Abstract
Thermal protection and insulation are important problems in many fields such as industry, agriculture and medicine. New composite materials with good thermal storage capacities have become important in the last few decades. The role of these materials is reflected in their ability to store energy and allow it to be reused in some other thermal systems. The aim of this study was to create a new material based on the basically activated bentonite clay. First, the clay was basically activated, resulting in a thick gel. Afterwards, stearic acid, Glauber’s salt and active carbon were added, and a heterogeneous gel was obtained as a finished final product. In order to obtain the best heterogeneous gel with satisfactory storage properties, the amount of stearic acid and Glauber’s salt was varied. The characterization of the resulting heterogeneous gel was performed by measuring the cooling rate of the gel samples. Compared with stearic acid, Glauber’s proved to be more effective. Heterogeneous gel cooling tests have shown that there was a certain proportional dependence between the concentration of stearic acid and the Glauber salt. However, it has been noticed the reduction in the cooling rate. Namely, the increase in stearic acid and Glauber’s salt concentration lead to slowing down the cooling rate of the gel. Adding active carbon to the heterogeneous gel also reduced the cooling rate, which indicated that the presence of active carbon in the heterogeneous gel should not be excluded in the future. The advantage of this system is the improvement of the gel thermal characteristics by the presence of water and clay. The gel was reversibly cooled and heated up to 100 °C without changing the homogeneous structure. This system can be used as a heat recovery pad, due to its flexible body pillow. It can be very quickly warmed up in a microwave oven if it is packaged in polyethylene packaging.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
1 Introduction
Thermal energy storages (TESs) in the last few decades become very popular, especially due to their important role in the processes of heat recovery processes as well as in improving the performance of the thermal systems [1,2,3]. TES contain mediums able to store the thermal energy (charging mode) and release the stored thermal energy (discharging mode) when it is necessary to compensate deficiency in the main thermal source [4,5,6]. The most common storage medium is a fluid or phase change material (PCM). National Aeronautics and Space Administration (NASA) has reported seven material categories with phase change properties: paraffins, non-paraffin organics, salt hydrates, metallic, fused salt eutectic mixtures, miscellaneous, and solid-solid [7, 8]. Each of these materials possesses some advantages and limitations, so the selection has to be done based on the application requirements [9, 10]. Generally, all these materials have a low thermal conductivity which induces slow charging and discharging rate [11]. In order to eliminate the existing lacks, scientists began to use nano-additives to enable continuous and linear energy exchange. Nanomaterials are used as additives to change the basic materials characteristics [12,13,14]. Once added to the fluid, produce mixture become nanofluids. Nanomaterials additions to the phase change materials provide nano-composites. Typical paraffin waxes are saturated hydrocarbon mixtures and usually consist of a mixture predominantly of alkanes of the real molecular chains such as CH3-(CH2)n-CH3. Crystallization (CH2)n chains can liberate a large amount of latent heat. Fatty acids are characterized by the formula CH3(CH2)2nCOOH. Paraffin waxes and fatty acids are organic supplements and are distinguished from inorganic PCM materials. As they are mostly chemically inert, stable, easy to recycle, these compounds show phase separation and corrosive behavior (with the exception of fatty acids). However, some disadvantages are low thermal conductivity, volumetric storage (less than 103 kg/m3), and flammability, unlike inorganic PCM materials [15, 16].
Results obtained by scanning electron microscopy (SEM) showed that the bentonite clay represent a good structural barrier for the organic PCMs homogenously dispersed onto its surface and interlayers [17, 18]. The chemical investigations made by using Fourier transform infrared (FT-IR) spectroscopy revealed that the attractions between the components of the composites were physical in nature and thus the PCM were hold by capillary forces [19, 20]. The advantage of this system is that the storage temperature may be higher than 100 °C, while pressure still remains very close to atmospheric pressure. Activations of clay by alkalis allows homogenization of the materials capable of melting heat change, whether an organic or inorganic nature [21, 22].
In the process of composites production, achieving the stable thermal cycles without loss of medium mass is the main goal. By combining the relationship of the Glauber’s salt and stearic acid into the selected composite, it is possible to influence the cooling kinetics. By increasing the content of the Glauber salt, it is possible to cool at temperatures close to its melting point. These are temperatures between 40 °C and 50 °C [23, 24].
