Abstract
Today, more than 95% of pituitary adenomas are removed using transsphenoidal surgery. The complication rates both for the traditional microscopic technique and for the more recently introduced endoscopic technique are comparably low. In acromegaly, the overall surgical cure rate of the transsphenoidal operation is approximately 50% in experienced hands. In Cushing’s disease, the cure rate is high if an adenoma is visible on MRI. In prolactinomas, surgery should be preferentially offered to patients with microadenomas (<10 mm) as their chance of surgical cure is >90%.
Adequate perioperative endocrinological management is pivotal. Replacement therapy for adrenal insufficiency must be adapted to the perioperative demand. Diabetes insipidus (DI) with impaired ADH secretion is encountered frequently on days 1–5 after surgery while the opposing syndrome of inappropriate antidiuretic hormone secretion (SIADH) with excessive ADH release typically presents on days 3–10. Thorough surveillance of water and electrolyte balance in the postoperative course is paramount for early detection and treatment of these typical postoperative dysregulations of the posterior pituitary lobe. Postoperative endocrine care includes early assessment of remission status and pituitary function. It is recommended that neuro-endocrine and neurosurgical follow-up appointments be scheduled prior to discharge to guarantee professional ongoing follow-up.
For non-functioning pituitary adenomas (NFPA), radiotherapy (RT) may be considered for invasive residual tumour after surgery. The timing of radiotherapy is still a subject of controversy. For functioning adenomas, radiotherapy is indicated if surgery and medical therapy cannot control hormonal oversecretion. Fractionated radiotherapy (fRT) is used for large adenoma volumes to minimize secondary injury to surrounding structures. Stereotactic radiosurgery (SRS) is used for small target volumes with a sufficient distance from the optic apparatus. These two principle techniques have different risk profiles. Both fRT and SRS are highly effective in preventing further adenoma growth. Biochemical cure is less frequent. Reportedly, the biochemical cure rates are slightly higher for Cushing’s disease than for acromegaly and are least favourable in prolactinomas. Biochemical remission is often delayed and the cure rates increase over the years after RT.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Pituitary surgery
- Pituitary adenoma
- Transsphenoidal
- Microscopic
- Endoscopic
- Fractionated radiotherapy
- Radiosurgery
-
Transsphenoidal: Surgery is performed through the nose and the sphenoid sinus.
-
Transcranial: Surgery is performed by removing a piece of skull bone.
-
Microscopic: The surgeon looks through a microscope during transsphenoidal surgery.
-
Endoscopic: The surgeon uses an endoscope to visualize during transsphenoidal surgery.
-
More than 95% of pituitary adenomas are removed through the transsphenoidal route. The complication rates both for the traditional microscopic technique and for the more recently introduced endoscopic technique are comparably low.
-
Pituitary surgery is the first line of treatment for large non-functioning adenomas and in functioning adenomas causing Cushing’s disease or acromegaly. Prolactinomas, by contrast, are primarily treated with dopamine-agonists (DAs). Transsphenoidal surgery, however, is an accepted alternative for small prolactinomas as cure rates greater than 90% can be achieved.
-
Adequate perioperative endocrinological management is pivotal. It includes perioperative hormonal replacement therapy, thorough surveillance of water and electrolyte balance, and assessment of postoperative remission status.
-
Radiotherapy is indicated if adenoma growth or hormonal hypersecretion is not controlled by surgery and/or medical therapy. Fractionated radiotherapy (fRT) is used for large adenoma volumes while stereotactic radiosurgery (SRS) is indicated for small target volumes with a sufficient distance from the optic nerves and chiasm.
-
Both fRT and SRS are effective in preventing further adenoma growth. Biochemical cure rates are slightly higher for Cushing’s disease than for acromegaly and are least favourable in prolactinomas.
1 Transsphenoidal Surgery
1.1 History of Transsphenoidal Surgery
The first successful operation for a pituitary tumour was performed by Victor Horsley in 1904 at Queen Square in London using a transcranial approach. Only a few years later, Hermann Schloffer, at University Clinic in Innsbruck, performed the very first transsphenoidal operation in 1907 (Schloffer 1907). He approached the sphenoid sinus and sella through an invasive rhinotomy incision along the lateral aspect of the nose (Fig. 22.1a). The nose was reflected to the side and the septum, medial wall of the orbit and portions of the maxillary sinus wall were removed. In the following years, transsphenoidal surgery was refined. Inferior nasal approaches were established with the advantage of less disfigurement and a better suprasellar view. The transnasal and sublabial approaches avoided an external incision (Fig. 22.1b). A major pioneer of transsphenoidal surgery was Harvey Cushing who performed 231 transsphenoidal operations in Boston between 1910 and 1925 with a mortality rate of 5.6%. However, he abandoned the transsphenoidal approach in the late 1920s because of the better results of transcranial surgery at that time. One must bear in mind that, at that time, imaging techniques were poor and it was impossible to know the true size and extent of pituitary tumours into the suprasellar space, making transsphenoidal surgery hazardous. Jules Hardy from Montreal who worked together with Gérard Guiot in Paris introduced the operating microscope for transsphenoidal surgery in the late 1960s (Hardy 1969). It offered two major advances that made the approach safer and more effective: First, it allowed better illumination of the operative field in the depth through a narrow approach. Second, selective adenomectomy with preservation of the pituitary gland and identification of small microadenomas became possible with magnification under the microscope. With the introduction of microscopy, the surgical morbidity and mortality of transsphenoidal surgery were significantly reduced and lead to worldwide recognition and adoption.
As early as in 1963, Gérard Guiot suggested the use of the endoscope at the end of a transsphenoidal operation for visualization. In the 1990s, Hae Dong Jho introduced the concept of pure endoscopic transnasal surgery (Jho et al. 1997). In the last two decades, endoscopy has become a generally accepted alternative to microscopy in pituitary surgery.
