Abstract
Since the inception of the microscope, optical imaging is serving the biological discovery for more than four centuries. With the recent emergence of methods appropriate for in vivo staining, such as bioluminescence, fluorescent molecular probes, and proteins, as well as nanoparticle-based targeted agents, significant attention has been shifted toward in vivo interrogations of different dynamic biological processes at the molecular level. This progress has been largely supported by the development of advanced optical tomographic imaging technologies suitable for obtaining volumetric visualization of biomarker distributions in small animals at a whole-body or whole-organ scale, an imaging frontier that is not accessible by the existing tissue-sectioning microscopic techniques due to intensive light scattering beyond the depth of a few hundred microns. Biomedical optoacoustics has also emerged in the recent decade as a powerful tool for high-resolution visualization of optical contrast, overcoming a variety of longstanding limitations imposed by light scattering in deep tissues. By detecting tiny sound vibrations, resulting from selective absorption of light at multiple wavelengths, multispectral optoacoustic tomography methods can now “hear color” in three dimensions, i.e., deliver volumetric spectrally enriched (color) images from deep living tissues at high spatial resolution and in real time. These new-found imaging abilities directly relate to preclinical screening applications in animal models and are foreseen to significantly impact clinical decision making as well
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Optical Imaging
- Light Attenuation
- Fluorescence Molecular Imaging
- Molecular Imaging Investigation
- Optoacoustic Imaging
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Introduction
Microscopy has been a major optical imaging tool for more than three centuries. Yet optical imaging is a rapidly emerging imaging science with remarkable new approaches continuously emerging to improve on the capabilities and application potential; ultimately impacting biological discovery and healthcare. A significant role in these developments has played the discovery, development, and propagation of fluorescent proteins and probes, as well as bioluminescence to in vivo imaging applications (Tsien 2005; Giepmans et al. 2006; Contag and Bachmann 2002). Linked to this progress is the ability to visualize cancer features and biomarkers with high versatility, spanning all areas of anatomic, functional, and molecular visualization.
While microscopic viewing of such markers is possible using advanced forms of microscopy such as confocal or two-photon/multi-photon microscopy (Denk et al. 1990; Webb 1999; Helmchen and Denk 2005), the penetration depths of even the most advanced forms of microscopy rarely exceed 500 microns in most tissues in vivo. While in optically cleared (transparent) samples greater depths can be reached, in vivo nontransparent tissues limit the penetration ability of modern microscopy due to strong photon scattering by various cellular organelles and membranes. Therefore imaging of various forms of optical contrast deeper than 500 microns requires the development of different optical imaging approaches that can handle the effects of scattering at greater depths.
Early optical imaging systems were based on photographic principles by simply utilizing a sensitive CCD camera to take pictures of animals at a wavelength range of preference, for example at a range where bioluminescence or fluorescence was emitted. Such systems offered no correction for scattering and for this reason yielded low resolution and generally inaccurate images, since they could not account for the effects of depth or of light attenuation as a function of the possible variation of the tissue’s optical properties. In response, optical imaging experienced a slow propagation into the biological practice with mixed experiences reporter depending on the user and the application.
Modern macroscopic optical imaging, however, moves away from these early attempts and utilizes tomographic hybrid approaches to offer highly robust and accurate imaging performance. Technologies such as hybrid Fluorescence Molecular Tomography-X-ray Computer Tomography (FMT-XCT) systems (Schulz et al. 2010) or multi-spectral optoacoustic tomography (MSOT) systems (Razansky et al. 2009; Ntziachristos and Razansky 2010) bring unprecedented levels of performance. This new found performance not only goes well beyond early attempts with photographic imaging but challenges even other established imaging modalities, within the operation range of optical imaging, i.e. 2–5 cm penetration in the near-infrared spectral region. The outcome is a technology that is well-suited for macroscopic imaging of small animals but also clinical endoscopic applications.
In the following we discuss new developments in optical imaging, in particular MSOT and FMT-XCT, which are expected to revolutionize the field of optical imaging in general. Whereas focus is given herein to small animal imaging, since a significant part of recent literature demonstrates these technologies with small animals, we stress again the significant potential for clinical translation of these methodologies.
2 Multi-Spectral Optoacoustic Tomography
Optoacoustic interrogations of biological tissues has been considered since the early 1970s (Rosencwaig 1973; Bowen et al. 1982; Oraevsky and Esenaliev 1994) and offers a powerful methodology for molecular imaging investigations. In vivo imaging of cellular and subcellular markers can be achieved by MSOT, an emerging field in the imaging sciences. MSOT overcomes major limitations of conventional optical imaging while it retains many of the advantages of photonic methods.
