Abstract
In well-studied aquatic systems such as surface waters and groundwater, noble gases are used extensively as natural tracers to reconstruct palaeoenvironmental conditions, to study transport and mixing, and to identify the geochemical origin of geogenic fluids. It has been suggested that less well-studied aquatic systems such as the porewaters of lacustrine and oceanic sediments and the fluid inclusions present in stalagmites might also be suitable as noble gas archives for environmental studies, but until recently the lack of adequate experimental techniques had hindered the development of noble gas geochemistry in these systems. This chapter reviews recent technical advances in this field and describes the scientific applications that these advances have made possible. The porewaters of lacustrine and oceanic sediments are now well established as noble gas archives in studies of temperature, salinity and mixing conditions that prevailed in the overlying water body in the past, as well as in studies of the transport and origin of solutes and pore fluids in the sediment. The geochemistry of noble gases in stalagmite fluid inclusions is still in the early stages of development. However, the results available to date suggest that stalagmite fluid inclusions have great potential as a noble gas archive in reconstructing palaeoclimatic conditions near caves with suitable stalagmites.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Introduction
Noble gases have been used extensively as environmental tracers in lakes, oceans and groundwater since the 1960s. They have proved highly useful in reconstructing palaeoenvironmental conditions, studying transport and mixing in aqueous environments, and in constraining the geochemical origin of geogenic fluids injected into natural water bodies (for reviews see Ballentine and Burnard 2002; Ballentine et al. 2002; Kipfer et al. 2002; Schlosser and Winckler 2002; Aeschbach-Hertig and Solomon 2012; Stanley and Jenkins 2012).
The concentrations of dissolved noble gases in natural water bodies are determined by various processes involving different noble gas reservoirs (Sect. 2). On the one hand, the partitioning of noble gases between air and water is governed by the exchange of these gases across the air-water interface and by the solubility equilibrium as described by Henry’s Law. This allows the concentrations of dissolved atmospheric noble gases (i.e., those derived from the atmosphere) in a water body to be used to reconstruct the temperature and salinity of the water as well as the atmospheric pressure that prevailed during gas exchange. On the other hand, non-atmospheric noble gas isotopes may accumulate in the water either as a result of their in-situ production by radioactive decay and other nuclear reactions or because of the addition of geogenic fluids to the water. The concentrations of such non-atmospheric noble gases can be used to determine the water age (defined as the time that elapsed since the last occurrence of gas exchange between the water and the atmosphere), to quantify the rates at which water and solutes are transported, and to study the accumulation and the geochemical origin of geogenic fluids. These concepts can, in principle, be adapted to other, more unconventional aquatic environments, such as the porewaters of lacustrine and oceanic sediments or water inclusions in stalagmites.
Lakes generally react sensitively to changes in climatic forcing. More than three decades ago, the porewater of lacustrine and oceanic sediments was therefore proposed as a potential noble gas archive for palaeoenvironmental reconstruction (Barnes 1979). In addition, the abundance and relative composition of non-atmospheric noble gases accumulating in the porewater as a result of in-situ production or the injection of geogenic fluids reflect the transport dynamics and the geochemical origin of the pore fluids (Sect. 3.1).
In caves, microscopic water inclusions embedded in the minerals of stalagmites contain a mixture of noble gases that were trapped at the time the inclusions were formed. It has been suggested that the elemental composition of these noble gases may reflect the cave temperature prevailing at that time. Because the ambient air temperature in caves remains relatively constant throughout the year at a value which corresponds closely to the local annual mean soil temperature and to the annual mean air temperature outside the cave, it may be possible to use the noble gases archived in stalagmite water inclusions to reconstruct air temperatures in the distant past (Sect. 4.1).
Until recently, however, a lack of suitable experimental techniques has hindered the development of noble gas geochemistry in lacustrine and oceanic sediment porewater and in stalagmite fluid inclusions. Various research groups have therefore begun to develop new methods of noble gas analysis for these more unconventional aquatic systems (Sects. 3.2 and 4.2).
The resulting experimental advances have meant that lacustrine and oceanic sediment porewaters are now well established as a new type of noble gas archive for environmental studies (Sect. 3.3). New experimental techniques have also allowed the scientific potential of stalagmite fluid inclusions as noble gas archives for palaeoenvironmental research to be assessed (Sect. 4.3).
2 Noble Gases in Aqueous Solution
2.1 Sources and Processes
The noble gas components found in natural waters are characterised in terms of the geochemical reservoirs from which they originate and in terms of the processes by which they enter the water (see also Aeschbach-Hertig and Solomon 2012). Figure 1 illustrates the terminology used in this chapter for the different noble gas components.
Terminology and classification of the different noble gas components found in natural waters. Terms associated with geochemical reservoirs are indicated by rectangles and terms associated with the processes governing noble gas abundance by ellipses. Note that radiogenic noble gases are often, but not always, of terrigenic origin (e.g., 3He produced by the radioactive decay of 3H in the atmosphere or the hydrosphere is not terrigenic)
Noble gases from the atmosphere enter the water by gas exchange across the air-water interface and, to a lesser extent, by the (partial) dissolution of air bubbles in the water. The atmospheric noble gas component in the water therefore comprises all noble gases (and their isotopes) that are found in the atmosphere (He, Ne, Ar, Kr and Xe). The non-atmospheric noble gas isotopes originate mainly from the solid earth (terrigenic noble gases), including the rock matrix within which the water is embedded, or from the radioactive decay of 3H in the water. Among the non-atmospheric noble gas isotopes, 3He and 4He have been used most frequently for environmental field investigations so far. Rn, the heaviest naturally occurring noble gas, may also be used as an environmental tracer. However, due to the rapid radioactive decay of the different Rn isotopes (which have half-lives ranging from seconds to days), Rn is most useful as a proxy for rapid transport and mixing processes in surface waters and groundwater (e.g., Hoehn and von Gunten 1989; Bertin and Bourg 1994; Huxol et al. 2012) rather than for the much slower processes dealt with in this chapter. Rn will therefore not be considered further here.
2.1.1 Atmospheric Noble Gases
In meteoric water, the concentrations of dissolved noble gases are dominated by the atmospheric component. When using noble gases as environmental proxies in meteoric water, the noble gas composition of the atmosphere (Table 1) is assumed to be constant in space and time. This assumption is justified because water renewal in the aquatic environment occurs much more rapidly than the evolution of the noble gas composition of the atmosphere (Kipfer et al. 2002). The abundance of atmospheric noble gases dissolved in water is therefore determined solely by the processes governing air-water gas partitioning and by the transport processes occurring within the water body.
2.1.1.1 Noble Gas Concentrations in Air-Saturated Water
At the water surface, noble gases are partitioned between the atmosphere and the water by exchange across the air-water interface. Because gas exchange usually occurs much faster than the mixing of the water immediately below the air-water interface with the bulk water, solubility equilibrium is attained at the water surface (e.g., Schwarzenbach et al. 2003). The resulting equilibrium concentrations \(C_{i}^*\) in the air-saturated water (ASW) are given by Henry’s Law (see also Fig. 2 and Kipfer et al. 2002):
where \(P_{i}\) is the partial pressure of the noble gas \(i\) in the gas phase; \(H_{i}\) is the Henry coefficient, which is a function of the water temperature \(T\) and salinity \(S\); and \(C_{i}^*\) is the equilibrium concentration of noble gas \(i\) in the water.
Noble gas equilibrium concentrations in air-saturated water (\(C_{i}^*\)) as a function of water temperature \(T\) (left) and salinity \(S\) (right, for a sea-salt solution). The concentrations are normalised to the ASW concentrations \(C_{i}^0\) at \(T=0\) \({}^\circ \text{C}\) and \(S=0\) g/kg, respectively. Concentrations were calculated using the solubility data recommended by Kipfer et al. (2002)
The partial pressure is given by \(P_{i} = (P_{\text{ atm}}- e_s(T)) v_{i}\), where \(P_{\text{ atm}}\) is the total atmospheric pressure, \(e_s(T)\) is the vapour pressure, and \(v_{i}\) is the volume fraction of gas \(i\) in dry air (Table 1).
2.1.1.2 Excess Air
Excess air is formed by the (partial) dissolution of air bubbles in water. Excess air is of paramount importance in groundwater, whereas it is usually negligible in surface waters (Sect. 3.1). Stalagmites usually contain many air inclusions in addition to water inclusions (Sect. 4.1). Although the air in these inclusions is not dissolved in water, the mathematical treatment of this air component in Sect. 2.1.3 is analogous to that of excess air.
2.1.2 Non-atmospheric Noble Gases
Noble gases that are not of atmospheric origin can also accumulate in the aquatic environment. Noble gas isotopes can be produced in situ by radioactive decay and other nuclear reactions, and they can also be released into the aquatic environment from the solid earth. The concentrations of these radiogenic or terrigenic noble gas isotopes can provide information on water residence times and the dynamics of relevant fluid transport processes.
Relative to the atmospheric noble gas concentrations, non-atmospheric He usually shows the highest concentrations of all terrigenic noble gas isotopes (Mamyrin and Tolstikhin 1984; Ozima and Podosek 2002; Solomon 2000). This is mainly because of (i) the high rate of He production by the radioactive decay of U and Th isotopes in the crust coupled with the low degree of He retention in rock (radiogenic He, typical 3He/4He ratio \(\approx \) \(10^{-8}\)), and (ii) the release of primordial and radiogenic He from the earth’s mantle (typical 3He/4He ratio \(\approx \) \(10^{-5}\)). In addition, the radioactive decay of 3H bound to water molecules produces considerable amounts of 3He (known as tritiogenic 3He; see, e.g., Solomon and Cook 2000). 3H is produced naturally by spallation in the atmosphere. It is also a by-product of earlier nuclear weapons testing in the atmosphere. The latter resulted in a distinct 3H maximum in the atmosphere in the 1960s, temporarily increasing the global inventory of terrestrial 3H by several orders of magnitude. On local spatial scales the use of 3H in technological applications can also affect 3H concentrations in the aquatic environment.
Apart from the isotopes of He, other isotopes of radiogenic origin found in the aquatic environment include 40Ar and, to a lesser extent, 21Ne. However, in most cases their presence is masked by higher concentrations of 40Ar and 21Ne of atmospheric origin (for an extended discussion see Kipfer et al. 2002).
2.1.3 Disentangling and Quantifying the Various Noble Gas Components
From the above discussion it follows that the overall concentration of each of the noble gases in water is given by the sum of the ASW concentration, the excess air component (“EA”), and the terrigenic He (“terr”) and tritiogenic 3He (“trit”) components (note that except for He, all non-atmospheric noble gas components are neglected as to their abundance is extremely low):
Experimental analysis yields only the overall concentrations (the left-hand side of Eq. 2), but not the individual components, which are needed to interpret the noble gas data in terms of \(T\), \(S\), \(P_{\text{ atm}}\), excess air, and the accumulation of terrigenic and radiogenic He isotopes.
Depending on the type of environment and archive (surface-water body and sediments, or cave and stalagmites), the values of some of these environmental parameters can be constrained using independent information. For instance, \(P_{\text{ atm}}\) can be determined from the altitude of the water surface of a lake or the altitude of the recharge area of a groundwater system. The values of the remaining unknown parameters can then be estimated by regression from the measured data analogously to the techniques used in surface waters and groundwater (Aeschbach-Hertig et al. 1999; Ballentine and Hall 1999). These techniques have been used widely to infer the groundwater temperature that prevailed during the last occurrence of gas exchange between groundwater and soil air. This temperature is known as the “noble gas temperature” and the procedures used to determine it are known as the “noble gas thermometer” (Mazor 1972).
2.2 Experimental Methods
Water samples taken in the field need to be transported to a laboratory that is specially equipped for the extraction and analysis of noble gases (see also Aeschbach-Hertig and Solomon 2012). In the laboratory, the concentrations of dissolved noble gases are determined from the amounts of each noble gas in the water sample and the mass of the water sample. Because noble gases are only slightly soluble in water, contact between the water samples and air (or any other gas reservoir) must be avoided during sampling, transport, storage and processing.
Conventional bulk water samples from surface waters or groundwater are allowed to expand into a vacuum system to extract the dissolved noble gases from the water (e.g., Bayer et al. 1989; Jean-Baptiste et al. 1992; Beyerle et al. 2000; Sültenfuss et al. 2009). The analysis of noble gases from sediment porewater or stalagmite water inclusions requires additional experimental steps to separate the water from its confining matrix prior to the vacuum extraction process. This critical step, which must be carried out in such a way that the water sample is not exposed to air or to any other gas, needs to be adapted to the type of sample (Sects. 3.2 and 4.2). After separation from the matrix, the dissolved gases are extracted from the water and transferred to a vacuum system to purify and separate out the different noble gases. The gas amounts and isotope ratios are then determined by static mass spectrometry using peak-height comparison (e.g., Beyerle et al. 2000).
The minimum sample size required for noble gas analysis is determined by the sensitivity of the mass spectrometer, the degree of dilution of the sample during the extraction and purification of the sample gas, and the detection limit of the analytical system. Taking these factors together, the practical minimum sample size required for a typical analytical system is approximately 0.1–1 mg of water.
3 Lacustrine and Oceanic Sediments
3.1 Noble Gases in Sediment Porewater
Because gas exchange across the air-water interface is rapid, noble gas concentrations at the surface of lakes and the ocean correspond closely to ASW concentrations as computed from the water temperature, salinity and atmospheric pressure (Sect. 2.1.1).