Today, the transsphenoidal approach is used in 96–99% of the patients for removal of pituitary adenomas (Honegger et al. 2007).
1.2 The Microscopic Transsphenoidal Approach
The preferred microscopic approach to the pituitary is the so-called “septum-pushover technique”. This technique is a uninostril-endonasal approach to the sphenoid sinus. The mucosa is incised over the nasal septum in the depth in front of the sphenoid sinus. The nasal septum is disconnected from the rostrum of the sphenoid sinus and displaced to the opposite side with the nasal speculum (Griffith and Veerapen 1987). At the end of the operation, the septum is brought back to the midline. The “septum-pushover technique” is a minimally invasive technique that is performed quickly and causes minimal postoperative discomfort and pain. In particular, the patients appreciate that no nasal packing is required and nasal breathing is possible immediately after surgery.
The microscopic approach (Figs. 22.2a, 22.3) offers the advantage of a 3-dimensional view. A speculum is necessary for visualization of the operative field, because the optic system is outside the nose. On the other hand, surgical manoeuvres are fast and straight-forward because the access to the operative field is held open by the speculum.
Schematic drawing of the microscopic and endoscopic setting. (a) Microscopy. The surgeon visualizes the surgical field through the microscope. The surgical corridor is held open by the nasal speculum. (b) Endoscopy. The endoscope is positioned inside the surgical corridor. The surgical field is visualized on the monitor. Copyright: Universitätsklinikum Tübingen
1.3 The Endoscopic Transsphenoidal Approach
For the endoscopic approach (Figs. 22.2b and 22.4), a binostril or uninostril approach is used to access the sphenoidal sinus (Juraschka et al. 2014). Endoscopic surgery is mostly performed in a “four-hand technique” where one surgeon performs the operation and the other surgeon holds and guides the endoscope. Once the sphenoid sinus is sufficiently opened, the endoscope is positioned in the sphenoid sinus. The position of the optic system inside offers the advantage of a panoramic view. In particular, lateral and suprasellar tumours can be directly visualized which can increase the extent of surgical resection.
One has to bear in mind that the microscope and endoscope are only instruments for visualization. The experience of the surgeon is most important for the success of surgery and not whether a microscopic or endoscopic technique is used.
1.4 Tumour Removal in Transsphenoidal Surgery
Once the anterior wall of the sphenoid sinus has been opened, the pituitary fossa becomes visible. The bony floor of the pituitary fossa is removed. For this surgical step, we use a diamond drill and punches. The next anatomical structure which lines the floor of the sella is the basal dura. Once this is opened in a Y-shaped manner, the pituitary adenoma is exposed. The adenoma is then removed with microinstruments. As adenomas are often soft, curettes are mostly used for adenomectomy. Once the intrasellar tumour is removed, the suprasellar portion can descend into the pituitary fossa and can be resected (Fatemi et al. 2008).
Approximately one-third of surgically treated pituitary adenomas show an invasive character. This means that the adenoma grows into the adjacent anatomical structures. The most frequent site of invasion is the cavernous sinus. Some soft adenomas may be removed from within the cavernous sinus without undue morbidity. However, invasion is clearly an adverse factor for complete resection and incurs risk to cranial nerves III, IV, VI, trigeminal nerve V2 (maxillary branch) and the internal carotid artery that traverse this area.
Under microscopic or endoscopic view, the pituitary gland can be differentiated from the adenoma and then preserved. With large adenomas, the gland has become flattened and displaced and lines the resection cavity.
The diaphragma sellae is the upper border of the pituitary fossa and protects the fossa from the cerebro-spinal fluid (CSF) space. Particularly in large adenomas, the diaphragma is thin and intraoperative CSF rhinorrhea can occur, requiring repair. For closure of a CSF leak, various techniques are used. Repair may be with autologous material from the patient (e.g. fascia late, abdominal fat) or with dural substitutes or both. For large CSF leaks, a vascularized naso-septal flat is often placed over the skull base defect (Hadad et al. 2006). If a large intraoperative leak occurs, an additional prophylactic postoperative lumbar drainage for 5–7 days may be placed to prevent formation of a nasal CSF fistula by lowering the intracranial pressure.
1.5 Risk of Transsphenoidal Surgery
The complication rate in transsphenoidal surgery for pituitary adenomas is relatively low. In a recent meta-analysis (Ammirati et al. 2013), the risk of a CSF leak was 6–7%. Meningitis occurred in 1–2% of cases. The frequency of these typical complications was similar if microscopic and endoscopic series were compared. The risk of death was 0.5% for microscopy and 1.58% for endoscopy. Only for vascular injury, was a significant difference between microscopy (0.23%) and endoscopy (0.49%) found.
Of course, the experience of the surgeons has major influence on the complication rates. In experienced hands, a risk of CSF leak requiring operative repair can be below 1%.
The risk of new postoperative hypopituitarism is about 10%. It is significantly correlated to adenoma size. On the other hand, the chance of postoperative improvement of pituitary function is 30–40%. While transient diabetes insipidus is frequently observed, the rate of permanent diabetes insipidus is only about 1% (Ammirati et al. 2013).
Postoperative deterioration of visual function and visual fields is rare (Fig. 22.5). It can be caused by postoperative bleeding. On the other hand, preoperative visual deficits often recover after surgery. The risk that chiasmal syndrome does not improve postoperatively is particularly high if preoperative deficits were long-standing and pronounced.
1.6 Special Considerations and Outcome in Different Adenoma Types
Pituitary adenomas are either non-functioning or hormone-secreting. The most frequent types of hormone-secreting adenomas are GH-secreting adenomas causing acromegaly or gigantism, ACTH-secreting adenomas causing Cushing’s disease and prolactin-secreting adenomas, which are called prolactinomas. In the following, the special surgical considerations and the surgical outcome of these frequent adenoma types are described.