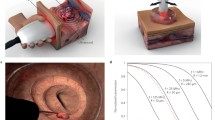
The MSOT principle of operation is shown on Fig. 1. Short laser pulses in the nanosecond range illuminate the tissue of interest over an area of interest. Absorption of the fast laser pulses by tissue photoabsorbers, such as oxy- and deoxy-hemoglobin, melanin, or extrinsically administered probes and agents creates a transient temperature increase which in turn leads to a thermoelastic expansion. This process creates acoustic waves in the 1–100 MHz range which can then be detected with multiple ultrasound elements also places around the illuminated area. By combining the ultrasonic measurements in the mathematical data inversion scheme, high resolution images of tissue can be produced. The amplitude of the generated broadband ultrasound waves reflects local optical absorption properties. The spatial resolution of the method is therefore solely determined by the diffraction limit of ultrasound waves or the available bandwidth of the ultrasonic detector.
Principle of MSOT operation. a Pulsed light of time-shared multiple wavelengths illuminates the tissue of interest and establishes transient photon fields in tissue. b In response to the fast absorption transients by tissue elements, acoustic responses are generated via the thermoacoustic phenomenon, which are then detected with acoustic detectors. By modeling photon and acoustic propagation in tissues and using inversion (tomographic) methods images can then be generated and spectrally unmixed to yield the biodistribution of reporter molecules and tissue biomarkers. Taken from Ref. (Ntziachristos and Razansky 2010)
MSOT further employs spectral identification of known reporter molecules, such as common fluorochrome or other chromophores, dyes and photoabsorbing nanoparticles. Molecules with spectra that are different than the ones of background tissue can be accurately resolved by MSOT with high specificity (Razansky et al. 2007). However, in practice, MSOT images obtained from tissues represent a mixed contribution of photon energy delivered in each volume element imaged and the total absorption contribution from the volume element. Consequently significant measures are taken to decompose the resulting image from the effects of inhomogeneous light attenuation in the tissue of interest (Rosenthal et al. 2009; Rosenthal et al. 2010).
The MSOT ability to detect reporter molecules from tissues has been showcased by visualizing common fluorochrome embedded deep in mice (Razansky et al. 2007; Buehler et al. 2010; Li et al. 2008). This was achieved without the need of baseline measurements obtained before the administration of the probe. This approach operates optimally by selecting fluorochromes (or possibly chromophores) with a steep-drop in their absorption spectrum and accordingly selects the imaging wavelengths to capture this absorption change (Fig. 2d). When utilizing fluorescent dyes, the emphasis is on low quantum-yield fluorochrome, which are particularly useful for optoacoustic signal excitation. The absorption spectrum of common organic fluorochromes drops significantly in the spectral window 750–850 nm, compared to the relatively smooth absorption variation of the spectra of common tissue chromophores in the NIR. Therefore intrinsic tissue contrast can be readily suppressed with a multi-spectral approach, yielding highly sensitive imaging of fluorochrome distribution in tissue obtained by spectral matching of photo-acoustic images acquired at several different adjacent wavelengths. Figure 2e shows spectrally-resolved MSOT image (in color), superimposed onto a single-wavelength anatomical image. The imaged is obtained by spectrally processing images at different wavelengths (Fig. 2) a–c for identifying the know spectrum of the AF750 molecule. While the simplest version of spectral matching operation can be achieved by image subtraction at two wavelengths, three- and overall multi-spectral imaging further suppress the background signals. Multi-spectral imaging further attains the potential to resolve multiple purely absorbing or absorbing/fluorescing dyes and probes in tissues and the overall method can be improved by more accurately considering the relative background absorption attenuation of tissue at each of the wavelengths used.
MSOT visualizes distribution of fluorescent molecular probe (AlexaFluor 750™) in a mouse leg13. a, b, and c are cross-sectional optoacoustic tomographic reconstructions acquired at 750, 770, and 790 nm, respectively. d Absorption as a function of wavelength for AF750 fluorescent probe as compared to some intrinsic tissue chromophores. Arrows indicate the three wavelengths used to spectrally resolve the probe location. e Spectrally-resolved MSOT image that incorporates measurements at all the three indicated wavelengths (in color), superimposed onto a single-wavelength anatomical image. f Corresponding ultrasonic image, acquired approximately at the same imaging plane, using 25 MHz high-resolution ultrasound system. g Planar epi-fluorescence image of dissected tissue confirms the fluorochrome location. Image and caption taken from Ref. (Ntziachristos and Razansky 2010)
Figure 3 shows an additional example of resolving the fluorescent dye ICG perfusing the mouse kidney of a nude female mouse in vivo. In this case MSOT has been implemented in video rate format (Buehler et al. 2010), where a single slice can be obtained out of a single laser pulse without data averaging. In this manner real-time data can be obtained with reduced sensitivity to motion artifacts.