For the purpose of this chapter, concentrations of excess air are assumed to be negligible in surface waters. Small amounts of excess air are observed only in large lakes and in the ocean, where excess air can be produced by the dissolution of bubbles entrained by breaking waves (Craig and Weiss 1971; Bieri 1971) or by the addition of meltwater from air-rich ice (Hohmann et al. 2002; Loose et al. 2009). The resulting noble gas excesses are usually \(\le \)5 % for Ne and even less for the heavier noble gases (Craig and Weiss 1971; Peeters et al. 2000).
The vertical distribution of heat and conservative solutes (e.g., noble gases and salts) in the water column is dominated by vertical transport resulting from advection and macroscopic turbulence (“eddy diffusion”, Lerman et al. 1995). The atmospheric noble gas concentrations observed at a given water depth are therefore coupled to the temperature and salinity of the water at that depth. They therefore correspond closely to the ASW concentrations calculated from this water temperature and salinity, as confirmed experimentally by, for instance, Aeschbach-Hertig et al. (1999) and Peeters et al. (2000).
During sedimentation, part of the open water just above the sediment-water interface is incorporated into the sediment pore space. The noble gas concentrations in this porewater therefore carry information about the temperature and salinity of the bottom water in the past (see examples discussed in Sect. 3.3.1). Analogously, dissolved chloride concentrations and the \(\updelta \)18O signature in the sediment porewater have been used to reconstruct palaeoenvironmental conditions in the ocean (Adkins et al. 2002; Schrag et al. 1996, 2002). However, geochemical alteration of the chloride concentrations in the porewater may mask the palaeosalinity information (e.g., Austin et al. 1986; Swart 2000). Also, the \(\updelta \)18O signature in sea water depends not only on water temperature and salinity, but also on the volumes of reservoirs of freshwater (ice, groundwater), which change in response to different climate conditions. Further, \(\updelta \)18O in the porewater may even be affected by the recrystallisation of dissolved carbonates (Swart 2000). By contrast, the concentrations of dissolved atmospheric noble gases are unaffected by such secondary processes and thus provide a direct measure of water temperature and salinity in the past.
While the noble gas signature in the bottom water is recorded and buried in the sediment, molecular diffusion acts at the same time to smooth out the noble gas profiles. This smoothing will, in principle, result in a loss of information with regard to the past noble gas signature in the water body, leading to a loss of information about the corresponding past environmental conditions. To assess the relative importance of sediment burial (advection) and diffusion for the archived noble gas signature, the distances by which solutes are transported by sediment burial or diffusion need to be compared (see also Strassmann et al. 2005, and references therein):
Sediment burial: The sediment burial velocity relative to the sediment surface is governed by the rates of sediment accumulation and compaction. For simplicity, compaction is assumed to be so small, that the vertical porewater offset relative to the sediment matrix is negligible. The porewater burial velocity is therefore approximately equal to the sediment accumulation rate (\(s\)). Within a given time interval \(\Delta t\), the porewater is transported downwards a distance \(\Delta z_\mathrm{adv}\):
$$\begin{aligned} \Delta z_\text{adv}\approx s \Delta t \end{aligned}$$(3)Diffusion: In contrast to advection, the characteristic transport length (\(\Delta z_{{\rm{diff}},{i}}\)) of a diffusive process increases with the square root of the elapsed time according to the Einstein-Smoluchowski equation (e.g., Einstein 1905; Grathwohl 1998; Schwarzenbach et al. 2003):
$$\begin{aligned} \Delta z_{{\rm{diff}},{i}} \approx \sqrt{\phi D_{i} \Delta t} \end{aligned}$$(4)where \(\phi \) is the sediment porosity and \(D_{i}\) is the effective diffusivity of the noble gas \(i\) in the sediment, given by:
$$\begin{aligned} D_{i} = D_{i}^0 / a \end{aligned}$$(5)where \(D_{i}^0\) is the molecular diffusivity of gas \(i\) in bulk water and \(a \ge 1\) is a scaling factor describing the effect of diffusion retardation in the sediment. This scaling factor takes into account the tortuosity of the sediment matrix, but also accounts for any other effects that may result in the retardation of diffusion in the porewater (Sect. 3.3.3).
The diffusive smoothing of vertical noble gas profiles can be considered negligible when the diffusive transport length (\(\Delta z_{{\rm{diff}},{i}}\)) is negligible compared to the advective transport length (\(\Delta z_\text{adv}\)); i.e., when \(\Delta z_{{\rm{diff}},{i}} \ll \Delta z_\text{adv}\). Combining Eqs. (3), (4) and (5), and assuming \(\phi \approx 1\) yields:
where \(Pe\) is the Peclet number and \(L\) is the sediment-depth range considered. With \(L \approx s \Delta t\) (still neglecting compaction), the importance of diffusion for smoothing the noble gas profiles therefore depends on the sediment accumulation rate and the time range considered. Strassmann et al. (2005) showed that in a “typical” sediment a step-like Xe profile would be smoothed out by diffusion over appoximately 2 m in \(10^{2}\) years, 5 m in \(10^{3}\) years, and 20 m in \(10^{4}\) years. A scaling factor \(a \approx 2\) was assumed in these calculations to reflect the tortuosity of the sediment matrix. These results illustrate that even if diffusion does smooth out the noble gas profiles, the palaeoenvironmental information is retained in the sediment over a large depth range \(L\) (i.e., if \(Pe \gg 1\)).
Apart from the reconstruction of palaeoenvironmental conditions, noble gases dissolved in sediment porewater have been shown to be sensitive tracers for the transport of solutes and pore fluids within the sediment column and for the exchange of solutes and fluids between the sediment and the overlying water body (see examples discussed in Sect. 3.3.2). The transient behaviour of radiogenic He isotopes accumulating in the porewater is especially suitable for studying the dynamics of the transport of solutes and fluids in the sediment. In addition, the 3He/4He ratio of the different He components reflects the geochemical origin and history of the pore fluids.
3.2 Experimental Methods
Peeper methods have been used to sample dissolved He in sediment porewater (Stephenson et al. 1994). A peeper is a dialysis sampler which is buried in the sediment. A gas-permeable membrane separates the sediment porewater from the sample cells, which are filled with gas or water. However, peepers need to be left at the sampling site for several days or weeks to allow the He in the sample cells to equilibrate with the He in the surrounding porewater (Dyck and Da Silva 1981). Because of their lower diffusivity, the heavier noble gases would require even longer equilibration times. Also, gases are lost rapidly from the sample cells during peeper retrieval and sample processing as a result of degassing caused by the decrease in hydrostatic pressure. Finally, peepers may not be suitable for sampling at great water depths because the membranes of the peeper cells collapse under the hydrostatic pressure exerted.
A wireline tool has been used for the in-situ sampling of porewater in marine sediment drill holes to quantify He fluxes through the sediment-water interface (Barnes 1973; Barnes and Bieri 1976; Barnes 1979, 1988; Sano and Wakita 1987). This non-standard sampler is complicated to operate and gas leakage may occur from the samples. Torres et al. (1995) and Winckler (1998) determined He, Ne, Ar, Kr and Xe concentrations in samples obtained using this tool, but it was also noted that gas bubbles may form in the system during sampling. Hence, the noble gas concentrations in the sample can vary in an uncontrollable manner because of gas exchange between the sample and the gas bubbles. Also, contamination with the drilling fluid or the water used to fill the in-situ sampler may occur (Winckler 1998).
To overcome the shortcomings of the methods described above, Brennwald et al. (2003), Chaduteau et al. (2007) and Tomonaga et al. (2011a) developed new experimental methods that rely on simple, robust, and standard sedimentological equipment. In the following, these state-of-the art methods will be described in detail.
3.2.1 Sampling
Sediment cores are collected in plastic tubes (liners) using a gravity corer or a similar coring device. After recovering the sediment core, about 30 g of bulk sediment is sampled from the desired sediment depths. Similar to noble gas sampling in bulk water (e.g., Beyerle et al. 2000), copper tubes are used as sample containers. The copper tubes are connected to the sediment core via fittings that penetrate into the core (Fig. 3a; Brennwald et al. 2003). Sampling ports can be drilled and covered with adhesive tape before coring to minimise the risk of contaminating the sediment with air.
Immediately after collecting the core, the sediment sample is transferred from the liner into the copper tubes utilising the squeezer set-up shown in Fig. 3a. The copper tubes are flushed with sediment to remove residual air and to expel the sediment fraction which may have exchanged noble gases with the atmosphere while the copper tubes were being mounted. The samples are closed and sealed by pinching off the copper tubes at both ends using the same standard clamps as those used with conventional water samples. This procedure allows the sampling of intact sediment cores and avoids air contamination and gas loss during sampling. By inspection of a layered sediment core after sampling, the uncertainty in sampling depth has been estimated to be less than 5 cm (Brennwald et al. 2004).
An alternative sampling method was developed by Chaduteau et al. (2007). Sediment is sampled in copper tubes by pushing the tubes into the sediment either parallel or perpendicular to the axis of the sediment core (Fig. 3b). This method has the advantage of being less destructive to the sediment core, and of requiring less sediment material because the copper tubes are not flushed with sediment. This “sub-coring” into the copper tubes causes hardly any interference with most other classical sedimentological analyses of the core. Also, the sampling depth within the sediment core is well defined, as there is no offset due to squeezing. This method works best with soft sediments that will easily fill the copper tubes. For other sediments, the squeezing method may be better suited. A disadvantage of the method of Chaduteau et al. (2007) is that it does not allow the copper tubes to be flushed with sediment material, which would help to avoid contamination of the sample with air.
3.2.2 Extraction and Analysis of Dissolved Noble Gases
The methods used to extract dissolved noble gases from conventional water samples (Bayer et al. 1989; Beyerle et al. 2000) cannot be applied directly to the porewater of unconsolidated sediments because the porewater is embedded and trapped in the sediment matrix. Brennwald et al. (2003), Chaduteau et al. (2007) and Tomonaga et al. (2011a) therefore developed other methods of extracting the gases from the porewater.
The method of Brennwald et al. (2003) for extracting the dissolved noble gases from the sediment porewater involves heating and opening the copper tube, which is attached to an evacuated extraction vessel. Heating increases the pressure in the copper tube, causing the whole sediment sample to be extruded explosively into the extraction vessel when the tube is opened. The dissolved noble gases are then extracted into the vacuum of the extraction vessel. The sample gases are transferred into a gas purification system, where the noble gases are cleansed of other gases which would interfere with the subsequent mass spectrometric analysis. This allows the noble gas concentrations to be determined with a standard error of \(<\)2 %. In some sediments, however, considerable amounts of radiogenic He can be released from the minerals when the sediment sample is heated during analysis, resulting in lower accuracy.
To reduce the release of radiogenic He from the sediment minerals by excessive heating, Chaduteau et al. (2007) developed an alternative method for the analysis of dissolved He in porewater. The sediment is transferred from the copper tube to an evacuated glass flask by first briefly flame-heating the copper tube and subsequently flushing it with degassed, hot water. The dissolved He is then extracted from the water in the glass flask at approximately 40 \({}^\circ \text{C}\) with the help of a magnetic stirrer, and is then transferred into the He analysis system. Although untested, this method would seem to be suitable for analysing not only dissolved He, but all other dissolved noble gases also.
Tomonaga et al. (2011a) optimised the extraction process of Brennwald et al. (2003) by centrifuging the sediment samples in the copper tubes to separate the porewater from the sediment matrix. After centrifuging, the part of the copper tube containing pure porewater is separated from the compacted sediment at the sediment-water interface using a third clamp. The porewater in the water-filled part of the copper tube is then analysed in the same way as a standard water sample (e.g., Beyerle et al. 2000). This method completely avoids heating the sediment and therefore guarantees reliable analysis of the concentration and isotopic signature of the He dissolved in the porewater.
Compared to the method of Chaduteau et al. (2007), the method of Tomonaga et al. (2011a) avoids the extra step of producing water free of noble gases, but requires that the sediment sample be centrifuged before analysis. Note also that one has to be careful to avoid gas fractionation when centrifuging gas-rich sediment samples. If gas bubbles form in the sample because of gas supersaturation at the temperature and pressure prevailing in the copper tube, the noble gases originally dissolved in the porewater will partially escape into these bubbles. The gas bubbles are quantitatively removed from the sediment matrix during centrifuging, whereas only a fraction of the porewater is separated from the sediment. Centrifuging gas-rich sediments may therefore result in an undesirable fractionation of noble gases between the compacted sediment and the porewater sample used for analysis, which includes the gas headspace.
3.3 Applications in Environmental Studies
3.3.1 Palaeoenvironmental Conditions
Barnes (1979) was the first to suggest that archived concentrations of atmospheric noble gases dissolved in sediment porewater might be useful as palaeotemperature proxies. Implementation of this idea, however, was long hampered by the lack of suitable experimental methods. This obstacle was removed by the recently developed methods described in Sect. 3.2.
These new methods were applied for the first time by Brennwald et al. (2004) to study the noble gas record in the sediments of Lake Issyk-Kul (Kyrgyzstan, central Asia), one of the worlds largest high-altitude lakes. The lake has no outflow and is therefore slightly saline (\(S= 6\) g/kg). Such closed lakes are known to exhibit large changes in lake level and salinity in response to changes in local climate (e.g., Fritz 1996). Analyses of the lake basin morphology and sedimentological proxies showed that during the mid-Holocene the salinity of the lake water was highly variable and that the lake level lay several hundred metres below its present level (e.g., De Batist et al. 2002; Giralt et al. 2003; Ricketts et al. 2001; Romanovsky 2002).