1.6.1 Transsphenoidal Surgery for Non-functioning Pituitary Adenomas (NFPA)
NFPA only become symptomatic if they cause local symptoms due to a space-occupying lesion. Visual deficits and hypopituitarism prevail and represent the indication for surgery. On the other hand, NFPA are often diagnosed incidentally during cranial imaging for other reasons (such as on evaluation of headaches or head injuries). The indication for treatment of asymptomatic adenomas is relative. Surgery is usually performed if the adenoma size is larger than 2 cm or some degree of chiasmal compression is found on MRI.
Despite their large size, more than 90% of NFPA can be removed by a transsphenoidal approach. Figure 22.6 shows the MRI of a large NFPA before and after transsphenoidal removal. If complete removal of a NFPA has been confirmed by MRI, the risk of recurrence is low (Chang et al. 2010). In contrast, residues of NFPA are at high risk for re-growth. Due to the low growth velocity of many pituitary adenomas, re-growth may be detected only after several years of observation.
1.6.2 Transsphenoidal Surgery and Outcome in Acromegaly
Transsphenoidal surgery is the first choice of treatment in acromegaly.
The anaesthetist must be prepared, as intubation might be difficult. Intubation can be hampered by macroglossia, goitre and spinal kyphosis.
The surgeon must be prepared that the nasal anatomy can be distorted due to overgrowth of the anatomical structures. The nasal septum is often deviated. An extra long nasal speculum may be needed because the approach can be abnormally deep in acromegaly.
Criteria for cure are normalized insulin-like growth factor 1 (IGF-1), normal basal growth hormone (GH) and adequate suppression of GH during an oral glucose tolerance test.
Strong adverse prognostic factors in terms of cure are large adenoma size, invasive character and high preoperative GH and IGF-1 levels. The data of the German Acromegaly Register showed a long-term cure rate of 38.8%, others report lower or similar remission rates (Schöfl et al. 2013; Minniti et al. 2003). In centres with a high case-load of acromegalic patients, the cure rate was 49.8–51% (Mortini et al. 2018). In those patients without complete surgical cure, a significant reduction of GH excess by surgery will improve the success rate of postoperative medical treatment or radiotherapy.
1.6.3 Transsphenoidal Surgery and Outcome in Cushing’s Disease
Cushing’s disease (CD) is a life-threatening disease that is mostly caused by microadenomas. CD is unique in that 30% of microadenomas are so small that they are not detected even with modern MRI of the pituitary.
Endocrinological diagnostics prove the pituitary origin and differentiate CD from ectopic and adrenal Cushing’s syndrome (CS). If the pituitary origin is unclear, inferior petrosal sinus sampling (IPSS) is performed as the pituitary blood is drained into the inferior petrosal sinus. Higher ACTH values in the petrosal sinus compared to ACTH in the peripheral vein confirm the pituitary origin. For prediction of the laterality of a microadenoma within the gland, IPSS only has a poor positive predictive value.
Transsphenoidal surgery is the treatment of first choice in CD. If no adenoma is detected by MRI, the pituitary gland is systematically explored by micro-incisions. A special small ultrasound probe for intraoperative detection of minute microadenomas is used in some specialized centres.
In large series with transsphenoidal surgery for microadenomas, postoperative remission rates in the range of 59–98% have been reported (Chandler et al. 2016). The cure rate is clearly superior if a microadenoma has been detected on preoperative MRI.
1.6.4 Transsphenoidal Surgery and Outcome in Prolactinomas
In prolactinomas, medical treatment with dopamine-agonists (DAs) is the first choice. However, a re-increase of prolactin occurs in 79% of prolactinomas after withdrawal of DA.
Transsphenoidal surgery is a second choice treatment for prolactinomas. The classical indications for surgery are resistance to DA or intolerable side-effects of DA. Further indications for surgery have emerged: The guidelines of the Pituitary Society from 2006 describe that the possibility of cure by surgery versus long-term DA therapy should be discussed with the patient, and patient preference is an indication for surgery (Casanueva et al. 2006). Surgery may be preferentially offered in microadenomas where the surgical cure rate is >90% (Casanueva et al. 2006; Kreutzer et al. 2008). The chance of prolactin normalization is still good in circumscribed intrasellar macroprolactinomas. Transsphenoidal surgery is usually not offered in large or invasive prolactinomas because postoperative normoprolactinaemia is unlikely. The risk of cardiac valvulopathy under DA is also a concern. Therefore, young patient age is an argument for surgery in order to avoid long-term DA. If acute visual loss occurs, it should not be hesitated to perform acute surgical decompression instead of awaiting the effect of DA (Kreutzer et al. 2008).
1.7 Modern Technologies in Transsphenoidal Surgery
Intraoperative MRI is available in some neurosurgical centres. The completeness of resection can be controlled intraoperatively. Identified residual adenoma can be removed during the same procedure avoiding a second operation.
Today, neuronavigation systems are widely used in neurosurgery and also in pituitary surgery. With neuronavigation, intraoperative tumour and major structure positions can be compared with the preoperative imaging data. With neuronavigation, the location of risk structures (i.e. carotid arteries) and the extent of resection can be verified intraoperatively.
2 Perioperative and Postoperative Care
2.1 Perioperative and Postoperative Care: Endocrinological
The postoperative endocrine care is demanding. Adequate management of postoperative endocrinological peculiarities is pivotal for the success of surgical treatment.
The details of postoperative management vary between different centres. The practices and therapeutic schemes that are provided in this section only have an exemplary nature.