MSOT of a fluorochrome (IndoCyanine Green; ICG) injected intravenously in a mouse leg in vivo. The images are cross-sectional optoacoustic images at different time-points of a the kidney anatomy before and as a function of ICG perfusion and b ICG distribution (in color) superimposed on the image of the kidneys before injection (top left image). The images are obtained from a female CD1 mouse injected with 0.33 μmoles of ICG. Images taken from Ref. (Buehler et al. 2010)
Since fluorescent proteins also have distinct spectra, it is possible to detect them using MSOT as was recently shown in images from the adult zebrafish (Razansky et al. 2009). It was shown that MSOT is possible through several 4–6 mm of developed non-transparent fish expressing the fluorescent protein mCherry in the brain and notochord. The resolution achieved in this case was 38 microns. Fluorescent proteins were resolved alongside anatomical images of the fish in three dimensions and in vivo. Overall, the development of fluorescent proteins with absorption spectral in the near-infrared opens exciting possibilities for the wide utilization of fluorescent protein MSOT imaging.
A particular strength of the MSOT technology is its ability to simultaneously deliver anatomical, functional, and molecular contrast from tissues, which is usually possible only if several different modalities are used for imaging. Moreover, MSOT scales well with different tissue sizes and, as demonstrated in phantom experiments, several centimeters of penetration with high spatial resolution can be achieved, especially when employing near-infrared light. Indeed, size of many relevant biological samples and model organisms, e.g. worms, developing and adult insects and vertebrates including small mammals and their extremities, lie in this range and could be visualized. Finally, optoacoustics is an inherently fast imaging technology (Buehler et al. 2010) therefore it holds great potential for real-time imaging of fast events and dynamic processes, such as pharmacokinetics, in living organisms.
Other molecules that can be used for MSOT applications include gold and carbon nanoparticles, nanoshells, nanocages, and carbon nanorods (La Zerda et al. 2008; Rayavarapu et al. 2007; Ntziachristos and Razansky 2010), all shown to increase optoacoustic signals in vivo. LacZ gene encoding for the X-gal chromogenic substrate expression and other possible chromogenic assays are also of potential interest (Li et al. 2007). These agents can improve the MSOT sensitivity but may offer other challenges associated with biodistribution and toxicity. Regardless they offer valuable mechanisms for MSOT contrast generation and their use is expected to significantly increase.
Many other dedicated contrast agents could potentially be developed for optoacoustic imaging applications, but additional studies will be required in order to address a variety of efficiency, dosing and safety, and toxicity concerns associated with the in vivo administration of these agents. Moreover, due to their relatively wide absorption spectra, nanoparticle-based contrast approaches typically require a background (before) image in order to attain specificity. On the other hand, MSOT has spectral mechanisms to differentiate molecules of distinct optical signatures, thus, many widely adopted optical contrast agents, such as fluorochromes, or gold nanoparticles can be efficiently used. Many fluorochromes, e. g. Alexa or Cy-based dyes, ICG, fluorescent proteins (GFP, RFP), or gold nano-rods exhibit sharp resonances in the vicinity of their peak excitation, making them convenient candidates for highly sensitive multi-wavelength imaging.
2.1 Sensitivity of Biomarker Detection
While the feasibility of different optoacoustic imaging implementations has been showcased, the overall sensitivity of the method for different experimental scenarios needs to be addressed from a theoretical and systematic stand-point (Razansky et al. 2009). This calculation and discussion, modified from Refs. (Razansky et al. 2009; Ntziachristos and Razansky 2010; Razansky and Ntziachristos 2010) considers the theoretically predicted sensitivity of the method over wide range of imaging-related parameters and considers the necessary experimental reference measurements for validating the theoretical findings. Overall, the determination of the MSOT detection sensitivity limits, as it relates to the sensitivity of optoacoustic tomography is not straightforward. This is because optoacoustics is a high resolution modality and an experimental determination of the sensitivity as a function of e.g. marker size or volume remains difficult since this would require reproducible creation of small volumes (e.g. 100 μm or less) containing well-defined concentrations of markers. Nevertheless, a prediction can alternatively be made by imaging larger amounts of the same marker and placing the measured value on the appropriate parameter-dependent signal intensity curve. A performance estimate of particular experimental system and the expected SNR for smaller amounts of markers can then be made (Razansky et al. 2009). To simulate optoacoustic signals emanating from a target biomarker, an absorbing sphere is considered embedded at different depths in tissue-mimicking scattering and absorbing phantoms. By accounting for diffuse light distribution and ultrasound dispersion as it occurs in tissues, system-dependent characteristics can be removed to yield a better understanding of performance and physical limitations of target detection using optoacoustics. In Fig. 4, a range of clinically relevant biomarker concentrations are examined, covering different target radii, and tissue dimensions.