Distinct minima in the concentrations of atmospheric noble gases in the sediment porewater were observed at approximately 90 cm sediment depth (Fig. 4). The effective diffusivities of the noble gases in the sediment porewater were found to be at least two orders of magnitude lower than in the bulk water, implying that the noble gas profiles in the sediment are virtually unaffected by vertical diffusion in the porewater. The ratio of the molecular diffusivity of Ar in water to that of Xe is 2.1. Assuming the diffusive pathways in the pore space to be the same for Ar and Xe, Eqs. (4) and (5) yield \(\Delta z_{\text{diff},Ar} / \Delta z_{\text{diff},Xe} \approx \sqrt{D_{Ar}^0 / D_{Xe}^0 } \approx 1.5\). Analogously, \(\Delta z_{\text{diff},Ar} / \Delta z_{\text{diff},Kr} \approx 1.3\). However, this diffusive separation disagrees with the measured noble gas data, because, within the vertical resolution of the measurements, the respective concentration minima are all found at the same sediment depth. Brennwald et al. (2004) therefore concluded that diffusion is strongly limited and is insufficient to decouple the Ar, Kr and Xe signals in the porewater from the sediment matrix. The noble gas concentrations at a given sediment depth are therefore essentially determined by the physical conditions prevailing in the overlying water at the time when the sediment was deposited.
The minima in the atmospheric noble gas concentrations at 90 cm sediment depth indicate that either the salinity of the water, its temperature, or both, were considerably higher during the mid-Holocene than they are now. A simple model linking the noble gas concentrations in the deep water of Lake Issyk-Kul to the volume of the lake, and hence to its water level, allowed Brennwald et al. (2004) to infer from the noble gas record of the porewater that the lake level was approximately 300 m lower in the mid-Holocene than it is today (Fig. 5).
Mineralogical sediment composition (\(M\)) and noble gas concentrations in the porewater (\(C_{i}\), normalised to present-day ASW noble gas concentrations \(C_{i,1}^*\) in the open water) as a function of sediment depth (Brennwald et al. 2004). Cc: calcite. Mg-Cc: magnesian calcite, which is precipitated in water of high salinity. Cc-H\(_2\)O: monohydrocalcite
Quantification of the change in the water level of Lake Issyk-Kul (Brennwald et al. 2004) based on the concentrations of dissolved Ar, Kr and Xe in the porewater. ASW concentrations in the water body (\(C_{i}^*\)) were calculated as a function of the change in lake level \(\Delta h\). The \(C_{i}^*\) were normalised to present-day ASW concentrations \(C_{i,1}^*\) calculated from the present-day temperature (\(T_1\)) and salinity (\(S_1\)) of the overlying water and the mean atmospheric pressure (\(P_1\)) at the water surface. \(T_2\), \(S_2\) and \(P_2\) are the respective values of temperature, salinity and pressure during a period of lower lake level in the past. The annual mean temperature profile in the lake (TP) was used to model \(C_{i}^*\) because the noble gas concentrations in the porewater reflect the annual mean temperature of the deep water (Brennwald et al. 2003). The solid parts of the curves represent the analytical 2\(\sigma \) uncertainty range of the measured minimum concentrations in Fig. 4. In the range 291 m \(<\) \(\Delta h\) \(<\) 307 m (horizontal grey bar), all modelled concentrations simultaneously match the measured minimum concentrations within the limits of analytical uncertainty
In Soppensee, a small Swiss freshwater lake with a maximum water depth of 27 m, noble gas concentration profiles in the sediment porewater were used to study and reconstruct the formation of methane bubbles in the sediment and their release into the overlying water body (Brennwald et al. 2005). The noble gas concentrations in the porewater of Soppensee were found to be much lower than the ASW concentrations of the overlying water (up to 75 % Ne depletion, and increasingly less for Ar, Kr and Xe, which are more soluble). This depletion is greatest just below the sediment surface and decreases steadily with sediment depth.
The decrease in noble gas depletion with the gas solubility is indicative of the loss of dissolved noble gases into a gas phase which was initially free of noble gases (Ballentine et al. 2002; Bosch and Mazor 1988; Holzner et al. 2008; Winckler et al. 2000). Brennwald et al. (2005) attributed this depletion pattern to the stripping of dissolved noble gases into gas bubbles forming in the sediment as a result of supersaturation of the porewater with biogenic \(\mathrm{CH}_{4}\). The escape of these gas bubbles from the sediment results in the observed noble gas depletion of the porewater.
The 20Ne/22Ne and 40Ar/36Ar ratios in the Soppensee porewater agree with their respective atmospheric equilibrium ratios. Brennwald et al. (2005) assumed that the diffusion of noble gases from the porewater into the gas bubbles would result in their isotopic fractionation in the porewater, as expected from Graham’s Law (\(D_i / D_j = \sqrt{m_j / m_i}\), where \(D_{i}\) and \(D_{j}\) are the diffusion coefficients of two isotopes \(i\) and \(j\) of the same element and \(m_{i}\) and \(m_{j}\) are their respective masses). The lack of such an isotopic fractionation indicates that the observed noble gas depletion is not controlled by the kinetics of gas partitioning between the porewater and the gas bubbles. In contrast, a secondary solubility equilibrium between porewater and gas bubbles is attained. Brennwald et al. (2005) therefore used a simple gas equilibration model to estimate the amount of exsolved \(\mathrm{CH}_{4}\), which was found to correspond to about 25 % of the total \(\mathrm{CH}_{4}\) that can be stored in dissolved form in the Soppensee sediments.
Using an argument similar to that used for Lake Issyk-Kul, Brennwald et al. (2005) showed that the lack of isotope fractionation also indicates that the noble gas signature in the porewater at a given sediment depth reflects the intensity of methane ebullition at the time when this sediment was deposited; i.e. that vertical diffusion had virtually no effect on the noble gas profiles in the porewater.
However, the molecular dynamics calculations of Bourg and Sposito (2008) suggest that isotopic fractionation owing to molecular diffusion in water might be much smaller than expected from Graham’s Law, which has been validated experimentally only for the diffusion coefficients of 3He and 4He (Jähne et al. 1987), but not for the diffusivities of the Ne or Ar isotopes. The lack of experimental data on the extent to which the isotopic fractionation of Ne and Ar is affected by diffusion in water does not allow the importance of diffusion for the noble gas profiles in the Soppensee porewater to be conclusively assessed.
3.3.2 Transport and Geochemical Origin of Pore Fluids
Dissolved noble gases make good tracers for the transport of solutes within the sediment porewater. The transient behaviour of radiogenic He isotopes accumulating in the porewater is particularly useful for studying the transport and geochemical origin of solutes and fluids in the sediment. In a few early studies (Barnes and Bieri 1976; Barnes 1987; Sano and Wakita 1987; Sayles and Jenkins 1982; Winckler 1998), the wireline tool discussed in Sect. 3.2 was used to study the fluxes of He isotopes through oceanic sediments. Stephenson et al. (1994) used a modified peeper (Sect. 3.2) to determine He concentrations when studying the injection of groundwater through the sediment into two small lakes.
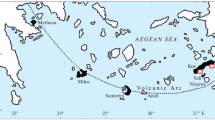
Noble gas signatures in the sediment porewater and in the overlying water body of the Black Sea have been used to study the injection of geogenic fluids into the deep water, to determine their geochemical origin and to characterise \(\mathrm{CH}_{4}\) emissions from high-intensity gas seeps in the vicinity of active mud volcanoes (Holzner et al. 2008). Both the He concentrations and their vertical gradients in the sediment porewater were found to be much greater near the gas seeps than at a reference site located approximately 200 km from the gas seeps that is unaffected by mud volcanism. The terrigenic He component of the pore fluids and of the open water body (Fig. 6) shows a 3He/4He ratio of approximately 3.5 \(\cdot \,10^{-7}\) near the gas seeps, whereas the terrigenic He at the reference site is enriched in 3He (3He/4He\(\approx\) \(10^{-6}\)). These differences in the abundance and in the isotopic signature of the terrigenic He in the sediment porewater illustrate the variability in the flux and the geochemical origin of the He (and the associated pore fluids) released from the Black Sea sediments.
Noble gas signatures in the sediment porewater (squares) and the open water body (circles, triangle) of the Black Sea, measured near active gas seeps at the Sorokin Trough (sediment cores GC17 and GC41, open-water profiles CTD109 and CTD110) and at a reference site not affected by outgassing (GC14 and CTD108). a He concentrations in the sediment porewater. b3He/4He isotope ratios in the sediment porewater. c The 3He/4He ratio as a function of the Ne/4He ratio for the sediment porewater, the open water at the seep area and the mean open-water reference profile (dashed line). Straight lines indicate mixing between ASW and a seafloor source with a 3He/4He ratio of \(10^{-6}\) (dotted line) as determined by Top et al. (1990) for the southern Black Sea, and with a mud-volcano source with an estimated 3He/4He ratio of 3.5\(\cdot 10^{-7}\) (solid line). After Holzner et al. (2008)
A similar approach was used to study the fluids released from cold \(\mathrm{CH}_{4}\) seeps off the coast of New Zealand (Tomonaga 2010). The He-Ne signatures in the porewater at two sampling sites at Omakere Ridge and one site at Rock Garden (near the Pacific-Australian subduction zone) show a mixture of ASW and terrigenic He. The 3He/4He ratio of the terrigenic He at the two sites at Omakere Ridge is approximately 6 \(\cdot\, 10^{-7}\), which indicates that the terrigenic He is mainly of crustal origin. At Rock Garden, the pore fluids have a 3He/4He ratio of approximately 2.5\(\cdot\,10^{-6}\), which suggests that these fluids are enriched with mantle-derived 3He.
At the Congo-Angola margin, the He dissolved in the sediment pore fluids near cold fluid seeps was found to be a mixture of seawater-derived He and radiogenic He produced in the sediment column or in the underlying crust (Chaduteau et al. 2009). The vertical He concentration profiles in the porewater and the pore-fluid temperature profiles were used to study pore-fluid circulation and to estimate pore-fluid advection rates in the sediment. The porewater He concentration profiles were shown to be more sensitive than the porewater temperature profiles to pore-fluid advection rates.
At the Mid-Okinawa Trough (Japan), 3He/4He and 4He/20Ne ratios in sediment porewater were used to study earth degassing at subduction zones by estimating the fluxes of terrigenic He isotopes through the sediment and into the ocean (Lan et al. 2010). The 3He fluxes observed at a hydrothermally active site amount to about 20 % of the 3He fluxes measured at the East Pacific Rise. Lan et al. (2010) argued that submarine hydrothermal systems in back-arc basins may therefore contribute a significant amount of helium to the ocean.
In Lake Van (eastern Anatolia, Turkey), a large, terminal, saline soda lake located in a region of high tectonic and volcanic activity, Tomonaga et al. (2011b) studied the flux of terrigenic He through the sediment. Analysis of the He profiles in 24 sediment cores taken at different sites in the lake showed that the He flux varies strongly within the lake basin, from 0.4\(\cdot \,10^{8}\) atoms/m2/s to 42\(\cdot\, 10^{8}\) atoms/m2/s. The largest He fluxes were found at the steep borders of the central, deep part of the lake basin (stations S1, S2, and S3 in Fig. 7). The isotope ratio for the terrigenic He is essentially the same in all sediment cores (3He/4He\(\approx \)(2.6–4.1)\(\cdot\, 10^{-6}\)), which suggests that this He is a mixture of crustal He and mantle He originating from a single, sub-continental source. Tomonaga et al. (2011b) argued that the release of He from the underlying lithosphere is fostered by fault structures that are most likely related to the ongoing subsidence of the circular main basin of the lake and to volcanic activity. This supports the hypothesis that the circular-shaped deep basin was formed by the collapse of an ancient caldera (Litt et al. 2009).
Geological map of Lake Van, showing the bathymetry of the lake basin and the sediment sampling sites (after Tomonaga et al. 2011b). (L) low He fluxes. (H) high He fluxes. (S) “hot spots” with highest He fluxes. (D) noble gas concentrations showing degassing artefacts, possibly caused by bubble formation during sampling
In the estuary of the St. Lawrence River (Québec, Canada), Pitre and Pinti (2010) studied the use of dissolved atmospheric noble gases in sediment porewater as proxies for the dynamics of dissolved or gaseous \(N _{2}\) produced by nitrate reduction in the sediment. In contrast to previous work, large excesses of atmospheric noble gases with respect to ASW were observed in the porewater samples of these estuarine sediments. The excess increased with increasing atomic mass, which led Pitre and Pinti (2010) to the hypothesis that the sediments were enriched with noble gases as a result of their sorption on to clay minerals or organic matter in the sediment.
3.3.3 Diffusion and Trapping Mechanisms
Some of the data discussed in Sect. 3.3.1 show that diffusion does not smooth out the noble gas profiles in the sediment enough to cause the information on the environmental history of the overlying water body to be lost, even over sediment depth ranges smaller than the diffusion lengths calculated by Strassmann et al. (2005) (Sect. 3.1). This can only be explained if the corresponding scaling factors \(a\) are several orders of magnitudes greater than would be expected from conventional tortuosity models of unconsolidated sediments.
Although a similar suppression of diffusion has been observed for He and \(\mathrm{H}_{2}\) in the porewater of compacted clays (Horseman et al. 1996), this phenomenon is not commonly reported for other gases dissolved in the porewater of slightly compacted lacustrine and oceanic sediments. While little is known about the mechanisms responsible for the suppression of the diffusion of noble gases in sediment porewater, the following conceptual hypotheses can be put forward as possible explanations:
- 1.