2.1.1 Postoperative Dysregulation of Water and Electrolyte Balance
Disturbances of water and electrolyte balance are frequently encountered during the early postoperative period due to dysregulation of the posterior pituitary lobe. The two major postoperative dysregulations are diabetes insipidus and syndrome of inappropriate antidiuretic hormone secretion (SIADH) which are opposing problems. Diabetes insipidus is caused by impaired secretion of antidiuretic hormone (ADH) from the posterior pituitary lobe. In contrast, SIADH is caused by postoperative degeneration of some ADH-secreting neurons with excessive release of ADH.
Surveillance of water and electrolyte balance plays a central role in postoperative management. (see Chap. 23: Pituitary Surgery: Part 2 Nursing Care) Monitoring incudes:
-
Balancing of daily fluid intake and output.
-
Specific gravity of every urine portion.
-
Daily measurement of serum sodium level.
-
Daily measurement of body weight.
2.1.2 Diagnosis and Treatment of Postoperative Diabetes Insipidus
Impaired ADH secretion of the posterior lobe results in diabetes insipidus which is frequently encountered in the first days after surgery:
-
40% of patients have polyuria (>2.5 l/24 h) at the first postoperative day after transsphenoidal surgery for a pituitary adenoma.
-
5% suffer from polyuria at the fifth postoperative day.
-
Permanent diabetes insipidus is only found in 1% of cases postoperatively.
Algorithm for the Management of Diabetes Insipidus: A Single Centre Protocol
Diagnosis
-
Fluid intake and output exceeds 3500 mL in 24 h.
-
Urine output exceeds 400 mL within 2 h.
-
Serum sodium above upper limit of normal.
Treatment
-
Desmopressin 2 μg subcutaneous or intramuscular after transsphenoidal surgery during the first postoperative days (because of reduced nasal uptake following transsphenoidal surgery).
-
Nasal application of desmopressin spray 1 puff can be commenced from postoperative day 6 onward.
-
Desmopressin tablets are also recommended for cases of mild or partial diabetes insipidus.
2.1.3 Diagnosis and Treatment of Postoperative Syndrome of Inappropriate Antidiuretic Hormone Secretion (SIADH)
SIADH usually occurs between postoperative day 3 and day 10 and is a potential life-threatening complication. This is reported in up to 30% of cases. SIADH results in hyponatremia. Therefore, our protocol is to regularly measure serum sodium level until postoperative day 10 but protocols may be site specific.
Asymptomatic SIADH can be treated with restriction of fluid intake to 1 L per day. In symptomatic SIADH, hyperosmolar sodium infusion is often required in addition to fluid restriction. Recently, the vasopressin antagonist tolvaptan became available. It allows efficient treatment of SIADH without fluid restriction. We start with a single dose of tolvaptan 7.5 mg. Mostly, a second dose is necessary 1–2 days later.
It is imperative that an unduly rapid correction of hyponatremia is avoided. A rapid correction can cause damage to the myelin sheath of the nerve cells in the brainstem called central pontine myelinolysis which is a severe neurological disorder with poor prognosis.
Signs and Symptoms of SIADH
-
Headache
-
Malaise
-
Agitation
-
Tiredness
-
Poor Concentration
-
Nausea and vomiting
-
Mental state changes and confusion
-
Epileptic seizure
2.1.4 Perioperative Replacement Therapy of Adrenal Insufficiency
Replacement therapy for adrenal insufficiency is of vital importance. A higher demand of cortisone is required during the stressful event of an operation. Two principle perioperative strategies can be pursued:
-
(a)
Replacement in every patient undergoing pituitary surgery.
-
(b)
Replacement only if:
-
Preoperative adrenal insufficiency exists or
-
Adrenal function is unknown (for example in emergency cases) or
-
New adrenal failure is anticipated based on intraoperative findings.
-
Table 22.1 shows our regime if glucocorticoid replacement is required during the perioperative period. Physiological doses are continued at discharge as indicated after assessment of HPA axis (Table 22.1).
In some countries, an emergency card is available or provided to the patient if postoperative adrenal insufficiency is found or if adrenal function is equivocal.
2.1.5 Early Postoperative Assessment of Remission Status
In functioning adenomas, postoperative assessment of the oversecreted hormone provides important information about the success of the surgery. Re-assessment should be performed as early as possible because the result is not only important for further treatment but also urgently awaited by the patient.
In prolactinomas, prolactin can be assessed on the first postoperative day. In acromegaly, we also assess GH on the first postoperative day. However, IGF-1 decline is delayed. Both prolactin and GH are peptide hormones with a short half-life period. Their measurement at the first postoperative day provides valuable information of surgical success regarding correction of the oversecreted hormone (see specific chapters for more information).
In Cushing’s disease, different methods for early assessment of the remission status are in use: Some centres withhold perioperative hydrocortisone replacement and measure cortisol daily after surgery. A drop in serum cortisol into the hypocortisolemic range indicates remission. As soon as remission is documented or the patient shows clinical signs of adrenal insufficiency, hydrocortisone replacement therapy must be commenced. This regimen allows for the earliest possible detection of remission. However, continuous clinical surveillance for symptoms of adrenal insufficiency is paramount to avoid adrenal crisis.
Other centres use perioperative hydrocortisone replacement and withdraw hydrocortisone some days after surgery for assessment of the remission status.
2.1.6 Early Postoperative Assessment of Anterior Pituitary Function
The regimen of postoperative endocrine re-assessment of pituitary function differs between centres. An orienting status of pituitary hormones prior to discharge should be the minimal standard.
2.2 Postoperative Care: Neurosurgical
2.2.1 Surveillance of Vision
The optic chiasm is in close proximity to the pituitary gland. Patients with pituitary tumours may suffer from chiasmal syndrome preoperatively. Assessment of visual acuity and visual fields is mandatory immediately and regularly postoperatively. Visual fields are tested with finger perimetry.
Knowledge of preoperative vision and visual fields is important for proper judgement of the postoperative state. Furthermore, the nurse must have information from the surgeon about which patients are at risk for visual deterioration.