Simulated optoacoustic signal strengths from an experimental and clinical point of view. a Signals detected from a 2 mm diameter target (Cy5.5 fluorescent dye) for increasing target depths at various concentrations. b Signals from increasing sized targets containing 5 μM of the dye at various depths. c Signals from the 2 mm diameter target containing 1 μM of fluorochrome at increasing depths of media simulating various human tissues. Graphs and the details of the study can be found in Ref. (Razansky et al. 2009)
Visualization of brain structure and function using photoacoustic tomography. Functional maps of brain activities corresponding to the left-side (a) and the right-side (b) whisker stimulations, respectively, acquired with the skin and skull intact. Images taken from review paper (Ntziachristos et al. 2005) originally published in Ref. (Wang et al. 2003)
Using such analysis, the optoacoustic detection limits are found constrained by the interplay of light penetration and ultrasonic frequency-dependent attenuation (dispersion) and exhibit a nonlinear performance in the detection limit, which invalidates simplistic linear predictions of optoacoustic sensitivity (Fig. 5). In particular, while optoacoustic signals depend linearly on probe concentration, they exhibit a nonlinear dependence not only as a function of target depth but also as a function of its volume. Naturally, the dependence of signal intensity on depth for a target of constant volume is determined by the nonlinear light attenuation with depth due to absorption and scattering and the corresponding sound attenuation. In particular, the steep attenuation of light causes a drop in the energy absorbed by targets as a function of depth and the corresponding detected optoacoustic signal intensity. On the other hand, when varying the lesion size instead, additional effects take place. First, as expected, the resulting light energy deposition is decreasing with target volume, simultaneously reducing the detected optoacoustic signal (voltage) as a function of square root of the deposited energy, i.e. as d 3/2 (d being the characteristic lesion size). It was, however, further noticed that for small targets (in practice, less than 0.2–0.5 mm in size), effects of ultrasonic dispersion start playing increasingly dominant role in the reduction of the detected optoacoustic signal intensity, owing to increased attenuation of high-frequency sound components. This important finding demonstrates that it would be inaccurate to linearly extrapolate the detection limits of optoacoustics from data obtained on larger lesions. All these effects create a complex estimation model on detection limits, which is defined also in the context of lesion size and depth, not only lesion concentration. Recent in vivo and phantom studies predicted detection limits on the order of 300 femtomoles for common fluorochromes in the near-infrared and 300 cells for red-shifted fluorescent proteins (Razansky et al. 2009).
2.2 Other Applications of Optoacoustic Imaging
While MSOT offers high potential for molecular imaging investigations, optoacoustic imaging is sensitive to all optical absorbers, the major one in tissues being hemoglobin. Therefore optoacoustic imaging has been extensively used to image vascularization, as shown in Fig. 4. As evident on the figure, it is not only the anatomy but importantly the functional information on oxygen saturation and blood oxygenation that can be measured, with further implications in resolving disease processes in oncology as well, in particularly relating to angiogenesis and hypoxia. In addition, other absorbing structures can be visualized, for example, in imaging anatomical images from other tissues, such as fat, bones, and other structures and organisms having no hemoglobin-based contrast (Fig. 6).
Images obtained with selective-plane optoacoustic tomography. Cross-sectional optoacoustic images of an intact Drosophila melanogaster pupae from a top part containing dark-color (highly absorbing) sensory organ of the pupa; and b salivary glands area. c Histological section of the pupa at the salivary gland area (blue-dapi staining; green—GFP fluorescence expressed in the fatty structures). Images from Lumbricus Terrestris (Earthworm) are shown in d Selective-plane optoacoustic image; e Anatomical diagram; and f The corresponding ultrasound image acquired using high-resolution ultrasound imaging system operating at 25 MHz. Images taken from Ref. (Razansky et al. 2009)
3 FMT-XCT
Fluorescence imaging deep in tissues is also possible using tomographic optical imaging approaches. In contrast to simple photographic imaging where the sample is illuminated with an expanded light beam and using a sensitive camera to collect fluorescence images from the same side as the illumination (epi-illumination) tomography is best implemented in transillumination, i.e. the light sources and the camera being on opposite sides of the tissue imaged or at some angle to each-other, and approach that allows sampling deeper parts of the tissue (Ntziachristos et al. 2005). Tissue of several centimeters thickness can be imaged when near-infrared light is used, due to the low attenuation of light by tissue in this range. The following discussion showcases progress with FMT, an optical tomography method developed for fluorescence molecular imaging that is combined with an anatomical modality to yield a better optical imaging tomographic system.