Because of the formation of new minerals or because of a geometric realignment of the sediment grains during sedimentation, compaction and early diagenesis, the diameter of the sediment pores or of the channels connecting them can be as small as a few μm or less (e.g., Horseman et al. 1996). In this case the Renkin effect (i.e., the increased viscosity of the water in the vicinity of the pore walls) will result in a reduction of molecular diffusion by several orders of magnitude (Renkin 1954; Grathwohl 1998; Schwarzenbach et al. 2003; Brennwald et al. 2004), as seems to be the case in firn (Beyerle et al. 2003; Huber et al. 2006).
- 2.
A considerable fraction of the pore space might consist of “dead” pores; i.e., pores that are not connected to the main pore space through which diffusive transport occurs on macroscopic scales. Solutes trapped in the dead pores cannot undergo macroscopic transport through the fully connected pore space. Note that conventional transport models axiomatically exclude the possibility of the occurrence of dead pores (e.g., Berner 1975; Imboden 1975; Strassmann et al. 2005), although the retention of solutes in dead pores results in a strong reduction in overall diffusivity within the sediment (e.g., Grathwohl 1998). To our knowledge, however, this axiomatic exclusion of dead pores appears not to be based on solid experimental evidence and may therefore represent an inappropriate model simplification.
- 3.
Microscopic gas bubbles might act as gas reservoirs. In the presence of such gas bubbles in the sediment, a large fraction of the noble gases initially dissolved in the porewater would escape into these bubbles, because noble gases are only slightly soluble in water. This fraction of the noble gas content of the sediment would be excluded from macroscopic diffusion through the porewater (see also the discussion by Tomonaga 2010). As in the case of the postulated dead-pore mechanism, this would result in the effective diffusion of noble gases, and possibly of other volatile gases, being strongly suppressed on a macroscopic scale.
- 4.
Noble gases might be adsorbed on to the sediment matrix; e.g., on to organic matter (Pitre and Pinti 2010). To our knowledge, the quantitative sorption of noble gases on to unconsolidated sediments has not yet been observed. However, if noble gases are indeed adsorbed quantitatively on to the sediment matrix, only the fraction of the noble gases that remain dissolved in the porewater would be subject to diffusion, as the adsorbed fraction would be retained by the sediment matrix. This would result in strong retardation of diffusive noble gas transport in the porewater.
Up until now, however, a systematic and conclusive assessment of these hypotheses has not been possible because of a lack of experimental evidence. Additional studies focusing on the mechanisms described above would be required to improve our understanding of how and why macroscopic noble gas diffusion in sediment porewater can be attenuated much more strongly than would be expected from conventional tortuosity models.
4 Stalagmites
4.1 Noble Gases in Stalagmite Fluid Inclusions
Stalagmites are formed in caves by the precipitation of calcite from drip water. The actively growing stalagmite surface is covered by a thin layer of drip water on the order of 0.1 mm thick (Dreybrodt 1980). Gas concentrations in this layer attain solubility equilibrium with the cave air within seconds to minutes. This rapid gas exchange allows the outgassing of excess \(\mathrm{CO}_{2}\), resulting in the precipitation of calcite in the water layer and promoting the growth of the stalagmite. Noble gas concentrations in the water layer, however, can be assumed to correspond closely to ASW concentrations (Sect. 2.1.1).
During stalagmite growth, minute quantities of drip water and cave air are trapped in the bulk calcite as fluid inclusions, which may contain water, cave air, or both. The abundance of the inclusions, and the volumetric ratio of air to water contained within them, vary strongly from stalagmite to stalagmite, and also differ considerably within individual stalagmites (e.g., Kluge et al. 2008). Microscopic investigations have shown that water and air inclusions differ in size and spatial arrangement within individual stalagmites (Scheidegger et al. 2010). Water inclusions are found mainly within the stalagmite calcite crystals, but only rarely at crystal boundaries (Fig. 8). The water inclusions usually do not exceed 50 μm in length. The water abundance per unit mass of calcite typically lies between 0.1 and 1 mg/g (Kendall and Broughton 1978; Schwarcz et al. 1976; Kluge et al. 2008). While air inclusions do occur within calcite crystals (intra-crystalline inclusions), they occur predominantly along grain boundaries between the calcite crystals (inter-crystalline inclusions). Intra-crystalline air inclusions are similar in size and shape to water inclusions (Fig. 8), whereas inter-crystalline air inclusions are larger (20–80 μm long) and more angular (Scheidegger et al. 2010). Air inclusions can account for up to 3 % of a stalagmite’s volume (Badertscher et al. 2007; Scheidegger et al. 2010).
Once a fluid inclusion has been formed, it is no longer in contact with the cave air. Using the He and Ar diffusion data of Musset (1969) and Copeland et al. (2007), Kluge (2008) estimated noble gas diffusivities in calcite at room temperature to be about \(10^{-25}\) m2/s. The diffusion of noble gases through the calcite surrounding a fluid inclusion therefore has a negligible effect on the noble gas concentrations in the inclusions even on time-scales of several 100 ka. The concentrations of dissolved noble gases in a given water inclusion can thus be assumed to reflect the temperature and salinity of the drip water at the time the inclusion was formed. The salinity of the drip water entering the cave is usually so low that it has no effect on noble gas solubilities. The concentrations of noble gases in water inclusions can therefore expected to be related directly and quantitatively to cave palaeotemperatures.
In most caves, temperatures remain constant throughout the year at a value corresponding approximately to the annual mean air temperature outside the cave (e.g., McDermott et al. 2005; Poulson and White 1969; Smithson 1991). Noble gas records in stalagmite water inclusions are thus expected to allow regional palaeotemperatures to be reconstructed over long periods of time. Stalagmites are especially valuable as a source of such information because of their global distribution. This is in contrast to ice cores, which can supply similar information, but only for polar or high-altitude regions.
Stalagmites have therefore become a major focus for palaeoclimate studies over the past few decades. Stalagmite calcite contains precise, high-resolution \(\updelta \)13C, \(\updelta \)2H and \(\updelta \)18O records covering time-scales of up to \(10^{5}\) years (e.g., Badertscher et al. 2011; Cheng et al. 2009; Fleitmann et al. 2009; Henderson 2006; Wang et al. 2008). Also, the signatures of \(\updelta \)2H and \(\updelta \)18O in water inclusions have been used to study palaeoclimate conditions (Fleitmann et al. 2003; Griffiths et al. 2010; van Breukelen et al. 2008; Wainer et al. 2011). These stable-isotope records are controlled by different environmental variables and processes such as temperature-dependent isotope fractionation during calcite formation, the local precipitation regime, the hydrological cycle, and amounts of precipitation. The complex interplay of these variables and processes makes it difficult to interpret stalagmite isotope records clearly and unequivocally (e.g., Lachniet 2009). Use of the noble gas thermometer to provide an independent estimate of the temperature at which stalagmite water inclusions were formed would therefore not only provide valuable palaeoclimate information in its own right; it would also help to overcome some of the limitations involved in interpreting stalagmite stable-isotope records, thereby contributing to exploiting their full potential.
Determination of the concentrations of noble gases dissolved in the water of stalagmite fluid inclusions was until recently not feasible, mainly because of the lack of adequate extraction methods that would allow water and air inclusions to be separated. As noble gases are much more abundant in air inclusions than in water inclusions, their concentrations in water inclusions are often masked by the comparatively large amounts of noble gases released from air inclusions during the extraction process. The noble gas excess from the air inclusions is mathematically analogous to the excess-air component in groundwater Eq. (2). Noble gas temperatures in groundwater may be determined even in the presence of moderate amounts of excess air (e.g., Aeschbach-Hertig et al. 1999). However, if the excess-air component is large (i.e., if \(\text{ Ne}\)EA/\(\text{ Ne}\)ASW\(\gtrsim \) 10 in Eqn. (2)), it will mask the ASW component to such a degree that the noble gas thermometer becomes unreliable. For the successful determination of noble gas temperatures from stalagmite water, it is of paramount importance to minimise the proportion of noble gases in the final sample that are derived from air inclusions.
4.2 Experimental Methods
Different approaches have been used to extract noble gases from stalagmite fluid inclusions (Sects. 4.2.2 and 4.2.3). Ayliffe et al. (1993) and Kluge et al. (2008) crushed stalagmites in vacuo to release noble gases from fluid inclusions. Kluge et al. (2008) applied this technique successfully to a stalagmite from Bunker Cave (Germany) that had an exceptionally low air-water volume ratio. However, the authors could not derive meaningful noble gas temperatures by applying their crushing method to more typical stalagmites with higher air-water volume ratios, because the noble gases released from the air inclusions masked the noble gas concentrations in the water inclusions.
Scheidegger et al. (2010, 2011) therefore explored alternative approaches that would reduce the contribution of noble gases derived from air inclusions to the final result. These approaches were all based on the authors’ observation that most of the air inclusions in stalagmites occur along grain boundaries and can be opened preferentially by gently crushing a sample to yield grains approximately the same size as that of its intrinsic crystals, whereas the intra-crystalline water inclusions remain mostly intact. The noble gases extracted from the calcite in a second extraction step are thus derived primarily from water inclusions. The resulting noble gas signatures indeed revealed a much lower contribution of noble gases from air inclusions, making feasible a mathematical correction of the remaining air-related noble gas component (Scheidegger et al. 2010). Unfortunately, however, the procedures used to reduce the amount of noble gases released from the air inclusions introduce other complexities into the noble gas signatures, thus still often preventing the straightforward calculation of meaningful noble gas temperatures (Sect. 4.3).
A seemingly obvious way of avoiding the problems associated with the release of noble gases from air inclusions would be to spot-drill individual water inclusions in vacuo with a laser. However, because of the small amount of water contained in a single inclusion and the low concentrations of noble gases in this water, the noble gas content of each inclusion is very small. To obtain amounts large enough for all stable noble gases to be analysed reliably, \(10^{4}\)–\(10^{5}\) water inclusions would have to be opened up (assuming typical detection limits for the mass-spectrometric noble gas analysis system), thus rendering this approach impracticable.
4.2.1 Sample Selection
As mentioned above, not all stalagmite samples are equally suited for the determination of noble gas temperatures. To be suitable, samples should have a water content of about 0.1 mg/g or higher (e.g. Kluge et al. 2008) and, equally importantly, the air-water volume ratio should not exceed a certain limit to avoid the ASW component from the water inclusions being masked by the noble gases released from the air inclusions. Kluge et al. (2008) give as an upper limit an air-water volume ratio of 0.5.
Petrographic information on stalagmites from Dimarshim Cave and Pit Cave (both Socotra Island, Yemen), Sofular Cave (Turkey), and Vallorbe Cave (Switzerland) (after Scheidegger 2011). I SEM images showing the calcite texture of freshly crushed fractions. II Photographs of thick sections taken using an optical microscope at low magnification in cross-polarised light. III Photographs of thick sections taken using an optical microscope showing typical water (W) and air (A) inclusions
Microscopic investigation of the samples to be analysed for noble gases is therefore highly recommended. Scheidegger et al. (2011) used scanning electron microscopy and optical microscopy to study samples of different stalagmites. A selection of the images obtained is illustrated in Fig. 9. In addition to yielding information on the size and spatial distribution of air and water inclusions, these images also provide information on the size of individual calcite crystals. This information is essential for determining the grain size to which a given stalagmite sample needs to be pre-crushed in order to separate air and water inclusions most effectively (Sect. 4.2.2). The size of water inclusions in comparison to the crystal size and texture of the host calcite can also provide information about the temperature required to assure quantitative gas extraction during heating (Sect. 4.2.3).
4.2.2 Crushing
4.2.2.1 Crushing in Vacuo
Several different devices for crushing a stalagmite sample in vacuo have been described in the literature. Kluge et al. (2008) used a cylindrical cell containing a steel ball, controlled by an external magnet, as a crusher. Badertscher et al. (2007) and Kluge et al. (2008) reported experiments in which a sample is placed in a copper tube which is evacuated and then squeezed with a vice to crush the sample. Scheidegger et al. (2010) used a pestle-operated vacuum crusher (Fig. 10) in which the pestle and the cylinder holding the sample are connected via a flexible bellows. The sample is crushed by striking the top of the pestle with a hammer. Following the crushing, the gases released from the sample are allowed to expand into a gas purification line leading to the noble gas mass spectrometer.
Stainless steel vacuum crusher used to extract gases from fluid inclusions (after Scheidegger 2011). The pestle is connected to the main chamber via a flexible bellows. The sample is crushed by striking the top of the pestle with a hammer
In an attempt to separate the gases released from the air inclusions from those released from the water inclusions, Scheidegger et al. (2010) applied several consecutive crushing steps to each sample, often followed by a final heating step (Sect. 4.2.3).
4.2.2.2 Crushing in a Gas Atmosphere
For stalagmites with typical air-water volume ratios, simple stepwise crushing in vacuo often does not allow the noble gases contained in water inclusions to be separated sufficiently well from the noble gases contained in air inclusions. Scheidegger et al. (2010, 2011) therefore explored the possibility of crushing stalagmite samples to a predefined grain size in different gas atmospheres. The appropriate grain size (e.g., 350–700 μm, Scheidegger et al. 2011), which should be close to the natural crystal size of the sample, is determined by microscopic inspection prior to crushing (Sect. 4.2.1). The sample is crushed using a stainless-steel mortar and pestle. After each gentle hammer stroke on the pestle, the sample is sieved to separate crystals of the desired size from larger crystal aggregates, and the large crystal aggregates are then crushed again. The crushing and sieving process is repeated until the whole sample consists of grains no larger than the predefined grain size. The air content of the resulting grains is \(10^{2}\)–\(10^{3}\) lower than that of a bulk stalagmite sample, because the air inclusions located at the grain boundaries are indeed preferentially opened during the crushing process (Scheidegger et al. 2011).