In addition to clay, activated carbon in composite structure is also popular. It has porous structure with many inner surfaces, which allows it to be easily saturated with the melted PCM [6, 13, 25]. The activated carbon is suitable to form carbonaceous layers, which creates a physical protective barrier on the composites surface. The protective barrier could confine the transfer of flammable molecules to the gas phase. This results in the improvement of composites thermal stability [12, 14, 26]. The high thermal conductivity of the graphite ligaments in the foam allows rapid transfer of heat throughout the PCM volume. The PCM is able to absorb a significant amount of heat without a great increase in temperature during the phase change [27,28,29]. The aim of this study is to make a composite by using the bentonite clay, Glauber’s salt, paraffin wax, stearic acid and activated carbon in order to determine the composite mixture with the largest heat storage capacity of 100 °C.
In this study we have integrated modified bentonite clay which in combination with water forms a gel miscible with a PCM. The resulting composite is nanofluid gel, with very pronounced thixotropy [30,31,32]. PCMs usually have a rather small values of thermal conductivity, in range of 0.1 to 0.3 W/(mK), but in combinations with other compounds their thermal conductivity could be significantly improved [11, 27].
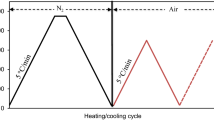
PCMs have a high density of storage of heat in a small temperature range. In this case, this is called apparent hysteresis. Figure 1 shows the different causes that can lead to hysteresis. Due to hysteresis, there are usually different data from charging and discharging experiments. Another aspect to be taken into account when it comes to heat transfer with solid-liquids phase change. In melted PCM is the effect of natural convection, because it is one of the main factors that influence the processes of phase transition. PCMs need to show a repeatable phase change in order to maintain the storage capacity of the heat needed for the designed application. It should be noted PCM showing phase separation will show a decrease in the enthalpy of melting after repetition cycling. For PCM-based elements, stability in cycling may also refer to the ability to avoid fluid leak after repeated melting and solidification cycles [33].
Real hysteresis as a material property caused by subcooling when: (a) the temperature rises again to the solidifying temperature of the PCM; (b) the temperature does not rise again to the solidifying temperature of the PCM. (c) Real hysteresis caused by slow heat release or a real difference between the phase-change temperatures. (d) Apparent hysteresis caused by non-isothermal conditions in the measurements [33].
2 Experimental
2.1 Materials
Bentonites clay was obtained from locations of Prisjan in the Pirot district (Serbia). Its chemical composition after drying at 110 °C is as follows: 51.82% SiO2, 0.34% TiO2, 26.86% Al2O3, 2.30% Fe2O3, 0.10% MnO, 1.27% MgO, 1.44% CaO, 0.75% Na2O and 2.07% K2O. Borax, Glauber’s salt, paraffin and stearic acid were obtained from ARIHEM (Nis, Serbia), while activated carbon was made by Miloje Zakic d.o.o. (Krusevac, Serbia). Temperature measurement was done using Digital thermometer DT-1 (Dalmacija, Dugi rat, Croatia).
2.2 Heterogeneous Gel Based on Alkali Bentonite Clay Activation Making Procedure
In the first phase, 100 ml of water was heated to the temperature of 90 °C and 60 g of bentonite clay was added. After intensive stirring, obtained liquid was concentrated by addition of 5 g NaOH. In the second phase, in easy flowable gel that was previously obtained by stirring activated carbon, Glauber’s salt, paraffin and stearic acid were added in appropriate amounts. In the last phase after the intensive stirring, easily mobile and homogeneous thixotropic gel was obtained, in which organic phase with the inorganic was emulsified.
2.3 Characterization of the Heterogeneous Gel
Heterogeneous gel in test tubes was heated in a temperature-regulated water bath to the temperature of 95 °C, and after completely melting the samples, the test tubes were removed from the hot water bath and left in an isolating vessel made of polystyrene (isolation diameter was 10 cm) which is shown in Fig. 2. All off experiments were done at ambient temperature for samples cooling to the room temperature. A temperature loss is measured every 60 min for a period of 9 h. In order to analyze the cooling behavior and the cooling degree of each sample, graphs of temperature changes during the time were plotted.
3 Results and Discussion
Results indicate a proportional decreasing of cooling speed with increasing of mass of Glauber’s salt in heterogeneous gel. At the relative ratio of Glauber’s salt and stearic acid of 1:2, the temperature storage effect is lower for about 5 to 10% compared to the 2:1 ratio in favor of Glauber’s salt (Figs. 1 and 2). According to results, Glauber’s salt is important for cooling speed decrease. Based on the obtained results it can be concluded that Glauber’s salt amount increasing may affect more favorable conditions for the heat storage.
Results of the stearic acid influence on temperature changes of heterogeneous gel are presented in a Fig. 3. Obtained results indicate that stearic acid has effect on cooling of heterogeneous gel, but that effect is not significant. Trend of temperature decreasing at stearic acid concentrations of 5, 10 and 15 g is almost identical, while at the highest stearic acid concentration (20 g) cooling trend of heterogeneous gel is lower compared to the previous investigated concentrations [34].