The principle of operation of FMT resembles that of XCT in that tissue is illuminated from different angles and at different positions and a mathematical formulation is used to describe photon propagation in tissue. However, a major difference between optical tomography and tomographic methods based on high energy rays is that photons in the optical range are highly scattered by tissue organelles and membranes. Photons do not propagate in straight lines when travelling through tissue, but become diffuse within a few millimeters of propagation. The diffusive nature of the light propagation through tissue limits the quantification ability and maximum resolution that can be achieved. Therefore, FMT attempts to localize and quantify fluorescent signals distributed in tissue. Yet the inversion problem is ill-posed, i.e. it does not have a unique or easy to find solution and for this reason leads to uncertainty that eventually limits the resolution and the overall image fidelity achieved, especially for complex problems such as a distributed fluorescence activity in optically heterogeneous tissue.
One approach to improve on the reconstruction ability is to combine FMT with another anatomical imaging method. The rationale is that by using co-registered anatomical information a hybrid FMT approach can build more accurate problems of photon propagation in tissues and restrain the inverse problems. For this reason there has been an emergence of hybrid imaging systems combining FMT with another modality. MRI can restrict the optical implementation due to the limited bore dimensions of an MRI magnet; eventually restricting the number of optical sources; and detectors that can be placed around an animal or tissue. On the other hand, XCT systems offer gantry implementation with the ability to place freely high quality and spatial sampling optical components, including mounting CCD cameras and scanning laser beams on the tissue surface offering a highly adept data set. This type of hybrid scanner allows therefore 360° projection viewing for both FMT and XCT and high spatial sampling of X-ray and photon fields leading to a high quality data set available for reconstructions. The combination of the FMT and XCT modality into a single system eliminates the need to transfer the imaging subject from one system to the other using elaborate methods for co-registration of images but allows for highly accurate image registration between the two modalities and for the possibility of simultaneous acquisition, although typically the FMT takes 2–10 times longer to acquire due to the laser scanning requirements.
An implementation of a gantry-based FMT-XCT system was proposed (Schulz et al. 2010) and shown in Fig. 7. In this implementation, diode lasers at different wavelengths in the near-infrared range are attached onto the gantry system and used for tissue illumination by employing a scanning mechanism based on motor-stages. The tissue illumination is based on a focused laser beam that established a point source on the animals surface. Using the motor stages this point source is scanned in one side of the animal. Data are collected on the other side of the animal using a CCD camera. By rotating the entire gantry around the animal, this process is repeated at different projections and a multi-projection data set is collected as raw data to feed an inversion algorithm for image reconstruction. An imaging example from reconstructing the fluorescence activity of a lung tumor, using the X-ray CT information into the optical inversion code is shown in Fig. 8. The particulars of the algorithm utilized for the reconstruction go beyond the scope of this chapter but can be found in detail in Ref. (Ale et al. 2010).
A hybrid FMT-XCT small animal imaging system implemented within the rotating gantry of a micro-CT scanner from General Electric. System details in Ref. (Schulz et al. 2010)
Imaging of a lung tumor implanted in a nude mouse using the hybrid FMT-XCT system and method. a XCT slice obtained from the thorax; the arrow points to the location of the tumor. b Fluorescence signal (in color) superimposed to the anatomical image in (a). The fluorochrome utilized here activates in the presence of proteases present in tumors. c Three dimensional rendering from the entire upper thorax scanned. Data from the studies included in Ref. (Schulz et al. 2010). The inversion algorithm used is described in Ref. (Ale et al. 2010)
Typically the illumination utilized in of constant intensity (constant wave or CW) since this approach leads to economical implementations and offers good signal-to-noise characteristics and is generally operationally simple and robust (Ntziachristos et al. 2005). One limitation of using CW light is the difficulty in separating scattering from absorption characteristics when such differentiation is required. This is not necessarily major complication in fluorescent imaging but becomes important when intrinsic tissue contrast is imaged. There are two other possible illumination strategies, one using light of modulated intensity (frequency domain), typically at frequencies of 100 MHz–1 GHz, or the use of ultrafast photon pulses (time-domain) in the 100 fs–100 ps range. In this case, the detection systems employed can measure changes in light attenuation and phase at different frequencies, for intensity modulating sources, or resolve the arrival of photons as a function of time, in time-resolved implementations. Frequency- and time domain methods can separate absorption from scattering and, in principle, also resolve fluorescence lifetime, although it remains difficult to measure the lifetime of fluorochrome deep inside tissues, since the broadening of the fluorescence response due to lifetime is mixed with the broadening of the fluorescence response due to tissue absorption and scattering and as a function of depth, which makes their separation very challenging.