Visual failure may indicate postoperative bleeding. The risk of bleeding is particularly high during the first postoperative day. On the other hand, improvement of preoperative visual deficits can often be detected immediately after surgery but may also be delayed. Formal ophthalmological re-assessment can be done 1 week after surgery.
Furthermore, optomotor nerves run lateral to the pituitary fossa within the cavernous sinus. Postoperative care includes alertness for double vision and ptosis.
2.2.2 Nasal Care After Transsphenoidal Surgery
Regular nasal application of decongestant nose drops enhances nasal breathing and avoids troublesome nasal secretion or painful retention of secretion in the paranasal sinuses. Some centres use xylometazoline 0.1% nasal spray or drops. This shrinks the swollen nasal mucosa via vasoconstriction. It is applied after transnasal surgery until there are minimal secretions and free ventilation is restored which is usually the case after 7–14 days. Other centres use sterile saline spray multiple times daily to achieve a similar outcome.
Medication for nasal pain is not routinely administered. Nonsteroidal anti-inflammatory drugs can be given if required. However, acetylsalicylic acid should be avoided during the first 10 postoperative days because of the anticoagulant effects.
It may be difficult to differentiate nasal secretion from cerebro-spinal fluid (CSF) rhinorrhea. No laboratory test is absolutely reliable. Measurement of glucose in nasal fluid is not helpful after transnasal surgery. Mucosal fluid is a hint for nasal secretion while clear fluid like water is suspicious of CSF. CSF rhinorrhea can be provoked if the patient is brought into a sitting position and the head bend forward.
The nasal conditions must be closely supervised. If CSF rhinorrhea occurs, lumbar drainage or operative repair in a timely fashion is indicated to avoid further complications such as meningitis.
2.3 Further Early Postoperative Care
The timing of discharge from the neurosurgical unit varies between centres. The postoperative stay is usually between 3 and 6 days. In some centres, the patients are transferred to the endocrine unit during the postoperative hospital stay. Prior to discharge, the patient is given both verbal and written instructions regarding postoperative home care. After transsphenoidal surgery, an increase of intracranial pressure must be avoided for a period of at least 4 weeks (Knappe et al. 2018). For that time, physical strain, sports activities, steam baths, saunas, and blowing the nose must be avoided. After 4 weeks, physical activities can be slowly resumed. Patients are advised to sneeze with an open mouth to avoid Valsalva pressure. The use of continuous positive airway pressure (CPAP) devices is not recommended immediately postoperatively and patients may need supplemental oxygen. Review with the Ear Nose and Throat (ENT) specialist 2–4 weeks postoperatively is recommended.
The patient’s case is booked for the tumour board where decisions of further management (for example requirement of postoperative medical treatment or radiotherapy) are made.
Most importantly, an appointment with the endocrinologist must be arranged prior to postoperative discharge. An exact date for the appointment is strongly recommended to guarantee an endocrinological follow-up. A first re-assessment by the endocrinologist is usually recommended 2–3 weeks after surgery. Some endocrinologists see their patients as early as 1 day after discharge from the neurosurgical unit.
Similarly, a neurosurgical follow-up appointment is mandatory. The first appointment at the neurosurgical outpatient department is usually scheduled 3–6 months postoperatively. The patient is instructed to bring along a recent postoperative MRI or is scheduled the same day for a postoperative MRI. Postoperative endocrine re-assessment may be scheduled simultaneously or independently and these results should be available to the neurosurgery at the time of this appointment. If the adenoma had suprasellar extension or the patient had suffered from chiasmal syndrome, an ophthalmological report should also be presented at the neurosurgical follow-up appointment.
3 Transcranial Surgery
3.1 Indications for Transcranial Surgery
Transcranial surgery is only required if a pituitary adenoma is not sufficiently accessible by a transsphenoidal operation. The decision for the appropriate approach depends on adenoma size and location. A multilobulated suprasellar extension points to a perforated diaphragm sellae. It means that the adenoma has grown out of the confines of the pituitary fossa into the intracranial (intradural) space. Under these circumstances, the risk of transsphenoidal surgery might be too high because it does not provide adequate control of intracranial neurovascular structures. Transcranial surgery is necessary in some of these adenomas. A predominant suprasellar adenoma with a small pituitary fossa, an eccentric suprasellar extension or a dumbbell shaped adenoma also hamper transsphenoidal resection and are reasons for transcranial surgery (Buchfelder and Kreutzer 2008). Today, less than 5% of pituitary adenomas require a transcranial operation.
3.2 Transcranial Approach and Tumour Removal
For a transcranial approach, the skin incision is made fronto-temporal behind the hairline to avoid an externally visible scar. The pterional approach and the fronto-lateral approach are most frequently used. The pterional approach exposes the Sylvian fissure between the frontal and temporal lobes. The arachnoid of the Sylvian fissure is opened for exposure of the adenoma. The adenoma can be visualized and removed through the prechiasmatic space or through the space between the optic nerve and carotid artery (so-called “optico-carotid triangle”). The fronto-lateral approach provides a more anterior view. It is less invasive but the lateral view through the optico-carotid triangle is limited.
3.3 Risk of Transcranial Surgery
The reported morbidity and mortality of transcranial surgery is certainly higher than of transsphenoidal surgery. However, one has to keep in mind a selection bias because the difficult adenomas with major intracranial, suprasellar extension require a transcranial operation. The mortality rate of transcranial surgery is approximately 2% (Buchfelder and Kreutzer 2008). Transcranial operations carry a particularly high risk of re-bleeding into the tumour bed. Another specific risk is hypothalamic dysfunction which may be caused by damage to small perforating arteries.
The risk of visual deterioration following transcranial surgery is approximately 15–22%. On the other hand, the chance that a pre-existing visual deficit improves after surgery is 50% (Bulters et al. 2009). In large adenomas with eccentric lateral extension, a significant risk of an oculomotor nerve palsy with ptosis and double vision exists.