Figure 1(a) and (b) shows the phase transitions with subcooling, which is reduced by the introduction of expanded clay. The gel based on expanded clay with water is high pH value. It provides a mild phase transition without subcooling [33].
Generally, thermal conductivity increase has positive influence on thermal storage properties of composite material. Namely, composite material will be effective as thermal storage material if during one cycle is fully charged (all PCM should melt) and then fully uncharged (all PCM should solidify), while the process of charging and discharging depends on the thermal conductivity of the composite. Clay activation by alkali in mixture with water creates a gel that has strong thixotropic properties. With minimal mixing, the gel becomes a fluid and easily maneuverable, that after a while becomes solid consistency. In this combination the presence of water induces primarily high specific heat [15]. It is known that water has a high coefficient of conduction and high specific heat, which is one of the preconditions for a high heat mixture capacity and high thermal conductivity. Additionally, increased thermal conductivity could be also achieved by clay particles presence in the mixture.
The influence of Glauber’s salt on heterogeneous gel temperature changes is presented in Fig. 4. Increasing the amount of Glauber’s salt is a longer period of heat transfer at a temperature of 50 °C (Fig. 4). This should be expected because the latent heat of the melting (vaporization) of the Galuber’s salt is greater than the latent heat of stearic acid melting [23].
In order to improve the thermodynamic properties in nanofluid gel, activated carbon was added. Besides the investigation of stearic acid and Glauber’s salt influence, effect of activated carbon on temperature changes was also investigated. It can be noted that a sample of heterogeneous gel with active carbon has proven to be more effective because of a slower cooling system for heat storage. For nine hours the heterogeneous gel temperature dropped from 95 to 48.2 °C, which is for about 10 °C higher compared to the heterogeneous gel in which activated carbon was not present (Fig. 5).
Activated carbon influence on temperature changes in heterogeneous gel. Heterogeneous gel composition: (A) 100 g of water, 60 g of clay, 20 g of Glauber’s salt, 5 g of NaOH, 10 g of stearic acid, 10 g of paraffin wax and 5 g of borax and; (B) 100 g of water, 60 g of clay, 20 g of Glauber’s salt, 5 g of NaOH, 10 g of stearic acid, 10 g of paraffin wax, 5 g of borax, and 10 g of activated carbon.
Activated carbon adsorbs water from salt and paraffin, which affects the reduction of vaporization energy, is significantly higher than the solubility of salt or paraffin. Activated carbon can affect the ratio of energy vaporization to the melting energy of the composite [3, 16]. As the mixture is being mixed and water is being added, a point is reached at which the water is sufficient to wet all the particles. Continued mixing compacts the powder, removing air and the compacted powder starts to agglomerate into lumps. Continued mixing resulted in these lumps becoming plastic. Excess water makes these lumps too soft for the forming process. Clays have been used as binders in carbon mixtures to impart strength to the carbon body formed there from. Typically, water contents of about 140% to about 180%, and more typically about 145% to about 160% based on the carbon and clay content impart good plasticity and handle ability to the mixture [27].
Active powder carbon is suitable in a mixture with water and clay, which can absorb gases at elevated temperatures close to 100 °C, thereby maintaining the volume of the same constant [10, 27].
Besides, the thermal conductivity in molten state was larger than that in solid state. It also indicated that the activated carbon was favorable to form carbonaceous layers, which created a physical protective barrier on the surface of the composites. The protective barrier could confine the transfer of flammable molecules to the gas phase, and thus the thermal stability of the composites was improved [7, 35].
For hydrocarbon adsorption applications, the preferred type of activated carbon is what is considered to be a collection of very small graphitic platelets which are bound together with an open structure leading to high surface area [5].
During phase changing, the solid-liquid interface moves away from the heat transfer surface, and the heat transfer resistance gradually increases due to the increased thickness of the molten/solidified medium. By introducing an expanded gel with bentonite clay, this effect is significantly reduced [36].
4 Conclusion
In this work, we managed to homogenize a heterogeneous mixture of inorganic and organic PCMs agents. In the homogeneous bentonite clay gel, 20–25% PCM was added. This gel contains about 50% water that significantly affects heat increase of the specific mixture. Obtained gel based on clay allows continuous temperature change and heat transfer, while Glauber’s salt, stearic acid and paraffin caused the latent heat of phase at different temperature intervals.