After crushing and sieving stalagmite samples in air and in a glove box flushed with \(\text{ N}_2\) (of purity 6.0), Scheidegger et al. (2010) found that in both cases residual atmospheric heavy noble gases were adsorbed on to the freshly produced grain surfaces. During the subsequent extraction of noble gases from these grains by heating (Sect. 4.2.3), the adsorbed noble gases were released together with the noble gases extracted from the fluid inclusions (Sect. 4.3.2). This increased the resulting concentrations of heavy noble gases considerably, so that in most cases, no meaningful noble gas temperature could be derived from the resulting data. Flushing the glove box with He (of purity 6.0) and further reducing the concentrations of residual heavy noble gases in the He atmosphere with a sorption pump installed in the glove box reduced the Kr and Xe residuals to negligible levels (Scheidegger et al. 2011). To prevent a crushed sample being exposed to air when transferring it to the noble gas purification line, it was first loaded into a stainless steel container sealed with a valve before removing it from the glove box.
4.2.3 Heating
Kluge et al. (2008) heated their vacuum-crushed samples to 50 \({}^\circ \text{C}\) to quantitatively transfer the extracted water into the analytical system (Sect. 4.2.4). In contrast, Scheidegger et al. (2010) heated their crushed samples up to 600 \({}^\circ \text{C}\) to open the intra-crystalline inclusions, which were assumed to be filled predominantly with water. Scheidegger et al. (2010) emphasised that the applied temperature must not exceed the threshold at which excessive amounts of gases such as \(\mathrm{CO}_{2}\) or \(\mathrm{H}_{2}\) are formed, as these prevent reliable determination of the amount of water released (Sect. 4.2.4). \(\mathrm{CO}_{2}\) is produced during the decomposition of calcite to CaO and \(\mathrm{CO}_{2}\) at temperatures \(\gtrsim 600\) \({}^\circ \text{C}\) (Faust 1950). \(\mathrm{H}_{2}\) is produced during the interaction of water released from the sample with the hot metal surfaces of the vacuum sample container. Scheidegger et al. (2010) found that at temperatures \(\gtrsim \)400 \({}^\circ \text{C}\), the partial pressure of \(\mathrm{H}_{2}\) and the corresponding (non-stoichiometric) loss of \(\mathrm{H}_{2}\mathrm{O}\) exceed the limits below which the amount of water released from the sample can be reliably determined (Sect. 4.2.4). Best results were achieved by heating the sample for 1 h to temperatures between 300 \({}^\circ \text{C}\) and 400 \({}^\circ \text{C}\) (Scheidegger et al. 2011).
4.2.4 Quantification of the Water Mass
Due to the low water content of stalagmites, typical stalagmite samples of 1–5 g yield no more than few milligrammes of water upon crushing or heating. Accurate determination of such a small mass of water is challenging and cannot easily be accomplished merely by weighing a sample before and after the water is extracted. Instead, the mass of water released is determined manometrically (Ayliffe et al. 1993; Kluge et al. 2008; Scheidegger et al. 2010). This requires the extracted water to be transferred quantitatively to a tempered expansion vessel large enough to prevent the water vapour from condensing. A pressure gauge is then used to determine the vapour pressure inside this vessel. Using the ideal-gas law, the water mass is then calculated from the vapour pressure, the temperature, and the volume of the expansion vessel.
To avoid bias in the determination of the mass of water, the gas pressure in the expansion vessel must be generated essentially by the water vapour only. Formation of excessive amounts of other gases (e.g., \(\mathrm{CO}_{2}\) or \(\mathrm{H}_{2}\)) must therefore be avoided during the extraction process (Sect. 4.2.3). If this requirement is met, the manometric method allows the mass of a few milligrammes of water to be determined with a standard error of about 1 % (Scheidegger et al. 2010).
4.3 First Applications of the Noble Gas Thermometer
The occurrence of both air and water inclusions in stalagmites suggests that the noble gas signatures in stalagmite samples should reflect a simple binary mixture of air and ASW. Noble gas data from Bunker Cave stalagmite samples with exceptionally low air-water volume ratios are indeed consistent with this expectation (Sect. 4.3.1). However, results from other stalagmite samples with more typical air-water volume ratios indicate a more complex picture involving additional gas components, such as fractionated air or lattice-trapped noble gases (Sect. 4.3.2).
4.3.1 Stalagmites from Bunker Cave (Germany)
Kluge et al. (2008) studied two stalagmites from Bunker Cave (Germany) with air-water volume ratios of 0.03–0.08, which is exceptionally low. It was therefore not necessary to reduce the air content of these samples by pre-crushing, and precise noble gas temperatures could be derived from the measured Ne, Ar, Kr and Xe concentrations as described in Sect. 2.1.3.
From six samples of one Bunker Cave stalagmite covering the time frame from 10.8–11.7 ka BP, a mean noble gas temperature of 2.9 \({}^\circ \text{C}\) with a standard deviation of 0.7 \({}^\circ \text{C}\) was determined. The noble gas temperatures derived from the different samples agreed with each other within their error limits. One sample from a second stalagmite with a U/Th age of 1.3 \(\pm \) 0.3 ka yielded a noble gas temperature of 7.1 \(\pm \) 0.8 \({}^\circ \text{C}\). The temperature difference of about 4 \({}^\circ \text{C}\) agrees with the results of other palaeotemperature reconstructions (e.g., Davis et al. 2003), which imply similar temperature changes in central and western Europe. However, while the absolute noble gas temperatures obtained are not unreasonable for central Europe, Kluge et al. (2008) could not exclude a possible bias, because their values seem to be slightly lower than would be expected from other palaeotemperature reconstructions in the Bunker Cave region. Kluge et al. (2008) therefore emphasised that the accuracy of noble gas temperatures derived from stalagmite fluid inclusions needs to be tested on modern stalagmites, for which a direct comparison with instrumental cave temperature records would be possible.
4.3.2 Stalagmites from Central Europe and the Middle East
To test different noble gas extraction methods, Scheidegger et al. (2010, 2011) determined noble gas concentrations in more than 50 stalagmite samples from Switzerland (Blättlerloch, Beatus Cave, Feés Cave, Vallorbe Cave), Germany (Bunker Cave), Turkey (Sofular Cave), Oman (Qunf Cave), and Yemen (Dimarshim Cave) covering a variety of climatic regions and time ranges (0–100 ka BP). The analytical errors of the noble gas concentrations ranged between 2 and 5 %.
Pre-crushing did indeed substantially reduce the noble gas contributions from air inclusions relative to those from water inclusions. However, it was still not possible to derive statistically acceptable noble gas temperatures by regression from the Ne, Ar, Kr, and Xe concentrations as described in Sect. 2.1.3. This was attributed to the fact that Eq. (2) assumes a binary mixture of ASW and air, whereas the results also showed contributions from additional noble gas components, such as adsorbed air (Sect. 4.2.2) or He and Ne trapped in the calcite lattice (see below). The elemental compositions of these additional components are poorly constrained and therefore cannot be included quantitatively in Eq. (2), thus precluding the robust determination of noble gas temperatures.
4.3.3 4.3.2.1 He and Ne Trapped in the Calcite Lattice?
In addition to the ASW and air components, excesses of He and Ne have been observed in various stalagmites (Ayliffe et al. 1993; Kluge 2008; Scheidegger et al. 2010) regardless of the extraction method employed (i.e., crushing in vacuo, crushing in a gas atmosphere, or heating; see Fig. 11). The He and Ne excesses therefore seem to be present naturally in the stalagmite samples.
Normalised elemental ratios \(r_i\) observed (a) after crushing stalagmite samples in vacuo (Sect. 4.2.2), and (b) after subsequently heating the same samples (Sect. 4.2.3), where \( r_i=(i/{\text{Ar}})_{\rm{sample}}/(i/{\text{Ar}})_{\rm{air}}\) with \(i=\text{He, Ne, Ar, Kr, Xe}\) (after Scheidegger et al. 2010). The noble gas compositions of ASW (water temperatures 0–30 \({}^\circ \text{C}\), grey areas) and of air (dashed lines) are shown for comparison
Ayliffe et al. (1993) and Scheidegger et al. (2010) hypothesised that the excesses of He and Ne might be due to the trapping of He and Ne atoms in crystallographic voids within the calcite structure during crystal growth. The maximum diameter of a sphere that would fit into these voids is approximately 3 Å (Scheidegger et al. 2010). Such voids would be large enough to accommodate an atom of He or Ne, but not of Ar, Kr or Xe, which have diameters >3 Å (Table 1). This hypothesis is supported by evidence from the Martian meteorite GaG 476, in which substantial Ne excesses (“extra Ne”) were found (Mohapatra et al. 2009). This extra Ne is likely to be of terrestrial atmospheric origin: during the process of recrystallisation that accompanied terrestrial weathering, atmospheric Ne was probably trapped in newly formed calcite crystals (Ulrich Ott, 2009, Max Planck Institute for Chemistry, Mainz, Germany, personal communication).
Relationship between the Ar/Kr and Xe/Kr ratios in the gas extracted from Dimarshim stalagmite samples using different pre-crushing methods (pre-crushing in air, in \(\mathrm{N}_{2}\), in extra-pure He, or in vacuo). The grey area indicates the Ar-Kr-Xe composition of air adsorbed on to calcite grains (data on adsorbed air extends to higher Xe/Kr ratios lying outside the range of the plot; see Scherer et al. 1994; Ozima and Podosek 2002)
Scheidegger et al. (2010) observed that the He/Ne ratio of the excess He and Ne resulting from gas extraction by heating was higher than that resulting from extraction by crushing (Fig. 11). They attributed this to He enrichment resulting from temperature-enhanced diffusion from the calcite, as the diffusivity of He is higher than that of Ne.
4.3.4 4.3.2.2 Ar-Kr-Xe Signatures in Fluid Inclusions
Figure 12 shows a typical example of the Ar-Kr-Xe signatures, based on samples from a Dimarshim Cave stalagmite, to which Scheidegger et al. (2010, 2011) had applied the whole suite of extraction methods discussed in Sects. 4.2.2 and 4.2.3. The Ar-Kr-Xe data from most samples are located within the mixing triangle of air, ASW, and adsorbed air (the latter with a broad range of elemental compositions). Samples pre-crushed in air clearly show the effect of the adsorption of the heavy noble gases on to the freshly produced grain surfaces (Scheidegger et al. 2010). Samples pre-crushed in \(\text{N}_2\) suffer from Ar excesses resulting from excessive residual Ar in the \(\text{N}_2\) protective gas. The Ar-Kr-Xe composition in the vacuum-crushed samples is similar to that of air because the air released from the air inclusions masks the ASW component from the water inclusions. The Ar-Kr-Xe composition of the samples pre-crushed in extra-pure He is closest to that of ASW, and many of these samples show Xe/Kr ratios indistinguishable from that of ASW. This led Scheidegger et al. (2010, 2011) to conclude that pre-crushing in extra-pure He substantially reduces the contribution of noble gases derived from air inclusions and also reduces the adsorption of Kr and Xe to negligible levels. They therefore considered this method to be the most suitable for reliable noble gas temperature determination, although artefacts cannot always be ruled out. Such artefacts can result, for instance, from the adsorption of residual heavy noble gases from the protective He atmosphere (Sofular Cave samples were crushed without using a sorption pump in the glove box), or from Kr and Xe deficits associated with the incomplete extraction of noble gases from the fluid inclusions (possibly because of insufficient heating). Nevertheless, 12 out of 30 noble gas temperatures calculated from the Kr and Xe concentrations in an extended Dimarshim Cave data set (Scheidegger 2011) agree to within analytical error with the modern cave temperature of 27 °C. This cave temperature has remained approximately constant since the mid-Holocene (e.g., Wanner et al. 2008). Likewise, 3 out of 9 noble gas temperatures derived from Kr and Xe concentrations in stalagmite samples from Sofular Cave are identical to the modern cave temperature of 12 °C. Also, Kr and Xe concentrations in 4 out of 7 samples from Vallorbe Cave are conceptually consistent with a binary mixture of air and ASW.
5 Summary, Outlook and Future Research
The recent methodological advances in the extraction of noble gases from the porewater of unconsolidated sediments (Sect. 3.2) represent an essential step forward in overcoming the experimental limitations that have hampered the development of terrestrial noble gas geochemistry in sediment porewater for decades. The experimental methods described in Sect. 3.2 can be employed by any noble gas laboratory equipped for the analysis of terrestrial water samples, thus allowing the laboratory to expand its activities to include the analysis of the porewater of unconsolidated sediments.
From a conceptual point of view, the key conclusion of recent sediment porewater studies is that in some sediments the noble gases diffusivities in the sediment porewater are similar to their corresponding molecular diffusivities in bulk water. In other sediments, however, the noble gases are quantitatively trapped in the sediment and diffusion is therefore strongly suppressed (Sects. 3.3.1 and 3.3.3). This trapping results in a stratigraphical control of the noble gas record in the sediment, which allows a time-scale to be associated with it. However, the mechanisms responsible for trapping the noble gases have yet to be identified. A mechanistic understanding of the suppression of diffusion would be required to fully establish the conceptual basis required for noble gases to be utilised as proxies for environmental conditions or as tracers of pore-fluid transport.