4 Overview of Performance Characteristics
Based on the results of recent studies, hybrid optical methods such as MSOT and FMT-XCT offer revolutionary performance characteristics over other optical imaging approaches. Both methods operate on very versatile molecular contrast, cost-effectiveness, and the use of non-ionizing radiation, as summarized in Fig. 9. MSOT in addition offers high resolution imaging and depending on the optical reporter used can also lead to high sensitivity imaging, in particular, using gold nanoparticles. FMT offers great sensitivity in resolving fluorochrome although with reduced resolution compared to MSOT. For these reasons both methods can find several applications in small animal imaging research, although their sensitivity drops exponentially with depth and it not expected to propagate to whole-body imaging of animals larger than a rat. However, they can still be employed for imaging dedicated organs not only in pre-clinical but also clinical applications, for example, in endoscopic imaging. When considering clinical applications, the methods described can find several niche focus points due to the high sensitivity, resolution, and portability, and could shift the paradigm of healthcare offering a safe point-of-care imaging modality for highly disseminated imaging. Both methods are expected to enter focused segments of the therapeutic efficacy and possibly the diagnostic segments, especially in areas not well served by current imaging modalities. Due to the limited penetration these methods do not compete with established modalities such as MRI or PET, but define new operational areas in interventional imaging.
5 Quantification
An important aspect of utilization of the methods discussed herein is their quantification ability. Typically, inaccurate algorithms can still offer images; however, with utility that does not fully capitalize on the potential of the methods considered. One important aspect in optical imaging methods is that of light attenuation with depth and as a function of the spatial variation of the optical properties of tissue. At the absence of accounting for the potentially strong spatial differences changes of photon intensity in tissue, the resulting images can offer significant artifacts, and lead to misleading conclusions.
For MSOT, it has been shown that backprojection-related artifacts can be avoided by use of the so-called model-based inverse methods (Rosenthal et al. 2010; Cox et al. 2006). The following discussion modified from Ref. (Ntziachristos and Razansky 2010) illuminates key aspects of MSOT artifacts and solutions for accurate inversion. In particular, in contrast to back-projection algorithms, model-based schemes are not based on an approximate analytical solution of the optoacoustic equation. Instead, the forward problem is solved numerically in an iterative optimization algorithm. In each iteration, the reconstructed optoacoustic image is changed to reduce the error between its corresponding acoustic signals and the measured signals. Since the forward problem is linear, the optimization problem has only a single minimum. Ideally, this approach can yield artifact-free quantified reconstructions. However, the computational complexity involved with model-based schemes has so far severely limited their achievable resolution. The two contributing factors to the high complexity were the low efficiency/accuracy of the numerical forward solution and the need of a high number of iterations. Recently, a novel semi-analytical model-based inversion scheme for quantitative optoacoustic image reconstruction was suggested (Rosenthal et al. 2010), where the presented semi-analytical solution is exact for piecewise planar acoustic-source functions, which significantly improves the accuracy and computational speed. The method eliminates image artifacts associated with the approximated back-projection formulations, i.e. no negative absorption values are produced and the reconstructed image corresponds to the true light attenuation and energy deposition within the object.
Clearly, since back-projection falsely emphasizes edges and fast image variations by producing large negative overshoots, it is capable of producing ‘good looking’ high-contrast images. However, due to its approximate formulation, it fails to reproduce the correct and quantitative image of the actual laser energy deposition in tissue and the underlining optical absorption values. This property is especially important for quantitative imaging applications, i.e. molecular imaging studies, in which obtaining the correct absorption maps is of high importance. Second, the model-based framework admits generalization of the forward solution to a more comprehensive acoustic propagation models without changing the inversion procedure. For instance, the frequency response of the acoustic detector as well as additional linear effects, such as the frequency-dependant acoustic attenuation and the detector’s focusing characteristics can also be conveniently and rigorously incorporated into the model. Finally and importantly, the model-based inversion can be seamlessly adapted to any detection geometry.