Expanded gel based on bentonite clay is a carrier by which the addition of PCMs can be used to change thermodynamic properties. Composite based on expanded bentonite and clay and PCMs has significantly higher specific heat from the mixture of PCMs. Addition of activated carbon in gel provides additional system homogenization and increased accumulation of heat. In addition, activated carbon significantly reduced the cooling of the gel, which indicates further investigation of activated carbon concentration increase in the future. It is also possible to insert micronized particles of some metal or salt in order to increase the thermal capacity of the system and to allow faster heating and cooling of the commercially available material.
The present-day composite gel based clay, active carbon and stearic acid gel is very suitable for thermotherapy in medicine packaged in elastic polyethylene bags that will be heated in a microwave oven. Due to its heterogeneous composition and possible corrosive properties, this composite should be encapsulated in polymeric capsules, tubes, spheres or some other geometric shapes.
References
Stojiljković, S.T., Todorović, B.Ž.: The adsorption-desorption power of bentonite based materials. LAMBERT Academic Publishing, Saarbrücken (2016)
Sadek, O.M., Mekhemer, W.K.: Na-montmorillonite clay as thermal energy storage material. Thermochim. Acta 370, 57–63 (2001)
Stojiljković, S., Savić, I., Mitković, P., Vasić, L., Marinković, A.: An urban planning approach to the climatization of space using natural resources based on ceramic clay, zeolite and bentonite clay. Sci. Sinter. 46(2), 259–268 (2014)
Al-Kayiem, H.H., Lin, S.C., Lukmon, A.: Review on nanomaterials for thermal energy storage technologies. Nanosci. Nanotechnol. Asia 3, 60–71 (2013)
Wuttig, M., Steimer, C.: Phase change materials: from material science to novel storage devices. Appl. Phys. A-Mater. Sci. Process. 87(3), 411–417 (2007)
Stojiljkovic, S., Miljkovic, V., Nikolic, G., Kostic, D., Arsic, B., Barber, J., Savic, I., Savic, I.: The influence of the addition of polymers on the physico-chemical properties of bentonite suspensions. Sci. Sinter. 46(1), 65–73.44 (2014)
Cao, L.: Properties evaluation and applications of thermal energy storage materials in buildings. Renew. Sustain. Energy Rev. 48, 500–522 (2015)
Todorović, B.Ž., Stojiljković, S.T., Stojiljković, D.T., Petrović, S.M., Takić Lj, M., Stojiljković, M.S.: Removal of As3+ cations from water by activated carbon, bentonite and zeolite in a batch system at different pH. J. Elementol. 22(2), 713–723 (2017)
Stojiljkovic, S.: Role of bentonite clay in the ecology of the human body. In: XIV International Conference on Medical Geology (GEOMED), pp. 20–25 (2011)
Veniale, F.: The role of microfabric in clay soil stability. Mineral. Petrogr. Acta 29, 101–119 (1985)
Chen, Z., Shan, F., Cao, L., Fang, G.Y.: Synthesis and thermal properties of shape stabilized lauric acid/activated carbon composites as phase change materials for thermal energy storage. Solar Energy Mater Solar Cells 102, 131–136 (2012)
Hamdan, M.A., Al-Hinti, I.: Analysis of heat transfer during the melting of a phase-change material. Appl. Therm. Eng. 24(13), 1935–1944 (2004)
Li, Z.B.: Paraffin/diatomite/multi-wall carbon nanotubes composite phase change material tailormade for thermal energy storage cement-based composites. Energy 72, 371–380 (2014)
Prekajski, M., Mirković, M., Todorović, B., Matković, A., Marinović-Cincović, M., Luković, J., Matović, B.: Ouzo effect – new simple nanoemulsion method for synthesis of strontium hydroxyapatite nanospheres. J. Eur. Ceram. Soc. 36(5), 1293–1298 (2016)
Mondal, S.: Phase change materials for smart textiles – an overview. Appl. Therm. Eng. 28, 1536–1550 (2008)
Nikolić, L., Ristić, I., Stojiljković, S., Vuković, Z., Stojiljković, D., Nikolić, V., Budinski-Simendić, J.: The influence of montmorillonite modification on the properties of composite material based on poly(methacrylic acid). J. Compos. Mater. 46, 921–928 (2012)
Wang, S.X., Li, Y., Hu, J.Y., Tokura, H., Song, Q.W.: Effect of phase change material on energy consumption of intelligent thermal protective clothing. Polym. Test. 25(5), 580587 (2006)