The physical mechanisms responsible for the trapping of noble gases in the porewater of unconsolidated sediments are not yet fully understood. However, the experimental tools necessary to study the noble noble gas isotopes dissolved in sediment porewater are now available. Specifically, it is now possible to assess the feasibility of using the concentrations of atmospheric noble gases in the porewater to reconstruct the geochemical evolution of the pore fluids, and to reconstruct past environmental conditions (such as the palaeosalinity of the ocean). Recently, during a scientific drilling programme in Lake Van (Litt et al. 2009, 2011), sediment samples for noble gas analysis were retrieved at depths of more than 200 m below the sediment surface. The highly compacted bulk sediments were squeezed into copper tubes using a hydraulic press. Such a hydraulic squeezer can, in principle, be used during any scientific drilling campaign in which lacustrine or oceanic sediments are recovered (e.g., the Integrated Ocean Drilling Program, IODP, or the International Continental Scientific Drilling Program, ICDP). Of particular interest are oceanic sediments from marginal seas, whose geochemical and physical conditions are expected to react very sensitively to climate change and tectonic activity. Preliminary results from the Sea of Japan (East Sea) suggest that the concentrations of atmospheric noble gases in the sediment porewater might reflect changes in freshwater input and in the mixing conditions of the water body during the last glaciation, as discussed by Lee and Nam (2003).
The sampling techniques developed for the analysis of noble gases in lacustrine and oceanic sediments might also be useful in groundwater studies. Up until now, groundwater dating using transient tracers (e.g., 3H/3He, terrigenic He, CFCs, SF6, etc.), and the reconstruction of environmental conditions from atmospheric noble gas concentrations, have been restricted to water samples taken from groundwater wells tapping flowing groundwater. However, available groundwater wells are often not located at the optimum position to address a specific scientific problem. Also, the groundwater flowing into the wells usually originates from a large and often poorly defined domain around the well. A better spatial resolution of the information on groundwater flow paths, water residence time and the environmental conditions pertaining throughout the aquifer is therefore desirable in many groundwater studies. For instance, studies on groundwater contaminants and their rates of natural attenuation and removal from the groundwater (e.g., Aeppli et al. 2010; Amaral et al. 2010; Klump et al. 2006) would greatly benefit from the availability of spatially accurate and highly resolved information on water residence time. One way forward in improving the spatial resolution would be to obtain drill cores from aquifers, or even adjacent aquicludes, at locations that are determined by the specific scientific problem to be addressed (see also Mazurek et al. 2011 for a review of similar porewater tracer analyses in drill cores from ‘tight’ rock formations). Porewater samples for the analysis of dissolved gases at high spatial resolution could then be taken from these drill cores at the exact depths required by adapting the sampling techniques developed for noble gas analysis in lacustrine and oceanic sediments; e.g., using a hydraulic porewater squeezer.
Stalagmite fluid inclusions, viewed as a noble gas archive, have great potential in palaeoenvironmental research. The recent experimental advances described in Sect. 4.2 now make possible the precise and accurate determination of the concentrations of noble gases dissolved in the minute amounts of water contained in these inclusions. It has become clear that the concentrations of dissolved noble gases in stalagmite water inclusions do indeed contain useful palaeotemperature information. This has been demonstrated convincingly for samples with very low air-water volume ratios and also for some samples with more typical air-water volume ratios (Sect. 4.3).
However, despite recent experimental progress it is not yet possible to use stalagmites with typical air-water volume ratios for the routine determination of noble gas temperatures that are of immediate relevance to state-of-the-art palaeotemperature reconstruction. This is because all extraction procedures that have been suggested so far either release too much air from the fluid inclusions or introduce artefacts that affect the determination of the noble gas concentrations in the water inclusions. In the case of Kr and Xe, contamination by adsorption on to the sample grains is effectively reduced by pre-crushing the stalagmite samples in extra-pure He. However, this is not true for Ar. Extracting the noble gases by crushing bulk stalagmite samples in vacuo would prevent such adsorption effects altogether. However, the large amounts of noble gases released from the air inclusions that are opened by crushing in vacuo usually mask the relatively small amounts of noble gases extracted from the water inclusions. This is because existing vacuum crushers do not allow the noble gases derived from air inclusions to be removed before extracting the noble gases from water inclusions. An obvious step towards routine noble gas analysis for the robust determination of noble gas temperatures would therefore be to pre-crush and then sieve out stalagmite grains of appropriate sizes in vacuo. Carrying out both pre-crushing and sieving in vacuo would allow uncontaminated calcite crystals of a suitable grain size to be prepared so that the noble gases can then be extracted from the intra-crystalline water inclusions (Vogel et al. 2012). Combining the crushing, sieving and subsequent heating steps in vacuo is likely to be technically challenging. However, in light of the great potential that stalagmite water inclusions have as a noble gas archive for palaeotemperature reconstruction, an attempt at implementing such an all-vacuum procedure would seem to be justified.
It is clear that the concentrations of dissolved noble gases in stalagmite water inclusions can provide valuable information on palaeotemperatures. However, preliminary studies have shown that the gases contained in stalagmite air inclusions should perhaps not be viewed merely as “contamination” that complicates the determination of noble gas concentrations in the water inclusions, but that they may possibly also contain additional information that can aid in interpreting the noble gas concentrations in the water inclusions. By applying the experimental techniques of trace-gas analysis in ice cores to stalagmite samples, Badertscher et al. (2007) and Scheidegger (2011) found the air inclusions to be enriched with \(\mathrm{CO}_{2}\) and \(\mathrm{CH}_{4}\) relative to atmospheric air. Gas inclusions in stalagmites from poorly ventilated caves showed volumetric \(\mathrm{CO}_{2}\) concentrations of up to \(\sim \)10 % (Badertscher 2007). An increased \(\mathrm{CO}_{2}\) partial pressure at the stalagmite surface would reduce the partial pressures of the noble gases during gas-water partitioning before separation of the water inclusions from the cave air. The increased \(\mathrm{CO}_{2}\) partial pressure would therefore result in lower noble gas concentrations, which would need to be taken into account when calculating noble gas temperatures.
In addition, the trace gases in air inclusions might contain useful information about the evolution of cave climates. Badertscher et al. (2007) and Scheidegger (2011) observed that the concentrations and isotopic compositions of \(\mathrm{CO}_{2}\) and \(\mathrm{CH}_{4}\) changed with the age of the stalagmite samples. The gas inclusions thus seem to record the \(\mathrm{CO}_{2}\) and \(\mathrm{CH}_{4}\) composition of the cave air, which is, for instance, related to the processes and rates of biogeochemical turnover in the soil overlying the cave.
It would seem obvious that the new experimental methods described here should also be applied to determine noble gas concentrations in minute amounts of water in other materials containing fluid inclusions. Papp et al. (2010) and Scheidegger (2011) applied these methods to analyse noble gases in fluid inclusions in mollusc shells and corals. However, Scheidegger (2011) found that such biogenic carbonates seem to have very low noble gas concentrations. Shells are known to contain organically bound water, which is formed by biochemical processes and has never been in contact with the surrounding water body. This organically bound water is therefore expected to exhibit lower noble gas concentrations than the surrounding water body. In order to exploit the noble gas concentrations in biological shells as proxies for environmental conditions, new methods would need to be developed to isolate those parts of the organism which contain water that is unaffected by biological processes from the parts of the organism which contain water that is affected by such processes.
The newly developed methods have also been applied to the porewater of consolidated clays that have been targeted as possible host rocks for nuclear waste deposits (Scheidegger 2011). This porewater was shown to be strongly enriched in radiogenic 4He and 40Ar, indicating a long residence time of the porewater within the clays. However, the atmospheric noble gas concentrations determined exceeded the expected atmospheric equilibrium concentrations. Scheidegger (2011) speculated that the clay samples lose water preferentially as a result of surface drying during storage and during evacuation of the gas extraction system, whereas the (heavy) noble gases are retained in the sample (see also, e.g., Osenbrück et al. 1998; Rübel et al. 2002; Mazurek et al. 2011). Although it is too early to draw final conclusions from these preliminary results, the data do seem to imply that the concentrations of noble gases dissolved in the porewater of consolidated sediments reflect the hydrogeochemical origin of the porewater and might allow the characteristic residence time of pore fluids in formations with very low permeability to be estimated. Such analyses might therefore provide assistance in studying the temporal evolution of sedimentary basins.
The new techniques for analysing noble gases in the porewater of unconsolidated sediments make possible a new experimental approach to the study of gas/liquid partitioning in other unconventional “aquatic” environments, such as blood or other body fluids. We sampled human blood in copper tubes, where the blood coagulated. The blood plasma was separated from the coagulated blood (erythrocytes) for noble gas analysis using the centrifuging method of Tomonaga et al. (2011a). For comparison, we also analysed the noble gas concentrations in the remaining erythrocyte fraction using the extrusion method of Brennwald et al. (2003). The concentrations of Kr and Xe in the plasma exceeded their respective ASW concentrations in fresh water, whereas He and Ne concentrations in the plasma were slightly lower than ASW concentrations (Fig. 13). In addition, the erythrocyte fraction showed an enrichment of all atmospheric noble gases relative to the plasma. These results indicate clearly the potential that noble gases possess as tracers to study gas partitioning in body liquids and living tissues; for instance, in analysing gas-exchange processes in the lung.
Noble gas concentrations in human blood plasma and in the erythrocyte fraction remaining after centrifuging (\(C_{i}\), see text), normalised to the relevant ASW concentrations in fresh water (\(C_{i}^*\)) computed using \(T = 36.5\) °C and \(P_{\text{ atm}}= 0.973\) atm (the atmospheric pressure at the altitude where the blood samples were taken)
These examples show that the new experimental methods are powerful tools for studying gas/liquid partitioning in new and sometimes unusual systems and environments. These new methods open up entirely new fields and applications in which terrestrial noble gas geochemistry can play an important role in tracing physical processes—we just have to free our minds and go and find them.
References
Adkins J, McIntyre K, Schrag P (2002) The salinity, temperature, and \(\delta^{18}\text{O}\) of the glacial deep ocean. Science 298:1769–1773. doi:10.1126/science.1076252
Aeppli C, Hofstetter TB, Amaral HIF, Kipfer R, Schwarzenbach RP, Berg M (2010) Quantifying in-situ transformation rates of chlorinated ethenes by combining compound-specific stable isotope analysis, groundwater dating, and carbon isotope mass balances. Environ Sci Technol 44(10):3705–3711. doi:10.1021/es903895b
Aeschbach-Hertig W, Solomon DK (2012) Noble gas thermometry in groundwater hydrology. In: Burnard PG (ed) The Noble Gases as geochemical tracers. Advances in Isotope Geochemistry Springer, New York.
Aeschbach-Hertig W, Peeters F, Beyerle U, Kipfer R (1999) Interpretation of dissolved atmospheric noble gases in natural waters. Water Resour Res 35(9):2779–2792. doi:10.1029/1999WR900130
Amaral H, Berg M, Brennwald MS, Hofer M, Kipfer R (2010) \(^{\rm13}\)C/\(^{\rm12}\)C analysis of ultra-trace amounts of volatile organic contaminants in groundwater by vacuum extraction. Environ Sci Technol 44:1023–1029. doi:10.1021/es901760q
Austin JA, Schlager W, Comet PA, Droxler AW, Eberli GP, Fourcade E, Freeman L, Fulthorpe C, Harwood G, Kuhn G, Lavoie D, Leckie M, Melillo AJ, Moore A, Mullins HT, Ravenne C, Sager WW, Swart P, Verbeek JW, Watkins DK, Williams C (1986) Site 628: Little Bahama Bank. In: Proceedings of the Ocean drilling program, part A: initial reports, College Station, TX (Ocean Drilling Program), vol 101, pp 213–270
Ayliffe LK, Turner G, Burnard PG (1993) Noble gas contents of speleothem inclusion fluids: potential as indicators of precipitation temperature. In: Terra Nova Abstracts, vol 5, p 646
Badertscher S, Fleitmann D, Cheng H, Edwards LR, Göktürk OM, Zumbühl A, Leuenberger M, Tüysüz O (2011) Pleistocene water intrusions from the Mediterranean and Caspian seas into the Black Sea. Nature Geosci 4:236–239. doi:10.1038/NGEO1106
Badertscher SV (2007) Charakterisierung von Einschlüssen in Stalagmiten zur Bestimmung der Paläotemperatur. Master’s thesis, ETH Zürich, Switzerland
Badertscher SV, Scheidegger Y, Leuenberger M, Nyfeler P, Fleitmann D, Wieler R, Kipfer R (2007) Trace gas content in air inclusions in speleothems as a new paleoclimate archive. In: Geophysical research abstracts, 4th EGU general assembly, European Geosciences Union, Vienna, Austria, vol 9, p A0491
Ballentine CJ, Burnard PG (2002) Production, release and transport of noble gases in the continental crust. In: Porcelli D, Ballentine C, Wieler R (eds) Noble gases in geochemistry and cosmochemistry, reviews in mineralogy and geochemistry, vol 47. Mineralogical Society of America, Geochemical Society, pp 481–538
Ballentine CJ, Hall CM (1999) Determining paleotemperature and other variables by using an error-weighted, nonlinear inversion of noble gas concentrations in water. Geochim Cosmochim Acta 63(16):2315–2336. doi:10.1016/S0016-7037(99)00131-3
Ballentine CJ, Burgess R, Marty B (2002) Tracing fluid origin, transport and interaction in the crust. In: Porcelli D, Ballentine C, Wieler R (eds) Noble gases in cosmochemistry and geochemistry, reviews in mineralogy and geochemistry, vol 47. Mineralogical Society of America, Geochemical Society, pp 539–614
Barnes R (1973) An in situ interstitial water sampler for use in unconsolidated sediments. Deep-Sea Res 20:1125–1128. doi:10.1016/0011-7471(73)90026-0
Barnes RO (1979) Operation of the IPOD in situ pore water sampler. In: Sibuet J, Ryan W (eds) Initial reports of the Deep Sea Drilling Project, vol 47, part 2, DSDP, Washington (U.S. Govt. Printing Office), pp 19–22
Barnes RO (1987) Fluid kinematics, fluid residence times, and rock degassing in oceanic crust determined from noble gas contents of Deep Sea Drilling Project pore waters. J Geophys Res 92(B12):12491–12506
Barnes RO (1988) ODP in-situ fluid sampling and measurement: a new wireline tool. In: Mascle A, Moore J (eds) Proceedings of the Ocean Drilling Program, initial reports (Part A), vol 110. ODP, College Station TX, pp 55–63
Barnes RO, Bieri RH (1976) Helium flux through marine sediments of the northern Pacific Ocean. Earth Planet Sci Lett 28(3):331–336. doi:10.1016/0012-821X(76)90194-1
Bayer R, Schlosser P, Bönisch G, Rupp H, Zaucker F, Zimmek G (1989) Performance and blank components of a mass spectrometric system for routine measurement of helium isotopes and tritium by the \(^3\)He ingrowth method. Sitzungsberichte der Heidelberger Akademie der Wissenschaften Mathemathisch-naturwissenschaftliche Klasse 5, University of Heidelberg, Germany.