Of equal importance are adept algorithms utilized in FMT that can correct for light attenuation and offer accurate, artifact free-images. One particular algorithm that has been shown appropriate for in vivo imaging normalizes raw fluorescent data by data obtained under the identical illumination and geometrical conditions by simply switching a filter in front of the camera utilized in order to capture an adjacent spectral window where excitation photons propagate (Ntziachristos and Weissleder 2001). Data obtained in the excitation wavelength contain information on the spatially dependent attenuation of light in tissues and the use of normalization in the data feeding the inversion helps reducing the sensitivity of the imaging method to the variation of optical properties. Regardless, this normalization is not perfect and contains different sensitivity to the variation of absorption vs. the variation of scattering (Soubret et al. 2005). To further improve on the accuracy of the method, it is possible to utilize the XCT information to develop a more accurate reconstruction problem (Hyde et al. 2009). In that respect the FMT-XCT system not only offers the ability of producing anatomical and molecular imaging data that are accurately registered, but overall leads to a more quantitative optical tomography system.
6 Optical Imaging Applications in Oncology
In addition to microscopy, macroscopic optical imaging has been considered in accelerating biological investigations at different system levels and in unperturbed host environments. While a limiting factor of conventional optical imaging has been the quantitative accuracy and the superficial nature of the images produced, MSOT and FMT-XCT come with performance that can rival many of the other molecular imaging techniques applied to macroscopic optical imaging. Therefore they can play a major role in biological discovery and pharmaceutical research.
Optical imaging significantly benefits from the rich intrinsic and extrinsic optical contrast of tissues in the visible and near-infrared spectra. In addition to specific absorption by natural chromophores (such as oxy- and deoxyhemoglobin or melanin) a large number of commercially available or investigational fluorescent probes and markers have shown ability to enable a highly potent field for biological imaging (Tsien 2005; Giepmans et al. 2006; Ntziachristos et al. 2005; Weissleder and Ntziachristos 2003). So far, optical probes were proven efficient in a number of clinical and small-animal applications, including probing of tissue hemodynamics or gene expression, detecting protease up-regulation associated with cancer growth and inflammation, monitoring apoptosis and the efficacy of anti-cancer treatments, and imaging specific markers involved in cancer growth and metastasis (Ntziachristos 2006).
MSOT in particular can deliver anatomical, functional, and molecular tissue biomarkers opening significant possibilities for a highly versatile interrogation of various tissue biomarkers linked to optical contrast. The method can image and separate different molecules from each other, based on their distinct spectral profiles. In that respect multiple targets and functions can be simultaneously imaged, potentially building a more accurate picture of the underlying biology. Optoacoustic imaging operates seamlessly within scales, within the penetration limits of light, i.e. 2–5 cm in muscle, deeper in lower attenuating tissues such as breast tissue in the near-infrared; the resolution achieved improving at shallower depths. Therefore MSOT offers a complementary method to microscopy—significantly extending the investigational depths available to optical imaging and linking macroscopic observation to microscopy. In addition, the technique is inherently fast, since images can form per single pulse. For this reason real-time imaging of fast dynamic processes is also possible, which allows accurate studies not only of pharmacokinetics but even faster functional events. Due to these features, MSOT can become an important tool in small animal imaging research. Conversely FMT-XCT can capitalize on existing fluorescence imaging probes and resolve them with accuracy and high sensitivity while significantly improving on the capacities of the combined systems, from offering a more accurate FMT method to adding molecular imaging capacity to XCT systems.
References
Ale A, Schulz R, Sarantopoulos A, Ntziachristos V (2010) Imaging performance of a hybrid x-ray computed tomography-fluorescence molecular tomography system using priors. Med Phys 37:1976–1986
Bowen T (1982) Radiation-induced thermoacoustic soft-tissue imaging. IEEE Trans on Son Ultrason 29: 187–187
Buehler A, Herzog E, Razansky D, Ntziachristos V (2010) Video rate optoacoustic tomography of mouse kidney perfusion. Opt Lett 35:2475–2477