The risk of postoperative hypopituitarism and diabetes insipidus is also higher in transcranial surgery than in transsphenoidal surgery and the chance of postoperative recovery of pre-existing endocrine deficits is low (Buchfelder and Kreutzer 2008).
As the adenomas requiring craniotomy are typically large and show invasive character, a gross total resection is mostly not feasible.
4 Surgery for Non-adenomatous Pituitary Lesions
4.1 Other Pathologies
The vast majority of surgically treated lesions of the pituitary area are adenomas. However, numerous other pathologies of the pituitary, pituitary stalk and hypothalamus are encountered (see Chap. 14). The appropriate surgical approach for other pituitary pathologies also depends on their location.
Among non-adenomatous lesions, tumours of neighbourhood origin that secondarily encroach upon the pituitary such as chordomas, chondosarcomas or perisellar meningiomas must also be considered.
4.2 Surgery for Craniopharyngiomas
The second most frequent pathology of pituitary or hypothalamic origin is the craniopharyngioma. In contrast to pituitary adenomas, only 30% of craniopharyngiomas show major intrasellar involvement allowing transsphenoidal surgery (Honegger and Tatagiba 2008). Many craniopharyngiomas are not confined to the pituitary fossa and grow in the suprasellar intradural space above the diaphragm sellae requiring craniotomy. Various transcranial approaches have to be considered as craniopharyngiomas may involve several intracranial compartments. Large craniopharyngioma cysts can be decompressed by stereotactic cyst puncture.
Recently, extended transsphenoidal operations have been suggested for purely suprasellar craniopharyngiomas (Kim et al. 2011) and other suprasellar tumours. The major disadvantage of such extended approaches is the large defect in the skull base and intraoperative CSF leak which is necessary for tumour exposure. It carries a high risk of a postoperative CSF fistula. Whether purely suprasellar tumours should be operated by craniotomy or extended transsphenoidal surgery is still a matter of debate (Jeswani et al. 2016).
Gross total resection of craniopharyngiomas offers a high chance of recurrence-free long-term survival. Some neurosurgeons recommend an attempt at total removal so long as there is no risk of hypothalamic damage (Honegger and Tatagiba 2008). Other surgeons recommend conservative resection (biopsy or partial resection) followed by radiotherapy. However, the tumour burden remains with this policy and the therapeutic options in the case of re-growth are limited.
5 Recommendations for Radiotherapy Postoperatively
5.1 Indications for Radiotherapy in Pituitary Tumours
5.1.1 Indications for Radiotherapy in Non-functioning Pituitary Adenomas (NFPA)
In NFPA, radiotherapy is indicated for postoperative adenoma remnants with invasive character that are not accessible surgically. The typical indication is a residual adenoma within the cavernous sinus (Fig. 22.7). The timing of radiation for NFPA is a current subject of controversy. Some radiotherapists recommend radiotherapy after initial surgery. Others use radiotherapy only if further growth of the residual adenoma is observed (Fig. 22.7).
5.1.2 Indications for Radiotherapy in Cushing’s Disease
Radiotherapy (RT) is a second treatment option in CD. Radiotherapy is indicated if CD persists after pituitary surgery. This can be the case after negative sellar exploration(s) or for non-resectable invasive residual adenoma within the cavernous sinus. RT is also an option for recurrent CD (Estrada et al. 1997).
Other second-line options are medical treatment or bilateral adrenalectomy. The treatment decision in the second-line therapy is usually made on an individual basis. Medical treatment might be required while awaiting the delayed effect of radiotherapy.
5.1.3 Indications for Radiotherapy in Acromegaly
In acromegaly, RT competes with medical treatment as the second-line treatment. However, medical treatment is favoured in some countries for second-line treatment and RT is used if GH hypersecretion cannot be controlled by surgery and medical treatment.
5.1.4 Indications for Radiotherapy in Prolactinomas
In prolactinomas, RT is used if hyperprolactinaemia persists under dopamine-agonist (DA) treatment and surgery is not successful or not indicated because of invasive adenoma extension. Radiotherapy is rarely used in prolactinomas because of the high efficacy of DAs.
5.1.5 Indications for Radiotherapy in Craniopharyngiomas
RT is indicated for residual or recurrent craniopharyngiomas. The ideal timing of RT is yet to be determined. An ongoing study in childhood craniopharyngiomas investigates whether adjunctive RT immediately after incomplete resection or salvage RT upon re-growth is superior.
5.2 Tumour Control and Remission Rates with Radiotherapy
Radiation techniques are explained in Chapter 24: Radiotherapy.
5.2.1 Tumour Control Rates in Non-functioning Pituitary Adenomas (NFPA)
The goal of radiotherapy in NFPA is tumour control which means that the adenoma remains stable in size or shrinks.
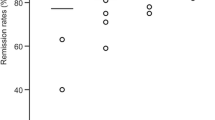
According to the literature, 80–98% of patients with NFPA are recurrence-free after fRT. The reported studies of SRS for NFPA showed tumour control rates of 83–100% (Sheehan et al. 2012) (Fig. 22.7). On average, the tumour control rate was 96%. However, long-term follow-up was not available in most of the studies on SRS.
Planning for stereotactic radiosurgery (SRS) with Gamma Knife of a left parasellar residual adenoma (arrow in a) within the cavernous sinus following transsphenoidal surgery. (a) Coronal view, (b) axial view, (c) 3D, (d) sagittal view. Yellow: 16 Gy isodose of the target volume; Green: 10 Gy isodose of the target volume. Blue: Optic chiasm (asterisk in a). Cyan: pituitary stalk and gland. The target volume has a sufficient distance to the optic chiasm to avoid visual compromise. Courtesy by Dr. G.A. Horstmann, Gamma Knife Centre, Krefeld, Germany
5.2.2 Remission Rates in Cushing’s Disease
With fRT, the reported remission rates with complete reversal of ACTH- and cortisol-oversecretion were between 46% and 100% (Estrada et al. 1997).