Berner RA (1975) Diagenetic models of dissolved species in the interstitial waters of compacting sediments. Am J Sci 275:88–96
Bertin C, Bourg ACM (1994) Rn-222 and chloride as natural tracers of the infiltration of river water into an alluvial aquifer in which there is significant river groundwater mixing. Environ Sci Technol 28(5):794–798
Beyerle U, Aeschbach-Hertig W, Imboden DM, Baur H, Graf T, Kipfer R (2000) A mass spectrometric system for the analysis of noble gases and tritium from water samples. Environ Sci Technol 34(10):2042–2050. doi:10.1021/es990840h
Beyerle U, Leuenberger M, Schwander J, Kipfer R (2003) Noble gas evidence for gas fractionation in firn. In: Abstracts of the 13th Annual V.M. Goldschmidt Conference 2003, Kurashiki, Japan, Geochim. Cosmochim. Acta, vol 67, p A38.
Bieri R (1971) Dissolved noble gases in marine waters. Earth Planet Sci Lett 10(3):329–333
Bosch A, Mazor E (1988) Natural gas association with water and oil as depicted by atmospheric noble gases: case studies from the Southeastern Mediterranean Coastal Plain. Earth Planet Sci Lett 87(3):338–346. doi:10.1016/0012-821X(88)90021-0
Bourg IC, Sposito G (2008) Isotopic fractionation of noble gases by diffusion in liquid water: molecular dynamics simulations and hydrologic applications. Geochim Cosmochim Acta 72:2237–2247. doi:10.1016/j.gca.2008.02.012
Brennwald MS, Hofer M, Peeters F, Aeschbach-Hertig W, Strassmann K, Kipfer R, Imboden DM (2003) Analysis of dissolved noble gases in the pore water of lacustrine sediments. Limnol Oceanogr Methods 1:51–62, http://aslo.org/lomethods/free/2003/0051.pdf
Brennwald MS, Peeters F, Imboden DM, Giralt S, Hofer M, Livingstone DM, Klump S, Strassmann K, Kipfer R (2004) Atmospheric noble gases in lake sediment pore water as proxies for environmental change. Geophys Res Lett 31(4):L04202. doi:10.1029/2003GL019153
Brennwald MS, Imboden DM, Kipfer R (2005) Release of gas bubbles from lake sediment traced by noble gas isotopes in the sediment pore water. Earth Planet Sci Lett 235(1–2):31–44. doi:10.1016/j.epsl.2005.03.004
van Breukelen MR, Vonhof HB, Hellstrom JC, Wester WCG, Kroon D (2008) Fossil dripwater in stalagmites reveals Holocene temperature and rainfall variation in Amazonia. Earth Planet Sci Lett 275(1–2):54–60. doi:10.1016/j.epsl.2008.07.060
Chaduteau C, Fourré E, Jean-Baptiste P, Dapoigny A, Baumier D, Charlou JL (2007) A new method for quantitative analysis of helium isotopes in sediment pore-waters. Limnol Oceanogr Methods 5:425–432. http://www.aslo.org/lomethods/free/2007/0425.pdf
Chaduteau C, Jean-Baptiste P, Fourré E, Charlou JL, Donval JP (2009) Helium transport in sediment pore fluids of the Congo-Angola margin. Geochem Geophys Geosyst 10(1). doi:10.1029/2007GC001897
Cheng H, Edwards RL, Broecker WS, Denton GH, Kong X, Wang Y, Zhang R, Wang X (2009) Ice age terminations. Science 326(5950):248–52. doi:10.1126/science.1177840
Copeland P, Watson EB, Urizar SC, Patterson D, Lapen TJ (2007) Alpha thermochronology of carbonates. Geochim Cosmochim Acta 71:4488–4511. doi:10.1016/j.gca.2007.07.004
Craig H, Weiss RF (1971) Dissolved gas saturation anomalies and excess helium in the ocean. Earth Planet Sci Lett 10(3):289–296. doi:10.1016/0012-821X(71)90033-1
Davis B, Brewer S, Stevenson A, Guiot J (2003) The temperature of Europe during the Holocene reconstructed from pollen data. Quaternary Sci Rev 22(15–17):1701–1716. doi:10.1016/S0277-3791(03)00173-2
De Batist M, Imbo Y, Vermeesch P, Klerkx J, Giralt S, Delvaux D, Lignier V, Beck C, Kalugin I, Abdrakhmatov K (2002) Bathymetry and sedimentary environment of Lake Issyk-Kul, Kyrgyz Republic (Central Asia): a large, high-altitude, tectonic lake. In: Klerkx J, Imanackunov B (eds) Lake Issyk-Kul: its natural environment, NATO science series IV: earth and environmental sciences, vol 13. Kluwer Academic Publishers, Boston, pp 101–123
Dreybrodt W (1980) Deposition of calcite from thin films of natural calcareous solutions and the growth of speleothems. Chem Geol 29:89–105
Dyck W, Da Silva FG (1981) The use of ping-pong balls and latex tubing for sampling the helium content of lake sediments. J Geochem Explor 14:41–48. doi:10.1016/0375-6742(81)90102-3
Einstein A (1905) Über die von der molekularkinetischen Theorie der Wärme geforderte Bewegung von in ruhenden Flüssigkeiten suspendierten Teilchen. Annalen der Physik 322(8):549–560. doi:10.1002/andp.19053220806
Faust GT (1950) Thermal analysis studies on carbonates: 1 aragonite and calcite. Am Mineral 35:207–224
Fleitmann D, Burns S, Neff U, Mangini A, Matter A (2003) Changing moisture sources over the last 333,000 years in Northern Oman from fluid inclusion evidence in speleothems. Quaternary Res 60:223–232. doi:10.1016/S0033-5894(03)00086-3
Fleitmann D, Cheng H, Badertscher S, Edwards RL, Mudelsee M, Goektuerk OM, Fankhauser A, Pickering R, Raible CC, Matter A, Kramers J, Tuysuz O (2009) Timing and climatic impact of Greenland interstadials recorded in stalagmites from northern Turkey. Geophys Res Lett 36: L10707. doi:10.1029/2009GL040050
Fritz SC (1996) Paleolimnological records of climatic change in North America. Limnol Oceanogr 41(5):882–889
Giralt S, Riera S, Leroy S, Buchaca T, Klerkx J, De Batist M, Beck C, Bobrov V, Brennwald MS, Catalan J, Gavshin V, Julia R, Kalugin I, Kipfer R, Lignier V, Lombardi S, Matychenkov V, Peeters F, Podsetchine V, Romanovsky V, Shukonikov F, Voltattorni N (2003) 1,000 years of environmental history of Lake Issyk-Kul. In: Nihoul J, Zavialov P, Micklin P (eds) Dying and dead seas: climatic versus anthropic causes, NATO science series IV: Earth and environmental sciences, vol 36. Kluwer Academic Publishers, Boston, pp 228–253
Grathwohl P (1998) Diffusion in natural porous media, topics in environmental fluid mechanics. Kluwer Academic Publishers, Boston
Griffiths ML, Drysdale RN, Vonhof HB, Gagan MK, Zhao Jx, Ayliffe LK, Hantoro WS, Hellstrom JC, Cartwright I, Frisia S, Suwargadi BW (2010) Younger Dryas-Holocene temperature and rainfall history of southern Indonesia from \(\delta ^{18}\text{O}\) in speleothem calcite and fluid inclusions. Earth Planet Sci Lett 295(1–2):30–36. doi: 10.1016/j.epsl.2010.03.018
Henderson GM (2006) Caving in to new chronologies. Science 313(5787):620–622. doi:10.1126/science.1128980
Hoehn E, von Gunten HR (1989) Radon in groundwater—a tool to assess infiltration from surface waters to aquifers. Water Resour Res 25(8):1795–1803
Hohmann R, Schlosser P, Jacobs S, Ludin A, Weppernig R (2002) Excess helium and neon in the southeast Pacific: Tracers for glacial meltwater. J Geophys Res-Oceans 107(C11):3198. doi:10.1029/2000JC000378
Holzner CP, McGinnis DF, Schubert CJ, Kipfer R, Imboden DM (2008) Noble gas anomalies related to high-intensity methane gas seeps in the Black Sea. Earth Planet Sci Lett 265(3–4):396–409. doi:10.1016/j.epsl.2007.10.029
Horseman S, Higgo J, Alexander J, Harrington J (1996) Water, gas and solute movement through argillaceaous media. Technical Report CC-96/1, OECD Nuclear Energy Agency, France
Huber C, Beyerle U, Leuenberger M, Schwander J, Kipfer R, Spahni R, Severinghaus JP, Weiler K (2006) Evidence for molecular size dependent gas fractionation in firn air derived from noble gases, oxygen, and nitrogen measurements. Earth Planet Sci Lett 243(1–2):61–73. doi:10.1016/j.epsl.2005.12.036
Huxol S, Brennwald MS, Hoehn E, Kipfer R (2012) On the fate of \(^{220}\)Rn in soil material in dependence of water content: Implications from field and laboratory experiments. Chem Geol 298–299:116–122. doi: 10.1016/j.chemgeo.2012.01.002
Imboden DM (1975) Interstitial transport of solutes in non-steady state accumulating and compacting sediments. Earth Planet Sci Lett 27(2):221–228. doi:10.1016/0012-821X(75)90033-3
Jähne B, Heinz G, Dietrich W (1987) Measurement of the diffusion coefficients of sparingly soluble gases in water. J Geophys Res 92(C10):10767–10776
Jean-Baptiste P, Mantisi F, Dapoigny A, Stievenard M (1992) Design and performance of a mass-spectrometric facility for measuring helium-isotopes in natural-waers and for low-level tritium determination by the He-3 ingrowth method. Appl Radiat Isotopes 43(7):881–891. doi:10.1016/0883-2889(92)90150-D
Kendall AC, Broughton PL (1978) Origin of fabrics in speleothems composed of columnar calcite crystals. J Sediment Petrol 48:519–538
Kipfer R, Aeschbach-Hertig W, Peeters F, Stute M (2002) Noble gases in lakes and ground waters. In: Porcelli D, Ballentine C, Wieler R (eds) Noble gases in geochemistry and cosmochemistry, reviews in mineralogy and geochemistry, vol 47. Mineralogical Society of America, Geochemical Society, pp 615–700
Kluge T (2008) Fluid inclusions in speleothems as a new archive for the noble gas thermometer. PhD thesis, University of Heidelberg, Germany
Kluge T, Marx T, Scholz D, Niggemann S, Mangini A, Aeschbach-Hertig W (2008) A new tool for palaeoclimate reconstruction: noble gas temperatures from fluid inclusions in speleothems. Earth Planet Sci Lett 269(3–4):408–415
Klump S, Kipfer R, Cirpka OA, Harvey CF, Brennwald MS, Ashfaque KN, Badruzzaman ABM, Hug SJ, Imboden DM (2006) Groundwater dynamics and arsenic mobilization in Bangladesh assessed using noble gases and tritium. Environ Sci Technol 40(1):243–250 doi:10.1021/es051284w
Lachniet MS (2009) Climatic and environmental controls on speleothem oxygen-isotope values. Quat Sci Rev 28(5–6):412–432. doi:10.1016/j.quascirev.2008.10.021
Lan TF, Sano Y, Yang TF, Takahata N, Shirai K, Pinti DL (2010) Evaluating earth degassing in subduction zones by measuring helium fluxes from the ocean floor. Earth Planet Sci Lett 298(3–4):317–322. doi:10.1016/j.epsl.2010.07.049
Lee E, Nam S (2003) Freshwater supply by Korean rivers to the East Sea during the last glacial maximum: a review and new evidence from the Korea Strait region. Geo-Marine Lett 23(1):1–6. doi:10.1007/s00367-003-0118-1
Lerman A, Imboden D, Gat J (1995) Physics and chemistry of lakes. Springer, Berlin. doi:10.1002/iroh.19960810312
Litt T, Krastel S, Sturm M, Kipfer R, Örcen S, Heumann G, Franz S, Ülgen U, Niessen F (2009) ’PALEOVAN’, International Continental Scientific Dilling Program (ICDP) site survey results and perspectives. Quaternary Sci Rev 28:1555–1567. doi:10.1016/j.quascirev.2009.03.002
Litt T, Anselmetti FS, Cagatay MN, Kipfer R, Krastel S, Schmincke HU (2011) A 500,000-year-long sediment archive drilled in Eastern Anatolia. EOS 92(51):477–479
Loose B, Schlosser P, Smethie WM, Jacobs S (2009) An optimized estimate of glacial melt from the ross ice shelf using noble gases, stable isotopes, and CFC transient tracers. J Geophys Res-Oceans 114:C08007. doi:10.1029/2008JC005048