Contag CH, Bachmann MH (2002) Advances in in vivo bioluminescence imaging of gene expression. Annu Rev Biomed Eng 4:235–260
Cox BT, Arridge SR, Kostli KP, Beard PC (2006) Two-dimensional quantitative photoacoustic image reconstruction of absorption distributions in scattering media by use of a simple iterative method. Appl Opt 45:1866–1875
De La Zerda A et al (2008) Carbon nano-tubes as photoacoustic molecular imaging agents in living mice. Nat Nanotechnol 3:557–562
Denk W, Strickler JH, Webb WW (1990) Two-photon laser scanning fluorescence microscopy. Science 248:73–76
Giepmans BNG, Adams SR, Ellisman MH, Tsien RY (2006) Review—The fluorescent toolbox for assessing protein location and function. Science 312:217–224
Helmchen F, Denk W (2005) Deep-tissue two-photon microscopy. Nat Methods 2:932–940
Hyde D, Schulz R, Brooks D, Miller E, Ntziachristos V (2009) Performance dependence of hybrid x-ray computed tomography/fluorescence molecular tomography on the optical forward problem. J Opt Soc Am A: 26:919–923
Li L, Zemp R, Lungu G, Stoica G, Wang L (2007) Photoacoustic imaging of lacZ gene expression in vivo. JBO Lett vol. 12
Li ML, Oh JT, Xie XY, Ku G, Wang W, Li C, Lungu G, Stoica G, Wang LV (2008) Simultaneous molecular and hypoxia imaging of brain tumors in vivo using spectroscopic photoacoustic tomography. Proc IEEE 96:481–489
Ntziachristos V (2006) Fluorescence Molecular Imaging. Annu Rev Biomed Eng 8:1–33
Ntziachristos V, Weissleder R (2001) Experimental three-dimensional fluorescence reconstruction of diffuse media using a normalized Born approximation. Opt Lett 26:893–895
Ntziachristos V, Razansky D (2010) Molecular imaging by means of multi-spectral opto-acoustic tomography (MSOT). ACR Chemical Review 110:2783–2794
Ntziachristos V, Ripoll J, Wang LHV, Weissleder R (2005) Looking and listening to light: the evolution of whole-body photonic imaging. Nat Biotechnol 23:313–320
Oraevsky AA, Esenaliev RO, Tittel FK (1994) Laser based optoacoustic imaging in biological tissues. Proc. SPIE 2134A: 122–128
Rayavarapu R, Petersen W, Ungureanu C, Post J, van Leeuwen T, Manohar S (2007) Synthesis and bioconjugation of gold nanoparticles as potential molecular probes for light-based imaging techniques. Int J Biomed Imaging p. 29817
Razansky D, Vinegoni C, Ntziachristos V (2007) Multispectral photoacoustic imaging of fluorochromes in small animals. Opt Lett 32:2891–2893
Razansky D, Ntziachristos V (2010) Multi-spectral optoacoustic tomography. In Knaeblein J (ed) Modern Biopharmaceuticals vol. in press, Wiley
Razansky D, Distel M, Vinegoni C, Ma R, Perrimon N, Koester R, Ntziachristos V (2009a) Going deeper than microscopy with multi-spectral optoacoustic tomography of fluorescent proteins in vivo. Nat Photonics 3:412–417
Razansky D, Baeten J, Ntziachristos V (2009b) Sensitivity of molecular target detection by multispectral optoacoustic tomography (MSOT). Med Phys 36:939–945
Razansky D, Vinegoni C, Ntziachristos V (2009c) Imaging of mesoscopic-scale organisms using selective-plane optoacoustic tomography. Phys Med Biol 54:2769–2777
Rosencwaig A (1973) Photoacoustic spectroscopy of biological materials. Science 181:657–658
Rosenthal A, Razansky D, Ntziachristos V (2009) Quantitative optoacoustic signal extraction using sparse signal representation. IEEE Trans Med Imag. 28:1997–2006
Rosenthal A, Razansky D, Ntziachristos V (2010) Fast semi-analytical model-based acoustic inversion for quantitative optoacoustic tomography. IEEE Trans Med Imaging 29:1275–1285
Schulz R, Ale A, Sarantopoulos A, Freyer M, Soehngen E, Zientkowska M, Ntziachristos V (2010) Hybrid system for simultaneous fluorescence and X-ray computed tomography. IEEE Trans Med Imag. 29:465–473
Soubret A, Ripoll J, Ntziachristos V (2005) Accuracy of fluorescent tomography in presence of heterogeneities: study of the normalized Born ratio. IEEE Med Imag. 24:1369–1376
Tsien RY (2005) Building and breeding molecules to spy on cells and tumors. FEBS Lett 579:927–932
Wang X, Pang Y, Ku G, Xie X, Stoica G, Wang LH (2003) Noninvasive laser-induced photoacoustic tomography for structural and functional in vivo imaging of the brain. Nat Biotechnol 21:803–806
Webb RH (1999) Theoretical basis of confocal microscopy. Methods Enzymol 307:3–20
Weissleder R, Ntziachristos V (2003) Shedding light onto live molecular targets. Nat Med 9:123–128
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2013 Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
Ntziachristos, V., Razansky, D. (2013). Optical and Opto-Acoustic Imaging. In: Schober, O., Riemann, B. (eds) Molecular Imaging in Oncology. Recent Results in Cancer Research, vol 187. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-642-10853-2_4
Download citation
DOI: https://doi.org/10.1007/978-3-642-10853-2_4
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-642-10852-5
Online ISBN: 978-3-642-10853-2
eBook Packages: MedicineMedicine (R0)