In published series of SRS with more than ten cases, the remission rates were highly variable with a range from 17% to 83%. Recent studies provide evidence that remission rates of 60–80% can be achieved today (Marek et al. 2015).
5.2.3 Remission Rates in Acromegaly
In the retrospective data collection of fRT for acromegaly from 14 centres throughout the United Kingdom, normalization of IGF-1 was achieved in 63% of the patients at 10 years (Jenkins et al. 2006).
The remission rates of SRS in acromegaly reported in the literature are heterogeneous. On average, a remission was achieved in approximately 50% of the patients (Pollock et al. 2008). Recent studies suggest that higher remission rates can be achieved today (Lee et al. 2015).
5.2.4 Remission and Control Rates in Prolactinomas
Both with fRT and with SRS, further tumour growth can be prevented in the vast majority of cases. However, remission with normal prolactin off dopamine-agonist treatment is only achieved in a minority of cases. Evidently, remission in prolactinomas is less frequent than in Cushing’s disease and acromegaly (Tanaka et al. 2010).
5.2.5 Control Rates in Craniopharyngiomas
In the main published studies on fRT, control rates at 10 years after RT were 56.5–100% (Minniti et al. 2009). Long-term results of SRS are still sparse. In the main published studies on SRS with mean follow-up periods between 16 months and 17 years, control rates of SRS were 34–88% (Minniti et al. 2009).
Abbreviations
- ACTH:
-
Adreno-corticotrophic hormone
- ADH:
-
Antidiuretic hormone
- CD:
-
Cushing’s disease
- CS:
-
Cushing’s syndrome
- CSF:
-
Cerebro-spinal fluid
- DA:
-
Dopamine-agonist
- DI:
-
Diabetes insipidus
- fRT:
-
Fractionated radiotherapy
- GH:
-
Growth hormone
- GKRS:
-
Gamma-knife radiosurgery
- Gy:
-
Gray
- IGF-1:
-
Insulin-like growth factor 1
- LINAC:
-
Linear accelerator based radiosurgery
- MRI:
-
Magnetic resonance imaging
- NFPA:
-
Non-functioning pituitary adenoma
- RT:
-
Radiotherapy
- SIADH:
-
Syndrome of inappropriate antidiuretic hormone secretion
- SRS:
-
Stereotactic radiosurgery
References
Ammirati M, Wei L, Ciric I. Short-term outcome of endoscopic versus microscopic pituitary adenoma surgery: a systematic review and meta-analysis. J Neurol Neurosurg Psychiatry. 2013;84:843–9.
Buchfelder M, Kreutzer J. Transcranial surgery for pituitary adenomas. Pituitary. 2008;11:375–84.
Bulters DO, Shenouda E, Evans BT, et al. Visual recovery following optic nerve decompression for chronic compressive neuropathy. Acta Neurochir. 2009;151:325.
Casanueva FF, Molitch ME, Schlechte JA, Abs R, Bonert V, Bronstein MD, Brue T, Cappabianca P, Colao A, Fahlbusch R, Fideleff H, Hadani M, Kelly P, Kleinberg D, Laws E, Marek J, Scanlon M, Sobrinho LG, Wass JAH, Giustina A. Guidelines of the Pituitary Society for the diagnosis and management of prolactinomas. Clin Endocrinol. 2006;65:265–73.
Chandler WF, Barkan AL, Hollon T, Sakharova A, Sack J, Brahma B, Schteingart DE. Outcome of transsphenoidal surgery for Cushing disease: a single-center experience over 32 years. Neurosurgery. 2016;78:216–23.
Chang EF, Sughrue ME, Zada G, Wilson CB, Blevins LS, Kunwar S. Long term outcome following repeat transsphenoidal surgery for recurrent endocrine-inactive pituitary adenomas. Pituitary. 2010;13(3):223–9. https://doi.org/10.1007/s11102-010-0221z.
Estrada J, Boronat M, Mielgo M, Magallón R, Millán I, Díez S, Lucas T, Barceló B. The long-term outcome of pituitary irradiation after unsuccessful transsphenoidal surgery in Cushing’s disease. N Engl J Med. 1997;336:172–7.
Fatemi N, Dusick JR, de Paiva Neto MA, Kelly DF. The endonasal microscopic approach for pituitary adenomas and other parasellar tumors: a 10-year experience. Neurosurgery. 2008;63(4 Suppl 2):244–56.
Griffith HB, Veerapen R. A direct transnasal approach to the sphenoid sinus. Technical note. J Neurosurg. 1987;66:140–2.
Hadad G, Bassagasteguy L, Carrau RL, Mataza JC, Kassam A, Snyderman CH, Mintz A. A novel reconstructive technique after endoscopic expanded endonasal approaches: vascular pedicle nasoseptal flap. Laryngoscope. 2006;116:1882–6.
Hardy J. Transsphenoidal microsurgery of the normal and pathological pituitary. Clin Neurosurg. 1969;16:185–217.
Honegger J, Tatagiba M. Craniopharyngioma surgery. Pituitary. 2008;11:361–73.
Honegger J, Ernemann U, Psaras T, Will B. Objective criteria for successful transsphenoidal removal of suprasellar nonfunctioning pituitary adenomas. A prospective study. Acta Neurochir. 2007;149:21–9.
Jenkins PJ, Bates P, Carson N, Stewart PM, Wass JAH, On behalf of the UK National Acromegaly Register Study Group. Conventional pituitary irradiation is effective in lowering serum growth hormone and insulin-like growth factor-I in patients with acromegaly. J Clin Endocrinol Metab. 2006;91:1239–45.
