim J, Quon MJ, Montagnani M (2007) EGCG, a green tea polyphenol, improves endothelial function and insulin sensitivity, reduces blood pressure, and protects against myocardial I/R injury in SHR. Am J Physiol Endocrinol Metab 292:E1378–E1387
Puddu P, Puddu GM, Cravero E, De Pascalis S, Muscari A (2009) The emerging role of cardiovascular risk factor-induced mitochondrial dysfunction in atherogenesis. J Biomed Sci 16:112–120
Rajagopalan S, Meng XP, Ramasamy S, Harrison DG, Galis ZS (1996) Reactive oxygen species produced by macrophage-derived foam cells regulate the activity of vascular matrix metalloproteinases in vitro: implications for atherosclerotic plaque stability. J Clin Invest 98:2572–2579
Rimm EB, Stampfer MJ, Ascherio A, Giovannucci E, Colditz GA, Willett WC (1993) Vitamin E consumption and the risk of coronary heart disease in men. N Engl J Med 328:1450–1456
Roberts JM, Myatt L, Spong CY, Thom EA, Hauth JC, Leveno KJ, Pearson GD, Wapner RJ, Varner MW, Thorp JM et al (2010) Vitamins C and E to prevent complications of pregnancy-associated hypertension. N Engl J Med 362:1282–1291
Romero JC, Reckelhoff JF (1999) Role of angiotensin and oxidative stress in essential hypertension. Hypertension 34:943–949
Ross R (1999) Atherosclerosis: an inflammatory disease. N Engl J Med 340:115–126
Sacks FM, Svetkey LP, Vollmer WM, Appel LJ, Bray GA, Harsha D, Obarzanek E, Conlin PR, Miller ER, Simons-Morton DG et al (2001) Effects on blood pressure of reduced dietary sodium and the Dietary Approaches to Stop Hypertension (DASH) diet. N Engl J Med 344:3–10
Salvemini D, Cuzzocrea S (2003) Therapeutic potential of superoxide dismutase mimetics as therapeutic agents in critical care medicine. Crit Care Med 31:S29–S38
Sesso HD, Buring JE, Christen WG, Kurth T, Belanger C, MacFadyen J, Bubes V, Manson JE, Glynn RJ, Gaziano JM (2008) Vitamins E and C in the prevention of cardiovascular disease in men: the physicians’ health study II randomized controlled trial. J Am Med Assoc 300:2123–2133
Shihabi A, Li WG, Miller FJ, Weintraub NL (2002) Antioxidant therapy for atherosclerotic vascular disease: the promise and the pitfalls. Am J Physiol 282:H797–H802
Sorescu D, Weiss D, Lassègue B, Clempus RE, Szöcs K, Sorescu GP, Valppu L, Quinn MT, Lambeth JD, Vega JD et al (2002) Superoxide production and expression of NOX family proteins in human atherosclerosis. Circulation 105:1429–1435
Srivastava S, Vladykovskaya E, Barski OQ, Spite M, Kaiserova K, Petrash JM, Chung SS, Hunt G, Dawn B, Bhatnagar A (2009) Aldose reductase protects against early atherosclerotic lesion formation in apolipoprotein e-null mice. Circ Res 105:793–802
Stampfer MJ, Hennekens CH, Manson JE, Colditz GA, Rosner B, Willett WC (1993) Vitamin E consumption and the risk of coronary heart disease in women. N Engl J Med 328:1444–1449
Stephens NG, Parsons A, Schofield PM, Kelly F, Cheeseman K, Mitchinson MJ (1996) Randomised controlled trial of vitamin E in patients with coronary disease: Cambridge Heart Antioxidant Study (CHAOS). Lancet 347:781–786
Sugiura T, Kondo T, Kureishi-Bando Y, Numaguchi Y, Yoshida O, Dohi Y, Kimura G, Ueda R, Rabelink TJ, Murohara T (2008) Nifedipine improves endothelial function: role of endothelial progenitor cells. Hypertension 52:491–498
Tabet F, Schiffrin EL, Callera GE, He Y, Yao G, Ostman A, Kappert K, Tonks NK, Touyz RM (2008) Redox-sensitive signaling by angiotensin II involves oxidative inactivation and blunted phosphorylation of protein tyrosine phosphatase SHP-2 in vascular smooth muscle cells from SHR. Circ Res 103:149–154
Tenaglia AN, Peters KG, Sketch MH, Annex BH (1998) Neovascularization in atherectomy specimens from patients with unstable angina: implications for pathogenesis of unstable angina. Am Heart J 135:10–14