Li, Y.: The science of clothing comfort. Text. Prog. 31, 1–135 (2001)
Shukla, N., Fallahi, A., Kosny, J.: Performance characterization of PCM impregnated gypsum board for building applications. Energy Procedia 30, 370–379 (2012)
Iten, M., Liu, S., Shukla, A.: Renew. Sustain. Energy Rev. 61, 175–186 (2016)
Sari, A.: Thermal energy storage characteristics of bentonite-based composite PCMs with enhanced thermal conductivity as novel thermal storage building materials. Energy Convers. Manag. 117, 132141 (2016)
Sharma, A., Tyagi, V.V., Chen, C.R., Buddhi, D.: Review on thermal energy storage with phase change materials and applications. Renew. Sustain. Energy Rev. 13(2), 318–345 (2009)
Maciej, J., Shady, A.: Thermal conductivity of gypsum with incorporated phase change material (PCM) for building applications. J. Power Technol. 91(2), 49–53 (2011)
Zastawna-Rumin, A.: Phase change materials vs. internal temperature in a building. Techn. Trans. 111(8-A), 207–213 (2014)
Pan, L., Tao, Q., Zhang, S., Wang, S., Zhang, J., Wang, S., Wang, Z., Zhang, Z.: Preparation, characterization and thermal properties of microencapsulated phase change materials. Sol. Energy Mater. Sol. Cells 98, 6670 (2012)
Marín, J.M., Zalba, B., Cabeza, L.F., Mehling, H.: Improvement of a thermal energy storage using plates with paraffin–graphite composite. Int. J. Heat Mass Transfer 48, 2561–2570 (2005)
Karaipekli, A., Sarı, A., Kaygusuz, K.: Thermal conductivity improvement of stearic acid using expanded graphite and carbon fiber for energy storage applications. Renew Eng. 32, 2201–2210 (2007)
Memon, S.A., Cui, H.Z., Zhang, H., Xing, F.: Appl. Energy 139, 43–55 (2015)
Cabeza, L.F., Barreneche, C., Martorell, I., Miró, L., Sari-Bey, Fois S.M., Paksoy, H.O., Sahan, N., Weber, R., Constantinescu, M., Anghel, E.M., Malikova, M., Krupa, I., Delgado, M., Dolado, P., Furmanski, P., Jaworski, M., Haussmann, T., Gschwander, S., Fernández, A.I.: Unconventional experimental technologies available for phase change materials (PCM) characterization. Part 1. Thermophysical properties. Renew. Sustain. Energy Rev. 43, 1399–1414 (2015)
Gschwander, S.: Standardization of PCM characterization via DSC. In: The 13th international Conference on Energy Storage, Greenstock, Beijing (2015)
De Garcia, A., Cabeza, L.F.: Phase change materials and thermal energy storage for buildings. Energy Build. 104, 414–419 (2015)
Stojiljkovic, S., Miljkovic, V., Nikolic, G., Kostic, D., Arsic, B., Barber, J., Savic, I.: The influence of the addition of polymers on the physico-chemical properties of bentonite suspensions. Sci. Sinter. 46(1), 65–73 (2014)
Soares, N.M.L.: (2016) Thermal energy storage with phase change materials (PCMs) for the improvement of the energy performance of buildings. Tese de doutoramento, Coimbra. Disponível na. www: http://hdl.handle.net/10316/29306
Lamberg, P.: Approximate analytical model for two phase solidification problem in a finned phase-change-material storage. Appl. Energy 77, 131–152 (2004)
Li, B.X., Liu, T.X., Hu, L.Y., Wang, Y.F., Nie, S.B.: Facile preparation and adjustable thermal property of stearic acid–graphene oxide composite as shapestabilized phase change material. Chem. Eng. J. 215, 819–826 (2013)
Stojiljković, S., Savić, I., Mitković, P., Vasić, L., Marinković, A.: An urban planning approach to the climatization of space using natural resources based on ceramic clay, zeolite and bentonite clay. Sci. Sinter. 46(2), 259–268 (2014)
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this paper
Cite this paper
Stojiljkovic, M., Stojiljkovic, S., Todorovic, B., Reljic, M., Savić, S., Petrovic, S. (2019). Thermal Energy Storage of Composite Materials Based on Clay, Stearic Acid, Paraffin and Glauber’s Salt as Phase Change Materials. In: Mitrovic, N., Milosevic, M., Mladenovic, G. (eds) Experimental and Numerical Investigations in Materials Science and Engineering. CNNTech CNNTech 2018 2018. Lecture Notes in Networks and Systems, vol 54. Springer, Cham. https://doi.org/10.1007/978-3-319-99620-2_3
Download citation
DOI: https://doi.org/10.1007/978-3-319-99620-2_3
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-99619-6
Online ISBN: 978-3-319-99620-2
eBook Packages: EngineeringEngineering (R0)