Mamyrin BA, Tolstikhin IN (1984) Helium isotopes in nature, developments in geochemistry, vol 3, 1st edn. Elsevier, Amsterdam, Oxford, New York, Tokyo
Mazor E (1972) Paleotemperatures and other hydrological parameters deduced from gases dissolved in groundwaters, Jordan Rift Valley, Israel. Geochim Cosmochim Acta 36(12):1321–1336. doi:10.1016/0016-7037(72)90065-8
Mazurek M, Alt-Epping P, Bath A, Gimmi T, Waber HN, Buschaert S, De Cannière P, De Craen M, Gautschi A, Savoye S, Vinsot A, Wemaere I, Wouters L (2011) Natural tracer profiles across argillaceous formations. Appl Geochem 26:1035–1064. doi:10.1016/j.apgeochem.2011.03.124
McDermott F, Schwarcz H, Rowe P (2005) Isotopes in speleothems. In: Leng M (ed) Isotopes in palaeoenvironmental research, developments in paleoenvironmental research, vol 10. Springer, pp 185–225
Mohapatra RK, Schwenzer SP, Herrmann S, Murty SVS, Ott U, Gilmour JD (2009) Noble gases and nitrogen in Martian meteorites Dar al Gani 476, Sayh al Uhaymir 005 and Lewis Cliff 88516: EFA and extra neon. Geochim Cosmochim Acta 73(5):1505–1522. doi:10.1016/j.gca.2008.11.030
Musset AE (1969) Diffusion measurements and the potassium-argon method of dating. Geophys J Roy Astron Soc 18(3):257–303. doi:10.1111/j.1365-246X.1969.tb03569.x
Osenbrück K, Lippmann J, Sonntag C (1998) Dating very old pore waters in impermeable rocks by noble gas isotopes. Geochim Cosmochim Acta 62(18):3041–3045. doi:10.1016/S0016-7037(98)00198-7
Ozima M, Podosek FA (2002) Noble gas geochemistry, 2nd edn. Cambridge University Press
Papp L, Palcsu L, Major Z (2010) Noble gas measurements from tiny water amounts: fluid inclusions in carbonates of speleothemes and coral skeletons. In: Geophysical Research Abstracts, 7th EGU General Assembly, European Geosciences Union, Vienna, Austria, vol 12, p A432
Peeters F, Kipfer R, Achermann D, Hofer M, Aeschbach-Hertig W, Beyerle U, Imboden DM, Rozanski K, Fröhlich K (2000) Analysis of deep-water exchange in the Caspian Sea based on environmental tracers. Deep-Sea Res I 47(4):621–654. doi:10.1016/S0967-0637(99)00066-7
Pitre F, Pinti DL (2010) Noble gas enrichments in porewater of estuarine sediments and their effect on the estimation of net denitrification rates. Geochim Cosmochim Acta 74:531–539. doi:10.1016/j.gca.2009.10.004
Poulson TL, White WB (1969) The cave environment. Science 165(3897):971–981. doi:10.1126/science.165.3897.971
Renkin EM (1954) Filtration, diffusion, and molecular sieving through porous cellulose membranes. J General Physiol 38:225–243
Ricketts RD, Johnson TC, Brown ET, Rasmussen KA, Romanovsky VA (2001) The Holocene paleolimnology of Lake Issyk-Kul, Kyrgyzstan: trace element and stable isotope composition of ostracodes. Palaeogeogr Palaeoclimatol Palaeoecol 176(1–4):207–227. doi:10.1016/S0031-0182(01)00339-X
Romanovsky VV (2002) Water level variations and water balance of Lake Issyk-Kul. In: Klerkx J, Imanackunov B (eds) Lake Issyk-Kul: its natural environment, NATO science series IV: earth and environmental sciences, vol 13. Kluwer Academic Publishers, London, pp 45–57
Rübel A, Sonntag C, Lippmann J, Pearson F, Gautschi A (2002) Solute transport in formations of very low permeability: profiles of stable isotope and dissolved noble gas contents of pore water in the Opalinus Clay. Mont Terri. Switzerland. Geochim Cosmochim Acta 66(8):1311–1321. doi:10.1016/S0016-7037(01)00859-6
Sano Y, Wakita H (1987) Helium isotopes and heat flow on the ocean floor. Chem Geol 66(3–4):217–226. doi:10.1016/0168-9622(87)90043-1
Sayles FL, Jenkins WJ (1982) Advection of pore fluids through sediments in the Equatorial East Pacific. Science 217:245–248
Scheidegger Y (2011) The use of noble gases in stalagmite fluid inclusions as proxies for the cave temperature. PhD thesis, Swiss Federal Institute of Technology Zurich (ETH), Switzerland. doi:10.3929/ethz-a-006551468
Scheidegger Y, Kluge T, Kipfer R, Aeschbach-Hertig W, Wieler R (2008) Paleotemperature reconstruction using noble gas concentrations in speleothem fluid inclusions. Pages News 16(3):10–12
Scheidegger Y, Baur H, Brennwald MS, Fleitmann D, Wieler R, Kipfer R (2010) Accurate analysis of noble gas concentrations in small water samples and its application to fluid inclusions in stalagmites. Chem Geol 272(1–4):31–39. doi:10.1016/j.chemgeo.2010.01.010
Scheidegger Y, Brennwald MS, Fleitmann D, Jeannin PY, Wieler R, Kipfer R (2011) Determination of Holocene cave temperatures from Kr and Xe concentrations in stalagmite fluid inclusions. Chem Geol 288(1–2):61–66. doi:10.1016/j.chemgeo.2011.07.002
Scherer P, Schultz L, Loeken T (1994) Weathering and atmospheric noble gases in chondrites. Matsuda J (ed) Noble gas geochemistry and cosmochemistry. Terra Scientific Publishing Company, pp 43–53
Schlosser P, Winckler G (2002) Noble gases in ocean waters and sediments. In: Porcelli D, Ballentine C, Wieler R (eds) Noble gases in geochemistry and cosmochemistry, reviews in mineralogy and geochemistry, vol 47. Mineralogical Society of America, Geochemical Society, pp 701–730
Schrag DP, Hampt G, Murray DW (1996) Pore fluid constraints on the temperature and oxygen isotopic composition of the glacial ocean. Science 272:1930–1932
Schrag DP, Adkins J, McIntyre K, Alexander J, Hodell A, Charles D, McManus J (2002) The oxygen isotopic composition of seawater during the Last Glacial Maxiumum. Quaternary Sci Rev 21(1–3):331–342. doi:10.1016/S0277-3791(01)00110-X
Schwarcz HP, Harmon RS, Thompson P, Ford DC (1976) Stable isotope studies of fluid inclusions in speleothems and their paleoclimatic significance. Geochim Cosmochim Acta 40:657–665
Schwarzenbach RP, Gschwend PM, Imboden DM (2003) Environmental Organic Chemistry, 2nd edn. Wiley, New York
Smithson PA (1991) Interrelationships between cave and outside air temperatures. Theoret Appl Climatology 44(1):65–73
Solomon DK (2000) \(^4\)He in groundwater. In: Cook P, Herczeg AL (eds) Environmental tracers in subsurface hydrology. Kluwer Academic Publishers, Boston, pp 425–439
Solomon DK, Cook PG (2000) \(^3\)H and \(^3\)He. In: Cook P, Herczeg AL (eds) Environmental tracers in subsurface hydrology. Kluwer Academic Publishers, Boston, pp 397–424
Stanley R, Jenkins W (2012) Noble gases in seawater as tracers for physical and biogeochemical ocean processes. In: Burnard PG (ed) The Noble Gases as geochemical tracers, Advances in isotope geochemistry. Springer, New York
Stephenson M, Schwartz WJ, Melnyk TW, Motycka MF (1994) Measurement of advective water velocity in lake sediment using natural helium gradients. J Hydrol 154(1–4):63–84. doi:10.1016/0022-1694(94)90212-7
Strassmann KM, Brennwald MS, Peeters F, Kipfer R (2005) Dissolved noble gases in porewater of lacustrine sediments as palaeolimnological proxies. Geochim Cosmochim Acta 69(7):1665–1674. doi:10.1016/j.gca.2004.07.037
Sültenfuss J, Roether W, Rhein M (2009) The Bremen mass spectrometric facility for the measurement of helium isotopes, neon, and tritium in water. Isotopes In Environmental and Health Studies 45(2):83–95. doi:10.1080/10256010902871929
Swart KS (2000) The oxygen isotopic composition of interstitial waters: evidence for fluid flow and recrystalization in the margin of the Great Bahama Bank. In: Proceedings of the Ocean Drilling Program, Scientific Results, vol 166, pp 91–98
Tomonaga Y (2010) Noble gases as tracers for transport of solutes and fluids in lake and ocean sediments. PhD thesis, Swiss Federal Institute of Technology Zurich (ETH), Switzerland. doi:10.3929/ethz-a-006129449
Tomonaga Y, Brennwald MS, Kipfer R (2011a) An improved method for the analysis of dissolved noble gases in the pore water of unconsolidated sediments. Limnol Oceanogr, Methods 9:42–49
Tomonaga Y, Brennwald MS, Kipfer R (2011b) Spatial distribution and flux of terrigenic He dissolved in the sediment pore water of Lake Van (Turkey). Geochim Cosmochim Acta 75(10):2848–2864. doi:10.1016/j.gca.2011.02.038
Top Z, Izdar E, Ergün M, Konuk T (1990) Evidence for tectonism from \(^3\)He and residence time of helium in the Black Sea. EOS 71:1020–1021
Torres M, Bayer R, Winckler G, Suckow A, Froelich P (1995) Elemental and isotopic abundance of noble gases in formation fluids recovered in situ from the Chile Triple Junction. In: Lewis S, Behrmann J, Musgrave R, Cande S (eds) Proceedings of the Ocean Drilling Program, Scientific Results, vol 141. ODP, College Station TX, pp 321–329
Vogel N, Scheidegger Y, Brennwald MS, Fleitmann D, Figura S, Wieler R, Kipfer R (2012) Noble gas paleotemperatures and water contents of stalagmites—a new extraction tool and a new paleoclimate proxy. In: Geophysical Research Abstracts, EGU General Assembly 2012, European Geosciences Union, Vienna, Austria, vol 14
Wainer K, Genty D, Blamart D, Daeron M, Bar-Matthews M, Vonhof H, Dublyansky Y, Pons-Branchu E, Thomas L, van Calsteren P, Quinif Y, Caillon N (2011) Speleothem record of the last 180 ka in Villars cave (SW France): Investigation of a large \(\delta ^{18}\text{O}\) shift between MIS6 and MIS5. Quaternary Sci Rev 30(1–2):130–146. doi:10.1016/j.quascirev.2010.07.004
Wang Y, Cheng H, Edwards RL, Kong X, Shao X, Chen S, Wu J, Jiang X, Wang X, An Z (2008) Millennial- and orbital-scale changes in the east asian monsoon over the past 224,000 years. Nature 451(7182):1090–1093. doi:10.1038/nature06692
Wanner H, Beer J, Bütikofer J, Crowley T, Cubasch U, Flückiger J, Goosse H, Grosjean M, Joos F, Kaplan J, Küttel M, Müller S, Prentice I, Solomina O, Stocker T, Tarasoc P, Wagner M, Widmann M (2008) Mid- to Late Holocene climate change: an overview. Quaternary Sci Rev 27:1791–1828
Winckler G (1998) Radiogenes Helium im Ozean: Drei Fallstudien. PhD thesis, University of Heidelberg, Germany
Winckler G, Kipfer R, Aeschbach-Hertig W, Botz R, Schmidt M, Schuler S, Bayer R (2000) Sub sea floor boiling of Red Sea brines: new indication from noble gas data. Geochim Cosmochim Acta 64(9):1567–1575. doi:10.1016/S0016-7037(99)00441-X
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2013 Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
Brennwald, M., Vogel, N., Scheidegger, Y., Tomonaga, Y., Livingstone, D., Kipfer, R. (2013). Noble Gases as Environmental Tracers in Sediment Porewaters and Stalagmite Fluid Inclusions. In: Burnard, P. (eds) The Noble Gases as Geochemical Tracers. Advances in Isotope Geochemistry. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-642-28836-4_6
Download citation
DOI: https://doi.org/10.1007/978-3-642-28836-4_6
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-642-28835-7
Online ISBN: 978-3-642-28836-4
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)