The HOPE, HOPE-TOO Trial Investigators (2005) Effects of long-term vitamin E supplementation on cardiovascular disease and cancer. J Am Med Assoc 293:1338–1347
Touyz RM, Schiffrin EL (2001) Increased generation of superoxide by angiotensin II in smooth muscle cells from resistance arteries of hypertensive patients: role of phospholipase D-dependent NAD(P)H oxidase-sensitive pathways. J Hypertens 19:1245–1254
Touyz RM, Schiffrin EL (2004) Reactive oxygen species in vascular biology: implications in hypertension. Histochem Cell Biol 122:339–352
Ungvari Z, Bagi Z, Feher A, Recchia FA, Sonntag WE, Pearson K, de Cabo R, Csiszar A (2010) Resveratrol confers endothelial protection via activation of the antioxidant transcription factor Nrf2. Am J Physiol Heart Circ Physiol 299:H18–H24
Virtamo J, Rapola JM, Ripati S, Heinonen OP, Taylor PR, Albanes D, Huttunen JK (1998) Effect of vitamin E and beta carotene on the incidence of primary nonfatal myocardial infarction and fatal coronary heart disease. Arch Intern Med 158:668–675
Vivekananthan DP, Penn MS, Sapp SK, Hsu A, Topol EJ (2003) Use of antioxidant vitamins for the prevention of cardiovascular disease: meta-analysis of randomized trials. Lancet 361:2017–2023
Wang D, Strandgaard S, Iversen J, Wilcox CS (2009) Asymmetric dimethylarginine, oxidative stress, and vascular nitric oxide synthase in essential hypertension. Am J Physiol 296:R195–R200
Weiss D, Kools JJ, Taylor WR (2001) Angiotensin II-induced hypertension accelerates the development of atherosclerosis in apoE-deficient mice. Circulation 103:448–454
Welch WJ, Chabrashvili T, Solis G, Chen Y, Gill PS, Aslam S, Wang X, Ji H, Sandberg K, Jose P et al (2006) Role of extracellular superoxide dismutase in the mouse angiotensin slow pressor response. Hypertension 48:934–941
Widder JD, Guzik TJ, Mueller CFH, Clempus RE, Schmidt HHHW, Dikalov SI, Griendling KK, Jones DP, Harrison DG (2007) Role of the multidrug resistance protein-1 in hypertension and vascular dysfunction caused by angiotensin II. Arterioscler Thromb Vasc Biol 27:762–768
Wilcox CS, Pearlman A (2008) Chemistry and antihypertensive effects of tempol and other nitroxides. Pharmacol Rev 60:418–469
Xu H, Perez-Cuevas R, Xiong X, Reyes H, Roy C, Julien P, Smith G, von Dadelszen P, Leduc L, Audibert F et al (2010) An international trial of antioxidants in the prevention of preeclampsia (INTAPP). Am J Obstet Gynecol 202:239.e1–239.e10
Yusuf S, Dagenais G, Pogue J, Bosch J, Sleight P (2000a) Vitamin E supplementation and cardiovascular events in high-risk patients. N Engl J Med 342:154–160
Yusuf S, Sleight P, Pogue J, Bosch J, Davies R, Dagenais G (2000b) Effects of an angiotensin-converting-enzyme, ramipril, on cardiovascular events. N Engl J Med 342:145–153
Zureik M, Galan P, Bertrais S, Mennen L, Czernichow S, Blacher J, Ducimetière P, Hercberg S (2004) Effects of long-term daily low-dose supplementation with antioxidant vitamins and minerals on structure and function of large arteries. Arterioscler Throm Vasc Biol 24:1485–1491
Acknowledgments
Studies from the author’s laboratory were supported by funds from the Canadian Institutes of Health Research (CIHR) [Grants 37917, 82790] and a Canada Research Chair (CRC) from the CIHR/Government of Canada CRC Program and by the Canada Fund for Innovation.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer-Verlag Berlin Heidelberg
About this entry
Cite this entry
Schiffrin, E.L. (2014). Reactive Oxygen Species in Hypertension and Atherosclerosis. In: Laher, I. (eds) Systems Biology of Free Radicals and Antioxidants. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-642-30018-9_58
Download citation
DOI: https://doi.org/10.1007/978-3-642-30018-9_58
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-642-30017-2
Online ISBN: 978-3-642-30018-9
eBook Packages: Biomedical and Life SciencesReference Module Biomedical and Life Sciences