Jeswani S, Nuno M, Wu A, Bonert V, Carmichael JD, Black KL, Chu R, King W, Mamelak AN. Comparative analysis of outcomes following craniotomy and expanded endoscopic endonasal transsphenoidal resection of craniopharyngioma and related tumors: a single-institution study. J Neurosurg. 2016;124:627–38.
Jho HD, Carrau RL, Ko Y, Daly MA. Endoscopic pituitary surgery: an early experience. Surg Neurol. 1997;47:213–23.
Juraschka K, Khan OH, Godoy BL, Monsalves E, Kilian A, Krischek B, Ghare A, Vescan A, Gentili F, Zadeh G. Endoscopic endonasal transsphenoidal approach to large and giant pituitary adenomas: institutional experience and predictors of extent of resection. J Neurosurg. 2014;121:75–83.
Kim EH, Ahn JY, Kim SH. Technique and outcome of endoscopy-assisted microscopic extended transsphenoidal surgery for suprasellar craniopharyngiomas. J Neurosurg. 2011;114:1138–349.
Knappe UJ, Moskopp D, Gerlach R, Conrad J, Flitsch J, Honegger JB. Consensus on postoperative recommendations after transsphenoidal surgery. Exp Clin Endocrinol Diabetes 2018 doi: 10.1055/a-0664-7710. [Epub ahead of print].
Kreutzer J, Buslei R, Wallaschofski H, Hofmann B, Nimsky C, Fahlbusch R, Buchfelder M. Operative treatment of prolactinomas: indications and results in a current consecutive series of 212 patients. Eur J Endocrinol. 2008;158:11–8.
Lee CC, Vance ML, Lopes MB, Xu Z, Chen CJ, Sheehan J. Stereotactic radiosurgery for acromegaly: outcomes by adenoma subtype. Pituitary. 2015;18(3):326–34. https://doi.org/10.1007/s11102-014-0578-5.
Marek J, Jezková J, Hána V, Krsek M, Liscák R, Vladyka V, Pecen L. Gamma knife radiosurgery for Cushing’s disease and Nelson’s syndrome. Pituitary. 2015;18:376–84.
Minniti G, Jaffrain-Rea ML, Esposito V, Santoro A, Tamburrano G, Cantore G. Evolving criteria for post-operative biochemical remission of acromegaly: can we achieve a definitive cure? An audit of surgical results on a large series and a review of the literature. Endocr Relat Cancer. 2003;10(4):611–9. https://doi.org/10.1677/erc.0.0100611.
Minniti G, Esposito V, Amichetti M, Enrici RM. The role of fractionated radiotherapy and radiosurgery in the management of patients with craniopharyngiomas. Neurosurg Rev. 2009;32:125–32.
Mortini P, Barzaghi LR, Albano L, Panni P, Losa M. Microsurgical therapy of pituitary adenomas. Endocrine. 2018;59(1):72–81. https://doi.org/10.1007/s12020-017-1458-3. Epub 2017 Oct 24.
Pollock BE, Brown PD, Nippoldt TB, Young WF. Pituitary tumor type affects the chance of biochemical remission after radiosurgery of hormone-secreting pituitary adenomas. Neurosurgery. 2008;62:1271–8.
Schloffer H. Erfolgreiche Operation eines Hypophysentumors auf nasalem Wege. Wien Klin Wochenschr. 1907;20:621–4.
Schöfl C, Franz H, Grussendorf M, Honegger J, Jaursch-Hancke C, Mayr B, Schopohl J. Long-term outcome in patients with acromegaly: analysis of 1344 patients from the German Acromegaly Register. Eur J Endocrinol. 2013;168:39–47.
Sheehan JP, Xu Z, Lobo MJ. External beam radiation therapy and stereotactic radiosurgery for pituitary adenomas. Neurosurg Clin N Am. 2012;23:571–86.
Tanaka S, Link MJ, Brown PD, Stafford SL, Young WF, Pollock BE. Gamma knife radiosurgery for patients with prolactin-secreting pituitary adenomas. World Neurosurg. 2010;74:147–52.
Key Reading
Ammirati M, Wei L, Ciric I. Short-term outcome of endoscopic versus microscopic pituitary adenoma surgery: a systematic review and meta-analysis. J Neurol Neurosurg Psychiatry. 2013;84:843–9.
Chandler WF, Barkan AL, Hollon T, Sakharova A, Sack J, Brahma B, Schteingart DE. Outcome of transsphenoidal surgery for Cushing disease: a single-center experience over 32 years. Neurosurgery. 2016;78:216–23.
Honegger J, Tatagiba M. Craniopharyngioma surgery. Pituitary. 2008;11:361–73.
Sheehan JP, Xu Z, Lobo MJ. External beam radiation therapy and stereotactic radiosurgery for pituitary adenomas. Neurosurg Clin N Am. 2012;23:571–86.
Schöfl C, Franz H, Grussendorf M, Honegger J, Jaursch-Hancke C, Mayr B, Schopohl J. Long-term outcome in patients with acromegaly: analysis of 1344 patients from the German Acromegaly Register. Eur J Endocrinol. 2013;168:39–47.
Acknowledgement
The editors acknowledge manuscript review by Justin Cetas MD who is the Associate Professor of Department of Neurosurgery, Oregon Health & Sciences University, Portland, Oregon, USA.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Honegger, J. (2019). Pituitary Surgery. In: Llahana, S., Follin, C., Yedinak, C., Grossman, A. (eds) Advanced Practice in Endocrinology Nursing. Springer, Cham. https://doi.org/10.1007/978-3-319-99817-6_22
Download citation
DOI: https://doi.org/10.1007/978-3-319-99817-6_22
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-99815-2
Online ISBN: 978-3-319-99817-6
eBook Packages: MedicineMedicine (R0)